Abstract
Aim
In the present study, we performed bioinformatics studies and in vitro functional assays to explore the underlying role of serpin family H member 1 (SERPINH1) in the diabetic retinopathy.
Methods
Common differentially expressed genes (DEGs) between diabetic retinal tissues and normal retinal tissues were analyzed using Gene Expression Omnibus (GEO) database. The proliferation and migration of human retinal endothelial cells (HRECs) was evaluated by MTS, EdU and wound healing assays, respectively; the miRNA and mRNAs expression levels of hub genes in HRECs were determined using quantitative real-time PCR (qRT-PCR). Protein levels were determined using a Western blot assay.
Results
A total of 189 common DEGs were screened between two GEO datasets (GSE60436 and GSE94019), and ten potential hub genes that may link to the progression of diabetic retinopathy were detected. The qRT-PCR results showed that collagen, type I, alpha 1 (COL1A1), Collagen, type I, alpha 2 (COL1A2) and serpin family H member 1 (SERPINH1) mRNA expression levels were up-regulated in the HRECs after being exposed to high glucose for 48 h. Silence of SERPINH1 repressed the high glucose-induced increase in proliferation and migration of HRECs. SERPINH1 was a target of miR-29b and was suppressed by miR-29 in HRECs. SERPINH1 overexpression promoted HREC proliferation and migration. Furthermore, miR-29b suppressed HREC proliferation and migration under high-glucose stimulation, which was significantly attenuated by enforced expression of SERPINH1.
Conclusion
In conclusion, by performing the integrated bioinformatics analysis, the present study suggested that 3 hub genes (COL1A1, COL1A2 and SERPINH1) may be associated with diabetic retinopathy pathophysiology. Further mechanistic studies indicated that miR-29b/SERPINH1 signaling participated in high glucose-induced enhancement in the proliferation and migration of HRECs.
Keywords: diabetic retinopathy, bioinformatics analysis, HRECs, SERPINH1, proliferation, migration
Introduction
Diabetic retinopathy is a type of microvascular complication featured by dysfunction in the retinal microvascular, and it can lead to impaired vision and loss of vision in diabetic patients.1 The incidence of diabetic retinopathy tends to increase annually in China with the increased aging population.2 The death of endothelial cells and pericytes are the main features at the early stage of diabetic retinopathy. With the progression of diabetic retinopathy, the increased vascular leakage will lead to diabetic macular edema, which may cause vision loss.3 The main treatments for diabetic retinopathy include intraocular injection of anti-neovascularization drug, panretinal photocoagulation and vitrectomy.1 There is growing evidence indicating that the main causative contributors to diabetic retinopathy include oxidative stress, cell apoptosis, inflammation and autophagy;3 whereas the molecular mechanisms underlying diabetic retinopathy progression remain unclear. In this regard, further understanding into the pathophysiology of diabetic retinopathy is of great importance for developing novel therapies for diabetic retinopathy.
With the development of high-throughput technologies, discovery of novel genes associated with specific diseases has been greatly accelerated.4 Recently, several key regulators in the pathophysiology of diabetic retinopathy have been uncovered via different high-throughput technologies. Lam et al identified that runt-related transcription factor 1 was involved in aberrant retinal angiogenesis by using transcriptomic analysis.5 Berdasco et al performed the genome-scale DNA methylation profiling using samples from normal human eye and five ocular-related diseases, and the studies found that three key genes including ETS proto-oncogene 1 and PR domain containing 16 participated in neuro-vascularization during diabetic retinopathy.6 With the aid of bioinformatics tool, analysis of public available datasets has revealed new mediators in the regulation of diabetic retinopathy progression. You et al performed the analysis of weighted genes in diabetic retinopathy from GSE19122 datasets and proposed that metastasis associated with lung adenocarcinoma transcript 1 might play important roles in diabetic retinopathy.7 Ishikawa et al performed microarray analysis (GSE60436) and found that extracellular matrix-related molecules, such as periostin, tenascin C, tumor growth factor beta, and angiogenic factors, have important roles in promoting the development of preretinal fibrovascular membranes associated with diabetic retinopathy.8 Lam et al performed the transcriptomic analysis and their results suggested that the preferential selection of inflammatory and angiogenic pathways using this gene list is highly consistent with diabetic retinopathy pathogenesis, which involves leaky and aberrant vessel growth.5 Platania et al performed Gene Expression Omnibus (GEO) datasets with an enrichment-information approach, which gave as output a series of complex gene-pathway and drug-gene networks. Analysis of these networks identified genes and biological pathways related to inflammation, fibrosis and G protein-coupled receptors that are potentially involved in the development of the disease.9
In the present study, we analyzed the differentially expressed genes (DEGs) from GSE60436 and GSE94019 datasets; further comprehensive bioinformatics analysis was performed to decipher potential hub genes in the pathophysiology of diabetic retinopathy. These hub genes expression levels were validated in an in vitro cellular diabetic retinopathy model. The mechanistic studies were further undertaken to uncover the potential role of serpin family H member 1 (SERPINH1) in the pathophysiology of diabetic retinopathy.
Materials and Methods
Collection of Microarray Data, Data Preprocessing and DEGs Screening
The GEO datasets including GSE60436 (3 normal retinal tissues and 6 retinal tissues with diabetic retinopathy) and GSE94019 (3 normal retinal tissues and 9 retinal tissues with diabetic retinopathy) were retrieved from the Gene Expression Omnibus database. The differentially expressed genes (DEGs) for GSE94019 were extracted using the Geo RNA-seq experiments Interactive Navigator tool;10 for GSE60634, the data were processed with log2 transforming by “Limma” R package and normalized by median normalization. Then, we also used the “Limma” R package to screen the DEGs. A |logFC| >1.5 and false discovery rate < 0.05 were chosen as an optimum fold change cutoff value for the identification of DEGs.
Functional Analysis of DEGs
Gene Ontology (GO) and the Kyoto encyclopedia of genes and genomes (KEGG) database were undertaken to classify the functionalities of these DEGs.11–13 A ontology-based tool, g:Profiler (https://biit.cs.ut.ee/gprofiler/gost), was used to perform Gene Ontology (GO) enrichment, and KEGG pathway, Reactome pathway, WikiPathway and miRNA pathway analysis for the DEGs.14
Protein–Protein Interaction (PPI) Network Analysis
The PPI network analysis was performed using the Search Tool for the Retrieval of Interacting Genes (STRING).15 The interactions between DEGs were evaluated using STRING and genes with a combined score > 0.7 were defined as hub DEGs. Subsequently, Cytoscape (version 3.6.1) was used to generate PPI network of hub DEGs that were identified. Molecular complex detection (MCODE) and cytoHubba, the Cytoscape plugins, were used with the default parameters to identify subset modules.
