Abstract
Androgens and estrogens influence skeletal development and maintenance in males. However, the relative contributions of the circulating sex steroid hormones that originate from testicular/adrenal secretion versus those produced locally in bone via intracrine action require further elucidation. Our novel hypothesis is that testosterone exerts direct protective effects on the adult male skeleton independently of the actions of 5α-reductase or aromatase.
Keywords: dihydrotestosterone, estradiol, 5α-reductase, aromatase, androgen, estrogen, dehydroepiandrosterone
INTRODUCTION
Androgens and estrogens influence skeletal development and maintenance in males (31). 17β-Hydroxyandrost-4-en-3-one (testosterone) is the most abundant bioactive sex steroid hormone in the circulation and the principal androgen producing biologic effects in tissues that do not express 5α-reductase (10). Testosterone (T) acts by binding androgen receptors (AR) directly and also is a prohormone for the localized tissue-specific synthesis of dihydrotestosterone (DHT) and estradiol (E2), which are considered essential for normal in utero and adolescent bone development (31). Bone expresses the complete array of sex steroid hormone–modifying enzymes necessary to synthesize DHT and E2 from T and from several other circulating androgens and estrogens (30). However, the relative contributions of the circulating bioactive sex steroid hormones versus those produced locally in bone via intracrine actions involving 5α-reductase and aromatase require further elucidation. Herein, we present an overview of sex steroid hormone metabolism, with particular focus on the ability of bone to synthesize sex steroid hormones from circulating androgenic and estrogenic precursors and the roles of the circulating and intraskeletal sex steroid hormone reservoirs in bone development and maintenance in males. We also will present evidence supporting our novel hypothesis that T exerts direct protective effects on the adult male skeleton and that this effect does not require actions of 5α-reductase or aromatase, at least in the presence of abundant T.
INTRACRINE SEX STEROID HORMONE SYNTHESIS
Endogenous sex steroid hormone synthesis is initiated via P450 side-chain cleavage of cholesterol and involves several subsequent enzymatic reactions that produce a variety of gonadally and adrenally derived androgens and estrogens (Fig. 1), each varying in biologic activity. Historically, the concentrations of sex steroid hormones present in the circulation were thought to estimate roughly that which is capable of exerting biologic actions in bone (34). This seems largely true for T (34,36), which is produced predominantly in the Leydig cells of the testes (37). However, less than 20% of circulating DHT (10) and E2 (9) are derived from testicular secretion, with the rest originating from localized metabolism in peripheral tissues expressing 5α-reductase and aromatase. Thus, circulating DHT and E2 concentrations may not represent hormone concentrations within these tissues. In addition, T, DHT, and E2 each circulate in several states (unbound (free), loosely bound to albumin, or tightly bound to sex hormone-binding globin (SHBG)), which influences the bioavailability of these sex steroid hormones. In this context, the relatively small amount of sex steroid hormones that circulate free or albumin bound represents the bioavailable sex steroid fractions capable of traversing cell membranes and exerting biological actions, whereas that bound to SHBG is considered largely inactive (10). The more recent identification of the full array of enzymes necessary for sex steroid hormone synthesis in osteocytes, osteoblasts, and osteoclasts (30) and the ability of freshly resected human bone to produce DHT (19) and E2 (17) has resulted in the understanding that bone (like many other tissues) is an intracrine organ capable of using circulating androgenic and estrogenic precursors for localized sex steroid hormone synthesis (11). In this manner, the reservoir of sex steroid hormones capable of inducing biologic actions in bone depends not only on the circulating concentrations of the bioavailable androgens and estrogens but also on localized intraskeletal sex steroid hormone metabolism.
Figure 1.
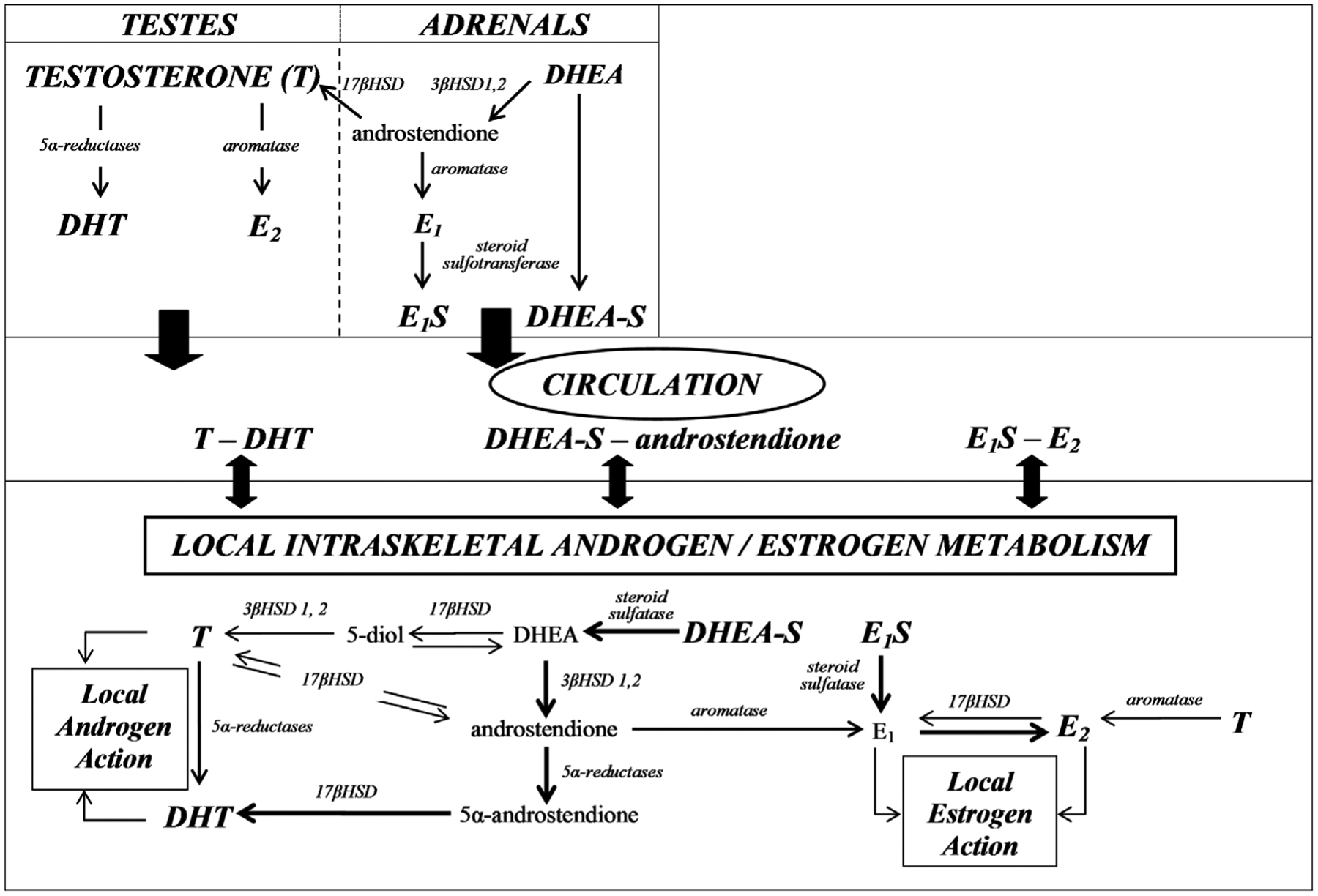
Schematic diagram of sex steroid hormone synthesis in the testes/adrenals and intracrine action in bone. Testosterone (T) exerts direct protective effects on the adult male skeleton, independently of the actions of 5α-reductase or aromatase. The testes and adrenals are the primary source of T and dehydroepiandosterone-sulfate (DHEA-S), respectively, whereas dihydrotestosterone (DHT) and estradiol (E2) are primarily produced in peripheral tissues from androgenic and estrogenic precursors present in the circulation. The individual unidirectional 17β-hydroxysteroid dehydrogenase (17βHSD) isozymes that catalyze the oxidation or reduction reactions are listed in the text. The thickness of the arrows represents the relative affinity of a substrate for the listed reaction and the importance of the pathway in producing the specified sex steroid hormones (14). E1S, estrone sulfate; 3βHSD, 3β-hydroxysteroid dehydrogenase.
