Abstract
Natural extracts are the source of many antioxidant substances. They have proven useful not only as supplements preventing diseases caused by oxidative stress and food additives preventing oxidation but also as system components for the production of metallic nanoparticles by the so-called green synthesis. This is important given the drastically increased demand for nanomaterials in biomedical fields. The source of ecological technology for producing nanoparticles can be plants or microorganisms (yeast, algae, cyanobacteria, fungi, and bacteria). This review presents recently published research on the green synthesis of nanoparticles. The conditions of biosynthesis and possible mechanisms of nanoparticle formation with the participation of bacteria are presented. The potential of natural extracts for biogenic synthesis depends on the content of reducing substances. The assessment of the antioxidant activity of extracts as multicomponent mixtures is still a challenge for analytical chemistry. There is still no universal test for measuring total antioxidant capacity (TAC). There are many in vitro chemical tests that quantify the antioxidant scavenging activity of free radicals and their ability to chelate metals and that reduce free radical damage. This paper presents the classification of antioxidants and non-enzymatic methods of testing antioxidant capacity in vitro, with particular emphasis on methods based on nanoparticles. Examples of recent studies on the antioxidant activity of natural extracts obtained from different species such as plants, fungi, bacteria, algae, lichens, actinomycetes were collected, giving evaluation methods, reference antioxidants, and details on the preparation of extracts.
Keywords: antioxidants, natural extracts, antioxidant activity/capacity, green synthesis, nanoparticles
1. Introduction
Recently, much research has been devoted to free radical chemistry. There are undeniable pieces of evidence that free radicals are responsible for the oxidative damage of biomolecules such as proteins, lipids, or nucleic acids in the structures of cell nuclei and molecular membranes. Maintaining the balance between free radicals and antioxidants is a prerequisite for staying healthy. Thus, the control of oxidative stress processes may turn out to be fundamental in both the prevention and treatment of many diseases, such as diabetes, atherosclerosis, coronary artery disease, cancer, inflammation, liver diseases, cardiovascular diseases, cataracts, nephrotoxicity, and neurodegenerative processes accompanying aging. In order to maintain redox homeostasis, excess free radicals are neutralized by enzymes and non-enzymatic antioxidants, which, with the exception of a few produced by the human body, e.g., glutathione, uric acid, and uricinol, must be supplied with the diet. Since synthetic antioxidants butylated hydroanisole (BHA), butylated hydrotoluene (BHT), n-propyl gallate (PG) pose a potential health risk due to contamination with chemical precursors, toxic solvents, and the formation of hazardous by-products, natural antioxidants are an attractive alternative. For this reason, there is an extensive search for effective, non-toxic, and natural antioxidants. According to PubMed, in the last 5 years, over three thousand review articles that prove the effectiveness of natural antioxidants in preventing diseases caused by oxidative stress have been published. Therefore, antioxidants have become co-adjuvants utilized in conventional therapies with the aim of combating oxidative stress. Many natural antioxidants have been shown to have strong antiviral effects. The efficacy of flavonoids, i.e., (+)—catechin, luteolin, apigenin, quercetin, and quercetin 7-rhamnoside, has been proven in coronavirus infections (Porcine epidemic diarrhea virus (PEDV), Transmissible gastroenteritis virus (TGEV) [1,2,3]. In the absence of effective therapies for the treatment of diseases caused by coronaviruses, antioxidants may prove to be an effective alternative to fight the SARS- and MERS-CoV pandemic [4]. The site of action of antioxidants is the oxidative stress pathway, which plays a key role in coronavirus-induced pathogenesis. Diniz et al. [4] reviewed different effects of natural antioxidants against coronavirus covering reduction nucleocapsid (N) protein expression, inhibition 3C-like protease (3CLpro) [5,6,7,8] enzyme responsible for replication of SARS-CoV (quercetin and its derivatives), papain-like protease (PLpro) (isobavachalcone and psoralidin) [9], and helicase protein by affected ATPase activity (myricetin and scutellarein) [10]. A recently published review demonstrated the usefulness of antioxidants in the treatment of neurological disorders caused by COVID-19 [11]. However, reports that do not confirm the effectiveness of antioxidants in vivo cannot be ignored [12]. The activity of antioxidants is mainly limited by ADMET (Absorption, Distribution, Metabolism, Excretion, and Toxicology)processes related to poor absorption caused by restrictions in the penetration of cell membranes and degradation that occurs in the stomach and intestines. It has also been reported that low molecular weight antioxidants lose their ability to scavenge free radicals inside cells. This is especially true for the scavenging of the hydroxyl radical (OH•), superoxide (O2•−), and H2O2 [13,14].
The sources of natural antioxidants are mainly plants, i.e., edible vegetables, fruits, spices, and herbs, which are rich in vitamins, phenolic compounds, carotenoids, and microelements [15,16,17]. However, it should be emphasized that the antioxidant activity is different for different varieties and morphological parts of natural resources. In addition, the activity of natural products is influenced by many other factors, such as climatic and soil conditions or harvest time. They hinder the standardization of natural products to a large extent. Due to the fact that natural antioxidants have the ability to inhibit the processes of oxidation and the growth of microorganisms, including many pathogenic ones, e.g., Salmonella spp. and Escherichia coli [18], they are more and more often used as preservatives in food products [19] or as packaging ingredients for food [20]. In recent years, a large body of evidence has been published that natural antioxidants increase the stability of edible oils [21,22,23], the stability of carotenoid dyes, and the aroma of fruit juices [24] and that they work well as additives in meat products [25,26] and even in bakery products [27], successfully replacing artificial preservatives and stabilizers. It should be emphasized that the choice of bioactive compounds for the food industry is significantly limited due to the obvious taste requirements [28] and the need for approval by EFSA (European Food Safety Authority) or FDA (American Food and Drug Administration). Currently, we can observe an interesting trend in the strategy of using by-products of the processing industry [29,30]. This is related not only to environmental protection or economic reasons but to the fact that they have a significant content of bioactive substances, exceeding that in the flesh of fruit, such as polyphenols in apple and olive pomace [31], lycopene in tomato pomace [32], phenolic compounds from the group of flavonoids (anthocyanins, catechins), and phenolic acids and stilbenes in grape skins [33] or citrus fruits [34].
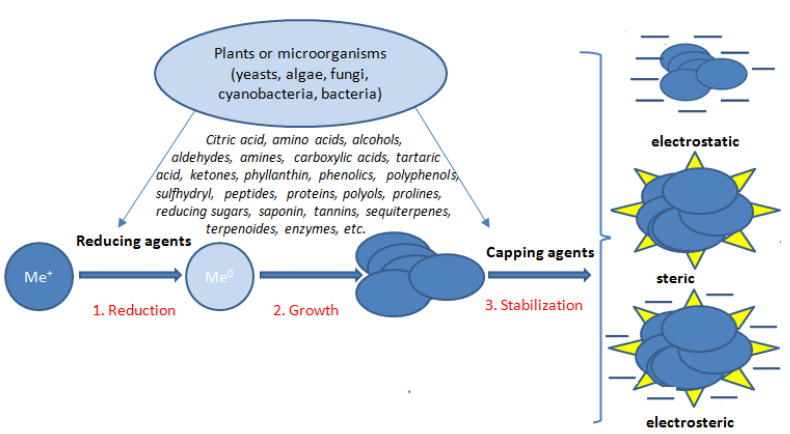
In recent years, natural extracts have become attractive, also due to the rapid development of nanotechnology. As a source of substances with reducing potential, they have replaced the toxic reagents used in chemical synthesis and ushered in the era of the so-called biogenic synthesis and nanobiotechnology [35].
Numerous spectroscopic, biochemical, and electrochemical assays are used to test antioxidant abilities, which are still modified so that they can effectively assess the potential of antioxidants, taking into account the variability of their mechanisms of action. They are usually based on a free radical scavenging reaction or the prevention of their formation by the addition of an antioxidant. Various techniques used for this purpose differ in terms of repeatability and costs associated with the necessity of using specialized equipment. Valuable reviews, published in recent years, describe problems related to (i) the mechanisms responsible for the antioxidant activity [17]; (ii) the antioxidant activity of natural extracts prepared from various plant species [36], microorganisms [37], and food ingredients [38]; and (iii) the preventive role of antioxidants in various diseases such as diabetes [39], human gut diseases [40], and cancer [41], as well as the use of natural extracts for the synthesis of nanoparticles [42].
This review gathers together issues related to antioxidants (classification, natural sources, measurement of antioxidant activity) as well as their application in nanotechnology. Within the review, two main issues can be distinguished:
-
(i)
The wide range of industrial and biomedical applications of antioxidants requires effective and rapid in vitro tests to evaluate total antioxidant activity. Various methods were collected in the review, i.e., chromatographic, spectrometric, and electrochemical. Particular attention was paid to the method based on metallic nanoparticles, which are used as optical probes (SNPAC). The method is useful for measuring the antioxidant activity of both simple chemical compounds and mixtures of natural origin. The SNAPC tests are effective in assessing electron transfer but are not used very often. The review includes information on the extracts from plants, lichens, fungi, algae, and actinomycetes (reference antioxidants, extraction process, antioxidant activity tests, and activity parameters).
-
(ii)
Natural extracts as a source of both reducing and stabilizing substances are used for the green synthesis of nanoparticles. The review includes examples of the synthesis of metallic/metal oxides of nanoparticles using extracts from various plant species and microorganisms (yeast, algae, cyanobacteria, fungi, and bacteria). The information collected allows us to trace the links between the type of antioxidant, its origin, activity, and suitability for the efficient synthesis of nanoparticles. Extensive data were collected on the methods of extract preparation, antioxidant activity tests, detection methods, NPs synthesis conditions, and the morphology of the obtained nanoparticles.
This review highlights recent trends in antioxidant research, measurement of antioxidant activity, biogenic nanoparticle synthesis, and nano-drug delivery systems.
2. Free Radicals/Antioxidants
2.1. Free Radicals vs. Oxidative Stress
Free radicals can be defined as highly reactive species that contain an unpaired electron in the valence shell. They can donate this electron but also accept it from other molecules, acting as an oxidant or reducing agent [43]. In the human body, reactive forms (RS) come from metabolic processes involved in the respiratory chain, phagocytosis, prostaglandin synthesis, and the cytochrome P-450 system [44].
The most reactive species found in biological systems include the hydroxyl radical (OH●), which is formed by attaching three electrons to an oxygen molecule, e.g., as a result of the Fenton reaction, and the superoxide radical (O2●−), which is formed mainly in mitochondria, as a byproduct of electron transport in the respiratory chain. Other reactive forms of oxygen (ROS), nitrogen (RNS), and chlorine occurring as free radicals and nonradicals that as oxidizing agents can be easily converted into radicals are listed in Table 1 [45,46].
Table 1.
Examples of reactive species. Reproduced with permission from Graves, D.B., [J. Phys. D Appl. Phys.]; published by IOP Publishing, 2012 [47].
| Reactive Species | Form | Example |
|---|---|---|
| Reactive oxygen species (ROS) | Radical | HO•, 1[O]2, O2•− HOO•, ROO•, RO•, CO2•−, CO3•− |
| Non-radical | O3, H2O2, HOCl, HOI, HOBr, ROOH, CO, ONOOH, ONOO−, O2NOO−, HOOCO2−, (O2 1Dg) | |
| Reactive nitrogen species (RNS) | Radical | NO•, NO2•, NO3• |
| Non-radical | ROONO, RO2ONO, CH3C(O)OONO2, N2O4, N2O3, N2O5, HNO2 NO2Cl NO−, NO+ |
|
| Reactive chlorine species | Radical | Cl• |
| Non-radical | ClBr, Cl2, ClO2 | |
| Reactive sulfur species | Radical | S• |
| Non-radical | H2S, RSSR, RS(O)SR, RSOH, RS(O)2SR, RSR’ |
ROS/RNS generated in oxygen metabolism are necessary in the regulation of gene expression, cell proliferation, apoptosis, the processes of protein phosphorylation or calcium concentration in cells, activation of proteins controlling cell division, and elimination of microorganisms. Free radicals are also generated under the influence of external sources, such as exposure to X-rays, ozone, smoking, air pollution, and industrial chemicals [48,49]. There is a balance in the cell between RS production and its neutralization by defense systems. Under physiological conditions, this balance is slightly shifted in favor of pro-oxidative conditions, providing continuous, mild oxidative stress [50].
Each disturbance of this particular balance may lead to the development of oxidative stress, i.e., a state in which the oxidizing potential increases to a level that threatens the stability of cellular structures [51]. Under oxidative stress, biologically important macromolecules such as DNA, proteins, carbohydrates, and lipids are damaged. The excess of free radicals changes their structure and thus the physiological functioning of the cell by disrupting redox signaling and the accumulation of cytotoxic compounds, such as malonyl dialdehyde or 4-hydroxynonenal [52,53].
There is evidence that free radicals can accumulate throughout the body with age, initiating the aging process, as well as various neurodegenerative diseases such as Alzheimer’s disease, Parkinson’s disease, muscular dystrophy, and atherosclerosis [54]. An imbalance between ROS and the antioxidant defense system has also been recognized in the induction of diabetes and age-related eye disease [55]. Currently, it is believed that oxidative stress has a significant negative impact also on inflammatory diseases, cancer, ischemic diseases, immunodeficiency syndrome, hypertension, alcoholism, smoking-related diseases, and many others [56,57,58,59,60,61]. Oxidative stress was first described and defined by Sies in 1991 [62].
The reasons for the occurrence of oxidative stress may be (i) an increase in the rate of ROS production, (ii) deficiencies of low-molecular-weight antioxidants, and (iii) inactivation of enzymes with antioxidant activity. Increased and/or prolonged state of oxidative stress may cause serious damage to the cell and even lead to its death [63]. Therefore, the current discussions focus on the role of free radicals in the pathogenesis of many diseases and the usefulness of antioxidants in their potential therapy [55,64,65].
Antioxidants are produced by the protective system of various organisms in order to respond to the destructive effects of free radicals. Antioxidants are able to reduce the damage caused by ROS/RNS and even chlorine. The action of the protective system may limit the negative effects of free radicals by preventing the formation of reactive radicals or by interrupting free radical reactions [66].
2.2. Antioxidants
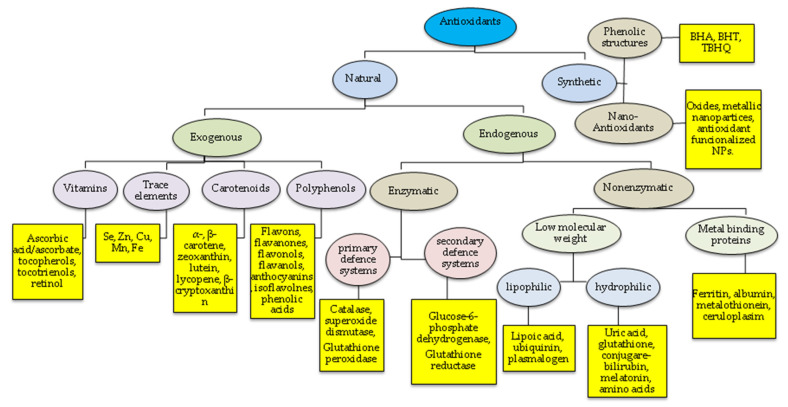
Antioxidants act by delaying or preventing the oxidation of other chemicals. The first studies on the role of antioxidants in biology focused on their use in preventing unsaturated fats from going rancid [67,68,69]. However, the milestone that led to the understanding of the role of antioxidants for living organisms was the identification of vitamins A, C, and E [70] and the understanding of the mechanism of lipid peroxidation prevention by vitamin E [71]. The classification of antioxidants, along with the most representative examples, is shown in the diagram (Figure 1). Antioxidants are usually classified into enzymatic and non-enzymatic. Among them, there are various compounds with different modes and places of action and different final effects. This diversity determines the individual role of each of them in the body. It should be emphasized that the network of interacting antioxidant enzymes, such as superoxide dismutase enzymes (SODs), catalase (CAT), glutathione peroxidase (GPx), and glutathione reductase (GRd), shows the highest antioxidant defense effectiveness [72].
Figure 1.
Antioxidants classification.
Low-molecular-weight antioxidants, including vitamin C, E, coenzyme Q, carotenes, glutathione, and trace elements, are also responsible for inactivating reactive radicals. Some of them, including glutathione, ubiquinone, albumin and metallothioneins, and uric acid, are produced in the body [73], but most are exogenous compounds derived from natural sources such as plants (flavonoids, phenolic acids, carotenoids, stilbenes, coumarins, lignans, organosulfur compounds, vitamins) or minerals (selenium, zinc, manganese) provided with the diet. When endogenous antioxidants involved in free radical defenses cannot protect the body against ROS, there is a need for exogenous antioxidants. Almost all living organisms, both prokaryotes and eukaryotes, are capable of producing bioactive compounds.
Many of the naturally occurring antioxidants are now isolated, fully characterized, and available for various applications as prophylactic and therapeutic agents to inhibit the adverse effects generated by ROS [74,75].
A good diet that includes fruit, tea, wine, vegetables, and grains is a rich source of antioxidants. Some drugs, apart from their therapeutic effect, also have antioxidant effects, e.g., captopril belonging to angiotensin-converting enzyme (ACE) inhibitors, N-acetylcysteine [76], or dihydropyridine calcium antagonists [77]. However, the concentrations used in the therapy do not provide antioxidant activity in vivo.
The source of antioxidants and other bioactive compounds are also microorganisms, including actinomycetes, bacteria [78], cyanobacteria, fungi, and lichens [79]. Compared to plants, these organisms can grow very quickly under strictly controlled conditions, which makes them a favorable source of natural bioactive molecules for industrial food, pharmaceuticals, nutraceuticals, and agricultural applications.
Antioxidants can also be delivered to the body in the form of dietary supplements. The synthetic forms of antioxidants are bioequivalent to their natural forms, e.g., biovitamin C vs. chemically synthesized L-ascorbic acid, or synthetic and natural R, R, R-α-tocopherol. Antioxidants are also used as additives to prevent the oxidation of unstable ingredients in the food, cosmetic, and pharmaceutical industries. This mainly concerns synthetic antioxidants with a phenolic structure, such as butylated hydroanisole (BHA), butylated hydrotoluene (BHT), and tert-butylated hydroquinone (TBHQ), which are added to foodstuffs to prevent lipid rancidity [80].
Antioxidants differ in their ability to scavenge free radicals. It has been shown that antioxidant activity can be significantly correlated with the number of active groups such as OH or NH2 and the position of these functional groups in the order ortho > para > meta, from the highest to the lowest active [81]. It should be remembered that antioxidants can act through various mechanisms, not only scavenging radicals, but also sequestering transition metal ions, decomposing hydrogen peroxide or hydroperoxides, quenching active pro-oxidants, and enhancing endogenous antioxidant defense but also by repairing the resulting cellular damage. Therefore, antioxidants are sometimes classified as primary or chain-breaking antioxidants and as secondary or preventive antioxidants [82]. Primary antioxidants actively inhibit oxidation reactions by scavenging ROS/RNS, while secondary antioxidants act indirectly through chelation of transition metal (iron) ions [83,84] and other specific actions such as anti-inflammatory, induction of protective factors, inhibition of NADPH oxidase (nicotinamide adenine dinucleotide phosphate oxidase), inhibition of xanthine oxidase, and regulation of redox-sensitive signal transduction pathways, including transcription factors and inhibition of poly (ADP-ribose) −1 (PARP-1) polymerase [81,85,86]. Another indirect way of antioxidant activity is the activation of transcription factors, including Nrf2, which in turn leads to the activation of endogenous antioxidant enzymes [87].
Currently, the role of exogenous antioxidants in preventing or delaying oxidative damage is becoming more and more controversial. The initial enthusiasm for their positive health effects was mainly based on in vitro experiments. In the initial studies, the in vivo bioavailability of the antioxidants, which is generally quite low, was neglected. In this context, the activity of scavenging free radicals by antioxidant metabolites seems to be more reliable [50,88]. The high in vitro chemical reactivity of the antioxidant is therefore not evidence of its effectiveness in vivo. Moreover, as shown by individual studies [89,90], supplementation with antioxidants may be ineffective and even very dangerous. An example may be the disappointing research on the effectiveness of vitamin E in the risk of cardiovascular disease or hemorrhagic stroke [91,92,93,94,95]. Reports that the use of antioxidants not only prevent cancer but may also provoke it are also alarming [96]. As it turns out, it is especially dangerous to supplement with antioxidants in doses exceeding the daily intake. For example, supplementation with β-carotene over ten times the daily intake increased the incidence of lung cancer in smoking men by 18% [97]. Vitamin C supplementation is particularly controversial. Linus Pauling recommended health-promoting use of a high daily dose of 1000 mg [98]. Unfortunately, it turned out that even at low concentrations of ascorbic acid, a pro-oxidative effect can occur in the presence of transition metals, e.g., iron. An example of this effect is the effect of ascorbic acid on iron-induced lipid peroxidation [99].
In the review by Hrelia and Angeloni [100], recent reports on new mechanisms of action of natural antioxidants are collected. Their study highlights the fact that natural antioxidants are heavily metabolized in vivo, a result of which is that their redox potential drops significantly at the physiological level.
The authors observed a growing interest in the scientific community in the interactions of natural antioxidants with proteins that are involved in intracellular signaling cascades and modulation of the gut microflora.
Currently, in research on natural antioxidants, research issues can be distinguished regarding (i) combination therapies using the synergistic effect of natural antioxidants, (ii) anti-aging effects of fermented preparations, (iii) enzyme research, (iv) genetic research, (v) studies on the effect of antioxidants on the intestinal microflora, and (vi) the effect of antioxidants on hormonal activity.
3. Antioxidant Capacity/Activity Measurements
Determination of antioxidant status attracts growing attention for clinical purposes [48,101]. However, the determination of antioxidative potential, in this case, is difficult to establish due to the complex mechanisms of action for the individual anti-oxidants. Some of them act by scavenging free radicals, some by preventing the formation of ROS or inducing the signaling pathways or by repairing the oxidative damage. Cellular protection is ensured mainly by enzymes (glutathione peroxidase, SOD, catalase), whereas the non-enzymatic antioxidants act in the plasma. Additionally, the status of redox homeostasis differs significantly between the individuals; therefore, the reference values have not been established so far [102,103]. Presently, there is also no direct method dedicated to accurate measurement of oxidative stress in vivo conditions. Therefore, oxidative stress is measured by the use of multiple in vitro assays [102], which can identify free radicals directly like electron paramagnetic resonance (EPR) or electron spin resonance (ESR) spectroscopy, fluorescent probes, or indirect methods enabled to identify the stable products which are created as a consequence of the free radical attack, like chromatography, colorimetry, and immune, or enzymatic tests [104].
There is also some misunderstanding regarding specific terms that are used to describe antioxidants measurement assays. Bunaciu et al., in a critical review [105], pointed out that the terms “antioxidant activity” and “antioxidant capacity” need some more clarification because they are often used interchangeably despite having different meanings. It should be emphasized that the term “antioxidant activity” refers to kinetic-based assays measuring the rate constant of a reaction between reactants or scavenging percentages per unit time. Thus, the term is characteristic of a specific antioxidant and oxidant, expressed as reaction rates value. In turn, the antioxidant capacity can be defined as the efficiency of antioxidants to inhibit the oxidative degradation of the various bio-compounds. The measurements are based on the reaction between studied antioxidants and free radicals (reactive species inactivation, quenching, or scavenging) or on the reaction of the sample with transition metals. Antioxidant capacity expresses the amount (in moles) of a given free radical that is scavenged by a sample.
In the case of a heterogeneous mixture, the antioxidant capacity of each individual component is not possible to measure as all antioxidants react simultaneously to produce the total scavenging ability of the sample. In the case of the complex samples, the most reasonable way of their antioxidant capacity is using a variety of methods that can address the different mechanisms of action of individual components [106,107]. The collaborative effect of all sample components (i.e., synergistic or antagonistic effects) is responsible for “total antioxidant capacity” (TAC) measured.
Antioxidants’ capacity can be estimated by considering the final effects of their presence, by the use of in vitro tests, or directly by more complex methods utilizing exogenic probes to detect oxidation. With such a variety of mechanisms involved in the action of antioxidants, determining the level of total antioxidant capacity (TAC) is one of the major challenges in antioxidant testing. Thus far, no universal method has been developed that would gain general and univocal acceptance. Therefore, when choosing a specific method, one should be aware of what kind of an antioxidant function is being measured [46,108].
The measured activity of primary antioxidants reflects their ability to scavenge ROS/RNS throughout hydrogen atom (H•) or electron (e−) transfer or both species simultaneously (i.e., proton-coupled electron transfer). Secondary antioxidants, which are known as preventive ones, are evaluated by the chelating ability of selected transition metal ions e.g., Fe(II) or Cu(I). Preventive antioxidants act by inhibiting Fenton reactions as a source of hydroxyl radicals or a Lewis acid-base neutralization (metal ion—antioxidant). In turn, endogenous antioxidative enzymes, being “first-line defense antioxidants” such as SOD, CAT, and GPx, which are able to scavenge superoxide anion radicals and hydrogen peroxides, require enzymatic methods for evaluation of the antioxidants activity [108].
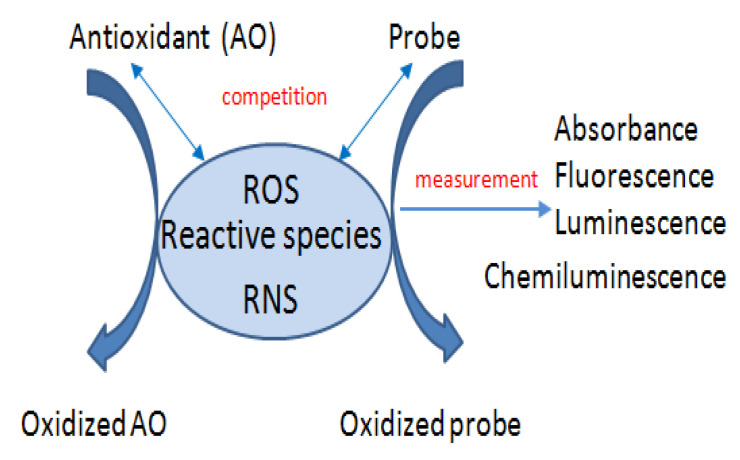
Nonenzymatic primary antioxidant assays can be non-competitive or competitive [109]. Competitive assays such as TRAP, ORAC, TOSC, crocin bleaching, peroxyl radical trapping antioxidant parameter, act due to the competition between a fluorogenic or chromogenic probe and antioxidants for the reactive species (ROS/RNS). In the presence of antioxidants, the probe undergoes weaker oxidation, which is reflected in the changes of its measurable properties (absorbance, fluorescence, luminescence) [110] (Figure 2).
Figure 2.
Schematic illustration of competitive antioxidant (AO) assay.
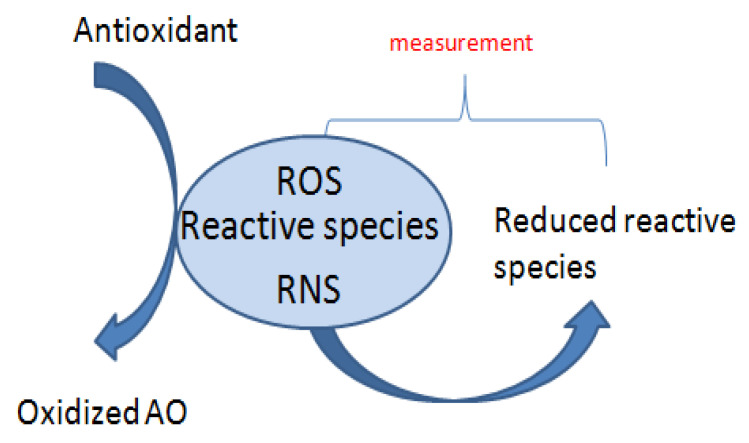
The non-competitive (Figure 3) ones based on Folin−Ciocalteu reaction, ABTS/TEAC, CUPRAC, FRAP, DPPH, ABTS differ in the lack of the presence of any competing target molecule. TAC measurements are considered to be noncompetitive if they rely on electron transfer (ET) mechanism, whereas competitive measurements are usually based on a hydrogen atom transfer (HAT) [46].
Figure 3.
Schematic representation of non-competitive antioxidant (AO) assay.
In certain circumstances, ET/HAT mechanisms may not be easily identified like for 2,2′-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid (ABTS), and 2,2-diphenyl-1-picrylhydrazyl (DPPH) assays, which are sometimes classified as mixed-mode assays (ET/HAT). Both free radicals react according to two mechanisms: HAT (1) and SET (single electron transfer) (2):
| HAT: DPPH● + A → DPPH-H + A●, | (1) |
| SET: DPPH● + AH → DPPH− + AH+●; AH+● → AH● +H+; DPPH− + H+ → DPPH-H, | (2) |
Experimental investigations [111,112] confirm that HAT and SET transformations may occur at the same time as a sequential proton-loss electron transfer (SPLET), which is also named as a proton-coupled electron transfer (PCET) [106]:
| SPLET: AH → AH− + H+; AH− + DPPH● → AH● + DPPH−; DPPH− + H+ → DPPH-H | (3) |
It has been proven that the HAT mechanism dominates in aqueous solutions. In turn, the SET and SPLET may dominate in non-aqueous solutions due to the possibility of organic solvents forming hydrogen bonds with molecules of antioxidants [113,114,115,116]. Among the SET methods, the most used are DPPH radical scavenging capacity assay, Trolox equivalent antioxidant capacity (TEAC or ABTS) assay, ferric reducing (FRAP) assay, reducing power assay (RP), and copper reduction (CUPRAC) assay. HAT assays include the total per-oxyl radical-trapping antioxidant parameter (TRAP) assay, the crocin bleaching assay, oxygen radical absorbance capacity (ORAC) assay, and total oxyradical scavenging capacity (TOSC) assay.
Antioxidant activity can also be estimated using nanoparticle-based assays utilizing nanoparticles probes exhibiting localized surface Plasmon resonance (LSPR) absorption [117,118]. It has been established that the LSPR absorption connected with the nanoparticles grove rises linearly depending on antioxidant concentration. Scampicchio et al. described such correlation for gold nanoparticles (AuNPs) generated under the influence of phenolic acid antioxidants being able to donate electrons. Özyürek et al. proved the same for silver nanoparticles (Ag-NPs), which were formed as a product of AgNO3 reduction with polyphenolic antioxidants.
Many studies are dedicated to the estimation of the antioxidant power of various individual chemicals, as well as food samples and natural extracts [119]. For this purpose, various tests were applied, including, among others, the oxygen radical absorbance capacity test, the Trolox equivalent antioxidant capacity, and the ability to reduce metal ions, such as copper or iron. Several reviews have been published that highlight the advantages and disadvantages of the available tests [120,121,122,123,124]. However, there is still no standard quantitative method for measuring antioxidant activity. Therefore, it is extremely difficult to compare the results obtained from different studies. The complexity and variety of research systems make it impossible to repeat and confirm experiments by independent laboratories. The most common methods related to the antioxidant assessment are summarized in Table 2.
Table 2.
