Highlights
-
•
Adolescents with asthma have similar aerobic fitness to their non-asthma peers.
-
•
High-intensity interval training may prevent increases in body mass index.
-
•
High-intensity interval training is equally effective at increasing aerobic fitness, regardless of asthma status.
Keywords: Body mass index, Cardiorespiratory fitness, Intermittent exercise, Intervention, Quality of life
Abstract
Background
Higher levels of cardiorespiratory fitness are associated with reduced asthma severity and increased quality of life in those with asthma. Therefore, the purpose of this study was to evaluate the effectiveness of a 6-month high-intensity interval training (HIIT) intervention in adolescents with and without asthma.
Methods
A total of 616 adolescents (334 boys; 13.0 ± 1.1 years, 1.57 ± 0.10 m, 52.6 ± 12.9 kg, mean ± SD), including 155 with asthma (78 boys), were recruited as part of a randomized controlled trial from 5 schools (4 control and 1 intervention). The 221 intervention participants (116 boys; 47 asthma) completed 6 months of school-based HIIT (30 min, 3 times per week, 10–30 s bouts at >90% age-predicted maximum heart rate with equal rest). At baseline, mid-intervention, post-intervention, and 3-month follow-up, measurements for 20-m shuttle run, body mass index (BMI), lung function, Pediatric Quality of Life Inventory, Paediatric Asthma Quality of Life Questionnaire, and Asthma Control Questionnaire were collected. Additionally, 69 adolescents (39 boys (of the 36 with asthma there were 21 boys)) also completed an incremental ramp test. For analysis, each group's data (intervention and control) were divided into those with and without asthma.
Results
Participants with asthma did not differ from their peers in any parameter of aerobic fitness, at any time-point, but were characterized by a greater BMI. The intervention elicited a significant improvement in maximal aerobic fitness but no change in sub-maximal parameters of aerobic fitness, lung function, or quality of life irrespective of asthma status. Those in the intervention group maintained their BMI, whereas BMI significantly increased in the control group throughout the 6-month period.
Conclusion
HIIT represents an effective tool for improving aerobic fitness and maintaining BMI in adolescents, irrespective of asthma status. HIIT was well-tolerated by those with asthma, who evidenced a similar aerobic fitness to their healthy peers and responded equally well to a HIIT program.
Graphical abstract
1. Introduction
The prevalence of asthma and obesity have both increased dramatically over the past few decades, making them two of the most common chronic conditions in the UK.1, 2 This concomitant increase has led to suggestions that the 2 conditions may be causatively linked,3, 4 with overweight and obesity more prevalent in those who suffer from asthma.5 Cardiorespiratory fitness has been suggested to be a key influential factor in the relationship between asthma and obesity,6 although the nature and extent of this influence remains to be elucidated. Indeed, the influence of asthma on cardiorespiratory fitness requires clarification, with little consensus currently available in the literature.5,7, 8, 9, 10 These equivocal findings may be attributable, at least in part, to the exercise testing methodologies used to determine cardiorespiratory fitness. Specifically, some studies reporting a lower aerobic fitness in those with asthma have used indirect estimates obtained from tests such as the 20-m shuttle run test.11, 12 Recent reports have highlighted the limitations associated with this measure,13 issues that may be exacerbated in those with asthma given the commonly cited fear of exercise-induced bronchoconstriction,14 leading to erroneous conclusions with regard to the pathophysiological influence of asthma. It is also pertinent to note the exclusive focus on peak oxygen uptake (VO2) in earlier studies concerning the influence of asthma on aerobic fitness. Whilst VO2 is accepted as a strong prognostic tool in many clinical conditions,15 it lacks direct applicability to many everyday functional abilities.
In addition to providing improvements in fitness,16, 17, 18 exercise may elicit additional health benefits in those with asthma, such as reduced symptoms and severity and an improved quality of life.19, 20, 21 Specifically, a higher level of aerobic fitness in children is associated with a better quality of life,22 while a greater body mass index (BMI) is related to a poorer quality of life.23, 24 Therefore, these measures should be targeted in future exercise interventions aimed at improving a population's quality of life. However, whilst adolescents with asthma have identified exercise as one of their favorite activities,14 few adolescents actively engage in exercise on a regular basis.5 This finding may be attributable to the use of conventional, moderate-intensity, continuous exercise in previous exercise interventions in children with asthma.20, 25 Winn et al.14 recently reported that adolescents with asthma prefer varied exercises, such as circuits or team games, with apprehension expressed towards long-distance running. Indeed, such variation would avoid monotony during sessions, which is associated with increased dropout rates.26
High-intensity interval training (HIIT) has received considerable attention in recent years because it has been identified as a time-efficient method of exercise that can elicit significant improvements in both cardiorespiratory fitness and body composition in youth.27, 28 Given the potential relationship between asthma, obesity, and fitness and the decreased likelihood of exercise-induced bronchoconstriction owing to its intermittent nature,29 HIIT represents a promising management strategy for those with asthma. However, it is important to acknowledge that some studies have raised concerns regarding the safety of HIIT, suggesting that it may be an inappropriate exercise modality for non-athlete populations.30 In contrast with these concerns, children with asthma have previously been reported to tolerate HIIT similarly to their healthy peers.16, 31 Furthermore, whilst comparable data is not available in youth with asthma, healthy children and adolescents perceive HIIT as being more enjoyable to participate in compared with constant-intensity exercise,32 with enjoyment a key component in eliciting the effort required for reaching high intensities.33 Indeed, in adults with asthma, interval exercise is associated with lower ratings of perceived exertion and dyspnea, which is likely due to the rest periods.34 Whether HIIT is similarly perceived to be enjoyable among adolescents with asthma remains to be elucidated, and the debate will continue regarding whether HIIT is associated with feelings of considerable discomfort that would prevent long-term adherence.35
Therefore, the aim of the present study was to ascertain the effectiveness of 6-month, field-based HIIT intervention in adolescents with asthma compared with their healthy peers. Furthermore, a secondary aim of this study was to use a 3-month follow-up to determine the sustainability of any adaptations elicited by the intervention. It was hypothesized that HIIT would lead to improvements in cardiorespiratory fitness and quality of life and a decrease in BMI in adolescents, irrespective of asthma, but that these beneficial adaptations would be lost within 3 months after the intervention cessation. This study is the first to implement a HIIT intervention in adolescents with asthma, which may be less monotonous than traditional continuous intensity exercise. If no differences are evident between participants with and without asthma, this finding will aid in informing future interventions for those with asthma and to decrease stigmatization and exclusion of those with asthma from everyday activities. Moreover, identifying a nonpharmacological intervention to reduce asthma symptoms and improve control and quality of life would be valuable.
2. Methods
2.1. Experimental design
The eXercise for Asthma with Commando Joe's® (X4ACJ) program used in this study was a randomized controlled trial. Cluster randomization was used to select 1 intervention and 4 control schools in South Wales, matched for free school meal status. The exercise intervention began at the start of the school year in September and ended in March, with data collection continuing to July. Ethical approval was granted by Swansea University Medical School and the College of Engineering research ethics committees (Ref: 140515 and PG/2014/29). Parent/guardian and head teacher written consent, in addition to child written assent, were obtained before participation.
2.2. Participants
To calculate the number of participants required to power the intervention study, the Paediatric Asthma Quality of Life Questionnaire was used as the primary outcome variable. It was calculated that, to achieve 80% power with an effect size of 0.5, which is deemed the minimal change considered clinically significant (confidence level 0.05), a sample of 132 participants would be required. Owing to the prevalence of asthma and the pragmatic nature of only being able to conduct the intervention in 1 school, 44 participants with asthma were required for the intervention, with the remaining 88 with asthma required as controls. To increase the statistical power of the study, 2 healthy participants were sought to be recruited for every adolescent with asthma from both the intervention and the control schools. For the more sensitive subsample measures, to achieve an 80% power and 0.05 confidence level, 8 participants were required in each group. In total, 616 adolescents (334 boys; Table 1), of which 155 had asthma (78 boys), agreed to participate in the study. A total of 221 participants (116 boys) were recruited from the intervention school, of which 47 suffered from asthma (24 boys). Asthma severity was assessed using the Global Initiative for Asthma guidelines36 and was classified as mild, moderate, or severe according to the medication step required to achieve asthma control. For the purpose of analysis, moderate and severe asthma were grouped to power the statistics. Participants were excluded if they did not have stable asthma (n = 3), if the participant had been admitted to hospital owing to their asthma in the last 6 weeks, visited their doctor because of their asthma becoming worse in the last 3 weeks, had a severe attack of asthma owing to exercise, or if they had ever been admitted to intensive care because of their asthma.
Table 1.
