Abstract
Epithelial tissues are the most rapidly dividing tissues in the body, holding a natural ability for renewal and regeneration. This ability is crucial for survival as epithelia are essential to provide the ultimate barrier against the external environment, protecting the underlying tissues. Tissue stem and progenitor cells are responsible for self-renewal and repair during homeostasis and following injury. Upon wounding, epithelial tissues undergo different phases of haemostasis, inflammation, proliferation and remodelling, often resulting in fibrosis and scarring. In this review, we explore the phenotypic differences between the skin, the oesophagus and the oral mucosa. We discuss the plasticity of these epithelial stem cells and contribution of different fibroblast subpopulations for tissue regeneration and wound healing. While these epithelial tissues share global mechanisms of stem cell behaviour for tissue renewal and regeneration, the oral mucosa is known for its outstanding healing potential with minimal scarring. We aim to provide an updated review of recent studies that combined cell therapy with bioengineering exporting the unique scarless properties of the oral mucosa to improve skin and oesophageal wound healing and to reduce fibrotic tissue formation. These advances open new avenues toward the ultimate goal of achieving scarless wound healing.
Keywords: oral mucosa, oesophagus, skin, homeostasis, wound repair, regenerative therapy, tissue engineering, regenerative medicine
Introduction
Epithelial tissues provide the body’s first line of protection from physical, chemical and biological damage. Mammalian epithelia vary in structure throughout the body according to their function and microenvironment. Skin is considered the largest organ of our body; however, it is not the only epithelium exposed to the external environment. The airways, digestive tract, as well as the urinary and reproductive systems, are all exposed to external stress and are lined by an epithelium, sharing some important structural and functional features.
In this review, we focus on three stratified squamous epithelial tissues – the skin, the oesophagus and the oral mucosa – and provide a comparative analysis of the architecture, cell composition and behaviour of these three different tissues during homeostasis and wound healing. We discuss the outstanding regenerative potential of the oral mucosa and how its scarless wound healing properties can be applied to the other tissues.
Comparative Analysis of Skin, Oesophagus, and Oral Mucosa
Adult epithelia harbour resident stem cells (SCs) responsible for homeostasis and tissue repair. The epithelial lining of the skin develops from the ectoderm, the oesophageal epithelium derives from the endoderm, while the oral epithelium derives both from ectoderm and endoderm (Wells and Melton, 1999; Fuchs, 2007; Que et al., 2007; Rothova et al., 2012). Skin, oesophagus and oral mucosa share global cellular architecture (Figure 1) and homeostasis, however several studies have highlighted different markers for their SCs and differentiated cells (Figure 2).
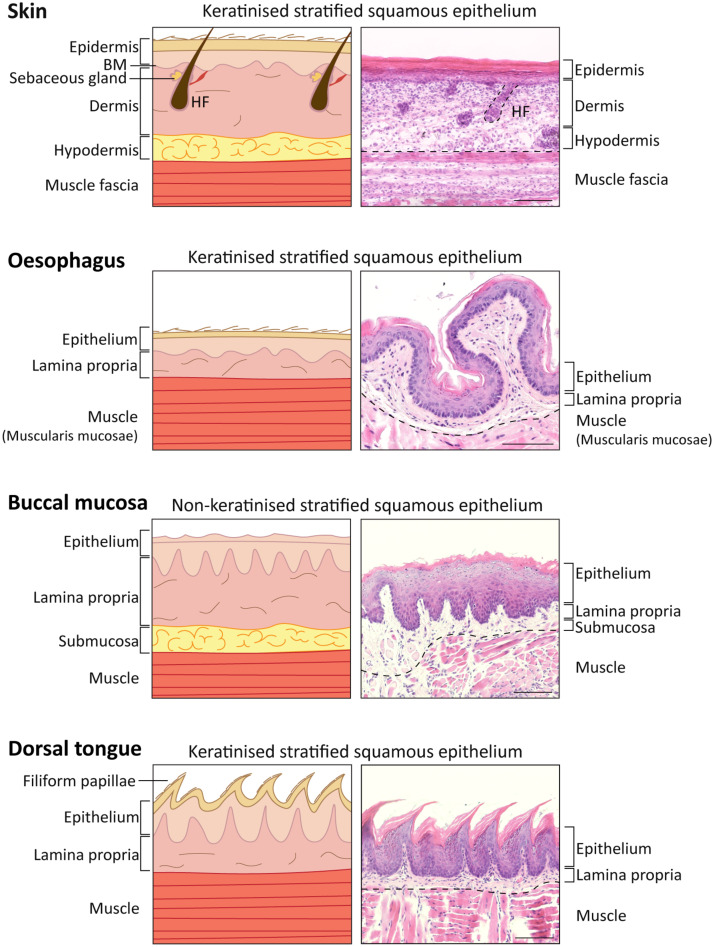
FIGURE 1.
Comparison of skin, oesophagus, oral mucosal tissue structure in Mus musculus. Diagram (Left) and representative histological images (Right) of skin, oesophagus and oral mucosa keratinised (dorsal tongue) and non-keratinised (buccal mucosa) tissues identifying the different layers. 5 μm-sections collected from a 16-week-old C57BL/6 mouse stained with haematoxylin and eosin staining (H&E). Scale bar: 100 μm. HF, hair follicle.
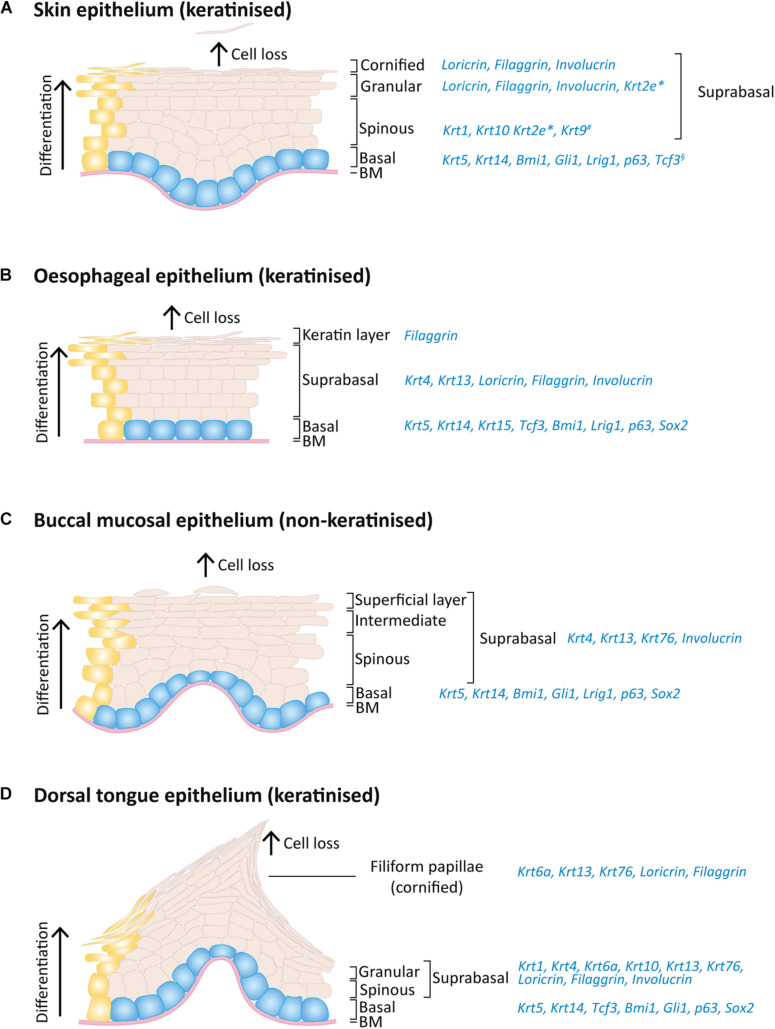
FIGURE 2.
Expression pattern of keratins and others markers on the adult mouse skin, oesophagus and oral epithelia. Schematic of epithelial layers and respective expression markers for (A) skin, (B) oesophageal, (C) buccal mucosa, and (D) dorsal tongue epithelia. During normal epithelial homeostasis, epithelial cells proliferate on the basal layer (blue) and keratinocyte differentiation (yellow) is accompanied by an upward migration through the suprabasal layers, replacing dead cells that shed from the epithelium surface. *Expressed only on ear, sole and tail skin; #expressed only on sole and palm skin; §expressed only on paw skin.
Skin, Oesophagus and Oral Mucosa Structural Comparison
The skin is comprised of three different layers: epidermis, dermis and hypodermis, and harbours additional appendages, such as hair follicles, nails, sweat and sebaceous glands (Watt, 2014). The interfollicular epidermis (IFE) is the outermost layer and is responsible not only for mechanical protection from the hostile environment, but also prevents from dehydration and invasion by microorganisms. The IFE is a multi-layered stratified squamous epithelium with four layers that have different degrees of differentiation: basal, spinous, granular and stratum corneum or cornified layer (Figures 1, 2A), and is composed of keratinocytes, Merkel cells, melanocytes, Langerhans cells and lymphocytes (Rushmer et al., 1966; Matoltsy, 1986; Odland, 1991; Holbrook, 1994; Joost et al., 2020). The IFE is separated from the underlying dermis by a basement membrane (Figure 1), an extracellular matrix (ECM) rich in type IV collagen and laminin (Timpl and Brown, 1996).
The dermis is the connective tissue layer that provides skin elasticity and tensile strength (Frantz et al., 2010) and it is mainly composed of fibroblasts, but also monocytes, macrophages, mast cells, lymphocytes, dermal adipocytes, as well as blood vessel- and sensory nerves-related cells (Lai-Cheong and McGrath, 2013). Using intra-vital imaging on normal mouse ear and paw skin showed that fibroblasts maintain a stable position, and that upon loss of neighbouring cells, the cell membranes extend to fill in the space in a Rac1-dependent process. This process is also conserved upon fibroblast loss in skin ageing (Marsh et al., 2018). However, it is the non-cellular component of the dermis – the ECM – that provides the scaffolding for the skin cellular constituents and that regulates the signalling required for tissue morphogenesis, differentiation and homeostasis (Sorrell and Caplan, 2004; Frantz et al., 2010).
The dermis can be separated into three spatially distinct layers with unique characteristics in development, regeneration and fibrosis: (1) the papillary layer, closest to the epidermis with a high cell density and loose connective tissue and expressing CD90+CD39+FAP+ in human; (2) the reticular layer, with lower cell density but rich in connective tissue and expressing FAP–CD90+ in human (and CD36+ for the lower reticular); and (3) the hypodermis which consists mainly of adipose tissue, loose connective tissue and is highly vascularised and rich in hormones and growth factors and expressing CD90+CD36+ in human (Figure 1; Harper and Grove, 1979; Azzarone and Macieira-Coelho, 1982; Schafer et al., 1985; Sorrell et al., 1996; Freinkel and Woodley, 2001; Sorrell and Caplan, 2004; Watt and Fujiwara, 2011; Driskell et al., 2013; Driskell and Watt, 2015; Sriram et al., 2015; Hiraoka et al., 2016; Philippeos et al., 2018; Korosec et al., 2019). Another fibroblast subpopulation associated with hair follicles lies in the dermal papilla and on the hair follicle dermal sheath, and belongs to the papillary lineage (Reynolds and Jahoda, 1991; Jahoda and Reynolds, 1996; Driskell et al., 2013; Joost et al., 2020). Several studies have highlighted the functional heterogeneity of fibroblasts with different healing potential (Driskell et al., 2013; Rinkevich et al., 2015; Mastrogiannaki et al., 2016; Jiang and Rinkevich, 2018; Jiang et al., 2018; Philippeos et al., 2018; Tabib et al., 2018; Correa-Gallegos et al., 2019; Guerrero-Juarez et al., 2019; Abbasi et al., 2020; Joost et al., 2020; Phan et al., 2021) as well as differences in the expression of collagen subtypes and proteoglycans (Meigel et al., 1977; Zimmermann et al., 1994; Sorrell et al., 1999; Sorrell and Caplan, 2004) and response to different signals originating from neoplastic epidermal SCs (Lichtenberger et al., 2016). Papillary fibroblasts are more proliferative than site-matched reticular fibroblasts, in both mouse and human skin (Harper and Grove, 1979; Azzarone and Macieira-Coelho, 1982; Schafer et al., 1985; Sorrell et al., 1996; Sorrell and Caplan, 2004) and more effectively support the formation of a multi-layered epithelium in two- and three-dimensional (3D) cultures (Higgins et al., 2017; Korosec and Lichtenberger, 2018; Philippeos et al., 2018). Reticular dermis is richer in fibrous connective tissue and, when in culture, reticular dermal fibroblasts contract collagen latices faster than papillary dermal fibroblasts (Schafer et al., 1985; Sorrell et al., 1996). According to lineage tracing and skin reconstitution assays in mice, reticular fibroblasts descending from PDGFRα+Dlk1+ progenitors are responsible for the first wave of dermal wound repair and produce the bulk of the ECM whereas papillary fibroblast lineage supports healthy skin regeneration and hair follicle development, namely through expression of the key transcription factor Lef1 (Driskell et al., 2013; Rognoni et al., 2016, 2018; Phan et al., 2020). More recently, the quiescence-associated factor hypermethylated in cancer 1 positive (Hic1+) progenitors, primarily distributed in the reticular dermis, was shown to robustly contribute to regenerate injured dermis and to populate neogenic hair follicles in adult mice (Abbasi et al., 2020). As for the hypodermis, the deepest layer of the mammalian skin that provides insulation and cushioning, is crucial for wound healing, re-epithelialisation and angiogenesis processes (Freinkel and Woodley, 2001; Rivera-Gonzalez et al., 2014; López et al., 2018; Zomer et al., 2020).
A recent study has identified an additional fibroblast subpopulation below the hypodermis called the fascia that contribute to skin scar formation (see section “The Outstanding Regenerative Potential of Oral Mucosa – Scarless Wound Healing”; Correa-Gallegos et al., 2019; Jiang et al., 2020a; Jiang and Rinkevich, 2021).
In continuity with the skin epithelium, the stratified oral mucosa provides an important barrier to the external challenges. The structure of the oral epithelium comprises a stratified squamous epithelium (keratinised or non-keratinised) and the underlying lamina propria, which is rich in connective tissue, fibroblasts, nerves, minor salivary glands and blood vessels (Jones and Klein, 2013; Hand and Frank, 2014; Figure 1).
The non-keratinised oral epithelia comprise basal, spinous, intermediate and superficial layers, while the keratinised oral epithelia resemble the skin epidermis and include basal, spinous, granular and cornified layers (example of keratinised vs. non-keratinised oral epithelia in Figures 1, 2; comparison between all mouse oral epithelia reviewed in Jones and Klein, 2013). Furthermore, the oral mucosa is subdivided in masticatory (hard palate and gingiva), specialised (dorsal tongue) and lining subtypes (soft palate, buccal mucosa, ventral tongue, intra-oral lips and alveolar mucosa) (Gartner, 1991; Jones and Klein, 2013), reflecting the different structures within the oral cavity. For instance, the cheek buccal mucosa and soft palate are covered by non-keratinised lining mucosa which confers flexibility (Figure 1). The hard palate and gingiva are characterised by a keratinised masticatory epithelium prepared for stresses caused by chewing food. The tongue presents two different phenotypes: the ventral surface displays a non-keratinised lining epithelium, and the dorsal surface is covered by a specialised keratinised epithelium (Figures 1, 2; Jones and Klein, 2013; Hand and Frank, 2014; Groeger and Meyle, 2019). The specialised epithelium of the dorsal tongue houses four types of lingual papillae, three gustatory papillae (fungiform, circumvallate and foliate) with taste buds for sensorial stimuli, and filiform papillae important to grip and process food (Mistretta and Kumari, 2017). Filiform papillae are found in large numbers through the dorsal tongue and present a spinous cone-shaped structure (Figures 1, 2D; Hume and Potten, 1976).
Oral (gingival) fibroblasts are known to resemble foetal skin regenerative potential, namely on their migratory capacity through production of MSF, a migration stimulating factor not present in adult skin (Irwin et al., 1994).
From a development perspective, dorsal skin and oral mucosal fibroblasts have different origins: while the non-cranial dorsal skin dermis has an Engrailed1-lineage-positive somitic origin, the oral mucosa lamina propria and cranial skin dermis originates from Wnt1-lineage-positive neural crest cells (Janebodin et al., 2011; Ishii et al., 2012; Rinkevich et al., 2015). This may be the basis for the intrinsic phenotypic differences between oral and skin fibroblasts in wound healing. For instance, CD90+CD26+ skin fibroblasts were linked to scarring in skin wound healing, however, in gingiva CD26+ fibroblasts are only residually present (Mah et al., 2017; Worthen et al., 2020). Oral mucosal fibroblasts are also primed with higher expression levels of hepatocyte growth factor and its most relevant isoform NK1, therefore more effectively resist to TGF-β1-driven myofibroblast differentiation when compared to dermal fibroblasts (Dally et al., 2017). Another crucial difference relies on the phenotypic activity of the matrix metalloproteinase (MMP) tissue inhibitors (TIMP), namely TIMP-1 and TIMP-2 production, which in the oral mucosa is reduced, therefore allowing for increased MMP-2 activity in the remodelling phase of oral wound healing (Stephens et al., 2001).
Importantly, the epithelial-stromal interaction is key determinant of the phenotypic dynamics of the epithelium in homeostasis and when challenged. The epithelium is affected by the underlying mesenchymal cells, as these produce keratinocyte growth factor and hepatocyte growth factor/scatter factor molecules, important for the regulation of epithelial growth and integrity (Grøn et al., 2002; Costea et al., 2003; McKeown et al., 2003; Shannon et al., 2006; Sa et al., 2019). Furthermore, the epithelial-stromal-immune cell crosstalk in gingival mucosa was recently described as determinant of inducing an immune response to environmental cues and in regulating mucosal immunity (Nowarski et al., 2017; Caetano et al., 2021; Williams et al., 2021).
The submucosal layer of the oral cavity can be compared to the hypodermis in skin, being composed of loose fatty or glandular connective tissue. The presence of a submucosal layer depends on the oral cavity region and is directly linked to the flexibility of the attachment of the oral mucosa to underlying structures. In regions of lining epithelium (such as the cheek buccal mucosa, lips and some hard palate regions) this layer separates the oral mucosa from the bone or muscle below (Figure 1), while regions of masticatory and specialised mucosa (such as gingiva and some hard palate regions) lack this layer (Squier and Kremer, 2001).
Compared to skin and oral mucosa, the oesophagus epithelium is relatively simpler. Given its physiological function of transferring food from the oral cavity to the stomach, this organ is extended from the upper to the lower oesophageal sphincters which are respectively overlapped by the pharyngoesophageal and gastroesophageal junctions. The sphincters open during swallowing and the oesophagus initiates the process of peristalsis to assure the unidirectional transport of the content to the stomach. The mouse oesophagus comprises a keratinised stratified squamous epithelium, differing from non-keratinised human oesophageal epithelium (Figure 1). There are a few other key aspects that differentiate the mouse and human oesophageal epithelia. In humans, the oesophageal epithelium is folded around structures called papillae, which separates the basal layer into either interpapillary or papillary basal layers; it is also characterised by the presence of submucosal glands. This contrasts with the simple epithelium found in mice, devoid of papillae and glands (Messier and Leblond, 1960; Seery, 2002; Doupé et al., 2012; Alcolea, 2017). Additionally, while in mice the oesophageal epithelium comprises a basal layer of proliferating cells (Goetsch, 1910; Messier and Leblond, 1960; Marques-Pereira and Leblond, 1965; Gavaghan, 1999), in humans, cycling cells extend to the 5th-6th suprabasal layers (Barbera et al., 2015).
The oesophagus mucosa is composed of two other layers: the lamina propria, which in this organ is a very thin layer of connective tissue supporting the epithelium, as well as a thin layer of longitudinally organised smooth muscle (Goetsch, 1910; Oezcelik and DeMeester, 2011). To add to this diversity, it is known that the human oesophagus is not only composed of squamous epithelium, but on the most distal area there is a 1-2cm transition to columnar epithelium, which is the same lining epithelium covering the stomach (Gavaghan, 1999). Furthermore, the muscularis mucosae thickness increases from the most proximal to the most distal part of the oesophagus (Goetsch, 1910; Oezcelik and DeMeester, 2011).
Both the oral and the oesophageal epithelia are devoid of appendages. Although they belong to the gastrointestinal tract, they share the same stratified epithelium architecture as the skin rather than the single layer of cells that line the stomach, the small intestine and the large intestine, important for greater absorption capacity (Goetsch, 1910; Gordon, 1994).
Comparison of the Keratin Expression Programme Between Different Epithelia
Keratins are intermediate filament proteins of epithelial cells providing mechanical integrity and structure to the epithelia and act as a scaffold that enables cells to resist stress and damage, which is essential for normal tissue function (Coulombe et al., 1991; Moll et al., 2008). Changes in keratin synthesis leads to alterations in cell movement or cell differentiation and, consequently, their function (Vassar et al., 1991; Singh and Gupta, 1994). Mutations that impair keratin assembly have been identified in a range of human skin or multifactorial disorders, such as epidermolysis bullosa, typically leading to loss of epithelial integrity, abnormal differentiation and affecting epithelial regeneration (Lane, 1994; Quinlan et al., 1994; Knöbel et al., 2015; Herrmann and Aebi, 2016; Bardhan et al., 2020).
While different epithelia exhibit different patterns of keratin expression (Franke et al., 1981) the keratin patterns are similar between the same anatomic regions of different species. During epithelial homeostasis, epithelial cells migrate from the basal into the suprabasal layer and progressively loose their proliferative potential and begin to synthesise a set of structural proteins (Candi et al., 2005). The switch in the keratin expression from proliferating basal cells to differentiated suprabasal cells indicates a change in the cell cytoskeleton organisation, influencing their functional properties.
In all skin, oesophageal and oral stratified squamous epithelia, the basal dividing cells produce keratin 5 (Krt5) and Krt14 (Squier and Kremer, 2001; Rosekrans et al., 2015; Gonzales and Fuchs, 2017). Krt15 is additionally expressed in the oesophageal basal cells (Rosekrans et al., 2015; Giroux et al., 2017). As cells leave the basal layer and start differentiating, the keratin expression suffers a transition to other keratins and differences arise between types of epithelia. For instance, mouse skin epidermal suprabasal cells switch to expressing Krt10 and Krt1 on interfollicular epidermis and Krt2e on the ears, soles and tail (Fuchs and Green, 1980; Candi et al., 2005; Fischer et al., 2016; Figure 2A). Interestingly, the epidermis of palms and soles, which are the thickest epidermis withstanding the highest degree of mechanical stress the body is exposed to, also express Krt9 in suprabasal layers to provide additional mechanical reinforcement (Knapp et al., 1986; Moll et al., 1987; Candi et al., 2005; Fu et al., 2014).
The heterogeneity of oral epithelia is reflected in its suprabasal keratin expression. The non-keratinised lining mucosa shares the expression of Krt4 and Krt13 (Dale et al., 1990), whereas the palatal and gingival masticatory epithelia are keratinised and share the expression of Krt1, Krt2p (now called Krt76) and Krt10 with skin (Dale et al., 1990; Collin et al., 1992). The gingiva itself is composed of a heterogeneous combination of keratin expression varying between the gingival epithelium mentioned above, the sulcular epithelium (expressing Krt4 and Krt13) and junctional epithelium (expressing Krt8, Krt13, Krt16, Krt18 and Krt19) (Dale et al., 1990; Groeger and Meyle, 2019). Regarding the specialised epithelium of the dorsal tongue, a heterogeneous pattern is also found: Krt4 and Krt13 are expressed in the interpapillary zone and anterior papillae, Krt1 and Krt6a are expressed in the anterior papillae and Krt1 and Krt10 are locally expressed in the posterior papillae (Dale et al., 1990; Howard et al., 2014; Nishiguchi et al., 2016). A recent study has also shown the expression of Krt76 in the palate, buccal mucosa and dorsal tongue suprabasal layers, including filiform papillae (Figures 2C,D; Sequeira et al., 2018).
With more similarities with oral than with skin epithelia, the oesophageal epithelium expresses Krt4 and Krt13 on the suprabasal layers (Figure 2B; Treuting et al., 2012; Rosekrans et al., 2015; Zhang et al., 2017). The mouse oesophagus contains an acellular layer of keratin on the top of the squamous epithelium, similar to the skin, however this keratin layer is absent in the human oesophagus (Treuting et al., 2012).
