Abstract
Background
The association between immune checkpoint inhibitors (ICIs) and Stevens-Johnsons syndrome (SJS) /toxic epidermal necrolysis (TEN) is unclear. We assessed the risk of SJS and TEN related to ICIs, via a systematic analysis of SJS/TEN cases reported in clinical trials and the FDA Adverse Event Reporting System (FAERS).
Methods
We explored ICIs related SJS/TEN events in randomized control trials available in ClinicalTrials.gov and electronic databases (Pubmed, Embase, the Cochrane Central Register of Controlled Trials) up to 12 January 2021. Meta-analysis was performed by using Peto odds ratios (ORs) with 95% CIs. In a separate retrospective pharmacovigilance study of FAERs, cases of ICIs related SJS/TEN were extracted between the first quarter (Q1) of 2004 and Q4 of 2020. Disproportionality was analyzed using the proportional reports reporting odds ratio (ROR) and information components (IC). PROSPERO registration number: CRD42021232399.
Findings
A total of 20 RCTs (11597 patients) were included. ICIs were associated with an increased risk of SJS/TEN (OR= 4.33, 95%CI:1.90–9.87). FAERS pharmacovigilance data identified 411 cases of SJS (n = 253) or TEN (n = 184) related to ICIs therapy. ICIs were significantly associated with SJS/TEN (n = 411; ROR=2.88, 95%CI:2.61–3.17; IC=1.49, 95%CI:1.35–1.65). The median onset time of SJS/TEN was 25.5 days (SJS:21.5 days; TEN:32 days) (n = 190), 97.5% of patients discontinued use of ICIs when suffering from SJS/TEN (n = 201). Of 305 cases that reported outcomes, 113 (37%) resulted in death (SJS:19.9%, TEN:61.6%).
Interpretation
These data suggest that ICIs were significantly associated with increased risk of SJS/TEN.
Research in context.
Evidence before this study
We searched PubMed for articles published until February 1, 2021, with the terms (“immune checkpoint inhibitors” OR “PD1” OR “PD-L1” OR “nivolumab” OR “pembrolizumab” OR “cemiplimab” OR “atezolizumab” OR “avelumab” OR “durvalumab” OR “ipilimumab” OR “tremelimumab”) AND (“Stevens-Johnson syndrome” and “toxic epidermal necrolysis”). Our search retrieved no reports of meta-analyses or pharmacovigilance studies investigated the between immune checkpoint inhibitors and Stevens-Johnsons syndrome /toxic epidermal necrolysis.
Added value of this study
To the best of our knowledge, this study is the largest and most extensive analysis of Stevens-Johnsons syndrome/toxic epidermal necrolysis associated with immune checkpoint inhibitors collected data from both clinical trials and a worldwide pharmacovigilance database to date. Meta-analysis of 20 RCTs suggested that immune checkpoint inhibitors are associated with increased risk of Stevens-Johnsons syndrome/toxic epidermal necrolysis. FAERS pharmacovigilance data of 411 cases also indicated that immune checkpoint inhibitors were significantly associated with over-reporting frequencies of Stevens-Johnsons syndrome/toxic epidermal necrolysis. The median onset time of events was 25.5 days, with a mortality rate of 37%.
Implications of all the available evidence
Both meta-analysis of RCT's and real-world FAERS pharmacovigilance data suggested that the immune checkpoint inhibitors may increase the risk of Stevens-Johnsons syndrome/toxic epidermal necrolysis. Further studies are needed to identify risk factors and optimal management of Stevens-Johnsons syndrome/toxic epidermal necrolysis-like reactions to immune checkpoint inhibitors.
Alt-text: Unlabelled box
1. Introduction
Immune checkpoint inhibitors (ICIs), include agents target programmed death-1 receptor (PD-1), programmed death-ligand 1 (PD-L1), and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), have radically transformed the therapeutic landscape for multiple cancer types [1]. Despite the survival benefits of ICIs therapy, these treatments can also lead to a variety of immune-related adverse events (irAEs), include cutaneous reactions, such as morbilliform, psoriasiform, lichenoid, eczematous, and rah are common in patients on these drugs [2,3].
Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) are rare and life threatening skin adverse reactions, characterized by flaccid blister formation, rapidly progressive and extensive necrosis with epidermal detachment [4,5], SJS involves <10% body surface area and TEN >30% body surface area [4]. Therefore, knowing the incidence and risk of SJS/TEN associated with ICIs will help practitioners to make appropriate measures to limit their effects. SJS/TEN events related to ICIs are reported occasionally in small case series and case reports [6,7], but no definitive data have been established.
Given the widespread and increasing use of ICIs in real world, and the potential risk of SJS/TEN induced by ICIs, performed a systematic review and meta-analysis of randomized controlled trials (RCTs) to estimate the risk of developing SJS/TEN related to ICIs. Furthermore, we also examine reported events of ICIs-associated SJS/TEN in clinical practice using real-world pharmacovigilance data from Food and Drug Administration (FDA) Adverse Event Reporting System (FAERS) [8].
2. Methods
2.1. Study design
Firstly, we did a meta-analysis of RCTs to investigate the risk of SJS/TEN in patients treated with ICIs. Furthermore, a retrospective data mining analysis was conducted using the FDA FAERS database to further examine the risk of SJS/TEN in clinical practice. J. W. had full access to all the data in the study
2.2. Systematic review procedures
This study was prospectively registered in PROSPERO (https://www.crd.york.ac.uk/PROSPERO/) (CRD42021232399). The meta-analysis was performed according to the PRISMA checklist [9]. A systematic literature review was performed in Pubmed, Embase, the Cochrane Central Register of Controlled Trials (CENTRAL) [10], and the ClinicalTrials.gov website to identify randomized clinical trials of immune checkpoint inhibitors (up to 12 January 2021), with no language restriction. Terms related to immune checkpoint inhibitors (nivolumab, pembrolizumab, cemiplimab, atezolizumab, avelumab, durvalumab, ipilimumab and tremelimumab) and study design (RCT) were used. RCTs that compared immune checkpoint inhibitors versus placebo or active controls in cancer patients and reported adverse events of SJS/TEN were eligible for inclusion.
Data were collected by two authors (J.Z. and G.C.) independently, and discrepancies were resolved by a third investigator (Z.H.). The number of patients with SJS/TEN events classified according to the Cancer Therapy Evaluation Program (CTEP) in trials reported on ClinicalTrials.gov was extracted [11]. We further collected SJS/TEN events according to the Common Terminology Criteria for Adverse Events (CTCAE) in published RCTs [12], if data were not available on ClinicalTrials.gov. For each included trial, additional data including NCT number, trial name, study design, ICIs regimen, control arm regimen, median age, median follow-up, sample size, and tumor type was also extracted. The risk of bias of all included RCTs was examined by two reviewers (Y.Z. and S.G.) concurrently according to the recommendations outlined in the Cochrane Handbook [13].