Anthropometric measures for participants within intervention and control for asthma and without asthma.
| Asthma |
Non-asthma |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Baseline | Mid-intervention | Post-intervention | Follow-up | n | Baseline | Mid-intervention | Post-intervention | Follow-up | |
| All | ||||||||||
| Age (year) | 155 | 13.0 ± 1.1 | 13.2 ± 1.1 | 13.5 ± 1.1 | 13.8 ± 1.1 | 461 | 13.0 ± 1.1 | 13.3 ± 1.1 | 13.6 ± 1.1 | 13.9 ± 1.1 |
| Stature (cm) | 100 | 157.4 ± 9.5 | 159.0 ± 9.7 | 160.7 ± 9.5 | 161.7 ± 9.5 | 255 | 157.9 ± 10.1 | 159.5 ± 10.0 | 161.1 ± 9.8 | 162.0 ± 9.8 |
| Body mass (kg) | 99 | 54.2 ± 14.0 | 55.4 ± 14.3 | 57.3 ± 14.7 | 58.2 ± 15.0 | 255 | 50.4 ± 12 | 51.5 ± 12.3 | 53.4 ± 12.4 | 54.3 ± 12.6 |
| Sitting stature (cm) | 99 | 78.9 ± 4.8 | 87.6 ± 74 | 81.2 ± 4.9 | 81.8 ± 4.8 | 255 | 79.5 ± 5.6 | 80.3 ± 8.6 | 81.3 ± 5.6 | 82.1 ± 5.5 |
| BMI (kg/m2) | 99 | 21.7 ± 4.4 | 21.7 ± 4.4 | 22.0 ± 4.4 | 22.1 ± 4.5# | 256 | 20.0 ± 3.4* | 20.0 ± 3.4 | 20.4 ± 3.4 | 20.5 ± 3.5# |
| Tanner stage | 93 | 3.2 ± 1.0 | 3.4 ± 1.0 | 3.6 ± 0.9 | 3.8 ± 0.9 | 242 | 3.3 ± 1.0 | 3.6 ± 1.0 | 3.7 ± 1.0 | 3.9 ± 0.9 |
| Age from PHV (year) | 98 | –0.1 ± 1.3 | 0.6 ± 1.3 | 0.4 ± 1.2 | 0.6 ± 1.2 | 255 | –0.2 ± 1.4 | 0.6 ± 1.4 | 0.3 ± 1.4 | 0.6 ± 1.4 |
| Intervention | ||||||||||
| Age (year) | 50 | 13.1 ± 1.0 | 13.4 ± 1.0 | 13.7 ± 1.0 | 14.0 ± 1.0 | 171 | 13.2 ± 1.1 | 13.5 ± 1.1 | 13.8 ± 1.1 | 14.1 ± 1.1 |
| Stature (cm) | 29 | 158.3 ± 10.5 | 160.1 ± 10.8 | 161.8 ± 10.9 | 163.1 ± 10.7 | 95 | 157.4 ± 9.9 | 159.1 ± 10.0 | 160.6 ± 9.9 | 161.9 ± 9.8 |
| Body mass (kg) | 28 | 57.4 ± 15.7 | 58.1 ± 15.8 | 59.8 ± 16.6 | 61.4 ± 17.0 | 95 | 51.3 ± 13.0 | 52.0 ± 13.2 | 53.7 ± 13.6 | 55.3 ± 13.8 |
| Sitting stature (cm) | 29 | 79.9 ± 4.8 | 80.9 ± 5.1 | 82.3 ± 5.3 | 83.2 ± 5.1 | 95 | 79.3 ± 5.7 | 80.0 ± 5.7 | 81.3 ± 5.8 | 82.2 ± 5.7 |
| BMI (kg/m2) | 28 | 22.6 ± 4.6 | 22.3 ± 4.5 | 22.5 ± 4.6 | 22.7 ± 4.7# | 95 | 20.4 ± 3.4* | 20.3 ± 3.5 | 20.6 ± 3.6 | 20.8 ± 3.7# |
| Tanner stage | 29 | 3.5 ± 1.1 | 3.4 ± 1.2 | 3.6 ± 1.0 | 3.9 ± 0.9 | 94 | 3.3 ± 1.2 | 3.6 ± 1.1 | 3.6 ± 1.0 | 3.9 ± 1.0 |
| Age from PHV (year) | 28 | 0.0 ± 1.2 | 0.2 ± 1.1 | 0.5 ± 1.3 | 0.8 ± 1.2 | 95 | –0.2 ± 1.4 | 0.1 ± 1.1 | 0.3 ± 1.4 | 0.6 ± 1.4 |
| Control | ||||||||||
| Age (year) | 105 | 12.9 ± 1.2 | 13.2 ± 1.2 | 13.4 ± 1.2 | 13.7 ± 1.2 | 290 | 12.9 ± 1.0 | 13.2 ± 1.0 | 13.5 ± 1.0 | 13.8 ± 1.0 |
| Stature (cm) | 71 | 157.0 ± 9.1 | 158.5 ± 9.2 | 160.3 ± 9.0 | 161.1 ± 8.9 | 160 | 158.2 ± 10.2 | 159.7 ± 10.1 | 161.3 ± 9.8 | 162.0 ± 9.8 |
| Body mass (kg) | 71 | 53.0 ± 13.2 | 54.3 ± 13.7 | 56.3 ± 13.9 | 56.9 ± 14.0 | 160 | 49.9 ± 11.4 | 51.2 ± 11.7 | 53.2 ± 11.7 | 53.7 ± 11.9 |
| Sitting stature (cm) | 70 | 78.5 ± 4.8 | 80.2 ± 4.9 | 80.7 ± 4.7 | 81.2 ± 4.6 | 160 | 79.6 ± 5.5 | 79.7 ± 6.6 | 81.3 ± 5.5 | 82.0 ± 5.4 |
| BMI (kg/m2) | 71 | 21.4 ± 4.4 | 21.5 ± 4.3 | 21.8 ± 4.4# | 21.8 ± 4.4# | 161 | 19.8 ± 3.3* | 19.9 ± 3.3 | 20.3 ± 3.4# | 20.4 ± 3.5# |
| Tanner stage | 64 | 3.0 ± 0.9 | 3.4 ± 1.0 | 3.6 ± 0.9 | 3.8 ± 0.9 | 148 | 3.4 ± 1.0 | 3.6 ± 1.0 | 3.7 ± 1.0 | 3.9 ± 0.9 |
| Age from PHV (year) | 70 | –0.2 ± 1.3 | 0.2 ± 1.4 | 0.3 ± 1.2 | 0.5 ± 1.2 | 160 | –0.2 ± 1.4 | 0.3 ± 1.5 | 0.3 ± 1.4 | 0.5 ± 1.4 |
Note: Data are presented as n or mean ± SD as indicated.
Abbreviations: BMI = body mass index; PHV = peak height velocity.
p < 0.05, significantly different from Asthma at baseline.
p < 0.05, significantly different compared with baseline.
2.3. Intervention
The intervention design was devised based on formative work.14 The intervention consisted of a 6-month HIIT program, delivered by a Commando Joe's® personal trainer, involving 30-min sessions 3 times per week (Monday, Wednesday, and Friday). Participants were able to attend sessions before or after school but were asked to attend only 1 session per day. The sessions consisted of a combination of circuits and game-based activities (Table 2) lasting between 10 s and 30 s, followed by an equal period of rest (1:1 work-to-rest ratio). Throughout each exercise bout, participants were asked to exercise maximally, with exercise activities designed to elicit a heart rate (HR) of >90% of HR maximum (HRmax).37 HRmax was predicted according to Tanaka et al.,38 whose predictions have been validated for use in children and adolescents.39 During each session the participants’ HR was continuously monitored (Activio Sport; Activio AB, Stockholm, Sweden), and those who were not achieving the target HRs were individually encouraged to do so. Attendance and effort were further incentivized by a reward-based system whereby those who regularly engaged were entered into a prize drawing at the mid-intervention and end-of-intervention points. Those in the control group engaged in their usual day-to-day activities.
Table 2.
Detailed examples of exercises.
| Activity | Example exercises |
|---|---|
| Static exercises | Participants stood in a space where they conducted exercises. Examples: |
| • Burpees | |
| • Jumping jacks | |
| • Squats | |
| • Press-ups | |
| Obstacle course | Created using resources such as nets, benches, cones, and hurdles. Examples: |
| • Agility | |
| • Crawling | |
| • Jumping | |
| • Rolling | |
| Speed, agility, and quickness | Activities included fast paced, low-skill agility exercises such as: |
| • Zig-zag sprints | |
| • Slalom sprints | |
| • Hurdles | |
| • Shuttles | |
| • Fast feet ladder drills | |
| Linear sprints | Participants moved between each side of the hall. Movement was varied, that is, forward, backwards, and sideways. Variations to the sprints included: |
| • Bear crawls | |
| • Gorilla walks | |
| • Crab walks |
2.4. Procedures
Measurements were taken from both intervention and control groups at 4 time-points (baseline, mid-intervention, post-intervention, and 3-month follow-up) irrespective of condition.
2.4.1. Anthropometrics
Stature and body mass were measured according to the techniques outlined by the International Society for the Advancement of Kinanthropometry.40 Stature, sitting stature and waist circumference were measured to the nearest 0.1 cm (Seca 213; Seca GmbH, Hamburg, Germany) and body mass to the nearest 0.1 kg (Seca 876; Seca GmbH). BMI was subsequently calculated and grouped using age- and sex-specific child percentiles.41 Maturity offset was calculated according to Mirwald et al.,42 and lower limb length was calculated as the difference between stature and sitting stature
2.4.2. Lung function
Forced expiratory volume in 1 s (FEV1), forced vital capacity (FVC), FEV1/FVC ratio, peak expiratory flow, and forced expiratory flow between 25% and 75% of vital capacity (FEF25–75) was measured using a portable dry spirometer (Alpha Spirometer; Vitalograph Ltd., Buckingham, UK). Participants were asked to sit up straight, breathe in as deeply as possible, place their lips around the mouthpiece tube and, when they were instructed, “blow out” into the mouthpiece as hard and as fast as possible until no further air could be exhaled; this was explained and demonstrated before the test. Each participant was asked to complete 3 acceptable tests, defined as each exhalation being within 5% of the other two. The best of the 3 acceptable measurements was used, as recommended by the American Thoracic Society guidelines43 and by the standardized protocol.44 The best value was then expressed as a percentage of the age–sex–stature predicted value.45
2.4.3. Fractional exhaled nitric oxide (FeNO)
FeNO was measured before spirometric testing. The FeNO test was performed in accordance with the American Thoracic Society guidelines.46 Participants were asked to completely exhale and then inhale to total lung capacity through the device (NIOX MINO; Aerocrine AB, Solna, Sweden) before immediately exhaling for 10 s at 50 ± 5 mL/s (mean ± SD). Visual and audio cues were provided by the computer software throughout. One test was completed at all time-points except the 3-month follow-up. The final 3 s of exhalation were evaluated.
2.4.4. Asthma control
Asthma control was assessed using the Asthma Control Questionnaire (ACQ),47 which consists of 7 items focusing on reliever inhaler use and symptoms over the previous week and the participants’ FEV1 score. Items on the ACQ are scored from 0 to 6, with ACQ scores of ≤0.75 or ≥1.5 indicating well-controlled or poorly controlled asthma, respectively. The ACQ has been validated in children between the ages of 6 and 16 years47 and was found to be responsive to change in asthma control with a minimal important difference (MID) of 0.52 ± 0.45. Internal consistency for the ACQ, measured using Cronbach's α coefficients,48 was deemed acceptable (α: 0.73–0.82).
