Abstract
Pancreatic islets are vital endocrine regulators of systemic metabolism, and recent investigations have increasingly focused on understanding human islet biology. Studies of isolated human islets have advanced understanding of the development, function, and regulation of cells comprising islets, especially pancreatic α- and β-cells. However, the multicellularity of the intact islet has stymied specific experimental approaches—particularly in genetics and cell signaling interrogation. This barrier has been circumvented by the observation that islet cells can survive dispersion and reaggregate to form “pseudoislets,” organoids that retain crucial physiological functions, including regulated insulin and glucagon secretion. Recently, exciting advances in the use of pseudoislets for genetics, genomics, islet cell transplantation, and studies of intraislet signaling and islet cell interactions have been reported by investigators worldwide. Here we review molecular and cellular mechanisms thought to promote islet cell reaggregation, summarize methods that optimize pseudoislet development, and detail recent insights about human islet biology from genetic and transplantation-based pseudoislet experiments. Owing to robust, international programs for procuring primary human pancreata, pseudoislets should serve as both a durable paradigm for primary organoid studies and as an engine of discovery for islet biology, diabetes, and metabolism research.
Introduction
Diabetes pathogenesis involves multiple mechanisms, reflected by a spectrum of manifestations, including type 1 diabetes, type 2 diabetes (T2D), and type 3c diabetes (1). Underlying this diabetes diversity is the unifying concept that pathology and dysfunction of the pancreatic islets of Langerhans are common to all types of diabetes. Thus, intensive efforts in diabetes research are focused on understanding of islet biology, genetics, signaling, and function in physiological or pathophysiological settings, framed by the goal of generating targeted therapies responsive to each form of diabetes.
Increasingly, these efforts have focused on investigations of human islets, which have multiple properties distinct from rodent islets: these include different endocrine cell composition and arrangement; physiological differences, including differences in glucose set point and basal and stimulated insulin and glucagon secretion (2,3); and molecular differences, including distinct transcriptomic, epigenomic, and chromatin signatures (4,5). Although the mechanism(s) underlying these species differences is not entirely clear, prior studies suggest that the predominance of heterologous contacts between α- and β-cells enhances regulation of insulin secretion by glucagon in human islets, compared with rodent islets (6). These specific features motivate studies of human islets.
Pancreatic islet architecture is complex, comprised of interspersed endocrine α-, β-, δ-, ε-, and PP islet cells comingled with multiple nonislet cell types, including endothelial cells, stellate cells, smooth muscle, fibroblasts, and resident immune cells (7). Pancreatic islets offer a strikingly broad set of experimental opportunities, stemming from the ability to isolate and purify them from the pancreas of multiple species, including humans. Once isolated, islets can be maintained in vitro with established culture regimes, permitting a range of phenotyping, including functional studies of insulin secretion by β-cells and glucagon secretion by α-cells. Unlike for most other organs and tissues, however, islet function can be assessed following transplantation to suitable animal hosts in experimentally convenient, heterotopic engraftment sites, like the subcapsular renal space (8–15). Transplanted islets revascularize and reacquire endocrine functions, including regulated insulin or glucagon secretion stimulated by physiological secretagogues like glucose or amino acids (13).
The impermeability of intact cultured islets to many experimental reagents presents an experimental challenge, especially for genetics. For example, exposure of intact islets to compounds or transgene vectors often leads to changes only in the most superficial islet cell layer, and failure to penetrate the innermost islet regions, limiting or precluding interpretation. To circumvent this, islets can be dispersed into single-cell suspensions and then reaggregated to form multicellular structures. This process of dispersion and reaggregation to form “pseudoislets” was first reported with canine islets (16), and subsequent work showed the feasibility of pseudoislet formation from diverse sources, including rodents, pigs, and humans (17–19). Remarkably, multiple studies have shown that pseudoislets retain the principal islet cell composition and endocrine functions of intact islets. For example, human pseudoislets reform gap junctions linking islet cells (20) and retain glucose-stimulated insulin secretion (GSIS) both in vitro (19,21) and in vivo after transplantation (8). This singular feature of islets among mature solid organs—tolerance of a transient dispersed state—provides powerful experimental opportunities to modulate the composition and genetics of islet cells prior to reaggregation into pseudoislets.
Here we briefly discuss molecular and cellular mechanisms underlying islet cell reaggregation, summarize methods that optimize pseudoislet formation, and detail recent insights about human islet biology from genetic and transplantation-based pseudoislet experiments. These investigations of gene expression, hormone production, stimulus-secretion coupling, and islet cell functional maturation capitalize on the multitude of unique experimental advantages for islet studies. Concurrent advances in genetics, genomics, and transplantation techniques have made it possible to use pseudoislets for interrogation of islet genetics, intraislet signaling, and islet cell interactions, studies less feasible with intact islets, in vitro cell lines, or transgenic mice. The advantages afforded by pseudoislet biology are demonstrated by a surge of recent studies from investigators worldwide.
Mechanisms Guiding Pseudoislet Formation
Cellular dissociation, reaggregation, and self-organization in vitro were the focus of classical studies of the 19th and early 20th centuries and helped to inspire studies of islet self-(re)assembly (16). The process of self-organization has been described in normal developmental processes, wound healing, regeneration, and pathologies in mammalian tissues. Strikingly, dissociated cells from vertebrate embryonic tissues display a propensity to reaggregate and reconstitute multicellular systems (22). Steinberg hypothesized that this self-organization reflects cellular rearrangement toward a thermodynamic equilibrium. Specifically, unbound adhesion sites on the surface of cells represent free energy, and reduction of unbound surface area via binding at the junction between two cells moves the system toward an energy minimum. Based on observations that “like” cells in his studies adhered more strongly to each other than “unlike cells,” Steinberg postulated the existence of selective and differential adhesion mechanisms (22). Investigations have since identified specific adhesion molecules that drive self-organization. While the scope of our article precludes an in-depth discussion of this field, we highlight several studies of these processes in the developing pancreas and intact postnatal islets that have improved the understanding and development of pseudoislets.
Cell-cell aggregation is driven primarily by calcium-dependent and calcium-independent cellular adhesion molecules (CAMs) displayed on the surfaces of vertebrate and mammalian cells (23). Among the best characterized CAMs in islets are members of the calcium-dependent cadherin superfamily, epithelial (E-) and neural (N-) cadherin. E-cadherin and N-cadherin are essential for embryonic clustering of pancreatic endocrine cells into islets and are expressed on the surface of mature pancreatic islet cells (24). These transmembrane proteins have an extracellular domain that facilitates cell-to-cell adhesions by forming homodimers with cadherins on neighboring cells and have a cytoplasmic domain that binds to intracellular α- and β-catenins, which bridge to the F-actin cytoskeleton (23). Calcium binding causes conformational changes in these cadherins that decrease their susceptibility to proteolytic cleavage and enable their presentation to mediate islet cell clustering (24). Additionally, E- and N-cadherin may directly regulate insulin secretion in response to glucose, proliferation, and cell viability (25,26).
