Summary
Background
Electronic nicotine delivery systems (ENDSs) are used by some smokers to reduce cigarette consumption, but their effectiveness is uncertain. We aimed to examine the extent to which ENDSs or a non-nicotine cigarette substitute influence tobacco-related toxicant exposure and cigarette consumption in smokers interested in smoking reduction.
Methods
We did a four-arm, parallel-group, randomised controlled trial at two sites in the USA (Penn State University, Hershey, PA, and Virginia Commonwealth University, Richmond, VA). We enrolled adults aged 21–65 years who smoked more than nine cigarettes per day (for at least the past year), with exhaled CO of more than 9 parts per million at screening, who were not currently using an ENDS, and who were interested in reducing smoking but not quitting. Participants were randomised (site-specific with allocation concealment; 1:1:1:1) to receive either a cartomiser-based, pen-style ENDS (eGo-style) paired with 0, 8, or 36 mg/mL liquid nicotine (participants and researchers masked to concentration) or a non-ENDS cigarette-shaped plastic tube that delivered no nicotine or aerosol (cigarette substitute; unmasked) for 24 weeks. Conditions were chosen to reflect a range of nicotine delivery including none (cigarette substitute and 0 mg/mL ENDS), low (8 mg/mL), and cigarette-like (36 mg/mL), and all conditions were paired with smoking reduction instructions. The primary outcome was concentration of the tobacco-specific carcinogen metabolite 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL; urinary total) collected at randomisation and at 4, 12, and 24 weeks. Multiple imputation with and without covariate adjustment was used in addition to sensitivity analyses. This trial is registered with ClinicalTrials.gov, NCT02342795.
Findings
Between July 22, 2015, and Nov 16, 2017, 684 individuals were screened and 520 (76%) were enrolled and randomised. 188 (36%) of 520 participants were lost to follow-up by week 24; attrition did not differ by study group (39 [30%] of 130 in the cigarette substitute group, 56 [43%] of 130 in the ENDS with 0 mg/mL nicotine group, 49 [38%] of 130 in the ENDS 8 mg/mL group, and 44 [34%] of 130 in the ENDS 36 mg/mL group). Urinary total NNAL at 24 weeks in the ENDS with 36 mg/mL nicotine group was 210∙80 pg/mg creatinine (95% CI 163·03–274·42) compared with 346·09 pg/mg creatinine (265·00–455·32) in the cigarette substitute group (p=0·0061). No other significant differences between groups were observed for any time point for urinary total NNAL. Serious adverse event frequency was similar across groups (12 events in 12 participants [9%] in the ENDS with 36 mg/mL nicotine group, seven events in six participants [5%] in the 8 mg/mL group, 11 events in ten participants [8%] in the 0 mg/mL group, and 13 events in 13 participants [10%] in the cigarette substitute group), and all of these were deemed unrelated or unlikely to be related to study product use. There was one death between randomisation and 24 weeks (suicide; in the ENDS with 0 mg/mL nicotine group).
Interpretation
Use of an ENDS with cigarette-like nicotine delivery can reduce exposure to a major pulmonary carcinogen, NNAL, even with concurrent smoking. Future ENDS trials should involve products with well characterised nicotine delivery, including those with nicotine delivery approaching that of a cigarette.
Introduction
Electronic nicotine delivery systems (ENDSs) heat a nicotine-containing liquid to form an inhalable aerosol.1 Although the health effects of long-term ENDS use remain unclear,2 ENDSs might reduce smokers’ consumption of cigarettes and associated toxicant exposure. Large-scale randomised controlled trials (ie, with >500 participants) of ENDSs have examined their effects on smoking cessation and indicate that ENDS use can result in modest improvements in cigarette smoking cessation rates when used alone or combined with a nicotine patch, compared with using a nicotine patch alone.3,4 When individual counselling is also provided, ENDS use can almost double cigarette smoking cessation rates relative to nicotine replacement therapy.5 Findings from other trials suggest that ENDS use can reduce cigarette smokers’ exposure to tobacco smoke toxicants such as the carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) or CO.6–10 A limitation of these previous randomised trials is that the nicotine delivery profile of the ENDSs used was either unknown4,5,8,9 or far less (eg, about one tenth) than that of a combustible cigarette.3,6,7,10 If the ENDS nicotine delivery is unknown or very low it is possible that larger effects could be obtained with use of an ENDS with higher or known nicotine delivery.
To our knowledge, no randomised, controlled trial has yet examined the extent to which an ENDS, with a cigarette-like nicotine delivery profile, is associated with reduced tobacco-related toxicant exposure in long-term smokers. There are many examples of ENDSs that deliver little or no nicotine.11,12 However, ENDSs that deliver nicotine at high levels are now easily accessible. For instance, in a previous study, when a commercially available approximately 7-watt ENDS was paired with a 36 mg/mL liquid nicotine, 64 participants who had ten inhalations from it showed a mean plasma nicotine concentration increase of 13·0 ng/mL (SD 14·3), approaching that from a cigarette smoked under similar conditions (eg, 15·0–20·0 ng/mL).13
We aimed to evaluate the effects of an ENDS paired with 0, 8, or 36 mg/mL liquid nicotine (as in the study by Hiler and colleagues13), or a non-ENDS cigarette substitute that delivers no nicotine or aerosol as a control, on measures of tobacco-related toxicant exposure, nicotine exposure, and cigarette consumption in smokers interested in cigarette smoking reduction.
Methods
Study design
We did a four-arm, parallel-group, randomised, controlled trial at two sites in the USA: Penn State University, Hershey, PA, and Virginia Commonwealth University, Richmond, VA. The study was approved by the institutional review board at each site. All authors vouch for the fidelity of the trial to the protocol, which has been published previously.14 There was one major protocol change approved by both sites in October, 2016, which was a change to the number of participants to be enrolled at each site (originally 260 participants each), in response to recruitment challenges at the Richmond site.
Participants
Participants were recruited via study advertisements that were distributed through a variety of print, radio, online, and other methods. Inclusion criteria were age 21–65 years, more than nine filtered cigarettes smoked per day for at least the past year, exhaled CO of more than 9 parts per million (ppm) at screening, no attempt to quit in the past 30 days, interest in reducing cigarette smoking but not planning to quit completely in the next 6 months, and ability to read and write English. All participants provided written informed consent before enrolling. Exclusion criteria included use of any tobacco cessation medication in the past 30 days, use of any non-cigarette nicotine delivery product in the past 7 days, use of an ENDS for 5 days or more in the past 28 days, use of hand-rolled cigarettes, pregnancy or breastfeeding, unstable or major medical condition in the past 12 months, disorders or use of medication that might affect safety or biomarker data, known allergy to propylene glycol or vegetable glycerin, and use of marijuana or any illicit or prescription drugs for non-medical use weekly or more frequently in the past 3 months (further details are published elsewhere).14
Randomisation and masking
The study statistician (M-SY) prepared site-specific randomisation lists using the sample function in R version 3.2.0 (blocks of eight). These lists were uploaded onto a study-specific website that interfaced with the data collection and management system (REDCap). Only unmasked researchers at each site with no participant contact accessed their list to prepare cartomisers for dispensing. Participants were randomly assigned in-person and in real-time using an electronic function within REDCap to one of four conditions (1:1:1:1): an ENDS paired with cartomisers containing 0, 8, or 36 mg/mL liquid nicotine (administered double-blind) or a cigarette substitute that delivered no nicotine or aerosol (unmasked). Participants and researchers who enrolled participants and who collected outcome data were masked to which liquid nicotine concentration was assigned among individuals in ENDS groups, and all cartomisers were identical except for the liquid placed within them. Masking success was not evaluated systematically. Researchers who analysed the data were not masked to condition assignment.
