Abstract
Background
The use of transvenous pacing leads is associated with the risk of developing tricuspid valve (TV) dysfunction. This develops through several mechanisms including the failure of leaflet coaptation or direct damage to the TV or to its sub-valvular apparatus and can result in significant tricuspid regurgitation (TR). Multiple approaches to pacemaker implantation after transvenous lead extraction (TLE) or surgical TV repair have been described. Placement of pacing leads across the TV is generally avoided in such circumstances.
Case summary
A 66-year-old woman presented with a year-long history of exertional dyspnoea, peripheral oedema, and postural neck pulsations. Her medical history included a dual-chamber pacemaker implantation for sinus node dysfunction 14 years ago. Echocardiography revealed severe lead-related TR. Her case was discussed in our multi-disciplinary team meeting. A decision was made to perform a TLE and implant a leadless pacemaker in an attempt to avoid open-heart surgery if possible. This was reserved as an option in the event of persistent severe TR. Transvenous extraction of the right ventricular lead was performed. The atrial lead was preserved and connected to and AAI device. A Micra AV was implanted allowing for atrioventricular (AV) synchronous pacing.
Discussion
We present the first case of successful implementation of AV sequential pacing using a dual-pacemaker approach involving the use of an AAI pacemaker and a Micra AV device. This was performed after TLE for severe lead-related TR.
Keywords: Atrioventricular synchrony, Leadless pacemaker, Micra AV, Transvenous lead extraction, Pacemaker complications, Case report
For the podcast associated with this article, please visit https://academic.oup.com/ehjcr/pages/podcast
Learning points:
Lead-related tricuspid valve dysfunction is an important and increasingly recognized complication.
The Micra™ AV is a second-generation leadless pacemaker which uses a three-axis accelerometer to sense the atrial mechanical contraction, thus enabling atrioventricular synchronous pacing.
Atrio-ventricular sequential pacing can be achieved using a dual-pacemaker approach involving the use of a Micra™ AV and a transvenous AAI.
Introduction
The use of transvenous pacing leads is associated with the risk of developing tricuspid valve (TV) dysfunction. This develops through a number of mechanisms including the failure of leaflet coaptation or direct damage to the TV or to its sub-valvular apparatus and can result in significant tricuspid regurgitation (TR).1–3 The development of significant lead-related TR is associated with right ventricular (RV) and atrial (RA) dilatation, an increased risk of heart failure hospitalization and all-cause mortality.4
Multiple approaches to pacemaker implantation after transvenous lead extraction (TLE) or surgical TV repair have been described. Placement of pacing leads across the TV is generally avoided in such circumstances. We describe a novel approach to maintaining atrioventricular (AV) sequential pacing in a patient with sinus node dysfunction, AV conduction disease, and severe lead-related TR.
Timeline
| March 2004 | Left atrial myxoma resection |
| July 2005 | Typical atrial flutter ablation |
| July 2006 | Dual-chamber pacemaker implant for sinus node dysfunction |
| May 2019 | Onset of symptoms |
| May 2020 | Referred to tertiary centre with diagnosis of severe lead related tricuspid regurgitation (TR) |
| 29 May 2020 | Multi-disciplinary team decision of lead RV lead extraction and leadless pacemaker implant. |
| 15 June 2020 | Transvenous right ventricular (RV) lead extraction + Micra AV Implant |
| 1 July 2020 | Satisfactory device checks. Persistent New York Heart Association III and transthoracic echocardiography showing persistent severe TR |
| 14 September 2020 | Assessed by cardiothoracic surgeon and pre-operative work-up organized |
Case presentation
A 66-year-old woman presented with a year-long history of exertional dyspnoea, peripheral oedema, and postural neck pulsations.
She had a history of hypertension, type II diabetes mellitus, hypothyroidism, previous excision of a benign left atrial myxoma, previous ablation for typical atrial flutter, and dual-chamber pacemaker implantation for sinus node dysfunction 14 years ago. This was a Medtronic™ Versa® dual-chamber pacemaker with active fixation Medtronic™ 5568 and 5076 leads to the RA appendage and RV apex, respectively. An echocardiogram performed prior to her initial pacemaker implant was normal apart for minor aortic valve sclerosis.
Pacemaker interrogation revealed that the patient was 100% atrially paced and had developed AV conduction disease with a 10% ventricular pacing requirement at a base rate of 60 b.p.m. despite the use of an algorithm to minimize ventricular pacing (AAI-DDD mode).
Clinical examination revealed peripheral oedema and an elevated jugular venous pressure with prominent V waves.
