Abstract
This study aimed to investigate the function of estrogen receptors (ERs) in myoregeneration and intermuscular adipogenesis. Ovariectomized (OVX) ERα knockout (KO) mice and ERβ KO mice were used to assess the effect of estrogen on the myoregenerative process. Tibialis anterior muscle was collected on days 7, 10, and 14 after cardiotoxin injection to assess myotube morphology and adipogenesis area. Regenerated myotubes from OVX-ERβ KO mice were consistently smaller in diameter than those from OVX-ERα KO and OVX-wild-type mice, whereas the adipogenesis area of OVX-ERβ KO mice was consistently greater than that of the other types. Therefore, ERβ may be an influential factor in promoting myoregeneration and adipogenesis inhibition compared to ERα.
Keywords: adipogenesis, estrogen, estrogen receptors, myoregeneration
Estrogen deficiency in women after menopause or ovariectomy affects not only reproductive dysfunction but also non-reproductive functions such as sarcopenia, obesity and predisposition to develop frailty [17, 25, 28, 31, 33]. So far, there have been several reports that low estrogen status inhibits regeneration of damaged muscle tissue [5, 22, 29]. Skeletal muscles express estrogen receptor α (ERα) and ERβ; however, the functional difference between the two types of ERs remains unclear, especially in the morphology of muscle regeneration. Collins et al. [5] reported that estrogen and muscle satellite cell expression of ERα is necessary to prevent apoptosis of satellite cells. On the other hand, Seko et al. [36] insisted that ERβ knockout (KO) mice exhibited impaired muscle regeneration following acute muscle injury, probably due to reduced proliferation and increased apoptosis of satellite cells. Thus, there are several reports on the involvement of both ERs in muscle tissue regeneration, but it was not concluded which ER action has a strong effect on myoregeneration [2, 9]. Moreover, estrogen has been reported to affect the regulation of adipocyte accumulation in regenerating muscle [6, 12, 29, 32]. When the estrogen concentration decreased, adipogenesis was accelerated in skeletal muscle tissue [3, 15, 18, 21]. However, the effect of ERs in the process of adipogenesis is poorly understood.
In this study, we chemically induced muscle damage in ER KO mice with low estrogen status and morphologically evaluated the effect of estrogen and ERs on myoregeneration and adipose tissue formation.
MATERIALS AND METHODS
Animals
Two subtypes of ERs KO (ERα KO and ERβ KO) and wild-type (WT; C57BL/6) mice were used in this study. ERα KO mice were obtained by mating mice of a mixed C57BL/6 background that were heterozygous for ERα gene disruption, as described previously [10]. Ers2tm1Unc/J, ERβ gene disruption mice were purchased from The Jackson Laboratory (Bar Harbor, MA, USA), and ERβ KO was obtained by mating mice of a mixed C57BL/6 background that were heterozygous [7, 23]. After 28 days of age, genotypes of both subtypes of ERs KO mice were determined by multiplex PCR. Female WT, ERα KO, and ERβ KO mice were fed a commercial diet (CLEA Rodent Diet CE-2; CLEA, Tokyo, Japan) and tap water ad libitum. Animals were kept in an environment of a temperature of 23 ± 1.0°C under 12 hr light/12 hr dark by artificial illumination (lights on from 7 am to 7 pm). The animal experiments were approved by the Animal Research Committee of Tottori University, Japan (approval number: 18-T-37, 32-040).
Animal operation and muscle injury procedure
All mice (aged 8 weeks old) underwent ovariectomy (OVX) under anesthesia by intraperitoneal administration of medetomidine (Kyoritsu Seiyaku, Tokyo, Japan), midazolam (Maruishi, Osaka, Japan), and butorphanol (Meiji Seika, Tokyo, Japan) [20]. OVX mice were divided into 3 groups (n=3): OVX-WT (control), OVX-ERα KO, and OVX-ERβ KO group. Four weeks after OVX (aged 12 weeks), 50 µl of 10 µM cardiotoxin (CTX) (Latoxan, Portes lès Valence, France) was injected into the right tibialis anterior (TA) muscles [13, 14, 27]. The contralateral left TA muscle was left intact and served as the non-injured control. The injured TA muscles were collected on after euthanasia on days 7, 10, and 14 (D7, D10, and D14, respectively) after CTX injection. In addition, the non-injured control (left) TA muscles were also collected at D7. All mice were sacrificed by inhalation of an overdose of isoflurane (MSD Animal Health, Osaka, Japan).
Measurement of estrogen level in serum
To measure the estradiol (E2) concentration in serum, blood was collected from the tail immediately before OVX (8 weeks old) and 4 weeks after OVX treatment (12 weeks old). Separated serum from the blood was stored at −30°C until use. The E2 concentration was measured with ELISA (Estradiol ELISA Kit; Cayman Chemical, Ann Arbor, MI, USA) and we confirmed the mice used in the experiment were in a low estrogen state.
Histological analysis
The collected muscle tissue was immediately immersed in 10% neutral buffered formalin and fixed at room temperature for 16 hr. Then, a series of procedures were performed to embed the tissue in paraffin. Hematoxylin and eosin (HE)-stained and Masson’s trichrome-stained paraffin sections (6 µm thickness) were examined using an inverted light microscope (IX71, Olympus, Tokyo, Japan). Digital images were obtained and used to evaluate morphological changes in muscle fibers, regenerated myotube diameter, adipocyte accumulation and connective tissue deposition.
To evaluate myogenesis, the regenerated muscle region was defined as follows: a region of newly formed myotubes with a central nucleus, residual region of necrotic muscle fibers, and a region of regenerated myotubes of various sizes with a central nucleus. In the myoregenerated region, we randomly selected approximately 150 myotubes (including the center region of the section) per specimen (at 200× magnification), and the minimum diameter of muscle fiber was measured as the axis diameter of the fibers [27, 29]. Adipogenesis area (%) was defined as the area of adipocytes distributed between and among individual regenerated myotubes with central nuclei at all time points post injection or non-injured muscle fibers. For the adipogenesis area (%) calculation, 4 images of non-overlapping areas at 200× magnification were chosen after manual outlining of the intermuscular adipocyte area and dividing by the total area of the image using the image analysis software (ImageJ; v1.46r, National Institutes of Health, Bethesda, MD, USA) [29, 34].
