Significance
The steady increase of electromagnetic radiation (EMR) in the environment, particularly the wireless signal, causes serious public concern over its potential negative impact on health. However, it is challenging to examine such impact on human subjects due to associated complex issues. In this study, we establish an experimental system for the investigation of EMR impact on mice. Using this system, we uncovered a causal relationship between 2.4-GHz EMR modulated by 100-Hz square pulses and increased wakefulness in mice. This result identifies sleep alteration as a potential consequence of exposure to excessive wireless signals.
Keywords: electromagnetic radiation, sleep, wireless signal, mouse model, public health
Abstract
Electromagnetic radiation (EMR) in the environment has increased sharply in recent decades. The effect of environmental EMR on living organisms remains poorly characterized. Here, we report the impact of wireless-range EMR on the sleep architecture of mouse. Prolonged exposure to 2.4-GHz EMR modulated by 100-Hz square pulses at a nonthermal output level results in markedly increased time of wakefulness in mice. These mice display corresponding decreased time of nonrapid eye movement (NREM) and rapid eye movement (REM). In contrast, prolonged exposure to unmodulated 2.4-GHz EMR at the same time-averaged output level has little impact on mouse sleep. These observations identify alteration of sleep architecture in mice as a specific physiological response to prolonged wireless-range EMR exposure.
Electromagnetic radiation (EMR) is omnipresent in the world. In the past several decades, EMR has been drastically increased in the environment. Wi-Fi, which mainly works in the dual frequencies around 2.4 and 5 GHz (1), is available in numerous households for local wireless network communication. 4G cell phones, Bluetooth, and microwave ovens also use frequencies around 2.4 GHz. Wireless equipment in major cities of the world has doubled in the last 5 y (2), exposing the public to a potential health risk that has yet to be adequately assessed.
Epidemiology studies have revealed worldwide rise of certain health conditions such as sleep disorder (3), infertility (4), psychiatric disorder (5), and cancer (6). The rise has been generally attributed to worsened environment such as work stress and air or water pollution. Although it remains unclear whether EMR constitutes one of these environmental factors, public concern is growing over the safety of EMR, particularly in the microwave frequency. Unfortunately, investigations on the effects of EMR have often been controversial. In 2011, a World Health Organization (WHO)-authorized agency classified 30-kHz to 300-GHz EMR as possibly carcinogenic (7). In 2014, however, a WHO project found no adverse health effects by mobile phone use (8). More recently, EMR was reported to adversely affect the central nervous system, causing sleep disorder (9) and learning/memory impairment in humans (10), stress and anxiety-like behavior in rats (11), and physical/cognitive abnormality (12). Increased incidences of malignant gliomas and schwannomas in male rats appear to be associated with prolonged exposure to modulated EMR at 900 MHz and 1.8 GHz (13–15).
Sleep is essential for attention and cognition (16–18). Sleep disorder has become a worldwide health challenge (19). The sleep–wake cycle of mammals is divided into three phases: wakefulness, rapid eye movement (REM) sleep, and non-REM (NREM) sleep (20). Several studies on pulse-modulated 900 MHz EMR suggest a potential effect on the architecture of human sleep, such as altered EEG spectral power at specific frequency bands or sleep phases (21–26). Small effects of mobile phone EMR on sleep EEG parameters are considered possible (27).
Previous studies reported an inconclusive effect of EMR on human sleep architecture (25, 28–31), in part because human volunteers are easily disturbed by environmental factors (e.g., coffee/alcohol/smoking/medication/mobile phone), and these studies may lack adequate control (25, 32). In addition, the effect of EMR may depend on exposure time, radiation intensity, modulation mode, and other parameters (21, 30, 31, 33–36). To address these issues, we establish an experimental system to investigate the EMR effect on mouse behavior. To our knowledge, such effort using a mouse model has not been previously attempted. We demonstrate that prolonged exposure to pulse-modulated 2.4-GHz EMR results in marked increase of the total time of wakefulness in mice.
Results
The Experimental System.
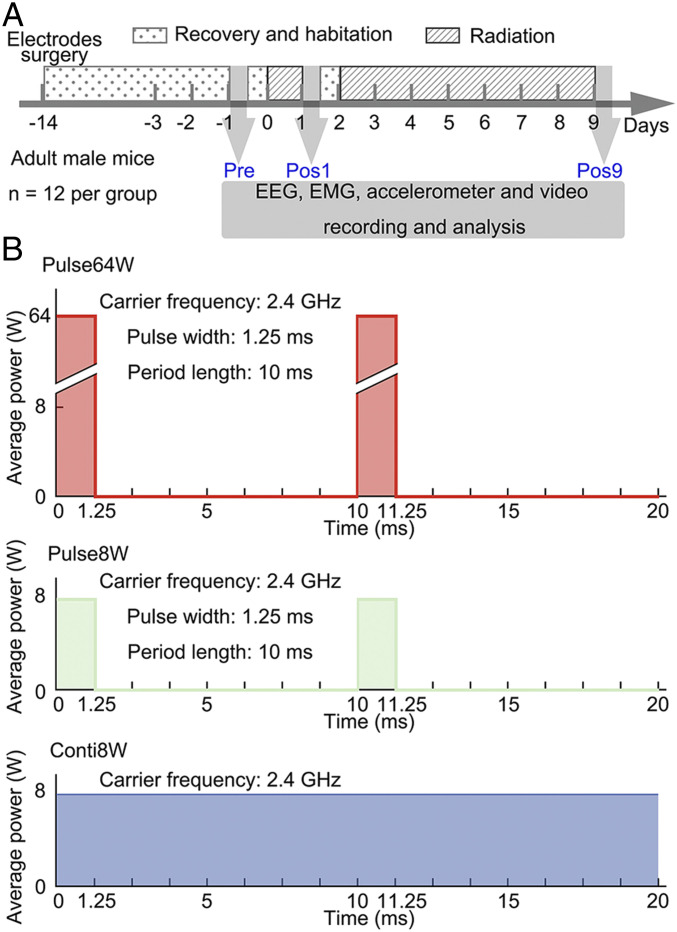
We designed a closed chamber with the EMR antennae on top and a mouse cage at the bottom (SI Appendix, Fig. S1 A and B). To record electroencephalogram (EEG), we planted four cranial electrodes in the skull of each mouse. To help identify the wakefulness phase of sleep, we planted two electrodes in the neck musculature for electromyography (EMG) and installed an accelerometer in the headstage of each mouse. In addition, we planted intracranial electrodes in three regions of the mouse brain: hippocampus, ventrolateral periaqueductal gray matter (vlPAG), and pedunculopontine tegmental nuclei (PPT). The surgery was performed 14 d ahead of data recording (day −14) to allow recovery and habitation. Three sets of the polysomnography, each lasting 12 h, were collected (Fig. 1A). The first recording begins on day −1 and serves as the reference (referred to as “Pre”). The second recording begins on day 1, immediately after 24 h of radiation (referred to as “Pos1”). The third recording begins on day 9, immediately after 7 d of radiation (referred to as “Pos9”).