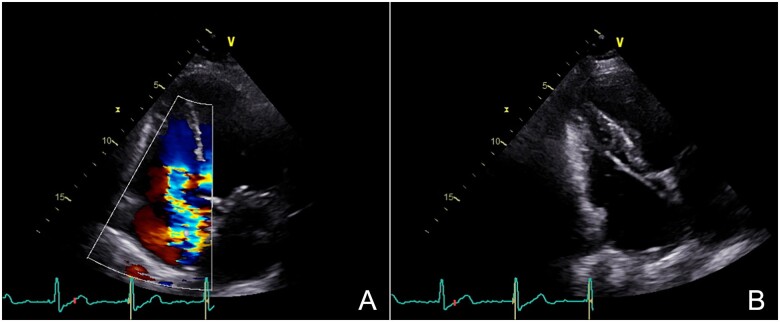
Transthoracic echocardiography (TTE) revealed severe TR due to apparent tethering of the septal leaflet of the TV by the RV lead (Figure 1A and B, Video 1A and B). The RV was non-dilated with preserved systolic function. Left ventricular systolic function was normal, and there were no other significant valvular abnormalities.
Figure 1.
(A) Apical four-chamber view showing severe tricuspid regurgitation. (B) Modified apical four-chamber view suggesting right ventricular lead impingement of the tricuspid valve septal leaflet.
The patient’s case was discussed in our cardiology/cardiothoracic multi-disciplinary team meeting. Her estimated risk of inpatient surgical mortality for TV replacement or repair was 4.1% (EUROSCORE II). Transvenous RV lead extraction with leadless pacemaker implantation was chosen as the first-line strategy, with a view to subsequent TV surgery (either via sternotomy or minimally invasive surgery) if severe TR persisted despite extraction of the RV lead.
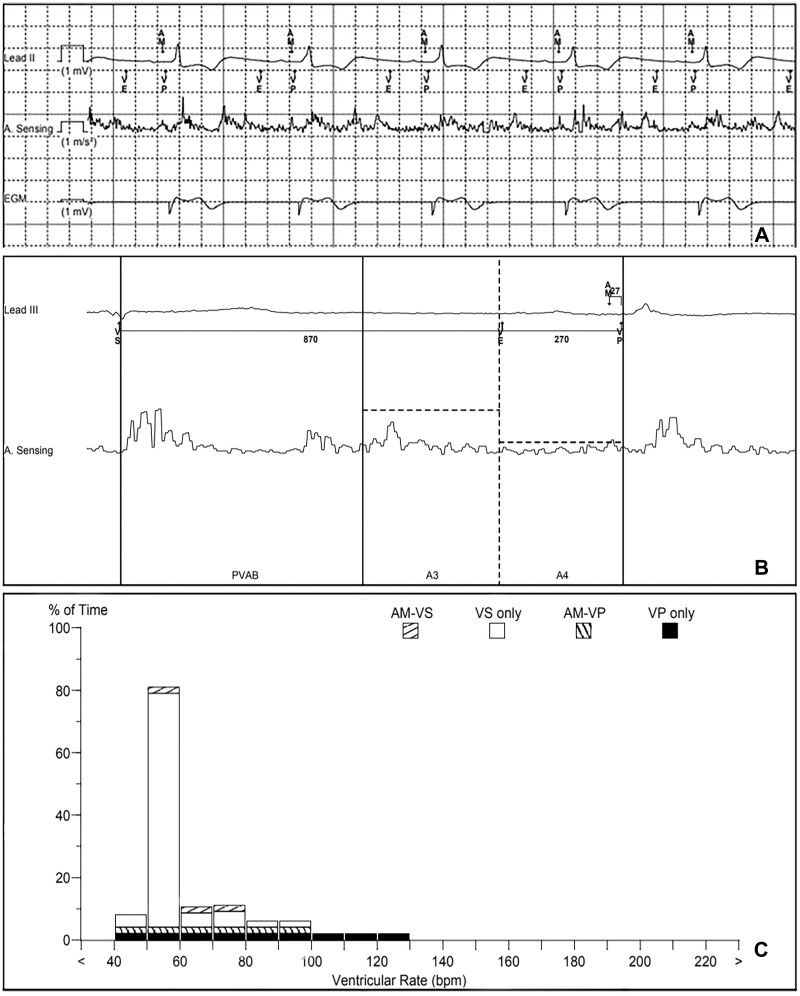
The patient was admitted electively for the procedure. This was performed under general anaesthesia with prophylactic antibiotic cover. A temporary pacing wire was placed through left femoral venous access. A left infra-clavicular horizontal incision was made through the old scar using a PlasmaBlade™ (Medtronic Plc, USA). Dissection was made to the pacemaker generator and it was liberated from the pre-pectoral pocket. The RV lead was extracted using a 9 Fr Evolution® RL sheath (Cook Medical, USA). The RA lead was tested then connected to an Accolade SR (Boston Scientific, Massachusetts, USA) device. The device was placed in a TYRX™ envelope (Medtronic Plc, USA) and the wound was closed in two layers. Right femoral venous access was obtained and a Micra AV™ (Medtronic Plc, USA) was implanted using the well-described implantation technique.5 A mid-septal location was chosen on this occasion. The atrial sensing setup feature was run determining an A4 threshold of 1.0 m/s2, A3 threshold of 3.7 m/s2, A3 window end of 860 ms, and a sensing vector of 1 + 3. Sequential AV pacing was successful (Figure 2A).