Muscle sections were stained with Masson’s trichrome stain to detect changes in tissue composition after CTX injury and quantify the areas of muscle (red), adipocytes (transparent) and connective tissue (blue). The stained sections were observed at a magnification of 200× with a light microscopy, 4 images of each section, 3 mice at each time point were randomly selected and analyzed with Image J software (National Institutes of Health). After adjusting the threshold, each area was displayed as a percentage of the total area [27].
Assessment of skeletal muscle recovery
The recovery ratio (%) of OVX mice to that of non-injured control was calculated by the average diameter of newly formed myotubes with a central nucleus of OVX mice in each group and divided by the average diameter of muscle fibers of non-injured control at each time point. Recovery ratio (%) of OVX-ER KO mice to compare with OVX-WT mice was calculated by the average diameter of muscle fibers or newly formed myotubes with a central nucleus in each group was divided by the average diameter of OVX-WT mice at each time point. The myoregeneration rate (µm/day) of OVX mice was calculated by the inclination is changed in average diameter of regenerated muscle fibers in each group was divided by the duration period of day after injury.
Statistical analysis
Data were expressed as average ± standard deviation (SD). Obtained data were analyzed with StatView software, version 5.0 (SAS Institute, Cary, NC, USA) using Student’s t-test, two-tailed to compare the E2 concentration in serum. One-way ANOVA followed by Bonferroni’s post-hoc test was used to compare the histometrical data. Significance level was set as P<0.05.
RESULTS
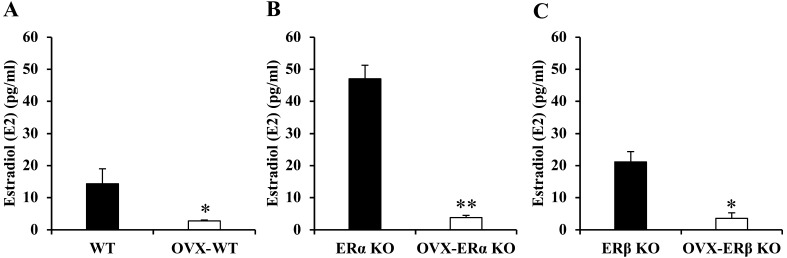
Before OVX treatment of the ERα KO group, E2 concentration in serum was particularly higher than that in the other groups. The E2 concentration in the serum of all groups (three types of OVX mice) 4 weeks after OVX treatment was significantly lower than that before treatment. For this reason, the mice used in the muscle regeneration experiment are in a “low estrogen state” (Fig. 1).
Fig. 1.
The estradiol (E2) concentration in serum before and after ovariectomy (OVX) treatment of all three types of mice. E2 concentration in wild-type (WT), estrogen receptor α knockout (ERα KO) and ERβ KO mice (A, B, C). Data were shown as average ± standard deviation (SD), n=3/groups, * indicates significant difference between group before and after OVX treatment, P<0.05, ** indicates significant difference between groups before and after OVX treatment, P<0.01, Student’s t-test, two-tailed.
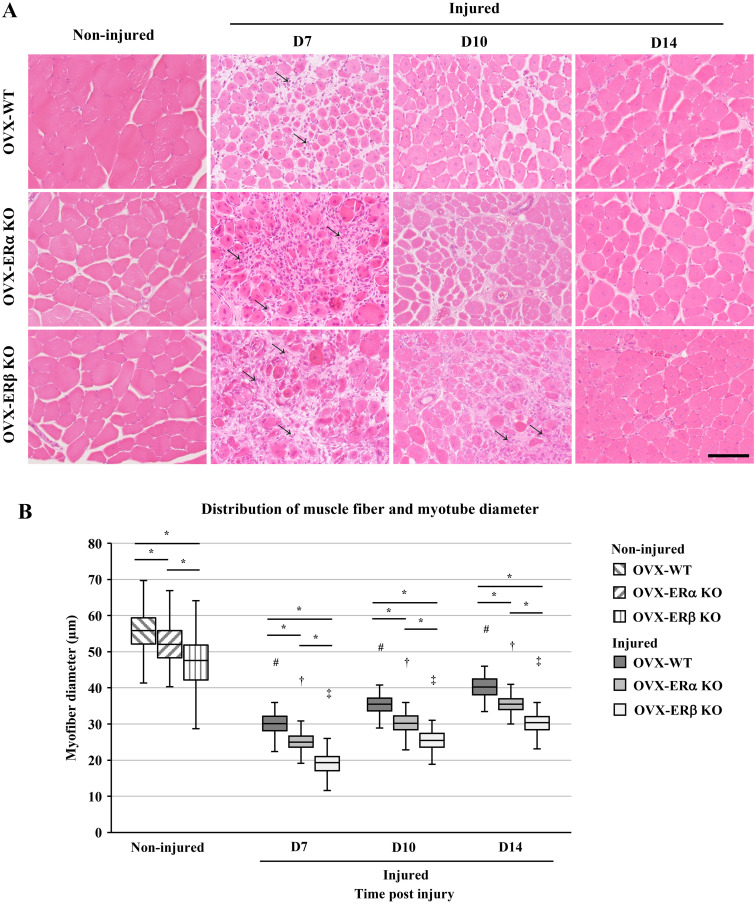
On D7 after CTX treatment, the muscle fibers with a central nucleus appeared, and the start of muscle regeneration was confirmed. Moreover, adipocytes appeared between the regenerated muscle fibers in all mice. That is, muscle regeneration and adipocyte differentiation were observed on the same specimen on D7 (Supplementary Fig. 1). Cell infiltration in connective tissue and regenerated muscle fibers appeared by D7 in all OVX treated mice. However, OVX-ERβ KO mice showed residual necrotic muscle fibers on D7. At D10, OVX-ERβ KO mice showed cell infiltration between the regenerated muscle fibers. Moreover, muscle fibers of all three types of OVX mice on D14 appeared to be well regenerated when compared with non-injured muscle fibers (Fig. 2A).
