Key Points
Question
Does treatment of moderately severe to severe nonproliferative diabetic retinopathy (NPDR) with intravitreal aflibercept injections result in 2-step or greater improvement on the Diabetic Retinopathy Severity Scale in more eyes, fewer vision-threatening complications, and fewer center-involved diabetic macular edema events from baseline through 100 weeks compared with sham injections?
Findings
In this randomized clinical trial of 402 patients with moderately severe to severe NPDR without diabetic macular edema, more eyes treated with intravitreal aflibercept injections showed a 2-step or greater improvement on the Diabetic Retinopathy Severity Scale at 24, 52, and 100 weeks, with significantly fewer vision-threatening complications and center-involved diabetic macular edema events. No differences in mean change in best-corrected visual acuity at weeks 52 and 100 were observed.
Meaning
In this study, anatomic improvement was more likely to occur in eyes with moderately severe to severe NPDR that were treated with intravitreal aflibercept injections; in year 2, fixed dosing appeared necessary to maintain anatomic benefit.
Abstract
Importance
Proactive treatment of nonproliferative diabetic retinopathy (NPDR) reduces the risk of progression to vision-threatening complications.
Objective
To evaluate vascular endothelial growth factor blockade therapy with intravitreal aflibercept injections in eyes with severe NPDR without diabetic macular edema (DME).
Design, Setting, and Participants
The Study of the Efficacy and Safety of Intravitreal Aflibercept for the Improvement of Moderately Severe to Severe Nonproliferative Diabetic Retinopathy (PANORAMA) was a double-masked 100-week randomized clinical trial conducted in multiple centers worldwide. The study included 402 adults with Diabetic Retinopathy Severity Scale (DRSS) level 47 or 53 with no DME and best-corrected visual acuity of 20/40 or better.
Interventions
Intravitreal injections of aflibercept, 2 mg, every 16 weeks after 3 initial monthly doses and one 8-week interval (aflibercept 2q16 group); intravitreal injections of aflibercept, 2 mg, every 8 weeks after 5 initial monthly doses, with pro re nata (PRN) dosing beginning at week 56 (aflibercept 2q8/PRN group); or sham injections (control group).
Main Outcomes and Measures
Proportions of eyes with a 2-step or greater improvement in DRSS level, vision-threatening complications, and center-involved DME from baseline to weeks 24, 52, and 100.
Results
Among 402 participants (1 eye per participant), the mean (SD) age was 55.7 (10.5) years; 225 (56.0%) were male, and 310 (77.1%) were White. A total of 135 were randomized to the aflibercept 2q16 group, 134 to the aflibercept 2q8/PRN group, and 133 to the control group. At 24 weeks, treatment with aflibercept resulted in a 2-step or greater improvement in DRSS level in 157 of 269 eyes (58.4%) in the combined aflibercept groups vs 8 of 133 eyes (6.0%) in the control group (adjusted difference, 52.3%; 95% CI, 45.2%-59.5%; P < .001). At 52 weeks, 88 of 135 eyes (65.2%) in the aflibercept 2q16 group (adjusted difference, 50.1%; 95% CI, 40.1%-60.1%) and 107 of 134 eyes (79.9%) in the aflibercept 2q8/PRN group (adjusted difference, 64.8%; 95% CI, 55.8%-73.9%) compared with 20 of 133 eyes (15.0%) in the control group (P < .001 for both comparisons) showed a 2-step or greater improvement in DRSS level. Fewer eyes treated with aflibercept vs sham injections developed vision-threatening complications and/or center-involved DME through week 100 (22 of 135 eyes [16.3%] in the 2q16 group [adjusted difference, −34.2%; 95% CI, −44.6 to −23.8] and 25 of 134 eyes [18.7%] in the 2q8/PRN group [adjusted difference, −31.7%; 95% CI, −42.5 to −20.9] compared with 67 of 133 eyes [50.4%] in the control group; P < .001 for both comparisons). No new safety signals were identified.
Conclusions and Relevance
In this study, significantly more eyes with moderately severe to severe NPDR that were treated with aflibercept showed a 2-step or greater improvement in DRSS level at 24, 52, and 100 weeks, and significantly fewer eyes treated with aflibercept vs sham developed vision-threatening complications and center-involved DME. Outcomes on the DRSS between year 1 and 2 emphasize the need for ongoing vascular endothelial growth factor suppression and adherence.
Trial Registration
ClinicalTrials.gov Identifier: NCT02718326
This randomized clinical trial evaluates whether treatment with intravitreal aflibercept vs sham injections results in anatomic improvement from baseline through 100 weeks in eyes with moderately severe to severe nonproliferative diabetic retinopathy and no diabetic macular edema.
Introduction
Diabetic retinopathy (DR) is a leading cause of blindness globally.1,2 Disease severity ranges from nonproliferative DR (NPDR) to proliferative DR (PDR), with 13 distinct categories on the Diabetic Retinopathy Severity Scale (DRSS) developed in the Early Treatment Diabetic Retinopathy Study (ETDRS).3 The microvascular ischemia associated with DR leads to vision loss, primarily through pathologic neovascularization of the retina and/or anterior segment and diabetic macular edema (DME). Increasing NPDR severity is associated with an increased risk of progression to PDR and vision loss.3
Among patients with untreated eyes in the landmark Diabetic Retinopathy Study, after 4 years, 12.8% of eyes with severe NPDR, 20.9% of eyes with non–high-risk PDR, and 44.0% of eyes with high-risk PDR developed severe vision loss.4 The Diabetic Retinopathy Study established panretinal photocoagulation (PRP) as the cornerstone of treatment for PDR, providing a 57% reduction in the development of severe vision loss.5,6 Several clinical trials, including the phase 2 CLARITY (Clinical Efficacy and Mechanistic Evaluation of Aflibercept for Proliferative Diabetic Retinopathy) study and the DRCR Network Protocol S (Prompt Panretinal Photocoagulation Versus Intravitreal Ranibizumab With Deferred Panretinal Photocoagulation for Proliferative Diabetic Retinopathy) study, have found vascular endothelial growth factor (VEGF) inhibition to be highly effective, with some indications of superiority in specific outcomes compared with PRP.7,8,9 For patients with DME, intravitreal VEGF inhibitors have delivered substantial long-term visual gains compared with focal laser photocoagulation, establishing anti-VEGF therapy as the standard of care.10,11,12,13
To date, DR treatment guidelines remain predominantly reactive,14,15 with treatment recommended only when center-involved DME (CI-DME) and/or PDR develop. However, once PDR has developed, retinal damage from fibrosis and contraction may not be reversible, even with intervention, and can be associated with permanent vision loss.7,9 Subsequent treatment with PRP leads to visual field loss because the procedure is inherently destructive.16 Therefore, prevention of progression to PDR and DME represents an important public health goal. Patients with NPDR (even those without DME) have experienced a progressive decrease in vision-related quality-of-life functioning as retinopathy severity worsens.17 The Study of the Efficacy and Safety of Intravitreal Aflibercept for the Improvement of Moderately Severe to Severe Nonproliferative Diabetic Retinopathy (PANORAMA) was a randomized clinical trial conducted to compare intravitreal aflibercept injections with sham injections in eyes with moderately severe to severe NPDR (referred to as severe NPDR hereinafter) and no DME.