In addition to keratins, important transcription factors are also expressed in the basal layer of different stratified epithelia: Lef/Tcf-family transcription factor Tcf3 was found in paw skin, dorsal tongue and oesophagus (Howard et al., 2014); Bmi1, Lrig1 and p63 are enriched as well in all these epithelial basal layers (Figure 2; Que et al., 2007; Senoo et al., 2007; Choy et al., 2012; Jones and Klein, 2013; Zhang et al., 2017; Byrd et al., 2019; Jones et al., 2019; Piedrafita et al., 2020); Gli1+ cells are present in oral mucosa and skin epithelial basal layer while Sox2 is in oesophageal and oral epithelia, including tongue taste bud cells (Que et al., 2007; Jones and Klein, 2013; Zhang et al., 2017; Jones et al., 2019; Ohmoto et al., 2020; Figure 2).
The proteins filaggrin, involucrin, and loricrin are also expressed in the suprabasal layers of these epithelia, being key differentiation proteins involved in the thickening of the cornified cell envelope (Figure 2; Mehrel et al., 1990; Squier and Kremer, 2001; Howard et al., 2014; Nishiguchi et al., 2016; Quiroz et al., 2020). Considering the lack a cornified layer in non-keratinised epithelia, keratinocytes retain their nucleus and despite presenting membrane-coating granules, the accumulation and aggregation of cytokeratins with formation of bundles of filaments seen in keratinised epithelia is much less pronounced (Squier, 1977).
Interestingly, keratin expression programmes can change when epithelial cells are exposed to a different environment. Epithelial cells respond to extrinsic signals and change their identity when placed in a different microenvironment, as observed when oesophageal, thymic or cornea epithelial are placed on skin microenvironment (Ferraris et al., 2000; Bonfanti et al., 2010; Bejar et al., 2021). For instance, when oesophageal epithelial cells are grafted into skin, the suprabasal layers loose Krt4 expression as it transforms into a skin identity (Bejar et al., 2021). The mechanisms regulating this identity change remain to be elucidated.
Epithelia Homeostasis and Cellular Differentiation
Tissues such as the squamous epithelia of the epidermis, oral cavity and oesophagus hold the natural capacity of self-renewal, with resident adult SCs actively replacing dying cells to accomplish homeostasis. The skin epidermis is by far the most studied epithelium, and this reflects the depth of the knowledge on SC behaviour and differentiation. In adult skin, different epithelia maintain homeostasis by their own pool of SC niches that are found in the basal layer of the IFE, as well as in the sweat glands, touch domes and hair follicle (Fuchs and Green, 1980; Cotsarelis et al., 1990; Blanpain et al., 2004; Morris et al., 2004; Ito et al., 2005; Legué and Nicolas, 2005; Clayton et al., 2007; Jaks et al., 2008; Jensen et al., 2009; Snippert et al., 2010b; Legué et al., 2012; Lu et al., 2012; Sequeira and Nicolas, 2012; Doucet et al., 2013; Page et al., 2013; Schepeler et al., 2014; Sada et al., 2016; Donati et al., 2017; Mesler et al., 2017; Yang et al., 2017).
There has been a great effort to understand the organisation and fate of stem cells in the basal layer that maintain tissue homeostasis. The first proposed model was the SC-transient amplifying cell hierarchy of the epidermal proliferative unit (EPU) (Potten, 1974; Figure 3A). The EPU model defends that each stack of cornified cells is maintained by a single slow-cycling SC basally located within the basal layer. The SC divides asymmetrically to generate another SC and a daughter transient amplifying cell, organised in 3D columns. The transient amplifying cells show high proliferative potential, undergo a fixed number of divisions prior upward migration and differentiation (Potten, 1974; Mackenzie, 1997). This model predicts clone size to rise into a plateau and then remain stable, although this was ruled out by lineage-tracing experiments (Clayton et al., 2007).
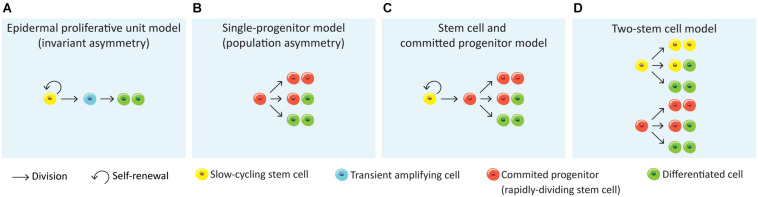
FIGURE 3.
Models for epithelial self-renewal. (A) Epidermal proliferative unit or invariant asymmetry model (Potten, 1974) suggests that epithelial renewal relies on quiescent slow-cycling SCs that generate transient amplifying actively cells, which in turn generate non-dividing, differentiated cells. (B) A second model defends that the epithelial renewal is achieved long-term by a single population of actively cycling and through stochastic fate of committed progenitor cells that directly generate differentiated cells (single-progenitor model) (Clayton et al., 2007; Doupé et al., 2010). (C) The stem cell and committed progenitor model aroused from the observation of a fast-cycling stem cell population (committed progenitor) within the basal layer that is generated by slow-cycling stem cells. These progenitors eventually produce differentiated cells, however due to its short lifespan their contribution to wound healing is limited (Mascré et al., 2012; Sánchez-Danés et al., 2016). (D) The two stem cell model suggests the co-existence of two stem cell populations independent from each other, with different division rates (Joost et al., 2016; Rompolas et al., 2016; Sada et al., 2016; Aragona et al., 2020; Piedrafita et al., 2020).
More recently, another study proposed the “population asymmetry” or “single-progenitor” model (Clayton et al., 2007), where the epidermal maintenance is achieved long-term through stochastic fate of a single committed keratinocyte progenitor in the basal layer from a pool of relatively fast cycling undifferentiated Krt5+Krt14+ epidermal SCs (Figure 3B). This pool is maintained by an autocrine mechanism of Wnt signalling (Lim et al., 2013). According to this model, SCs divide to generate one basal cell that attaches to the basement membrane and one committed progenitor cell which will be prone to leave the basal layer to enter an upward differentiation process. The progenitor population will continuously divide wile committed cells leave the basal layer and differentiate. This model suggests that the progenitor population randomly undergoes either asymmetrical or symmetrical divisions, the latter giving two progenitors or two differentiated cells (Clayton et al., 2007; Doupé et al., 2010; Lim et al., 2013; Rompolas et al., 2016; Figure 3B). Lineage-tracing experiments have shown that following this stochastic choice between symmetric or asymmetric SC division, the mean clone size progressively increases with time (Clayton et al., 2007).
An alternative to this model is the “Stem cell – committed progenitor model” (Figure 3C) that proposes a hierarchy of slow-cycling SCs that will give rise to active SCs (progenitors) which will then follow symmetric of asymmetric divisions to self-renew or to generate the differentiated cells (Mascré et al., 2012; Sánchez-Danés et al., 2016; Figure 3C).
Finally, a fourth model proposes the existence of two SC populations that differ in their proliferative dynamics, their gene-expression profile and their ability to repair the epidermis after injury (Figure 3D; Rompolas et al., 2016; Sada et al., 2016; Piedrafita et al., 2020). Some studies have already demonstrated heterogeneity within the mouse IFE basal cells. Joost and colleagues found two basal subpopulations in mouse dorsal IFE basal I and basal II, differing in the additional expression of Avpi1, Krt16, Thbs1, and the transcription factor Bhlhe40 by IFE basal I (Joost et al., 2016). Furthermore, the IFE progenitors found in different regions of the body were slow-cycling cells able to both self-renew and give rise to intermediate progenitors with a shorter lifespan and greater tendency to differentiation (Mascré et al., 2012; Sada et al., 2016; Sánchez-Danés et al., 2016; Piedrafita et al., 2020). The different observations on IFE basal cell populations in relation to the anatomical position were proposed to be dependent on hair follicle density in those regions. Both the distance to the hair follicles and its cycling status were shown to influence clonal progression reflecting fast- and slow-cycling progenitors (Roy et al., 2016; Gonzales and Fuchs, 2017). This two-stem cell model (Figure 3D) was very recently reinforced by Aragona and colleagues through the study of cellular and molecular mechanisms underlying stretch-mediated expansion in vivo (Aragona et al., 2020). The authors show that stretching induces changes in the renewal activity of a subset of epidermal SCs, which is crucial for expansion, while a second progenitor subpopulation committed to differentiation is preserved. These events were shown to be more consistently governed by the two-stem cell model when compared to the single-progenitor model (Figures 3B,D). Interestingly, a recent single-cell RNA-sequencing analysis of human neonatal foreskin discovered four basal SC populations with differential spatial distribution on the rete ridges of the epidermis, agreeing with a model of multiple SC pools that differ in their proliferation capacity (Wang et al., 2020). Future lineage-tracing, single-cell and microscopic analysis will be needed to further elucidate the basal layer cellular heterogeneity as well as novel markers and regulators.
The hair follicle has separate pools of long-term SCs [CD34+ (Blanpain et al., 2004), Gata6+ (Donati et al., 2017), Lgr5+ (Jaks et al., 2008), Lgr6+ (Snippert et al., 2010a), Lrig1+ (Jensen et al., 2009)] that are responsible for the homeostasis and the cycling regeneration of the hair follicle; and some of these subpopulations can contribute to the IFE for wounding regeneration, although they do not contribute to normal homeostasis maintenance of the IFE (Ito et al., 2005; Legué et al., 2012; Mesa et al., 2015; Liakath-Ali et al., 2018; Dekoninck and Blanpain, 2019; Abbasi et al., 2020).
IFE and oesophageal epithelia appear to share common homeostasis mechanisms (Piedrafita et al., 2020). As for the IFE, oesophageal homeostasis mechanisms of cell behaviour remain controversial. Some studies postulated that a hierarchy of stem and transient amplifying cells maintains homeostasis. Croagh and colleagues reported the existence of three basal cell subpopulations, according to the expression profiles of α6integrin and transferrin receptor (CD71): one α6briCD71dim is a putative oesophageal SC population, the α6briCD71bri represents the transient amplifying cell population and the third population α6dim which is a population of early differentiating cells (Croagh et al., 2007). In agreement to the postulated heterogeneity of basal cells, DeWard and colleagues used a combination of cell-surface markers and labelled proliferating basal epithelial cells in vivo to infer cell-cycle profiles and proliferation kinetics. Differences on the expression of α6 integrin (Itgα6, also known as CD49f) and β4 integrin (Itgβ4, CD104) in Sox2+ basal cells, combined with CD73 and Krt14, Krt13and Krt4 revealed three different basal subpopulations: Itgα6/Itgβ4HighCD73+ is a SC population, the faster dividing Itgα6/Itgβ4HighCD73– is a transient-amplifying population and Itgα6/Itgβ4Low represented the more differentiated basal cell population (DeWard et al., 2014). However, more studies argue that proliferation of a single progenitor population is confined to the basal layer in contact to the basement membrane and as progenitors are committed to differentiation, they withdraw from the cell cycle and migrate from this layer toward the epithelial surface. The fate of a dividing cell is randomly assigned, however the probabilities are balanced, so equal proportions of progenitor and differentiated cells are generated to maintain cellular homeostasis (Piedrafita et al., 2020). How this balance is maintained is not yet clear (Jankowski, 1993; Doupé et al., 2010, 2012; Alcolea et al., 2014; Frede et al., 2016). Recently, Giroux and colleagues defended the existence of a long-lived Krt15+ population with stem/progenitor cell characteristics through in vivo lineage-tracing and pointed against the single-progenitor model (Giroux et al., 2017).
All these paradigms around the proposed models of skin and oesophageal epithelia cell dynamics prompted Piedrafita and colleagues to conduct an in-depth study of nine lineage-tracing datasets in both oesophagus and various skin regions (paw, ear, back, tail scale and tail interscale) (Doupé et al., 2010, 2012; Mascré et al., 2012; Lim et al., 2013; Füllgrabe et al., 2015; Sada et al., 2016; Sánchez-Danés et al., 2016; Giroux et al., 2017; Murai et al., 2018), defending that divergent hypothesis result from distinct datasets analysis through distinct interpreting and suitable procedures, lacking alternative hypotheses tests. The authors used cell-cycle properties from the H2B-GFP dilution data to fit lineage-tracing results by maximum likelihood parameter inference. The results show that all these datasets are in unison with the single-progenitor model (Figure 3B), with the exception of the tail inter-scale region of the skin, defending that skin and oesophageal epithelia homeostasis is equally controlled by this model of basal cell behaviour (Piedrafita et al., 2020).
Besides intrinsic ability for division, the factors that drive basal cells to make the decision to proliferate or differentiate were not yet disclosed. For instance, upon skin wounding different SC populations were shown to contribute to different compartments and change their behaviour in order to increase proliferation over differentiation until complete wound closure, only then reverting to homeostasis (Jaks et al., 2008; Lim et al., 2013; Roshan et al., 2016; Donati et al., 2017). This highlights their plasticity when challenged. More recently the concepts of local fate coordination and epidermal cell competition were brought into discussion as key players of epithelial cell dynamics (Lei and Chuong, 2018; Mesa et al., 2018; Murai et al., 2018; Piedrafita et al., 2020). SC self-renewal was shown to be driven by differentiation of neighbouring cells, supporting the concept of local fate coordination, needed to achieve a precise balance of SC activity (Mesa et al., 2018). Upon differentiation, the space left is occupied by one of the directly neighbouring progenitors which competes with the others for filling the space. Cell competition is the process of elimination of less fit cells that regulates tissue homeostasis and defence against mutant populations which ultimately could evolve to tumours (Murai et al., 2018). Cell competition has been found in different tissues, such as skin, oral mucosa, intestine and oesophagus, and it is often associated with differential gene expression between competing cells (Klein et al., 2010; Snippert et al., 2010b; Klein and Simons, 2011; Alcolea et al., 2014; Lynch et al., 2017; Martincorena et al., 2018; Corominas-Murtra et al., 2020). Clone growth is restricted by the limited size of the proliferating compartment; therefore, since the epithelial progenitors reside in a continuous sheet with no barriers, the mutant clones can expand and collide with other surrounding progenitors. When these encounter similar competitive cells, the fate of the mutant clones reverts to a homeostatic behaviour (Hall et al., 2018; Martincorena et al., 2018; Colom et al., 2020). Both the skin and oesophageal local fate coordination and competition events were shown to be compatible with the single-progenitor model, regulating epithelial cell dynamics governed by stochastic, but, also biased progenitor fates (Piedrafita et al., 2020).
The oral epithelia SCs remain largely uncharacterised and the attribution of the EPU model of homeostasis was often assumed from studies performed in other epithelia, mainly skin (Alonso and Fuchs, 2003; Dabelsteen and Mackenzie, 2006; Thomson, 2020). More recent studies have been exploring different regions of the oral cavity and pointing to which model of epithelial homeostasis suits best with the results. Some studies have defended the EPU model for mouse tongue SC patterns (Luo et al., 2009; Tanaka et al., 2013; Tang et al., 2013). The specialised epithelium of the tongue was demonstrated to house two different SC niches, one in the basal layer where long-term progenitors characterised as Krt14+Krt5+Trp63+Sox2Low maintain the physiology of filiform and fungiform papillae, circumvallate papilla and soft palate, and the other is located outside the taste buds and is a Krt14+Krt5+Trp63+Sox2+ population of bipotential progenitor cells which give rise to both taste pore keratinocytes and receptor cells of the taste buds (Okubo et al., 2009). Jones and colleagues presented a study using lineage-tracing, label retention and single-cell RNA-sequencing that argues against the EPU model, stating that both the dorsal tongue and buccal mucosa epithelia are maintained by the single-progenitor model of homeostasis (Figure 3B). Additionally, the oral epithelial progenitor cells responded to epithelial damage by amending their daughter cell fates (Jones et al., 2019). Label-retaining cells were not found in tongue and oropharynx epithelia, however they observed that the hard palate displays a heterogeneous pattern of proliferation. The palate rugae junctional zone was proposed to hold a reserve SC niche, as these present characteristics of quiescence, self-renewal by symmetric cell divisions, Lrig1 expression, and activation after injury (Byrd et al., 2019). Furthermore, Lrig1 plays a critical role in regulating the oral epithelial SCs of the hard palate: upon decrease of Lrig1 expression, cells exit their quiescence mode, inducing proliferation in response to stress and injury (Byrd et al., 2019). Another study points to a Wnt-responsive long-lived SC population in the hard and soft palates basally located, responsible for homeostasis and response to injury. However, the soft palate showed a more robust and faster re-epithelialisation (Yuan et al., 2019).
Overall, the oral cavity is composed of a variety of types of epithelia with different lineage origins (Rothova et al., 2012) that serve distinct functions. More studies are needed to unveil the mechanisms underlying normal physiology of these tissues.
The main differences between epithelia of the skin, oesophagus and oral cavity, are their function, external microenvironment and differentiation markers. Their SCs are also estimated to divide at different rates: proliferating cells on the oesophagus and the oral mucosa divide on average every 2.4 days, while on the epidermis on average between 3.5 and 6 days, depending on the body region (Jones et al., 2019; Piedrafita et al., 2020). Furthermore, tissue expansion during growth or in adulthood (for example, ventral skin during pregnancy) also regulates SC division rate and global behaviour. This further supports the notion that SC behaviour is regulated by a combination of molecular and mechanical cues that regulate tissue microenvironment and cell behaviour (Vining and Mooney, 2017; Li et al., 2018; Shyer et al., 2018; Aragona et al., 2020; Biggs et al., 2020; Mcginn et al., 2021). Importantly, one of the crucial components of the microenvironment surrounding epithelial progenitor cells are fibroblasts. It has been shown that different fibroblast subpopulations which carry regionally intrinsic signals, determine the behaviour of adult epithelial cells, namely in skin and oral mucosa (Locke et al., 2008; Rinkevich et al., 2015; Yang et al., 2017; Abbasi et al., 2020). For instance, in the gingiva structure, the gingival and the junctional epithelia are phenotypically distinct. This is in part due to heterogeneous resident fibroblasts that provide different support to the epithelial growth and differentiation (Locke et al., 2008). These interactions between epithelial and subepithelial tissues hold a key role in tissue repair (McKeown et al., 2003).
Wound Repair Mechanisms in Skin, Oesophagus, and Oral Epithelia
Skin Wound Healing Process
Mammalian epithelia are prepared to respond to assaults to the normal tissue homeostasis, including physical, chemical and biological stress that often result in wounding. Skin wound healing response has been extensively studied giving cues to what may also be happening in the process of wound healing in other tissues.
Wound healing response begins right after injury and comprises a series of coordinated events that make part of a highly dynamic process. Although there are variations among different species, the mammalian wound healing follows a general pattern organised in four main phases: haemostasis, inflammation, proliferation and remodelling. As a very tightly regulated mechanism, minor changes could lead to impaired healing (Gurtner et al., 2008; Shaw and Martin, 2009).
The first phase, haemostasis, is triggered by damaged blood vessels leading to bleeding. At first, blood vessels constrict to stop blood flow, platelets are activated and aggregate in order to seal the ruptured blood vessel wall. Consequently, a fibrin clot is formed to keep the platelets and blood cells in the wound site. The clot holds a role as an initial matrix scaffold rich in growth factors that will recruit cells for further wound healing stages (Etulain, 2018). Platelets were also shown to produce a positive effect on mouse skin wound healing by enhancing the angiogenic potential of mesenchymal SCs (Levoux et al., 2021). Upon activation, platelets release respiration-competent mitochondria that are internalised by recipient mesenchymal SCs, where it stimulates their metabolism to produce increased levels of certain metabolites. Particularly citrate, which works as the main fuel for de novo fatty acid synthesis that in turn increase secretion of pro-angiogenic factors by mesenchymal SCs (Levoux et al., 2021). The inflammatory phase of wounding response starts with the recruitment of immune cells that travel to the injury site in order to remove pathogenic microbes. Following the platelets, neutrophils and monocytes, which differentiate into macrophages, are recruited. These have been shown to also participate in later phases of wound healing, contributing largely to cytokines and growth factors secretion, which activates and recruits other cells important for the wound healing process (Park and Barbul, 2004). The proliferation phase of wound healing comprises the rebuild of the wound site where new tissue is generated. In skin, it starts from 2 to 10 days after injury and can last for up to 3 weeks. This phase is characterised by abundant formation of a highly vascularised granulation tissue through deposition of ECM by fibroblasts (mainly composed of type III collagen), replacing the fibrin matrix (Rognoni et al., 2018). Keratinocytes and endothelial cells are recruited and activated in the wound site, actively promoting re-epithelialisation and neovascularization. Fibroblasts in the wound bed will transition to an activated state, myofibroblasts, which will not only contribute for ECM deposition but also to allow wound closure through contraction (Hinz, 2007; Velnar et al., 2009; Darby et al., 2014; Rognoni et al., 2018; DesJardins-Park et al., 2019). Importantly, in mice the presence of a thin muscular layer, the panniculus carnosus, promotes skin contraction and union of the wound edges; while human skin is devoid of this muscular layer (Zomer and Trentin, 2018).
The last and longest phase of wound healing is the remodelling phase which starts around week 3 and can last for up to more than 1 year. During this phase the type III collagen is actively remodelled to type I collagen by fibroblasts, macrophages and endothelial cells, which secrete MMPs (Martins et al., 2013). This rearrangement of collagen fibres allows the new skin area to become stronger and reduces scar thickness over time; however, the tensile strength of the wound area can only reach 80% compared to unwounded tissue (Gurtner et al., 2008; Xue and Jackson, 2015; Marshall et al., 2018; DesJardins-Park et al., 2019). Recent findings highlighted the role of two fibroblast-expressing transcription factors in wound healing impairment and scarring of the skin: the cyclin-dependent kinase inhibitor p21 and the gap junction alpha-1 protein Connexin43 (Jiang et al., 2020b; Wan et al., 2021).
Wound healing in the oral cavity has a different timeline from the skin. Epithelial cells start migrating and proliferating 24h post wounding and, for wound areas up to 5mm, a complete re-epithelialisation is reached by day 2 to 3 in oral mucosa, while in skin it would take up to 7 days (Szpaderska et al., 2003; Chen et al., 2010; Larjava, 2012; Glim et al., 2013; Iglesias-Bartolome et al., 2018). Inflammation peaks at days 2 to 3 as well and is resolved by day 6 (Bodner et al., 1993; Szpaderska et al., 2003; Iglesias-Bartolome et al., 2018). The further proliferation phase takes place very early from day 2 to 7, being followed by the remodelling of collagen (Bodner et al., 1993; Nikoloudaki et al., 2020).
The cellular and molecular mechanisms underlying oesophageal wound healing have recently attracted attention. Despite comparisons with gastric healing, the similarities to the epidermis have also prompted studies to disclose possible critical players in oesophageal response to wounding (Baatar et al., 2002a, b; Chai et al., 2007; Tarnawski and Ahluwalia, 2012; Jönsson et al., 2016; Tabola et al., 2016; Cai et al., 2018; Komaki et al., 2019; Boudaka et al., 2020).
The Multifaceted Outcomes of Scarring
The regeneration of a skin wound will lead to fine scar formation in superficial injuries. However, there are more complex outcomes for scarring including widespread scars, atrophic scars, scar contractures, hypertrophic scars, and keloid scars (Karppinen et al., 2019). Hypertrophic and keloid scars are pathological outcomes that come with devastating consequences for patients, such as pain and itching. Hypertrophic scars are lifted, erythematous, pruritic lesions confined to the wound boundaries while keloids are benign fibroproliferative dermal scars, growing beyond the wound margins (Bayat et al., 2003; Brown et al., 2008; Karppinen et al., 2019). Given their quasi-neoplastic tendencies, it has been argued that keloids should be classified as a pathological disease rather than a scar (Ud-Din and Bayat, 2020). Besides minor traumatic wounds and acne, other cases can arise from clinical surgeries, chemical and thermal burns or in consequence of allergic reactions. Self-harm scarring and combat wounds also a matter of concern (Mitchell et al., 2019; Johnson et al., 2020). The traumatic wounds in the hostility of war context come with exposure of bone, ligaments and tendons, as well as contamination, and the limited available resources in conflict zones’ hospitals impede the treatment of these wounds (Johnson et al., 2020).