Calcium-independent CAMs are also present on islet cells and play crucial roles in islet cell clustering and organization. Such CAMs include immunoglobulins, integrins, and claudins (27–29). Neural CAM (NCAM) is one example of a calcium-independent immunoglobulin expressed in islet cells. NCAM expression becomes concentrated in islet cells during pancreatic ontogeny and mediates both homophilic and heterophilic cell-cell interactions. Such interactions are necessary for proper cadherin clustering and islet cell type segregation in development (27).
Gap junctions are another critical element in guiding the aggregation, adhesion, and proper function of islet endocrine cells. The expression and roles of gap junction proteins in endocrine secretion have been extensively reviewed by Meda and others (30). Briefly, these intercellular channels, composed of 12 connexin subunits, are crucial for shuttling ions, metabolites, and other small molecules between cells (31). In islets, these gap junctions are composed of connexin36 (Cx36) and regulate electrical activity and insulin secretion between β-cells (30). Other connexin species have also been identified at the transcriptome level, though their roles are much less understood (32). These studies have been complemented by those of connexin regulators, such as the EphA receptor tyrosine kinases, and their role in β-cell bidirectional cell-cell communication for proper insulin secretion (33).
The in vitro aggregation of pseudoislets is likely modulated by the same native adhesion molecules governing in vivo islet cell aggregation. Expression of E-cadherin is increased in MIN6 cell pseudoislets relative to monolayers (26,34), and E-cadherin expression correlates with glucose-regulated insulin secretion (35). Additionally, studies in rat pseudoislets demonstrate that E-cadherin is necessary for proper islet cell organization (24) and that differences in NCAM expression between islet cell types also contribute to cell localization within multicellular clusters (36). Murine studies revealed that β-cells promote pseudoislet reaggregation: cultures of mouse α-cells alone or δ-cells alone failed to form pseudoislets, indicating that β-cells may provide key cell-to-cell contacts that drive islet cell clustering (37). Additionally, lentiviral vector targeting of specific connexins demonstrated that modulation of gap junction proteins negatively affected insulin secretion, indicating that appropriate construction of gap junctions between islet cells is critical to proper function (20).
Methods for Human Pseudoislet Formation
Distinct methods have capitalized on the intrinsic islet cell-cell adhesion processes to promote formation of organoid-like multicellular pseudoislet clusters from dispersed human islet cells. To date, well-characterized approaches for achieving human pseudoislet formation include spontaneous, hanging-drop, and microwell reaggregation techniques (summarized in Table 1).
Table 1.
Summary of established methods for human pseudoislet formation
| Method | Approach | Advantages | Disadvantages | Key references |
|---|---|---|---|---|
| Spontaneous reaggregation | After dissociation, islet cells are introduced to ultra-low attachment plates and reaggregate spontaneously into pseudoislets | Accessible and widely validated in transduction experiments | Produces pseudoislets of heterogeneous size (although greater homogeneity can be achieved by reducing the volume of culture media and using gentle centrifugation) | Liu et al. (2019), Zaldumbide et al. (2013), Arda et al. (2016), Peiris et al. (2018), Bevacqua et al. (2021a & 2021b) |
| Microwell | After dissociation, islet cells are concentrated into small-diameter wells in customized microwell plates to achieve size-controlled clusters | Achieves pseudoislets of uniform size and efficient for large-scale experiments | Limited by cost of microwell plates | Liu et al. (2019), Hilderink et al. (2015), Yu et al. (2018) |
| Hanging drop | After dissociation, islet cells are suspended in small liquid droplets (for example, distributed across inverted tissue-culture dish lids), and gravity facilitates the formation of spherical clusters | Accessible and achieves pseudoislets of uniform size | Laborious (although modified hanging-drop platforms that are compatible with automated cell seeding have facilitated larger-scale studies) | Zuellig et al. (2017), van Krieken et al. (2019), Walker et al. (2020) |
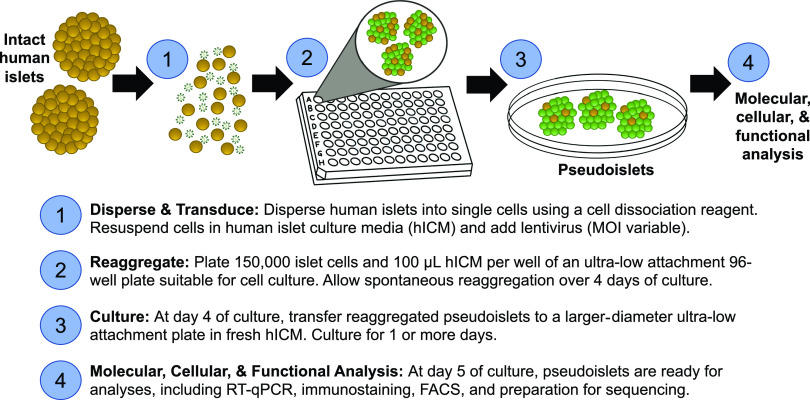
Spontaneous reaggregation is the most accessible and least laborious approach for formation of human pseudoislets. After dissociation, islet cells are introduced to ultra-low attachment plates and reaggregate stochastically into pseudoislets over 5–7 days (see Fig. 1). Approximately one pseudoislet is generated per 5–10 input human islet equivalents (38 and R.J.B., S.K.K., unpublished data). While this approach typically yields pseudoislets that are heterogeneous in size, greater homogeneity can be achieved by reduction of the volume of culture media and by use of gentle centrifugation to prompt clustering (38). Our group and others have demonstrated that spontaneous reaggregation is an effective approach for efficient viral transduction and genetic modification (8,9,20,21,38,39).
Figure 1.
A visual protocol for pseudoislet formation with viral transduction for genetic modification studies. hICM, human islet culture media; MOI, multiplicity of infection; RT-qPCR, quantitative RT-PCR.
The microwell and hanging-drop reaggregation methods were developed to control the size and composition of pseudoislets. In the hanging-drop approach, islet cells are suspended in small liquid droplets (for example, distributed across inverted tissue-culture dish lids) and reaggregated into spheroid pseudoislets during culture (40). This approach can be laborious, but modified hanging-drop platforms that are compatible with automated cell seeding have facilitated larger-scale studies (41,42). Customized microwell plates also concentrate cells into small-diameter wells to achieve size-controlled clusters (10), and an inverse pyramidal microwell system validated in human pseudoislet studies is commercially available under the name of AggreWell (11,38). Each well can create thousands of pseudoislets with a single pipetting and centrifugation step, a platform efficient for larger-scale studies. In a direct comparison of these methods, Walker et al. (41) found that glucose-dependent insulin or glucagon secretion was comparable in pseudoislets prepared with hanging drops or microwells. Thus, distinct reaggregation methods can produce pseudoislets with similar in vitro endocrine functions.
