Abstract
Background
Frailty is a multifactorial physiological syndrome most often associated with age but which has received increasing recognition as a component of chronic illnesses such as heart failure. Patients with heart failure are likely to be frail, irrespective of their age. Adipokine dysregulation, which is associated with frailty, occurs in patients with heart failure. In this study, we tested the hypothesis that adipokines are associated with skeletal muscle and bone mineral density that change lead to frailty in patients with heart failure.
Methods
Thirty-five patients with heart failure (age, 67 ± 14 years; 25 males; left ventricular ejection fraction, 45 ± 19%) were included. Serum adipokine levels, physical performance, and body composition were measured.
Results
Adiponectin and leptin were inversely correlated with grip strength. Adiponectin was inversely correlated with bone mineral density. Leptin was positively correlated with fat mass. Adipokines were not correlated with skeletal muscle mass.
Conclusions
Adipokines were associated with grip strength and bone mineral density in patients with heart failure. Adipokine dysregulation may play a role in the development of frailty in heart failure.
Keywords: adipokines, frailty, heart failure, leptin, osteoporosis
Introduction
Frailty is a clinically recognizable state of increased vulnerability mainly resulting from aging-associated decline in reserve and function across multiple physiologic systems. Heart failure is associated with accelerated biological aging (1) and patients with heart failure are likely to be frail irrespective of their age (2). A previous systematic review has reported that the prevalence of frailty in heart failure ranges from 18 to 54%, and this has been reported as 45% in a recent meta-analysis (3, 4). Frailty is an independent predictor of all-cause mortality and hospital readmissions in patients with heart failure (5). Frailty also increases mortality in patients with acute decompensated heart failure attending an emergency department (6). Furthermore, frailty has a negative impact on the physical activity, mental state, and quality of life of patients with heart failure (7, 8).
Frailty is affected by age-related changes in body composition. Skeletal muscle mass, grip strength, and bone density are important factors related to frailty, and sarcopenia and osteoporosis patients have significantly reduced physical activity (9). Muscle and bone tissues are modified by various factors other than age, such as inflammation.
Inflammation has been implicated in the pathogenesis of both frailty and heart failure, although the pathophysiology of both disorders is complex and includes multiple derangement pathways, which require further elucidation (10-13). Adipokines secreted by adipose tissue play a role in the regulation of metabolic functions such as lipid metabolism and inflammation, and recent evidence has shown that adipokine dysregulation is associated with frailty in old age (10, 14-17). Furthermore, adipokine dysregulation has also been reported in heart failure (18-20). Here, we tested the hypothesis that adipokines are associated with skeletal muscle and bone mineral density in patients with heart failure.
Methods
Study population
We prospectively included patients admitted with congestive heart failure who underwent phase 2 cardiac rehabilitation in our hospitals (Niigata University Graduate School of Medical and Dental Sciences, Niigata Minami Hospital, Shinrakuen Hospital, Niigata Medical Center, and Saiseikai Niigata Daini Hospital) from February to August 2014. Patients with cardiovascular events such as myocardial infarction, stroke, and thromboembolism within 6 weeks before baseline examination, heart transplantation, or pregnancy were excluded. Patients on dialysis were also excluded. Written informed consent was obtained from all patients. This study was conducted in accordance with the guidelines of the Declaration of Helsinki. The study protocol was reviewed and approved by the Ethics Committee of the Niigata University Graduate School of Medicine (application number 1788).
Assessment of body composition
Dual-energy X-ray absorptiometry (DEXA; Hologic Discovery QDR Series; Hologic Inc., Bedford, MA) was used to evaluate bone mineral density, lean mass, and fat mass. The lean mass was defined as skeletal muscle mass, and appendicular muscle mass was defined as the combined lean mass of both arms and legs. Appendicular skeletal muscle mass index (ASMI), appendicular fat mass index (AFMI), and appendicular bone mineral density (ABMD) were calculated as each measurement divided by height in meters squared. Grip strength was measured using a Smedley-type handgrip dynamometer.