Examples of the non-enzymatic assays used for in vitro determination of antioxidant capacity with distinguished chromogenic agents, observed changes, the principle, mode, and mechanism of the assay (Mech).
| Assay | The Chromogenic Agents |
Observed Changes | Principle of Assay | Mode | Mech | Ref |
|---|---|---|---|---|---|---|
| Total antioxidant capacities | ||||||
| Crocin bleaching | crocin | bleaching of crocin | The ability of AOs to inhibit oxidation of crocin. | Abs. 443 nm pH = 7.0–7.5 |
HAT | [125,126] |
| ORAC (Oxygen radical absorbance capacity) |
fluorescein, dichloro- fluorescein | fluorescence decay | The fluorescence caused by oxidation of the probe by peroxyl-radical initiated by thermal decomposition of AAPH, is delayed/inhibited by AOs. | Fl. λex = 485 nm λem = 538 nm pH = 7.4 |
HAT | [127] |
| TRAP (Total peroxyl radical trapping antioxidant parameter) | β-phycoerythrin | fluorescence decay | Fluorescence decay along time due to oxidation of the probe is delayed by AOs. | Fl. λex = 495 nm λem = 575 nm pH = 7.5 |
HAT | [128,129] |
| β-carotene bleaching assay | β-carotene | bleaching yellow color of β-carotene | The ability of AOs to slow down the rate of β-carotene bleaching due to its reaction with peroxyl radicals, which are formed by linolenic acid oxidation. | Abs. 470 nm pH = 5.5–7.5 |
HAT | [130,131] |
| PCL (Photochemiluminescence) | luminol | blue light emision | An AO-sensitive inhibition of a photo-induced, chemiluminescence accompanying autooxidation of luminol. | Cl. 360 nm pH = 10.5 |
HAT | [132,133,134] |
| Reducing antioxidant power (RP) | ||||||
| FRAP (Ferric reducing antioxidant potential) |
ferric tripyridyl triazine | yellow color to blue | AOs as reductant at low pH can reduce ferric tripyridyl triazine to ferrous form, causing absorbance increase. | Abs. 593 nm pH = 3.6 |
ET | [135] |
| CUPRAC (cupric ion reducing antioxidant capacity) |
Cu(II) complex | light blue to orange-yellow | Ability of AO for the reduction of Cu(II) in bathocuproine(2,9-dimethyl-4,7-diphenyl-1,10-phenanthroline) or neocuproine (2,9-dimethyl-1,10-phenanthroline) complexes to Cu(I) forms. | Abs. 490 nm 450 nm pH = 7 |
ET | [136] |
| CERAC (Ce(IV)-based reducing capacity) |
Ce (IV) | fluorescence | The ability of AO to reduce Ce(IV) to Ce(III) accopanied with fluorescence elevation. | Fl. λex = 256 nm λem = 360 nm pH acidic |
ET | [137,138] |
| CHROMAC (Chromium reducing antioxidant capacity) | Cr (VI) with DPC | red–violet product | The reduction of chromate(VI) to Cr(III) in acidic solution. The remaining Cr(VI) reacts with DPC to produce a chelate complex. The Cr(VI) consumption was correlated with AO’ concentration. | Abs. 540 nm. pH = 2.8 |
ET | [139] |
| Phosphomolybdenum assay | Phosphormolybdenum complex |
green product | The reduction of Mo(Vl) to Mo(V) by AO. | Abs. 695 nm pH acidic |
ET | [140] |
| The Folin–Ciocalteu (FC) assay | Tungstate–molybdate complexes | from yellow to dark blue | FC reagent in a basic medium is able to oxidize reducing substances, mainly phenolic and polyphenolic AOs. The change in color is connected with transformation of Mo(VI) to Mo(V), causing absorbance increase. | Abs. 750–765 nm pH = 10 |
ET | [109] |
| PFRAP (Potassium ferricyanide reducing power assay) |
Ferricyanide reagent: Fe(III), Fe(CN)63− |
prussian blue | The AOs react with potassium ferricyanide Fe(CN)63−) forming potassium ferrocyanide Fe(CN)64− which further reacts with FeCl3 to form prussian blue KFe[Fe(CN)6]. | Abs. 700 nm pH = 6.6 |
ET | [141] |
| FTC (Ferric thiocyanate) |
Fe(S-CN)2 | red color | A hydroperoxide formed from a lipid (linoleic acid) oxidizes a ferrous ion to a ferric ion. The AO causes an inhibitory effect on hydroperoxide formation or by its ability to donate an electron to ferric ion. | Abs. 500 nm |
ET | [142,143] |
| FOX (Ferrous Oxidation-Xylenol Orange Assay) | ferric-XO complex |
blue-purple color | The presence of hydroperoxides that oxidize ferrous ion to ferric ion, which subsequently react with xylenol orange (XO). | Abs. 550 nm. |
ET | [144] |
| Assays associated with lipid peroxidations | ||||||
| LPO (Lipid peroxidation inhibition assay) |
N-methyl-2-phenylindole | dye product | AOs delay radical-induced malonyl dialdehyde generation. MDA and HAE are measured as an indicator of lipid peroxidation. The product -MDA with chromogenic reagent gives carbocyanine adduct. | Abs. 586 nm |
ET | [138,145] |
| TBARS (Thiobarbituric acid reactive substances assay) | TBARS | red-pink color | The reaction of lipid peroxidation products (MDA), with TBA, leads to the formation of MDA-TBA adducts (TBARS). | Abs. 532 nm pH = 4 |
ET | [130,131,146,147] |
| Conjugated diene assay | linoleic acid | UV absorbance | Antioxidants delay conjugated dienes formation. The AO effect can be evaluated by monitoring the conjugated diene formation. | Abs. 234 nm |
[148] | |
| Radical scavenging assays | ||||||
| DPPH | 2,2-diphenyl-1-picrylhydrazyl radical | deep violet to pale yellow or colorless | The decrease in DPPH absorbance depends linearly on AO’ concentration. | Abs. 515–517 nm pH = 7 |
HAT/ET | [130] |
| ABTS | 2,2’-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid (ABTS+.) | bluish-green to colorless | ABTS treated with Na/K persulphate or MnO2 gives a radical cation (ABTS+). ABTS+. is reduced by antioxidants. The decrease in absorbance depends linearly on AO’concentration. | Abs. 734 nm pH = 7.4 |
HAT/ET | [149] |
| DMPD (N,N-dimethyl-p-phenylene-diamine) |
DMPD·+ radical cation | reduction of purple color | DMPD·+ is generated through a reaction between DMPD and potassium persulphate the assay measures scavenging of free radicals by AOs. | Abs. 517 nm pH = 5.25 |
HAT | [150,151] |
| SOSA (Superoxide Anion Radical Scavenging Capacity) | NBT | yellow to blue | The ability of the AO to compete with NBT to scavenge O2•− generated by an enzymatic HPX-XOD, X-XOD or PMS/NADH systems. | Abs. 560 nm pH = 7.4 |
ET | [135,152] |
| Nitric oxide free radical scavenging activity | Griess reagent | colorless to light pink to deep purple | NO was generated from sodium nitroprusside and measured by the Greiss reaction. AO reduces the amount of nitrite. | Abs. 546 nm pH = 7.4 |
ET | [153] |
| Peroxynitrite Scavenging Capacity Assay | Evans Blue | dye bleaching | The percentage of scavenging of ONOO− by the Evans Blue was measured in presence of AO. | Abs. 611 nm pH < 7 |
ET | [154] |
| HORAC (Hydroxyl Radical Averting Capacity Assay) | fluorescein | fluorescence decay | OH radicals are generated by a Co(II)-mediated Fenton-like reaction. The reaction is confirmed by the hydroxylation of p-hydroxybenzoic acid. Metal ion-induced OH radical generation reaction can be monitored by the fluorescence decay of fluorescein. In the presence of AO, the formation of OH radicals can be inhibited because the metal is deactivated due to coordination with AO. | Fl. λex = 493 λem = 515 nm |
HAT | [130,135,146,147,148,149,150,151,152,153,154,155] |
| HRS (Deoxyribose Degradation Assay) | MDA-TBA adducts | pink | A mixture of Fe(III)-EDTA, H2O2, vit.C generates OH radical, is able to degrade deoxyribose. The products heated under acidic conditions form MDA detected by adduct with TBA. AO can inhibit deoxyribose damage. | Abs. 532 nm pH = 7.4 |
ET | [156] |
| Hydroxyl Radical Scavenging Capacity Assay | Fenton-like system Fe(II)/H2O2 | - | The Fenton system generates a constant flux of pure OH radicals. ESR measurements evaluate the OH radicals scavenging capacity of AOs. | Electron spin resonance (ESR) |
ET | [157] |
| CAA(Cellular Antioxidant Activity Assays) | DCFH-DA | fluorescence decay |
The ability of AOs to prevent oxidation of DCFH by azide generated peroxyl radicals in human hepatocarcinoma HepG2 cells. | Fl. λexc.502 nm, λem 520 nm |
ET | [158] |
| Nonradical reactive oxygen species scavenging assay | ||||||
| Hydrogen peroxide scavenging activity | hydrogen peroxide | UV absorbance | Hydroxyl radicals are the byproducts of H2O2 decomposition. They initiate lipid peroxidation. After the addition of AO, the absorbance is measured against blank (phosphate buffer). | Abs. 230 m pH = 7.4 |
ET | [159] |
| Singlet oxygen scavenger | RNO | bleaching of RNO | Production of singlet oxygen (1O2) was achieved by monitoring RNO bleaching. Singlet oxygen was generated by a reaction between NaOCl and H2O2. | Abs. 440 pH = 7.1 |
ET | [160] |
| ACA (Aldehyde/carboxylic acid assay) | Alkylaldehyde/ alkylcarboxylic acid | - | The stoichiometric conversion from alkylaldehyde (hexanal) to alkylcarboxylic acid in the presence of radicals induced by heat, O2, or H2O2. | GC | ET | [161] |
| Metal chelating capacity assays (MCA) | ||||||
| Ferrous ions chelating assay | Fe(II) with 2,2-bipyridine or ferrozine |
blue | The capacity to chelate ferrous ion can be disturbed by the presence of other complexing agents (AOs), which cause a decrease intensity of the complex (Fe(II) and ferrozine). | Abs. 562 nm 522 nm pH = 4–10 |
ET | [162] |
| Copper(II) chelating capacity assay | Cu(II)- PV | dark to yellow |
The chelating activity can be estimated by the measurement of the rate of color reduction. | Abs. 632 nm pH = 6 |
ET | [163] |
| Nanoparticles (NPs)-based assays | ||||||
| Gold nanoparticles (Au-NPs) | NPs | No color into dark red |
The highest capacity of reducing gold(III) to gold NPs corresponds to the highest antioxidant activity. Alternatively, cyclic voltammetry measures anodic peak potentials | Abs. 555 nm pH = 8 |
ET | [117,118] |
| Silver nanoparticles (Ag-NPs) | NPs | no color into pale yellow | Nanoparticles generated from metal salts upon reduction with antioxidants in the presence of citrate-stabilized silver seeds. | Abs. 423 nm pH = 7 |
ET | [118] |
Abbreviations: AAPH (2,2′-azobis–2–methyl-propanimidamide,dihydrochloride); Abs. (Absorbance); DPC (1,5-diphenylcarbazide); XO (xylenol orange); MDA (malondialdehyde); HAE (4-hydroxyalkenals); NBT (nitroblue tetrazolium); Griess reagent (1% sulfanilamide, 2% H3PO4, and 0.1% naphthylethylenediamine dihydrochloride); DCFH (dichlorofluorescein); Luminol (5-amino-2,3-dihydrophthalazine-1,4-dione); Cl. (Chemiluminescence), Abs. (Absorbance); Fl. (Fluorescence); HPX-XOD (hypoxanthine–xanthine oxidase); X-XOD (xanthine–xanthine oxidase); PMS/NADH (phenazine methosulphate systems); AO (antioxidant); PV (pyrocatechol violet); RNO (N, N-dimethyl-p-nitrosoaniline); DCFH-DA (2’,7’-dichloro-dihydrofluorescein diacetate); triazine (2,3,5- triphenyl-1,3,4-triaza-azoniacyclopenta-1,4-diene chloride).
3.1. Techniques Used to Assess Antioxidant Capacity
A number of techniques are used to assess antioxidant capacities, such as UV-Vis spectroscopy, fluorescence spectroscopy, chemiluminescence, electron paramagnetic resonance (EPR), enzyme-catalyzed assays [164,165,166,167,168], and cell culture assays. Moreover, there are some electrochemical techniques, including controlled potential techniques, electrochemical sensors, and biosensors, which are commonly applied [169]. However, the most widely used techniques for evaluating the ability of an antioxidant to scavenge e.g., ABTS●+, DPPH●, O2●−, H2O2, a total antioxidant reducing capacity, e.g., TEAC, ORAC, and FRAP belong to spectrometric techniques. These methods have been commonly used to determine the antioxidant capacity of many plant extracts, foods, and dietary supplements [170,171,172,173,174]. These assays despite some drawbacks [129] are easy to use.
3.1.1. DPPH Free Radical Scavenging Assay
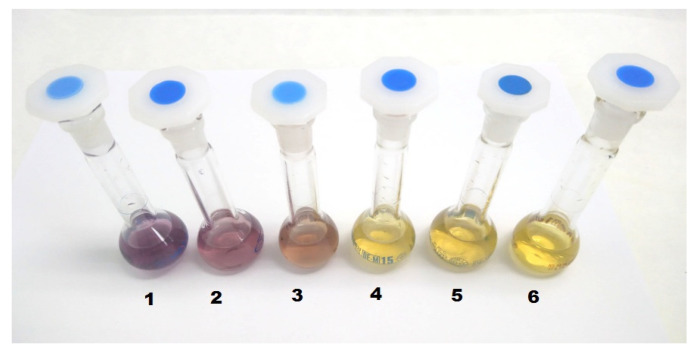
To measure antioxidants’ power, their ability to deactivate free radicals was used. One of the most frequently used stable free radicals is DPPH (1,1-diphenyl-2-picrylhydrazyl) discovered by Goldsmith and Renn in 1922 [175]. Due to the relocation of the unpaired electron, DPPH forms a stable radical cation and does not form dimers in alcohol solutions [176,177]. The DPPH solution has a dark purple color with maximum absorbance at wavelength = 517 nm. By reaction with a substance that gives off a hydrogen atom, a reduced form of DPPH 2,2-diphenyl-1-picrylhydrazine is formed, and then the purple color of the solution changes to yellow with a concomitant decrease in absorbance (Figure 4).
Figure 4.
1 mM DPPH solutions containing an increasing amount of Salvia officinalis extract: 1–20 μL, 2–30 μL, 3–50 μL, 4–80 μL, 5–100 μL, 6–120 μL.
The drop in absorbance is proportional to the amount of DPPH oxidized form that remains in solution. The color change from purple to yellow can be monitored spectrophotometrically and utilized for the assessment of the free radical scavenging potential of many antioxidants and natural products. For the first time, the colorimetric method was described by Blois [177] for the evaluation of the antioxidant properties of the thiol-containing amino acid cysteine as the model antioxidant. Since that time, an easy and convenient colorimetric method has been extensively used to evaluate the antioxidant capacity of many products of natural origin [178,179,180,181,182,183]. The reaction of DPPH with antioxidants was adapted for illustration and measuring the kinetics of radical quenching [184,185]. Since the beginning of the 1960s, the method, as well as antioxidant activity calculations, have evolved into numerous modifications [186,187].
DPPH Free Radical Scavenging Kinetics
DPPH free radical scavenging has been conducted by using at least two commonly practiced procedures (a) fixed reaction time, when the researcher imposes reaction times of 15, 30, or 60 min, and (b) steady-state saturation one, when the reaction time is related to the reaction kinetics. The reaction of DPPH radicals with antioxidants is a kinetically driven process. It has been proven that the time required to reach saturation state, i.e., the highest decrease in DPPH absorbance depends on concentration and the kind of antioxidant. To check out the kinetic behavior of the disappearance of DPPH radicals with individual antioxidants, kinetic scans should be performed at different concentration levels. Although at higher concentrations, the scavenging capacity is higher, sometimes the reaction cannot be completed quickly because of slow kinetics. For instance, the reaction of DPPH with ascorbic acid is fast and achieves completion within a minute [188], whereas even 3 h is not enough to finish the reaction for curcumin at so small a concentration as from 5 to 15 µM. In turn, the reaction time for BHT was found to be around 6 h. Such antioxidants as lipoic acid, melatonin, and pentoxifylline demonstrate slow reaction with DPPH radical up to 2 mM. Such kinetic measurements have been performed for different chemicals used as reference antioxidants. Considering the time duration of reaction to achieve the steady-state, antioxidants can be divided into categories of fast (<30 min), medium (30 min to 1 h), and slow (>1 h) kinetics. In 2012, Mishra et al. [178] established the nature of individual chemicals such as alpha-tocopherol, ascorbic acid, sesamol, gallic acid, ferulic acid, and BHT-butylated hydroxytoluene, which are commonly used as references in the comparative evaluation of antioxidant properties. Among these reagents, there are examples of fast (ascorbic acid), medium (gallic acid), and slow reaction kinetics, which is observed for BHT. Despite the fact that the time to attain an equilibrium state depends on the nature of antioxidants, researchers have usually chosen a fixed reaction time mode where reaction time is pre-imposed to be 20–30 min instead of the real-time required to attain completion of the redox reaction [176], ignoring their kinetic behavior and the fact that many antioxidants might react with different kinetics or might not react at all. Furthermore, some authors emphasize the reversibility of the free radical reduction by antioxidants, which results in underestimation of the antioxidant capacity of many antioxidants [106,189].
Considering numerous methodologies of DPPH assay described in the literature, involving variation in (i) concentrations of reagents, (ii) sample’ volume, (iii) the kind of reference molecules, (iv) antiradical parameters used, (v) units of applied parameters, and (vi) the kind of sample environment (methanol or semi-aqueous media), the antiradical potential of any sample assessed by DPPH assay, it is very difficult to compare results between laboratories. Mishra et al. [134] collected IC50 values of reference standards such as butylated hydroxyl anisole (BHA), ascorbic acid, gallic acid, BHT, and Trolox that determined by different authors. It appeared that the reported IC50 value of ascorbic acid was in the range from 11.85 to 629 µM. Unfortunately, such a large variation in IC50 values was also observed for remaining antioxidants. Recently, Xie and Schaich [190] have reevaluated the DPPH assay considering the solvent kind and pH values.
Parameters Used to Express the Antioxidant Potential
The DPPH free radical scavenging activity is commonly expressed in terms of the percentage of inhibition of the free radical by examined antioxidants. The EC50 value relates to the antioxidant concentration required to achieve a 50% decrease in the DPPH absorbance. This parameter is typically employed not only to express the antioxidant capacity but also to compare the activity of different compounds with each other. To find the above parameter, antiradical curves are plotted, representing the relationship between the concentration of antioxidants on the x-axis and relative scavenging capacity (E%) on the y-axis. The radical scavenging capacity can be calculated using the following equation:
| (4) |
However, to find the most credible EC50 value, an assay should be done using several antioxidant concentrations located near the estimated ED50 value. The above graph looks like a typical rectangular hyperbole, but it can be changed into a sigmoidal curve after the logarithmic transformation of the x-axis (log[mol/L]). The EC50 value is usually located in a short linear range, and it may be calculated by the use of the right-angled triangle [191,192]. This mathematical method must meet two assumptions: reaching the maximum response and recording at least two points located near the targeted point of the 50% maximal response. The following equation enables EC50 value calculation:
| (5) |
It should be noted that sigmoid curves based on the Hill equation are easier to interpret [193]. The logarithmic curve does not have to be symmetrical around its midpoint, thanks to the model using the Richards equation which provides a fitting thanks to the introduction of the S parameter, quantifying the asymmetry. Chen et al. [192] conducted a comparative study of several specialized computer programs based on various regression models towards the aim of EC50 estimation. The EC50 values obtained by the use of the statistical programs were similar to each other; however, GraphPad Prism@ five-parameter analysis showed the smallest variance in relation to the experimental estimated EC50. The authors claim that the observed differences in the results between the statistical processing programs GraphPad and SigmaPlot are due to the fact that the first one calculates actual EC50 values, while the second gives the inflection point as the EC50.
Antiradical power (ARP) is another parameter that can be used to define antioxidant activity. This parameter is defined as a reciprocal of EC50, which is why the higher value of EC50 is related to smaller antiradical power:
| (6) |
The antioxidant capacity can be expressed as reference chemical equivalent such as Trolox (µmol TE/g), ascorbic acid, gallic acid (GAE/g), etc. Unfortunately, comparison of results presented by different studies is difficult because of the variety of units used for the above recalculations. We can find mass/mass units such as milligrams per gram of dry material, µmol/g, or mass/volume ones.
DPPH Assay Approaches
In the original DPPH assay, provided by batch experiments, several automation approaches based on flow injection analysis (FIA) [194,195] and sequential injection analysis (SIA) [196] have been proposed in recent decades. An interesting approach inspired by HPLC-FIA [197] has been elaborated on by Koleva [198]. In this method, the HPLC-separated analytes react postcolumn with the DPPH solution, and the induced bleaching is detected as a negative peak by the second detector at 517 nm. Cerda et al. [199] described multi-syringe flow injection analysis (MSFIA) for determining the total antioxidant capacity of several food products. Flow injection analysis (FIA), similarly to sequential injection analysis (SIA), is beneficial for rapid testing of antioxidation/radical scavenging activity of large series of multicomponent samples [177]. Another advantage of automatic approaches in comparison to the standard spectrophotometric batch experiments lies in the visible improvement of measurement reproducibility. Another assay suitable for screening of either hydrophilic or lipophilic antioxidants is a high-throughput relative DPPH radical scavenging capacity (RDSC) assay elaborated by Cheng et al. [189]. The assay, which can be performed in aqueous and organic environments, utilizes a 96-well microplate reader with the spectrophotometric detector, ensuring acceptable accuracy, precision, and reproducibility.
The sophisticated instruments are required not only for the rapid determination of the antioxidant activity of complex mixtures but also for providing separation and identification of the selected antioxidant compounds. The HPLC method appears to be the method of choice in this case. For this purpose, HPLC should be used in combination with an appropriate detector, which is usually connected online to chromatographic apparatus. However, simultaneous determining of antioxidant capacity requires additional coupling with another radical scavenging detection mode. Such systems have been described in the literature; unfortunately, they are not adopted commonly due to their complexity and the lack of commercial availability. As an example, in 2007, Wu et al. [200] developed HPLC-ESI-MS and NMR for estimation of antioxidant capacity of polyphenolic acids in the plant extract. In turn, Nuengchamnong et al. [201] proposed RP-HPLC coupled with an electrospray ionization MS/MS system for the identification of antioxidant compounds in an extract of a Thai medicinal plant. An interesting HPLC approach, suitable for searching natural antioxidants in plant extract of Flos Lonicerae Japonicae, was developed by Tang et al. in 2008 [202]. The method’s idea assumes that the peak areas of compounds with antioxidant activity undergo reduction after reaction with DPPH. The authors performed additional identification of antioxidants by the HPLC-DAD-TOF/MS hyphenated technique.
Traditional thin-layer chromatography with post chromatographic derivatization using DPPH solution for free radical scavenging activity evaluation, discovered by Glavind and Holmer in 1967 [203], exists nowadays in the modern version owing to video scanning technology [204].
3.1.2. Electrochemical Methods
Electrochemical measurements possess some major advantages in comparison to spectrophotometric methods mainly due to the fact that they are fast, less tedious, cheaper, and safer for the environment. They include electrochemical techniques of antioxidant characterization as potentiometry, amperometry, biamperometry, cyclic voltammetry (CV), square-wave voltammetry (SWV), and differential pulse (DPV). These methods utilize the fact that antioxidants are involved in redox reactions acting as reducing agents. The electrochemical techniques are able to measure their redox potentials.
The Cyclic Voltammetry Method
The cyclic voltammetry method is applied to screen the reducing capacity of the samples. Cyclic voltammetry (CV) operates due to the combination of three electrodes, namely working electrode, reference, and auxiliary electrode. A polarogram representing the relationship between current intensity and an increasing potential applied to the working electrode is recorded. The obtained voltammograms show well-defined voltammetric peaks corresponding to the oxidation and reduction processes. Lower Epa values are associated with the higher reducing activity of the tested sample. Therefore, considering the first oxidation potential, the following classes of chemical compounds can be distinguished: if Ep is lower than 0.8 V, antioxidant power is high, and if Ep is between 0.8 and 1.3 V, antioxidant power is low [205]. The area under the curve of the voltammetric peak (AUC) corresponds to the concentration of antioxidants. Broad anodic peaks are usually observed due to the response of multiple reducing agents with different oxidation potentials present in the respective extracts. In such cases, Chevion et al. [206]. Martinez et al. [207], and Zielińska and Zieliński [208] suggested that the area under the anodic current wave should be used for the evaluation of reducing the power of the samples. Lower AUC indicates a lower reducing capacity of the investigated extract. Usually, the reducing capacity is statistically significantly correlated with the active components of the extracts. Zielińska et al. [209] found the existence of a significant positive correlation between the total phenolic content (r = 0.867; p < 0.01) and total flavonoid content (r = 0.752, p < 0.01) with the reducing capacity of peels of the investigated apple cultivars.
Biamperometry
Determination of the antioxidant activity by biamperometric measurements is based on a high degree of reversibility redox couple potential, including Fe3+/Fe2+, I2/I−, Br2/Br−, VO3−/VO2− Fe(CN)63−/Fe(CN)64−, and Ce(IV)/Ce(III). The DPPH•/DPPH couple is also suitable for this purpose. The current intensity is proportional to the decreasing concentration of free radicals after reaction with the antioxidants. The obtained results of antioxidant activity are usually in very good agreement with those determined by the use of other conventional methods such as spectroscopic measurements. The biamperometric technique was applied by Milardovic et al. [210] for evaluation of the selected standard antioxidants (ascorbic acid, uric acid, gallic acid, N-acetyl-l-cysteine, glutathione, caffeic acid, ferulic acid, sinapic acid, catechin hydrate, quercetin) and food samples such as coffee, tea, wine, and juices.
3.1.3. Nanoparticle-Based Approach for the Antioxidant Activity Measurement
More recently, the new nanoparticle-based approach for evaluation of antioxidant activity has been reported. This approach utilizes the unique optical, electronic, and catalytic properties of metallic nanoparticles (1–100 nm) [211,212,213,214].
For the first time, Scampicchio et al. [117] described a nanoparticle-based method for measuring antioxidant activity. The idea of the method was based on the catalytic growth of gold (Au) NPs mediated by phenolic acids as active reducing agents (vanillic acid, propyl gallate, protocatechuic acid, caffeic acid, ferulic acid). It appeared that the antioxidant (reducing) power of the phenolic acids was correlated with the optical properties of generated nanoparticles. The absorbance characteristic of the plasmon of the Au NPs (555 nm) was linearly dependent upon the concentration of the investigated phenolic acids. The authors confirmed the good agreement between the total phenolic content estimated by the Folin-Cicolteau spectrophotometric determination and the results of the Au NPs protocol.
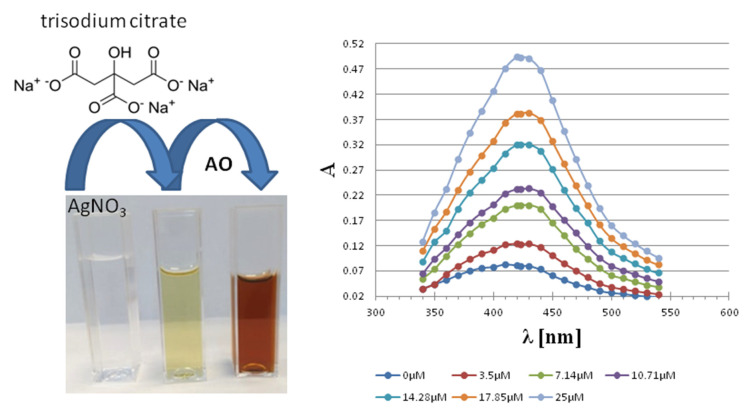
A few years later, Özyürek et al. [118] elaborated on a sensitive colorimetric method based on the reduction of Ag+ ions to silver nanoparticles (AgNPs) for the detection of polyphenols. The AgNPs revealed the absorption band at 423 nm, allowing the quantification of the polyphenols. The initial seeds were formed by the reduction of silver ions with trisodium citrate. The addition of antioxidants as secondary reductants caused the reduction of Ag+ ions on silver seeds and the deposition of more Ag atoms on the seeds, resulting in the final core−shell AgNP structures. The growth of AgNPs on monodisperse seed particles caused a linear, concentration-dependent absorbance increase. The method was named by the research group “Silver NanoParticle Antioxidant Capacity”, abbreviated as the SNPAC method, which is recommended for measuring the total antioxidant capacity (TAC) of a wide range of plant samples (Figure 5).
Figure 5.
Scheme illustrating the idea of Silver NanoParticle Antioxidant Capacity (SNAPC) Assay. On the left side, sample preparation steps; on the right side, surface plasmon resonance absorption of citrate-stabilized AgNPs. Absorption is intensified by the addition of increasing ascorbic acid (AO) concentration, which corresponds to NPs growth.
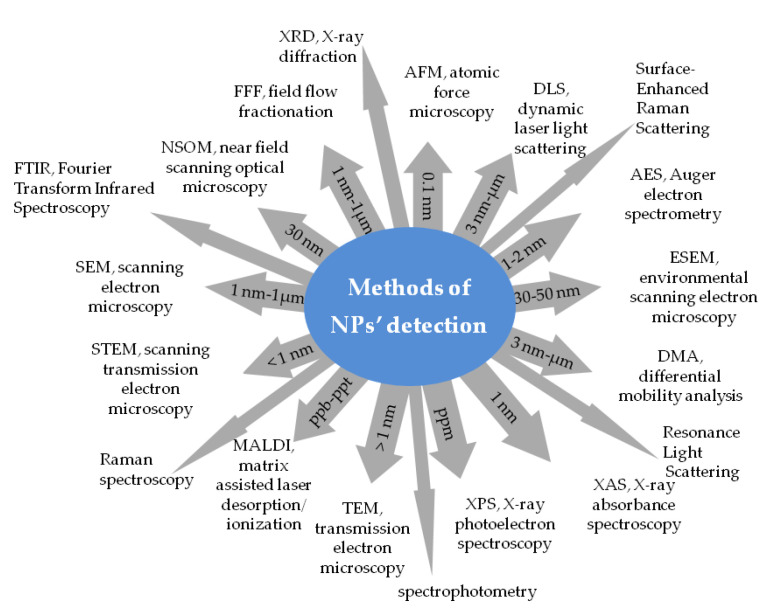
Until now, most assays applied for antioxidant capacity determination have involved the use of NPS of gold, silver, Fe3O4, quantum dots, and titania nanoparticles. The estimation of antioxidant activity relies on the antioxidant-mediated growth of NPs, monitoring changes in NPs size, changes in surface oxidation states, the degree of agglomeration of nanostructures, and optical monitoring of the plasmon absorption bands. AuNPs are still the most commonly used for that purpose. AuNPs have a very characteristic absorbance peak at 517 nm. AuNPs are soluble and stable in different solvents such as water, dichloromethane, or methanol. NPs formation can be monitored visually owing to AuNPs’ color, which depends on their shape and size, but also surface-adsorbed species, the refractive index of the dispersion medium, and interparticle interactions [215]. Different techniques have been engaged for detection and characterization of NPs such as the localized surface plasmon resonance (SPR), Surface-Enhanced Raman Scattering, spectrophotometry, Fourier Transform Infrared Spectroscopy (FTIR), Resonance Light Scattering, Raman spectroscopy, X-ray diffraction (XRD), and transmission electron microscopy (TEM) [216]. Selected methods suitable for measuring size, electric and mechanical properties, size distribution, hydrodynamic radius, elemental composition, and quantitative analysis of nanoparticles together with the methods’ detection limits are illustrated in Figure 6.
Figure 6.
Selected methods applied to detection of nanoparticles together with detection limits [217].
Since nanoparticles-based assay is a new analytical tool, calibration is usually performed using control antioxidants [218,219,220,221], and additionally, the assay is compared with reference methods, e.g., ORAC and TEAC. Many authors achieved very good agreement between the TAC values obtained by the nanoparticle-based approach and the Trolox Equivalent Antioxidant Capacity (TEAC), CUPRAC [222], Folin-Ciocalteu, FRAP, and DPPH [218] as reference tests.
Antioxidant capacity determination by nanoparticles-based method also involves other metallic or metal oxide NPs. Gatselou et al., in 2016 [223], reported that phenolic compounds (i.e., gallates, catechins, dihydroxybenzoic acids, and cinnamates) generate changes in the localized surface plasmon resonance of rhodium NPs, causing characteristic spectral and color transitions in their suspensions. Under the influence of the reaction between phenolic compounds and rhodium, absorbance at 450 nm and 580 nm increased linearly together with increasing concentration of antioxidants in the range of 0–500 μM.
Recently, antioxidant activity (AOA) assays using cerium oxide nanoparticles (CeO-NPs) as a novel colorimetric sensor were developed. Cerium oxide nanoparticles (CeO-NPs) may act as both an oxidant and an anti-oxidant, switching between trivalent and tetravalent oxidation states [224]. In 2018, Ozdemir Olgun [225] elaborated on a novel colorimetric sensor consisting of the poly(acrylic acid) sodium salt (PAANa)-coated CeO-NPs which oxidized a peroxidase substrate, namely tetramethyl benzidine (TMB) in acidic conditions to charge-transfer complex of a blue color. The analytical wavelength of the colored product was estimated at 651 nm. The antioxidant activity evaluation was based on the measurement of decreasing intensity of the nanoceria suspension absorbance caused by antioxidants. The authors demonstrated that the antioxidant capacities of hydrophilic and lipophilic antioxidants such as rutin, tetramethyl benzidine, quercetin, ascorbic acid, Trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid), ferulic acid, BHT, caffeic acid, and catechin estimated by the above procedure were compatible with those of reference assays ABTS, CUPRAC, and CERAC [225]. Currently, portable nanoparticle-based tests for rapid detection of food antioxidants (NanoCerac) are being developed, e.g., for nanoparticles of immobilized cerium oxide [226], or nanoparticles of metal oxides TiO2, Fe2O3, ZrO2, ZnO, and SiO2, which are immobilized on cellulose [227]. Several reviews regarding TAC determination by using NPs can be found in the literature [228,229,230,231,232].
4. Antioxidant Capacity of Extracts from Natural Sources
Epidemiological research confirms that the conditions related to oxidative stress can be improved by the consumption of food products rich in numerous compounds with high antioxidant activity [233,234]. Natural products containing at least 0.1% of antioxidants can be accepted as dietary supplements with antioxidant properties.
As the total antioxidant capacity (TAC) covers the additive (synergistic/antagonistic) action of different antioxidants of complex samples, most researchers use this parameter to assess plant-based extracts rather than the separate determination of the concentrations of the individual constituents. It should be emphasized that antioxidant capacity reflects the thermodynamic conversion efficiency of reactive species by antioxidants in contrast to the antioxidant activity, which is related to the kinetics of this reaction, usually expressed scavenging percentages per unit time. Unfortunately, many phytochemical studies have reported conflicting results, which is why TAC assays still require consideration and standardization in the following issues: (i) procedures of sample preparation, (ii) expressing results, (iii) statistical validation (e.g., using certified reference compounds that take into account the different reaction kinetics), and (iv) establishing effects of solvent, concentration, pH, etc.
The choice of extraction techniques has the greatest impact on the composition and concentration of the bio-composition of both active compounds and matrix components obtained from a wide range of plant materials (herbs, vegetables, berries, and fruits) [235]. It has been shown that different extraction methods lead to different extraction yields on the same plant material [236]. For example, Lisitsyn et al. [237] studied the plant extracts (rosemary, black pepper, thyme, and sage) obtained by the use of supercritical CO2 extraction. Owing to this extraction method, they produced extracts with a significantly different composition in comparison to those obtained in traditional ways. It appeared that supercritical extracts were rich in a variety of substances with high antioxidant, and antimicrobial activities such as alkaloids, terpene, phytosterols, waxes, pigments, high molecular weight unsaturated and saturated fatty acids, and vitamins.
Currently, classic extraction techniques, i.e., Soxhlet extraction, maceration, percolation, and distillation, which use large amounts of volatile organic solvents, or elevated temperature, are less frequently used due to the requirements of the so-called “green chemistry”, poor efficiency, and possible thermolability of extracted analytes [238]. High extraction efficiency and effectiveness are possible thanks to the use of unconventional techniques, i.e., Solid Phase Microextraction (SPME), Supercritical Fluid Extraction (SFE), Microwave-Assisted Extraction (MAE), Pulsed-Electric Field (PEF) Extraction, Ultrasound-Assisted Extraction (UAE), and Enzymatic Treatment or Pressurized Liquid Extraction (PLE). It should be remembered that antioxidant capacity changes not only in relation to the extraction techniques but varies with the growth period and drying methods and between plant parts. These factors’ influence has been confirmed by a multivariate analysis performed by Buitrago et al. [239] using Chenopodium quinoa Willd.
Plant-derived compounds possess well-known and established antioxidant activity. However, the microbes are also efficient producers of primary and secondary metabolites with specific antioxidant potential [240]. Thus far, microbial metabolites have been recognized as efficient remedies against fungal and bacterial infections (tetracyclines, amphotericin, penicillins, erythromycins, streptomycin, and vancomycin), cancer (daunorubicin, bleomycin, mitomycin, doxorubicin,), transplant rejection (rapamycin, cyclosporine), or high cholesterol (mevastatin, lovastatin) [241].
Almost all eubacteria possess the ability to produce a variety of extracellular metabolites with significant antioxidant activity, such as thiazostatins A, phenazoviridin, (Z)-1-((1-hydroxypenta-2,4-dien-1-Yl)oxy)anthracene-9,10-dione, 5-(2,4-dimethyl benzyl) pyrrolidin-2-one, benthophoenin, benzastatins C, benthocyanins A, B, C, exopolysaccharides (EPS), and benzastatins A [78]. Exopolysaccharides (EPS) are produced by numerous strains of microorganisms belonging to the genera Lactobacillus, Leuconostoc, Lactococcus, and Streptococcus, which are abbreviated LAB (Lactic Acid Bacteria) [54]. EPS are characterized by the presence of reactive functional groups including aldehyde, hydroxyl, and ketone groups. They can efficiently react with free radicals. These compounds have a polymeric structure. They are made up of repeating subunits of connected carbohydrates α- and β-glycosidic bonds. Homopolysaccharides are composed of one type of simple sugar, i.e., glucose or fructose, while heteropolysaccharides are more complex. The structure of individual heteropolysaccharides produced by particular species and strains of these bacteria can significantly vary. A common feature of most of them is occurrence in the composition of sugars e.g., rhamnose, arabinose, mannose, xylose, fructose, glucose, and galactose, in various ratios [242]. The EPS may exist in two forms: a cell-bound exopolysaccharide (c-EPS) that strongly binds to the bacterial surface and a released exopolysaccharide (r-EPS) that can be released into the medium [243].