The primary outcome was the summary risk of SJS/TEN related to immune checkpoint inhibitors, we performed a meta-analysis to compute Peto odds ratios (ORs) with 95% CIs. Between-study heterogeneity was determined by using the inconsistency index I2, with I2 values over 50% suggest substantial between-study heterogeneity [14]. Prespecified subgroup analyses were done according to the type of ICIs (ICIs treatment schedule), case of events (SJS vs TEN) or source of data (published paper vs ClinicalTrials.gov). In addition, if more than 10 studies were included in one meta-analysis, we also accessed the publication bias represented by the Egger's test [15,16]. A two-sided p value of less than 0.05 in all analyses was considered statistically significant. Statistical analyses were performed using Stata software (version 12.0).
2.3. Pharmacovigilance study procedures
Real-world pharmacovigilance data from FAERS were searched for SJS/TEN events from January 1, 2011 to December 31, 2020, with the MedDRA (version 23.1) preferred terms “Stevens-Johnson syndrome” (Preferred Term) and “toxic epidermal necrolysis” (Preferred Term), generic name of ICIs (nivolumab, pembrolizumab, cemiplimab, atezolizumab, avelumab, durvalumab, ipilimumab and tremelimumab) were used to identify cases notified as suspected to be caused by ICIs [17]. When available, data of clinical characteristics was also collected: reporter (health-care professional or non-health-care professional), sex, age, report countries, indication, reporting year, ICIs characteristics (regimen, start and end date, and treatment modifications), and SJS/TEN characteristics (date of event and reaction outcome). To avoid bias, data mining was performed by independent researchers by using the OpenVigil FDA tool [18]. Before performing statistical analysis, duplicate reports were removed by reviewing the unique ID and the case characteristics.
Two data mining methods using proportional reports reporting odds ratio (ROR) and Bayesian confidence propagation neural networks of information components (IC) were used to preform disproportionality analysis [14,19], with all other drugs/events recorded in FAERS as a comparator. For ROR, the lower limit of the 95% confidence interval (CI) of the ROR (ROR025) >1 with at least three case indicate a significant signal. For IC, the lower end of a 95% CI of the IC(IC025) >0 suggest a significant signal. Data analyses were performed using the Microsoft Excel (2010, Microsoft).
2.4. Role of the funding source
The funder had no role in the study design, data collection, data analysis, data interpretation, and writing of the report.
3. Results
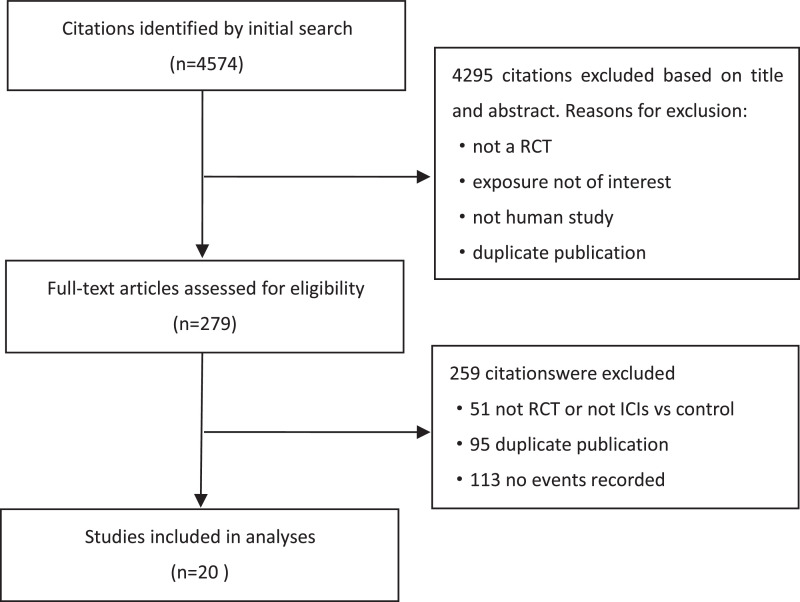
Of 4574 articles obtained from the initial search of the databases, 20 RCTs were included in the final analysis (Fig 1). Detailed baseline characteristics of the included trials are summarised in Table 1. Six trials were conducted in patients with lung cancer, 4 in patients with melanoma, 2 in patients with renal cell carcinoma, 2 in patients with multiple myeloma, 2 in patients with urothelial carcinoma, 2 in patients with gastroesophageal, one in head and neck, and one in hepatocellular carcinoma patients. Nivolumab used in 7 trials, ipilimumab used in 5 trials, pembrolizumab used in 5 trials, atezolizumab in 3 trials, durvalumab in 2 trials, and tremelimumab in 2 trials. Five trials used a combination of anti-PD-1/PDL-1 plus CTLA-4 treatment. The duration of the follow-up ranged from 9 to 61.6 months. Events of SJS/TEN collected from 9 published trials (supplementary material p.1), and the others were from ClinicalTrials.gov. Seven studies used blinding methods were judged to have a low risk of bias according to the Cochrane instrument (supplementary material p.2-p.3).
Fig 1.
Flow diagram of trial selection.
Table 1.
Characteristics of included randomised controlled trials.