Procedures
Following successful pre-screening via telephone, participants completed an in-person screening session to obtain informed consent and confirm eligibility. Approximately 1 week later, eligible individuals completed a baseline visit (week 0) and were randomly assigned to one of the four study conditions and asked to attend subsequent clinic visits at 1, 2, 4, 8, 12, 16, 20, 24, 28, and 36 weeks. Provision of the condition-specific product lasted for 24 weeks (intervention period). There was a 12-week follow-up period after the intervention period for each condition (appendix p 13). Participants were instructed to use study products as often as desired in place of cigarettes during the intervention period in combination with smoking reduction instructions (advised to reduce by 50% in weeks 0–2, by 75% in weeks 3–8, and continued maintenance or reduction in weeks 9–24). Following the intervention period, participants were advised to cease all cigarette smoking in weeks 25–36. Compliance with smoking reduction instructions was encouraged but not incentivised or penalised. Study product provision concluded at 24 weeks, and at this visit participants were eligible to receive the alternate product to that which they were originally assigned (appendix p 9). Eligible participants completing all visits could receive up to US$400 via gift cards.
ENDS conditions involved an eGo-style 3·3–4·1 volt, 1100 milliampere-h battery with an integrated automatic inhalation counter paired with a 1·5-ohm, dual-coil, 510-style opaque cartomiser (approximately 7-watt; both manufactured by SmokTech, Shenzhen, China). Each cartomiser contained 1 mL of a flavoured liquid (participant-selected at randomisation: tobacco or menthol; selected flavour could not be changed during the intervention period) with a 70:30 propylene glycol to vegetable glycerin ratio and containing 0, 8, or 36 mg/mL freebase nicotine (AVAIL Vapor, Richmond, VA, USA). The nicotine delivery profile of these ENDS and liquid nicotine combinations was characterised in the clinical laboratory (ie, ten directed inhalations) with a mean plasma nicotine increase of –0·1 ng/mL (SD 1·5) for 0 mg/mL liquid nicotine, 6·0 ng/mL (SD 6·6) for 8 mg/mL, and 13·0 ng/mL (SD 14·3) for 36 mg/mL.13 Cartomisers were provided in child-proof containers that allowed for use of three cartomisers per day initially. Cartomiser supply was modified at the 4-week study visit based on individual use behaviour during the previous weeks (ie, according to the number of returned used and unused cartomisers) for the remaining intervention period.
The cigarette substitute condition involved a non-combustible, unflavoured, plastic, tube-shaped, patented product designed to provide a draw resistance and physical appearance like a cigarette (QuitSmart; Hillsborough, NC, USA), and was similar to a product tested as part of a quitline provision (Better Quit).15 The cigarette substitute contained no nicotine or tobacco and produced no aerosol. Of note, this similar product (Better Quit) tested as part of a quitline provision did not significantly increase rates of smoking abstinence.15 In this group, participants were provided with two cigarette substitutes at randomisation, which were replaced as needed.
In all groups, participants were instructed to avoid using tobacco products other than their study product or their usual cigarettes. Brief directions regarding best practices for cigarette substitution were provided to all participants. There was no penalty or incentive for non-compliance or compliance regarding participant engagement in other tobacco use. Participants could discontinue study product use or request additional study product at any time during the intervention period without penalty. Participants who reported being unable to use their study product for more than 2 weeks not by choice (eg, restricted in the home or other environment) were considered for withdrawal by the principal investigator. Additional details regarding study product conditions, instructions, and blinding procedures are provided in the appendix (p 9).
Outcomes
The primary outcome was tobacco-related toxicant exposure to the potent lung carcinogen NNK, as indexed by the sum of its urinary metabolite 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) and its glucuronides (total NNAL; pg/mg creatinine) collected at randomisation and at 4, 12, and 24 weeks. The long half-life of NNAL (eg, terminal half-life 10–18 days)16 and its status as a carcinogen provide distinct advantages over other measures of tobacco-related exposure such as exhaled CO, including the ability of NNAL to serve as a correlate of tobacco’s carcinogenic effects. A secondary outcome was nicotine exposure via urine cotinine (ng/mg creatinine) collected at the same timepoints. A single void (spot) urine sample was collected at each visit for these outcomes. Time of collection varied between and within participants. Urine samples for total NNAL and cotinine were analysed using liquid chromatography tandem mass spectrometry techniques derived from previously validated methods.17,18 Other secondary outcomes included glutathione (via blood samples) and 8-Isoprostanes (urine and exhaled breath condensate samples); these results will be reported elsewhere. Exhaled CO was measured at each in-person visit and used as a quantitative indicator of recent tobacco smoke exposure (Vitalograph BreathCO monitor; Vitalograph, Lenexa, KS, USA; measured in ppm). Pulmonary function tests were done at randomisation, 4, 12, 24, and 36 weeks, and baseline measures were included in adjusted analyses as described in the statistical analysis section.
Self-reported number of cigarettes smoked per day and daily study product use (ENDS inhalations; cigarette substitute use in times, defined approximately as a period of use lasting about 15 inhalations or 10 min)19 were assessed at each in-person visit using a 7-day timeline follow-back procedure (adapted from the literature)20 supplemented with paper diaries completed daily by participants between visits. In addition, various measures assessing, for example, nicotine dependence, tobacco abstinence symptoms, and environmental tobacco smoke exposure were administered throughout the study (appendix p 9). Some of these variables were included in the adjusted analyses as described in the statistical analysis section.
Adverse events were recorded at and between visits, when participants reported any negative changes to their physical or mental health, including hospitalisation or emergency care after the first in-person visit. All adverse events were reviewed by a physician at each site and were characterised by symptom, time course, severity, expectedness, relatedness, and seriousness.
Statistical analysis
The complete statistical analysis plan is provided in the appendix (pp 172–187). Sample size determination was based on previous urinary total NNAL data.21 To detect an effect size of 58·59 pg/mL (SD 125·54) for pairwise comparisons between groups, 130 participants per group were needed to provide 80% power at a significance level of 0·008, which accounts for the six pairwise comparisons planned between groups, and for 20% attrition.
Baseline characteristics were first compared between groups to identify imbalances following randomisation (appendix p 27). We also examined baseline characteristics by site, which indicated significant differences, leading to the inclusion of site as a covariate in all adjusted analyses (appendix p 31). Data normality was examined for all four outcomes and only urinary total NNAL and urinary cotinine were adjusted using Box-Cox transformation.22 Missing data for urinary total NNAL, urinary cotinine, exhaled CO, and cigarettes smoked per day collected during the intervention period were imputed using multiple imputation. We first assessed baseline covariates associated with missing urinary total NNAL. The conditional regression model used to impute missing data consisted of the urinary total NNAL, urinary cotinine, exhaled CO, number of cigarettes smoked per day, covariates associated with missing NNAL, and covariates of interest (appendix pp 9–10).23,24 Five completed imputation datasets were created. The same linear mixed-effect models were applied to every dataset, and results from the datasets were pooled to construct statistical inferences.
The primary unadjusted analysis used linear mixed-effect models. The dependent variables were urinary total NNAL, urinary cotinine, exhaled CO, and number of cigarettes smoked per day. Fixed effects for the primary analysis included study condition, time, and their interaction. A random effect was included for within-participant dependence. Pairwise comparisons with Bonferroni adjustment were done, first between groups at each timepoint (six comparisons; p<0·0083), and second within groups relative to baseline (number of comparisons varies by outcome). The secondary adjusted analysis used linear mixed-effect models with the same fixed and random effects. Adjusted analyses included covariates from participant demo graphics (including age, sex, race and ethnicity, and site) and other baseline measures that were selected through examination of their associations with the outcome of interest during preliminary screening. We did sensitivity analyses (unadjusted and adjusted) with all available data (intention-to-treat population); with participants who completed visits at randomisation, 4, 12, and 24 weeks (per-protocol population); with baseline-carried-forward data; and with last-observation-carried-forward data. All point estimates and 95% CIs at every visit were obtained from the results of linear mixed-effect model analyses. Back-transformation was done through the inverse of the normalisation for estimates and 95% CI of urinary total NNAL and urinary cotinine.