Assessment of serum levels of adipokines
Serum levels of tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) were measured using chemiluminescence enzyme immunoassays (LSI Medience Corporation, Tokyo, Japan). Serum levels of adiponectin and leptin were measured using enzyme-linked immunosorbent assay (R&D Systems, Minneapolis, MN).
Statistical analysis
Continuous variables and categorical variables are expressed as mean ± standard deviation (SD) and numbers (percentages), respectively. Linear regression analysis was used to study the relationship between variables. All statistical analyses were performed using SPSS 23.0 (IBM Corporation, Armonk, NY). A two-tailed p-value less than 0.05 was considered statistically significant.
Results
Fifty-six patients underwent phase 2 cardiac rehabilitation during the study period, and 35 patients fulfilled the study inclusion criteria. The baseline characteristics of patients are shown in Table 1. The study cohort consisted of 25 males (71%). The mean age was 67 ± 14 years and 10 patients (29%) were younger than 60 years. The most common cause of heart failure was ischemic heart disease. The mean left ventricular ejection fraction was 45 ± 19% and there were 29 patients (83%) with New York Heart Association (NYHA) functional class less than III. Physical characteristics are shown in Table. There were 5 overweight or obese patients (14%) (body mass index (BMI) > 25 kg/m2) and 6 underweight patients (17%) (BMI < than 18.5 kg/m2). Weight classification by BMI was defined according to World Health Organization criteria.
Table 1.
Clinical characteristics of 35 patients
| Age, years | 67 ± 14 |
| Male, n (%) | 25 (71) |
| Cardiovascular diseases | |
| Ischemic heart disease, n (%) | 14 (40) |
| Dilated cardiomyopathy, n (%) | 10 (29) |
| Hypertrophic cardiomyopathy, n (%) | 4 (11) |
| Valvular heart disease, n (%) | 5 (14) |
| Congenital heart disease, n (%) | 2 (6) |
| Comorbidities | |
| Hypertension, n (%) | 16 (46) |
| Dyslipidemia, n (%) | 17 (49) |
| Diabetes mellitus, n (%) | 14 (40) |
| Arterial fibrillation, n (%) | 12 (34) |
| NYHA functional class | |
| I, n (%) | 10 (29) |
| II, n (%) | 19 (54) |
| III, n (%) | 6 (17) |
| Brain natriuretic peptide, pg/mL | 427 ± 422 |
| Left ventricular ejection fraction, % | 45 ± 19 |
| Medication | |
| Beta-blocker, n (%) | 27 (77) |
| ACE-I or ARB, n (%) | 22 (63) |
| Spironolactone, n (%) | 16 (46) |
| Loop diuretics, n (%) | 28 (80) |
| Physical characteristics | |
| Body height, cm | 161 ± 9 |
| Body weight, kg | 56 ± 14 |
| Body mass index, kg/m2 | 22 ± 4 |
| Skeletal muscle mass index, kg/m2 | 6.38 ± 1.25 |
| Fat mass index, kg/m2 | 2.17 ± 1.05 |
| Bone mineral density, kg/m2 | 0.38 ± 0.07 |
| Hand grip strength, kg | 29 ± 11 |
ACE-I, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker. Data are mean ± standard deviation or number (%).
We investigated the associations of adipokines with physical parameters. Adiponectin and leptin were inversely associated with grip strength (Figure 1 and 2).
Figure 1.

Association of adiponectin with grip strength and body composition. ASMI, appendicular skeletal muscle mass index; AFMI, appendicular fat mass index; ABMD, appendicular bone mineral density.
Figure 2.
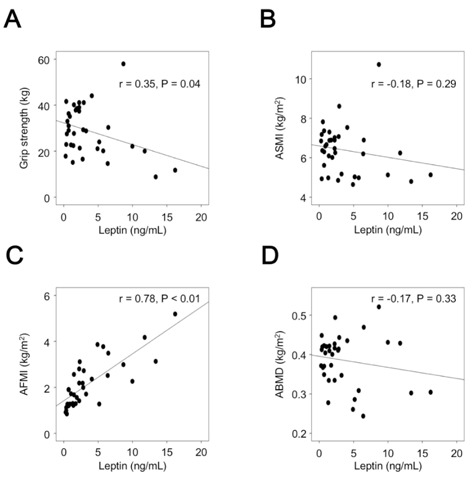
Association of leptin with grip strength and body composition. ASMI, appendicular skeletal muscle mass index; AFMI, appendicular fat mass index; ABMD, appendicular bone mineral density.