Fig. 2.
Regeneration of muscle in ovariectomy (OVX) treated wild-type (WT), estrogen receptor α knockout (ERα KO) and ERβ KO mice by cardiotoxin (CTX) injection. (A) Sections of the tibialis anterior (TA) muscle of non-injured control of OVX-WT mice, OVX-ERα KO and OVX-ERβ KO. Sections of the TA muscle injected with CTX at day 7, 10 and 14 (D7, D10 and D14) post injection. Arrows indicate cell infiltration among regenerated muscle fibers. (B) Distribution of non-injured muscle fiber diameter and newly formed muscle fiber diameter at D7, D10 and D14 post CTX injection. Data are expressed as average ± standard deviation (SD), # indicates significant differences from non-injured control in OVX-WT mice, † indicates significant differences from non-injured control in OVX-ERα KO mice, ‡ indicates significant differences from non-injured control in OVX-ERβ KO mice, P<0.01, * indicates significant difference between groups at time post injury, P<0.01. Scale bar=100 µm.
When comparing the diameters of muscle fibers of the non-injured control, there was a significant difference between each group, and the diameters of non-injured control of OVX-ERα KO mice and -ERβ KO mice were significantly smaller than those of non-injured control of OVX-WT. Moreover, when comparing the ER KO mice, OVX-ERβ KO mice showed a significantly lower value than that of OVX-ERα KO mice (Fig. 2B). Throughout the 14 days of the experimental period after CTX treatment, the diameter of newly formed myotubes increased over time in all groups. The average diameter of the myotube at D14 was OVX-WT mice: 40.2 ± 2.83 µm, OVX-ERα KO mice: 35.4 ± 2.69 µm and OVX-ERβ KO mice: 30.2 ± 2.81 µm, with a recovery ratio from 65.4 ± 0.37% to 71.5 ± 0.92% of the average diameter of non-injured control in each group.
The ratio of the average diameter of muscle fibers in each group to non-injured control at each time point is shown in Table 1. The value of OVX-ERβ KO group was significantly lower than those of OVX-WT and OVX-ERα KO group, respectively, but all values tended to increase. On the other hand, Table 2 shows the ratio (%) of the average diameter of muscle fibers in each group to the average diameter of OVX-WT mice at each time point. From D7 to D14, the value of OVX-ERα KO mice changed from 83.5 ± 1.39% to 88.0 ± 0.50%, and the value of OVX-ERβ KO mice changed from 63.6 ± 1.47% to 75.2 ± 0.42%. In both groups, their values increased with the passage of days. However, the value of OVX-ERβ KO mice group was significantly lower than OVX-ERα KO mice. Table 3 shows the rate of increase in muscle fiber diameter during the 7 days (first half period: D7 to D10, second half period: D10 to D14) after injury, that is, repair speed of regenerated muscle fibers. At the same duration period, there was no significant difference between groups, but OVX-ERβ KO mice was higher than the other groups on first half period and OVX-ERα KO mice was higher than the other groups on second half period. Of the three groups, the OVX-ERβ KO mice indicated the highest value through the period (D7 to D14).
Table 1. Ratio (%) of the average diameter of muscle fibers in each group to the average diameter of non-injured control at each time point.
| Type of mice | Time post injury |
||
|---|---|---|---|
| D7 | D10 | D14 | |
| OVX-WT | 53.4 ± 0.08 | 62.9 ± 1.57 | 71.5 ± 0.92 |
| OVX-ERα KO | 49.1 ± 0.82** | 58.8 ± 1.16* | 69.1 ± 0.39** |
| OVX-ERβ KO | 41.5 ± 0.96**,‡ | 55.0 ± 1.04**,† | 65.4 ± 0.37**,‡ |
Average ratio percentage values of each group were compared using the Bonferroni/Dunn post-hoc tests. Average ± standard deviation; n=3. *P<0.05, **P<0.01 vs. OVX-WT, respectively, †P<0.05, ‡P<0.01 vs. OVX-ERα KO, respectively. D, day; OVX, ovariectomized; WT, wild-type; ER, estrogen receptor; KO, knockout.
Table 2. Ratio (%) of the average diameter of muscle fibers in each group to the average diameter of wild-type mice at each time point.
| Type of mice | Non-injured | Time post injury |
||
|---|---|---|---|---|
| OVX | ||||
| D7 | D10 | D14 | ||
| ERα KO | 90.9 ± 1.06 | 83.5 ± 1.39 | 85.0 ± 1.68 | 88.0 ± 0.50 |
| ERβ KO | 82.1 ± 1.09** | 63.6 ± 1.47** | 71.7 ± 1.35** | 75.2 ± 0.42** |
Data were shown as average ratio percentage ± standard deviation. ** indicates significant difference from ERα KO mice, P<0.01. OVX, ovariectomized; D, day; ER, estrogen receptor; KO, knockout.
Table 3. Repair speed (µm/day) of regenerated muscle fibers in each group at several duration periods of days after injury.
| Type of mice | Duration period |
||
|---|---|---|---|
| D7 to D10 | D10 to D14 | D7 to D14 | |
| OVX-WT | 1.79 ± 0.29 | 1.20 ± 0.13 | 1.45 ± 0.07 |
| OVX-ERα KO | 1.66 ± 0.20 | 1.32 ± 0.05 | 1.47 ± 0.03 |
| OVX-ERβ KO | 2.08 ± 0.16 | 1.21 ± 0.04 | 1.58 ± 0.02 |
Data were shown as average/day ± standard deviation. D, day; OVX, ovariectomized; WT, wild-type; ER, estrogen receptor; KO, knockout.
