Abstract
Background
Sarcopenia is an independent risk factor not only for advanced‐stage non‐alcoholic fatty liver disease (NAFLD) but also for mortality. We investigated the association of sarcopenia and/or NAFLD with mortality among the Korean general population.
Methods
Individuals aged 35–75 years without any history of cancer, ischaemic heart disease, ischaemic stroke, or secondary causes of chronic liver disease were selected from the Korean National Health and Nutrition Examination Surveys from 2008 to 2015. Their mortality data until December 2018 were retrieved from the National Death Registry. NAFLD and sarcopenia were defined by hepatic steatosis index and appendicular skeletal muscle mass divided by body mass index (BMI), respectively.
Results
A total of 28 060 subjects were analysed [mean age, 50.6 (standard error, 0.1) years, 48.2 (0.3) % men]; the median follow‐up duration was of 6.8 (interquartile range, 4.8, 8.4) years. NAFLD predicted mortality after adjustment for age, sex, BMI, hypertension, dyslipidaemia, and smoking (HR 1.32, 95% CI 1.03–1.70), but this prediction lost its statistical significance after additional adjustment for diabetes mellitus. In contrast, NAFLD with advanced fibrosis independently increased the risk of mortality after adjustment for all covariates (HR 1.68, 95% CI 1.02–2.79). Stratified analysis revealed that NAFLD and sarcopenia additively increased the risk of mortality as an ordinal scale (HR 1.46, 95% CI 1.18–1.81, P for trend = 0.001). The coexistence of NAFLD and sarcopenia increased the risk of mortality by almost twice as much, even after adjustment for advanced fibrosis (HR 2.18, 95% CI 1.38–3.44).
Conclusions
Concurrent NAFLD and sarcopenia conferred a two‐fold higher risk of mortality. The observation that NAFLD and sarcopenia additively increase mortality suggests that risk stratification would be helpful in predicting mortality among those with metabolic derangement.
Keywords: Non‐alcoholic fatty liver disease, Sarcopenia, Mortality, Nationwide survey
Introduction
Non‐alcoholic fatty liver disease (NAFLD) affects a quarter of the global population, and its prevalence continues to increase with age. 1 , 2 NAFLD, which is associated with a higher cardiovascular risk 3 and increased fibrosis leading to cirrhosis and hepatocellular carcinoma, 4 is an umbrella term that ranges from non‐alcoholic fatty liver (NAFL) to non‐alcoholic steatohepatitis (NASH) and advanced fibrosis. In particular, advanced fibrosis is an independent risk factor for liver‐related and non‐liver‐related mortality among subjects with NAFLD. 5 , 6 , 7 , 8
The prevalence of sarcopenia is also rising as the global population ages, 9 , 10 and this significantly affects overall mortality not only in the elderly population but also in young adults. 11 , 12 , 13 Sarcopenia is also an independent risk factor for advanced‐stage NAFLD (i.e. NASH and significant fibrosis) independent of obesity and insulin resistance. 14 , 15 Given that both NAFLD and sarcopenia prominently contribute to serious health consequences and share a common pathophysiology (e.g. insulin resistance and chronic inflammation), 16 , 17 , 18 it is of paramount importance to delineate the prognostic value of each disease entity using population‐level data.
Here, we used a Korean nationwide survey and the National Death Registry to investigate the associations of NAFLD and sarcopenia with mortality to determine whether the presence of NAFLD and/or sarcopenia affects the mortality rate in the general population. We also assessed whether risk stratification based on subpopulation analysis could help clinicians predict prognosis in subjects with NAFLD and/or sarcopenia.
Methods
Study participants
The Korean Ministry of Health and Welfare designed the Korean National Health and Nutrition Examination Surveys (KNHANES), which has been conducted since 1998, to be representative of the Korean population using a stratified multistage probability sampling method; the survey has been described in detail elsewhere. 19 Briefly, the KNHANES recruit participants using a stratified multistage probability‐based sampling design, and sampling weights are assigned to each respondent to ensure that the results are representative of the whole Korean population. The use of the KNHANES data was approved by the Institutional Review Board (IRB) of the Korea Centers for Disease Control and Prevention (IRB No. 2008‐04EXP‐01‐C, 2009‐01CON‐03‐2C, 2010‐02CON‐21‐C, 2011‐02CON‐06‐C, 2012‐01EXP‐01‐2C, 2013‐07CON‐03‐4C, 2013‐12EXP‐03‐5C). IRB approval was not required for the use of KNHANES data for 2015 under the Bioethics Act. The study was conducted following the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Individuals, 35 to 75 years of age who were enrolled in the KNHANES from January 2008 to December 2015 were eligible for this study. The exclusion criteria were as follows: alcohol consumption >210 g/week for men and >140 g/week for women 20 ; positive serological markers for hepatitis B or C virus; history of cancer; history of ischaemic heart disease; and/or history of stroke. After this filtering, a total of 28 060 subjects were included in this study.
Definition of outcomes
Korean National Health and Nutrition Examination Surveys data were matched with those in the National Death Registry from the Korea National Statistical Office. The specific cause of death according to the International Classification of Diseases‐10 (ICD‐10) and date of death of the participants of KNHANES were available up to December 2018. Cause‐specific mortality was identified by the following ICD‐10 codes: cancer‐specific mortality, C00–D48; cardiovascular disease (CVD)‐specific mortality, I00–I99.
Assessment of metabolic parameters
Blood samples were drawn from the antecubital vein in the morning after subjects had fasted for at least 8 h. Samples were properly processed, immediately refrigerated at 2 to 8°C, and sent to a central laboratory. Fasting glucose, lipid profile (total cholesterol, high‐ and low‐density lipoprotein cholesterol, and triglycerides), serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), gamma‐glutamyl transferase, and creatinine were measured enzymatically (Hitachi Automatic Analyser 7600, Hitachi, Japan). Glycated haemoglobin was measured by high‐performance liquid chromatography (HLC‐723G7; Tosoh, Japan). Vitamin D was measured using a radioimmunoassay (1470 WIZARD gamma‐Counter; PerkinElmer, Finland). Complete blood count was measured using an XE‐2100D (Sysmex, Japan). Hepatitis B antigen was detected using an electrochemiluminescence immune assay (E‐170; Roche, Germany), and hepatitis C antibody was detected using a chemiluminescent microparticle immunoassay (ARCHITECT i4000Sr; ABBOTT, Germany).