| Study | Intervention | Cancer type | Sex, Male | Sample Size | Age years Mean (Standard Deviation) |
Median follow-up | Events reported |
|---|---|---|---|---|---|---|---|
| NCT02576509 | Nivolumab | Hepatocellular Carcinoma | 84.9% | 367 | 63.9 (10.61) | 3.5y | ClinicalTrials.gov |
| Sorafenib | 363 | 64.5 (10.91) | |||||
| NCT02231749 | Nivolumab + Ipilimumab | Renal Cell Carcinoma | 73.7% | 547 | 61.1 (9.76) | 31m | ClinicalTrials.gov |
| Sunitinib | 535 | 60.7 (10.10) | |||||
| NCT02481830 | Nivolumab | Small-cell Lung Cancer | 61.7% | 282 | 61.5 (9.2) | NR | ClinicalTrials.gov |
| Chemotherapy | 265 | 61.6 (8.4) | |||||
| NCT02041533 | Nivolumab | NSCLC | 61.4% | 267 | 62.8 (10.25) | 18m | ClinicalTrials.gov |
| Carbone 2017 | Chemotherapy | 263 | 63.4 (9.63) | Carbone 2017 | |||
| NCT03215706 | Nivolumab + Ipilimumab + Chemotherapy | NSCLC | 70.1% | 358 | 65.0 (8.3) | 23m | ClinicalTrials.gov |
| Chemotherapy | 349 | 65.0 (10.3) | |||||
| NCT03950674 | Nivolumab + Chemotherapy | NSCLC | 77.5% | 25 | 59.6 (12.7) | NR | ClinicalTrials.gov |
| Chemotherapy | 15 | 60.9 (11.8) | |||||
| NCT02579863 | Pembrolizumab + Lenalidomide + Dexamethasone | Multiple Myeloma | 46.5% | 154 | 74.4 (6.0) | 30m | Usmani 2019 |
| Usmani 2019 | Lenalidomide + Dexamethasone | 148 | 74.3 (5.9) | ||||
| NCT02576977 | Pembrolizumab+Pomalidomide+Dexamethasone | Multiple Myeloma | 62.5% | 122 | 65.5 (9.3) | 33m | Mateos 2019 |
| Pomalidomide+Dexamethasone | 123 | 66.4 (10.0) | |||||
| NCT02494583 | Pembrolizumab + Chemotherapy | Gastric or Gastroesophageal Junction | 72.6% | 250 | 60.9 (11.6) | 42m | ClinicalTrials.gov |
| Pembrolizumab | 254 | 59.9 (11.6) | |||||
| Chemotherapy | 244 | 60.7 (12.7) | |||||
| NCT02252042 | Pembrolizumab | Head and Neck | 83.2% | 246 | 60.3 (9.8) | 27m | Cohen 2019 |
| Standard Treatment | 234 | 60.2 (8.6) | |||||
| NCT01704287 | Pembrolizumab 2 mg/kg | Melanoma | 60.6% | 178 | 59.5 (14.9) | 75 m | ClinicalTrials.gov |
| Pembrolizumab 10 mg/kg | 179 | 60.1 (13.3) | |||||
| Chemotherapy | 171 | 60.5 (12.7) | |||||
| NCT02302807 | Atezolizumab 1200mg every 21d | Urothelial Bladder | 77.1% | 459 | 65.9 (9.6) | 46m | Powles 2018 |
| Chemotherapy | 443 | 66.1 (9.3) | |||||
| NCT02420821 | Atezolizumab + Bevacizumab | Renal Cell Carcinoma | 73.1% | ClinicalTrials.gov | |||
| Sunitinib | |||||||
| NCT02908672 | Atezolizumab + Cobimetinib + Vemurafenib | Melanoma | 58.2% | 230 | 54.0 (14.2) | 9m | ClinicalTrials.gov |
| Cobimetinib + Vemurafenib | 281 | 53.2 (14.1) | |||||
| NCT00094653 | Ipilimumab Plus gp100 | Melanoma | 59.3% | 380 | 55.6 | 61.6m | ClinicalTrials.gov |
| Ipilimumab | 131 | 56.8 | |||||
| gp100 | 132 | 57.4 | |||||
| NCT02516241 | Durvalumab±tremelimumab | Urothelial carcinoma | 75.5% | 340 | 67 | 41.2m | Powles 2020 |
| Powles 2020 | Durvalumab | 348 | 67 | ||||
| Chemotherapy | 313 | 68 | |||||
| NCT03043872 | Durvalumab±tremelimumab +Chemotherapy | SCLC | 71.6% | 266 | 63 | 25.1m | Goldman 2021 |
| Durvalumab +Chemotherapy | 265 | 62 | |||||
| Chemotherapy | 266 | 63 | |||||
| NCT02569242 | Nivolumab | Oesophageal squamous cell carcinoma | 89% | 209 | 64 | Kato 2019 | |
| Kato 2019 | Chemotherapy | 208 | 67 | ||||
| NCT02523313 | Ipilimumab+Nivolumab | Melanoma | 57% | 55 | 52 | 28.4m | Zimmer 2020 |
| Zimmer 2020 | Nivolumab | 56 | 57 | ||||
| Placebo | 51 | 58.5 | |||||
| NCT00527735 | Ipilimumab + Paclitaxel/Carboplatin | NSCLC/SCLC | 74.6% | 222 | / | NR | ClinicalTrials.gov |
| Paclitaxel/Carboplatin | 109 | / |
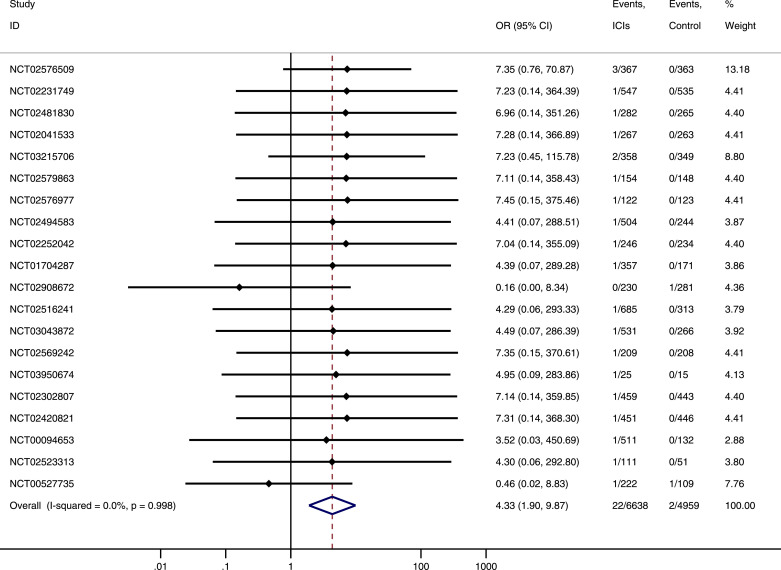
Pooled data from 20 trials, SJS/TEN occurred in 22 of 6638 participants randomized to the ICIs group and 2 of 4959 participants randomized to the control group. Meta-analysis showed that ICIs was associated with increased risk of SJS/TEN. The OR of SJS/TEN associated with ICIs was 4.33 (95% CI: 1.90–9.87, P < 0.001) with insignificant heterogeneity (I2 = 0%) (Fig 2). Results of subgroup analysis indicated that OR of SJS/TEN associated with ICIs did not vary significantly by type of ICIs (ICIs treatment schedule, Pinteraction=0.92), case of events (SJS vs TEN, Pinteraction=0.504) or source of data (published paper vs ClinicalTrials.gov, Pinteraction=0.703) (supplementary material p.4–p.6). No publication bias was observed among the included studies for the meta-analysis of SJS/TEN in the present study (Egger's test P = 0.974).
Fig 2.
Odds ratios of SJS/TEN in cancer patients treatment with ICIs compare with control.
From January 1, 2011 to December 31, 2020, there were 10 840 246 total adverse reactions of any drug reported, with 101 328 related to ICIs. Of these reports with ICIs, 411 SJS/TEN reactions were reported. Patient characteristics are summarised in Table 2. Most cases were reported by health professionals (77.3%), with median onset age of 66 years, and the male cases were slightly more than female cases (58.7% vs 41.3%). Most cases were observed in patients with lung cancer (46.6%), melanoma (17.3%), renal cancer (8.1%), lymphoma (4.7%) and head and neck cancer (4.5%). The number of cases increased during the 2011–2020 period, mainly from United States, Japan and Europe. Life-threatening events were higher (All cases:37%, SJS:19.9%, TEN:61.6%), almost all patients discontinued use of ICIs when suffered from SJS/TEN (97.5%). The median onset time of SJS/TEN was 25.5 days (SJS:21.5 days; TEN:32 days).
Table 2.