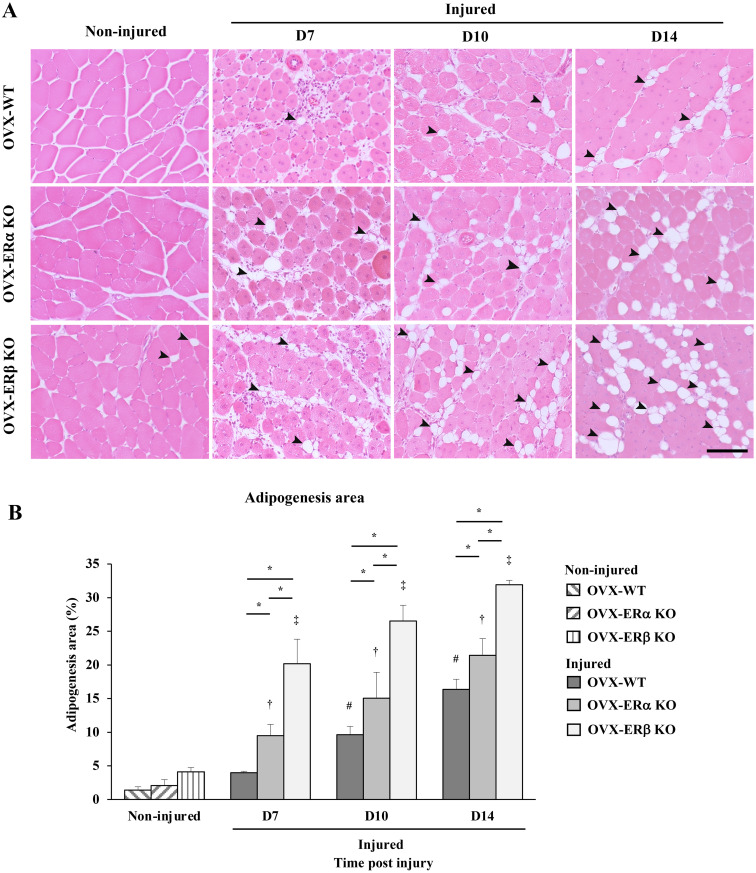
Intermuscular adipocytes appeared among the regenerated myotubes in OVX mice at D7. Adipocyte differentiation was observed within the regenerated muscle in OVX mice at D14 (Fig. 3A). The adipogenesis area (%) in OVX-ERβ KO mice was the largest compared to other types at all time points. At D14, the adipogenesis area in OVX-ERβ KO mice was about 1.49 and 1.95 times higher than that in OVX-ERα KO and OVX-WT mice, respectively (Fig. 3B).
Fig. 3.
Intermuscular adipogenesis in ovariectomy (OVX) treated wild-type (WT), estrogen receptor α knockout (ERα KO) and ERβ KO mice after cardiotoxin (CTX) injection. (A) Sections of the non-injured tibialis anterior (TA) muscle of OVX-WT mice, OVX-ERα KO mice and OVX-ERβ KO mice. Sections of the TA muscle injected with CTX in OVX-WT mice, OVX-ERα KO mice and OVX-ERβ KO mice at day 7, 10 and 14 (D7, D10 and D14) post injection. Arrow heads indicate adipocytes among non-injured muscle fibers and regenerated muscle fibers. (B) Adipogenesis area (%) of all three types of non-injured OVX mice and OVX mice injected CTX at D7, D10 and D14 post injection. Data are expressed as average ± standard deviation (SD), # indicates significant differences from non-injured control in OVX-WT mice, † indicates significant differences from non-injured in OVX-ERα KO mice, ‡ indicates significant differences from non-injured control in OVX-ERβ KO mice, P<0.05, * indicates significant difference between groups at time post injury, P<0.05. Scale bar=100 µm.
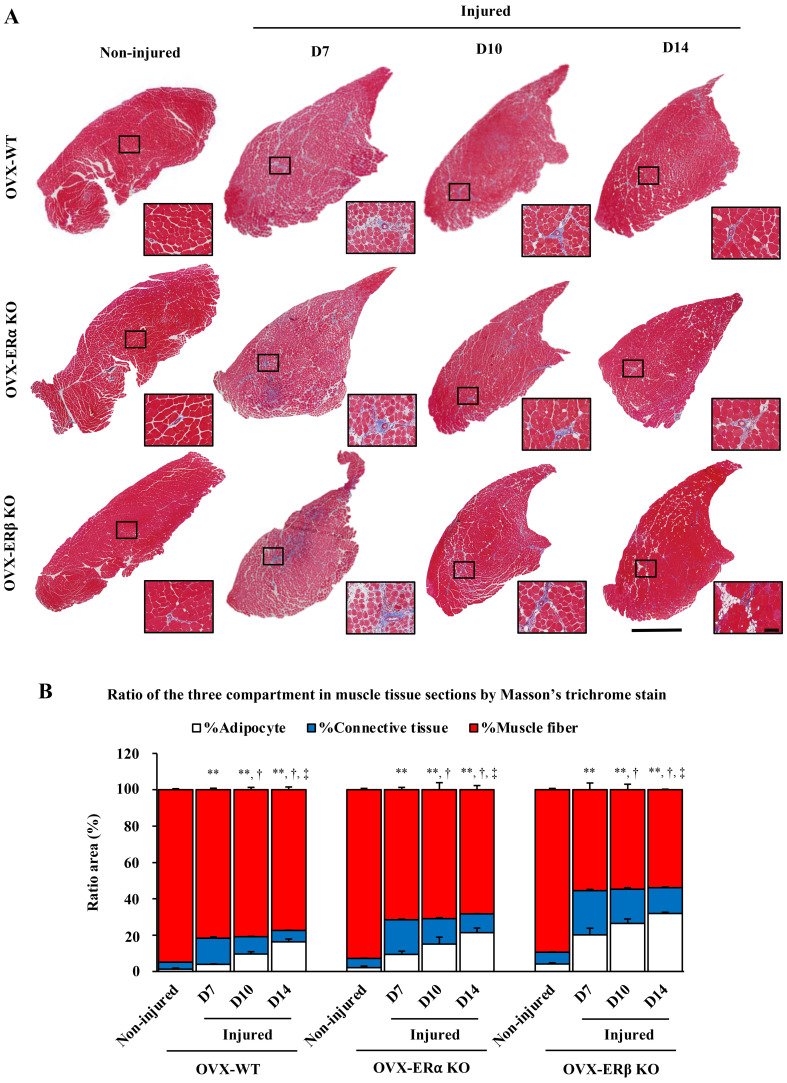
Masson’s trichrome staining revealed the ratio of muscle, fat and connective tissue. Connective tissue was originally present between muscle fibers, but in D7, the ratio (%) of connective tissue in all three types of OVX mice increased sharply compared to non-injured control. As the days went by, the ratio of connective tissue decreased with D10 and D14, and on the contrary, the ratio of fat increased. Interestingly, the ratio of muscle in each group remained almost constant regardless of the time after injury (Fig. 4).