Figure 2.
(A) Pacing system analysis demonstrating atrial mechanical sensing (AM) and atrioventricular sequential pacing. (B) Micra™ AV accelerometer waveform showing a small but well-sensed A4 signal. (C) Micra ™ AV rate histogram.
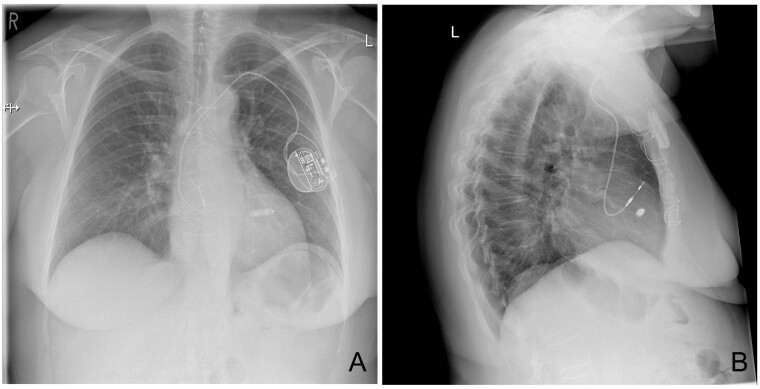
Post-procedural checks including a chest X-ray were satisfactory (Figure 3). The patient was allowed home the same day.
Figure 3.
(A) Post-procedural postero-anterior chest X-ray excluding pneumothorax/haemothorax. (B) Lateral chest X-ray showing correct lead and device positions.
The patient remained markedly breathless (New York Heart Association III) and described ongoing pedal oedema at her 2-week follow-up appointment. A repeat TTE was performed which confirmed severe residual eccentric TR. It showed RV dilatation with basal and mid-RV diameters of 4.5 cm and 3.7 cm, respectively, which may have developed secondary to persistent severe TR. The patient was referred to the cardiothoracic surgical team for TV surgery (repair or replacement). The operation is yet to be performed at the time of writing this manuscript, delayed by the current pandemic.
Interrogation of the Micra™ AV revealed satisfactory parameters with a sensed R wave of 19.7 mV, the impedance of 950 Ω, and threshold of 0.38 V @ 0.24 ms. Manual atrial mechanical testing revealed a small atrial mechanical marker (A4) signal, though this was well sensed (Figure 2B). The device was in VVI+ mode 87% of the time, favouring intrinsic AV conduction. Ventricular pacing (VP) was delivered 9.9% of the time. This was divided into 4.4% AMVP and 5.5% VP (Figure 2C). The projected battery longevity was 14 years. The interrogation of the AAI device was also satisfactory.
Discussion
Pacing leads may cause TR through several mechanisms including leaflet perforation, transection of the chordae tendinae or of the papillary muscle, or through failure of leaflet coaptation.1–3 Transvenous lead extraction can result in a significant reduction in TR severity and improved symptoms even in cases where a new RV lead is implanted.6 Park et al.7 found that TLE resulted in a significant reduction in TR severity in approximately one-third of patients with severe TR pre-extraction. It must be recognized, however, that effect of TLE on TV dysfunction is variable and highly dependent on the mechanism of TV dysfunction and that results are less favourable in the presence of tricuspid annular dilatation.8 Careful selection of patients prior to TLE is therefore essential. We routinely discuss such cases in a multi-disciplinary team setting involving cardiac device specialists, imaging specialists, and cardiothoracic surgeons. In this case, we decided to perform TLE in the first instance in an attempt to avert open-heart surgery. Unfortunately, TLE did not result in a reduction in TR, and the patient was referred for TV surgery.