Fig. 1.
Experimental design and radiation dosage. (A) Temporal design of the experiments. The electrodes are implanted in mice on day −14. Radiation is applied for 24 h on day 0 and continuously on days 2 through 9. With radiation off, the polysomnography is recorded in the light phase of day −1 (Pre), day 1 (Pos1), and day 9 (Pos9), each for 12 h. (B) Schematic diagram of three radiation regimens. With a carrier frequency of 2.4 GHz, EMR is given in three regimens: Pulse64W, 10-ms repeats, each with 1.25-ms radiation at an output power of 64 W (Top); Pulse8W, 10-ms repeats each with 1.25-ms radiation at an output power of 8 W (Middle); Conti8W, continuous radiation at an output power of 8 W (Bottom).
With a carrier frequency of 2.4 GHz, three distinct EMR regimens were employed: 100-Hz square modulation with a duty cycle of 1/8 and a maximal output of 64 W radiated through a horn antenna (referred to as Pulse64W); 100-Hz square modulation with a duty cycle of 1/8 and a maximal output of 8 W radiated through a horn antenna (referred to as Pulse8W); and continuous radiation with an output of 8 W radiated through a horn antenna (referred to as Conti8W) (Fig. 1B). The Pulse64W regimen has the same time-averaged output as that of Conti8W, whereas Pulse8W has 1/8 the total output as that of Conti8W or Pulse64W.
The experiments were performed on a cohort of four mice for each cycle. These four mice were simultaneously exposed to distinct EMR regimens: Pulse64W, Pulse8W, Conti8W, and no radiation (Control). In the end, we obtained valid data on 12 cohorts of 48 mice, with 12 mice exposed to each regimen.
The EMG, accelerometer, and EEG data were analyzed by the SleepSign software (37, 38) (SI Appendix, Fig. S2A). The results were visually validated and manually corrected using the same criteria for all data of the four experimental groups. Wakefulness, REM sleep, and NREM sleep each have their own distinct features in the EEG, EMG, and accelerometer recordings (SI Appendix, Fig. S2B). Fast Fourier transformation of a 4-s EEG epoch reveals distinct frequency features for the three phases of sleep architecture (SI Appendix, Fig. S2C). These data were processed to generate the total time of wakefulness, REM sleep, and NREM sleep for each mouse in each 12-h recording period. Double blindness between acquisition and processing of the data were strictly enforced; the individual who processed the data had no knowledge of the conditions of data acquisition.
Increased Wakefulness by Pulse64W Regimen.
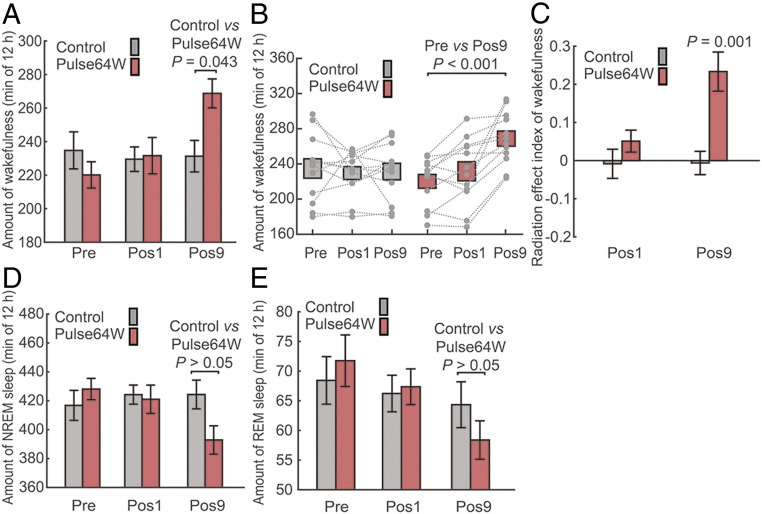
We first investigated potential effect of the Pulse64W regimen. The processed data at Pre, Pos1, and Pos9 were compared between the Control and the Pulse64W groups. Out of the 12-h sleep period, the average time of wakefulness for the Control group of 12 mice is 234.8 ± 11.1, 229.5 ± 7.3, and 231.3 ± 9.4 min, respectively, for the Pre, Pos1, and Pos9 recordings (Fig. 2 A and B). These values are close to each other. In contrast, the average time of wakefulness for the Pulse64W group displays a trend of marked increase: 220.1 ± 7.8 min for Pre, 231.6 ± 10.9 min for Pos1, and 268.8 ± 8.6 min for Pos9. Compared to the Control, the average time of wakefulness for the Pulse64W group is 16.2% more at Pos9, with a P value of 0.043 (Fig. 2A). The statistically significant increase at Pos9, but not at Pos1, suggests prolonged radiation as the key factor. A scatter plot for individual mouse confirms the increase of wakefulness time for the Pulse64W group (Fig. 2B). Within the Pulse64W group, the wakefulness time at Pos9 is 22.1% more than that at Pre, with a P value of less than 0.001.