To characterise study product use, 7-day timeline follow-back data were used to dichotomise participants into two groups on the basis of whether they used the study product in the past week before the visit (yes or no). Frequencies were compared between groups at each timepoint and within groups relative to the 1-week visit using χ2 tests or Fisher’s exact tests as appropriate. Three analyses were done: intention-to-treat with imputation, using the assumption that participants with missing data on study product use (ie, due to not attending that visit) were not using their study product; intention-to-treat with no imputation; and per-protocol. Bonferroni adjustment was applied to all pairwise comparisons.
All adverse events were examined descriptively by serious or severe status (severity was graded using National Cancer Institute Common Terminology Criteria for Adverse Events version 4 and 5), symptom, condition, and time (at randomisation, intervention period, and follow-up period).
For all analyses, the overall type I error was set at 0·05. SAS (version 9.4) was used for all analyses. This trial is registered with ClinicalTrials.gov, NCT02342795, and was monitored by an independent data and safety monitoring board (appendix p 8).
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
Between July 22, 2015, and Nov 16, 2017, 684 individuals were screened and 520 (76%; 320 from Penn State University and 200 from Virginia Commonwealth University) were randomised to one of the four groups (130 [25%] per group; figure 1, table, appendix pp 27–30). Baseline characteristics were balanced between the groups. 188 (36%) of 520 participants were lost to follow-up by week 24; attrition did not differ by group (39 [30%] of 130 in the cigarette substitute group, 56 [43%] of 130 in the ENDS with 0 mg/mL nicotine group, 49 [38%] of 130 in the ENDS with 8 mg/mL nicotine group, and 44 [34%] of 130 in the ENDS with 36 mg/mL nicotine group; p=0·15). Compared with participants who completed the study, the 188 participants who did not attend the 24-week visit were more likely to be younger (mean age 43·6 years [SD 11·8] vs 47·7 years [SD 11·3]; p=0·0001), have a lower level of education (p=0·030), have smoked for fewer years (mean 14·5 years [SD 12·3] vs 17·6 years [SD 13·5]; p=0·012), have started smoking at an earlier age (mean age 16·3 years [SD 3·7] vs 17·4 years [SD 4·7]; p=0·0032), and be more cigarette dependent (Penn State Cigarette Dependence Index mean 13·8 [SD 2·7] vs 13·2 [SD 3·1]; p=0·013; appendix p 35). Those who did not complete the study also had higher forced vital capacity indexed via pulmonary function tests at baseline compared to those who completed the study (p=0·043), likely to be due to their younger age. Reasons for study withdrawal by group and study period are provided in the appendix (p 39).
Figure 1: Trial profile.
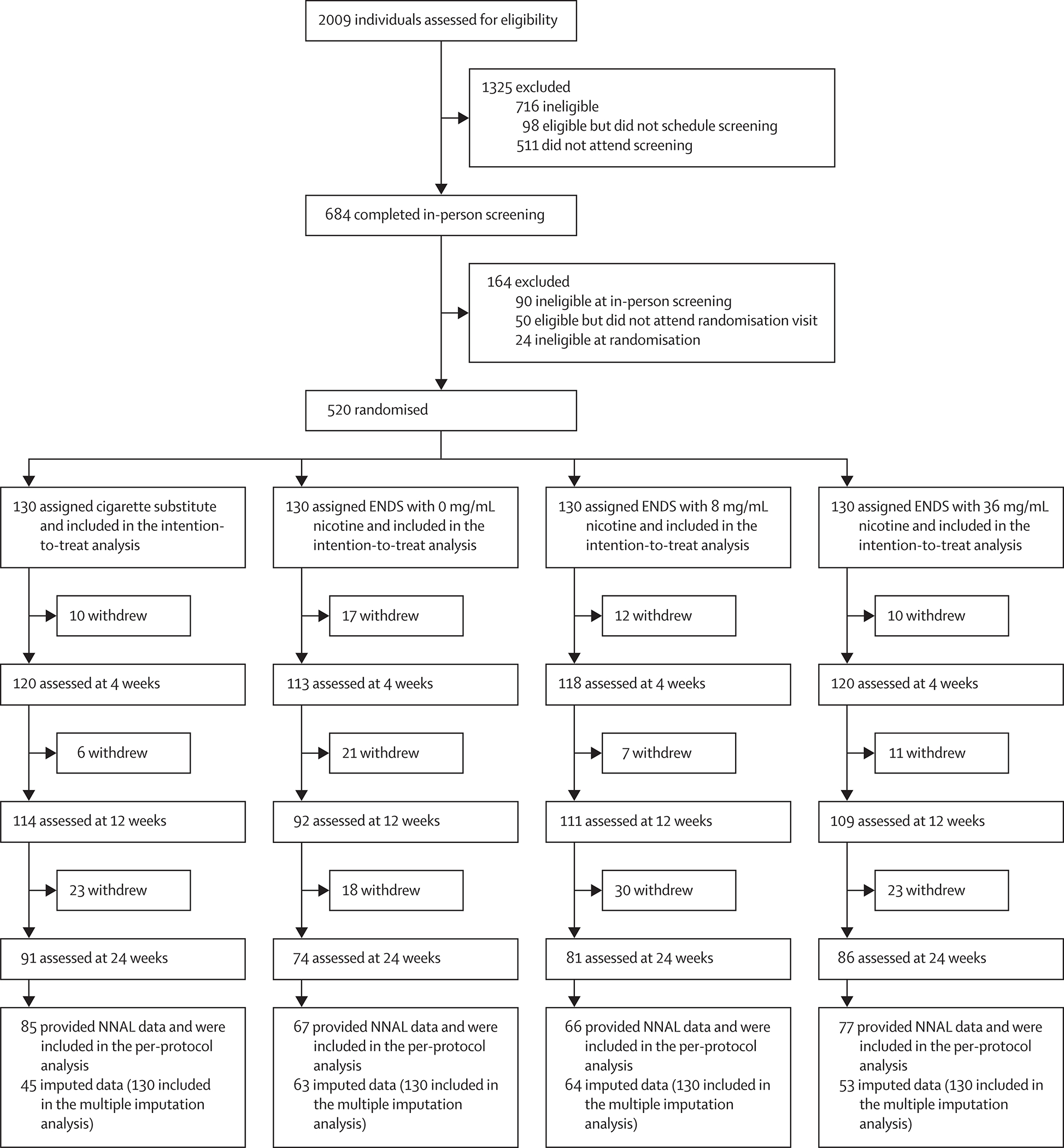
ENDS=electronic nicotine delivery system. NNAL=4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol.