Cell Culture
Human retinal endothelial cells (HRECs) were purchased from ScienCell (Carlsbad, USA). Cells were kept in the endothelial cell medium supplied with 5% fetal bovine serum (FBS; Thermo Fisher Scientific, Waltham, USA) and 1% endothelial cell growth supplement (ScienCell) as suggested by the manufacturer. The HRECs were kept in a humidified atmosphere with 5% CO2 at 37°C.
Hyperglycemia Treatment and Cell Transfections
HRECs were seeded in the 6-well plates with a density of 2×105 cells/well and were treated with 25 mM glucose (high glucose group), 5.5 mM glucose (normal glucose group) or 19.5 mM mannitol together with 5.5 mM glucose (osmotic group) for 48 h under normoxic conditions. The culture medium was refreshed every 24 h during the culturing process.
The SERPINH1 siRNA and scrambled siRNA were obtained from RiboBio (Guangzhou, China). MiR-29b mimics, miR-29b inhibitor and the corresponding negative controls (NCs: mimics NC and inhibitor NC) were also purchased from RiboBio. For SERPINH1-overexpressing vector, pcDNA-SERPINH1 and its NC (pcDNA) were Sangon Biotech Co., Ltd. (Shanghai, China). The HRECs were transfected with siRNAs, miRNAs or plasmids by using the Lipofectamine 3000 reagent (Invitrogen, Carlsbad, USA).
MTS Assay
The 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxy-methoxyphenyl)-2-(4-sulfophenyl)-2H–tetrazolium (MTS) kit (Beyotime, Beijing, China) was used to evaluate the cell viability of HRECs by following by the manufacturer’s protocol. HRECs cells were seeded in the 96-well plates at a density of 2×104 cells/well. After different treatments for 48 h, the cells were incubated with 20 μL of MTS for 1.5 h at 37 °C. After that, the cell proliferative index was evaluated by detecting the absorbance at 490 nm.
Quantitative Real-Time PCR
Total RNA was extracted from HERCs with different treatments for 48 h using the TRIzol reagent (Invitrogen, Carlsbad, USA) according to the manufacturer’s protocol, and RNA concentration and purity were measured on a NanoDrop 2000c spectrophotometer (Thermo Fisher Scientific). A total of 500 ng RNA was reversely transcribed using a Perfect real-time RT reagent kit (Takara Bio, Beijing, China). The real-time PCR reactions were performed on a LightCycler 480 (Roche Diagnostics, Basel, Switzerland). Gene expression was detected using 2−ΔΔCt method. U6 and GAPDH were used as the internal controls for miRNA and mRNA expression, respectively. The sequences for the primers are listed in Supplemental Table S1.
EdU Assay
The HREC proliferation was accessed using a 5-ethynyl-2ʹ-deoxyuridine (EdU) detection kit (Beyotime, Beijing, China). Briefly, HRECs with different treatments were incubated with 50 μM EdU for 2 h at 37°C. After that, HRECs were fixed with 4% paraformaldehyde for 15 min at room temperature followed by EdU staining at room temperature for 30 min in the dark. After that, the cells were incubated with 5 μg/mL Hoechst 33,342 dye for 30 min at room temperature for 20 min. The cell proliferation was assessed by percentage of EdU-positive cells.
Wound Healing Assay
HRECs with different treatments were cultured to full confluence, and a 1 mm wide wound was created by a pipette tip. Cell debris was removed by rinsing with phosphate buffered saline and the cell monolayer was further cultured at 37°C with 5% CO2 for another 48 h. Wound width was measured under a microscope at 0 and 48 h after treatments.
Western Blot Assay
The Western blot assay was performed according to previous studies.16 Briefly, cell lysates were extracted from Protein Lysis Buffer (Sigma-Aldrich, St. Louis, USA). The primary antibodies, including SERPINH1 (1:1000 in dilution) and β-actin (1:3000 in dilution) and horseradish conjugated secondary antibody (1:3000 in dilution), were purchased from Cell Signaling Technology (Danvers, USA). β-actin was used as the reference control.
Dual-Luciferase Reporter Assay
Wild type (wt) SERPINH1 3ʹ untranslated region (3ʹUTR) sequences targeted by miR-29b were subcloned into a pGL3-report vector (Promega, Madison, USA), named SERPINH1 3ʹUTR-wt. Mutant (mut) SERPINH1 3′-UTR bearing a substitution of 4 nucleotides (GGUG to CCAC) in the predicted sites named SERPINH1 3ʹUTR-mut. For dual-luciferase reporter assay, HEK293 cells were co-transfected with reporter vectors (SERPINH1 3ʹUTR-wt or SERPINH1 3ʹUTR-mut) and miRNAs (mimics NC or miR-29b mimics) using Lipofectamine 3000 reagent. At 48 h after transfection, the luciferase activity was determined by using the Dual-Luciferase Reporter Assay kit (Promega).
Statistical Analysis
Results are presented as means ± standard deviation. Each in vitro experiment was repeated at least three times. Prism 6.0 software (GraphPad Software, San Diego, CA, USA) was used for the analysis of statistical significance. Unpaired Student’s t-test or one-way analysis of variance followed by the Bonferroni’s post hoc test was used to assess statistical differences between/among groups in multiple comparisons. The presented p values are two-sided, and results were considered statistically significant at a p value less than 0.05.