On the one hand, skin scars carry long-term psychosocial effects, including anxiety and avoiding social interaction. This behaviour will interfere with future work life and relationships. In some contexts, scars result from traumatising events and bury a psychological meaning (Brown et al., 2008; Gibson et al., 2018; Mitchell et al., 2019). On the other hand, while visible skin scarring implies a social burden, oral and oesophagus scarring result in difficulties swallowing food and weight loss (Campos et al., 2020).
The fibrous tissues formed upon oesophageal injury are named oesophageal strictures and are mainly a consequence of various benign and malignant disorders. Some other causes include radiation therapy and caustic ingestions. Peptic strictures are caused by gastroesophageal reflux disease when stomach acid damages the oesophagus epithelium over time (Yamasaki et al., 2016). Stricture formation may result from extended endoscopic mucosal resection and submucosal dissection, two techniques used for treatment of superficial gastrointestinal neoplasia, gastric cancer and superficial Barrett’s oesophagus (Yang et al., 2019; Huang et al., 2020). The oesophageal stricture may be persistent or recurrent despite application of several therapies. These can cause complications such as solid and liquid dysphagia, regurgitations or aspiration, abdominal and chest pain as well as obstruction of the oesophagus (Ferguson, 2005).
Compared to the skin and oesophagus, the oral mucosa has an exceptional regenerative ability, being much less prone to scar formation. Despite owning this scar-free healing capacity, there are some particular cases of scar formation. The mucosal trauma applied by oral and perioral piercings may in some rare cases cause complications. Moreover, the oral mucosa may form a keloid or hypertrophic scar as a consequence of medication or of systematic disease (Escudero-Castaño et al., 2008). Additional scar formation may be a consequence of the cleft lip, palate and gum reconstruction, as well as removal of benign and malignant oral tumours (Goodacre and Swan, 2008; Chang et al., 2012; Fierz et al., 2013; Botticelli et al., 2019). Some diseases are also associated with oral mucosal fibrosis, including submucous fibrosis, pemphigus vulgaris and cicatricial pemphigoid, lichen planus, epidermolysis bullosa and proliferative verrucous leukoplakia (Evans, 2017). These can lead to failure in normal growth and restricted oral aperture (Wright, 2010). The molecular mechanisms underpinning these changes in oral wound healing are a subject of ongoing research.
The Outstanding Regenerative Potential of Oral Mucosa – Scarless Wound Healing
The only adult tissue with the potential to heal with minimal scar formation is the oral mucosa. This capacity is comparable to foetal skin scarless healing, occurring during the first and second trimesters of pregnancy (Rowlatt, 1979; Colwell et al., 2005; Karppinen et al., 2019). Several studies have evidenced that oral mucosa heals faster than skin (Szpaderska et al., 2003; Mak et al., 2009; Chen et al., 2010; Iglesias-Bartolome et al., 2018). Studies exploring the mechanisms of oral repair have allowed to point key differences responsible for the superior outcome when compared to skin (Figure 4). The main differences are:
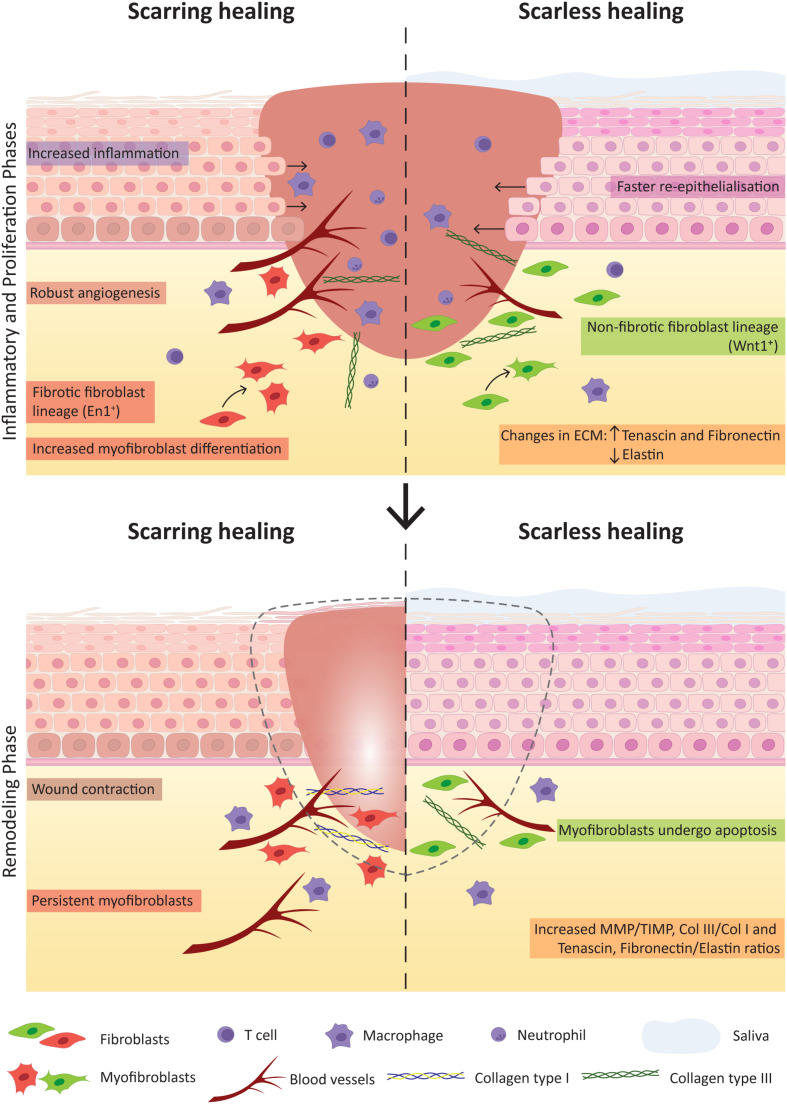
FIGURE 4.
Key factors contributing for scarring and scarless wound healing. The comparison of the inflammatory and proliferation phases (Top) and the remodelling phase (Bottom) of wound healing highlight crucial factors that notably contribute to the distinct healing outcome of skin (with scar formation) and oral mucosa (scarless).
-
(1)
Environment: the oral mucosa comes in contact with a very different environment compared to skin. The external factors such as saliva and the oral microbiota have been shown to play a role in oral wound healing (Hutson et al., 1979; Bodner et al., 1993; Su et al., 2018). The oral microbiota was shown to affect wound repair through secretion of lipopolysaccharides which maintains oral mesenchymal SCs homeostasis via miRNA-21/Sp1/telomerase reverse transcriptase pathway (Su et al., 2018). Bacteria may accelerate wound healing with beneficial effects in the immune response, granulation tissue and collagen formation (Jones et al., 2004). The positive role of saliva in wound repair has been explained by it being composed of growth factors such as the epidermal growth factor and peptides as histatins with antimicrobial function, responsible for enhanced oral keratinocyte and fibroblast migration. Therefore, saliva modulates oral and eventually skin wound healing mediating the inflammatory response (Figure 4; Zelles et al., 1995; Oudhoff et al., 2008; Boink et al., 2016; Neves et al., 2019).
-
(2)
Inflammation: the inflammatory response in oral wounds was shown to be reduced and to be concluded earlier than in skin wounds (Mak et al., 2009). In fact, there is much evidence linking excessive fibrosis with a strong inflammatory response to injury (Shaw et al., 2010; Wang et al., 2015). The number of immune cells such as neutrophils, macrophages and T cells in oral wound response is reduced when compared to skin, and linked with reduced levels of inflammatory cytokines [as interleukin (IL)-23, IL-24, IL-6, IL-8, tumour necrosis factor alpha (TNF-α)] and pro-fibrotic cytokines [transforming growth factor β1 (TGF-β1)], leading to decreased recruitment of inflammatory cells, and elevated anti-fibrotic cytokine TGF-β3 (Szpaderska et al., 2003; Schrementi et al., 2008; Chen et al., 2010; Glim et al., 2013). The reduced inflammation observed in the oral tissues during wound healing is a reflection of a tissue with the right tools to respond more efficiently. The local oral defences are constantly stimulated by the commensal microbiota and mastication, which trigger cellular crosstalk essential for homeostasis maintenance (Moutsopoulos and Konkel, 2018; Caetano et al., 2021; Williams et al., 2021). Another key immunosuppressive population in the mouth is Foxp3+ regulatory T cells (Park et al., 2018). Inflammatory response in the oral mucosa can be significantly amplified in cases of chemotherapy treatment or as a consequence of systemic conditions involving autoimmune responses, as of lichen planus, leading to increased probability of fibrotic tissue formation (Roopashree et al., 2010; Park et al., 2018; Basile et al., 2019). In fact the local oral tissue immunity can affect and be affected by extra-oral diseases (Moutsopoulos and Konkel, 2018; Kitamoto et al., 2020).
-
(3)
Angiogenesis: reduced angiogenesis could be expected to impair healing, though some studies have proven that inhibition of the angiogenic response in oral wounds is linked to reduced scar formation (Szpaderska et al., 2003; Wilgus et al., 2008). Angiogenesis can directly affect scar formation through oedema, apoptosis and transition of recruited pericytes to an activated fibroblast phenotype (Dulmovits and Herman, 2012; Johnson and Di Pietro, 2013; DiPietro, 2016). Angiogenesis and the inflammatory response act together as inflammatory cells release pro-angiogenic molecules (vascular endothelial growth factor (VEGF) and CXC chemokines) to promote capillary growth, which in turn will support the inflammatory response (Lucas et al., 2010; DiPietro, 2016).
-
(4)
Keratinocyte proliferation: the oral epithelia present faster re-epithelialisation (Szpaderska et al., 2003; Chen et al., 2010; Glim et al., 2014). Oral keratinocytes present higher proliferative potential and are less differentiated than skin keratinocytes therefore contributing with a greater regenerative potential (Glim et al., 2014; Turabelidze et al., 2014; Iglesias-Bartolome et al., 2018).
-
(5)
Fibroblasts: the major players of the proliferative phase of wound healing are fibroblasts that are responsible for collagen deposition and wound contraction, being critical players in the process of scarring. Several studies have investigated how different fibroblast lineages contribute to oral and to skin wound healing (Rinkevich et al., 2015; Gölz et al., 2016; Jiang et al., 2020a). Apart from the Engrailed1-lineage-positive fibroblast subpopulation, the study from Rinkevich and colleagues reports a Wnt1-lineage-positive population in the oral dermis tightly linked to the non-fibrotic healing that characterises the oral mucosa. A reciprocal transplantation of these oral mucosal- and skin-derived fibroblast populations performed in mice revealed that these mimic the response of the tissue of origin. Thus, the grafting of Wnt1-lineage-positive oral fibroblasts in skin resulted in decreased scar tissue formation while skin fibroblasts contributed for a scar-like tissue formation in the oral wound site, proving that the oral fibroblast lineage is determinant for the scarless healing of the oral mucosa (Rinkevich et al., 2015). Comparison between dermal and gingival fibroblasts showed that the latter have increased in vitro proliferation, migration and efficiency in remodelling connective tissue (Chaussain et al., 2002; Boink et al., 2016; Isaac et al., 2018), however contradictory results were reported in regard to the contraction capacity of oral fibroblasts (Lygoe et al., 2007; Mak et al., 2009). Recent studies in mice revealed the contribution of subcutaneous fascia fibroblasts to large deep skin wound healing through deposition of matrix and further contraction into a more exuberant scar matrix architecture (Correa-Gallegos et al., 2019). This is mediated by migration and swarming to the surface involving N-cadherin-mediated cell-cell adhesion. Further experiments with an ex vivo explant technique termed scar-like tissue in a dish (SCAD) using oral mucosa (without fascia) showed that swarming is absent and N-cadherin is minimally expressed, agreeing with its typical scarless healing phenotype (Jiang et al., 2020a).
Several studies explored the differential response of oral and dermal fibroblasts to TGFβ1, a cytokine known to mediate fibroblast to myofibroblast differentiation and up-regulating the α-smooth muscle actin (αSMA) in these cells. Oral fibroblasts were shown not only to express higher basal levels of αSMA but also have higher number of αSMA-positive myofibroblasts in oral mucosal wounds (Lygoe et al., 2007; Mak et al., 2009). However, these were shown to resist more to TGFβ1-controlled myofibroblast differentiation, together with decreased levels of TGFβ1 in oral wounds, and supporting its non-scarring phenotype (Meran et al., 2007). This can be regulated by the increased expression of the hepatocyte growth factor (Dally et al., 2017).
-
(6)
ECM: compared to cutaneous wounds, the ECM composition of oral wounds diverges and is a key determinant for the scarless phenotype. Oral wounds showed increased expression of hyaluronic acid, tenascin and fibronectin and decreased expression of elastin (Glim et al., 2013, 2014. MMP mediate ECM remodelling and are regulated by MMP tissue inhibitors. The balance between these two molecules was shown to be important for the final healing outcome. In oral wounds the ratio between MMP and MMP tissue inhibitors is high, namely the levels of MMP 2 and 3 (Stephens et al., 2001; Glim et al., 2013). Also, the collagen III to collagen I ratio is increased in oral wounds (Glim et al., 2013; Figure 4). The pro-fibrotic matricellular protein periostin was recently shown to be involved in ECM synthesis regulation in gingival wound healing, while in skin it appears as a mediator of myofibroblast differentiation through β1 integrin-focal adhesion kinase (FAK) signalling (Nikoloudaki et al., 2020). Another study related the activation of autophagic pathways with an increase in myofibroblast differentiation and noted heterogeneity within the oral cavity, namely between buccal mucosa and gingiva. The gingival tissue showed no autophagic process upon wound repair therefore leading to less myofibroblast differentiation when comparing to buccal mucosal tissue (Vescarelli et al., 2017). It would be interesting to deepen our knowledge on the different wound healing responses associated with different tissues of the oral cavity. Overall, the surrounding environment is capable of eliciting various responses that contribute for the scarless potential of oral mucosa, nevertheless, also inside the cells molecular differences can be pointed between skin and oral mucosa.
-
(7)
Molecular cues: transcriptomic analysis have uncovered the molecular differences between skin and oral mucosal wound healing (Chen et al., 2010; Turabelidze et al., 2014; Iglesias-Bartolome et al., 2018). Healthy oral mucosa is primed with transcriptional networks readily prepared to respond to wounding, suggesting that the oral epithelia is equipped with a specially prepared intrinsic genetic response, particularly for cellular growth and proliferation and inflammatory response (Turabelidze et al., 2014; Iglesias-Bartolome et al., 2018). Importantly, the discovery of key players in transcriptional networks directly working for a scarless healing is of major importance. For instance, the Sox2 and Pitx1 transcription factors were shown to be the master regulators of the oral mucosal wound healing response (Iglesias-Bartolome et al., 2018). However, the intrinsic features playing to scarless healing are not restricted to the protein coding genes; microRNAs were differentially expressed between skin and oral wound healing, highlighting that genetic and epigenetic response of oral mucosa through growth factor production, SC levels and cellular proliferation capacity gives this epithelium its superior final repair (Simões et al., 2019).
To conclude, the ability of the oral mucosa to heal without scarring cannot be attributed to a single feature but to key extrinsic and intrinsic factors present in all stages of the wound healing process, which are crucial to the final improved outcome.
Exporting the Properties of Oral Epithelia – the Source for Future Therapies in Wound Repair?
Improving wound healing in skin is an unmet need. Chronic skin wounds have devastating consequences for patients and treating chronic wounds costs the UK National Health Service £5 billion per annum (Guest et al., 2015). Development of more efficient wound treatments is urgently needed to increase the quality of life of patients and to effectively reduce healthcare costs.
Reconstruction of skin or oral mucosal tissues using tissue-engineering methods resembles wound healing processes. It requires active SCs, epithelial proliferation, epithelial and fibroblast cell migration and ECM production, all processes coordinated to regenerate the new 3D tissue with similar properties and functions.
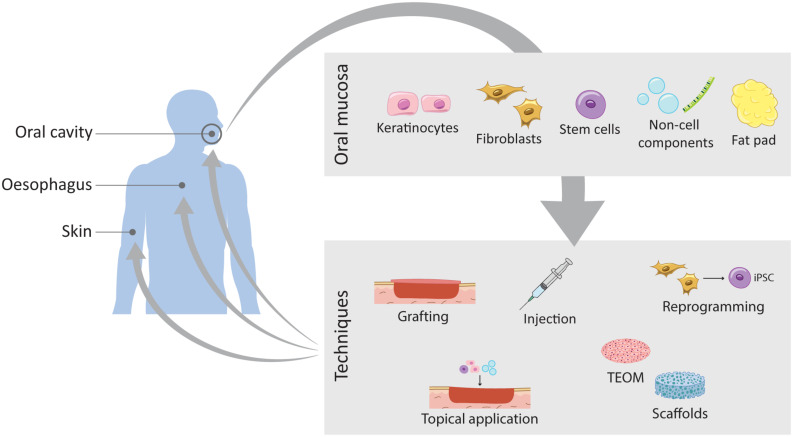
A large number of studies have been exploring SC therapies to improve skin regeneration. A major breakthrough recently published has used autologous transgenic skin epithelial cultures to regenerate an entire, fully functional epidermis from a patient with an epidermolysis bullosa disease caused by a mutation in laminin 332 usually expressed in skin’s basement membrane (Hirsch et al., 2017). Using retrovirus bearing healthy copies of the needed gene, LAMB3, epithelial cells from the patient were corrected, expanded in culture and grafted back to the patient. By combining cell and gene therapy, this clinical study demonstrated a life-saving regeneration of virtually the entire epidermis. This study inspires the use of other tissues for skin regeneration. Oral mucosal cells present advantages over skin cells in therapeutic applications due to their unique scarless properties and are an easy source to harvest reducing time for surgical procedures and accelerating patient’s recovery time (Izumi et al., 2015; Chapple, 2020). However, the direct use of mucosal grafts comes with various disadvantages associated with availability of sufficient amount of donor tissue as well as other graft-associated problems, such as donor site morbidity, recipient site, pain and risk of infection (Llames et al., 2014). To overcome these problems, the clinical use of tissue-engineered oral mucosa (TEOM) is the most adopted method (Figure 5).
FIGURE 5.
Schematic representation of the current work on wound healing improvement using oral mucosa. The oral mucosa represents a valuable source of different components that translates into different routes of exploration and expansion of its unique healing potential. Through different techniques these components can be applied to different tissues such as the oral mucosa itself, the skin and the oesophagus.
TEOMs are based on a scaffold matrix that provides structural support for the cells to seed, or as a scaffold used to deliver drugs or growth factors directly into the injured tissue, upon transplantation. The key factors are the optimal choice of the scaffold and the cells to seed. Collagen scaffolds are the golden standard, but advances in tissue engineering are proposing other synthetic scaffolds such as biodegradable hydrogels, as well as decellularised dermis (Figure 5). TEOM is a potential technique to reconstruct the oral cavity after tumour excision or after injury, and to repair congenital defects, such as cleft palate. Furthermore, it is a great model for in vitro testing of oral care products efficiency and safety, for evaluating cigarette smoke effects and to analyse cellular and molecular mechanisms of infection in the oral cavity (Chen et al., 2020; Wang et al., 2020; Zhong et al., 2020; Huang et al., 2021).
The TEOM explores the outstanding regenerative potential of the oral mucosal to reconstruct the oral cavity itself or in other tissues of the body. The following subchapters cover pre-clinical and clinical studies on the use of the oral mucosal tissue to improve the healing outcome of other intra- and extra-oral tissues (Tables 1, 2).
TABLE 1.
Clinical application of cellular therapy using human oral mucosa cells to regenerate oral tissues or other recipient tissues.
| Intra-oral | |||||
| Cell type | Method | Donor tissue | Recipient tissue | Outcome | Reference(s) |
Keratinocytes
|
TEOM
|
Hard palate | Tongue (intra-oral wound) | Improved tissue adhesion, speech and tongue mobility | Lauer and Schimming, 2001 |
| Keratinised oral mucosa on human dermis (AlloDerm®) | Tongue, alveolar gingiva, buccal mucosa, floor of mouth and Oropharyngeal mucosa | No postoperative pain, excellent adhesiveness and good epithelial coverage | Izumi et al., 2003 | ||
| Gingiva keratinocytes on human dermis (AlloDerm®) | Tongue, gingiva, buccal mucosa and alveolar ridge | Faster healing, negligible scar contracture | Hotta et al., 2007 | ||
| Hard palate keratinocytes on human dermis (AlloDerm®) | Gingiva | Good adhesiveness, increased gingival tissue | Izumi et al., 2013 | ||
Keratinocytes and fibroblasts
|
TEOM
|
Buccal mucosa | Tongue | Good mobility of tongue, satisfactory speech, residual fibrosis | Llames et al., 2014 |
| Palatal mucosa | Fibula flaps for maxillary and mandibular reconstruction | Granulation tissue formation in one patient, good restoring outcome | Gil et al., 2015 | ||
Fat pad
|
Grafting
|
Buccal fat | Posterior alveolus and hard palate | Full recovery | Egyedi, 1977 |
| Buccal fat | Mid-palatal and posterior palatal fistulas | Full recovery | Ashtiani et al., 2011 | ||
| Buccal fat | Palatal fistulas | Full recovery | Yaguchi et al., 2021 | ||
Fibroblasts
|
Injection
|
Gingiva | Gingiva | Test treatment improved papillary tissue augmentation | McGuire and Scheyer, 2007 |
Scaffold
|
Gingiva | Gingiva | Increased gingival width, keratinised epithelium supported by dense connective tissue | Mohammadi et al., 2011 | |
| Gingiva | Gingiva | Efficient gingival augmentation | Dominiak et al., 2012 | ||
| Extra-oral | |||||
| Epithelial flap | TEOM
|
Buccal mucosa | Trachea | Faster healing, buccal mucosa and fascia form an optimised tissue combination | Delaere et al., 2001 |
Keratinocytes
|
TEOM 
|
Buccal mucosa | Eye | Vision restored, no complications | Nishida et al., 2004 |
| Lip | Skin (scalp) | 30% success of engraftment due to local infection | Iida et al., 2005 | ||
| Buccal mucosa | Oesophagus | Effective re-epithelialisation, no dysphagia or stricture formation | Ohki et al., 2012 | ||
| Buccal mucosa | Oesophagus | Safe, reduced risk for post-ESD stricture formation | Jonas et al., 2016 | ||
| Buccal mucosa | Oesophagus | Short post-ESD ulcer healing period, successful cell sheet fabrication, transport and transplantation. | Yamaguchi et al., 2017 | ||
| Lingual tissue | Grafting
|
Ventrolateral tongue | Urethra | Good success rates of reconstruction of short strictures, combination with buccal mucosa for longer grafts | Simonato et al., 2008 |
| Buccal mucosal cells | TEOM
|
Buccal mucosa | Urethra | Safe and effective anterior urethroplasty | Barbagli et al., 2018 |
Comparison of the outcomes according to the tissue of origin, the therapeutic method used and the recipient tissue. TEOM, tissue-engineered oral mucosa; ESD, endoscopic submucosal dissection.
TABLE 2.