Diabetes mellitus (DM) was defined as a fasting plasma glucose concentration ≥7.0 mmol/L (126 mg/dL) or glycated haemoglobin ≥48 mmol/mol (6.5%), or reported use of anti‐diabetic medication including insulin. 21 Hypertension was defined as a systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mmHg, or reported use of antihypertensive medication. Dyslipidaemia was defined as non‐high‐density lipoprotein cholesterol ≥190 mg/dL or reported use of lipid‐lowering medication. Obesity was defined as a BMI ≥ 25 kg/m2 based on the World Health Organization (WHO) Asia–Pacific criteria and the Korean Society for the Study of Obesity guideline. 22 , 23 Central obesity was defined as waist circumference ≥90 cm in men and ≥85 cm in women. Metabolic syndrome was diagnosed if ≥3 of the criteria were met, according to the National Cholesterol Education Program (NCEP) Adult Treatment panel (ATP) III revised criteria, with central obesity defined as above. 24 Chronic kidney disease was defined as an estimated glomerular filtration rate (eGFR) < 60 mL/min/1.73 m2, as calculated by the Modification of Diet in Renal Disease (MDRD) Study equation. 25 Vitamin D deficiency was defined as serum 25‐hydroxyvitamin D < 20 ng/ml.
Assessment of non‐alcoholic fatty liver disease, fibrosis, and sarcopenia
Non‐alcoholic fatty liver disease was defined as a hepatic steatosis index (HSI) > 36 using the previously validated prediction model calculated as: 8 × ALT [U/L]/AST [U/L] + body mass index (BMI) [+2, if DM; +2 if female]. 26 Liver fibrosis was defined as a fibrosis‐4 index (FIB‐4) ≥ 1.3 for age ≤65 and FIB‐4 ≥ 2.0 for age >65; this was calculated as follows: age (years) × AST [U/L]/(platelet [109/L] × (ALT [U/L])1/2. 27 , 28
Appendicular skeletal muscle mass (ASM) was calculated as the sum of the lean mass in both arms and legs, which was assessed by dual energy X‐ray absorptiometry (DXA; QDR Discovery; Hologic, Inc., Bedford, MA). 29 Sarcopenia was defined by the Foundation for the National Institutes of Health criteria as: ASM divided by BMI ≤ 0.789 for men and ≤0.512 for women. 30 DXA was performed between 2008 and 2011; thus, sarcopenia could be evaluated in 11 005 subjects.
Statistical analysis
The data are expressed as the mean (standard error, SE) or prevalence (SE) (%). Stratification variables and sampling weights were used as designated in KNHANES. A linear regression or logistic linear regression model was used to compare the clinical variables according to NAFLD, sarcopenia, or survival status. The Cox proportional hazards model was used to determine whether the presence of NAFLD, advanced fibrosis, or sarcopenia was independently associated with mortality. For sensitivity analysis, participants were categorized into four subgroups based on the presence of NAFLD or sarcopenia (Model 1, adjusted for age, sex, and BMI; Model 2, further adjusted for hypertension, dyslipidaemia, and smoking; Model 3, further adjusted for DM, chronic kidney disease, vitamin D status, and dyslipidaemia medication; and Model 4, further adjusted for advanced fibrosis). Hazard ratios (HRs) are presented with corresponding 95% confidence intervals (CIs) and P‐values. P‐values for trends were calculated assuming that NAFLD and sarcopenia corresponded to 1 point each as an ordinal scale. All statistical analyses were performed using a complex sample design and applying SPSS version 25.0 (IBM, Armonk, NY). P < 0.05 was considered statistically significant.
Results
Baseline characteristics of study subjects according to non‐alcoholic fatty liver disease and advanced fibrosis status
A total of 28 060 subjects were included in the analysis; among them, 24.1 (SE, 0.3) % (unweighted N = 6488) were classified as NAFLD (Table 1). The mean age of the study population was 50.6 (SE, 0.1) years, and 48.2 (SE, 0.3) % were male.
Table 1.