Results
Analysis of DEGs from GSE60436 and GSE94019
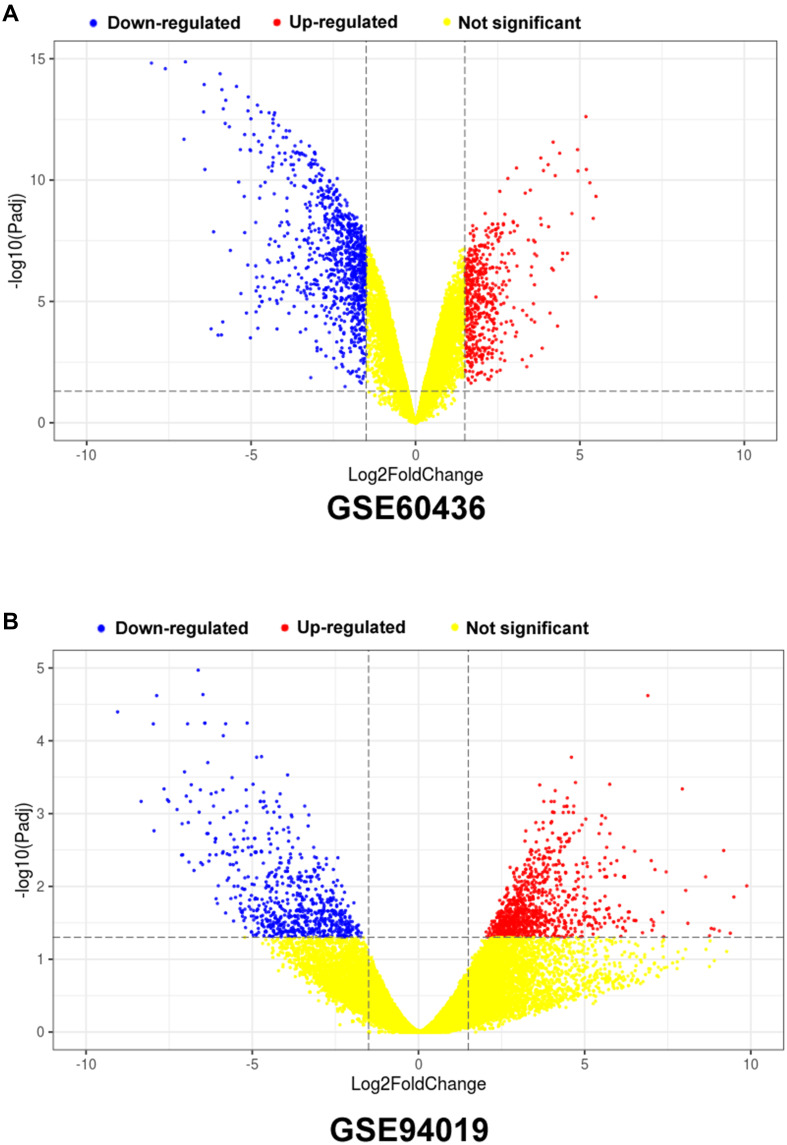
In the present study, we used different bioinformatics tools to extract the DEGs from database GSE60436 and GSE94019. As shown in Figure 1A, a total of 1281 DEGs were identified in the GSE60436, and among these DEGs, 454 genes were up-regulated and 827 genes were down-regulated (Figure 1A). For the GSE94019 dataset, a total of 1655 genes were identified with 966 up-regulated genes and 689 down-regulated genes (Figure 1B).
Figure 1.
Volcano plots of the DEGs in the GSE60436 and GSE94019 datasets. (A) Volcano plot of DEGs from GSE60436; (B) Volcano plot of DEGs from GSE94019.
Analysis of Common DEGS Between GSE60436 and GSE94019
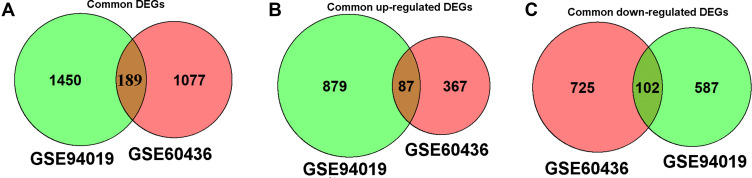
As shown in Figure 2A, a total of 189 common DEGs were detected between GSE60436 and GSE94019 datasets. In a further analysis, 87 commonly up-regulated DEGs were identified between these two datasets (Figure 2B); on the other hand, 102 commonly down-regulated DEGs were detected in these two datasets (Figure 2C). As such, a total of 189 DEGs were chosen for further analysis.
Figure 2.
Venn diagram showing the common DEGs between GSE60436 and GSE94019 datasets. (A) Venn diagram of the common DEGs between GSE60436 and GSE94019. (B) Venn diagram of the common up-regulated DEGs between GSE60436 and GSE94019. (C) Venn diagram of the common down-regulated DEGs between GSE60436 and GSE941919.
GO Analysis of DEGs
The GO analysis results were illustrated in Supplemental Figure S1. In biological process, DEGs were clustered in “visual perception”, “sensory perception of light stimulus”, “sensory perception”, “rhodopsin mediated signaling pathway” and so on (Supplemental Figure S1A). In cellular component, DEGs were clustered in “photoreceptor outer segment”, “photoreceptor cell cilium”, “9+0 non-motile cilium”, “extracellular matrix” and so on (Supplemental Figure S1B). For molecular function, DEGs were clustered in “extracellular matrix structural constituent”, “structural molecule activity”, “extracellular matrix structural constituent conferring tensile strength”, “collagen binding” and so on (Supplemental Figure S1C).
Pathways Analysis of Common DEGs Between GSE60436 and GSE94019
For KEGG analysis, DEGs were classified into the pathways associated with “phototransduction”, “ECM-receptor interaction”, “PI3K-Akt signaling pathway”, “AGE-RAGE signaling pathway in diabetic complications” and so on (Supplemental Figure S2A). For the Reactome analysis, the DEGs were classified into the pathways associated with “visual phototransduction”, “The phototransduction cascade”, “signaling by receptor tyrosine kinases” and so on (Supplemental Figure S2B). For the WikiPathways, the DEGS were classified into “miRNA targets in the EMC and membrane receptors”, “Focal Adhesion-PI3K-Akt-mTOR-signaling pathway”, “PI3K-Akt signaling pathway”, “inflammatory response pathway” and so on (Supplemental Figure S2C). For the miRNA pathway, miR-29b-3p, miR-29c-3p and miR-29a-3p were found to interact with these DEGs (Supplemental Figure S2D).