Each method for pseudoislet development in vitro has benefits and challenges. While microwells are efficient for larger-scale experiments (10,11), spontaneous or hanging-drop methods are more cost-effective for smaller-scale studies (38,40). Modified hanging-drop systems can be automated (41,42), but exposure to reagents with biohazardous potential (such as adenovirus or lentivirus for transduction experiments) is more easily contained within ultra-low attachment and microwell plates. Spontaneous reaggregation is widely validated in transduction experiments (8,9,21,38,39,43) and less costly, but this approach yields more heterogeneous pseudoislets. Finally, each laboratory may have empirical experiences that inform preferences in pseudoislet formation platforms: our group has found good formation of porcine and murine pseudoislets in microwell platforms, while human pseudoislet development is most effective with use of spontaneous aggregation. Each of these methods is compatible with subsequent molecular, cellular, and functional assays.
Pseudoislet Physiology and Function
The Importance of Islet and Pseudoislet Composition for Normal Function
For improvement of interpretation of most outcomes, the pseudoislet platform should ideally reconstitute important intrinsic and cell-cell mechanisms that modulate hormone secretion from islet cells. In humans, insulin-secreting β-cells, glucagon-secreting α-cells, and somatostatin-secreting δ-cells, respectively, constitute 50–75%, 15–30%, and 3–10% of the endocrine pancreas (2), though this composition appears to be age-dependent (44). Regulation of hormone secretion from islet cells is mediated by intrinsic, paracrine, and juxtacrine signaling, which is linked to islet architecture. For example, β-cell function is modulated by glucose metabolism (45) and electrical coupling between β-cells (37). In addition, intraislet signaling mediated by glucagon, somatostatin, and urocortin 3 modulates the “tone” of insulin output (46). Insulin secretion from β-cells is dysregulated upon dispersion, but reaggregation of islet cells restores GSIS (37,47). Moreover, these hallmark β-cell functions improve after birth and correlate with transcriptomic and epigenomic differences between juvenile and adult islets (21). Therefore, pseudoislet studies should ideally be adaptable for age-dependent islet studies. In our experience, pseudoislets made from juvenile donor islets retain properties of those donor islets, as assessed by transcriptomic and functional (GSIS) assessment (21,39). This is consistent with prior studies demonstrating that pseudoislets reconstitute native islet composition, cellular adhesions and connections, gene expression, hormone secretion, and developmental stage (11,21,39–41,48,49).
Cells and Gene Expression in Pseudoislets
Histological studies by us and others demonstrate that the ratio of input α-, β-, and δ-cells is preserved in cultured pseudoislets (39–41) (Fig. 2A and B). These findings are consistent with the observation that total insulin and glucagon content in pseudoislets and intact islets is similar (41,49). Additionally, after 5–6 days’ hanging drop development or spontaneous reaggregation, pseudoislet α-, β-, and δ-cells are found intermingled, as they are in native islets (14,39,40).
Figure 2.
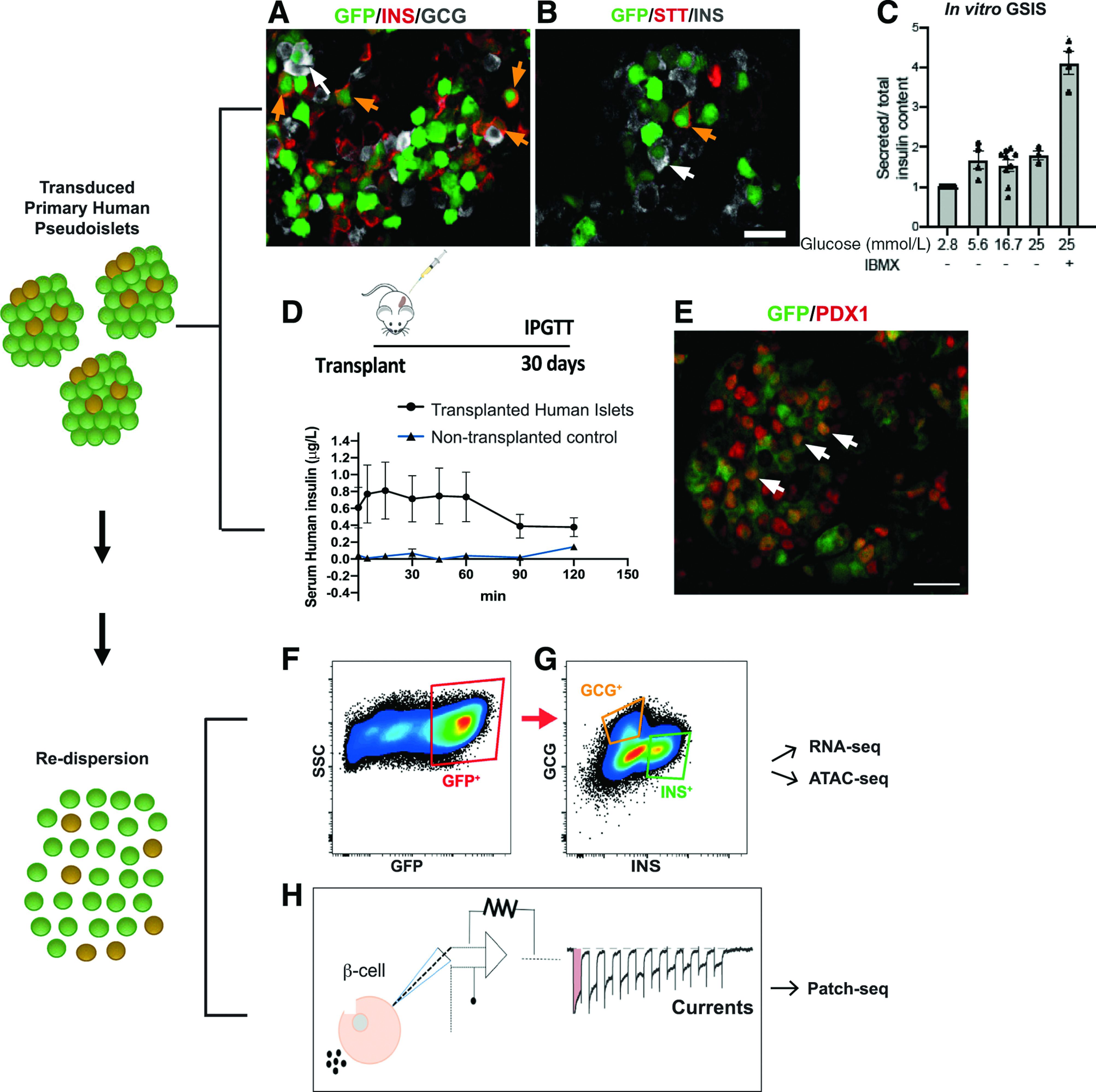
The human pseudoislet genetics platform allows assessment of islet-specific gene functionalities in vitro and in vivo. A and B: Dispersed human islet cells were transduced with lentiviruses coding for GFP under a constitutive promoter to show that all islet cell types can be efficiently transduced; immunostaining of human pseudoislets showing β (INS+, orange arrows) (A and B), α (GCG+, white arrows) (A), and δ (STT+, orange arrows) (B) efficiently transduced (GFP+) islet cells. Scale bar: 20 μm. C: Functionality of transduced pseudoislets can be assessed through GSIS in vitro. The data are normalized to 2.8 mmol/L glucose insulin level. D: Transduced pseudoislets (500/mouse) can be transplanted under the kidney capsule of immunocompromised mice and subjected to an intraperitoneal glucose tolerance test (IPGTT) in vivo, allowing assessment of longer-term effects of genetic manipulations. E: Grafts can be recovered and transduced cells (in this case, GFP+ PDX+ [white arrows]) further evaluated by immunostaining. Scale bar: 40 μm. F and G: Molecular analysis can also be performed following redispersion of pseudoislets transduced with lentiviruses coding for GFP under a constitutive promoter and FACS sorting of infected, GFP+ (F) specific cell types (α: GCG+; β: INS+) (G). H: Following redispersion, single cells can also be used for patch-clamp or patch-seq. ATAC-seq, assay for transposase-accessible chromatin sequencing; RNA-seq, RNA sequencing.