Several approaches to pacemaker implantation have been described following TLE for lead-related TR. These include ventricular pacing using epicardial leads, transvenous techniques that avoid placing a lead through the TV (e.g. LV pacing via the coronary sinus or His-bundle pacing), and the use of leadless pacemakers. We strongly considered using either an active fixation LV lead or His-bundle pacing, and both may have been effective alternatives, but we felt that the risk of raised thresholds or loss of ventricular pacing due to displacement or dislodgement during surgery made leadless pacing the most effective long-term option. We did not consider epicardial pacing to be a suitable first-line pacing strategy as epicardial leads can only be placed at the time of cardiac surgery.
Leadless pacemaker implantation is associated with high procedural success rates and a low complication rate.9 Their widespread use has, until recently, been limited by their inability to provide AV synchronous pacing. Atrial-based pacing has been demonstrated to reduce the risk of atrial fibrillation and stroke in patients with sinus node dysfunction.10 Some studies have also found reductions in the risk of pacemaker syndrome and improvements in quality of life with the use of dual-chamber devices.11
The second-generation leadless Micra™ AV has been released recently. It uses a three-axis accelerometer to sense the atrial mechanical contraction and allow AV synchronous pacing. This accelerometer produces waveforms, designated A1–A4, which correspond to the heart sounds S1–S4. Sensing of signals A1–A3 (corresponding to ventricular systole and passive ventricular filling) is avoided by the device as they fall during the post-ventricular atrial blanking period (PVAB) and A3 window (Figure 2B). The device seeks to sense the A4 signal (corresponding to atrial mechanical contraction) during the A4 window. This window starts at the end of the A3 window and is marked as ventricular end on the marker channel (Figure 2A and B). The A4 signal (designated AM on the marker channel) is followed by ventricular pacing after a brief ventriculo-atrial interval (Figure 2A and B).
The Micra™ AV was found to achieve ≥70% AV synchrony in 38 out of 40 patients with intact sinus node function and complete AV block.12 In our case, the patient had sinus node dysfunction requiring 100% atrial pacing prior to implantation of the Micra™ AV which can provide VDD pacing, but not atrial pacing. Our solution was to connect the existing atrial lead from the DDD system to a single chamber pulse generator to provide AAI pacing. The Micra™ AV sensed and tracked atrial activity thus achieving AV synchrony.
Another important point to consider is the impact of leadless pacing on TV function. Beurskens et al.,13 in an observational study assessing the impact of leadless pacemakers on cardiac and AV valve function, found comparable rates of worsening TR in 53 leadless pacemaker recipients and 53 age and sex-matched controls. Limitations included the fact that controls were recipients of dual (as opposed to single) -chamber pacemakers. This resulted in important differences in pacing indications between the two groups and higher rates of atrial arrhythmias in the leadless pacing group. Additionally, patients in the leadless pacing group did not benefit from AV synchrony. The available data, therefore, does not allow us to make any firm conclusions about the relative impact of leadless vs. conventional pacemakers on worsening TR. Importantly, Beurskens et al. found a five-fold increase in TR with septal as opposed to apical leadless pacing. The authors postulated that this was due to mechanical interactions between the leadless pacemakers and the TV and its sub-valvular apparatus. This striking finding clearly merits further investigation.
With regards to our patient’s long-term pacing strategy, her leadless pacemaker’s projected battery longevity (at current pacing requirements and outputs) is ∼14 years. Battery longevity would be expected to reduce to 9.5 years should a 50% pacing requirement develop. Depending on the clinical status when the Micra™ AV reaches the end of service, we may consider several pacing options including the addition of a further leadless pacemaker adjacent to the current device or the use of an LV lead.
In conclusion, our case demonstrates that a dual-pacemaker strategy using a single chamber atrial pacemaker and VDD leadless pacemaker can successfully deliver AV sequential pacing. Our case also illustrates the fact that the reduction in lead-related TR severity is variable, even in carefully selected cases.
Lead author biography

Dr Elhosseyn Guella is a subspecialist trainee in heart failure and cardiac implantable electronic devices in the North-Western Deanery of England. He completed a B.Sc. in basic medical sciences in 2010 and Doctorate in Medicine (M.D.) from the Arabian Gulf University in Bahrain in 2012. He undertook his Foundation and Core Medical Training in the north of England and became a member of the Royal College of Physicians (MRCP, London) in 2015. He completed a post-graduate diploma in medical education from the University of Manchester and joined the North-Western Deanery’s cardiology training programme in 2016. He was accredited as a Certified Cardiac Device Specialist by the IBHRE in 2020.
Supplementary material
Supplementary material is available at European Heart Journal - Case Reports online.
Supplementary Material
Acknowledgements
We thank Dr L. Darwich for her help with figure preparation.