Fig. 2.
The pulsed radiation of Pulse64W significantly increases the wakefulness time in mice. (A) The Pulse64W regimen markedly increases the wakefulness time at Pos9 in mice. Shown here is the average time of wakefulness for the Control (gray) and Pulse64W (salmon) groups at Pre, Pos1, and Pos9. The error bars throughout the manuscript are SEM. (B) A scatter plot of the wakefulness for individual mouse. Each dot represents the total time of wakefulness for one mouse, and the dotted line connects the data for the same mouse. (C) Evaluation of the change of wakefulness through analysis of the REI. (D) The Pulse64W regimen decreases the average time of NREM sleep at Pos9 in mice. (E) The Pulse64W regimen decreases the average time of REM sleep at Pos9 in mice. n = 12 per group.
Next, we developed another criteria for evaluation of the wakefulness change by defining the radiation effect index (REI) for the Pos1 and Pos9 data of the same mouse. For each mouse, REI at Pos1 or Pos9 is defined as the ratio of the difference of the total wakefulness time at Pos1 (TPos1) or Pos9 (TPos9) relative to that at Pre (TPre) over that at Pre. Therefore, REIPos1 = (TPos1 – TPre)/TPre; REIPos9 = (TPos9 – TPre)/TPre. An REI value of 0 indicates no change of wakefulness compared to the Pre data, and a value of 0.2 means 20% increase of wakefulness. For each group of 12 mice, the average REI value was calculated using the simple formula ∑REIi/12 (i = 1, 2, …, 12). The average REIPos1 and REIPos9 values for the Control group of 12 mice are −0.008 ± 0.038 and −0.006 ± 0.031, respectively (Fig. 2C). In contrast, the average REIPos1 and REIPos9 values for the Pulse64W group are 0.051 ± 0.029 and 0.234 ± 0.051, respectively. In particular, the REIPos9 value of 0.234 ± 0.051 indicates a statistically significant increase of wakefulness for the Pulse64W group at Pos9, with a P value of 0.001.
Decreased NREM and REM Sleep by Pulse64W Regimen.
Given the fixed 12-h period, increased wakefulness must be compensated by decreased NREM sleep and/or REM sleep. In contrast to the Control group that maintained a relatively steady average time of NREM sleep, the Pulse64W group at Pos9 exhibited 7.4% decrease of NREM sleep compared to the Control, but with a P value of greater than 0.05 (Fig. 2D). A scatter plot of the total time of NREM sleep for individual mouse confirms the decreasing trend from Pre to Pos9 within the Pulse64W group (SI Appendix, Fig. S3A). Notably, the NREM time at Pos9 is 8.2% less than that at Pre, with a P value of less than 0.001. The REI analysis reveals an average REIPos9 value of −0.081 ± 0.024 for the NREM sleep of the Pulse64W group (SI Appendix, Fig. S3B). With a P value of 0.006, this result indicates a statistically significant decrease of NREM sleep for the Pulse64W group at Pos9.
An analogous analysis on the REM sleep reveals a similar conclusion. Compared to the Control group, the Pulse64W group exhibits a 9.2% decrease of the REM sleep time, but with a P value of greater than 0.05 (Fig. 2E). In the scatter plot, however, the REM time at Pos9 is 18.6% less than that at Pre, with a P value of 0.003 (SI Appendix, Fig. S3C). The REI analysis reveals an average REIPos9 value of −0.170 ± 0.048 for the REM sleep of the Pulse64W group, with a P value of 0.004 (SI Appendix, Fig. S3D). This result indicates a statistically significant decrease of REM sleep for the Pulse64W group at Pos9.
Impact on Sleep Architecture by Pulse8W Regimen.
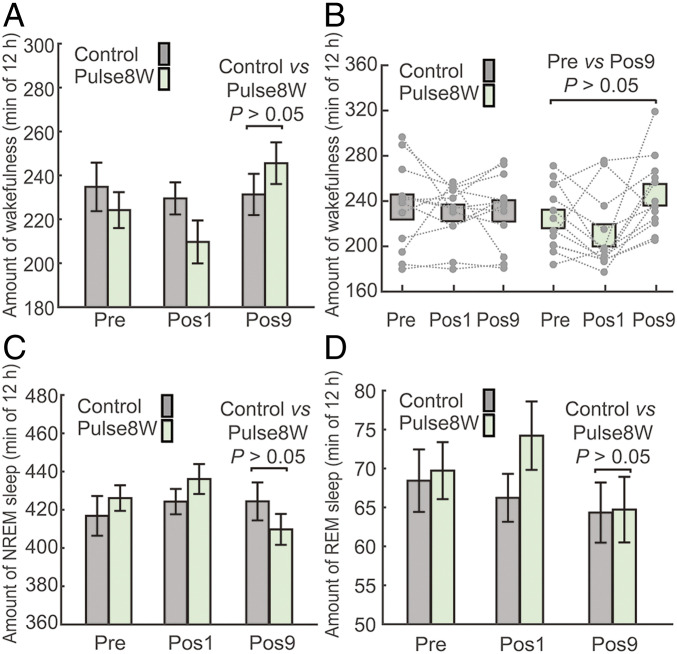
We then assessed the impact on sleep architecture by the Pulse8W treatment, which has one-eighth the radiation level compared to Pulse64W treatment. The average time of wakefulness for the Pulse8W group is 224.2 ± 8.2 min for Pre, 209.7 ± 9.8 min for Pos1, and 245.6 ± 9.5 min for Pos9 (Fig. 3A). Compared to the Control group, the average time of wakefulness for the Pulse8W group is 6.2% more at Pos9, with a P value of greater than 0.05. In the scatter plot, the wakefulness time at Pos9 is 9.5% more than that at Pre, with a P value of greater than 0.05 (Fig. 3B). Therefore, in neither case, the increased value of wakefulness is statistically significant. Next, we calculated the REI for the Pos1 and Pos9 data. Compared to the Pre data, the average REIPos1 and REIPos9 values for the Pulse8W group are −0.058 ± 0.043 and 0.103 ± 0.042, respectively (SI Appendix, Fig. S4C). In particular, the P value for the Pos9 over Pre data of the Pulse8W group is 0.031.
