Abstract
Vaccination is the most economic and effective measure to deal with infectious diseases and protect public health. Nowadays, due to the spread of COVID-19 and the ensuing pandemic, safe, effective vaccines are in urgent need. However, due to concerns about vaccine safety, there is still reluctance to vaccinate. In China, in response to the Changchun Changsheng Vaccine incident, the National People’s Congress Standing Committee passed the Vaccine Administration Law in 2019, which marks China’s first comprehensive piece of legislation on vaccine regulation. The law establishes a regulatory system covering the entire life cycle of vaccines, introduces the vaccine marketing authorization holder system, stipulates the legal responsibilities of all parties, and further clarifies the compensation system for any individuals who exhibit abnormal reactions to vaccination. In addition, it emphasizes the use of modern technology to build a national vaccine electronic platform for tracing. To balance vaccine efficacy and safety, it is necessary to further improve the vaccine risk management mechanism, promote cooperation between government and non-governmental actors, and avoid improper interventions in the vaccine market.
Keywords: Vaccine safety, Regulation system, Public health, China
1. Introduction
A vaccine is a biological product that can be administered to prevent certain diseases. Even today, as access to medicines and healthcare continues to increase, there is an ongoing push to develop vaccines to ward off various new influenza pandemics. 1 In the past few decades, the United States has supported several congressional vaccination programs to deal with infectious diseases. 2 In 2020, the unprecedented global crisis posed by the COVID-19 pandemic once again underscored the importance of vaccines. Accordingly, in the context of the high politicized pandemic, suggestions have been put forward for mandatory vaccination programs. 3 Due to their important status, many countries, both developed and developing, have put rules in place to regulate the entire vaccine life cycle as a means to ensure their safety and boost public confidence. 4
Nevertheless, despite the implementation of far-reaching regulatory frameworks, vaccine scandals do still occur. For example, in 2016, a police force in China’s Shandong Province uncovered an operation illegally selling large numbers of vaccines. By the time the scandal came to light, the illegal vaccines had spread across 24 of China’s provinces, with the total illegal income generated from vaccine sales reaching 570 million RMB. 5 More recently, in July 2018, the vaccines produced by Changchun Changsheng Biotechnology Co., Ltd (Changsheng Company below), a Chinese company, were reported to be substandard. At this point, over 65 million vaccines were implicated, thus catching the attention of the media and the public. 6
Reflecting on the series of vaccine incidents in China, 7on June 29th, 2019, the Standing Committee of the National People's Congress of China passed the Vaccine Administration Law of the People's Republic of China (hereafter, the Vaccine Administration Law). This law, which took effect on December 1st, 2019, marks the first time China has specifically regulated vaccines and the vaccine sector more broadly. This law aims to refine the vaccine regulation system in China and reestablish society's confidence in the safety of vaccines. 8
As a newly enacted law, it has its own unique characteristics and analyzing the law from a contemporary context may shed some light on how the law functions in relation to COVID-19 vaccinations. As for the structure of this article, it will first briefly describe the background of the Vaccine Administration Law before analyzing its substantive content. After introducing the basic framework, the article will evaluate the law, including both its breakthroughs and its shortcomings.
2. Background: The former vaccine regulation system and its defects
Prior to the enactment of the Vaccine Administration Law, the diffuse rules regulating vaccines in China were found in many different laws, such as the Drug Administration Law of the People's Republic of China (hereafter, the Drug Administration Law), the Law of the People's Republic of China on Prevention and Treatment of Infectious Diseases (Infectious Diseases Prevention law, below), and the Regulation on the Administration of Circulation and Vaccination of Vaccines (Vaccines Circulation Regulation, below) amongst others. Although many of the provisions set out rules in these pieces of legislation have covered the subject of vaccines, each law has its own application scope, none of which directly focus on the regulation of vaccines. By analyzing these rules, the former vaccine regulation system in China can be better understood and its defects can be identified.
2.1. Different departments involved in the chain of supervision
Currently, a number of government departments are involved in the regulation system for vaccines, with each taking responsibility for certain aspects (see Table 1 below). According to the Vaccines Circulation Regulation, the Department of Health, operating under the State Council, is responsible for supervising vaccinations throughout the country, whilst the Drug Administration Department, also operating under the State Council, is charged with supervising the quality of vaccines and administering them throughout the country. As for the qualification assessment of vaccine companies, this falls within the remit of each province's industrial and commercial departments. In one sense, having many different key parties involved in the regulatory system can allow for input from a wide-range of professionals and fully exert the potentials of the preset restriction mechanism. Thus, these departments can cumulatively contribute to the regulation process.
Table 1.
Main departments involved and their responsibilities.
| Department | Responsibilities for vaccine regulation | |
|---|---|---|
| National-level | National Medical Products Administration |
|
| National Health Commission |
|
|
| Provincial-level |
|
|
| Health department of provinces |
|
However, the issue with an uncoordinated system such as this is the lack of collaboration among different departments. This lack of unity also then introduces the possibility of a break in the supervision chain, which will also inevitably lead to many loop-holes in the vaccine regulation framework. For instance, traditionally, the departments have paid greater attention to vaccine production, whilst the custody aspect has been largely ignored. The custody process, however, does have a notable impact on the final quality of the vaccine. A typical example of this is the Shandong vaccine incident detailed above. According to the official investigation, although the vaccines involved were produced by licensed manufacturers, their quality was questionable as they were stored and transported without proper refrigeration. 9
2.2. Limited supervision resources
During the supervision process, the limited amount of resources and the difficult task of regulation mean the problems are unavoidable. An interview revealed that by 2017, in China, there were only 3478 Centers for Disease Control and Prevention at all levels, but more than 200,000 vaccination centers need to be supervised by them. 10 From these figures, it is readily apparent that it is a near-impossible task to effectively supervise such a huge number of vaccination centers. Additionally, some vaccination centers are located in remote areas of China, which further frustrates supervision efforts. 11 In practice, not every vaccination center will be inspected. 12
In addition to the issues related to the limited availability of human resources, there is also a large gap between the professional knowledge needed to effectively regulate the industry and the educational background of the relevant officials currently supervising the sector. The data in Dr. Hu's article shows that there is an urgent need for qualified supervisors to work in vaccine regulation. It should be noted that in the whole country, less than 500 people hold the necessary qualifications to conduct medical regulation. 13Due to the lack of knowledge and human resources, the phenomenon of power rent-seeking occurs, which is evidenced by the large number of criminal cases related to vaccines in the database of Chinese Judgments 14. In these cases, the parties are typically charged with bribery, abuse of power, illegal business activities, and so on. According to statistics, more than half of the corruption cases related to vaccine regulation judged by the court between 2014 and 2018 involved officials from the grass-roots levels Centers for Disease Control and Prevention accepting bribes in the vaccine procurement process. 15 Vaccine sellers usually pay kickbacks to the staff responsible for vaccine procurement to secure a sale. In the Chen Dexin bribery case, Chen Dexin, the former director of the Center for Disease Control and Prevention in Dafang County, Bijie City, Guizhou Province, accepted RMB 585,000 in bribes from the sales teams of Jiangxi Linyuan Biotechnology Co., Ltd from 2012 to 2015, in return for purchasing the company's varicella vaccine and typhoid vaccine. 16
2.3. Incomplete rules
By and large, regulation of the full vaccine life cycle has yet to be established in China. Typically, there are five phases of vaccine regulation: development, registration, production, circulation, and vaccination. The Vaccines Circulation Regulation focuses solely on circulation and vaccination, without touching on the development, registration and monitoring aspects. The main subject of the Infectious Diseases Prevention Law is to prevent and control the spread of infectious diseases. However, despite the huge scope of its responsibilities, its provisions only mention that “vaccines used for prophylactic vaccination shall conform to the quality standards of the State.”