Gene expression studies of pseudoislets also demonstrate the presence of the principal islet cell types and provide further understanding of pseudoislet signaling, function, and regulation. After 48 h of culture, pseudoislets and intact islets demonstrated similar transcript levels of genes involved in cell communication (ITGB1, CDH1, LAMB1, GJA1, ITGB7), secretory function (INS, PCSK2, PDX1, GLP1R, PCSK1), oxidative stress (SOD1, SOD2, CAT), and apoptosis (NFKB1, MAPK8, APAF1, MAPK10, NOS3) by quantitative RT-PCR (11). In a subsequent bulk RNA-sequencing study, it was found that expression levels of factors involved in GSIS (including GCK, SUR1, KCNJ11, CACNA1A, and GLP1R) and β-cell identity (including INS, MAFA, and NEUROD1) were indistinguishable between islets and pseudoislets (49). Immunostaining confirmed pseudoislet expression of β-cell PDX1 and NKX6.1, α-cell ARX, and MAFB, PAX6, and NKX2.2 in both β- and α-cells, like in native human islets (41).
Some studies report altered islet composition after pseudoislet formation. For example, insulin+ cells can localize to the periphery of cultured pseudoislets (10–12,41), possibly reflecting a centrifugation step during reaggregation (10–12) or specific culture media (41). Additionally, bulk RNA-sequencing methods revealed that mRNAs encoding extracellular matrix (ECM), endothelial, exocrine, and ductal cell markers were reduced in pseudoislets compared with primary islets, indicating enrichment of endocrine populations and potential loss of nonendocrine components during dissociation and culture (49). In standard culture media, like CMRL-1066 or RPMI-1640 supplemented with serum, the reduction of ECM components after reaggregation of pseudoislets has been postulated to facilitate diffusion of nutrients and oxygen to endocrine cells in vitro or after transplantation (11). In addition, modulation of input cell types could enhance pseudoislet survival and function after transplantation (14,50). For example, in one study it was found that inclusion of mesenchymal stem cells improved survival and enhanced insulin secretion from pseudoislets (14). This effect was dependent on N-cadherin–mediated cell adhesion, reinforcing the importance of adhesion and cell-to-cell contact in pseudoislet function.
Islet Cell Hormone Secretion in Pseudoislets
Studies at “basal” glucose levels (2.8 mmol/L) show comparable insulin secretion in control intact islets and pseudoislets after 5 days of in vitro culture. However, with use of GSIS assays, some studies have observed that a step increase of glucose to 12 or 20 mmol/L evokes higher insulin secretion in size-controlled pseudoislets than in intact islets (40,49); the basis for these findings has not been determined. In our experience, static batch GSIS after genetic manipulation of pseudoislets, like lentiviral transduction, leads to a 1.5-fold to 2.0-fold increase of insulin output for up to 10 days of culture (8,39 and A. Urizar, Y. Hang, S.K.K., unpublished data) (Fig. 2C), and this output is enhanced by the addition of the potentiator 3-isobutyl-1-methylxanthine (IBMX), consistent with reports by others (10,37).
With use of dynamic perifusion, biphasic GSIS by pseudoislets has also been characterized (40,41,48). In most studies, there was no significant difference between intact human islets and human pseudoislets in first-phase, second-phase, or total insulin secretion after 6–7 days of culture (40,41), although one report observed a higher first-phase–to–second-phase GSIS ratio in pseudoislets relative to intact islets cultured for the same period (48). In addition to preservation of the biphasic insulin response to glucose, pseudoislets retain physiologic responses to the secretion potentiators glibenclamide (48), potassium (41), IBMX (8,21), palmitate, and forskolin (49). Pseudoislets and intact islets also have similar glucagon secretion, which is inhibited by high glucose and potentiated by IBMX, epinephrine, potassium, and l-arginine (8,41). Moreover, direct comparison of pseudoislets prepared via hanging drop or ultra-low attachment microwells reported very similar insulin and glucagon secretion at low and high glucose levels (41), demonstrating that distinct reaggregation methods yield similar function in pseudoislets.
Relatively longer-term culture of pseudoislets (>6 days in vitro) can enhance experimental assessment of drug exposure (51) or genetic modification (43). Batch GSIS, insulin content, and glucagon content remain stable in pseudoislets from 6 to 14 days of standard culture (49). In contrast to analyses of intact islets (52,53), three studies reported that pseudoislet function improved with extended culture (11,37,40). Furthermore, relatively smaller pseudoislets had enhanced GSIS in comparison with native intact islets in static batch assays up to 15 days under standard culture conditions (11). The functional advantage of smaller pseudoislets was attributed to greater resistance to hypoxia, as smaller diameter facilitates diffusion of nutrients to cells within the pseudoislet core (11). By a similar logic, it has been proposed that size-matched pseudoislets may tolerate hypoxia and long-term culture better than native islets because of the relative paucity of ECM encasing the multicellular structure (11). Thus, pseudoislet studies demonstrate how simple manipulation of pancreatic islets, such as dispersion and reaggregation, may be useful for understanding endocrine function and optimizing cell performance in clinical islet transplantation.
