Abstract
Background and aim
The therapeutic procedures used in the treatment of caries do not always eliminate all the microorganisms. Persisting cariogenic bacteria can cause recurrent caries and failure of restoration. Incorporation of an antimicrobial agent in the restorative material may be of paramount significance. The purpose of this study was to evaluate and compare the antibacterial effect of glass ionomer cement (GIC) containing CHX and miswak extract on Streptococcus mutans and Streptococcus sobrinus in ECC children using polymerase chain reaction (PCR).
Materials and methods
Forty-five children with ECC in the age-group 3–6 years were selected. The children were randomly allocated into three groups. Supragingival plaque samples (S1) were collected from sound buccal or labial surfaces of primary teeth. Cavity preparation was done and the teeth were restored according to the group to which the child had been allotted. The second plaque sample (S2) was collected 1 month and the final sample after 3 months of restoring all the decayed teeth. All the samples were sent for PCR analysis.
Results
Intergroup analysis was done using Kruskal–Wallis test followed by Mann–Whitney post hoc test showed statistically significant difference in S. mutans and S. sobrinus count between group I (CHX) and group III (control) and group II (miswak) and group III (control) but no statistically significant difference between group I (CHX) and group II (miswak) in S. mutans and S. sobrinus count.
Conclusion
1% chlorhexidine digluconate and aqueous extract of miswak are equally effective against S. mutans and S. sobrinus. Miswak can be used as an alternative herbal antimicrobial that can be incorporated in anhydrous GIC.
How to cite this article
Kalpavriksha AJ, Siddaiah SB, Bilichodmath S, et al. Comparative Evaluation of Antibacterial Effect of GIC Containing Chlorhexidine and Miswak on Streptococcus mutans and Streptococcus sobrinus in Early Childhood Caries Children: A PCR Study. Int J Clin Pediatr Dent 2021;14(2):229–234.
Keywords: Chlorhexidine, Early childhood caries, PCR
Introduction
Dental caries is a multifactorial disease that is ubiquitous and is as old as mankind.1 It is an international public health challenge, especially among infants and toddlers, and is known as early childhood caries.2,3 The disease of ECC is the presence of 1 or more decayed (cavitated or non-cavitated lesions), missing (due to caries), or filled tooth surfaces in any primary tooth in a child who is 71 months of age or younger.4
ECC begins early in life, progresses rapidly, and its consequences can have both immediate and long-term effects not only on the child but also the family.4 Dental caries continues to remain a major dental health problem despite the numerous advances made in the field of preventive dentistry. A positive correlation has been observed between the levels of mutans streptococci and caries experience among individuals. Out of seven species of MS that exhibit variable virulence and adherence properties, Streptococcus mutans and Streptococcus sobrinus are strongly implicated with the occurrence of dental caries.5
Streptococcus mutans are the principal cariogenic bacteria for caries initiation. Streptococcus sobrinus although are less frequently detected is associated with enhanced caries activity on the smooth surfaces causing caries increment, development, and progression.6,7 In ECC, MS comprises 10% of salivary flora and 30–50% of plaque flora whereas <1% of the of the flora in low caries risk children is MS.7
Once the caries process sets in restoring the tooth become mandatory to prevent further damage to the tooth structure. The most widely used restorative material for restoring deciduous teeth is GIC.8 Several modifications have been made in the chemistry of conventional GIC's to improve their properties. One such modification is anhydrous GIC. This cement not only has improved physical properties but also better mixing and handling characteristics.9
The therapeutic procedure involved in the treatment of caries will not always eliminate all the microorganisms and the persisting cariogenic bacteria can further cause recurrent caries.10 This can be reduced by improving the antibacterial properties of the cement either by making advances of the material or modifications of the existing one. The fact that GIC can readily permit ion exchange with its local environment offers the advantage to dope the cement with other soluble antimicrobials.11,12
CHX, a bisbiguanide, is a gold standard for caries and gingivitis control.13 Owing to its antimicrobial properties, low cost, and well understood pharmacological properties CHX can be easily incorporated into several dental materials including GIC.12 Due to an increase in the side effects and development of antibiotic resistance to some antimicrobials, on one hand; safety, easy availability, and low cost of natural products, on the other hand, several natural and herbal products used for caries prevention, can be incorporated into dental products.14 One such material is Miswak. Miswak also known as Siwak is derived from the twigs or roots of Salvadora persica or Arak tree, commonly found in Asia and Africa.14 It possesses properties like anti-caries, antifungal, antibacterial, anti-periopathic, anti-inflammatory, and helps in wound healing.14,15
To understand the part played by the microorganisms in causing caries efficient methods are needed to detect and identify them. qRT-PCR uses primers that are specific for a given species, thus providing a sensitive and accurate method to detect and quantify individual species of bacteria and also the total bacteria.5,6 Not many studies have compared the antibacterial properties of GIC with CHX and miswak in children against the mentioned microorganisms using qRT-PCR. Hence, the present study aimed to evaluate the effect of adding CHX and miswak extract to GIC on S. mutans and S. sobrinus when used as a restorative material in ECC.
