Abstract
Background:
Right ventricular hypertrophy (RVH) provides a key remodeling index alterable by pulmonary hypertension (PH). While echo commonly integrates linear wall thickness and chamber dimensions to quantify left ventricular remodeling, utility of an equivalent RV-based approach is unknown.
Methods:
This was a retrospective analysis of 200 patients undergoing transthoracic echo and CMR within 30 days (median=3 days, IQR=15 days), stratified by echo-quantified pulmonary artery systolic pressure (PASP <35│35 to <55│ 55 to <75│ ≥75mmHg). Echo assessment included RV linear dimensions in parasternal long axis (PLAX) and apical 4-chamber, and wall thicknesses in PLAX, 4-chamber, and subcostal views. Subcostal wall thickness was integrated with chamber diameters to calculate RV mass, which was tested in relation to CMR-quantified RV mass, and all-cause mortality.
Results:
Echo-based quantification of all linear dimensions was feasible in 95% of patients (190/200). RV wall thicknesses in all orientations increased in relation to PASP (p<0.001) and was greater among patients with, versus those without, CMR-evidenced RVH (all p<0.001). Correlations between echo and CMR were greatest for RV basal diameter (r=0.73), RV subcostal wall thickness (r=0.71) and global RV mass (r=0.82; all p<0.001). Echo-derived global RV mass cutoffs were established in a derivation cohort and tested in a validation cohort. Results demonstrated good sensitivity and specificity (75.5% and 74.0%, respectively) in relation to CMR-quantified RVH. During follow-up (median 4.2 years), 18% (n=36) of patients died. Echo-evidenced RVH (HR 1.98, [95% CI: 1.09–3.88], p=0.048) conferred similar mortality risk compared to RVH on CMR (HR 2.41, [95% CI: 1.22–4.78], p=0.01).
Conclusions:
Echo-quantified RV parameters provide a robust index of RV afterload. Global RV mass calculated using a novel echo formula based on readily available linear indices yields good diagnostic performance for CMR evidenced RVH and confers increased mortality risk.
Keywords: right ventricular hypertrophy, echocardiography, cardiac magnetic resonance
Introduction
Right ventricular hypertrophy (RVH) provides a physiologic marker of adverse remodeling that can be induced by pulmonary hypertension (PH). Whereas volumetric imaging techniques such as cardiac magnetic resonance (CMR) have been well-validated to quantify RVH,(1–4) echo-based approaches are more challenging due to complex RV chamber geometry. Given that echo is widely used as the primary non-invasive modality to assess patients with known or suspected PH, lack of validation data for echo-based diagnosis of RVH is a key shortcoming.
Whereas the RV is irregularly contoured, PH-induced increments in RV afterload would be expected to be uniformly transmitted to RV myocardium – providing a rationale for the use of discrete linear indices as markers of global RVH. Recent data by our group and others have supported the concept that linear RV chamber dimensions provide utility as surrogates of global (volumetric) RV size and function (5–8). Prior research has shown RV chamber dimensions to increase in proportion to RV size (6, 8) and RV linear fractional shortening to decrease in proportion to contractile dysfunction (7) as quantified volumetrically on CMR. Wall thickness integration with chamber dimension is widely used to quantify left ventricular (LV) remodeling.(9, 10) However, whereas RV mass extrapolated from linear dimensions is noted in consensus echo guidelines,(11) validation literature supporting this approach is uncertain. For example, guidelines recommend measurement of RV free wall thickness in subcostal orientation. However, relative utility of this approach in relation to other orientations is unknown, as are specific cutoffs that should be used to define RVH on echo. More broadly, a widely applicable echo-based formula for global RV myocardial mass – integrating RV wall thickness and chamber size as can be readily measured on echo – has yet to be validated.
This study tested echo-quantified RV wall thickness as an index of RVH among a broad cohort of patients stratified by presence and severity of PH. Study aims were to: (1) validate linear RV wall thickness in relation to RV afterload as measured by PA pressure, (2) test diagnostic performance of RV wall thickness alone, as well as an integrated echo formula for global RV mass (based on linear wall thickness and chamber diameter) in relation to volumetric RV mass on CMR, and (3) determine prognostic utility of echo-quantified RVH (based on global RV mass).
Methods
Population
The study population was derived from a retrospective dataset of patients undergoing clinically ordered transthoracic echo and CMR within a 30-day interval (September 2015-June 2018), for whom RV afterload (pulmonary arterial systolic pressure, [PASP]) was quantifiable on echo. To study a broad distribution of RV remodeling, inclusive of both normal and increased RV mass constituting a wide range of RV afterload states, the study cohort consisted of consecutive patients within pre-defined PH strata (PASP 35 to <55|55 to <75|≥ 75mmHg), as well as controls without PH (PASP<35 mmHg). Controls and PH strata were included in a 2:1:1:1 manner (80 controls, 40 in each PH sub-group). Patients were then randomly assigned to derivation and validation cohorts (n=100 each), stratified by PASP. Patients with congenital heart disease were excluded; no patients were excluded from this study based on image quality or other clinical indices.
Comprehensive demographic data including PH-related risk factors, WHO pulmonary hypertension classification, and medication regimen were categorized in a uniform manner. Mortality status was assessed using the U.S. Social Security Death Index supplemented by electronic medical record review.
This study was conducted with approval of the Institutional Review Board at Weill Cornell Medicine (New York, NY), which provided approval for analysis of pre-existing data for study purposes.
Imaging Protocol
Echo
Two-dimensional transthoracic echoes were performed using commercial equipment (General Electric Vivid-7, Siemens SC2000, Philips iE33/EPIQ7). Exams included evaluation of the RV in parasternal long axis, apical 4-chamber, and subcostal 4-chamber orientations as specified in consensus guidelines.(11) LV apical and parasternal images were also acquired, as was continuous wave Doppler assessment of tricuspid regurgitant velocity for quantification of PASP.
CMR
CMR exams were performed using commercial (1.5 and 3.0T) scanners, inclusive of cine-CMR for assessment of cardiac structure/function. Cine-CMR utilized a steady-state free precession pulse sequence; images were acquired in conventional LV (2, 3, 4-chamber) long- and short-axis orientations, the latter of which were acquired contiguously from the RV outflow tract (pulmonic valve annulus) to RV apex.
RV Chamber Quantification
Echo and CMR analysis were supervised by experienced physicians (echo: JK, CMR: JWW) for whom reproducibility for RV analyses has been previously reported. (6, 12) Key analysis methods are detailed below:
Echocardiography
RV chamber dimensions were measured at end-diastole in RV-focused apical views in accordance with methodologies employed in our prior work and as specified in consensus echo guidelines. (6, 11) Measurements were made on the apical image demonstrating the maximum diameter of the right ventricle without foreshortening. All chamber measurements were made inner-edge to inner-edge. Trabeculations, papillary muscles and moderator band were included within internal chamber dimensions. In accordance with consensus guidelines, trabeculae, papillary muscle and epicardial fat were excluded from right ventricular wall thickness measurement:
RV outflow tract dimension (RVOTd) was quantified in parasternal long axis images, on which it was defined as the maximal (perpendicular) diameter between the RV free wall and septal-aortic junction.
RV basal dimension (RVBd) was quantified using apical 4-chamber images, on which it was defined as the maximal diameter in the basal third of the RV (perpendicular to septum and free wall).