Consent: The authors confirm that written consent for submission and publication of this case report including images and associated text has been obtained from the patient in line with COPE guidance.
Slide sets: A fully edited slide set detailing these cases and suitable for local presentation is available online as Supplementary data.
Funding: None declared.
Conflict of interest: F.Z.A. has previously received a research grant funded by Medtronic. F.Z.A. has received honoraria from Medtronic, Vifor, Astrazeneca, and Servier. C.C. has received honoraria and speaking fees from Abbott, Boston Scientific and Medtronic. A.Z. has received honoraria from Medtronic, Abbott, and Boston Scientific. The remaining authors have nothing to disclose.
References
- 1. Höke U, Auger D, Thijssen J, Wolterbeek R, van der Velde E, Holman E. et al. Significant lead-induced tricuspid regurgitation is associated with poor prognosis at long-term follow-up. Heart 2014;100:960–968. [DOI] [PubMed] [Google Scholar]
- 2. Chang J, Manning W, Ebrille E, Zimetbaum T.. Tricuspid valve dysfunction following pacemaker or cardioverter-defibrillator implantation. J Am Coll Cardiol 2017;69:2331–2341. [DOI] [PubMed] [Google Scholar]
- 3. Lin G, Nishimura RA, Connolly HM, Dearani JA, Sundt TM, Hayes DL.. Severe symptomatic tricuspid valve regurgitation due to permanent pacemaker or implantable cardioverter-defibrillator leads. ACC Curr J Rev 2005;14:52. [DOI] [PubMed] [Google Scholar]
- 4. Delling FN, Hassan ZK, Piatkowski G, Tsao CW, Rajabali A, Markson LJ. et al. Tricuspid regurgitation and mortality in patients with transvenous permanent pacemaker leads. Am J Cardiol 2016;117:988–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. EL-Chami M, Roberts P, Kypta A, Omdahl P, Bonner M, Kowal R. et al. How to implant a leadless pacemaker with a tine-based fixation. J Cardiovasc Electrophysiol 2016;27:1495–1501. [DOI] [PubMed] [Google Scholar]
- 6. Polewczyk A, Kutarski A, Tomaszewski A, Brzozowski W, Czajkowski M, Polewczyk M. et al. Lead dependent tricuspid dysfunction: analysis of the mechanism and management in patients referred for transvenous lead extraction. Cardiol J 2013;20:402–410. [DOI] [PubMed] [Google Scholar]
- 7. Park S-J, Gentry JL, Varma N, Wazni O, Tarakji KG, Mehta A. et al. transvenous extraction of pacemaker and defibrillator leads and the risk of tricuspid valve regurgitation. JACC Clin Electrophysiol 2018;4:1421–1428. [DOI] [PubMed] [Google Scholar]
- 8. Nazmul M, Cha Y, Lin G, Asirvatham S, Powell B.. Percutaneous pacemaker or implantable cardioverter-defibrillator lead removal in an attempt to improve symptomatic tricuspid regurgitation. Europace 2013;15:409–413. [DOI] [PubMed] [Google Scholar]
- 9. El-Chami M, Al-Samadi F, Clementy N, Garweg C, Martinez-Sande J, Piccini J. et al. Updated performance of the Micra transcatheter pacemaker in the real-world setting: a comparison to the investigational study and a transvenous historical control. Heart Rhythm 2018;15:1800–1807. [DOI] [PubMed] [Google Scholar]
- 10. Healey J, Toff W, Lamas G, Andersen H, Thorpe K, Ellenbogen K. et al. Cardiovascular outcomes with atrial-based pacing compared with ventricular pacing. Circulation 2006;114:11–17. [DOI] [PubMed] [Google Scholar]
- 11. Lamas G, Lee K, Sweeney M, Silverman R, Leon A, Yee R. et al. Ventricular pacing or dual-chamber pacing for sinus-node dysfunction. N Engl J Med 2002;346:1854–1862. [DOI] [PubMed] [Google Scholar]
- 12. Steinwender C, Khelae S, Garweg C, Chan J, Ritter P, Johansen J. et al. Atrioventricular synchronous pacing using a leadless ventricular pacemaker. JACC: Clin Electrophysiol 2020;6:94–106. [DOI] [PubMed] [Google Scholar]
- 13. Beurskens N, Tjong F, de Bruin-Bon R, Dasselaar K, Kuijt W, Wilde A. et al. Impact of leadless pacemaker therapy on cardiac and atrioventricular valve function through 12 months of follow-up. Circ: Arrhythmia Electrophysiol 2019;12:e007124. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.