Baseline clinical characteristics according to non‐alcoholic fatty liver disease
| Total | NAFLD (−) | NAFLD (+) | P value* | P value** | |
|---|---|---|---|---|---|
| Unweighted, N | 28 060 | 21 572 | 6488 | ||
| Age (years) | 50.6 (0.1) | 50.7 (0.1) | 50.1 (0.2) | <0.001 | ‐ |
| Gender (men, %) | 48.2 (0.3) | 46.9 (0.3) | 52.1 (0.7) | <0.001 | ‐ |
| BMI (kg/m2) | 24.0 (0.0) | 22.9 (0.0) | 27.6 (0.0) | <0.001 | <0.001 |
| Waist circumference (cm) a | 82.1 (0.1) | 79.2 (0.1) | 91.2 (0.1) | <0.001 | <0.001 |
| HbA1c (%) a | 5.89 (0.01) | 5.74 (0.01) | 6.34 (0.02) | <0.001 | <0.001 |
| Total cholesterol (mg/dL) | 193.5 (0.3) | 191.2 (0.3) | 200.6 (0.6) | <0.001 | <0.001 |
| Triglycerides (mg/dL) a | 144.3 (0.9) | 130.3 (0.9) | 188.4 (2.1) | <0.001 | <0.001 |
| HDL‐C (mg/dL) a | 49.0 (0.1) | 50.4 (0.1) | 44.7 (0.2) | <0.001 | <0.001 |
| AST (U/L) a | 22.4 (0.1) | 21.3 (0.1) | 25.6 (0.2) | <0.001 | <0.001 |
| ALT (U/L) a | 22.2 (0.1) | 18.1 (0.1) | 35.2 (0.4) | <0.001 | <0.001 |
| GGT (U/L) a | 35.7 (0.7) | 32.1 (0.8) | 47.5 (1.3) | <0.001 | <0.001 |
| Platelets (109/L) | 255.4 (0.4) | 253.5 (0.5) | 261.4 (0.9) | <0.001 | <0.001 |
| eGFR (mL/min/1.73 m2) a | 92.3 (0.2) | 92.6 (0.2) | 91.7 (0.3) | 0.001 | <0.001 |
| SBP (mmHg) | 119.0 (0.2) | 117.5 (0.2) | 123.5 (0.2) | <0.001 | <0.001 |
| DBP (mmHg) | 77.5 (0.1) | 76.4 (0.1) | 81.0 (0.2) | <0.001 | <0.001 |
| Diabetes mellitus (%) | 11.5 (0.2) | 6.6 (0.2) | 26.8 (0.7) | <0.001 | <0.001 |
| Hypertension (%) | 29.8 (0.4) | 25.5 (0.4) | 43.3 (0.8) | <0.001 | <0.001 |
| Dyslipidaemia (%) | 15.4 (0.3) | 12.4 (0.3) | 24.9 (0.6) | <0.001 | <0.001 |
| Obesity (%) | 34.8 (0.4) | 19.0 (0.3) | 84.7 (0.5) | <0.001 | <0.001 |
| Sarcopenia (%) | 9.1 (0.4) | 7.0 (0.4) | 15.7 (1.0) | <0.001 | <0.001 |
| Chronic kidney disease (%) | 1.8 (0.1) | 1.7 (0.1) | 2.2 (0.2) | 0.012 | <0.001 |
| Vitamin D deficiency (%) | 66.1 (0.7) | 65.3 (0.7) | 68.7 (1.0) | <0.001 | <0.001 |
| Smoking (%) | |||||
| Never | 73.0 (0.3) | 74.0 (0.4) | 69.9 (0.7) | <0.001 | 0.262 |
| Past | 6.5 (0.2) | 6.4 (0.2) | 6.8 (0.4) | ||
| Active | 20.5 (0.;3) | 19.6 (0.3) | 23.3 (0.6) |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; GGT, gamma‐glutamyl transferase; HDL‐C, high‐density lipoprotein cholesterol; NAFLD, non‐alcoholic fatty liver disease; SBP, systolic blood pressure.
Values are presented as mean or % (standard error).
Presented as median values (standard error).
Without adjustment.
With adjustment for age and sex.
Subjects with NAFLD had a higher prevalence of DM, hypertension, dyslipidaemia, obesity, and sarcopenia (age‐ and sex‐adjusted P < 0.001 in all; Table 1). Their AST and ALT levels and eGFR were also worse than subjects without NAFLD (age‐ and sex‐adjusted P < 0.001 in all; Table 1). Among subjects with NAFLD, those with advanced fibrosis were older and more obese and had higher AST, ALT, and GGT levels than those without advanced fibrosis (Supporting Information, Table S1).
Comparison of clinical characteristics according to survival status
Overall mortality was observed in 2.5 (SE, 0.1) % (unweighted N = 939) of all study subjects during the median follow‐up period of 6.8 (interquartile range [IQR], 4.8, 8.4) years. Cancer‐related mortality and CVD‐related mortality were reported to be 0.9 (SE, 0.1) % and 0.5 (SE, 0.0) %, respectively. Deceased subjects were predominantly male (64.1 [SE, 2.0] % vs. 47.8 [SE, 0.3] %) and significantly older (mean age: 62.1 [SE, 0.5] years, vs. 50.3 [SE, 0.1] years) compared with survivors (Supporting Information, Table S2). After adjustment for age and sex, DM, dyslipidaemia, CKD, and smokers were more frequently found in the deceased subjects compared to survivors, whereas the prevalence of obesity was significantly lower in the deceased subjects (P < 0.001; Supporting Information, Table S2). The prevalence of sarcopenia was higher in the deceased subjects compared with survivors (21.0 [SE, 2.2] % vs. 8.6 [SE, 0.4] %; P < 0.001 in crude analysis), but this did not retain significance after adjustment for age and sex (P = 0.087; Supporting Information, Table S2).
Risk of mortality according to non‐alcoholic fatty liver disease, advanced fibrosis, and sarcopenic status
Non‐alcoholic fatty liver disease increased the risk of mortality by 42% in the age‐, sex‐, and BMI‐adjusted model (HR, 1.42; 95% CI, 1.11–1.82; Model 1 in Table 2) and maintained its statistical significance after adjustment for hypertension, dyslipidaemia, and smoking status (HR 1.32, 95% CI, 1.03–1.70, Model 2 in Table 2). However, after additional adjustment for DM, NAFLD was not associated with overall mortality (Model 3 in Table 2; Figure 1A). In contrast, NAFLD with advanced fibrosis significantly increased overall mortality in the fully adjusted model (HR 1.68, 95% CI, 1.02–2.76; Model 3 in Table 2). Sarcopenia was also an independent risk factor for overall mortality in the fully adjusted model (HR 1.48, 95% CI, 1.11–1.98, Model 3 in Table 2).
Table 2.
Hazard ratios for mortality according to non‐alcoholic fatty liver disease, advanced fibrosis, and sarcopenia
| NAFLD | NAFLD with advanced fibrosis | Sarcopenia | ||||
|---|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |
| Model 1 | 1.42 (1.11–1.82) | 0.005 | 2.11 (1.30–3.43) | 0.003 | 1.56 (1.17–2.08) | 0.002 |
| Model 2 | 1.32 (1.03–1.70) | 0.027 | 2.10 (1.29–3.41) | 0.003 | 1.53 (1.15–2.04) | 0.004 |
| Model 3 | 1.10 (0.85–1.42) | 0.474 | 1.68 (1.02–2.76) | 0.040 | 1.48 (1.11–1.98) | 0.008 |
Hazard ratio for 6.8‐year mortality was evaluated using multivariate Cox analysis. Model 1: Adjusted for age, sex, and BMI. Model 2: Adjusted for hypertension, dyslipidaemia, and smoking status in addition to the adjustments listed for Model 1. Model 3: Adjusted for diabetes mellitus in addition to the adjustments listed for Model 2.