Testosterone-Dependent Pathways of DHT and E2 Synthesis
In addition to acting as a hormone, T represents a substrate for DHT synthesis via actions of any of the three known 5α-reductase isozymes (types 1–3) (37) (Fig. 1). 5α-Reductase expression amplifies androgen signaling in a localized tissue-specific manner in at least two ways: 1) DHT has nearly three times greater affinity for the AR than T; and 2) T undergoes rapid tissue-specific conversion to androstendione (a weaker androgen) via several 17β-hydroxysteroid dehydrogenase (17βHSD) isozymes and/or irreversible conversion to E2 (via aromatase), whereas DHT is not metabolized by these enzymes and thus maintains a longer presence within tissues (37). DHT is the primary and most potent androgen in tissues that highly express any of the 5α reductase isozymes (e.g., prostate and skin), whereas T remains the primary androgen in tissues with relatively low 5α reductase activity (37). Importantly, T and DHT bind to the same AR, implying that the tissue-specific expression and/or activity of 5α-reductase is the primary means of locally modifying androgen action in tissues that express AR. For example, ground spongiosa from resected bone of normal and osteoporotic men produces DHT in culture (19), most likely via action of the type 1 5α-reductase isozyme, which is present in much higher concentrations in bone than that of the type 2 isozyme (32). Bone also may express the more recently identified type 3 5α-reductase isozyme, although we are unaware of any research evaluating the role of the type 3 isozyme in skeletal biology. Importantly, AR are expressed in the outer dense compact (cortical) bone and the inner spongelike trabecular (cancellous) bone spicules that are present in the medullary canal near the end of long bones and within vertebrae (31), indicating that androgens can exert genomic effects in male bone. In fact, AR activation seems to be the primary factor underlying the larger cortical bone circumference in males versus females primarily caused by expansion along the periosteal surface of the cortical bone (31), although the AR-mediated signaling pathway responsible for radial bone expansion remains to be identified (15,31).
As mentioned previously, T also can be converted to E2 via aromatase (Fig. 1), a membrane-bound protein that is expressed in human bone and a variety of other tissues (9). The primary source of circulating E2 in adult males seems to be adipose tissue (10). However, several bone cells also express aromatase (30), as discussed earlier, and freshly resected human bone synthesizes E2 in culture, demonstrating bone is an intracrine tissue (17). In addition, two distinct estrogen receptors (ERα and ERβ) are present in humans and rodents, indicating that estrogen action is influenced not only by aromatase expression but also by the localized expression and activity of the ER (31). In this regard, the ER seem to exert divergent effects on the male skeleton, with ERα (expressed in both cancellous and cortical bone) positively influencing peak bone mass and stimulating epiphyseal closure during human and rodent male adolescent bone development (for in-depth reviews, see (15,31)). In contrast, ERβ (predominantly expressed in cancellous bone) seems to play little role in male bone development, as evidenced by male ERβ knockout mice that exhibit normal skeletal development (15,31).
Testosterone-Independent Pathways of DHT and E2 Synthesis
Several T-independent pathways of DHT and E2 synthesis also have been identified (for in-depth reviews, see (11,14)). These pathways use dehydroepiandrosterone (DHEA) as an initial substrate, bypassing the necessity of T as a metabolic intermediate but continue to require actions of 5α-reductase and aromatase. DHEA is produced primarily in the adrenals of humans and circulates principally as DHEA-sulfate (DHEA-S) following the reversible actions of steroid sulfotransferase. In adult men, circulating DHEA-S concentrations are 100 to 500 times greater than that of T and upward of 20,000 times higher than that of E2 (10), demonstrating the availability of this substrate.
In target tissues (e.g., bone), DHEA-S can be converted to DHEA, via steroid sulfatase (11) (Fig. 1). DHEA can then undergo unidirectional actions of 3β-hydroxysteroid dehydrogenase type 1 or 2 to produce androstendione, which exhibits a higher affinity than T for both 5α-reductase and aromatase (14). Androstendione circulates in concentrations that are 80% to 90% lower than T (10), although concentrations in several tissues are elevated because of localized sex steroid metabolism. As such, tissues that express 5α-reductase or aromatase can convert androstendione to 5α-androstendione or to estrone (E1), respectively, which can then undergo reduction by several 17βHSD isozymes to produce DHT or E2.
At least 15 (types 1–15) unidirectional 17βHSD isozymes exist with varying tissue-specific expression and activity. In particular, 17βHSD types 2, 4, and 14 catalyze the oxidation of E2 to E1 and type 2 catalyzes the oxidation of T to androstendione (14), which diminishes local androgen/estrogen signaling. In contrast, 17βHSD types 1, 7, and 12 catalyze the reduction of E1 to E2, types 3 and 5 catalyze the reduction of androstendione to T, and types 5 and 15 catalyze the reduction of 5α-androstendione to DHT (14), which amplifies local androgen/estrogen signaling. As such, it is important to consider carefully the tissue-specific “reversibility” of 17βHSD activity, which is based on the localized expression of the various 17βHSD isozymes and the differing oxidation/reduction reactions catalyzed.
Research evaluating the intraskeletal expression of the various 17βHSD isozymes remains limited, although bone has the ability to both oxidize and reduce sex steroid hormones to less and more bioactive states, respectively. In addition, determination of the direct roles of the various 17βHSD isozymes in skeletal development has been difficult because of the early fetal lethality occurring in some 17βHSD knockout models (20). However, ubiquitous/constitutive overexpression of human 17βHSD type 2 in young male mice reduces serum and intratesticular T, retards longitudinal bone growth, and produces low cancellous and cortical bone mass in comparison with wild types (21), demonstrating a direct role of this enzyme in adolescent bone development, although it remains unclear whether the observed skeletal phenotype resulted from systemic or local intraskeletal 17βHSD2-mediated sex steroid hormone inactivation. The ability of the aforementioned T-independent pathways to alter the endogenous sex steroid hormone milieu and exert biologic effects also is evidenced by the ability of supplemental DHEA to increase circulating androstendione, E1, E2, and DHT in those with panhypopituitarism (i.e., the total absence of gonadally and adrenally derived sex steroid hormones) to the levels present in young eugonadal men (38) and to suppress longitudinal bone growth in culture through ER-dependent mechanisms (25).