RV longitudinal dimension (RVLd) was quantified using apical 4-chamber images, on which it was measured as the diameter from the mid-point of the tricuspid annulus to the RV apex.
RV wall thickness was measured in discrete locations corresponding to chamber dimensions:
In the apical 4-chamber view, maximal RV wall thickness was measured in the basal third of the RV (4-chamber RV free wall thickness, 4C-FWt) where endocardial and epicardial borders were most clearly delineated.
Maximal RV wall thickness was also measured in the subcostal 4-chamber (subcostal RV free wall thickness, SC-FWt) orientations in the basal third of the RV where endocardial and epicardial borders were most clearly delineated, and in the parasternal long axis (proximal RVOT thickness, RVOT-Wt).
Figure 1 provides representative examples of linear RV indices acquired in this study. PASP was calculated using the modified Bernoulli equation (4 [peak tricuspid regurgitant velocity]2 + right atrial pressure), for which tricuspid regurgitant peak velocity was measured via Doppler, and right atrial pressure determined based on size and collapsibility of the inferior vena cava with sniff performed in all patients. LV function, size, and mass were quantified via linear dimensions, consistent with methods validated in previous necropsy comparison and outcomes studies.(9, 13) RV systolic function was assessed via TAPSE and S’ which were acquired in accordance with consensus guidelines. (9, 13, 14). Advanced (≥moderate) tricuspid regurgitation was defined based on semi-quantitative criteria (jet >1/3 right atrial area in 4-chamber orientation).
Figure 1. Echo-based linear right ventricular dimensions.
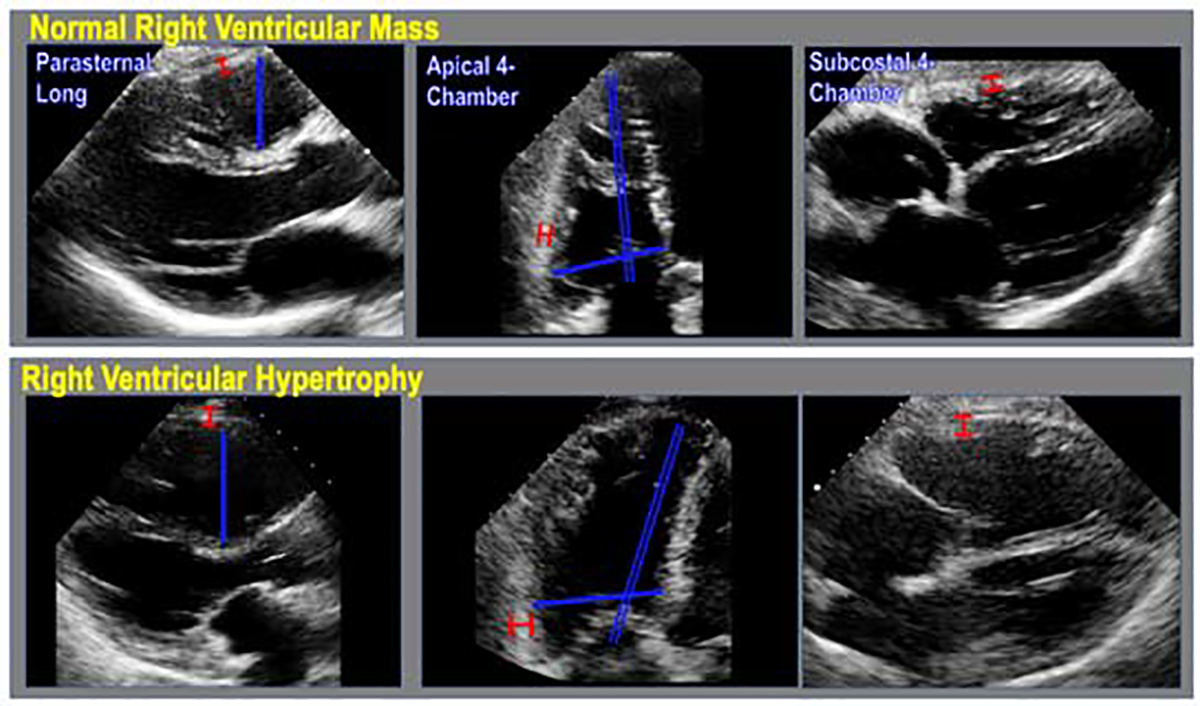
Representative examples of echo-based linear dimensions (blue) and wall thicknesses (red), as acquired in parasternal long axis (left), apical 4-chamber (middle) and subcostal 4-chamber (right) axis images, in patients without (top) and with (bottom) right ventricular hypertrophy.
Intra- and inter-reader reproducibility for key echocardiographic indices was tested in a random cohort comprising 15% (n=30) of the study population including each level of PASP. Inter-reader reproducibility was tested via a designated reader (JC). Readers were otherwise blinded to clinical history, results of other imaging modalities, and initial measurements.
CMR
Cine-CMR short axis datasets were manually planimetered to quantify RV end-diastolic and end-systolic chamber volumes. Epicardial contours were drawn at RV end-diastole to calculate RV mass (myocardial volume x specific gravity [1.05g/ml]). Established gender-based normative (>95% CI) cutoffs (males: >31.9g, females: >26.4g) were used to define RVH.(1)
Echocardiographic RV Mass Quantification
Global RV mass was calculated using a geometric formula for which chamber volume was approximated as a ¼ prolate ellipsoid. A ¼ prolate ellipsoid is the simplest geometrical shape that can be approximated using standard ASE-guideline recommended measurements, which visually approximates the shape of the body of the right ventricle. This approach has been used in prior studies demonstrating similar ¼ prolate ellipse geometrical assumptions to estimate RV volumes and RV mass.(15,16) The model is defined by distance measurements in three axes (Figure 2), that can be approximated using linear indices encompassed by basal RV (RVBd), RV longitudinal (RVLd), and LV basal (LVEDd) diameters. RV surface area was approximated based on geometric mathematical modeling estimations of the surface area of a prolate ellipsoid,(15) further simplified by Knud Thomsen:
RV mass was approximated as the product of RV surface area and free wall thickness (i.e. myocardial volume), multiplied by myocardial specific gravity (1.05g/ml):
Echo-calculated RV mass values were analyzed against CMR-quantified RV mass in the derivation cohort via linear regression to model a slope (β) and correction factor (α) for the relationship. These values were used to derive an adjusted echo-quantified RV mass for the full population:
Figure 2. Ellipsoid model of the right ventricle.

One-quarter prolate ellipsoid model used for quantifying for RV mass. The 3 radii are defined by the RV basal diameter (a), LV end diastolic diameter/2 (b), and RV longitudinal diameter (c). The surface area is calculated from these dimensions, and then multiplied by the RV free wall thickness in the subcostal view and by myocardial density to quantify RV free wall mass.