Materials and Methods
The current study was carried on 45 children in the age-group 3–6 years visiting the Department of Pediatric and Preventive Dentistry, RajaRajeswari Dental College and Hospital, Bengaluru. The ethical clearance was obtained from institutional review board. Children with 4 or more than 4 cavitated restorable lesions were included in the study. Children who had taken antibiotics in the past 3 months, children who received fluoride topical application during last 48 hours, children who require special health care needs, children with restored teeth, pulpally involved teeth and children undergoing any kind of interceptive orthodontic treatment were excluded from the study.
The decayed, missing, or filled teeth were given scores according to WHO criteria. Children were randomly allotted one of the groups:-
Experimental group I: (N = 15)
Children where all the decayed teeth were restored using GIC with 1% CHX digluconate.
Experimental group II: (N = 15)
Children where all the decayed teeth were restored using GIC with miswak extract.
Experimental group III (control): (N = 15)
Children where all the decayed teeth were restored using GIC with deionized water.
Preparation of Study Materials
Chlorhexidine Digluconate Solution
20% CHX digluconate solution was purchased from Sigma-Aldrich. This was further diluted to 1% working stock solution. To obtain 50 mL solution of 1% CHX digluconate, 2.5 mL of 20% CHX digluconate was added to 47.5 mL of sterile distilled water. This 1% solution was filter sterilized using 0.23 μm syringe filters and sterile plastic 50 mL containers. The filtered 1% CHX digluconate was stored at 4°C until used.
Aqueous Extract of Miswak
Miswak sticks were chopped into thin strands using a knife and dried in shade for 4 days. The thin strand was further chopped into 0.3–0.5 cm pieces using strong cutters and was dried for 2 days in the shade. The small miswak pieces were grounded into a fine powder using a blender. The powder was sieved to remove the coarse particles. The cold maceration protocol was followed by soaking 10 g of sieved fine powder of miswak in 100 mL of deionized sterile (autoclaved) water. The mixture was incubated for 48 hours at 4°C in a refrigerator with customized setup for continuous stirring using magnetic stirrer. After incubation, the mixture was then centrifuged at 2,000 rpm for 15 minutes. The supernatant was collected in new 50 mL tubes and initially filtered using Whatman qualitative filter paper No. 1. Then, the filtrate was again filtered with 0.45 μm membrane filters using vacuum filtration apparatus. The final miswak extract was aliquot in 1 mL which was stored at −20°C until use. The prepared and stored extract was used within 7 days.
Sample Collection
Supragingival plaque samples (S1) were obtained from sound buccal/labial surfaces of primary teeth using a sterile dental explorer. The collected plaque sample was aseptically transferred to the transport media TE buffer and the sample was stored at −20°C until the time qRT-PCR analysis was done. Similar method was followed for all the collected samples.
All the carious dentin was removed using a sterile spoon excavator. Cavity preparation was done using sterile #330 round bur in a high-speed handpiece. All the infected dentin was removed leaving behind the affected dentin. Any leftover unsupported enamel was removed using an enamel hatchet. The cavity was then cleaned and dried by blotting with a dry cotton pellet. All the decayed teeth were then restored according to the assigned study group (group I/II/III). All the clinical procedures were performed by a single examiner.
The water dispenser supplied by the manufacturer was filled with the liquid (1% CHX digluconate, aqueous extract of miswak or deionized water) and the plastic insert was placed onto the neck. To dispense the liquid, the bottle was held vertically above the mixing pad and squeezed gently. The powder bottle was inverted before use to fluff the powder. The powder liquid ratio was used according to the manufacturer's instructions, i.e., two scoops powder: two drops liquid. The cement was mixed using an agate spatula. Mixing was done by dividing the powder into two equal halves on the mixing pad. The first half of the powder was mixed with the liquid in 5 seconds and then the second half was incorporated and mixed for 10 seconds with mixing time not exceeding 20 seconds. The final mix should have a glossy surface.