Fig. 3.
The pulsed radiation of Pulse8W results in statistically insignificant increase of wakefulness in mice. (A) The Pulse8W regimen results in modest increase of wakefulness at Pos9 in mice. Shown here is the average time of wakefulness at Pre, Pos1, and Pos9 for the Control (gray) and Pulse8W (light green) groups. (B) A scatter plot of the wakefulness for individual mouse. (C) The impact of the Pulse8W regimen on the NREM sleep. Shown here is the average time of NREM sleep at Pre, Pos1, and Pos9. (D) The impact of the Pulse8W regimen on the REM sleep. n = 12 per group.
These analyses identify a mild increase of wakefulness in mice by the Pulse8W regimen. However, the extent of increase is considerably smaller compared to that by the Pulse64W regimen. Such a modest increase of wakefulness at Pos9 is compensated by the 3.5% decrease of NREM sleep compared to Control, with a P value of greater than 0.05 (Fig. 3C). Notably, there is little change (0.6%) for the REM time at Pos9 between Pulse8W and Control (Fig. 3D). These results are confirmed by the scatter plots for individual mouse (SI Appendix, Fig. S4 A and B) and by the REI analysis (SI Appendix, Fig. S4 D and E). Compared to Pre, the Pulse8W group exhibits no statistically significant changes on NREM sleep or REM sleep at either Pos1 or Pos9 (SI Appendix, Fig. S4 A and B). The average REIPos1 and REIPos9 values carry no statistical significance (SI Appendix, Fig. S4 D and E).
Impact on Sleep Architecture by Conti8W Regimen.
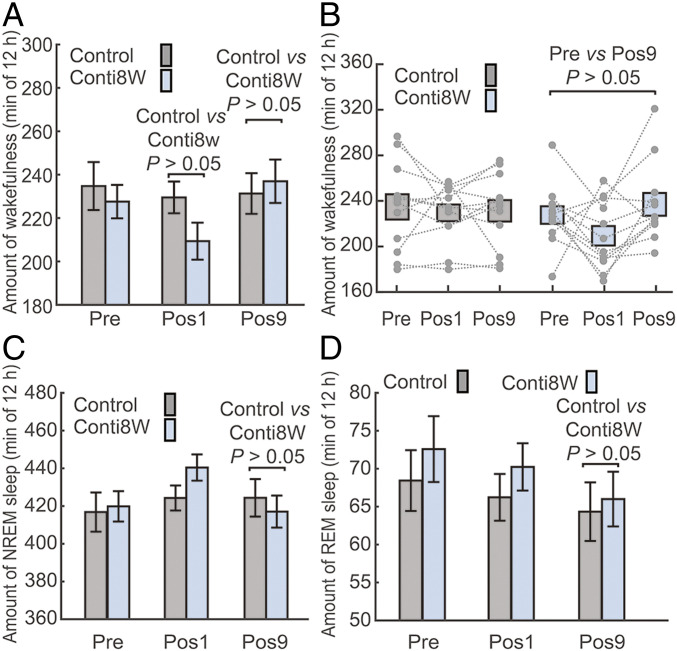
Our analyses thus far indicate that Pulse64W, and to a much lesser extent Pulse8W, affects the sleep architecture of mouse. Both Pulse64W and Pulse8W are pulse-modulated, although the latter has one-eighth the radiation level of the former. To examine the potential contribution by pulse modulation, we compared the data of the Conti8W treatment to that of Pulse64W. Conti8W and Pulse64W have the same time-averaged radiation level. The average time of wakefulness for the Conti8W group is 227.6 ± 7.7 min for Pre, 209.4 ± 8.5 min for Pos1, and 237.0 ± 10.0 min for Pos9 (Fig. 4 A and B). Compared to the Control, the wakefulness time for the Conti8W group is 8.8% less at Pos1 and 2.5% more at Pos9, both with P values of greater than 0.05 (Fig. 4A). Compared to Pre, the average REIPos1 and REIPos9 values for the Conti8W group are −0.064 ± 0.061 and 0.048 ± 0.042, respectively, both with P values of greater than 0.05 (SI Appendix, Fig. S5C).
Fig. 4.
Impact of the continuous radiation Conti8W on the sleep architecture of mice. (A) The Conti8W regimen shows no obvious impact on the wakefulness in mice. Shown here is the average time of wakefulness at Pre, Pos1, and Pos9 for the Control (gray) and Conti8W (light blue) groups. (B) A scatter plot of the wakefulness for individual mouse. (C) The Conti8W regimen has no obvious impact on the average time of NREM sleep. (D) The Conti8W regimen has no obvious impact on the average time for REM sleep at Pos9 in mice. n = 12 per group.