Pre-clinical studies with oral mucosa.
| In vivo | ||||||
| Species | Cell type or component | Method | Donor tissue | Recipient tissue | Outcome | Reference(s) |
| Mouse | SCs | Injection | Deciduous teeth | Skin | Accelerated wound healing | Nishino et al., 2011 |
| Keratinocytes | Topical application | Human gingiva | Skin | Rapid re-epithelialisation | Kim et al., 2013 | |
| Fibroblasts | Injection | Buccal mucosa | Skin | Reduced scarring, lineage-dependent behaviour | Rinkevich et al., 2015 | |
| SC/progenitor cells | Salisphere cell transplantation | Human submandibular salivary gland | Mouse submandibular salivary gland | Rescue of saliva production | Pringle et al., 2016 | |
| Keratinocytes and fibroblasts | TEOM | Oral mucosa (non-specified) | Skin | Faster wound healing, reduced scarring | Roh et al., 2017 | |
| miRNA-31 mimic | Injection | Hard palate | Skin | Significant acceleration of wound closure | Chen et al., 2019 | |
| Keratinocytes and fibroblasts | TEOM | Human oral mucosa | Skin | Accelerated wound healing, reduced scarring | Lee et al., 2019 | |
| Exosomes | Injection | Human saliva | Skin | Efficient wound healing through promotion of angiogenesis | Mi et al., 2020 | |
| SCs | Injection | Oral mucosa (non-specified) | Skin | Accelerated wound healing | Kuperman et al., 2020 | |
| Rat | Keratinocytes | TEOM | Oral mucosa (non-specified) | Uterus | Highly effective against intrauterine adhesions | Kuramoto et al., 2015 |
| Keratinocytes and fibroblasts | pre-vascularized TEOM | Oral mucosa (non-specified) | Buccal mucosa | Accelerated and more efficient healing | Lee et al., 2017 | |
| Exosomes | Hydrogel topical application | Human gingival mesenchymal SCs | Skin | Promotion of re-epithelialisation, deposition and remodelling of ECM | Shi et al., 2017 | |
| Keratinocytes and fibroblasts | TEOM | Buccal mucosa | Skin | Accelerated wound healing, reduced scarring | Lee et al., 2018 | |
| Dental pulp SCs | Injection via tail vein | Upper and lower incisors | Oesophagus | Improved healing | Zhang et al., 2018 | |
| EGF, HA, bFGF and lysozyme | Biomimetic hydrogel | Commercial | Skin | Accelerated wound healing, reduced scarring | Kong et al., 2019 | |
| Mucosal tissue | Grafting | Tongue | Skin | Lower levels of EGF and VEGF-C | Qi et al., 2019 | |
| Exosomes | Topical application | Human buccal epithelial cell sheets | Skin | Significant acceleration of wound closure | Sjöqvist et al., 2019 | |
| Dog | Keratinocytes | TEOM | Buccal mucosa | Oesophagus | Complete faster wound healing, no stenosis | Ohki et al., 2006 |
| Keratinocytes and fibroblasts | TEOM | Oral mucosa (non-specified) | Oesophagus | Good distensibility and epithelial thickness, successful oesophageal replacement | Nakase et al., 2008 | |
| Keratinocytes | TEOM | Buccal mucosa | Oesophagus | Successful attachment and re-epithelisation | Takagi et al., 2010 | |
| Rabbit | Dental pulp SCs | TEOM | Human deciduous teeth | Eye | Corneal reconstruction | Gomes et al., 2010 |
| Keratinocytes | TEOM | Buccal mucosa | Urethra | Urethroplasty reconstruction | Yudintceva et al., 2020 | |
| Goat | Epithelial graft | Grafting | Oral mucosa (non-specified) | Trachea | Coverage of the constructed trachea lumen | Li et al., 2019 |
| Pig | Keratinocytes | Injection | Buccal mucosa | Oesophagus | Improved re-epithelisation, reduced risk of stenosis and contraction | Sakurai et al., 2007 |
| In vitro | ||||||
| Species | Cell type | Method | Donor tissue | Outcome | Reference(s) | |
| Human | Keratinocytes | TEOM | Gingiva | Fabrication of oral mucosal equivalent similar to the native tissue | Yoshizawa et al., 2004 | |
| Fibroblasts | Reprogramming | Buccal mucosa | Efficient reprogramming into induced pluripotent SCs | Miyoshi et al., 2010 | ||
| Keratinocytes | TEOM | Cryopreserved lip mucosa | Successful fabrication of oral mucosa equivalents | Xiong et al., 2010 | ||
| Fibroblasts | Low-level laser therapy | Cell line | Increased cell number and migration | Basso et al., 2012 | ||
| Keratinocytes | TEOM | Lip | Fabrication of 3D human lip skin equivalent | Peramo et al., 2012 | ||
| Keratinocytes | TEOM | Keratinised oral mucosa | Development of large TEOM | Kato et al., 2015 | ||
| Fibroblasts and immortalised OKF6/TERET-2 oral keratinocytes | TEOM | Gingiva | Development of 3D bone-oral mucosa model | Almela et al., 2016 | ||
| Fibroblasts | Feeder cells | Gingiva | Improved cell proliferation, promising candidate feeder cells | Yu et al., 2016 | ||
| Fibroblasts | In vitro differentiation, feeder cells | Oral mucosa (non-specified) | Fabrication of corneal epithelial sheets, multipotent differentiation into mesenchymal or neural crest-derived cells, good source of feeder cells | Higa et al., 2017 | ||
| Keratinocytes and fibroblasts | Scaffolds | Buccal mucosa | Tri-layer micro-nano-3D porous synthetic scaffold mimics normal human oral mucosa, minimal contraction, good mechanical properties | Simsek et al., 2018 | ||
| Keratinocytes and fibroblasts | TEOM | Gingiva | Development of 3D epithelium and lamina propria | Nishiyama et al., 2019 | ||
| Pig | Keratinocytes | TEOM | Buccal mucosa | Culture on acellular scaffolds | Poghosyan et al., 2013 | |
| Dog | Keratinocytes | TEOM | Buccal mucosa | Successful construction of TEOM with adipose derived SCs and small intestine submucosa | Zhang et al., 2021 | |
Comparison of the outcomes according to the animal species, the cell type or non-cell component, the method used and the recipient tissue. TEOM, tissue-engineered oral mucosa; EGF, epidermal growth factor; HA, hyaluronic acid; bFGF, basic fibroblast growth factor; VEGF-C, vascular endothelial growth factor C.
Exploring the Use of Oral Mucosa for Oral Tissue Repair
The human clinical application of oral mucosal scarless potential and exceptional properties for repair is still scarce, however the number of case reports and pilot studies has been growing (Figure 5 and Table 1). TEOM produced ex vivo from autologous keratinocytes from the hard palate or gingiva were successfully used for reconstruction of intra-oral lining tissues and periodontal plastic surgeries (Lauer and Schimming, 2001; Izumi et al., 2003; Hotta et al., 2007), while full-thickness TEOM combined with fibula flap allowed for the lining reconstruction of maxilla and mandible (Gil et al., 2015). Other cases of congenital anomalies such as hemifacial microsomia, ankyloglossia (tongue-tie) and cleft palate were treated with TEOM yielding satisfactory outcomes (Llames et al., 2014; Hixon et al., 2019). The use of TEOM to repair mucogingival defects demonstrated its capacity to integrate and vascularise (Izumi et al., 2013), however this technique still needs to be improved to avoid postoperative wound shrinkage.
The buccal fat pad flap is reported to be a reliable and effective flap with clinical application in reconstruction of oral defects due to its high vascularity, reducing tissue hypoxia and improving graft survival. This has been used to treat oroantral fistula, congenital defects such as the cleft palate, osteonecrosis of the jawbone and defects induced by removal of tumours or cysts (Egyedi, 1977; Ashtiani et al., 2011; Kim et al., 2017; Yaguchi et al., 2021).
The clinical use of oral-derived SCs is still limited. Oral SCs have been derived from dental pulp, periodontal ligament, exfoliated deciduous teeth, apical papilla, dental follicle, gingiva, oral mucosa, salivary glands and alveolar bone (Kanwal et al., 2017; Bryja et al., 2019; Sanz et al., 2019). The work with oral SCs for hard and soft tissue regeneration within the oral cavity has focused on the use of oral SCs for reconstructing periodontal, bone, dentin and pulp tissues (Seo et al., 2004; Feng et al., 2010; Giuliani et al., 2013; Shiehzadeh et al., 2014; Surendran and Sivamurthy, 2015; Chen et al., 2016; Kanwal et al., 2017). Human gingival and mouse palatal epithelial cells were used to develop teeth in combination with mouse embryonic tooth mesenchyme following transplantation into renal capsules (Nakagawa et al., 2009; Volponi et al., 2013). The combination of human oral epithelial cells and dental pulp SCs using a matrigel as scaffold allowed the 3D construction of an epithelial invagination model, an important feature of early tooth development (Xiao and Tsutsui, 2012). Furthermore, human salivary gland-derived SCs were used to restore saliva production after radiation of salivary glands, opening doors for the treatment of hyposalivation resulting from head and neck cancer radiotherapy (Pringle et al., 2016).
Several clinical studies explored the potential of using oral mucosal fibroblasts for gingival tissue augmentation. Autologous gingival fibroblasts seeded in different scaffolds improved keratinised tissue formation (Mohammadi et al., 2011; Dominiak et al., 2012). Additionally, the injection of autologous fibroblasts harvested from keratinised tissue from the maxillary tuberosity in interdental papillary recession defects improved the papillary tissue augmentation (McGuire and Scheyer, 2007; Table 1).
In addition to clinical studies, in vitro and in vivo research is progressing with more viable alternatives (Table 2). A large size ex vivo fabricated oral mucosal equivalent was successfully achieved using higher cell seeding density of oral keratinocytes and a thinner AlloDerm scaffold, reaching a final 15cm2 size, which can be applied in the reconstruction of significant soft tissue defects (Kato et al., 2015). Cryopreservation of abundant lip mucosa tissues harvested upon cleft lip repair proved to be a useful approach to biobank oral keratinocytes for TEOMs. The 4- to 6-month cryopreservation did not affect the characteristics of the TEOM when compared with the equivalents engineered from fresh lip and palate (Xiong et al., 2010). Furthermore, pre-vascularised oral mucosal cell sheets grafted into deep wounds in the buccal region of rats healed more rapidly and without fibrosis (Lee et al., 2017).
Using cell surface coating through layer-by-layer assembled ECM films succeeded in creating the 3D oral mucosal equivalents composed of epithelium, lamina propria and blood capillaries, recreating the tissue cellular heterogeneity (Nishiyama et al., 2019). Furthermore, the in vitro incorporation of oral mucosa and bone components in a composite scaffold model that mimics the natural structure of alveolar bone with an overlying oral mucosa was achieved and is a possible future application in cleft palate repair (Almela et al., 2016; Hixon et al., 2019).
Oral mucosal fibroblasts were recently reprogrammed into induced pluripotent SCs (Miyoshi et al., 2010) and were shown to be efficient feeders for induced pluripotent SCs expansion (Yu et al., 2016; Figure 5). This represents a promising tool for ex vivo cell expansion.
Recent technologies have been exploring the use of cell-free therapies for oral maxillofacial regeneration, particularly the use of extracellular vesicles (Figure 5; reviewed in Lv et al., 2019; Shi et al., 2020). This technology overcomes the shortcoming of cells and instead explores the cell paracrine effects that can activate endogenous repair pathways (Jiang et al., 2017; Zheng et al., 2018; Ren, 2019). As in TEOMs, it remains important to consider the origin and the differentiation status of the secreting cells to guarantee not only improved outcome but also safe clinical applications.
Exploring Oral Mucosal Properties to Improve Skin and Oesophageal Repair
It is well known that adult skin wounds are frequently accompanied by scar formation that can become fibrotic, while oral mucosal wounds heal in an accelerated fashion, displaying minimal scar formation (see section “Wound Repair Mechanisms in Skin, Oesophagus, and Oral Epithelia”). While surgical reconstruction of the oral cavity with skin grafts has been easily and routinely accomplished for a long time, particularly after tumour resection, the clinical application of oral mucosa grafts into skin has been less explored (Schramm and Myers, 1980; Schramm et al., 1983; de Bree et al., 2008). However, exploring the scarless potential of the oral mucosa offers an exciting strategy to accelerate skin wound healing and to improve the quality of life for patients with chronic wounds. There have been promising studies around this topic, but few clinical studies. Iida and colleagues used TEOMs based on an acellular allogeneic dermal matrix grafted into scalp skin with extensive deep skin burn showing 30% of the graft efficacy (Iida et al., 2005; Table 1).
In vitro studies have demonstrated the potential of oral mucosal fibroblasts, to improve wound healing after biostimulation with low-level laser therapy (Basso et al., 2012). A recent study by Kong and colleagues engineered a biomimetic gel inspired by the characteristics of oral mucosal wound healing. The hydrogel was loaded with epidermal growth factor, basic fibroblast growth factor, lysozyme and hyaluronic acid, as these were observed to be highly expressed in the oral mucosal wound healing when comparing to skin (Kong et al., 2019). The authors were able to simulate the oral mucosal trauma microenvironment through controlled release of these molecules resorting to microspheres and chitosan thermo-sensitive gels. The use of this biomimetic hydrogel in skin wounds of rats resulted in rapid wound healing and reduced scar formation (Kong et al., 2019).
As for pre-clinical studies, topical grafting of human oral keratinocytes onto skin wounds in nude mice improved regeneration of skin wounds, with increased production of keratinocyte growth factor and cytokines IL-6, and IL-1α (Kim et al., 2013).
SCs from human exfoliated deciduous teeth and oral mucosa were shown to improve skin wound healing in mice when injected around the wound or topically applied onto the wound bed highlighting the plasticity of different SC types that could be used to regenerate skin (Nishino et al., 2011; Kuperman et al., 2020). Other studies have explored the potential of oral mucosal engineered cell sheets to apply on skin excisional and burn wounds. All reported results indicated the plasticity of the cell sheets in adapting to the skin wounds, the contribution to accelerated healing and limited scar formation (Roh et al., 2017; Lee et al., 2018, 2019).
The implementation of recent knowledge in the prevention of oesophageal stricture after endoscopic submucosal dissection has attracted increasing attention. Autologous oral mucosal keratinocytes were endoscopically implanted after oesophageal resection in a porcine model, resulting in accelerated re-epithelialisation and wound healing (Sakurai et al., 2007). On a “bench to bedside” approach, tissue-engineered autologous oral epithelial cell sheets and full-thickness substitutes were endoscopically transplanted into an oesophageal ulcer immediately after a large endoscopic submucosal dissection, showing excellent results with no cases of dysphagia or stricture formation (Ohki et al., 2006, 2012, 2015; Arakelian et al., 2018; Ohki and Yamamoto, 2020). The first clinical trial using cell sheet technology for oesophageal reconstruction in Europe used cell sheets from autologous oral epithelial cells, transplanted right after endoscopic submucosal dissection of Barrett’s neoplasms, resulting in decreased risk and extent of strictures (Jonas et al., 2016). On a canine model, the replacement of the full-circumference and full-thickness intrathoracic oesophagus was achieved using autologous oral keratinocytes and fibroblasts seeded on amniotic membrane, sheeted on polyglycolic acid filled with smooth muscle tissue (Nakase et al., 2008), a technique later also achieved without animal-derived materials that could compromise future human clinical trials (Takagi et al., 2010). On a porcine model, an in vitro engineered oesophageal substitute was obtained with an acellular small intestinal submucosa scaffold seeded with autologous skeletal myoblasts, covered with a human amniotic membrane and seeded with autologous oral epithelial cells (Poghosyan et al., 2013).
Other non-cellular-based approaches have also emerged which explore the benefits of oral microRNA and exosomes for skin and oesophageal wound repair (Shi et al., 2017; Chen et al., 2019; Sjöqvist et al., 2019; Mi et al., 2020).
Finally, an interesting and challenging study tested the safety and feasibility of transplanting engineered autologous oral mucosal cell sheets in patients who had undergone extensive endoscopic submucosal dissection for oesophageal squamous cell carcinoma removal. The challenge of this study was the use on non-autologous cell sheets produced 1200km away from the patients that were transported by air for 7h before transplantation (Yamaguchi et al., 2017). Such studies are of major relevance when thinking about future tissue-engineering therapies reaching remote hospitals with no tissue engineering facilities, or hospitals in conflict zones.
Exploring the Use of Oral Mucosa for the Repair of Other Tissues
The use of oral mucosa to improve wound healing in other tissues has taken its first steps with successful applications in the clinical field, such as in urethral reconstruction and stricture repair through grafting, with recent research focused on improving the TEOM for better outcomes (Simonato et al., 2008; Barbagli and Lazzeri, 2015; Horiguchi, 2017; Barbagli et al., 2018; Simsek et al., 2018; Chapple, 2020; Yudintceva et al., 2020; Zhang et al., 2021). The advantages of using buccal mucosa instead of other donor sites, such as skin, are the fact that the buccal mucosa is a non-keratinised squamous epithelium that lacks hair (Figure 1) and has low associated morbidity and short harvest time.
Tissue-engineered cell sheets of autologous oral epithelial cells alone, or combined with other tissues have been used for ocular diseases treatment, including eyelid and corneal reconstructions (Nishida et al., 2004; Yoshizawa et al., 2004; Oliva et al., 2020; Sasaki et al., 2020). Using an in vivo rabbit model of total limbal SC deficiency, dental pulp SCs were used in a tissue-engineered cell sheet for ocular surface reconstruction (Gomes et al., 2010) highlighting the plasticity of these cells. Furthermore, oral mucosal fibroblasts containing neural crest-origin cells showed plasticity in differentiating into mesenchymal and neural cell lineages, being proposed as an autologous cell source for establishing human corneal epithelial cell sheets suitable for corneal regeneration (Higa et al., 2017). Other applications of the oral mucosa comprise the treatment of tracheal defects (Delaere et al., 2001; Li et al., 2019) and prevention of intrauterine adhesions caused by endometrial damage (Kuramoto et al., 2015).
While in full-thickness skin transplant into the oral cavity (to reconstruct tongue or buccal mucosa), the recipient organ keeps characteristics of the donor tissue (Vural and Suen, 2000; Sebastian et al., 2008; Amin et al., 2011; Aslam-Pervez et al., 2018), there is mounting evidence that grafted epithelial cells can adopt the phenotype of the recipient tissue, stressing the plasticity of these cells and the importance of the underlying connective tissue and fibroblasts to determine epithelial cells phenotype. Recent animal work has successfully grafted oesophageal tissue into skin demonstrating that when exposed to the adult skin dermis, oesophageal epithelial cells transition to a skin identity following a cell fate conversion process (Bejar et al., 2021). This highlights how the stromal cells influence the final epithelial phenotype in a homeostatic tissue. However, this is a topic of much controversy and variability of results over the years, especially when considering clinical and in vivo applications (Billingham and Silvers, 1967; Mackenzie and Hill, 1984; Luca et al., 1990; Katou et al., 2003). Whether the epithelium is transplanted alone or with subepithelial tissue as full thickness flaps, or applied as single-cell suspensions of fully differentiated cells or stem cells alone might contribute for the observed heterogenous responses. Additionally, the majority of these events lack a more in depth study of the molecular mechanisms driving the final outcomes. Nonetheless, we can’t discard the intrinsic identity and programmes that epithelial and fibroblastic cells carry themselves, which differ between oral mucosa and skin (Turabelidze et al., 2014; Rinkevich et al., 2015; Iglesias-Bartolome et al., 2018). This heterogeneity could as well explain the predisposition of a tissue to resemble the origin characteristics or to be more influenced by the recipient. Regardless, the studies presented in this final chapter highlight a very promising venue for using these intrinsic cellular properties into other tissue wounds, mainly through improved in vitro tissue engineering.
Conclusion
Techniques to improve skin wound healing are currently under development and are an unmet need, particularly following large burns and war injuries, where treatment is still primarily performed by split-thickness skin grafting and accompanied by problems associated with limited donor tissue, pain and scarring (Singh et al., 2015; Connolly et al., 2016).
Mounting evidence over the last two decades has demonstrated a remarkable plasticity in adult epithelial cell fate while in in different niches, leaving behind the concept of strict SC fates (Bonfanti et al., 2010; Blanpain and Fuchs, 2014; Chacón-Martínez et al., 2018; Yuan et al., 2019). While epithelial cells and fibroblasts reciprocal grafting have dissected their contribution to wound healing, the plasticity of these cells in the new microenvironment and their cellular behaviour need further investigation. There is an exciting future for collaborative efforts to understand the heterogeneity and plasticity between these tissues and yet their common features and the mechanisms behind the cell fate conversion, in order to improve the use of heterotypic transplants for future therapeutic strategies.
In this review, we elucidate the intrinsic properties that prime the oral mucosa with scarless wound healing response. Lessons should be learned from the oral mucosa to apply on other tissues, exploring these unique properties in future innovative therapies combining cell therapy with bioengineering to prevent pathologies associated with impaired wound healing and scar formation in both skin and oesophagus.
Author Contributions
DP and IS: conceptualisation, writing, editing, and figures. Both authors contributed to the article and approved the submitted version.
Conflict of Interest
IS is a consultant for L’Oréal Research and Innovation. The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to acknowledge Beate Lichtenberger and Oliver Culley for comments on the manuscript.
Footnotes
Funding. IS was funded by a Barts Charity Lectureship (MGU045) and Royal Society (RGS\R2\202291). DP was funded by INOV Contacto (AICEP, Portugal) and Fundação para a Ciência e a Tecnologia (2020.08715.BD).