Fig. 4.
Masson’s trichrome stain. (A) Sections of tibialis anterior (TA) muscle injected with cardiotoxin (CTX) and without CTX (non-injured as control) in ovariectomized-wild-type (OVX-WT) mice, OVX- estrogen receptor α knockout (OVX-ERα KO) mice and OVX-ERβ KO mice all time post CTX injection at day 7, 10 and 14 (D7, D10 and D14). Square areas within low magnification of whole section images are magnified in the right bottom panels. (B) Ratio of the three compartments in muscle tissue sections by Masson’s trichrome stain. Muscle fiber area (red), adipocyte area (white), and connective tissue area (blue) (%) of all three types of non-injured OVX mice and OVX mice injected CTX at several time points post injection at day 7, 10 and 14. Data are expressed as average ± standard deviation (SD), ** indicates significant differences from non-injured control of connective tissue area (%), † indicates significant differences from D7 of connective tissue area (%), ‡ indicates significant differences from D10 of connective tissue area (%), P<0.01, Scale bar=1 mm (low magnification), 100 µm (high magnification).
DISCUSSION
In this study, we measured the diameter of regenerated muscle fibers in ER KO mice after injury in low estrogen status. The diameter of muscle fibers in ER KO mice was significantly smaller than that in WT mice. To maintain muscle fiber formation, the following steps should proceed in an orderly manner: 1) proliferation of satellite cells, 2) differentiation into myoblasts, 3) fusion myoblasts, and 4) elongation of myotubes [11, 19, 35]. However, in ER KO (both subtypes) mice, the muscle fiber diameter was significantly low. Satellite cells are stem cells of muscle fibers, and their initial cell growth is important for muscle regeneration smoothly. Several researchers mentioned that estrogen and ERs are necessary for satellite cell survival and proliferation [5, 22, 24, 36, 43]. If satellite cell survival and proliferation were inhibited due to low estrogen status, this experiment may imply that the low number of satellite cells at early stages reflects the delay of myoregeneration.
The muscle fiber diameter was significantly smaller in ERβ KO mice than in ERα KO mice. Based on these results, it can be inferred that the action of estrogen via ERβ has a stronger effect on the maintenance of muscle tissue homeostasis than ERα. ERβ is thought to regulate not only satellite cell growth, but also prevent apoptosis in the differentiation steps of muscle fiber formation [36]. However, ERα is thought to be involved in the maintenance of the number of satellite cells in the proliferation process [5]. Our results indicated that the recovery ratio of ERβ KO mice was lower than that of ERα KO mice, indicating that the loss of ERβ could not accelerate muscle fiber formation in several steps. On the other hand, ERα KO mice showed better regeneration of muscle fiber formation than ERβ KO mice. Taken together, ERβ is likely to play a more important role in regulating myoregeneration than ERα in muscle tissue.
A difference was observed in the muscle regeneration speed between ERα KO mice and ERβ KO mice. That is, the muscle regeneration speed of ERβ KO mice tended to be high in the first half period (D7 to D10) and that of ERα KO tended to be high in the second half period (D10 to D14). It is considered that this difference in myoregeneration rate depending in the timing after injury may reflect the functional regulation by each ER at each stage of myoregeneration process such as proliferation and differentiation of satellite cells, and myotube formation. The detail of functional regulation by ER need to be further examined.
All types of OVX mice after CTX injury were significantly increased in the adipogenesis area (%) at D7, D10, and D14 after injury compared with non-injured control. OVX-ERβ KO mice showed a larger adipogenesis area (%) than OVX-ERα and OVX-WT mice. Delay of myoregeneration and induction of intermuscular adipogenesis in CTX-injured OVX-ER KO mice may alter the muscle and fat metabolism pathways and alter gene expression in muscle tissue. For example, myostatin, a member of the transforming growth factor-β superfamily, is a protein synthesized and released by myocytes that act as muscle cells to suppress myogenesis, such as muscle cell growth and differentiation [8, 30]. In a previous study, myostatin inhibited intermuscular preadipocyte differentiation in vitro [26, 39]. However, in a low estrogen state, muscle regeneration itself was suppressed, resulting in a decrease in muscle mass and production and expression of myostatin, which may have promoted the proliferation and differentiation of adipocytes. In contrast, it has been reported that adipocyte stimulated interleukin-6 expression in muscle cells could suppress muscle cell differentiation [37]. Platelet-derived growth factor receptor α (PDGFRα) -positive mesenchymal progenitor cells are thought to be the origin of intermuscular adipocytes [42]. In our experiments, adipocytes increased between muscle fibers after tissue damage, but the area of adipocytes formed varied significantly between ERα KO mice and ERβ KO mice. It is easy to imagine that the number and proliferation of PDGFRα-positive mesenchymal progenitor cells have a great influence on the proliferation of adipocytes after muscle tissue damage, but to our best knowledge, there are no reports of ER expression in PDGFRα-positive mesenchymal progenitor cells. By elucidating the relationship between ER and PDGFRα-positive mesenchymal progenitor cells, we will be able to get closer to elucidating the mechanism of ectopic adipocyte accumulation after postmenopausal muscle injury.