We also compared the NREM and REM data. The Conti8W group exhibits no statistically significant change in either NREM sleep (Fig. 4C) or REM sleep (Fig. 4D), compared to the Control group. Compared to Pre, the Conti8W group exhibits no statistically significant changes on NREM or REM sleep at either Pos1 or Pos9 (SI Appendix, Fig. S5 A and B). The average REIPos1 and REIPos9 values carry no statistical significance (SI Appendix, Fig. S5 D and E).
Confirmation of Increased Wakefulness by Pulse64W.
All above experiments were performed in mice with intracranial electrodes planted in specific sleep-related brain regions in addition to cranial electrodes in the skull. Although our simulation experiments indicate otherwise, there is a remote possibility that the local electric field generated by the intracranial electrodes may affect the sleep architecture of the mice. To scrutinize this possibility, we repeated the experiments on two groups of mice with only cranial electrodes: the Control without EMR (referred to as Control-R, R for repeat) and the EMR group with Pulse64W regimen (Pulse64W-R). Each group has 12 mice.
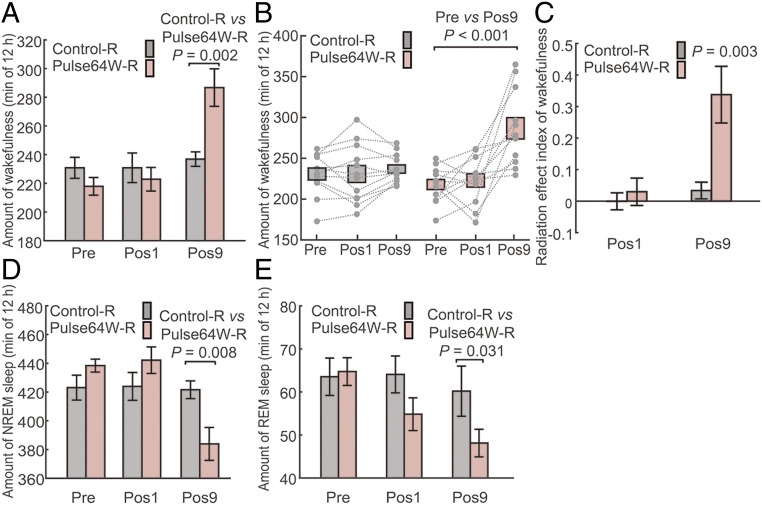
For the Control-R group, the average time of wakefulness is very similar for the three recording periods, Pre, Pos1, and Pos9 (Fig. 5A). In contrast, the average time of wakefulness for the Pulse64W-R group increased from 217.9 ± 6.2 min for Pre and 222.9 ± 8.2 min for Pos1, to 286.7 ± 13.1 min for Pos9. In particular, the average wakefulness of the Pulse64W-R group at Pos9 is 21.0% more than that of the Control-R group, with a P value of 0.002. This result qualitatively agrees with the conclusion associated with the Pulse64W group, except that the extent of wakefulness increase for the Pulse64W-R group is even greater. This result is also clearly shown in the scatter plot (Fig. 5B). Within the Pulse64W-R group, the average wakefulness at Pos9 is 31.6% more than that at Pre, with a P value of less than 0.001.
Fig. 5.
Confirmation of increased wakefulness in mice induced by the pulsed radiation of Pulse64W. (A) The Pulse64W regimen markedly increases wakefulness at Pos9 in mice of the Pulse64W-R group (light salmon) compared to the Control-R group (gray). The Pulse64W-R group of 12 mice and the Control-R group of 12 mice only have cranial electrodes in their skulls. (B) A scatter plot of the wakefulness for individual mouse. (C) Evaluation of the change of wakefulness through analysis of the REI. (D) The Pulse64W regimen results in a marked decrease of the NREM sleep at Pos9 in the Pulse64W-R group. (E) The Pulse64W regimen results in a marked decrease of the REM sleep at Pos9 in the Pulse64W-R group. n = 12 per group.
The REIPos9 value for the Pulse64W-R group is 0.338 ± 0.090 with a P value of 0.003 (Fig. 5C), again confirming the increased wakefulness at Pos9. Similar to prior findings, the increased wakefulness is compensated by corresponding decreased NREM sleep (Fig. 5D) and REM sleep (Fig. 5E). Compared to the Control-R group, the NREM sleep and REM sleep for the Pulse64W-R group are decreased by 8.9% and 19.8% at Pos9, respectively, with P values of 0.008 and 0.031. Within the Pulse64W-R group, the NREM sleep and REM sleep at Pos9 are decreased by 12.4% and 22.6% compared to that at Pre, with P values of less than 0.001 and 0.005, respectively (SI Appendix, Fig. S6 A and C). The decrease of NREM sleep and REM sleep is confirmed by the REI analysis (SI Appendix, Fig. S6 B and D).
A direct comparison between the Pulse64W and Pulse64W-R groups reveals a highly similar pattern of changes in sleep architecture, as revealed by the REI analysis (SI Appendix, Fig. S7). The REIPos9 values for the wakefulness changes of the Pulse64W and Pulse64W-R groups are 0.234 and 0.338, respectively (SI Appendix, Fig. S7A). This is compensated by corresponding decrease of the NREM sleep (SI Appendix, Fig. S7B) and REM sleep (SI Appendix, Fig. S7C). These results further indicate that the implanted intracranial electrodes have little impact on the observed increase of wakefulness due to the Pulse64W regimen. Therefore, with the caveat of the intracranial electrodes, the observed alteration of sleep architecture is a direct result of the EMR on mice.