With regard to the Drug Administration Law, it is formulated to guarantee the quality and safety of drugs. This law views vaccines as an indistinct type of drug, it emphasizes the production aspects of the vaccine life cycle whilst ignoring the vaccination procedure. However, in contrast with typical forms of drugs, vaccines have their unique features and thus special procedures need to be followed. 17 Regarding the uniqueness of vaccines, the Drug Administration Law does almost nothing to attend to these aspects of vaccines.
It can clearly be seen that prior to the implementation of the Vaccine Administration Law, the regulatory framework consisted of a patchwork of different rules and pieces of legislation, none of which could adequately take responsibility for or effectively oversee the regulation of vaccines (See Table 2 , below). “In order to prepare for a pandemic, or any other public health emergency, public health officials must know the laws under which they operate.” 18 In practice, the disparate rules will undoubtedly lead to confusion amongst the parties responsible for supervising vaccine production and administration.
Table 2.
The former vaccine regulation system.
| The Former Vaccine Regulation System | ||
|---|---|---|
| Laws and Regulations | Content related to vaccine regulation | Shortcomings |
| Drug Administration Law |
|
Failure to make specific provisions for vaccine regulation |
| Infectious Diseases Prevention Law |
|
The purpose is to prevent, control and eliminate the occurrence and prevalence of infectious diseases, not to strengthen systems for the effective regulation of vaccine |
| Vaccines Circulation Regulation |
|
Failure to consider the whole life cycle regulation of vaccines |
| ||
| Regulations on Administration of Vaccine Storage and Transportation |
|
Failure to consider the whole life cycle regulation of vaccines |
| ||
2.4. Difficulties in tracing
Technically, the location of substandard vaccines 19 cannot be effectively traced. Broadly speaking, in China, there are two classes of vaccines: those vaccines belonging to the first class are provided to citizens free of charge, whilst for the vaccines in the second class, citizens are voluntarily inoculated at their own expense. Since 2006, the former State Food and Drug Administration has explored the use of a 20-digit Electronic Drug Monitoring Code to ensure every vaccine can be tracked and located. 20 Unfortunately, this endeavor has ended in failure. 21
There are many factors contributing to this outcome: on the one hand, the systems used by different departments are incompatible, and different provinces and regions in China have different vaccination plans. Therefore, it is a technical challenge to trace vaccines. On the other hand, due to the lack of mandatory legal requirements, some Centers for Disease Control and Prevention did not scan the Electronic Drug Monitoring Code, which made it impossible to record key information on vaccine circulation and subsequent vaccination. Although the price of the second class of vaccines is determined and set by the government, it is the vaccination centers that have the power to choose providers. Due to the limited budget provided by the government, the local centers for disease control and prevention are incentivized to pursue profit by selling the vaccines in the second class to individuals who wish to be inoculated. In order to maximize their profits, vaccination centers purchase vaccines that are approaching their expiration date and they lack the proper custody for from agents at a low price. 22 Whilst this method increases profits, it goes without saying that such practices may negatively impact the quality of the vaccines customers receive.
3. The main contents of the vaccine administration law
Following the Changsheng incident, both the Chinese Communist Party Central Committee and the State Council of China became acutely aware of the importance of vaccine regulation and ordered that a long-term mechanism for vaccine regulation should be devised and implemented. On November 11, 2018, the draft of Vaccine Administration Law and a draft explanation were published on the government's website to solicit public opinions on the law. 23 On December 23, 2018, the draft of Vaccine Administration Law was referred to the Standing Committee of the National People's Congress for deliberation. Finally, on June 29th, 2019, it was passed into law, taking only six months from the time of drafting to enactment.
The final version of the Vaccine Administration Law spans eleven chapters and consists of one hundred provisions. It covers the entirety of the vaccine life cycle, with provisions regulating vaccine development, registration, production, circulation, vaccination, monitoring, safeguarding measures, and legal liability, amongst others, the legislation’s main innovations can be generalized as follows.
3.1. Setting up a whole-process regulatory system
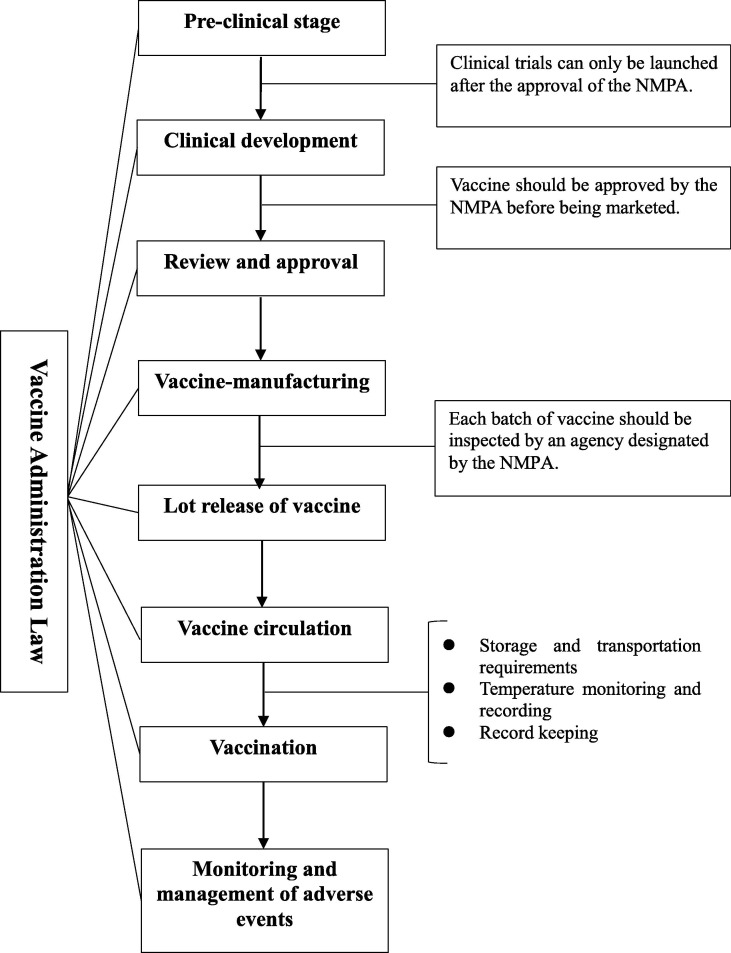
To avoid regulatory gaps, the Vaccine Administrative Law has established a regulatory system that covers the entire process from vaccine development to post-marketing management (see Fig. 1 below).