Alternatively, E1 also can be converted reversibly to E1-sulfate (E1S) via steroid sulfotransferase, which provides a storage form of E1 for subsequent tissue-specific interconversion to E2 through the pathway discussed (14). Interestingly, E1S is the most abundant circulating estrogen in men and seems to be the primary substrate for E2 synthesis, at least in bone. As evidence, E2 formation in freshly resected human bone fragments and cultured osteoblast-like cells is nearly 30 times greater from E1S than from androstendione and nearly 50 times greater than from the aromatization of T (17). Regardless, bone expresses all necessary enzymes for the conversion of DHEA-S to DHT and E2, which demonstrates the viability of these T-independent sex steroid synthesis pathways and provides additional evidence supporting the concept that T is not the sole (and perhaps not even the primary) substrate for localized intraskeletal synthesis of DHT and E2. However, the biological influences of the T-independent sex steroid synthesis pathways on the adult male skeleton remain to be determined, given that supplemental DHEA produces no discernible improvement in bone mineral density (BMD) in men (7).
Intraskeletal Sex Steroid Hormones
Because bone is an intracrine tissue that locally synthesizes bioactive sex steroid hormones, it seems logical to assume that the intraskeletal concentrations of DHT and E2 differ from circulating concentrations. In an effort to assess this supposition directly, we developed methods to extract sex steroid hormones from intact bone (34) and observed that the T concentrations in serum and bone were roughly similar, whereas DHT was approximately 40-fold higher in bone compared with the circulation (36) (Fig. 2A, B). Similar findings also were observed when bone was evaluated with and without marrow/cancellous bone, indicating that calcified cortical bone represents a relatively consistent and perhaps independent sex steroid hormone reservoir (34). The higher intraskeletal DHT versus intraskeletal T concentrations that we observed may suggest that DHT is present in nonbioavailable forms that would prevent interactions with AR, although this remains speculative. In addition, the higher intraskeletal DHT concentrations that we observed, in comparison with that in the circulation, seem to support findings from Schweikert et al. (19) indicating that ground human spongiosa synthesizes large quantities of DHT in culture. However, resected bone also synthesizes androstendione (19), and this androgenic precursor exhibits a higher affinity for 5α-reductase than T (14) and can be converted to DHT via T-independent pathways present in bone. Interestingly, Turner et al. (27) reported that periosteal cells isolated from tibiae of skeletally mature male rats do not produce DHT in culture when incubated with T, perhaps suggesting that androstendione is the primary substrate for intraskeletal DHT synthesis, although this remains to be determined. In contrast, E2 concentrations in bone were more than 50% lower than in the circulation (34), regardless of sex (36), which seems somewhat at odds with a predominant hypothesis in the literature, suggesting that the skeletal effects of T are at least partially mediated via intraskeletal aromatization to E2 and subsequent ER activation (9,10,31). We also have observed that intraskeletal T and DHT were 60% to 300% higher in long bones of intact male rats compared with those in intact females, whereas intraskeletal E2 was approximately fivefold higher in females (36), demonstrating that differences exist between sexes.
Figure 2.
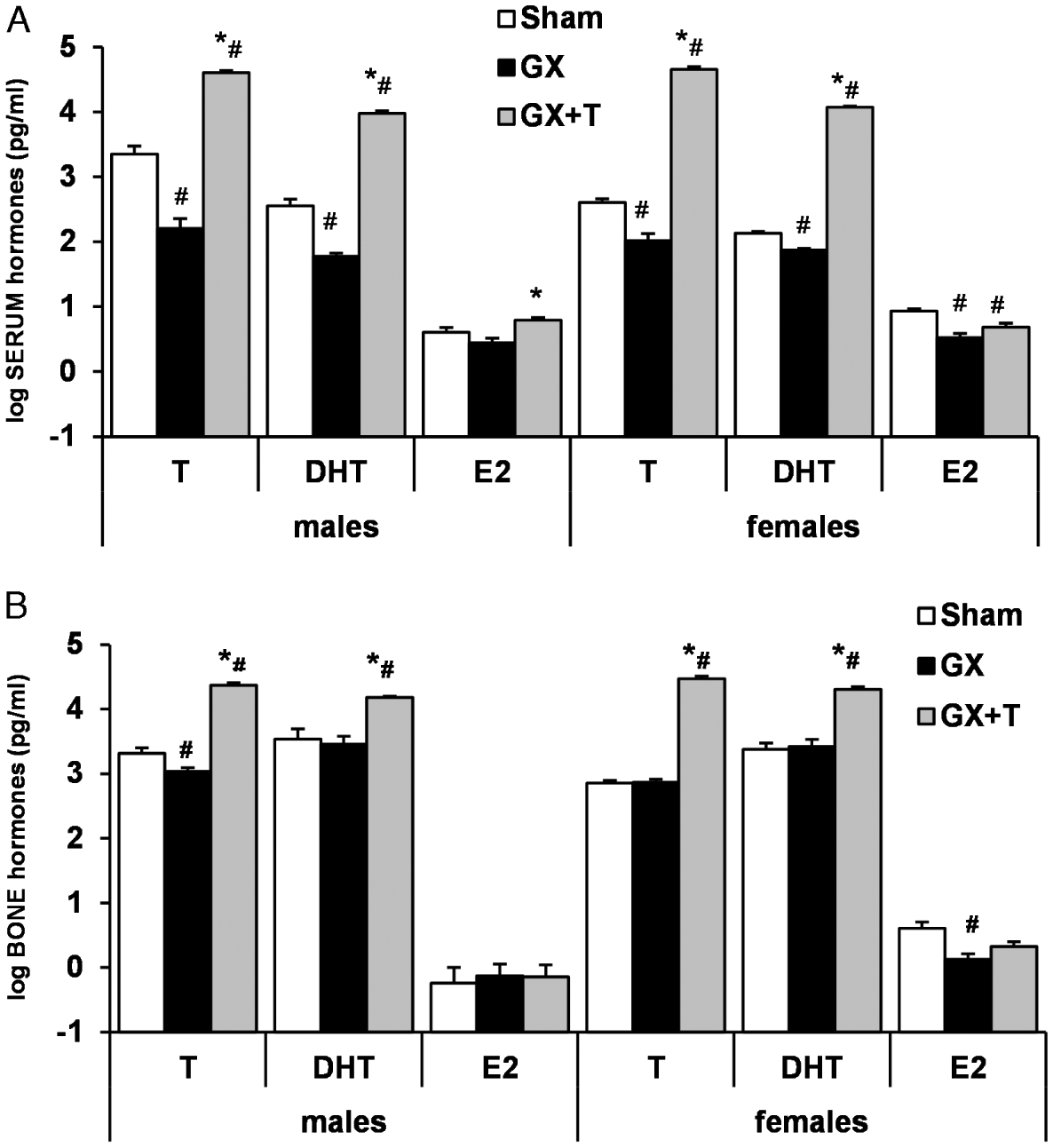
Effects of gonadectomy (GX) and testosterone (T) administration on (A) serum and (B) bone (tibial) T, dihydrotestosterone (DHT), and estradiol (E2) concentrations in male and female rats. Rats received GX vs sham surgery on day 0 and were injected with 7.0 mg T-enanthate wk−1 intramuscularly. Bone T was reduced in GX males and bone E2 was reduced in GX females, whereas bone androgens were increased with T-enanthate treatment in both sexes. Values are log means ± SE; n = 9–10 per group (androgens) and 4–7 per group (E2). #P < 0.05 vs sham, *P < 0.05 vs GX. [Adapted from (36). Copyright © 2008 The Physiological Society. Used with permission.]