Discussion
Prolonged radiation of mice (Pos9) using the Pulse64W regimen, but not the Conti8W regimen, results in statistically significant increase of wakefulness (Figs. 2, 4, and5). Notably, these two regimens have the same time-averaged radiation level over the 12-h sleep period, suggesting a key role for pulse modulation. The increase at Pos9 is estimated to be about 16.2% compared to the Control (Fig. 2A) and 23.4% compared to Pre (Fig. 2B). Consistent with our conclusion, 1-mo exposure (1 h/d) to modulated 900-MHz EMR, but not the unmodulated EMR, was found to affect the sleep power spectra of Wistar rats (39). Exposure of Wistar rats to unmodulated 900-MHz EMR for several weeks affected the sleep macrostructure marginally (40, 41), although the EMR intensity is less than that of our Conti8W regimen.
On the other hand, the Pulse8W regimen, with one-eighth the radiation level of Pulse64W, only induced insignificant increase of wakefulness compared to the Control (Fig. 3). A side-by-side comparison between the Pulse64W and Pulse8W groups illustrates a similar trend but distinct consequences on sleep architecture (SI Appendix, Fig. S8). In contrast, the comparison between the Pulse64W and Conti8W groups reveals little similarity (SI Appendix, Fig. S9).
For both Pulse64W and Pulse64W-R groups, the increase of wakefulness is insignificant at Pos1 but becomes quite prominent at Pos9 (Figs. 2 and 5). The decrease of NREM sleep follows a similar pattern (SI Appendix, Figs. S3 and S6). For Pulse8W group, however, the increase of wakefulness is insignificant at both Pos1 and Pos9 (Fig. 3). Our observed dose-dependent effects of EMR on mouse sleep are consistent with a previous study, which identified a dose-dependent relationship between pulsed EMR field intensity and NREM sleep EEG for human (31).
A distinct implication of this study is the possibility that prolonged exposure to modulated 2.4-GHz radiation, such as the wireless signal, might also increase wakefulness for humans. However, assessing this possibility may take extraordinary effort because of the complex issues for human volunteers. Consistent with our conclusion on the Conti8W group, previous studies found no significant effect on human sleep by unmodulated radiation (33, 36). Whole-night exposure to Wi-Fi was found to affect the sleep microstructure of humans but had no significant acute effects on the sleep macrostructure (42). This result is consistent with prior studies on the effect of mobile phone and base station radiation on human sleep (24, 28, 33). Another study on human subjects found no significant effects of 900-MHz EMR with global system for mobile communications modulation, either on conventional sleep parameters or on power spectra (30).
A relatively small sample size of 12 mice per group was used in this study. However, this should not be a concern for the conclusion because statistical analysis takes into account the sample size. In fact, a decreased sample size is unfavorable for reaching a conclusion as it tends to make the conclusion less convincing. This may be the case for the Pulse8W group, which shows moderate, but statistically insignificant, increase of wakefulness for the Pos9 data. With a larger sample size, the increase at Pos9 may become statistically significant for Pulse8W.
One-way ANOVA analysis of the baseline (Pre) data for the time of wakefulness, NREM, and REM reveals no significant differences among the Control, Pulse64W, Pulse8W, and Conti8W groups (in all cases, P > 0.05). To examine whether the circadian rhythm of mice is normal before radiation, we combined the baseline data of all 48 mice. The results of the average time spent in wakefulness, NREM sleep, and REM sleep per hour at Pre-2, Pre-1, and Pre (days −3, −2, and −1) confirmed the normal sleep behavior of mice (SI Appendix, Fig. S10).
The implanted intracranial electrodes in hippocampus, vlPAG, and PPT allow investigation of possible neural mechanisms of EMR effect on sleep. Analysis of the average normalized local field potential power density during wakefulness, NREM sleep, and REM sleep reveals no significant differences among the Pre, Pos1, and Pos9 data (SI Appendix, Figs. S11–S13). In this study, we focus on the effect of EMR on mouse sleep architecture in the light period. In the future, we plan to extend such effort into the dark period and scrutinize other parameters that influence the sleep/wake cycle.
In a collective exposure scenario, the average power density at close proximity is about 0.037 W/m2 for a smartphone, 0.013 W/m2 for a laptop, and 0.13 W/m2 near the Wi-Fi router (1). These values are considerably lower than the time- and whole-body–averaged general public exposure limit of 10 W/m2 or occupational exposure limit of 50 W/m2 for 2–300 GHz suggested by International Commission on Non-Ionizing Radiation Protection (43). In our experiments, the measured spatial averaged power density for Conti8W is 36.80 ± 0.92 W/m2. Pulse64W is expected to have the same power density. Importantly, the effective EMR dose for inducing a biological response in mice is likely to be different from that in humans. Therefore, the relatively high EMR dose of the Pulse64W regimen that causes increased wakefulness in mice could be markedly reduced in humans. An epidemiological survey among those who work under either very high or very low doses of wireless radiation may reveal some clues.
In this study, 2.4-GHz EMR is modulated by 100-Hz square pulses, which have sharp edges and thus might have some unanticipated impact on neural activity in the brain. Additional experiments should be performed to examine whether other modulation functions such as sinusoidal modulation can induce similar increase of wakefulness in mice. In addition, other modulation frequencies such as 10 and 1,000 Hz should be investigated to answer the question of whether increased wakefulness is specific to certain modulation frequencies. Finally, both the intensity and the frequency of the carrier EMR (2.4 GHz in this study) should be scrutinized.
Materials and Methods
All methods are detailed in SI Appendix and briefly described here.
Radiation Equipment Setup.
A MXG Vector signal generator together with an amplifier were used to generate 2.4-GHz EMR of three distinct patterns: Pulse64W, Pulse8W, and Conti8W. The signal was emitted through a horn antenna. Absorbing materials were used in the animal container to drastically reduce reflection.
Polysomnographic Recording and Analysis.