Characteristics of ICIs related SJS/TEN cases from FAERs database.
| All cases (n = 411) | SJS(n = 253) | TEN (n = 184) | |
|---|---|---|---|
| Reporter | |||
| Total data | 406 | 250 | 181 |
| Health-care professional | 314(77.3%) | 191(76.4%) | 141(77.9%) |
| Non-health-care professional | 92(22.7%) | 59(23.6%) | 40(22.1%) |
| Sex | |||
| Total data | 361 | 229 | 155 |
| Male | 212(58.7%) | 139(60.7%) | 88(56.8%) |
| Female | 149(41.3%) | 90(39.3%) | 67(43.2%) |
| Age | |||
| Total data | 305 | 190 | 132 |
| Median | 66years | 65years | 66years |
| Range | 32–89years | 32–87years | 36–89years |
| Indication | |||
| Total data | 358 | 217 | 161 |
| Lung cancer | 167(46.6%) | 101(46.5%) | 77(47.8%) |
| Melanoma | 62(17.3%) | 31(14.3%) | 32(19.9%) |
| Renal Cancer | 29(8.1%) | 15(6.9%) | 15(9.3%) |
| Lymphoma | 17(4.7%) | 7(3.2%) | 12(7.5%) |
| Head And Neck Cancer | 16(4.5%) | 15(6.9%) | 2(1.2%) |
| Other cancer/Unknown Indication | 67(18.7%) | 48(22.1%) | 23(14.3%) |
| Report countries | |||
| Total data | 409 | 251 | 184 |
| Japan,JP | 119(29.1%) | 94(37.5%) | 31(16.8%) |
| United States,US | 109(26.7%) | 75(29.9%) | 45(24.5%) |
| Germany,DE | 35(8.6%) | 13(5.2%) | 26(14.1%) |
| Canada, CA | 27(6.6%) | 7(2.8%) | 20(10.9%) |
| United Kingdom,GB | 27(6.6%) | 6(2.4%) | 22(11.9%) |
| Other countries | 92(22.5%) | 56(22.3%) | 40(21.7%) |
| Reporting year | |||
| Total data | 411 | 253 | 184 |
| 2020 | 132(32.1%) | 82(32.4%) | 61(33.2%) |
| 2019 | 119(28.9%) | 70(27.7%) | 58(31.5%) |
| 2018 | 68(16.5%) | 43(17%) | 26(14.1%) |
| 2017 | 51(12.4%) | 34(13.4%) | 20(10.9%) |
| 2016 | 26(6.3%) | 18(7.1%) | 10(5.4%) |
| 2015 | 9(2.2%) | 3(1.2%) | 6(3.3%) |
| 2014 | 1(0.2%) | 1(0.4%) | 0(0%) |
| 2013 | 4(1%) | 1(0.4%) | 3(1.6%) |
| 2012 | 0(0%) | 0(0%) | 0(0%) |
| 2011 | 1(0.2%) | 1(0.4%) | 0(0%) |
| Reaction Outcome | |||
| Total data | 305 | 176 | 146 |
| Recovered/resolved | 77(25.2%) | 56(31.8%) | 24(16.4%) |
| Recovering/resolving | 73(23.9%) | 50(28.4%) | 24(16.4%) |
| Not recovered/not resolved | 39(12.8%) | 33(18.7%) | 7(4.8%) |
| Recovered/resolved with sequelae | 3(1%) | 2(1.1%) | 1(0.7%) |
| Fatal | 113(37.0%) | 35(19.9%) | 90(61.6%) |
| Treatment modifications | |||
| Total data | 201 | 143 | 67 |
| Drug withdrawn | 196(97.5%) | 140(97.9%) | 65(97.0%) |
| Dose reduced | 0(0%) | 0(0%) | 0(0%) |
| Dose not changed | 5(2.5%) | 3(2.1%) | 2(3.0%) |
| Latency period | |||
| Total data | 190 | 130 | 70 |
| Median | 25.5 days | 21.5days | 32days |
| Range | 0-844 days | 0-844 days | 0-500 days |
| Within 3 weeks | 86(45.3%) | 65(50%) | 26(37.1%) |
| <24 h | 7(3.7%) | 6(46.2%) | 1(1.4%) |
| 1–7 days | 31(16.3%) | 23(17.7%) | 11(15.7%) |
| 8-30days | 73(38.4%) | 54(41.5%) | 22(31.4%) |
| 31-60days | 38(20%) | 17(13.1%) | 24(34.3%) |
| >61 days | 41(21.6%) | 30(23.1%) | 12(17.1%) |
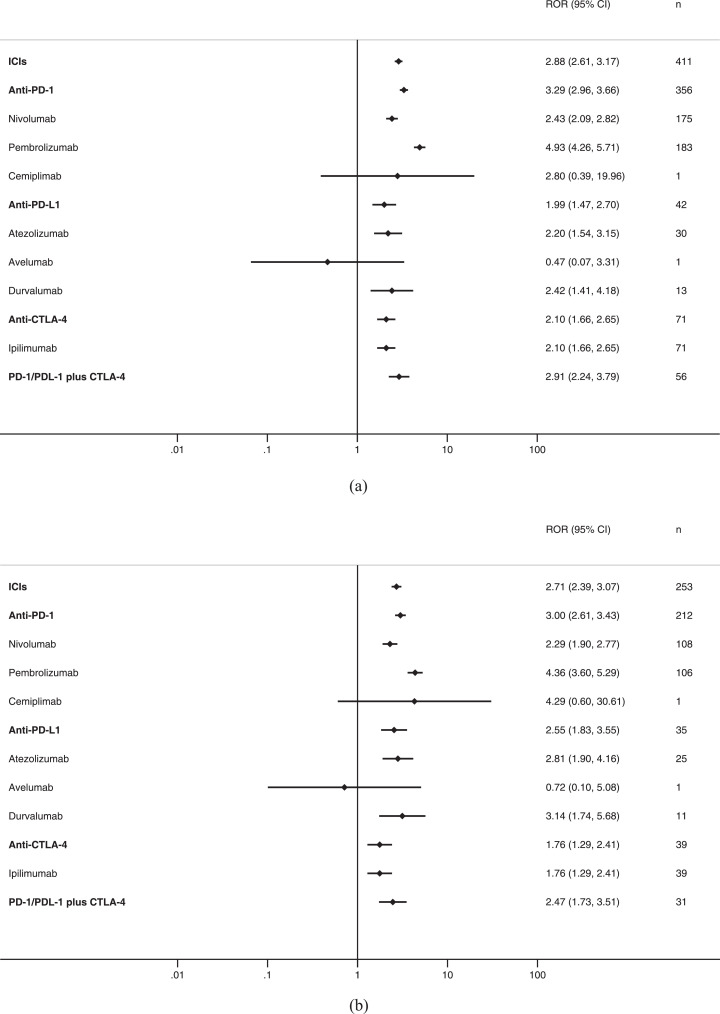
For all cases of SJS/TEN, ICIs as a class had an ROR of 2.88 (95% CI 2.61–3.17) and a IC of 1.49 (95% CI 1.35–1.65) compared with all other medications (Table 3, Fig. 3A). Significant associations were also observed for anti-PD-1 as a class, nivolumab, pembrolizumab, anti-PD-L1, atezolizumab, durvalumab, anti-CTLA-4 as a class, ipilimumab, and anti-PD-1/PDL-1 plus anti-CTLA-4, and not reported with tremelimumab.