The intraskeletal sex steroid hormone reservoir also seems modifiable in a manner that is somewhat distinct from the circulation. In particular, orchiectomy (ORX) reduces circulating and intraskeletal T and circulating DHT in males but does not alter intraskeletal DHT (Fig. 2A, B) (36), providing further evidence that intraskeletal DHT may be derived primarily from T-independent sources. Similarly, in females, ovariectomy (OVX) reduces T, DHT, and E2 in the circulation, whereas only intraskeletal E2 is reduced (36). The relative stability of intraskeletal DHT and E2 in ORX males and of intraskeletal T and DHT in OVX females is notable, especially given that systemic sex steroid hormone deficiency induces rapid and dramatic cancellous bone loss in both sexes (36). These results provide preliminary evidence that gonadectomy-induced cancellous bone losses occur primarily via an intraskeletal T deficit in males and an intraskeletal E2 deficit in females or that the intraskeletal sex steroid hormones are present in nonbioavailable forms. In contrast, (supraphysiologic) T administration dramatically elevates serum and intraskeletal T concentrations in both sexes but does not increase intraskeletal E2 (Fig. 2A, B) (36), which supports the findings of Muir et al. (17) that T is a relatively weak substrate for E2 formation in human bone. Regardless, it is apparent that T exerts divergent effects in the adult male and female skeleton because T administration elevates serum and intraskeletal T concentrations in an identical magnitude in both sexes but only provides full protection against sex steroid deficiency-induced cancellous bone loss in males (Fig. 3A–F) (36). Certainly, future research evaluating intraskeletal concentrations of precursor androgens (e.g., DHEA, DHEA-S, androstendione, and 5α-androstendione) and estrogens (e.g., E1 and E1S) would assist in further elucidating the intracrine potential of bone and the influence of intraskeletal sex steroid hormones on skeletal biology.
Figure 3.
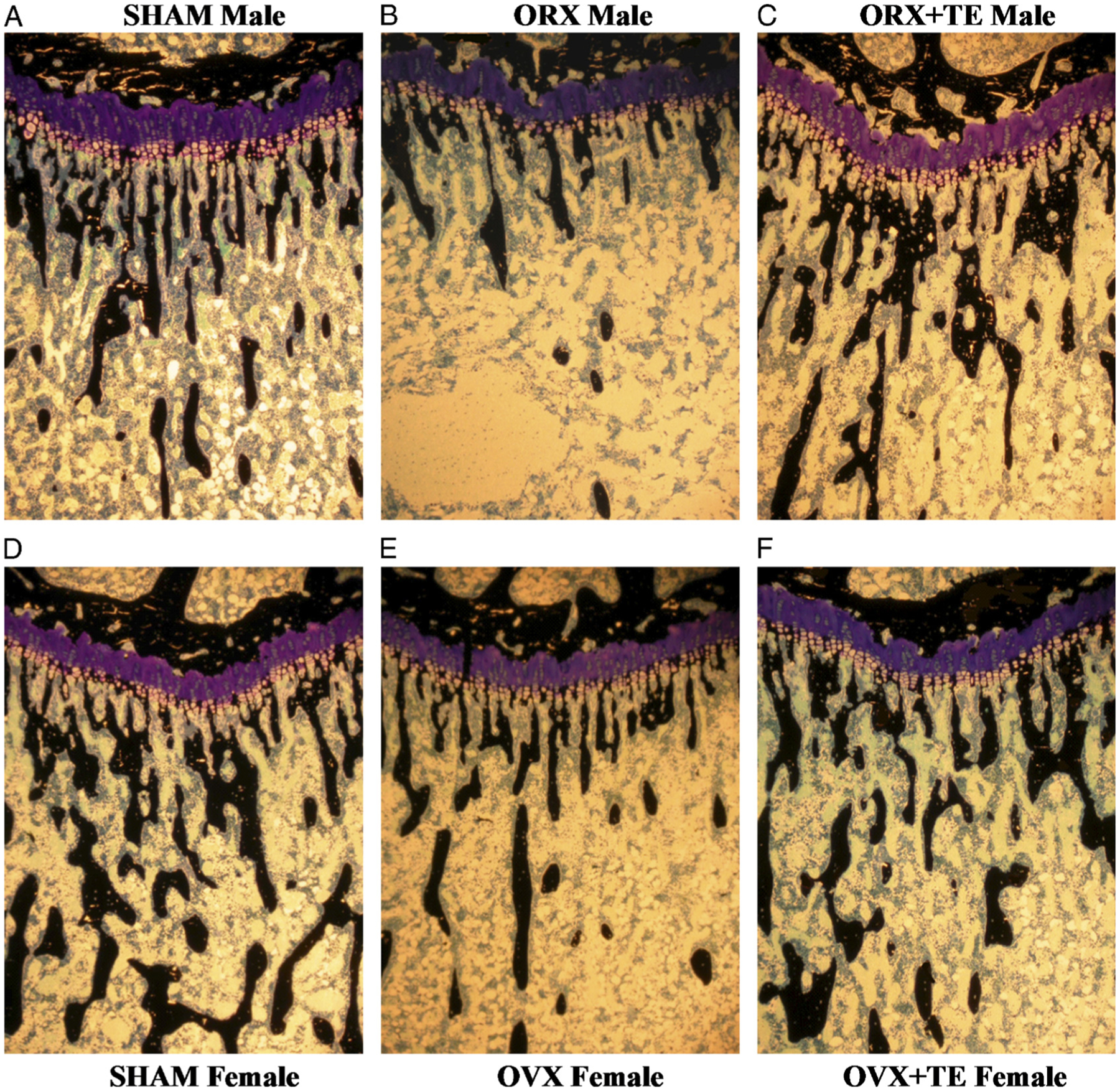
Proximal tibial metaphyses from male sham (A), orchiectomy (ORX) (B), and ORX + T (C) and female sham (D), ovariectomy (OVX) (E), and OVX + T (F) rats. Note reduced mass of black cancellous bone spicules, indicative of cancellous osteopenia in GX rats of both sexes. Testosterone (T) prevented loss of cancellous bone in ORX males and partially prevented bone loss in OVX females (×40). [Adapted from (36). Copyright © 2008 The Physiological Society. Used with permission.]