Cyanobacteria and blue-green algae are sources of a significant amount of free radical scavengers such as carotenoids, phycocynanin which are water-soluble pigments possessing N-H reactive groups. Astaxanthin produced by microalga Haematococcus pluvialis possessing several times higher antioxidant activity in comparison to vitamin E [244,245]. Algae are able to produce also other phenolic compounds with reactive OH moieties responsible for an antioxidant activity like carrageenan, bromophenol, fucophlorethols, galactan sulphate, phlorotannins, fucoxanthin, phycoerythrin, shinorine, catechin, por-phyran, epicatechin, gallate, laminaran, vitamin A, alginic acid, phloroglucinol, eckol, fucodiphlorethol G, 7-phloroeckol, dieckol, phlorofucofuroeckol A, 6,60-bieckol, 2,70-phloroglucinol-6,60-bieckol, and triphlorethol-A, [246,247,248]. As a potential antioxidant, phycobilins rich with groups i.e., N–H, COOH, C–O, and O–H produced by cyanobacteria have been described. However, efficient extraction and purification are required for the recovery of phycobiliproteins on an industrial scale [249]. In 2019, the Special Issue of Antioxidants focused on recent investigations concerning marine algal antioxidants and specific antioxidant networks functioning in algae [248,249,250,251,252,253,254,255,256,257,258,259].
Lichens produce various extracellular, secondary metabolites that can be used as potential sources of natural antioxidants [260]. At the beginning of the 21st century, the significant free radical scavenging activity of Cetraria islandica aqueous extracts [261] and Usnea ghattensis [262] methanolic extracts of Platismatia glauca, Parmelia saxatilis, Ramalina pollinaria, Umbilicaria nylanderiana, and Ramalina polymorpha [263] were described. Fernández-Morianoet et al. [264] prepared a systematic review concerning the key antioxidant compounds in lichens extracts. It appeared that flavonoids and phenols are mainly responsible for the antioxidant activity of the examined extracts [265,266,267,268]. Some of them also exhibited beneficial antimicrobial and anticancer activities [269,270].
Actinomycetes also produce chemically diverse and pharmaceutically useful compounds with antifungal, antibacterial, diabetogenic, antiviral, immunosuppressive, antiparasitic, antitumor, insecticidal, antioxidant, anti-inflammatory, enzyme inhibitory, and others [271]. Actinomycetes originating from different habitats usually manifest very different antioxidant activity [272,273,274,275,276,277]. Published studies show that nitrogen-containing metabolites, such as the carbazole and phenazinylhetero cycles, constitute the main group of antioxidant compounds produced by Streptomyces spp. Stealthins contain OH, NH, and CO groups isolated from S. Aeriouvifer, S. Violaceus, and S. viridochromogenes showed even several dozen times stronger activity than vitamin E [278].
Other examples of antioxidant capacity assessment of the different extracts from plants, lichens, fungi, algae, and actinomycetes are collected in Table 3.
Table 3.
Examples of antioxidant capacity assessment of extracts obtained from different species.
| Analysed Product | Antioxidant Assays |
Positive Control |
Extraction Procedure | Ref. |
|---|---|---|---|---|
| Plants | ||||
| Bryonia alba L. | DPPH, CUPRAC, FRAP, TEAC, SNPAC |
quercetine, BHT, Trolox | Fifty grams of powder was macerated with 500 mL methanol for 24 h. After percolation, extract was evaporated under vacuum at 40 °C. | [279] |
| Cistus ladanifer L., Cistus salvifolius L., Cistus albidus L., Erica australis L., Arbutus unedo L., Pistacia lentiscus L. | DPPH, FRAP, ABTS, RP |
quercetin | Twenty grams of grounded leaves was mixed with 200 mL of methanol. The mixture was kept for 24 h at RT. Then, it was filtered. | [280] |
| Prunus avium, Prunus persica, Prunus domestica, Olea europeae, Pirus communis, Pirus maus, Pistacia verra, Castanea sativa | DPPH, FRAP, TAC |
Trolox, ascorbic acid | Two grams of sample was mixed with 60% methanol and kept 1 h at dark at RT. The procedure was repeated two times. The combined extracts were centrifuged and filled to 50 mL by aqueous methanol. | [281] |
|
Vitis vinifera L. (Maraština, Pošip; Lasin, Merlot, Syrah, Vranac) |
DPPH, FRAP | Trolox | The dry plant material (20 g) was extracted using 100 mL of ethanol/water 80/20, (v/v) at 60 °C, for 60 min. The extract was filtered and dried under a vacuum at 50 °C. The dry residues were redissolved with 50% methanol-water and centrifuged at 5000 rpm for 10 min. | [282] |
| Ornithogalum billardieri | β-carotene-linoleic acid assay, ABTS, MCA/ferrous ion, TAC/H2SO4, Na3PO4, (NH4)2MoO4) | ascorbic acid | The combined maceration with sonication either at 25 °C for 50% ultra-sound (US) treatment or the extract obtained under the optimal conditions: extraction time: 37.1 min, temperature: 44.2 °C, water volume-to-mass ratio: 33.8 mL/g, and US%:51.7% | [283] |
| Apple cultivars: antonówka, delikates, Early Geneva, papierówka, Paulared, Sunrise, Quinte Gloster, Jonagored, Ligol, Rubinola |
DPPH, FRAP, MCA/ferrous ion | Trolox, catechin | The material was lyophilized. A total of 250 mg of sample was extracted by sonication using 1 mL of 80% methanol for 30 s. Then, the mixture was vortexed, centrifuged for 5 min (13,200 rpm), and sonicated. The extraction procedure was repeated five times. The supernatants were collected together. | [209] |
| Fraxinus angustifolia Vahl | TAC/Folin-Ciocalteu, FRAP, ABTS, DPPH | α-tocopherol | Manna samples (10 g) were dissolved in methanol-water (2:1) and extraction carried out at 25 ± 2 °C and in darkness for 1 h., and centrifuged (3000 g, 10 min). The supernatants were filtered and evaporated at 35 °C. Dried samples were resuspended in 5 mM phosphate buffer saline pH 7.4. | [284] |
| Chenopodium quinoa | DPPH, FRAP | Trolox, gallic acid | Dry plant materials (roots, leaves, stems, flowers, and seeds) were extracted by the use of ultrasound-assisted extraction with 96% ethanol. The extracts were concentrated under reduced pressure in a rotary evaporator. | [239] |
|
Lycium barbarum, Lyciumchinense |
DPPH, ABTS | - | Samples were dried, powdered, and dissolved in distilled water. | [285] |
| Aegopodium podagraria L. | DPPH | GSH, ascorbic acid | A total of 2.5 g of air-dried or fresh aerial parts was extracted by 100 mL of 80% (v/v) ethanol. The samples were kept at RT for 3 days, three months in dark, or in an ultrasonic bath for 60 min. Then, the extracts were filtered. | [286] |
| Fungi | ||||
| Achaetomium sp. | DPPH | ascorbic acid, BHT, gallic acid, pyrogallol | The organic ethyl acetate extract was evaporated. The crude extract was dissolved in DMSO. | [287] |
| Acremonium charticola, Rhizopus oryzae | ABTS | ascorbic acid | Fungi were cultured in potato dextrose broth at 37 °C. After 3 days, the cultures were centrifugated at 5000 rpm for 10 min. The filtrate (1 g) was mixed with 100 mL methanol and ultrasonicated for 30 min. The homogenate was created for three days at RT. Then, it was evaporated with a rotary vacuum evaporator (50 °C, 100 rpm) to the volume of 25 mL. | [288] |
| Agaricus bisporus, Pleurotus ostreatus, Pleurotus eryngii, Lentinula edodes | TPC/Folin–Ciocalteu, Ferricyanide/prussian blue, DPPH, TBARS β-carotene/linoleate |
Trolox, gallic acid | The product was lyophilized. The obtained powder was mixed with methanol and kept at 25 °C at 150 rpm for 1 h. Then, the mixture was and filtered. The extracts were evaporated under reduced pressure and redissolved in methanol at a concentration of 20 mg/mL. | [171] |
| Aspergillus wentii, A. wentii, Penicillium citrinum, Penicillium granulatum | DPPH, FRAP, MCA/ferrous ion, NO• scavenging activity, RP/potassium ferricyanide |
ascorbic acid, BHT, rutin, catechin |
The fungal mycelia were grown on agar plates with extracts of yeast and glucose. After 6 to 7 days, the Czapek–Dox’s broth was inoculated of fungal mycelia. After 10 days of incubation at 25 °C, the culture broth was filtered. | [289] |
|
Aspergillus niger, Aspergillus peyronelii |
DPPH, RP H2O2 scavenging activity |
ascorbic acid | The dried samples were extracted by the use of ethyl acetate (1:10) applying cold percolation for 48–72 h. Then, obtained extracts were filtered, and concentrated under vacuum at 40 °C. | [290] |
| Aspergillus versicolor | ABTS, DPPH | Trolox | Aspergillus versicolor was cultivated on rice for 30 days. The EtOAc extracts of solid fermentation were fractioned through slica gel and Sephadex LH-20 column chromatography (CC), and were further purified by semi-preparative HPLC. | [291] |
| Auricularia auricular | ABTS, O2●−, OH• scavenging activity, lipid peroxidation |
- | A.auricula was extracted by hot water and ultrasonic-assisted extraction. The supernatants were precipitated with absolute ethanol (95%) and maintained at 4 °C overnight. The precipitate was centrifugated, dissolved in DI, and dialyzed. The non-dialyzed portion was lyophilized to give a crude polysaccharide extract. The separation of polysaccharides was performed using CTAB or CPC. | [292] |
| Cephalosporium sp. | DPPH | gallic acid | The fermented material (1.75 kg) was extracted twice with EtOAc (9.0 L) for 3 days at RT, and the extract was evaporated under vacuum. | [293] |
| Cerrena unicolor | ABTS, DPPH | Trolox, ascorbic acid | Ten-day-old cultures were filtered and washed with DW. The fungal biomass was used for the polysaccharides extraction by hot water (90 °C, 4 h). The proteins were separated by anion exchange chromatography on a DEAE Sepharose column with a linear gradient of NaCl (0.1–0.5 M). The culture liquid was subdivided into two fractions by ultrafiltration: substances above 10 kDa (rude lactase) and substances below 10 kDa with low molecular weight metabolites. | [294] |
|
Flammulina velutipes, Hypsizygus tessellatus |
DPPH, H2O2 scavenging activity, FRAP |
gallic acid, quercetin | The stems of the mushrooms were dried at 60 °C and pulverized. Samples (180 g) were extracted overnight with 500 mL of water, absolute methanol, 95% acetone, or 95% ethyl acetate at RT. The extracts were filtered, evaporated to dryness. Aqueous fractions were concentrated to 50 mL, freeze-dried, and stored at 4 °C. | [295] |
| Grifola frondosa | DPPH, β-carotene bleaching assay, inhibition of lipid peroxidation, RP, CUPRAC | quercetin | Mushrooms were boiled in DW at the ratio of 1:10 (w/v) for 30 min. Then, the extract was filtered, and freeze-dried. | [296] |
| Penicillium expansum | DPPH, RP, FRAP MCA/ferrous ion, NO• scavenging activity |
- | The fungi were grown on CDM, MEM, PDM, and YEM. After incubation at 25 °C for 10 days, the culture broth was filtered through filter paper. The culture broth was extracted with petroleum ether, chloroform, ethyl acetate, and butanol. Then, the extracts were then evaporated to dryness in a vacuum, and the precipitates were dissolved in DMSO. | [297] |
|
Phlebia brevispora, Phlebia floridensis, Phlebia radiate, Phlebia fascicularia |
DPPH, FRAP, RP, MCA, NO• scavenging activity |
- | Five grams of dried wheat straw was ground, washed, and dried at 90 °C. Next, it was moistened by the use of 25 mL of malt extract (0.5%, w/v) and inoculated with three mycelial discs (8 mm), which were grown on YGA plates for 6 days. The inoculated flasks were kept at 25 °C for 30 days. Then, they were homogenized, filtered, and dried at 90 °C. | [298] |
|
Pleurotus florida, Pleurotus sajor-caju, Pleurotus cystidiosus, Pleurotus djamor |
FRAP, DPPH, RP, MCA, H2O2, O2●− scavenging activity |
- | Sporophores were cleaned and dried at 40 ± 2 °C for 10–12 h. The obtained powders (100 g) were refluxing with light petrol (60–80 °C) for 6 h with the aim of defatting. Then, the material was extracted with 95% ethanol (500 mL × 3) by refluxing for 6 h. The extracts were combined, filtered, and evaporated to dryness at 40 °C. The dried extracts were redissolved in methanol at a concentration of 20 mg/mL for analysis. | [299] |
|
Trametes versicolor, Trametes hirsuta, Trametes gibbosa |
ABTS, FRAP | ascorbic acid | Dried material (3.0 g) was grounded. Extraction was carried out in 96% ethanol during 72 h. The extracts were centrifuged and supernatants filtrated. The filtrates were concentrated under reduced pressure at 40 °C to dryness and redissolved in 96% ethanol | [300] |
| Bacteria | ||||
| Bacillus coagulans RK-02 | β-carotene-linoleate, O2●−, OH• scavenging activity, DPPH |
ascorbic acid, α-tocopherol | The 36 h culture was centrifuged at 10,000× g for 20 min at RT. The supernatant was filtered. The proteins were isolated by the addition of 10% TCA. After 12 h at 4 °C, the mixture was centrifugated. Four volumes of 95% ethanol were added to the supernatant and centrifuged. The pellet was lyophilized, dissolved in 5 mL DW and dialyzed (MWCO 12,000 Da). | [301] |
| Weissella cibaria GA44 | DPPH, RP, O2●−, OH• scavenging activity | ascorbic acid | The cultures were heated at 100 °C for 10 min. The cells were removed by centrifugation at 12,000× g for 15 min at 4 °C. The supernatant was precipitated with double volume of chilled ethanol, shaken, and centrifuged at 98 5000× g for 30 min at 4 °C. The precipitate was dried at 50 °C, and dissolved in water. This step was repeated three times and dialyzed against distilled water for two days at 4 °C using 10 kDa dialysis membrane and then lyophilized. | [302] |
| Lactobacillus plantarum C88 | OH• scavenging activity, DPPH, the LPC-1 on H2O2− induced oxidative stress in Caco-2 cells | ascorbic acid | After 20 h of the incubation period, TCA was added to achieve 4% (w/v). The mixture was stirred for 30 min at RT. Cells were removed by centrifugation (10,000× g, 4 °C, 15 min). Crude EPS was precipitated by the addition of 2 volumes of cold ethanol. Crude EPS was collected by centrifugation. The pellet was dissolved in deionized water and dialyzed (MW cut-off 3500 Da) for 24 h against distilled water at 4 °C and then lyophilized. | [303] |
|
Pseudomonas hibiscicola, Macrococcus caseolyticus, Enterobacter ludwigii, Bacillus anthracis |
DPPH | ascorbic acid | The bacteria were cultured in 500 mL broth at 35 °C. Then, the culture was centrifuged at 8000× g for 5 min. The supernatant was extracted with ethyl acetate (ratio of 1:1). Then, the extract was concentrated to dryness in a rotatory evaporator at 37 °C. The solids were re-dissolved in 20% DMSO and filtered. | [304] |
|
Alteromonas sp. Shewanella sp. Serratia sp., Citricoccus sp., Cellulophaga sp., Ruegeria sp. Staphylococcus sp. |
DPPH, ORAC | BHT, Trolox | Isolated bacteria were cultured in 500 mL of Marine Broth for 3 days at 25 °C. Bacteria cells were centrifuged, and the pellet was lyophilized. Then, the lyophilizes were extracted with methanol and dichloromethane (1:1) for 12 h. The solvents were evaporated at 40 °C, and the extracts were re-dissolved in DMSO. | [305] |
|
Pseudomonas sp. (HR04) |
anti-lipoperoxidative activity | BHT, α-tocopherol | The mycelial cake was extracted with acetone followed by purification by column chromatography on silica gel and Sephadex LH-20. | [306] |
|
Lactobacillus casei CRL 431 (IC431) |
LPO, ABTS, ORAC, CAT, GPx |
Trolox | An aliquot (10 mL) of bacteria suspended in PBS was mixed with lysozyme (1 mg/mL) and incubated at 37 °C for 150 min. Then, cells were disrupted by sonication in an ultrasonic processor at 10 °C. After centrifugation (3600× g, 4 °C, 10 min), the supernatant was collected and stored in dark at 4 °C. | [307] |
| Lactobacillus fermentum | DPPH, ABTS, FRAP, OH• scavenging activity | ascorbic acid | The cells were washed with 0.85% NaCl and sonicated. The obtained fluid was mixed with 75% ethanol. The precipitate was collected, redissolved, deproteinized, purified on an anion exchange column eluting with deionized water, 0.1 M, and 0.3 M NaCl and subsequently loaded onto a Sephadex G-100 column and eluted with DW. The collected fractions were lyophilized. | [308] |
|
Lactobacillus plantarum (LAU103) |
ABTS, DPPH, ORAC, MCA/ferrous ion, OH• scavenging activity | ascorbic acid | A total of 5 mL of crude EPS solution (20 mg/mL) was separated with DEAE-cellulose column using deionized water, 0.2 and 0.5 M NaCl as eluent. Peak fractions containing polysaccharides were pooled, dialyzed, and lyophilized. Then, the fraction was further purified on a Sepharose CL-6B gel column and eluted with 0.9 M NaCl solution. | [242] |
|
Lactobacillus paracasei subsp. paracasei NTU 101 (101EP), Lactobacillus plantarum NTU 102 (102EP) |
DPPH, MCA/ferrous ion, inhibition of linoleic acid peroxidation, RP |
ascorbic acid | The cultures were centrifugated at 5000× g at 4 °C for 15 min. The supernatant was added to 0.4 TCA at 4 °C for 3 h. Then, the supernatant was mixed with ethanol at 4 °C for 24 h, followed by centrifugation. The obtained precipitate was dialyzed for 24 h and lyophilized. | [309] |
| Algae | ||||
| Astaxanthin | FRAP, TEAC ORAC, DPPH |
tert-Butyl alcohol | Astaxanthin was dissolved in tetrahydrofuran (THF). | [244] |
|
Desmarestia antarctica, Iridaea cordata |
RP, TPC, DPPH | gallic acid, ascorbic acid | The samples were rinsed with Milli-Q water, cut into fine pieces, then boiled at reflux for 15 min. The flask was moved to an ice bath to complete the extraction. The extract was thus centrifuged at 4500 rpm for 10 min, and the supernatant was filtered and stored at 4 °C. | [310] |
|
Haematococcus pluvialis, synthetic astaxanthin |
ABTS, ORAC, CAA | Trolox | The extracts were obtained by solvent using DMSO or supercritical extraction (AstaCO2). | [311] |
| astaxanthin | ORAC-EPR | - | The acetonitrile solutions of Catechin, epicatechin, epigallocatechin gallate, kaempferol, myricetin, resveratrol, and astaxanthin were diluted with phosphate buffer containing DM-β-CD. | [312] |
|
Dunaliella salina, Tetraselmis chuii, Isochrysis galbana clone Tahiti. |
DPPH | - | Methanolic extracts were prepared in different concentrations (50, 100, 250, 500, and 1000 ppm). | [313] |
|
Galderia sulphuraria, Neochloris texensis, Stichococcus bacillaris, Ettlia carotinosa, Chlorella minutissima, Schizochytrium limacinum, Crypthecodinium cohnii, Chlorella vulgaris |
DPPH | - | Methanol extraction: 20 mL methanol was added to 0.5 g dry biomass and sonicated (9 cycles, 50% power) for 20 min. Then, samples were centrifuged at 3500 rpm for 5 min. Pellets were re-extracted in 20 mL methanol 3 times and the supernatants were collected. The samples were filtered and evaporated at 40 °C. Hot water extraction: 1 g of dry sample was added to 100 mL DW and boiled for 30 min. After cooling, extracts were centrifuged at 3500 rpm for 10 min, and supernatants were freeze-dried. | [314] |
|
Chlorella vulgaris
Spirulina platensis |
ORAC, DPPH, FRAP | Trolox | The extracts were obtained by the use of ultrasound-assisted extraction by water/ethanol (50:50, v/v). | [315] |
| Scenedesmus subspicatus | DPPH | catequin, gallic acid | Different solvents such as ethanol, methanol, butanol, acetone, DMSO, and water were used for extraction. One gram of dried samples were mixed with 10 mL for each solvent. The extraction was carried out for 30 min by sonication (40 kHz) in an ultrasonic bath followed by a 2 h shake, and centrifugation for 10 min. | [316] |
| Tetraselmis suecica | DPPH | α-tocopherol | A total of 100 mg of freeze-dried biomass was extracted with 1 mL ethanol/water (3:1, v/v) for 30 min. The mixture was centrifuged at 4500× g, for 10 min, at 20 °C. Then, the ethanolic phase was dried. | [317] |
| Lichens | ||||
| Cetraria islandica (L) Ach. | DPPH, the thiocyanate method, RP, O2●− scavenging activity | α-tocopherol, BHT, BHA |
For water extraction, 20 g sample was mixed with 400 mL boiled DW and stirred for 15 min. Then, the extract was filtered. The obtained filtrates were frozen and lyophilized. | [261] |
| Usnea ghattensis | ABTS, O2●− scavenging activity, lipid peroxidation/linoleic acid | Trolox BHT, BHA, quercetin | Cell mass (14.8 g dry wt) was extracted using 20 mL of 10% (v/v) acetone, dimethyl sulphoxide (DMSO), methanol or light petroleum (40–60 °C) at RT. The extracts were then filtered, concentrated 4-fold under vacuum, and freeze-dried and then dissolved in 1 mL of acetone, DMSO, methanol, or water for the preparation of test stock solutions. | [262] |
|
Parmelia saxatilis, Platismatia glauca, Ramalina pollinaria, Ramalina polymorpha Umbilicaria nylanderiana |
DPPH, the inhibition of linoleic acid oxidation | gallic acid | Air-dried and powdered lichens (10 g) were mixed with 250 mL of methanol. The extraction was conducted in the Soxhlet apparatus for 72 h at a temperature of the boiling point of the solvent. The extracts were filtered and then concentrated in vacuo at 40 °C. | [263] |
|
Anaptychya ciliaris, Nephroma parile, Ochrolechia tartarea Parmelia centrifuga |
MCA/ferrous ion, TPC/Folin-Ciocalteu reagent, RP | Trolox, ascorbic acid | One hundred grams of pulverized dried lichen were extracted with 1 L of methanol using a Soxhlet apparatus for 72 h. The obtained extracts were filtered and then concentrated under reduced pressure. | [266] |
|
Parmotrema praesorediosum, P. rampoddense, P. tinctorum P. reticulatum |
DPPH | - | Powdered lichen samples (250 g) were subjected to soxhlet extraction using acetone and methanol. The extracts were then filtered through filter paper, concentrated in vacuo, and air-dried. | [268] |
| Actinomycetes | ||||
|
Streptomyces (R56-07) |
DPPH | α-tocopherol | The ethyl acetate extract of the fermentation broth was subjected to silica gel MPLC and for further purification to a Sephadex LH-20 column and RP-HPLC. | [271] |
| Streptomyces chromofuscus | the inhibition of lipid peroxidation, DPPH | α-tocopherol BHT | Carbazole compounds, carazostatin, carbazomycin B and their chemically modified derivatives were isolated from the culture of Streptomyces chromofuscus by the use of chromatography on silica gel with hexane-EtOAc (20:1) as an eluent. | [318] |
| Streptomyces sp. (CL190) | the inhibition of lipid peroxidation |
α-tocopherol | The mycelial cake was stirred with acetone. The extract was concentrated in vacuo and extracted twice with ethyl acetate. The extract was dried and concentrated in vacuo. The fraction was applied to a silica gel column with n-hexane and ethyl acetate (4:1). The fraction was concentrated to dryness. The dry residue was rechromatographed on a silica gel column with chloroform, methanol, and ammonia (200:20:1). The elute was concentrated in vacuo and the residue was dissolved in chloroform and methanol (1:1) and purified by column chromatography on Sephadex LH-20 with the same mixture. The fraction was evaporated to dryness in vacuo and dissolved in ethyl acetate. | [319] |
|
Streptomyces LK-3 (JF710608) |
DPPH, MCA/ferrous ion, FRAP, β-carotene assay, NO• scavenging activity | gallic acid | The crude extracts were diluted in water containing daidzein- 8-C-glucoside (puerarin), (−) gallocatechin gallate, sesamol, cyanidin-3-O-rutinoside, and delphinidinas. | [320] |
Abbreviations: DPPH (2,2-diphenyl-1-picrylhydrazyl), CUPRAC (cupric reducing antioxidant capacity), FRAP (ferric reducing ability of plasma), TEAC (Trolox equivalent antioxidant capacity), EPR (electron paramagnetic resonance method), SNPAC (silver nanoparticles antioxidant capacity), BHT (butyl-hydroxytoluene), TE (Trolox equivalents), RP (reducing power assay), TPC (Total Phenolic Content), MCA (metal chelating activity assays), TBARS (Thiobarbituric Acid Reactive Substances), AAP (A. auricula-judae polysaccharide), EPS (Exopolysaccharide), SDM (A semi-defined medium), LPO (lipid peroxidation–hepatic lipid peroxidation), CAT (Antioxidant Enzymes Activity–catalase), GPx (glutathione peroxidase), TCA (trichloroacetic acid), ESR (electron spin resonance), XO (xanthine oxidase), GR (glutathione reductase), PMSF (phenylmethanesulfonylfluoride), CAA (cellular antioxidant activity), RT (room temperature), GSH (Reduced glutathione), CTAB (Cetyl Trimethyl Ammonium Bromide), CPC (Cetylpyridinium Chloride), CDM (Czapek Dox’s Medium), MEM (Malt Extract Medium), PDM (Potato Dextrose Medium), YEM (yeast extract glucose medium), YGA (yeast extract glucose agar), Astaxanthin (3,31-dihydroxy-β,β 1-carotene-4,41–dione), DMSO (dimethyl sulfoxide).
5. Synthesis of Nanoparticles (NPs) by Natural Extracts
The various types of metallic/metal oxides nanoparticles composed of silver, gold, platinum, palladium, cerium, copper, nickel, selenium, or iron have been described in the literature. Their unique physicochemical properties make them advanced materials for industry and biomedical applications [321]. There is also evidence that, besides natural extracts, some nanoparticles such as carbon nanotubes, metal, and metal oxides, and various types of polymer-loaded nanoparticles also possess antioxidant activity and can scavenge the reactive nitrogen and reactive oxygen species (RNS/ROS) [322,323]. The iron nanoparticles (INPs), due to high catalytic activity, low toxicity, high magnetism, and microwave absorption ability [324,325,326], have already found varied applications in pharmacy (drug delivery), clinical diagnostic (magnetic targeting, negative MRI contrast enhancement, pigments, stem cell sorting), therapy (gene therapy), and analytical chemistry (bio-separation), bioprocesses (environmental remediation, food preservation), industry (lithium-ion batteries) [247,327].
Unfortunately, nanoparticles synthesized by chemical methods often require toxic reducing and stabilizing agents. These toxic substances adsorbed on the surfaces of the nanoparticles limit their applications in biomedical fields [143]. Thus to obtain nanomaterials, the natural synthesis methods involving the reduction of metallic cations by plant extracts, yeasts, fungus, and bacteria are used more and more often. The formation of NPs is achieved via two steps: in the first one, metal ions are reduced, and in the second one the agglomeration of colloidal suspension causing the formation of the oligomeric clusters [328]. So-called “green synthesis” or “biogenic synthesis” has gained more and more attention as an eco-friendly approach useful for synthesis of not only metal/metal oxide nanoparticles but also the production of other nanomaterials, such as hybrid materials, or a variety of bioinspired materials. Nanoparticles produced by green synthesis methods may be less stable compared to nanoparticles obtained as a product of chemical synthesis [329,330,331]. The stabilization of nanoparticles is mainly achieved by electrostatic repulsion. Unfortunately, this type of stabilization is only effective with low ionic strength extracts where the repulsion is facilitated by the highly dispersed double layer. In the case of high ionic strength, aggregation occurs under the influence of strong van der Waals interactions [332]. Another type of stabilization is the creation of an additional barrier on the surface of the NPs. Steric stabilization is provided by proteins, if they are components of the extracts, or by coating the surface with polymers such as PEG or PVP (polyethylene glycol, polyvinylpyrrolidone). Steric stoppers, thanks to their hydrophilic properties, provide an additional stabilizing element in the form of short-distance repulsive forces. The stages of NPs formation and stabilization are schematically illustrated in Figure 7.
Figure 7.
Schematic diagram illustrating the mechanisms of the biogenic synthesis of metallic nanoparticles.
Presently, one can observe an increasing interest in studies on the reactivity of nanoparticles compared to macroscopic objects and their cytotoxicity [333,334,335,336,337] accumulation in the body, which can generate reactive oxygen species (ROS) [338,339,340,341]. A relatively new area of research is the use of nanoparticles with redox-active potential as radical scavengers. For example, cerium and yttrium oxides either act as antioxidants [342] or can prevent the increase of ROS [343,344] by mimicking the activity of the oxidative enzymes, catalase, or superoxide dismutase [345]. It has been proven that silver nanoparticles (AgNPs) inhibit cell proliferation and modulate the activity of antioxidant enzymes [340,346,347]. Hirst et al. confirmed by an in vivo test on mice the effectiveness of cerium oxide nanoparticles (CONPs) in treating oxidative stress [348]. A comparative study conducted by Caputo et al. (2015) revealed that the antioxidant potential of N-acetyl-cysteine and Trolox (soluble analogues of vitamin E) was significantly lower in comparison to CONPs [349]. The authors highlighted the CONPs regenerative redox cycle influencing the stability of the antioxidants molecules.
5.1. Microbial Synthesis of NPs
The first experiments on AgNPs biosynthesis using bacteria were carried out in the culture of Pseudomonas stutzeri AG 259, Morganella sp. Bacillus subtilis [350]. Using microscopic and spectral techniques SEM, TEM, EDX, and EDS, it was possible to identify various shapes of nanoparticles, i.e., triangular, hexagonal, and spherical with sizes ranging from a few to several hundred nm. The synthesis process was initiated within the first hour of cultivation. The obtained NPs were coated with protein, which allowed them to maintain stability and avoid their aggregation. It has been shown that the enzyme nitrate reductase is responsible for the reduction of silver ions. Thus far, the participation of other groups of enzymes, whose role is electron donation and their further transfer, has been described and proven, i.e., nitrate and iron reductases, dependent on the nicotinamide adenine dinucleotide (NAD+)/NADH and the nicotinamide adenine dinucleotide phosphate NADP+/NADPH redox couples hydrogenase, and oxidase. Silver ions, due to their interaction with cytochromes and inhibition of electron transport, lead to disturbances in the functioning of the respiratory chain. The mechanism of silver nanoparticle synthesis in lactic acid bacteria was investigated in detail [351]. It was noted that the alkaline environment clearly favors the formation of nanoparticles as it catalyzes the enolization of monosaccharides. The resulting aldehyde is oxidized to carboxylic acid, while the metal ions are reduced to nanoparticles.
The effect of nanoparticles on bacteria is complex and not fully understood due to the existence of numerous mechanisms of action (Figure 8). Bacteria and other microorganisms such as viruses, fungi, flagella, yeasts, and actinomycetes possess the ability to produce metallic NPs intracellularly as well as extracellularly. The studies showed the action of AgNPs on Escherichia coli and Staphylococcus ureus [352]. The appearance of pits in the bacterial envelope has been observed, which lead to a change in the electrostatic potential, an increase in the permeability of membranes, and damage to the DNA of the cell. Later, research was extended to other species of bacteria, which allowed for the emergence of two more mechanisms of action in the form of overproduction of free radicals (ROS) and the formation of complexes with various intracellular compounds, i.e., nucleic acids [353,354]. It is particularly interesting that even a short incubation with nanoparticles leads to the accumulation of chaperones and the S6 protein [355] and inhibition of the bacterial communication system (quorum sensing, QS), which is associated with a change in gene expression controlled by transcription regulators. It is known that disturbances in the functioning of these genes cause a change in the behavior of cells in the environment, e.g., the ability to create biofilms. This applies even to pathogenic bacterial strains such as Pseudomonas aeruginosa and Staphylococcus aureus. Biofilms create populations of microorganisms (bacteria, fungi, protozoa) that live at the interface [356]. They are surrounded by a protective substance composed of polysaccharides, proteins and nucleic acids, called a matrix. Biofilm formation is a multi-step process but is always initiated by adhesion. Within the biofilm, there may be synergistic or antagonistic interactions between the species inhabiting it, which may lead to the matrix disintegration [357]. The most dangerous are biofilms composed of pathogenic bacteria E. faecalis, S. aureus, Staphylococcus epidermidis, E. coli, Klebsiella pneumoniae, and P. aeruginosa [358], which are mostly responsible for hospital infections that are difficult to cure and are characterized by increased resistance to therapies [359]. The antibiotic resistance of biophimes is the result of, among other things, the presence of a matrix that is a barrier to drug penetration and the production of enzymes responsible for the hydrolysis of ß-lactam antibiotics [360,361]. Research on the potential of nanoparticles to combat biofilms meets the expectations of modern medicine. However, the number of publications on this topic has so far been rather small. It was shown that P. aeruginosa and S. epidermidis biofilms were inhibited in over 95% of cases by silver nanoparticles with a spherical shape and an average diameter of 50 nm [362]. The inhibition of biofilms formed by Multidrug-Resistant Klebsiella pneumoniae [363], Methicillin-Resistant Staphylococcus aureus [364], and Mycobacterium tuberculosis [365] has been proven. Unfortunately, the aggregation of nanoparticles inhibits their effective activity. Consequently, various stabilizers such as starch, citrate, and amino silica are used, and numerous composites composed of nanoparticles and other compounds have been identified. There are also reports on the inhibition of biofilm formation by AgNPs on medical devices, i.e., urological catheters, the surface of which was covered with nanosilver, were characterized by resistance to E. coli, S. aureus, and Candida albicans, even under continuous fluid flow conditions. [366]. Metallic/metal oxide nanoparticles, i.e., silver, gold, magnesium, titanium, zinc, aluminum, tantalum, and zirconium have been tested in orthopedics [367,368,369,370]. Nanoparticles embedded in implants and orthopedic scaffolds provide mechanical strength and antimicrobial protection. However, it should be remembered that many nanoparticles exhibit cytotoxicity and genotoxicity, especially in the case of their small size and higher concentrations [371].