EEG and EMG signals were collected using a digital headstage. The data acquisition system receives the digital signal at a sample rate of 1,000 Hz. All data were amplified and filtered. All video signals were monitored as an auxiliary method. Using the sleep analysis software, we analyzed the filtered EEG data (bandpass, 0.5–100 Hz) using fast Fourier transformation. The spectral signatures of EEG, EMG, and acceleration signals were used to score brain states into wakefulness, NREM, and REM for each 4-s epoch (38).
Information of animal, electrode implantation, and statistical analysis are described in SI Appendix.
Supplementary Material
Acknowledgments
This work was funded by the National Key R&D Program (2020YFA0509300) from the Ministry of Science and Technology of China, the National Natural Science Foundation of China (Projects 31930059 and 81920108015), and the Key R&D Program of Zhejiang Province (2020C04001), Beijing Advanced Innovation Center for Structural Biology and Frontier Research Center for Biological Structure, and startup funds from Westlake University.
Footnotes
The authors declare no competing interest.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2105838118/-/DCSupplemental.
Data Availability
All study data are included in the article and/or supporting information.
References
- 1.Pereda D. G., Fernandez Andres M., Pena Valverde I., Gil Abaunza U., Arrinda Sanzberro A., “Time variability of electromagnetic exposure due to Wi Fi networks” in 2019 International Conference on Electromagnetics in Advanced Applications (ICEAA) (IEEE, 2019), pp. 1199–1202.
- 2.Alsop T., WLAN connected devices worldwide 2016–2021. https://www.statista.com/statistics/802706/world-wlan-connected-device/. Accessed 29 December 2020.
- 3.Kocevska D., et al., Sleep characteristics across the lifespan in 1.1 million people from the Netherlands, United Kingdom and United States: A systematic review and meta-analysis. Nat. Hum. Behav. 5, 113–122 (2021). [DOI] [PubMed] [Google Scholar]
- 4.Vander Borght M., Wyns C., Fertility and infertility: Definition and epidemiology. Clin. Biochem. 62, 2–10 (2018). [DOI] [PubMed] [Google Scholar]
- 5.Baxter A. J., Scott K. M., Vos T., Whiteford H. A., Global prevalence of anxiety disorders: A systematic review and meta-regression. Psychol. Med. 43, 897–910 (2013). [DOI] [PubMed] [Google Scholar]
- 6.Torre L. A., Siegel R. L., Ward E. M., Jemal A., Global cancer incidence and mortality rates and trends—an update. Cancer Epidemiol. Biomarkers Prev. 25, 16–27 (2015). [DOI] [PubMed] [Google Scholar]
- 7.Baan R.et al.; WHO International Agency for Research on Cancer Monograph Working Group , Carcinogenicity of radiofrequency electromagnetic fields. Lancet Oncol. 12, 624–626 (2011). [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization , Fact sheet no. 193 on electromagnetic fields and public health: Mobile phones. https://www.who.int/news-room/fact-sheets/detail/electromagnetic-fields-and-public-health-mobile-phones. Accessed 27 April 2020.
- 9.Danker-Hopfe H., et al., Effects of mobile phone exposure (GSM 900 and WCDMA/UMTS) on polysomnography based sleep quality: An intra- and inter-individual perspective. Environ. Res. 145, 50–60 (2016). [DOI] [PubMed] [Google Scholar]
- 10.Shahin S., Banerjee S., Swarup V., Singh S. P., Chaturvedi C. M., From the cover: 2.45-GHz microwave radiation impairs hippocampal learning and spatial memory: Involvement of local stress mechanism-induced suppression of iGluR/ERK/CREB signaling. Toxicol. Sci. 161, 349–374 (2018). [DOI] [PubMed] [Google Scholar]
- 11.Gupta S. K., et al., Long-term exposure of 2450 MHz electromagnetic radiation induces stress and anxiety like behavior in rats. Neurochem. Int. 128, 1–13 (2019). [DOI] [PubMed] [Google Scholar]
- 12.Bogers R. P., et al., Individual variation in temporal relationships between exposure to radiofrequency electromagnetic fields and non-specific physical symptoms: A new approach in studying “electrosensitivity.” Environ. Int. 121, 297–307 (2018). [DOI] [PubMed] [Google Scholar]
- 13.Michael Wyde M. C., et al., Report of partial findings from the National Toxicology Program carcinogenesis studies of cell phone radiofrequency radiation in Hsd: Sprague Dawley SD rats (whole body exposures). bioRxiv [Preprint] (2016). 10.1101/055699 (Accessed 28 April 2020). [DOI]
- 14.Falcioni L., et al., Report of final results regarding brain and heart tumors in Sprague-Dawley rats exposed from prenatal life until natural death to mobile phone radiofrequency field representative of a 1.8 GHz GSM base station environmental emission. Environ. Res. 165, 496–503 (2018). [DOI] [PubMed] [Google Scholar]
- 15.Smith-Roe S. L., et al., Evaluation of the genotoxicity of cell phone radiofrequency radiation in male and female rats and mice following subchronic exposure. Environ. Mol. Mutagen. 61, 276–290 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Killgore W. D., Effects of sleep deprivation on cognition. Prog. Brain Res. 185, 105–129 (2010). [DOI] [PubMed] [Google Scholar]
- 17.McCoy J. G., Strecker R. E., The cognitive cost of sleep lost. Neurobiol. Learn. Mem. 96, 564–582 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weber F., Dan Y., Circuit-based interrogation of sleep control. Nature 538, 51–59 (2016). [DOI] [PubMed] [Google Scholar]