Methods
Study Design
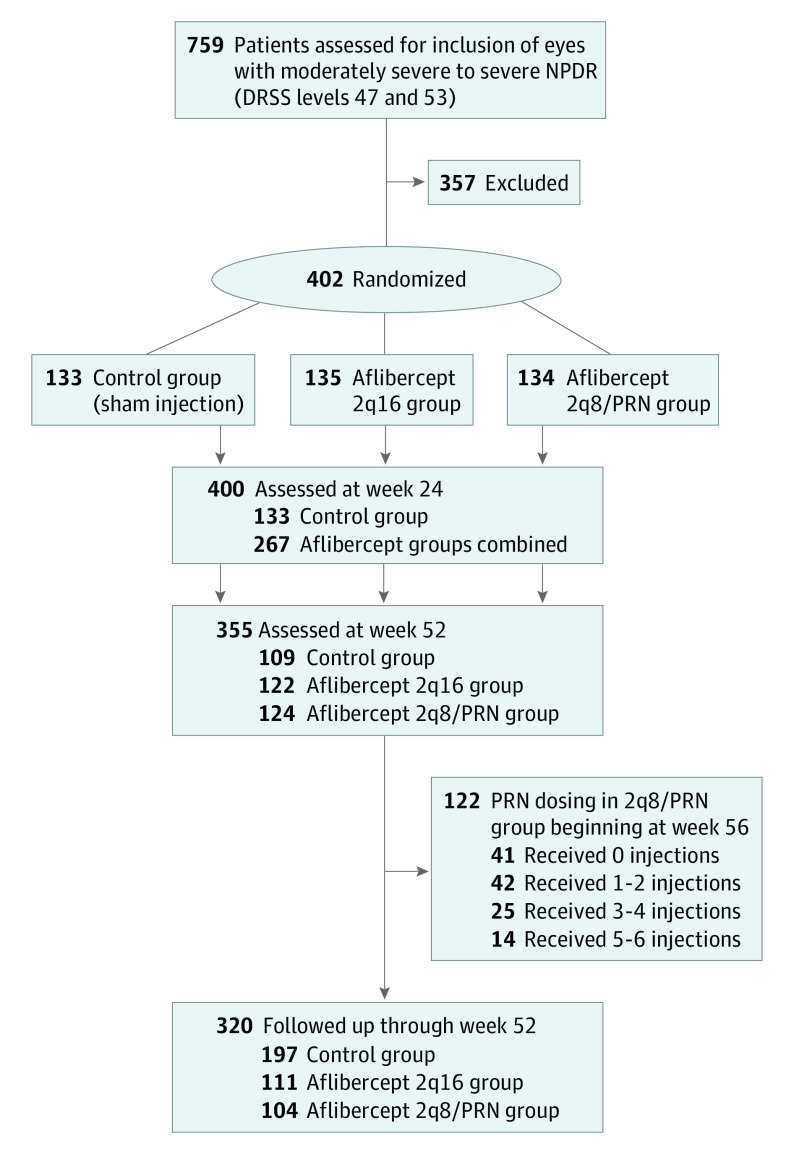
The PANORAMA study was a phase 3 double-masked 100-week randomized clinical trial of adult participants who had diabetes and severe treatment-naive NPDR (DRSS level of 47 or 53) with no DME and best-corrected visual acuity (BCVA) of 20/40 (Snellen equivalent) or better. The study was conducted at 87 sites in the US, Japan, Germany, Hungary, and the United Kingdom. The trial protocol was approved by the institutional review board and/or ethics committee at each participating site, and the study was performed in adherence with the principles of the Declaration of Helsinki18 and local regulations. All participants provided written informed consent. This study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline for randomized clinical trials.
Data for this article were collected between March 2016 and July 2019. Full details of the clinical trial design are provided in Supplement 1, and information about the PANORAMA investigators and patient eligibility is available in eMethods 1 and eMethods 2 in Supplement 2.
Treatments
Eyes were randomized to 1 of 3 groups. The first group received intravitreal injections of aflibercept, 2 mg, every 16 weeks after 3 initial monthly doses and one 8-week interval (aflibercept 2q16 group). The second group received intravitreal injections of aflibercept, 2 mg, every 8 weeks after 5 initial monthly doses during year 1, with flexible pro re nata (PRN) dosing in year 2 if the investigator (D.M.B., C.C.W., W.L.C., A.E., or P.M.H.) determined the DRSS level was worse than 35 (aflibercept 2q8/PRN group). The third group received sham injections with observation (control group) (Figure 1). Randomization was stratified by baseline DRSS level (47 or 53). All participants were evaluated monthly through week 16, then every 8 weeks through week 96, with additional evaluations at weeks 52 and 100 (eFigure 1 in Supplement 2).
Figure 1. Study Design.
One eye was assessed per participant. Aflibercept 2q16 group: intravitreal injection of aflibercept, 2 mg, every 16 weeks after 3 initial monthly doses and one 8-week interval. Aflibercept 2q8/PRN group: intravitreal injection of aflibercept, 2 mg every 8 weeks after 5 initial monthly doses, with pro re nata (PRN) dosing beginning at week 56. DRSS indicates Diabetic Retinopathy Severity Scale; NPDR, nonproliferative diabetic retinopathy; and PRN, pro re nata.
Participants in all groups could receive rescue treatment at the discretion of the masked investigator (D.M.B., C.W., W.L.C., A.E., or P.M.H.). Those with vision-threatening complications (including events of PDR and/or anterior segment neovascularization) received 1 intravitreal aflibercept injection, PRP, and/or vitrectomy; those with CI-DME received PRN intravitreal aflibercept injections or laser photocoagulation. After rescue treatment, randomized treatment was discontinued for patients with CI-DME but continued for those with vision-threatening complications.
Outcome Measures
The primary efficacy end point for the PANORAMA study was the proportion of eyes with a 2-step or greater improvement in DRSS level from baseline to week 24 (both aflibercept groups combined vs the control group) and week 52 (each aflibercept group vs the control group) (Figure 1). Secondary end points included the proportion of eyes that developed vision-threatening complications or CI-DME at week 52. Additional end points included the proportion of eyes with a 2-step or greater worsening in DRSS level from baseline to week 52 and week 100. This article presents the 24-week, 52-week, and 100-week results. Further methodologic details are available in eMethods 2 in Supplement 2.
Statistical Analysis
All efficacy end points for week 100 were exploratory, and P values were nominal. Missing or nongradable postbaseline values were imputed using the last observation carried forward procedure. Baseline values were carried forward if all postbaseline observations were missing or nongradable. For participants receiving rescue treatment, data from the last observation before administration of rescue treatment were carried forward. The primary end point analyses were tested at a significance level of 1.67% (P < .0167); P values for primary end points were calculated using a 2-sided Cochran-Mantel-Haenszel test adjusted by baseline DRSS stratification variable. The significance thresholds for the secondary end points were dependent on the outcomes of the primary end points. For the sensitivity analysis, the primary efficacy analysis was also performed using all observed measurements (regardless of whether rescue treatment was administered). All analyses were performed using SAS software, version 9.4 (SAS Institute Inc).
Results
Of 759 participants assessed for eligibility, 402 participants with severe NPDR (1 eye per participant) were randomized. The mean (SD) age was 55.7 (10.5) years; 225 participants (56.0%) were male, 177 (44.0%) were female, 310 (77.1%) were White, 41 (10.2%) were Black or African American, and 23 (5.7%) were Asian. Baseline characteristics were similar across treatment groups (Table). A total of 135 participants were randomly assigned to the aflibercept 2q16 group, 134 participants to the aflibercept 2q8/PRN group, and 133 participants to the control group (eFigure 2 in Supplement 2). Enrolled eyes had near-normal mean vision (Snellen equivalent, 20/25) and central subfield thickness (CST; mean [SD], 247.4 [34.8] μm). Overall, 355 participants (88.3%) completed week 52, and 320 participants (79.6%) completed week 100, with higher completion rates in the aflibercept groups vs the control group at weeks 52 and 100 (Table; eFigure 2 and eTable 1 in Supplement 2). The most common reasons for discontinuation were loss to follow-up and participant withdrawal from the study (eFigure 2 in Supplement 2).