However, TNF-α and IL-6 were not associated with grip strength. There was no association of four cytokines tested with ASMI (Figure 1-4). Leptin was positively associated with AFMI, but there was no association of AFMI with the other three cytokines evaluated (Figure 1-4). Adiponectin was inversely associated with ABMD (Figure 1).
Figure 4.

Association of adiponectin with grip strength and body composition. ASMI, appendicular skeletal muscle mass index; AFMI, appendicular fat mass index; ABMD, appendicular bone mineral density.
Figure 3.

Association of TNF-α with grip strength and body composition. ASMI, appendicular skeletal muscle mass index; AFMI, appendicular fat mass index; ABMD, appendicular bone mineral density.
Discussion
In this study, we found that adipokines were associated with physical performance and body composition in patients with heart failure. This association suggests that deranged inflammatory pathways in adipose tissues may play a role in frailty in patients with heart failure.
Adipose tissue secretes anti-inflammatory adipokines such as adiponectin and pro-inflammatory ones such as TNF-α, IL-6, and leptin (21). Recent studies have revealed that a decline in immune function with age is associated with frailty, and adipokine dysregulation plays an important role in this association (10, 14-17). C-reactive protein is positively associated with the severity of frailty in individuals aged over 75 years, and increasing frailty has also been shown with increasing TNF-α and IL-6 levels (14). Adiponectin and IL-6 are increased in older adults with frailty compared to those without frailty, and are inversely associated with grip strength and gait speed (17). Leptin is also inversely associated with physical function (16). Adipokine dysregulation has also been reported in patients with heart failure (18-20) and in this study we found an inverse association of adiponectin and leptin with grip strength, but there was no association of TNF-α and IL-6 with grip strength. This suggests that adipokine dysregulation, which occurs in association with heart failure, may play a role in development of frailty.
The prevalence of frailty in heart failure patients is high irrespective of age, and frailty is associated with acute exacerbation of heart failure and increased mortality (2-6). Decreased skeletal muscle mass is associated with impaired cardiorespiratory fitness and quality of life, in addition to weak muscle strength in patients with heart failure (22). A previous study of patients with heart failure has found that IL-6 is associated with decreased skeletal muscle mass (22), while another has demonstrated an inverse association of both adiponectin and leptin with lean mass (23). In this study, adiponectin and leptin were inversely associated with grip strength, thus suggesting a role of adipokines in frailty in heart failure. However, in this study, the association was negative for adipokines including adiponectin, TNF-α, IL-6, and leptin. These inconsistent results may be explained by the small size of the study cohort and the differences in patient characteristics.
This study also identified an association of adipokines with fat mass and bone mineral density, in addition to that with muscle mass. Leptin is increased in obese patients with heart failure and is correlated with BMI, percentage body fat, and waist circumference (24, 25). The mean BMI of patients with heart failure in our study group was 22±4, and in these patients, leptin was positively associated with fat mass. Furthermore, leptin is associated with epicardial fat thickness in non-cachectic patients with heart failure (26). The increased levels of leptin may play a role in disrupted lipid metabolism in heart failure.
Adiponectin has been shown to increase bone turnover, and therefore, has been implicated in the pathogenesis of osteoporosis (27). A previous study has demonstrated that adiponectin levels increase as heart failure severity worsens, and that adiponectin was inversely associated with bone mineral density (28). Patients with heart failure have various risk factors for osteoporosis such as older age, physical inactivity, and therapy with loop diuretics. The increased levels of adiponectin may further contribute to the pathogenesis of osteoporosis in heart failure.
Conclusion
Adipokines were associated with grip strength and bone mineral density in patients with heart failure. Adipokine dysregulation may play a role in the development of frailty in heart failure.