2.4.5. Asthma-related quality of life
The Paediatric Asthma Quality of Life Questionnaire (PAQLQ) was used to compare the asthma-specific quality of life between those in the intervention and the control groups, as well as to assess the changes over the course of the intervention. Specifically, the participants were asked to recall the previous week in response to 23 questions (scored on a Likert scale from 1 to 7), with a higher score indicative of a better asthma status. The PAQLQ questions are divided into 3 domains, including activity limitations (5 questions), symptoms (10 questions), and emotional function (8 questions), with a mean score for each domain and a total overall score. The PAQLQ has been validated in children between the ages of 6 and 16 years49 and was found to be responsive to change in quality of life with a MID of 0.5. Internal reliability for the PAQLQ was deemed excellent (α: 0.96–0.97).
2.4.6. Quality of life
The Pediatric Quality of Life Inventory (PedsQL) Teenager Report Version 4.050 was used to compare the perceived quality of life between those participants with and without asthma and to assess any changes throughout the intervention. The participants were asked to recall their previous week and answer questions accordingly. A widely validated measure in adolescents aged 12–18 years,51, 52, 53 the 23-item PedsQL consists of domains on the participants’ physical, emotional, social, and school functioning quality, with higher scores indicating a better quality of life. Internal reliability for the PedsQL was deemed excellent (α: 0.89–0.90).
2.4.7. Cardiorespiratory fitness
2.4.7.1. Twenty-meter shuttle run
Cardiorespiratory fitness was estimated using the 20-m progressive shuttle run test, a previously validated field measure in children.11 The test required participants to walk or run between 2 lines 20-m apart in time with pre-recorded beeps that progressively increased in speed throughout the test. The number of shuttles completed before voluntary exhaustion was recorded.
2.4.7.2. Peak VO2
A total of 69 adolescents (39 boys) inclusive of 36 with asthma (21 boys) were selected using stratified randomization to complete incremental ramp tests. The groups were stratified for age, sex, and condition to provide a representative sample of the wider population. Participants performed an incremental ramp exercise test to volitional exhaustion on an electromagnetically braked cycle ergometer (Ergoselect 200; Ergoline GmbH, Lindenstrasse, Germany), with seat and handlebar height individually adjusted for each participant. The ramp protocol consisted of 3 min of “unloaded” pedaling (0 watt (W)) followed by an increase in work rate of 12–24 W/min depending on the age and height of the participant. Participants were asked to maintain a constant cadence (75 ± 5 revolutions per min) until voluntary exhaustion. Breath-by-breath pulmonary ventilation and gas exchange (VO2 and VCO2) were recorded throughout (Oxycon Mobile; Jaeger GmbH, Hoechberg, Germany).
2.5. Data analysis
The peak VO2 was taken as the highest 10-s mean attained before the end of the test. The gas exchange threshold (GET) was determined using the V-slope method.54 The GET was also expressed relative to peak VO2 (GET%VO2). To account for the influence of body mass on peak VO2, data were log transformed and population-specific power function ratios calculated using analysis of covariance (ANCOVA). Breath-by-breath data were then averaged into 10-s time bins, and the mean response time (MRT) and gain (ΔVO2/ΔW) were calculated according to the methods reported by Barstow et al.55 Specifically, the gain was determined by linear regression over 3 segments: S1, from 1 min into the ramp to GET; S2, from GET to peak VO2; and ST, over the total range of S1 + S2. The baseline VO2 was taken as the mean of the first 45 s of the last minute before the increase in work rate. The MRT was calculated as the point of intersection between the baseline VO2 and a backwards linear extrapolation of the VO2 by time slope from the onset of the ramp protocol. The MRT was also determined using 2 segments, S1 (MRT1) and ST (MRTT).
2.6. Statistical analysis
Shapiro–Wilk tests were used to assess normality. After identification of normal distribution, the influence of asthma and the intervention, and their interaction, was assessed using a mixed-model analysis of variance (ANOVA) (groups were asthma intervention, non-asthma intervention, asthma control, and non-asthma control; time was collected as baseline, mid-intervention, post-intervention, and follow-up). Tukey's post hoc analyses were conducted to ascertain where differences in time were found. If significant differences were found, mixed-design ANCOVA tests were run to adjust for baseline maturity because this factor may in part explain any changes in parameters. Baseline maturity was used in the ANCOVA because the test did not allow for time-varying covariates. Asthma-specific measures were analyzed using repeated measures ANOVAs. Data presented within the tables include the number of participants providing data at every time-point; therefore, the number of participants differ between measurements. All analyses were conducted using an intention-to-treat approach, thus including all participants with measures at any time-point. Data were subsequently analyzed using sensitivity analyses on participants who participated in the majority of the intervention sessions (>70%). Eta-squared (ηp2) effect sizes were determined from baseline to 3-month follow-up. All statistical analyses were conducted using SPSS Version 22.0 (IBM Corp., Armonk, NY, USA). All data are presented as mean ± SD, with statistical significance accepted as p < 0.05.
3. Results
The participants with asthma in the intervention group consisted of 87.2% with mild persistent asthma and 12.8% with moderate or severe asthma. The percentages of participants with mild persistent asthma and moderate or severe asthma in the control group asthma population were 77.1% and 22.9%, respectively. These percentages were similar in both the intention-to-treat and sensitivity analyses. Where no differences between the intention-to-treat and sensitivity analyses were found, results refer to the results obtained from the intention-to-treat analysis. Furthermore, no differences were observed when covarying for maturity offset or Tanner stages and are therefore not reported in this article.
3.1. Lung function
Lower FEV1% and FEF25–75 values were found in participants with asthma at baseline, indicating more airway obstruction and more marked small airways obstruction, respectively. Those with asthma did not have an obstructed FEV1/FVC ratio. There were no changes in lung function over time in any group as highlighted by the mixed methods ANOVAs, which revealed no differences between intervention and control, asthma and non-asthma for lung function (p > 0.05), according to group or time or time-by-group interaction. There was, however, a trend for FeNO to reduce in the intervention asthma group (Table 3).
Table 3.
Lung function measures for participants within the intervention and control groups with asthma and without asthma.
| Asthma |
Non-asthma |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Baseline | Mid-intervention | Post-intervention | Follow-up | n | Baseline | Mid-intervention | Post-intervention | Follow-up | |
| Intervention | ||||||||||
| FEV1 (% predicted) | 29 | 93.5 ± 15.5 | 94.7 ± 14.1 | 92.2 ± 14.8 | 88.1 ± 15.8 | 90 | 93.9 ± 16.5* | 95.7 ± 13.8 | 97.1 ± 13.4 | 91.3 ± 15.4 |
| FVC (% predicted) | 29 | 102.4 ± 13.2 | 102.5 ± 13 | 100.4 ± 14.7 | 94.9 ± 17.7 | 90 | 99.5 ± 13.8 | 100.8 ± 13.1 | 101.5 ± 18.3 | 94.5 ± 16.4 |
| FEV1/FVC | 29 | 0.8 ± 0.1 | 0.8 ± 0.1 | 0.8 ± 0.1 | 0.8 ± 0.1 | 90 | 0.8 ± 0.1 | 0.8 ± 0.1 | 0.8 ± 0.1 | 0.8 ± 0.1 |
| FEF25–75 (% predicted) | 29 | 78.2 ± 28.0 | 81.7 ± 22.5 | 77.7 ± 31.3 | 77.1 ± 24.1 | 90 | 83.1 ± 23.8* | 86.9 ± 23.4 | 89.2 ± 26.7 | 85.2 ± 25.4 |
| PEF (% predicted) | 29 | 90.0 ± 18.5 | 89.5 ± 20.9 | 91.4 ± 19.1 | 85.7 ± 22.8 | 90 | 87.8 ± 19.8 | 90.0 ± 19.4 | 93.9 ± 17.9 | 88.5 ± 20.7 |
| FeNO (ppb) | 29 | 40.3 ± 31.2 | 38.5 ± 31.3 | 38.6 ± 27.4 | – | 0 | – | – | – | – |
| Control | ||||||||||
| FEV1 (% predicted) | 68 | 90.1 ± 14.7 | 87.9 ± 19.4 | 92.8 ± 18.4 | 91.7 ± 13.5 | 155 | 96.1 ± 17.2* | 90.8 ± 19.5 | 96.1 ± 14.3 | 97.1 ± 16.6 |
| FVC (% predicted) | 68 | 97.3 ± 14.6 | 96.0 ± 20.5 | 98.5 ± 20.1 | 97.0 ± 14.0 | 155 | 99.7 ± 15.0 | 92.9 ± 19.0 | 97.8 ± 13.7 | 98.1 ± 13.4 |
| FEV1/FVC | 68 | 0.8 ± 0.1 | 0.8 ± 0.1 | 0.8 ± 0.1 | 0.8 ± 0.1 | 155 | 0.8 ± 0.1 | 0.9 ± 0.1 | 0.9 ± 0.1 | 0.9 ± 0.1 |
| FEF25–75 (% predicted) | 68 | 75.6 ± 24.8 | 74.6 ± 29.0 | 79.0 ± 23.9 | 80.4 ± 21.3 | 155 | 88.2 ± 26.2* | 85.6 ± 26.2 | 91.9 ± 23.3 | 92.5 ± 24.5 |
| PEF (% predicted) | 68 | 93.0 ± 21.1 | 89.7 ± 25.1 | 91.0 ± 15.2 | 91.5 ± 17.9 | 155 | 91.1 ± 21.4 | 87.6 ± 24.1 | 92.8 ± 17.0 | 92.8 ± 18.9 |
| FeNO (ppb) | 70 | 43.6 ± 38.8 | 41.9 ± 39.7 | 41.4 ± 38.1 | – | 0 | – | – | – | – |
Note: Data are presented as n or mean ± SD as indicated.
Abbreviations: % predicted = expressed as a percentage of the age–sex–stature predicted value; FeNO = fractional exhaled nitric oxide; FEF25–75 = forced expiratory flow between 25% and 75% of vital capacity; FEV1 = forced expiratory volume in 1 s; FVC = forced vital capacity; PEF = peak expiratory flow; ppb = parts per billion.
p < 0.05, significantly different from Asthma at baseline.