Statistical Methods
Continuous variables are summarized as means ± standard deviations. Comparisons of continuous variables between groups were made using Student’s t-test. Categorical variables were summarized as frequencies and percentages. Categorical variables were compared using chi-square tests. Correlation coefficients and linear regression were used to evaluate associations between continuous variables. Generalized linear models were fit to characterize the association of wall thicknesses and echo-quantified RV mass with PASP strata; linear tests of trends were done by specifying the relevant contrasts. Univariable linear regression was used to derive a slope (β) and correction factor (α) for the relation between RV mass on echo and CMR; agreement between modalities was assessed using the method of Bland and Altman,(16) yielding the mean difference as well as limits of agreement (mean ± 1.96 SD). The coefficient of variation was calculated as the standard deviation of the absolute difference between two modalities divided by the mean (expressed as a percentage). Reproducibility was similarly assessed using the method of Bland and Altman. Coefficient of variation was calculated as the standard deviation of the absolute difference between two acquisitions divided by the mean of the repeated acquisitions. Inter-rater reliability among the two raters was estimated using the intra-class correlation coefficient. Receiver operating characteristic (ROC) analysis was used to evaluate diagnostic performance of echo methods in relation to the reference of CMR-quantified RVH. RVH on echo and CMR were tested in relation to survival using the Kaplan-Meier method; patients were considered at risk starting at the date of the imaging study until death. Cox proportional hazard analysis was used to evaluate associations with mortality. Statistical calculations were performed using SPSS 22.0 (SPSS Inc. [Chicago, IL]). Two-sided p<0.05 was considered indicative of statistical significance.
Results
Population Characteristics
Patients underwent echo and CMR within a median 3-day interval [IQR=15 days]). RVH as defined by the reference standard of CMR was present in 47% (n=93) of patients; prevalence increased in proportion to RV afterload as quantified by PASP (< 35mmHg: 21%|35 to <55mmHg: 28%|55 to <75mmHg: 70%| ≥ 75%: 93%; p<0.001). Among patients with pulmonary hypertension, WHO class II was the most common categorization (58%, n=70), followed by WHO class I (30%, n=36), WHO class IV (6%, n =7), WHO class III (4%, n=5), and least common was WHO class V (2%, n=2).
Table 1 details population characteristics, including comparisons between patients with and without CMR-quantified RVH. As shown, RVH was less common in patients with CAD, but groups were similar with respect to LV chamber size and function. Regarding RV parameters, patients with RVH had greater impairments in RV function, as evidenced by lower S’ (p=0.001) and a trend towards decreased tricuspid annular plane systolic excursion (p=0.08), paralleled by lower RV ejection fraction on CMR (p<0.001).
Table 1.
Clinical and Imaging Characteristics
| Overall (n=200) | RVH* + (n=93) | RVH* − (n=107) | P | |
|---|---|---|---|---|
| CLINICAL | ||||
| Age (year) | 59.9 ± 16.2 | 57.7 ± 16.8 | 61.9 ± 15.5 | 0.06 |
| Male sex | 53% (106) | 53% (49) | 53% (57) | 0.94 |
| Coronary Artery Disease Risk Factors | ||||
| Hypertension | 59% (118) | 57% (52) | 61% (65) | 0.59 |
| Hypercholesterolemia | 52% (103) | 45% (42) | 57% (61) | 0.09 |
| Diabetes Mellitus | 24% (47) | 25% (23) | 22% (24) | 0.70 |
| Tobacco Use | 37% (74) | 34% (32) | 39% (42) | 0.48 |
| Family History | 21% (41) | 20% (19) | 21% (22) | 0.98 |
| Coronary Artery Disease | 34% (67) | 22% (20) | 44% (47) | 0.001 |
| Prior Myocardial Infarction | 27% (53) | 15% (14) | 36% (39) | 0.001 |
| Cardiovascular Medications | ||||
| Beta-blocker | 55% (109) | 52% (48) | 57% (61) | 0.45 |
| ACE-Inhibitor/Angiotensin Receptor Blocker | 42% (83) | 36% (33) | 47% (50) | 0.11 |
| Loop diuretic | 38% (76) | 53% (49) | 25% (27) | <0.001 |
| HMG CoA-Reductase Inhibitor | 49% (98) | 41% (38) | 56% (60) | 0.03 |
| Aspirin | 51% (101) | 44% (41) | 56% (60) | 0.09 |
| CARDIAC MORPHOLOGY AND FUNCTION | ||||
| Echocardiography | ||||
| Right Ventricle | ||||
| Tricuspid annular plane systolic excursion (cm) | 1.87 ± 0.56 | 1.79 ± 0.58 | 1.94 ± 0.53 | 0.08 |
| RV peak systolic annular velocity – S’ (mm/s) | 11.8 ± 3.4 | 10.9 ± 3.0 | 12.5 ± 3.5 | 0.001 |
| RV dysfunction† | 20% (34) | 25% (21) | 14% (13) | 0.07 |
| Advanced (≥ moderate) tricuspid regurgitation | 17% (33) | 32% (29) | 4 (4%) | <0.001 |
| Left Ventricle | ||||
| Ejection Fraction (%) | 52.6 ± 17.2 | 53.2 ± 17.7 | 52.1 ± 16.9 | 0.67 |
| End-diastolic diameter (cm) | 5.3 ± 0.9 | 5.3 ± 1.0 | 5.2 ± 0.7 | 0.42 |
| End-systolic diameter (cm) | 3.9 ± 1.1 | 3.9 ± 1.2 | 3.8 ± 1.0 | 0.52 |
| Myocardial Mass (g) | 175.8 ± 70.5 | 176.9 ± 76.8 | 174.8 ± 64.2 | 0.64 |
| (g/m2) | 93.8 ± 35.4 | 92.6 ± 38.0 | 95.1 ± 32.9 | 0.84 |
| Pulmonary Arterial Systolic Pressure (mmHg) | 51.1 ± 27.1 | 68.0 ± 28.6 | 36.4 ± 14.3 | <0.001 |
| Pulmonary hypertension (PASP ≥35mmHg) | 60% (120) | 82% (76) | 41% (44) | <0.001 |
| Cardiac Magnetic Resonance | ||||
| Right Ventricle | ||||
| Ejection fraction (%) | 46.6 ± 13.2 | 39.4 ± 13.3 | 53.0 ± 9.2 | <0.001 |
| End-diastolic volume (ml) | 170.8 ± 63.2 | 216.7 ± 57.7 | 130.9 ± 34.2 | <0.001 |
| (ml/m2) | 92.1 ± 32.9 | 115.2 ± 31.6 | 72.0 ± 16.9 | <0.001 |
| End-systolic volume (ml) | 95.3 ± 54.5 | 133.4 ± 56.0 | 62.2 ± 22.1 | <0.001 |
| (ml/m2) | 51.4 ± 29.4 | 71.4 ± 31.3 | 34.1 ± 11.2 | <0.001 |
| Myocardial Mass (g) | 32.2 ± 13.2 | 42.8 ± 11.7 | 23.0 ± 4.6 | <0.001 |
| (g/m2) | 17.4 ± 7.0 | 22.9 ± 6.7 | 12.7 ± 2.3 | <0.001 |
| RV mass/volume ratio (g/ml) | 0.19 ± 0.04 | 0.20 ± 0.04 | 0.18 ± 0.04 | 0.001 |
| Left Ventricle | ||||
| Ejection fraction (%) | 55.2 ± 16.5 | 55.9 ± 17.6 | 54.7 ± 15.5 | 0.61 |
| End-diastolic volume (ml) | 154.3 ± 70.1 | 165.4 ± 85.3 | 144.7 ± 52.1 | 0.04 |
| (ml/m2) | 82.7 ± 34.3 | 86.6 ± 41.4 | 79.4 ± 26.3 | 0.15 |
| End-systolic volume (ml) | 75.6 ± 64.4 | 81.7 ± 80.4 | 70.4 ± 46.1 | 0.23 |
| (ml/m2) | 40.4 ± 32.8 | 42.9 ± 40.6 | 38.3 ± 24.0 | 0.35 |
| Myocardial Mass (g) | 140.4 ± 52.5 | 150.7 ± 62.8 | 131.4 ± 39.9 | 0.02 |
| (g/m2) | 75.2 ± 25.4 | 79.2 ± 30.3 | 71.8 ± 19.9 | 0.054 |
Data presented as mean ± standard deviation (data in parentheses refer to range for each respective variable)
Defined by the reference standard of CMR using established criteria(1) (males: >31.9g, females: >26.4g in females)
Defined by tricuspid annular plane systolic excursion <1.6cm and RV peak systolic annular velocity <10mm/sec
RV Wall Thickness and Chamber Dimensions
Quantification of all echo linear dimensions (RV wall thicknesses, chamber dimensions) was feasible in 95% (190/200) of patients. Figure 3 stratifies echo-quantified RV wall thickness in relation to PA pressure. As shown, subcostal mean wall thickness increased stepwise in relation to RV afterload and was more than 1.5-fold higher among the top quartile of PASP as compared to patients without PH (p<0.001). Importantly, associations between RV wall thickness and PA pressure were similar irrespective of orientation used for echo analysis: Paralleling subcostal analyses, RV free wall thickness as measured in both apical 4-chamber and parasternal long axis orientation similarly increased between patient sub-group stratified by PASP (p<0.001 for all).
