Abstract
Background
Transcatheter aortic valve replacement (TAVR) has become the standard of care in the majority of patients with symptomatic severe aortic stenosis. Data on long-term mortality and durability of transcatheter heart valves (THVs) beyond 5 years are limited. Our study aimed to assess elderly and high-risk patients’ long-term outcomes treated with TAVR in a prospective single-centre registry focusing on the durability of THVs.
Methods
We included 795 patients with severe calcific aortic stenosis treated by transfemoral TAVR between 2006 and 2011. Echocardiography was performed at baseline; discharge; 1 year; and afterward, annually, until the longest available follow-up. Mortality rates were estimated for 1, 5, 6, 7, and 8 years. The rates of structural valve deterioration (SVD) and bioprosthetic valve failure (BVF) were assessed in accordance with consensus definitions. Outcome measures were adjudicated according to Valve Academic Research Consortium-2 (VARC-2).
Results
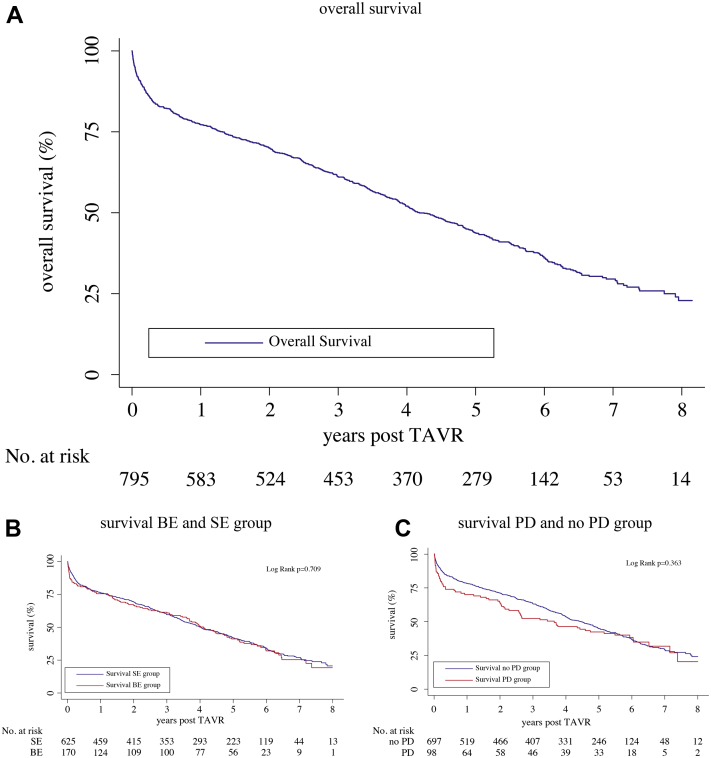
Median (interquartile range) follow-up time was 1345 (316; 2015) days. One-year, 5-year, 6-year, 7-year, and 8-year overall mortality was 25.4%, 59.0%, 64.6%, 67.9%, and 69.2%, respectively. At 8 years, no significant differences in mortality were found comparing self-expanding vs balloon-expandable valves (69.5% vs 68.0%, P = 0.709) and postdilatation (PD) vs no-PD (69.4% vs 69.2%, P = 0.363). SVD was detected in 26 patients (3.3%), and 19 (2.4%) of the 795 patients had evidence of BVF during follow-up.
Conclusions
Our study demonstrates good long-term results for high-risk patients who were alive up to 8 years after TAVR.
Résumé
Contexte
Le remplacement valvulaire aortique par cathéter (RVAC) est maintenant la norme de soins dans la majorité des cas de sténose de l’aorte grave symptomatique. On dispose de peu de données sur la mortalité à long terme et sur la durabilité des valves cardiaques transcathéter (VCT) au-delà de 5 ans. Nous avons donc évalué les résultats à long terme, notamment la durabilité des VCT, chez les patients âgés et les patients exposés à un risque élevé traités par RVAC figurant dans un registre prospectif unicentrique.
Méthodologie
Notre étude comprenait 795 patients présentant une sténose calcifiée de l’aorte grave traitée par RVAC transfémoral entre 2006 et 2011. Une échocardiographie a été réalisée au départ, à la sortie de l’hôpital, 1 an plus tard et tous les ans par la suite, jusqu’au suivi le plus long disponible. Les taux de mortalité à 1, 5, 6, 7 et 8 ans ont été estimés. Les taux de détérioration structurelle des valves (DSV) et de défaillance de la bioprothèse valvulaire (DBV) ont été évalués selon des définitions établies par consensus. Les mesures des résultats ont été confirmées selon les critères du VARC-2 (Valve Academic Research Consortium-2).
Résultats
La durée médiane (intervalle interquartile) du suivi était de 1 345 (316 à 2 015) jours. Les taux de mortalité globaux s’établissaient comme suit : taux à 1 an : 25,4 %; à 5 ans : 59,0 %; à 6 ans : 64,6 %; à 7 ans : 67,9 % et à 8 ans : 69,2 %. À 8 ans, on n’a noté aucune différence significative en ce qui concerne la mortalité chez les patients ayant reçu une valve auto-expansible comparativement à ceux ayant reçu une valve expansible par ballonnet (69,5 % vs 68,0 %; p = 0,709) et chez ceux ayant subi une post-dilatation ou non (69,4 % vs 69,2 %; p = 0,363). Une DSV a été détectée chez 26 patients (3,3 %), et 19 (2,4 %) des 795 patients ont présenté des signes de DBV pendant la période de suivi.
Conclusions
Notre étude a révélé de bons résultats à long terme chez les patients présentant un risque élevé qui étaient toujours en vie 8 ans après le RVAC.