- 19.Van Someren E. J., et al., Disrupted sleep: From molecules to cognition. J. Neurosci. 35, 13889–13895 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu D., Dan Y., A motor theory of sleep-wake control: Arousal-action circuit. Annu. Rev. Neurosci. 42, 27–46 (2019). [DOI] [PubMed] [Google Scholar]
- 21.Mann K., Röschke J., Effects of pulsed high-frequency electromagnetic fields on human sleep. Neuropsychobiology 33, 41–47 (1996). [DOI] [PubMed] [Google Scholar]
- 22.Wagner P., et al., Human sleep EEG under the influence of pulsed radio frequency electromagnetic fields. Results from polysomnographies using submaximal high power flux densities. Neuropsychobiology 42, 207–212 (2000). [DOI] [PubMed] [Google Scholar]
- 23.Huber R., et al., Exposure to pulsed high-frequency electromagnetic field during waking affects human sleep EEG. Neuroreport 11, 3321–3325 (2000). [DOI] [PubMed] [Google Scholar]
- 24.Borbély A. A., et al., Pulsed high-frequency electromagnetic field affects human sleep and sleep electroencephalogram. Neurosci. Lett. 275, 207–210 (1999). [DOI] [PubMed] [Google Scholar]
- 25.Wallace J., Selmaoui B., Effect of mobile phone radiofrequency signal on the alpha rhythm of human waking EEG: A review. Environ. Res. 175, 274–286 (2019). [DOI] [PubMed] [Google Scholar]
- 26.Lowden A., et al., Sleep after mobile phone exposure in subjects with mobile phone-related symptoms. Bioelectromagnetics 32, 4–14 (2011). [DOI] [PubMed] [Google Scholar]
- 27.Scientific Committee on Emerging Newly Identified Health Risks , Opinion on potential health effects of exposure to electromagnetic fields. Bioelectromagnetics 36, 480–484 (2015).26179386 [Google Scholar]
- 28.Schmid M. R., et al., Sleep EEG alterations: Effects of different pulse-modulated radio frequency electromagnetic fields. J. Sleep Res. 21, 50–58 (2012). [DOI] [PubMed] [Google Scholar]
- 29.Lustenberger C., et al., Stimulation of the brain with radiofrequency electromagnetic field pulses affects sleep-dependent performance improvement. Brain Stimul. 6, 805–811 (2013). [DOI] [PubMed] [Google Scholar]
- 30.Fritzer G., et al., Effects of short- and long-term pulsed radiofrequency electromagnetic fields on night sleep and cognitive functions in healthy subjects. Bioelectromagnetics 28, 316–325 (2007). [DOI] [PubMed] [Google Scholar]
- 31.Regel S. J., et al., Pulsed radio-frequency electromagnetic fields: Dose-dependent effects on sleep, the sleep EEG and cognitive performance. J. Sleep Res. 16, 253–258 (2007). [DOI] [PubMed] [Google Scholar]
- 32.Ohayon M. M., Stolc V., Freund F. T., Milesi C., Sullivan S. S., The potential for impact of man-made super low and extremely low frequency electromagnetic fields on sleep. Sleep Med. Rev. 47, 28–38 (2019). [DOI] [PubMed] [Google Scholar]
- 33.Huber R., et al., Electromagnetic fields, such as those from mobile phones, alter regional cerebral blood flow and sleep and waking EEG. J. Sleep Res. 11, 289–295 (2002). [DOI] [PubMed] [Google Scholar]
- 34.Regel S. J., et al., Pulsed radio frequency radiation affects cognitive performance and the waking electroencephalogram. Neuroreport 18, 803–807 (2007). [DOI] [PubMed] [Google Scholar]
- 35.Perentos N., Croft R. J., McKenzie R. J., Cosic I., The alpha band of the resting electroencephalogram under pulsed and continuous radio frequency exposures. IEEE Trans. Biomed. Eng. 60, 1702–1710 (2013). [DOI] [PubMed] [Google Scholar]
- 36.Perentos N., Croft R. J., McKenzie R. J., Cvetkovic D., Cosic I., Comparison of the effects of continuous and pulsed mobile phone like RF exposure on the human EEG. Australas. Phys. Eng. Sci. Med. 30, 274–280 (2007). [DOI] [PubMed] [Google Scholar]
- 37.Kohtoh S., et al., Algorithm for sleep scoring in experimental animals based on fast Fourier transform power spectrum analysis of the electroencephalogram. Sleep Biol. Rhythms 6, 163–171 (2008). [Google Scholar]
- 38.Ren S., et al., The paraventricular thalamus is a critical thalamic area for wakefulness. Science 362, 429–434 (2018). [DOI] [PubMed] [Google Scholar]
- 39.Mohammed H. S., Fahmy H. M., Radwan N. M., Elsayed A. A., Non-thermal continuous and modulated electromagnetic radiation fields effects on sleep EEG of rats. J. Adv. Res. 4, 181–187 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pelletier A., et al., Effects of chronic exposure to radiofrequency electromagnetic fields on energy balance in developing rats. Environ. Sci. Pollut. Res. Int. 20, 2735–2746 (2013). [DOI] [PubMed] [Google Scholar]
- 41.de Jenlis A. B., Del Vecchio F., Delanaud S., Bach V., Pelletier A., Effects of co-exposure to 900 MHz radiofrequency electromagnetic fields and high-level noise on sleep, weight, and food intake parameters in juvenile rats. Environ. Pollut. 256, 113461 (2020). [DOI] [PubMed] [Google Scholar]
- 42.Danker-Hopfe H., et al., Spending the night next to a router—results from the first human experimental study investigating the impact of Wi-Fi exposure on sleep. Int. J. Hyg. Environ. Health 228, 113550 (2020). [DOI] [PubMed] [Google Scholar]
- 43.International Commission on Non-Ionizing Radiation Protection , Guidelines for limiting exposure to electromagnetic fields (100 kHz to 300 GHz). Health Phys. 118, 483–524 (2020). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and/or supporting information.