The cement mix was packed into the cavity using the plastic filling instrument. It was then pressed into the cavity for 30 seconds using a gloved finger coated with petroleum jelly. Occlusion was checked and excess material was removed using a sterile carver. All the participants in all the groups were advised not to drink or eat anything for an hour.
The second plaque sample (S2) was collected after 1 month and the final sample was collected after 3 months of restoring the final decayed tooth from the buccal surfaces of primary molars or labial surfaces of primary anteriors and was stored until PCR analysis was done.
qRT-PCR
Extraction of DNA from the plaque samples was done by using a highly efficient Invitrogen DNA isolation kit (Purelink™ DNA extraction kit, Applied BioSystems, India). The extracted DNA was then purified. In the current study, Custom SYBR Green assay reagents (Applied BioSystems, India) were used. The primer sequences are as follows:
Streptococcus mutans Forward: 5′-GCCTACAGCTCAGAGATGCTATTCT-3′ Reverse: 5′GCCATACACCACTCATGAATTGA-3′
Streptococcus sobrinus Forward: 5′-TGC TAT CTT TCC CTA GCA TG-3′ Reverse: 5′-GGT ATT CGG TTT GAC TGC-3′
16S RNA (2 sets) Forward Primer: 3′-TCCTACGGGAGGCAGCAGT-5′ Reverse Primer: 5′-GGACTACCAGGGTATCTAATCCTGTT-3′
Pure link genomic DNA extraction kit (135 samples).
PCR Protocol
A reaction solution composed of SYBR Green Universal PCR Master Mix (10 μL), forward primer (1 μL) and reverse primer (1 μL) for the specific organism, extracted DNA of unknown sample (3 μL) and water that is nucleus free to make a complete reaction volume of 20 μL. The conditions for real-time PCR were as follows: holding stage at 95°C for 10 seconds followed by 40 cycles of shuttle heating at 95°C for 15 seconds and at 60°C for 1 minute. The melt curve stage was at 95°C for 15 seconds, 60°C for 1 minute, and 95°C for 15 seconds. 16S RNA was used as an endogenous control (SYBR Green assay reagents, Applied Biosystems, India). Relative quantification (RQ) for S. mutans and S. sobrinus was based on the Ct (the number of PCR cycles necessary to obtain the threshold signal of fluorescence) values. All the calculations were done using Applied Biosystems Software.
Statistical Analysis
Statistical Package for Social Sciences (SPSS) for Windows Version 22.0 Released 2013. Armonk, NY: IBM Corp., was used to perform statistical analyzes.
Descriptive Statistics
It includes expression of the S. mutans and S. sobrinus levels in terms of mean and standard deviation.
Inferential Statistics
Friedman's test followed by the Wilcoxon signed post hoc test was used to compare the mean S. mutans and S. sobrinus count between different time intervals in each study groups. Kruskal–Wallis test followed by Mann–Whitney post hoc test was used to compare the mean S. mutans and S. sobrinus count between the three groups at different time intervals. The level of significance will be set at p < 0.05.
Results
The intergroup comparison of three groups showed a statistically significant difference in the count of both the studied microorganisms between group I (GIC + CHX) and group III (GIC + deionized water) at 1 month and 3 months and group II (GIC + miswak) and group III (GIC + deionized water) at 1 month and 3 months. The difference in the mean count of both the studied microorganisms between group I (GIC + CHX) and group II (GIC + miswak) was not statistically significant suggestive of similar antimicrobial potential against S. mutans and S. sobrinus (Figs 1 and 2).
Fig. 1.
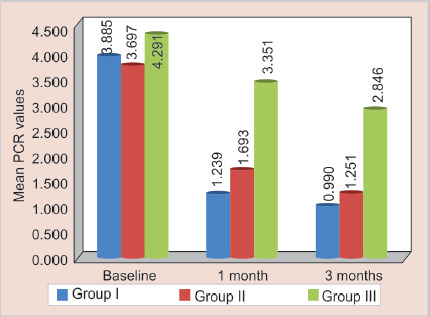
Mean PCR values of S. mutans between three groups at different time intervals
Fig. 2.