SEX STEROID HORMONE DEFICIENCY AND BONE
Circulating T peaks during puberty and into early adulthood in males, with a relatively large interindividual T variability present among men (normal eugonadal range ~315–1000 ng dL−1 or 11–335 nmol L−1) (10). Subsequently, a gradual reduction in serum T persists throughout life, such that values of men aged in the mid-70s are roughly two thirds lower than that of men in their mid-20s (10). In older men, hypogonadism is associated with a low BMD and an increased bone fracture risk (10), despite elderly men experiencing an elevated E2/T ratio in the circulation (9) and relatively little reduction in circulating DHT (10). Interestingly, SHBG is elevated in elderly men and E2 exhibits approximately 50% lower affinity than T for SHBG (10), which seems to at least partially explain why aging lowers bioavailable T while producing relatively less change in bioavailable E2. However, low-dose (replacement) T (administered transdermally via gel or patch) produces little improvement in BMD in hypogonadal men. In contrast, higher doses of long-acting T esters (e.g., T-enanthate or T-cypionate that are administered intramuscularly) produce clear BMD improvements (2,5,26), suggesting that T exerts a dose-response effect on bone with higher-than-replacement administration required for robust skeletal effects. However, T replacement therapy also elevates circulating DHT and E2 (2,5), which raises the possibility that these bioactive sex steroid hormones influence the skeletal effects of T replacement therapy.
ANDROGENS AND BONE
Androgens exert a profound influence on skeletal development (for in-depth reviews, see (10, 15, 31)). As evidence, men with complete androgen insufficiency resulting from a loss-of-function mutation in the AR exhibit low bone mass despite normal circulating T and DHT concentrations (24). Male mice with a global AR deletion also exhibit high turnover osteopenia with reduced cancellous and cortical bone mass (15). The primary site of AR action seems to be the osteoblast and/or osteocyte, as evidenced by male mice with AR deletions in the complete mesenchymal (osteoblast) lineage (15) or targeted AR deletions in mature osteoblasts or osteocytes that exhibit reduced cancellous volume but normal cortical bone volume (15). Similarly, AR overexpression in mature osteoblast/osteocyte populations produces male mice with a high cancellous bone volume resulting from exaggerated androgen action (33). In contrast, male mice with AR deletion in the complete myeloid (osteoclast) lineage exhibit relatively normal cancellous and cortical bone volume (15), indicating that androgenic effects on the skeleton are not mediated via osteoclast precursor cells or mature osteoclasts. However, extrapolation of these findings to that of the adult human or animal male skeleton should be approached with caution because transgenic alterations produce skeletal phenotypes that are distinct from that of normal bone development, which results from lifelong androgen exposure. Nevertheless, androgens clearly influence bone maintenance in adult male rodents, as evidenced by the ability of nonaromatizable androgens to prevent ORX-induced cancellous (3,16) and cortical (29) bone loss in rats completely.
5α-Reductase and Bone
As previously discussed, ground spongiosa from human bone actively synthesizes DHT (19) via localized T-dependent and/or T-independent DHT synthesis pathways that require 5α-reductase (Fig. 1). Considering that DHT is a more potent androgen than T, some have suggested that DHT is the primary androgen acting in bone (19,32). In this regard, inactivation of 5α-reductase type 1 in male Srd5a1−/− mice results in several skeletal maladaptations, including reduced cancellous and cortical bone mass, demonstrating the essential nature of this isozyme in male skeletal development (32). In addition, skeletally mature male Srd5a1−/− mice exhibit reduced responsiveness to the effects of T on cortical bone parameters (32). In contrast, men with 5α-reductase type 2 deficiency seem to have normal BMD (24) likely because type 1 5α-reductase is more highly expressed in bone (32) and/or because actions of the type 1 isozyme can override any deficiency in type 2 isozyme during adolescent bone development.
Despite the known influence of the type 1 5α-reductase isozyme on male skeletal development (32), it seems that neither the type 1 nor 2 5α-reductase isozyme is required for bone maintenance in adult men. For example, a recent meta-analysis has reported no association between use of finasteride (a type 2 5α-reductase inhibitor) or dutasteride (a dual type 1/2 5α-reductase inhibitor) and bone fracture risk (13). In addition, several randomized clinical trials have reported that neither finasteride (5) (which produces a 50%–70% reduction in circulating DHT) nor dutasteride (which produces a >90% reduction in circulating DHT) adversely affects BMD or bone turnover in adult men (1). Further evidence for no discernible role of 5α-reductase on the adult male skeleton stems from our research, which demonstrates that T, when administered alone or in combination with MK-434 (a dual type 1/2 5α reductase inhibitor), completely prevents ORX-induced cancellous bone loss in male rats (4). More recently, we have expanded on these findings, evaluating the individual and combined effects of T-enanthate and finasteride administration in hypogonadal elderly men (5). T (alone) increased hip and lumbar spine BMD by 2% and 4%, respectively, increased lean mass and muscle strength, and reduced visceral adiposity for 12 months but resulted in approximately 43% enlargement of the prostate. Coadministration of T and finasteride resulted in near identical improvements in BMD, lean mass, muscle strength, and adiposity and also prevented the T-induced prostate enlargement completely (5). Similarly, Amory et al. (2) treated older hypogonadal men for 36 months with T-enanthate ± finasteride and reported that finasteride did not reduce the T-induced improvements in lumbar spine and hip in BMD. However, elevated circulating E2 also has been observed in hypogonadal men receiving T-enanthate (alone) or in combination with finasteride (2,5), suggesting that a closer examination of the effects of E2 on T-induced skeletal protection is warranted. In this regard, we have reported that 17β-hydroxyestra-4,9,11-trien-3-one (trenbolone), a non-5α-reducible nonaromatizable synthetic T analog completely prevents cancellous bone loss in skeletally mature ORX rats (3, 16) (Fig. 4) but only partially prevents bone loss in young ORX rats (35). These results support the concept that 5α-reductase exerts divergent effects on the young versus the adult male skeleton, with the type 1 5α-reductase isozyme influencing bone development before skeletal maturity but neither the type 1 nor 2 isozyme exerting skeletal effects in adulthood. Interestingly, this finding has important clinical ramifications because 5α-reductase activity is known to mediate several of the adverse effects of T replacement therapy, including prostate enlargement and male-pattern balding in adult men (37). As such, coadministration of finasteride may represent a means of improving the safety profile of T replacement therapy while maintaining the beneficial musculoskeletal and lipolytic effects of this treatment (5), although further clinical evaluation of this concept is warranted.
Figure 4.