Figure 1.
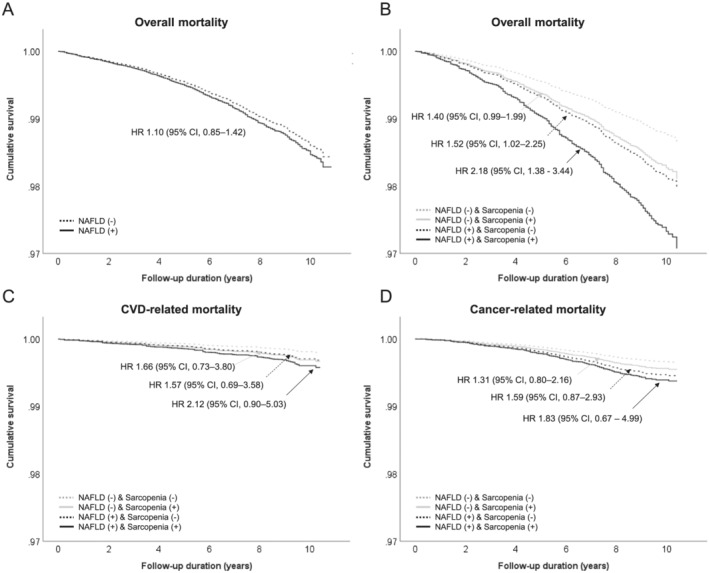
Overall and cause‐specific mortality by NAFLD and sarcopenic status. Cumulative survivals according to (A) overall mortality in subjects with (solid black line) and without (dashed black line) NAFLD; and (B) overall, (C) CVD‐related, and (D) cancer‐related mortality by the presence of either NAFLD and/or sarcopenia for median 6.8 years of follow‐up were analysed using Cox proportional hazards analysis. (1) No NAFLD and no sarcopenia (dashed grey line), (2) no NAFLD but sarcopenia (solid grey line), (3) NAFLD without sarcopenia (dashed black line), and (4) NAFLD with sarcopenia (solid black line) (unweighted N = 28 060; overall mortality, 2.5% (SE, 0.1%); CVD‐related mortality, 0.5% (SE, 0.0%); cancer‐related mortality, 0.9% (SE, 0.1%)). Hazard ratios (95% confidence intervals) were calculated after adjustment for age, sex, BMI, hypertension, dyslipidemia, smoking, and diabetes mellitus for Figure 1A (Model 3 in Table 2) or further adjustment for chronic kidney disease, vitamin D, dyslipidaemia medication, and advanced fibrosis for Figure 1B–D (Model 4 in Table 3 or Supporting Information, Table S5).
Considering that sarcopenia was more prevalent in subjects with NAFLD (15.7% vs. 7.0%; Table 1), we stratified the study population into four subgroups by NAFLD and sarcopenia status to evaluate whether sarcopenia affects mortality in association with NAFLD. Compared with subjects with NAFLD but without sarcopenia, subjects with NAFLD and sarcopenia were older and had higher BMI (Supporting Information, Table S3). Stratified analysis according to sarcopenia and NAFLD confirmed that NAFLD without sarcopenia increased the risk of overall mortality significantly in the age‐, sex‐, and BMI‐adjusted model compared with the reference group (those without NAFLD or sarcopenia; HR, 1.86, 95% CI, 1.27–2.74; Model 1 in Table 3). The statistical significance of this increase was maintained upon further adjustment for hypertension, dyslipidaemia, DM, chronic kidney disease, smoking, vitamin D, and advanced fibrosis (HR, 1.52, 95% CI, 1.02–2.25; Model 4 in Table 3). The coexistence of NAFLD and sarcopenia approximately doubled the risk of overall mortality (HR, 2.18, 95% CI, 1.38–3.44 Model 4 in Table 3; Figure 1B). In this model, advanced fibrosis was also an independent risk factor for overall mortality (HR, 1.44, 95% CI, 1.13–1.84, Supporting Information, Table S4). Moreover, NAFLD and sarcopenia additively increased the risk of mortality as an ordinal scale (HR 1.46, 95% CI 1.18–1.81, P for trend = 0.001). However, neither CVD‐related mortality nor cancer‐related mortality were significantly increased in subjects with NAFLD and sarcopenia (Supporting Information, Table S5; Figure 1C,D).
Table 3.
Hazard ratios for mortality according to non‐alcoholic fatty liver disease and sarcopenia
| NAFLD(−) Sarcopenia(−) | NAFLD(−) Sarcopenia(+) | NAFLD(+) Sarcopenia(−) | NAFLD(+) Sarcopenia(+) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | HR | 95% CI | P value | HR | 95% CI | P value | HR | 95% CI | P value | P for trends | |
| Model 1 | 1.00 (ref.) | 1.53 | 1.09–2.15 | 0.013 | 1.86 | 1.27–2.74 | 0.002 | 2.85 | 1.83–4.44 | <0.001 | <0.001 |
| Model 2 | 1.00 (ref.) | 1.50 | 1.07–2.11 | 0.019 | 1.73 | 1.18–2.55 | 0.006 | 2.68 | 1.73–4.17 | <0.001 | <0.001 |
| Model 3 | 1.00 (ref.) | 1.39 | 0.98–1.98 | 0.065 | 1.45 | 0.98–2.14 | 0.062 | 2.08 | 1.32–3.27 | 0.002 | 0.001 |
| Model 4 | 1.00 (ref.) | 1.40 | 0.99–1.99 | 0.059 | 1.52 | 1.02–2.25 | 0.038 | 2.18 | 1.38–3.44 | 0.001 | 0.001 |
Hazard ratio for 6.8‐year mortality was evaluated using multivariate Cox regression analysis. Model 1: Adjusted for age, sex, and BMI. Model 2: Adjusted for hypertension, dyslipidaemia, and smoking status in addition to the adjustments listed for Model 1 Model 3: Adjusted for diabetes mellitus, chronic kidney disease, vitamin D status, and dyslipidaemia medication in addition to the adjustments listed for Model 2. Model 4: Adjusted for advanced fibrosis in addition to the adjustments listed for Model 3.