Figure 3. Right ventricular wall thicknesses and echo-quantified RV mass in relation to pulmonary arterial systolic pressure.
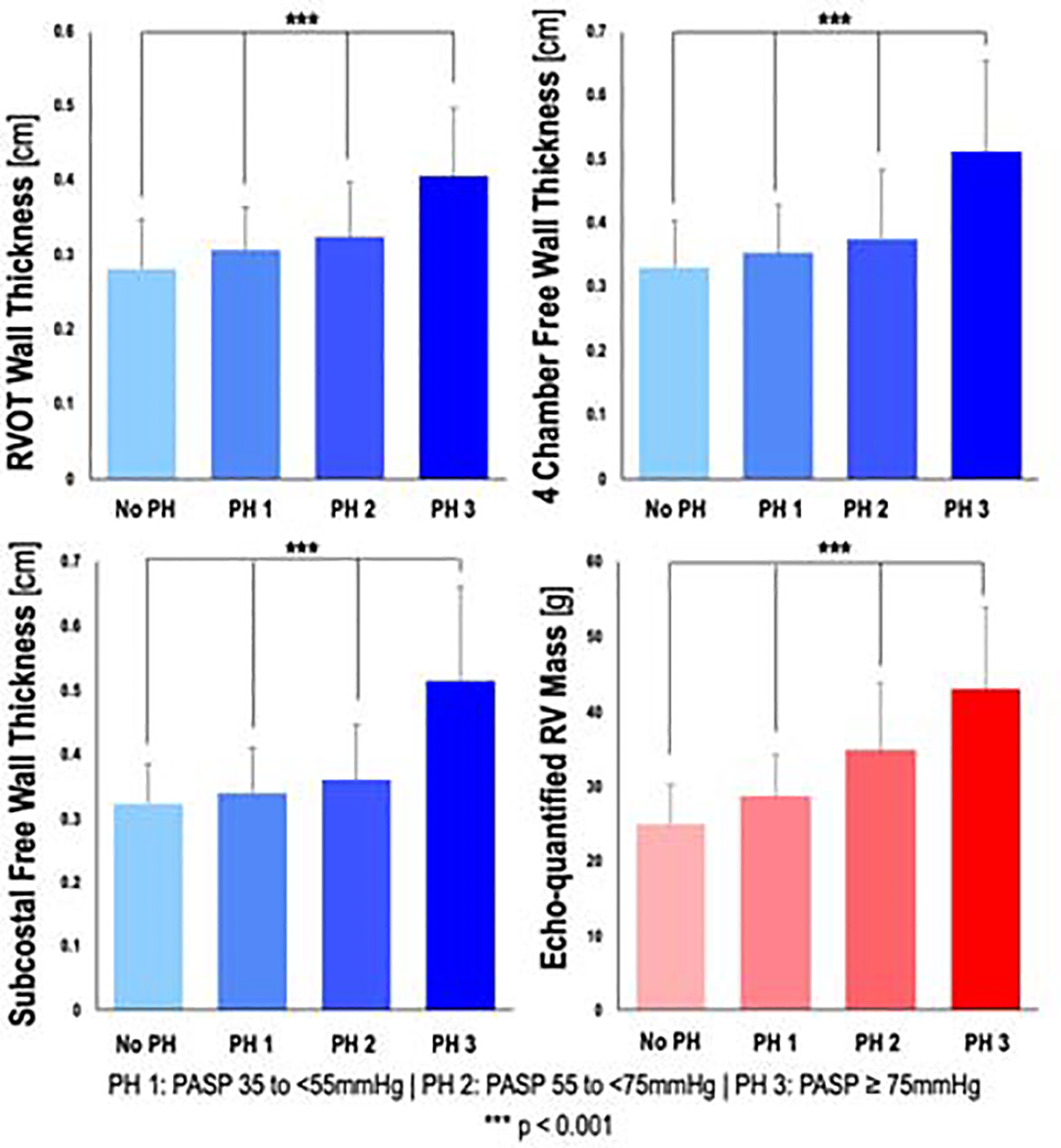
Bar graphs depicting RV wall thicknesses in the right ventricular outflow tract, and RV free wall as measured in the apical 4-chamber and subcostal views, as well as echo-quantified RV mass, stratified by presence and severity of pulmonary hypertension. P-values are for linear tests of trends.
Table 2a compares echo-derived linear dimensions among patients with and without CMR-evidenced RVH. Consistent with its above noted relationships between PA pressure, RV wall thickness increased among patients with RVH irrespective of echo orientation used for analyses (p<0.001 for all). Regarding RV chamber size, RVH was accompanied by geometric remodeling, as evidenced by increased volumetric chamber size on CMR (previously shown in Table 1) as well as linear chamber dimensions in apical 4-chamber (RV basilar, RV longitudinal) or parasternal (RVOT) long axis orientations (all p<0.01). Table 2b reports correlations between RV mass on CMR and echo-quantified linear indices, demonstrating geometry independent associations for echo derived variables (all p<0.001), among which the highest correlations were observed for subcostal free wall thickness (r=0.71) and apical 4-chamber basal RV diameter (r=0.73).
Table 2a.
Linear Dimensions Stratified by CMR-Evidenced RV Hypertrophy
| Derivation Cohort (n=100) | ||||
|---|---|---|---|---|
| Total (n=100) | RVH* + (n=43) | RVH* − (n=57) | P | |
| RVOT dimension (cm) | 3.46 ± 0.58 | 3.66 ± 0.60 | 3.31 ± 0.52 | 0.002 |
| RV basal dimension (cm) | 4.18 ± 0.74 | 4.68 ± 0.60 | 3.80 ± 0.60 | <0.001 |
| RV longitudinal dimension (cm) | 7.70 ± 0.94 | 8.06 ± 0.81 | 7.42 ± 0.95 | 0.001 |
| Proximal RVOT wall thickness (cm) | 0.34 ± 0.09 | 0.40 ± 0.09 | 0.30 ± 0.05 | <0.001 |
| 4-chamber RV free wall thickness (cm) | 0.40 ± 0.13 | 0.49 ± 0.14 | 0.32 ± 0.06 | <0.001 |
| Subcostal RV free wall thickness (cm) | 0.39 ± 0.12 | 0.48 ± 0.12 | 0.31 ± 0.04 | <0.001 |
Defined by the reference standard of CMR using established criteria(1) (males: >31.9g, females: >26.4g in females)
Table 2b.