3.2. Asthma control and quality of life
The intervention had no effect on asthma control or asthma-related quality of life. The MID for both the ACQ and PAQLQ was a change in score of 0.5. Both intervention and control asthma participants demonstrated similar results, with 33.4% and 35.1% scoring above the MID for the ACQ and 18.6% and 16.4% scoring above the MID for the PAQLQ. The results of the PedsQL revealed no significant differences between those with and without asthma in either the intervention or control group. The intervention was not associated with any significant change at any time-point in any of the groups (Table 4).
Table 4.
Twenty-meter shuttle run and quality of life questionnaire data for participants within the intervention and control groups with asthma and without asthma.
| Asthma |
Non-asthma |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Baseline | Mid-intervention | Post-intervention | Follow-up | n | Baseline | Mid-intervention | Post-intervention | Follow-up | |
| Intervention | ||||||||||
| 20-m shuttle run (shuttle) | 20 | 35.8 ± 21.2 | 38.7 ± 15.8 | 40.8 ± 22.3# | 39.4 ± 22.4 | 72 | 47.5 ± 24.0* | 53.6 ± 22.9# | 51.8 ± 24.1* | 47.5 ± 24.2 |
| PedsQL PhH | 22 | 76.8 ± 14.3 | 79.4 ± 13.8 | 83.8 ± 12.3 | 85.1 ± 13.0 | 85 | 81.6 ± 12.5 | 82.9 ± 11.9 | 83.7 ± 14.3 | 82.6 ± 12.9 |
| PedsQL PsH | 22 | 76.4 ± 11.8 | 74.6 ± 14.1 | 80.5 ± 15.1 | 82.0 ± 13.7 | 85 | 79.7 ± 14.9 | 76.4 ± 18.3 | 78.8 ± 17.9 | 79.0 ± 16.9 |
| PedsQL total | 22 | 76.5 ± 11.5 | 76.3 ± 12.3 | 81.6 ± 12.7 | 83.1 ± 12.3 | 85 | 80.3 ± 13.0* | 78.6 ± 14.6 | 80.5 ± 15.6 | 80.4 ± 14.4 |
| ACQ | 32 | 1.0 ± 0.9 | 1.2 ± 0.8 | 1.0 ± 0.7 | 1 ± 0.8 | 0 | — | — | — | — |
| PAQLQ symptoms | 29 | 5.6 ± 1.4 | 5.7 ± 1.3 | 6.0 ± 0.9 | 6.2 ± 1.2 | 0 | — | — | — | — |
| PAQLQ activities | 29 | 5.7 ± 1.4 | 5.9 ± 1.0 | 6.2 ± 0.9 | 6.2 ± 1.1 | 0 | — | — | — | — |
| PAQLQ emotions | 29 | 5.9 ± 1.4 | 6 ± 1.1 | 6.5 ± 0.8 | 6.5 ± 1.0 | 0 | — | — | — | — |
| PAQLQ | 29 | 5.7 ± 1.3 | 5.9 ± 1.1 | 6.2 ± 0.8 | 6.3 ± 1.0# | 0 | — | — | — | — |
| Control | ||||||||||
| 20-m shuttle run (shuttle) | 22 | 44.5 ± 21.9 | 44.6 ± 17.0 | 42.3 ± 20.4 | 42.6 ± 22.4 | 84 | 52.7 ± 26.0* | 52.4 ± 23.7 | 54.9 ± 24.0 | 54.8 ± 25.6 |
| PedsQL PhH | 62 | 75.1 ± 19.2 | 76.1 ± 17.4 | 80.3 ± 15.4# | 81.9 ± 14.8# | 159 | 79.5 ± 14.9 | 80.9 ± 14.0 | 82.1 ± 13.6 | 83.6 ± 13.0# |
| PedsQL PsH | 62 | 72.4 ± 19.2 | 72.2 ± 16.0 | 77.1 ± 14.3 | 80.5 ± 14.5# | 159 | 75.2 ± 16.9 | 76.1 ± 16.0 | 76.9 ± 15.2 | 80.5 ± 13.9# |
| PedsQL total | 62 | 73.3 ± 17.8 | 73.6 ± 15.1 | 78.2 ± 12.4# | 81.0 ± 13.2# | 159 | 76.7 ± 14.8* | 77.8 ± 14.1 | 78.7 ± 13.2 | 81.7 ± 12.3# |
| ACQ | 84 | 1.4 ± 1.0 | 1.2 ± 0.9 | 1.1 ± 0.9 | 0.9 ± 0.8# | 0 | — | — | — | — |
| PAQLQ symptoms | 69 | 5.5 ± 1.2 | 5.6 ± 1.3 | 5.9 ± 1.2# | 6.2 ± 1.1# | 0 | — | — | — | — |
| PAQLQ activities | 69 | 5.5 ± 1.2 | 5.7 ± 1.2 | 5.8 ± 1.3 | 6.2 ± 1.2# | 0 | — | — | — | — |
| PAQLQ emotions | 69 | 6.0 ± 1.2 | 6.0 ± 1.1 | 6.2 ± 1.1 | 6.3 ± 1.1 | 0 | — | — | — | — |
| PAQLQ | 69 | 5.7 ± 1.2 | 5.8 ± 1.1 | 6.0 ± 1.1 | 6.2 ± 1.1# | 0 | — | — | — | — |
Note: Data are presented as n or mean ± SD as indicated.
Abbreviations: ACQ = Asthma Control Questionnaire; PAQLA = Paediatric Asthma Quality of Life Questionnaire; PedsQL = Pediatric Quality of Life Inventory; PhH = Physical score; PsH = Psychological score.
p < 0.05, significantly different from Asthma at baseline.
p < 0.05, significantly different from baseline.
3.3. BMI
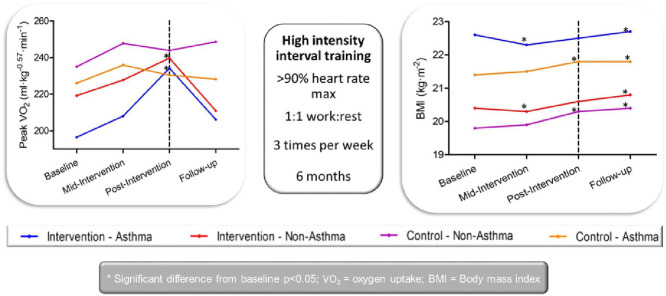
BMI was found to be significantly greater in participants with asthma at baseline in comparison with their peers (21.7 ± 4.4 kg/m2 vs. 20.0 ± 3.4 kg/m2). There was a significant effect of time on BMI (F(2.23, 782) = 15.4, p < 0.05, ηp2 = 0.04) and a significant difference between groups (F(3, 351) = 5.29, p < 0.05, ηp2 = 0.04), but no interaction between time and group (F(6.68, 782) = 1.16, p = 0.33, ηp2 = 0.01). Specifically, although the intervention participants maintained their baseline BMI to post-intervention, BMI in the control participants, both with and without asthma, increased throughout the intervention (asthma: 21.4 ± 4.4 kg/m2 to 21.8 ± 4.4 kg/m2 vs. non-asthma: 19.8 ± 3.3 kg/m2 to 20.3 ± 3.4 kg/m2, p < 0.05). At the 3-month follow-up, all groups (intervention and control, asthma and non-asthma) had significantly greater BMI than they had at baseline (Table 1).
3.4. Twenty-meter shuttle run
No significant effects were found for group or time and no interaction was observed between group and time for the 20-m shuttle run. However, when applying sensitivity analysis, there was a significant effect of time (F(3, 386) = 5.44, p < 0.05, ηp2 = 0.04) and a significant interaction of group by time (F(9, 386) = 3.23, p < 0.05, ηp2 = 0.06). Post hoc analyses revealed a significant increase in the number of shuttles completed in both asthma and non-asthma intervention participants with time, which returned to baseline at the 3-month follow-up (Table 4).
3.5. Incremental ramp test
A significant effect of time and interaction between time and the group was observed, with no significant effect of group on peak VO2. When scaled for body size, these differences were maintained with time (F(3, 138) = 8.47, p < 0.05, ηp2 = 0.16), group by time (F(9, 138) = 2.70, p < 0.05, ηp2 = 0.15), and group (F(3, 46) = 1.55, p = 0.22, ηp2 = 0.09). Post hoc analyses revealed significant increases in scaled peak VO2 in both asthma and non-asthma intervention groups, with 3-month follow-up results showing a return to baseline levels. No differences were observed in either the asthma or non-asthma control groups across the intervention for peak or scaled peak VO2 (Table 5).
Table 5.