Discussion
Non‐alcoholic fatty liver disease and sarcopenia adversely affect metabolic health outcomes and pose an increasing global health and economic burden. 2 , 31 Therefore, in the current study, we assessed the prognostic values of NAFLD and sarcopenia in predicting mortality among the general population using a Korean nationwide survey and the National Death Registry. Using HSI ≥ 36 to define hepatic steatosis, we diagnosed a quarter of study subjects with NAFLD. We found that both sarcopenia and advanced fibrosis independently predicted mortality during the follow‐up of 6.8 years, whereas NAFLD alone did not. The coexistence of NAFLD and sarcopenia was associated with an approximately 2.2‐fold higher risk of mortality relative to the reference group without these conditions, and each condition additively contributed to an increased mortality.
Sarcopenia and NAFLD share common pathophysiological mechanisms, including insulin resistance and chronic inflammation. 32 Insulin resistance induces lipolysis in the adipose tissue and is associated with a higher circulating free fatty acid level, 33 which eventually leads to ectopic fat deposition in the muscle tissue (i.e. myosteatosis) and liver (i.e. simple steatosis). Insulin resistance promotes both de novo lipogenesis and gluconeogenesis in the liver through transcriptional activation of SREBP‐1c and ChREBP, 34 and the subsequent increases in glucose flux and lipotoxicity exacerbate proteolysis in muscle. 32 , 35 Pro‐inflammatory conditions associated with insulin resistance may directly stimulate protein catabolism, leading to a loss of muscle mass. 36 Myokines, 37 hepatokines, 38 and pro‐inflammatory cytokines 36 have been suggested to contribute to the interplay between muscle and liver. 37 , 38 It thus appears that liver and muscle interact with each other to exacerbate the progression of both NAFLD and sarcopenia, although the underlying mechanisms and biological sequences responsible for such phenotypic changes need to be further elucidated. A more severe inflammatory response and/or insulin resistance may be driven by the coexistence of NAFLD and sarcopenia via changes in the interplay between liver and muscle, which might explain why the prevalence of sarcopenia exhibits a stepwise increase with increasing severity of NAFLD. 14 , 20 , 39 Furthermore, improvement in skeletal muscle mass during a 7‐year follow‐up period was associated with a decrease in the incidence of NAFLD, 40 which suggests that sarcopenia may be a predictor of NAFLD progression as well as a risk factor for NAFLD development.
Previous studies have yielded inconsistent results regarding the effect of NAFLD on mortality: NAFLD predicted mortality in some studies 41 , 42 but failed to do so in others. 43 Rather, advanced fibrosis was reported to be an independent risk factor for mortality among NAFLD subjects. 5 , 6 In the current study, stratified analysis according to the presence of NAFLD and/or sarcopenia indicated that subjects with NAFLD but without sarcopenia had a significantly increased risk of mortality compared with the reference group without NAFLD or sarcopenia, independent of advanced fibrosis. In the no‐NAFLD population, the proportion of sarcopenic subjects was 7.0%; these individuals were significantly older and had a higher mortality rate compared with the reference group, which might attenuate the effect of NAFLD on mortality. Considering the conflicting data regarding the effect of NAFLD on mortality, 41 , 42 , 43 it is important to define the healthy reference group properly, and careful evaluation of metabolic and other risk factors should be made for the analysis. The apparent dose–response relationship between the risk of mortality and the presence of sarcopenia and/or NAFLD likely reflects that sarcopenia might increase the risk of mortality irrespective of the presence of NAFLD and vice versa.
In the current study, the coexistence of NAFLD and sarcopenia did not predict CVD‐ or cancer‐related mortality although it significantly increased the overall mortality, which might be from the relatively small number of events studied in the present work. Recently, Golabi et al. reported sarcopenia independently contributed cardiac and cancer‐specific mortality as well as overall mortality in the individuals with NAFLD using the US NHANES data. 44 In that study, 587 mortalities out of 4611 subjects were observed for 13.5 years; their long duration of follow‐up might disclose the effect of sarcopenia with NAFLD more clearly. However, they did not exclude subjects with cancer or CVD at the baseline, although sarcopenia is closely associated with the risk of cancer and CVD. 45 , 46 The current study excluded subjects with previous history of cancer or CVD and adjusted for advanced fibrosis and BMI, well‐known risk factors for mortality, which made it robust to demonstrate the predictive value of NAFLD and sarcopenia for mortality. Welch et al. also demonstrated that decreased initial muscle mass and the extent of the reduction in muscle mass measured by consecutive computed tomography scans are associated with increased risk of mortality in 83 patients with biopsy‐proven cirrhosis. 47 These studies implicate that sarcopenia should be evaluated for risk stratification of mortality in subjects with the full spectrum of NAFLD. Further studies are needed to investigate whether the interplay between NAFLD and sarcopenia may exacerbate each and/or additively increase the risk of cancer or CVD in the diverse populations.