Limitation
There are several limitations to this study. The number of patients was small, and multivariate analysis could not be performed. The etiologies of heart failure in patients were inconsistent. Analysis stratified by age and gender could not be performed. Comorbidities varied and the duration was unknown. The patient’s conditions of heart failure were consistent at the time of blood sampling, DEXA and grip strength, although prior therapy and rehabilitation periods were inconsistent.
Acknowledgments:
The authors would like to thank all the staffs and patients who are participating in this trial.
Conflicts of interest:
Each author declares that he or she has no commercial associations (e.g. consultancies, stock ownership, equity interest, patent/licensing arrangement etc.) that might pose a conflict of interest in connection with the submitted article.
References
- Wong LS, van der Harst P, de Boer RA, et al. Aging, telomeres and heart failure. Heart failure reviews. 2010;15:479–86. doi: 10.1007/s10741-010-9173-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodson JA, Chaudhry SI. Geriatric conditions in heart failure. Current cardiovascular risk reports. 2012;6:404–410. doi: 10.1007/s12170-012-0259-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha SR, Ha HS, Hickman LD, et al. Frailty in advanced heart failure: a systematic review. Heart failure reviews. 2015;20:553–60. doi: 10.1007/s10741-015-9493-8. [DOI] [PubMed] [Google Scholar]
- Denfeld QE, Winters-Stone K, Mudd JO, et al. The prevalence of frailty in heart failure: A systematic review and meta-analysis. International journal of cardiology. 2017;236:283–289. doi: 10.1016/j.ijcard.2017.01.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Yuan M, Gong M, Tse G, et al. Frailty and Clinical Outcomes in Heart Failure: A Systematic Review and Meta-analysis. Journal of the American Medical Directors Association. 2018;19:1003–1008.e1. doi: 10.1016/j.jamda.2018.06.009. [DOI] [PubMed] [Google Scholar]
- Martín-Sánchez FJ, Rodríguez-Adrada E, Vidan MT, et al. Impact of Frailty and Disability on 30-Day Mortality in Older Patients With Acute Heart Failure. The American journal of cardiology. 2017;120:1151–1157. doi: 10.1016/j.amjcard.2017.06.059. [DOI] [PubMed] [Google Scholar]
- Uchmanowicz I, Gobbens RJ. The relationship between frailty, anxiety and depression, and health-related quality of life in elderly patients with heart failure. Clinical interventions in aging. 2015;10:1595–600. doi: 10.2147/CIA.S90077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidán MT, Blaya-Novakova V, Sánchez E, et al. Prevalence and prognostic impact of frailty and its components in non-dependent elderly patients with heart failure. European journal of heart failure. 2016;18:869–75. doi: 10.1002/ejhf.518. [DOI] [PubMed] [Google Scholar]
- Sepúlveda-Loyola W, Phu S, Bani Hassan E, et al. The Joint Occurrence of Osteoporosis and Sarcopenia (Osteosarcopenia): Definitions and Characteristics. J. Am. Med. Dir. Assoc. 2020;21:220–225. doi: 10.1016/j.jamda.2019.09.005. [DOI] [PubMed] [Google Scholar]
- Wilson D, Jackson T, Sapey E, et al. Frailty and sarcopenia: The potential role of an aged immune system. Ageing research reviews. 2017;36:1–10. doi: 10.1016/j.arr.2017.01.006. [DOI] [PubMed] [Google Scholar]
- Mann DL. Innate immunity and the failing heart: the cytokine hypothesis revisited. Circulation research. 2015;116:1254–68. doi: 10.1161/CIRCRESAHA.116.302317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frantz S, Falcao-Pires I, Balligand JL, et al. The innate immune system in chronic cardiomyopathy: a European Society of Cardiology (ESC) scientific statement from the Working Group on Myocardial Function of the ESC. European journal of heart failure. 2018;20:445–459. doi: 10.1002/ejhf.