Table. Participant Characteristics.
| Characteristic | No. (%) | |||
|---|---|---|---|---|
| Total | Control groupa | Aflibercept 2q16 groupb | Aflibercept 2q8/PRN groupc | |
| Total participants, No. | 402 | 133 | 135 | 134 |
| Participants completing week 52 | 355 (88.3) | 109 (82.0) | 122 (90.4) | 124 (92.5) |
| Participants completing week 100 | 320 (79.6) | 97 (72.9) | 111 (82.2) | 112 (83.6) |
| Age, mean (SD), y | 55.7 (10.5) | 55.8 (10.3) | 55.4 (11.1) | 55.8 (10.2) |
| Sex | ||||
| Male | 225 (56.0) | 69 (51.9) | 75 (55.6) | 81 (60.4) |
| Female | 177 (44.0) | 64 (48.1) | 60 (44.4) | 53 (39.6) |
| Race | ||||
| White | 310 (77.1) | 107 (80.5) | 99 (73.3) | 104 (77.6) |
| Black or African American | 41 (10.2) | 13 (9.8) | 16 (11.9) | 12 (9.0) |
| Asian | 23 (5.7) | 4 (3.0) | 12 (8.9) | 7 (5.2) |
| Otherd | 28 (7.0) | 9 (6.8) | 8 (5.9) | 11 (8.2) |
| Glycated hemoglobin A1c, mean (SD), % | 8.5 (1.6) | 8.5 (1.5) | 8.6 (1.7) | 8.4 (1.6) |
| Duration of diabetes, mean (SD), y | 14.4 (9.2) | 15.5 (9.3) | 13.7 (8.6) | 14.0 (9.7) |
| Diabetes | ||||
| Type 1 | 34 (8.5) | 10 (7.5) | 14 (10.4) | 10 (7.5) |
| Type 2 | 368 (91.5) | 123 (92.5) | 121 (89.6) | 124 (92.5) |
| Best-corrected visual acuity ETDRS letter score, mean (SD) | 82.4 (6.0) | 82.7 (6.0) | 82.2 (6.6) | 82.3 (5.2) |
| Snellen equivalent | 20/25 | 20/25 | 20/25 | 20/25 |
| Central subfield thickness, mean (SD), μm | 247.4 (34.8) | 249.4 (38.4) | 246.0 (34.3) | 246.8 (31.6) |
| DRSS level | ||||
| 47 | 302 (75.1) | 99 (74.4) | 102 (75.6) | 101 (75.4) |
| 53 | 100 (24.9) | 34 (25.6) | 33 (24.4) | 33 (24.6) |
Abbreviations: DRSS, Diabetic Retinopathy Severity Scale; ETDRS, Early Treatment Diabetic Retinopathy Study; PRN, pro re nata.
The control group received sham injections.
The aflibercept 2q16 group received intravitreal injections of aflibercept, 2 mg, every 16 weeks after 3 initial monthly doses and one 8-week interval.
The aflibercept 2q8/PRN group received intravitreal injections of aflibercept, 2 mg, every 8 weeks after 5 initial monthly doses, with PRN dosing beginning at week 56.
Specific races included in this category were not specified.
Efficacy Outcomes
Change in DRSS Level
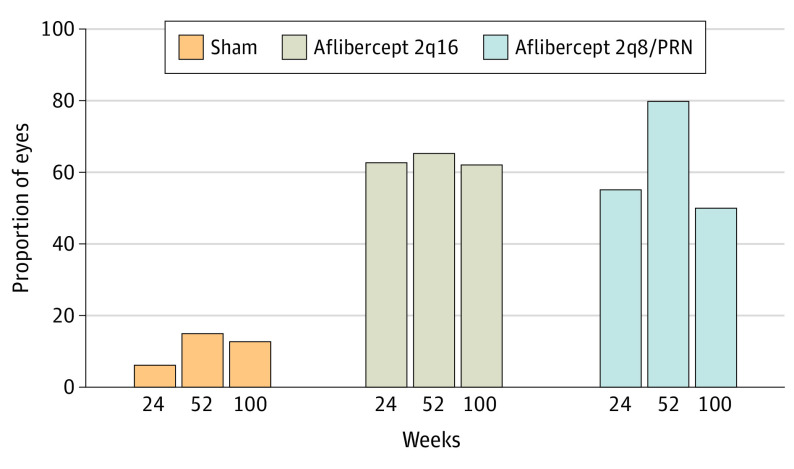
The proportion of eyes with a 2-step or greater improvement in DRSS level from baseline was significantly higher in the combined aflibercept groups vs the control group at week 24 (157 of 269 eyes [58.4%] vs 8 of 133 eyes [6.0%], respectively; [adjusted difference, 52.3%; 95% CI, 45.2%-59.5%]; P < .001) and in the aflibercept groups individually (88 of 135 eyes [65.2%] in the 2q16 group [adjusted difference, 50.1%; 95% CI, 40.1%-60.1%] and 107 of 134 eyes [79.9%] in the 2q8/PRN group [adjusted difference, 64.8%; 95% CI, 55.8%-73.9%] vs 20 of 133 eyes [15.0%] in the control group; P < .001 for both comparisons) and at week 100 (84 of 135 eyes [62.2%] in the 2q16 group vs 67 of 134 eyes [50.0%] in the 2q8/PRN group vs 17 of 133 eyes [12.8%] in the control group; nominal P < .001 for both comparisons) (Figure 2; eTable 2 in Supplement 2). The results of the sensitivity analyses were consistent with those of the primary analyses (eTables 3-8 in Supplement 2).
Figure 2. Proportion of Eyes With a 2-Step or Greater Improvement on Diabetic Retinopathy Severity Scale From Baseline Through Week 100.
Eyes in the aflibercept 2q16 group (n = 135) received intravitreal injections of aflibercept, 2 mg, every 16 weeks after 3 initial monthly doses and one 8-week interval; eyes in the aflibercept 2q8/PRN group (n = 134) received intravitreal injections of aflibercept, 2 mg, every 8 weeks after 5 initial monthly doses, with pro re nata (PRN) dosing beginning at week 56; and eyes in the control group (n = 133) received sham injections. The full analysis set was analyzed using the last observation carried forward method. At week 52, P < .001 for aflibercept 2q16 vs sham (65.2% vs 15.0%) and aflibercept 2q8/PRN vs sham. At week 100, P values for aflibercept 2q16 vs sham and aflibercept 2q8/PRN vs sham were nominal.
At week 52, the event rate for a 2-step or greater worsening in DRSS level was 1.6% in the aflibercept 2q16 group and 0% in the aflibercept 2q8/PRN group compared with 11.9% in the control group. The event rate for worsening was also observed at week 100, with rates of 4.5% in the aflibercept 2q16 group and 2.4% in the aflibercept 2q8/PRN group compared with 20.2% in the control group (nominal P < .001 for both comparisons). The risk of a 2-step or greater worsening in DRSS level was significantly reduced by 89% at week 52 and 81% at week 100 in the aflibercept 2q16 group and by 100% at week 52 and 93% at week 100 in the aflibercept 2q8/PRN group vs the control group.