Incremental ramp test results for participants within the intervention and control groups with asthma and without asthma.
| Asthma |
Non-asthma |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Baseline | Mid-intervention | Post-intervention | Follow-up | n | Baseline | Mid-intervention | Post-intervention | Follow-up | |
| Intervention | ||||||||||
| Baseline VO2(L/min) | 14 | 0.6 ± 0.1 | 0.6 ± 0.1 | 0.7 ± 0.1 | 0.6 ± 0.1 | 14 | 0.6 ± 0.1 | 0.6 ± 0.1 | 0.6 ± 0.1 | 0.5 ± 0.1 |
| Peak VO2 (L/min) | 14 | 2.0 ± 0.5 | 2.1 ± 0.6 | 2.5 ± 0.8* | 2.2 ± 0.6* | 14 | 2.1 ± 0.5 | 2.2 ± 0.6 | 2.3 ± 0.5* | 2.1 ± 0.5 |
| Scaled peak VO2 (mL/kg0.57/min) | 14 | 196.4 ± 41.1 | 208.0 ± 42.1 | 234.2 ± 48.5* | 206.1 ± 40.8 | 14 | 219.2 ± 29.6 | 227.7 ± 36.5 | 239.7 ± 31.0* | 210.9 ± 33.9 |
| GET (L/min) | 14 | 1.0 ± 0.3 | 1.1 ± 0.3 | 1.3 ± 0.4* | 1.3 ± 0.3* | 14 | 1.0 ± 0.2 | 1.2 ± 0.3* | 1.3 ± 0.3* | 1.2 ± 0.3 |
| GET (%VO2) | 14 | 51.5 ± 8.4 | 54.4 ± 4.5 | 55.0 ± 5.2 | 58.3 ± 5.0* | 14 | 50.5 ± 6.5 | 56.0 ± 6.8 | 56.9 ± 3.7* | 58.9 ± 3.5* |
| S1 gain (mL/min/W) | 14 | 8.3 ± 1.6 | 9.8 ± 2.3 | 9.6 ± 2.7 | 9.7 ± 2.6 | 14 | 10.1 ± 2.6 | 9.6 ± 1.9 | 9.5 ± 1.3 | 9.4 ± 1.5 |
| S2 gain (mL/min/W) | 14 | 10.0 ± 1.6 | 10.4 ± 2.2 | 11.4 ± 1.9* | 9.7 ± 1.8 | 14 | 10.9 ± 1.2 | 10.9 ± 1.5 | 11.2 ± 1.4 | 9.1 ± 1.8* |
| ST gain (mL/min/W) | 14 | 9.4 ± 1.5 | 10.0 ± 1.9 | 10.6 ± 1.4* | 9.6 ± 1.3 | 14 | 10.6 ± 1.1 | 10.4 ± 1.1 | 10.4 ± 1.1 | 9.1 ± 1.2* |
| MRT1 (s) | 14 | 69.6 ± 15.4 | 61.0 ± 17.9 | 65.1 ± 17.2 | 72.8 ± 13.7 | 14 | 72.8 ± 18.4 | 64.4 ± 13.3 | 61.5 ± 12.1 | 60.6 ± 13.1 |
| MRTT (s) | 14 | 69.8 ± 22.2 | 61.9 ± 21.1 | 74.7 ± 21.1 | 74.0 ± 9.9 | 14 | 78.0 ± 19.9 | 71.1 ± 21.4 | 66.8 ± 16.4 | 55.1 ± 17.3 |
| Time to exhaustion (min) | 14 | 9.9 ± 1.5 | 10.1 ± 1.7 | 9.8 ± 1.2 | 9.6 ± 1.4 | 14 | 9.8 ± 1.5 | 9.8 ± 1.7 | 9.5 ± 1.1 | 9.2 ± 1.0 |
| Peak HR (beat/min) | 14 | 193.0 ± 9.0 | 191.0 ± 9.0 | 191.0 ± 7.0 | 191.0 ± 11.0 | 14 | 196.0 ± 9.0 | 192.0 ± 8.0 | 192.0 ± 11.0 | 190.0 ± 7.0 |
| Control | ||||||||||
| Baseline VO2(L/min) | 10 | 0.6 ± 0.1 | 0.6 ± 0.1 | 0.6 ± 0.1 | 0.6 ± 0.1 | 12 | 0.6 ± 0.1 | 0.6 ± 0.1 | 0.6 ± 0.1 | 0.6 ± 0.1 |
| Peak VO2(L/min) | 10 | 2.2 ± 0.5 | 2.3 ± 0.6 | 2.3 ± 0.6 | 2.3 ± 0.5 | 12 | 2.2 ± 0.6 | 2.4 ± 0.7 | 2.4 ± 0.6 | 2.4 ± 0.7 |
| Scaled peak VO2 (mL/kg0.57/min) | 10 | 226.0 ± 39.4 | 235.9 ± 49.3 | 230.4 ± 43.0 | 228.2 ± 42.9 | 12 | 235.0 ± 47.0 | 247.7 ± 51.2 | 243.9 ± 43.9 | 248.6 ± 54.4 |
| GET (L/min) | 10 | 1.1 ± 0.2 | 1.3 ± 0.3 | 1.5 ± 0.3* | 1.4 ± 0.3* | 12 | 1.1 ± 0.4 | 1.4 ± 0.4* | 1.5 ± 0.4* | 1.4 ± 0.4* |
| GET (%VO2) | 10 | 52.5 ± 8.5 | 58.1 ± 8.2 | 63.3 ± 4.3* | 62.1 ± 3.8* | 12 | 50.4 ± 7.5 | 57.6 ± 4.4* | 62.4 ± 3.7* | 59.4 ± 6.9* |
| S1 gain (mL/min/W) | 10 | 9.4 ± 1.5 | 11.8 ± 2.4 | 10.1 ± 1.8 | 9.8 ± 0.7 | 12 | 8.4 ± 2.9 | 9.5 ± 1.1 | 9.8 ± 1.2 | 10.1 ± 1.9 |
| S2 gain (mL/min/W) | 10 | 12.3 ± 1.8 | 11.7 ± 1.4 | 10.5 ± 1.2 | 10.7 ± 2.1 | 12 | 11.5 ± 1.5 | 10.9 ± 1.9 | 11.0 ± 2.3 | 10.8 ± 2.3 |
| ST gain (mL/min/W) | 10 | 11.0 ± 1.0 | 11.5 ± 1.2 | 10.2 ± 1.0 | 10.2 ± 0.9 | 12 | 10.4 ± 1.7 | 10.3 ± 1.3 | 10.2 ± 1.0 | 10.2 ± 1.4 |
| MRT1 (s) | 10 | 72.3 ± 13.0 | 73.0 ± 19.6 | 54.9 ± 15.8 | 61.8 ± 8.6 | 12 | 78.3 ± 13.6 | 63.5 ± 15.6 | 60.0 ± 18.7 | 65.3 ± 7.4 |
| MRTT (s) | 10 | 80.0 ± 15.9 | 76.4 ± 16.1 | 64.9 ± 15.4 | 64.8 ± 13.1 | 12 | 85.6 ± 17.3 | 65.8 ± 19.0 | 62.4 ± 16.7 | 63.6 ± 10.5 |
| Time to exhaustion (min) | 10 | 9.9 ± 1.6 | 9.5 ± 0.8 | 9.7 ± 0.9 | 9.2 ± 1.1 | 12 | 10.6 ± 1.7 | 9.5 ± 1.1 | 9.9 ± 0.8 | 9.6 ± 0.9 |
| Peak HR (beat/min) | 10 | 194.0 ± 12.0 | 196.0 ± 10.0 | 192.0 ± 10.0 | 188.0 ± 11.0 | 12 | 191.0 ± 11.0 | 190.0 ± 8.0 | 191.0 ± 10.0 | 189.0 ± 10.0 |
Note: Data are presented as n or mean ± SD as indicated.
Abbreviations: GET = gas exchange threshold; HR = heart rate; MRT1 = mean response time for S1; MRTT = mean response time for S1 + S2; S1 gain = gain from 1 min into ramp to GET; S2 gain = gain from GET to peak VO2; ST gain = gain over the total range S1 + S2; VO2 = oxygen uptake; W = watt.
p < 0.05, significantly different from baseline.
There were no differences in GET between groups; however, there was a significant increase over time in all groups (F(2.23, 138) = 41.56, p < 0.05, ηp2 = 0.48). There was no significant between-group difference for GET as a percentage of peak VO2. Post hoc analyses showed significant increases at post-intervention for the non-asthma intervention group and for both the asthma and non-asthma control groups. However, inclusive of the asthma intervention group, all groups significantly increased GET from baseline to 3-month follow-up. A sensitivity analysis also showed that there were no significant increases throughout the intervention in GET%VO2 for participants in the non-asthma intervention group. There were no significant differences to either section of the MRT according to time, group, or time-by-group interaction across all time-points. The gain, however, was found to significantly increase in the intervention asthma group for both S2 and ST, with no significant differences observed in any of the other groups (Table 5).
3.6. Intervention intensity
Throughout the intervention sessions, exclusive of warm-up and cool-down, participants’ mean HR (155 ± 18 beats/min; 78%HRmax ± 9%HRmax) and mean HRmax (188 ± 18 beats/min; 95%HRmax ± 6%HRmax) were calculated for each session. During the main body of the sessions, inclusive of both the exercise and rest intervals, HR exceeded the threshold (>90%HRmax) for 24% of the total time.
3.7. Correlations
All measures were positively correlated with themselves between baseline and post-intervention, with the exception of the MRT and gain. A weak negative correlation was observed between BMI and fitness (r = –0.34, p < 0.05), quality of life (r = –0.11, p < 0.05), and lung function (r = –0.21, p < 0.05) at baseline, but only between BMI and fitness (r = –0.33, p < 0.05) at post-intervention. Fitness was also weakly correlated with quality of life (r = 0.26, p < 0.05) and lung function (r = 0.34, p < 0.05) at all time-points. However, scaled peak VO2 was not associated with quality of life or lung function (p > 0.05).
4. Discussion
This study was the first to evaluate the effectiveness of a 6-month field-based HIIT intervention in adolescents with asthma compared with their healthy peers. The main findings of this study were that (1) adolescents with asthma did not differ from their healthy counterparts in cardiorespiratory fitness at baseline, despite having a greater BMI, and (2) adolescents with asthma and their healthy peers demonstrated a similar response to the HIIT intervention. Specifically, HIIT elicited significant improvements in cardiorespiratory fitness and maintained BMI in adolescents, irrespective of whether participants had asthma. However, HIIT did not elicit significant improvements in lung function, asthma control, or quality of life. These findings have important implications for the design of future interventions for those with asthma, highlighting that those with asthma are able to tolerate, and benefit from, similar exercise stimuli recommended for their healthy counterparts. This study demonstrates the fallacy of the perception that adolescents with asthma should be excluded from exercise, including HIIT because they are unable to participate and keep up with their peers when involved in similar activities.56, 57
In accord with previous research and recent systematic reviews,27, 58 the present study found that HIIT elicited increases in cardiorespiratory fitness in adolescents. Specifically, in the overall population, 20-m shuttle run scores significantly improved, irrespective of condition, with no significant changes noted for the controls. Furthermore, both absolute and body size-scaled peak VO2 increased throughout the intervention, providing evidence of true physiological improvements in cardiorespiratory fitness. Interestingly, participants in the asthma intervention group increased their scaled VO2 to a greater extent than their non-asthma peers (19.2% vs. 9.4%) and increased it considerably more than previously reported through conventional training programs for healthy adolescents.59 This greater increase may be related to the (non-significantly) lower baseline fitness in those with asthma since baseline fitness has been reported to influence the magnitude of change elicited by an intervention in youth.60, 61, 62 Although improvements in peak VO2 after moderate-intensity exercise over a shorter time-frame16, 17, 63, 64 have been noted in those with asthma, the suitability of continuous exercise for those with asthma is questionable. Indeed, research has suggested that prolonged continuous exercise is not enjoyable26 and may trigger the onset of asthma symptoms,29 both of which are key barriers to exercise for those with asthma.14 Furthermore, traditional endurance training, which typically involves a greater time commitment than HIIT, may also be less appealing than the suggested HIIT format to time-poor adolescents.27 Importantly, the beneficial adaptations in the peak VO2 for those with asthma were sustained in the 3 months after intervention cessation. Although it is beyond the scope of the present study to ascertain whether this was because these participants maintained a higher level of exercise post-intervention, this finding is encouraging for the long-term efficacy of HIIT in adolescents with asthma.