Echo-Derived RV Parameters in Relation to CMR-Quantified RV Mass
| Correlation Coefficient (r) | Coefficient of Determination (r2) | P | |
|---|---|---|---|
| Linear Dimensions | |||
| RVOT dimension (cm) | 0.454 | 0.206 | <0.001 |
| RV basal dimension (cm) | 0.725 | 0.526 | <0.001 |
| RV longitudinal dimension (cm) | 0.521 | 0.271 | <0.001 |
| Wall Thickness | |||
| Proximal RVOT (mm) | 0.601 | 0.361 | <0.001 |
| 4-chamber RV free wall (mm) | 0.638 | 0.407 | <0.001 |
| Subcostal RV free wall (mm) | 0.707 | 0.500 | <0.001 |
Global RV Mass
To test utility of linear wall thickness cutoffs alone – as well as an integrated formula for global RV mass incorporating wall thickness and chamber dimensions – in diagnosis of CMR-evidenced RVH, analyses were performed in separate derivation and validation subgroups (each n=100), which were partitioned based on equivalent distributions of PH severity.
Figure 4A displays a scatterplot of CMR and echo-quantified global RV mass (based on geometric assumption of a ¼ prolate ellipsoid) in the derivation cohort, demonstrating a linear relationship (r=0.82, r2=0.68). Linear regression was performed to derive an empiric correction factor (regression coefficient: β=0.779) and constant (intercept: α=8.227g) for the relationship. As for RV wall thickness, Echo-estimated RV mass increased stepwise with increasing PA pressures (see Figure 3). The relationship between CMR and echo-quantified RV mass was not modified by the presence of advanced (≥ moderate) tricuspid regurgitation or WHO pulmonary hypertension classification (p=NS for interaction for both). Regression was applied to the validation cohort to produce an adjusted echo-quantified RV mass. Bland-Altman analysis comparing this adjusted estimate to CMR-quantified RV mass in the validation cohort is shown in Figure 4B, demonstrating mean difference of −1.64g (limits of agreement: −18.49g, 15.22g | coefficient of variation: 26.7%). Table 3 details inter- and intra-observer reproducibility for echo-derived RV variables incorporated into the echocardiographic RV mass formula. Findings demonstrate good reproducibility for all measurements.
Figure 4. Scatterplot relating echo-quantified to CMR quantified right ventricular mass in the derivation cohort (A) and Bland-Altman plot comparing Echo-quantified right ventricular mass to CMR-quantified right ventricular mass in the validation cohort (B).
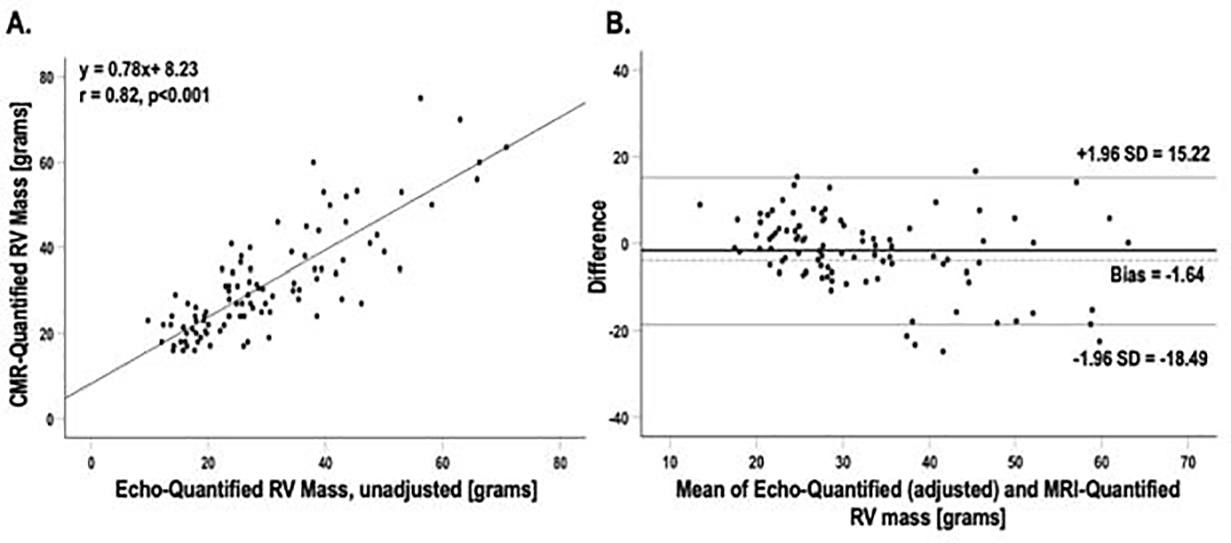
Correlation coefficients and corresponding linear regression line for scatter plot. Middle line on Bland-Altman plot line denotes mean. Outer lines denote ±1.96 standard deviations.
Table 3.
Methodological Reproducibility
| Δ, Limits of Agreement | Intraclass correlation coefficient | Coefficient of Variation (%) | |
|---|---|---|---|
| Intra-Observer | |||
| RV Basal Dimension (cm) | 0.09 (0.71, 1.35) | 0.870 | 7.64 |
| RV Length (cm) | 0.12 (1.03, 1.96) | 0.873 | 5.71 |
| Subcostal Wall Thickness (cm) | 0.05 (0.16, 0.27) | 0.740 | 19.86 |
| Inter-Observer | |||
| RV Basal Dimension (cm) | 0.12 (0.76, 1.4) | 0.831 | 8.72 |
| RV Length (cm) | 0.11 (1.04, 1.97) | 0.857 | 6.36 |
| Subcostal Wall Thickness (cm) | 0.05 (0.16, 0.27) | 0.750 | 18.79 |
To assess reliability of our echocardiographic RV mass assessment in patients with thin RV walls, we assessed performance of our formula among the 38 patients with subcostal wall thickness <3.0mm. A scatterplot and Bland-Altman analysis comparing unadjusted echo-quantified RV mass versus CMR-quantified mass are shown in the Supplement. Findings demonstrate narrower limits of agreement with smaller coefficient of variation than for the overall cohort.
Echo-derived RV wall thicknesses and adjusted echo-quantified RV mass cutoffs were tested in relation to diagnostic test performance for CMR-evidenced RVH. ROC analysis was first performed in the derivation cohort (Table 4a); cutoffs were chosen to maximize sensitivity while providing a minimum specificity of 80%.
Table 4a.