Development of Vision-Threatening Complications and CI-DME
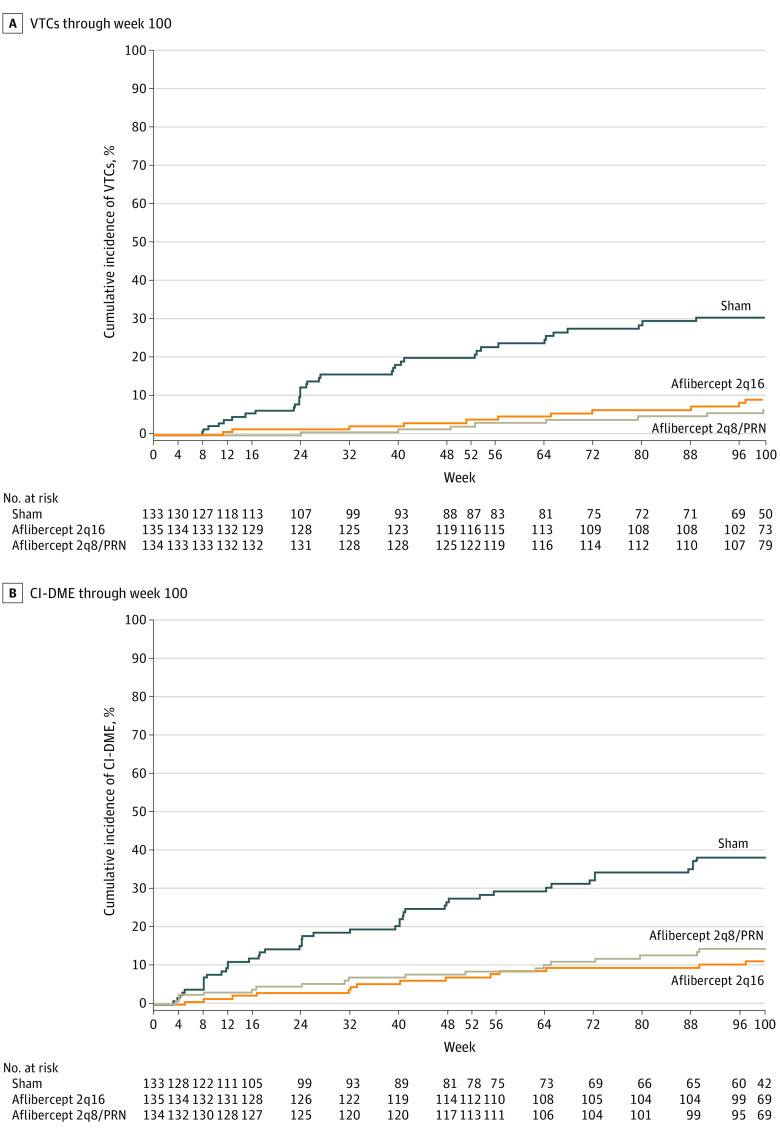
Overall, fewer eyes treated with intravitreal aflibercept vs sham injections developed vision-threatening complications and/or CI- DME through week 100 (22 of 135 eyes [16.3%] in the 2q16 group [adjusted difference, −34.2%; 95% CI, −44.6 to −23.8] and 25 of 134 eyes [18.7%] in the 2q8/PRN group [adjusted difference, −31.7%; 95% CI, −42.5 to −20.9] compared with 67 of 133 eyes [50.4%] in the control group; P < .001 for both comparisons). In the control group, a total of 27 of 133 eyes (20.3%) and 36 of 133 eyes (27.1%) developed vision-threatening complications by weeks 52 and 100, respectively. Significantly fewer eyes in the aflibercept groups vs the control group developed vision-threatening complications at 52 weeks (5 of 135 eyes [3.7%] in the 2q16 group and 4 of 134 eyes [3.0%] in the 2q8/PRN group vs 27 of 133 [20.3%] eyes in the control group) and 100 weeks (11 of 135 eyes [8.1%] in the 2q16 group and 8 of 134 eyes [6.0%] in the 2q8/PRN group vs 36 of 133 eyes [27.1%] in the control group) (Figure 3A; eTable 9 in Supplement 2). The event rate for vision-threatening complications (derived from Kaplan-Meier analyses) was lower through week 52 in the aflibercept groups (4.0% in the 2q16 group and 2.4% in the 2q8/PRN group) compared with the control group (20.1%; P < .001 for both comparisons); this lower event rate was also observed in week 100 (9.1% in the 2q16 group and 6.9% in the 2q8/PRN group vs 30.6% in the control group; nominal P < .001 for both comparisons) (Figure 3B and Figure 4A). Compared with the control group, the risk of developing a vision-threatening complication was significantly reduced in the aflibercept groups through week 52 (reduction of 85% in the 2q16 group and 88% in the 2q8/PRN group) and week 100 (reduction of 77% in the 2q16 group and 83% in the 2q8/PRN group) (Figure 3B).
Figure 3. Analysis of Events Indicative of Diabetic Retinopathy Progression.
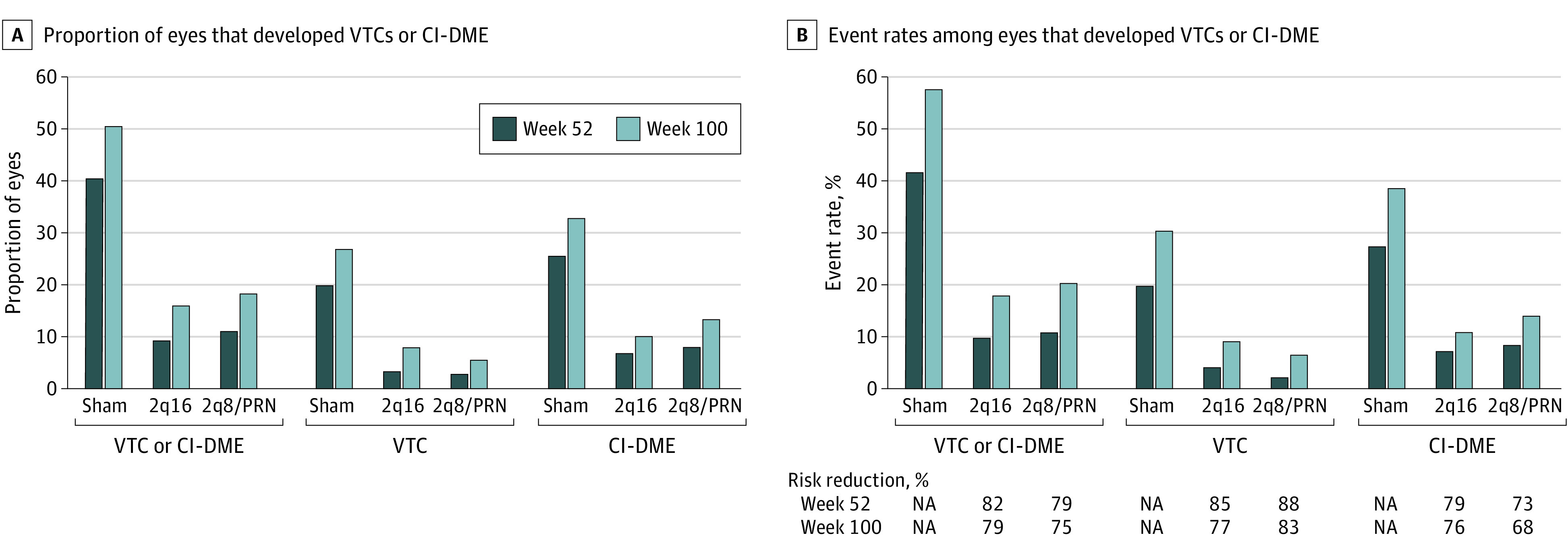
Eyes in the aflibercept 2q16 group (n = 135) received intravitreal injections of aflibercept, 2 mg, every 16 weeks after 3 initial monthly doses and one 8-week interval; eyes in the aflibercept 2q8/PRN group (n = 134) received intravitreal injections of aflibercept, 2 mg, every 8 weeks after 5 initial monthly doses, with pro re nata (PRN) dosing beginning at week 56; and eyes in the control group (n = 133) received sham injections. A, Proportion of eyes that developed VTCs and/or CI-DME through weeks 52 and 100. At week 52, P < .001 for aflibercept 2q16 vs sham (9.6% vs 40.6%) and aflibercept 2q8/PRN vs sham (11.2% vs 40.6%) in eyes that developed VTCs and/or CI-DME, aflibercept 2q16 vs sham (3.7% vs 20.3%) and aflibercept 2q8/PRN vs sham (3.0% vs 20.3%) in eyes that developed VTCs, and aflibercept 2q16 vs sham (6.7% vs 25.6%) and aflibercept 2q8/PRN vs sham (8.2% vs 25.6%) in eyes that developed CI-DME. At week 100, nominal P < .001 for aflibercept 2q16 vs sham (16.3% vs 50.4%) and aflibercept 2q8/PRN vs sham (18.7% vs 50.4%) in eyes that developed VTCs and/or CI-DME, aflibercept 2q16 vs sham (8.1% vs 27.1%) and aflibercept 2q8/PRN vs sham (6.0% vs 27.1%) in eyes that developed VTCs, and aflibercept 2q16 vs sham (10.4% vs 33.1%) and aflibercept 2q8/PRN vs sham (13.4% vs 33.1%) in eyes that developed CI-DME. B, Event rates among eyes that developed VTCs and/or CI-DME through weeks 52 and 100. At week 52, P < .001 for aflibercept 2q16 vs sham (10.1% vs 41.8%) and aflibercept 2q8/PRN vs sham (10.8% vs 41.8%) in eyes that developed VTCs and/or CI-DME, aflibercept 2q16 vs sham (4.0% vs 20.1%) and aflibercept 2q8/PRN vs sham (2.4% vs 20.1%) in eyes that developed VTCs, and aflibercept 2q16 vs sham (7.0% vs 27.6%) and aflibercept 2q8/PRN vs sham (8.5% vs 27.6%) in eyes that developed CI-DME. At week 100, nominal P < .001 for aflibercept 2q16 vs sham (17.9% vs 57.7%) and aflibercept 2q8/PRN vs sham (20.5% vs 57.7%) in eyes that developed VTCs and/or CI-DME, aflibercept 2q16 vs sham (9.1% vs 30.6%) and aflibercept 2q8/PRN vs sham (6.9% vs 30.6%) in eyes that developed VTCs, and aflibercept 2q16 vs sham (11.3% vs 38.4%) and aflibercept 2q8/PRN vs sham (14.4% vs 38.4%) in eyes that developed CI-DME. CI-DME indicates center-involved diabetic macular edema (diagnosed by the investigator); NA, not applicable; and VTC, vision-threatening complication (including proliferative diabetic retinopathy and/or anterior segment neovascularization diagnosed by the investigator and/or the reading center).