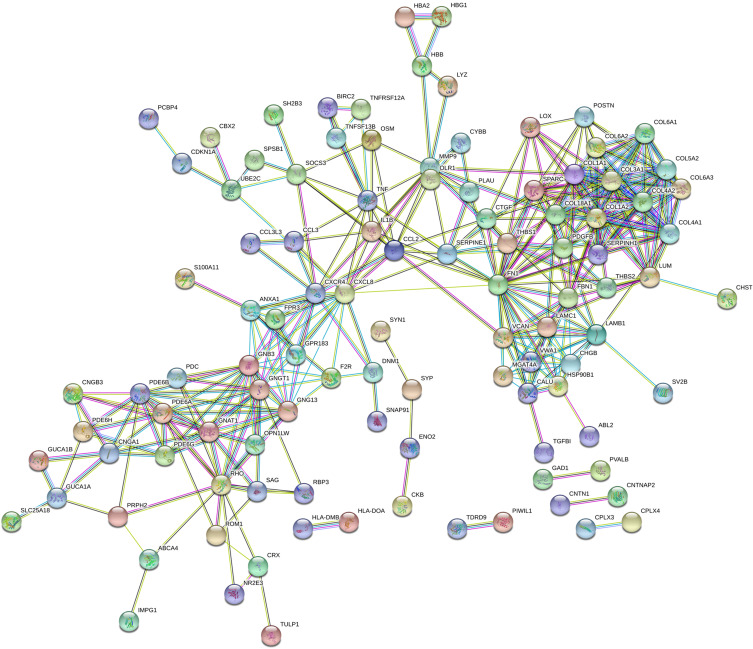
PPI Network of DEGs
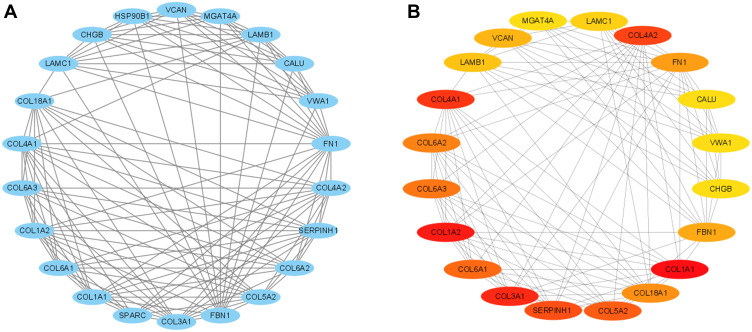
The String PPI network database was used to analyze the PPI for these DEGs, and the results showed 186 nodes and 363 edges with an average node degree of 3.9 in the PPI network (Figure 3). The further analysis using CytoScape was performed to reconstruct the PPI network. Subsequently, when “score greater than 2” was defined as the cut-off criterion in MCODE, 4 clusters were identified from PPI network, and the most significant clusters consisted of 22 nodes and 123 edges with 22 hub genes (Figure 4A). Furthermore, by using the cytoHubba, 20 genes were ranked by MMC mode, and top 20 hub genes are shown in Figure 4B. In a further attempt, we chose the top 10 overlapped hub genes screened by MCODE and cytoHubba based on the highest scores for validation analysis.
Figure 3.
PPI network of the DEGs.
Figure 4.
Identification of hub gene modules using Cytoscape. (A) Module of PPI network constructed by MCODE. (B) Module of PPI network constructed by CytoHubba.
Validation of Hub Genes in HREC Cells After Exposing to High Glucose
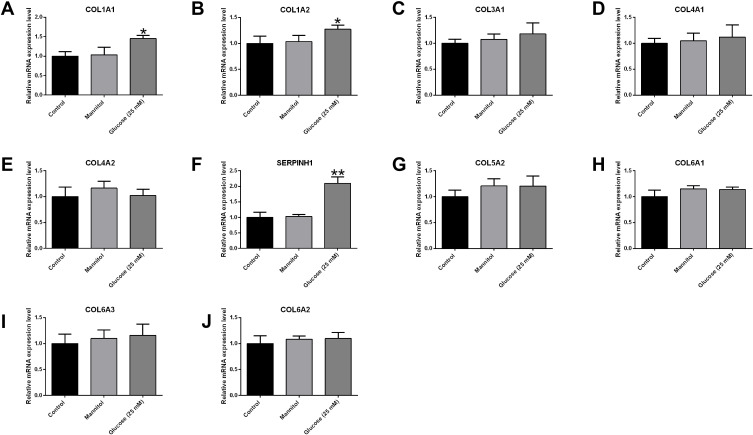
In order to confirm the potential roles of these hub genes in the pathophysiology of diabetic retinopathy, we performed the PCR validation studies in the HREC cells after exposing to high glucose. The qRT-PCR results showed that high glucose treatment significantly increased the mRNA expression levels of COL1A1, COL1A2 and SERPINH1 (Figure 5); while the mRNA expression levels of other genes were not affected by glucose treatment in the HRECs (Figure 5).
Figure 5.
Effects of high glucose treatment on the hub genes in the HRECs. The mRNA expression levels of (A) COL1A1, (B) COL1A2, (C) COL3A1, (D) COL4A1, (E) COL4A2, (F) SERPINH1, (G) COL5A2, (H) COL6A1, (I) COL6A3 and (J) COL6A2 in the HRECs after different treatments were determined by qRT-PCR. *p<0.05 and **p<0.01.
SERPINH1 Silence Attenuated the High Glucose-Induced Increase in Cell Proliferation and Migration of HRECs
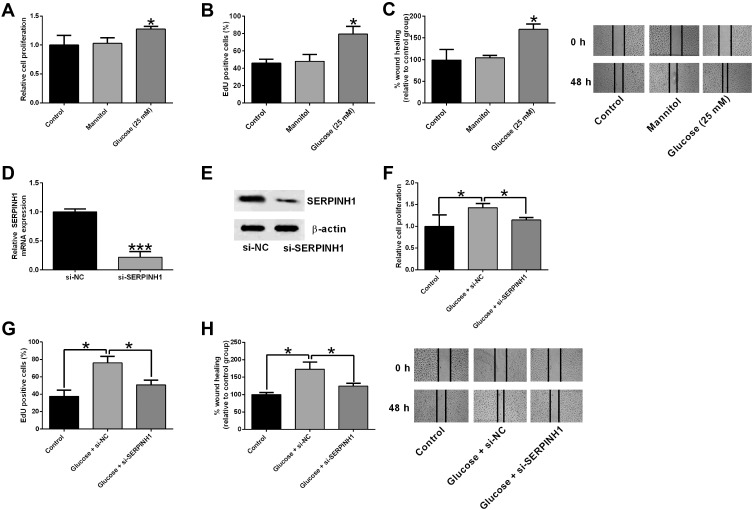
Furthermore, we further performed in vitro assays to determine the role of SERPINH1 in regulating high glucose-stimulated HREC proliferation and migration. As shown in Figure 6A and 6B, high glucose treatment for 48 h significantly enhanced the HREC viability and proliferation as determined by MTS and EdU assays. Consistently, high glucose also promoted HREC migration as assessed by wound healing assay (Figure 6C). The RNAi study showed that SERPINH1 siRNA transfection significantly repressed the SERPINH1 mRNA and protein expression levels in HRECs when compared to si-NC group (Figure 6D and E). The in vitro functional assays revealed that SERPINH1 silence significantly attenuated the high-glucose induced increase in the proliferation and migration of HRECs (Figure 6F–H).
Figure 6.