Interestingly, obesity played a protective role in the mortality in the currently study. The J‐shaped relationship between BMI and mortality has been reported across the diverse population. 48 In the Korean population, the lowest mortality rate was observed in obese subjects (25–29.9 kg/m2), 49 , 50 which suggests that among the Korean population with BMI < 30 kg/m2, there might be an inverse association between their BMI and mortality rate. In addition, Asians have a relatively higher proportion of non‐obese NAFLD, which exhibits distinct metabolic features and genetic alterations in PNPLA3 and TM6SF2 compared with obese NAFLD in Western population. 51 , 52 , 53 A recent study from the U.S. NHANES reported a higher mortality rate in subjects with non‐obese NAFLD. Therefore, NAFLD subjects with a low to normal BMI should be carefully assessed for their long‐term prognosis, although they may present with favourable metabolic profiles. 54
A strength of our study is that we used nationwide survey data representing the Korean general population, and mortality data were retrieved from the National Death Registry from the Statistics Office of Korea. ICD‐10 codes were used to define the cause of death. This study therefore has the unique advantages of utilizing survey data organized by the Korean government, and combining analysis of cross‐sectional surveys (KNHANES) and the death registry to enable longitudinal outcome research. Our observation that the adverse joint effect of NAFLD and sarcopenia on mortality remained markedly significant even after adjustment for other metabolic comorbidities and advanced fibrosis emphasizes the need to perform stratified analysis by risk factors when seeking to predict long‐term prognoses in subjects with metabolic derangements.
The current study has some limitations. First, we alternatively used the HSI and FIB‐4 to define hepatic steatosis and advanced fibrosis, although histological or radiological assessment might be more accurate for confirming NAFLD. However, a number of studies on the diagnostic and prognostic performance of the HSI and FIB‐4 indicated that they are acceptable surrogates for use in epidemiological studies. 26 , 28 , 55 , 56 , 57 Second, the follow‐up duration varied among study participants due to the inherent nature in the study design, where ongoing annual surveys were sequentially incorporated into the current study with a single end‐point. Third, we could not analyse liver‐related mortality in the current study. The KNHANES did not allow us to obtain or analyse data on liver‐related mortality due to the small number of liver‐related deaths. Finally, sarcopenia was not evaluable in all subjects, as DXA has not been performed since 2012. However, we could draw a relatively clear conclusion from this portion of the analysis.
With these caveats in mind, we herein report that concurrent NAFLD and sarcopenia additively increased the risk of mortality in the Korean general population. Our results are relevant to clinical practitioners because they may help guide the risk stratification of patients with NAFLD. Our finding that NAFLD increased the risk of mortality when stratified by sarcopenic status conveys an important message to clinicians, and may facilitate the prediction of clinical outcomes in subjects with metabolic abnormalities, including NAFLD and sarcopenia. Our results also provide a basis for stimulating further research into the role of crosstalk between liver and muscle in predicting the long‐term prognoses of patients with NAFLD and/or sarcopenia.
Funding
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean Government (MEST) (2021R1A2C2005820, 2021M3A9E4021818, and 2021R1C1C1009875), the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI) funded by the Ministry of Health & Welfare, Republic of Korea (HI21C0538), and a public clinical research grant‐in‐aid from the Seoul Metropolitan Government Seoul National University (SMG‐SNU) Boramae Medical Center (04‐2021‐0041).
Author contributions
All authors did the conception and/or design; the acquisition, analysis, and/or interpretation of data; the drafting and/or revising of the work; and the final approval of the manuscript.
Conflict of interest
The authors have no conflicts of interest to disclose.
Supporting information
Table S1. Baseline clinical characteristics according to nonalcoholic fatty liver disease and advanced fibrosis
Table S2. Baseline clinical characteristics according to survival status
Table S3. Baseline clinical characteristics according to nonalcoholic fatty liver disease and sarcopenia
Table S4. Hazard ratios for mortality according to nonalcoholic fatty liver disease and sarcopenia in the fully adjusted model (Model 4 in Table 3)
Table S5. Hazard ratios for cardiovascular disease‐specific and cancer‐specific mortality according to nonalcoholic fatty liver disease and sarcopenia in the fully adjusted model (Model 4 in Table 3)
Acknowledgements
The authors of this manuscript certify that they comply with the ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle. 58
Moon J. H., Koo B. K., and Kim W. (2021) Non‐alcoholic fatty liver disease and sarcopenia additively increase mortality: a Korean nationwide survey, Journal of Cachexia, Sarcopenia and Muscle, 12, 964–972, 10.1002/jcsm.12719
Contributor Information
Bo Kyung Koo, Email: bokyungkoomd@gmail.com.
Won Kim, Email: drwon1@snu.ac.kr.