In the first years, transcatheter aortic valve replacement (TAVR) has been a treatment option for patients with symptomatic severe aortic stenosis who were at high risk or prohibitive risk for surgical aortic valve replacement (SAVR). Meanwhile, TAVR has become the standard of care in patients across all risk categories.1, 2, 3 Since the introduction of the TAVR concept in 2002, experience and techniques have constantly improved over the last years.4,5 In addition, the design of newer generation transcatheter heart valves (THVs) has addressed issues of their predecessors: special sealing mechanisms to reduce residual perivalvular aortic regurgitation (PAR). As a result, complication rates decreased, survival rates increased, and there is a noticeable shift toward the treatment of younger and lower-risk patients.6 Self-expanding (SE) and balloon-expandable (BE) valves are available for patient treatment. Analyzing outcomes of 12,381 patients from 10 registries and clinical trials (the CENTER collaboration), lower rates for pacemaker (PM) implantation and stroke were found in patients treated with BE compared with SE valves. Patients treated with new-generation SE valves less likely suffered from major or life-threatening bleedings compared with patients with new-generation BE valves. Overall, 30-day mortality was not significantly different across valve types.7 In a recent randomized controlled trial (RCT), the primary composite endpoint (all-cause mortality, moderate to severe prosthetic valve regurgitation, stroke, and permanent pacemaker implantation) at 30 days was not significantly different comparing SE and BE valves.8 However, there are concerns about valve longevity in both valve types, especially if TAVR treatment is considered in younger patients. Data on long-term durability of THVs are limited.9, 10, 11
To assess short- and long-term valve performance in a standardized way, the European Association of Percutaneous Cardiovascular Interventions (EAPCI), the European Society of Cardiology (ESC), and the European Association for Cardio-Thoracic Surgery (EACTS) published standardized definitions for valve durability outcomes. In addition to the definition of structural valve deterioration (SVD), the consortium defined a new clinical endpoint—bioprosthetic valve failure (BVF)—to assess the clinical relevance of SVD. SVD is a valve dysfunction caused by intrinsic changes of the prosthesis (ie, calcification, flail), resulting in aortic regurgitation (AR) or stenosis. Hemodynamic SVD is defined by transprosthetic gradients and intraprosthetic AR. Morphological SVD can be caused by leaflet structure or leaflet-function abnormalities or strut/frame abnormalities. BVF is the clinical correlate that might be caused by SVD but also by nonstructural valve deterioration (ie, patient-prosthesis mismatch, late embolization), thrombosis, and endocarditis.12
PAR after TAVR is a known risk factor for a worse outcome.13 Five-year mortality in the Placement of Aortic Transcatheter Valves-2 (PARTNER-2) trial was higher in patients with moderate to severe PAR after TAVR.14 However, even mild PAR after TAVR is associated with a poorer patient outcome. Laakso et al. report a rate of mild PAR in 21.7% of TAVR patients. Compared with patients with none-to-trace PAR, patients with mild PAR had significantly higher mortality at 4 years. A significantly lower rate of PAR was found in new-generation THVs.15
A postdilatation (PD) is frequently performed to reduce PAR, but there are concerns regarding earlier valve degeneration caused by PD. In the PARTNER-2 SAPIEN 3 (Edwards Lifesciences, Irvine, California) registry, 208 of 1661 patients had undergone PD. PD was more likely performed in patients with lower oversizing and a greater calcium burden. However, the 1-year outcomes regarding death, rehospitalization, or stroke were not different between patients with or without PD.16 Little is known about the long-term impact of PD.
Our study aimed to assess the long-term outcomes of elderly and high-risk TAVR patients in a prospective single-centre registry as well as the durability of THVs. The impact of PD on the outcome was determined.
Methods
Patient selection and study design
Between January 2006 and December 2011, 795 consecutive patients with symptomatic severe native aortic stenosis treated by transfemoral TAVR were included in this analysis. According to the current definitions for device success, a lower success rate of valve-in-valve procedures is anticipated.17,18 Thus, patients with failed bioprostheses in aortic position were excluded from the analysis. Patients were considered to be at high risk for surgery based on heart-team decisions. This heart team included cardiologists, cardiovascular surgeons, and cardiovascular anesthesiologists who made decisions based on a quantitative assessment of the expected operative mortality using the Logistic European System for Cardiac Operative Risk Evaluation (logEuroSCORE) and the Society of Thoracic Surgeons score (STS score).19 Other factors considered for the heart team decision were frailty, porcelain aorta, hostile chest, and severe liver disease. Mortality rates were assessed at 1 year, 5 years, 6 years, 7 years, and 8 years. Telephone visits were scheduled for patients who were not able to visit our centre. Echocardiography was performed at our centre at baseline, discharge, 1 year, and afterward annually until the longest available follow-up. The rates of SVD and BVF were assessed in accordance with the 2017 published consensus definitions.12 In short, definitions for hemodynamic SVD are moderate SVD (mean gradient ≥ 20 mm Hg and ≤ 40 mm Hg, mean gradient ≥ 10 and ≤ 20 mm Hg change from baseline, moderate valvular AR, or change of AR ≥ 1 grade from baseline); severe SVD (mean gradient ≥ (40 mm Hg, mean gradient ≥ 020 mm Hg change from baseline, severe valvular AR, or change of AR ≥ 02 grades from baseline).
A BVF occurring up to 30 days after the index procedure is an early BVF. BVFs occurring later than 30 days are defined as late BVFs. Our echocardiographic reports and films were reviewed again to be in line with consensus definitions. Device success and other outcome measures are fully adjudicated according to the Valve Academic Research Consortium-2 (VARC-2) criteria.17
TAVR procedures
All patients who were considered for treatment with TAVR underwent systematic assessment, including transthoracic and transesophageal echocardiography, coronary angiography, right-heart catheterization, computed tomography (CT) scan, pulmonary function testing, carotid artery ultrasonography, and frailty assessment.
The TAVR procedure was performed via transfemoral access using the Medtronic CoreValve system (MCV; Medtronic, Minneapolis, Minnesota) and the Edwards SAPIEN valve system (ES) after predilation of the native aortic valve in the majority of cases. Valve sizes used were 26, 29, 31 for MCV, and 23, 26 for ES. Size 29 was used in most of the MCV cases (57.1%), and size 26 in most of the ES cases (66.9%). Further details and correlating annulus sizes are shown in Supplemental Table S5. Two ProGlides (Abbott Vascular, Santa Clara, CA) and 1 AngioSeal (6F or 8F; St. Jude Medical, St. Paul, MN) were used for femoral access-site closure in nearly all cases. All procedures were performed under conscious sedation, guided by fluoroscopy. Only in cases of emergency “rescue” have surgery patients been under general anesthesia. All patients received perioperative antibiotic prophylaxis, using cefazolin, in most of the cases.