Fig. 1.
The Regulatory System set out in the Vaccine Administration Law.
Formal regulation begins with vaccine testing: a sponsor who wishes to conduct clinical trials for a vaccine must obtain approval from the National Medical Products Administration (NMPA). 24 If the clinical trials demonstrate that the vaccine is safe and effective, the applicant can submit a registration application to the NMPA. 25 NMPA shall review the production technology and quality control standards of the vaccines applied for marketing. 26 After obtaining a drug registration certificate, the vaccine can then enter the market.
In contrast with the general rules which apply to drugs 27, the Vaccine Administration Law imposes strict requirements for vaccine production. On the one hand, in addition to the conditions of drug manufacturing prescribed in the Drug Administration Law, vaccine manufacturing must also meet the following requirements: being equipped with appropriate scale and sufficient capacity reserves; possessing systems, facilities and equipment to ensure biosafety; and meeting the needs of disease prevention and control. 28 On the other hand, the whole vaccine production process shall comply with the standards of good manufacturing practice (GMP). 29 It should be noted that the National Medical Products Administration revised the GMP for biological products in light of relevant provisions of the Vaccine Administration Law.
All parties involved in the vaccine circulation process must strictly comply with vaccine storage and transportation management requirements, particularly the necessary temperature and light requirements which must be maintained to ensure the safety and effectiveness of the vaccines. 30 Moreover, the parties must establish honest, accurate, and complete records to ensure transparency and also allow for the whole process to be tracked and monitored. Also, the Vaccine Administration Law extends the minimum record-keeping period from 2 years to 5 years after the shelf life of the relevant vaccines. 31
Furthermore, the Vaccine Administration Law also sets out specific requirements for disease control and prevention institutions, vaccination providers, and medical personnel. Finally, and most importantly, this piece of legislation marks the first time that the risk management requirements have been clearly specified for post-marketing of vaccines.
3.2. Introducing the vaccine marketing authorization holder system
For the first time, the Vaccine Administrative Law specifies a marketing authorization holder (MAH) system for vaccines. In recent years, a pilot drug MAH regime has been implemented in select areas across China. The General Office of the State Council issued a notice on June 26, 2016, formally authorizing a pilot program for drug MAH systems in ten provinces. However, vaccines were notably excluded from the pilot plan. 32
Under the law, the MAH regime will hold the vaccine MAH responsible for the safety, effectiveness and quality of vaccines throughout the vaccine’s whole life cycle. 33 In practice, this means that vaccine safety no longer depends so heavily on the government department. Instead, vaccine companies will assume a more active role throughout the entire chain and vaccine life cycle.
The regulations on vaccine MAHs are more stringent than those imposed on drug MAHs. On the one hand, the conditions for becoming a vaccine MAH are stricter than those for general drugs. According to the 2016 Pilot Plan, drug research institutions and personnel within the pilot regions are eligible to become drug MAHs. The 2019 revision of the Drug Administration Law stipulates that the drug MAH will be implemented nationwide and defines a drug MAH as an enterprise or research institution that has obtained a drug registration certificate. Significantly, the Vaccine Administration Law further restricts vaccine MAHs to enterprises that have obtained both a vaccine registration certificate and a drug manufacturing license. 34 On this basis, research institutions will not be qualified to become vaccine MAHs.
On the other hand, unlike the requirements for drug MAHs, vaccine MAHs must have vaccines manufacturing capacity, and usually they should produce vaccines by themselves. According to Article 32 of the revised Drug Administration Law, a drug MAH may produce the drug by itself or mandate another drug manufacturing enterprise to produce the drug. However, the vaccine MAHs can only mandate other entities to produce vaccines after obtaining the approval of the NMPA. 35
3.3. Establishing the national electronic vaccine tracking system
From a technical perspective, due to the proliferation of Internet and big data technologies, it is easier than ever before to effectively trace vaccines. According to Article 10 of the Vaccine Administration Law, a national vaccine electronic tracking platform is to be established by the National Medical Products Administration and the National Health Commission. In accordance with the design, this platform will be linked to the electronic tracking systems for vaccines set up by the vaccine producers to allow for the integration of production, circulation, and vaccination information. In this way, the whole-life trace of vaccines will be realized and the data barriers between different departments can be eliminated.
In order to promote the establishment of the vaccine tracking system, the National Medical Products Administration and the National Health Commission jointly issued a circular on December 6, 2019, 36 which requires Beijing, Tianjin, Inner Mongolia, Shanghai, Jiangsu, Hainan, and Chongqing to take the lead in establishing provincial-level vaccine tracking system, and then connect with the national vaccine tracking system under construction. In Shanghai, for example, a comprehensive information management system has already been established. In this system, once the code is scanned, almost every phase of vaccine regulation (purchase, custody, circulation, etc.) can be tracked and investigated in the preset program. Besides gathering the information about vaccines, the identities of vaccine recipients and qualifications of doctors will also be verified at the same time. Meanwhile, parents can check their child's previous vaccination records through this system and in the instance that a vaccine is found to be substandard, the system will immediately send out an alert.