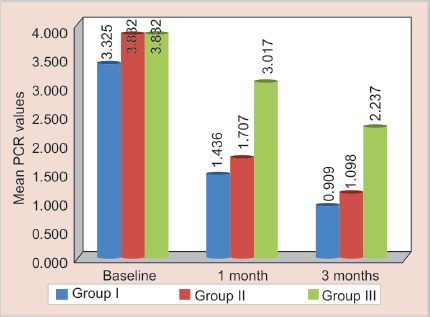
Mean PCR values of S. sobrinus between three groups at different time intervals
Discussion
Despite several advances in the field of preventive dentistry, dental caries still continues to be the most common oral disease in mankind.7 Dental caries in infants and toddlers have a distinctive pattern affecting the maxillary primary incisors and the first primary molars in a way reflecting the pattern of eruption and is collectively called ECC.4,16 Although the etiology of ECC is multifactorial, S. mutans and S. sobrinus play a key role.3 Children with ECC were included in the current study, as the acquisition of MS has been found to be more common during the window period that occurs around the age of 26 months.7 Thus for a thorough caries prediction and its subsequent treatment, detection of the presence of MS in early years is important. Many studies have been conducted to understand the association between caries activity and microorganisms, the results of which have revealed children with elevated counts of S. mutans and S. sobrinus have a higher incidence of dental caries.3,5,6,17
The cariogenicity of S. mutans can be assigned to its characteristics of adhesiveness, acidogenicity, and acid tolerance.18 Streptococcus mutans adhere within dental plaque via sucrose-independent and sucrose-dependent means using pellicle directed and specific surface antigens, respectively.18,19 These organisms produce lactate, acetate, formate, and ethanol using the glycolytic pathway. The acids produced by these organisms at a pH range from 7.0 to 5.0 are at a velocity that exceeds that of other oral streptococci. F1F0-ATPase proton pump and an adaptation occurring due to the change in gene and protein expression in these organisms make them acid tolerant.18 Streptococcus sobrinus adhere to the tooth surface mainly by using glucans and its virulence factor is due to the presence of seven genes encoding for glucosyltransferase.19 Considering the virulence of both these microorganisms in the initiation and progressions of ECC they were used as a test organism in the present study.
In the current study, organisms were detected from dental plaque rather than saliva as the salivary sample does not help in establishing an effective association between cariogenic bacteria and caries. Higher counts of S. mutans are found in saliva but are lower on the tooth's surface, where they actually produce acids responsible for demineralization.3 Their quantification and identification are relevant for epidemiological and early intervention studies. Detection of MS can be done by various methods like microbial culture technique, biochemical identification, genetic and immunologic methods using DNA probes, ELISA, molecular methods like PCR, etc.5,6
In the present study, PCR was used to amplify the gene sequences of S. mutans and S. sobrinus. PCR is not only rapid but also a highly sensitive method and takes 6–7 hours for the detection of microorganisms.3 In the present study, the mean score of S. mutans at baseline was 4.291 and maximum count of 8.28 in all the three groups was observed. The mean score of S. sobrinus at baseline was 3.832 and maximum count about 8.27 in all the three groups was observed. These findings are in agreement with various studies, that both these organisms present in dental plaque are strongly associated with the development of ECC.3,5,6,17 In contrast to the findings of the present study, Matee et al. reported high levels of MS in some caries free children. They also suggested that in children with rampant caries in the age-group of 1–2.5 years S. sobrinus did not play a key role.17
In the current study, anhydrous GIC was used as a restorative material as the ions can readily travel in and out of the material; hence, the cement can be doped with other soluble antimicrobials. Second, this type of GIC is normally mixed with distilled water allowing easy usage of different antimicrobial agents for mixing the cement. Third, conventional high viscosity GIC easily releases the antibacterial product whereas resin-modified GIC may keep the same product in the matrix for a longer time delaying its release.20 Turkun et al. and Kabil et al. have also used anhydrous GIC in their studies and found that anhydrous GIC allows easy incorporation of antimicrobials agents to GIC without altering powder liquid ratio.13,21
CHX is a bisbiguanide and has cationic properties. It is composed of two chlorophenyl rings and two biguanide groups that are connected by a central hexamethylene chain.22 Based on its concentration, it can act as a bacteriostatic or bactericidal agent. At lower concentrations, it affects the integrity of the cell wall further attacking the cytoplasmic membrane.21 In high concentrations, CHX causes the cytoplasm to solidify.23 CHX is a gold standard for caries and gingivitis control.13 Because of its property of substantivity, it is slowly released into the oral cavity and hence its antibacterial effect lasts for a longer time compared with other agents. Hence, in this study, CHX was used as one of the antimicrobial agents. Several authors have also incorporated CHX into GIC to improve the antimicrobial properties of GIC and have found that CHX has potent antibacterial activity and is effective in reducing S. mutans counts.8,13,21,24,25
CHX is commercially available in the form of—CHX diacetate, CHX dihydrochloride, or CHX digluconate. Digluconate salt is commercially available as 20% (wt/vol) concentration and is diluted (2%) for clinical use.26 Increased and rapid release of CHX is noted from the digluconate form compared with CHX diacetate and CHX dihydrochloride.21 Due to this advantage, CHX digluconate was used in this study. CHX digluconate was also used by several other authors in their studies and they have concluded that CHX digluconate can prevent secondary caries for an extended period of time.13,20,21,27
The addition of antibacterial agents can affect the mechanical properties of GIC and also reduce the F release from the set cement. According to Jedrychowski, Caputo, and Kerper, the properties of GIC deteriorate after the addition of CHX at concentrations above 5%. According to Hoszek et al. after addition of 10% CHX, the F release of GIC decreases over time.20 Turkun et al. in their study have shown that addition of 1% CHX did not affect the physical and mechanical properties of GIC.21 Hence, 1% CHX digluconate solution was used in the present study.