In contrast with suggestions that submaximal parameters of aerobic fitness may demonstrate greater sensitivity to exercise stimuli than peak VO2, but in agreement with previous studies,65 the absolute GET was unaffected by the intervention in the present study, irrespective of asthma status. This finding may indicate that training above the GET for short intermittent periods is not an effective strategy for increasing the GET in youth. These apparent age- and/or maturation-related changes in the relative GET are in contrast to previous reports26; thus, further research that ascertains the influence of growth and maturation on the GET is required.
Like the GET, the MRT did not show significant improvement following HIIT, irrespective of condition. These findings are perhaps surprising in that HIIT involves repeated transitions from rest to vigorous-intensity exercise. The MRT in the present study was longer than previously reported in healthy children,66, 67 but did not differ between those with and without asthma. The longer MRT may reflect a lower level of aerobic fitness, although, given that aerobic fitness increased throughout the intervention with no concomitant speeding of the MRT, this explanation seems unlikely. The lack of effect of asthma in the present study is in contrast with the slower MRT reported in those with cystic fibrosis.68 This finding may be attributable to the different etiologies of the 2 diseases and their influences on exercise tolerance. However, it may also be related to the relatively mild asthma of the majority of the participants in the present study. Additional inter-study comparisons are precluded because the ramp rate of the incremental test, which differs significantly between studies, profoundly affects the MRT.69 Interestingly, there were no differences in the MRT between participants with and without asthma, suggesting that asthma does not impede the response to exercise.
In participants with asthma, the increase in gain observed over the intervention is suggestive of a positive adaptation in the delivery and utilization of oxygen by the muscles during exercise.70 Although no differences in gain were observed at baseline, it is of note that S2 and ST gain increased post-intervention for participants with asthma to levels similar to those reported elsewhere in healthy adolescents.68 This increase in gain may indicate that HIIT elicits different adaptations in those with and without asthma, although it may also be a function of the lower baseline level of gain in those with asthma, allowing greater capacity for improvement. The lower levels of aerobic efficiency in participants with asthma may be related to a decreased lung function and may be a contributory mechanism for the onset of early fatigue and the perception that people with asthma are not as fit as their peers, although it is worth noting that gain and measures of lung function were not correlated in the current study. Indeed, Fielding et al.68 found a reduced gain in patients with cystic fibrosis patients and suggested that this explained, at least in part, the reduced exercise intolerance in individuals with cystic fibrosis compared with their healthy peers. Importantly, the current study demonstrates that the gain for participants with asthma, but not for adolescents without asthma, can be improved with a HIIT program.
The findings in the present study are in accord with previous findings in non-asthma populations71 in that cardiorespiratory fitness at baseline for those with asthma was found to have a weak but significant correlation with quality of life, highlighting the importance of exercise as a management strategy for those with asthma. However, despite this correlation and the increase in cardiorespiratory fitness observed in the current study, quality of life did not change over time, irrespective of treatment group or asthma status, which was contrary to the findings from previous studies involving exercise interventions.20, 21 Furthermore, in the present study there was no change over time for perceived asthma-related quality of life, symptoms, or asthma control. It could be postulated that the lack of improvement in asthma-related quality of life may be due to the mildness of the participants’ asthma or to participants having high baseline values for quality of life,1, 21, 72 which decreased the likelihood of an effect, or indeed the need for an effect. Finally, the lack of improvement in quality of life in the present study may have been due to the fact that HIIT decreased, or rather may not have increased, the participants’ total time in physical activity as a result of the compensation effect.73 This suggests that increased global physical activity may be more beneficial for quality of life rather than a specific HIIT intervention or increases in cardiorespiratory fitness.
Although our study is consistent with the majority of the literature in that we found that exercise did not affect lung function,1 it is pertinent to note that 2 studies reported a significant increase in FEV1% (8%–20%) and that both studies implemented intermittent training.29, 31 This discrepancy may be related to the mildness of asthma among participants in our study or to the duration of our HIIT intervention. Although the duration in our study was longer than in many previous studies,72, 74 6 months may not have been sufficient to elicit significant adaptations in lung function. It is interesting to note that both studies that previously reported beneficial adaptations in lung function involved younger, largely pre-pubertal, children.29, 31 Furthermore, the actual exercise time, despite being based on intermittent bouts, was significantly longer in Latorre-Román et al.,31 although the participants in Sidiropoulou et al.29 had exercise-induced bronchoconstriction rather than asthma per se. These factors therefore limit additional conclusions that can be drawn as to the discrepancy in these findings with regards to lung function.
In accordance with previous studies, the current findings suggest that BMI increases with age in youth.75 It is important to note that the intervention was able to maintain the baseline BMI and prevent an increase in BMI in those with and without asthma. Given that childhood obesity is known to track strongly into adolescence and adulthood, with evidence suggesting that 80% of obese adolescents will become obese adults,76 the current findings may have important implications in terms of effective exercise interventions that may help to ameliorate this increase. Furthermore, exercise and physical activity have previously been suggested to be influential in the self-management of asthma.77 The present study is the first to address whether HIIT may aid in the non-pharmacological management of asthma. Although the maintenance of BMI and increased fitness are promising findings, HIIT did not improve lung function, asthma control, and quality of life. Therefore, taking all findings together, 6 months of HIIT may not be effective at improving mild asthma in adolescents. Nevertheless, maintenance of BMI and increased fitness as a result of HIIT may make it an important non-pharmacological strategy in the management of asthma.
There were no differences in fitness or trainability between adolescents with and without asthma; these findings have important practical implications for the design of future exercise interventions. Indeed, this study should decrease stigmatization of adolescents with asthma. Moreover, there were no exacerbations throughout the study, demonstrating that HIIT is safe to use in adolescents with mild asthma. A key strength of the present study was the use of more sensitive measures of aerobic fitness (GET, MRT, and gain), which have not previously been assessed across multiple time-points in adolescents with asthma. Nonetheless, several limitations to the study should be acknowledged. As with any exercise intervention, there may have been a self-selection bias since recruited participants volunteered to participate. Furthermore, although the HIIT intervention used formative research in its design,14 participants who signed up for the intervention either committed fully to attending a large proportion of the sessions throughout the 6 months or attended only minimally over the intervention. This finding may indicate that the HIIT intervention is effective for those who fully engage in it, but that it is not acceptable to everyone. Although the utility of the intervention may be questioned, it may be that the timing of the exercise sessions (both before and after school) reduced participation and that if more optimal timing was possible, a stronger adherence could be achieved.
5. Conclusion
HIIT, a previously underused method of managing asthma in adolescents, may be an effective tool for increasing peak aerobic fitness and preventing an increase in BMI in adolescents, irrespective of asthma. This study adds to the literature by demonstrating that adolescents with asthma elicited similar physiological adaptations in comparison to their healthy peers, thereby demonstrating that asthma does not influence aerobic fitness or trainability in adolescents. Furthermore, the lack of exercise-induced asthma attacks suggests that HIIT is safe for, and well-tolerated by, adolescents with asthma.
Acknowledgments
Acknowledgments
The authors thank the students and staff of the schools involved with the planning and execution of the study. Also, the authors would like to thank Nicholas Wade and all others that assisted with data collection. This work was funded by the Asthma UK Centre for Applied Research (AUK-AC-2012-01) and Swansea University Medical School. Commando Joe's® implemented the intervention and also assisted in funding for co-author WTBE.
Authors’ contributions
CONW conceptualized and designed the study, collected data, conducted the statistical analysis, interpreted the data, and drafted the manuscript; MAM, KAM, and GAD conceptualized and designed the study, supervised the work, interpreted the data, and critically revised the manuscript; GS and AMW interpreted the data and critically revised the manuscript; WTBE collected data, interpreted the data, and critically revised the manuscript. All authors have read and approved the final version of the manuscript, and agree with the order of presentation of the authors.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Peer review under responsibility of Shanghai University of Sport.
Contributor Information
Melitta A. McNarry, Email: m.mcnarry@swansea.ac.uk.
Gwyneth A. Davies, Email: gwyneth.davies@swansea.ac.uk.
Supplementary materials
Supplementary material associated with this article can be found in the online version at https://doi.org/10.1016/j.jshs.2019.05.009.