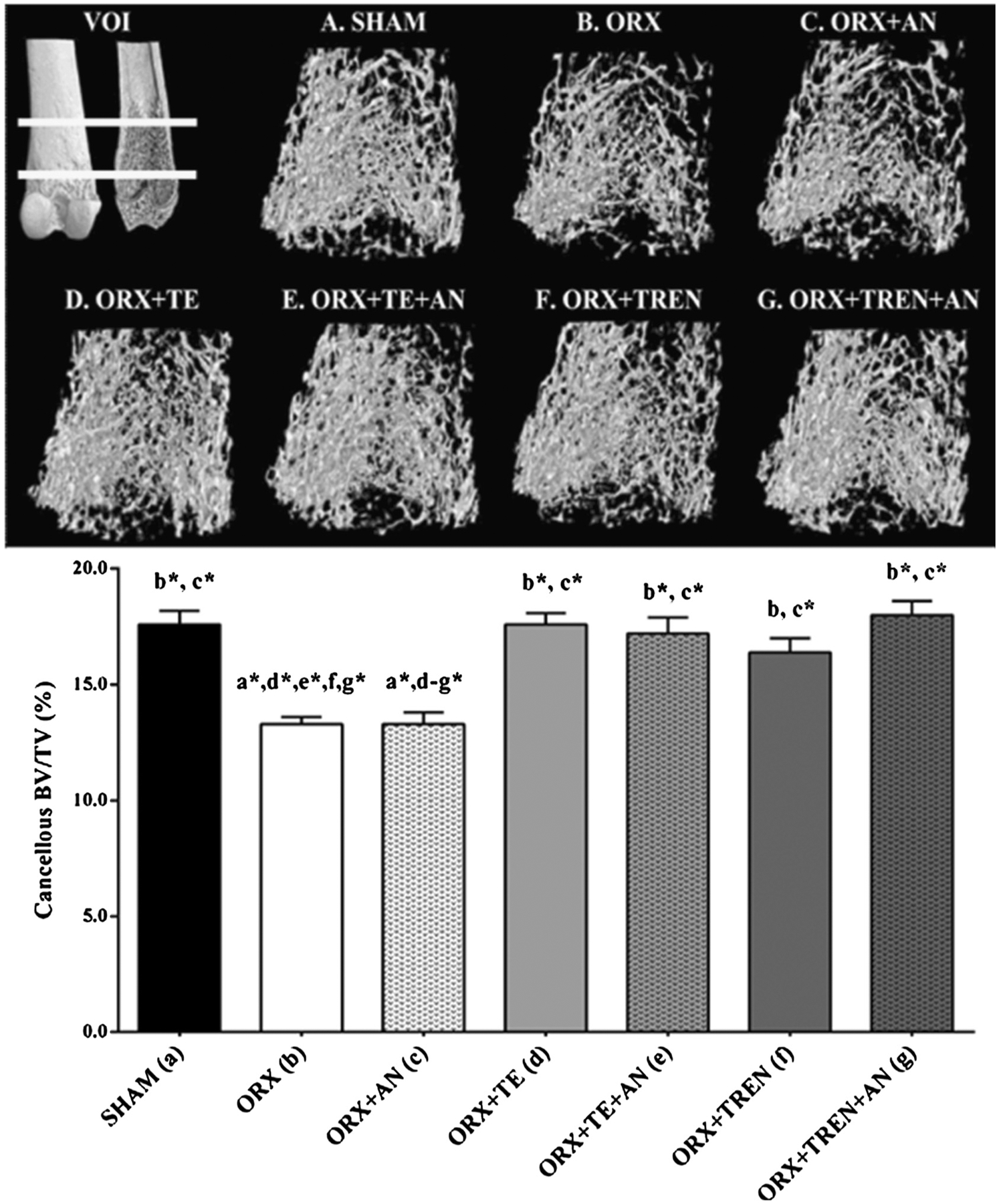
A–G. Microcomputed tomography images of cancellous bone at the distal femoral metaphysis from adult male rats receiving sham surgery or orchiectomy (ORX) in combination with anastrozole (AN), T-enanthate (TE), or trenbolone-enanthate (TREN). Values represent mean ± SE of 8 to 11 per group. Letters a to g indicated differences between respective groups at P < 0.05 or *P < 0.01 ((A) vs SHAM; (B) vs ORX; (C) vs ORX + AN; (D) vs ORX + TE; (E) vs ORX + TE + AN; (F) vs ORX + TREN; (G) vs ORX + TREN + AN). VOI, volume of interests; BV/TV, cancellous bone volume. [Adapted from (3). Copyright © 2014 John Wiley and Sons. Used with permission.]
ESTROGENS AND BONE
The influence of aromatase on male adolescent bone development is well established (for an in-depth review, see (31)), as demonstrated by several men diagnosed with congenital aromatase deficiency exhibiting a tall stature because of delayed epiphyseal closure and severe osteopenia, which is treatable with E2 but not T (9). Male aromatase knockout (ArKO) mice also exhibit adverse skeletal development, including severe osteopenia (18). In addition, a direct effect of intraskeletally derived E2 has been demonstrated in mice with osteoblast-specific overexpression of aromatase that exhibit increased bone mass without elevations of circulating E2 (23).
Both ERα and ERβ also are expressed throughout the mesenchymal and myeloid lineages of bone cells, although estrogenic effects on the developing male skeleton seem to be mediated primarily by ERα (for in-depth reviews, see (15,31)). As evidence, germline (global) ERβ deletion produces little effect on longitudinal bone growth or cancellous/cortical bone mass in males. In contrast, germline ERα deletion produces a complex skeletal phenotype in males that is composed of reduced longitudinal and radial bone growth with unfused growth plates, low cortical bone area, and high trabecular bone mass (15,31), demonstrating influence of ERα signaling on the male skeleton. However, determination of the exact site of estrogenic effects on the male skeleton has proven difficult because male mice with targeted ERα deletion in cartilage chondrocytes exhibit normal longitudinal growth, and ERα deletion from mature osteoblasts, mature osteocytes, or the complete mesenchymal or myeloid lineages produces no cancellous or cortical bone deficits (15). Furthermore, administration of the potent antiestrogen ICI 182,700 produces minimal skeletal effects in young gonadally intact male rats (28) despite the ability of this agent to mimic OVX-induced high-turnover cancellous osteopenia in young gonadally intact female rats (22). As such, it seems that E2 produces indirect effects on cancellous/cortical bone development and longitudinal bone growth in males (at least in rodents) perhaps through ERα-mediated effects in other cell types or via alternative signaling pathways (15). However, as previously discussed, extrapolation of these findings to the human or rodent adult male skeleton should be approached with caution.