Another important value of the collaboration platform is that it can help to improve regulatory oversight through the effective use of the program. “Information is the life's blood of the regulatory process.” 37 This platform can not only collate information throughout the whole vaccine life cycle in the smallest packaging unit, but also can break down the information barriers between different regulatory agencies. By analyzing various data on the platform, regulators can develop a better understanding of the life cycle of vaccines, issue early warnings for vaccine stocks in different provinces, and understand the growth rate of vaccine production. More importantly, regulators are able to monitor the validity period of vaccines and ensure vaccine quality as part of their efforts to protect public health and safety.
3.4. Clearly establishing the legal responsibilities of all parties
According to the Vaccine Administration Law, the relevant party will be subject to severe penalties if certain requirements have not been met or strictly adhered to. For instance, if a vaccine is found to be counterfeit, a fine of no less than 15 times(but no more than 50 times), the value of the relevant vaccine will be imposed. Meanwhile, where the quality of a vaccine is found to be inferior, a fine can be issued which is as much as 30 times the value. 38
In addition to the high fines, several other penalties can also be imposed on the relevant bodies, such as confiscating materials, suspending business operations, revoking the drug registration certificates, and so on. If certain acts have violated the criminal law, the person will also be charged under the relevant criminal provisions. The deterrence enforcement strategy assumes that those regulated are rational actors, and claims that the severity of punishment and the frequency of inspection are the main factors that affect the behavior of the regulated object. In this sense, increasingly severe sanctions, coupled with effective supervision, will function to deter illegal behaviors, to some extent. It should also be noted that, in addition to the company producing the vaccine, any officials or personnel in the government departments that have contravened the Vaccine Administration Law will also be punished.
3.5. Implementing the compensation system for abnormal reactions to vaccination
Vaccines are an excellent means for protection from illness for most individuals and also for wider society. However, they are not universally effective nor are they completely safe. 39 “Abnormal reactions to vaccination” refers to the adverse reactions of qualified vaccines which cause damage to the vaccinated person's tissues, organs or functions in the process of or following standardized vaccination, and for which no relevant party has any fault. 40 In practice, the number of abnormal reactions to vaccination is relatively low, but once it occurs, it may be disastrous for the whole family. In China, the vaccine adverse event reporting system was established in 2005. Due to the ongoing pandemic, following the administration of COVID-19 vaccines, serious adverse reactions should be reported in this system within two hours. According to the data released by the Chinese Center for Disease Control and Prevention, by April 30, 2021, 265 million vaccines doses have been administered, with just 31,434 abnormal reactions being reported. 41
To compensate individuals who experience adverse reactions, the Vaccine Administration Law ascribes different responsibilities to various parties for different categories of vaccines. 42 This marks the first time that an abnormal reaction compensation system has been clearly established in Chinese Law. As for the scope of the compensation offered, the Vaccine Administration Law stipulates that “the scope of compensation shall be subject to management by catalogue and dynamically adjusted according to actual circumstances.” On December 7, 2020, the National Health Commission issued the “Reference Catalogue and Description of Compensation Scope for Abnormal Vaccination Reactions.” 43 As a new compensation management method, the compensation catalogue helps to make the scope of compensation clearer and easier to carry out specific compensation. However, it should be noted that the compensation catalogue is only for reference. If the damage is not within the scope listed in the catalogue, but there is a causal relationship between the damage and the vaccination, compensation should also be given.
It should also be noted that the compensation system is somewhat different from the established western no-fault compensation schemes. A no-fault compensation scheme does not use legal fault as the basis for determining liability. Instead, the dispositive issue usually turns on whether the doctor caused the injury. 44 Meanwhile, no-fault compensation schemes would examine several factors, including whether the injury falls within the scope of compensable damages, as defined by statute. 45 But in China, the compensation catalogue is only for reference. Besides, if it falls under abnormal reactions to vaccination or abnormal reactions cannot be excluded, compensation shall also be made. In this sense, the scope for compensation is broader than that of no-fault compensation schemes.
3.6. Emphasizing the development of new vaccines
China has the largest population of any country in the world and produces the largest number of vaccines globally each year. 46 However, most of the vaccines registered in China are traditional vaccines 47and there is still a large gap in the development of novel vaccines when compared with developed countries. Projects to develop new vaccines typically require large investments of time and capital and the potential market for the vaccine is a major consideration for companies when determining whether to proceed.
In the past, on average, Chinese biotechnology companies devoted about 5% of their annual income to development), which is much lower than the amount channeled into development in similar countries abroad. 48 According to the Vaccine Administration Law, government funds will support the development of polyvalent, multivalent, and other new-type vaccines. This goal will be achieved in two ways. The routine path is to encourage vaccine marketing license holders to increase investment in R&D and optimize production processes through favorable policies or economic incentive mechanisms. Due to the length of the R&D cycle, the large capital investment required, and the high risk of failure, the market mechanism may fail. Following the outbreak of infectious diseases, it is difficult to rely solely on enterprises to develop vaccine in a timely manner. Therefore, for vaccines urgently needed for epidemic prevention and control, the state will organize vaccine marketing license holders, scientific research entities, medical and health institutions and other actors to cooperate in vaccine R&D. 49
Government support of this kind will undoubtedly be a catalyst to speed up the development of novel vaccines. In the past year, “The COVID-19 pandemic has illustrated the need for the swift development of new vaccines targeting emerging pathogens causing outbreaks of infectious diseases.” 50 In order to organize and mobilize resources promptly in state of emergency, 12 departments, including the Ministry of Science and Technology and the National Health Commission jointly established a COVID-19 vaccine task force, which is affiliated with the Joint Prevention and Control Mechanism of the State Council and directly reports to the Vice Premier of China. 51 China has issued conditional market approval to four domestically made COVID-19 vaccines, and two of which vaccines have been approved for emergency use by the World Health Organization (WHO). 52 A report reveals that by May 18th, 2021, more than 435,689,000 doses of vaccines for COVID-19 have already been administered in China. 53 This achievement can, to some extent, be attributed to the new mechanism established by the Vaccine Administration Law.
4. Evaluation
A scientific regulatory system can be an impetus for the healthy development of the vaccine industry. Briefly speaking, the Vaccine Administration Law has demonstrated the Chinese government's sincere determination to regulate the vaccine sectors effectively and the implementation of this legislation will affect the current vaccine regulation system in China. Although it has yielded many benefits and proven to be effective, further efforts are still needed to further refine the regulatory system.
4.1. Improving the risk management system
The public’s tolerance to risk from vaccines is lower than for other pharmaceutical products since vaccine recipients are mainly from healthy populations which include infants and children. 54 Risk management 55 is therefore not just directly related to the development of the vaccine industry, but also is closely related to vaccine regulation.