SERPINH1 silence attenuated the high glucose-induced increase in cell proliferation and migration of HRECs. (A) The cell proliferation of HRECs after different treatments (control, mannitol, 25 mM glucose) was determined by CCK-8 assay. (B) EdU assay was used to determined HREC proliferation after different treatments (control, mannitol, 25 mM glucose). (C) The HREC migration after different treatments (control, mannitol, 25 mM glucose) was determined by wound healing assay. (D) The mRNA and (E) protein expression levels of SERPINH1 in HRECs after being transfected with si-NC or si-SERPINH1 were determined by qRT-PCR and Western blot assay, respectively. (F–H) HRECs were transfected with si-NC or si-SERPINH1 followed by treating with 25 mM glucose, the cell proliferation and migration of HRECs were determined by CCK-8, EdU and wound healing assay, respectively. *p<0.05.
miR-29b Repressed the Expression of SERPINH1 in HRECs
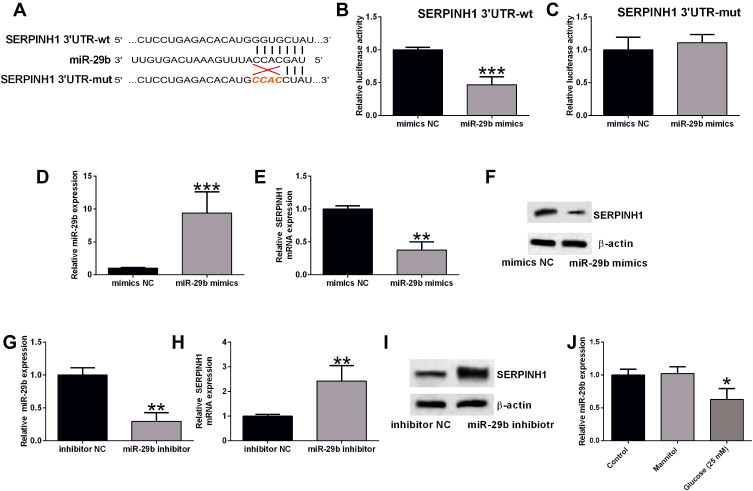
As previous studies have demonstrated that SERPINH1 is modulated by miR-29b in different types of cancers,17 we explored if SERPINH1 was regulated miR-29b in high glucose-treated HRECs. The complementary binding sites between miR-29b and SERPINH1 3ʹUTR were illustrated by TargetScan (Figure 7A). The luciferase reporter assay showed that miR-29b overexpression remarkedly repressed the luciferase activity of SERPINH1 3ʹUTR-wt but had no effect on the SERPINH1 3ʹUTR-mut luciferase activity (Figure 7B–D). MiR-29b overexpression down-regulated the mRNA and protein levels of SERPINH1 in HRECs (Figure 7E and F). Moreover, HRECs transfected with miR-29b inhibitor showed down-regulated expression of miR-29b when compared to those transfected with inhibitor NC (Figure 7G), and miR-29b inhibition up-regulated the SERPINH1 mRNA and protein expression levels in HRECs (Figure 7H and I). In addition, high glucose treatment significantly decreased the miR-29b expression level in HRECs (Figure 7J).
Figure 7.
MiR-29b repressed the expression of SERPINH1 in HRECs. (A) The predicted complementary sequences between miR-29b and SERPINH1 3ʹUTR. (B and C) The luciferase reporter activities of SERPINH1 3ʹUTR-wt (B) and SERPINH1 3ʹUTR-mut (C) in HRECs after being transfected with mimics NC or miR-29b mimics were determined by Dual-Luciferase Reporter assay. (D) The miR-29b expression level in HRECs after being transfected with mimics NC or miR-29b mimics were determined by qRT-PCR. (E and F) The mRNA and protein expression levels of SERPINH1 in HRECs after being transfected with mimics NC or miR-29b mimics were determined by qRT-PCR. (G) The miR-29b expression level in HRECs after being transfected with inhibitor NC or miR-29b inhibitor was determined by qRT-PCR. (H and I) The mRNA and protein expression levels of SERPINH1 in HRECs after being transfected with inhibitor NC or miR-29b inhibitor were determined by qRT-PCR. (J) The mRNA expression levels of miR-29b in the HRECs after different treatments were determined by qRT-PCR. *p<0.05, **p<0.01 and ***p<0.001.
miR-29b/SERPINH1 Axis Participated in the Glucose-Induced Increase in HREC Proliferation and Migration
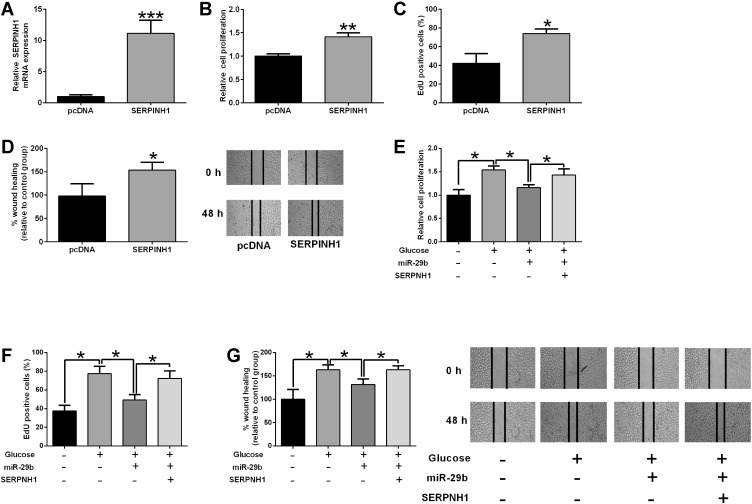
As shown in Figure 8A, pcDNA-SERPINH1 transfection dramatically increased the mRNA level of SERPINH1 in HRECs when compared to pcDNA transfection (Figure 8A). The in vitro functional assays showed that SERPINH1 overexpression promoted the proliferation and migration of HRECs (Figure 8B–D). The rescue experiments showed that miR-29b overexpression partially reversed high glucose-induced increase in cell proliferation and migration of HRECs (Figure 8E–G). On the other hand, SERPINH1 overexpression significantly attenuated the inhibitory effects of miR-29b overexpression on the proliferation and migration of high glucose-treated HRECs (Figure 8E–G).
Figure 8.
MiR-29b/SERPINH1 axis participated in the glucose-induced increase in HREC proliferation and migration. (A) The mRNA expression level of SERPINH1 in HRECs after being transfected with pcDNA3.1 or pcDNA3.1-SERPINH1 was determined by qRT-PCR. (B–D) HRECs were transfected with pcDNA3.1 or pcDNA3.1-SERPINH1, the cell viability, proliferation and migration of HRECs were determined by MTS (B), EdU (C) and wound healing assay (D), respectively. (E–G) HRECs were transfected with different miRNAs or plasmids followed by treatment with 25 mM glucose, the cell viability, proliferation and migration of HRECs were determined by MTS, EdU and wound healing assay, respectively. *p<0.05, **p<0.01 and ***p<0.001.