Table 3.
Disproportionality analysis of ICIs and SJS/TEN.
| Category | N. of case | ROR | ROR025 | ROR975 | IC | IC025 | IC975 |
|---|---|---|---|---|---|---|---|
| All cases (SJS or TEN) | |||||||
| ICIs | 411 | 2.88 | 2.61 | 3.17 | 1.49 | 1.35 | 1.65 |
| Anti-PD-1 | 356 | 3.29 | 2.96 | 3.66 | 1.69 | 1.52 | 1.87 |
| Nivolumab | 175 | 2.43 | 2.09 | 2.82 | 1.26 | 1.09 | 1.46 |
| Pembrolizumab | 183 | 4.93 | 4.26 | 5.71 | 2.27 | 1.96 | 2.62 |
| Cemiplimab | 1 | 2.80 | 0.39 | 19.96 | 0.81 | 0.11 | 5.74 |
| Anti-PD-L1 | 42 | 1.99 | 1.47 | 2.70 | 0.97 | 0.72 | 1.32 |
| Atezolizumab | 30 | 2.20 | 1.54 | 3.15 | 1.10 | 0.77 | 1.58 |
| Avelumab | 1 | 0.47 | 0.07 | 3.31 | -0.82 | -0.11 | -5.80 |
| Durvalumab | 13 | 2.42 | 1.41 | 4.18 | 1.20 | 0.70 | 2.07 |
| Anti-CTLA-4 | 71 | 2.10 | 1.66 | 2.65 | 1.05 | 0.83 | 1.33 |
| Ipilimumab | 71 | 2.10 | 1.66 | 2.65 | 1.05 | 0.83 | 1.33 |
| Tremelimumab | / | ||||||
| PD-1/PDL-1 plus CTLA-4 | 56 | 2.91 | 2.24 | 3.79 | 1.51 | 1.16 | 1.96 |
| SJS | |||||||
| ICIs | 253 | 2.71 | 2.39 | 3.07 | 1.41 | 1.24 | 1.59 |
| Anti-PD-1 | 212 | 3.00 | 2.61 | 3.43 | 1.55 | 1.36 | 1.78 |
| Nivolumab | 108 | 2.29 | 1.90 | 2.77 | 1.18 | 0.97 | 1.42 |
| Pembrolizumab | 106 | 4.36 | 3.60 | 5.29 | 2.09 | 1.72 | 2.53 |
| Cemiplimab | 1 | 4.29 | 0.60 | 30.61 | 1.03 | 0.14 | 7.36 |
| Anti-PD-L1 | 35 | 2.55 | 1.83 | 3.55 | 1.31 | 0.94 | 1.83 |
| Atezolizumab | 25 | 2.81 | 1.90 | 4.16 | 1.44 | 0.97 | 2.13 |
| Avelumab | 1 | 0.72 | 0.10 | 5.08 | -0.34 | -0.05 | -2.41 |
| Durvalumab | 11 | 3.14 | 1.74 | 5.68 | 1.52 | 0.84 | 2.75 |
| Anti-CTLA-4 | 39 | 1.76 | 1.29 | 2.41 | 0.80 | 0.58 | 1.10 |
| Ipilimumab | 39 | 1.76 | 1.29 | 2.41 | 0.80 | 0.58 | 1.10 |
| Tremelimumab | / | ||||||
| PD-1/PDL-1 plus CTLA-4 | 31 | 2.47 | 1.73 | 3.51 | 1.26 | 0.89 | 1.80 |
| TEN | |||||||
| ICIs | 184 | 2.82 | 2.43 | 3.26 | 1.46 | 1.26 | 1.69 |
| Anti-PD-1 | 166 | 3.36 | 2.88 | 3.92 | 1.71 | 1.47 | 2.00 |
| Nivolumab | 73 | 2.21 | 1.76 | 2.79 | 1.13 | 0.89 | 1.42 |
| Pembrolizumab | 93 | 5.49 | 4.47 | 6.74 | 2.40 | 1.96 | 2.95 |
| Cemiplimab | / | ||||||
| Anti-PD-L1 | 10 | 1.04 | 0.56 | 1.93 | 0.05 | 0.03 | 0.09 |
| Atezolizumab | 7 | 1.12 | 0.53 | 2.35 | 0.15 | 0.07 | 0.32 |
| Avelumab | 0 | ||||||
| Durvalumab | 3 | 1.22 | 0.39 | 3.80 | 0.25 | 0.08 | 0.76 |
| Anti-CTLA-4 | 34 | 2.20 | 1.57 | 3.08 | 1.11 | 0.79 | 1.55 |
| Ipilimumab | 34 | 2.20 | 1.57 | 3.08 | 1.11 | 0.79 | 1.55 |
| Tremelimumab | / | ||||||
| PD-1/PDL-1 plus CTLA-4 | 26 | 2.96 | 2.01 | 4.35 | 1.51 | 1.03 | 2.22 |
| SJS/TEN overlap* | |||||||
| ICIs | 26 | 1.65 | 1.12 | 2.44 | 0.70 | 0.47 | 1.03 |
| Anti-PD-1 | 22 | 1.85 | 1.22 | 2.82 | 0.85 | 0.56 | 1.30 |
| Nivolumab | 6 | 0.76 | 0.34 | 1.69 | -0.37 | -0.17 | -0.82 |
| Pembrolizumab | 16 | 3.95 | 2.41 | 6.46 | 1.85 | 1.13 | 3.02 |
| Cemiplimab | / | ||||||
| Anti-PD-L1 | 3 | 1.31 | 0.42 | 4.06 | 0.32 | 0.10 | 1.01 |
| Atezolizumab | 2 | 1.35 | 0.34 | 5.39 | 0.33 | 0.08 | 1.33 |
| Avelumab | / | ||||||
| Durvalumab | 1 | 1.71 | 0.24 | 12.18 | 0.47 | 0.07 | 3.33 |
| Anti-CTLA-4 | 2 | 0.54 | 0.14 | 2.17 | -0.74 | -0.19 | -2.98 |
| Ipilimumab | 2 | 0.54 | 0.14 | 2.17 | -0.74 | -0.19 | -2.98 |
| Tremelimumab | / | ||||||
| PD-1/PDL-1 plus CTLA-4 | 1 | 0.48 | 0.07 | 3.38 | -0.79 | -0.11 | -5.63 |
*SJS and TEN reported in the same case
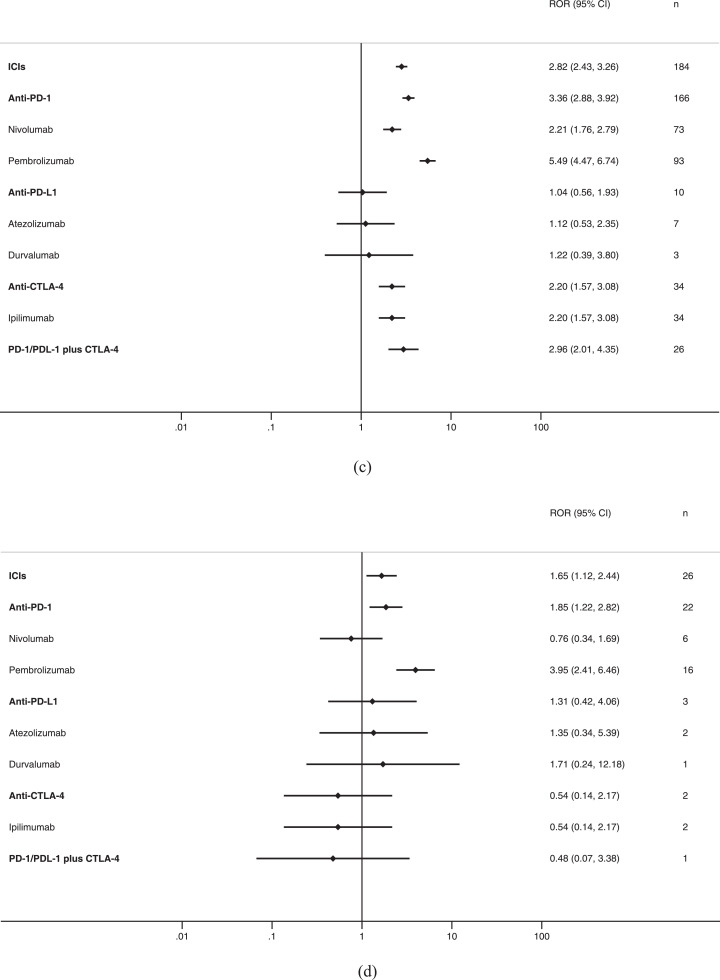
Fig 3.
