Abstract
Background
Depression is one of the most disabling disorders worldwide, yet it often remains undetected. One promising approach to address both early detection and disease burden is depression screening followed by direct feedback to patients. Evidence suggests that individuals often seek information regarding mental health on the internet. Thus, internet-based screening with automated feedback has great potential to address individuals with undetected depression.
Objectives
To determine whether automated feedback after internet-based depression screening reduces depression severity as compared to no feedback.
Methods
The internet-based, observer-blinded DISCOVER RCT aims to recruit a total of 1074 individuals. Participants will be screened for depression using the Patient Health Questionnaire (PHQ-9). In case of a positive screening result (PHQ-9 ≥ 10), participants with undetected depression will be randomised into one of three balanced study arms to receive either (a) no feedback (control arm), (b) standard feedback, or (c) tailored feedback on their screening result. The tailored feedback version will be adapted to participants' characteristics, i.e. symptom profile, preferences, and demographic characteristics. The primary hypothesis is that feedback reduces depression severity six months after screening compared to no feedback. The secondary hypothesis is that tailored feedback is more efficacious compared to standard feedback. Further outcomes are depression care, help-seeking behaviour, health-related quality of life, anxiety, somatic symptom severity, intervention acceptance, illness beliefs, adverse events, and a health economic evaluation. Follow-ups will be conducted one month and six months after screening by self-report questionnaires and clinical interviews. According to a statistical analysis plan, the primary outcome will be analysed on an intention-to-treat basis applying multilevel modelling.
Discussion
The results of the DISCOVER RCT will inform about how automated feedback after internet-based screening could improve early detection and resolution of depression. Ways of dissemination and how the trial can contribute to an understanding of help-seeking behaviour processes will be discussed. If the results show that automated feedback after internet-based depression screening can reduce depression severity, the intervention could be easily implemented and might substantially reduce the disease burden of individuals with undetected depression.
Ethical approval
The study is approved by the Ethics Committee of the Hamburg Medical Association.
Trial registration
The trial was registered at ClinicalTrials.gov in November 2020 (identifier: NCT04633096).
Keywords: Depression screening, Early detection, Tailored feedback, Patient engagement, Internet-based intervention, Randomised controlled trial protocol
Highlights
-
•
DISCOVER is an internet-based three-armed randomised controlled trial.
-
•
The efficacy of feedback after internet-based depression screening is unknown.
-
•
The feedback intervention was developed in a multistage process and is open-source.
-
•
DISCOVER tests a novel approach to detect depression early via internet.
1. Background
Major depression is one of the most disabling disorders worldwide and affects one out of ten individuals over their lifetime (Busch et al., 2013; Vos et al., 2020). Untreated depression leads to rising healthcare costs, has an increased likelihood of a chronic course and treatment resistance and, most importantly, results in an increased disease burden (Chisholm et al., 2016; Fichter et al., 2010; Ghio et al., 2014). Nevertheless, depression often remains undetected: in primary care, for example, it is estimated that only 50% of depressed patients are correctly diagnosed as such (Mitchell et al., 2009; Trautmann and Beesdo-Baum, 2017). One promising approach to address early detection of depression is widely accessible depression screening.
Standardised depression screening alone, however, appears to be insufficient to alter disease burden (Gilbody et al., 2008; Thombs et al., 2014). A worthwhile approach to increase the efficacy of depression screening is to enhance patient engagement by feedback provided directly to the individual. In line with self-regulation theories of health behaviour (e.g. Leventhal et al., 2003), feedback allows individuals to recognise that they suffer from depression and motivates individuals to actively engage in functional health behaviour such as help-seeking and depression care. In turn, this should reduce depression severity in the long run. Indeed, the results of our preceding DEPSCREEN-INFO RCT indicate that a feedback intervention - including the screening result as well as recommendations on further diagnostic consultation and help-seeking – can increase patients' engagement in seeking information on depression and, most importantly, reduce depression severity after six months in patients with coronary heart disease (Löwe et al., 2016).
To expand the evidence on feedback after depression screening to the primary care setting, we currently run the multicentre RCT GET.FEEDBACK.GP (Kohlmann et al., 2020). Yet, barriers such as fear of stigmatisation or the desire to handle the problem on one's own often deter professional help-seeking in depression (Boerema et al., 2016; Schomerus and Angermeyer, 2008). Whereas individuals with stigmatised symptoms may be reluctant to present to a health professional, however, the internet has increasingly become a source for individuals with elevated depression severity to actively seek mental health information (Berger et al., 2005). In Germany, for example, one of four individuals would consider seeking help for mental health online (Eichenberg et al., 2013). Conducting the feedback intervention as an internet-based intervention, therefore, appears to have a great potential to reach a large population of affected individuals outside of the medical system.