Figure 8.
Schematic representation of intra- and extracellular NPs synthesis together with their possible mechanisms of antibacterial action.
Interesting examples of NPs that were produced by microorganisms are iron oxide nanoparticles which were produced by aquatic magnetotactic bacteria (MTB). These bacteria are able to biomineralize, magnetic magnetite, or greigite nanocrystallites called magnetosomes. When isolated from the MTB, magnetosomes exceed synthetic magnetic nanoparticles exhibiting promising anti-tumor efficacy against glioblastoma tumors in vivo tests [372,373]. It should be emphasized, however, that the anticancer activity is based on various mechanisms of action (heat, the release of chemotherapeutic drugs under a pH variation, nanoparticle excitation by radiation, and apoptotic tumor cell death). Magnetic nanoparticles are useful for targeted cancer therapies because they can be manipulated by external magnetic fields. Moreover, they are attracted toward hypoxic areas, such as the tumor regions, while retaining the therapeutic and imaging capacities of the isolated magnetosomes [374]. In nature, we can find other examples of a variety of nanomaterials synthesized by biological processes like example diatoms, which synthesize siliceous materials or S-layer bacteria forming NPs of gypsum and calcium carbonate layers.
5.2. Plant Extracts-Mediated NPs Synthesis
Plant extracts contain diverse compounds, which can be utilized as potent reducing agents, stabilizing agents, and precursor molecules for NPs formation [375,376]. In order to prepare the extracts, both the biomass of the whole plant and selected parts such as leaves, fruit, seeds, and above-ground parts can be used. The plant material can be fresh or powder-dried. Various techniques are used to prepare the extracts, but most consist of classical maceration with various solvents including water or water–alcohol mixtures. Nanoparticle synthesis is mediated by extract components with reducing potential including alkaloids, terpenoids, polyphenols, phenols, flavonoids, and proteins, which have additionally been identified as nanoparticle stabilizers. As compared with ordinary metal salts or initial materials alone, biologically synthesized nanoparticles have been found to be better scavengers of free radicals [377]. The antioxidant activity of NPs frequently depends on their size [378,379] as well as shape [380,381].
So far, many examples of the phytogenic synthesis of NPs have been described, including, among others, copper oxide and copper nanoparticles by the use of the leaf extract of Cissus arnotiana with antioxidant ability [382,383]. Apart from zinc oxide (ZnONPs), selenium (SeNPs), and nickel oxide nanoparticles (NiONPs), one of the biggest groups of plant-mediated NPs is iron nanoparticles (INPs). This group is divided into (a) iron oxide nanoparticles (IONs), (b) iron oxide hydroxide (FeOOH) nanoparticles, and (c) zero-valent iron (ZVI) nanoparticles [384,385,386,387]. Iron oxide (magnetite Fe3O4, magemite Fe2O3) NPs of certain sizes have superparamagnetic properties; therefore, they are useful as contrast agents and drug carriers.
The main problems encountered in the biogenic synthesis of nanoparticles concern achieving their appropriate shape, size, and monodispersity in the solution phase. Undoubtedly, the size and shape of NPs depend on the synthesis conditions and the chemical composition of the extract. Usually, optimization of synthesis conditions concerns such factors as the extract concentration, pH, temperature, and reaction or incubation time [388,389,390,391]. The reports on a plant-mediated approach to synthesize NPs by the use of different extracts are collected in Table 4.
Table 4.
Examples of biosynthesis of nanoparticles (NPs).
| Shape/Size | Activity Assay/ Control |
Biological Material |
Effective Molecules |
Preparation of Extract | Bio-Synthesis of NPs | Ref. |
|---|---|---|---|---|---|---|
| Silver (Ag) NPs, Absorbance at 430–450 nm | ||||||
| spherical 410–450 nm | DPPH/ ascorbic acid |
Lantana camara L. | terpenes | Powder (10 gm) of dried leaves was extracted with petroleum ether (30 mL) at RT for 6 h with shaking. It was treated with 30 mL of warm 10% aqueous KOH, shaken and two layers were separated. The petroleum ether layer was concentrated to dryness under reduced pressure. | One milliliter of concentrated extract was added to 6 mL of 1 mM AgNO3 at RT, and kept in the dark for 24 h. The slurry was dried under vacuum. | [392] |
| spherical 5–38 nm | DPPH/ ascorbic acid | Costus afer | Carbohydrates flavonoids, phenolics, alkaloids, organic acids |
Fresh leaves were air-dried. Two grams of the powder was macerated with 150 mL DW and heated at 90 °C for 1 h. | Eighty milliliters of filtered extract was mixed with 400 mL of 1 mM AgNO3. The mixture was stirred at 90 °C for 120 min. | [393] |
| spherical 5–30 nm |
ABTS, DPPH, NO f.r.s.a. |
Taraxacum
officinale |
flavonoids, primary aromatic amines, terpenoids, triterpenes |
The dried leaves were powdered and sieved. Five grams of powder was added to 50 mL of DW and boiled at 60 °C for 15 min, followed by cooling and filtration. | The extract was mixed with AgNO3 (1 mM) with a 1:5 ratio for 15 min. at pH 6.0, at RT. | [394] |
| spherical 12–40 nm. |
DPPH, ABTS, O2●−, NO• f.r.s.a. | Morus alba | carbohydratesproteins, secondary metabolites | Ten grams of the chopped leaves were refluxed with 100 mL DDW for 60 min. The product was filtered and centrifuged at 2000 rpm for 5 min. | Ten milliliters of extract was mixed with 90 mL AgNO3 solution with stirring for 10 min. | [395] |
| spherical 50–60 nm | DPPH/BHT | Thymus kotschyanus | phenolic, flavonoid compounds | The plant was washed, dried at 25 °C, powdered with mortar. Two grams of powder was added to 300 mL of boiling water and kept for 30 min. The obtained extract was filtered. | The extract (10 mL) was mixed with 100 mL of 1 m M aqueous solution of AgNO3 at RT and stirred for 30 min in a dark place. | [396] |
| spherical 5–45 nm |
DPPH, H2O2, OH•, O2●− f.r.s.a. | Cestrum nocturnum | phenolic compounds, amines, amides, aldehydes, nitriles, flavonoids, tannins | The leaves were dried and powdered. Eight grams of powder was added to 100 mL DI and heated at 70 °C for 2 h. The extract was centrifuged at 3000 rpm for 5 min followed the filtration. | Twenty milliliters extract was stirred with 180 mL 1 mM AgNO3 solution for 5 min at RT. | [397] |
| spherical 5–50 nm |
DPPH, FRAP, TAC/ascorbic acid | Streptomyces naganishii (MA7) | Proteins, enzymes | The strain was inoculated into 50 mL of ISP 2. The mycelium was centrifugated at 5000 rpm for 30 min. | Five grams of wet biomass was exposed to 50 mL of 1 mM AgNO3. The mixture was incubated for 28 °C at 120 rpm, and then ultracentrifugation. | [398] |
| variables 150–250 nm |
DPPH, FRAP, TAC |
Parmeliopsis ambigua, Punctelia subrudecta Evernia mesomorpha, Xanthoparmelia plitti mycelia mats |
polyphenols, native proteins | The cultures were inoculated on MYE. The plates were incubated at 28 °C. After 7–10 days, the isolated mycobiont was subcultured into a fresh medium. The mycobiont was grown aerobically in MYE at 28 °C with shaking at 150 rpm. After 10 days, mycelia were separated by filtration. | The mycelia mats were mixed separately with 100 mL SDW and 1 mM AgNO3, and incubated at RT on a rotary shaker at 150 rpm. The reaction was carried out in bright conditions for 24 h. | [399] |
| spherical 15–30 nm | DPPH | marine algae Ecklonia cava | polyphenols, polysaccharides, amine, amide species | Five grams of powder and 500 mL of DW were kept at 100 °C for 1 h. Then, the mixture was centrifuged at 3000 rpm for 20 min, and filtered by a filter paper. | Ten milliliters of aqueous extract was mixed with 90 mL of 1 mM AgNO3 solution and stirred for 72 h. AgNPs were lyophilized. | [400] |
| spherical 2–10 nm | DPPH, H2O2 f.r.s.a. | Pestalotiopsis microspora VJ1/VS1 | phenolic compounds, proteins | The culture was cultivated in 100 mL of PDB at 25 °C. After 6 days fungal biomass was transferred to 100 mL of SDDW, boiled, and filtered. | 10 mL of filtrate was incubated with 90 mL of 1 mM AgNO3 in darkness for 24 h at RT. | [401] |
| 100 nm | DPPH/ ascorbic acid |
Cladosporium cladosporioides - | NADPH-dependent reductase, phenolic compounds, proteins | The mycelial was grown in PDB for 72 h. The biomass was filtered and then incubated at RT for 48 h in 100 mL DW. | Ten milliliters of filtrate was added to 90 mL of 1 mM AgNO3. | [402] |
| spherical 3–40 nm | DPPH/ ascorbic acid |
Aspergillus versicolor ENT7-isolated from the ethnomedicinal plant Centella asiatica. | - | The fungal isolate was grown in 100 mL of PDB at 26 °C with shaking at a speed of 100 rpm. After the seventh day, the fungal biomass was separated and washed with SDDW. 10 g of biomass was mixed with 100 mL SDDW and kept at 28 °C for 72 h in a constant shaking. | The aqueous solution was filtered (100 mL) and added to 100 mL of 1 mM of silver nitrate and incubated at 28 °C for 24 h in dark condition. | [403] |
| 15–25 nm | DPPH | Trichoderma atroviride KNUP001 | - | The freshly prepared mycelial filtrate was prepared by aerobically growing in PDB with the agitation of 180 rpm at 28 °C for 4 days. Then, the biomass was filtrated and washed SDDW. The biomass (20 g) was ground in 100 mL of deionized water and filtered. | The filtrate (100 mL) was mixed with AgNO3 (5 mM or 10 mM) and the solution was kept at 40 °C under darkness. | [404] |
| spherical 65 nm |
DPPH, FRAP/ascorbic acid | endophytic fungi, Penicillium species of Glycosmis mauritiana | tannins, saponins, terpenoids flavonoids, | Sterilized (HgCl2, 1 mg ml−1) bark material was incubated in PDA at RT for 7–8 days. The isolated fungi were cultured in PDB for 10 days. The mycelial mat was centrifuged (6000 rpm, 10 min) and the supernatant was shaken for 24 h. | Eighty milliliters of 3 mM AgNO3 was added to 20 mL of extract. NPs were centrifugated at 7000 rpm for 10 min. | [405] |
| spherical 15–35 nm | ABTS/BHT | Inonotus obliquus | proteins | Ten grams of mushrooms were washed, crushed mixed with 200 mL DDW, and stirred for about half an hour. | Five milliliters of the filtered solution was mixed with 95 mL of 1 mM AgNO3 at RT for 80 min. | [406] |
| spherical | FRAP, DPPH, /ascorbic acid | Cladosporium | carbohydratestannin, phenolic glycosides, terpenoids, alkaloids, phenol anthraquinones, flavanones | The species was cultured using PDB for 15 days at RT. | Five grams of dried and milled mycelia mat was mixed with 20 mL of SDDW, The mixture was heated to 100 °C for 10 min. Then, 10 mL of 5 mM AgNO3 was added. | [407] |
| 10–80 nm | DPPH |
Agaricus bisporus, Ganoderma lucidum |
flavoproteins, lysine, tryptophan, glutamic acid, riboflavin | Fresh mushrooms were washed with DDW, dried for 4 days, powdered. 1 g of powder was added to100 mL of DDW, and stirred for 60 min. | The filtered extract (10 mL) was added to 90 mL of 1 mM AgNO3. This solution was kept at RT for 12 h or heated at 60 °C for 5 h. | [408] |
| spherical 15–22 nm | DPPH/trolox, ascorbic acid | Ganoderma lucidum | proteins, steroids, nucleotides, amino acids, terpenoids, phenols, vitamins, glycoproteins, poly-saccharides | Five grams of powdered mushrooms were added to 100 mL of 70% ethanol solution. The extract was prepared by the microwave-assisted process. | Twenty milliliters of filtered extract was diluted to 100 mL by DDW, and then 15 mg of AgNO3 was added and mixed by the magnetic stirrer system. | [409] |
| spherical 10–30 nm | DPPH | Ganoderma lucidum | polyphenol, carbonyl species, amino acid | The sample was washed with DW and dried at 40 °C for 3 days. The dried sample was grounded into a powder. 5 g of powder was extracted using water (20 mL via Soxhlet extractor at 80 °C for 8 h. The extract was filtered, and concentrated to 100 mL under 60 °C in a rotary evaporator. | Ten millilitres of extract was added to 90 mL of 1 mM AgNO3 solution and incubated at 60 °C in dark, with an interval the stirring for 4 h of incubation. Ag-NPs were collected by centrifugation at 10,000 rpm for 30 min at 4 °C. The pellet was washed and dried at 60 °C. | [410] |
| spherical 5–20 nm | DPPH/ ascorbic acid |
Streptomyces griseorubens AU2 | - | The pure culture was inoculated on ISP-2 broth and incubated at 28 °C and 130 rpm for 7 days. After that, the culture was centrifuged at 4000 rpm for 20 min and the biomass was washed with DW, suspended in DW, and incubated at 28 °C and 130 rpm for 48 h, and finally centrifuged at 4000 rpm. | Ten milliliters of supernatant with 50 mL of 1 mM AgNO3 were incubated at 28 °C and 130 rpm for 48 h. | [411] |
| spherical 12–16 nm | DPPH, ABTS, FRAP | Raphanus sativus L. | - | The fresh leaves were washed, pat dried, and chopped, shade-dried to constant mass at RT. Ratio: product/solvent was kept at 1:12 w:v, extraction time: 3 h. mechanical stirring, temperature: 70 °C (hydroalcoholic mixture), 67 °C (ethanol); microwave-assisted extraction: time:10 min. at 140 °C, max. power 1000 W. | One hundred milliliters of each filtered extract were mixed with 100 mL of 10 mM aqueous AgNO3 solution and incubated at RT for 30 min. | [412] |
| Gold (Au) NPs, Absorbance at 530–535 nm | ||||||
| multiply twinned quasi- spherical 5–35 nm |
DPPH |
Acroscyphus sphaerophoroides Lev, Sticta nylanderiana |
carboxylic acids, esters, phenols, quinones | The samples were cleaned with DDW, shade dried, and ground in a glass mortar. | One gram of powder was stirred with 100 mL aqueous solution (10−3 M) of HAuCl4, at RT for 12 h. The supernatant was centrifugated (10,000 rpm). The biomass was washed with DDW and dried. | [413] |
| Spherical 5–15 nm | DPPH | Lemanea fluviatilis (L.) | proteins | The red alga samples were cleaned by DW and then dried for a one week in a dark place. | One gram of powder was stirred with 100 mL aqueous solution (10−3 M) of HAuCl4, at RT for 12 h. The supernatant was centrifugated (10,000 rpm). The biomass was washed with DDW and dried. | [143] |
| spherical 79 nm | - | Tetraselmis suecica | water-soluble heterocyclic compounds | The cultures were harvested on the sixth day, then centrifuged at 2000 g for 10 min at 4 °C. The biomass was washed with 0.9% NaCl and centrifuged. The cells were damaged in a mortar in a presence of liquid N2 and then again centrifuged under the same conditions. | The cell extracts were added to 4 mL of 1 mM HAuCl4. The mixtures were incubated in a water bath. The recommended conditions: 1 mL of extract, 5 min of incubation, 90 °C of incubation temperature. | [414] |
| triangular, circular, hexagonal | DPPH/ ascorbic acid |
Escherichia coli | - | E. coli was grown in a nutrient broth at 25 °C under agitation at 180 rpm. The biomass sieved and washed with DW. 1 mg of biomass mixed with 50 mL of SDW, and after 24 h, precipitated by NH4(SO4)2. The pellet was dissolved in phosphate buffer (0.05 M, pH 8.0) and dialyzed. | Five milliliters of solution (50 mg of HAuCl4 in 250 mL of water) was mixed with 24 or 30 mL of the protein solution and vigorously stirred for 4 h. | [415] |
| spherical | DPPH, OH, O2●−, NO• f.r.s.a. | Solanum torvum | - | The dried fruit was made to a fine powder. The 1% of aqueous extract was obtained by using soxhlet apparatus. | Eight milliliters of extract was mixed with 2 mL of 1 mM HAuCl4 and incubated at RT for 24 h, then the mixture was centrifuged at 10,000 rpm for 10 min. The pellet was re-suspended in ethanol. | [416] |
| spherical 13–15 nm |
- |
Phormidium valderianum, P.tenue, Microcoleus chthonoplastes, Rhizoclonium fontinale, Ulva intestinalis, Chara zeylanica, Pithophora oedogoniana |
- | The samples were cultured in an artificial seawater medium. Algal biomass was mixed with betadine and antibiotic mixtures. After 12 the biomass was washed with SDW. | Au-loaded biomass was obtained by its expose to 15 ppm Au (III) solution at pH (5, 7, 9). After 72 h, it was washed with SDDW, and dried on air. The biomass was sonicated for 30 min with 7.5 mM sodium citrate, followed by centrifugation of 5 min at 3000 rpm. | [417] |
| 100 nm | DPPH, FRAP | Cladosporium cladosporioides | NADPH- dependent reductase, phenolic compounds |
The endophytic fungal isolates were cultured using PDB for 21 days at 25 to 28 °C. The biomass was filtered and washed with DW. This biomass was incubated at RT for 48 h in 100 mL DW. | A 1 mM HAuCl4 solution was mixed with the fungal suspension filtrate. | [402] |
| spherical, triangle, hexagonal rod 23 nm |
ABTS | Inonotus obliquus | proteins | Ten grams of cut mushrooms were stirred with 100 mL of DDW, for 30 min. Then, the solution was filtered through Whatman filter paper. | The extract (5 mL) was added to 95 mL, 1.0 mM HAuCl4. The mixture was stirred at RT for 30 min. | [418] |
| spherical 5–30 nm | DPPH | Lactobacillus kimchicus DCY51T 19 | - | Bacterial cells isolated from kimchi were inoculated into 100 mL MRS broth and incubated at 37 °C for 24 h. After incubation, the broth was centrifuged at 6300× g for 5 min. | The biomass was washed with SDW and resuspended in 15 mL of SDW. Then, 1 mM of gold salt was added. The mixture was incubated at 30 °C and shaken at 150× g in darkness. The product was centrifuged at 2500× g for 5 min. | [419] |
| spherical 8–50 nm | DPPH | Enterococcus species | proteins and other nitrogenous molecules | A distinct colony of each strain was used to inoculate 10 mL of sterile broth and incubated at 37 °C for 18 h. Then, the cultures were centrifuged at 4000 rpm at 10 °C for 15 min. | One milliliter of the cell-free extract and 30 mL of 1 mM HAuCl4 solution were mixed. | [420] |
| 8–12 nm | - | Sargassum wightii | - | Seaweed was cleaned, dried for 3–5 days, ground to powder. | One gram of seaweed powder was added to 100 mL of 1 mM HAuCl4 solution within 12 h in a stirring condition. | [421] |
| Spherical, cubic 15–60 nm | - | S. platensis | - | The strain was cultivated in a standard Zaroukh water-salt nutrient medium. After 5–6 days of cultivation, the bacterial cells were harvested and then were washed in DW. | The wet biomass (1 g) was mixed with 100 mL of HAuCl4 solution (10−2–10−4 M). The mixture was shaken for 5 days at RT. | [422] |
| 20–70 nm | DPPH, NO• f.r.s.a. | Vitex negundo | Flavonoids, polyphenols |
Leaves were dried for 3 days in a dark place. The biomass (10 g) was stirred with DDW (50 mL) for 12 h at 500 rpm. The extracts were filtered and lyophilized. | Lyophilized extract (0.5 g) was reconstituted in 5 mL DDW at 100 µgmL−1. To 1 mL of extract, 20 mL of HAuCl4 (0.01 M) was added drop-wise and stirred at 500 rpm. The solution was kept overnight. | [423] |
| Zinc oxide (ZnO) NPs, Absorbance at 340–360 nm. | ||||||
| hexagonal 10–61 nm |
DPPH | Pichia kudriavzevii | amino acids | The yeast was grown on PDB in a vibrating incubator at 150 rpm for 72 h at 28 °C. Mycelia were centrifugated (10,000 rpm, 10 min, 4 °C), washed with SDW. 20 g of biomass was suspended in 100 mL of SDW and incubated for 72 h. Then, biomass was filtrated. | One hundred milliliters of filtrate was added to10 mL of 10 mM Zn(Ac)2·2H2O, incubated at 35 °C with agitation at 150 rpm for 12–36 h. The biomass was centrifugated at 10,000 rpm for 10 min and dried at 150 °C for 6 h. | [424] |
| 20–40 nm | DPPH | Berberis aristata | polyphenols, alcohol, carboxylic acid, ether ester amino acid | The leaves were washed, dry at RT. Later 10 g of leaves were cut, soaked in 100 mL of DDW, heated at 50 °C for 10 min., and filtered. | Sixty milliliters of extract was heated to 70 °C and stirred with 0.1 M Zn(Ac)2·2H2O) at basic conditions. Then, the solution was centrifuged at 6000 rpm for 20–25 min. | [425] |
| Selenium (Se) NPs, Absorbance at 510 nm | ||||||
| 10–250 nm | DPPH, FRAP, TAC |
Streptomyces minutiscleroticus (M10A62) |
protein, peptide, amine, amide compounds |
A 0.1 g soil sample was plated in starch casein agar plates enriched with nystatin (100 µg/mL) and nalidixic acid (20 µg/mL). The strain was transferred to 100 mL of MYE broth and incubated in a rotator shaker (200 rpm) for 5 days, and centrifugated at 5000 rpm for 30 min | Five grams of biomass washed with SDDW was mixed with 100 mL of an aqueous solution of 1 mM Na2SeO3 and kept in a rotator shaker for 72 h. | [426] |
| 30–300 nm | DCF in HUVEC | Pantoea agglomerans strain UC-32 | - | Bacterial cells were cultivated in TSB enriched with 1 mM Na2SeO3 at 25 °C. | Cell suspensions were sonicated at 100 W for 2 min and centrifuged at 10,000× g for 10 min. Pellets were suspended in SDS 0.1%/1 M NaOH, and centrifuged. | [427] |
| spherical tetragonal 14–26 nm. | DPPH/ ascorbic acid | Ephedra aphylla | phenolic compounds, flavonoid tannin | Twenty grams of the dried plants were shaken with 200 mL DW for 30 min in a water bath at 70 °C. The mixture was filtered. | Twenty milliliters of 1 mM selenium sulfate was stirred with 20 mL of the plant extract for 2 h at RT. | [428] |
| Copper (Cu) NPs Absorbance at 350–380 nm. | ||||||
| spherical 60–90 nm | DPPH/ ascorbic acid |
Cissus arnotiana | - | One gram of the powder of the dried leaves was added to 100 mL DDW, boiled at 70 °C for 30 min. The mixture was filtered. | Ten milliliters of the extract was stirred with 90 mL of 10 mM of CuSO4, for 4 h at RT. The mixture was centrifugated at 10,000 rpm for 5 min., The pellet was washed with DDW, and ethanol. | [382] |
| 12–16 nm | DPPH, NO•, O2●− f.r.s.a. |
Dioscorea bulbifera | ascorbic acid | The washed and sliced tubers were dried in a dark place for 3 days. Five grams of the obtained powder and 100 mL of SDW were boiled for 5 min. The extract was filtered | Five milliliters of extract was shaken at 150 rpm in the dark place at 40 °C with 95 mL of 1 mM CuSO4·5H2O. | [429] |
| Copper oxide (CuO) NPs Absorbance at 280–360 nm | ||||||
| 1.5–20 nm | - | Lens culinaris | primary and secondary amines, aldehydes, phenols, proteins | The plant was homogenized in mortar. After that, 100 mL of distilled water was added. | 1 mM CuSO4 was stirred with the filtrated extract (ratio: 1:5, v/v) for 1 h at 37 °C (pH 9). Then, it was centrifuged at 12,000× g for 15 min. The pellet was washed with DW, re-suspended in DW and ultra-sonicated. | [430] |
| 10 nm | DPPH | Galeopsidis herba | flavonoids, phenolic acids,poly- saccharides |
A total of 4.5 g powdered plants were mixed with 300 mL DDW, and stirred for 50 min at 85 °C. Then, the mixture was filtered. | The extract was mixed with Cu(NO3)2 in the proportion: 90:1 (w/w), and vigorously stirred for 4 h at 80 °C. | [431] |
| Spherical, agglomerated | - | Terminalia phanerophlebia | - | Extract from the oven-dried leaves was prepared from 2 g of the ground powdered and 150 mL deionized water, ethanol, or acetone. The extracts were filtered. | Thirty milliliters of CuSO4·5H2O (0.1 M) was stirred with 10 mL of the plant extract, and heated at 90 °C for 5 h. The solution was kept overnight at RT. The CuO NPs were centrifuged, washed with DW, dried in hot air. | [432] |
| Iron (Fe) NPs Absorbance at 214 nm | ||||||
| Spherical, cubic 43–220 nm |
DPPH | Amaranthus dubius | amaranthine, phenolic compounds | The leaves were cleaned, chopped into small pieces. 20 g of leaves were mixed with 100 mL DW and keep at 50 °C for 45 min. The mixture was filtered. | The leaf extract (pH 6) was added a drop to 0.5 M FeCl3 with stirring for 90 min. | [433] |
| 20–25 nm | DPPH, ABTS, H2O2 f.r.s.a. | Asphodelus aestivus Brot. | phenolic compounds, poly- sachharides |
The infusion was prepared in a ratio of 5%. The filtrate was concentrated using a vacuum evaporator. | Five milliliters of extract was mixed with 5 mL of 1 mM aqueous FeCl3. The mixture was kept at 50–60 °C for 20 min with shaking. Then, it was centrifuged at 5000 rpm for 30 min. | [434] |
| Iron oxide (FeO) NPs Absorbance at 290 nm | ||||||
| spherical 58–530 nm |
DPPH | Amaranthus spinosus L. | amaranthine, compounds with hydroxyl or amines groups, free amino, carboxylic moieties |
Ten grams of fresh leaves were washed with DW, and chopped into pieces, and mixed with 50 mL water, and keep at 50 °C for 45 min. The supernatant was filtered. | The leaf extract (pH 6) was added to 50 mL of 0.5 M FeCl3 stirring at 37 ± 1 °C for 90 min. The precipitate (FeO NPs) was washed with ethanol and dried at 60 °C for 180 min. | [383] |
| Nickel oxide (NiO) NPs Absorbance at 305 nm | ||||||
| spherical agglomerated NPs 20–50 nm |
DPPH | stevia leaf broth | terpenoids, polyphenols, proteins, aldoses | To 5 g of dried leaves, 100 mL DW was added and boiled (2 min.), and finally filtered. | One gram of nickel acetate in 200 mL DW was stirred with 25 mL extract for 2 h. The mixture was then heated at 100 °C, and then at 500 °C for 2 h. | [435] |
| agglomerated NPs | TAC/phosphomolybdenum, DPPH | Berberis balochistanica | polyphenols, carboxylic acids, alcohols, sulfur compounds | The material was washed, oven-dried for 10 h at 40 °C. 20.66 g of powder was stirred with 200 mL of DW for 12 h. Then, the extract was filtered and centrifuged at 3000 rpm for 30 min. | A 50 mL extract was added drop by drop to the solution of NiNO3 (0.3 M). The mixture was heated at 60 °C with stirred at 500 rpm for 3 h. | [436] |
| Manganese (Mn) NPs Absorbance at 415–417 nm | ||||||
| spherical granular 57–69 nm |
- |
Ctenolepis garcini (Burm. f.) |
native proteins | To 2 g air-dried sample, 30 mL of SDW was added, and boiled (2 min.). | Five milliliters of the filtered extract was added to 25 mL of 1 mM KMnO4 solution and stored in RT for 24 h. | [437] |
| Manganese oxide (MnO) NPs Absorbance at 460 nm | ||||||
| spherical 80± 0.5 nm |
- | Abutilon indicum | - | Twenty grams of leaves powder was mixed with 50% methanol. It was placed on a magnetic hot plate and underwent stirring for about 30 min at 55 °C and allowed to settle overnight. | One hundred milliliters of 0.1 M MnSO4·H2O was mixed with 100 mL of plant extract. A total of 0.1 M NaOH solution was added dropwise to the beaker with constant stirring for about 1 h at pH 8.0 and 50 °C. | [438] |
| Magnesium Oxide Nanoparticles (MgO) NPs Absorbance at 250 nm | ||||||
| Spherical 7–40 nm | - | Penicillium chrysogenum | polysaccharides hydrocarbons, amines, carboxylate, amino groups | The fungal strains were inoculated into MAB, and incubated for 5 days at 30 ± 2 °C and shaking state at 150 rpm. Then, the biomass was centrifuged and resuspended in 100 mL in DDW. | A total of 76.9 mg of Mg(NO3)2.6H2O was dissolved in 10 mL DW, mixed with 90 mL of biomass filtrate and incubated for 24 h. The white precipitate was collected and rinsed with DW, and oven-dried at 400 °C for 3 h. | [439] |
Abbreviations: room temperature (RT); double-distilled water (DDW), tryptic soy broth (TSB), human umbilical vein endothelial cells (HUVEC), starch casein agar medium (SCA), malt extract broth media (MAB), Malt Yeast Extract medium (MYE), sterile double distilled water (SDDW), double distilled water (DDW), sterile distilled water (DW), deionized water (DI), potato dextrose broth (PDB), potato dextrose agar (PDA), International Streptomyces (ISP 2), dichlorofluorescein (DCF), free radical scavenging activity (f.r.s.a).
5.3. Trends of NPs Modification
Increasing attention is paid to nanoparticles functionalized by various antioxidants obtained from various natural sources, such as algae, bacteria, fungi, lichens, and plants. It should be emphasized that most authors reported that the functionalized NPs exhibit a few times greater antiradical activity. The effective transport across the cell membrane through pinocytosis and the possibility of targeted localization give rise possibility of NPs utilization also as carriers for antioxidants. In those cases, inert metalcore and antioxidants attached to the nanoparticle surface can exert also independent activity [63]. In 2020, a review on antioxidant functionalized NPs was published [247]. Most papers present the synthesis of gold and silver nanoparticles that are easily functionalized with different small molecules of antioxidants, for instance, gold nanoparticles functionalized with tocopherol [440,441], gold nanoparticles coated with chitosan [442], silver NPs with glutathione [443], or more complex ones like graphite layered 30 nm cobalt nanomagnets with attached tocopherol derivatives [444]. Konopko et al. [440] and Nie et al. [441] prepared and characterized gold nanoparticles (AuNPs) coated with α-tocopherol-like residues. Both research groups proved that the assembly of chromanol groups on gold nanoparticles could efficiently enhance the activity of the vitamin E-derived antioxidant. In 2019, Mohd Taib et al. [445] synthesized Au-NPs utilizing water extract of Hibiscus sabdariffa leaves. Owing to the UV–VIS, FTIR, and HPLC analysis, chlorogenic acid was identified as the major antioxidant compound involved in the reduction of Au3+ ions. Moreover, the thiol groups can interact directly with the gold core to form gold–sulfur bonds (Au-S) responsible for the mucoadhesion properties of the synthetized AuNPs [446]. In 2017, Choi et al. [447] described nanoparticles modified by caffeic acid, which was immobilized on the surfaces of micro-dielectric barrier discharge (DBD) plasma-treated ZnO nanoparticles. Obtained nanoparticles showed strong antioxidant (ABTS), antibacterial activity against Gram-positive bacteria (Staphylococcus aureus), including resistant bacteria such as methicillin-resistant S. aureus, and against Gram-negative bacteria (Escherichia coli). Nanostructural materials such as nanotubes have been described as novel synergistic nano-antioxidants [448], for example, ascorbic acid loaded into the inner lumen of natural halloysite nanotubes [449] or halloysite externally deco-rated with tocopherol-like moieties and containing quercetin inside the nanotube [450]. Many studies have described functionalized silver and gold nanoparticles derived from fungal or bacterial extracts obtained from species Ganoderma lucidium [408,409,410], Aspergillus versicolor, Cladosporium cladosporioides, Pestalotiopsis microspore [401,403,451], and bacteria Lactobacillus kimchicus [419].
Another promising trend of nanobiotechnology represents the development of nano-drug delivery systems composed of biocompatible and biodegradable polymeric nanomaterials (polylactide-PLA, poly-lactic-co-glycolic acid- PLGA) that are able to encapsulate the therapeutic agent and progressively release it at the target site. Chlorogenic acid entrapped in hybrid materials composed of SiO2 and polyethylene glycol has been identified as a system able to control the overproduction of RNS/ROS [452]. Another example is curcumin encapsulated in a nanocarrier and covered with chitosan. Authors observed a protective effect of chitosan on the antioxidant activity of curcumin [453,454]. While inorganic nanoparticles, especially those with semiconductor properties, have found applications in in vitro diagnostics and imaging, nano-drugs ensure effective biodistribution thanks to the ability to overcome biological barriers. Thus far, many medicinal preparations in the form of nanoparticles have been developed, belonging to different classes of NPs (polymeric, inorganic, and lipid-based), such as polymer-drug conjugate, protein–drug conjugate, polymer–protein conjugate, antibody–drug conjugate, dendimeric drug, polymeric micelle, polymersome, liposome, PEGylated liposome, organic/inorganic colloid, quantum dot, Si-NPs, Au-NPs, and INPs. Extensive reviews on this subject have already been published [455,456,457,458]; unfortunately, it is beyond the scope of the current study.
Interesting nano-formulas are also doubly hydrophilic self-assembling block copolymers (DHBC), which in recent years have aroused more and more interest not only for the production of nanoparticles, but also as controlled drug distribution systems. A valuable review on DHBC was published in 2020 by Jundi et al. [459].
6. Concluding Remarks and Future Perspectives
Considering the key role of antioxidants to treat oxidative diseases, the development of reliable antioxidant activity assays of different products with high antioxidant content, as potential drugs or supplements, is needed. Several analytical techniques can be applied for this purpose such as spectroscopic, chromatographic, and electrochemical ones. At the beginning of the 21st century, antioxidant assays based on NPs were developed. The use of NPs as optical or electrochemical probes appears to be a very promising approach; however, this technique has still been scarcely followed. Over 5 years of research on the NPs-based method has resulted in a negligible number of publications, which illustrates the fact that in the PubMed database, the phrases “antioxidant capacity”, “nanoparticles”, and “plant extracts” are associated with no more than 70 scientific papers. One should emphasize that performing the comparative analysis of antioxidant potentials on the basis of results published by different research groups is very difficult. The antioxidant potential of natural products, and even single chemicals, depends on many factors such as conditions of samples collections, as well as the extracts preparation method and the way of expressing results.