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellumkonda L, Tyrrell D, Hummel SL, et al. Pathophysiology of heart failure and frailty: a common inflammatory origin? Aging cell. 2017;16:444–450. doi: 10.1111/acel.12581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard RE, O’Mahony MS, Savva GM, et al. Inflammation and frailty measures in older people. Journal of cellular and molecular medicine. 2009;13:3103–9. doi: 10.1111/j.1582-4934.2009.00733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai JS, Wu CH, Chen SC, et al. Plasma adiponectin levels correlate positively with an increasing number of components of frailty in male elders. PloS one. 2013;8:e56250. doi: 10.1371/journal.pone.0056250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibasaki K, Yamada S, Akishita M, et al. Plasma Leptin Concentration and Sympathetic Nervous Activity in Older Adults With Physical Dysfunction. Journal of the Endocrine Society. 2018;2:1040–1049. doi: 10.1210/js.2018-00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Sha G, Zhang Y, et al. Elevated serum IL-6 and adiponectin levels are associated with frailty and physical function in Chinese older adults. Clinical interventions in aging. 2018;13:2013–2020. doi: 10.2147/CIA.S180934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiedermann CJ, Beimpold H, Herold M, et al. Increased levels of serum neopterin and decreased production of neutrophil superoxide anions in chronic heart failure with elevated levels of tumor necrosis factor-alpha. Journal of the American College of Cardiology. 1993;22:1897–901. doi: 10.1016/0735-1097(93)90776-w. [DOI] [PubMed] [Google Scholar]
- Leyva F, Anker SD, Egerer K, et al. Hyperleptinaemia in chronic heart failure. Relationships with insulin. European heart journal. 1998;19:1547–51. doi: 10.1053/euhj.1998.1045. [DOI] [PubMed] [Google Scholar]
- Kistorp C, Faber J, Galatius S, et al. Plasma adiponectin, body mass index, and mortality in patients with chronic heart failure. Circulation. 2005;112:1756–62. doi: 10.1161/CIRCULATIONAHA.104.530972. [DOI] [PubMed] [Google Scholar]
- Ouchi N, Parker JL, Lugus JJ, et al. Adipokines in inflammation and metabolic disease. Nature reviews. Immunology. 2011;11:85–97. doi: 10.1038/nri2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emami A, Saitoh M, Valentova M, et al. Comparison of sarcopenia and cachexia in men with chronic heart failure: results from the Studies Investigating Co-morbidities Aggravating Heart Failure (SICA-HF) Eur. J. Heart Fail. 2018;20:1580–1587. doi: 10.1002/ejhf.1304. [DOI] [PubMed] [Google Scholar]
- Loncar G, Bozic B, von Haehling S, et al. Association of adiponectin with peripheral muscle status in elderly patients with heart failure. Eur. J. Intern. Med. 2013;24:818–23. doi: 10.1016/j.ejim.2013.09.011. [DOI] [PubMed] [Google Scholar]
- Motie M, Evangelista LS, Horwich T, et al. Association between inflammatory biomarkers and adiposity in obese patients with heart failure and metabolic syndrome. Experimental and therapeutic medicine. 2014;8:181–186. doi: 10.3892/etm.2014.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEntegart MB, Awede B, Petrie MC, et al. Increase in serum adiponectin concentration in patients with heart failure and cachexia: relationship with leptin, other cytokines, and B-type natriuretic peptide. European heart journal. 2007;28:829–35. doi: 10.1093/eurheartj/ehm033. [DOI] [PubMed] [Google Scholar]
- Karayannis G, Giamouzis G, Tziolas N, et al. Association between epicardial fat thickness and weight homeostasis hormones in patients with noncachectic heart failure. Angiology. 2013;64:173–80. doi: 10.1177/0003319712447978. [DOI] [PubMed] [Google Scholar]
- Naot D, Musson DS, Cornish J. The Activity of Adiponectin in Bone. Calcified tissue international. 2017;100:486–499. doi: 10.1007/s00223-016-0216-5. [DOI] [PubMed] [Google Scholar]
- Tsutamoto T, Tanaka T, Sakai H, et al. Total and high molecular weight adiponectin, haemodynamics, and mortality in patients with chronic heart failure. European heart journal. 2007;28:1723–30. doi: 10.1093/eurheartj/ehm154. [DOI] [PubMed] [Google Scholar]