Relatively less work has evaluated the role of the aromatase enzyme in adult male bone maintenance directly, although circulating and locally synthesized estrogens certainly seem capable of influencing the adult male skeleton (for an in-depth review, see (9)). For example, anastrozole (a nonsteroidal aromatase inhibitor) partially inhibits T-induced suppression of circulating bone resorption markers in older men treated with a gonadotropin-releasing hormone (GnRH) agonist to suppress gonadal sex steroid synthesis (12). Furthermore, administered E2 suppresses circulating bone resorption markers more than transdermally administered T in elderly men cotreated with a long-acting GnRH and the aromatase inhibitor letrozole to suppress endogenous T and E2 production (8). However, two caveats should be noted about the aforementioned studies (8,12): 1) both studies administered T transdermally in low (replacement) doses, whereas only higher T doses administered intramuscularly have been shown by meta-analysis to produce a skeletal benefit in adult men (26); and 2) a full suppression of GnRH-mediated bone turnover occurred only when T and E2 were administered simultaneously in these studies, suggesting that T and E2 may coregulate bone turnover in adult men. Despite the aforementioned findings, anastrozole (alone) does not alter circulating bone turnover markers and produces only very minor bone loss (or, in some cases, no bone loss) in elderly men with low T (6) perhaps because aromatase inhibition dramatically increases circulating T or because the aromatization of T is not the primary pathway of intraskeletal E2 synthesis (17). In this regard, we have reported that trenbolone (non-5α-reducible, nonaromatizable, nonestrogenic synthetic T analog) fully prevents ORX-induced cancellous bone loss in skeletally mature male rodents (16) and that anastrozole does not inhibit the ability of administered (supraphysiologic) T or trenbolone to prevent bone loss in this rodent model (3), demonstrating that administered T exerts skeletal preservation independent of the effects of aromatase in adult male rats. However, verification of these findings in adult men remains to be completed.
CONCLUSIONS
In summary, sex steroid hormones influence bone development and maintenance throughout the life span (10,31). Historically, the skeletal effects of T were thought to be mediated partially by the localized 5α reduction (32) or aromatization (9) of T to DHT or E2, respectively, within bone. However, T is a relatively weak substrate for 5α-reductase and aromatase in comparison with several other androgens and estrogens that circulate in abundance or which can be synthesized locally within bone, independent of T (11,14). Moreover, the predominant pathway of intraskeletal E2 synthesis uses E1S as the initial substrate and occurs independent of local aromatase action (17). As such, careful consideration of the relative contributions of the circulating versus intraskeletal sex steroid hormone reservoirs must be taken when evaluating the influences of 5α-reductase and aromatase on the skeleton. In this regard, future research is warranted to determine the biological role of the intraskeletal sex steroid hormone reservoir and what, if any, role the T-independent pathways of DHT and E2 synthesis play in bone maintenance.
Regardless, the findings presented herein support the novel hypothesis that T exerts direct protective effects on the adult male skeleton that are independent of the actions of 5α-reductase or aromatase. The primary evidence supporting this hypothesis stems from the following research conducted in our laboratory. First, ORX-induced cancellous bone loss is accompanied by a reduction in intraskeletal T but not intraskeletal DHT or E2 (36). Second, systemic supraphysiological T-enanthate administration elevates intraskeletal T and completely prevents ORX-induced cancellous bone loss in male rats but does not alter intraskeletal E2 (34,36). Third, pharmacologic 5α-reductase inhibition does not diminish the ability of administered T to completely prevent cancellous bone loss in ORX rats (4) or to improve BMD in hypogonadal elderly men (5). Fourth, pharmacologic aromatase inhibition does not diminish the bone-protective effects of aromatizable (i.e., T) or nonaromatizable androgens (i.e., trenbolone) in skeletally mature ORX rats (3,16). Nevertheless, additional research is needed to better understand the influences of sex steroid hormones on the adult male skeleton. We suggest that a particular emphasis should be placed on evaluating the effects of androgens and estrogens in bone cell-specific inducible knockout models where target genes can be inactivated selectively after skeletal maturity. In addition, further clinical evaluation of the effects of T in combination with pharmacologic 5α-reductase or aromatase inhibitors would advance knowledge related to the influence of these enzymes on T-mediated bone maintenance and other systemic androgenic/estrogenic effects in adult men.
Acknowledgments
This work was supported by resources provided by the North Florida/South Georgia Veterans Health System, Gainesville, FL, and by work supported by a SPiRE (1I21RX001371-01) to J.F. Yarrow and Merit Award (AR688R) to S.E. Borst from the US Department of Veterans Affairs, Rehabilitation Research and Development Programs. The work reported herein does not represent the views of the US Department of Veterans Affairs or of the US Government.
References
- 1.Amory JK, Anawalt BD, Matsumoto AM, et al. The effect of 5alpha-reductase inhibition with dutasteride and finasteride on bone mineral density, serum lipoproteins, hemoglobin, prostate specific antigen and sexual function in healthy young men. J. Urol 2008; 179:2333–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amory JK, Watts NB, Easley KA, et al. Exogenous testosterone or testosterone with finasteride increases bone mineral density in older men with low serum testosterone. J. Clin. Endocrinol. Metab 2004; 89:503–10. [DOI] [PubMed] [Google Scholar]
- 3.Beck DT, Yarrow JF, Beggs LA, et al. Influence of aromatase inhibition on the bone-protective effects of testosterone. J. Bone Miner. Res 2014; 29:2405–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borst SE, Conover CF, Carter CS, et al. Anabolic effects of testosterone are preserved during inhibition of 5alpha-reductase. Am. J. Physiol. Endocrinol. Metab 2007; 293:E507–14. [DOI] [PubMed] [Google Scholar]
- 5.Borst SE, Yarrow JF, Conover CF, et al. Musculoskeletal and prostate effects of combined testosterone and finasteride administration in older hypogonadal men: a randomized, controlled trial. Am. J. Physiol. Endocrinol. Metab 2014; 306:E433–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burnett-Bowie SA, McKay EA, Lee H, Leder BZ. Effects of aromatase inhibition on bone mineral density and bone turnover in older men with low testosterone levels. J. Clin. Endocrinol. Metab 2009; 94:4785–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Corona G, Rastrelli G, Giagulli VA, et al. Dehydroepiandrosterone supplementation in elderly men: a meta-analysis study of placebo-controlled trials. J. Clin. Endocrinol. Metab 2013; 98:3615–26. [DOI] [PubMed] [Google Scholar]