References
- 1. Williams CD, Stengel J, Asike MI, Torres DM, Shaw J, Contreras M, et al. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle‐aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology 2011;140:124–131. [DOI] [PubMed] [Google Scholar]
- 2. Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol 2018;15:11–20. [DOI] [PubMed] [Google Scholar]
- 3. Targher G, Byrne CD, Lonardo A, Zoppini G, Barbui C. Non‐alcoholic fatty liver disease and risk of incident cardiovascular disease: a meta‐analysis. J Hepatol 2016;65:589–600. [DOI] [PubMed] [Google Scholar]
- 4. Torres DM, Williams CD, Harrison SA. Features, diagnosis, and treatment of nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 2012;10:837–858. [DOI] [PubMed] [Google Scholar]
- 5. Angulo P, Kleiner DE, Dam‐Larsen S, Adams LA, Bjornsson ES, Charatcharoenwitthaya P, et al. Liver fibrosis, but no other histologic features, is associated with long‐term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology 2015;149:389–397, e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kim D, Kim W, Kim HJ, Therneau TM. Association between noninvasive fibrosis markers and mortality among adults with nonalcoholic fatty liver disease in the United States. Hepatology 2013;57:1357–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dulai PS, Singh S, Patel J, Soni M, Prokop LJ, Younossi Z, et al. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: systematic review and meta‐analysis. Hepatology 2017;65:1557–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Taylor RS, Taylor RJ, Bayliss S, Hagstrom H, Nasr P, Schattenberg JM, et al. Association between fibrosis stage and outcomes of patients with nonalcoholic fatty liver disease: a systematic review and meta‐analysis. Gastroenterology 2020;158:1611–1625, e12. [DOI] [PubMed] [Google Scholar]
- 9. Bae EJ, Kim YH. Factors affecting sarcopenia in korean adults by age groups. Osong Public Health Res Perspect 2017;8:169–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yazar T, Yazar HO. Prevalance of sarcopenia according to decade. Clin Nutr ESPEN 2019;29:137–141. [DOI] [PubMed] [Google Scholar]
- 11. Cho YJ, Lim YH, Yun JM, Yoon HJ, Park M. Sex‐ and age‐specific effects of energy intake and physical activity on sarcopenia. Sci Rep 2020;10:9822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pasco JA, Mohebbi M, Holloway KL, Brennan‐Olsen SL, Hyde NK, Kotowicz MA. Musculoskeletal decline and mortality: prospective data from the Geelong Osteoporosis Study. J Cachexia Sarcopenia Muscle 2017;8:482–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Moon JH, Kim KM, Kim JH, Moon JH, Choi SH, Lim S, et al. Predictive values of the new sarcopenia index by the Foundation for the National Institutes of Health Sarcopenia project for mortality among older korean adults. PLoS ONE 2016;11:e0166344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Koo BK, Kim D, Joo SK, Kim JH, Chang MS, Kim BG, et al. Sarcopenia is an independent risk factor for non‐alcoholic steatohepatitis and significant fibrosis. J Hepatol 2017;66:123–131. [DOI] [PubMed] [Google Scholar]
- 15. Kang S, Moon MK, Kim W, Koo BK. Association between muscle strength and advanced fibrosis in non‐alcoholic fatty liver disease: a Korean nationwide survey. J Cachexia Sarcopenia Muscle 2020;11:1232–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dalle S, Rossmeislova L, Koppo K. The role of inflammation in age‐related sarcopenia. Front Physiol 2017;8:1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bano G, Trevisan C, Carraro S, Solmi M, Luchini C, Stubbs B, et al. Inflammation and sarcopenia: a systematic review and meta‐analysis. Maturitas 2017;96:10–15. [DOI] [PubMed] [Google Scholar]
- 18. Friedman SL, Neuschwander‐Tetri BA, Rinella M, Sanyal AJ. Mechanisms of NAFLD development and therapeutic strategies. Nat Med 2018;24:908–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kweon S, Kim Y, Jang MJ, Kim Y, Kim K, Choi S, et al. Data resource profile: the Korea National Health and Nutrition Examination Survey (KNHANES). Int J Epidemiol 2014;43:69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018;67:328–357. [DOI] [PubMed] [Google Scholar]
- 21. American Diabetes Association . 2. Classification and diagnosis of diabetes: standards of medical care in diabetes‐2020. Diabetes Care 2020;43:S14–S31. [DOI] [PubMed] [Google Scholar]
- 22. WHO Expert Consultation . Appropriate body‐mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004;363:157–163. [DOI] [PubMed] [Google Scholar]
- 23. Seo MH, Lee WY, Kim SS, Kang JH, Kang JH, Kim KK, et al. 2018 Korean Society for the Study of Obesity Guideline for the management of obesity in Korea. J Obes Metab Syndr 2019;28:40–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute scientific statement. Circulation 2005;112:2735–2752. [DOI] [PubMed] [Google Scholar]
- 25. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 1999;130:461–470. [DOI] [PubMed] [Google Scholar]
- 26. Lee JH, Kim D, Kim HJ, Lee CH, Yang JI, Kim W, et al. Hepatic steatosis index: a simple screening tool reflecting nonalcoholic fatty liver disease. Dig Liver Dis 2010;42:503–508. [DOI] [PubMed] [Google Scholar]
- 27. McPherson S, Hardy T, Dufour JF, Petta S, Romero‐Gomez M, Allison M, et al. Age as a confounding factor for the accurate non‐invasive diagnosis of advanced NAFLD fibrosis. Am J Gastroenterol 2017;112:740–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. McPherson S, Stewart SF, Henderson E, Burt AD, Day CP. Simple non‐invasive fibrosis scoring systems can reliably exclude advanced fibrosis in patients with non‐alcoholic fatty liver disease. Gut 2010;59:1265–1269. [DOI] [PubMed] [Google Scholar]
- 29. Hong S, Oh HJ, Choi H, Kim JG, Lim SK, Kim EK, et al. Characteristics of body fat, body fat percentage and other body composition for Koreans from KNHANES IV. J Korean Med Sci 2011;26:1599–1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Studenski SA, Peters KW, Alley DE, Cawthon PM, McLean RR, Harris TB, et al. The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. J Gerontol A Biol Sci Med Sci 2014;69:547–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Beaudart C, Rizzoli R, Bruyere O, Reginster JY, Biver E. Sarcopenia: burden and challenges for public health. Arch Public Health 2014;72:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bhanji RA, Narayanan P, Allen AM, Malhi H, Watt KD. Sarcopenia in hiding: the risk and consequence of underestimating muscle dysfunction in nonalcoholic steatohepatitis. Hepatology 2017;66:2055–2065. [DOI] [PubMed] [Google Scholar]