Procedural complications and successes, need for blood transfusions, pacemaker implantations, hospital stay, and renal replacement therapy were recorded.
Statistical analysis
Continuous parameters are reported as mean ± standard deviation (SD) and were compared using Student’s t-test, if normally distributed. Continuous variables not normally distributed are reported as median and interquartile ranges (IQRs) and were compared using the Mann–Whitney test. Categorical variables are presented as numbers and percentages of the total and were compared by χ2 or Fisher’s exact test, as indicated.
The 1-, 5-, 6-, 7-, and 8-year mortality rates were analyzed according to the method of Kaplan-Meier, and group comparisons were made applying the log-rank test.
Cox multivariate regression analysis with stepwise forward selection was performed to adjust for significant differences in baseline and procedural characteristics. Clinically relevant variables with P < 0.1 in univariate analysis were included in the model.
Results were considered significant for 2-sided P values < 0.05. All analyses were performed using SPSS software (version 25.0; IBM, SPSS Software, Chicago, IL).
Results
Baseline and procedural characteristics
Between January 2006 and December 2011, 795 consecutive patients were treated with transfemoral TAVR at our institution. Patient demographics and baseline characteristics are provided in Table 1. The characteristics of the patient subgroup analysis (BE compared with SE valves and PD vs no PD) are provided in the supplementary material (Supplemental Tables S1 and S2).
Table 1.
Baseline characteristics of the study population
| Characteristics | All patients n = 795 |
|---|---|
| Age (years) | 81.0 (77.0; 85.0) |
| Male sex (%) | 330 (41.5%) |
| Body mass index (kg/m2) | 27.3 (24.1; 30.9) |
| Logistic EuroSCORE | 18.5 (12.8; 29.0) |
| STS score risk of mortality | 8.1 (5.4; 12.3) |
| eGFR (mL/min per 1.73 m2) | 53 (39; 69) |
| NYHA class, number (%) | |
| II | 124 (15.6%) |
| III | 494 (62.1%) |
| IV | 132 (16.6%) |
| Diabetes mellitus , number (%) | 345 (43.4%) |
| Hypertension, number (%) | 717 (90.2%) |
| Coronary artery disease, number (%) | 368 (46.3%) |
| Previous MI, number (%) | 98 (12.3%) |
| Previous stroke, number (%) | 63 (7.9%) |
| Peripheral artery disease, number (%) | 90 (11.3%) |
| COPD, number (%) | 289 (36.4%) |
| Atrial fibrillation, number (%) | 274 (34.5%) |
| Previous CABG, number (%) | 117 (14.7%) |
| Previous PCI, number (%) | 182 (22.9%) |
| Grip strength | 18.0 (13.0; 24.5) |
| Gait speed | 7.0 (6.0; 9.0) |
| Echocardiographic findings | |
| LVEF < 55%, number (%) | 314 (39.5%) |
| Peak velocity baseline, m/s | 4.2 (3.7; 4.7) |
| Maximum gradient, mm Hg | 71 (56;88) |
| Mean gradient, mm Hg | 44 (35;56) |
| AVA, cm2 | 0.6 (0.5;0.8) |
| Systolic PAP > 60 mm Hg, number (%) | 291 (36.6%) |
| MR ≥ 2 - no. (%) | 89 (11.2%) |
All values are given in median (interquartile range) or number (%) unless otherwise stated.
AVA, aortic valve area, CABG, coronary artery bypass graft; COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate; MI, myocardial infarction; PAP, pulmonary artery pressure; LVEF, left-ventricular ejection fraction; MR, mitral regurgitation; NYHA, New York Heart Association; PCI, percutaneous coronary intervention; STS, Society of Thoracic Surgeons.
We treated elderly and high-risk patients (median [IQR] age 81.0 [77.0; 85.0] years, STS score 8.1 [5.4; 12.3], logEuroScore 18.5 [12.8; 29.0]). Patients received SE valves more often (n = 625) than BE valves (n = 170). The distribution of valve sizes can be found in Supplemental Table S5. Patients in the BE group were older compared with the SE group (82.0 [78.0; 85.0] vs 81.0 [77.0; 85.0], P = 0.041), and more female patients were treated with BE valves (82.4% female patients in the BE group vs 52.0% female patients in the SE group, P < 0.001). There were no significant differences between BE and SE groups with regard to the risk scores (Supplemental Table S1).
Procedural data are listed in Table 2 for all patients and in Supplemental Table S3 and S4 for the subgroups. Device success was achieved in 92.1% of the cases. Higher rates of new pacemaker implantations were observed in the SE group (SE 23.2%; BE 11.6%, P = 0.003). PD was more frequently performed in the SE group than in the BE group (14.8% vs 5.9%, P = 0.004). Patients in the PD group had a longer procedural time (47.0 [38.5; 66.0] minutes vs 43.0 [35.0; 60.0] minutes, P = 0.011), and a larger amount of contrast medium was used in patients undergoing PD (140 [115; 190] cc vs 125 [105; 150] cc; P < 0.001). The device success rate was lower in patients with PD (78.6% vs 94.0%, P < 0.001), which was mainly because of the remaining PAR, despite the fact that PD was performed. At 30 days, the rate of stroke was higher in patients with PD (10.2% vs 4.6%, P = 0.019).
Table 2.