References
- Abbasi S., Sinha S., Labit E., Rosin N. L., Yoon G., Rahmani W., et al. (2020). Distinct regulatory programs control the latent regenerative potential of dermal fibroblasts during wound healing. Cell Stem Cell 27 396–412.e6. 10.1016/j.stem.2020.07.008 [DOI] [PubMed] [Google Scholar]
- Alcolea M. P. (2017). “Oesophageal stem cells and cancer,” in Stem Cell Microenvironments and Beyond, ed. Birbrair A. (Cham: Springer; ), 187–206. 10.1007/978-3-319-69194-7_10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcolea M. P., Greulich P., Wabik A., Frede J., Simons B. D., Jones P. H. (2014). Differentiation imbalance in single oesophageal progenitor cells causes clonal immortalization and field change. Nat. Cell Biol. 16 612–619. 10.1038/ncb2963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almela T., Brook I. M., Moharamzadeh K. (2016). Development of three-dimensional tissue engineered bone-oral mucosal composite models. J. Mater. Sci. Mater. Med. 27:4. 10.1007/s10856-016-5676-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso L., Fuchs E. (2003). Stem cells of the skin epithelium. Proc. Natl. Acad. Sci. U.S.A. 100(Suppl. 1) 11830–11835. 10.1073/pnas.1734203100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin A. A., Sakkary M. A., Khalil A. A., Rifaat M. A., Zayed S. B. (2011). The submental flap for oral cavity reconstruction: extended indications and technical refinements. Head Neck Oncol. 3 1–7. 10.1186/1758-3284-3-51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragona M., Sifrim A., Malfait M., Song Y., Van Herck J., Dekoninck S., et al. (2020). Mechanisms of stretch-mediated skin expansion at single-cell resolution. Nature 584 268–273. 10.1038/s41586-020-2555-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arakelian L., Kanai N., Dua K., Durand M., Cattan P., Ohki T. (2018). Esophageal tissue engineering: from bench to bedside. Ann. N. Y. Acad. Sci. 1434 156–163. 10.1111/nyas.13951 [DOI] [PubMed] [Google Scholar]
- Ashtiani A. K., Bohluli B., Motamedi M. H. K., Fatemi M. J., Moharamnejad N. (2011). Effectiveness of buccal fat in closing residual midpalatal and posterior palatal fistulas in patients previously treated for clefts. Int. J. Oral Maxillofac. Surg. 69 e416–e419. 10.1016/j.joms.2011.02.010 [DOI] [PubMed] [Google Scholar]
- Aslam-Pervez N., Caldroney S. J., Isaiah A., Lubek J. E. (2018). A retrospective volume matched analysis of the submental artery island pedicled flap as compared to the forearm free flap: is it a good alternative choice for the reconstruction of defects of the oral cavity and oropharynx? J. Oral Maxillofac. Surg. 76 656–663. 10.1016/j.joms.2017.08.003 [DOI] [PubMed] [Google Scholar]
- Azzarone B., Macieira-Coelho A. (1982). Heterogeneity of the kinetics of proliferation within the human skin fibroblastic cell populations. J. Cell Sci. 57 177–187. [DOI] [PubMed] [Google Scholar]
- Baatar D., Jones M. K., Tsugawa K., Pai R., Moon W. S., Koh G. Y., et al. (2002a). Esophageal ulceration triggers expression of hypoxia-inducible factor-1α and activates vascular endothelial growth factor gene: implications for angiogenesis and ulcer healing. Am. J. Pathol. 161 1449–1457. 10.1016/S0002-9440(10)64420-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baatar D., Kawanaka H., Szabo I. L., Pai R., Jones M. K., Kitano S., et al. (2002b). Esophageal ulceration activates keratinocyte growth factor and its receptor in rats: implications for ulcer healing. Gastroenterology 122 458–468. 10.1053/gast.2002.31004 [DOI] [PubMed] [Google Scholar]
- Barbagli G., Akbarov I., Heidenreich A., Zugor V., Olianas R., Aragona M., et al. (2018). Anterior urethroplasty using a new tissue engineered oral mucosa graft: surgical techniques and outcomes. J. Urol. 200 448–456. 10.1016/j.juro.2018.02.3102 [DOI] [PubMed] [Google Scholar]
- Barbagli G., Lazzeri M. (2015). Clinical experience with urethral reconstruction using tissue-engineered oral mucosa: a quiet revolution. Eur. Urol. 68 917–918. 10.1016/j.eururo.2015.05.043 [DOI] [PubMed] [Google Scholar]
- Barbera M., Pietro M. D., Walker E., Brierley C., MacRae S., Simons B. D., et al. (2015). The human squamous oesophagus has widespread capacity for clonal expansion from cells at diverse stages of differentiation. Gut 64 11–19. 10.1136/gutjnl-2013-306171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardhan A., Bruckner-Tuderman L., Chapple I. L. C., Fine J. D., Harper N., Has C., et al. (2020). Epidermolysis bullosa. Nat. Rev. Dis. Primers 6:1. 10.1038/s41572-020-0210-0 [DOI] [PubMed] [Google Scholar]
- Basile D., Di Nardo P., Corvaja C., Garattini S. K., Pelizzari G., Lisanti C., et al. (2019). Mucosal injury during anti-cancer treatment: from pathobiology to bedside. Cancers 11 1–22. 10.3390/cancers11060857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basso F. G., Pansani T. N., Turrioni A. P. S., Bagnato V. S., Hebling J., Costa C. A. S. (2012). In vitro wound healing improvement by low-level laser therapy application in cultured gingival fibroblasts. Int. J. Dent. 2012:719452. 10.1155/2012/719452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayat A., McGrouther D. A., Ferguson M. W. J. (2003). Skin scarring: clinical review. BMJ 326 88–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bejar M. T., Jimenez-gomez P., Moutsopoulos I., Colom B., Han S. (2021). Defining the transcriptional signature of esophageal-to-skin lineage conversion. bioRxiv [Preprint] 10.1101/2021.02.19.431899 [DOI] [Google Scholar]
- Biggs L. C., Kim C. S., Miroshnikova Y. A., Wickström S. A. (2020). Mechanical forces in the skin: roles in tissue architecture, stability, and function. J. Invest. Dermatol. 140 284–290. 10.1016/j.jid.2019.06.137 [DOI] [PubMed] [Google Scholar]
- Billingham R. E., Silvers W. K. (1967). Studies on the conservation of epidermal specificities of skin and certain mucosas in adult mammals. J. Exp. Med. 125 429–446. 10.1084/jem.125.3.429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanpain C., Fuchs E. (2014). Plasticity of epithelial stem cells in tissue regeneration. Science 344:1242281. 10.1126/science.1242281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanpain C., Lowry W. E., Geoghegan A., Polak L., Fuchs E. (2004). Self-renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche. Cell 118 635–648. 10.1016/j.cell.2004.08.012 [DOI] [PubMed] [Google Scholar]
- Bodner L., Dayan D., Pinto Y., Hammel I. (1993). Characteristics of palatal wound healing in desalivated rats. Arch. Oral Biol. 38 17–21. 10.1016/0003-9969(93)90149-G [DOI] [PubMed] [Google Scholar]
- Boink M. A., van den Broek L. J., Roffel S., Nazmi K., Bolscher J. G. M., Gefen A., et al. (2016). Different wound healing properties of dermis, adipose, and gingiva mesenchymal stromal cells. Wound Repair Regen. 24 100–109. 10.1111/wrr.12380 [DOI] [PubMed] [Google Scholar]
- Bonfanti P., Claudinot S., Amici A. W., Farley A., Blackburn C. C., Barrandon Y. (2010). Microenvironmental reprogramming of thymic epithelial cells to skin multipotent stem cells. Nature 466 978–982. 10.1038/nature09269 [DOI] [PubMed] [Google Scholar]
- Botticelli S., Küseler A., Mølsted K., Ovsenik M., Nørholt S. E., Dalstra M., et al. (2019). Palatal morphology in unilateral cleft lip and palate patients: association with infant cleft dimensions and timing of hard palate repair. Orthod. Craniofac. Res. 22 270–280. 10.1111/ocr.12318 [DOI] [PubMed] [Google Scholar]
- Boudaka A., Saito C. T., Tominaga M. (2020). Deletion of TRPV4 enhances in vitro wound healing of murine esophageal keratinocytes. Sci. Rep. 10:11349. 10.1038/s41598-020-68269-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown B. C., McKenna S. P., Siddhi K., McGrouther D. A., Bayat A. (2008). The hidden cost of skin scars: quality of life after skin scarring. J. Plast. Reconstr. Aesthetic Surg. 61 1049–1058. 10.1016/j.bjps.2008.03.020 [DOI] [PubMed] [Google Scholar]
- Bryja A., Stefañska K., Budna-Tukan J., Kempisty B., Dyszkiewicz-Konwiñska M. (2019). Oral derived stem cells potential – current status. CPQ Dent. 1:4. [Google Scholar]
- Byrd K. M., Piehl N. C., Patel J. H., Huh W. J., Sequeira I., Lough K. J., et al. (2019). Heterogeneity within stratified epithelial stem cell populations maintains the oral mucosa in response to physiological stress. Cell Stem Cell 25 814–829.e6. 10.1016/j.stem.2019.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caetano A. J., Yianni V., Volponi A., Booth V., D’Agostino E. M., Sharpe P. (2021). Defining human mesenchymal and epithelial heterogeneity in response to oral inflammatory disease. ELife 10:e62810. 10.7554/eLife.62810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai H., Li Y., Wang R., Cui Y. (2018). The effects of NF-K B signal pathway on the process of anastomotic stricture after the radical resection of esophageal carcinoma. Eur. J. Inflamm. 16 1–8. 10.1177/2058739218777593 [DOI] [Google Scholar]
- Campos J., Tanny S. P. T., Kuyruk S., Sekaran P., Hawley A., Brooks J. A., et al. (2020). The burden of esophageal dilatations following repair of esophageal atresia. J. Pediatr. Surg. 55 2329–2334. 10.1016/j.jpedsurg.2020.02.018 [DOI] [PubMed] [Google Scholar]
- Candi E., Schmidt R., Melino G. (2005). The cornified envelope: a model of cell death in the skin. Nat. Rev. Mol. Cell Biol. 6 328–340. 10.1038/nrm1619 [DOI] [PubMed] [Google Scholar]
- Chacón-Martínez C. A., Koester J., Wickström S. A. (2018). Signaling in the stem cell niche: regulating cell fate, function and plasticity. Development 145:15. 10.1242/dev.165399 [DOI] [PubMed] [Google Scholar]
- Chai J., Norng M., Tarnawski A. S., Chow J. (2007). A critical role of serum response factor in myofibroblast differentiation during experimental oesophageal ulcer healing in rats. Gut 56 621–630. 10.1136/gut.2006.106674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C. S., Wong A., Rohde C. H., Ascherman J. A., Wu J. K. (2012). Management of lip hemangiomas: minimizing peri-oral scars. J. Plast. Reconstr. Aesthet. Surg. 65 163–168. 10.1016/j.bjps.2011.08.033 [DOI] [PubMed] [Google Scholar]
- Chapple C. (2020). Tissue engineering of the urethra: where are we in 2019? World J. Urol. 38 2101–2105. 10.1007/s00345-019-02826-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaussain C., Septier D., Bonnefoix M., Lecolle S., Lebreton-Decoster C., Coulomb B., et al. (2002). Human dermal and gingival fibroblasts in a three-dimensional culture: a comparative study on matrix remodeling. Clin. Oral Investig. 6 39–50. 10.1007/s00784-001-0143-2 [DOI] [PubMed] [Google Scholar]
- Chen F. M., Gao L. N., Tian B. M., Zhang X. Y., Zhang Y. J., Dong G. Y., et al. (2016). Treatment of periodontal intrabony defects using autologous periodontal ligament stem cells: a randomized clinical trial. Stem Cell Res. Ther. 7 1–11. 10.1186/s13287-016-0288-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Arbieva Z. H., Guo S., Marucha P. T., Mustoe T. A., DiPietro L. A. (2010). Positional differences in the wound transcriptome of skin and oral mucosa. BMC Genomics 11:47. 10.1186/1471-2164-11-47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Simões A., Chen Z., Zhao Y., Wu X., Dai Y., et al. (2019). Overexpression of the oral mucosa-specific microRNA-31 promotes skin wound closure. Int. J. Mol. Sci. 20:3679. 10.3390/ijms20153679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Zhao J., Peng J., Li X., Deng X., Geng Z., et al. (2020). Detection of 2019-NCoV in Saliva and Characterization of Oral Symptoms in COVID-19 Patients. Available online at: https://ssrn.com/abstract=3556665 (accessed February 27, 2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choy B., Bandla S., Xia Y., Tan D., Pennathur A., Luketich J. D., et al. (2012). Clinicopathologic characteristics of high expression of Bmi-1 in esophageal adenocarcinoma and squamous cell carcinoma. BMC Gastroenterol. 12:146. 10.1186/1471-230X-12-146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton E., Doupé D. P., Klein A. M., Winton D. J., Simons B. D., Jones P. H. (2007). A single type of progenitor cell maintains normal epidermis. Nature 446 185–189. 10.1038/nature05574 [DOI] [PubMed] [Google Scholar]
- Collin C., Ouhayoun J. P., Grund C., Franke W. W. (1992). Suprabasal marker proteins distinguishing keratinizing squamous epithelia: cytokeratin 2 polypeptides of oral masticatory epithelium and epidermis are different. Differentiation 51 137–148. 10.1111/j.1432-0436.1992.tb00690.x [DOI] [PubMed] [Google Scholar]
- Colom B., Alcolea M. P., Piedrafita G., Hall M. W. J., Wabik A., Dentro S. C., et al. (2020). Spatial competition shapes the dynamic mutational landscape of normal esophageal epithelium. Nat. Genet. 52 604–614. 10.1038/s41588-020-0624-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colwell A. S., Longaker M. T., Lorenz H. P. (2005). “Mammalian fetal organ regeneration,” in Regenerative Medicine I, Vol. 93 ed. Yannas I. V. (Berlin: Springer; ), 83–100. 10.1007/b99972 [DOI] [PubMed] [Google Scholar]
- Connolly M., Ibrahim Z. R., Johnson O. N. (2016). changing paradigms in lower extremity reconstruction in war-related injuries. Mil. Med. Res. 3 1–6. 10.1186/S40779-016-0080-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corominas-Murtra B., Scheele C. L. G. J., Kishi K., Ellenbroek S. I. J., Simons B. D., Van Rheenen J., et al. (2020). Stem cell lineage survival as a noisy competition for niche access. Proc. Natl. Acad. Sci. U.S.A. 117 16969–16975. 10.1073/pnas.1921205117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa-Gallegos D., Jiang D., Christ S., Ramesh P., Ye H., Wannemacher J., et al. (2019). Patch repair of deep wounds by mobilized fascia. Nature 576 287–292. 10.1038/s41586-019-1794-y [DOI] [PubMed] [Google Scholar]
- Costea D. E., Loro L. L., Dimba E. A. O., Vintermyr O. K., Johannessen A. C. (2003). Crucial effects of fibroblasts and keratinocyte growth factor on morphogenesis of reconstituted human oral epithelium. J. Invest. Dermatol. 121 1479–1486. 10.1111/j.1523-1747.2003.12616.x [DOI] [PubMed] [Google Scholar]
- Cotsarelis G., Sun T. T., Lavker R. M. (1990). Label-retaining cells reside in the bulge area of pilosebaceous unit: implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell 61 1329–1337. 10.1016/0092-8674(90)90696-C [DOI] [PubMed] [Google Scholar]
- Coulombe P. A., Hutton M. E., Vassar R., Fuchs E. (1991). A function for keratins and a common thread among different types of epidermolysis bullosa simplex diseases. J. Cell Biol. 115 1661–1674. 10.1083/jcb.115.6.1661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croagh D., Phillips W. A., Redvers R., Thomas R. J. S., Kaur P. (2007). Identification of candidate murine esophageal stem cells using a combination of cell kinetic studies and cell surface markers. Stem Cells 25 313–318. 10.1634/stemcells.2006-0421 [DOI] [PubMed] [Google Scholar]
- Dabelsteen S., Mackenzie I. C. (2006). The stem cell concept in oral mucosa and in cancer. Nor. Tannlegeforen. Tid. 116 6–11. [Google Scholar]
- Dale B. A., Salonen J., Jones A. H. (1990). New approaches and concepts in the study of differentiation of oral epithelia. Crit. Rev. Oral Biol. Med. 1 167–190. 10.1177/10454411900010030201 [DOI] [PubMed] [Google Scholar]
- Dally J., Khan J. S., Voisey A., Charalambous C., John H. L., Woods E. L., et al. (2017). Hepatocyte growth factor mediates enhanced wound healing responses and resistance to transforming growth factor-B 1-driven myo?broblast differentiation in oral mucosal fibroblasts. Int. J. Mol. Sci. 18 1–15. 10.3390/ijms18091843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darby I. A., Laverdet B., Bonté F., Desmoulière A. (2014). Clinical, cosmetic and investigational dermatology dovepress fibroblasts and myofibroblasts in wound healing. Clin. Cosmet. Investig. Dermatol. 4 301–311. 10.2147/CCID.S50046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bree R., Rinaldo A., Genden E. M., Suárez C., Rodrigo J. P., Fagan J. J., et al. (2008). Modern reconstruction techniques for oral and pharyngeal defects after tumor resection. Eur. Arch. Otorhinolaryngol. 265 1–9. 10.1007/s00405-007-0413-y [DOI] [PubMed] [Google Scholar]
- Dekoninck S., Blanpain C. (2019). Stem cell dynamics, migration and plasticity during wound healing. Nat. Cell Biol. 21 18–24. 10.1038/s41556-018-0237-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaere P. R., Hermans R., Hardillo J., Van Den Hof B. (2001). Prefabrication of composite tissue for improved tracheal reconstruction. Ann. Otol. Rhinol. Laryngol. 110 849–860. 10.1177/000348940111000909 [DOI] [PubMed] [Google Scholar]
- DesJardins-Park H. E., Mascharak S., Chinta M. S., Wan D. C., Longaker M. T. (2019). The spectrum of scarring in craniofacial wound repair. Front. Physiol. 10:322. 10.3389/fphys.2019.00322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWard A. D., Cramer J., Lagasse E. (2014). Cellular heterogeneity in the mouse esophagus implicates the presence of a nonquiescent epithelial stem cell population. Cell Rep. 9 701–711. 10.1016/j.celrep.2014.09.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPietro L. A. (2016). Angiogenesis and wound repair: when enough is enough. J. Leukoc. Biol. 100 979–984. 10.1189/jlb.4mr0316-102r [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominiak M., Łysiak-Drwal K., Saczko J., Kunert-Keil C., Gedrange T. (2012). The clinical efficacy of primary culture of human fibroblasts in gingival augmentation procedures-a preliminary report. Ann. Anat. 194 502–507. 10.1016/j.aanat.2012.03.011 [DOI] [PubMed] [Google Scholar]
- Donati G., Rognoni E., Hiratsuka T., Liakath-Ali K., Hoste E., Kar G., et al. (2017). Wounding induces dedifferentiation of epidermal gata6 + cells and acquisition of stem cell properties. Nat. Cell Biol. 19 603–613. 10.1038/ncb3532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doucet Y. S., Woo S. H., Ruiz M. E., Owens D. M. (2013). The touch dome defines an epidermal niche specialized for mechanosensory signaling. Cell Rep. 3 1759–1765. 10.1016/j.celrep.2013.04.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doupé D. P., Alcolea M. P., Roshan A., Zhang G., Klein A. M., Simons B. D., et al. (2012). A single progenitor population switches behavior to maintain and repair esophageal epithelium. Science 337 1091–1093. 10.1126/science.1218835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doupé D. P., Klein A. M., Simons B. D., Jones P. H. (2010). The ordered architecture of murine ear epidermis is maintained by progenitor cells with random fate. Dev. Cell 18 317–323. 10.1016/j.devcel.2009.12.016 [DOI] [PubMed] [Google Scholar]
- Driskell R. R., Watt F. M. (2015). Understanding fibroblast heterogeneity in the skin. Trends Cell Biol. 25 92–99. 10.1016/j.tcb.2014.10.001 [DOI] [PubMed] [Google Scholar]
- Driskell R. R., Lichtenberger B. M., Hoste E., Kretzschmar K., Simons B. D., Charalambous M., et al. (2013). Distinct fibroblast lineages determine dermal architecture in skin development and repair. Nature 504 277–281. 10.1038/nature12783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulmovits B. M., Herman I. M. (2012). Microvascular remodeling and wound healing: a role for pericytes. Int. J. Biochem. Cell Biol. 44 1800–1812. 10.1016/j.biocel.2012.06.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egyedi P. (1977). Utilization of the buccal fat pad for closure of oro-antral and/or oro-nasal communications. J. Maxillofac. Surg. 5 241–244. 10.1016/S0301-0503(77)80117-3 [DOI] [PubMed] [Google Scholar]
- Escudero-Castaño N., Perea-García M. A., Campo-Trapero J., Sánchez C., Bascones-Martínez A. (2008). Oral and perioral piercing complications. Open Dent. J. 2 133–136. 10.2174/1874210600802010133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etulain J. (2018). Platelets in wound healing and regenerative medicine. Platelets 29 556–568. 10.1080/09537104.2018.1430357 [DOI] [PubMed] [Google Scholar]
- Evans E. W. (2017). Treating scars on the oral mucosa. Facial Plast. Surg. Clin. North. Am. 25 89–97. 10.1016/j.fsc.2016.08.008 [DOI] [PubMed] [Google Scholar]
- Feng F., Akiyama K., Liu Y., Yamaza T., Wang T. M., Chen J. H., et al. (2010). Utility of PDL progenitors for in vivo tissue regeneration: a report of 3 cases. Oral Dis. 16 20–28. 10.1111/j.1601-0825.2009.01593.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson D. D. (2005). Evaluation and management of benign esophageal strictures. Dis. Esophagus 18 359–364. 10.1111/j.1442-2050.2005.00516.x [DOI] [PubMed] [Google Scholar]
- Ferraris C., Chevalier G., Favier B., Jahoda C. A. B., Dhouailly D. (2000). Adult corneal epithelium basal cells possess the capacity to activate epidermal, pilosebaceous and sweat gland genetic programs in response to embryonic dermal stimuli. Development 127 5487–5495. [DOI] [PubMed] [Google Scholar]
- Fierz J., Hallermann W., Mericske-Stern R. (2013). Patients with oral tumors. Part 1: prosthetic rehabilitation following tumor resection. Schweiz. Monatsschr. Zahnmed. 123 91–105. 10.7892/boris.40796 [DOI] [PubMed] [Google Scholar]
- Fischer H., Langbein L., Reichelt J., Buchberger M., Tschachler E., Eckhart L. (2016). Keratins K2 and K10 are essential for the epidermal integrity of plantar skin. J. Dermatol. Sci. 81 10–16. 10.1016/j.jdermsci.2015.10.008 [DOI] [PubMed] [Google Scholar]
- Franke W. W., Schiller D. L., Moll R., Winter S., Schmid E., Engelbrecht I., et al. (1981). Diversity of cytokeratins. differentiation specific expression of cytokeratin polypeptides in epithelial cells and tissues. J. Mol. Biol. 153 933–959. 10.1016/0022-2836(81)90460-5 [DOI] [PubMed] [Google Scholar]
- Frantz C., Stewart K., Weaver V. (2010). The extracellular matrix at a glance. J. Cell Sci. 123 4195–4200. 10.1242/jcs.023820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frede J., Greulich P., Nagy T., Simons B. D., Jones P. H. (2016). A single dividing cell population with imbalanced fate drives oesophageal tumour growth. Nat. Cell Biol. 18 967–978. 10.1038/ncb3400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freinkel R. K., Woodley D. T. (eds) (2001). The Biology of the Skin. Boca Raton, FL: CRC Press. [Google Scholar]
- Fu D. J., Thomson C., Lunny D. P., Dopping-Hepenstal P. J., McGrath J. A., Smith F. J. D., et al. (2014). Keratin 9 is required for the structural integrity and terminal differentiation of the palmoplantar epidermis. J. Invest. Dermatol. 134 754–763. 10.1038/jid.2013.356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs E. (2007). Scratching the surface of skin development. Nature 445 834–842. 10.1038/nature05659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs E., Green H. (1980). Changes in keratin gene expression during terminal differentiation of the keratinocyte. Cell 19 1033–1042. 10.1016/0092-8674(80)90094-X [DOI] [PubMed] [Google Scholar]
- Füllgrabe A., Joost S., Are A., Jacob T., Sivan U., Haegebarth A., et al. (2015). Dynamics of Lgr6+ progenitor cells in the hair follicle, sebaceous gland, and interfollicular epidermis. Stem Cell Rep. 5 843–855. 10.1016/j.stemcr.2015.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartner L. P. (1991). Oral anatomy and tissue types. Semin. Dermatol. 13 68–73. [PubMed] [Google Scholar]