ICIs and Stevens-Johnson syndrome (SJS)/toxic epidermal necrolysis (TEN), reporting odds ratios: A, all cases (SJS or TEN); B, SJS; C, TEN; D, SJS/TEN overlap.
For SJS, ICIs as a class had an ROR of 2.71 (95% CI 2.39–3.07) and a IC of 1.41 (95% CI 1.24–1.59) compared with all other medications (Table 3, Fig. 3B). Significant associations were also observed for anti-PD-1 as a class, nivolumab, pembrolizumab, anti-PD-L1, atezolizumab, durvalumab, anti-CTLA-4 as a class, ipilimumab, and anti-PD-1/PDL-1 plus anti-CTLA-4, and not reported with tremelimumab.
For TEN, ICIs as a class had an ROR of 2.82 (95% CI 2.43–3.26) and a IC of 1.46 (95% CI 1.26–1.69) compared with all other medications (Table 3, Fig. 3C). Significant associations were also observed for Anti-PD-1 as a class, nivolumab pembrolizumab, anti-CTLA-4, ipilimumab, anti-PD-1/PDL-1 plus anti-CTLA-4, but unassociated with anti-PD-L1, atezolizumab, durvalumab, and not reported with cemiplimab, avelumab, tremelimumab.
For SJS/TEN overlap, ICIs as a class had an ROR of 1.65 (95% CI 1.12–2.44) and a IC of 1.48 (95% CI 1.27–1.72) compared with all other medications (Table 3, Fig. 3D), it was also observed strongly associated with anti-PD-1 as a class and pembrolizumab.
4. Discussion
Following increasing use of ICIs, rare serious adverse events have been reported which prompted additional investigations. To our knowledge, the present study is the largest and most extensive analysis of SJS and TEN associated with ICIs collected data from both clinical trials and a worldwide pharmacovigilance database, and results suggested that ICIs was associated with significantly increased risk of SJS/TEN.
Due to the rare incidence of SJS and TEN, early RCTs did not identify any concerning safety signals. A small number of case reports were published6-7, but no causal association of ICIs with SJS and TEN could be established. Our results, based on meta-analysis of 20 RCTS (11597 patients), suggested that ICIs as class significantly increased the risk of SJS/TEN. We also performed a real-world analysis of 411 cases from FAERS, and results indicated that ICIs had a statistically significant positive signal with SJS/TEN. Previous studies suggested that the safety profiles of anti-PD-1, anti-PD-L1 and anti-CTLA-4 differed. Our results observed the safety signal for SJS/TEN was differed between different ICI therapies, with pembrolizumab presented the strongest signal for both SJS (ROR=4.36) and TEN (ROR=5.49), and the signal of TEN was differed between anti-PD-1(ROR=3.36) and anti-PD-L1(ROR=1.04) (Pinteraction<0.01). However, our subgroup analysis of RCTs did not find significant difference between different ICI therapies. Inconsistent with previous studies [20], combination therapy seemed to increase the risk of immune related cutaneous adverse events was not observed in our analysis, but this could be confounded by the tumors where combination therapy is used. It is interesting that there were proportionately more cases from Japan from FAERS, this may reflect the differences in the reporting structure, since anti-PD-1 drug was first approved in Japan. However, we did a post-hot analysis by excluding reportes from Japan, the signal for SJS/TEN seems to remain robust (ROR=2.58; IC:1.34). Due to some drugs (cemiplimab, avelumab, tremelimumab) are approved later, it is difficult to compare the safety profiles of different ICI therapies. Future research needed to be further investigated.
SJS/TEN are often triggered by particular medications, other medications that have been shown to increase these reactions include nonsteroidal antiinflammatory drugs, antiepileptic drugs, and certain antibiotics [21], [22], [23]. About 80% of the SJS/TEN cases occurred within the first 2 months of initiation treatment, with median onset time was 25.5 days (SJS:21.5 days; TEN:32 days), matching other studies [21], [22], [23]. Mortality rates of SJS/TEN among ICIs related cases (All cases:37%, SJS:19.9%, TEN:61.6%) seems to be higher than other medications, as previous study reported that mortality from SJS is approximately 5%, and mortality from TEN is approximately 30% [24]. One possible reason for this is the concurrent malignancy and older age as compared to other studies, which are specific predictor factors for mortality according to score for toxic epidermal necrolysis (SCORTEN) [25]. In addition, ICIs were mostly used as later line therapy in the past years, the mortality rate due to SJS/TEN may decrease with the increasing use of ICIs in first line and adjuvant therapy setting. Therefore, a high index of suspicion is necessary to identify SJS/TEN in its early stages in order to minimize the potential for both morbidity and mortality in patients using ICIs [7].
The mechanism of immune checkpoint inhibitor results to SJS/TEN is not clear. One mechanism is thought to be due to cytotoxicity induced by ICIs resulting in T-cell targeting of keratinocytes leading to apoptosis [5,26]. Currently, there is a consensus recommendation for permanent discontinuation of the offending drug, considering the high mortality rate associated with SJS/TEN. This was observed in our study, with 97.5% cases discontinue the ICIs. Serious skin toxicity usually requires systemic immunomodulating drugs, some evidence suggested that pulse steroids may have benefit [27]. Therefore, close collaboration between oncologists and dermatologists are encouraged in order to optimize treatment strategy, since immunomodulating drugs may impede the action of ICIs [27]. Subsequently, best supportive care should be provided to maintain fluid and electrolyte balance, minimize infectious, and prevent other complications risks. Further studies are needed to identify risk factors, pathogenesis and optimal management of SJS/TEN-like reactions to ICIs.