Procedural characteristics of the study population
| Characteristics | All patients n = 795 |
|---|---|
| Time of procedure, minutes | 44.0 (35.0; 60.0) |
| Amount of CM for procedure, mL | 125 (105; 150) |
| Postdilatation | 98 (12.3%) |
| Self-expandable valve number (%) | 625 (78.6%) |
| Balloon-expandable valve number (%) | 170 (21.4%) |
| Device success | 732 (92.1%) |
| Concomitant PCI | 2 (0.3%) |
| Postprocedural variables | |
| Myocardial infarction, number (%) | 7 (0.9%) |
| Stroke, number (%) | 42 (5.3%) |
| Death within 24 hours, number (%) | 11 (1.4%) |
| New pacemaker implantation | 165 (20.7%) |
| Closure device failure | 76 (9.6%) |
| Life-threatening or major bleeding | 255 (32.1%) |
| Cardiac tamponade | 31 (3.9%) |
| AKI (any stage) | 156 (19.6%) |
| AKI Stage I, number (%) | 77 (9.7%) |
| AKI Stage II, number (%) | 18 (2.3%) |
| AKI Stage III, number (%) | 61 (7.7%) |
| Need for hemodialysis, number (%) | 63 (7.9%) |
| Hospitalization length of stay, days | 20 (15; 27) |
All values are given in median (interquartile range) or number (%) unless otherwise stated.
AKI, acute kidney injury; CM, contrast medium; PCI, percutaneous coronary intervention.
Survival and long-term clinical outcomes
Median (IQR) follow-up time was 1345 (316; 2015) days. The longest follow-up time was nearly 10 years (3635 days). Median (IQR) follow-up time for BE and SE group were 1344 (284; 1924) and 1350 (321; 2041) days (P = 0.327). Median (IQR) follow-up time for PD and no-PD group were 955 (79; 2087) and 1407 (359; 2014) days (P = 0.104). We performed a telephone interview for clinical evaluation in 211 patients who could not visit our centre.
Clinical follow-up rates did not differ significantly between the PD and no-PD group (P = 0.744). More patients were treated with SE valves, and significantly more patients in this group had clinical follow-up at 8 years (P = 0.036). One-year, 5-year, 6-year, 7-year, and 8-year all-cause mortality was 25.4%, 59.0%, 64.6%, 67.9%, and 69.2%, respectively (Fig. 1A). At 8 years, no significant differences in survival were found comparing SE/BE and PD/no-PD groups (Fig. 1B and C). These findings remained consistent after adjusting for differences in baseline and procedural characteristics (Supplemental Fig. S1, Supplemental Table S6). Patients with AR ≥ 2 had higher mortality rates compared with patients with AR < 2 (Supplemental Fig. S2). However, patients in the PD group with successful reduction of PAR (lower than moderate) had similar survival rates to patients in the no-PD group with no or mild PAR after TAVR (38.2% vs 34.7% survival at 8 years, P = 0.835).
Figure 1.
Kaplan-Meier survival estimates. (A) Overall survival for the whole cohort (795 patients): 1-year, 5-year, 6-year, 7-year, and 8-year overall mortality was 25.4%, 59.0%, 64.6%, 67.9%, and 69.2%, respectively. (B) Survival for balloon-expandable and self-expanding groups: 1-year, 5-year, 6-year, 7-year, and 8-year mortality for self-expanding valve group was 25.2%, 58.9%, 64.7%, 68.2%, and 69.5%, respectively; 1-year, 5-year, 6-year, 7-year, and 8-year mortality for balloon-expandable valve group were 26.0%, 59.2%, 64.5%, 66.9%, and 68.0%, respectively. There were no significant differences between the 2 groups at 8 years after TAVR (log-rank test, P = 0.709). (C) Survival for postdilatation and no postdilatation groups: 1-year, 5-year, 6-year, 7-year, and 8-year mortality for postdilatation group was 32.7%, 61.2%, 64.3%, 67.3%, and 69.4%, respectively; 1-year, 5-year, 6-year, 7-year, and 8-year mortality for no-postdilatation group was 24.4%, 58.6%, 64.7%, 68.0%, and 69.2%, respectively. There were no significant differences between the 2 groups at 8 years after TAVR (log-rank test, P = 0.363). TAVR, transcatheter aortic valve replacement.
Patients improved in New York Heart Association (NYHA) functional class after TAVR. At 1-year follow-up, 89.3% of the patients were in NYHA class I or II, as were 74.6%, 57.8%, and 50.0% of the patients at 5-, 6-, and 7-year follow-up, respectively. More than one-half (56.7%) of the patients were alive at 7 years and later were in NYHA class I or II. Further details are shown in Supplemental Fig. S3.
Echocardiographic follow-up
Echocardiographic data are given in Table 3 for the whole cohort and in Supplemental Tables S7 and S8 for the subgroups. Echocardiographic data were available for 402, 176, 123, 96, 86, 56, and 32 patients for 1-, 2-, 3-, 4-, 5-, 6-, and ≥ 7-year follow-up, respectively. Median (IQR) echocardiographic follow-up time was 831 (366; 1827) days. The longest echocardiographic follow-up time was 3635 days. Median (IQR) follow-up time for BE and SE group were 606 (365; 1777) and 933 (367; 1828) days (P = 0.101). Median (IQR) follow-up time for PD and no-PD group were 1120 (465; 2004) and 803 (366; 1827) days (P = 0.120). The main reasons for incomplete echocardiographic follow-up were death before the next visit or inability to visit our centre. Patients who were alive at 7 years and beyond had median aortic valve area (AVA) of 2.1 (1.5; 2.6) cm2 and low mean gradients (7.8 [5.3; 12.0] mm Hg). The number of patients with remaining AR ≥ 2 decreased over the years because of the higher mortality in this subgroup. The median ejection fraction was ≥ 54% during follow-up. No significant differences in AVA, EF, or remaining AR were found comparing BE and SE valves at ≥ 7 years (Supplemental Table S7). No patient who had received PD had echocardiographic follow-up beyond 5 years. Thus, comparison of echocardiographic findings for PD/no-PD subgroups was only possible up to 5 years (Supplemental Table S8). More patients in the PD group had remaining perivalvular AR (18.8% vs 7.1%, P < 0.001) at discharge. No statistically significant differences between the PD and no-PD groups were apparent at 5-year follow-up (Supplemental Table S8).
Table 3.