- Gavaghan M. (1999). Anatomy and physiology of the esophagus. AORN J. 69 370–386. 10.1016/s0001-2092(07)68909-1 [DOI] [PubMed] [Google Scholar]
- Gibson J. A. G., Ackling E., Bisson J., I, Dobbs T. D., Whitaker I. S. (2018). The association of affective disorders and facial scarring: systematic review and meta-analysis. J. Affect. Disord. 239 1–10. 10.1016/j.jad.2018.06.013 [DOI] [PubMed] [Google Scholar]
- Gil S. R., Pagés C. M., Díez E. G., Llames S., Fuertes A. F., Vilagran J. L. (2015). Tissue-engineered oral mucosa grafts for intraoral lining reconstruction of the maxilla and mandible with a fibula flap. J. Oral Maxillofac. Surg. 73 195.e1–195.e16. 10.1016/j.joms.2014.09.001 [DOI] [PubMed] [Google Scholar]
- Giroux V., Lento A. A., Islam M., Pitarresi J. R., Kharbanda A., Hamilton K. E., et al. (2017). Long-lived keratin 15+ esophageal progenitor cells contribute to homeostasis and regeneration. J. Clin. Invest. 127 2378–2391. 10.1172/JCI88941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuliani A., Manescu A., Langer M., Rustichelli F., Desiderio V., Paino F., et al. (2013). Three years after transplants in human mandibles, histological and in-line holotomography revealed that stem cells regenerated a compact rather than a spongy bone: biological and clinical implications. Stem Cells Transl. Med. 2 316–324. 10.5966/sctm.2012-0136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glim J. E., Everts V., Niessen F. B., Ulrich M. M., Beelen R. H. J. (2014). Extracellular matrix components of oral mucosa differ from skin and resemble that of foetal skin. Arch. Oral Biol. 50 1048–1055. 10.1016/j.archoralbio.2014.05.019 [DOI] [PubMed] [Google Scholar]
- Glim J. E., van Egmond M., Niessen F. B., Everts V., Beelen R. H. J. (2013). Detrimental dermal wound healing: what can we learn from the oral mucosa? Wound Repair Regen. 21 648–660. 10.1111/wrr.12072 [DOI] [PubMed] [Google Scholar]
- Goetsch E. (1910). The structure of the mammalian oesophagus. Am. J. Anat. 10 1–40. 10.1002/aja.1000100102 [DOI] [Google Scholar]
- Gölz L., Vestewig E., Blankart M., Kraus D., Appel T., Frede S., et al. (2016). Differences in human gingival and dermal fibroblasts may contribute to oral-induced tolerance against nickel. J. Allergy Clin. Immunol. 138 1202–1205.e3. 10.1016/j.jaci.2016.03.036 [DOI] [PubMed] [Google Scholar]
- Gomes J. ÁP., Monteiro B. G., Melo G. B., Smith R. L., Silva M. C. P., Lizier N. F., et al. (2010). Corneal reconstruction with tissue-engineered cell sheets composed of human immature dental pulp stem cells. Investig. Ophthalmol. Vis. Sci. 51 1408–1414. 10.1167/iovs.09-4029 [DOI] [PubMed] [Google Scholar]
- Gonzales K. A. U., Fuchs E. (2017). Skin and its regenerative powers: an alliance between stem cells and their niche. Dev. Cell 43 387–401. 10.1016/j.devcel.2017.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodacre T., Swan M. C. (2008). Cleft lip and palate: current management. J. Paediatr. Child Health 18 283–292. 10.1016/j.paed.2008.03.008 [DOI] [Google Scholar]
- Gordon J. I. (1994). Differentiation and self-renewal in the mouse gastrointestinal epithelium. Curr. Opin. Cell Biol. 6 795–803. 10.1016/0955-0674(94)90047-7 [DOI] [PubMed] [Google Scholar]
- Groeger S., Meyle J. (2019). Oral mucosal epithelial cells. Front. Immunol. 10:208. 10.3389/fimmu.2019.00208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grøn B., Stoltze K., Andersson A., Dabelsteen E. (2002). Oral fibroblasts produce more HGF and KGF than skin fibroblasts in response to co-culture with keratinocytes. APMIS 110 892–898. 10.1034/j.1600-0463.2002.1101208.x [DOI] [PubMed] [Google Scholar]
- Guerrero-Juarez C. F., Dedhia P. H., Jin S., Ruiz-Vega R., Ma D., Liu Y., et al. (2019). Single-cell analysis reveals fibroblast heterogeneity and myeloid-derived adipocyte progenitors in murine skin wounds. Nat. Commun. 10:650. 10.1038/s41467-018-08247-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guest J. F., Ayoub N., McIlwraith T., Uchegbu I., Gerrish A., Weidlich D., et al. (2015). Health economic burden that wounds impose on the national health service in the UK. BMJ Open 5:e009283. 10.1136/bmjopen-2015-009283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurtner G. C., Werner S., Barrandon Y., Longaker M. T. (2008). Wound repair and regeneration. Nature 453 314–321. 10.1038/nature07039 [DOI] [PubMed] [Google Scholar]
- Hall M. W. J., Jones P. H., Hall B. A. (2018). Relating evolutionary selection and mutant clonal dynamics in normal epithelia. bioRxiv [Preprint] 10.1101/480756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hand A. R., Frank M. E. (2014). Fundamentals of Oral Histology and Physiology. Hoboken, NJ: John Wiley & Sons, Inc. [Google Scholar]
- Harper R. A., Grove G. (1979). Human skin fibroblasts derived from papillary and reticular dermis: differences in growth potential in vitro. Science 204 526–527. 10.1126/science.432659 [DOI] [PubMed] [Google Scholar]
- Herrmann H., Aebi U. (2016). Intermediate filaments: structure and assembly. Cold Spring Harb. Perspect. Biol. 8:a018242. 10.1101/cshperspect.a018242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higa K., Satake Y., Shimazaki J. (2017). The characterization of human oral mucosal fibroblasts and their use as feeder cells in cultivated epithelial sheets. Future Sci. OA 3:FSO243. 10.4155/fsoa-2017-0074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins C. A., Roger M. F., Hill R. P., Ali-Khan A. S., Garlick J. A., Christiano A. M., et al. (2017). Multifaceted role of hair follicle dermal cells in bioengineered skins. Br. J. Dermatol. 176 1259–1269. 10.1111/bjd.15087 [DOI] [PubMed] [Google Scholar]
- Hinz B. (2007). Formation and function of the myofibroblast during tissue repair. J. Invest. Dermatol. 127 526–537. 10.1038/sj.jid.5700613 [DOI] [PubMed] [Google Scholar]
- Hiraoka C., Toki F., Shiraishi K., Sayama K., Nishimura E. K., Miura H., et al. (2016). Two clonal types of human skin fibroblasts with different potentials for proliferation and tissue remodeling ability. J. Dermatol. Sci. 82 84–94. 10.1016/j.jdermsci.2016.01.009 [DOI] [PubMed] [Google Scholar]
- Hirsch T., Rothoeft T., Teig N., Bauer J. W., Pellegrini G., De Rosa L., et al. (2017). Regeneration of the entire human epidermis using transgenic stem cells. Nature 551 327–332. 10.1038/nature24487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hixon K. R., Lin A. Y., Sell S. A. (2019). “Scaffolds for cleft lip and cleft palate reconstruction,” in Handbook of Tissue Engineering Scaffolds, Vol. 1 eds Mozafari M., Sefat F., Atala A. (Sawston: Woodhead Publishing; ), 421–435. 10.1016/B978-0-08-102563-5.00020-4 [DOI] [Google Scholar]
- Holbrook K. (1994). “Ultrastructure of the epidermis,” in The Keratinocyte Handbook, eds Leigh I. M., Lane E. B., Watt F. M. (Cambridge: Cambridge University Press; ), 3–39. [Google Scholar]
- Horiguchi A. (2017). Substitution urethroplasty using oral mucosa graft for male anterior urethral stricture disease: current topics and reviews. Int. J. Urol. 24 493–503. 10.1111/iju.13356 [DOI] [PubMed] [Google Scholar]
- Hotta T., Yokoo S., Terashi H., Komori T. (2007). Clinical and histopathological analysis of healing process of intraoral reconstruction with ex vivo produced oral mucosa equivalent. Kobe J. Med. Sci. 53 1–14. [PubMed] [Google Scholar]
- Howard J. M., Nuguid J. M., Ngole D., Nguyen H. (2014). Tcf3 expression marks both stem and progenitor cells in multiple epithelia. Development 141 3143–3152. 10.1242/dev.106989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang N., Perez P., Kato T., Mikami Y., Okuda K., Gilmore R. C., et al. (2021). SARS-CoV-2 infection of the oral cavity and saliva. Nat. Med. 27 892–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z., Wei W., Cheng F. (2020). Endoscopic radial incision method for two strictures of the esophagus after endoscopic submucosal dissection: a case report. World J. Surg. Oncol. 18 1–6. 10.1186/s12957-020-01812-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hume W. J., Potten C. S. (1976). The ordered columnar structure of mouse filiform papillae. J. Cell Sci. 22 149–160. [DOI] [PubMed] [Google Scholar]
- Hutson J. M., Niall M., Evans D., Fowler R. (1979). Effect of salivary glands on wound contraction in mice. Nature 279 793–795. 10.1038/279793a0 [DOI] [PubMed] [Google Scholar]
- Iglesias-Bartolome R., Uchiyama A., Molinolo A. A., Abusleme L., Brooks S. R., Callejas-Valera J. L., et al. (2018). Transcriptional signature primes human oral mucosa for rapid wound healing. Sci. Transl. Med. 10:451. 10.1126/scitranslmed.aap8798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iida T., Takami Y., Yamaguchi R., Shimazaki S., Harii K. (2005). Development of a tissue-engineered human oral mucosa equivalent based on an acellular allogeneic dermal matrix: a preliminary report of clinical application to burn wounds. Scand. J. Plast. Reconstr. Surg. Hand Surg. 39 138–146. 10.1080/0284431051006376 [DOI] [PubMed] [Google Scholar]
- Irwin C. R., Picardo M., Ellis I., Sloan P., Grey A. M., McGurk M., et al. (1994). Inter- and Intra-site heterogeneity in the expression of fetal-like phenotypic characteristics by gingival fibroblasts: potential significance for wound healing. J. Cell Sci. 107 1333–1346. [DOI] [PubMed] [Google Scholar]
- Isaac J., Nassif A., Asselin A., Taïhi I., Fohrer-Ting H., Klein C., et al. (2018). Involvement of neural crest and paraxial mesoderm in oral mucosal development and healing. Biomaterials 172 41–53. 10.1016/j.biomaterials.2018.04.036 [DOI] [PubMed] [Google Scholar]
- Ishii M., Arias A. C., Liu L., Chen Y., Bronner M. E., Maxson R. E. (2012). A stable cranial neural crest cell line from mouse. Stem Cells Dev. 21 3069–3080. 10.1089/scd.2012.0155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M., Liu Y., Yang Z., Nguyen J., Liang F., Morris R. J., et al. (2005). Stem cells in the hair follicle bulge contribute to wound repair but not to homeostasis of the epidermis. Nat. Med. 11 1351–1354. 10.1038/nm1328 [DOI] [PubMed] [Google Scholar]
- Izumi K., Feinberg S. E., Iida A., Yoshizawa M. (2003). Intraoral grafting of an Ex vivo produced oral mucosa equivalent: a preliminary report. Int. J. Oral Maxillofac. Surg. 32 188–197. 10.1054/ijom.2002.0365 [DOI] [PubMed] [Google Scholar]
- Izumi K., Kato H., Feinberg S. E. (2015). “Tissue engineered oral mucosa,” in Stem Cell Biology and Tissue Engineering in Dental Sciences, eds Vishwakarma A., Sharpe P., Shi S., Ramalingam M. (Amsterdam: Elsevier Inc.), 721–731. 10.1016/B978-0-12-397157-9.00077-1 [DOI] [Google Scholar]
- Izumi K., Neiva R. F., Feinberg S. E. (2013). Intraoral grafting of tissue-engineered human oral mucosa. Int. J. Oral Maxillofac. Implants 28 e295–e303. 10.11607/jomi.te11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahoda C. A. B., Reynolds A. J. (1996). Dermal-epidermal interactions. Adult follicle-derived cell populations and hair growth. Dermatol. Clin. 14 573–583. 10.1016/s0733-8635(05)70385-5 [DOI] [PubMed] [Google Scholar]
- Jaks V., Barker N., Kasper M., Van Es J. H., Snippert H. J., Clevers H., et al. (2008). Lgr5 marks cycling, yet long-lived, hair follicle stem cells. Nat. Genet. 40 1291–1299. 10.1038/ng.239 [DOI] [PubMed] [Google Scholar]
- Janebodin K., Horst O. V., Ieronimakis N., Balasundaram G., Reesukumal K., Pratumvinit B., et al. (2011). Isolation and characterization of neural crest-derived stem cells from dental pulp of neonatal mice. PLoS One 6:e27526. 10.1371/journal.pone.0027526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowski J. (1993). Gene expression in Barrett’s mucosa: acute and chronic adaptive responses in the oesophagus. Gut 32 1649–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen K. B., Collins C. A., Nascimento E., Tan D. W., Frye M., Itami S., et al. (2009). Lrig1 expression defines a distinct multipotent stem cell population in mammalian epidermis. Cell Stem Cell 4 427–439. 10.1016/j.stem.2009.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang D., Rinkevich Y. (2018). Defining skin fibroblastic cell types beyond CD90. Front. Cell Dev. Biol. 6:133. 10.3389/fcell.2018.00133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang D., Rinkevich Y. (2021). Distinct fibroblasts in scars and regeneration. Curr. Opin. Genet. Dev. 70 7–14. 10.1016/j.gde.2021.04.005 [DOI] [PubMed] [Google Scholar]
- Jiang D., Christ S., Correa-Gallegos D., Ramesh P., Gopal S. K., Wannemacher J., et al. (2020a). Injury triggers fascia fibroblast collective cell migration to drive scar formation through N-cadherin. Nat. Commun. 11:5653. 10.1038/s41467-020-19425-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang D., Correa-Gallegos D., Christ S., Stefanska A., Liu J., Ramesh P., et al. (2018). Two succeeding fibroblastic lineages drive dermal development and the transition from regeneration to scarring. Nat. Cell Biol. 20 422–431. 10.1038/s41556-018-0073-8 [DOI] [PubMed] [Google Scholar]
- Jiang D., de Vries J. C., Muschhammer J., Schatz S., Ye H., Hein T., et al. (2020b). Local and transient inhibition of P21 expression ameliorates age-related delayed wound healing. Wound Repair Regen. 28 49–60. 10.1111/wrr.12763 [DOI] [PubMed] [Google Scholar]
- Jiang N., Xiang L., He L., Yang G., Zheng J., Wang C., et al. (2017). Exosomes mediate epithelium-mesenchyme crosstalk in organ development. ACS Nano 11 7736–7746. 10.1021/acsnano.7b01087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson A., Di Pietro L. A. (2013). Apoptosis and angiogenesis: an evolving mechanism for fibrosis. FASEB J. 27 3893–3901. 10.1096/fj.12-214189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson O. N., Nelson M., Estabrooke I., Sopko N., Swanson E. W. (2020). Successful treatment of war zone traumatic lower extremity wound with exposed tendons using an autologous homologous skin construct. Cureus 12 1–7. 10.7759/cureus.7952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas E., Sjöqvist S., Elbe P., Kanai N., Enger J., Haas S. L., et al. (2016). Transplantation of tissue-engineered cell sheets for stricture prevention after endoscopic submucosal dissection of the oesophagus. United Eur. Gastroenterol. J. 4 741–753. 10.1177/2050640616631205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones K. B., Klein O. D. (2013). Oral epithelial stem cells in tissue maintenance and disease: the first steps in a long journey. Int. J. Oral Sci. 5 121–129. 10.1038/ijos.2013.46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones K. B., Furukawa S., Marangoni P., Ma H., Pinkard H., D’Urso R., et al. (2019). Quantitative clonal analysis and single-cell transcriptomics reveal division kinetics, hierarchy, and fate of oral epithelial progenitor cells. Cell Stem Cell 24 183–192.e8. 10.1016/j.stem.2018.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S. G., Edwards R., Thomas D. W. (2004). Inflammation and wound healing: the role of bacteria in the immuno-regulation of wound healing. Int. J. Low Extrem. Wounds 3 201–208. 10.1177/1534734604271810 [DOI] [PubMed] [Google Scholar]
- Jönsson L., Blom M. D., Friberg L., Gatzinsky V., Holmquist O., Jennische E., et al. (2016). Macrophage phenotype is associated with the regenerative response in experimental replacement of the porcine esophagus. Artif. Organs 40 950–958. 10.1111/aor.12652 [DOI] [PubMed] [Google Scholar]
- Joost S., Annusver K., Jacob T., Sun X., Dalessandri T., Sivan U., et al. (2020). The molecular anatomy of mouse skin during hair growth and rest. Cell Stem Cell 26 441–457.e7. 10.1016/j.stem.2020.01.012 [DOI] [PubMed] [Google Scholar]
- Joost S., Zeisel A., Jacob T., Sun X., La Manno G., Lönnerberg P., et al. (2016). Single-cell transcriptomics reveals that differentiation and spatial signatures shape epidermal and hair follicle heterogeneity. Cell Syst. 3 221–237.e9. 10.1016/j.cels.2016.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwal A., D’souza J., Muthiah L., Srividya S. (2017). The current status of stem cell regeneration in intra oral applications—a systematic review. Open J. Stomatol. 7 197–224. 10.4236/ojst.2017.74015 [DOI] [Google Scholar]
- Karppinen S. M., Heljasvaara R., Gullberg D., Tasanen K., Pihlajaniemi T. (2019). Toward understanding scarless skin wound healing and pathological scarring. F1000Res. 8:787. 10.12688/f1000research.18293.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato H., Marcelo C. L., Washington J. B., Bingham E. L., Feinberg S. E. (2015). Fabrication of large size ex vivo-produced oral mucosal equivalents for clinical application. Tissue Eng. Part C Methods 21 872–880. 10.1089/ten.tec.2014.0600 [DOI] [PubMed] [Google Scholar]
- Katou F., Shirai N., Kamakura S., Tagami H., Nagura H., Motegi K. (2003). Differential expression of cornified cell envelope precursors in normal skin, intraorally transplanted skin and normal oral mucosa. Br. J. Dermatol. 148 898–905. 10.1046/j.1365-2133.2003.05288.x [DOI] [PubMed] [Google Scholar]
- Kim H. S., Kim N. H., Kim J., Cha I. H. (2013). Inducing re-epithelialization in skin wound through cultured oral mucosal keratinocytes. J. Korean Assoc. Oral Maxillofac. Surg. 39:63. 10.5125/jkaoms.2013.39.2.63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M., Han W., Kim S. (2017). The use of the buccal fat pad flap for oral reconstruction. Maxillofac. Plast. Reconstr. Surg. 39 1–9. 10.1186/s40902-017-0105-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamoto S., Nagao-Kitamoto H., Jiao Y., Gillilland M. G., Hayashi A., Imai J., et al. (2020). The intermucosal connection between the mouth and gut in commensal pathobiont-driven colitis. Cell 182 447–462.e14. 10.1016/j.cell.2020.05.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein A. M., Simons B. D. (2011). Universal patterns of stem cell fate in cycling adult tissues. Development 138 3103–3111. 10.1242/dev.060103 [DOI] [PubMed] [Google Scholar]
- Klein A. M., Brash D. E., Jones P. H., Simons B. D. (2010). Stochastic fate of P53-mutant epidermal progenitor cells is tilted toward proliferation by UV B during preneoplasia. Proc. Natl. Acad. Sci. U.S.A. 107 270–275. 10.1073/pnas.0909738107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp A. C., Franke W. W., Heid H., Hatzfeld M., Jorcano J. L., Moll R. (1986). Cytokeratin no. 9, an epidermal type i keratin characteristic of a special program of keratinocyte differentiation displaying body site specificity. J. Cell Biol. 103 657–667. 10.1083/jcb.103.2.657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knöbel M., O’Toole E. A., Smith F. J. D. (2015). Keratins and skin disease. Cell Tissue Res. 360 583–589. 10.1007/s00441-014-2105-4 [DOI] [PubMed] [Google Scholar]
- Komaki Y., Kanmura S., Sasaki F., Maeda H., Oda K., Arima S., et al. (2019). Hepatocyte growth factor facilitates esophageal mucosal repair and inhibits the submucosal fibrosis in a rat model of esophageal ulcer. Digestion 99 227–238. 10.1159/000491876 [DOI] [PubMed] [Google Scholar]
- Kong X., Fu J., Shao K., Wang L., Lan X., Shi J. (2019). Biomimetic hydrogel for rapid and scar-free healing of skin wounds inspired by the healing process of oral mucosa. Acta Biomater. 100 255–269. 10.1016/j.actbio.2019.10.011 [DOI] [PubMed] [Google Scholar]
- Korosec A., Lichtenberger B. M. (2018). “12 in vitro models to study hair follicle generation,” in Skin Tissue Models, eds Marques A., Reis R., Pirraco R., Cerqueira M. (Cambridge, MA: Academic Press; ), 279–301. [Google Scholar]
- Korosec A., Frech S., Gesslbauer B., Vierhapper M., Radtke C., Petzelbauer P., et al. (2019). Lineage identity and location within the dermis determine the function of papillary and reticular fibroblasts in human skin. J. Invest. Dermatol. 139 342–351. 10.1016/j.jid.2018.07.033 [DOI] [PubMed] [Google Scholar]
- Kuperman S., Efraty R., Arie I., Rahmanov A., Gavrielov M. R., Noff M., et al. (2020). Examination of the therapeutic potential of mouse oral mucosa stem cells in a wound-healing diabetic mice model. Int. J. Environ. Res. Public Health 17 1–10. 10.3390/ijerph17134854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuramoto G., Takagi S., Ishitani K., Shimizu T., Okano T., Matsui H. (2015). Preventive effect of oral mucosal epithelial cell sheets on intrauterine adhesions. Hum. Reprod. 30 406–416. 10.1093/humrep/deu326 [DOI] [PubMed] [Google Scholar]
- Lai-Cheong J. E., McGrath J. A. (2013). Structure and function of skin, hair and nails. Medcine 41 317–320. 10.1016/j.mpmed.2013.04.017 [DOI] [Google Scholar]
- Lane E. B. (1994). Keratin diseases. Curr. Opin. Genet. Dev. 4 412–418. 10.1016/0959-437X(94)90030-2 [DOI] [PubMed] [Google Scholar]
- Larjava H. (ed.) (2012). Oral Wound Healing: Cell Biology and Clinical Management. West Sussex: John Wiley & Sons, Inc. [Google Scholar]
- Lauer G., Schimming R. (2001). Tissue-engineered mucosa graft for reconstruction of the intraoral lining after freeing of the tongue: a clinical and immunohistologic study. J. Oral Maxillofac. Surg. 59 169–175. 10.1053/joms.2001.20489 [DOI] [PubMed] [Google Scholar]
- Lee J., Kim E. H., Shin D., Roh J. L. (2017). Accelerated oral wound healing using a pre-vascularized mucosal cell sheet. Sci. Rep. 7:10667. 10.1038/s41598-017-10991-x [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Lee J., Shin D., Roh J. L. (2018). Use of a pre-vascularised oral mucosal cell sheet for promoting cutaneous burn wound healing. Theranostics 8 5703–5712. 10.7150/thno.28754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J., Shin D., Roh J. L. (2019). Promotion of skin wound healing using prevascularized oral mucosal cell sheet. Head Neck 41 774–779. 10.1002/hed.25432 [DOI] [PubMed] [Google Scholar]
- Legué E., Nicolas J. F. (2005). Hair follicle renewal: organization of stem cells in the matrix and the role of stereotyped lineages and behaviors. Development 132 4143–4154. 10.1242/dev.01975 [DOI] [PubMed] [Google Scholar]
- Legué E., Sequeira I., Nicolas J. (2012). “Hair follicle stem cells,” in Stem Cells and Cancer Stem Cells, ed. Hayat M. (Dordrecht: Springer; ), 35–47. 10.1007/978-94-007-2415-0 [DOI] [Google Scholar]
- Lei M., Chuong C. M. (2018). Epidermal darwinism and competitive equilibrium within the epidermis. Cell Stem Cell 23 627–629. 10.1016/j.stem.2018.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levoux J., Prola A., Lafuste P., Gervais M., Chevallier N., Koumaiha Z., et al. (2021). Platelets facilitate the wound-healing capability of mesenchymal stem cells by mitochondrial transfer and metabolic reprogramming. Cell Metab. 33 283–299.e9. 10.1016/j.cmet.2020.12.006 [DOI] [PubMed] [Google Scholar]
- Li D., Yin Z., Liu Y., Feng S., Liu Y., Lu F., et al. (2019). Regeneration of trachea graft with cartilage support, vascularization, and epithelization. Acta Biomater. 89 206–216. 10.1016/j.actbio.2019.03.003 [DOI] [PubMed] [Google Scholar]
- Li J., Wang Z., Chu Q., Jiang K., Li J., Tang N. (2018). The strength of mechanical forces determines the differentiation of alveolar epithelial cells. Dev. Cell 44 297–312.e5. 10.1016/j.devcel.2018.01.008 [DOI] [PubMed] [Google Scholar]