On the other hand, the plant extracts rich in antioxidants that act as both reducing and stabilizing agents appear to be useful for creating metallic nanoparticles. Green synthesis surpasses classical methods, providing such benefits as low-cost, environmentally friendly strategies not requiring high pressure, energy, temperature, or external toxic chemical agents. Furthermore, green synthesis ensures the formation of nanoparticles free of toxic contaminants, which makes them suitable in therapeutic applications such as antimicrobial agents in bandages, applications in targeted drug delivery, or clinical diagnostics as contrast agents (MRI-Magnetic Resonance Imaging). The popularity of green nanoparticle synthesis toward bio and medical applications is reflected in the number of around 5000 publications that have appeared in the PubMed database in the last five years.
A promising trend that has been developing dynamically in recent years is the synthesis of antioxidant functionalized nanoparticles. Such modification improves the bioavailability of antioxidants providing the benefits of biocompatibility, high stability, and targeted delivery.
Author Contributions
J.F. and W.F. wrote—original draft preparation, J.B. and W.F. collected and reviewed the data; W.F. edited the paper and prepared visualization of the paper., J.F. and R.M. wrote—review and edited, R.M. was responsible for supervision, R.M. was responsible for project administration, and J.F., J.B., and R.M. dealt with funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Choi H.J., Kim J.H., Lee C.H., Ahn Y.J., Song J.H., Baek S.H., Kwon D.H. Antiviral activity of quercetin 7-rhamnoside against porcine epidemic diarrhea virus. Antiviral Res. 2009;81:77–81. doi: 10.1016/j.antiviral.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Song J.H., Shim J.K., Choi H.J. Quercetin 7-rhamnoside reduces porcine epidemic diarrhea virus replication via independent pathway of viral induced reactive oxygen species. Virol. J. 2011;8:460. doi: 10.1186/1743-422X-8-460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liang W., He L., Ning P., Lin J., Li H., Lin Z., Kang K., Zhang Y. (+)-Catechin inhibition of transmissible gastroenteritis coronavirus in swine testicular cells is involved its antioxidation. Res. Vet. Sci. 2015;103:28–33. doi: 10.1016/j.rvsc.2015.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Diniz L.R.L., Bezerra Filho C.D.S.M., Fielding B.C., de Sousa D.P. Natural Antioxidants: A Review of Studies on Human and Animal Coronavirus. Oxid. Med. Cell. Longev. 2020:1–14. doi: 10.1155/2020/3173281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Delgado-Roche L., Mesta F. Oxidative Stress as Key Player in Severe Acute Respiratory Syndrome Coronavirus (SARS-CoV) Infection. Arch. Med. Res. 2020;51:384–387. doi: 10.1016/j.arcmed.2020.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jo S., Kim S., Shin D.H., Kim M.S. Inhibition of SARS-CoV 3CL protease by flavonoids. J. Enzyme Inhib. Med. Chem. 2020;35:145–151. doi: 10.1080/14756366.2019.1690480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen L., Li J., Luo C., Liu H., Xu W., Chen G., Liew O.W., Zhu W., Puah C.M., Shen X., et al. Binding interaction of quercetin-3-beta-galactoside and its synthetic derivatives with SARS-CoV 3CL(pro): Structure-activity relationship studies reveal salient pharmacophore features. Bioorg. Med. Chem. 2006;14:8295–8306. doi: 10.1016/j.bmc.2006.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ryu Y.B., Jeong H.J., Kim J.H., Kim Y.M., Park J.Y., Kim D., Nguyen T.T., Park S.J., Chang J.S., Park K.H., et al. Biflavonoids from Torreya nucifera displaying SARS-CoV 3CL(pro) inhibition. Bioorg. Med. Chem. 2010;18:7940–7947. doi: 10.1016/j.bmc.2010.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim D.W., Seo K.H., Curtis-Long M.J., Oh K.Y., Oh J.W., Cho J.K., Lee K.H., Park K.H. Phenolic phytochemical displaying SARS-CoV papain-like protease inhibition from the seeds of Psoralea corylifolia. J. Enzyme Inhib. Med. Chem. 2014;29:59–63. doi: 10.3109/14756366.2012.753591. [DOI] [PubMed] [Google Scholar]
- 10.Yu M.S., Lee J., Lee J.M., Kim Y., Chin Y.W., Jee J.G., Keum Y.S., Jeong Y.J. Identification of myricetin and scutellarein as novel chemical inhibitors of the SARS coronavirus helicase, nsP13. Bioorg. Med. Chem. Lett. 2012;22:4049–4054. doi: 10.1016/j.bmcl.2012.04.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cárdenas-Rodríguez N., Bandala C., Vanoye-Carlo A., Ignacio-Mejía I., Gómez-Manzo S., Hernández-Cruz E.Y., Pedraza-Chaverri J., Carmona-Aparicio L., Hernández-Ochoa B. Use of Antioxidants for the Neuro-Therapeutic Management of COVID-19. Antioxidants. 2021;10:971. doi: 10.3390/antiox10060971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Forman H.J., Zhang H. Targeting oxidative stress in disease: Promise and limitations of antioxidant therapy. Nat. Rev. Drug Discov. 2021 doi: 10.1038/s41573-021-00233-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Forman H.J., Davies K.J., Ursini F. How do nutritional antioxidants really work: Nucleophilic tone and para-hormesis versus free radical scavenging in vivo. Free Radic. Biol. Med. 2014;66:24–35. doi: 10.1016/j.freeradbiomed.2013.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sies H. Strategies of antioxidant defense. Eur. J. Biochem. 1993;215:213–219. doi: 10.1111/j.1432-1033.1993.tb18025.x. [DOI] [PubMed] [Google Scholar]
- 15.Bansal S., Choudhary S., Sharma M., Kumar S.S., Lohan S., Bhardwaj V., Syan N., Jyoti S. Tea: A native source of antimicrobial agents. Food Res. Int. 2013;53:568–584. doi: 10.1016/j.foodres.2013.01.032. [DOI] [Google Scholar]
- 16.Jiang J., Xiong Y.L. Natural antioxidants as food and feed additives to promote health benefits and quality of meat products: A review. Meat Sci. 2016;120:107–117. doi: 10.1016/j.meatsci.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 17.Lü J.-M., Lin P.H., Yao Q., Chen C. Chemical and molecular mechanisms of antioxidants: Experimental approaches and model systems. J. Cell. Mol. Med. 2009;14:840–860. doi: 10.1111/j.1582-4934.2009.00897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cetin-Karaca H., Newman M.C. Antimicrobial efficacy of plant phenolic compounds against Salmonella and Escherichia coli. Food Biosci. 2015;11:8–16. doi: 10.1016/j.fbio.2015.03.002. [DOI] [Google Scholar]
- 19.Caleja C., Barros L., Antonio A.L., Ćirić A., Barreira J.C., Sokovic M., Oliveira M.B.P., Santos-Buelga C., Ferreira I.C. Development of a functional dairy food: Exploring bioactive and preservation effects of chamomile (Matricaria recutita L.) J. Funct. Foods. 2015;16:114–124. doi: 10.1016/j.jff.2015.04.033. [DOI] [Google Scholar]
- 20.De Dicastillo C.L., Rodríguez F., Guarda A., Galotto M.J. Antioxidant films based on cross-linked methyl cellulose and native Chilean berry for food packaging applications. Carbohydr. Polym. 2016;136:1052–1060. doi: 10.1016/j.carbpol.2015.10.013. [DOI] [PubMed] [Google Scholar]
- 21.Galanakis C.M., Tsatalas P., Charalambous Z., Galanakis I.M., Charalambous Z. Polyphenols recovered from olive mill wastewater as natural preservatives in extra virgin olive oils and refined olive kernel oils. Environ. Technol. Innov. 2018;10:62–70. doi: 10.1016/j.eti.2018.01.012. [DOI] [Google Scholar]
- 22.Bodoira R.M., Penci M.C., Ribotta P.D., Martinez M.L. Chia (Salvia hispanica L.) oil stability: Study of the effect of natural antioxidants. LWT. 2017;75:107–113. doi: 10.1016/j.lwt.2016.08.031. [DOI] [Google Scholar]
- 23.Özcan M.M., Arslan D. Antioxidant effect of essential oils of rosemary, clove and cinnamon on hazelnut and poppy oils. Food Chem. 2011;129:171–174. doi: 10.1016/j.foodchem.2011.01.055. [DOI] [Google Scholar]
- 24.Wibowo S., Vervoort L., Tomic J., Santiago J.S., Lemmens L., Panozzo A., Grauwet T., Hendrickx M., Van Loey A. Colour and carotenoid changes of pasteurised orange juice during storage. Food Chem. 2015;171:330–340. doi: 10.1016/j.foodchem.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 25.Oswell N.J., Thippareddi H., Pegg R.B. Practical use of natural antioxidants in meat products in the U.S.: A review. Meat Sci. 2018;145:469–479. doi: 10.1016/j.meatsci.2018.07.020. [DOI] [PubMed] [Google Scholar]
- 26.Das A.K., Rajkumar V., Nanda P.K., Chauhan P., Pradhan S.R., Biswas S. Antioxidant Efficacy of Litchi (Litchi chinensis Sonn.) Pericarp Extract in Sheep Meat Nuggets. Antioxidants. 2016;5:16. doi: 10.3390/antiox5020016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Caleja C., Barros L., Antonio A.L., Oliveira M.B.P., Ferreira I.C. A comparative study between natural and synthetic antioxidants: Evaluation of their performance after incorporation into biscuits. Food Chem. 2017;216:342–346. doi: 10.1016/j.foodchem.2016.08.075. [DOI] [PubMed] [Google Scholar]
- 28.Embuscado M.E. Spices and herbs: Natural sources of antioxidants—A mini review. J. Funct. Foods. 2015;18:811–819. doi: 10.1016/j.jff.2015.03.005. [DOI] [Google Scholar]
- 29.Lourenço S.C., Moldão-Martins M., Alves V.D. Antioxidants of Natural Plant Origins: From Sources to Food Industry Applications. Molecules. 2019;24:4132. doi: 10.3390/molecules24224132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Freitas A., Moldão-Martins M., Costa H.S., Albuquerque T.G., Valente A., Sanches-Silva A. Effect of UV-C radiation on bioactive compounds of pineapple (Ananas comosus L. Merr.) by-products. J. Sci. Food Agric. 2015;95:44–52. doi: 10.1002/jsfa.6751. [DOI] [PubMed] [Google Scholar]
- 31.Panić M., Stojković M.R., Kraljić K., Škevin D., Redovniković I.R., Srček V.G., Radošević K. Ready-to-use green polyphenolic extracts from food by-products. Food Chem. 2019;283:628–636. doi: 10.1016/j.foodchem.2019.01.061. [DOI] [PubMed] [Google Scholar]
- 32.Corrêa-Filho L.C., Lourenço S.C., Duarte D.F., Moldão-Martins M., Alves V.D. Microencapsulation of Tomato (Solanum lycopersicum L.) Pomace Ethanolic Extract by Spray Drying: Optimization of Process Conditions. Appl. Sci. 2019;9:612. doi: 10.3390/app9030612. [DOI] [Google Scholar]
- 33.Brezoiu A.-M., Matei C., Deaconu M., Stanciuc A.-M., Trifan A., Gaspar-Pintiliescu A., Berger D. Polyphenols extract from grape pomace. Characterization and valorisation through encapsulation into mesoporous silica-type matrices. Food Chem. Toxicol. 2019;133:110787. doi: 10.1016/j.fct.2019.110787. [DOI] [PubMed] [Google Scholar]
- 34.Da Silva D.I., Nogueira G.D., Duzzioni A.G., Barrozo M.A. Changes of antioxidant constituents in pineapple (Ananas comosus) residue during drying process. Ind. Crop. Prod. 2013;50:557–562. doi: 10.1016/j.indcrop.2013.08.001. [DOI] [Google Scholar]
- 35.Goodsell D.S. Bionanotechnology: Lessons from Nature. John Wiley & Sons, Inc.; Hoboken, NJ, USA: 2004. Bionanomedicine in Action. [Google Scholar]
- 36.Chrząszcz M., Krzemińska B., Celiński R., Szewczyk K. Phenolic Composition and Antioxidant Activity of Plants Belonging to the Cephalaria (Caprifoliaceae) Genus. Plants. 2021;10:952. doi: 10.3390/plants10050952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rani A., Saini K.C., Bast F., Mehariya S., Bhatia S.K., Lavecchia R., Zuorro A. Microorganisms: A Potential Source of Bioactive Molecules for Antioxidant Applications. Molecules. 2021;26:1142. doi: 10.3390/molecules26041142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gülçin İ. Antioxidant activity of food constituents: An overview. Arch. Toxicol. 2011;86:345–391. doi: 10.1007/s00204-011-0774-2. [DOI] [PubMed] [Google Scholar]
- 39.Marcelino G., Machate D.J., Freitas K.d.C., Hiane P.A., Maldonade I.R., Pott A., Asato M.A., Candido C.J., Guimarães R.d.C.A. β-Carotene: Preventive Role for Type 2 Diabetes Mellitus and Obesity: A Review. Molecules. 2020;25:5803. doi: 10.3390/molecules25245803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scarano A., Chieppa M., Santino A. Plant Polyphenols-Biofortified Foods as a Novel Tool for the Prevention of Human Gut Diseases. Antioxidants. 2020;9:1225. doi: 10.3390/antiox9121225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Talib W.H., AL-Ataby I.A., Mahmod A.I., Jawarneh S., Al Kury L.T., AL-Yasari I.H. The Impact of Herbal Infusion Consumption on Oxidative Stress and Cancer: The Good, the Bad, the Misunderstood. Molecules. 2020;25:4207. doi: 10.3390/molecules25184207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mittal A.K., Chisti Y., Banerjee U.C. Synthesis of metallic nanoparticles using plant extracts. Biotechnol. Adv. 2013;31:346–356. doi: 10.1016/j.biotechadv.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 43.Cheeseman K.H., Slater T.F. An introduction to free radicals chemistry. Br. Med. Bull. 1993;49:481–493. doi: 10.1093/oxfordjournals.bmb.a072625. [DOI] [PubMed] [Google Scholar]
- 44.Liu T., Stern A., Roberts L.J., Morrow J.D. The isoprostanes: Novel prostanglandin-like products of the free radical catalyzed peroxidation of arachidonic acid. J. Biomed. Sci. 1999;6:226–235. doi: 10.1007/BF02253564. [DOI] [PubMed] [Google Scholar]
- 45.Beers R.F., Sizer I.W. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J. Biol. Chem. 1952;195:133–140. doi: 10.1016/S0021-9258(19)50881-X. [DOI] [PubMed] [Google Scholar]
- 46.Apak R., Özyürek M., Güçlü K., Çapanoğlu E. Antioxidant Activity/Capacity Measurement. 1. Classification, Physicochemical Principles, Mechanisms, and Electron Transfer (ET)-Based Assays. J. Agric. Food Chem. 2016;64:997–1027. doi: 10.1021/acs.jafc.5b04739. [DOI] [PubMed] [Google Scholar]
- 47.Graves D.B. The emerging role of reactive oxygen and nitrogen species in redox biology and some implications for plasma applications to medicine and biology. J. Phys. D Appl. Phys. 2012;45:263001. doi: 10.1088/0022-3727/45/26/263001. [DOI] [Google Scholar]
- 48.Poljsak B., Šuput D., Milisav I. Achieving the Balance between ROS and Antioxidants: When to Use the Synthetic Antioxidants. Oxidative Med. Cell. Longev. 2013:1–11. doi: 10.1155/2013/956792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bagchi K., Puri S. Free radicals and antioxidants in health and disease. East Mediterr. Health J. 1998;4:350–360. [Google Scholar]
- 50.Hollman P.C., Cassidy A., Comte B., Heinonen M., Richelle M., Richling E., Serafini M., Scalbert A., Sies H., Vidry S. The biological relevance of direct antioxidant effects of polyphenols for cardiovascular health in humans is not established. J. Nutr. 2011;141:989S–1009S. doi: 10.3945/jn.110.131490. [DOI] [PubMed] [Google Scholar]
- 51.Roediger B., Armati P.J. Oxidative stress induces axonal beading in cultured human brain tissue. Neurobiol. Dis. 2003;13:222–229. doi: 10.1016/S0969-9961(03)00038-X. [DOI] [PubMed] [Google Scholar]
- 52.Young I.S., Woodside J.V. Antioxidants in health and disease. J. Clin. Pathol. 2001;54:176–186. doi: 10.1136/jcp.54.3.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pisoschi A.M., Pop A. The role of antioxidants in the chemistry of oxidative stress: A review. Eur. J. Med. Chem. 2015;97:55–74. doi: 10.1016/j.ejmech.2015.04.040. [DOI] [PubMed] [Google Scholar]
- 54.Singh U., Jialal I. Oxidative stress and atherosclerosis. Pathophysiology. 2006;13:129–142. doi: 10.1016/j.pathophys.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 55.Rao A.L., Bharani M., Pallavi V. Role of antioxidants and free radicals in health and disease. Adv. Pharmacol. Toxicol. 2006;7:29–38. [Google Scholar]
- 56.Stefanis L., Burke R.E., Greene L.A. Apoptosis in neurodegenerative disorders. Curr. Opin. Neurol. 1997;10:299–305. doi: 10.1097/00019052-199708000-00004. [DOI] [PubMed] [Google Scholar]
- 57.Lobo V., Patil A., Phatak A., Chandra N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010;4:118–126. doi: 10.4103/0973-7847.70902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sas K., Robotka H., Toldi J., Vécsei L. Mitochondria, metabolic disturbances, oxidative stress and the kynurenine system, with focus on neurodegenerative disorders. J. Neurol. Sci. 2007;257:221–239. doi: 10.1016/j.jns.2007.01.033. [DOI] [PubMed] [Google Scholar]
- 59.Beal M.F. Therapeutic approaches to mitochondrial dysfunction in Parkinson’s disease. Parkinsonism Relat. Disord. 2009;15:S189–S194. doi: 10.1016/S1353-8020(09)70812-0. [DOI] [PubMed] [Google Scholar]
- 60.Chong Z.Z., Li F., Maiese K. Oxidative stress in the brain: Novel cellular targets that govern survival during neurodegenerative disease. Prog. Neurobiol. 2005;75:207–246. doi: 10.1016/j.pneurobio.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 61.Halliwell B. Oxidative stress and neurodegeneration: Where are we now? J. Neurochem. 2006;97:1634–1658. doi: 10.1111/j.1471-4159.2006.03907.x. [DOI] [PubMed] [Google Scholar]
- 62.Sies H. Oxidative stress: From basic research to clinical application. Am. J. Med. 1991;91:31S–38S. doi: 10.1016/0002-9343(91)90281-2. [DOI] [PubMed] [Google Scholar]
- 63.Brown G.C., Borutaite V. Nitric oxide, mitochondria, and cell death. IUBMB Life. 2001;52:189–195. doi: 10.1080/15216540152845993. [DOI] [PubMed] [Google Scholar]
- 64.Aruoma O.I. Nutrition and health aspects of free radicals and antioxidants. Food Chem. Toxicol. 1994;32:671–683. doi: 10.1016/0278-6915(94)90011-6. [DOI] [PubMed] [Google Scholar]
- 65.McCord J.M. The evolution of free radicals and oxidative stress. Am. J. Med. 2000;108:652–659. doi: 10.1016/S0002-9343(00)00412-5. [DOI] [PubMed] [Google Scholar]
- 66.Fang Y.Z., Yang S., Wu G. Free radicals, antioxidants, and nutrition. Nutrition. 2002;18:872–879. doi: 10.1016/S0899-9007(02)00916-4. [DOI] [PubMed] [Google Scholar]
- 67.Knight J.A. Free radicals: Their history and current status in aging and disease. Ann. Clin. Lab. Sci. 1998;28:331–346. [PubMed] [Google Scholar]
- 68.Jacob R.A. Three eras of vitamin C discovery. Subcell. Biochem. 1996;25:1–16. [PubMed] [Google Scholar]
- 69.Moreau D. Comptes Rendus des Séances et Mémoires de la Société de Biologie. Volume 86. Au Bureau de la Gazette médicale; Paris, France: 1922. p. 321. [Google Scholar]
- 70.Mattill H.A. Antioxidants. Annu. Rev. Biochem. 1947;16:177–192. doi: 10.1146/annurev.bi.16.070147.001141. [DOI] [PubMed] [Google Scholar]
- 71.German J.B. Food processing and lipid oxidation. Adv. Exp. Med. Biol. 1999;459:23–50. doi: 10.1007/978-1-4615-4853-9_3. [DOI] [PubMed] [Google Scholar]
- 72.Sies H. Oxidative stress: Oxidants and antioxidants. Exp. Physiol. 1997;82:291–295. doi: 10.1113/expphysiol.1997.sp004024. [DOI] [PubMed] [Google Scholar]
- 73.Shi H., Noguchi N., Niki E. Comparative study on dynamics of antioxidative action of alpha-tocopheryl hydroquinone, ubiquinol, and alpha-tocopherol against lipid peroxidation. Free Radic. Biol. Med. 1999;27:334–346. doi: 10.1016/S0891-5849(99)00053-2. [DOI] [PubMed] [Google Scholar]
- 74.Weiss J.F., Landauer M.R. Protection against ionizing radiation by antioxidant nutrients and phytochemicals. Toxicology. 2003;189:1–20. doi: 10.1016/S0300-483X(03)00149-5. [DOI] [PubMed] [Google Scholar]
- 75.Blasko G., Cordell G.A. Recent developments in chemistry of plant derived anticancer agents. In: Wagner H., Hiroshi H., Farnsworth N.R., editors. Economic and Medicinal Plant Research. Academic Press; New York, NY, USA: 1998. [Google Scholar]
- 76.Decramer M., Rutten-van Mölken M., Dekhuijzen P.N., Troosters T., van Herwaarden C., Pellegrino R., van Schayck C.P., Olivieri D., Del Donno M., De Backer W., et al. Effects of N-acetylcysteine on outcomes in chronic obstructive pulmonary disease (Bronchitis Randomized on NAC Cost-Utility Study, BRONCUS): A randomised placebo-controlled trial. Lancet. 2005;365:1552–1560. doi: 10.1016/S0140-6736(05)66456-2. [DOI] [PubMed] [Google Scholar]
- 77.Bast A. Antioxidant pharmacotherapy. Drug News Perspect. 1994;7:465–472. [Google Scholar]
- 78.Rahbar Saadat Y., Yari Khosroushahi A., Pourghassem Gargari B. A comprehensive review of anticancer, immunomodulatory and health beneficial effects of the lactic acid bacteria exopolysaccharides. Carbohydr. Polym. 2019;217:79–89. doi: 10.1016/j.carbpol.2019.04.025. [DOI] [PubMed] [Google Scholar]
- 79.Chandra P., Sharma R.K., Arora D.S. Antioxidant compounds from microbial sources: A review. Food Res. Int. 2020;129:108849. doi: 10.1016/j.foodres.2019.108849. [DOI] [PubMed] [Google Scholar]
- 80.Yang M.-H., Lin H.-J., Choong Y.-M. A rapid gas chromatographic method for direct determination of BHA, BHT and TBHQ in edible oils and fats. Food Res. Int. 2002;35:627–633. doi: 10.1016/S0963-9969(01)00164-8. [DOI] [Google Scholar]
- 81.Bendary E., Francis R.R., Ali H.M.G., Sarwat M.I., El Hady S. Antioxidant and structure–activity relationships (SARs) of some phenolic and anilines compounds. Ann. Agric. Sci. 2013;58:173–181. doi: 10.1016/j.aoas.2013.07.002. [DOI] [Google Scholar]
- 82.Madhavi D.L., Deshpande S.S., Salunkhe D.K. Introduction. In: Madhavi D.L., Deshpande S.S., Salunkhe D.K., editors. Food Antioxidants: Technological, Toxicological, and Health Perspectives. Dekker; New York, NY, USA: 1996. pp. 1–4. [Google Scholar]
- 83.Craft B.D., Kerrihard A.L., Amarowicz R., Pegg R.B. Phenol-based antioxidants and the in vitro methods used for their assessment. Compr. Rev. Food Sci. Food Saf. 2012;11:148–173. doi: 10.1111/j.1541-4337.2011.00173.x. [DOI] [Google Scholar]
- 84.Amarowicz R., Pegg R.B. Natural antioxidants of plant origin. Adv. Food Nutr. Res. 2019;90:1–81. doi: 10.1016/bs.afnr.2019.02.011. [DOI] [PubMed] [Google Scholar]
- 85.Weseler A.R., Bast A. Oxidative stress and vascular function: Implications for pharmacologic treatments. Curr. Hypertens. Rep. 2010;12:154–161. doi: 10.1007/s11906-010-0103-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bast A., Haenen G.R. Ten misconceptions about antioxidants. Trends Pharmacol. Sci. 2013;34:430–436. doi: 10.1016/j.tips.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 87.Reuland D.J., McCord J.M., Hamilton K.L. The role of Nrf2 in the attenuation of cardiovascular disease. Exerc. Sport Sci. Rev. 2013;41:162–168. doi: 10.1097/JES.0b013e3182948a1e. [DOI] [PubMed] [Google Scholar]
- 88.Anson N.M., Selinheimo E., Havenaar R., Aura A.M., Mattila I., Lehtinen P., Bast A., Poutanen K., Haenen G.R. Bioprocessing of wheat bran improves in vitro bioaccessibility and colonic metabolism of phenolic compounds. J. Agric. Food Chem. 2009;57:6148–6155. doi: 10.1021/jf900492h. [DOI] [PubMed] [Google Scholar]
- 89.Bjelakovic G., Nikolova D., Gluud L.L., Simonetti R.G., Gluud C. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: Systematic review and meta-analysis. JAMA. 2007;297:842–857. doi: 10.1001/jama.297.8.842. [DOI] [PubMed] [Google Scholar]
- 90.Biesalski H.K., Grune T., Tinz J., Zöllner I., Blumberg J.B. Reexamination of a meta-analysis of the effect of antioxidant supplementation on mortality and health in randomized trials. Nutrients. 2010;2:929–949. doi: 10.3390/nu2090929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Stampfer M.J., Hennekens C.H., Manson J.E., Colditz G.A., Rosner B., Willett W.C. Vitamin E consumption and the risk of coronary disease in women. N. Engl. J. Med. 1993;328:1444–1449. doi: 10.1056/NEJM199305203282003. [DOI] [PubMed] [Google Scholar]
- 92.Vivekananthan D.P., Penn M.S., Sapp S.K., Hsu A., Topol E.J. Use of antioxidant vitamins for the prevention of cardiovascular disease: Meta-analysis of randomised trials. Lancet. 2003;361:2017–2023. doi: 10.1016/S0140-6736(03)13637-9. [DOI] [PubMed] [Google Scholar]
- 93.Eidelman R.S., Hollar D., Hebert P.R., Lamas G.A., Hennekens C.H. Randomized trials of vitamin E in the treatment and prevention of cardiovascular disease. Arch. Intern. Med. 2004;164:1552–1556. doi: 10.1001/archinte.164.14.1552. [DOI] [PubMed] [Google Scholar]
- 94.Halliwell B. The antioxidant paradox. Lancet. 2000;355:1179–1180. doi: 10.1016/S0140-6736(00)02075-4. [DOI] [PubMed] [Google Scholar]
- 95.Schürks M., Glynn R.J., Rist P.M., Tzourio C., Kurth T. Effects of vitamin E on stroke subtypes: Meta-analysis of randomised controlled trials. BMJ. 2010;341:c5702. doi: 10.1136/bmj.c5702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Watson J. Oxidants, antioxidants and the current incurability of metastatic cancers. Open Biol. 2013;3:120144. doi: 10.1098/rsob.120144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Alpha-Tocopherol Beta Carotene Cancer Prevention Study Group The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. N. Engl. J. Med. 1994;330:1029–1035. doi: 10.1056/NEJM199404143301501. [DOI] [PubMed] [Google Scholar]
- 98.Pauling L. Vitamin C and Common Cold. JAMA. 1971;216:332. doi: 10.1001/jama.1971.03180280086025. [DOI] [PubMed] [Google Scholar]
- 99.Bast A., Haenen G.R., Doelman C.J. Oxidants and antioxidants: State of the art. Am. J. Med. 1991;91:2S–13S. doi: 10.1016/0002-9343(91)90278-6. [DOI] [PubMed] [Google Scholar]
- 100.Hrelia S., Angeloni C. New Mechanisms of Action of Natural Antioxidants in Health and Disease. Antioxidants. 2020;9:344. doi: 10.3390/antiox9040344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lunec J., Griffits H.R. Measuring In Vivo Oxidative Damage. John Willey and Sons; New York, NY, USA: 2000. [Google Scholar]
- 102.Poljsak B., Jamik P. Methodology for oxidative state detection in biological systems. In: Kozyrev D., Slutsky V., editors. Handbook of Free Radicals: Formation, Types and Effects. Nova Science; New York, NY, USA: 2010. Cell Biology Research Progress Series. [Google Scholar]
- 103.Argüelles S., Gómez A., Machado A., Ayala A. A preliminary analysis of within-subject variation in human serum oxidative stress parameters as a function of time. Rejuvenation Res. 2007;10:621–636. doi: 10.1089/rej.2006.0528. [DOI] [PubMed] [Google Scholar]
- 104.Sahnoun Z., Jamoussi K., Zeghal K.M. Free radicals and antioxidants: Human physiology, pathology and therapeutic aspects. Therapie. 1997;52:251–270. [PubMed] [Google Scholar]
- 105.Bunaciu A.A., Danet A.F., Fleschin Ş., Aboul-Enein H.Y. Recent Applications for in Vitro Antioxidant Activity Assay. Crit. Rev. Anal. Chem. 2016;46:389–399. doi: 10.1080/10408347.2015.1101369. [DOI] [PubMed] [Google Scholar]
- 106.Huang D., Ou B., Prior R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005;53:1841–1856. doi: 10.1021/jf030723c. [DOI] [PubMed] [Google Scholar]
- 107.Pérez-Jiménez J., Arranz S., Tabernero M., Díaz- Rubio M.E., Serrano J., Goñi I., Saura-Calixto F. Updated methodology to determine antioxidant capacity in plant foods, oils and beverages: Extraction, measurement and expression of results. Food Res. Int. 2008;41:274–285. doi: 10.1016/j.foodres.2007.12.004. [DOI] [Google Scholar]
- 108.Niki E., Noguchi N. Evaluation of antioxidant capacity. What capacity is being measured by which method? IUBMB Life. 2000;50:323–329. doi: 10.1080/15216540051081119. [DOI] [PubMed] [Google Scholar]
- 109.Magalhães L.M., Segundo M.A., Reis S., Lima J.L. Methodological aspects about in vitro evaluation of antioxidant properties. Anal. Chim. Acta. 2008;613:1–19. doi: 10.1016/j.aca.2008.02.047. [DOI] [PubMed] [Google Scholar]
- 110.Bartosz G. Total antioxidant capacity. In: Spiegel H.E., editor. Advances in Clinical Chemistry. 1st ed. Volume 37. Academic Press; New York, NY, USA: 2003. pp. 219–292. [DOI] [PubMed] [Google Scholar]
- 111.Leopoldini M., Marino T., Russo N., Toscano M. Antioxidant properties of phenolic compounds: H-atom versus electron transfer mechanism. J. Phys. Chem. A. 2004;108:4916–4922. doi: 10.1021/jp037247d. [DOI] [Google Scholar]
- 112.Wright J.S., Johnson E.R., DiLabio G.A. Predicting the activity of phenolic antioxidants: Theoretical method, analysis of substituent effects, and application to major families of antioxidants. J. Am. Chem. Soc. 2001;123:1173–1183. doi: 10.1021/ja002455u. [DOI] [PubMed] [Google Scholar]
- 113.Noipa T., Srijaranai S., Tuntulani T., Ngeontae W. New approach for evaluation of the antioxidant capacity based on scavenging DPPH free radical in micelle systems. Food Res. Int. 2011;44:798–806. doi: 10.1016/j.foodres.2011.01.034. [DOI] [Google Scholar]
- 114.Boudier A., Tournebize J., Bartosz G., El Hani S., Bengueddour R., Sapin-Minet A., Leroy P. High performance liquid chromatographic method to evaluate the hydrogen atom transfer during reaction between 1,1-diphenyl-2-picryl-hydrazyl radical and antioxidants. Anal. Chim. Acta. 2012;711:97–106. doi: 10.1016/j.aca.2011.10.063. [DOI] [PubMed] [Google Scholar]
- 115.Shahidi F., Ambigaipalan P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects—A review. J. Funct. Foods. 2015;18:820–897. doi: 10.1016/j.jff.2015.06.018. [DOI] [Google Scholar]
- 116.Shahidi F., Zhong Y. Measurement of antioxidant activity. J. Funct. Foods. 2015;18:757–781. doi: 10.1016/j.jff.2015.01.047. [DOI] [Google Scholar]
- 117.Scampicchio M., Wang J., Blasco A.J., Sanchez Arribas A., Mannino S., Escarpa A. Nanoparticle-based assays of antioxidant activity. Anal. Chem. 2006;78:2060–2063. doi: 10.1021/ac052007a. [DOI] [PubMed] [Google Scholar]
- 118.Özyürek M., Güngör N., Baki S., Güçlü K., Apak R. Development of a Silver Nanoparticle-Based Method for the Antioxidant Capacity Measurement of Polyphenols. Anal. Chem. 2012;84:8052–8059. doi: 10.1021/ac301925b. [DOI] [PubMed] [Google Scholar]
- 119.Antolovich M., Prenzler P.D., Patsalides E., McDonald S., Robards K. Methods for testing antioxidant activity. Analyst. 2002;127:183–198. doi: 10.1039/b009171p. [DOI] [PubMed] [Google Scholar]
- 120.Arnao M.B. Some methodological problems in the determination of antioxidant activity using chromogen radicals: A practical case. Trends Food Sci. Technol. 2000;11:419–421. doi: 10.1016/S0924-2244(01)00027-9. [DOI] [Google Scholar]
- 121.Iveković D., Milardović S., Roboz M., Grabarić B.S. Evaluation of the antioxidant activity by flow injection analysis method with electrochemically generated ABTS radical cation. Analyst. 2005;130:708. doi: 10.1039/b415939j. [DOI] [PubMed] [Google Scholar]
- 122.Apak R., Güçlü K., Ozyürek M., Karademir S.E. Novel total antioxidant capacity index for dietary polyphenols and vitamins C and E, using their cupric ion reducing capability in the presence of neocuproine: CUPRAC method. J. Agric. Food Chem. 2004;52:7970–7981. doi: 10.1021/jf048741x. [DOI] [PubMed] [Google Scholar]