- 8.Falahati-Nini A, Riggs BL, Atkinson EJ, O’Fallon WM, Eastell R, Khoslam S. Relative contributions of testosterone and estrogen in regulating bone resorption and formation in normal elderly men. J. Clin. Invest 2000; 106:1553–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gennari L, Nuti R, Bilezikian JP. Aromatase activity and bone homeostasis in men. J. Clin. Endocrinol. Metab 2004; 89:5898–907. [DOI] [PubMed] [Google Scholar]
- 10.Kaufman JM, Vermeulen A. The decline of androgen levels in elderly men and its clinical and therapeutic implications. Endocr. Rev 2005; 26:833–76. [DOI] [PubMed] [Google Scholar]
- 11.Labrie F, Luu-The V, Bélanger A, et al. Is dehydroepiandrosterone a hormone? J. Endocrinol 2005; 187:169–96. [DOI] [PubMed] [Google Scholar]
- 12.Leder BZ, LeBlanc KM, Schoenfeld DA, Eastell R, Finkelstein JS. Differential effects of androgens and estrogens on bone turnover in normal men. J. Clin. Endocrinol. Metab 2003; 88:204–10. [DOI] [PubMed] [Google Scholar]
- 13.Lim SY, Laengvejkal P, Panikkath R, Nugent K. The association of alpha-blockers and 5-alpha reductase inhibitors in benign prostatic hyperplasia with fractures. Am. J. Med. Sci 2014; 347:463–71. [DOI] [PubMed] [Google Scholar]
- 14.Luu-The V Assessment of steroidogenesis and steroidogenic enzyme functions. J. Steroid Biochem. Mol. Biol 2013; 137:176–82. [DOI] [PubMed] [Google Scholar]
- 15.Manolagas SC, O’Brien CA, Almeida M. The role of estrogen and androgen receptors in bone health and disease. Nat. Rev. Endocrinol 2013; 9:699–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCoy SC, Yarrow JF, Conover CF, et al. 17beta-Hydroxyestra-4,9,11-trien-3-one (Trenbolone) preserves bone mineral density in skeletally mature orchiectomized rats without prostate enlargement. Bone. 2012; 51:667–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muir M, Romalo G, Wolf L, Elger W, Schweikert HU. Estrone sulfate is a major source of local estrogen formation in human bone. J. Clin. Endocrinol. Metab 2004; 89:4685–92. [DOI] [PubMed] [Google Scholar]
- 18.Oz OK, Zerwekh JE, Fisher C, et al. Bone has a sexually dimorphic response to aromatase deficiency. J. Bone Miner. Res 2000; 15:507–14. [DOI] [PubMed] [Google Scholar]
- 19.Schweikert HU, Rulf W, Niederle N, Schäfer HE, Keck E, Krück F. Testosterone metabolism in human bone. Acta Endocrinol. (Copenh) 1980; 95:258–64. [DOI] [PubMed] [Google Scholar]
- 20.Shehu A, Mao J, Gibori GB, et al. Prolactin receptor-associated protein/17beta-hydroxysteroid dehydrogenase type 7 gene (Hsd17b7) plays a crucial role in embryonic development and fetal survival. Mol. Endocrinol 2008; 22:2268–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shen Z, Peng Z, Sun Y, Väänänen HK, Poutanen M. Overexpression of human hydroxysteroid (17beta) dehydrogenase 2 induces disturbance in skeletal development in young male mice. J. Bone Miner. Res 2008; 23:1217–26. [DOI] [PubMed] [Google Scholar]
- 22.Sibonga JD, Dobnig H, Harden RM, Turner RT. Effect of the high-affinity estrogen receptor ligand ICI 182,780 on the rat tibia. Endocrinology. 1998; 139:3736–42. [DOI] [PubMed] [Google Scholar]
- 23.Sjogren K, Lagerquist M, Moverare-Skrtic S, et al. Elevated aromatase expression in osteoblasts leads to increased bone mass without systemic adverse effects. J. Bone Miner. Res 2009; 24:1263–70. [DOI] [PubMed] [Google Scholar]
- 24.Sobel V, Schwartz B, Zhu YS, Cordero JJ, Imperato-McGinley J. Bone mineral density in the complete androgen insensitivity and 5alpha-reductase-2 deficiency syndromes. J. Clin. Endocrinol. Metab 2006; 91:3017–23. [DOI] [PubMed] [Google Scholar]
- 25.Sun H, Zang W, Zhou B, Xu L, Wu S. DHEA suppresses longitudinal bone growth by acting directly at growth plate through estrogen receptors. Endocrinology. 2011; 152:1423–33. [DOI] [PubMed] [Google Scholar]
- 26.Tracz MJ, Sideras K, Boloña ER, et al. Testosterone use in men and its effects on bone health. A systematic review and meta-analysis of randomized placebo-controlled trials. J. Clin. Endocrinol. Metab 2006; 91:2011–6. [DOI] [PubMed] [Google Scholar]
- 27.Turner RT, Bleiberg B, Colvard DS, Keeting PE, Evans G, Spelsberg TC. Failure of isolated rat tibial periosteal cells to 5 alpha reduce testosterone to 5 alpha-dihydrotestosterone. J. Bone Miner. Res 1990; 5:775–9. [DOI] [PubMed] [Google Scholar]
- 28.Turner RT, Evans GL, Dobnig H. The high-affinity estrogen receptor antagonist ICI 182,780 has no effect on bone growth in young male rats. Calcif. Tissue Int 2000; 66:461–4. [DOI] [PubMed] [Google Scholar]
- 29.Turner RT, Wakley GK, Hannon KS. Differential effects of androgens on cortical bone histomorphometry in gonadectomized male and female rats. J. Orthop. Res 1990; 8:612–7. [DOI] [PubMed] [Google Scholar]
- 30.van der Eerden BC, Löwik CW, Wit JM, Karperien M. Expression of estrogen receptors and enzymes involved in sex steroid metabolism in the rat tibia during sexual maturation. J. Endocrinol 2004; 180:457–67. [DOI] [PubMed] [Google Scholar]
- 31.Vanderschueren D, Laurent MR, Claessens F, et al. Sex steroid actions in male bone. Endocr. Rev 2014; 35:906–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Windahl SH, Andersson N, Borjesson AE, et al. Reduced bone mass and muscle strength in male 5α-reductase type 1 inactivated mice. PLoS One. 2011; 6:e21402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wiren KM, Semirale AA, Zhang XW, et al. Targeting of androgen receptor in bone reveals a lack of androgen anabolic action and inhibition of osteogenesis: a model for compartment-specific androgen action in the skeleton. Bone. 2008; 43:440–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yarrow JF, Conover CF, Lipinska JA, Santillana CA, Wronski TJ, Borst SE. Methods to quantify sex steroid hormones in bone: applications to the study of androgen .ablation and administration. Am. J. Physiol. Endocrinol. Metab 2010; 299:E841–7. [DOI] [PubMed] [Google Scholar]
- 35.Yarrow JF, Conover CF, McCoy SC, et al. 17β-Hydroxyestra-4,9,11-trien-3-one (trenbolone) exhibits tissue selective anabolic activity: effects on muscle, bone, adiposity, hemoglobin, and prostate. Am. J. Physiol. Endocrinol. Metab 2011; 300:E650–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yarrow JF, Conover CF, Purandare AV, et al. Supraphysiological testosterone enanthate administration prevents bone loss and augments bone strength in gonadectomized male and female rats. Am. J. Physiol. Endocrinol. Metab 2008; 295:E1213–22. [DOI] [PubMed] [Google Scholar]
- 37.Yarrow JF, McCoy SC, Borst SE. Intracrine and myotrophic roles of 5α-reductase and androgens: a review. Med. Sci. Sports Exerc 2012; 44:818–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Young J, Couzinet B, Nahoul K, et al. Panhypopituitarism as a model to study the metabolism of dehydroepiandrosterone (DHEA) in humans. J. Clin. Endocrinol. Metab 1997; 82:2578–85. [DOI] [PubMed] [Google Scholar]