Table:
Baseline characteristics
| Cigarette substitute group (n=130) | ENDS with 0 mg/mL nicotine group (n=130) | ENDS with 8 mg/mL nicotine group (n=130) | ENDS with 36 mg/mL nicotine group (n=130) | Overall (n=520) | |
|---|---|---|---|---|---|
| Age, years | 46·1 (12·4) | 45·7 (11·4) | 45·6 (11·7) | 47·4 (11·1) | 46·2 (11·6) |
| Sex | |||||
| Female | 79 (61%) | 80 (62%) | 80 (62%) | 67 (52%) | 306 (59%) |
| Male | 51 (39%) | 50 (38%) | 50 (38%) | 63 (48%) | 214 (41%) |
| Ethnicity | |||||
| White non-Hispanic | 83 (64%) | 92 (71%) | 87 (67%) | 88 (68%) | 350 (67%) |
| Black non-Hispanic | 39 (30%) | 33 (25%) | 37 (28%) | 36 (28%) | 145 (28%) |
| Other | 8 (6%) | 5 (4%) | 6 (5%) | 6 (5%) | 25 (5%) |
| Some college education or higher | 76 (58%) | 72 (55%) | 83 (64%) | 79 (61%) | 310 (60%) |
| Income >US$39 999* | 51 (40%) | 47 (37%) | 51 (41%) | 60 (46%) | 209 (41%) |
| Cigarettes smoked per day, mean over 7 days† | 18·37 (7·13) | 18·79 (8·35) | 19·45 (8·73) | 17·76 (6·52) | 18·59 (7·74) |
| Exhaled CO, ppm | 23·65 (12·54) | 23·35 (12·23) | 21·84 (9·80) | 21·94 (10·48) | 22·69 (11·32) |
| Penn State Cigarette Dependence Index score | 13·4 (3·0) | 13·7 (2·7) | 13·2 (3·0) | 13·2 (3·2) | 13·4 (3·0) |
| Menthol cigarette smoking | 87 (67%) | 88 (68%) | 81 (62%) | 77 (59%) | 333 (64%) |
| Use of other tobacco products in past 30 days | 4 (3%) | 9 (7%) | 10 (8%) | 8 (6%) | 31 (6%) |
| Urinary total NNAL, pg/mg creatinine | |||||
| Median (IQR) | 441·42 (683·19) | 499·76 (612·20) | 419·97 (835·64) | 544·38 (825·33) | 473·18 (710·61) |
| Geometric mean (95% CI) | 404·22 (33047–49444) | 440·69 (366·42–530·01) | 425·04 (347·34–520·11) | 432·73 (339·33–551·83) | 425·45 (383·64–471·81) |
| Urinary cotinine, ng/mg creatinine | |||||
| Median (IQR) | 1641·21 (2018·30) | 1593·17 (1564·13) | 1733·97 (2057·46) | 1871·47 (2137·68) | 1662·45 (1908·58) |
| Geometric mean (95% CI) | 1441·56 (1227·93–1692·35) | 1444·53 (1241·45–1680·83) | 1729·53 (1493·97–2002·23) | 1658·74 (1411·23–1949·67) | 1563·39 (1447·38–1688·70) |
Data are mean (SD) or n (%) unless otherwise stated. Demographic characteristics and use of other tobacco products were assessed by self-report at the in-person screening visit (1 week before randomisation). Cigarettes smoked per day, exhaled CO, Penn State Cigarette Dependence Index score, menthol cigarette smoking status, urinary total NNAL, and urinary cotinine were assessed at the baseline visit (at randomisation). There were no significant differences in baseline characteristics between groups (p>0·05 for each characteristic). ENDS=electronic nicotine delivery system. ppm=parts per million. NNAL=4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol.
Two participants in the cigarette substitute group, three in the 0 mg/mL group, and five in the 8 mg/mL group had missing income data.
Calculated using self-reported timeline follow-back for the 7 days before the randomisation visit (collected in-person at randomisation).
At the 24-week visit, urinary total NNAL was significantly lower for the ENDS with 36 mg/mL nicotine group (210∙80 pg/mg creatinine [95% CI 163·03–274·42]) than in the cigarette substitute group (346·09 pg/mg creatinine [265·00–455·32]; p=0·0061; figure 2A; appendix p 62); no other significant differences in urinary total NNAL between groups were observed at this timepoint (332·43 pg/mg creatinine [95% CI 254·31–437·78] in the 0 mg/mL nicotine group; 271·21 pg/mg creatinine [95% CI 196·99–377·36] in the 8 mg/mL nicotine group) or any other timepoint. For the ENDS with 36 mg/mL nicotine group, compared with baseline (389·13 pg/mg creatinine [95% CI 305·93–497·90]), NNAL decreased significantly at 12 weeks (238·96 pg/mg creatinine [188·35–304·93]; p=0·0014) and 24 weeks (210∙80 pg/mg creatinine [95% CI 163·03–274·42]; p=0·0005; appendix p 72). After adjusting for relevant covariates, these significant differences (in the 36 mg/mL group vs cigarette substitute and 36 mg/mL group vs baseline) persisted (appendix pp 14, 62, 72). Sensitivity analyses were largely consistent with the multiple imputation analyses. In unadjusted models for the intention-to-treat, per-protocol, and baseline-carried-forward analyses and unadjusted and adjusted models for last-observation-carried-forward analyses, total NNAL at 24 weeks did not differ between the ENDS with 36 mg/mL nicotine and cigarette substitute groups. For the adjusted model for the baseline-carried-forward analysis and unadjusted and adjusted models for the last-observation-carried-forward analyses, the ENDS with 36 mg/mL nicotine group had significantly lower urinary total NNAL than the 0 mg/mL group at 24 weeks (appendix pp 15–16, 68–71). There were no significant differences at any timepoint between groups for urinary cotinine (figure 2B; appendix p 82). For the ENDS with 8 mg/mL nicotine group, compared with baseline (1729·53 ng/mg creatinine [95% CI 1423·04–2102·03]), urinary cotinine significantly decreased at 4 weeks (1279·92 ng/mg creatinine [1045·16–1567·42]; p=0·0054), 12 weeks (1092·94 ng/mg creatinine [876·82–1362·31]; p=0·0009), and 24 weeks (1157·95 ng/mg creatinine [910·95–1471·93]; p=0·0099; appendix p 92). After adjusting for relevant covariates, reductions compared with baseline for the ENDS with 8 mg/mL nicotine group persisted, and one additional significant reduction compared with baseline was observed at 12 weeks for the cigarette substitute group (appendix pp 17, 92). Sensitivity analyses were relatively consistent, with significant reductions compared with baseline for the ENDS with 8 mg/mL nicotine group observed at varying timepoints in unadjusted and adjusted models (appendix pp 93–96). For the adjusted models for the intention-to-treat and baseline-carried-forward analyses at 4 weeks, the ENDS with 8 mg/mL nicotine group had significantly lower cotinine than the 36 mg/mL group (appendix pp 18–19, 93, 95).
Figure 2: Biomarkers of tobacco-related exposure.