Transplantation Studies of Pseudoislet Function
Pseudoislet transplantation has been valuable for investigating durable in vivo properties, including after pseudoislet genetic manipulation (Fig. 2D and E). To date, the most common site for human pseudoislet transplantation studies is the renal subcapsular space of immunodeficient mice (8–15 [this is not an exhaustive reference listing]). In this setting, human pseudoislets and intact islets engrafted with equivalent efficiency, and pseudoislets performed at least as well as intact islets in rescuing hyperglycemia in streptozotocin-induced diabetic mice (11). Moreover, pseudoislet function was durable: glucose clearance after intraperitoneal glucose challenge 2 months after pseudoislet transplantation was comparable with that of untreated nondiabetic controls. In some cases, in vivo function of the transplanted pseudoislets was confirmed by reversion to diabetes after pseudoislet graft removal (11). Immunostaining of recovered grafts revealed that morphology, hormone expression, and cell localization of transplanted human pseudoislets resembled those of islets in situ (8,11,13,43). Additionally, pseudoislets appeared well revascularized in vivo, even potentially to a greater extent than intact islets. This observation has been attributed to the reduced or absent ECM capsule in pseudoislets that otherwise might impede neovascularization (11,13).
Novel Functional and Molecular Approaches for Characterizing Pseudoislets
Whole-cell patch clamp can be used for assessment of electrophysiological function and hormone exocytosis from single cells recovered from pseudoislets (43) (Fig. 2H). Recent advances combining whole-cell patch-clamp measurements and single-cell RNA sequencing (“patch-seq”) have allowed simultaneous assessment and correlation of quantitative physiologic measurements and gene expression in human islet cells (54). In combination with novel genetic pseudoislet approaches, patch-seq may illuminate genetic and functional pathways critical for pancreatic endocrine cell function.
Genetic Studies With Pseudoislet-Based Approaches
The application of genetics, especially loss-of-function approaches, to primary human islet cells has been an enduring challenge. Human stem cell–derived insulin-producing cells that resemble β-cells (47) and immortalized β-cell lines derived from human fetal pancreas like EndoC-βH3 (55,56) have provided surrogate systems for investigating human islet genetics. Despite the value of these models, they are fundamentally different from genuine pancreatic islet cells in terms of gene regulation, maturation, function, proliferation (57), and representation of β- and non-β-cell types, limiting their interpretation. Still, the quiescence and restricted duration of primary human islet cell in vitro culture impose restrictions on genetic studies that require efficient gene-delivery systems for transduction. Lentiviruses, retroviruses, adenoviruses, and adeno-associated viruses (AAVs) carrying cDNA-expressing constructs have been used for overexpression experiments in intact islets of mice and humans (58–62). However, overall cellular transduction in intact islets was low and inconsistent, ranging from 2% to 50%, reflecting the relative impermeability of the intact islet to viral transduction (58,59). Moreover, variable results from viral transduction of intact islets may arise from the viral type or subtype and the species origin of islets used (9,60).
Viral transduction studies showed that dispersion of murine islets improved transduction efficiency of pancreatic endocrine cells by retroviruses and lentiviruses (59). Interestingly, only lentiviruses resulted in efficient transduction of β-cells (25% transduced insulin+ cells for lentiviruses, vs. <1% by retroviruses). However, islet cells were cultured as a dispersed monolayer following infection in this prior publication, precluding analysis in vivo. Multiple subsequent studies showed that viral transduction of pseudoislets could be applied for genetic studies of islets from rodents (20,42) and humans (8,9,21,39,41,43,48,63,64) (see Table 2). More than 70–80% of all islet cells can be transduced for gain- or loss-of-function studies (8,41,48,63) (see Fig. 2A and B). This approach can be adapted in multiple ways to achieve islet cell targeting, including use of cis-regulatory elements that promote specific transgene expression in β-cells (9,48) or α-cells (65). Likewise, this general method has been useful for studies of cadaveric human islets from a range of subjects, including neonates, children, adults, and subjects with diabetes, particularly when combined with cell-subtype transcriptome and chromatin analyses (8,21,39,43) (Fig. 2F and G).
Table 2.
Comprehensive list of studies with use of genetics in pseudoislets
| Delivery | Genetic modification | Species | Reference(s) | Application |
|---|---|---|---|---|
| Lentiviruses | Gain of function | Human | Zaldumbide et al. (2013) | Misexpression of immune-evasion proteins |
| Arda et al. (2016) Peiris et al. (2018) Bevacqua et al. (2021a) |
Misexpression of transcription factors Misexpression of transcription factors Misexpression of transcription factors |
|||
| Rats | Caton et al. (2003) | Misexpression of various connexins | ||
| Lentiviruses | Loss of function (shRNAs) | Human | Peiris et al. (2018) | Silencing of transcription factor |
| Harata et al. (2018) | Silencing of glucokinase | |||
| Liu et al. (2019) | Technical report | |||
| Liu et al. (2020) | Silencing of adipose triglyceride lipase | |||
| Bevacqua et al. (2021a) | Silencing of transcription factor | |||
| Lentiviruses | Knockout (indels/deletions) | Human | Bevacqua et al. (2021b) | CRISPR/Cas9 knockout of transcription factors and noncoding regulatory DNA |
| Lentiviruses | Endogenous gene activation | Human | Bevacqua et al. (2021b) | CRISPR-A of a transcription factor and enhancer |
| Adenoviruses | Gain of function | Human | Walker et al. (2020) | Misexpression of biosensor (GCAMP), study of cell-cell interactions |
| Furuyama et al. (2019) | Reprogramming of α-cells via ectopic expression of β-cell transcription factors | |||
| Mice | van Krieken et al. (2019) | Amplification of the V1b receptor signaling | ||
| Herpes simplex virus | Gain of function | Human | Rabinovitch et al. (1999) | Misexpression of antiapoptotic gene (bcl-2) |
For example, a study from our group (21) showed that misexpression of the transcription factor SIX3 in juvenile human islets enhanced GSIS. Normally, SIX3 expression in human islets is restricted to β-cells and not detected before age 10–11 years (21) or in rodent islets (4), suggesting that this transcription factor might play a key, species-specific role in functional maturation of β-cells, a possibility supported by recent studies with SIX3 knockdown in human pseudoislets (39). In another study (8), we revealed—using gain- and loss-of-function studies in human pseudoislets—that the transcriptional repressor BCL11A had previously uncharacterized roles in regulating human β-cell function. Both SIX3 and BCL11A have been implicated by genome-wide association studies as candidate risk regulators in T2D (8,39,66,67). Thus, genetic studies in human pseudoislets have broken new ground in the long-standing drive to identify genetic regulators of human diabetes risk. Future application of pseudoislet genetics will benefit from methods to measure additional pseudoislet phenotypes (summarized in Fig. 2), including signaling pathways and cell-cell interactions that govern islet cell function (20,41,51).