Discussion
The pathophysiology of diabetic retinopathy involves complex signaling pathways, which largely hinder the development of effective therapies.18 With the aid of high-throughput technologies, various novel biomarkers have been identified for certain types of diseases.19,20 This study performed an integrated bioinformatics analysis using the GEO datasets (GSE60436 and GSE94019). A total of 189 common DEGs were identified between these two datasets. In addition, the GO enrichment analysis, KEGG pathway analysis, Reactome pathway analysis, WikiPathways analysis and miRNA pathways analysis were performed to explore the regulatory network of these DEGs. The PPI network analysis revealed 10 potential hub genes that may link to diabetic retinopathy. The qRT-PCR validation results showed that COL1A1, COL1A2 and SERPINH1 mRNA expression levels were up-regulated in the HRECs exposed to high glucose stimulation for 48 h. Silence of SERPINH1 repressed the proliferation and migration of HRECs under high glucose stimulation. SERPINH1 was a direct target of miR-29b and was suppressed by miR-29b in HRECs. SERPINH1 overexpression promoted HREC proliferation and migration. Furthermore, miR-29b suppressed HREC proliferation and migration under high glucose stimulation, which was significantly attenuated by enforced expression of SERPINH1. Collectively, our data suggested the potential role of SERPINH1 in diabetic retinopathy development.
By analyzing these two datasets, the functional enrichment analysis found that the common DEGs were associated with phototransduction, EMC-receptor interaction, PI3K-Akt signaling pathway and so on. Microarray analysis of GSE60436 has revealed that extracellular matrix-related molecules and angiogenic factors participate in promoting the development of preretinal fibrovascular membranes associated with diabetic retinopathy.7 Microarray analysis of GSE94019 showed that the DEGs were enriched in inflammatory and angiogenic pathways that are highly consistent with diabetic retinopathy pathogenesis, which involves leaky and aberrant vessel growth.5 As a matter of fact, large amount of studies have shown that impairment of phototransduction is one of the main features of diabetic retinopathy;21–23 EMC–receptor interaction has been proposed to play an important role in retinal vascular related pathology.24,25 In addition, the role of PI3K-Akt signaling in the pathogenesis of diabetic retinopathy has been elucidated in several studies.26,27 Analysis of the microarray datasets (GSE52257 and GSE60436) indicated that inflammation, fibrosis and G protein-coupled receptors were potentially involved in the development of diabetic retinopathy.9 However, how these DEGs regulated the apoptosis in diabetic retinopathy still requires further examination.
Based on the PPI network analysis and qRT-PCR validation, we found that 3 hub genes (COL1A1, COL1A2 and SERPINH1) were up-regulated in the HRECs under high glucose stimulation for 48 h. COL1A1 and COL1A2 encode major components of type I collagen.28 High glucose was found to induce up-regulation of type I collagen mRNA expression in cardiac fibroblasts;29 Han et al demonstrated that high glucose stimulated proliferation and collagen type I synthesis in renal cortical fibroblasts.30 Recent findings showed high glucose-induced type I collagen up-regulation in human umbilical vein endothelial cells.31 In our studies, we consistently showed the up-regulation of COL1A1 and COL1A2 in high glucose-treated HRECs, whereas whether up-regulation of COL1A1 and COL1A2 contributed to the enhanced synthesis of type I collagen in HRECs under high glucose condition still requires further examination.
In the present study, we found that SERPINH1 was one of the most-regulated hub genes in HRECs under high glucose stimulation. SERPINH1, also known as heat shock protein 47, acts as a human chaperone protein for collagen. The plasma SERPINH1 level was increased in the diabetic foot.32,33 In our studies, we found that high glucose enhanced the HREC proliferation and migration was accompanied by SERPINH1 up-regulation, suggesting that SERPINH1 may participate in the HREC proliferation and migration. As expected, the functional studies showed that SERPINH1 knockdown attenuated the enhanced effects of high glucose on the HREC proliferation and migration. Yamamoto et al showed that SERPINH1 promoted the proliferation, invasion and migration of cervical squamous cell carcinoma cells;34 Zhao et al showed that SERPINH1 was regulated by miR-29a to promote glioma tumor growth, invasion and angiogenesis.35,36 A recent study showed that SERPINH1 promoted cancer metastasis.37 TGF-beta signaling has been regarded as an important player in the retinal neurodevelopment.38 In the visual system, TGF-beta2 treatment-induced cellular stress in the optic nerve head astrocytes was accompanied by increased SERPINH1 expression,39 and TGFβ1 has been suggested as a biomarker and pharmacological target of diabetic retinopathy.38 In our results, we also showed SERPINH1 overexpression promoted HREC proliferation and migration. Collectively, these data may imply that high glucose induced the increase in HREC proliferation and migration may be correlated with the up-regulation of SERPINH1.
Based on the previous studies, several studies have confirmed the interaction between SERPINH1 and miR-29b in skin wound healing and tumor biology,40–42 thus, we further explored if miR-29b/SERPINH1 participated in the HREC proliferation and migration under high glucose stimulation. In our study, the luciferase reporter assay confirmed the interaction between miR-29b and SERPINH1 3ʹUTR. Furthermore, miR-29b overexpression suppressed the SERPINH1 expression, while miR-29b inhibition up-regulated SERPINH1 expression in HRECs. In addition, high glucose treatment caused the down-regulation of miR-29b in HRECs. These results indicated that high glucose up-regulated SERPINH1 expression by partly repressing miR-29b expression in HRECs. In terms of miR-29b, studies found that miR-29b promoted HREC apoptosis via blocking sirtuin 1 in diabetic retinopathy,43 and a recent study by Tang et al showed that miR-29b inhibited cell proliferation and angiogenesis by targeting vascular endothelial growth factor A and platelet-derived growth factor subunit B in HRECs.44 Consistently, miR-29b also suppressed HREC proliferation and migration, which was significantly attenuated by enforced expression of SERPINH1. Collectively, these results imply that there is a high glucose-induced increase in HREC proliferation and migration via modulating the miR-29b/SERPINH1 axis.
In this study, several limitations exist. The present study used MTS assay to evaluate cell HERC viability. However, metabolic activity may be changed by different conditions or chemical treatments which can cause considerable variation in results reported from the assay.45 In this study, two GEO datasets were included for the analysis, and future studies may explore more datasets to further decipher more potential mediators in regulating diabetic retinopathy progression. The examination into the role of SERPINH1 is still at an early stage in in vitro studies, and future in vivo studies may be considered to further understand the significance of this gene in diabetic retinopathy.