Figure 4. Cumulative Incidence of Events Indicative of Diabetic Retinopathy Progression.
Eyes in the aflibercept 2q16 group (n = 135) received intravitreal injections of aflibercept, 2 mg, every 16 weeks after 3 initial monthly doses and one 8-week interval; eyes in the aflibercept 2q8/PRN group (n = 134) received intravitreal injections of aflibercept, 2 mg, every 8 weeks after 5 initial monthly doses, with pro re nata (PRN) dosing beginning at week 56; and eyes in the control group (n = 133) received sham injections. A, VTCs through week 100. The cumulative event rate at week 100 was 9.1% in the aflibercept 2q16 group (hazard ratio [HR], 0.23; P < .001) and 6.9% in the aflibercept 2q8/PRN group (HR, 0.17; P < .001) compared with 30.6% in the control group. B, CI-DME through week 100. The cumulative event rate at week 100 was 11.3% in the aflibercept 2q16 group (HR, 0.24; P < .001) and 14.4% in the aflibercept 2q8/PRN group (HR, 0.32; P < .001) compared with 38.4% in the control group. CI-DME indicates center-involved diabetic macular edema (diagnosed by the investigator); VTC, vision-threatening complication (including proliferative diabetic retinopathy and/or anterior segment neovascularization diagnosed by the investigator and/or the reading center).
The proportion of eyes in the aflibercept groups that developed CI-DME through week 52 was also significantly lower than that in the control group (9 of 135 eyes [6.7%] in the 2q16 group and 11 of 134 eyes [8.2%] in the 2q8/PRN group vs 34 of 133 eyes [25.6%] in the control group; P < .001 for both comparisons). This outcome continued through week 100 (14 of 135 eyes [10.4%] in the 2q16 group and 18 of 134 eyes [13.4%] in the 2q8/PRN group vs 44 of 133 eyes [33.1%] in the control group; nominal P < .001 for both comparisons) (Figure 3A; eTable 10 in Supplement 2). The CI-DME event rate was also significantly lower in the aflibercept groups vs the control group through week 52 (7.0% in the 2q16 group and 8.5% in the 2q8/PRN group vs 27.6% in the control group; P < .001 for both comparisons) and week 100 (11.3% in the 2q16 group and 14.4% in the 2q8/PRN group vs 38.4% in the control group; nominal P < .001 for both comparisons) (Figure 3B and Figure 4B).
Rescue Treatment
Of 133 eyes in the control group, 19 eyes (14.3%) received rescue treatment for vision-threatening complications, and 39 eyes (29.3%) received rescue treatment for CI-DME. Of 135 eyes in the aflibercept 2q16 group, 4 eyes (3.0%) received rescue treatment for vision-threatening complications, and 10 eyes (7.4%) received rescue treatment for CI-DME. Of 134 eyes in the aflibercept 2q8/PRN group, 5 eyes (3.7%) received rescue treatment for vision-threatening complications, and 11 eyes (8.2%) received rescue treatment for CI-DME. The proportion of participants who underwent PRP or vitrectomy was lower in the aflibercept groups compared with the control group (eTable 11 in Supplement 2).
Changes in CST and BCVA
At week 52, changes in CST were −18.9 μm in the aflibercept 2q16 group and −24.9 μm in the aflibercept 2q8/PRN group compared with 5.3 μm in the control group (nominal P < .001 for both comparisons). At week 100, changes in CST were −18.6 μm in the aflibercept 2q16 group and −15.2 μm in the aflibercept 2q8/PRN group compared with 10.3 μm in the control group (nominal P < .001 for both comparisons).
At week 52, the mean (SD) area under the curve (AUC) for the change in BCVA (measured in ETDRS letters) from baseline was 1.7 (3.5) letters in the aflibercept 2q16 group (P = .006) and 1.3 (3.5) letters in the aflibercept 2q8/PRN group (P = .05) compared with 0.5 (3.0) letters in the control group. At week 100, the mean (SD) AUC for the change in BCVA from baseline was 1.5 (3.6) letters in the aflibercept 2q16 group (nominal P = .02) and 0.8 (3.8) letters in the aflibercept 2q8/PRN group (nominal P = .54) compared with 0.6 (3.2) letters in the control group.