Pseudoislet biology and genetic studies have also been useful for investigating islet transplantation outcomes (Fig. 2D and E). In one study, islet cells transduced with the BCL2 gene were protected from cytokine-induced accumulation of toxic oxygen radicals and DNA fragmentation (63). Zaldumbide et al. (9) showed that human β-cells can be efficiently transduced with human cytomegalovirus-encoded US2 protein and serine proteinase inhibitor 9, and protected from autoreactive T cells, while retaining their hallmark functions. Lentiviral-mediated misexpression of the V1b receptor (a GPCR member of the vasopressin-oxytocin family) in mouse pseudoislets can improve function after engraftment into immunocompromised mice (42). These studies illuminate how pseudoislet studies might lead to translational applications in islet transplantation.
Conclusions and Outlook
Pseudoislets offer multiple experimental advantages, while maintaining crucial physiological functions of intact islets, features that can be exploited to address unsolved mysteries in human islet biology and diabetes. However, there are limitations that may be addressed in the future to optimize the use of this strategy. For example, cell loss during pseudoislet formation and the cost of human islet procurement could limit investigations requiring significant pseudoislet input. Continued optimization of islet isolation, islet culture, and pseudoislet formation methods will ameliorate these limitations. It will also be important to clarify how donor age or disease status impacts studies of pseudoislets, as most studies to date have focused on pseudoislets from previously healthy adult donors. Our group recently generated genetically modified pseudoislets from juvenile (21,39) and T2D human donors (R.J.B., S.K.K., unpublished data), but additional studies are needed to clarify the impact of age, disease status, and other clinical variables on possible molecular and functional differences of pseudoislet biology.
Pseudoislet biology offers many tantalizing possibilities for future studies—including the exciting possibilities of using nuclease-based site-specific gene editing on specific islet cell types and building chimeric pseudoislets to unveil signals that enhance islet cell differentiation (57). While space limits preclude extensive discussion, we foresee that pseudoislet approaches will be coupled with emerging approaches for high-throughput sequencing (55,68), specialized proteomics (69), islet electrophysiology (54), and studies of islets from large animal models with experimental advantages, including sheep and pigs (70–72). The pseudoislet organoid platform provides a strategy for incisive in vitro and in vivo studies of islet genetics, signaling, pharmacology, and integrated function. Pseudoislet studies could transform approaches for studying human islets in physiological and disease settings and promote relevant translational efforts for ameliorating or curing diabetes.
Article Information
Acknowledgments. The authors thank past and present members of the Kim group for pioneering genetic studies of pseudoislets, especially Drs. Efsun Arda, Yan Hang, Krissie Tellez, Harini Chakravarthy, and Heshan Peiris, and Dr. Vincenzo Cirulli (University of Washington) for helpful suggestions, advice, and encouragement.
Funding. M.S.H.F. is a student in the Stanford Medical Scientist Training Program and Knight-Hennessey Scholars Program and was supported by the Stanford Medical Scholars Program and Howard Hughes Medical Institute Medical Research Fellows Program. V.M.N. was supported by a National Institutes of Health (NIH) T32 training grant (5T32GM007790). R.J.B. was supported by a postdoctoral fellowship from JDRF (3-PDF-2018-584-A-N) and is on leave from the Animal Biotechnology Laboratory, Facultad de Agronomía, Universidad de Buenos Aires/INPA CONICET, Ciudad Autonoma de Buenos Aires, Argentina. Work here was supported by NIH awards (R01 DK107507, R01 DK108817, U01 DK123743, and R01 DK126482 to S.K.K.) and JDRF Center of Excellence (to S.K.K. and M. Hebrok). Work here was also supported by NIH grant P30 DK116074 (to S.K.K.), by the Snyder Foundation and Elser Trust, and by the Stanford Islet Research Core and Diabetes Genomics and Analysis Core of the Stanford Diabetes Research Center.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. M.S.H.F., V.M.N., S.K.K., and R.J.B. researched and wrote, discussed, and reviewed the manuscript. M.S.H.F. and R.J.B. also created figures and tables, which were reviewed and edited by all authors.
Footnotes
This article contains supplementary material online at https://doi.org/10.2337/figshare.14075618.
M.S.H.F. and V.M.N. made equal contributions.
References
- 1. American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2020 . Diabetes Care 2020;43(Suppl. 1):S14–S31 [DOI] [PubMed] [Google Scholar]
- 2. Cabrera O, Berman DM, Kenyon NS, Ricordi C, Berggren PO, Caicedo A.. The unique cytoarchitecture of human pancreatic islets has implications for islet cell function. Proc Natl Acad Sci U S A 2006;103:2334–2339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rodriguez-Diaz R, Molano RD, Weitz JR, et al. Paracrine interactions within the pancreatic islet determine the glycemic set point. Cell Metab 2018;27:549–558.e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Benner C, van der Meulen T, Cacéres E, Tigyi K, Donaldson CJ, Huising MO.. The transcriptional landscape of mouse beta cells compared to human beta cells reveals notable species differences in long non-coding RNA and protein-coding gene expression. BMC Genomics 2014;15:620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Baron M, Veres A, Wolock SL, et al. A single-cell transcriptomic map of the human and mouse pancreas reveals inter- and intra-cell population structure. Cell Syst 2016;3:346–360.e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bosco D, Armanet M, Morel P, et al. Unique arrangement of α- and β-cells in human islets of Langerhans. Diabetes 2010;59:1202–1210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Segerstolpe Å, Palasantza A, Eliasson P, et al. Single-cell transcriptome profiling of human pancreatic islets in health and type 2 diabetes. Cell Metab 2016;24:593–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Peiris H, Park S, Louis S, et al. Discovering human diabetes-risk gene function with genetics and physiological assays. Nat Commun 2018;9:3855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zaldumbide A, Alkemade G, Carlotti F, et al. Genetically engineered human islets protected from CD8-mediated autoimmune destruction in vivo. Mol Ther 2013;21:1592–1601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hilderink J, Spijker S, Carlotti F, et al. Controlled aggregation of primary human pancreatic islet cells leads to glucose-responsive pseudoislets comparable to native islets. J Cell Mol Med 2015;19:1836–1846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yu Y, Gamble A, Pawlick R, et al. Bioengineered human pseudoislets form efficiently from donated tissue, compare favourably with native islets in vitro and restore normoglycaemia in mice. Diabetologia 2018;61:2016–2029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Spijker HS, Ravelli RBG, Mommaas-Kienhuis AM, et al. Conversion of mature human β-cells into glucagon-producing α-cells. Diabetes 2013;62:2471–2480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lavallard V, Armanet M, Parnaud G, et al. Cell rearrangement in transplanted human islets. FASEB J 2016;30:748–760 [DOI] [PubMed] [Google Scholar]