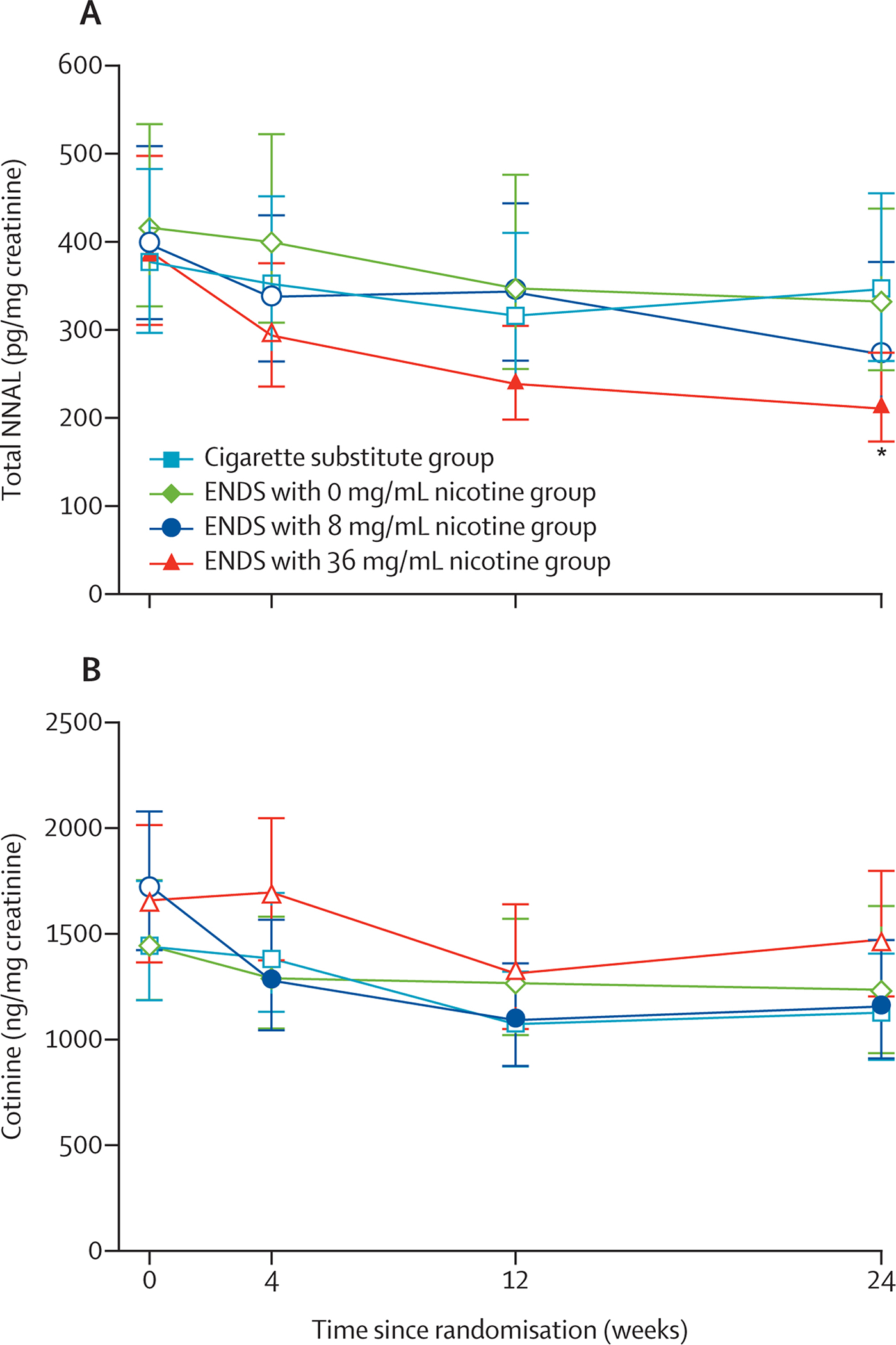
(A) Urinary total NNAL over time. (B) Urinary cotinine over time. ENDS=electronic nicotine delivery system. NNAL=4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol. Analyses were unadjusted. Estimates with 95% CI were obtained via back-transformation to the original scale following Box-Cox transformation and multiple imputation. Filled symbols indicate a significant difference relative to week 0 within that group (Bonferroni correction α=0·017; three comparisons with week 0 were done for each group). Six between-group comparisons were done at each timepoint (Bonferroni correction α=0·0083); asterisks indicate a significant difference relative to the cigarette substitute group for that group at that timepoint.
Significant differences between groups were observed at every post-randomisation timepoint for exhaled CO (figure 3A, appendix p 102). Exhaled CO in the ENDS with 8 mg/mL nicotine group was significantly lower than in the cigarette substitute group at 1, 2, 4, 8, 16, and 20 weeks and lower than in the 0 mg/mL group at 1, 2, 4, 12, 16, and 20 weeks (all p values <0·0083); estimates at 20 weeks were 16·56 ppm (95% CI 14·21–18·91) in the ENDS with 8 mg/mL nicotine group versus 20·71 ppm (18·78–22·65) in the cigarette substitute group and 21·02 ppm (18·74–23·29) in the 0 mg/mL group. Exhaled CO in the ENDS with 36 mg/mL nicotine group was significantly lower than in the cigarette substitute group at 1, 4, 8, 16, 20, and 24 weeks and lower than in the 0 mg/mL group at 4, 12, 16, 20, and 24 weeks (all p values <0·0083); estimates at 24 weeks were 16·93 ppm (14·50–19·35) in the 36 mg/mL group versus 20·79 ppm (18·67–22·90) in the cigarette substitute group and 21·31 ppm (19·27–23·36) in the 0 mg/mL group. Within groups, exhaled CO was significantly lower for the ENDS with 8 mg/mL nicotine and 36 mg/mL groups at all post-randomisation timepoints compared with baseline, with the exception of at 24 weeks for the 8 mg/mL group (p values <0·003; appendix p 117). A significant decrease in exhaled CO compared with baseline also was observed for the 0 mg/mL group at 2 weeks (p=0·0062). After adjusting for relevant covariates, trends in exhaled CO between and within groups were relatively consistent, but there were several additional significant reductions within the ENDS with 0 mg/mL nicotine and cigarette substitute groups compared with baseline (appendix pp 20, 103, 117). Sensitivity analyses had similar overall trends, with some variation in the significance of differences between the ENDS with 36 mg/mL nicotine group vs 0 mg/mL and vs cigarette substitute groups at 24 weeks (appendix pp 21–22, 105–116, 118–120).
Figure 3: Exhaled CO and cigarettes smoked per day.

(A) Exhaled CO over time. (B) Number of cigarettes smoked per day over time (average of the previous 7 days). ENDS=electronic nicotine delivery system. ppm=parts per million. Analyses were unadjusted and represent estimated means with 95% CI following multiple imputation. Filled symbols indicate a significant difference relative to week 0 within that group (Bonferroni correction α=0·0063; eight comparisons with week 0 were done for each group). Six between-group comparisons were done at each timepoint (Bonferroni correction α=0·0083); asterisks indicate a significant difference relative to the cigarette substitute group for that group at that timepoint; number signs indicate a significant difference relative to the 0 mg/mL group for that group at that timepoint.
Significant differences between groups in number of cigarettes smoked per day were observed at every post-randomisation timepoint (figure 3B; appendix p 127). The number of cigarettes smoked per day in the ENDS with 36 mg/mL nicotine group was significantly lower than in the cigarette substitute group at 1–24 weeks (p values <0·0003); estimates at 24 weeks were 7·43 cigarettes per day (95% CI 6·14–8·71) in the ENDS with 36 mg/mL nicotine group versus 11·23 (9·76–12·71) in the cigarette substitute group. The number of cigarettes smoked per day in the ENDS with 0 mg/mL nicotine group was significantly lower than in the cigarette substitute group at 4, 8, and 16 weeks (p values <0·0083); estimates at 16 weeks were 9·26 cigarettes per day (7·92–10·60) in the ENDS with 0 mg/mL nicotine group versus 11·77 (10·46–13·08) in the cigarette substitute group. At 4 weeks, the number of cigarettes smoked per day was significantly lower in the 8 mg/mL group (10·41 [95% CI 9·11–11·71]) than in the cigarette substitute group (13·26 [12·03–14·50]; p=0·0021). Within all groups, the number of cigarettes smoked per day significantly decreased over time compared with baseline (p values <0·0001; appendix p 142). After adjusting for relevant covariates, the numbers of cigarettes smoked per day in all ENDS groups were significantly lower than in the cigarette substitute group at all post-randomisation timepoints, with the exception of the ENDS with 8 mg/mL nicotine group at 12 weeks (p values <0·0083; appendix pp 23, 128). Sensitivity analyses showed similar patterns, with stronger effects noted for nicotine-containing ENDS groups compared with the cigarette substitute group in unadjusted models; following adjustment, there were more between-group differences for all ENDS groups compared with the cigarette substitute group (appendix pp 24–25, 130–141, 143–146).