Among participants who experienced vision-threatening complications and/or CI-DME and had vision loss of 5 or more ETDRS letters from baseline at any point during the study (regardless of whether rescue treatment was administered), a post hoc analysis identified a greater number of participants experiencing 5 or more, 10 or more, and 15 or more ETDRS letter losses from baseline in the control group (38 participants [28.6%] lost ≥5 letters, 18 participants [13.5%] lost ≥10 letters, and 11 participants [8.3%] lost ≥15 letters) compared with the aflibercept 2q16 group (12 participants [9.0%] lost ≥5 letters [nominal P < .001], 7 participants [5.2%] lost ≥10 letters [P = .02], and 4 participants [3.0%] lost ≥15 letters [P = .07]) and the aflibercept 2q8/PRN group (12 participants [9.0%] lost ≥5 letters [nominal P < .001], 6 participants [4.5%] lost ≥10 letters [P = .01], and 4 participants [3.0%] lost ≥15 letters [P = .07]).
Efficacy Outcomes in Control Group Participants Receiving Rescue Treatment
At week 100, among 53 eyes in the control group that received rescue treatment, 21 eyes (39.6%) had a 2-step or greater improvement in DRSS level from baseline. At baseline, the mean (SD) BCVA was 82 (5.69) letters, and the mean (SD) CST was 259.6 (48.2) μm among eyes receiving rescue treatment, with a mean (SD) change from baseline to week 100 of 0.38 (6.76) letters in BCVA and −0.94 (62.21) μm in CST.
Flexible (PRN) Dosing
Beginning at week 56, eyes in the aflibercept 2q8/PRN group received an average of 1.8 PRN injections (maximum injections = 6). Of 122 eyes, 41 eyes (33.6%) received 0 injections, 42 eyes (34.4%) received 1 to 2 injections, 25 eyes (20.5%) received 3 to 4 injections, and 14 eyes (11.5%) received 5 to 6 injections.
Of the 41 eyes in the aflibercept 2q8/PRN group that received no injections in year 2, 4 eyes (9.8%) developed a vision-threatening complication. No vision-threatening complications occurred in eyes that received 1 or more injection in year 2. During year 2, 4 eyes (9.8%) that received 0 injections and 3 (of 42) eyes (7.1%) that received 1 or 2 injections developed CI-DME. No incidences of CI-DME in eyes that received 3 or more injections were observed.
Adverse Events
Through week 100, ocular adverse events occurring in more than 7.5% of eyes in the control and aflibercept groups included conjunctival hemorrhage (8 eyes [6.0%] in the control group, 18 eyes [13.3%] in the 2q16 group, and 25 eyes [18.7%] in the 2q8/PRN group), DME (43 eyes [32.3%] in the control group, 14 eyes [10.4%] in the 2q16 group, and 19 eyes [14.2%] in the 2q8/PRN group), vitreous floaters (3 eyes [2.3%] in the control group, 7 eyes [5.2%] in the 2q16 group, and 13 eyes [9.7%] in the 2q8/PRN group), eye pain (6 eyes [4.5%] in the control group, 11 eyes [8.1%] in the 2q16 group, and 5 eyes [3.7%] in the 2q8/PRN group), and DR (22 eyes [16.5%] in the control group, 3 eyes [2.2%] in the 2q16 group, and 5 eyes [3.7%] in the 2q8/PRN group). Seven ocular serious adverse events (iris neovascularization, DR, and retinal neovascularization) were reported in 3 participants in the control group. Reduced visual acuity, vitreous hemorrhage, and cystoid macular edema and orbital fracture were reported in 3 participants in the aflibercept 2q8/PRN group. No cases of occlusive retinal vasculitis or endophthalmitis were observed. Nonocular serious adverse events were similar across treatment groups (eTable 12 in Supplement 2). Arterial thromboembolic events (as defined by the Antiplatelet Trialists’ Collaboration19) occurred in 7 participants (5.3%) in the control group, 8 participants (5.9%) in the aflibercept 2q16 group, and 4 participants (3.0%) in the aflibercept 2q8/PRN group; of those, 4 events (3.0%) in the control group, 0 events in the aflibercept 2q16 group, and 1 event (0.7%) in the aflibercept 2q8/PRN group were vascular deaths.
Discussion
The PANORAMA randomized clinical trial evaluated VEGF blockade therapy with intravitreal aflibercept injections compared with sham injections for the treatment of severe NPDR without DME. The primary end point, the proportion of eyes with a 2-step or greater improvement in DRSS level from baseline in the aflibercept groups compared with the control group, was met at weeks 24 and 52. The development of PDR or CI-DME was reduced among eyes that received intravitreal aflibercept injections compared with sham injections by more than 70% through weeks 52 and 100. Improvements in DRSS levels suggested a dose-dependent effect, with 2-step or greater improvements achieved in 79.9% of the aflibercept 2q8/PRN group and 65.2% of the aflibercept 2q16 group at week 52.
At week 100, significant DRSS level improvements were maintained with both aflibercept dosing strategies, and both treatment groups had a significantly lower incidence of vision-threatening complications and CI-DME compared with the control group. No differences in visual acuity were observed at 2 years. However, in a post hoc analysis of participants who had vision-threatening complications and/or CI-DME, 3 times as many participants (28.6%) who received sham injections lost 5 or more ETDRS letters at some point over the course of the study compared with those who received intravitreal aflibercept injections (9.0% in each treatment group).
However, aflibercept dosing in year 2 in the 2q8/PRN group was not fixed, with an average of only 1.8 injections; the proportion of participants with a 2-step or greater improvement in DRSS level decreased from 79.9% at week 52 to 50.0% at week 100. In addition, through the second year, the incidence of vision-threatening complications increased to 9.8% among the 33.6% of participants in the aflibercept 2q8/PRN group who received no PRN injections. Thus, although 1 year of aflibercept therapy substantially decreased the occurrence of PDR and CI-DME compared with sham treatment and observation in the PANORAMA study, discontinuation or reduction in the frequency of therapy may lead to PDR-related complications. In both treated groups, fewer participants experienced both vision-threatening complications and CI-DME plus vision loss of 5 or more letters compared with the control group (regardless of whether rescue treatment was administered), indicating a greater avoidance of both these events and clinically substantial vision loss with proactive treatment.