- Liakath-Ali K., Mills E. W., Sequeira I., Lichtenberger B. M., Pisco A. O., Sipilä K. H., et al. (2018). An evolutionarily conserved ribosome-rescue pathway maintains epidermal homeostasis. Nature 556 367–380. 10.1038/s41586-018-0032-3 [DOI] [PubMed] [Google Scholar]
- Lichtenberger B. M., Mastrogiannaki M., Watt F. M. (2016). Epidermal β-catenin activation remodels the dermis via paracrine signalling to distinct fibroblast lineages. Nat. Commun. 7:10537. 10.1038/ncomms10537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim X., Tan S. H., Koh W. L. C., Chau R. M. W., Yan K. S., Kuo C. J., et al. (2013). Interfollicular epidermal stem cells self-renew via autocrine wnt signaling. Science 342 1226–1230. 10.1126/science.1239730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llames S., Recuero I., Romance A., García E., Peña I., Del Valle ÁF., et al. (2014). Tissue-engineered oral mucosa for mucosal reconstruction in a pediatric patient with hemifacial microsomia and ankyloglossia. Cleft Palate Craniofac. J. 51 246–251. 10.1597/12-245 [DOI] [PubMed] [Google Scholar]
- Locke M., Hyland P. L., Irwin C. R., Mackenzie I. C. (2008). Modulation of gingival epithelial phenotypes by interactions with regionally defined populations of fibroblasts. J. Periodontal Res. 43 279–289. 10.1111/j.1600-0765.2007.01028.x [DOI] [PubMed] [Google Scholar]
- López J. F., Sarkanen J. R., Huttala O., Kaartinen I. S., Kuokkanen H. O., Ylikomi T. (2018). Adipose tissue extract shows potential for wound healing: in vitro proliferation and migration of cell types contributing to wound healing in the presence of adipose tissue preparation and platelet rich plasma. Cytotechnology 70 1193–1204. 10.1007/s10616-018-0211-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C. P., Polak L., Rocha A. S., Pasolli H. A., Chen S. C., Sharma N., et al. (2012). Identification of stem cell populations in sweat glands and ducts reveals roles in homeostasis and wound repair. Cell 150 136–150. 10.1016/j.cell.2012.04.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luca M., Albanese E., Megna M., Cancedda R., Mangiante P. E., Cadoni A., et al. (1990). Evidence that human oral epithelium reconstituted in vitro and transplanted onto patients with defects in the oral mucosa retains properties of the original donor site. Transplantation 50 454–459. 10.1097/00007890-199009000-00019 [DOI] [PubMed] [Google Scholar]
- Lucas T., Waisman A., Ranjan R., Roes J., Krieg T., Müller W., et al. (2010). Differential roles of macrophages in diverse phases of skin repair. J. Immunol. Res. 184 3964–3977. 10.4049/jimmunol.0903356 [DOI] [PubMed] [Google Scholar]
- Luo X., Okubo T., Randell S., Hogan B. L. M. (2009). Culture of endodermal stem/progenitor cells of the mouse tongue. In Vitro Cell. Dev. Biol. Anim. 45 44–54. 10.1007/s11626-008-9149-2 [DOI] [PubMed] [Google Scholar]
- Lv L., Sheng C., Zhou Y. (2019). Extracellular vesicles as a novel therapeutic tool for cell-free regenerative medicine in oral rehabilitation. J. Oral Rehabil. 47 29–54. 10.1111/joor.12885 [DOI] [PubMed] [Google Scholar]
- Lygoe K. A., Wall I., Stephens P., Lewis M. P. (2007). Role of vitronectin and fibronectin receptors in oral mucosal and dermal myofibroblast differentiation. Biol. Cell 99 601–614. 10.1042/bc20070008 [DOI] [PubMed] [Google Scholar]
- Lynch M. D., Lynch C. N. S., Craythorne E., Liakath-Ali K., Mallipeddi R., Barker J. N., et al. (2017). Spatial constraints govern competition of mutant clones in human epidermis. Nat. Commun. 8:1119. 10.1038/s41467-017-00993-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie I. C. (1997). Retroviral transduction of murine epidermal stem cells demonstrates clonal units of epidermal structure. J. Invest. Dermatol. 109 377–383. 10.1111/1523-1747.ep12336255 [DOI] [PubMed] [Google Scholar]
- Mackenzie I. C., Hill M. W. (1984). Connective tissue influences on patterns of epithelial architecture and keratinization in skin and oral mucosa of the adult mouse. Cell Tissue Res. 235 551–559. 10.1007/BF00226952 [DOI] [PubMed] [Google Scholar]
- Mah W., Jiang G., Olver D., Gallant-Behm C., Wiebe C., Hart D. A., et al. (2017). Elevated CD26 expression by skin fibroblasts distinguishes a profibrotic phenotype involved in scar formation compared to gingival fibroblasts. Am. J. Pathol. 187 1717–1735. 10.1016/j.ajpath.2017.04.017 [DOI] [PubMed] [Google Scholar]
- Mak K., Manji A., Gallant-Behm C., Wiebe C., Hart D. A., Larjava H., et al. (2009). Scarless healing of oral mucosa is characterized by faster resolution of inflammation and control of myofibroblast action compared to skin wounds in the red duroc pig model. J. Dermatol. Sci. 56 168–180. 10.1016/j.jdermsci.2009.09.005 [DOI] [PubMed] [Google Scholar]
- Marques-Pereira J. P., Leblond C. P. (1965). Mitosis and differentiation in the stratified squamous epithelium of the rat esophagus. Am. J. Anat. 117 73–87. 10.1002/aja.1001170106 [DOI] [PubMed] [Google Scholar]
- Marsh E., Gonzalez D. G., Lathrop E. A., Boucher J., Greco V. (2018). Positional stability and membrane occupancy define skin fibroblast homeostasis in vivo. Cell 175 1620–1633.e13. 10.1016/j.cell.2018.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall C. D., Hu M. S., Leavitt T., Barnes L. A., Lorenz H. P., Longaker M. T. (2018). Cutaneous scarring: basic science, current treatments, and future directions. Adv. Wound Care 7 29–45. 10.1089/wound.2016.0696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martincorena I., Fowler J. C., Wabik A., Lawson A. R. J., Abascal F., Hall M. W. J., et al. (2018). Somatic mutant clones colonize the human esophagus with age. Science 362 911–917. 10.1126/science.aau3879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins V. L., Caley M., O’Toole E. A. (2013). Matrix metalloproteinases and epidermal wound repair. Cell Tissue Res. 351 255–268. 10.1007/s00441-012-1410-z [DOI] [PubMed] [Google Scholar]
- Mascré G., Dekoninck S., Drogat B., Youssef K. K., Brohée S., Sotiropoulou P. A., et al. (2012). Distinct contribution of stem and progenitor cells to epidermal maintenance. Nature 489 257–262. 10.1038/nature11393 [DOI] [PubMed] [Google Scholar]
- Mastrogiannaki M., Lichtenberger B. M., Reimer A., Collins C. A., Driskell R. R., Watt F. M. (2016). β-catenin stabilization in skin fibroblasts causes fibrotic lesions by preventing adipocyte differentiation of the reticular dermis. J. Invest. Dermatol. 136 1130–1142. 10.1016/j.jid.2016.01.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matoltsy A. G. (1986). “Structure and function of the mammalian epidermis,” in Biology of the Integument, eds Bereiter-Hahn J., Matoltsy A. G., Richards K. S. (Berlin: Springer; ), 255–271. 10.1007/978-3-662-00989-5_14 [DOI] [Google Scholar]
- Mcginn J., Hallou A., Han S., Krizic K., Ulyanchenko S., Iglesias-Bartolome R., et al. (2021). A biomechanical switch regulates the transition towards homeostasis in esophageal epithelium. bioRxiv [Preprint] Available online at: https://www.biorxiv.org/content/10.1101/2021.02.03.428820v1 (accessed March 2, 2021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire M. K., Scheyer E. T. (2007). A randomized, double-blind, placebo-controlled study to determine the safety and efficacy of cultured and expanded autologous fibroblast injections for the treatment of interdental papillary insufficiency associated with the papilla priming procedure. J. Periodontol. 78 4–17. 10.1902/jop.2007.060105 [DOI] [PubMed] [Google Scholar]
- McKeown S. T. W., Hyland P. L., Locke M., Mackenzie I. C., Irwin C. R. (2003). Keratinocyte growth factor and scatter factor expression by regionally defined oral fibroblasts. Eur. J. Oral Sci. 111 42–50. 10.1034/j.1600-0722.2003.00002.x [DOI] [PubMed] [Google Scholar]
- Mehrel T., Hohl D., Rothnagel J. A., Longley M. A., Bundman D., Cheng C., et al. (1990). Identification of a major keratinocyte cell envelope protein, loricrin. Cell 61 1103–1112. 10.1016/0092-8674(90)90073-N [DOI] [PubMed] [Google Scholar]
- Meigel W. N., Gay S., Weber L. (1977). Dermal architecture and collagen type distribution. Arch. Dermatol. Res. 259 1–10. 10.1007/BF00562732 [DOI] [PubMed] [Google Scholar]
- Meran S., Thomas D., Stephens P., Martin J., Bowen T., Phillips A., et al. (2007). Involvement of hyaluronan in regulation of fibroblast phenotype. J. Biol. Chem. 282 25687–25697. 10.1074/jbc.M700773200 [DOI] [PubMed] [Google Scholar]
- Mesa K. R., Kawaguchi K., Cockburn K., Gonzalez D., Boucher J., Xin T., et al. (2018). Homeostatic epidermal stem cell self-renewal is driven by local differentiation. Cell Stem Cell 23 677.e–686.e. 10.1016/j.stem.2018.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesa K. R., Rompolas P., Greco V. (2015). The dynamic duo: niche/stem cell interdependency. Stem Cell Rep. 4 961–966. 10.1016/j.stemcr.2015.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesler A. L., Veniaminova N. A., Lull M. V., Wong S. Y. (2017). Hair follicle terminal differentiation is orchestrated by distinct early and late matrix progenitors. Cell Rep. 19 809–821. 10.1016/j.celrep.2017.03.077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messier B., Leblond C. P. (1960). Cell proliferation and migration as revealed by radioautography after injection of thymidine-H3 into male rats and mice. Am. J. Anat. 106 247–285. 10.1002/aja.1001060305 [DOI] [PubMed] [Google Scholar]
- Mi B., Chen L., Xiong Y., Yan C., Xue H., Panayi A. C., et al. (2020). Saliva exosomes-derived UBE2O MRNA promotes angiogenesis in cutaneous wounds by targeting SMAD6. J. Nanobiotechnol. 18 1–14. 10.1186/s12951-020-00624-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mistretta C. M., Kumari A. (2017). Tongue and taste organ biology and function: homeostasis maintained by hedgehog signaling. Annu. Rev. Physiol. 79 335–356. 10.1146/annurev-physiol-022516-034202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell H., Abel K. M., Dunlop B. J., Walker T., Ranote S., Robinson L., et al. (2019). Acceptability and feasibility pilot randomised controlled trial of medical skin camouflage for recovery of women prisoners with self-harm scarring (COVER): the study protocol. BMJ Open 9:e021891. 10.1136/bmjopen-2018-021891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi K., Tsuji D., Kudoh K., Satomura K., Muto T., Itoh K., et al. (2010). Generation of human induced pluripotent stem cells from oral mucosa. J. Biosci. Bioeng. 110 345–350. 10.1016/j.jbiosc.2010.03.004 [DOI] [PubMed] [Google Scholar]
- Mohammadi M., Mofid R., Shokrgozar M. A. (2011). peri-implant soft tissue management through use of cultured gingival graft: a case report. Acta Med. Iran. 49 319–324. [PubMed] [Google Scholar]
- Moll I., Heid H., Franke W. W., Moll R. (1987). Distribution of a special subset of keratinocytes characterized by the expression of cytokeratin 9 in adult and fetal human epidermis of various body sites. Differentiation 33 254–265. 10.1111/j.1432-0436.1987.tb01565.x [DOI] [PubMed] [Google Scholar]
- Moll R., Divo M., Langbein L. (2008). The human keratins: biology and pathology. Histochem. Cell Biol. 129 705–733. 10.1007/s00418-008-0435-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris R. J., Liu Y., Marles L., Yang Z., Trempus C., Li S., et al. (2004). Capturing and profiling adult hair follicle stem cells. Nat. Biotechnol. 22 411–417. 10.1038/nbt950 [DOI] [PubMed] [Google Scholar]
- Moutsopoulos N. M., Konkel J. E. (2018). Tissue-specific immunity at the oral mucosal barrier. Trends Immunol. 39 276–287. 10.1016/j.it.2017.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murai K., Skrupskelyte G., Piedrafita G., Hall M., Kostiou V., Ong S. H., et al. (2018). Epidermal Tissue adapts to restrain progenitors carrying clonal P53 mutations. Cell Stem Cell 23 687.e–699.e. 10.1016/j.stem.2018.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa E., Itoh T., Yoshie H., Satokata I. (2009). Odontogenic potential of post-natal oral mucosal epithelium. J. Dent. Res. 88 219–223. 10.1177/0022034509333198 [DOI] [PubMed] [Google Scholar]
- Nakase Y., Nakamura T., Kin S., Nakashima S., Yoshikawa T., Kuriu Y., et al. (2008). Intrathoracic esophageal replacement by in situ tissue-engineered esophagus. J. Thorac. Cardiovasc. Surg. 136 850–859. 10.1016/j.jtcvs.2008.05.027 [DOI] [PubMed] [Google Scholar]
- Neves C. R., Buskermolen J., Roffel S., Waaijman T., Thon M., Veerman E., et al. (2019). Human saliva stimulates skin and oral wound healing in vitro. J. Tissue Eng. Regen. Med. 13 1079–1092. 10.1002/term.2865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikoloudaki G., Creber K., Hamilton D. W. (2020). Wound healing and fibrosis: a contrasting role for periostin in skin and the oral mucosa. Am. J. Physiol. Cell Physiol. 318 C1065–C1077. 10.1152/ajpcell.00035.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida K., Yamato M., Hayashida Y., Watanabe K., Yamamoto K., Adachi E., et al. (2004). Corneal reconstruction with tissue-engineered cell sheets composed of autologous oral mucosal epithelium. N. Engl. J. Med. 351 1187–1196. 10.1056/nejmoa040455 [DOI] [PubMed] [Google Scholar]
- Nishiguchi Y., Ohmoto M., Koki J., Enomoto T., Kominami R., Matsumoto I., et al. (2016). Bcl11b/Ctip2 is required for development of lingual papillae in mice. Dev. Biol. 416 98–110. 10.1016/j.ydbio.2016.06.001 [DOI] [PubMed] [Google Scholar]
- Nishino Y., Yamada Y., Ebisawa K., Nakamura S., Okabe K., Umemura E., et al. (2011). Stem cells from human exfoliated deciduous teeth (SHED) enhance wound healing and the possibility of novel cell therapy. Cytotherapy 13 598–605. 10.3109/14653249.2010.542462 [DOI] [PubMed] [Google Scholar]
- Nishiyama K., Akagi T., Iwai S., Akashi M. (2019). Construction of vascularized oral mucosa equivalents using a layer-by-layer cell coating technology. Tissue Eng. Part C Methods 25 262–275. 10.1089/ten.tec.2018.0337 [DOI] [PubMed] [Google Scholar]
- Nowarski R., Jackson R., Flavell R. A. (2017). The stromal intervention: regulation of immunity and inflammation at the epithelial-mesenchymal barrier. Cell 168 362–375. 10.1016/j.cell.2016.11.040 [DOI] [PubMed] [Google Scholar]
- Odland G. F. (1991). “Structure of the skin,” in Physiology, Biochemistry, and Molecular Biology of the Skin, ed. Goldsmith L. A. (Oxford: Oxford University Press; ), 3–62. [Google Scholar]
- Oezcelik A., DeMeester S. R. (2011). General anatomy of the esophagus. Thorac. Surg. Clin. 21 289–297. 10.1016/j.thorsurg.2011.01.003 [DOI] [PubMed] [Google Scholar]
- Ohki T., Yamamoto M. (2020). Esophageal regenerative therapy using cell sheet technology. Regen. Ther. 13 8–17. 10.1016/j.reth.2020.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohki T., Yamato M., Murakami D., Takagi R., Yang J., Namiki H., et al. (2006). Treatment of oesophageal ulcerations using endoscopic transplantation of tissue-engineered autologous oral mucosal epithelial cell sheets in a canine model. Gut 55 1704–1710. 10.1136/gut.2005.088518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohki T., Yamato M., Ota M., Takagi R., Kondo M., Kanai N., et al. (2015). Application of regenerative medical technology using tissue-engineered cell sheets for endoscopic submucosal dissection of esophageal neoplasms. Dig. Endosc. 27 182–188. 10.1111/den.12354 [DOI] [PubMed] [Google Scholar]
- Ohki T., Yamato M., Ota M., Takagi R., Murakami D., Kondo M., et al. (2012). Prevention of esophageal stricture after endoscopic submucosal dissection using tissue-engineered cell sheets. Gastroenterology 143 582–588.e2. 10.1053/j.gastro.2012.04.050 [DOI] [PubMed] [Google Scholar]
- Ohmoto M., Lei W., Yamashita J., Hirota J., Jiang P., Matsumoto I. (2020). SOX2 regulates homeostasis of taste bud cells and lingual epithelial cells in posterior tongue. Edited by Hiroaki Matsunami. PLoS One 15:e0240848. 10.1371/journal.pone.0240848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okubo T., Clark C., Hogan B. L. M. (2009). Cell lineage mapping of taste bud cells and keratinocytes in the mouse tongue and soft palate. Stem Cells 27 442–450. 10.1634/stemcells.2008-0611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliva J., Bardag-Gorce F., Niihara Y. (2020). Clinical trials of limbal stem cell deficiency treated with oral mucosal epithelial cells. Int. J. Mol. Sci. 21:411. 10.3390/ijms21020411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oudhoff M. J., Bolscher J. G. M., Nazmi K., Kalay H., Hof W., Amerongen A. V. N., et al. (2008). Histatins are the major wound-closure stimulating factors in human saliva as identified in a cell culture assay. FASEB J. 22 3805–3812. 10.1096/fj.08-112003 [DOI] [PubMed] [Google Scholar]
- Page M. E., Lombard P., Ng F., Göttgens B., Jensen K. B. (2013). The epidermis comprises autonomous compartments maintained by distinct stem cell populations. Cell Stem Cell 13 471–482. 10.1016/j.stem.2013.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J. E., Barbul A. (2004). Understanding the role of immune regulation in wound healing. Am. J. Surg. 187 S11–S16. 10.1016/S0002-9610(03)00296-4 [DOI] [PubMed] [Google Scholar]
- Park J. Y., Chung H., Dipalma D. T., Tai X., Park J. H. (2018). Immune quiescence in the oral mucosa is maintained by a uniquely large population of highly activated Foxp3+ regulatory T cells article. Mucosal Immunol. 11 1092–1102. 10.1038/s41385-018-0027-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peramo A., Marcelo C. L., Feinberg S. E. (2012). Tissue engineering of lips and muco-cutaneous junctions: in vitro development of tissue engineered constructs of oral mucosa and skin for lip reconstruction. Tissue Eng. Part C Methods 18 273–282. 10.1089/ten.tec.2011.0406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan Q. M., Fine G. M., Salz L., Herrera G. G., Wildman B., Driskell I. M., et al. (2020). Lef1 expression in fibroblasts maintains developmental potential in adult skin to regenerate wounds. Elife 9:e60066. 10.7554/ELIFE.60066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan Q. M., Sinha S., Biernaskie J., Driskell R. R. (2021). Single-cell transcriptomic analysis of small and large wounds reveals the distinct spatial organization of regenerative fibroblasts. Exp. Dermatol. 30 92–101. 10.1111/exd.14244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippeos C., Telerman S. B., Oulès B., Pisco A. O., Shaw T. J., Elgueta R., et al. (2018). Spatial and single-cell transcriptional profiling identifies functionally distinct human dermal fibroblast subpopulations. J. Invest. Dermatol. 138 811–825. 10.1016/j.jid.2018.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piedrafita G., Kostiou V., Wabik A., Colom B., Fernandez-Antoran D., Herms A., et al. (2020). A single-progenitor model as the unifying paradigm of epidermal and esophageal epithelial maintenance in mice. Nat. Commun. 11:1429. 10.1038/s41467-020-15258-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poghosyan T., Gaujoux S., Vanneaux V., Bruneval P., Domet T., Lecourt S., et al. (2013). In vitro development and characterization of a tissue-engineered conduit resembling esophageal wall using human and pig skeletal myoblast, oral epithelial cells, and biologic scaffolds. Tissue Eng. Part A 19 2242–2252. 10.1089/ten.tea.2012.0565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potten C. S. (1974). The epidermal proliferative unit: the possible role of the central basal cell. Cell Prolif. 7 77–88. 10.1111/j.1365-2184.1974.tb00401.x [DOI] [PubMed] [Google Scholar]
- Pringle S., Maimets M., van der Zwaag M., Stokman M. A., van Gosliga D., Zwart E., et al. (2016). Human salivary gland stem cells functionally restore radiation damaged salivary glands. Stem Cells 34 640–652. 10.1002/stem.2278 [DOI] [PubMed] [Google Scholar]
- Qi S., Yang C., Zhu M., Chen H. (2019). Effect of oral mucosal transplantation on the expression of EGF and VEGF−C during skin wound repair. Exp. Ther. Med. 18 320–325. 10.3892/etm.2019.7546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Que J., Okubo T., Goldenring J. R., Nam K. T., Kurotani R., Morrisey E. E., et al. (2007). Multiple dose-dependent roles for Sox2 in the patterning and differentiation of anterior foregut endoderm. Development 134 2521–2531. 10.1242/dev.003855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan R., Hutchison C., Lane B. (1994). Intermediate filament proteins. Protein Profile 1 779–911. [PubMed] [Google Scholar]
- Quiroz F. G., Fiore V. F., Levorse J., Polak L., Wong E., Pasolli H. A., et al. (2020). Liquid-liquid phase separation drives skin barrier formation. Science 367:6483. 10.1126/science.aax9554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren K. (2019). Exosomes in perspective: a potential surrogate for stem cell therapy. Odontology 107 271–284. 10.1007/s10266-018-0395-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds A. J., Jahoda C. A. B. (1991). Inductive properties of hair follicle cells. Ann. N. Y. Acad. Sci. 641 226–241. 10.1111/j.1749-6632.1991.tb24390.x [DOI] [PubMed] [Google Scholar]
- Rinkevich Y., Walmsley G. G., Hu M. S., Maan Z. N., Newman A. M., Drukker M., et al. (2015). Identification and isolation of a dermal lineage with intrinsic fibrogenic potential. Science 348:aaa2151. 10.1126/science.aaa2151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera-Gonzalez G., Shook B., Horsley V. (2014). Adipocytes in skin health and disease. Cold Spring Harb. Perspect. Med. 4:3. 10.1101/cshperspect.a015271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rognoni E., Gomez C., Pisco A. O., Rawlins E. L., Simons B. D., Watt F. M., et al. (2016). Inhibition of β-catenin signalling in dermal fibroblasts enhances hair follicle regeneration during wound healing. Development 143 2522–2535. 10.1242/dev.131797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rognoni E., Pisco A. O., Hiratsuka T., Sipilä K. H., Belmonte J. M., Mobasseri S. A., et al. (2018). Fibroblast state switching orchestrates dermal maturation and wound healing. Mol. Syst. Biol. 14:8. 10.15252/msb.20178174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roh J. L., Lee J., Kim E. H., Shin D. (2017). Plasticity of oral mucosal cell sheets for accelerated and scarless skin wound healing. Oral Oncol. 75 81–88. 10.1016/j.oraloncology.2017.10.024 [DOI] [PubMed] [Google Scholar]
- Rompolas P., Mesa K. R., Kawaguchi K., Park S., Gonzalez D., Brown S., et al. (2016). Spatiotemporal coordination of stem cell commitment during epidermal homeostasis. Science 352 1471–1474. 10.1126/science.aaf7012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roopashree M. R., Gondhalekar R. V., Shashikanth M. C., George J., Thippeswamy S. H., Shukla A. (2010). Pathogenesis of oral lichen planus – a review. J. Oral Pathol. Med. 39 729–734. 10.1111/j.1600-0714.2010.00946.x [DOI] [PubMed] [Google Scholar]
- Rosekrans S. L., Baan B., Muncan V., Van Den Brink G. R. (2015). Esophageal development and epithelial homeostasis. Am. J. Physiol. Gastrointest. Liver Physiol. 309 G216–G228. 10.1152/ajpgi.00088.2015 [DOI] [PubMed] [Google Scholar]
- Roshan A., Murai K., Fowler J., Simons B. D., Nikolaidou-Neokosmidou V., Jones P. H. (2016). Human keratinocytes have two interconvertible modes of proliferation. Nat. Cell Biol. 18 145–156. 10.1038/ncb3282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothova M., Thompson H., Lickert H., Tucker A. S. (2012). Lineage tracing of the endoderm during oral development. Dev. Dyn. 241 1183–1191. 10.1002/dvdy.23804 [DOI] [PubMed] [Google Scholar]