The present study has some limitations. Limitation for data from trials: First, adverse events generally reportable only up to 30 days following the last treatment date according to CTCAEs. Therefore, events occurring after 30 days following last treatment would not have been captured, which create a bias in the analysis [28]. Second, We collected events of SJS and TEN from both published data (from published literature) and unpublished data (from ClinicalTrials.gov), we chose to include events from the ClinicalTrials.gov, as it comprehensively reports all serious adverse events and updates events reporting even after publication [29].There might be bias surrounding the inclusion of unpublished data, as this data was not peer-reviewed. Our subgroup analysis by data source showed no significant difference, possibly explained by SJS and TEN being considered as serious conditions in almost every case. However, the interpretation of results from subgroup analyses should be cautious, in view of limited patients included, and subgroup analysis according to study quality was not performed. Limitation for pharmacovigilance data: First, the actual incidence of SJS/TEN due to ICIs cannot be determined, as the total number of patients using these medications is undetermined [30]. A high number of reported cases for SJS/TEN in the FAERS database were from healthcare providers (77.3%), which may improve the quality of reporting. Second, due to the voluntary nature of reporting to FAERS, the relationship between target drug and suspected adverse event was not clear and definite [30]. Third, drugs with frequent adverse events can increase the total number of reaction reports and therefore influence the value of ROR and IC [30]. Therefore, the magnitude and statistical significance of these associations may change with more reports are submitted. In addition, misclassification bias is highly probable for the definition of SJS/TEN, and data on SCORTEN score was not provided.
In conclusion, both meta-analysis of RCT's and real-world FAERS pharmacovigilance data suggested that the ICIs may increase the risk of SJS/TEN. Further studies are needed to identify risk factors and optimal management of SJS/TEN-like reactions to ICIs.
Author contributions
Junyan Wu had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Jianhong Zhu and Junyan Wu.
Acquisition of data: Jianhong Zhu, Guanghui Chen and Zhichao He
Analysis and interpretation of data: Jianhong Zhu, Guanghui Chen, Zhichao He, Yayuan Zheng, Siyuan Gao, Jianfang Li, Yin Ling, Xiaoxia Yu, Kaifeng Qiu, Junyan Wu.
Drafting of the manuscript: Jianhong Zhu, Guanghui Chen, Zhichao He, Yayuan Zheng, Siyuan Gao, Jianfang Li, Yin Ling, Xiaoxia Yu, Kaifeng Qiu, Junyan Wu.
Statistical analysis: Jianhong Zhu, Guanghui Chen, Zhichao He, Yayuan Zheng, Siyuan Gao.
Data sharing statement
Data from the meta-analysis are freely and publicly available on published literature and ClinicalTrials.gov. Data of retrospective pharmacovigilance study are collected from the FDA Adverse Event Reporting System, and can be gained via the OpenVigil website (http://openvigil.pharmacology.uni-kiel.de/openvigilfda.php).
Declaration of Competing Interest
Dr. Wu reports grants from Research Fund of Guangdong Pharmaceutical Association (2018LR18), during the conduct of the study. Dr. Zhu reports grants from Research Fund of Guangdong Pharmaceutical Association (2021ZX01), during the conduct of the study, the funder had no role in the study design, data collection, data analysis, data interpretation, and writing of the report. All other authors have nothing to declare.
Funding
Research Fund of Guangdong Pharmaceutical Association (2021ZX01; 2018LR18).
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2021.100951.
Appendix. Supplementary materials
References
- 1.Johnson D.B., Reynolds K.L., Sullivan R.J., Balko J.M., Patrinely J.R., Cappelli L.C., Naidoo J., Moslehi J.J. Immune checkpoint inhibitor toxicities: systems-based approaches to improve patient care and research. Lancet Oncol. 2020;21(8):e398–e404. doi: 10.1016/S1470-2045(20)30107-8. Aug. [DOI] [PubMed] [Google Scholar]
- 2.Xu C., Chen Y.P., Du X.J., Liu J.Q., Huang C.L., Chen L., Zhou G.Q., Li W.F., Mao Y.P., Hsu C., Liu Q., Lin A.H., Tang L.L., Sun Y., Ma J. Comparative safety of immune checkpoint inhibitors in cancer: systematic review and network meta-analysis. BMJ. 2018;363:k4226. doi: 10.1136/bmj.k4226. Nov 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ji H.H., Tang X.W., Dong Z., Song L., Jia Y.T. Adverse event profiles of anti-CTLA-4 and Anti-PD-1 monoclonal antibodies alone or in combination: analysis of spontaneous reports submitted to FAERS. Clin Drug Investig. 2019;39(3):319–330. doi: 10.1007/s40261-018-0735-0. Mar. [DOI] [PubMed] [Google Scholar]
- 4.Roujeau J.C., Stern R.S. Severe adverse cutaneous reactions to drugs. N Engl J Med. 1994;331(19):1272–1285. doi: 10.1056/NEJM199411103311906. Nov 10. [DOI] [PubMed] [Google Scholar]
- 5.Muntyanu A., Netchiporouk E., Gerstein W., Gniadecki R., Litvinov I.V. Cutaneous Immune-Related Adverse Events (irAEs) to immune checkpoint inhibitors: a dermatology perspective on management. J Cutan Med Surg. 2020 doi: 10.1177/1203475420943260. Aug 3. [DOI] [PubMed] [Google Scholar]
- 6.Phillips G.S., Wu J., Hellmann M.D., Postow M.A., Rizvi N.A., Freites-Martinez A., Chan D., Dusza S., Motzer R.J., Rosenberg J.E., Callahan M.K., Chapman P.B., Geskin L., Lopez A.T., Reed V.A., Fabbrocini G., Annunziata M.C., Kukoyi O., Pabani A., Yang C.H., Chung W.H., Markova A., Lacouture M.E. Treatment outcomes of immune-related cutaneous adverse events. J Clin Oncol. 2019;37(30):2746–2758. doi: 10.1200/JCO.18.02141. Oct 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maloney N.J., Ravi V., Cheng K., Bach D.Q., Worswick S. Stevens-Johnson syndrome and toxic epidermal necrolysis-like reactions to checkpoint inhibitors: a systematic review. Int J Dermatol. 2020;59(6):e183–e188. doi: 10.1111/ijd.14811. Jun. [DOI] [PubMed] [Google Scholar]
- 8.8Sarangdhar M., Tabar S., Schmidt C., Kushwaha A., Shah K., Dahlquist J.E., Jegga A.G., Aronow B.J. Data mining differential clinical outcomes associated with drug regimens using adverse event reporting data. Nat Biotechnol. 2016;34(7):697–700. doi: 10.1038/nbt.3623. Jul 12. [DOI] [PubMed] [Google Scholar]
- 9.Liberati A., Altman D.G., Tetzlaff J., Mulrow C., Gøtzsche P.C., Ioannidis J.P., Clarke M., Devereaux P.J., Kleijnen J., Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. Jul 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tudor Car L., Li L., Smith H., Atun R. Cochrane review: search strategies to identify observational studies in MEDLINE and EMBASE. J Evid Based Med. 2019;12(3):225–226. doi: 10.1111/jebm.12358. Aug. [DOI] [PubMed] [Google Scholar]
- 11.National Cancer Institute. NCI guidelines: adverse event reporting requirements. Sept 16, 2013. https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/aeguidelines.pdf
- 12.National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE). Version 4.0. 2010 (http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf).