Appendix. Supplementary materials
References
- 1.Wanrooij V.H., Willeboordse M., Dompeling E., van de Kant K.D. Exercise training in children with asthma: A systematic review. Br J Sports Med. 2014;48:1024–1031. doi: 10.1136/bjsports-2012-091347. [DOI] [PubMed] [Google Scholar]
- 2.Ng M., Fleming T., Robinson M. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: A systematic analysis for the Global Burden of Disease Study 2013. The Lancet. 2014;384:766–781. doi: 10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Farah C.S., Salome C.M. Asthma and obesity: A known association but unknown mechanism. Respirology. 2012;17:412–421. doi: 10.1111/j.1440-1843.2011.02080.x. [DOI] [PubMed] [Google Scholar]
- 4.Lucas S.R., Platts-Mills T.A. Paediatric asthma and obesity. Paediatr Respir Rev. 2006;7:233–238. doi: 10.1016/j.prrv.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 5.Vahlkvist S., Pedersen S. Fitness, daily activity and body composition in children with newly diagnosed, untreated asthma. Allergy. 2009;64:1649–1655. doi: 10.1111/j.1398-9995.2009.02081.x. [DOI] [PubMed] [Google Scholar]
- 6.Lochte L., Angermann M., Larsson B. Cardiorespiratory fitness of asthmatic children and validation of predicted aerobic capacity. Clin Respir J. 2009;3:42–50. doi: 10.1111/j.1752-699X.2008.00107.x. [DOI] [PubMed] [Google Scholar]
- 7.Santuz P., Baraldi E., Filippone M., Zacchello F. Exercise performance in children with asthma: Is it different from that of healthy controls? Eur Respir J. 1997;10:1254–1260. doi: 10.1183/09031936.97.10061254. [DOI] [PubMed] [Google Scholar]
- 8.Pianosi P.T., Davis H.S. Determinants of physical fitness in children with asthma. Pediatrics. 2004;113:e225–e229. doi: 10.1542/peds.113.3.e225. [DOI] [PubMed] [Google Scholar]
- 9.Berntsen S., Carlsen K.C., Anderssen S.A. Norwegian adolescents with asthma are physical active and fit. Allergy. 2009;64:421–426. doi: 10.1111/j.1398-9995.2008.01845.x. [DOI] [PubMed] [Google Scholar]
- 10.Villa F., Castro A.P., Pastorino A.C. Aerobic capacity and skeletal muscle function in children with asthma. Arch Dis Child. 2011;96:554–559. doi: 10.1136/adc.2011.212431. [DOI] [PubMed] [Google Scholar]
- 11.Mayorga-Vega D., Aguilar-Soto P., Viciana J. Criterion-related validity of the 20-M shuttle run test for estimating cardiorespiratory fitness: A meta-analysis. J Sports Sci Med. 2015;14:536–547. [PMC free article] [PubMed] [Google Scholar]
- 12.Cairney J., Hay J.A., Faught B.E., Léger L., Mathers B. Generalized self-efficacy and performance on the 20-metre shuttle run in children. Am J Hum Biol. 2008;20:132–138. doi: 10.1002/ajhb.20690. [DOI] [PubMed] [Google Scholar]
- 13.Armstrong N., Welsman J. Twenty-metre shuttle run: (mis)representation, (mis)interpretation and (mis)use. Br J Sports Med. 2019;53:1199. doi: 10.1136/bjsports-2018-100082. [DOI] [PubMed] [Google Scholar]
- 14.Winn C.O.N., Mackintosh K.A., Eddolls W.T.B. Perceptions of asthma and exercise in adolescents with and without asthma. J Asthma. 2017;55:868–876. doi: 10.1080/02770903.2017.1369992. [DOI] [PubMed] [Google Scholar]
- 15.Pianosi P.T., Liem R.I., McMurray R.G., Cerny F.J., Falk B., Kemper H.C. Pediatric exercise testing: Value and implications of peak oxygen uptake. Children(Basel) 2017;4:6. doi: 10.3390/children4010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Counil F.P., Varray A., Matecki S. Training of aerobic and anaerobic fitness in children with asthma. J Pediatr. 2003;142:179–184. doi: 10.1067/mpd.2003.83. [DOI] [PubMed] [Google Scholar]
- 17.Ahmaidi S.B., Varray A.L., Savy-Pacaux A.M., Prefaut C.G. Cardiorespiratory fitness evaluation by the shuttle test in asthmatic subjects during aerobic training. Chest. 1993;103:1135–1141. doi: 10.1378/chest.103.4.1135. [DOI] [PubMed] [Google Scholar]
- 18.van Veldhoven N.H., Vermeer A., Bogaard J.M. Children with asthma and physical exercise: Effects of an exercise programme. Clin Rehabil. 2001;15:360–370. doi: 10.1191/026921501678310162. [DOI] [PubMed] [Google Scholar]
- 19.Andrade L.B., Britto M.C., Lucena-Silva N., Gomes R.G., Figueroa J.N. The efficacy of aerobic training in improving the inflammatory component of asthmatic children. Randomized trial. Respir Med. 2014;108:1438–1445. doi: 10.1016/j.rmed.2014.07.009. [DOI] [PubMed] [Google Scholar]
- 20.Basaran S., Guler-Uysal F., Ergen N., Seydaoglu G., Bingol-Karakoç G., Ufuk Altintas D. Effects of physical exercise on quality of life, exercise capacity and pulmonary function in children with asthma. J Rehabil Med. 2006;38:130–135. doi: 10.1080/16501970500476142. [DOI] [PubMed] [Google Scholar]
- 21.Fanelli A., Cabral A.L., Neder J.A., Martins M.A., Carvalho C.R. Exercise training on disease control and quality of life in asthmatic children. Med Sci Sports Exerc. 2007;39:1474–1480. doi: 10.1249/mss.0b013e3180d099ad. [DOI] [PubMed] [Google Scholar]
- 22.Andersen J.R., Natvig G.K., Aadland E. Associations between health-related quality of life, cardiorespiratory fitness, muscle strength, physical activity and waist circumference in 10-year-old children: The ASK study. Qual Life Res. 2017;26:3421–3428. doi: 10.1007/s11136-017-1634-1. [DOI] [PubMed] [Google Scholar]
- 23.Swallen K.C., Reither E.N., Haas S.A., Meier A.M. Overweight, obesity, and health-related quality of life among adolescents: The national longitudinal study of adolescent health. Pediatrics. 2005;115:340–347. doi: 10.1542/peds.2004-0678. [DOI] [PubMed] [Google Scholar]
- 24.Williams J.W., Canterford L., Hesketh K.D. Changes in body mass index and health related quality of life from childhood to adolescence. Int J Pediatr Obes. 2011;6:e442–e448. doi: 10.3109/17477166.2010.526226. [DOI] [PubMed] [Google Scholar]
- 25.Onur E., Kabaroğlu C., Günay O. The beneficial effects of physical exercise on antioxidant status in asthmatic children. Allergol Immunopathol. 2011;39:90–95. doi: 10.1016/j.aller.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 26.McNarry M.A., Lambrick D., Westrupp N., Faulkner J. The influence of a six-week, high-intensity games intervention on the pulmonary oxygen uptake kinetics in prepubertal obese and normal-weight children. Appl Physiol Nutr Metab. 2015;40:1012–1018. doi: 10.1139/apnm-2015-0051. [DOI] [PubMed] [Google Scholar]
- 27.Costigan S.A., Eather N., Plotnikoff R.C., Taaffe D.R., Lubans D.R. High-intensity interval training for improving health-related fitness in adolescents: A systematic review and meta-analysis. Br J Sports Med. 2015;49:1253–1261. doi: 10.1136/bjsports-2014-094490. [DOI] [PubMed] [Google Scholar]
- 28.Logan G.R., Harris N., Duncan S., Schofield G. A review of adolescent high-intensity interval training. Sports Med. 2014;44:1071–1085. doi: 10.1007/s40279-014-0187-5. [DOI] [PubMed] [Google Scholar]
- 29.Sidiropoulou M.P., Fotiadou E.G., Tsimaras V.K., Zakas A.P., Angelopoulou N.A. The effect of interval training in children with exercise-induced asthma competing in soccer. J Strength Cond Res. 2007;21:446–450. doi: 10.1519/R-17825.1. [DOI] [PubMed] [Google Scholar]
- 30.MacDonald M.J., Currie K.D. Interval exercise is a path to good health, but how much, how often and for whom? Clin Sci (Lond) 2009;116:315–316. doi: 10.1042/CS20080632. [DOI] [PubMed] [Google Scholar]
- 31.Latorre-Román P.Á., Navarro-Martinez A.V., Garcia-Pinillos F. The effectiveness of an indoor intermittent training program for improving lung function, physical capacity, body composition and quality of life in children with asthma. J Asthma. 2014;51:544–551. doi: 10.3109/02770903.2014.888573. [DOI] [PubMed] [Google Scholar]
- 32.Malik A.A., Williams C.A., Bond B., Weston K.L., Barker A.R. Acute cardiorespiratory, perceptual and enjoyment responses to high-intensity interval exercise in adolescents. Eur J Sport Sci. 2017;17:1335–1342. doi: 10.1080/17461391.2017.1364300. [DOI] [PubMed] [Google Scholar]
- 33.Westergren T., Fegran L., Nilsen T. et al. Active play exercise intervention in children with asthma: A pilot study. BMJ Open. 2016;6 doi: 10.1136/bmjopen-2015-009721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O'Neill C., Dogra S. Subjective Responses to interval and continuous exercise in adults with exercise-induced bronchoconstriction. J Phys Act Health. 2017;14:486–491. doi: 10.1123/jpah.2016-0221. [DOI] [PubMed] [Google Scholar]
- 35.Ekkekakis P. Pleasure and displeasure from the body: Perspectives from exercise. Cogn Emot. 2003;17:213–239. doi: 10.1080/02699930302292. [DOI] [PubMed] [Google Scholar]
- 36.The Global Initiative for Asthma. Global strategy for asthma management and prevention. Available at: http://www.ginasthma.org. [accessed 18.01.2019].