- 14. Montanari E, Meier RPH, Mahou R, et al. Multipotent mesenchymal stromal cells enhance insulin secretion from human islets via N-cadherin interaction and prolong function of transplanted encapsulated islets in mice. Stem Cell Res Ther 2017;8:199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Furuyama K, Chera S, van Gurp L, et al. Diabetes relief in mice by glucose-sensing insulin-secreting human α-cells. Nature 2019;567:43–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Scharp DW, Downing R, Merrell RC, Greider M.. Isolating the elusive islet. Diabetes 1980;29(Suppl. 1):19–30 [DOI] [PubMed] [Google Scholar]
- 17. Schröder D, Wegner U, Hehmke B, Besch W, Zühlke H.. Pancreatic islet cell suspensions of newborn rats and the formation of pseudo-islets in culture. Acta Biol Med Ger 1982;41:1145–1150 [PubMed] [Google Scholar]
- 18. Britt LD, Stojeba PC, Scharp CR, Greider MH, Scharp DW.. Neonatal pig pseudo-islets. A product of selective aggregation. Diabetes 1981;30:580–583 [DOI] [PubMed] [Google Scholar]
- 19. Kuo CY, Herrod HG, Burghen GA.. Formation of pseudoislets from human pancreatic cultures. Pancreas 1992;7:320–325 [DOI] [PubMed] [Google Scholar]
- 20. Caton D, Calabrese A, Mas C, et al. Lentivirus-mediated transduction of connexin cDNAs shows level- and isoform-specific alterations in insulin secretion of primary pancreatic beta-cells. J Cell Sci 2003;116:2285–2294 [DOI] [PubMed] [Google Scholar]
- 21. Arda HE, Li L, Tsai J, et al. Age-dependent pancreatic gene regulation reveals mechanisms governing human β cell function. Cell Metab 2016;23:909–920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Steinberg MS. Reconstruction of tissues by dissociated cells. Some morphogenetic tissue movements and the sorting out of embryonic cells may have a common explanation. Science 1963;141:401–408 [DOI] [PubMed] [Google Scholar]
- 23. Tiwari P, Mrigwani A, Kaur H, Kaila P, Kumar R, Guptasarma P.. Structural-mechanical and biochemical functions of classical cadherins at cellular junctions: a review and some hypotheses. Adv Exp Med Biol 2018;1112:107–138 [DOI] [PubMed] [Google Scholar]
- 24. Rouiller DG, Cirulli V, Halban PA.. Uvomorulin mediates calcium-dependent aggregation of islet cells, whereas calcium-independent cell adhesion molecules distinguish between islet cell types. Dev Biol 1991;148:233–242 [DOI] [PubMed] [Google Scholar]
- 25. Parnaud G, Lavallard V, Bedat B, et al. Cadherin engagement improves insulin secretion of single human β-cells. Diabetes 2015;64:887–896 [DOI] [PubMed] [Google Scholar]
- 26. Carvell MJ, Marsh PJ, Persaud SJ, Jones PM.. E-cadherin interactions regulate beta-cell proliferation in islet-like structures. Cell Physiol Biochem 2007;20:617–626 [DOI] [PubMed] [Google Scholar]
- 27. Esni F, Täljedal I-B, Perl A-K, Cremer H, Christofori G, Semb H.. Neural cell adhesion molecule (N-CAM) is required for cell type segregation and normal ultrastructure in pancreatic islets. J Cell Biol 1999;144:325–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yebra M, Diaferia GR, Montgomery AMP, et al. Endothelium-derived Netrin-4 supports pancreatic epithelial cell adhesion and differentiation through integrins α2β1 and α3β1. PLoS One 2011;6:e22750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Li H, Neelankal John A, Nagatake T, Hamazaki Y, Jiang F-X.. Claudin 4 in pancreatic β cells is involved in regulating the functional state of adult islets. FEBS Open Bio 2020;10:28–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Meda P. Gap junction proteins are key drivers of endocrine function. Biochim Biophys Acta Biomembr 2018;1860:124–140 [DOI] [PubMed] [Google Scholar]
- 31. Goldberg GS, Lampe PD, Nicholson BJ.. Selective transfer of endogenous metabolites through gap junctions composed of different connexins. Nat Cell Biol 1999;1:457–459 [DOI] [PubMed] [Google Scholar]
- 32. Eiberger J, Degen J, Romualdi A, Deutsch U, Willecke K, Söhl G.. Connexin genes in the mouse and human genome. Cell Commun Adhes 2001;8:163–165 [DOI] [PubMed] [Google Scholar]
- 33. Konstantinova I, Nikolova G, Ohara-Imaizumi M, et al. EphA-Ephrin-A-mediated β cell communication regulates insulin secretion from pancreatic islets. Cell 2007;129:359–370 [DOI] [PubMed] [Google Scholar]
- 34. Hauge-Evans AC, Squires PE, Persaud SJ, Jones PM.. Pancreatic beta-cell-to-beta-cell interactions are required for integrated responses to nutrient stimuli: enhanced Ca2+ and insulin secretory responses of MIN6 pseudoislets. Diabetes 1999;48:1402–1408 [DOI] [PubMed] [Google Scholar]
- 35. Jaques F, Jousset H, Tomas A, et al. Dual effect of cell-cell contact disruption on cytosolic calcium and insulin secretion. Endocrinology 2008;149:2494–2505 [DOI] [PubMed] [Google Scholar]
- 36. Rouiller DG, Cirulli V, Halban PA.. Differences in aggregation properties and levels of the neural cell adhesion molecule (NCAM) between islet cell types. Exp Cell Res 1990;191:305–312 [DOI] [PubMed] [Google Scholar]
- 37. Reissaus CA, Piston DW.. Reestablishment of glucose inhibition of glucagon secretion in small pseudoislets. Diabetes 2017;66:960–969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Liu S, Harata M, Promes JA, Burand A, Ankrum JA, Imai Y. Lentiviral mediated gene silencing in human pseudoislet prepared in low attachment plates. J Vis Exp 2019;147:e59578 [DOI] [PMC free article] [PubMed]
- 39. Bevacqua RJ, Lam JY, Peiris H, et al. SIX2 and SIX3 coordinately regulate functional maturity and fate of human pancreatic β cells. Genes Dev 2021;35:234–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zuellig RA, Cavallari G, Gerber P, et al. Improved physiological properties of gravity-enforced reassembled rat and human pancreatic pseudo-islets. J Tissue Eng Regen Med 2017;11:109–120 [DOI] [PubMed] [Google Scholar]
- 41. Walker JT, Haliyur R, Nelson HA, et al. Integrated human pseudoislet system and microfluidic platform demonstrate differences in GPCR signaling in islet cells. JCI Insight 2020;5:e137017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. van Krieken PP, Voznesenskaya A, Dicker A, et al. Translational assessment of a genetic engineering methodology to improve islet function for transplantation. EBioMedicine 2019;45:529–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bevacqua RJ, Dai X, Lam J, et al. CRISPR-based genome editing in primary human pancreatic islet cells. Nature Communications. In press [DOI] [PMC free article] [PubMed]