Initially, this study aimed to clarify whether estrogen and ER affect muscle regeneration. The results of the comparison in ER KO mice revealed that ER function in myoregeneration is more important for ERβ than ERα. Also, it has been reported that estrogen and ER are important factors in the proliferation and differentiation of satellite cells [5, 22, 36, 43]. However, it is interesting that even in OVX-ER KO mice, in which the low estrogen status and the absence of ER, muscle regeneration was delayed. This phenomenon may indicate that there are other mechanisms besides estrogen-ER that maintain the progression of myoregeneration. Insulin-like growth factor-1 (IGF-1), which is known to be a major factor in the increase in skeletal muscle mass [1], and IGF-1 and its receptor (IGF-1R) appear to be linked to estrogen and ER. Temporary interruption of estrogen supply weakened ER signaling and dramatically increased IGF-1 compared to normal conditions [16]. In addition, administration of estrogen to OVX animals suppressed IGF-1 production [41]. There are other cases where IGF-1 signaling is regulated by ER pathway. For example, in lung cancer cells, estrogen has been shown to elevate the IGF-1 signaling pathway via ERβ [40]. In addition, there is an interaction between IGF-1R and ERα, which act synergistically to promote the proliferation of nucleus pulposus cells [4]. However, there are still many uncertainties about the relationship between estrogen and IGF-1, and their receptors in muscle tissue. Focusing on these factors, it is necessary to elucidate the detailed mechanism of myogenesis and myoregeneration. Of course, it is quite possible that factors other than those mentioned above are involved in the formation of muscle, fat and connective tissue after muscle injury. It is also reasonable to assume that the many number of infiltrated cells observed in the connective tissue between muscle fibers after muscle injury perform a variety of functions in a complex manner. Therefore, further research is needed to determine the biological significance of these cells and phenomena.
In this experiment, adipose accumulation was observed in the skeletal muscle tissue of OVX mice after CTX injury (Figs. 3 and 4). Moreover, muscle fiber diameter and cross-sectional area of TA muscle did not recover until the CTX pre-injury level (Figs. 2 and 4, Table 1). In sarcopenia, accumulation of ectopic adipose tissue is observed in skeletal muscle, as well as atrophy of muscle fibers and deterioration of muscle strength and physical function [17, 38]. Comparing the CTX-injured and sarcopenic muscle, there are many morphologically similar parts. We have not investigated the function of CTX-injured mice, but it can be easily inferred that the muscle mass and strength of TA muscle of these mice are reduced. The mice used in this experiment may be a good model candidate for pathophysiological studies of sarcopenia.
In conclusion, our data may indicate that low estrogen affects myoregeneration via the ER. In addition, its action was more remarkable via ERβ rather than by ERα, and it was morphologically shown that removal of these ERs delayed muscle regeneration and promoted adipose tissue formation. Targeting estrogen and ER may provide clues for maintaining muscle tissue homeostasis.
POTENTIAL CONFLICTS OF INTEREST
The authors have nothing to disclose.
Supplementary
Acknowledgments
The Authors thank Dr. Pierre Chambon, Institute for Genetics and Cellular and Molecular Biology, France, for providing ERα KO mice. This work was supported by JSPS Grant-in-Aid for Scientific Research (B) Grant Number JP17H03934.
REFERENCES
- 1.Adams G. R., McCue S. A.1998. Localized infusion of IGF-I results in skeletal muscle hypertrophy in rats. J. Appl. Physiol. (1985) 84: 1716–1722. doi: 10.1152/jappl.1998.84.5.1716 [DOI] [PubMed] [Google Scholar]
- 2.Brown M., Ning J., Ferreira J. A., Bogener J. L., Lubahn D. B.2009. Estrogen receptor-α and -β and aromatase knockout effects on lower limb muscle mass and contractile function in female mice. Am. J. Physiol. Endocrinol. Metab. 296: E854–E861. doi: 10.1152/ajpendo.90696.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Campbell S. E., Febbraio M. A.2001. Effect of ovarian hormones on mitochondrial enzyme activity in the fat oxidation pathway of skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 281: E803–E808. doi: 10.1152/ajpendo.2001.281.4.E803 [DOI] [PubMed] [Google Scholar]
- 4.Chen R. S., Zhang X. B., Zhu X. T., Wang C. S.2020. The crosstalk between IGF-1R and ER-α in the proliferation and anti-inflammation of nucleus pulposus cells. Eur. Rev. Med. Pharmacol. Sci. 24: 5886–5894. [DOI] [PubMed] [Google Scholar]