One of the main advancements of the Vaccine Administration Law is to establish the risk management mechanism. The Vaccine Administration Law explicitly lists “risk management” as the main principle of the vaccine regulatory system, and stipulates a series of specific requirements on this basis. Article 60 of Vaccine Administration Law requires vaccine MAHs to establish quality retrospective analysis and risk reporting systems, and annually report on vaccine production, circulation, post-marketing research, risk management, and other aspects to the National Medical Products Administration. In terms of vaccine lot release, inspection items and frequency will be dynamically adjusted based on a vaccine risk assessment.
The surveillance of adverse events following immunization (AEFI) is an important part of the risk management process after the vaccine is marketed. Taking the COVID-19 vaccine as an example, the statement issued by the Chinese Center of Disease Control and Prevention(China CDC) pointed out that a total of 31,434 AEFI cases were reported between Dec.15,2020 and April 30,2021 when the country administered 265 million vaccine doses. Abnormal reactions accounted for 17.04%, of which 188 cases were deemed severe. The serious abnormal reactions reporting ratios is 0.07/100,000 doses, which is extremely rare (less than 1 in 10,000). 56
However, compared with other countries, China’s AEFI surveillance system still has room for improvement. First, the AEFI surveillance system should be open to the public. Unlike the United Kingdom's Yellow Card Scheme, 57 China's AEFI information system does not accept adverse reaction reports submitted directly by the public. According to the Vaccine Administration Law and China’s national AEFI guidelines, 58healthcare facilities, vaccination centers, Centers for Disease Control and Prevention, adverse drug reaction monitoring agencies, and vaccine MAHs are responsible reporters of AEFIs. If the vaccine recipient or guardian suspects that the symptoms may be related to vaccination, they can only notify the above authorized reporters, and then the authorized reporters will submit the AEFI data to the National AEFI Information System. This makes it difficult for the recipients of vaccines to directly report suspected AEFIs in a timely manner. In order to accurately estimate the incidence of adverse events, the public needs to be provided with different ways to report AEFIs that are easy and convenient. Second, in addition to passively receiving adverse reaction reports, vaccination providers can take a more proactive approach, such as issuing reminder cards or sending out text messages and phone reminders to guide the public to rationally understand the risks of vaccination and reduce people's fear of AEFIs. 59 Finally, drawing on the experience of the vaccine risk monitoring database in the United States, 60 bringing together and integrating information on vaccines, medical illnesses and other scattered data can aid in tracking adverse reactions to vaccines, which can further help to continually evaluate the safety of vaccines.
4.2. Strengthening the government's regulatory capabilities
As mentioned above, due to the rapid development of the pharmaceutical industry, including the vaccine sectors, government regulators are increasingly faced with issues such as heavy inspection burdens, inadequate staffing, and insufficient professional capabilities.
In response to these difficulties, the State Council issued a circular on July 8, 2019, proposing the establishment of professional drug-inspection teams both at the national and provincial levels in the next 3 to 5 years, which will be responsible for conducting compliance inspections and risk assessments in relation to the places and activities related to drug R&D and production, with a particular emphasis on strengthening the inspection of high-risk drugs, such as vaccines. This is beneficial as it will boost the number of drug inspectors, whilst also helping to increase the professionalism of inspectors. Based on their professional skills, and other factors, drug inspectors will be divided into four levels: junior, intermediate, senior, and expert inspectors. Whilst the qualifications and responsibilities of drug inspectors at each level are different, all inspectors must undergo training in business, legal, and regulatory training for no less than 60 h per year. Given the limited number of positions and the low level of remuneration, it remains to be seen whether enough professionals can be attracted to join the drug inspection team.
Another useful way to strengthen the government’s ability to regulate vaccines is to refer to specialists from universities or research centers when faced with complex professional problems. When confronted with vaccine risks with inherent uncertainties, it is often necessary to make judgments on scientific frontier issues. 61 By holding meetings and consultations with expert, a consensus can gradually be gathered from the scientific community to ensure that vaccines are as safe and effective as possible. In recent years, government regulators have increasingly relied on the advices provided by expert groups in their drug regulation activities. According to Article 17 of Drug Registration Regulation of 2019, the Center of Drug Evaluation shall, as needed for work, form an expert advisory committee, and request experts’ opinions on major issues during the process of evaluation, inspection, examination, and confirmation of common names. Meanwhile, Article 42 of the Vaccine Administration Law obliges the National Health Commission to establish an expert advisory committee for national immunization programs. Drawing on the experience of the World Health Organization and other countries, it is necessary to further specify the expert selection mechanisms, meeting procedures, rights and obligations, and information disclosure requirements to ensure the independence of the expert advisory committee. Furthermore, an advisory committee can be set up to specifically attend to vaccine safety issues. For example, in Australia, the Vaccine Advisory Committee is responsible for providing independent medical and scientific advice to the Minister for Health and the Therapeutic Goods Administration on issues relating to vaccine safety, quality, and effectiveness, including problems that may arise at various stages of the vaccine life-cycle such as pre-market evaluation and post-market surveillance. 62
Communication and cooperation with international organizations will also help strengthen the capabilities of the regulatory authority, improve the China’s vaccine regulation system, and align the country’s vaccine sector with international technical standards to ensure the safe and quality of vaccines. From a global regulatory network perspective, even though there are many differences between countries, cross-border exchange of information may still trigger both rational learning and normative emulation. 63 For example, by becoming a regulatory member of the International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use (ICH), China's drug regulatory authority can learn the latest regulatory scientific outcomes and advanced regulatory concepts globally. Since joining ICH in 2017, China has transformed and implemented 46 ICH guidelines, which has promoted the reform of China's drug review rules and procedures. 64
4.3. Paying attention to the role of non-governmental actors
Regulatory resources are dispersed amongst different actors, rather than being concentrated in the government. Accordingly, the regulatory capabilities of non-governmental actors are increasingly recognized by the state. 65 The report delivered by President Xi Jinping at the 19th National Congress of the Communist Party of China pointed out that strengthening the cooperation between multiple actors is an important element of promoting the modernization of the national governance system and its governance capabilities. 66 In view of the conflict between the limited pool of government resources and the heavy regulatory tasks demanded to ensure public safety, it is important that the resources of non-governmental actors are effectively utilized to perform certain vaccine regulatory functions.