Echocardiographic data (all patients)
| discharge n = 713 |
1 year n = 402 | 2 years n = 176 | 3 years n = 123 | 4 years n = 96 | 5 years n = 86 | 6 years n = 56 | ≥ 7 years n = 32 | |
|---|---|---|---|---|---|---|---|---|
| AVA (cm2) | 1.9 (1.6; 2.2) | 1.8 (1.6;2.2) | 1.9 (1.5;2.1) | 1.8 (1.5;2.2) | 1.8 (1.5;2.1) | 1.7 (1.4;2.0) | 1.6 (1.3;2.1) | 2.1 (1.5;2.6) |
| vmax (m/s) | 2.0 (1.7; 2.3) | 1.9 (1.6;2.3) | 1.8 (1.6;2.2) | 1.9 (1.6;2.3) | 1.8 (1.4;2.1) | 1.9 (1.4;2.2) | 1.9 (1.6;2.2) | 2.2 (1.6;2.4) |
| pmax (mm Hg) | 16.0 (12.0; 21.0) | 15.0 (11.0; 21.0) | 14.0 (10.1; 19.0) | 15.0 (10.5; 21.5) | 14.9 (9.9; 20.0) | 15.0 (9.0; 20.1) | 16.0 (12.0; 22.0) | 14.0 (8.0; 20.0) |
| pmean (mm Hg) | 9.0 (6.0; 12.0) | 8.0 (6.0; 11.0) | 7.8 (5.0; 10.0) | 8.0 (6.0; 12.0) | 8.0 (5.1; 10.9) | 8.0 (5.0; 11.0) | 9.0 (7.0; 12.0) | 7.8 (5.3; 12.0) |
| EF (%) | 60 (52; 66) | 60 (54; 65) | 59 (50; 65) | 59 (50; 65) | 55 (50; 65) | 57 (50; 62) | 54 (43; 63) | 55 (45; 64) |
| AR ≥ 2, number (%) | 61 (8.6%) | 25 (6.2%) | 10 (5.7%) | 7 (5.9%) | 6 (6.3%) | 4 (4.7%) | 1 (1.8%) | 3 (9.3%) |
| MR ≥ 2, number (%) | 60 (8.4%) | 26 (6.5%) | 16 (9.1%) | 14 (11.4 %) | 13 (13.5%) | 5 (5.8%) | 7 (12.5%) | 3 (9.3%) |
All values are given in median (interquartile range) or number (%) unless otherwise stated.
AR, aortic regurgitation; AVA, aortic valve area; EF, ejection fraction; MR, mitral regurgitation; pmax, maximum pressure gradient; pmean, mean pressure gradient; vmax, maximum velocity.
Structural valve deterioration and bioprosthetic valve failure
SVD was detected in 26 patients (3.3%). Twenty (2.5%) patients had moderate and 6 (0.8%) patients severe SVD. Median time (IQR) to the occurrence of moderate SVD was 1111 (368; 1952) days compared with 2117 (1403; 2423) days for the occurrence of severe SVD (P = 0.053). Comparing SE/BE group and PD/no-PD group, no significant differences were found in the rate of SVD (Supplemental Table S9). Nineteen (2.4%) of 795 patients had evidence of BVF during the follow-up period, 2 cases of early BVF, and 17 cases of late BVF. Four cases of BVF occurred in the BE group (2.4% of patients in the BE group) and 15 cases in the SE group (2.4% of patients in the SE group), P = 0.972. Also, there were no significant differences in the rates of BVF in the PD group compared with no-PD (2 [2.0%] vs 17 [2.4%], P = 0.809). The 2 early BVF cases occurred after 17 and 14 days in a female and a male patient. Both BVFs were related to endocarditis and not to SVD. In both patients, a 29 MCV was successfully implanted. Both patients had acute kidney injuries after the index procedures (Stage 1 and 3). Dialysis was needed in the patient with Stage 3 acute kidney injury. The other patient received a pacemaker after TAVR, and endocarditis was also evident on the pacemaker leads. In both cases, endocarditis was treated with antibiotics, which was successful in the patient without PM. The patient with successful endocarditis treatment died after 105 days out of our hospital, the other patient after 22 days in hospital.
In 4 cases of late BVF, a TAVR-in-TAVR procedure was successfully performed. In 2 of these cases, severe SVD was caused by severe restenosis of an Edwards SAPIEN 26 and Edwards SAPIEN 23, 1971 and 2327 days after initial TAVR, respectively. The patient with the Edwards SAPIEN 26 mm was treated with an Edwards SAPIEN XT 26 mm; the other patient with a Medtronic CoreValve Evolut R 26 as TAVR-in-TAVR procedures. Each of the 2 other BVF cases was caused by severe valvular AR of a Medtronic Corevalve 29, 56, and 542 days after initial TAVR. There was no evidence of active endocarditis in either case. Thus, TAVR-in-TAVR was successfully performed using a Medtronic CoreValve 29 in both cases.
Eight cases of late BVF were caused by infective endocarditis. One patient with infective endocarditis was treated by surgery. One-year, 5-year, and 8-year mortality rates for patients with BVF were 21.1%, 57.9%, and 73.7%, respectively.
Discussion
The main outcomes of this long-term analysis of patients who had received transfemoral TAVR at a high-volume center in Germany from 2006 to 2011 are that (1) mortality was nearly 60% at 5 years of follow-up, leaving only 40% of patients for analysis beyond 5 years; (2) comparing SE vs BE or PD vs no-PD, no differences in mortality were evident during long-term follow up; (3) echocardiographic follow-up revealed a stable valve function during follow-up with single-digit mean gradients in this cohort of patients comprising SE and BE valves; (4) applying standardized definitions for SVD and BVF, those events were rare during long-term follow-up with no significant differences between valve types or PD vs no-PD; and (5) the of stroke at 30 days was higher in patients with PD (10.2% vs 4.6%, P = 0.019).