- 123.Mannino S., Brenna O., Buratti S., Cosio M.S. A New Method for the Evaluation of the “Antioxidant Power” of Wines. Electroanalysis. 1998;10:908–912. doi: 10.1002/(SICI)1521-4109(199810)10:13<908::AID-ELAN908>3.0.CO;2-L. [DOI] [Google Scholar]
- 124.Amorati R., Valgimigli L. Methods To Measure the Antioxidant Activity of Phytochemicals and Plant Extracts. J. Agric. Food Chem. 2018;66:3324–3329. doi: 10.1021/acs.jafc.8b01079. [DOI] [PubMed] [Google Scholar]
- 125.Ordoudi S.A., Tsimidou M.Z. Crocin bleaching assay step by step: Observations and suggestions for an alternative validated protocol. J. Agric. Food Chem. 2006;54:1663–1671. doi: 10.1021/jf052731u. [DOI] [PubMed] [Google Scholar]
- 126.Prieto M.A., Vázquez J.A., Murado M.A. Crocin bleaching antioxidant assay revisited: Application to microplate to analyse antioxidant and pro-oxidant activities. Food Chem. 2015;167:299–310. doi: 10.1016/j.foodchem.2014.06.114. [DOI] [PubMed] [Google Scholar]
- 127.Lussignoli S., Fraccaroli M., Andrioli G., Brocco G., Bellavite P. A microplate-based colorimetric assay of the total peroxyl radical trapping capability of human plasma. Anal. Biochem. 1999;269:38–44. doi: 10.1006/abio.1999.4010. [DOI] [PubMed] [Google Scholar]
- 128.Gupta D. Methods for Determination of Antioxidant Capacity: A Review. Int. J. Pharm. Sci. Res. 2015;6:546–566. doi: 10.13040/IJPSR.0975-8232.6. [DOI] [Google Scholar]
- 129.Pisoschi A.M., Negulescu G.P. Methods for Total Antioxidant Activity Determination: A Review. Biochem. Anal. Biochem. 2011;1:106. doi: 10.4172/2161-1009.1000106. [DOI] [Google Scholar]
- 130.Ndhlala A.R., Moyo M., Van Staden J. Natural antioxidants: Fascinating or mythical biomolecules? Molecules. 2010;15:6905–6930. doi: 10.3390/molecules15106905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kaur I.P., Geetha T. Screening methods for antioxidants-a review. Mini Rev. Med. Chem. 2006;6:305–312. doi: 10.2174/138955706776073448. [DOI] [PubMed] [Google Scholar]
- 132.Christodouleas D., Fotakis C., Papadopoulos K., Dimotikali D., Calokerinos A.C. Luminescent Methods in the Analysis of Untreated Edible Oils: A Review. Anal. Lett. 2012;45:625–641. doi: 10.1080/00032719.2011.649461. [DOI] [Google Scholar]
- 133.Gámiz-Gracia L., García-Campaña A.M., Huertas-Pérez J.F., Lara F.J. Chemiluminescence detection in liquid chromatography: Applications to clinical, pharmaceutical, environmental and food analysis—A review. Anal. Chim. Acta. 2009;640:7–28. doi: 10.1016/j.aca.2009.03.017. [DOI] [PubMed] [Google Scholar]
- 134.Popov I., Lewin G. Antioxidative homeostasis: Characterization by means of chemiluminescent technique. Methods Enzymol. 1999;300:437–456. doi: 10.1016/s0076-6879(99)00149-4. [DOI] [PubMed] [Google Scholar]
- 135.Robak J., Gryglewski R.J. Flavonoids are scavengers of superoxide anions. Biochem. Pharmacol. 1988;37:837–841. doi: 10.1016/0006-2952(88)90169-4. [DOI] [PubMed] [Google Scholar]
- 136.Apak R., Güçlü K., Ozyürek M., Karademir S.E., Altun M. Total antioxidant capacity assay of human serum using copper(II)-neocuproine as chromogenic oxidant: The CUPRAC method. Free Radic. Res. 2005;39:949–961. doi: 10.1080/10715760500210145. [DOI] [PubMed] [Google Scholar]
- 137.Ozyurt D., Demirata B., Apak R. Determination of total antioxidant capacity by a new spectrophotometric method based on Ce(IV) reducing capacity measurement. Talanta. 2007;71:1155–1165. doi: 10.1016/j.talanta.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 138.Ozyurt D., Demirata B., Apak R. Modified cerium(IV)-based antioxidant capacity (CERAC) assay with selectivity over citric acid and simple sugars. J. Food Compos. Anal. 2010;23:282–288. doi: 10.1016/j.jfca.2009.09.005. [DOI] [Google Scholar]
- 139.Işık E., Şahin S., Demir C. Development of a new chromium reducing antioxidant capacity (CHROMAC) assay for plants and fruits. Talanta. 2013;111:119–124. doi: 10.1016/j.talanta.2013.02.053. [DOI] [PubMed] [Google Scholar]
- 140.Priya D., Rajaram K., Suresh-kumar P. In vitro antioxidant and preliminary phytochemical studies of Caralluma fimbriata wall. Int. J. Pharm. Res. 2012;4:44–48. [Google Scholar]
- 141.Kumar C.S., Loh W.S., Ooi C.W., Quah C.K., Fun H.K. Structural correlation of some heterocyclic chalcone analogues and evaluation of their antioxidant potential. Molecules. 2013;18:11996–12011. doi: 10.3390/molecules181011996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kikuzaki H., Nakatani N. Antioxidant Effects of Some Ginger Constituents. J. Food Sci. 1993;58:1407–1410. doi: 10.1111/j.1365-2621.1993.tb06194.x. [DOI] [Google Scholar]
- 143.Sharma B., Purkayastha D.D., Hazra S., Thajamanbi M., Bhattacharjee C.R., Ghosh N.N., Rout J. Biosynthesis of fluorescent gold nanoparticles using an edible freshwater red alga, Lemanea fluviatilis (L.) C.Ag. and antioxidant activity of biomatrix loaded nanoparticles. Bioprocess Biosyst. Eng. 2014;37:2559–2565. doi: 10.1007/s00449-014-1233-2. [DOI] [PubMed] [Google Scholar]
- 144.Friel J.K., Diehl-Jones W.L., Suh M., Tsopmo A., Shirwadkar V.P. Impact of iron and vitamin C-containing supplements on preterm human milk: In vitro. Free Radic. Biol. Med. 2007;42:1591–1598. doi: 10.1016/j.freeradbiomed.2007.02.022. [DOI] [PubMed] [Google Scholar]
- 145.Özyürek M., Güçlü K., Tütem E., Başkan K.S., Erçağ E., Esin Çelik S., Baki S., Yildiz L., Karaman S., Apak R. A comprehensive review of CUPRAC methodology. Anal. Methods. 2011;3:2439–2453. doi: 10.1039/c1ay05320e. [DOI] [Google Scholar]
- 146.Kunchandy E., Rao M.N.A. Oxygen radical scavenging activity of curcumin. Int. J. Pharm. 1990;58:237–240. doi: 10.1016/0378-5173(90)90201-E. [DOI] [Google Scholar]
- 147.Aguilar Diaz De Leon J., Borges C.R. Evaluation of Oxidative Stress in Biological Samples Using the Thiobarbituric Acid Reactive Substances Assay. J. Vis. Exp. 2020;159 doi: 10.3791/61122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Moon J.K., Shibamoto T. Antioxidant Assays for Plant and Food Components. J. Agric. Food Chem. 2009;57:1655–1666. doi: 10.1021/jf803537k. [DOI] [PubMed] [Google Scholar]
- 149.Re R., Pellegrini N., Proteggente A., Pannala A., Yang M., Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999;26:1231–1237. doi: 10.1016/S0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 150.Asghar M.N., Khan I.U., Arshad M.N., Sherin L. Evaluation of Antioxidant Activity Using an Improved DMPD Radical Cation Decolorization Assay. Acta Chim. Slov. 2007;54:295–300. [Google Scholar]
- 151.Damien Dorman H.J., Shikov A.N., Pozharitskaya O.N., Hiltunen R. Antioxidant and pro-oxidant evaluation of a Potentilla alba L. rhizome extract. Chem. Biodivers. 2011;8:1344–1356. doi: 10.1002/cbdv.201100043. [DOI] [PubMed] [Google Scholar]
- 152.Fontana M., Mosca L., Rosei M.A. Interaction of enkephalins with oxyradicals. Biochem. Pharmacol. 2001;61:1253–1257. doi: 10.1016/S0006-2952(01)00565-2. [DOI] [PubMed] [Google Scholar]
- 153.Habu J.B., Ibeh B.O. In vitro antioxidant capacity and free radical scavenging evaluation of active metabolite constituents of Newbouldia laevis ethanolic leaf extract. Biol. Res. 2015;48:16. doi: 10.1186/s40659-015-0007-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Bailly F., Zoete V., Vamecq J., Catteau J.P., Bernier J.L. Antioxidant actions of ovothiol-derived 4-mercaptoimidazoles: Glutathione peroxidase activity and protection against peroxynitrite-induced damage. FEBS Lett. 2000;486:19–22. doi: 10.1016/S0014-5793(00)02234-1. [DOI] [PubMed] [Google Scholar]
- 155.Ou B., Hampsch-Woodill M., Flanagan J., Deemer E.K., Prior R.L., Huang D. Novel Fluorometric Assay for Hydroxyl Radical Prevention Capacity Using Fluorescein as the Probe. J. Agric. Food Chem. 2002;50:2772–2777. doi: 10.1021/jf011480w. [DOI] [PubMed] [Google Scholar]
- 156.Chu Y.H., Chang C.L., Hsu H.F. Flavonoid content of several vegetables and their antioxidant activity. J. Sci. Food Agric. 2000;80:561–566. doi: 10.1002/(SICI)1097-0010(200004)80:5<561::AID-JSFA574>3.0.CO;2-#. [DOI] [Google Scholar]
- 157.Cheng Z., Zhou H., Yin J., Yu L. Electron spin resonance estimation of hydroxyl radical scavenging capacity for lipophilic antioxidants. J. Agric. Food Chem. 2007;55:3325–3333. doi: 10.1021/jf0634808. [DOI] [PubMed] [Google Scholar]
- 158.Carini M., Aldini G., Piccone M., Facino R.M. Fluorescent probes as markers of oxidative stress in keratinocyte cell lines following UVB exposure. Farmaco. 2000;55:526–534. doi: 10.1016/S0014-827X(00)00037-9. [DOI] [PubMed] [Google Scholar]
- 159.Nabavi S.M., Ebrahimzadeh M.A., Nabavi S.F., Hamidinia A., Bekhradnia A.R. Determination of antioxidant activity, phenol and flavonoids content of Parrotia persica Mey. Pharmacologyonline. 2008;2:560–567. [Google Scholar]
- 160.Pedraza-Chaverri J., Barrera D., Maldonado P.D., Chirino Y.I., Macias-Ruvalcaba N.A., Medina-Campos O.N., Castro L., Salcedo M.I., Hernandez-Pando R. S-allylmercaptocysteine scavenges hydroxyl radical and singlet oxygen in vitro and attenuates gentamicin-induced oxidative and nitrosative stress and renal damage in vivo. BMC Clin. Pharmacol. 2004;4:5. doi: 10.1186/1472-6904-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Macku C., Shibamoto T. Volatile antioxidants produced from heated corn oil/glycine model system. J. Agric. Food Chem. 1991;39:1990–1993. doi: 10.1021/jf00011a022. [DOI] [Google Scholar]
- 162.Sudan R., Bhagat M., Gupta S., Singh J., Koul A. Iron (FeII) Chelation, Ferric Reducing Antioxidant Power, and Immune Modulating Potential of Arisaema jacquemontii (Himalayan Cobra Lily) BioMed. Res. Int. 2014 doi: 10.1155/2014/179865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Sousa S.J., Brizola V.R.A., Granato D. High-throughput assay comparison and standardization for metal chelating capacity screening: A proposal and application. Food Chem. 2017;214:515–522. doi: 10.1016/j.foodchem.2016.07.091. [DOI] [PubMed] [Google Scholar]
- 164.Rodriguez-Amaya D.B. Quantitative analysis, in vitro assessment of bioavailability and antioxidant activity of food carotenoids -A review. J. Food Compos. Anal. 2010;23:726–740. doi: 10.1016/j.jfca.2010.03.008. [DOI] [Google Scholar]
- 165.Fukai T., Ushio-Fukai M. Superoxide dismutases: Role in redox signaling, vascular function, and diseases. Antioxid. Redox Signal. 2011;15:1583–1606. doi: 10.1089/ars.2011.3999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Brigelius-Flohé R., Maiorino M. Glutathione peroxidases. Biochim. Biophys. Acta. 2013;1830:3289–3303. doi: 10.1016/j.bbagen.2012.11.020. [DOI] [PubMed] [Google Scholar]
- 167.Rhee S.G., Kang S.W., Chang T.S., Jeong W., Kim K. Peroxiredoxin, a novel family of peroxidases. IUBMB Life. 2001;52:35–41. doi: 10.1080/15216540252774748. [DOI] [PubMed] [Google Scholar]
- 168.Arnér E.S., Holmgren A. Physiological functions of thioredoxin and thioredoxin reductase. Eur. J. Biochem. 2000;267:6102–6109. doi: 10.1046/j.1432-1327.2000.01701.x. [DOI] [PubMed] [Google Scholar]
- 169.Pellegrini N., Serafini M., Colombi B., Del Rio D., Salvatore S., Bianchi M., Brighenti F. Total antioxidant capacity of plant foods, beverages and oils consumed in Italy assessed by three different in vitro assays. J. Nutr. 2003;133:2812–2819. doi: 10.1093/jn/133.9.2812. [DOI] [PubMed] [Google Scholar]
- 170.Gómez-Alonso S., Mancebo-Campos V., Salvador M.D., Fregapane G. Evolution of major and minor components and oxidation indices of virgin olive oil during 21 months storage at room temperature. Food Chem. 2007;100:36–42. doi: 10.1016/j.foodchem.2005.09.006. [DOI] [Google Scholar]
- 171.Reis F.S., Martins A., Barros L., Ferreira I.C. Antioxidant properties and phenolic profile of the most widely appreciated cultivated mushrooms: A comparative study between in vivo and in vitro samples. Food Chem. Toxicol. 2012;50:1201–1207. doi: 10.1016/j.fct.2012.02.013. [DOI] [PubMed] [Google Scholar]
- 172.Marcocci L., Maguire J.J., Droy-Lefaix M.T., Packer L. The nitric oxide-scavenging properties of Ginkgo biloba extract EGb 761. Biochem. Biophys. Res. Commun. 1994;201:748–755. doi: 10.1006/bbrc.1994.1764. [DOI] [PubMed] [Google Scholar]
- 173.Prior R.L., Wu X., Schaich K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005;53:4290–4302. doi: 10.1021/jf0502698. [DOI] [PubMed] [Google Scholar]
- 174.Prieto P., Pineda M., Aguilar M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of vitamin E. Anal. Biochem. 1999;269:337–341. doi: 10.1006/abio.1999.4019. [DOI] [PubMed] [Google Scholar]
- 175.Goldschmidt S., Renn K. Zweiwertiger Stickstoff: Über das α, α-Diphenyl-β-trinitrophenyl-hydrazyl. (IV. Mitteilung über Amin-Oxydation) Chem. Ber. 1922;55:628–643. doi: 10.1002/cber.19220550308. [DOI] [Google Scholar]
- 176.Molyneux P. The use of the stable free radical diphenylpicrylhydrazyl (DPPH) for estimating antioxidant activity. Songklanakarin. J. Sci. Technol. 2004;26:211–219. [Google Scholar]
- 177.Blois M. Antioxidant Determinations by the Use of a Stable Free Radical. Nature. 1958;181:1199–1200. doi: 10.1038/1811199a0. [DOI] [Google Scholar]
- 178.Mishra K., Ojha H., Chaudhury N.K. Estimation of antiradical properties of antioxidants using DPPH• assay: A critical review and results. Food Chem. 2012;130:1036–1043. doi: 10.1016/j.foodchem.2011.07.127. [DOI] [Google Scholar]
- 179.Osman A.M. Multiple pathways of the reaction of 2,2-diphenyl-1-picrylhydrazyl radical (DPPH•) with (+)-catechin: Evidence for the formation of a covalent adduct between (DPPH•) and the oxidized form of the polyphenol. Biochem. Biophys. Res. Commun. 2011;412:473–478. doi: 10.1016/j.bbrc.2011.07.123. [DOI] [PubMed] [Google Scholar]
- 180.Passari A.K., Leo V.V., Singh G., Samanta L., Ram H., Siddaiah C.N., Hashem A., Al-Arjani A.-B.F., Alqarawi A.A., Fathi Abd_Allah E., et al. In Vivo Studies of Inoculated Plants and In Vitro Studies Utilizing Methanolic Extracts of Endophytic Streptomyces sp. Strain DBT34 Obtained from Mirabilis jalapa L. Exhibit ROS-Scavenging and Other Bioactive Properties. Int. J. Mol. Sci. 2020;21:7364. doi: 10.3390/ijms21197364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Ražná K., Sawinska Z., Ivanišová E., Vukovic N., Terentjeva M., Stričík M., Kowalczewski P.Ł., Hlavačková L., Rovná K., Žiarovská J., et al. Properties of Ginkgo biloba L.: Antioxidant Characterization, Antimicrobial Activities, and Genomic MicroRNA Based Marker Fingerprints. Int. J. Mol. Sci. 2020;21:3087. doi: 10.3390/ijms21093087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Gois Ruivo da Silva M., Skrt M., Komes D., Poklar Ulrih N., Pogačnik L. Enhanced Yield of Bioactivities from Onion (Allium cepa L.) Skin and Their Antioxidant and Anti-α-Amylase Activities. Int. J. Mol. Sci. 2020;21:2909. doi: 10.3390/ijms21082909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Pisoschi A.M., Cheregi M.C., Danet A.F. Total Antioxidant Capacity of Some Commercial Fruit Juices: Electrochemical and Spectrophotometrical Approaches. Molecules. 2009;14:480–493. doi: 10.3390/molecules14010480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.McGowan J.C., Powell T., Raw R. The rates of reaction of α,α-diphenyl-β-picrylhydrazyl with certain amines and phenols. J. Chem. Soc. 1959;1959:3103–3110. doi: 10.1039/JR9590003103. [DOI] [Google Scholar]
- 185.Hogg J.S., Lohmann D.H., Russell K.S. The kinetics of reaction of 2,2-diphenyl-1-picrylhydrazyl with phenols. Can. J. Chem. 1961;39:1588–1594. doi: 10.1139/v61-202. [DOI] [Google Scholar]
- 186.Apak R., Gorinstein S., Böhm V., Schaich K.M., Özyürek M., Güçlü K. Methods of measurement and evaluation of natural antioxidant capacity/activity. Pure Appl. Chem. 2013;85:957–998. doi: 10.1351/PAC-REP-12-07-15. [DOI] [Google Scholar]
- 187.Brand-Williams W., Cuvelier M.E., Berset C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995;28:25–30. doi: 10.1016/S0023-6438(95)80008-5. [DOI] [Google Scholar]
- 188.Cheng Z., Moore J., Yu L. High-throughput relative DPPH radical scavenging capacity assay. J. Agric Food Chem. 2006;54:7429–7436. doi: 10.1021/jf0611668. [DOI] [PubMed] [Google Scholar]
- 189.Bondet V., Brand-Williams W., Berset C. Kinetics and mechanisms of antioxidant activity using the DPPH radical method. LWT-Food Sci. Technol. 1997;30:609–615. doi: 10.1006/fstl.1997.0240. [DOI] [Google Scholar]
- 190.Xie J., Schaich K.M. Re-evaluation of the 2,2-Diphenyl-1-picrylhydrazyl Free Radical (DPPH) Assay for Antioxidant Activity. J. Agric. Food Chem. 2014;62:4251–4260. doi: 10.1021/jf500180u. [DOI] [PubMed] [Google Scholar]
- 191.Alexander B., Browse D.J., Reading S.J., Benjamin I.S. A simple and accurate mathematical method for calculation of the EC50. J. Pharmacol. Toxicol. Methods. 1999;41:55–58. doi: 10.1016/S1056-8719(98)00038-0. [DOI] [PubMed] [Google Scholar]
- 192.Chen Z., Bertin R., Froldi G. EC50 estimation of antioxidant activity in DPPH·assay using several statistical programs. Food Chem. 2013;138:414–420. doi: 10.1016/j.foodchem.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 193.Van der Graaf P.H., Schoemaker R.C. Analysis of asymmetry of agonist concentration-effect curves. J. Pharmacol. Toxicol. Methods. 1999;41:107–115. doi: 10.1016/S1056-8719(99)00026-X. [DOI] [PubMed] [Google Scholar]
- 194.Ukeda H., Adachi Y., Sawamura M. Flow injection analysis of DPPH radical based on electron spin resonance. Talanta. 2002;58:1279–1283. doi: 10.1016/S0039-9140(02)00411-3. [DOI] [PubMed] [Google Scholar]
- 195.Ukeda H. Flow-injection analytical system for the evaluation of antioxidative activity. Bunseki Kagaku. 2004;53:221. doi: 10.2116/bunsekikagaku.53.221. [DOI] [Google Scholar]
- 196.Polásek M., Skála P., Opletal L., Jahodár L. Rapid automated assay of anti-oxidation/radical-scavenging activity of natural substances by sequential injection technique (SIA) using spectrophotometric detection. Anal. Bioanal. Chem. 2004;379:754–758. doi: 10.1007/s00216-004-2559-4. [DOI] [PubMed] [Google Scholar]
- 197.Yamaguchi T., Takamura H., Matoba T., Terao J. HPLC method for evaluation of the free radical-scavenging activity of foods by using 1,1-diphenyl-2-picrylhydrazyl. Biosci. Biotechnol. Biochem. 1998;62:1201–1204. doi: 10.1271/bbb.62.1201. [DOI] [PubMed] [Google Scholar]
- 198.Koleva I.I., Niederländer H.A., van Been T.A. An on-line HPLC method for detection of radical scavenging compounds in complex mixtures. Anal. Chem. 2000;72:2323–2328. doi: 10.1021/ac9912451. [DOI] [PubMed] [Google Scholar]
- 199.Cerdà V., Estela J.M., Forteza R., Cladera A., Becerra E., Altimira P., Sitjar P. Flow techniques in water analysis. Talanta. 1999;50:695–705. doi: 10.1016/S0039-9140(99)00196-4. [DOI] [PubMed] [Google Scholar]
- 200.Wu C., Chen F., Wang X., Wu Y., Dong M., He G., Galyean R.D., He L., Huang G. Tanacetum parthenium by HPLC-ESI-MS/MS and NMR. Phytochem. Anal. 2007;18:401–410. doi: 10.1002/pca.995. [DOI] [PubMed] [Google Scholar]
- 201.Nuengchamnong N., de Jong C.F., Bruyneel B., Niessen W.M., Irth H., Ingkaninan K. HPLC coupled on-line to ESI-MS and a DPPH-based assay for the rapid identification of anti-oxidants in Butea superba. Phytochem. Anal. 2005;16:422–428. doi: 10.1002/pca.865. [DOI] [PubMed] [Google Scholar]
- 202.Tang D., Li H.J., Chen J., Guo C.W., Li P. Rapid and simple method for screening of natural antioxidants from Chinese herb Flos Lonicerae Japonicae by DPPH-HPLC-DAD-TOF/MS. J. Sep. Sci. 2008;31:3519–3526. doi: 10.1002/jssc.200800173. [DOI] [PubMed] [Google Scholar]
- 203.Glavind J., Holmer G. Thin-Layer Chromatographic Determination of Antioxidants by the Stable Free Radical Diphenyl-picrylhydrazyl. J. Am. Oil Chem. Soc. 1967;44:539–542. doi: 10.1007/BF02679243. [DOI] [Google Scholar]
- 204.Li P., Anu H., Jari S., Teijo Y., Heikki V. TLC Method for Evaluation of Free Radical Scavenging Activity of Rapeseed Meal by Video Scanning Technology; Proceedings of the 10th International Rapseed Congress; Canberra, Australia. 26–29 September 1999; [(accessed on 30 September 2008)]. Available online: http://www.regional.org.au/au/gcirc/1/551.htm. [Google Scholar]
- 205.Blasco A.J., Rogerio M.C., Gonzalez M.C., Escarpa A. “Electrochemical Index” as a screening method to determine “total polyphenolics” in foods: A proposal. Anal. Chim. Acta. 2005;539:237–244. doi: 10.1016/j.aca.2005.02.056. [DOI] [Google Scholar]
- 206.Chevion S., Roberts M.A., Chevion M. The use of cyclic voltammetry for the evaluation of antioxidant capacity. Free Radic. Biol. Med. 2000;28:860–870. doi: 10.1016/S0891-5849(00)00178-7. [DOI] [PubMed] [Google Scholar]
- 207.Martinez S., Valek L., Resetic J., Rusic D.F. Cyclic voltammetry study of plasma antioxidant capacity–comparison with the DPPH and TAS spectrophotometric methods. J. Electroanal. Chem. 2006;588:68–73. doi: 10.1016/j.jelechem.2005.12.016. [DOI] [Google Scholar]
- 208.Zielińska D., Zieliński H. Antioxidant activity of flavone C-glucosides determined by updated analytical strategies. Food Chem. 2011;124:672–678. doi: 10.1016/j.foodchem.2010.06.051. [DOI] [Google Scholar]
- 209.Zielińska D., Turemko M. Electroactive Phenolic Contributors and Antioxidant Capacity of Flesh and Peel of 11 Apple Cultivars Measured by Cyclic Voltammetry and HPLC–DAD–MS/MS. Antioxidants. 2020;9:1054. doi: 10.3390/antiox9111054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Milardovic S., Ivekovic D., Rumenjak V., Bozidar S.G. Use of DPPH./DPPH redox couple for biamperometric determination of antioxidant activity. Electroanalysis. 2005;17:1847–1853. doi: 10.1002/elan.200503312. [DOI] [Google Scholar]
- 211.Baig N., Kammakakam I., Falath W. Nanomaterials: A review of synthesis methods, properties, recent progress, and challenges. Mater. Adv. 2021;2:1821–1871. doi: 10.1039/D0MA00807A. [DOI] [Google Scholar]
- 212.Katz E., Willner I. Integrated nanoparticle-biomolecule hybrid systems: Synthesis, properties, and applications. Angew. Chem. Int. Ed. Engl. 2004;43:6042–6108. doi: 10.1002/anie.200400651. [DOI] [PubMed] [Google Scholar]
- 213.Katz E., Willner I., Wang J. Electroanalytical and Bioelectroanalytical Systems Based on Metal and Semiconductor Nanoparticles. Electroanalysis. 2004;16:19–44. doi: 10.1002/elan.200302930. [DOI] [Google Scholar]
- 214.Rosi N.L., Mirkin C.A. Nanostructures in biodiagnostics. Chem. Rev. 2005;105:1547–1562. doi: 10.1021/cr030067f. [DOI] [PubMed] [Google Scholar]
- 215.Vasilescu A., Sharpe E., Andreescu S. Nanoparticle-Based Technologies for the Detection of Food Antioxidants. Curr. Anal. Chem. 2012;8:495–505. doi: 10.2174/157341112803216780. [DOI] [Google Scholar]
- 216.Mourdikoudis S., Pallares R.M., Thanh N.T.K. Characterization techniques for nanoparticles: Comparison and complementarity upon studying nanoparticle properties. Nanoscale. 2018;10:12871–12934. doi: 10.1039/C8NR02278J. [DOI] [PubMed] [Google Scholar]
- 217.Hendrickson O.D., Safenkova I.V., Zherdev A.V., Dzantiev B.B., Popov V.O. Methods of detection and identification of manufactured nanoparticles. Biophysics. 2011;56:961–986. doi: 10.1134/S0006350911060066. [DOI] [PubMed] [Google Scholar]
- 218.Szydlowska-Czerniak A., Tulodziecka A. Comparison of a silver nanoparticle-based method and the modified spectrophotometric methods for assessing antioxidant capacity of rapeseed varieties. Food Chem. 2013;141:1865–1871. doi: 10.1016/j.foodchem.2013.04.111. [DOI] [PubMed] [Google Scholar]
- 219.Rao Y., Chen Q., Dong J., Qian W. Growth-sensitive 3D ordered gold nanoshells precursor composite arrays as SERS nanoprobes for assessing hydrogen peroxide scavenging activity. Analyst. 2011;136:769–774. doi: 10.1039/C0AN00725K. [DOI] [PubMed] [Google Scholar]
- 220.Zhao W., Brook M.A., Li Y.F. Design of Gold NanoparticleBased Colorimetric Biosensing Assays. ChemBioChem. 2008;9:2363–2371. doi: 10.1002/cbic.200800282. [DOI] [PubMed] [Google Scholar]
- 221.Ma X., Qian W. Phenolic acid induced growth of gold nanoshells precursor composites and their application in antioxidant capacity assay. Biosens. Bioelectron. 2010;26:1049–1055. doi: 10.1016/j.bios.2010.08.055. [DOI] [PubMed] [Google Scholar]
- 222.Alzahrani E. Colorimetric Detection Based on Localised Surface Plasmon Resonance Optical Characteristics for the Detection of Hydrogen Peroxide Using Acacia Gum–Stabilised Silver Nanoparticles. Anal. Chem. Insights. 2017 doi: 10.1177/1177390116684686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Gatselou V., Christodouleas D.C., Kouloumpis A., Gournis D., Giokas D.L. Determination of phenolic compounds using spectral and color transitions of rhodium nanoparticles. Anal. Chim. Acta. 2016;932:80–87. doi: 10.1016/j.aca.2016.05.029. [DOI] [PubMed] [Google Scholar]
- 224.Dahle T., Arai Y. Environmental geochemistry of cerium: Applications and toxicology of cerium oxide nanoparticles. Int. J. Environ. Res. Public Health. 2015;12:1253–1278. doi: 10.3390/ijerph120201253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Ozdemir Olgun F.A., Üzer A., Ozturk B.D., Apak R. A novel cerium oxide nanoparticles-based colorimetric sensor using tetramethyl benzidine reagent for antioxidant activity assay. Talanta. 2018;182:55–61. doi: 10.1016/j.talanta.2018.01.047. [DOI] [PubMed] [Google Scholar]
- 226.Sharpe E., Frasco T., Andreescu D., Andreescu S. Portable ceria nanoparticle-based assay for rapid detection of food antioxidants (NanoCerac) Analyst. 2013;138:249–262. doi: 10.1039/C2AN36205H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Sharpe E., Bradley R., Frasco T., Jayathilaka D., Marsh A., Andreescu S. Metal oxide-based multisensor array and portable database for field analysis of antioxidants. Sens. Actuators B-Chem. 2014;193:552–562. doi: 10.1016/j.snb.2013.11.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Lopez-Alarcon C., Denicola A. Evaluating the antioxidant capacity of natural products: A review on chemical and cellular-based assays. Anal. Chim. Acta. 2013;763:1–10. doi: 10.1016/j.aca.2012.11.051. [DOI] [PubMed] [Google Scholar]
- 229.Alarcon-Angeles G., Alvarez-Romero G.A., Merkoci A. Emerging Nanomaterials for Analytical Detection. Biosensors for Sustainable Food—New Opportunities and Technical Challenges. Compr. Anal. Chem. 2016;74:195–246. doi: 10.1016/bs.coac.2016.03.022. [DOI] [Google Scholar]
- 230.Della Pelle F., Compagnone D. Nanomaterial-Based Sensing and Biosensing of Phenolic Compounds and Related Antioxidant Capacity in Food. Sensors. 2018;18:462. doi: 10.3390/s18020462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Bedlovičová Z., Strapáč I., Baláž M., Salayová A. A Brief Overview on Antioxidant Activity Determination of Silver Nanoparticles. Molecules. 2020;25:3191. doi: 10.3390/molecules25143191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Vilela D., Gonzalez M.C., Escarpa A. Nanoparticles as analytical tools for in-vitro antioxidant-capacity assessment and beyond. TrAC. 2015;64:1–16. doi: 10.1016/j.trac.2014.07.017. [DOI] [Google Scholar]
- 233.Ganesan K., Kumar K.S., Rao P.V.S. Comparative assessment of antioxidant activity in three edible species of green seaweed, Enteromorpha from Okha, northwest coast of India. Innov. Food Sci. Emerg. Technol. 2011;12:73–78. doi: 10.1016/j.ifset.2010.11.005. [DOI] [Google Scholar]
- 234.Pisoschi A.M., Pop A., Cimpeanu C., Predoi G. Antioxidant Capacity Determination in Plants and Plant-Derived Products: A Review. Oxid. Med. Cell Longev. 2016;2016:9130976. doi: 10.1155/2016/9130976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Smith R.M. Before the injection--modern methods of sample preparation for separation techniques. J. Chromatogr. A. 2003;1000:3–27. doi: 10.1016/S0021-9673(03)00511-9. [DOI] [PubMed] [Google Scholar]
- 236.Gupta A., Naraniwal M., Kothari V. Modern extraction methods for preparation of bioactive plant extracts. Int. J. Appl. Nat. Sci. 2012;1:8–26. [Google Scholar]
- 237.Lisitsyn A.B., Semenova A.A., Gundyreva M.I. A study of the antioxidant properties of supercritical CO2 extracts. Meat Ind. 2006;3:30–35. [Google Scholar]
- 238.Luque de Castro M.D., García-Ayuso L.E. Soxhlet extraction of solid materials: An outdated technique with a promising innovative future. Anal. Chim. Acta. 1998;369:1–10. doi: 10.1016/S0003-2670(98)00233-5. [DOI] [Google Scholar]
- 239.Buitrago D., Buitrago-Villanueva I., Barbosa-Cornelio R., Coy-Barrera E. Comparative Examination of Antioxidant Capacity and Fingerprinting of Unfractionated Extracts from Different Plant Parts of Quinoa (Chenopodium quinoa) Grown under Greenhouse Conditions. Antioxidants. 2019;8:238. doi: 10.3390/antiox8080238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Singh B.P., Rateb M.E., Rodriguez-Couto S., Polizeli M.L.T.M., Li W.J. Editorial: Microbial secondary metabolites: Recent developments and technological challenges. Front. Microbiol. 2019;10:914. doi: 10.3389/fmicb.2019.00914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Demain A.L. Importance of microbial natural products and the need to revitalize their discovery. J. Ind. Microbiol. Biotechnol. 2014;41:185–201. doi: 10.1007/s10295-013-1325-z. [DOI] [PubMed] [Google Scholar]
- 242.Min W.H., Fang X.B., Wu T., Fang L., Liu C.L., Wang J. Characterization and antioxidant activity of an acidic exopolysaccharide from Lactobacillus plantarum JLAU103. J. Biosci. Bioeng. 2019;127:758–766. doi: 10.1016/j.jbiosc.2018.12.004. [DOI] [PubMed] [Google Scholar]
- 243.Yang Z., Li S., Zhang X., Zeng X., Li D., Zhao Y., Zhang J. Capsular and slime-polysaccharide production by Lactobacillus rhamnosus JAAS8 isolated from Chinese sauerkraut: Potential application in fermented milk products. J. Biosci. Bioeng. 2010;110:53–57. doi: 10.1016/j.jbiosc.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 244.Dose J., Matsugo S., Yokokawa H., Koshida Y., Okazaki S., Seidel U., Eggersdorfer M., Rimbach G., Esatbeyoglu T. Free radical scavenging and cellular antioxidant properties of astaxanthin. Int. J. Mol. Sci. 2016;17:103. doi: 10.3390/ijms17010103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Naguib Y.M.A. Antioxidant activities of astaxanthin and related carotenoids. J. Agric. Food Chem. 2000;48:1150–1154. doi: 10.1021/jf991106k. [DOI] [PubMed] [Google Scholar]