Diagnostic Performance of Echo RV Linear Dimensions for Right Ventricular Hypertrophy (Derivation Cohort)
| AUC (95% CI) | Cutoff | Sensitivity | Specificity | Accuracy | PPV | NPV | ||
|---|---|---|---|---|---|---|---|---|
| Cohort-Derived Wall Thickness Cutoffs | ||||||||
| Proximal RVOT wall thickness (mm) | Male | 0.79 (0.65–0.93) | 3.55mm | 61.9% | 83.9% | 75.0% | 72.2% | 76.5% |
| Female | 0.87 (0.78–0.97) | 3.40mm | 72.7% | 84.6% | 79.2% | 80.0% | 78.6% | |
| 4-chamber RV free wall thickness (mm) | Male | 0.86 (0.75–0.97) | 3.75mm | 76.2% | 83.9% | 80.8% | 76.2% | 83.9% |
| Female | 0.90 (0.82–0.99) | 3.60mm | 81.8% | 88.5% | 85.4% | 85.7% | 85.2% | |
| Subcostal RV free wall thickness (mm) | Male | 0.93 (0.86–1.00) | 3.65mm | 85.7% | 83.9% | 84.6% | 78.3% | 89.7% |
| Female | 0.94 (0.87–1.00) | 3.65mm | 86.4% | 92.0% | 89.4% | 90.5% | 88.5% | |
| Echo-quantified RV mass | ||||||||
| Echo-quantified RV mass (grams) | Male | 0.89 (0.80–0.98) | 31.9g | 76.2% | 87.1% | 82.3% | 80.0% | 84.4% |
| Female | 0.96 (0.91–1.00) | 25.3g | 100% | 84.0% | 91.5% | 84.6% | 100% | |
ASE-guideline and cohort-derived cutoffs were subsequently tested in a validation cohort. As shown in Table 4b, application of the SC-FWt cutoff recommended in ASE guidelines (RVH if ≥ 5mm) yielded modest diagnostic performance for CMR-evidenced RVH (sensitivity 30.6%, specificity 98.0%, accuracy 64.6%). Application of echo-quantified RV mass cutoffs [males: >31.9g, females: >26.4g] improved sensitivity (75.5% vs. 30.6%, p<0.001) at the expense of specificity (74.0% vs. 98.0%, p<0.001), with a trend towards increased accuracy (74.7% vs. 64.6%, p =0.12).
Table 4b.
Diagnostic Performance of Echo RV Parameters for Right Ventricular Hypertrophy (Validation Cohort)
| AUC (95% CI) | Cutoff | Sensitivity | Specificity | Accuracy | PPV | NPV | ||
|---|---|---|---|---|---|---|---|---|
| ASE Guideline Recommended Wall Thickness Cutoff | ||||||||
| Subcostal RV free wall (cm) | - | 5.00mm | 30.6% | 98.0% | 64.6% | 93.8% | 59.0% | |
| Cohort-Derived Wall Thickness Cutoffs | ||||||||
| Proximal RVOT wall thickness (mm) | Male | 0.74 (0.60–0.88) | 3.55mm | 47.9% | 81.6% | 64.9% | 71.9% | 61.5% |
| Female | 0.77 (0.63–0.90) | 3.40mm | ||||||
| 4-chamber RV free wall thickness (mm) | Male | 0.64 (0.49–0.79) | 3.75mm | 60.4% | 68.0% | 64.2% | 64.4% | 64.2% |
| Female | 0.80 (0.66–0.93) | 3.60mm | ||||||
| Subcostal RV free wall thickness (mm) | Male | 0.67 (0.52–0.82) | 3.65mm | 65.3% | 76.0% | 70.7% | 72.7% | 69.1% |
| Female | 0.84 (0.73–0.95) | 3.65mm | ||||||
| Echo-quantified RV mass | ||||||||
| Echo-quantified RV mass (grams) | Male | 0.85 (0.74–0.95) | 31.9g | 75.5% | 74.0% | 74.7% | 74.0% | 75.5% |
| Female | 0.88 (0.78–0.97) | 25.3g | ||||||
Clinical Prognosis
Mortality was assessed to determine the prognostic impact of echo-derived RV mass. During an interval of 4.2 (IQR 3.0, 4.5) years following imaging, 18% (n=36) of patients died. As shown in Figure 5, echo-derived RVH stratified risk for death (HR 1.98, [1.09–3.88], p=0.048), paralleling similar risk for CMR-quantified RVH (HR 2.41, [1.22–4.78], p=0.01). In multivariable analysis adjusted for age, echo-derived RVH was found to be significantly associated with death (HR 2.07, [95% CI: 1.06–4.07], p=0.03), and yielded similar degree of association as to CMR-derived RVH (HR 2.70, [95% CI: 1.36–5.37], p=0.01) as well as echo-quantified PH (HR 2.11, [95% CI: 0.99–4.55], p=0.05). Hazard ratios for RVH as assessed by wall thickness or RV mass overlap both in adjusted and unadjusted conditions. Regarding components of RV mass quantification, increased RV wall thickness stratified risk for death (HR 2.44 [1.16–5.12], p=0.018) whereas RV basal dimension did not (HR .97 [0.50–1.88], p=0.92), thereby highlighting the concept that the association of RV mass with death is driven primarily by RV wall thickness, rather than RV chamber size.
Figure 5. Mortality in Relation to right ventricular hypertrophy.

Kaplan-Meier survival curves depicting all-cause mortality among groups stratified based on CMR-quantified (A) or Echo-quantified (B) right ventricular hypertrophy ([males: >31.9g, females: >26.4g], by either modality).
Discussion
This is the first study to systematically test RV linear dimensions and wall thicknesses in relation to CMR-quantified RV mass for assessment of RVH, and the first to evaluate an echo-based geometric formula for quantification of RV mass across a wide range of RV conditions and to show that echo-based RV mass quantification stratifies mortality. There are several key findings: (1) Complete echo linear dimensions and wall thicknesses were measurable in nearly all patients (95%). (2) RV linear dimensions and wall thicknesses were significantly larger among patients with CMR-evidenced RVH (p<0.001 for all), and the greatest correlation with RV mass was observed between the RV basal dimension (r=0.73) and subcostal wall thickness (r=0.71). In addition, echo-quantified RV mass – based on the geometric assumption of a ¼ prolate ellipsoid – correlated strongly with RV mass (r=0.82). (3) ASE guideline recommended subcostal wall thickness cutoffs (5.0mm) yielded high specificity but low sensitivity for CMR-evidenced RVH. (4) RVH as assessed by echo and CMR quantified RV mass was similarly associated with mortality [(HR 1.98, [95% CI: 1.01–3.88], p= 0.048) and (HR 2.41, [95% CI: 1.22–4.78], p=0.01)]. Taken together, these findings indicate that RV mass quantified on routine 2D echo provides a physiologic index of RV loading conditions with prognostic value similar to that of less widely available volumetric modalities such as CMR.
It is important to recognize that RV linear assessment, especially that of the RV free wall, is known to be challenging due to the anterior and retrosternal location of the RV, as well as its thin-walled nature adjacent to epicardial fat. Current ASE guidelines recommend RV wall thickness to be measured in the subcostal or parasternal long axis orientation. However, the feasibility and incremental value of these methodologies in relation to one another has not been methodologically tested and current assessment does not take into account RV chamber size which is known to affect aggregate RV mass quantification. To address these knowledge gaps, our study tested RV wall thickness in multiple views – including the parasternal long axis, apical 4C, and subcostal views. In our study, wall thickness derived in the subcostal orientation provided highest diagnostic accuracy – likely reflecting higher axial resolution in the subcostal view with perpendicular orientation of ultrasound beams to the RV free wall – and therefore was incorporated into the RV mass formula along with RV basal and longitudinal linear dimension. Our findings using this approach indicate that RV wall thickness in combination with RV linear dimensions on conventional echo provides incremental information regarding RV geometry. Whereas RV subcostal wall thickness and RV basal linear chamber dimension correlated reasonably well with CMR derived RV mass (r=0.71–0.73), correlations increased when both indices were considered in our prolate ellipsoid model for echo quantified RV mass (r=0.82).