- Rowlatt U. (1979). Intrauterine wound healing in a 20 week human fetus. Virchows Arch. A Pathol. Anat. Histopathol. 381 353–361. 10.1007/BF00432477 [DOI] [PubMed] [Google Scholar]
- Roy E., Neufeld Z., Cerone L., Wong H. Y., Hodgson S., Livet J., et al. (2016). Bimodal behaviour of interfollicular epidermal progenitors regulated by hair follicle position and cycling. EMBO J. 35 2658–2670. 10.15252/embj.201693806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushmer R. F., Buettner K. J., Short J. M., Odland G. F. (1966). The skin. Am. Assoc. Adv. Sci. 154 343–348. 10.1177/002205741508100313 [DOI] [PubMed] [Google Scholar]
- Sa G., Liu Z., Ren J., Wan Q., Xiong X., Yu Z., et al. (2019). Keratinocyte growth factor (KGF) induces podosome formation via integrin-Erk1/2 signaling in human immortalized oral epithelial cells. Cell. Signal. 61 39–47. 10.1016/j.cellsig.2019.05.007 [DOI] [PubMed] [Google Scholar]
- Sada A., Jacob F., Leung E., Wang S., White B. S., Tumbar T. (2016). Defining the cellular lineage hierarchy in the interfollicular epidermis of adult skin. Nat. Cell Biol. 18 619–631. 10.1038/ncb3359.Defining [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai T., Miyazaki S., Miyata G., Satomi S., Hori Y. (2007). Autologous buccal keratinocyte implantation for the prevention of stenosis after emr of the esophagus. Gastrointest. Endosc. 66 167–173. 10.1016/j.gie.2006.12.062 [DOI] [PubMed] [Google Scholar]
- Sánchez-Danés A., Hannezo E., Larsimont J. C., Liagre M., Youssef K. K., Simons B. D., et al. (2016). Defining the clonal dynamics leading to mouse skin tumour initiation. Nature 536 298–303. 10.1038/nature19069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz M., Dahlin C., Apatzidou D., Artzi Z., Bozic D., Calciolari E., et al. (2019). Biomaterials and regenerative technologies used in bone regeneration in the craniomaxillofacial region: consensus report of group 2 of the 15th european workshop on periodontology on bone regeneration. J. Clin. Periodontol. 46(Suppl. 21) 82–91. 10.1111/jcpe.13123 [DOI] [PubMed] [Google Scholar]
- Sasaki K., Fujita Y., Imai Y., Oshima J., Sasaki M., Aihara Y., et al. (2020). Total upper and lower eyelid reconstruction using periosteal flap canthoplasty combined with auricular cartilage and oral mucosa grafts. Eur. J. Plast. Surg. 43 83–86. 10.1007/s00238-019-01556-4 [DOI] [Google Scholar]
- Schafer I. A., Pandy M., Ferguson R., Davis B. R. (1985). Comparative observation of fibroblasts derived from the papillary and reticular dermis of infants and adults: growth kinetics, packing density at confluence and surface morphology. Mech. Ageing Dev. 31 275–293. 10.1016/0047-6374(85)90095-8 [DOI] [PubMed] [Google Scholar]
- Schepeler T., Page M. E., Jensen K. B. (2014). Heterogeneity and plasticity of epidermal stem cells. Development 141 2559–2567. 10.1242/dev.104588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schramm V. L., Myers E. N. (1980). Skin grafts in oral cavity reconstruction. Arch. Otolaryngol. 106 528–532. 10.1001/archotol.1980.00790330008005 [DOI] [PubMed] [Google Scholar]
- Schramm V. L., Johnson J. T., Myers E. N. (1983). Skin grafts and flaps in oral cavity reconstruction. Arch. Otolaryngol. 109 175–177. 10.1001/archotol.1983.00800170041011 [DOI] [PubMed] [Google Scholar]
- Schrementi M. E., Ferreira A. M., Zender C., DiPietro L. A. (2008). Site-specific production of TGF-β in oral mucosal and cutaneous wounds. Wound Repair Regen. 16 80–86. 10.1111/j.1524-475X.2007.00320.x [DOI] [PubMed] [Google Scholar]
- Sebastian P., Thomas S., Varghese B. T., Iype E. M., Balagopal P. G., Mathew P. C. (2008). The submental island flap for reconstruction of intraoral defects in oral cancer patients. Oral Oncol. 44 1014–1018. 10.1016/j.oraloncology.2008.02.013 [DOI] [PubMed] [Google Scholar]
- Seery J. P. (2002). Stem cells of the oesophageal epithelium. J. Cell Sci. 115 1783–1789. [DOI] [PubMed] [Google Scholar]
- Senoo M., Pinto F., Crum C. P., McKeon F. (2007). P63 is essential for the proliferative potential of stem cells in stratified epithelia. Cell 129 523–536. 10.1016/j.cell.2007.02.045 [DOI] [PubMed] [Google Scholar]
- Seo B. M., Miura M., Gronthos S., Bartold P. M., Batouli S., Brahim J., et al. (2004). Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet 364 149–155. 10.1016/S0140-6736(04)16627-0 [DOI] [PubMed] [Google Scholar]
- Sequeira I., Nicolas J. F. (2012). Redefining the structure of the hair follicle by 3D clonal analysis. Development 139 3741–3751. 10.1242/dev.081091 [DOI] [PubMed] [Google Scholar]
- Sequeira I., Neves J. F., Carrero D., Peng Q., Palasz N., Liakath-Ali K., et al. (2018). Immunomodulatory role of keratin 76 in oral and gastric cancer. Nat. Commun. 9:3437. 10.1038/s41467-018-05872-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon D. B., McKeown S. T. W., Lundy F. T., Irwin C. R. (2006). Phenotypic differences between oral and skin fibroblasts in wound contraction and growth factor expression. Wound Repair Regen. 14 172–178. 10.1111/j.1743-6109.2006.00107.x [DOI] [PubMed] [Google Scholar]
- Shaw T. J., Martin P. (2009). Wound repair at a glance. J. Cell Sci. 122 3209–3213. 10.1242/jcs.031187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw T. J., Kishi K., Mori R. (2010). Wound-associated skin fibrosis: mechanisms and treatments based on modulating the inflammatory response. Endocr. Metab. Immune Disord. Drug Targets 10 320–330. 10.2174/1871530311006040320 [DOI] [PubMed] [Google Scholar]
- Shi Q., Huo N., Wang X., Yang S., Wang J., Zhang T. (2020). Exosomes from oral tissue stem cells: biological effects and applications. Cell Biosci. 10:108. 10.1186/s13578-020-00471-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Q., Qian Z., Liu D., Sun J., Wang X., Liu H., et al. (2017). GMSC-derived exosomes combined with a chitosan/silk hydrogel sponge accelerates wound healing in a diabetic rat skin defect model. Front. Physiol. 8:904. 10.3389/fphys.2017.00904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiehzadeh V., Aghmasheh F., Shiehzadeh F., Joulae M., Kosarieh E., Shiehzadeh F. (2014). Healing of large periapical lesions following delivery of dental stem cells with an injectable scaffold: new method and three case reports. Indian J. Dent. Res. 25 248–253. 10.4103/0970-9290.135937 [DOI] [PubMed] [Google Scholar]
- Shyer A. E., Rodrigues A. R., Schroeder G. G., Kassianidou E., Harland R. M. (2018). Initiate follicle pattern in the avian skin. Science 357 811–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simões A., Chen L., Chen Z., Zhao Y., Gao S., Marucha P. T., et al. (2019). Differential microRNA profile underlies the divergent healing responses in skin and oral mucosal wounds. Sci. Rep. 9:7160. 10.1038/s41598-019-43682-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonato A., Gregori A., Ambruosi C., Venzano F., Varca V., Romagnoli A., et al. (2008). Lingual mucosal graft urethroplasty for anterior urethral reconstruction. Eur. Urol. 54 79–87. 10.1016/j.eururo.2008.01.023 [DOI] [PubMed] [Google Scholar]
- Simsek A., Bullock A. J., Roman S., Chapple C. R., MacNeil S. (2018). Developing improved tissue-engineered buccal mucosa grafts for urethral reconstruction. Can. Urol. Assoc. J. 12 E234–E242. 10.5489/cuaj.4826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh M., Nuutila K., Kruse C., Robson M. C., Caterson E., Eriksson E. (2015). Challenging the conventional therapy: emerging skin graft techniques for wound healing. Plast. Reconstr. Surg. 136 524e–530e. 10.1097/PRS.0000000000001634 [DOI] [PubMed] [Google Scholar]
- Singh S., Gupta P. D. (1994). Tampering with cytokeratin expression results in cell dysfunction. Epithelial Cell Biol. 3 79–83. [PubMed] [Google Scholar]
- Sjöqvist S., Ishikawa T., Shimura D., Kasai Y., Imafuku A., Bou-Ghannam S., et al. (2019). Exosomes derived from clinical-grade oral mucosal epithelial cell sheets promote wound healing. J. Extracell. Vesicles 8:1. 10.1080/20013078.2019.1565264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snippert H. J., Haegebarth A., Kasper M., Jaks V., Van Es J. H., Barker N., et al. (2010a). Lgr6 marks stem cells in the hair follicle that generate all cell lineages of the skin. Science 327 1385–1389. 10.1126/science.1184733 [DOI] [PubMed] [Google Scholar]
- Snippert H. J., van der Flier L. G., Sato T., van Es J. H., van den Born M., Kroon-Veenboer C., et al. (2010b). Intestinal crypt homeostasis results from neutral competition between symmetrically dividing Lgr5 stem cells. Cell 143 134–144. 10.1016/j.cell.2010.09.016 [DOI] [PubMed] [Google Scholar]
- Sorrell J. M., Caplan A. I. (2004). Fibroblast heterogeneity: more than skin deep. J. Cell Sci. 117(Pt 5) 667–675. 10.1242/jcs.01005 [DOI] [PubMed] [Google Scholar]
- Sorrell J. M., Baber M. A., Caplan A. I. (1996). Construction of a bilayered dermal equivalent containing human papillary and reticular dermal fibroblasts: use of fluorescent vital dyes. Tissue Eng. 2 39–50. 10.1089/ten.1996.2.39 [DOI] [PubMed] [Google Scholar]
- Sorrell J. M., Carrino D. A., Baber M. A., Assclineau D., Caplan A. I. (1999). A monoclonal antibody which recognizes a glycosaminoglycan epitope in both dermatan sulfate and chondroitin sulfate proteoglycans of human skin. Histochem. J. 31 549–558. 10.1023/A:1003896124595 [DOI] [PubMed] [Google Scholar]
- Squier C. A. (1977). Membrane coating granules in nonkeratinizing oral epithelium. J. Ultrastruct. Res. 60 212–220. 10.1016/s0022-5320(77)80066-x [DOI] [PubMed] [Google Scholar]
- Squier C. A., Kremer M. J. (2001). Biology of oral mucosa and esophagus. J. Natl. Cancer Inst. Monogr. 52242 7–15. 10.1093/oxfordjournals.jncimonographs.a003443 [DOI] [PubMed] [Google Scholar]
- Sriram G., Bigliardi P. L., Bigliardi-Qi M. (2015). Fibroblast heterogeneity and its implications for engineering organotypic skin models in vitro. Eur. J. Cell Biol. 94 483–512. 10.1016/j.ejcb.2015.08.001 [DOI] [PubMed] [Google Scholar]
- Stephens P., Davies K. J., Occleston N., Pleass R. D., Kon C., Daniels J., et al. (2001). Skin and oral fibroblasts exhibit phenotypic differences in extracellular matrix reorganization and matrix metalloproteinase activity. Br. J. Dermatol. 144 229–237. 10.1046/j.1365-2133.2001.04006.x [DOI] [PubMed] [Google Scholar]
- Su Y., Chen C., Guo L., Du J., Li X., Liu Y. (2018). Ecological balance of oral microbiota is required to maintain oral mesenchymal stem cell homeostasis. Stem Cells 36 551–561. 10.1002/stem.2762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surendran S., Sivamurthy G. (2015). Current applications and future prospects of stem cells in dentistry. Dent. Update 42 556–561. 10.12968/denu.2015.42.6.556 [DOI] [PubMed] [Google Scholar]
- Szpaderska A. M., Zuckerman J. D., DiPietro L. A. (2003). Differential injury responses in oral mucosal and cutaneous wounds. J. Dent. Res. 82 621–626. 10.1177/154405910308200810 [DOI] [PubMed] [Google Scholar]
- Tabib T., Morse C., Wang T., Chen W., Lafyatis R. (2018). SFRP2/DPP4 and FMO1/LSP1 define major fibroblast populations in human skin. J. Invest. Dermatol. 138 802–810. 10.1016/j.jid.2017.09.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabola R., Augoff K., Lewandowski A., Ziolkowski P., Szelachowski P., Grabowski K. (2016). Esophageal anastomosis how the granulation phase of wound healing improves the incidence of anastomotic leakage. Oncol. Lett. 12 2038–2044. 10.3892/ol.2016.4873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi R., Murakami D., Kondo M., Ohki T., Sasaki R., Mizutani M., et al. (2010). Fabrication of human oral mucosal epithelial cell sheets for treatment of esophageal ulceration by endoscopic submucosal dissection. Gastrointest. Endosc. 72 1253–1259. 10.1016/j.gie.2010.08.007 [DOI] [PubMed] [Google Scholar]
- Tanaka T., Komai Y., Tokuyama Y., Yanai H., Ohe S., Okazaki K., et al. (2013). Identification of stem cells that maintain and regenerate lingual keratinized epithelial cells. Nat. Cell Biol. 15 511–518. 10.1038/ncb2719 [DOI] [PubMed] [Google Scholar]
- Tang X. H., Scognamiglio T., Gudas L. J. (2013). Basal stem cells contribute to squamous cell carcinomas in the oral cavity. Carcinogenesis 34 1158–1164. 10.1093/carcin/bgt021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarnawski A. S., Ahluwalia A. (2012). Molecular mechanisms of epithelial regeneration and neovascularization during healing of gastric and esophageal ulcers. Curr. Med. Chem. 19 16–27. 10.2174/092986712803414088 [DOI] [PubMed] [Google Scholar]
- Thomson P. (2020). Back to the future: revisiting oral carcinogenesis, stem cells and epithelial cell proliferation. Fac. Dent. J. 11 30–34. 10.1308/rcsfdj.2020.30 [DOI] [Google Scholar]
- Timpl R., Brown J. C. (1996). Supramolecular assembly of basement membranes. Bioessays 18 123–132. [DOI] [PubMed] [Google Scholar]
- Treuting P. M., Dintzis S. M., Liggitt D., Frevert C. W. (eds) (2012). Comparative Anatomy and Histology: A Mouse and Human Atlas (Expert Consult). Cambridge, MA: Academic Press. [Google Scholar]
- Turabelidze A., Guo S., Chung A. Y., Chen L., Dai Y., Marucha P. T., et al. (2014). Intrinsic differences between oral and skin keratinocytes. PLoS One 9:e101480. 10.1371/journal.pone.0101480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ud-Din S., Bayat A. (2020). Keloid scarring or disease: unresolved quasi-neoplastic tendencies in the human skin. Wound Repair Regen. 28 422–426. 10.1111/wrr.12793 [DOI] [PubMed] [Google Scholar]
- Vassar R., Coulombe P. A., Degenstein L., Albers K., Fuchs E. (1991). Mutant keratin expression in transgenic mice causes marked abnormalities resembling a human genetic skin disease. Cell 64 365–380. 10.1016/0092-8674(91)90645-F [DOI] [PubMed] [Google Scholar]
- Velnar T., Bailey T., Smrkolj V. (2009). The wound healing process: an overview of the cellular and molecular mechanisms. Int. J. Med. Res. 37 1528–1542. 10.1177/147323000903700531 [DOI] [PubMed] [Google Scholar]
- Vescarelli E., Pilloni A., Dominici F., Pontecorvi P., Angeloni A., Polimeni A., et al. (2017). Autophagy activation is required for myofibroblast differentiation during healing of oral mucosa. J. Clin. Periodontol. 44 1039–1050. 10.1111/jcpe.12767 [DOI] [PubMed] [Google Scholar]
- Vining K. H., Mooney D. J. (2017). Mechanical forces direct stem cell behaviour in development and regeneration. Nat. Rev. Mol. Cell Biol. 18 728–742. 10.1038/nrm.2017.108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volponi A. A., Kawasaki M., Sharpe P. T. (2013). Adult human gingival epithelial cells as a source for whole-tooth bioengineering. J. Dent. Res. 92 329–334. 10.1177/0022034513481041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vural E., Suen J. Y. (2000). The submental island flap in head and neck reconstruction. Head Neck 22 572–578. [DOI] [PubMed] [Google Scholar]
- Wan L., Jiang D., Correa-Gallegos D., Ramesh P., Zhao J., Ye H., et al. (2021). Connexin43 gap junction drives fascia mobilization and repair of deep skin wounds. Matrix Biol. 97 58–71. 10.1016/j.matbio.2021.01.005 [DOI] [PubMed] [Google Scholar]
- Wang B., Kovalchuk A., Li D., Rodriguez-Juarez R., Ilnytskyy Y., Kovalchuk I., et al. (2020). In search of preventive strategies: novel high-CBD cannabis sativa extracts modulate ACE2 expression in COVID-19 gateway tissues. PrePrints [Preprint] 10.20944/preprints202004.0315.v1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Chen Z., Li X. J., Ma L., Tang Y. L. (2015). Anti-inflammatory cytokine TSG-6 inhibits hypertrophic scar formation in a rabbit ear model. Eur. J. Pharmacol. 751 42–49. 10.1016/j.ejphar.2015.01.040 [DOI] [PubMed] [Google Scholar]
- Wang S., Drummond M. L., Guerrero-Juarez C. F., Tarapore E., MacLean A. L., Stabell A. R., et al. (2020). Single cell transcriptomics of human epidermis identifies basal stem cell transition states. Nat. Commun. 11:4239. 10.1038/s41467-020-18075-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt F. M. (2014). Mammalian skin cell biology: at the interface between laboratory and clinic. Science 346 937–940. 10.1126/science.1253734 [DOI] [PubMed] [Google Scholar]
- Watt F. M., Fujiwara H. (2011). Cell-extracellular matrix interactions in normal and diseased skin. Cold Spring Harb. Perspect. Biol. 3:a005124. 10.1101/cshperspect.a005124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells J. M., Melton D. (1999). Vertebrate endoderm development. Annu. Rev. Cell Dev. Biol. 15 393–410. [DOI] [PubMed] [Google Scholar]
- Wilgus T. A., Ferreira A. M., Oberyszyn T. M., Bergdall V. K., DiPietro L. A. (2008). Regulation of scar formation by vascular endothelial growth factor. Lab. Invest. 88 579–590. 10.1038/labinvest.2008.36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D. W., Greenwell-Wild T., Brenchley L., Dutzan N., Overmiller A., Sawaya A. P., et al. (2021). Human oral mucosa cell atlas reveals a stromal-neutrophil axis regulating tissue immunity. Cell 184 1–15. 10.1016/j.cell.2021.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worthen C. A., Cui Y., Orringer J. S., Johnson T. M., Voorhees J. J., Fisher G. J. (2020). CD26 identifies a subpopulation of fibroblasts that produce the majority of collagen during wound healing in human skin. J. Invest. Dermatol. 140 2515.e–2524.e. 10.1016/j.jid.2020.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright J. T. (2010). Oral manifestations in the epidermolysis bullosa spectrum. Dermatol. Clin. 28 159–164. 10.1016/j.det.2009.10.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao L., Tsutsui T. (2012). Three-dimensional epithelial and mesenchymal cell co-cultures form early tooth epithelium invagination-like structures: expression patterns of relevant molecules. J. Cell. Biochem. 113 1875–1885. 10.1002/jcb.24056 [DOI] [PubMed] [Google Scholar]
- Xiong X., Jia J., He S., Zhao Y. (2010). Cryopreserved lip mucosa tissue derived keratinocytes can fabricate tissue engineered palatal mucosa equivalent. J. Biomed. Mater. Res. Part B Appl. Biomater. 94 165–170. 10.1002/jbm.b.31637 [DOI] [PubMed] [Google Scholar]
- Xue M., Jackson C. J. (2015). Extracellular matrix reorganization during wound healing and its impact on abnormal scarring. Adv. Wound Care 4 119–136. 10.1089/wound.2013.0485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaguchi K., Fujita K., Noguchi M., Nagai F., Yuzuriha S. (2021). The palatal fistula closure using buccal fat graft after palatoplasty for cleft palate: two case reports. Cleft Palate Craniofac. J. 10.1177/10556656211007000 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Yamaguchi N., Isomoto H., Kobayashi S., Kanai N., Kanetaka K., Sakai Y., et al. (2017). Oral epithelial cell sheets engraftment for esophageal strictures after endoscopic submucosal dissection of squamous cell carcinoma and airplane transportation. Sci. Rep. 7:17460. 10.1038/s41598-017-17663-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki Y., Ozawa S., Oguma J., Kazuno A., Ninomiya Y. (2016). Long peptic strictures of the esophagus due to reflux esophagitis: a case report. Surg. Case. Rep. 2:64. 10.1186/s40792-016-0190-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D., Othman M., Draganov P. V. (2019). Endoscopic mucosal resection vs endoscopic submucosal dissection for Barrett’s esophagus and colorectal neoplasia. Clin. Gastroenterol. Hepatol. 17 1019–1028. 10.1016/j.cgh.2018.09.030 [DOI] [PubMed] [Google Scholar]
- Yang H., Adam R. C., Ge Y., Hua Z. L., Fuchs E. (2017). Epithelial-mesenchymal micro-niches govern stem cell lineage choices. Cell 169 483–496.e13. 10.1016/j.cell.2017.03.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshizawa M., Feinberg S. E., Marcelo C. L., Elner V. M. (2004). Ex vivo produced human conjunctiva and oral mucosa equivalents grown in a serum-free culture system. J. Oral Maxillofac. Surg. 62 980–988. 10.1016/j.joms.2004.02.010 [DOI] [PubMed] [Google Scholar]
- Yu G., Okawa H., Okita K., Kamano Y., Wang F., Saeki M., et al. (2016). Gingival fibroblasts as autologous feeders for induced pluripotent stem cells. J. Dent. Res. 95 110–118. 10.1177/0022034515611602 [DOI] [PubMed] [Google Scholar]
- Yuan X., Xu Q., Zhang X., Van Brunt L. A., Ticha P., Helms J. A. (2019). Wnt-responsive stem cell fates in the oral mucosa. IScience 21 84–94. 10.1016/j.isci.2019.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yudintceva N. M., Nashchekina Y. A., Shevtsov M. A., Mikhailova N. A., Vinogradova T. I., Gorelova A. A., et al. (2020). Application of tissue engineering construct seeded with buccal epithelium cells for replacement urethroplasty. Cell Tissue Biol. 14 481–491. 10.1134/S1990519X20060103 [DOI] [Google Scholar]
- Zelles T., Purushotham K. R., Macauley S. P., Oxford G. E., Humphreys-Beher M. G. (1995). Concise review: saliva and growth factors: the fountain of youth resides in us all. J. Dent. Res. 74 1826–1832. 10.1177/00220345950740120301 [DOI] [PubMed] [Google Scholar]
- Zhang C., Zhang Y., Feng Z., Zhang F., Liu Z., Sun X., et al. (2018). Therapeutic effect of dental pulp stem cell transplantation on a rat model of radioactivity-induced esophageal injury. Cell Death Dis. 9 1–13. 10.1038/s41419-018-0753-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Tian Y., Ding T., Femg D. (2021). A preliminary study of constructing tissue engineered oral mucosa material and methods. Research Square [Preprint] 10.21203/rs.3.rs-299660/v1 [DOI] [Google Scholar]
- Zhang Y., Jiang M., Kim E., Lin S., Liu K., Lan X., et al. (2017). Development and stem cells of the esophagus. Semin. Cell Dev. Biol. 66 25–35. 10.1016/j.semcdb.2016.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng G., Huang R., Qiu G., Ge M., Wang J., Shu Q., et al. (2018). Mesenchymal stromal cell-derived extracellular vesicles: regenerative and immunomodulatory effects and potential applications in sepsis. Cell Tissue Res. 374 1–15. 10.1007/s00441-018-2871-5 [DOI] [PubMed] [Google Scholar]
- Zhong M., Lin B., Pathak J. L., Gao H., Young A. J., Wang X., et al. (2020). ACE2 and furin expressions in oral epithelial cells possibly facilitate COVID-19 infection via respiratory and fecal–oral routes. Front. Med. 7:580796. 10.3389/fmed.2020.580796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann D. R., Dours-Zimmermann M. T., Schubert M., Bruckner-Tuderman L. (1994). Versican is expressed in the proliferating zone in the epidermis and in association with the elastic network of the dermis. J. Cell Biol. 124 817–825. 10.1083/jcb.124.5.817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zomer H. D., Trentin A. G. (2018). Skin wound healing in humans and mice: challenges in translational research. J. Dermatol. Sci. 90 3–12. 10.1016/j.jdermsci.2017.12.009 [DOI] [PubMed] [Google Scholar]
- Zomer H. D., Jeremias T. S., Ratner B., Trentin A. G. (2020). Mesenchymal stromal cells from dermal and adipose tissues induce macrophage polarization to a pro-repair phenotype and improve skin wound healing. Cytotherapy 22 247–260. 10.1016/j.jcyt.2020.02.003 [DOI] [PubMed] [Google Scholar]