Long-term mortality
Current data on long-term outcome and valve durability after TAVR are still circumscribed, with valid data up to 5 years but limited information beyond that point.9,20 In our analysis, the longest follow-up time was nearly 10 years, and the 5-year mortality rate was 59.0%, which is comparable with data reported in other studies.9,11,21, 22, 23, 24 Mortality at 7 years was 67.9% and thus lower than 76.8% reported by Deutsch et al. and slightly higher than 65% reported by Holy et al.11,24 Notably, there was no significant difference in long-term mortality between patients treated with SE vs BE valves or those in need for PD vs no-PD. However, patients with remaining PAR ≥ 2 had worse outcomes compared with patients with PAR < 2, which is in line with the pooled data analysis of Athappan et al.13 Patients with significant PAR who were successfully treated by PD had the same outcome as patients with initial no or mild PAR. Noteworthy, patients receiving PD had a significantly higher rate of stroke, which is in line with a previous work examining the efficacy and safety of PD in BE valves, which showed a clear tendency toward increased rates of stroke in patients receiving PD.25 However, it is still unclear whether PD per se is responsible for an increase in rate of stroke, rather than being associated with a high calcium burden that causes more embolic events.
In a recent long-term follow-up of the Nordic Aortic Valve Intervention (NOTION) trial, which was the first randomized trial comparing TAVR vs SAVR in lower-risk patients, mortality at 6 years was approximately 40%, with no significant difference among treatment modalities.26 This mortality difference between trials and registries, including patients at increased surgical risk vs those having lower surgical-risk patients, reveals the current problem regarding the analysis of long-term outcome and valve durability beyond 5 years of follow-up, which is limited because of a high rate of attrition in high-risk patients.
Valve function and SVD
Today, there are significant data from randomized controlled trials and registries showing good overall function and hemodynamics of transcatheter valves up to 5 years. In PARTNER 1, a randomized trial including 699 patients treated either by TAVR with a BE valves or by SAVR, no SVD was reported at the 5-year follow-up.22 No SVD and no valve-related explants were detected in a pooled analysis of 3 TAVR trials including 410 patients treated with BE valves with 114 surviving patients at 5 years (27.8%); no SVD and no valve-related explants were detected.27 In the Action in Diabetes and Vascular Disease: Preterax and Diamicron MR-Controlled Evaluation (ADVANCE) study, 996 patients received the self-expanding CoreValve prosthesis with rates of SVD of 0.2% and 0.9% at 1 and 5 years, respectively.28 In our analysis, the rates of SVD comparing BE and SE valves were not different after a median follow-up of 1345 days, indicating that both systems are associated with a low rate of SVD. Beyond that, PD was also not associated with an increased risk of SVD, which is reassuring, as it was hypothesized that PD could affect the integrity of the prostheses. The low rates (6 patients, 0.8%) of severe SVD and the late occurrence of severe SVD (median time of 5.8 years after TAVR) further indicate good durability and performance beyond 5 years.
Data from surgical cohorts suggest that SVD is rare within the first 5 years, but that failure increases thereafter. However, those results are hampered by the lack of standardized definitions of SVD, which typically has been described as death or reoperation. Current data suggest that the incidence of SVD in surgical prostheses—defined as death, reoperation, or clinical reinvestigation caused by suspected SVD—is < 1% in the first 5 years but increases to 10% at 10 years in patients > 65 years of age.29 Those data are typically highly dependent on the prosthesis type with a mean time to SVD of 3.8 ± 1.4 years for the Sorin Mitroflow valve (Sorin Group, Milan, Italy) and up to 19 years for the Carpentier-Edwards PERIMOUNT prosthesis (Edwards Lifesciences).30,31
Our study demonstrated excellent valve durability and performance in patients who were alive up to 8 years after TAVR. Patients who were alive beyond 7 years after TAVR had a median (IQR) AVA of 2.0 (1.5; 2.6) cm2 and single-digit mean gradients. These echocardiographic results translated into good clinical outcomes, with the majority of patients being in NYHA class I or II. Overall rates of SVD (3.3%) and BVF (2.4%) were low and comparable with other publications.11 The main cause of BVF (10 patients) was infective endocarditis (2 early and 8 late BVF). Four cases of SVD were caused by restenosis and AR. In those 4 cases, TAVR-in-TAVR procedures were successfully performed.
In an analysis of the UK-TAVI registry, 241 patients had paired postprocedure and late echocardiography.32 There was 1 case (0.4%) of severe SVD 5.3 years after implantation (new severe AR) and 21 cases (8.7%) of moderate SVD (mean 6.1 years postimplantation; range: 4.9 to 8.6 years). Twelve of these (57%) were caused by new AR and 9 (43%) by restenosis, a distribution comparable with our data. Moreover, as in our study, SE valves had lower gradients and greater AVA than BE valves at follow-up. However, significant differences in AVA, EF, or remaining AR were no longer seen comparing BE and SE valves at ≥ 7 years.
Study limitations
Although the presented data are derived from a large number of patients enrolled in this study, there are several limitations. First, it is an observational study with no randomization for the compared groups. Thus, there is the possibility of unknown confounding factors that might not be excluded by performing a Cox regression analysis. Second, there was no echocardiographic core laboratory involved. Thus, it is difficult to define and compare valvular degeneration, which should entail visualization and thickness of the leaflets on follow-up. The sonographers were not aware of the endpoints for this study to reduce possible bias. Third, only 40% of patients were alive beyond 5 years, and patients may have died before the next study visit. Thus, there is a possibility that BVF and SVD occurred more frequently and were not detected and under-reported in our cohort. Although it is a prospective study registry, standardized definitions for SVD and BVF were published in 2017, so the analysis of these endpoints was performed retrospectively.12 Fourth, the data for long-term results beyond 5 years are derived from a relatively low number of patients. No echocardiographic follow-up beyond 5 years was available for patients with PD. Thus, a long-term comparison of echocardiographic outcomes in this group is impossible. Fifth, our study reflects TAVI experience from the years 2006 to 2011, and first-generation THVs were used in a large proportion of patients. The first-generation THVs (MCV and ES) had no anticalcification strategies. Meanwhile, the first-generation valve systems have been replaced in most centres by newer valves from the same industry (ie, EvolutR and EvolutPro or SAPIEN XT and, now, SAPIEN 3). The newer-generation valves are equipped with special sealing mechanisms to reduce PAR. Thus, the rapid evolution of THV devices and continuous improvement of techniques reduces the overall applicability of our results to current scenarios. Sixth, there was a relatively high rate of new pacemaker implantations in our study because of a low threshold for implanting a pacemaker after TAVR in the first years of TAVR experience (ie, new left bundle branch block and new first-degree AV-block). The risk of infection might be higher because of the pacemaker implantation, and, thus, some cases of BVF caused by endocarditis might be related to infected PM leads in the first place. Finally, this registry reflects solely the experience of our centre, where we treated relatively old patients with a high STS scores and relatively high 1-year mortality. Thus, results are not necessarily transferrable to other cohorts and do not resemble the considerations in young patients, who are associated with more longevity and who are more at risk for valve degeneration, which we treat more commonly now. However, even in our old high-risk cohort, with high rates of bleedings and pacemaker implantations, survival at 8 years was 30.8%.