The Vaccine Administration Law specifically stipulates the role of vaccine industry associations and news media. Following the implementation of the Vaccine Administration Law, the China Association for Vaccines (CAV) was created on the basis of the original “Chinese Pharmaceutical Enterprise Development Promotion Association”. 67 The CAV, which is the first national-level vaccine and biological products industry organization to be created in China, not only aims to promote the high-quality development of the vaccine industry, but also implement effective self-regulation to ensure vaccine safety. Similarly, the role of the media cannot be ignored. On the one hand, the media can monitor the misconduct of vaccine manufacturers, vaccination providers, the government, and other relevant actors to a certain extent. The news media sometimes plays an important policing role and can expose the illegal activities of vaccine manufacturers before the government takes action. Accordingly, the pressure of public opinion caused by media exposure will force the government and vaccine manufacturers to actively respond. On the other hand, vaccine information can be fully exchanged through social media, which helps people rationally assess the benefits and potential harms of vaccination.
To achieve the goal of vaccine regulation, it is critical to effectively tap into the resources of non-governmental actors and form a cooperative regulatory space through complementary advantages. In this regard, it is necessary for the government to implement some specific mechanisms, such as actively requesting the opinions of non-governmental entities in the process of vaccine policy formulation, and encouraging them to monitor vaccine quality. For example, Canadian vaccine regulators often engage in open dialogues with non-governmental bodies on relevant immunization issues, which not only contributes to the formation of effective vaccine policies, but also enhances the transparency and accountability of the vaccine review process. 68 In addition, the mechanism of public interest litigation can be further improved so as to allow for a more effective use of non-governmental actors to ensure vaccine safety. The Administrative Litigation Law currently only allows People’s Procuratorates to bring public interest litigation on the basis of the government‘s misconduct or nonfeasance in drug regulation. 69 In order to promote participation by civil society and its actors in the enforcement of the Vaccine Administration Law, non-governmental organizations (NGOs) should be granted the ability to initiate public interest litigation.
4.4. Avoiding excessive government intervention
The implementation of the Vaccine Administration Law can help to upgrade the industrial structure of vaccine sectors. 70 After analyzing the relevant provisions in the law, it can be concluded that the new law has imposed a high burden and exacting standards on vaccine manufacturers. Following the implementation of these strict requirements, small vaccine companies that cannot comply with the legislation will gradually be removed from the sector by operation of the law. Besides, “the critical importance of vaccines highlights the need to ensure robust vaccine innovation to combat global health threats.” 71 Compared with small companies, the vaccine companies that have the necessary foundation for innovative development will be given greater access to new opportunities due to government financial support, as well as the technological benefits from the research institutions. In this way, those companies that cannot gain from the policy will be marginalized by the market.
Whilst strict legislation does help to promote the high-quality development of the vaccine industry, it is worth noting that government regulators should avoid excessive intervention in the vaccine market, especially for the price of vaccines in the second class. There is a certain degree of administrative monopoly in the procurement of vaccines, and there is a clear imbalance in the relative negotiating positions of vaccine manufacturers and the government in terms of pricing. Meanwhile, vaccine manufacturers cannot exceed the maximum retail price set by the vaccine bidder, otherwise, they will lose their market share. Narrow profit margins force vaccine companies to continuously lower their production costs which may be detrimental to product quality and will also inhibit their enthusiasm for innovation. 72 For example, an investigation by the State Council team into the Changsheng Biotechnology Company vaccine incident indicated that the company violated the approved production process in order to reduce costs. 73
5. Conclusion
As a special form of drug, vaccines are the basic public product that can be used to protect public health. At the same time, it is also the most economical and effective measure to deal with infectious diseases. Frequent vaccine incidents have severely damaged people’s confidence in vaccines and immunization programs more broadly. 74 In response, the 2019 Vaccine Administration Law provides for the “strictest” vaccine management with tough penalties, which is of particular significance for ensuring vaccine safety and promoting the development of the vaccine industry. The effectiveness of the vaccine regulatory system depends on the proper implementation of the vaccine management law. To this end, it is necessary to further improve risk management mechanisms, enhance the regulatory capacity of government regulators, effectively use the resources of non-governmental actors, and avoid improper government interventions in the vaccine market. At the time of writing, the COVID-19 pandemic is still ongoing. Despite the implementation of various measures to stop the spread of the virus, it appears almost certain that COVID-19 will not be controlled globally without the development of a vaccine. 75 By making necessary improvements to its vaccine regulation system, China can certainly make a greater contribution to curbing the spread of the virus and reducing its associated illness and mortality.
Funding
This research was supported by the Major Program of the National Social Science Foundation of China (Grant No. 20 & ZD188).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Eva B. Stensvad, Immunity for Vaccine Manufactures: The Vaccine Act and Preemption of Design Defect Claims, 95 Minn. L. Rev. 315, 316, 2010.
These congressional vaccination programs include The National Swine Flu Immunization Program of 1976, The National Childhood Vaccine Injury Act, The Smallpox Vaccination Program, Public Readiness and Emergency Preparedness Act, and so on. See Joanna B. Apolinskya and Jeffrey A. Van Detta, Rethinking Liability for Vaccine Injury, 19 Cornell J.L. & Pub. Pol'y 537, 546–558, 2010.
Madison N. Heckel, Do I Have To?: Mandating a Vaccine in a Politicized Pandemic, 30 Ann. Health L. Advance Directive 183,183(2020).
See Julie Milstien, Lahouari Belgharbi, Regulatory Pathways for Vaccines for Developing Countries, Bulletin of the World Health Organization 2004 ; 82(2) : 128–133.
See http://sd.people.com.cn/n2/2016/0320/c166192-27971184.html(last visited:2019–09-29).
Report on the Changchun Changsheng Vaccine Incident,http://finance.ifeng.com/a/20180722/16397506_0.shtml(last visited:2019–09-29).
In the past decade or so, there have been three serious vaccine incidents in China. In addition to the two vaccine incidents mentioned in this article, the other one is the Shanxi vaccine incident. In 2010, media reports questioned that dozens of children in Shanxi were disabled due to the vaccination of vaccines exposed to high temperatures. Although the official survey results showed that only three children were confirmed to be abnormal vaccine reactions, this report still triggered a crisis of confidence in the vaccine.
Wangchen, To Provide Legal Guarantee for People's Safety, The People's Daily, 2019–07-31.
National Medical Products Administration, https://www.nmpa.gov.cn/directory/web/nmpa/zhuanti/lshzht/shdffyma/20160413231201533.html(last visited:2021–06-09).
In 2017, Dr. Hu YingLian interviewed an official from the Organs of the State Health and Family Planning Commission, and the data was provided by this official. See Hu YingLian, Administration Absorbs Market: The Governance Dilemma in Medicine Safety and Public Health, Social Sciences in Guangdong, 2017(5), p209.