- 246.Blagojević D., Babić O., Rašeta M., Šibul F., Janjušević L., Simeunović J. Antioxidant activity and phenolic profile in filamentous cyanobacteria: The impact of nitrogen. J. Appl. Phycol. 2018;30:2337–2346. doi: 10.1007/s10811-018-1476-4. [DOI] [Google Scholar]
- 247.Kumar H., Bhardwaj K., Nepovimova E., Kuča K., Singh Dhanjal D., Bhardwaj S., Bhatia S.K., Verma R., Kumar D. Antioxidant Functionalized Nanoparticles: A Combat against Oxidative Stress. Nanomaterials. 2020;10:1334. doi: 10.3390/nano10071334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Sansone C., Brunet C. Promises and Challenges of Microalgal Antioxidant Production. Antioxidants. 2019;8:199. doi: 10.3390/antiox8070199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Pagels F., Guedes A.C., Amaro H.M., Kijjoa A., Vasconcelos V. Phycobiliproteins from cyanobacteria: Chemistry and biotechnological applications. Biotechnol. Adv. 2019;37:422–443. doi: 10.1016/j.biotechadv.2019.02.010. [DOI] [PubMed] [Google Scholar]
- 250.Wekre M.E., Kåsin K., Underhaug J., Holmelid B., Jordheim M. Quantification of Polyphenols in Seaweeds: A Case Study of Ulva intestinalis. Antioxidants. 2019;8:612. doi: 10.3390/antiox8120612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Maroneze M.M., Zepka L.Q., Lopes E.J., Pérez-Gálvez A., Roca M. Chlorophyll Oxidative Metabolism During the Phototrophic and Heterotrophic Growth of Scenedesmus obliquus. Antioxidants. 2019;8:600. doi: 10.3390/antiox8120600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Rajauria G. In-Vitro Antioxidant Properties of Lipophilic Antioxidant Compounds from 3 Brown Seaweed. Antioxidants. 2019;8:596. doi: 10.3390/antiox8120596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Nègre D., Aite M., Belcour A., Frioux C., Brillet-Guéguen L., Liu X., Bordron P., Godfroy O., Lipinska A.P., Leblanc C., et al. Genome–Scale Metabolic Networks Shed Light on the Carotenoid Biosynthesis Pathway in the Brown Algae Saccharina japonica and Cladosiphon okamuranus. Antioxidants. 2019;8:564. doi: 10.3390/antiox8110564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Neumann U., Derwenskus F., Flaiz Flister V., Schmid-Staiger U., Hirth T., Bischoff S.C., Fucoxanthin A. Carotenoid Derived from Phaeodactylum tricornutum Exerts Antiproliferative and Antioxidant Activities In Vitro. Antioxidants. 2019;8:183. doi: 10.3390/antiox8060183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Smerilli A., Balzano S., Maselli M., Blasio M., Orefice I., Galasso C., Sansone C., Brunet C. Antioxidant and Photoprotection Networking in the Coastal Diatom Skeletonema marinoi. Antioxidants. 2019;8:154. doi: 10.3390/antiox8060154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Xu Y., Harvey P.J. Red Light Control of β-Carotene Isomerisation to 9-cis β-Carotene and Carotenoid Accumulation in Dunaliella salina. Antioxidants. 2019;8:148. doi: 10.3390/antiox8050148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Le B., Golokhvast K.S., Yang S.H., Sun S. Optimization of Microwave-Assisted Extraction of Polysaccharides from Ulva pertusa and Evaluation of Their Antioxidant Activity. Antioxidants. 2019;8:129. doi: 10.3390/antiox8050129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Xu Y., Harvey P.J. Carotenoid Production by Dunaliella salina under Red Light. Antioxidants. 2019;8:123. doi: 10.3390/antiox8050123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Merino J.J., Parmigiani-Izquierdo J.M., Toledano Gasca A., Cabaña-Muñoz M.E. The Long-Term Algae Extract (Chlorella and Fucus sp.) and Aminosulphurate Supplementation Modulate SOD-1 Activity and Decrease Heavy Metals (Hg++, Sn) Levels in Patients with Long-Term Dental Titanium Implants and Amalgam Fillings Restorations. Antioxidants. 2019;8:101. doi: 10.3390/antiox8040101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Ranković B., Kosanić M. Lichens as a potential source of bioactive secondary metabolites. In: Ranković B., editor. Lichen Secondary Metabolites: Bioactive Properties and Pharmaceutical Potential. Springer International Publishing; Berlin/Heidelberg, Germany: 2015. [DOI] [Google Scholar]
- 261.Gülçin İ., Oktay M., Küfrevioğlu Ö.İ., Aslan A. Determination of antioxidant activity of lichen Cetraria islandica (L.) Ach. J. Ethnopharmacol. 2002;79:325–329. doi: 10.1016/S0378-8741(01)00396-8. [DOI] [PubMed] [Google Scholar]
- 262.Behera B.C., Verma N., Sonone A., Makhija U. Antioxidant and antibacterial activities of lichen Usnea ghattensis in vitro. Biotechnol. Lett. 2005;27:991–995. doi: 10.1007/s10529-005-7847-3. [DOI] [PubMed] [Google Scholar]
- 263.Gulluce M., Aslan A., Sokmen M., Sahin F., Adiguzel A., Agar G., Sokmen A. Screening the antioxidant and antimicrobial properties of the lichens Parmelia saxatilis, Platismatia glauca, Ramalina pollinaria, Ramalina polymorpha and Umbilicaria nylanderiana. Phytomedicine. 2006;13:515–521. doi: 10.1016/j.phymed.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 264.Fernández-Moriano C., Gómez-Serranillos M.P., Crespo A. Antioxidant potential of lichen species and their secondary metabolites. A systematic review. Pharm. Biol. 2016;54:1–17. doi: 10.3109/13880209.2014.1003354. [DOI] [PubMed] [Google Scholar]
- 265.Kosanić M., Ranković B. Lichens as possible sources of antioxidants. Pak. J. Pharm. Sci. 2011;24:165–170. [PubMed] [Google Scholar]
- 266.Ranković B., Ranković D., Kosanić M., Marić D. Antioxidant and antimicrobial properties of the lichens Anaptychya ciliaris, Nephroma parile, Ochrolechia tartarea and Parmelia centrifuga. Cent. Eur. J. Biol. 2010;5:649–655. doi: 10.2478/s11535-010-0043-z. [DOI] [Google Scholar]
- 267.Bhattarai H.D., Paudel B., Hong S.G., Lee H.K., Yim J.H. Thin layer chromatography analysis of antioxidant constituents of lichens from Antarctica. J. Nat. Med. 2008;62:481–484. doi: 10.1007/s11418-008-0257-9. [DOI] [PubMed] [Google Scholar]
- 268.Rajan V.P., Gunasekaran S., Ramanathan S., Murugaiyah V., Samsudin M.W., Din L.B. Biological activities of four Parmotrema species of Malaysian origin and their chemical constituents. J. Appl. Pharm. Sci. 2016;6:36–43. doi: 10.7324/JAPS.2016.60806. [DOI] [Google Scholar]
- 269.Tomović J., Kosanić M., Ristić S., Ranković B., Stanojković T., Manojlović N. Chemical composition and bioactive properties of the lichen, Pleurosticta acetabulum. Trop. J. Pharm. Res. 2017;16:2977–2984. doi: 10.4314/tjpr.v16i12.23. [DOI] [Google Scholar]
- 270.Sisodia R., Geol M., Verma S., Rani A., Dureja P. Antibacterial and antioxidant activity of lichen species Ramalina roesleri. Nat. Prod. Res. 2013;27:2235–2239. doi: 10.1080/14786419.2013.811410. [DOI] [PubMed] [Google Scholar]
- 271.Kawahara T., Izumikawa M., Otoguro M., Yamamura H., Hayakawa M., Takagi M., Shin Ya K. JBIR-94 and JBIR-125, antioxidative phenolic compounds from Streptomyces sp. R56-07. J. Nat. Prod. 2012;75:107–110. doi: 10.1021/np200734p. [DOI] [PubMed] [Google Scholar]
- 272.Lertcanawanichakul M., Pondet K., Kwantep J. In vitro antimicrobial and antioxidant activities of bioactive compounds (secondary metabolites) extracted from Streptomyces lydicus A2. J. Appl. Pharm. Sci. 2015;5:17–21. doi: 10.7324/JAPS.2015.50204. [DOI] [Google Scholar]
- 273.Kiruthika P., Durairaju Nisshanthini S., Angayarkanni J. In vitro antimicrobial and antioxidant profile of Streptomyces spp. isolated from coromandel coast region, India. Int. J. Pharma Bio Sci. 2013;4:127–136. [Google Scholar]
- 274.Lee D.R., Lee S.K., Choi B.K., Cheng J., Lee Y.S., Yang S.H., Suh J.W. Antioxidant activity and free radical scavenging activities of Streptomyces sp. strain MJM 10778. Asian Pac. J. Trop. Med. 2014;7:962–967. doi: 10.1016/S1995-7645(14)60170-X. [DOI] [PubMed] [Google Scholar]
- 275.Janardhan A., Kumar A.P., Viswanath B., Saigopal D.V.R., Narasimha G. Production of Bioactive Compounds by Actinomycetes and Their Antioxidant Properties. Biotechnol. Res. Int. 2014:1–8. doi: 10.1155/2014/217030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Thenmozhi M., Sindhura S., Kannabiran K. Characterization of antioxidant activity of Streptomyces species VITTK3 isolated from Puducherry coast, India. J. Adv. Sci. Res. 2010;1:46–52. [Google Scholar]
- 277.Saurav K., Kannabiran K. Cytotoxicity and antioxidant activity of 5-(2,4-dimethylbenzyl)pyrrolidin-2-one extracted from marine Streptomyces VITSVK5 spp. Saudi J. Biol. Sci. 2012;19:81–86. doi: 10.1016/j.sjbs.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Komiyama K., Funayama S., Anraku Y., Mita A., Takahashi Y., Omura S., Shimasaki H. Isolation of isoflavonoids possessing antioxidant activity from the fermentation broth of Streptomyces spp. J. Antibiot. 1989;42:1344–1349. doi: 10.7164/antibiotics.42.1344. [DOI] [PubMed] [Google Scholar]
- 279.Ielciu I., Frédérich M., Hanganu D., Angenot L., Olah N.-K., Ledoux A., Crișan G., Păltinean R. Flavonoid Analysis and Antioxidant Activities of the Bryonia alba L. Aerial Parts. Antioxidants. 2019;8:108. doi: 10.3390/antiox8040108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Chaves N., Santiago A., Alías J.C. Quantification of the Antioxidant Activity of Plant Extracts: Analysis of Sensitivity and Hierarchization Based on the Method Used. Antioxidants. 2020;9:76. doi: 10.3390/antiox9010076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Gougoulias N. Evaluation of antioxidant activity and polyphenol content of leaves from some fruit species. Oxid. Commun. 2015;38:35–45. [Google Scholar]
- 282.Katalinic V., Mozina S.S., Generalic I., Skroza D., Ljubenkov I., Klancnik A. Phenolic Profile, Antioxidant Capacity, and Antimicrobial Activity of Leaf Extracts from Six Vitis vinifera, L. Varieties. Int. J. Food Prop. 2013;16:45–60. doi: 10.1080/10942912.2010.526274. [DOI] [Google Scholar]
- 283.Medlej M.K., Batoul C., Olleik H., Li S., Hijazi A., Nasser G., Maresca M., Pochat-Bohatier C. Antioxidant Activity and Biocompatibility of Fructo-Polysaccharides Extracted from a Wild Species of Ornithogalum from Lebanon. Antioxidants. 2021;10:68. doi: 10.3390/antiox10010068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Attanzio A., D’Anneo A., Pappalardo F., Bonina F.P., Livrea M.A., Allegra M., Tesoriere L. Phenolic Composition of Hydrophilic Extract of Manna from Sicilian Fraxinus angustifolia Vahl and its Reducing, Antioxidant and Anti-Inflammatory Activity in Vitro. Antioxidants. 2019;8:494. doi: 10.3390/antiox8100494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Skenderidis P., Lampakis D., Giavasis I., Leontopoulos S., Petrotos K., Hadjichristodoulou C., Tsakalof A. Chemical Properties, Fatty-Acid Composition, and Antioxidant Activity of Goji Berry (Lycium barbarum L. and Lycium chinense Mill.) Fruits. Antioxidants. 2019;8:60. doi: 10.3390/antiox8030060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Flieger J., Flieger M. The [DPPH●/DPPH-H]-HPLC-DAD Method on Tracking the Antioxidant Activity of Pure Antioxidants and Goutweed (Aegopodium podagraria L.) Hydroalcoholic Extracts. Molecules. 2020;25:6005. doi: 10.3390/molecules25246005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Uma Anitha K.P.G., Mythili S. Antioxidant and hepatoprotective potentials of novel endophytic fungus Achaetomium sp., from Euphorbia hirta. Asian Pac. J. Trop. Med. 2017;10:588–593. doi: 10.1016/j.apjtm.2017.06.008. [DOI] [PubMed] [Google Scholar]
- 288.Sugiharto S., Yudiarti T., Isroli I. Assay of Antioxidant Potential of Two Filamentous Fungi Isolated from the Indonesian Fermented Dried Cassava. Antioxidants. 2016;5:6. doi: 10.3390/antiox5010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Arora D.S., Chandra P. In vitro antioxidant potential of some soil fungi: Screening of functional compounds and their purification from Penicillium citrinum. Appl. Biochem. Biotechnol. 2011;165:639–651. doi: 10.1007/s12010-011-9282-3. [DOI] [PubMed] [Google Scholar]
- 290.Yadav M., Yadav A., Yadav J.P. In vitro antioxidant activity and total phenolic content of endophytic fungi isolated from Eugenia jambolana Lam. Asian Pac. J. Trop. Med. 2014;7S1:S256–S261. doi: 10.1016/S1995-7645(14)60242-X. [DOI] [PubMed] [Google Scholar]
- 291.Li T.X., Meng D.D., Wang Y., An J.L., Bai J.F., Jia X.W., Xu C.P. Antioxidant coumarin and pyrone derivatives from the insect-associated fungus Aspergillus versicolor. Nat. Prod. Res. 2020;34:1360–1365. doi: 10.1080/14786419.2018.1509334. [DOI] [PubMed] [Google Scholar]
- 292.Zhang H., Wang Z.Y., Yang L., Yang X., Wang X., Zhang Z. In vitro antioxidant activities of sulfated derivatives of polysaccharides extracted from Auricularia auricular. Int. J. Mol. Sci. 2011;12:3288–3302. doi: 10.3390/ijms12053288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Huang X.Z., Zhu Y., Guan X.L., Tian K., Guo J.M., Wang H.B., Fu G.M. A novel antioxidant isobenzofuranone derivative from fungus Cephalosporium sp.AL031. Molecules. 2012;17:4219–4224. doi: 10.3390/molecules17044219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Jaszek M., Osińska-Jaroszuk M., Janusz G., Matuszewska A., Stefaniuk D., Sulej J., Polak J., Ruminowicz M., Grzywnowicz K., Jarosz-Wilkołazka A. New bioactive fungal molecules with high antioxidant and antimicrobial capacity isolated from Cerrena unicolor idiophasic cultures. Biomed. Res. Int. 2013;2013:1–11. doi: 10.1155/2013/497492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Shah S.R., Ukaegbu C.I., Hamid H.A., Alara O.R. Evaluation of antioxidant and antibacterial activities of the stems of Flammulina velutipes and Hypsizygus tessellates (white and brown var.) extracted with different solvents. J. Food Meas. Charact. 2018;12:1947–1961. doi: 10.1007/s11694-018-9810-8. [DOI] [Google Scholar]
- 296.Abdullah N., Ismail S.M., Aminudin N., Shuib A.S., Lau B.F. Evaluation of Selected Culinary-Medicinal Mushrooms for Antioxidant and ACE Inhibitory Activities. Evid. Based Complement. Alternat. Med. 2012;2012:464238. doi: 10.1155/2012/464238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Chandra P., Arora D.S. Antioxidant Potential of Penicillium expansum and Purification of its Functional Compound. Asian J. Biotechnol. 2017;9:24–34. doi: 10.3923/ajbkr.2017.24.34. [DOI] [Google Scholar]
- 298.Arora D.S., Sharma R.K., Chandra P. Biodelignification of wheat straw and its effect on in vitro digestibility and antioxidant properties. Int. Biodeterior. Biodegrad. 2011;65:352–358. doi: 10.1016/j.ibiod.2010.12.009. [DOI] [Google Scholar]
- 299.Babu D.R., Pandey M., Rao G.N. Antioxidant and electrochemical properties of cultivated Pleurotus spp. and their sporeless/low sporing mutants. J. Food Sci. Technol. 2012;51:3317–3324. doi: 10.1007/s13197-012-0822-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Knežević A., Stajić M., Sofrenić I., Stanojković T., Milovanović I., Tešević V., Vukojević J. Antioxidative, antifungal, cytotoxic and antineurodegenerative activity of selected Trametes species from Serbia. PLoS ONE. 2018;13:e0203064. doi: 10.1371/journal.pone.0203064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Kodali V.P., Sen R. Antioxidant and free radical scavenging activities of an exopolysaccharide from a probiotic bacterium. Biotechnol. J. 2008;3:245–251. doi: 10.1002/biot.200700208. [DOI] [PubMed] [Google Scholar]
- 302.Adesulu-Dahunsi A., Sanni A., Jeyaram K. Production, characterization and in vitro antioxidant activities of exopolysaccharide from Weissella cibaria GA44. LWT Food Sci. Technol. 2018;87:432–442. doi: 10.1016/j.lwt.2017.09.013. [DOI] [Google Scholar]
- 303.Zhang L., Liu C., Li D., Zhao Y., Zhang X., Zeng X., Yang Z., Li S. Antioxidant activity of an exopolysaccharide isolated from Lactobacillus plantarum C88. Int. J. Biol. Macromol. 2013;54:270–275. doi: 10.1016/j.ijbiomac.2012.12.037. [DOI] [PubMed] [Google Scholar]
- 304.Akinsanya M.A., Goh J.K., Lim S.P., Ting A.S. Diversity, antimicrobial and antioxidant activities of culturable bacterial endophyte communities in Aloe vera. FEMS Microbiol. Lett. 2015;362:fnv184. doi: 10.1093/femsle/fnv184. [DOI] [PubMed] [Google Scholar]
- 305.Horta A., Pinteus S., Alves C., Fino N., Silva J., Fernandez S., Rodrigues A., Pedrosa R. Antioxidant and antimicrobial potential of the Bifurcaria bifurcata epiphytic bacteria. Mar. Drugs. 2014;12:1676–1689. doi: 10.3390/md12031676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Kato S., Shindo K., Yamagishi Y., Matsuoka M., Kawai H., Mochizuki J. Phenazoviridin, a novel free radical scavenger from Streptomyces sp. Taxonomy, fermentation, isolation, structure elucidation and biological properties. J. Antibiot. 1993;46:1485–1493. doi: 10.7164/antibiotics.46.1485. [DOI] [PubMed] [Google Scholar]
- 307.Aguilar-Toalá J.E., Astiazarán-García H., Estrada-Montoya M.C., Garcia H.S., Vallejo-Cordoba B., González-Córdova A.F., Hernández-Mendoza A. Modulatory Effect of the Intracellular Content of Lactobacillus casei CRL 431 Against the Aflatoxin B1-Induced Oxidative Stress in Rats. Probiotics Antimicrob. Proteins. 2019;11:470–477. doi: 10.1007/s12602-018-9433-8. [DOI] [PubMed] [Google Scholar]
- 308.Wang K., Niu M., Yao D., Zhao J., Wu Y., Lu B., Zheng X. Physicochemical characteristics and in vitro and in vivo antioxidant activity of a cell-bound exopolysaccharide produced by Lactobacillus fermentum S1. Int. J. Biol. Macromol. 2019;139:252–261. doi: 10.1016/j.ijbiomac.2019.07.200. [DOI] [PubMed] [Google Scholar]
- 309.Liu C.F., Tseng K.C., Chiang S.S., Lee B.H., Hsu W.H., Pan T.M. Immunomodulatory and antioxidant potential of Lactobacillus exopolysaccharides. J. Sci. Food Agric. 2011;91:2284–2291. doi: 10.1002/jsfa.4456. [DOI] [PubMed] [Google Scholar]
- 310.González-Ballesteros N., Rodríguez-Argüelles M.C., Lastra-Valdor M. Evaluation of the Antioxidant Capacities of Antarctic Macroalgae and Their Use for Nanoparticles Production. Molecules. 2021;26:1182. doi: 10.3390/molecules26041182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Régnier P., Bastias J., Rodriguez-Ruiz V., Caballero-Casero N., Caballo C., Sicilia D., Fuentes A., Maire M., Crepin M., Letourneur D., et al. Astaxanthin from Haematococcus pluvialis Prevents Oxidative Stress on Human Endothelial Cells without Toxicity. Mar. Drugs. 2015;13:2857–2874. doi: 10.3390/md13052857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Sueishi Y., Ishikawa M., Yoshioka D., Endoh N., Oowada S., Shimmei M., Fujii H., Kotake Y. Oxygen radical absorbance capacity (ORAC) of cyclodextrin-solubilized flavonoids, resveratrol and astaxanthin as measured with the ORAC-EPR method. J. Clin. Biochem. Nutr. 2012;50:127–132. doi: 10.3164/jcbn.11-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Widowati I., Zainuri M., Kusumaningrum H.P., Susilowati R., Hardivillier Y., Leignel V., Bourgougnon N., Mouget J.L. Antioxidant activity of three microalgae Dunaliella salina, Tetraselmis chuii and Isochrysis galbana clone Tahiti. IOP Conf. Ser. Earth Environ. Sci. 2017;55:012067. doi: 10.1088/1755-1315/55/1/012067. [DOI] [Google Scholar]
- 314.Gürlek C., Yarkent C., Köse A., Oral I., Öncel S.S., Elibol M. Evaluation of several microalgal extracts as bioactive metabolites as potential pharmaceutical compounds; Proceedings of the CMBEBIH 2019: Proceedings of the International Conference on Medical and Biological Engineering; Banja Luka, Bosnia and Herzegovina. 16–18 May 2019. [Google Scholar]
- 315.Agregán R., Munekata P.E.S., Franco D., Carballo J., Barba F.J., Lorenzo J.M. Antioxidant Potential of Extracts Obtained from Macro- (Ascophyllum nodosum, Fucus vesiculosus and Bifurcaria bifurcata) and Micro-Algae (Chlorella vulgaris and Spirulina platensis) Assisted by Ultrasound. Medicines. 2018;5:33. doi: 10.3390/medicines5020033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Dantas D.M.M., Oliveira C.Y.B., Costa R.M.P.B., Carneiro-da-Cunha M.D.G., Gálvez A.O., Bezerra R.S. Evaluation of antioxidant and antibacterial capacity of green microalgae Scenedesmus subspicatus. Food Sci. Technol. Int. 2019;25:318–326. doi: 10.1177/1082013218825024. [DOI] [PubMed] [Google Scholar]
- 317.Sansone C., Galasso C., Orefice I., Nuzzo G., Luongo E., Cutignano A., Romano G., Brunet C., Fontana A., Esposito F., et al. The green microalga Tetraselmis suecica reduces oxidative stress and induces repairing mechanisms in human cells. Sci. Rep. 2017;7:41215. doi: 10.1038/srep41215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Kato S., Kawasaki T., Urata T., Mochizuki J. In vitro and ex vivo free radical scavenging activities of carazostatin, carbazomycin b and their derivatives. J. Antibiot. 1993;46:1859–1865. doi: 10.7164/antibiotics.46.1859. [DOI] [PubMed] [Google Scholar]
- 319.Shin-Ya K., Imai S., Furihata K., Hayakawa K., Hayakawa Y., Kato Y., Vanduyne G.D., Clardy J., Seto H. Isolation and structural elucidation of an antioxidative agent, naphterpin. J. Antibiot. 1990;43:444–447. doi: 10.7164/antibiotics.43.444. [DOI] [PubMed] [Google Scholar]
- 320.Karthik L., Kumar G., Rao K.V.B. Antioxidant activity of newly discovered lineage of marine actinobacteria. Asian Pac. J. Trop. Med. 2013;6:325–332. doi: 10.1016/S1995-7645(13)60065-6. [DOI] [PubMed] [Google Scholar]
- 321.Boisselier E., Astruc D. Gold nanoparticles in nanomedicine: Preparations, imaging, diagnostics, therapies and toxicity. Chem. Soc. Rev. 2009;38:1759–1782. doi: 10.1039/b806051g. [DOI] [PubMed] [Google Scholar]
- 322.Eftekhari A., Dizaj S.M., Chodari L., Sunar S., Hasanzadeh A., Ahmadian E., Hasanzadeh M. The promising future of nano-antioxidant therapy against environmental pollutants induced-toxicities. Biomed. Pharmacother. 2018;103:1018–1027. doi: 10.1016/j.biopha.2018.04.126. [DOI] [PubMed] [Google Scholar]
- 323.Nelson B.C., Johnson M.E., Walker M.L., Riley K.R., Sims C.M. Antioxidant Cerium Oxide Nanoparticles in Biology and Medicine. Antioxidants. 2016;5:15. doi: 10.3390/antiox5020015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Herlekar M., Barve S., Kumar R. Plant-mediated green synthesis of iron nanoparticles. J. Nanopart. Res. 2014;2014:140614. doi: 10.1155/2014/140614. [DOI] [Google Scholar]
- 325.Huber D.L. Synthesis, properties, and applications of iron nanoparticles. Small. 2005;1:482–501. doi: 10.1002/smll.200500006. [DOI] [PubMed] [Google Scholar]
- 326.Guo J., Wang R., Tjiu W.W., Pan J., Liu T. Synthesis of Fe nanoparticles@ graphene composites for environmental applications. J. Hazard. Mater. 2012;225:63–73. doi: 10.1016/j.jhazmat.2012.04.065. [DOI] [PubMed] [Google Scholar]
- 327.Ebrahiminezhad A., Zare-Hoseinabadi A., Sarmah A.K., Taghizadeh S., Ghasemi Y., Berenjian A. Plant-mediated synthesis and applications of iron nanoparticles. Mol. Biotechnol. 2018;60:154–168. doi: 10.1007/s12033-017-0053-4. [DOI] [PubMed] [Google Scholar]
- 328.Kumar H., Bhardwaj K., Kuča K., Kalia A., Nepovimova E., Verma R., Kumar D. Flower-Based Green Synthesis of Metallic Nanoparticles: Applications beyond Fragrance. Nanomaterials. 2020;10:766. doi: 10.3390/nano10040766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Henriksen-Lacey M., Carregal-Romero S., Liz-Marzán L.M. Current Challenges toward In Vitro Cellular Validation of Inorganic Nanoparticles. Bioconjug. Chem. 2017;28:212–221. doi: 10.1021/acs.bioconjchem.6b00514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Ariga K., Minami K., Ebara M., Nakanishi J. What are the emerging concepts and challenges in NANO Nanoarchitectonics, hand-operating nanotechnology and mechanobiology. Polym. J. 2016;48:371–389. doi: 10.1038/pj.2016.8. [DOI] [Google Scholar]
- 331.Jessl S., Tebbe M., Guerrini L., Fery A., Alvarez-Puebla R.A., Pazos-Perez N. Silver-Assisted Synthesis of Gold Nanorods: The Relation between Silver Additive and Iodide Impurities. Small. 2018;14:e1703879. doi: 10.1002/smll.201703879. [DOI] [PubMed] [Google Scholar]
- 332.Boström M., Williams D.R., Ninham B.W. Specific ion effects: Why DLVO theory fails for biology and colloid systems. Phys. Rev. Lett. 2001;87:168103. doi: 10.1103/PhysRevLett.87.168103. [DOI] [PubMed] [Google Scholar]
- 333.Nel A., Xia T., Mädler L., Li N. Toxic potential of materials at the nanolevel. Science. 2006;311:622–627. doi: 10.1126/science.1114397. [DOI] [PubMed] [Google Scholar]
- 334.Oberdörster G., Oberdörster E., Oberdörster J. Nanotoxicology: An emerging discipline evolving from studies of ultrafine particles. Environ. Health Perspect. 2005;113:823–839. doi: 10.1289/ehp.7339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Gurunathan S., Jeyaraj M., La H., Yoo H., Choi Y., Do J.T., Park C., Kim J.H., Hong K. Anisotropic Platinum Nanoparticle-Induced Cytotoxicity, Apoptosis, Inflammatory Response, and Transcriptomic and Molecular Pathways in Human Acute Monocytic Leukemia Cells. Int. J. Mol. Sci. 2020;21:440. doi: 10.3390/ijms21020440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Giordo R., Nasrallah G.K., Al-Jamal O., Paliogiannis P., Pintus G. Resveratrol Inhibits Oxidative Stress and Prevents Mitochondrial Damage Induced by Zinc Oxide Nanoparticles in Zebrafish (Danio rerio) Int. J. Mol. Sci. 2020;21:3838. doi: 10.3390/ijms21113838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Zhou X., Zhao L., Luo J., Tang H., Xu M., Wang Y., Yang X., Chen H., Li Y., Ye G., et al. The Toxic Effects and Mechanisms of Nano-Cu on the Spleen of Rats. Int. J. Mol. Sci. 2019;20:1469. doi: 10.3390/ijms20061469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Khanna P., Ong C., Bay B.H., Baeg G.H. Nanotoxicity: An Interplay of Oxidative Stress, Inflammation and Cell Death. Nanomaterials. 2015;5:1163–1180. doi: 10.3390/nano5031163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Fubini B., Hubbard A. Reactive oxygen species (ROS) and reactive nitrogen species (RNS) generation by silica in inflammation and fibrosis. Free Radic. Biol. Med. 2003;34:1507–1516. doi: 10.1016/S0891-5849(03)00149-7. [DOI] [PubMed] [Google Scholar]
- 340.Manke A., Wang L., Rojanasakul Y. Mechanisms of nanoparticle-induced oxidative stress and toxicity. Biomed. Res. Int. 2013;2013:942916. doi: 10.1155/2013/942916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Sims C.M., Hanna S.K., Heller D.A., Horoszko C.P., Johnson M.E., Montoro Bustos A.R., Reipa V., Riley K.R., Nelson B.C. Redox-active nanomaterials for nanomedicine applications. Nanoscale. 2017;9:15226–15251. doi: 10.1039/C7NR05429G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Schubert D., Dargusch R., Raitano J., Chan S.W. Cerium and yttrium oxide nanoparticles are neuroprotective. Biochem. Biophys. Res. Commun. 2006;342:86–91. doi: 10.1016/j.bbrc.2006.01.129. [DOI] [PubMed] [Google Scholar]
- 343.Chen J., Patil S., Seal S., McGinnis J.F. Rare earth nanoparticles prevent retinal degeneration induced by intracellular peroxides. Nat. Nanotechnol. 2006;1:142–150. doi: 10.1038/nnano.2006.91. [DOI] [PubMed] [Google Scholar]
- 344.Das M., Patil S., Bhargava N., Kang J.F., Riedel L.M., Seal S., Hickman J.J. Auto-catalytic ceria nanoparticles offer neuroprotection to adult rat spinal cord neurons. Biomaterials. 2007;28:1918–1925. doi: 10.1016/j.biomaterials.2006.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Korsvik C., Patil S., Seal S., Self W.T. Superoxide dismutase mimetic properties exhibited by vacancy engineered ceria nanoparticles. Chem. Commun. 2007;10:1056–1058. doi: 10.1039/b615134e. [DOI] [PubMed] [Google Scholar]
- 346.Reidy B., Haase A., Luch A., Dawson K.A., Lynch I. Mechanisms of Silver Nanoparticle Release, Transformation and Toxicity: A Critical Review of Current Knowledge and Recommendations for Future Studies and Applications. Materials. 2013;6:2295–2350. doi: 10.3390/ma6062295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.AshaRani P.V., Low Kah Mun G., Hande M.P., Valiyaveettil S. Cytotoxicity and genotoxicity of silver nanoparticles in human cells. ACS Nano. 2009;3:279–290. doi: 10.1021/nn800596w. [DOI] [PubMed] [Google Scholar]
- 348.Hirst S.M., Karakoti A., Singh S., Self W., Tyler R., Seal S., Reilly C.M. Bio-distribution and in vivo antioxidant effects of cerium oxide nanoparticles in mice. Environ. Toxicol. 2013;28:107–118. doi: 10.1002/tox.20704. [DOI] [PubMed] [Google Scholar]
- 349.Caputo F., De Nicola M., Sienkiewicz A., Giovanetti A., Bejarano I., Licoccia S., Traversa E., Ghibelli L. Cerium oxide nanoparticles, combining antioxidant and UV shielding properties, prevent UV-induced cell damage and mutagenesis. Nanoscale. 2015;7:15643–15656. doi: 10.1039/C5NR03767K. [DOI] [PubMed] [Google Scholar]
- 350.Koul B., Poonia A.K., Yadav D., Jin J.-O. Microbe-Mediated Biosynthesis of Nanoparticles: Applications and Future Prospects. Biomolecules. 2021;11:886. doi: 10.3390/biom11060886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Garmasheva I., Kovalenko N., Voychuk S., Ostapchuk A., Livins’ka O., Oleschenko L. Lactobacillus species mediated synthesis of silver nanoparticles and their antibacterial activity against opportunistic pathogens in vitro. BioImpacts. 2016;6:219–223. doi: 10.15171/bi.2016.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Vu X.H., Duong T.T.T., Pham T.T.H., Trinh D.K., Nguyen X.H., Dang V.-S. Synthesis and study of silver nanoparticles for antibacterial activity against Escherichia coli and Staphylococcus aureus. Adv. Nat. Sci. Nanosci. Nanotechnol. 2018;9:025019. doi: 10.1088/2043-6254/aac58f. [DOI] [Google Scholar]
- 353.Rai M., Yadav A., Gade A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol. Adv. 2009;27:76–83. doi: 10.1016/j.biotechadv.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 354.Lemire J.A., Harrison J.J., Turner R.J. Antimicrobial activity of metals: Mechanisms, molecular targets and applications. Nat. Rev. Microbiol. 2013;11:371–384. doi: 10.1038/nrmicro3028. [DOI] [PubMed] [Google Scholar]
- 355.Lok C.N., Ho C.M., Chen R., He Q.Y., Yu W.Y., Sun H., Tam P.K., Chiu J.F., Che C.M. Silver nanoparticles: Partial oxidation and antibacterial activities. J. Biol. Inorg. Chem. 2007;12:527–534. doi: 10.1007/s00775-007-0208-z. [DOI] [PubMed] [Google Scholar]