Conclusions
Our study demonstrates an 8-year survival of 30.8% in a high-risk TAVR cohort. Patients who were alive up to 8 years after treatment with SE and BE valves had good long-term valve function. In cases with successful PD for reducing PAR, patients had similar outcomes to patients without PD and no or AR only minor AR. PD did not lead to increased rates of SVD or BVF.
Funding Sources
No funding has been provided for this article.
Disclosures
N.M. has received personal fees from Edwards Lifesciences, Medtronic, Biotronik, Novartis, Sanofi Genzyme, and AstraZeneca. S.L. has received personal fees from St. Jude Medical and Medtronic. D.H. has received personal fees from Symetis and Medtronic. M.A.B. has received speakersʹ honoraria and consulting fees from Edwards Lifesciences, Medtronic, and CryoLife. A.L. has received grants from Novartis; personal fees from Medtronic, Abbott, Edwards Lifesciences, Boston Scientific, Astra Zeneca, Novartis, Pfizer, Abiomed, Bayer, and Boehringer; and stock options from Picardia, Transverse Medical, and Claret Medical. The other authors have no conflicts of interest to disclose.
Footnotes
Ethics Statement: The study was conducted according to the Declaration of Helsinki and approved by the local ethical board (Registration number 167-10-12072010).
See page 851 for disclosure information.
To access the supplementary material accompanying this article, visit CJC Open at https://www.cjcopen.ca/ and at https://doi.org/10.1016/j.cjco.2021.01.012.
Supplementary Material
References
- 1.Leon M.B., Smith C.R., Mack M. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363:1597–1607. doi: 10.1056/NEJMoa1008232. [DOI] [PubMed] [Google Scholar]
- 2.Smith C.R., Leon M.B., Mack M.J. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. 2011;364:2187–2198. doi: 10.1056/NEJMoa1103510. [DOI] [PubMed] [Google Scholar]
- 3.Reyes M., Reardon M.J. Transcatheter valve replacement: risk levels and contemporary outcomes. Methodist Debakey Cardiovasc J. 2017;13:126–131. doi: 10.14797/mdcj-13-3-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cribier A., Eltchaninoff H., Bash A. Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: first human case description. Circulation. 2002;106:3006–3008. doi: 10.1161/01.cir.0000047200.36165.b8. [DOI] [PubMed] [Google Scholar]
- 5.Walther T., Hamm C.W., Schuler G. Perioperative results and complications in 15,964 transcatheter aortic valve replacements: prospective data from the GARY registry. J Am Coll Cardiol. 2015;65:2173–2180. doi: 10.1016/j.jacc.2015.03.034. [DOI] [PubMed] [Google Scholar]
- 6.Adams D.H., Popma J.J., Reardon M.J. Transcatheter aortic-valve replacement with a self-expanding prosthesis. N Engl J Med. 2014;370:1790–1798. doi: 10.1056/NEJMoa1400590. [DOI] [PubMed] [Google Scholar]
- 7.Vlastra W., Quevedo P.J., Tchetche D. Predictors, Incidence, and Outcomes of Patients Undergoing Transfemoral Transcatheter Aortic Valve Implantation Complicated by Stroke. Circ Cardiovasc Interv. 2019;12 doi: 10.1161/CIRCINTERVENTIONS.118.007546. [DOI] [PubMed] [Google Scholar]
- 8.Thiele H., Kurz T., Feistritzer H.-J. Comparison of newer generation self-expandable vs. balloon-expandable valves in transcatheter aortic valve implantation: the randomized SOLVE-TAVI trial. Eur Heart J. 2020;41:1890–1899. doi: 10.1093/eurheartj/ehaa036. [DOI] [PubMed] [Google Scholar]
- 9.Toggweiler S., Humphries K.H., Lee M. 5-year outcome after transcatheter aortic valve implantation. J Am Coll Cardiol. 2013;61:413–419. doi: 10.1016/j.jacc.2012.11.010. [DOI] [PubMed] [Google Scholar]
- 10.Barbanti M., Petronio A.S., Ettori F. 5-year outcomes after transcatheter aortic valve implantation with CoreValve prosthesis. JACC Cardiovasc Interv. 2015;8:1084–1091. doi: 10.1016/j.jcin.2015.03.024. [DOI] [PubMed] [Google Scholar]
- 11.Holy E.W., Kebernik J., Abdelghani M. Long-term durability and hemodynamic performance of a self-expanding transcatheter heart valve beyond 5 years after implantation: a prospective observational study applying the standardized definitions of structural deterioration and valve failure. EuroIntervention. 2018;14:e390–e396. doi: 10.4244/EIJ-D-18-00041. [DOI] [PubMed] [Google Scholar]
- 12.Capodanno D., Petronio A.S., Prendergast B. Standardized definitions of structural deterioration and valve failure in assessing long-term durability of transcatheter and surgical aortic bioprosthetic valves: a consensus statement from the European Association of Percutaneous Cardiovascular Interventions (EAPCI) endorsed by the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) Eur Heart J. 2017;38:3382–3390. doi: 10.1093/eurheartj/ehx303. [DOI] [PubMed] [Google Scholar]
- 13.Athappan G., Patvardhan E., Tuzcu E.M. Incidence, predictors, and outcomes of aortic regurgitation after transcatheter aortic valve replacement: meta-analysis and systematic review of literature. J Am Coll Cardiol. 2013;61:1585–1595. doi: 10.1016/j.jacc.2013.01.047. [DOI] [PubMed] [Google Scholar]