According to Article 21 of Vaccines Circulation Regulation, a lawful vaccination center should meet three requirements: (1) a medical institution practicing permit; (2) qualified doctors or nurses; (3) qualified refrigerating equipment. From these provisions, it can be seen that it is not difficult to set up a vaccination center.
Unannounced inspection means that the responsible departments do on-the-spot inspections without notifying those being inspected in advance. The unannounced inspection model has its advantages as well as its disadvantages. Under this model, there is a great possibility that vaccine producers and the huge volumes of vaccines they produce cannot be effectively supervised.
Hu YingLian, Administration Absorbs Market: The Governance Dilemma in Medicine Safety and Public Health, Social Sciences in Guangdong, 2017(5), p209. As is mentioned above, less than 500 people had the necessary qualifications in China. Besides, due to the high standard of educational background, such positions are sometimes in vacancy.
The database of Chinese Judgments is owned by the Supreme People's Court Of China and it is the largest judgment database in China. As of October 10, 2019, it had indexed more than 78 million judgments and has been visited over 35.6 billion times. The database can be accessed at http://wenshu.court.gov.cn/.
Zhang Jian, Dozens of judgments expose corruption in the vaccine industry, https://www.yicai.com/news/100001009.html (last visited:2021–06-09).
Court Judgment: Weining People’s Procuratorate vs. Chen Dexin, 2nd instance, Criminal Division, Bijie Intermediate Court [2016] No.495.
For example, general drugs are normally administered to people who are ill, whilst vaccines are typically administered to healthy people to prevent diseases. By using drugs, symptoms can be treated; however, with the use of vaccines, the disease can ultimately be eliminated. As for their ingredients, general drugs can be composed of herbal drugs, chemical-based compounds, amongst others; contrastingly, all vaccines are biological products.
Wendy E. Parmet, Pandemics, Populism and the Role of Law in the H1N1 Vaccine Campaign, 4 St. Louis U. J. Health L. & Pol'y 113,153,2010.
A substandard vaccine is one that has not been stored in approved conditions or has expired.
Center for information, NMPA, https://www.nmpaic.org.cn/yp/gzdt/202103/t20210329_300770.html (last visited:2021–06-09).
Reduced scanning rate in circulation leads to failure of Electronic Drug Monitoring Code, http://www.yygcj.com/info/2016–06/36412.html (last visited:2021–07-09).
Hu YingLian, Administration Absorbs Market: The Governance Dilemma in Medicine Safety and Public Health, Social Sciences in Guangdong, 2017(5), p210.
The draft of Vaccine Administration Law, See http://www.gov.cn/hudong/2018–11/12/content_5339460.htm (last visited:2021–06-09).
See Article 16 of the Vaccine Administration Law.
See Article 19 of the Vaccine Administration Law.
See Article 21 of the Vaccine Administration Law.
In China, the Drug Administration Law was adopted in 1984, with its most recent revision coming into force in 2019. According to this law, drug administration departments in China are responsible for the supervision and administration of drugs. The responsibility covers the phrases of development, registration, production, distribution, post-market management and so on. As for its application scope, this regulatory system applies to Chinese drugs, chemical drugs, and biological products, including vaccines. This means that under the current regulation system, the regulation of vaccines should follow the rules in Drug Administration Law ; however, if more stringent requirements are set out in the Vaccine Administration Law, then the latter should be followed.
See Article 22 of the Vaccine Administration Law.
See Article 24 of the Vaccine Administration Law.
See Article 37 of the Vaccine Administration Law. Vaccines are sensitive biological products that may become less effective, or even denatured (rendering them useless), when exposed to temperatures outside the recommended range and/or to direct sunlight or fluorescent light.
See Article 39 of the Vaccine Administration Law.
The ten provinces authorized to implement the drug MAH system are Beijing, Tianjin, Hebei, Shanghai, Jiangsu, Zhejiang, Fujian, Shandong, Guangdong, and Sichuan.
See Article 5 of the Vaccine Administration Law.
See Article 97 of the Vaccine Administration Law.
See Article 22 of the Vaccine Administration Law.
Promoting the construction of vaccine information traceability system, https://www.nmpa.gov.cn/xxgk/fgwj/gzwj/gzwjyp/20191212191401334.html (last visited: 2021–06-09).
Peter L. Strauss, Administrative Justice in the United States, Carolina Academic Press, 2002, p43.
See Article 80 of the Vaccine Administration Law. According to the Drug Administration Law, if a person produces and sells fake drugs, a fine of no less than 15 times(but no more than 50 times)will be imposed. If the drug is found to be inferior, the body will be fined no more than 20 times of the unlawful income. By comparing these two articles, it can be concluded that fines relating to vaccines are more severe than those related to normal drugs.
Efthimios Parasidis, Recalibrating Vaccination Laws, 97B.U. L. Rev. 2153, p2241, 2017.
See Article 52 of the Vaccine Administration Law.
See The Report of Abnormal Reactions to Vaccines for COVID-19 in China, http://www.chinacdc.cn/jkzt/ymyjz/ymyjjz_6758/202105/t20210528_230911.html (last visited:2021–05-21).
According to Article 56 of the Vaccine Administration Law, the compensation fees required for vaccines under immunization programs shall be allocated from vaccination funds by public finance departments of the People's Governments of provinces, autonomous regions, and municipalities directly under the Central Government; the compensation fees required for vaccines not covered by immunization programs shall be assumed by the relevant vaccine marketing license holders.
Reference Catalogue and Description of Compensation Scope for Abnormal Vaccination Reactions, http://www.nhc.gov.cn/jkj/s3581/202012/58eb4a4b47694e5b81248b29cc315feb.shtml (last visited: 2021–06-09).
David M. Studdert & Troyen A. Brennan, No-Fault Compensation for Medical Injuries: The Prospect of Error Prevention, 286 J. OF THE AM. MED. ASS'N 217, 219 (2001).
Jill Horwitz & Troyen A. Brennan, No-Fault Compensation for Medical Injury: A Case Study, 14 HEALTH AFF. 164, 167 (1995).
As of July 22, 2018, there were 45 vaccine manufacturing enterprises in China producing 63 kinds of vaccines to prevent 34 infectious diseases. Each year, more than 1 billion vaccines are manufactured, which constitutes more than 95% of all the vaccines needed. See the website of National Medical Products Administration, The head of National Medical Products Administration Introduces the Changsheng Case, http://www.nmpa.gov.cn/WS04/CL2056/329653.html(Last visited:2019–10-13).