In group I (GIC + CHX), a reduction in the study microorganism was observed from baseline to 1 month, from 1 to 3 months, and between baseline and 3 months and this difference was statistically significant. This suggests that CHX possesses antibacterial activity against both the studied microorganisms (Tables 1 and 2). Study conducted by Duque et al. concluded that incorporation of 1.25% CHX significantly reduced S. mutans counts without changing the mechanical properties and F release of GIC.20
Table 1.
Comparison of mean S. mutans PCR values between different time intervals in each group using Friedman's test followed by Wilcoxon signed post hoc test
| Groups | Time | N | Mean | SD | Min | Max | p value | Sig. diff. | p value |
|---|---|---|---|---|---|---|---|---|---|
| Group I | Baseline | 15 | 3.885 | 2.262 | 1.20 | 7.60 | <0.001* | T1 vs T2 | 0.001* |
| 1 month | 15 | 1.239 | 0.900 | 0.39 | 3.80 | T1 vs T3 | 0.001* | ||
| 3 months | 15 | 0.990 | 0.625 | 0.29 | 2.50 | T2 vs T3 | 0.01* | ||
| Group II | Baseline | 15 | 3.697 | 2.094 | 1.18 | 8.28 | <0.001* | T1 vs T2 | 0.001* |
| 1 month | 15 | 1.693 | 0.935 | 0.53 | 4.10 | T1 vs T3 | 0.001* | ||
| 3 months | 15 | 1.251 | 0.643 | 0.42 | 2.40 | T2 vs T3 | 0.01* | ||
| Group III | Baseline | 15 | 4.291 | 2.668 | 1.30 | 8.24 | <0.001* | T1 vs T2 | 0.001* |
| 1 month | 15 | 3.351 | 2.322 | 0.60 | 7.29 | T1 vs T3 | 0.001* | ||
| 3 month | 15 | 2.846 | 2.254 | 0.40 | 6.51 | T2 vs T3 | 0.01* |
Statistically significant
T1-Baseline, T2-1 month, T3-3 months
Table 2.
Comparison of mean S. sobrinus PCR values between different time intervals in each group using Friedman's test followed by Wilcoxon signed post hoc test
| Groups | Time | N | Mean | SD | Min | Max | p value | Sig. diff. | p value |
|---|---|---|---|---|---|---|---|---|---|
| Group I | Baseline | 15 | 3.325 | 1.954 | 0.55 | 6.53 | <0.001* | T1 vs T2 | 0.001* |
| 1 month | 15 | 1.436 | 0.816 | 0.12 | 3.40 | T1 vs T3 | 0.001* | ||
| 3 months | 15 | 0.909 | 0.607 | 0.00 | 1.90 | T2 vs T3 | 0.001* | ||
| Group II | Baseline | 15 | 3.832 | 1.804 | 1.57 | 7.73 | <0.001* | T1 vs T2 | 0.001* |
| 1 month | 15 | 1.707 | 0.747 | 0.99 | 3.90 | T1 vs T3 | 0.001* | ||
| 3 months | 15 | 1.098 | 0.277 | 0.50 | 1.50 | T2 vs T3 | 0.001* | ||
| Group III | Baseline | 15 | 3.832 | 2.580 | 1.10 | 8.27 | <0.001* | T1 vs T2 | 0.001* |
| 1 month | 15 | 3.017 | 2.366 | 0.67 | 7.51 | T1 vs T3 | 0.001* | ||
| 3 months | 15 | 2.237 | 1.603 | 0.50 | 5.33 | T2 vs T3 | 0.002* |
Statistically significant
T1-Baseline, T2-1 month, T3-3 months
In contrast, Sandham et al. in his study have stated that eliminating MS even for a shorter period of time using CHX is difficult in some individuals and that faster recolonization of oral surfaces occur in subjects whose infections are eliminated easily.28
Plant-derived phytochemicals have been gaining a lot of attention as an alternative to commercial antimicrobials due to their added benefits of being used in traditional medicines and being economical without causing antibacterial resistance.29 Miswak is one such herbal antimicrobial derived from the plant Arak (Salvadora persica).30
Salvadora persica extract can be obtained either in aqueous or alcoholic form.31 Studies have shown that aqueous extract of miswak has growth inhibitory effects on the primary colonizers responsible for early stages of plaque formation and bacterial species associated with dental caries and periodontal diseases.32 Hence, in the present study, aqueous extract of miswak was used as an antimicrobial agent.