- 37.Hood M.S., Little J.P., Tarnopolsky M.A., Myslik F., Gibala M.J. Low-volume interval training improves muscle oxidative capacity in sedentary adults. Med Sci Sports Exerc. 2011;43:1849–1856. doi: 10.1249/MSS.0b013e3182199834. [DOI] [PubMed] [Google Scholar]
- 38.Tanaka H., Monahan K.D., Seals D.R. Age-predicted maximal heart rate revisited. J Am Coll Cardiol. 2001;37:153–156. doi: 10.1016/s0735-1097(00)01054-8. [DOI] [PubMed] [Google Scholar]
- 39.Mahon A.D., Marjerrison A.D., Lee J.D., Woodruff M.E., Hanna L.E. Evaluating the prediction of maximal heart rate in children and adolescents. Res Q Exerc Sport. 2010;81:466–471. doi: 10.1080/02701367.2010.10599707. [DOI] [PubMed] [Google Scholar]
- 40.Stewart A., Marfell-Jones M., Olds T., de Ridder H. International Society for the Advancement of Kinanthropometry; Lower Hutt, New Zealand: 2011. International standards for anthropometric assessment; pp. 53–56. [Google Scholar]
- 41.Barlow S.E. Expert Committee. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: Summary report. Pediatrics. 2007;120(Suppl. 4):S164–S192. doi: 10.1542/peds.2007-2329C. [DOI] [PubMed] [Google Scholar]
- 42.Mirwald R.L., Baxter-Jones A.D., Bailey D.A., Beunen G.P. An assessment of maturity from anthropometric measurements. Med Sci Sports Exerc. 2002;34:689–694. doi: 10.1097/00005768-200204000-00020. [DOI] [PubMed] [Google Scholar]
- 43.American Thoracic Society Standardization of spirometry, 1994 update. Am J Respir Crit Care Med. 1995;152:1107–1136. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- 44.Miller M.R., Hankinson J., Brusasco V. Standardisation of spirometry. Eur Respir J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 45.Rosenthal M., Bain S.H., Cramer D. Lung function in white children aged 4 to 19 years: I-Spirometry. Thorax. 1993;48:794–802. doi: 10.1136/thx.48.8.794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dweik R.A., Boggs P.B., Erzurum S.C. An official ATS clinical practice guideline: Interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am J Respir Crit Care Med. 2011;184:602–615. doi: 10.1164/rccm.9120-11ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Juniper E.F., Gruffydd-Jones K., Ward S., Svensson K. Asthma Control Questionnaire in children: Validation, measurement properties, interpretation. Eur Respir J. 2010;36:1410–1416. doi: 10.1183/09031936.00117509. [DOI] [PubMed] [Google Scholar]
- 48.Cronbach L.J. Coefficient alpha and the internal structure of tests. Psychometrika. 1951;16:297–334. [Google Scholar]
- 49.Juniper E.F., Guyatt G.H., Feeny D.H., Ferrie P.J., Griffith L.E., Townsend M. Measuring quality of life in children with asthma. Qual Life Res. 1996;5:35–46. doi: 10.1007/BF00435967. [DOI] [PubMed] [Google Scholar]
- 50.Varni J.W., Seid M., Rode C.A. The PedsQL: Measurement model for the Pediatric Quality of Life Inventory. Med Care. 1999;37:126–139. doi: 10.1097/00005650-199902000-00003. [DOI] [PubMed] [Google Scholar]
- 51.Varni J.W., Burwinkle T.M., Seid M., Skarr D. The PedsQL 4.0 as a pediatric population health measure: Feasibility, reliability, and validity. Ambul Pediatr. 2003;3:329–341. doi: 10.1367/1539-4409(2003)003<0329:tpaapp>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 52.Varni J.W., Burwinkle T.M., Seid M. The PedsQL 4.0 as a school population health measure: Feasibility, reliability, and validity. Qual Life Res. 2006;15:203–215. doi: 10.1007/s11136-005-1388-z. [DOI] [PubMed] [Google Scholar]
- 53.Varni J.W., Seid M., Kurtin P.S. PedsQL 4.0: Reliability and validity of the Pediatric Quality of Life Inventory version 4.0 generic core scales in healthy and patient populations. Med Care. 2001;39:800–812. doi: 10.1097/00005650-200108000-00006. [DOI] [PubMed] [Google Scholar]
- 54.Beaver W.L., Wasserman K., Whipp B.J. A new method for detecting anaerobic threshold by gas exchange. J Appl Physiol (1985) 1986;60:2020–2027. doi: 10.1152/jappl.1986.60.6.2020. [DOI] [PubMed] [Google Scholar]
- 55.Barstow T.J., Scremin A.M., Mutton D.L., Kunkel C.F., Cagle T.G., Whipp B.J. Peak and kinetic cardiorespiratory responses during arm and leg exercise in patients with spinal cord injury. Spinal Cord. 2000;38:340–345. doi: 10.1038/sj.sc.3101014. [DOI] [PubMed] [Google Scholar]
- 56.Trollvik A., Nordbach R., Silén C., Ringsberg K.C. Children's experiences of living with asthma: Fear of exacerbations and being ostracized. J Pediatr Nurs. 2011;26:295–303. doi: 10.1016/j.pedn.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 57.van den Bemt L., Kooijman S., Linssen V. How does asthma influence the daily life of children? Results of focus group interviews. Health Qual Life Outcomes. 2010;8:5. doi: 10.1186/1477-7525-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Taylor K.L., Weston M., Batterham A.M. Evaluating intervention fidelity: An example from a high-intensity interval training study. PLoS One. 2015;10 doi: 10.1371/journal.pone.0125166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Armstrong N., Barker A.R. Endurance training and the elite young athlete. Med Sci Sport. 2011;56:59–83. doi: 10.1159/000320633. [DOI] [PubMed] [Google Scholar]
- 60.Mandigout S., Lecoq A.M., Courteix D., Guenon P., Obert P. Effect of gender in response to an aerobic training programme in prepubertal children. Acta Paediatr. 2001;90:9–15. doi: 10.1080/080352501750064815. [DOI] [PubMed] [Google Scholar]
- 61.Eliakim A., Barstow T.J., Brasel J.A. Effect of exercise training on energy expenditure, muscle volume, and maximal oxygen uptake in female adolescents. J Pediatr. 1996;129:537–543. doi: 10.1016/s0022-3476(96)70118-x. [DOI] [PubMed] [Google Scholar]
- 62.Tolfrey K., Campbell I.G., Batterham A.M. Aerobic trainability of prepubertal boys and girls. Pediatr Exerc Sci. 1998;10:248–263. [Google Scholar]
- 63.Varray A.L., Mercier J.G., Prefaut C.G. Individualized training reduces excessive exercise hyperventilation in asthmatics. Int J Rehabil Res. 1995;18:297–312. doi: 10.1097/00004356-199512000-00002. [DOI] [PubMed] [Google Scholar]
- 64.Varray A.L., Mercier J.G., Terral C.M., Prefaut C.G. Individualized aerobic and high intensity training for asthmatic children in an exercise readaptation program. Is training always helpful for better adaptation to exercise? Chest. 1991;99:579–586. doi: 10.1378/chest.99.3.579. [DOI] [PubMed] [Google Scholar]
- 65.Baquet G., Berthoin S., Gerbeaux M., Van Praagh E. High-intensity aerobic training during a 10 week one-hour physical education cycle: Effects on physical fitness of adolescents aged 11 to 16. Int J Sports Med. 2001;22:295–300. doi: 10.1055/s-2001-14343. [DOI] [PubMed] [Google Scholar]
- 66.Barstow T.J., Jones A.M., Nguyen P.H., Casaburi R. Influence of muscle fibre type and fitness on the oxygen uptake/power output slope during incremental exercise in humans. Exp Physiol. 2000;85:109–116. [PubMed] [Google Scholar]
- 67.McNarry M.A., Welsman J.R., Jones A.M. The influence of training and maturity status on girls' responses to short-term, high-intensity upper- and lower-body exercise. Appl Physiol Nutr Metab. 2011;36:344–352. doi: 10.1139/h11-019. [DOI] [PubMed] [Google Scholar]
- 68.Fielding J., Brantley L., Seigler N., McKie K.T., Davison G.W., Harris R.A. Oxygen uptake kinetics and exercise capacity in children with cystic fibrosis. Pediatr Pulmonol. 2015;50:647–654. doi: 10.1002/ppul.23189. [DOI] [PubMed] [Google Scholar]
- 69.Wilcox S.L., Broxterman R.M., Barstow T.J. Constructing quasi-linear VO2 responses from nonlinear parameters. J Appl Physiol (1985) 2016;120:121–129. doi: 10.1152/japplphysiol.00507.2015. [DOI] [PubMed] [Google Scholar]
- 70.Neder J.A., Nery L.E., Peres C., Whipp B.J. Reference values for dynamic responses to incremental cycle ergometry in males and females aged 20 to 80. Am J Respir Crit Care Med. 2001;164:1481–1486. doi: 10.1164/ajrccm.164.8.2103007. [DOI] [PubMed] [Google Scholar]
- 71.Wu X.Y., Han L.H., Zhang J.H., Luo S., Hu J.W., Sun K. The influence of physical activity, sedentary behavior on health-related quality of life among the general population of children and adolescents: a systematic review. PLoS One. 2017;12 doi: 10.1371/journal.pone.0187668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Moreira A., Delgado L., Haahtela T. Physical training does not increase allergic inflammation in asthmatic children. Eur Respir J. 2008;32:1570–1575. doi: 10.1183/09031936.00171707. [DOI] [PubMed] [Google Scholar]
- 73.Ridgers N.D., Timperio A., Cerin E., Salmon J. Compensation of physical activity and sedentary time in primary school children. Med Sci Sports Exerc. 2014;46:1564–1569. doi: 10.1249/MSS.0000000000000275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wicher I., Ribeiro M.A., Marmo D.B. Effects of swimming on spirometric parameters and bronchial hyperresponsiveness in children and adolescents with moderate persistent atopic asthma. J Pediatr (Rio J) 2010;86:384–390. doi: 10.2223/JPED.2022. [DOI] [PubMed] [Google Scholar]
- 75.de Souza M.C., Eisenmann J.C., e Santos D.V., de Chaves R.N., de Moraes Forjaz C.L., Maia J.A. Modeling the dynamics of BMI changes during adolescence. Int J Obes (Lond) 2015;39:1063–1069. doi: 10.1038/ijo.2015.60. [DOI] [PubMed] [Google Scholar]
- 76.Schonfeld-Warden N., Warden C.H. Pediatric obesity: An overview of etiology and treatment. Pediatr Clin North Am. 1997;44:339–361. doi: 10.1016/s0031-3955(05)70480-6. [DOI] [PubMed] [Google Scholar]
- 77.Scottish Intercollegiate Guidelines Network, Healthcare Improvement Scotland. British Guideline for the management of asthma. Available at:http://www.sign.ac.uk/sign-153-british-guideline-on-the-management-of-asthma.html. [accessed 18.01.2019].
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.