- 356.Percival S.L., Hill K.E., Williams D.W., Hooper S.J., Thomas D.W., Costerton J.W. A review of the scientific evidence for biofilms in wounds. Wound Repair. Regen. 2012;20:647–657. doi: 10.1111/j.1524-475X.2012.00836.x. [DOI] [PubMed] [Google Scholar]
- 357.Rendueles O., Ghigo J.M. Multi-species biofilms: How to avoid unfriendly neighbors. FEMS Microbiol. Rev. 2012;36:972–989. doi: 10.1111/j.1574-6976.2012.00328.x. [DOI] [PubMed] [Google Scholar]
- 358.Donlan R.M., Costerton J.W. Biofilms: Survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 2002;15:167–193. doi: 10.1128/CMR.15.2.167-193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Mah T.F., O’Toole G.A. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 2001;9:34–39. doi: 10.1016/S0966-842X(00)01913-2. [DOI] [PubMed] [Google Scholar]
- 360.Kakoullis L., Papachristodoulou E., Chra P., Panos G. Mechanisms of Antibiotic Resistance in Important Gram-Positive and Gram-Negative Pathogens and Novel Antibiotic Solutions. Antibiotics. 2021;10:415. doi: 10.3390/antibiotics10040415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Amanatidou E., Matthews A.C., Kuhlicke U., Neu T.R., McEvoy J.P., Raymond B. Biofilms facilitate cheating and social exploitation of β-lactam resistance in Escherichia coli. NPJ Biofilms Microbiomes. 2019;5 doi: 10.1038/s41522-019-0109-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Kalishwaralal K., Barath Mani Kanth S., Pandian S.R.K., Deepak V., Gurunathan S. Silver nanoparticles impede the biofilm formation by Pseudomonas aeruginosa and Staphylococcus epidermidis. Colloids Sur. B Biointerfaces. 2010;79:340–344. doi: 10.1016/j.colsurfb.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 363.Siddique M.H., Aslam B., Imran M., Ashraf A., Nadeem H., Hayat S., Khurshid M., Afzal M., Malik I.R., Shahzad M., et al. Effect of Silver Nanoparticles on Biofilm Formation and EPS Production of Multidrug-Resistant Klebsiella pneumoniae. Biomed. Res. Int. 2020;2020:6398165. doi: 10.1155/2020/6398165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Hamida R.S., Ali M.A., Goda D.A., Khalil M.I., Al-Zaban M.I. Novel Biogenic Silver Nanoparticle-Induced Reactive Oxygen Species Inhibit the Biofilm Formation and Virulence Activities of Methicillin-Resistant Staphylococcus aureus (MRSA) Strain. Front. Bioeng. Biotechnol. 2020;8 doi: 10.3389/fbioe.2020.00433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Jafari A., Nagheli A., Alavi Foumani A., Soltani B., Goswami R. The Role of Metallic Nanoparticles in Inhibition of Mycobacterium Tuberculosis and Enhances Phagosome Maturation into the Infected Macrophage. Oman Med. J. 2020;35:e194. doi: 10.5001/omj.2020.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Li X., Sun L., Zhang P., Wang Y. Novel Approaches to Combat Medical Device-Associated BioFilms. Coatings. 2021;11:294. doi: 10.3390/coatings11030294. [DOI] [Google Scholar]
- 367.Liu Z., Liu X., Ramakrishna S. Surface engineering of biomaterials in orthopedic and dental implants: Strategies to improve osteointegration, bacteriostatic and bactericidal activities. Biotechnol. J. 2021:e2000116. doi: 10.1002/biot.202000116. [DOI] [PubMed] [Google Scholar]
- 368.Filipović U., Dahmane R.G., Ghannouchi S., Zore A., Bohinc K. Bacterial adhesion on orthopedic implants. Adv. Colloid Interface Sci. 2020;283:102228. doi: 10.1016/j.cis.2020.102228. [DOI] [PubMed] [Google Scholar]
- 369.Chouirfa H., Bouloussa H., Migonney V., Falentin-Daudré C. Review of titanium surface modification techniques and coatings for antibacterial applications. Acta Biomater. 2019;83:37–54. doi: 10.1016/j.actbio.2018.10.036. [DOI] [PubMed] [Google Scholar]
- 370.Wang N., Fuh J.Y.H., Dheen S.T., Senthil Kumar A. Functions and applications of metallic and metallic oxide nanoparticles in orthopedic implants and scaffolds. J. Biomed. Mater. Res. B Appl. Biomater. 2021;109:160–179. doi: 10.1002/jbm.b.34688. [DOI] [PubMed] [Google Scholar]
- 371.Sukhanova A., Bozrova S., Sokolov P., Berestovoy M., Karaulov A., Nabiev I. Dependence of Nanoparticle Toxicity on Their Physical and Chemical Properties. Nanoscale Res. Lett. 2018;13:44. doi: 10.1186/s11671-018-2457-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Alphandéry E. Natural Metallic Nanoparticles for Application in Nano-Oncology. Int. J. Mol. Sci. 2020;21:4412. doi: 10.3390/ijms21124412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Alphandéry E. Bio-synthesized iron oxide nanoparticles for cancer treatment. Int. J. Pharm. 2020;586:119472. doi: 10.1016/j.ijpharm.2020.119472. [DOI] [PubMed] [Google Scholar]
- 374.Fdez-Gubieda M.L., Alonso J., García-Prieto A., García-Arribas A., Barquín L.F., Muela A. Magnetotactic bacteria for cancer therapy. J. Appl. Phys. 2020;128:070902. doi: 10.1063/5.0018036. [DOI] [Google Scholar]
- 375.Bai Y., Qiu X., Zhang Q., Qiu S., Qin Y., Wang T. Green Synthesis of Highly Dispersed Ni/SiO2 Catalysts Using Natural Biomass of Sesbania Powder. Ind. Eng. Chem. Res. 2020;59:17399–17407. doi: 10.1021/acs.iecr.0c02005. [DOI] [Google Scholar]
- 376.Nasrullah M., Gul F.Z., Hanif S., Mannan A., Naz S., Ali J.S., Zia M. Green and Chemical Syntheses of CdO NPs: A Comparative Study for Yield Attributes, Biological Characteristics, and Toxicity Concerns. ACS Omega. 2020;5:5739–5747. doi: 10.1021/acsomega.9b03769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Sriranjani R., Srinithya B., Vellingiri V., Brindha P., Anthony S.P., Sivasubramanian A., Muthuraman M.S. Silver nanoparticle synthesis using Clerodendrum phlomidis leaf extract and preliminary investigation of its antioxidant and anticancer activities. J. Mol. Liq. 2016;220:926–930. doi: 10.1016/j.molliq.2016.05.042. [DOI] [Google Scholar]
- 378.Misawa M., Takahashi J. Generation of reactive oxygen species induced by gold nanoparticles under X-ray and UV Irradiations. Nanomedicine. 2011;7:604–614. doi: 10.1016/j.nano.2011.01.014. [DOI] [PubMed] [Google Scholar]
- 379.Avalos A., Haza A.I., Mateo D., Morales P. Cytotoxicity and ROS production of manufactured silver nanoparticles of different sizes in hepatoma and leukemia cells. J. Appl. Toxicol. 2014;34:413–423. doi: 10.1002/jat.2957. [DOI] [PubMed] [Google Scholar]
- 380.Flores-López L.Z., Espinoza-Gómez H., Somanathan R. Silver nanoparticles: Electron transfer, reactive oxygen species, oxidative stress, beneficial and toxicological effects. Mini review. J. Appl. Toxicol. 2019;39:16–26. doi: 10.1002/jat.3654. [DOI] [PubMed] [Google Scholar]
- 381.Chithrani B.D., Ghazani A.A., Chan W.C. Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano. Lett. 2006;6:662–668. doi: 10.1021/nl052396o. [DOI] [PubMed] [Google Scholar]
- 382.Rajeshkumar S., Menon S., Kumar S.V., Tambuwala M.M., Bakshi H.A., Mehta M., Satija S., Gupta G., Chellappan D.K., Thangavelu L., et al. Antibacterial and antioxidant potential of biosynthesized copper nanoparticles mediated through Cissus arnotiana plant extract. J. Photochem. Photobiol. 2019;197:111531. doi: 10.1016/j.jphotobiol.2019.111531. [DOI] [PubMed] [Google Scholar]
- 383.Muthukumar H., Manickam M. Amaranthus spinosus leaf extract mediated FeO nanoparticles: Physicochemical traits, photocatalytic and antioxidant activity. ACS Sustain. Chem. Eng. 2015;3:3149–3156. doi: 10.1021/acssuschemeng.5b00722. [DOI] [Google Scholar]
- 384.Babay S., Mhiri T., Toumi M. Synthesis, structural and spectroscopic characterizations of maghemite-Fe2O3 prepared by one-step coprecipitation route. J. Mol. Struct. 2015;1085:286–293. doi: 10.1016/j.molstruc.2014.12.067. [DOI] [Google Scholar]
- 385.Saleh N., Kim H.J., Phenrat T., Matyjaszewski K., Tilton R.D., Lowry G.V. Ionic strength and composition a_ect the mobility of surface-modified Fe0 nanoparticles in water-saturated sand columns. Environ. Sci. Technol. 2008;42:3349–3355. doi: 10.1021/es071936b. [DOI] [PubMed] [Google Scholar]
- 386.Kim H.J., Kim D.G., Yoon H., Choi Y.S., Yoon J., Lee J.C. Polyphenol/FeIII complex coated membranes having multifunctional properties prepared by a one-step fast assembly. Adv. Mater. Interfaces. 2015;2:1500298. doi: 10.1002/admi.201500298. [DOI] [Google Scholar]
- 387.Yang L., Cao Z., Sajja H.K., Mao H., Wang L., Geng H., Xu H., Jiang T., Wood W.C., Nie S., et al. Development of receptor targeted magnetic iron oxide nanoparticles for effcient drug delivery and tumor imaging. J. Biomed. Nanotechnol. 2008;4:439–449. doi: 10.1166/jbn.2008.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Akhtar M.S., Panwar J., Yun Y.S. Biogenic Synthesis of Metallic Nanoparticles by Plant Extracts. ACS Sustain. Chem. Eng. 2013;1:591–602. doi: 10.1021/sc300118u. [DOI] [Google Scholar]
- 389.Gericke M., Pinches A. Biological synthesis of metal nanoparticles. Hydrometallurgy. 2006;83:132–140. doi: 10.1016/j.hydromet.2006.03.019. [DOI] [Google Scholar]
- 390.Suresh A.K., Pelletier D.A., Wang W., Broich M.L., Moon J.W., Gu B., Allison D.P., Joy D.C., Phelps T.J., Doktycz M.J. Biofabrication of discrete spherical gold nanoparticles using the metal-reducing bacterium Shewanella oneidensis. Acta Biomater. 2011;7:2148–2152. doi: 10.1016/j.actbio.2011.01.023. [DOI] [PubMed] [Google Scholar]
- 391.Grzelczak M., Pérez-Juste J., Mulvaney P., Liz-Marzán L.M. Shape control in gold nanoparticle synthesis. Chem. Soc. Rev. 2008;37:1783–1791. doi: 10.1039/b711490g. [DOI] [PubMed] [Google Scholar]
- 392.Patil S.P., Kumbhar S.T. Antioxidant, antibacterial and cytotoxic potential of silver nanoparticles synthesized using terpenes rich extract of Lantana camara L. leaves. Biochem. Biophys. Rep. 2017;10:76–81. doi: 10.1016/j.bbrep.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Elemike E.E., Fayemi O.E., Ekennia A.C., Onwudiwe D.C., Ebenso E.E. Silver Nanoparticles Mediated by Costus afer Leaf Extract: Synthesis, Antibacterial, Antioxidant and Electrochemical Properties. Molecules. 2017;22:701. doi: 10.3390/molecules22050701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Saratale R.G., Benelli G., Kumar G., Kim D.S., Saratale G.D. Bio-fabrication of silver nanoparticles using the leaf extract of an ancient herbal medicine, dandelion (Taraxacum o_cinale), evaluation of their antioxidant, anticancer potential, and antimicrobial activity against phytopathogens. Environ. Sci. Pollut. Res. 2018;25:10392–10406. doi: 10.1007/s11356-017-9581-5. [DOI] [PubMed] [Google Scholar]
- 395.Das D., Ghosh R., Mandal P. Biogenic synthesis of silver nanoparticles using S1 genotype of Morus alba leaf extract: Characterization, antimicrobial and antioxidant potential assessment. SN Appl. Sci. 2019;1:498. doi: 10.1007/s42452-019-0527-z. [DOI] [Google Scholar]
- 396.Hamelian M., Zangeneh M.M., Amisama A., Varmira K., Veisi H. Green synthesis of silver nanoparticlesusing Thymus kotschyanus extract and evaluation of their antioxidant, antibacterial and cytotoxic effects. Appl. Organomet. Chem. 2018;32:e4458. doi: 10.1002/aoc.4458. [DOI] [Google Scholar]
- 397.Keshari A.K., Srivastava R., Singh P., Yadav V.B., Nath G. Antioxidant and antibacterial activity of silver nanoparticles synthesized by Cestrum nocturnum. J. Ayurveda Integr. Med. 2020;11:37–44. doi: 10.1016/j.jaim.2017.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Shanmugasundaram T., Radhakrishnan M., Gopikrishnan V., Pazhanimurugan R., Balagurunathan R. A study of the bactericidal, anti-biofouling, cytotoxic and antioxidant properties of actinobacterially synthesised silver nanoparticles. Colloids Surf. B Biointerfaces. 2013;111:680–687. doi: 10.1016/j.colsurfb.2013.06.045. [DOI] [PubMed] [Google Scholar]
- 399.Dasari S., Suresh K.A., Rajesh M., Reddy C.S.S., Hemalatha C.S., Wudayagiri R., Valluru L. Biosynthesis, characterization, antibacterial and antioxidant activity of silver nanoparticles produced by lichens. J. Bionanosci. 2013;7:237–244. doi: 10.1166/jbns.2013.1140. [DOI] [Google Scholar]
- 400.Venkatesan J., Kim S.K., Shim S.K. Antimicrobial, antioxidant, and anticancer activities of biosynthesized silver nanoparticles using marine algae Ecklonia cava. Nanomaterials. 2016;6:235. doi: 10.3390/nano6120235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Netala V.R., Bethu M.S., Pushpalatha B., Baki V.B., Aishwarya S., Rao J.V., Tartte V. Biogenesis of silver nanoparticles using endophytic fungus Pestalotiopsis microspora and evaluation of their antioxidant and anticancer activities. Int. J. Nanomed. 2016;11:5683–5696. doi: 10.2147/IJN.S112857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Manjunath H.M., Joshi C.G., Danagoudar A., Poyya J., Kudva A.K., Dhananjaya B.L. Biogenic synthesis of gold nanoparticles by marine endophytic fungus-Cladosporium cladosporioides isolated from seaweed and evaluation of their antioxidant and antimicrobial properties. Process Biochem. 2017;63:137–144. doi: 10.1016/j.procbio.2019.04.011. [DOI] [Google Scholar]
- 403.Netala V.R., Kotakadi V.S., Bobbu P., Gaddam S.A., Tartte V. Endophytic fungal isolate mediated biosynthesis of silver nanoparticles and their free radical scavenging activity and anti microbial studies. 3 Biotech. 2016;6:132. doi: 10.1007/s13205-016-0433-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Saravanakumar K., Wang M.H. Trichoderma based synthesis of anti-pathogenic silver nanoparticles and their characterization, antioxidant and cytotoxicity properties. Microb. Pathog. 2018;114:269–273. doi: 10.1016/j.micpath.2017.12.005. [DOI] [PubMed] [Google Scholar]
- 405.Govindappa M., Farheen H., Chandrappa C.P., Rai R.V., Raghavendra V.B. Mycosynthesis of silver nanoparticles using extract of endophytic fungi, Penicillium species of Glycosmis mauritiana, and its antioxidant, antimicrobial, anti-inflammatory and tyrokinase inhibitory activity. Adv. Nat. Sci. Nanosci. Nanotechnol. 2016;7:035014. doi: 10.1088/2043-6262/7/3/035014. [DOI] [Google Scholar]
- 406.Nagajyothi P.C., Sreekanth T.V.M., Lee J.I., Lee K.D. Mycosynthesis: Antibacterial, antioxidant and antiproliferative activities of silver nanoparticles synthesized from Inonotus obliquus (Chaga mushroom) extract. J. Photochem. Photobiol. B. 2014;130:299–304. doi: 10.1016/j.jphotobiol.2013.11.022. [DOI] [PubMed] [Google Scholar]
- 407.Popli D., Anil V., Subramanyam A.B., Namratha M.N., Ranjitha V.R., Rao S.N., Rai R.V., Govindappa M. Endophyte fungi, Cladosporium species-mediated synthesis of silver nanoparticles possessing in vitro antioxidant, anti-diabetic and anti-Alzheimer activity. Artif. Cells Nanomed. Biotechnol. 2018;46:676–683. doi: 10.1080/21691401.2018.1434188. [DOI] [PubMed] [Google Scholar]
- 408.Sriramulu M., Sumathi S. Photocatalytic, antioxidant, antibacterial and anti-inflammatory activity of silver nanoparticles synthesised using forest and edible mushroom. Adv. Nat. Sci. Nanosci. Nanotechnol. 2017;8:045012. doi: 10.1088/2043-6254/aa92b5. [DOI] [Google Scholar]
- 409.Aygün A., Özdemir S., Gülcan M., Cellat K., Sen F. Synthesis and characterization of Reishi mushroom-mediated green synthesis of silver nanoparticles for the biochemical applications. J. Pharm. Bimed. Anal. 2020;178:112970. doi: 10.1016/j.jpba.2019.112970. [DOI] [PubMed] [Google Scholar]
- 410.Poudel M., Pokharel R., Sudip K.C., Awal S.C., Pradhananga R. Biosynthesis of silver nanoparticles using Ganoderma Lucidum and assessment of antioxidant and antibacterial activity. Int. J. Appl. Sci. Biotechnol. 2017;5:523–531. doi: 10.3126/ijasbt.v5i4.18776. [DOI] [Google Scholar]
- 411.Baygar T., Ugur A. Biosynthesis of silver nanoparticles by Streptomyces griseorubens isolated from soil and their antioxidant activity. IET Nanobiotechnol. 2017;11:286–291. doi: 10.1049/iet-nbt.2015.0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Ungureanu C., Fierascu I., Fierascu R.C., Costea T., Avramescu S.M., Călinescu M.F., Somoghi R., Pirvu C. In Vitro and In Vivo Evaluation of Silver Nanoparticles Phytosynthesized Using Raphanus sativus L. Waste Extracts. Materials. 2021;14:1845. doi: 10.3390/ma14081845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Debnath R., Purkayastha D.D., Hazra S., Ghosh N.N., Bhattacharjee C.R., Rout J. Biogenic synthesis of antioxidant, shape selective gold nanomaterials mediated by high altitude lichens. Mater. Lett. 2016;169:58–61. doi: 10.1016/j.matlet.2016.01.072. [DOI] [Google Scholar]
- 414.Shakibaie M., Forootanfar H., Mollazadeh-Moghaddam K., Bagherzadeh Z., Nafissi-Varcheh N., Shahverdi A.R., Faramarzi M.A.J.B., Biochemistry A. Green synthesis of gold nanoparticles by the marine microalga Tetraselmis suecica. Biotechnol. Appl. Biochem. 2010;57:71–75. doi: 10.1042/BA20100196. [DOI] [PubMed] [Google Scholar]
- 415.Veeraapandian S., Sawant S.N., Doble M. Antibacterial and antioxidant activity of protein capped silver and gold nanoparticles synthesized with Escherichia coli. J. Biomed. Nanotechnol. 2012;8:140–148. doi: 10.1166/jbn.2012.1356. [DOI] [PubMed] [Google Scholar]
- 416.Ramamurthy C.H., Padma M., Samadanam I.D., Mareeswaran R., Suyavaran A., Kumar M.S., Premkumar K., Thirunavukkarasu C. The extra cellular synthesis of gold and silver nanoparticles and their free radical scavenging and antibacterial properties. Colloids Surf. B Biointerfaces. 2013;102:808–815. doi: 10.1016/j.colsurfb.2012.09.025. [DOI] [PubMed] [Google Scholar]
- 417.Parial D., Patra H.K., Dasgupta A.K., Pal R. Screening of different algae for green synthesis of gold nanoparticles. Eur. J. Phycol. 2012;47:22–29. doi: 10.1080/09670262.2011.653406. [DOI] [Google Scholar]
- 418.Lee K.D., Nagajyothi P.C., Sreekanth T.V.M., Park S. Eco-friendly synthesis of gold nanoparticles (AuNPs) using Inonotus obliquus and their antibacterial, antioxidant and cytotoxic activities. J. Ind. Eng. Chem. 2015;26:67–72. doi: 10.1016/j.jiec.2014.11.016. [DOI] [Google Scholar]
- 419.Markus J., Mathiyalagan R., Kim Y.J., Abbai R., Singh P., Ahn S., Perez Z.E.J., Hurh J., Yang D.C. Intracellularsynthesis of goldnanoparticles with antioxidantactivity by probiotic Lactobacillus kimchicus DCY51T isolated from Koreankimchi. Enzym. Microb. Technol. 2016;95:85–93. doi: 10.1016/j.enzmictec.2016.08.018. [DOI] [PubMed] [Google Scholar]
- 420.Oladipo I.C., Lateef A., Elegbede J.A., Azeez M.A., Asafa T.M., Yekeen T.A., Akinboro A., Gueguim-Kana E.B., Beukes L.S., Oluyide T.O., et al. Enterococcus species for the one-pot biofabrication of gold nanoparticles: Characterization and nanobiotechnological applications. J. Photochem. Photobiol. B. 2017;173:250–257. doi: 10.1016/j.jphotobiol.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 421.Singaravelu G., Arockiamary J., Kumar V.G., Govindaraju K. A novel extracellular synthesis of monodisperse gold nanoparticles using marine alga, Sargassum wightii Greville. Colloids Surf. B Biointerfaces. 2007;57:97–101. doi: 10.1016/j.colsurfb.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 422.Kalabegishvili T., Kirkesali E.I., Rcheulishvili A.N., Ginturi E., Murusidze I., Kuchava N., Bagdavadze N., Tsertsvadze G., Gabunia V., Frontasyeva M., et al. Synthesis of gold nanoparticles by blue-green algae Spirulina platensis. Adv. Sci. Eng. Med. 2012;4:1–7. [Google Scholar]
- 423.Veena S., Devasena T., Sathak S.S.M., Yasasve M., Vishal L.A. Green synthesis of gold nanoparticles from Vitex negundo leaf extract: Characterization and in vitro evaluation of antioxidant-antibacterial activity. J. Clust. Sci. 2019;30:1591–1597. doi: 10.1007/s10876-019-01601-z. [DOI] [Google Scholar]
- 424.Moghaddam A.B., Moniri M., Azizi S., Rahim R.A., Ari_ A.B., Saad W.Z., Namvar F., Navaderi M., Mohamad R. Biosynthesis of ZnO nanoparticles by a new Pichia kudriavzevii yeast strain and evaluation of their antimicrobial and antioxidant activities. Molecules. 2017;22:872. doi: 10.3390/molecules22060872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425.Chandra H., Patel D., Kumari P., Jangwan J.S., Yadav S. Phyto-mediated synthesis of zinc oxide nanoparticles of Berberis aristata: Characterization, antioxidant activity and antibacterial activity with special reference to urinary tract pathogens. Mater. Sci. Eng. C Mater. Biol. Appl. 2019;102:212–220. doi: 10.1016/j.msec.2019.04.035. [DOI] [PubMed] [Google Scholar]
- 426.Ramya S., Shanmugasundaram T., Balagurunathan R. Biomedical potential of actinobacterially synthesized selenium nanoparticles with special reference to anti-biofilm, anti-oxidant, wound healing, cytotoxic and anti-viral activities. J. Trace Elem. Med. Biol. 2015;32:30–39. doi: 10.1016/j.jtemb.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 427.Torres S.K., Campos V.L., León C.G., Rodríguez-Llamazares S.M., Rajos S.M., González M., Smith C., Mondaca M.A. Biosynthesis of selenium nanoparticles by Pantoea agglomerans and their antioxidant activity. J. Nanopart. Res. 2012;14:1236. doi: 10.1007/s11051-012-1236-3. [DOI] [Google Scholar]
- 428.El-Zayat M.M., Eraqi M.M., Alrefai H., El-Khateeb A.Y., Ibrahim M.A., Aljohani H.M., Aljohani M.M., Elshaer M.M. The Antimicrobial, Antioxidant, and Anticancer Activity of Greenly Synthesized Selenium and Zinc Composite Nanoparticles Using Ephedra aphylla Extract. Biomolecules. 2021;11:470. doi: 10.3390/biom11030470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429.Ghosh S., More P., Nitnavare R., Jagtap S., Chippalkatti R., Derle A., Kitture R., Asok A., Kale S., Singh S., et al. Antidiabetic and antioxidant properties of copper nanoparticles synthesized by medicinal plant Dioscorea bulbifera. J. Nanomed. Nanotechnol. 2015;S6:007. doi: 10.4172/2157-7439.S6-007. [DOI] [Google Scholar]
- 430.Sarkar J., Chakraborty N., Chatterjee A., Bhattacharjee A., Dasgupta D., Acharya K. Green synthesized copper oxide nanoparticles ameliorate defence and antioxidant enzymes in Lens culinaris. Nanomaterials. 2020;10:312. doi: 10.3390/nano10020312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431.Dobrucka R. Antioxidant and catalytic activity of biosynthesized CuO nanoparticles using extract of Galeopsidis herba. J. Inorg. Organomet. Polym. Mater. 2018;28:812–819. doi: 10.1007/s10904-017-0750-2. [DOI] [Google Scholar]
- 432.Keabadile O.P., Aremu A.O., Elugoke S.E., Fayemi O.E. Green and Traditional Synthesis of Copper Oxide Nanoparticles—Comparative Study. Nanomaterials. 2020;10:2502. doi: 10.3390/nano10122502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Harshiny M., Iswarya C.N., Matheswaran M. Biogenic synthesis of iron nanoparticles using Amaranthus dubius leaves extract as reducing agents. Powder Technol. 2015;286:744–749. doi: 10.1016/j.powtec.2015.09.021. [DOI] [Google Scholar]
- 434.Tuzun B.S., Fafal T., Tastan P., Kivcak B., Yelken B.O., Kayabasi C., Susluer S.Y., Gunduz C. Structural characterization, antioxidant and cytotoxic e_ects of iron nanoparticles synthesized using Asphodelus aestivus Brot. aqueous extract. Green Process. Synth. 2020;9:153–163. doi: 10.1515/gps-2020-0016. [DOI] [Google Scholar]
- 435.Srihasam S., Thyagarajan K., Korivi M., Lebaka V.R., Mallem S.P.R. Phytogenic generation of NiO nanoparticles using Stevia leaf extract and evaluation of their in-vitro antioxidant and antimicrobial properties. Biomolecules. 2020;10:89. doi: 10.3390/biom10010089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436.Uddin S., Safdar L.B., Anwar S., Iqbal J., Laila S., Abbasi B.A., Saif M.S., Ali M., Rehman A., Basit A., et al. Green Synthesis of Nickel Oxide Nanoparticles from Berberis balochistanica Stem for Investigating Bioactivities. Molecules. 2021;26:1548. doi: 10.3390/molecules26061548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.Paul J.J.P., Sakunthala M., Udhaya I. Green synthesis of Manganese nanoparticles using the aqueous extract of Ctenolepis garcini (Burm. f.) C.B Clarke. Int. J. Bot. Stud. 2017;2:71–75. [Google Scholar]
- 438.Khan S.A., Shahid S., Shahid B., Fatima U., Abbasi S.A. Green Synthesis of MnO Nanoparticles Using Abutilon indicum Leaf Extract for Biological, Photocatalytic, and Adsorption Activities. Biomolecules. 2020;10:785. doi: 10.3390/biom10050785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439.Fouda A., Awad M.A., Eid A.M., Saied E., Barghoth M.G., Hamza M.F., Awad M.F., Abdelbary S., Hassan S.E.-D. An Eco-Friendly Approach to the Control of Pathogenic Microbes and Anopheles stephensi Malarial Vector Using Magnesium Oxide Nanoparticles (Mg-NPs) Fabricated by Penicillium chrysogenum. Int. J. Mol. Sci. 2021;22:5096. doi: 10.3390/ijms22105096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440.Konopko A., Kusio J., Litwinienko G. Antioxidant Activity of Metal Nanoparticles Coated with Tocopherol-Like Residues-The Importance of Studies in Homo- and Heterogeneous Systems. Antioxidants. 2019;9:5. doi: 10.3390/antiox9010005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441.Nie Z., Liu K.J., Zhong C.J., Wang L.F., Yang Y., Tian Q., Liu Y. Enhanced radical scavenging activity by antioxidant-functionalized gold nanoparticles: A novel inspiration for development of new artificial antioxidants. Free Radic. Biol. Med. 2007;43:1243–1254. doi: 10.1016/j.freeradbiomed.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 442.Esumi K., Takei N., Yoshimura T. Antioxidant-potentiality of gold-chitosan nanocomposites. Colloids Surf. B Biointerfaces. 2003;32:117–123. doi: 10.1016/S0927-7765(03)00151-6. [DOI] [Google Scholar]
- 443.Baruwati B., Polshettiwar V., Varma R.S. Glutathione promoted expeditious green synthesis of silver nanoparticles in water using microwaves. Green Chem. 2009;11:926–930. doi: 10.1039/b902184a. [DOI] [Google Scholar]
- 444.Viglianisi C., Di Pilla V., Menichetti S., Rotello V.M., Candiani G., Malloggi C., Amorati R. Linking an α-tocopherol derivative to cobalt(0) nanomagnets: Magnetically responsive antioxidants with superior radical trapping activity and reduced cytotoxicity. Chemistry. 2014;20:6857–6860. doi: 10.1002/chem.201402289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445.Mohd Taib S.H., Shameli K., Moozarm Nia P., Etesami M., Miyake M., Rasit Ali R., Abouzari-Lotf E., Izadiyan Z. Electrooxidation of nitrite based on green synthesis of gold nanoparticles using Hibiscus sabdariffa leaves. J. Taiwan Inst. Chem. Eng. 2018;95:616–626. doi: 10.1016/j.jtice.2018.09.021. [DOI] [Google Scholar]
- 446.Ouellette M., Masse F., Lefebvre-Demers M., Maestracci Q., Grenier P., Millar R., Bertrand N., Prieto M., Boisselier E. Insights into gold nanoparticles as a mucoadhesive system. Sci. Rep. 2018;8:14357. doi: 10.1038/s41598-018-32699-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 447.Choi K.H., Nam K.C., Lee S.Y., Cho G., Jung J.S., Kim H.J., Park B.J. Antioxidant Potential and Antibacterial Efficiency of Caffeic Acid-Functionalized ZnO Nanoparticles. Nanomaterials. 2017;7:148. doi: 10.3390/nano7060148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448.Valgimigli L., Baschieri A., Amorati R. Antioxidant activity of nanomaterials. J. Mater. Chem. B. 2018;6:2036–2051. doi: 10.1039/C8TB00107C. [DOI] [PubMed] [Google Scholar]
- 449.Baschieri A., Amorati R., Benelli T., Mazzocchetti L., D’Angelo E., Valgimigli L. Enhanced Antioxidant Activity under Biomimetic Settings of Ascorbic Acid Included in Halloysite Nanotubes. Antioxidants. 2019;8:30. doi: 10.3390/antiox8020030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 450.Massaro M., Riela S., Guernelli S., Parisi F., Lazzara G., Baschieri A., Valgimigli L., Amorati R. A synergic nanoantioxidant based on covalently modified halloysite-trolox nanotubes with intra-lumen loaded quercetin. J. Mater. Chem. B. 2016;4:2229–2241. doi: 10.1039/C6TB00126B. [DOI] [PubMed] [Google Scholar]
- 451.Manjunath H.M., Joshi C.G. Characterization, antioxidant and antimicrobial activity of silver nanoparticles synthesized using marine endophytic fungus- Cladosporium cladosporioides. Process Biochem. 2019;82:199–204. doi: 10.1016/j.procbio.2019.04.011. [DOI] [Google Scholar]
- 452.Catauro M., Barrino F., Dal Poggetto G., Crescente G., Piccolella S., Pacifico S. Chlorogenic Acid Entrapped in Hybrid Materials with High PEG Content: A Strategy to Obtain Antioxidant Functionalized Biomaterials? Materials. 2019;12:148. doi: 10.3390/ma12010148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 453.Shah B.R., Zhang C., Li Y., Li B. Bioaccessibility and antioxidant activity of curcumin after encapsulated by nano and Pickering emulsion based on chitosan-tripolyphosphate nanoparticles. Food Res. Int. 2016;89 (Pt 1):399–407. doi: 10.1016/j.foodres.2016.08.022. [DOI] [PubMed] [Google Scholar]
- 454.Pu H.L., Chiang W.L., Maiti B., Liao Z.X., Ho Y.C., Shim M.S., Chuang E.Y., Xia Y., Sung H.W. Nanoparticles with dual responses to oxidative stress and reduced ph for drug release and anti-inflammatory applications. ACS Nano. 2014;8:1213–1221. doi: 10.1021/nn4058787. [DOI] [PubMed] [Google Scholar]
- 455.Talelli M., Barz M., Rijcken C.J., Kiessling F., Hennink W.E., Lammers T. Core-Crosslinked Polymeric Micelles: Principles, Preparation, Biomedical Applications and Clinical Translation. Nano Today. 2015;10:93–117. doi: 10.1016/j.nantod.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 456.Kwon G.S., Kataoka K. Block copolymer micelles as long-circulating drug vehicles. Adv. Drug Deliv. Rev. 2012;64:237–245. doi: 10.1016/j.addr.2012.09.016. [DOI] [Google Scholar]
- 457.Biswas S., Kumari P., Lakhani P.M., Ghosh B. Recent advances in polymeric micelles for anti-cancer drug delivery. Eur. J. Pharm. Sci. 2016;83:184–202. doi: 10.1016/j.ejps.2015.12.031. [DOI] [PubMed] [Google Scholar]
- 458.Mitchell M.J., Billingsley M.M., Haley R.M., Wechsler M.E., Peppas N.A., Langer R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021;20:101–124. doi: 10.1038/s41573-020-0090-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 459.El Jundi A., Buwalda S.J., Bakkour Y., Garric X., Nottelet B. Double hydrophilic block copolymers self-assemblies in biomedical applications. Adv. Colloid Interface Sci. 2020;283:102213. doi: 10.1016/j.cis.2020.102213. [DOI] [PubMed] [Google Scholar]