Our findings build upon prior research by our group and others on quantitative echo methods to assess RV chamber remodeling. Prior studies have employed two-dimensional echocardiography for RV mass quantification. Joyce et al(17) derived a formula for RV mass based on a similar ¼ prolate ellipsoid assumption in predominantly congenital heart disease pediatric patients who underwent echo and CMR in close proximity; quantified RV mass formula correlated highly (r=0.97) with CMR-derived mass. However, it should be noted that measurements in a pediatric – predominantly congenital, where geometric relationships may be altered – may not be directly applicable to a broader adult cohort. Pontes et al(18) used bullet and trapezium geometric assumptions to estimate mass in 20 human patients before transplant and demonstrated good correlation with explanted RV free wall mass (r = 0.84 and 0.81, respectively). However, these approaches leveraged measurements not routinely obtained in standard echocardiography practice, thus limiting clinical adoptability. Our approach achieves similar correlation (r = 0.82) using standardized ASE-guideline concordant measurements in a broadly generalizable adult cohort. Notably, while echo and CMR-quantified RV mass were tightly associated at the lower range of RV mass, heteroscedasticity was seen at higher values, likely reflecting distortion of the geometric relationship between echocardiographic linear dimensions and RV surface area with right ventricular dilation.
Regarding prognosis, it is important to note that in our study, survival curves comparing patients with and without RVH do not separate until 2 years. This finding may be due to the fact study inclusion did not necessitate a new diagnosis of pulmonary hypertension – patients were studied based only on the PA pressure at a prespecified time window within our institution. Prior data examining patients with primary pulmonary hypertension(19) demonstrates an initial precipitous survival drop over the first year of diagnosis followed by a survival plateau. In this context, it should also be noted that in our population, patients without elevated pulmonary pressure were not “normal” controls, but instead a population of patients without pulmonary hypertension who were referred for echo and MRI exams for a variety of indications which may themselves be associated with mortality.
It should also be noted that as stated in the guidelines, widespread utilization of linear RV wall thickness measurements has been limited by sparse prognostic data available in select cohorts. Steiner et al studied a series of 152 patients with echo-evidenced pulmonary hypertension, showing that increased RV wall thickness was associated with mortality independent of age and RV dysfunction.(20) Katz et al similarly studied a series of 419 patients with heart failure with preserved ejection fraction and demonstrated RV free wall thickness in the subcostal view >4.8mm to be predictive of mortality.(21) On the other hand, RVH as defined using CMR – a less widely available imaging modality – is an established prognostic marker in a broad array of cardiovascular conditions including pulmonary hypertension,(22) hypertrophic cardiomyopathy,(23) and tetralogy of Fallot(24, 25), as well as in normative population cohorts.(1, 26) Importantly, in our population RVH by CMR was seen in 21% of patients with normal PASP (n=17), only one of whom had subcostal wall thickness >5.0 mm supporting the notion that comprehensive assessment of RV wall thickness and chamber size is important for assessment of right ventricular hypertrophy. Our study is the first to assess the prognostic impact of increased RV mass as quantified by echo across a wide spectrum of PH strata and to demonstrate that mortality stratification on echo paralleled that on CMR.
Study Limitations
Several limitations should be noted. First, our formula does not consider myocardial mass encompassed in the RV outflow tract, which is an important region where RV remodeling can occur as measured by CMR. Attempts to incorporate this geometry into our formula were limited by inconsistent imaging of the distal RVOT dimension on echocardiography, which is a known challenge with sonographic technique. Additionally, while complete right ventricular measurements were feasible in nearly all (95%) patients in the cohort, it is important to note that image quality may have been favorably biased by the study inclusion criteria of a quantifiable PASP. It should also be noted that tricuspid regurgitation was assessed by semi-quantitative methodology; future studies using quantitative techniques (e.g. PISA and volumetric Doppler) are needed to more rigorously test the impact of tricuspid regurgitation on loading conditions, RVH and mortality. Finally, our echo-quantified mass RVH cutoffs were derived from established CMR normative values. Application of this approach in a normative echo population to test a more broadly applicable RV mass cutoff has the potential to further optimize accuracy of this approach for RVH assessment. Additionally, further research is warranted to test the relative prognostic utility of echo derived RVH and RV function in larger-scale epidemiologic cohorts and to prospectively examine the temporal relationship between RVH and survival.
Conclusions
In conclusion, the current study of patients across a wide range of RV afterload states demonstrates that integrated assessment incorporating conventional linear chamber dimensions with subcostal wall thickness allows for RV mass quantification that correlates strongly with CMR, with good diagnostic performance for detection of RVH. Echo-derived RVH stratified risk for death, paralleling similar risk for CMR-quantified RVH.
Supplementary Material
Highlights.
This study tested echo-derived parameters for RV mass quantification.
Echo-quantified RV mass has good diagnostic performance in relation to RVH on CMR.
Echo-derived RVH confers similar mortality risk compared to RVH on CMR.
Echo RV parameters are important diagnostic and prognostic indices of RV afterload.
Sources of Funding:
This work was supported by the NIH (1K23 HL140092, 1R01HL128278, 5T32 HL7854–23) and Glorney-Raisbeck Fellowship Program via the Corlette Glorney Foundation and the New York Academy of Medicine
Abbreviations
- 4C-FWt
4-chamber free wall thickness
- ASE
American Society of Echocardiography
- CMR
cardiac magnetic resonance
- Echo
echocardiography
- ESV
end-systolic volume
- EDV
end-diastolic volume
- LV
left ventricle
- LVEDd
left ventricular basal dimension in end-diastole
- PASP
pulmonary arterial systolic pressure
- PH
pulmonary hypertension
- ROC
receiver operating characteristic
- RVBd
right ventricular basal dimension
- RVH
right ventricular hypertrophy
- RVLd
right ventricular longitudinal dimension
- RVOTd
right ventricle outflow tract dimension
- RVOT-Wt
right ventricular outflow tract wall thickness
- SC-FWt
subcostal free wall thickness
Footnotes