Miswak contains sulfur, alkaloids, and butanediamide which have antimicrobial activity, chlorides and fluorides help in enamel remineralization, Vitamin C helps in tissue healing and repair, tannins reduce plaque and gingivitis, silica removes the stains, benzyl isothiocyanate prevents cariogenic and genotoxic compounds and essential oils improve the flow and buffering action of saliva.33 Miswak is readily available, cost-effective, and has a wide range of actions and hence it was used in the present study.
In group II (GIC + Miswak), a reduction in the study microorganism was observed from baseline to 1 month, from 1 to 3 months, and between baseline and 3 months and this difference was statistically significant. This suggests that miswak extract possesses antibacterial activity against both the studied microorganisms (Tables 1 and 2).
The results derived from the present study are in unison with the results obtained by Sofrata et al. who reported the antibacterial effect of S. persica against oral microorganisms like S. mutans, L. acidophillus.34 Al-Bayata et al., Shingare and Chaugule, and Masoumeh et al. had also found miswak extract as an effective antimicrobial agent which is comparable to the present study results. In contrast, Almas et al. in their study concluded that aqueous miswak extract has no effect on S. mutans, S. aureus, and C. albicans. This difference could be attributed to the variation in microbial strains and the type of method employed.35 El-Tatari in his study concluded that the addition of 1% SPE did not affect the compressive strength and diametral tensile strength, and the addition of 4% SPE although decreased the physical properties of GIC gave promising results regarding antimicrobial activity against S. mutans, S. sanguis, and C. albicans.36
In the current study, deionized water was used as a control to mix GIC. In group III (GIC + deionized water), there was a drop in the count of studied microorganism from baseline to 3 months. The difference in the microorganism count between baseline to 1 month, from 1 to 3 months, and between baseline and 3 months was statistically significant (Tables 1 and 2).
Conclusion
All three groups had an antimicrobial effect against S. mutans and S. sobrinus. CHX was the most effective antimicrobial agent among the three materials. The antimicrobial efficacy of CHX and miswak extract was similar at 1 and 3 months intervals. Miswak can be used as an alternative herbal antimicrobial agent against both S. mutans and S. sobrinus. Conventional anhydrous GIC was least effective in reducing the mean count of S. mutans and S. sobrinus.
Footnotes
Source of support: Department of Pedodontics and Preventive Dentistry, RajaRajeswari Dental College and Hospital, Mysore road, Bengaluru, Karnataka, India
Conflict of interest: None
References
- 1.Fejerskov O, Nyvad B, Kidd E. The disease and its clinical management. 3rd ed., West Sussex (UK): Wiley Blackwell; 2015. Dental caries. [Google Scholar]
- 2.Bariker RH, Mandroli PS. An in-vitro evaluation of antibacterial effect of Amalgomer CR and Fuji VII against bacteria causing severe early childhood caries. J Indian Soc Pedod Prev Dent. 2016;34(1):23–29. doi: 10.4103/0970-4388.175506. DOI: [DOI] [PubMed] [Google Scholar]
- 3.Soni H, Vasavada M. Distribution of S. mutans and S. sorbinus in caries active and caries free children by PCR approach. Int J Oral Craniofac Sci. 2015;1(1):027–030. doi: 10.17352/2455-4634.000005. DOI: [DOI] [Google Scholar]
- 4.Çolak H, Dulgergil ÇT, Dalli M, et al. Early childhood caries update: a review of causes, diagnoses, and treatments. J Nat Sci Biol Med. 2013;4(1):29–38. doi: 10.4103/0976-9668.107257. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singla D, Sharma A, Sachdev V, et al. Distribution of Streptococcus mutans and Streptococcus sobrinus in dental plaque of indian pre-school children using PCR and SB-20M agar medium. J Clin Diagn Res. 2016;10(11):60–63. doi: 10.7860/JCDR/2016/19256.8909. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choi EJ, Lee SH, Kim YJ. Quantitative real‐time polymerase chain reaction for Streptococcus mutans and Streptococcus sobrinus in dental plaque samples and its association with early childhood caries. Int Paediatr Dent. 2009;19(2):141–147. doi: 10.1111/j.1365-263X.2008.00942.x. DOI: [DOI] [PubMed] [Google Scholar]