- 14.Makkar R.R., Thourani V.H., Mack M.J. Five-year outcomes of transcatheter or surgical aortic-valve replacement. N Engl J Med. 2020;382:799–809. doi: 10.1056/NEJMoa1910555. [DOI] [PubMed] [Google Scholar]
- 15.Laakso T., Laine M., Moriyama N. Impact of paravalvular regurgitation on the mid-term outcome after transcatheter and surgical aortic valve replacement. Eur J Cardiothorac. 2020;58:1145–1152. doi: 10.1093/ejcts/ezaa254. [DOI] [PubMed] [Google Scholar]
- 16.Hahn R.T., Pibarot P., Leipsic J. The effect of post-dilatation on outcomes in the PARTNER 2 SAPIEN 3 registry. JACC Cardiovasc Interv. 2018;11:1710–1718. doi: 10.1016/j.jcin.2018.05.035. [DOI] [PubMed] [Google Scholar]
- 17.Kappetein A.P., Head S.J., Généreux P. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document. J Am Coll Cardiol. 2012;60:1438–1454. doi: 10.1016/j.jacc.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 18.Seiffert M., Conradi L., Baldus S. Impact of patient-prosthesis mismatch after transcatheter aortic valve-in-valve implantation in degenerated bioprostheses. J Thorac Cardiovasc Surg. 2012;143:617–624. doi: 10.1016/j.jtcvs.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 19.Nashef S.A., Roques F., Michel P., Gauducheau E., Lemeshow S., Salamon R. European system for cardiac operative risk evaluation (EuroSCORE) Eur J Cardiothorac Surg. 1999;16:9–13. doi: 10.1016/s1010-7940(99)00134-7. [DOI] [PubMed] [Google Scholar]
- 20.Daubert M.A., Weissman N.J., Hahn R.T. Long-term valve performance of TAVR and SAVR: a report from the PARTNER I trial. JACC Cardiovasc Img. 2016;10:15–25. doi: 10.1016/j.jcmg.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 21.Kapadia S.R., Leon M.B., Makkar R.R. 5-year outcomes of transcatheter aortic valve replacement compared with standard treatment for patients with inoperable aortic stenosis (PARTNER 1): a randomised controlled trial. Lancet. 2015;385:2485–2491. doi: 10.1016/S0140-6736(15)60290-2. [DOI] [PubMed] [Google Scholar]
- 22.Mack M.J., Leon M.B., Smith C.R. 5-year outcomes of transcatheter aortic valve replacement or surgical aortic valve replacement for high surgical risk patients with aortic stenosis (PARTNER 1): a randomised controlled trial. Lancet. 2015;385:2477–2484. doi: 10.1016/S0140-6736(15)60308-7. [DOI] [PubMed] [Google Scholar]
- 23.Duncan A., Ludman P., Banya W. Long-term outcomes after transcatheter aortic valve replacement in high-risk patients with severe aortic stenosis: the U.K. Transcatheter Aortic Valve Implantation Registry. JACC Cardiovasc Interv. 2015;8:645–653. doi: 10.1016/j.jcin.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 24.Marcus-Andre D., Magdalena E., Melchior B. Beyond the five-year horizon: long-term outcome of high-risk and inoperable patients undergoing TAVR with first-generation devices. EuroIntervention. 2018;14:41–49. doi: 10.4244/EIJ-D-17-00603. [DOI] [PubMed] [Google Scholar]
- 25.Daneault B., Koss E., Hahn R.T. Efficacy and safety of postdilatation to reduce paravalvular regurgitation during balloon-expandable transcatheter aortic valve replacement. Circ Cardiovasc Interv. 2013;6:85–91. doi: 10.1161/CIRCINTERVENTIONS.112.971614. [DOI] [PubMed] [Google Scholar]
- 26.Søndergaard L., Ihlemann N., Capodanno D. Durability of transcatheter and surgical bioprosthetic aortic valves in patients at lower surgical risk. J Am Coll Cardiol. 2019;73:546–553. doi: 10.1016/j.jacc.2018.10.083. [DOI] [PubMed] [Google Scholar]
- 27.Sawaya F., Kappetein A.P., Wisser W. Five-year haemodynamic outcomes of the first-generation SAPIEN balloon-expandable transcatheter heart valve. EuroIntervention. 2016;12:775–782. doi: 10.4244/EIJV12I6A126. [DOI] [PubMed] [Google Scholar]
- 28.Gerckens U., Tamburino C., Bleiziffer S. Final 5-year clinical and echocardiographic results for treatment of severe aortic stenosis with a self-expanding bioprosthesis from the ADVANCE study. Eur Heart J. 2017;38:2729–2738. doi: 10.1093/eurheartj/ehx295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nishida T., Tominaga R. A look at recent improvements in the durability of tissue valves. Gen Thorac Cardiovasc Surg. 2013;61:182–190. doi: 10.1007/s11748-013-0202-z. [DOI] [PubMed] [Google Scholar]
- 30.Yankah C.A., Pasic M., Musci M. Aortic valve replacement with the Mitroflow pericardial bioprosthesis: durability results up to 21 years. J Thorac Cardiovasc Surg. 2008;136:688–696. doi: 10.1016/j.jtcvs.2008.05.022. [DOI] [PubMed] [Google Scholar]
- 31.Forcillo J., Pellerin M., Perrault L.P. Carpentier-Edwards pericardial valve in the aortic position: 25-years experience. Ann Thorac Surg. 2013;96:486–493. doi: 10.1016/j.athoracsur.2013.03.032. [DOI] [PubMed] [Google Scholar]
- 32.Blackman D.J., Saraf S., MacCarthy P.A. Long-term durability of transcatheter aortic valve prostheses. J Am Coll Cardiol. 2019;73:537–545. doi: 10.1016/j.jacc.2018.10.078. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.