- 5.Collins B. C., Arpke R. W., Larson A. A., Baumann C. W., Xie N., Cabelka C. A., Nash N. L., Juppi H. K., Laakkonen E. K., Sipilä S., Kovanen V., Spangenburg E. E., Kyba M., Lowe D. A.2019. Estrogen regulates the satellite cell compartment in females. Cell Rep. 28: 368–381.e6. doi: 10.1016/j.celrep.2019.06.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Contreras-Shannon V., Ochoa O., Reyes-Reyna S. M., Sun D., Michalek J. E., Kuziel W. A., McManus L. M., Shireman P. K.2007. Fat accumulation with altered inflammation and regeneration in skeletal muscle of CCR2-/- mice following ischemic injury. Am. J. Physiol. Cell Physiol. 292: C953–C967. doi: 10.1152/ajpcell.00154.2006 [DOI] [PubMed] [Google Scholar]
- 7.Curtis Hewitt S., Couse J. F., Korach K. S.2000. Estrogen receptor transcription and transactivation: Estrogen receptor knockout mice: what their phenotypes reveal about mechanisms of estrogen action. Breast Cancer Res. 2: 345–352. doi: 10.1186/bcr79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deng B., Zhang F., Wen J., Ye S., Wang L., Yang Y., Gong P., Jiang S.2017. The function of myostatin in the regulation of fat mass in mammals. Nutr. Metab. (Lond.) 14: 29. doi: 10.1186/s12986-017-0179-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diel P.2014. The role of the estrogen receptor in skeletal muscle mass homeostasis and regeneration. Acta Physiol. (Oxf.) 212: 14–16. doi: 10.1111/apha.12341 [DOI] [PubMed] [Google Scholar]
- 10.Dupont S., Krust A., Gansmuller A., Dierich A., Chambon P., Mark M.2000. Effect of single and compound knockouts of estrogen receptors α (ERalpha) and β (ERbeta) on mouse reproductive phenotypes. Development 127: 4277–4291. doi: 10.1242/dev.127.19.4277 [DOI] [PubMed] [Google Scholar]
- 11.Forcina L., Cosentino M., Musarò A.2020. Mechanisms regulating muscle regeneration: insights into the interrelated and time-dependent phases of tissue healing. Cells 9: 1297. doi: 10.3390/cells9051297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Girousse A., Gil-Ortega M., Bourlier V., Bergeaud C., Sastourné-Arrey Q., Moro C., Barreau C., Guissard C., Vion J., Arnaud E., Pradère J. P., Juin N., Casteilla L., Sengenès C.2019. The release of adipose stromal cells from subcutaneous adipose tissue regulates ectopic intramuscular adipocyte deposition. Cell Rep. 27: 323–333.e5. doi: 10.1016/j.celrep.2019.03.038 [DOI] [PubMed] [Google Scholar]
- 13.Hardy D., Besnard A., Latil M., Jouvion G., Briand D., Thépenier C., Pascal Q., Guguin A., Gayraud-Morel B., Cavaillon J. M., Tajbakhsh S., Rocheteau P., Chrétien F.2016. Comparative study of injury models for studying muscle regeneration in mice. PLoS One 11: e0147198. doi: 10.1371/journal.pone.0147198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harris J. B.2003. Myotoxic phospholipases A2 and the regeneration of skeletal muscles. Toxicon 42: 933–945. doi: 10.1016/j.toxicon.2003.11.011 [DOI] [PubMed] [Google Scholar]
- 15.Hausman G. J., Basu U., Du M., Fernyhough-Culver M., Dodson M. V.2014. Intermuscular and intramuscular adipose tissues: Bad vs. good adipose tissues. Adipocyte 3: 242–255. doi: 10.4161/adip.28546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iida M., Tsuboi K., Niwa T., Ishida T., Hayashi S. I.2019. Compensatory role of insulin-like growth factor 1 receptor in estrogen receptor signaling pathway and possible therapeutic target for hormone therapy-resistant breast cancer. Breast Cancer 26: 272–281. doi: 10.1007/s12282-018-0922-0 [DOI] [PubMed] [Google Scholar]
- 17.Ikeda K., Horie-Inoue K., Inoue S.2019. Functions of estrogen and estrogen receptor signaling on skeletal muscle. J. Steroid Biochem. Mol. Biol. 191: 105375. doi: 10.1016/j.jsbmb.2019.105375 [DOI] [PubMed] [Google Scholar]
- 18.Jackson K. C., Wohlers L. M., Lovering R. M., Schuh R. A., Maher A. C., Bonen A., Koves T. R., Ilkayeva O., Thomson D. M., Muoio D. M., Spangenburg E. E.2013. Ectopic lipid deposition and the metabolic profile of skeletal muscle in ovariectomized mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 304: R206–R217. doi: 10.1152/ajpregu.00428.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karalaki M., Fili S., Philippou A., Koutsilieris M.2009. Muscle regeneration: cellular and molecular events. In Vivo 23: 779–796. [PubMed] [Google Scholar]
- 20.Kawai S., Takagi Y., Kaneko S., Kurosawa T.2011. Effect of three types of mixed anesthetic agents alternate to ketamine in mice. Exp. Anim. 60: 481–487. doi: 10.1538/expanim.60.481 [DOI] [PubMed] [Google Scholar]
- 21.Kiens B.2006. Skeletal muscle lipid metabolism in exercise and insulin resistance. Physiol. Rev. 86: 205–243. doi: 10.1152/physrev.00023.2004 [DOI] [PubMed] [Google Scholar]
- 22.Kitajima Y., Ono Y.2016. Estrogens maintain skeletal muscle and satellite cell functions. J. Endocrinol. 229: 267–275. doi: 10.1530/JOE-15-0476 [DOI] [PubMed] [Google Scholar]
- 23.Krege J. H., Hodgin J. B., Couse J. F., Enmark E., Warner M., Mahler J. F., Sar M., Korach K. S., Gustafsson J. Å., Smithies O.1998. Generation and reproductive phenotypes of mice lacking estrogen receptor β. Proc. Natl. Acad. Sci. USA 95: 15677–15682. doi: 10.1073/pnas.95.26.15677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.LaBarge S., McDonald M., Smith-Powell L., Auwerx J., Huss J. M.2014. Estrogen-related receptor-α (ERRα) deficiency in skeletal muscle impairs regeneration in response to injury. FASEB J. 28: 1082–1097. doi: 10.1096/fj.13-229211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.La Colla A., Pronsato L., Milanesi L., Vasconsuelo A.2015. 17β-Estradiol and testosterone in sarcopenia: Role of satellite cells. Ageing Res. Rev. 24 Pt B: 166–177. doi: 10.1016/j.arr.2015.07.011 [DOI] [PubMed] [Google Scholar]