- 33. Samuel VT, Shulman GI. The pathogenesis of insulin resistance: integrating signaling pathways and substrate flux. J Clin Invest 2016;126:12–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Petersen MC, Shulman GI. Mechanisms of insulin action and insulin resistance. Physiol Rev 2018;98:2133–2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Meex RCR, Blaak EE, van Loon LJC. Lipotoxicity plays a key role in the development of both insulin resistance and muscle atrophy in patients with type 2 diabetes. Obes Rev 2019;20:1205–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Phillips T, Leeuwenburgh C. Muscle fiber specific apoptosis and TNF‐alpha signaling in sarcopenia are attenuated by life‐long calorie restriction. FASEB J 2005;19:668–670. [DOI] [PubMed] [Google Scholar]
- 37. Zhang HJ, Zhang XF, Ma ZM, Pan LL, Chen Z, Han HW, et al. Irisin is inversely associated with intrahepatic triglyceride contents in obese adults. J Hepatol 2013;59:557–562. [DOI] [PubMed] [Google Scholar]
- 38. Stefan N, Haring HU. The role of hepatokines in metabolism. Nat Rev Endocrinol 2013;9:144–152. [DOI] [PubMed] [Google Scholar]
- 39. Issa D, Alkhouri N, Tsien C, Shah S, Lopez R, McCullough A, et al. Presence of sarcopenia (muscle wasting) in patients with nonalcoholic steatohepatitis. Hepatology 2014;60:428–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kim G, Lee SE, Lee YB, Jun JE, Ahn J, Bae JC, et al. Relationship between relative skeletal muscle mass and nonalcoholic fatty liver disease: a 7‐year longitudinal study. Hepatology 2018;68:1755–1768. [DOI] [PubMed] [Google Scholar]
- 41. Adams LA, Lymp JF, St Sauver J, Sanderson SO, Lindor KD, Feldstein A, et al. The natural history of nonalcoholic fatty liver disease: a population‐based cohort study. Gastroenterology 2005;129:113–121. [DOI] [PubMed] [Google Scholar]
- 42. Ong JP, Pitts A, Younossi ZM. Increased overall mortality and liver‐related mortality in non‐alcoholic fatty liver disease. J Hepatol 2008;49:608–612. [DOI] [PubMed] [Google Scholar]
- 43. Lazo M, Hernaez R, Bonekamp S, Kamel IR, Brancati FL, Guallar E, et al. Non‐alcoholic fatty liver disease and mortality among US adults: prospective cohort study. BMJ 2011;343:d6891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Golabi P, Gerber L, Paik JM, Deshpande R, de Avila L, Younossi ZM. Contribution of sarcopenia and physical inactivity to mortality in people with non‐alcoholic fatty liver disease. JHEP Rep 2020;2:100171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Williams GR, Chen Y, Kenzik KM, McDonald A, Shachar SS, Klepin HD, et al. Assessment of sarcopenia measures, survival, and disability in older adults before and after diagnosis with cancer. JAMA Netw Open 2020;3:e204783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Chin SO, Rhee SY, Chon S, Hwang YC, Jeong IK, Oh S, et al. Sarcopenia is independently associated with cardiovascular disease in older Korean adults: the Korea National Health and Nutrition Examination Survey (KNHANES) from 2009. PLoS ONE 2013;8:e60119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Welch N, Dasarathy J, Runkana A, Penumatsa R, Bellar A, Reen J, et al. Continued muscle loss increases mortality in cirrhosis: impact of aetiology of liver disease. Liver Int 2020;40:1178–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Romero‐Corral A, Montori VM, Somers VK, Korinek J, Thomas RJ, Allison TG, et al. Association of bodyweight with total mortality and with cardiovascular events in coronary artery disease: a systematic review of cohort studies. Lancet 2006;368:666–678. [DOI] [PubMed] [Google Scholar]
- 49. Kong KA, Park J, Hong SH, Hong YS, Sung YA, Lee H. Associations between body mass index and mortality or cardiovascular events in a general Korean population. PLoS ONE 2017;12:e0185024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Oh H, Kwak S‐Y, Jo G, Lee J, Park D, Lee DH, et al. Adiposity and mortality in Korean adults: a population‐based prospective cohort study. Am J Clin Nutr 2020;113:142–153. [DOI] [PubMed] [Google Scholar]
- 51. Younossi Z, Tacke F, Arrese M, Chander Sharma B, Mostafa I, Bugianesi E, et al. Global perspectives on nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Hepatology 2019;69:2672–2682. [DOI] [PubMed] [Google Scholar]
- 52. Chen F, Esmaili S, Rogers GB, Bugianesi E, Petta S, Marchesini G, et al. Lean NAFLD: a distinct entity shaped by differential metabolic adaptation. Hepatology 2020;71:1213–1227. [DOI] [PubMed] [Google Scholar]
- 53. Wei JL, Leung JC, Loong TC, Wong GL, Yeung DK, Chan RS, et al. Prevalence and severity of nonalcoholic fatty liver disease in non‐obese patients: a population study using proton‐magnetic resonance spectroscopy. Am J Gastroenterol 2015;110:1306–1314. [DOI] [PubMed] [Google Scholar]
- 54. Younossi ZM, Stepanova M, Negro F, Hallaji S, Younossi Y, Lam B, et al. Nonalcoholic fatty liver disease in lean individuals in the United States. Medicine (Baltimore) 2012;91:319–327. [DOI] [PubMed] [Google Scholar]
- 55. Kim BK, Kim DY, Park JY, Ahn SH, Chon CY, Kim JK, et al. Validation of FIB‐4 and comparison with other simple noninvasive indices for predicting liver fibrosis and cirrhosis in hepatitis B virus‐infected patients. Liver Int 2010;30:546–553. [DOI] [PubMed] [Google Scholar]
- 56. Kahl S, Strassburger K, Nowotny B, Livingstone R, Kluppelholz B, Kessel K, et al. Comparison of liver fat indices for the diagnosis of hepatic steatosis and insulin resistance. PLoS ONE 2014;9:e94059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lee YH, Cho Y, Lee BW, Park CY, Lee DH, Cha BS, et al. Nonalcoholic fatty liver disease in diabetes. Part I: epidemiology and diagnosis. Diabetes Metab J 2019;43:31–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2019. J Cachexia Sarcopenia Muscle 2019;10:1143–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Baseline clinical characteristics according to nonalcoholic fatty liver disease and advanced fibrosis
Table S2. Baseline clinical characteristics according to survival status
Table S3. Baseline clinical characteristics according to nonalcoholic fatty liver disease and sarcopenia
Table S4. Hazard ratios for mortality according to nonalcoholic fatty liver disease and sarcopenia in the fully adjusted model (Model 4 in Table 3)
Table S5. Hazard ratios for cardiovascular disease‐specific and cancer‐specific mortality according to nonalcoholic fatty liver disease and sarcopenia in the fully adjusted model (Model 4 in Table 3)