- 44. Mizukami H, Takahashi K, Inaba W, et al. Age-associated changes of islet endocrine cells and the effects of body mass index in Japanese. J Diabetes Investig 2014;5:38–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Benninger RKP, Remedi MS, Head WS, Ustione A, Piston DW, Nichols CG.. Defects in beta cell Ca2+ signalling, glucose metabolism and insulin secretion in a murine model of K(ATP) channel-induced neonatal diabetes mellitus. Diabetologia 2011;54:1087–1097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Noguchi GM, Huising MO.. Integrating the inputs that shape pancreatic islet hormone release. Nat Metab 2019;1:1189–1201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Nair GG, Liu JS, Russ HA, et al. Recapitulating endocrine cell clustering in culture promotes maturation of human stem-cell-derived β cells. Nat Cell Biol 2019;21:263–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Harata M, Liu S, Promes JA, Burand AJ, Ankrum JA, Imai Y.. Delivery of shRNA via lentivirus in human pseudoislets provides a model to test dynamic regulation of insulin secretion and gene function in human islets. Physiol Rep 2018;6:e13907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lorza-Gil E, Gerst F, Oquendo MB, et al. Glucose, adrenaline and palmitate antagonistically regulate insulin and glucagon secretion in human pseudoislets. Sci Rep 2019;9:10261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Nalbach L, Roma LP, Schmitt BM, et al. Improvement of islet transplantation by the fusion of islet cells with functional blood vessels. EMBO Mol Med 2021;13:e12616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lorza-Gil E, Kaiser G, Rexen Ulven E, et al. FFA2-, but not FFA3-agonists inhibit GSIS of human pseudoislets: a comparative study with mouse islets and rat INS-1E cells. Sci Rep 2020;10:16497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Paraskevas S, Maysinger D, Wang R, Duguid TP, Rosenberg L.. Cell loss in isolated human islets occurs by apoptosis. Pancreas 2000;20:270–276 [DOI] [PubMed] [Google Scholar]
- 53. Kin T, Senior P, O’Gorman D, Richer B, Salam A, Shapiro AM.. Risk factors for islet loss during culture prior to transplantation. Transpl Int 2008;21:1029–1035 [DOI] [PubMed] [Google Scholar]
- 54. Camunas-Soler J, Dai X-Q, Hang Y, et al. Patch-Seq links single-cell transcriptomes to human islet dysfunction in diabetes. Cell Metab 2020;31:1017–1031.e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Miguel-Escalada I, Bonàs-Guarch S, Cebola I, et al. Human pancreatic islet three-dimensional chromatin architecture provides insights into the genetics of type 2 diabetes. Nat Genet 2019;51:1137–1148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Grotz AK, Abaitua F, Navarro-Guerrero E, Hastoy B, Ebner D, Gloyn AL.. A CRISPR/Cas9 genome editing pipeline in the EndoC-βH1 cell line to study genes implicated in beta cell function. Wellcome Open Res 2020;4:150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Sneddon JB, Tang Q, Stock P, et al. Stem cell therapies for treating diabetes: progress and remaining challenges. Cell Stem Cell 2018;22:810–823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Sigalla J, David A, Anegon I, et al. Adenovirus-mediated gene transfer into isolated mouse adult pancreatic islets: normal beta-cell function despite induction of an anti-adenovirus immune response. Hum Gene Ther 1997;8:1625–1634 [DOI] [PubMed] [Google Scholar]
- 59. Leibowitz G, Beattie GM, Kafri T, et al. Gene transfer to human pancreatic endocrine cells using viral vectors. Diabetes 1999;48:745–753 [DOI] [PubMed] [Google Scholar]
- 60. Giannoukakis N, Rudert WA, Ghivizzani SC, et al. Adenoviral gene transfer of the interleukin-1 receptor antagonist protein to human islets prevents IL-1beta-induced beta-cell impairment and activation of islet cell apoptosis in vitro. Diabetes 1999;48:1730–1736 [DOI] [PubMed] [Google Scholar]
- 61. Gallichan WS, Kafri T, Krahl T, Verma IM, Sarvetnick N.. Lentivirus-mediated transduction of islet grafts with interleukin 4 results in sustained gene expression and protection from insulitis. Hum Gene Ther 1998;9:2717–2726 [DOI] [PubMed] [Google Scholar]
- 62. Pekrun K, De Alencastro G, Luo Q-J, et al. Using a barcoded AAV capsid library to select for clinically relevant gene therapy vectors. JCI Insight 2019;4:e131610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Rabinovitch A, Suarez-Pinzon W, Strynadka K, et al. Transfection of human pancreatic islets with an anti-apoptotic gene (bcl-2) protects beta-cells from cytokine-induced destruction. Diabetes 1999;48:1223–1229 [DOI] [PubMed] [Google Scholar]
- 64. Liu S, Promes JA, Harata M, et al. Adipose triglyceride lipase is a key lipase for the mobilization of lipid droplets in human β-cells and critical for the maintenance of syntaxin 1a levels in β-cells. Diabetes 2020;69:1178–1192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Pauerstein PT, Park KM, Peiris HS, Wang J, Kim SK.. Research resource: genetic labeling of human islet alpha cells. Mol Endocrinol 2016;30:248–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kim YJ, Go MJ, Hu C, et al.; MAGIC consortium. Large-scale genome-wide association studies in East Asians identify new genetic loci influencing metabolic traits. Nat Genet 2011;43:990–995 [DOI] [PubMed] [Google Scholar]
- 67. Zeggini E, Scott LJ, Saxena R, et al.; Wellcome Trust Case Control Consortium. Meta-analysis of genome-wide association data and large-scale replication identifies additional susceptibility loci for type 2 diabetes. Nat Genet 2008;40:638–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Enge M, Arda HE, Mignardi M, et al. Single-cell analysis of human pancreas reveals transcriptional signatures of aging and somatic mutation patterns. Cell 2017;171:321–330.e14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Marx V. A dream of single-cell proteomics. Nat Methods 2019;16:809–812 [DOI] [PubMed] [Google Scholar]
- 70. Vilarino M, Rashid ST, Suchy FP, et al. CRISPR/Cas9 microinjection in oocytes disables pancreas development in sheep. Sci Rep 2017;7:17472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Kuppan P, Seeberger K, Kelly S, et al. Co-transplantation of human adipose-derived mesenchymal stem cells with neonatal porcine islets within a prevascularized subcutaneous space augments the xenograft function. Xenotransplantation 2020;27:e12581 [DOI] [PubMed] [Google Scholar]
- 72. Kim S, Whitener RL, Peiris H, et al. Molecular and genetic regulation of pig pancreatic islet cell development. Development 2020;147:dev186213 [DOI] [PMC free article] [PubMed] [Google Scholar]