Traditional vaccines are ones that require relatively weak innovation, and insufficient investment in R&D.
See Bai Tiantian, China's Vaccine Industry is Still in the Early Stage of the Industry, Economic Information, 2012–02-17.
For example, currently, the Chinese government has sponsored many research projects developing vaccines for COVID-19. Many scientific institutions and vaccine manufactures have cooperated in this research, including Sinovac Research & Development Co., Ltd., and Sinopharm, amongst others. Meanwhile, at the same time, cooperation among different countries is also encouraged. See http://www.nhc.gov.cn/xcs/s3574/202010/a95d956c39cf4393b400b42aa8433033.shtml (last visited:2021–05-21).
Ana Santos Rutschman: The COVID-19 Vaccine Race: Intellectual Property, Collaboration(s), Nationalism and Misinformation, 64 Wash. U. J.L. & Pol'y 167,169(2021).
See Yinglian Hu and Simiao Chen, What Can We Learn from COVID-19 Vaccine R&D in China? A Discussion from a Public Policy Perspective, Journal of Travel Medicine 2021; 28(4):1–3.
WHO lists additional COVID-19 vaccine for emergency use and issues interim policy recommendations, See https://www.who.int/news/item/07–05-2021-who-lists-additional-covid-19-vaccine-for-emergency-use-and-issues-interim-policy-recommendations (last visited:2021–06-08); WHO validates Sinovac COVID-19 vaccine for emergency use and issues interim policy recommendations, See https://www.who.int/news/item/01–06-2021-who-validates-sinovac-covid-19-vaccine-for-emergency-use-and-issues-interim-policy-recommendations(last visited:2021–06-08).
See the official website of National Health Commission of the People’s Republic of China: http://www.nhc.gov.cn/xcs/yqfkdt/202105/beda31b9f97b4c3cae8afba056330f98.shtml (last visited:2021–05-20).
See World Health Organization, Vaccine Safety Basics Learning Manual, 2013, p14.
Article 12 of China’s Drug Administration Law revised in 2019 clearly stipulates that “The state shall establish a pharmaco-vigilance system to monitor, identify, assess, and control adverse pharmaceutical reactions and other harmful reactions associated with the use of pharmaceuticals.”
See the website of Chinese Center of Disease Control and Prevention, http://www.chinacdc.cn/jkzt/ymyjz/ymyjjz_6758/202105/t20210528_230911.html (last visited:2021–07-18).
The Yellow Card Scheme is the UK system for collecting and monitoring information on suspected safety concerns or incidents involving medicines and medical devices. Anyone can report a suspected side effect of vaccination to the MHRA through the Yellow Card Scheme. For details about yellow card, see https://yellowcard.mhra.gov.uk/the-yellow-card-scheme/ (Last visited:2020–08-08).
National guideline for the surveillance of adverse events following immunization,2010. http://www.nhc.gov.cn/zwgk/wtwj/201402/5dd5633d93174a7c8e93d8af7579a613.shtml (last visited:2021–06-08).
See Ding Xue-jiao, Shi Nian-min, Li Li, et al. Investigation on Model of Active Surveillance of AEFI in Chaoyang District, Beijing Taking DTaP as An Example, Chinese Journal of Biologicals, 2016 (3), p289.
Michael M McNeil, Julianne Gee, Eric S Weintraub, et al. The Vaccine Safety Datalink: successes and challenges monitoring vaccine safety, Vaccine. 2014;32(42):5390–5398.
See Vern R. Walker, Risk Regulation and the “Faces” of Uncertainty, 9 Risk 29–34 (1998).
For a detailed introduction to the Vaccine Advisory Committee, see https://www.tga.gov.au/committee/advisory-committee-vaccines-acv (last visited: 2020–08-08).
See Mathias Koenig‐Archibugi, Global Regulation, in The Oxford Handbook of Regulation, edited by Robert Baldwin, Martin Cave, and Martin Lodge, Oxford University Press, 2010, p412.
NMPA holds symposium on process and prospects of ICH in China, See http://english.nmpa.gov.cn/2021–04/10/c_610224.htm (last visited:2021–06-08).
See Peter N. Grabosky, Using Non-Governmental Resources to Foster Regulatory Compliance, 8 Governance 527, 1995.
Xi Jinping's report at 19th CPC National Congress, See http://www.gov.cn/zhuanti/2017–10/27/content_5234876.htm (last visited:2021–06-08).
See http://www.cujin.org/Content-3–8.aspx (last visited: 2020–08-08).
See BIOTECanada Vaccine industry Committee, Building on the Legacy of Vaccines in Canada: Value, Opportunities, and Challenges Series, 2010, p26.
According to Article 25 of Administrative Litigation Law, if a People’s Procuratorate identifies that the responsible department did not act or acted illegally in the area of the drug, the People's Procuratorate can send procuratorial proposals to the department and urge it to perform its duties. Where the department fails to perform its duties or reply to the proposal, the People's Procuratorate can file a lawsuit.
By 2020, there were 37 vaccine sectors in China, whilst the number was 45 in 2018.The vaccine sectors for vaccines in the first class are always state-owned enterprises. This market is stable and there is very little competition. However, the vaccine sectors in the second class are composed of state-owned enterprises, transnational corporations and private-owned companies. In this market, the competition is fierce and the functions of private-owned companies have enhanced gradually. See https://www.askci.com/news/chanye/20200706/1659091163175_4.shtml (last visited:2021–05-23); See also https://www.nmpa.gov.cn/yaowen/ypjgyw/20210420210207140.html (last visited:2021–05-23).
John D. Winter, Cassye Cole and Jonah Wacholder, Toward A Global Solution on Vaccine Liability and Compensation, 74 Food & Drug L.J. 1,2,2019.
See Research Group of CPAS, Balance Regulation and Market: Ideology and Approach of Drug Safety Governance in China——A Case Study of Changsheng Vaccine Incident, Chinese Public Administration, 2018(10), p7.
For the investigation results of Changsheng Biotechnology Company's vaccine incident, see http://www.gov.cn/xinwen/2018–07/27/content_5309928.htm (last visited:2020–08-08).
See Liu, Baohua, Ruohui Chen, et al., Vaccine Confidence in China after the Changsheng Vaccine Incident: a Cross-sectional Study, 19 BMC Public Health 1564, 2019.
Lawrence O. Gostin, Safura Abdool Karim, and Benjamin Mason Meier: Facilitating Access to a COVID-19 Vaccine through Global Health Law: Global Health Law, 48 J.L. Med. & Ethics 622,622(2020).