- 7.Law V, Seow WK, Townsend G. Factors influencing oral colonization of mutans streptococci in young children. Aust Dent J. 2007;52(2):93–100. doi: 10.1111/j.1834-7819.2007.tb00471.x. DOI: [DOI] [PubMed] [Google Scholar]
- 8.Jaidka S, Somani R, Singh DJ, et al. Comparative evaluation of compressive strength, diametral tensile strength and shear bond strength of GIC type IX, chlorhexidine-incorporated GIC and triclosan-incorporated GIC: an in vitro study. J Int Soc Prev Commun Dent. 2016;6(Suppl 1):S64–S69. doi: 10.4103/2231-0762.181188. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tobias RS, Plant CG, Rippin JW, et al. Pulpal response to an anhydrous glass ionomer luting cement. Endod Dent Traumatol. 1989;5(5):242–252. doi: 10.1111/j.1600-9657.1989.tb00369.x. DOI: [DOI] [PubMed] [Google Scholar]
- 10.Deepalakshmi M, Poorni S, Miglani R, et al. Evaluation of the antibacterial and physical properties of glass ionomer cements containing chlorhexidine and cetrimide: an in-vitro study. Indian J Dent Res. 2010;21(4):552–556. doi: 10.4103/0970-9290.74217. DOI: [DOI] [PubMed] [Google Scholar]
- 11.Mittal S, Soni H, Sharma DK, et al. Comparative evaluation of the antibacterial and physical properties of conventional glass ionomer cement containing chlorhexidine and antibiotics. J Int Soc Prev Community Dent. 2015;5(4):268–275. doi: 10.4103/2231-0762.161754. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bellis CA, Addison O, Nobbs AH, et al. Glass ionomer cements with milled, dry chlorhexidine hexametaphosphate filler particles to provide long-term antimicrobial properties with recharge capacity. Dent Mater. 2018;34(12):1717–1726. doi: 10.1016/j.dental.2018.09.003. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kabil NS, Badran AS, Wassel MO. Effect of the addition of chlorhexidine and miswak extract on the clinical performance and antibacterial properties of conventional glass ionomer: an in vivo study. Int J Paediatr Dent. 2016;27(5):380–387. doi: 10.1111/ipd.12273. DOI: [DOI] [PubMed] [Google Scholar]
- 14.Wassel MO, Khattab MA. Antibacterial activity against Streptococcus mutans and inhibition of bacterial induced enamel demineralization of propolis, miswak, and chitosan nanoparticles based dental varnishes. J Adv Res. 2017;8(4):387–392. doi: 10.1016/j.jare.2017.05.006. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bawazeer TM, Alsoufi MS, Katowah D, et al. Effect of aqueous extracts of Salvadora persica “Miswak” on the acid eroded enamel surface at nano-mechanical scale. Mater Sci Appl. 2016;7(11):754–771. [Google Scholar]
- 16.Kawashita Y, Kitamura M, Saito T. Early childhood caries. Int J Dent. 2011;2011:725320. doi: 10.1155/2011/725320. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Okada M, Soda Y, Hayashi F, et al. PCR detection of Streptococcus mutans and S. sobrinus in dental plaque samples from Japanese pre-school children. J Med Microbiol. 2002;51(5):443–447. doi: 10.1099/0022-1317-51-5-443. DOI: [DOI] [PubMed] [Google Scholar]
- 18.Banas JA. Virulence properties of Streptococcus mutans. Front Biosci. 2004;9(10):1267–1277. doi: 10.2741/1305. DOI: [DOI] [PubMed] [Google Scholar]