Conclusions
In conclusion, by performing the integrated bioinformatics analysis, the present study suggested that 3 hub genes (COL1A1, COL1A2 and SERPINH1) may be associated with diabetic retinopathy pathophysiology. Further mechanistic studies indicated that miR-29b/SERPINH1 signaling participated in high glucose-induced enhancement in the proliferation and migration of HRECs. Future studies are warranted to further explore the detailed roles of SERPINH1 in diabetic retinopathy.
Funding Statement
There is no funding to report.
Data Sharing Statement
All the data are available.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Amoaku WM, Ghanchi F, Bailey C, et al. Diabetic retinopathy and diabetic macular oedema pathways and management: UK Consensus Working Group. Eye. 2020;34(Suppl 1):1–51. doi: 10.1038/s41433-020-0961-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu Y, Li J, Ma J, Tong N. The threshold of the severity of diabetic retinopathy below which intensive glycemic control is beneficial in diabetic patients: estimation using data from large randomized clinical trials. J Diabetes Res. 2020;2020:8765139. doi: 10.1155/2020/8765139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahmad I, Hoda M. Attenuation of diabetic retinopathy and neuropathy by resveratrol: review on its molecular mechanisms of action. Life Sci. 2020;245:117350. doi: 10.1016/j.lfs.2020.117350 [DOI] [PubMed] [Google Scholar]
- 4.Singh RR. Next-generation sequencing in high-sensitive detection of mutations in tumors: challenges, advances, and applications. J Mol Diagn. 2020;22(8):994–1007. doi: 10.1016/j.jmoldx.2020.04.213 [DOI] [PubMed] [Google Scholar]
- 5.Lam JD, Oh DJ, Wong LL, et al. Identification of RUNX1 as a mediator of aberrant retinal angiogenesis. Diabetes. 2017;66(7):1950–1956. doi: 10.2337/db16-1035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berdasco M, Gómez A, Rubio MJ, et al. DNA methylomes reveal biological networks involved in human eye development, functions and associated disorders. Sci Rep. 2017;7(1):11762. doi: 10.1038/s41598-017-12084-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.You ZP, Zhang YL, Li BY, Zhu XG, Shi K. Bioinformatics analysis of weighted genes in diabetic retinopathy. Invest Ophthalmol Vis Sci. 2018;59(13):5558–5563. doi: 10.1167/iovs.18-25515 [DOI] [PubMed] [Google Scholar]
- 8.Ishikawa K, Yoshida S, Kobayashi Y, et al. Microarray analysis of gene expression in fibrovascular membranes excised from patients with proliferative diabetic retinopathy. Invest Ophthalmol Vis Sci. 2015;56(2):932–946. doi: 10.1167/iovs.14-15589 [DOI] [PubMed] [Google Scholar]
- 9.Platania CBM, Leggio GM, Drago F, Salomone S, Bucolo C. Computational systems biology approach to identify novel pharmacological targets for diabetic retinopathy. Biochem Pharmacol. 2018;158:13–26. doi: 10.1016/j.bcp.2018.09.016 [DOI] [PubMed] [Google Scholar]
- 10.Mahi NA, Najafabadi MF, Pilarczyk M, Kouril M, Medvedovic M. GREIN: an interactive web platform for re-analyzing GEO RNA-seq data. Sci Rep. 2019;9(1):7580. doi: 10.1038/s41598-019-43935-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tatusov RL, Fedorova ND, Jackson JD, et al. The COG database: an updated version includes eukaryotes. BMC Bioinform. 2003;4(1):41. doi: 10.1186/1471-2105-4-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ashburner M, Ball CA, Blake JA, et al. Gene ontology: tool for the unification of biology. The gene ontology consortium. Nat Genet. 2000;25(1):25–29. doi: 10.1038/75556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mao X, Cai T, Olyarchuk JG, Wei L. Automated genome annotation and pathway identification using the KEGG Orthology (KO) as a controlled vocabulary. Bioinformatics. 2005;21(19):3787–3793. doi: 10.1093/bioinformatics/bti430 [DOI] [PubMed] [Google Scholar]
- 14.Kuleshov MV, Jones MR, Rouillard AD, et al. Enrichr: a comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res. 2016;44(W1):W90–W97. doi: 10.1093/nar/gkw377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Szklarczyk D, Morris JH, Cook H, et al. The STRING database in 2017: quality-controlled protein-protein association networks, made broadly accessible. Nucleic Acids Res. 2017;45(D1):D362–D368. doi: 10.1093/nar/gkw937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kumar R, Harris-Hooker S, Kumar R, Sanford G. Co-culture of retinal and endothelial cells results in the modulation of genes critical to retinal neovascularization. Vasc Cell. 2011;3(1):27. doi: 10.1186/2045-824x-3-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Falzone L, Romano GL, Salemi R, et al. Prognostic significance of deregulated microRNAs in uveal melanomas. Mol Med Rep. 2019;19(4):2599–2610. doi: 10.3892/mmr.2019.9949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vergmann AS, Grauslund J. Changes of visual fields in treatment of proliferative diabetic retinopathy: a systematic review. Acta Ophthalmol. 2020;98(8):763–773. doi: 10.1111/aos.14474 [DOI] [PubMed] [Google Scholar]
- 19.Syu GD, Dunn J, Zhu H. Developments and applications of functional protein microarrays. Mol Cell Proteomics. 2020;19(6):916–927. doi: 10.1074/mcp.R120.001936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang X, Kui L, Tang M, et al. High-throughput transcriptome profiling in drug and biomarker discovery. Front Genet. 2020;11:19. doi: 10.3389/fgene.2020.00019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barber AJ, Gardner TW, Abcouwer SF. The significance of vascular and neural apoptosis to the pathology of diabetic retinopathy. Invest Ophthalmol Vis Sci. 2011;52(2):1156–1163. doi: 10.1167/iovs.10-6293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cecilia OM, José Alberto CG, José NP, et al. Oxidative stress as the main target in diabetic retinopathy pathophysiology. J Diabetes Res. 2019;2019:8562408. doi: 10.1155/2019/8562408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McAnany JJ, Park JC. Cone photoreceptor dysfunction in early-stage diabetic retinopathy: association between the activation phase of cone phototransduction and the flicker electroretinogram. Invest Ophthalmol Vis Sci. 2019;60(1):64–72. doi: 10.1167/iovs.18-25946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Farnoodian M, Halbach C, Slinger C, Pattnaik BR, Sorenson CM, Sheibani N. High glucose promotes the migration of retinal pigment epithelial cells through increased oxidative stress and PEDF expression. Am J Physiol Cell Physiol. 2016;311(3):C418–C436. doi: 10.1152/ajpcell.00001.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gnanaguru G, Brunken WJ. The cell-matrix interface: a possible target for treating retinal vascular related pathologies. J Ophthalmic Vis Res. 2012;7(4):316–327. [PMC free article] [PubMed] [Google Scholar]