With continued dosing of intravitreal aflibercept at a fixed interval of every 16 weeks, the proportion of participants with a 2-step or greater improvement in DRSS level at week 52 (65.2%) was maintained through week 100 (62.2%), with a similar reduction in vision-threatening complications. This finding suggests that, at a population level, to maintain DRSS improvement and minimize the risk of vision-threatening events, treatment at a fixed frequency (eg, 3-4 times annually) allows alteration of the natural history of disease progression in this patient population without increasing the management burden relative to the recommended follow-up of every 3 to 4 months.14
These results are consistent with previous studies reporting DRSS level improvements in eyes with PDR or DME that were treated with anti-VEGF therapeutics7,12,20 and demonstrate that treatment with intravitreal aflibercept injections improves DRSS levels and prevents disease progression in eyes with NPDR without concomitant DME. A recent single-masked randomized clinical trial investigated aflibercept therapy every 16 weeks vs sham treatment for the prevention of PDR and DME in a similar patient population, although the study included patients with milder forms of NPDR (DRSS level of 43).20 Consistent with findings from the PANORAMA study, the proportion of eyes that developed PDR or CI-DME with vision loss was significantly lower with aflibercept vs sham treatment through at least 2 years. The proportion of eyes with a 2-step or greater improvement in DRSS level was also significantly higher with aflibercept vs sham treatment.20
Once PDR has developed, damage to the retina may no longer be reversible, as suggested in several studies.7,9 In the Protocol S study, 70% and 65% of eyes treated with PRP and intravitreal ranibizumab, respectively, remained at the PDR level (DRSS level of 61 or worse) and did not improve to the NPDR level after 2 years of treatment.7 In the CLARITY study, 90% and 78% of eyes treated with PRP and intravitreal aflibercept injections, respectively, remained at the PDR level (DRSS level of 61 or worse) and did not improve to the NPDR level after 1 year of treatment.9 Although these results may represent undertreatment (both studies used PRN dosing regimens), the evidence suggests that there is value in targeting the disease process at the nonproliferative stage to reduce the development of PDR and CI-DME.
Without intervention, proliferative disease can cause rapid, irreversible vision loss. To halt progression and prevent vision loss, clinicians typically initiate treatment once PDR develops. Many ophthalmologists employ PRP treatment at this stage and do not treat PDR with anti-VEGF monotherapy because of concerns about patient adherence, although it remains unknown whether outcomes are generally better if PRP is administered as initial treatment (eg, the Protocol S study found that 15% of eyes needed vitrectomy and 30% of eyes needed anti-VEGF therapy for CI-DME with vision loss by 2 years after PRP7). The Diabetic Retinopathy Study found that untreated eyes with PDR have a 37% rate of severe vision loss at 72 months, and a patient who does not adhere to the treatment regimen may have a similar outcome.5 Although the current standard of care for severe NPDR is observation, the risk of patients with severe NPDR developing potentially irreversible PDR and associated vision loss (approximately 30% at 2 years in the control group of PANORAMA) suggests that implementing anti-VEGF therapy should be considered and discussed with these patients.
Although the underlying mechanisms of DR are not yet completely understood, vascular damage from diabetes is a well-established mechanism that leads to vision loss. Anti-VEGF treatment can improve or reverse many of the clinical findings associated with assessing DR severity; however, it is not yet clear what impact these improvements may have on the underlying disease processes. Of the photographic features that compose DRSS grading, it is likely that reductions in dot/blot hemorrhage do not correlate with actual improvements in retinal vascular perfusion; however, reductions in cotton-wool spots and resolution of vascular tortuosity appear more likely to represent physiologically relevant improvement in retinal ischemia. Despite these unanswered and important questions, the PANORAMA study has demonstrated that treatment with aflibercept meaningfully reduced the risk of clinically relevant manifestations of DR and that this benefit was seen with an injection frequency consistent with current practice guidelines for patient evaluation.
The overall safety of intravitreal aflibercept injections through 100 weeks in the PANORAMA study was consistent with the known safety profile.21 The most frequent ocular adverse events were consistent with those associated with intravitreal injections and the progression of DR, and there were no cases of endophthalmitis.
Limitations
This study has limitations. The study population was limited to eyes with moderately severe to severe NPDR. It would be useful to know whether eyes with more or less severe retinopathy respond differently to either dosing regimen. In addition, the group receiving 2 mg of aflibercept every 8 weeks was switched to PRN dosing in the second year, limiting the ability to determine whether continued dosing every 8 weeks through week 100 has additional benefit. Participants were evaluated on a fixed schedule, with more frequent visits than are typically used in a clinical setting outside of a clinical trial; thus, the diagnosis and treatment of NPDR and anterior segment neovascularization and/or DME may have occurred earlier than in a typical clinical setting, in which visits may be less frequent. In addition, the 100-week clinical trial design provided a relatively short duration of follow-up for a chronic disease.
Conclusions
Treatment of severe NPDR with intravitreal aflibercept injections in the PANORAMA clinical trial improved the severity of retinopathy and reduced the risk of progression to CI-DME and vision-threatening complications vs sham treatment with observation. No differences in visual acuity were noted at the 2-year visit. However, in a post hoc analysis of participants who had vision-threatening complications and/or CI-DME, more participants in the control group experienced a letter loss of 5 or more over the course of the clinical trial, although differences across groups were not seen for 10 or more or 15 or more letters lost over the course of the study. Nonproliferative DR represents a potential window of opportunity to slow disease progression, thereby preventing the development of potentially irreversible damage that may lead to vision loss and have a meaningful impact on quality of life. Treatment at a fixed interval may be an optimal strategy compared with reactive PRN dosing. Fixed intravitreal aflibercept injection dosing in a patient population who adhered to the treatment regimen was demonstrated to be safe and effective in preventing vision-threatening complications and CI-DME in eyes with severe NPDR; however, no differences in visual acuity were noted, at least at the 2-year visit, suggesting that longer-term follow-up of this approach may be insightful.
Trial Protocol
eMethods 1. List of PANORAMA Study Investigators
eMethods 2. Patient Inclusion/Exclusion Criteria, Randomization and Masking, Methods to Assess Efficacy and Safety Outcomes, Protocol Amendments, Sample Size Calculation, and Statistical Analyses
eTable 1. Treatment Experience Through Week 100
eTable 2. Proportion of Patients With a 2-Step or Greater Improvement in DRSS Score From Baseline to Weeks 24, 52, and 100 Using LOCF Method
eTable 3. Proportion of Patients With a 2-Step or Greater Improvement in DRSS Score From Baseline to Week 52 Using aLOCF Method
eTable 4. Proportion of Patients With a 2-Step or Greater Improvement in DRSS Score From Baseline to Week 100 Using aLOCF Method
eTable 5. Proportion of Patients With a 2-Step or Greater Improvement in DRSS Score From Baseline to Week 52 Using aOC Method
eTable 6. Proportion of Patients With a 2-Step or Greater Improvement in DRSS Score From Baseline to Week 100 Using aOC Method