Study product use (ENDS or cigarette substitute) declined significantly relative to baseline in all groups by the 12-week visit across all analysis methods (p values <0·0071; appendix pp 26, 153–155). When individuals with missing data for each visit were assumed not to be using study product, few differences between groups emerged, but when analyses were restricted to only available data or individuals who attended visits at baseline and 4, 12, and 24 weeks, study product use rates were significantly greater for ENDS groups, particularly the 36 mg/mL nicotine group, compared with the cigarette substitute group (p values <0·0083; appendix pp 150–152).
Serious adverse events and associated withdrawals from the study were similar in frequency between groups over 36 weeks: 13 events (13 participants [10%], two withdrawals) in the cigarette substitute group; 11 events (ten participants [8%], four withdrawals) in the ENDS with 0 mg/mL nicotine group; seven events (six participants [5%], two withdrawals) in the 8 mg/mL group; and 12 events (12 participants [9%], one withdrawal) in the 36 mg/mL group (appendix pp 167–168). All serious adverse events were deemed unrelated or unlikely to be related to study product use. One participant died (0 mg/mL group; suicide). The frequency of adverse events graded as severe or higher was also similar across groups over 36 weeks: 26 events (22 participants [17%]) in the cigarette substitute group; 34 events (24 participants [18%]) in the ENDS 0 mg/mL nicotine group; 22 events (16 participants [12%]) in the 8 mg/mL group; and 31 events (25 participants [19%]) in the 36 mg/mL group (appendix p 167). Only four severe adverse events were judged as possibly, probably, or definitely related to study product use: hypertension (0 mg/mL group), dyspnoea (8 mg/mL group), cough and syncope (8 mg/mL group), and cough (36 mg/mL group; appendix p 168–171). The most common severe or life-threatening adverse events were hypertension (37 events [32 participants], hypertriglyceridaemia (12 events [12 participants]), pneumonia (five events [five participants]), and depression (five events [four participants]).
Discussion
Provision of an ENDS with a cigarette-like nicotine delivery profile (36 mg/mL liquid nicotine)13 was associated with reduced urinary total NNAL, exhaled CO, and cigarette consumption over 24 weeks among smokers who were encouraged to reduce their smoking. Participants who received an ENDS with 8 mg/mL liquid nicotine showed some similar effects but not to the same extent, highlighting the importance of ENDS nicotine delivery capability. In the 0 mg/mL and cigarette substitute groups there was little change in tobacco-related toxicant exposure. Study product use declined during the intervention period but did not differ significantly between ENDS groups. Serious adverse events that occurred during the trial were judged not related or not likely to be related to study product use. These findings are consistent with previous randomised, controlled trial results regarding the ability of ENDS to reduce tobacco smoke toxicant exposure with few safety concerns.6–9
To our knowledge, the demonstration of ENDS nicotine delivery profile or dose-related effects on toxicant exposure is unique to this study. Greater effects for nicotine-containing ENDSs compared with placebo ENDSs have been noted among other randomised trials,3,4,25,26 but only two have tested differences in tobacco smoke exposure and behaviour between two active ENDS nicotine concentrations, with few or no significant differences noted.10,27 The use of ENDS conditions with known nicotine delivery profiles strengthens our study conclusions regarding nicotine dose-related effects similar to that observed for nicotine replacement therapy for cessation-related outcomes28 (ie, benefits of combination nicotine replacement therapy using the patch and a fast-acting form and higher-dose [21 mg] nicotine patches). Our findings also highlight the importance of characterising ENDS nicotine delivery before testing in a trial. Importantly, because ENDS nicotine delivery is a function of the interaction between device design, liquid constituents, and user behaviour,29 simply putting 36 mg/mL liquid nicotine into any ENDS is not sufficient to ensure a cigarette-like nicotine delivery profile. Indeed, some ENDS device and liquid combinations might use lower or higher power, or lower or higher liquid nicotine concentration, to obtain what this study reveals as a potentially crucial component of using ENDSs effectively as a harm-reduction strategy: cigarette-like nicotine delivery.
A limitation of our study was participant attrition. To mitigate bias, we used multiple imputation,30 did sensitivity analyses to detect any underlying patterns in missing data, and examined the influence of adjustment for baseline covariates. Another limitation was that study product use declined during the trial, and use of other nicotine or tobacco products during the intervention was discouraged but possible. However, we asked about product use at each visit and did not penalise non-compliance. In addition, as previous ENDS use was not an exclusion criterion, it is possible that participants assigned to an ENDS might have guessed their condition assignment, particularly in the 0 mg/mL nicotine group due to the absence of oral or throat sensations or other physiological effects. Perceived condition-related effects or absence of such effects could have influenced tobacco use behaviours and related toxicant exposure. Individuals assigned to ENDS conditions were also restricted to tobacco or menthol flavoured liquid for the duration of the trial; inability to switch or vary liquid flavours as well as other less user-friendly features of the ENDS or cartomiser used might have decreased the likelihood for these study products to substitute for cigarettes most effectively. There was inconsistency between self-reported cigarette consumption, which was lower in all groups, and urinary total NNAL (lower in the 36 mg/mL nicotine group only) and exhaled CO (primarily lower in the 8 mg/mL and 36 mg/mL groups). Nonetheless, sustained reductions in cigarette smoking among those assigned to 36 mg/mL (58% reduction at week 24 compared with baseline; 7·43 cigarettes per day at week 24 vs 17·76 at baseline) were accompanied by significant reductions in urinary total NNAL (46% reduction; 210∙80 pg/mg creatinine at week 24 vs 389·13 pg/mg creatinine at baseline) and exhaled CO (23% reduction; 16·93 ppm at week 24 vs 21·94 ppm at baseline). Thus, despite participant attrition and reduced study product use over time, condition-related toxicant exposure reductions persisted. The participants in this trial also attended numerous visits and received encouragement to reduce their cigarette smoking. It is therefore unclear if these results would also apply to smokers using ENDSs for cigarette reduction without such support. Lastly, study participants were daily smokers who were interested in smoking reduction but not quitting, and they did not have unstable medical or psychiatric conditions. Given that smokers are disproportionately affected by mental health problems, these population characteristics might limit generalisability to the wider population of smokers.
There are concerns from some in the public health and scientific community that many smokers who try ENDSs continue to smoke cigarettes at the same time, referred to as dual use. One of the main concerns is that dual use might result in greater exposure to nicotine and other harmful toxicants found in cigarette smoke and ENDS aerosol. Our results are somewhat reassuring on this point, as we found no evidence of greater nicotine, NNAL, exhaled CO exposure, or serious adverse events among participants practicing dual use while trying to reduce their cigarette consumption.
In this trial, where smoking reduction was encouraged over 24 weeks with multiple visits and assessments, we found that an ENDS was most likely to help smokers reduce toxicant exposure and cigarette consumption when it was capable of delivering nicotine at levels similar to that of a cigarette. The pattern of adverse events and toxicant exposure suggests there is little basis for concern regarding the use of these ENDS and liquid nicotine combinations over 24 weeks, even when participants also smoked cigarettes. Future ENDS trials should involve products with well characterised nicotine delivery, including ENDSs with nicotine delivery approaching that of a cigarette.
Supplementary Material
Research in context.