- 19.Conrads G, de Soet JJ, Song L, et al. Comparing the cariogenic species Streptococcus sobrinus and S. mutans on whole genome level. J Oral Microbiol. 2014;6(1):26189. doi: 10.3402/jom.v6.26189. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duque C, Aida KL, Pereira JA, et al. In vitro and in vivo evaluations of glass-ionomer cement containing chlorhexidine for atraumatic restorative treatment. J Appl Oral Sci. 2017;25(5):541–550. doi: 10.1590/1678-7757-2016-0195. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Turkun LS, Turkun M, Ertug Rul FA, et al. Long‐term antibacterial effects and physical properties of a chlorhexidine‐containing glass ionomer cement. J Esthet Restor Dent. 2008;20(1):29–44. doi: 10.1111/j.1708-8240.2008.00146.x. DOI: [DOI] [PubMed] [Google Scholar]
- 22.Sajjan P, Laxminarayan N, Kar PP, et al. Chlorhexidine as an antimicrobial agent in dentistry–a review. Oral Health Dent Manag. 2016;15(2):93–100. [Google Scholar]
- 23.Kumar SB. Chlorhexidine mouthwash — a review. J Pharm Sci Res. 2017;9(9):1450–1452. [Google Scholar]
- 24.Prabhakar AR, Agarwal S, Basappa N. Comparative evaluation of antibacterial effect and physical properties of conventional glass-ionomer cement containing 1% chlorhexidine and 1% xylitol. Int J Oral Health Sci. 2014;4(2):63–69. doi: 10.4103/2231-6027.165103. DOI: [DOI] [Google Scholar]
- 25.Mishra A, Pandey RK, Manickam N. Antibacterial effect and physical properties of chitosan and chlorhexidine-cetrimide-modified glass ionomer cements. J Indian Soc Pedodontics Prev Dent. 2017;35(1):28–33. doi: 10.4103/0970-4388.199224. DOI: [DOI] [PubMed] [Google Scholar]
- 26.Zeng P, Rao A, Wiedmann TS, et al. Solubility properties of chlorhexidine salts. Drug Dev Ind Pharm. 2009;35(2):172–176. doi: 10.1080/03639040802220318. DOI: [DOI] [PubMed] [Google Scholar]
- 27.Marti LM, Mata MD, Ferraz-Santos B, et al. Addition of chlorhexidine gluconate to a glass ionomer cement: a study on mechanical, physical and antibacterial properties. Braz Dent J. 2014;25(1):33–37. doi: 10.1590/0103-6440201302328. DOI: [DOI] [PubMed] [Google Scholar]
- 28.Grönroos L, Mättö J, Saarela M, et al. Chlorhexidine susceptibilities of mutans streptococcal serotypes and ribotypes. Antimicrob Agents Chemother. 1995;39(4):894–898. doi: 10.1128/AAC.39.4.894. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khalil AK, Qarani SM, Omer AG. In vitro antimicrobial activity of Miswak extracts against some oral pathogenic isolates. Zanco J Med Sci. 2010;14(1):71–78. [Google Scholar]
- 30.El-Latif Hesham A, Alrumman SA. Antibacterial activity of miswak Salvadora persica extracts against isolated and genetically identified oral cavity pathogens. Technol Health Care. 2016;24(s2):S841–S848. doi: 10.3233/THC-161214. DOI: [DOI] [PubMed] [Google Scholar]
- 31.Balto H, Al-Sanie I, Al-Beshri S, et al. Effectiveness of Salvadora persica extracts against common oral pathogens. Saudi Dent J. 2017;29(1):1–6. doi: 10.1016/j.sdentj.2016.11.001. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Darout IA, Albandar JM, Skaug N, et al. Salivary microbiota levels in relation to periodontal status, experience of caries and miswak use in Sudanese adults. J Clin Periodontol. 2002;29(5):411–420. doi: 10.1034/j.1600-051x.2002.290505.x. DOI: [DOI] [PubMed] [Google Scholar]
- 33.Abhary M, Al-Hazmi AA. Antibacterial activity of Miswak (Salvadora persica L.) extracts on oral hygiene. J Taibah Univ Sci. 2015;10(4):513–520. doi: 10.1016/j.jtusci.2015.09.007. DOI: [DOI] [Google Scholar]
- 34.Sofrata AH, Claesson RL, Lingström PK, et al. Strong antibacterial effect of miswak against oral microorganisms associated with periodontitis and caries. J Periodontol. 2008;79(8):1474–1479. doi: 10.1902/jop.2008.070506. DOI: [DOI] [PubMed] [Google Scholar]
- 35.Naseem S, Hashmi K, Fasih F, et al. In vitro evaluation of antimicrobial effect of miswak against common oral pathogens. Pak J Med Sci. 2014;30(2):398–403. doi: 10.12669/pjms.302.4284. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.El-Tatari A, De Soet JJ, De Gee AJ, et al. Influence of Salvadora persica (miswak) extract on physical and antimicrobial properties of glass ionomer cement. Eur Arch Paediatri Dent. 2011;12(1):22–25. doi: 10.1007/BF03262774. [DOI] [PubMed] [Google Scholar]