- 13.Higgins J.P., Altman D.G., Gøtzsche P.C., Jüni P., Moher D., Oxman A.D. Cochrane Bias Methods Group; Cochrane Statistical Methods Group. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. Oct 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zheng Y., Zhu J., Liu Y., Lai W., Lin C., Qiu K., Wu J., Yao W. Triple therapy in the management of chronic obstructive pulmonary disease: systematic review and meta-analysis. BMJ. 2018;363:k4388. doi: 10.1136/bmj.k4388. Nov 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu J., Yu X., Zheng Y., Li J., Wang Y., Lin Y., He Z., Zhao W., Chen C., Qiu K., Wu J. Association of glucose-lowering medications with cardiovascular outcomes: an umbrella review and evidence map. Lancet Diabetes Endocrinol. 2020;8(3):192–205. doi: 10.1016/S2213-8587(19)30422-X. Mar. [DOI] [PubMed] [Google Scholar]
- 16.Guyatt G., Oxman A.D., Akl E.A., Kunz R., Vist G., Brozek J., Norris S., Falck-Ytter Y., Glasziou P., DeBeer H., Jaeschke R., Rind D., Meerpohl J., Dahm P., Schünemann H.J. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64(4):383–394. doi: 10.1016/j.jclinepi.2010.04.026. Apr. [DOI] [PubMed] [Google Scholar]
- 17.Zhai Y., Ye X., Hu F., Xu J., Guo X., Zhuang Y., He J. Endocrine toxicity of immune checkpoint inhibitors: a real-world study leveraging US Food and Drug Administration adverse events reporting system. J Immunother Cancer. 2019;7(1):286. doi: 10.1186/s40425-019-0754-2. Nov 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Böhm R., von Hehn L., Herdegen T., Klein H.J., Bruhn O., Petri H. OpenVigil FDA – inspection of U.S. American adverse drug events pharmacovigilance data and novel clinical applications. PLoS One. 2016;11(6) doi: 10.1371/journal.pone.0157753. Jun 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Norén G.N., Hopstadius J., Bate A. Shrinkage observed-to-expected ratios for robust and transparent large-scale pattern discovery. Stat Methods Med Res. 2013;22(1):57–69. doi: 10.1177/0962280211403604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khoja L., Day D., Wei-Wu Chen T., Siu L.L., Hansen A.R. Tumour- and class-specific patterns of immune-related adverse events of immune checkpoint inhibitors: a systematic review. Ann Oncol. 2017;28(10):2377–2385. doi: 10.1093/annonc/mdx286. Oct 1. [DOI] [PubMed] [Google Scholar]
- 21.Barvaliya M., Sanmukhani J., Patel T., Paliwal N., Shah H., Tripathi C. Drug-induced Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), and SJS-TEN overlap: a multicentric retrospective study. J Postgrad Med. 2011;57(2):115–119. doi: 10.4103/0022-3859.81865. Apr-Jun. [DOI] [PubMed] [Google Scholar]
- 22.Oshikoya K.A., Ogunyinka I.A., Ogar C.K., Abiola A., Ibrahim A., Oreagba I.A. Severe cutaneous adverse drug reactions manifesting as Stevens-Johnson syndrome and toxic epidermal necrolysis reported to the national pharmacovigilance center in Nigeria: a database review from 2004 to 2017. Ther Adv Drug Saf. 2020;11 doi: 10.1177/2042098620905998. Feb 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abe J., Umetsu R., Mataki K., Kato Y., Ueda N., Nakayama Y., Hane Y., Matsui T., Hatahira H., Sasaoka S., Motooka Y., Hara H., Kato Z., Kinosada Y., Inagaki N., Nakamura M. Analysis of Stevens-Johnson syndrome and toxic epidermal necrolysis using the Japanese Adverse Drug Event Report database. J Pharm Health Care Sci. 2016;2:14. doi: 10.1186/s40780-016-0048-5. Jun 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bastuji-Garin S., Fouchard N., Bertocchi M., Roujeau J.C., Revuz J., Wolkenstein P. SCORTEN: a severity-of-illness score for toxic epidermal necrolysis. J Invest Dermatol. 2000;115(2):149–153. doi: 10.1046/j.1523-1747.2000.00061.x. Aug. [DOI] [PubMed] [Google Scholar]
- 25.Goldinger S.M., Stieger P., Meier B., Micaletto S., Contassot E., French L.E., Dummer R. Cytotoxic cutaneous adverse drug reactions during anti-PD-1 therapy. Clin Cancer Res. 2016;22(16):4023–4029. doi: 10.1158/1078-0432.CCR-15-2872. Aug 15. [DOI] [PubMed] [Google Scholar]
- 26.Apalla Z., Papageorgiou C., Lallas A., Delli F., Fotiadou C., Kemanetzi C., Lazaridou E. Cutaneous adverse events of immune checkpoint inhibitors: a literature review. Dermatol Pract Concept. 2021;11(1) doi: 10.5826/dpc.1101a155. Jan 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu J., Wu J., Li G., Li J., Lin Y., He Z., Su C., Zhao W., Wu Q., Chen Z., Qiu K. Meta-analysis of randomized controlled trials for the incidence and risk of fatal adverse events in cancer patients treated with ipilimumab. Expert Opin Drug Saf. 2017;16(4):423–428. doi: 10.1080/14740338.2017.1297420. Apr. [DOI] [PubMed] [Google Scholar]
- 28.Morice P.M., Leary A., Dolladille C., Chrétien B., Poulain L., González-Martín A., Moore K., O'Reilly E.M., Ray-Coquard I., Alexandre J. Myelodysplastic syndrome and acute myeloid leukaemia in patients treated with PARP inhibitors: a safety meta-analysis of randomised controlled trials and a retrospective study of the WHO pharmacovigilance database. Lancet Haematol. 2021;8(2):e122–e134. doi: 10.1016/S2352-3026(20)30360-4. Feb. [DOI] [PubMed] [Google Scholar]
- 29.Borrelli E.P., Lee E.Y., Descoteaux A.M., Kogut S.J., Caffrey A.R. Stevens-Johnson syndrome and toxic epidermal necrolysis with antiepileptic drugs: an analysis of the US Food and Drug Administration Adverse Event Reporting System. Epilepsia. 2018;59(12):2318–2324. doi: 10.1111/epi.14591. Dec. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.