Evidence before this study
A systematic search of all available electronic nicotine delivery system (ENDS) literature was done by the Committee on the Review of the Health Effects of Electronic Nicotine Delivery Systems organised by the National Academies of Sciences, Engineering, and Medicine in 2017. On the subject of harm reduction, the committee identified studies that prospectively evaluated changes in health outcomes (including toxicant exposure) among smokers who completely switched from cigarettes to ENDS use (15 longitudinal observational studies or crossover experimental studies) and among concurrent cigarette and ENDS users (two longitudinal observational studies, one laboratory study, and one cross-sectional study). The committee concluded that there was conclusive evidence that use of an ENDS as complete substitute for cigarettes reduces exposure to cigarette-associated toxicants and carcinogens. Dual cigarette and ENDS use was not associated with smoking fewer cigarettes compared with smoking cigarettes only, but there was some indication that ENDS use could help maintain smoking reduction. To add to this previous evidence, we sought to identify studies that more closely approximated our study design (randomised controlled trials) and evaluated toxicant exposure and smoking behaviour outcomes. On Sept 25, 2020, we searched MEDLINE using the same keywords as the previous committee search: “e-cig*”, “ecig*”, “electronic nicotine*”, “electronic cigarette*”, “vape*”, “vaper*”, “vaping*”, and “e-liquid*” with the additional criteria of “randomized controlled trial” (publication type).
We identified 137 articles, of which 19 were trials that involved ENDS provision under non-acute or laboratory conditions and assessed tobacco-related toxicant exposure or smoking behaviour. Few of the studies involved large samples (four studies had >500 participants) or extended ENDS provision (eight studies had ≥12 weeks), and among this subset, all studies measured changes in smoking behaviour but only one reported ENDS-associated changes in tobacco-related toxicant exposure other than exhaled CO. In this latter trial, daily smokers were randomly assigned to use either a 3·0–4·2 voltage ENDS loaded with 2·0% nicotine cartridges (and asked to avoid cigarettes, although smoking was permitted) or their preferred brand of cigarettes for 12 weeks with periodic in-person assessments. Urinary total 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) was significantly lower in the ENDS group compared with the cigarette group at 4, 8, and 12 weeks of follow-up. Compliance in the ENDS group was relatively high with 123 (40%) of 306 participants meeting the criteria of reporting no cigarettes smoked via diary and having exhaled CO ≤8 ppm) on 80% or more of study days. Thus, this report showed that use of a nicotine-containing ENDS was associated with reduced exposure to tobacco-related toxicants among smokers who were fairly intervention adherent. Across many randomised trials, ENDS use has been associated with smoking cessation or reduction and with decreases in exhaled CO. Several reports aimed to address the role of ENDS nicotine content on measures of smoking behaviour change and exposure with some suggestion that a nicotine-containing ENDS can increase rates of smoking cessation or reduction compared with a nicotine-free or placebo ENDS. Often these comparisons have not found differences by ENDS nicotine content. Crucially, there are few data on nicotine delivery profile for the majority of nicotine-containing ENDSs that have been tested in these trials, which further challenges the interpretation of the role of ENDS nicotine content.
Added value of this study
This study addresses the need for a large-scale randomised, controlled trial of an ENDS with known nicotine delivery capability. Our study design builds on specific gaps in the literature including the use of an extended intervention period (24 weeks), multiple tobacco-related biomarkers of exposure (urinary total NNAL, urinary cotinine, and exhaled CO), ENDS conditions with varying nicotine delivery profile, and a non-nicotine and non-aerosol control condition (cigarette substitute). Our ability to make comparisons between groups and across time on multiple outcome measures strengthens our conclusion that an ENDS with cigarette-like nicotine delivery is more effective than an ENDS with lower nicotine in reducing tobacco-related toxicant exposure or cigarette consumption. This information is crucial to inform future studies and randomised trials aiming to test ENDS-related effectiveness in smokers and for regulators considering the effect of restrictions on ENDS-related nicotine content or delivery.
Implications of all the available evidence
Previous trials and our findings indicate that ENDSs with nicotine can help some smokers cease or reduce their smoking and associated toxicant exposure. This study suggests that an ENDS with cigarette-like nicotine delivery might provide greater benefits than an ENDS with lower nicotine delivery for smokers interested in behaviour change under specific conditions. Future studies should use ENDSs with well characterised nicotine delivery to allow for more informed public health and regulatory recommendations.
Acknowledgments
This research was supported by grants P50DA036105 and U54DA036105 from the National Institute on Drug Abuse of the National Institutes of Health and the Center for Tobacco Products of the US Food and Drug Administration. Data collection was supported by UL1TR002649 at Virginia Commonwealth University and by UL1TR002014 at Penn State University from the National Center for Advancing Translational Sciences of the National Institutes of Health. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the US Food and Drug Administration. We thank all the study participants, students, fellows, faculty, and staff members at Virginia Commonwealth University and Penn State University who were involved in this study. A full list of acknowledgments is provided in the appendix.
Funding
National Institutes of Health, US Food and Drug Administration.
Footnotes
Declaration of interests
COC reports grants from National Institute on Drug Abuse and US Food and Drug Administration, during the conduct of the study. JF reports grants from National Institute on Drug Abuse and US Food and Drug Administration, during the conduct of the study, and grants, personal fees, and non-financial support from Pfizer, outside of the submitted work. AAL reports grants from National Institute on Drug Abuse and US Food and Drug Administration, during the conduct of the study. JMY reports grants from National Institute on Drug Abuse and US Food and Drug Administration, during the conduct of the study. LK reports grants from National Institute on Drug Abuse and US Food and Drug Administration, during the conduct of the study. SV reports grants from National Institute on Drug Abuse and US Food and Drug Administration, during the conduct of the study. CB has previously undertaken trials of electronic cigarettes for smoking cessation (with electronic cigarettes purchased from an online retailer [NZVAPOR], electronic cigarette liquid for one trial purchased from Nicopharm, Australia, and nicotine patches supplied by the New Zealand Government via their contract with Novartis [Sydney, Australia]). Neither NZVAPOR nor Nicopharm have links with the tobacco industry. None of these parties had any role in the design, conduct, analysis, or interpretation of the trial findings, or writing of this publication. TE reports grants from National Institute on Drug Abuse and US Food and Drug Administration, during the conduct of the study, and is a paid consultant in litigation against the tobacco industry and also the electronic cigarette industry and is named on one patent for a device that measures the puffing behavior of electronic cigarette users and on another patent for a smartphone application that determines electronic cigarette device and liquid characteristics. M-SY reports grants from the National Institute on Drug Abuse and the US Food and Drug Administration, during the conduct of the study.
Data sharing
Upon publication, requests for deidentified individual participant data or study documents (eg, data dictionary; protocol; statistical analysis plan; measures, manuals, or informed consent documentation) will be considered. The requestor must submit a one-page abstract of their proposed research, including the purpose, analytical plan, and dissemination plans. The Executive Leadership Committee (Virginia Commonwealth University and Penn State University) will review the abstract and decide on the basis of the individual merits. Review criteria and prioritisation of projects include potential of the proposed work to advance public health, qualifications of the applicant, the potential for publication, the potential for future funding, and enhancing the scientific, geographic, and demographic diversity of the research portfolio. Following abstract approval, requestors must receive institutional ethics approval or confirmation of exempt status for the proposed research. An executed data use agreement must be completed before data distribution. Requests should be made to the corresponding author.
See Online for appendix
Contributor Information
Caroline O Cobb, Department of Psychology, Virginia Commonwealth University, Richmond, VA, USA; Center for the Study of Tobacco Products, Virginia Commonwealth University, Richmond, VA, USA.
Jonathan Foulds, Center for Research on Tobacco and Health, Penn State University College of Medicine, Hershey, PA, USA; Department of Public Health Sciences, Penn State University College of Medicine, Hershey, PA, USA.
Miao-Shan Yen, Department of Biostatistics, Virginia Commonwealth University, Richmond, VA, USA; Center for the Study of Tobacco Products, Virginia Commonwealth University, Richmond, VA, USA.
Susan Veldheer, Center for Research on Tobacco and Health, Penn State University College of Medicine, Hershey, PA, USA; Department of Public Health Sciences, Penn State University College of Medicine, Hershey, PA, USA; Department of Family and Community Medicine, Penn State University College of Medicine, Hershey, PA, USA.