- 26.Li F., Yang H., Duan Y., Yin Y.2011. Myostatin regulates preadipocyte differentiation and lipid metabolism of adipocyte via ERK1/2. Cell Biol. Int. 35: 1141–1146. doi: 10.1042/CBI20110112 [DOI] [PubMed] [Google Scholar]
- 27.Mahdy M. A. A., Lei H. Y., Wakamatsu J., Hosaka Y. Z., Nishimura T.2015. Comparative study of muscle regeneration following cardiotoxin and glycerol injury. Ann. Anat. 202: 18–27. doi: 10.1016/j.aanat.2015.07.002 [DOI] [PubMed] [Google Scholar]
- 28.Maher A. C., Akhtar M., Tarnopolsky M. A.2010. Men supplemented with 17β-estradiol have increased β-oxidation capacity in skeletal muscle. Physiol. Genomics 42: 342–347. doi: 10.1152/physiolgenomics.00016.2010 [DOI] [PubMed] [Google Scholar]
- 29.McHale M. J., Sarwar Z. U., Cardenas D. P., Porter L., Salinas A. S., Michalek J. E., McManus L. M., Shireman P. K.2012. Increased fat deposition in injured skeletal muscle is regulated by sex-specific hormones. Am. J. Physiol. Regul. Integr. Comp. Physiol. 302: R331–R339. doi: 10.1152/ajpregu.00427.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McPherron A. C., Lee S. J.1997. Double muscling in cattle due to mutations in the myostatin gene. Proc. Natl. Acad. Sci. USA 94: 12457–12461. doi: 10.1073/pnas.94.23.12457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muramatsu M., Inoue S.2000. Estrogen receptors: how do they control reproductive and nonreproductive functions? Biochem. Biophys. Res. Commun. 270: 1–10. doi: 10.1006/bbrc.2000.2214 [DOI] [PubMed] [Google Scholar]
- 32.Nagai S., Ikeda K., Horie-Inoue K., Takeda S., Inoue S.2018. Estrogen signaling increases nuclear receptor subfamily 4 group A member 1 expression and energy production in skeletal muscle cells. Endocr. J. 65: 1209–1218. doi: 10.1507/endocrj.EJ17-0548 [DOI] [PubMed] [Google Scholar]
- 33.Nilsson S., Gustafsson J. Å.2011. Estrogen receptors: therapies targeted to receptor subtypes. Clin. Pharmacol. Ther. 89: 44–55. doi: 10.1038/clpt.2010.226 [DOI] [PubMed] [Google Scholar]
- 34.Ochoa O., Sun D., Reyes-Reyna S. M., Waite L. L., Michalek J. E., McManus L. M., Shireman P. K.2007. Delayed angiogenesis and VEGF production in CCR2-/- mice during impaired skeletal muscle regeneration. Am. J. Physiol. Regul. Integr. Comp. Physiol. 293: R651–R661. doi: 10.1152/ajpregu.00069.2007 [DOI] [PubMed] [Google Scholar]
- 35.Relaix F., Zammit P. S.2012. Satellite cells are essential for skeletal muscle regeneration: the cell on the edge returns centre stage. Development 139: 2845–2856. doi: 10.1242/dev.069088 [DOI] [PubMed] [Google Scholar]
- 36.Seko D., Fujita R., Kitajima Y., Nakamura K., Imai Y., Ono Y.2020. Estrogen receptor β controls muscle growth and regeneration in young female mice. Stem Cell Reports 15: 577–586. doi: 10.1016/j.stemcr.2020.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seo K., Suzuki T., Kobayashi K., Nishimura T.2019. Adipocytes suppress differentiation of muscle cells in a co-culture system. Anim. Sci. J. 90: 423–434. doi: 10.1111/asj.13145 [DOI] [PubMed] [Google Scholar]
- 38.Srikanthan P., Hevener A. L., Karlamangla A. S.2010. Sarcopenia exacerbates obesity-associated insulin resistance and dysglycemia: findings from the National Health and Nutrition Examination Survey III. PLoS One 5: e10805. doi: 10.1371/journal.pone.0010805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sun W. X., Dodson M. V., Jiang Z. H., Yu S. G., Chu W. W., Chen J.2016. Myostatin inhibits porcine intramuscular preadipocyte differentiation in vitro. Domest. Anim. Endocrinol. 55: 25–31. doi: 10.1016/j.domaniend.2015.10.005 [DOI] [PubMed] [Google Scholar]
- 40.Tang H., Liao Y., Chen G., Xu L., Zhang C., Ju S., Zhou S.2012. Estrogen upregulates the IGF-1 signaling pathway in lung cancer through estrogen receptor-β. Med. Oncol. 29: 2640–2648. doi: 10.1007/s12032-012-0198-8 [DOI] [PubMed] [Google Scholar]
- 41.Tsai W. J., McCormick K. M., Brazeau D. A., Brazeau G. A.2007. Estrogen effects on skeletal muscle insulin-like growth factor 1 and myostatin in ovariectomized rats. Exp. Biol. Med. (Maywood) 232: 1314–1325. doi: 10.3181/0704-RM-92 [DOI] [PubMed] [Google Scholar]
- 42.Uezumi A., Fukada S., Yamamoto N., Takeda S., Tsuchida K.2010. Mesenchymal progenitors distinct from satellite cells contribute to ectopic fat cell formation in skeletal muscle. Nat. Cell Biol. 12: 143–152. doi: 10.1038/ncb2014 [DOI] [PubMed] [Google Scholar]
- 43.Velders M., Schleipen B., Fritzemeier K. H., Zierau O., Diel P.2012. Selective estrogen receptor-β activation stimulates skeletal muscle growth and regeneration. FASEB J. 26: 1909–1920. doi: 10.1096/fj.11-194779 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.