Conflicts of Interests Disclosure: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kawut SM, Barr RG, Lima JA, Praestgaard A, Johnson WC, Chahal H, et al. Right ventricular structure is associated with the risk of heart failure and cardiovascular death: the Multi-Ethnic Study of Atherosclerosis (MESA)--right ventricle study. Circulation. 2012;126(14):1681–8. Epub 2012/08/31. doi: 10.1161/CIRCULATIONAHA.112.095216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mooij CF, de Wit CJ, Graham DA, Powell AJ, Geva T. Reproducibility of MRI measurements of right ventricular size and function in patients with normal and dilated ventricles. J Magn Reson Imaging. 2008;28(1):67–73. Epub 2008/06/27. doi: 10.1002/jmri.21407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grothues F, Moon JC, Bellenger NG, Smith GS, Klein HU, Pennell DJ. Interstudy reproducibility of right ventricular volumes, function, and mass with cardiovascular magnetic resonance. Am Heart J 2004;147(2):218–23. Epub 2004/02/05. doi: 10.1016/j.ahj.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 4.Farber NJ, Reddy ST, Doyle M, Rayarao G, Thompson DV, Olson P, et al. Ex vivo cardiovascular magnetic resonance measurements of right and left ventricular mass compared with direct mass measurement in excised hearts after transplantation: a first human SSFP comparison. J Cardiovasc Magn Reson. 2014;16:74. Epub 2014/10/16. doi: 10.1186/s12968-014-0074-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lindqvist P, Henein M, Kazzam E. Right ventricular outflow-tract fractional shortening: an applicable measure of right ventricular systolic function. Eur J Echocardiogr 2003;4(1):29–35. Epub 2003/02/05. doi: 10.1053/euje.2002.0177. [DOI] [PubMed] [Google Scholar]
- 6.Kim J, Srinivasan A, Seoane T, Di Franco A, Peskin CS, McQueen DM, et al. Echocardiographic Linear Dimensions for Assessment of Right Ventricular Chamber Volume as Demonstrated by Cardiac Magnetic Resonance. J Am Soc Echocardiogr 2016;29(9):861–70. Epub 2016/06/15. doi: 10.1016/j.echo.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Srinivasan A, Kim J, Khalique O, Geevarghese A, Rusli M, Shah T, et al. Echocardiographic linear fractional shortening for quantification of right ventricular systolic function-A cardiac magnetic resonance validation study. Echocardiography. 2017;34(3):348–58. Epub 2017/03/02. doi: 10.1111/echo.13438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van der Zwaan HB, Geleijnse ML, McGhie JS, Boersma E, Helbing WA, Meijboom FJ, et al. Right ventricular quantification in clinical practice: two-dimensional vs. three-dimensional echocardiography compared with cardiac magnetic resonance imaging. European Journal of Echocardiography. 2011;12(9):656–64. doi: 10.1093/ejechocard/jer107. [DOI] [PubMed] [Google Scholar]
- 9.Devereux RB, Alonso DR, Lutas EM, Gottlieb GJ, Campo E, Sachs I, et al. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol 1986;57(6):450–8. Epub 1986/02/15. doi: 10.1016/0002-9149(86)90771-x. [DOI] [PubMed] [Google Scholar]
- 10.Devereux RB, Reichek N. Echocardiographic determination of left ventricular mass in man. Anatomic validation of the method. Circulation. 1977;55(4):613–8. Epub 1977/04/01. doi: 10.1161/01.cir.55.4.613. [DOI] [PubMed] [Google Scholar]
- 11.Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr 2010;23(7):685–713; quiz 86–8. Epub 2010/07/14. doi: 10.1016/j.echo.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 12.Kim J, Krichevsky S, Xie L, Palumbo MC, Rodriguez-Diego S, Yum B, et al. Incremental Utility of Right Ventricular Dysfunction in Patients With Myeloproliferative Neoplasm-Associated Pulmonary Hypertension. J Am Soc Echocardiogr 2019;32(12):1574–85. Epub 2019/10/08. doi: 10.1016/j.echo.2019.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Devereux RB, Roman MJ, Palmieri V, Liu JE, Lee ET, Best LG, et al. Prognostic implications of ejection fraction from linear echocardiographic dimensions: the Strong Heart Study. Am Heart J 2003;146(3):527–34. Epub 2003/08/30. doi: 10.1016/s0002-8703(03)00229-1. [DOI] [PubMed] [Google Scholar]
- 14.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015;28(1):1–39.e14. Epub 2015/01/07. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 15.Klamkin MS. Elementary Approximations to the Area of N-Dimensional Ellipsoids. The American Mathematical Monthly. 1971;78(3):280–3. doi: 10.2307/2317530. [DOI] [Google Scholar]
- 16.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1(8476):307–10. [PubMed] [Google Scholar]
- 17.Joyce JJ, Denslow S, Kline CH, Baylen BG, Wiles HB. Estimation of right ventricular free-wall mass using two-dimensional echocardiography. Pediatr Cardiol 2001;22(4):306–14. Epub 2001/07/17. doi: 10.1007/s002460010235. [DOI] [PubMed] [Google Scholar]
- 18.Pontes SC Jr., Assef JE, Barretto RB, Chaccur P, Moreira DA, Da SNVJ, et al. Estimation of right ventricular mass by two-dimensional echocardiography. J Am Soc Echocardiogr 2005;18(5):427–34. Epub 2005/05/14. doi: 10.1016/j.echo.2005.01.036. [DOI] [PubMed] [Google Scholar]
- 19.D’Alonzo GE, Barst RJ, Ayres SM, Bergofsky EH, Brundage BH, Detre KM, et al. Survival in patients with primary pulmonary hypertension. Results from a national prospective registry. Ann Intern Med 1991;115(5):343–9. Epub 1991/09/01. doi: 10.7326/0003-4819-115-5-343. [DOI] [PubMed] [Google Scholar]
- 20.Steiner J, Wu WC, Jankowich M, Maron BA, Sharma S, Choudhary G. Echocardiographic predictors of mortality in patients with pulmonary hypertension and cardiopulmonary comorbidities. PLoS One. 2015;10(3):e0119277. Epub 2015/03/17. doi: 10.1371/journal.pone.0119277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burke MA, Katz DH, Beussink L, Selvaraj S, Gupta DK, Fox J, et al. Prognostic importance of pathophysiologic markers in patients with heart failure and preserved ejection fraction. Circ Heart Fail. 2014;7(2):288–99. Epub 2013/12/25. doi: 10.1161/CIRCHEARTFAILURE.113.000854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Badagliacca R, Poscia R, Pezzuto B, Nocioni M, Mezzapesa M, Francone M, et al. Right ventricular remodeling in idiopathic pulmonary arterial hypertension: adaptive versus maladaptive morphology. J Heart Lung Transplant. 2015;34(3):395–403. Epub 2014/12/17. doi: 10.1016/j.healun.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 23.Nagata Y, Konno T, Fujino N, Hodatsu A, Nomura A, Hayashi K, et al. Right ventricular hypertrophy is associated with cardiovascular events in hypertrophic cardiomyopathy: evidence from study with magnetic resonance imaging. Can J Cardiol 2015;31(6):702–8. Epub 2015/05/04. doi: 10.1016/j.cjca.2014.12.036. [DOI] [PubMed] [Google Scholar]
- 24.Valente AM, Gauvreau K, Assenza GE, Babu-Narayan SV, Schreier J, Gatzoulis MA, et al. Contemporary predictors of death and sustained ventricular tachycardia in patients with repaired tetralogy of Fallot enrolled in the INDICATOR cohort. Heart. 2014;100(3):247–53. Epub 2013/11/02. doi: 10.1136/heartjnl-2013-304958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beurskens NEG, Hagdorn QAJ, Gorter TM, Berger RMF, Vermeulen KM, van Melle JP, et al. Risk of cardiac tachyarrhythmia in patients with repaired tetralogy of Fallot: a multicenter cardiac MRI based study. Int J Cardiovasc Imaging. 2019;35(1):143–51. Epub 2018/08/11. doi: 10.1007/s10554-018-1435-9. [DOI] [PubMed] [Google Scholar]
- 26.Chatterjee NA, Shah RV, Murthy VL, Praestgaard A, Shah SJ, Ventetuolo CE, et al. Right Ventricular Structure and Function Are Associated With Incident Atrial Fibrillation: MESA-RV Study (Multi-Ethnic Study of Atherosclerosis-Right Ventricle). Circ Arrhythm Electrophysiol 2017;10(1). Epub 2017/01/14. doi: 10.1161/CIRCEP.116.004738. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