Alexa A Lopez, University of Wisconsin-Milwaukee, Milwaukee, WI, USA.
Jessica M Yingst, Center for Research on Tobacco and Health, Penn State University College of Medicine, Hershey, PA, USA; Department of Public Health Sciences, Penn State University College of Medicine, Hershey, PA, USA.
Christopher Bullen, University of Auckland, Auckland, New Zealand.
Le Kang, Department of Biostatistics, Virginia Commonwealth University, Richmond, VA, USA.
Thomas Eissenberg, Department of Psychology, Virginia Commonwealth University, Richmond, VA, USA; Center for the Study of Tobacco Products, Virginia Commonwealth University, Richmond, VA, USA.
References
- 1.Breland A, Soule E, Lopez A, Ramôa C, El-Hellani A, Eissenberg T. Electronic cigarettes: what are they and what do they do? Ann N Y Acad Sci 2017; 1394: 5–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Population Health and Public Health Practice; Committee on the Review of the Health Effects of Electronic Nicotine Delivery Systems. Public health consequences of e-cigarettes. Washington, DC: National Academies Press, 2018. [Google Scholar]
- 3.Bullen C, Howe C, Laugesen M, et al. Electronic cigarettes for smoking cessation: a randomised controlled trial. Lancet 2013; 382: 1629–37. [DOI] [PubMed] [Google Scholar]
- 4.Walker N, Parag V, Verbiest M, Laking G, Laugesen M, Bullen C. Nicotine patches used in combination with e-cigarettes (with and without nicotine) for smoking cessation: a pragmatic, randomised trial. Lancet Respir Med 2020; 8: 54–64. [DOI] [PubMed] [Google Scholar]
- 5.Hajek P, Phillips-Waller A, Przulj D, et al. A randomized trial of e-cigarettes versus nicotine-replacement therapy. N Engl J Med 2019; 380: 629–37. [DOI] [PubMed] [Google Scholar]
- 6.D’Ruiz CD, Graff DW, Robinson E. Reductions in biomarkers of exposure, impacts on smoking urge and assessment of product use and tolerability in adult smokers following partial or complete substitution of cigarettes with electronic cigarettes. BMC Public Health 2016; 16: 543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cravo AS, Bush J, Sharma G, et al. A randomised, parallel group study to evaluate the safety profile of an electronic vapour product over 12 weeks. Regul Toxicol Pharmacol 2016; 81 (suppl 1): S1–14. [DOI] [PubMed] [Google Scholar]
- 8.Round EK, Chen P, Taylor AK, Schmidt E. Biomarkers of tobacco exposure decrease after smokers switch to an e-cigarette or nicotine gum. Nicotine Tob Res 2019; 21: 1239–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hatsukami DK, Meier E, Lindgren BR, et al. A randomized clinical trial examining the effects of instructions for electronic cigarette use on smoking-related behaviors and biomarkers of exposure. Nicotine Tob Res 2020; 22: 1524–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carpenter MJ, Heckman BW, Wahlquist AE, et al. A naturalistic, randomized pilot trial of e-cigarettes: uptake, exposure, and behavioral effects. Cancer Epidemiol Biomarkers Prev 2017; 26: 1795–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yingst JM, Foulds J, Veldheer S, et al. Nicotine absorption during electronic cigarette use among regular users. PLoS One 2019; 14: e0220300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vansickel AR, Cobb CO, Weaver MF, Eissenberg TE. A clinical laboratory model for evaluating the acute effects of electronic “cigarettes”: nicotine delivery profile and cardiovascular and subjective effects. Cancer Epidemiol Biomarkers Prev 2010; 19: 1945–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hiler M, Breland A, Spindle T, et al. Electronic cigarette user plasma nicotine concentration, puff topography, heart rate, and subjective effects: influence of liquid nicotine concentration and user experience. Exp Clin Psychopharmacol 2017; 25: 380–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lopez AA, Cobb CO, Yingst JM, et al. A transdisciplinary model to inform randomized clinical trial methods for electronic cigarette evaluation. BMC Public Health 2016; 16: 217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bauer JE, Carlin-Menter SM, Celestino PB, Hyland A, Cummings KM. Giving away free nicotine medications and a cigarette substitute (Better Quit) to promote calls to a quitline. J Public Health Manag Pract 2006; 12: 60–67. [DOI] [PubMed] [Google Scholar]
- 16.Goniewicz ML, Havel CM, Peng MW, et al. Elimination kinetics of the tobacco-specific biomarker and lung carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol. Cancer Epidemiol Biomarkers Prev 2009; 18: 3421–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shah KA, Peoples MC, Halquist MS, Rutan SC, Karnes HT. Microfluidic direct injection method for analysis of urinary 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) using molecularly imprinted polymers coupled on-line with LC-MS/MS. J Pharm Biomed Anal 2011; 54: 368–78. [DOI] [PubMed] [Google Scholar]
- 18.Cappendijk SL, Pirvan DF, Miller GL, et al. In vivo nicotine exposure in the zebra finch: a promising innovative animal model to use in neurodegenerative disorders related research. Pharmacol Biochem Behav 2010; 96: 152–59. [DOI] [PubMed] [Google Scholar]
- 19.Foulds J, Veldheer S, Yingst J, et al. Development of a questionnaire for assessing dependence on electronic cigarettes among a large sample of ex-smoking E-cigarette users. Nicotine Tob Res 2015; 17: 186–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sobell LC, Sobell MB. Timeline follow-back: a technique for assessing self-reported alcohol consumption. In: Litten RZ, Allen J, eds. Measuring alcohol consumption: psychosocial and biological methods. Totowa, NJ: Humana Press, 1992: 41–72. [Google Scholar]
- 21.Breland AB, Kleykamp BA, Eissenberg T. Clinical laboratory evaluation of potential reduced exposure products for smokers. Nicotine Tob Res 2006; 8: 727–38. [DOI] [PubMed] [Google Scholar]
- 22.Box GEP, Cox DR. An analysis of transformations. J R Stat Soc Series B Stat Methodol 1964; 26: 211–52. [Google Scholar]
- 23.Little R, Rubin D. Statistical analysis with missing data, 2nd edn. Hoboken, NJ: John Wiley & Sons, 2002. [Google Scholar]
- 24.Schafer JL. The analysis of incomplete multivariate data. London: Chapman & Hall, 1997. [Google Scholar]
- 25.Tseng TY, Ostroff JS, Campo A, et al. A randomized trial comparing the effect of nicotine versus placebo electronic cigarettes on smoking reduction among young adult smokers. Nicotine Tob Res 2016; 18: 1937–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Masiero M, Lucchiari C, Mazzocco K, et al. E-cigarettes may support smokers with high smoking-related risk awareness to stop smoking in the short run: preliminary results by randomized controlled trial. Nicotine Tob Res 2019; 21: 119–26. [DOI] [PubMed] [Google Scholar]
- 27.Caponnetto P, Campagna D, Cibella F, et al. Efficiency and safety of an electronic cigarette (ECLAT) as tobacco cigarettes substitute: a prospective 12-month randomized control design study. PLoS One 2013; 8: e66317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lindson N, Chepkin SC, Ye W, Fanshawe TR, Bullen C, Hartmann-Boyce J. Different doses, durations and modes of delivery of nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev 2019; 4: CD013308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shihadeh A, Eissenberg T. Electronic cigarette effectiveness and abuse liability: predicting and regulating nicotine flux. Nicotine Tob Res 2015; 17: 158–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Newgard CD, Lewis RJ. Missing data: how to best account for what is not known. JAMA 2015; 314: 940–41. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