eTable 7. Proportion of Patients With a 2-Step or Greater Improvement in DRSS Score From Baseline to Week 52 Using Multiple Imputation
eTable 8. Proportion of Patients With a 2-Step or Greater Improvement in DRSS Score From Baseline to Week 100 Using Multiple Imputation
eTable 9. Proportion of Patients Who Developed Any Vision-Threatening Complication (PDR/ASNV) Through Week 52 and Week 100
eTable 10. Proportion of Patients Who Developed CI-DME Through Week 52 and Week 100
eTable 11. Proportion of Patients With Panretinal Photocoagulation or Vitrectomy Through Week 100
eTable 12. Nonocular SAEs Occurring in 1% of Patients or More in Any Treatment Group from Baseline Through Week 100
eFigure 1. Visit and Dosing Schedule of the Phase 3 Double-Masked PANORAMA Randomized Clinical Trial
eFigure 2. Patient Disposition
eReferences
Data Sharing Statement
References
- 1.Duh EJ, Sun JK, Stitt AW. Diabetic retinopathy: current understanding, mechanisms, and treatment strategies. JCI Insight. 2017;2(14):e93751. doi: 10.1172/jci.insight.93751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee R, Wong TY, Sabanayagam C. Epidemiology of diabetic retinopathy, diabetic macular edema and related vision loss. Eye Vis (Lond). 2015;2:17. doi: 10.1186/s40662-015-0026-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Early Treatment Diabetic Retinopathy Study Research Group . Fundus photographic risk factors for progression of diabetic retinopathy. ETDRS report number 12. Ophthalmology. 1991;98(5)(suppl):823-833. doi: 10.1016/S0161-6420(13)38014-2 [DOI] [PubMed] [Google Scholar]
- 4.Diabetic Retinopathy Study Research Group . Indications for photocoagulation treatment of diabetic retinopathy: Diabetic Retinopathy Study report no. 14. Int Ophthalmol Clin. 1987;27(4):239-253. doi: 10.1097/00004397-198702740-00004 [DOI] [PubMed] [Google Scholar]
- 5.Diabetic Retinopathy Study Research Group . Photocoagulation treatment of proliferative diabetic retinopathy. clinical application of Diabetic Retinopathy Study (DRS) findings, DRS report number 8. Ophthalmology. 1981;88(7):583-600. [PubMed] [Google Scholar]
- 6.Diabetic Retinopathy Study Research Group . Preliminary report on effects of photocoagulation therapy. Am J Ophthalmol. 1976;81(4):383-396. doi: 10.1016/0002-9394(76)90292-0 [DOI] [PubMed] [Google Scholar]
- 7.Gross JG, Glassman AR, Jampol LM, et al. ; Writing Committee for the Diabetic Retinopathy Clinical Research Network . Panretinal photocoagulation vs intravitreous ranibizumab for proliferative diabetic retinopathy: a randomized clinical trial. JAMA. 2015;314(20):2137-2146. doi: 10.1001/jama.2015.15217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nguyen QD, Brown DM, Marcus DM, et al. ; RISE and RIDE Research Group . Ranibizumab for diabetic macular edema: results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology. 2012;119(4):789-801. doi: 10.1016/j.ophtha.2011.12.039 [DOI] [PubMed] [Google Scholar]
- 9.Sivaprasad S, Prevost AT, Vasconcelos JC, et al. ; CLARITY Study Group . Clinical efficacy of intravitreal aflibercept versus panretinal photocoagulation for best corrected visual acuity in patients with proliferative diabetic retinopathy at 52 weeks (CLARITY): a multicentre, single-blinded, randomised, controlled, phase 2b, non-inferiority trial. Lancet. 2017;389(10085):2193-2203. doi: 10.1016/S0140-6736(17)31193-5 [DOI] [PubMed] [Google Scholar]
- 10.Brown DM, Nguyen QD, Marcus DM, et al. ; RIDE and RISE Research Group . Long-term outcomes of ranibizumab therapy for diabetic macular edema: the 36-month results from two phase III trials: RISE and RIDE. Ophthalmology. 2013;120(10):2013-2022. doi: 10.1016/j.ophtha.2013.02.034 [DOI] [PubMed] [Google Scholar]
- 11.Elman MJ, Aiello LP, Beck RW, et al. ; Diabetic Retinopathy Clinical Research Network . Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology. 2010;117(6):1064-1077. doi: 10.1016/j.ophtha.2010.02.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heier JS, Korobelnik JF, Brown DM, et al. Intravitreal aflibercept for diabetic macular edema: 148-week results from the VISTA and VIVID studies. Ophthalmology. 2016;123(11):2376-2385. doi: 10.1016/j.ophtha.2016.07.032 [DOI] [PubMed] [Google Scholar]
- 13.Wells JA, Glassman AR, Ayala AR, et al. ; Diabetic Retinopathy Clinical Research Network . Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema: two-year results from a comparative effectiveness randomized clinical trial. Ophthalmology. 2016;123(6):1351-1359. doi: 10.1016/j.ophtha.2016.02.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.American Academy of Ophthalmology . Diabetic retinopathy preferred practice pattern 2019. October 2019. Accessed January 22, 2021. https://www.aao.org/preferred-practice-pattern/diabetic-retinopathy-ppp
- 15.Solomon SD, Chew E, Duh EJ, et al. Diabetic retinopathy: a position statement by the American Diabetes Association. Diabetes Care. 2017;40(3):412-418. doi: 10.2337/dc16-2641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reddy SV, Husain D. Panretinal photocoagulation: a review of complications. Semin Ophthalmol. 2018;33(1):83-88. doi: 10.1080/08820538.2017.1353820 [DOI] [PubMed] [Google Scholar]
- 17.Mazhar K, Varma R, Choudhury F, McKean-Cowdin R, Shtir CJ, Azen SP; Los Angeles Latino Eye Study Group . Severity of diabetic retinopathy and health-related quality of life: the Los Angeles Latino Eye Study. Ophthalmology. 2011;118(4):649-655. doi: 10.1016/j.ophtha.2010.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 19.Antiplatelet Trialists’ Collaboration. Collaborative overview of randomised trials of antiplatelet therapy—I: prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients. BMJ. 1994;308(6921):81-106. [PMC free article] [PubMed] [Google Scholar]
- 20.Maturi RK, Glassman AR, Josic K, et al. ; DRCR Retina Network . Effect of intravitreous anti-vascular endothelial growth factor vs sham treatment for prevention of vision-threatening complications of diabetic retinopathy: the Protocol W randomized clinical trial. JAMA Ophthalmol. Published online March 30, 2021. doi: 10.1001/jamaophthalmol.2021.0606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kitchens JW, Do DV, Boyer DS, et al. Comprehensive review of ocular and systemic safety events with intravitreal aflibercept injection in randomized controlled trials. Ophthalmology. 2016;123(7):1511-1520. doi: 10.1016/j.ophtha.2016.02.046 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eMethods 1. List of PANORAMA Study Investigators
eMethods 2. Patient Inclusion/Exclusion Criteria, Randomization and Masking, Methods to Assess Efficacy and Safety Outcomes, Protocol Amendments, Sample Size Calculation, and Statistical Analyses
eTable 1. Treatment Experience Through Week 100
eTable 2. Proportion of Patients With a 2-Step or Greater Improvement in DRSS Score From Baseline to Weeks 24, 52, and 100 Using LOCF Method
eTable 3. Proportion of Patients With a 2-Step or Greater Improvement in DRSS Score From Baseline to Week 52 Using aLOCF Method
eTable 4. Proportion of Patients With a 2-Step or Greater Improvement in DRSS Score From Baseline to Week 100 Using aLOCF Method
eTable 5. Proportion of Patients With a 2-Step or Greater Improvement in DRSS Score From Baseline to Week 52 Using aOC Method
eTable 6. Proportion of Patients With a 2-Step or Greater Improvement in DRSS Score From Baseline to Week 100 Using aOC Method
eTable 7. Proportion of Patients With a 2-Step or Greater Improvement in DRSS Score From Baseline to Week 52 Using Multiple Imputation
eTable 8. Proportion of Patients With a 2-Step or Greater Improvement in DRSS Score From Baseline to Week 100 Using Multiple Imputation
eTable 9. Proportion of Patients Who Developed Any Vision-Threatening Complication (PDR/ASNV) Through Week 52 and Week 100
eTable 10. Proportion of Patients Who Developed CI-DME Through Week 52 and Week 100
eTable 11. Proportion of Patients With Panretinal Photocoagulation or Vitrectomy Through Week 100
eTable 12. Nonocular SAEs Occurring in 1% of Patients or More in Any Treatment Group from Baseline Through Week 100
eFigure 1. Visit and Dosing Schedule of the Phase 3 Double-Masked PANORAMA Randomized Clinical Trial
eFigure 2. Patient Disposition
eReferences
Data Sharing Statement