- 26.Lu JM, Zhang ZZ, Ma X, Fang SF, Qin XH. Repression of microRNA-21 inhibits retinal vascular endothelial cell growth and angiogenesis via PTEN dependent-PI3K/Akt/VEGF signaling pathway in diabetic retinopathy. Exp Eye Res. 2020;190:107886. doi: 10.1016/j.exer.2019.107886 [DOI] [PubMed] [Google Scholar]
- 27.Xie W, Zhou P, Qu M, et al. Ginsenoside re attenuates high glucose-induced RF/6A injury via regulating PI3K/AKT inhibited HIF-1α/VEGF signaling pathway. Front Pharmacol. 2020;11:695. doi: 10.3389/fphar.2020.00695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu Y, Zhang S, Wang Y, Ren X, Han J. Molecular mechanisms and clinical manifestations of rare genetic disorders associated with type I collagen. Intractable Rare Dis Res. 2019;8(2):98–107. doi: 10.5582/irdr.2019.01064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang R, Chang L, Liu S, Jin X, Li Y. High glucose induces Rho/ROCK-dependent visfatin and type I procollagen expression in rat primary cardiac fibroblasts. Mol Med Rep. 2014;10(4):1992–1998. doi: 10.3892/mmr.2014.2408 [DOI] [PubMed] [Google Scholar]
- 30.Han DC, Isono M, Hoffman BB, Ziyadeh FN. High glucose stimulates proliferation and collagen type I synthesis in renal cortical fibroblasts: mediation by autocrine activation of TGF-beta. J Am Soc Nephrol. 1999;10(9):1891–1899. doi: 10.1681/ASN.V1091891 [DOI] [PubMed] [Google Scholar]
- 31.Chen Y, Yang Q, Zhan Y, Ke J, Lv P, Huang J. The role of miR-328 in high glucose-induced endothelial-to-mesenchymal transition in human umbilical vein endothelial cells. Life Sci. 2018;207:110–116. doi: 10.1016/j.lfs.2018.05.055 [DOI] [PubMed] [Google Scholar]
- 32.Zubair M, Ahmad J. Plasma Heat Shock Proteins (HSPs) 70 and 47 levels in diabetic foot and its possible correlation with clinical variables in a North Indian Tertiary care hospital. Diabetes Metab Syndr. 2015;9(4):237–243. doi: 10.1016/j.dsx.2015.02.015 [DOI] [PubMed] [Google Scholar]
- 33.Bellini S, Barutta F, Mastrocola R, Imperatore L, Bruno G, Gruden G. Heat shock proteins in vascular diabetic complications: review and future perspective. Int J Mol Sci. 2017;18(12):2709. doi: 10.3390/ijms18122709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yamamoto N, Kinoshita T, Nohata N, et al. Tumor-suppressive microRNA-29a inhibits cancer cell migration and invasion via targeting HSP47 in cervical squamous cell carcinoma. Int J Oncol. 2013;43(6):1855–1863. doi: 10.3892/ijo.2013.2145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao D, Jiang X, Yao C, et al. Heat shock protein 47 regulated by miR-29a to enhance glioma tumor growth and invasion. J Neurooncol. 2014;118(1):39–47. doi: 10.1007/s11060-014-1412-7 [DOI] [PubMed] [Google Scholar]
- 36.Wu ZB, Cai L, Lin SJ, et al. Heat shock protein 47 promotes glioma angiogenesis. Brain Pathol. 2016;26(1):31–42. doi: 10.1111/bpa.12256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xiong G, Chen J, Zhang G, et al. Hsp47 promotes cancer metastasis by enhancing collagen-dependent cancer cell-platelet interaction. Proc Natl Acad Sci U S A. 2020;117(7):3748–3758. doi: 10.1073/pnas.1911951117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Romano GL, Platania CBM, Leggio GM, et al. Retinal biomarkers and pharmacological targets for hermansky-pudlak syndrome 7. Sci Rep. 2020;10(1):3972. doi: 10.1038/s41598-020-60931-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yu AL, Moriniere J, Birke M, et al. Reactivation of optic nerve head astrocytes by TGF-beta2 and H2O2 is accompanied by increased Hsp32 and Hsp47 expression. Invest Ophthalmol Vis Sci. 2009;50(4):1707–1717. doi: 10.1167/iovs.08-1961 [DOI] [PubMed] [Google Scholar]
- 40.Yamada Y, Sugawara S, Arai T, et al. Molecular pathogenesis of renal cell carcinoma: impact of the anti-tumor miR-29 family on gene regulation. Int J Urol. 2018;25(11):953–965. doi: 10.1111/iju.13783 [DOI] [PubMed] [Google Scholar]
- 41.Zhu J, Xiong G, Fu H, Evers BM, Zhou BP, Xu R. Chaperone Hsp47 drives malignant growth and invasion by modulating an ECM gene network. Cancer Res. 2015;75(8):1580–1591. doi: 10.1158/0008-5472.can-14-1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhu Y, Li Z, Wang Y, et al. Overexpression of miR-29b reduces collagen biosynthesis by inhibiting heat shock protein 47 during skin wound healing. Transl Res. 2016;178:38–53.e36. doi: 10.1016/j.trsl.2016.07.001 [DOI] [PubMed] [Google Scholar]
- 43.Zeng Y, Cui Z, Liu J, Chen J, Tang S. MicroRNA-29b-3p promotes human retinal microvascular endothelial cell apoptosis via blocking SIRT1 in diabetic retinopathy. Front Physiol. 2019;10:1621. doi: 10.3389/fphys.2019.01621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tang W, Guo J, Gu R, et al. MicroRNA-29b-3p inhibits cell proliferation and angiogenesis by targeting VEGFA and PDGFB in retinal microvascular endothelial cells. Mol Vis. 2020;26:64–75. [PMC free article] [PubMed] [Google Scholar]
- 45.Wang P, Henning SM, Heber D. Limitations of MTT and MTS-based assays for measurement of antiproliferative activity of green tea polyphenols. PLoS One. 2010;5(4):e10202. doi: 10.1371/journal.pone.0010202 [DOI] [PMC free article] [PubMed] [Google Scholar]