In other domains such as prevention and intervention of mental disorders, internet-based interventions have already been shown to be effective (e.g. Ebert et al., 2017; Karyotaki et al., 2017; Richards and Richardson, 2012). Additionally, they can bring the benefits of fostering anonymity, of being cost-effective, and of being scalable, thus allowing for large populations to be reached (Andersson, 2016; Andersson and Titov, 2014; Ebert et al., 2017). Notably, the internet-based format also offers the possibility to individually tailor the feedback according to individuals' characteristics (Andersson and Titov, 2014). This is promising, as compared to standard health messages, tailored messages are more frequently read, better remembered and perceived as more relevant (Ryan et al., 2001). Regarding depression, tailored health messages motivate patients to engage in depression care and can help to reduce depression severity (Levesque et al., 2011; Shah et al., 2014). Tailored feedback after depression screening offers the opportunity to match depression-related information to individuals' characteristics with the aim to make it more salient. Accordingly, tailored feedback has the potential to enhance the effect on patient engagement and depression severity compared to standardised feedback.
Here, we describe the three-armed DISCOVER RCT to address early detection and resolution of depression by testing the efficacy of automated feedback after internet-based depression screening, as compared to no feedback. In addition, we will compare the efficacy of a standardised version of the feedback with a version that is tailored to participants' symptom profiles, preferences, and sociodemographic characteristics. The primary outcome will be depression severity six months after internet-based screening. To allow for a comprehensive evaluation, further secondary outcomes and process variables will be examined.
1.1. Trial hypotheses
The primary hypothesis is that depression severity six months after screening is lower in each of the two feedback study arms (STANDARD FEEDBACK and TAILORED FEEDBACK) as compared to the NO FEEDBACK study arm. As we assume that tailored feedback can maximise the efficacy of standardised feedback, the secondary hypothesis is that depression severity six months after screening is lower in the TAILORED FEEDBACK arm as compared to the STANDARD FEEDBACK arm.
2. Methods
2.1. Design
The DISCOVER trial is designed as an internet-based, observer-blinded, randomised controlled clinical trial with three parallel groups, which is conducted nationwide in Germany. After undergoing an online depression screening with the Patient Health Questionnaire-9 (PHQ-9; Kroenke et al., 2001; Löwe et al. (2004a), Löwe et al. (2004b)), participants with suspected depressive disorder (PHQ-9 ≥ 10 points) will be randomised into one of three balanced study arms: (a) NO FEEDBACK, (b) STANDARD FEEDBACK, or (c) TAILORED FEEDBACK on their screening result. Assessments will be conducted online and via telephone and will be scheduled at baseline (before randomisation: T0; 2 days after randomisation: T1), at 1-month (T2), and at 6-months follow-up (T3). The primary objective of the trial is to show superiority of both feedback arms compared to the control arm regarding depression severity 6 months after screening.
The trial (protocol) will be conducted and reported according to adequate CONSORT 2010 extensions and the CONSORT E-HEALTH statement (Boutron et al., 2017; Eysenbach, and Group, 2011; Moher et al., 2010; Montgomery et al., 2018; Schulz et al., 2010), as well as the SPIRIT 2013 statement (Chan et al., 2013).
2.2. Inclusion and exclusion criteria
Eligibility criteria will be assessed within a self-report online survey at T0. Participants will be required to (a) be aged 18 years or above, (b) have sufficient German language proficiency, (c) show an indication for at least moderate depression (PHQ-9 ≥ 10 points), (d) provide contact details, (e) have internet access, (f) have sufficient computer/internet literacy and (f) be willing to give informed consent. Participants will be excluded (a) if they were diagnosed with depression within the past 12 months or (b) if they currently are or were receiving depression treatment within the past 12 months.
2.3. Recruitment and procedure
The trial will be publicly promoted as a study ‘on stress and psychological well-being’. Study participants will be recruited from the general population through traditional and social media campaigns (e.g. advertisement on related websites/newsletters and Google, posts on Facebook, Instagram and Twitter) and through print advertisement in public areas of several German cities (e.g. flyers, posters). To reach a sample that strives for representativeness of the German population with respect to age and gender, a marketing company will further advertise the study via a population wide online access survey panel. Recruitment success and sample characteristics (i.e. age, gender) will be monitored on an ongoing basis and strategies will be adapted, if necessary. Recruitment has started in January 2021 and is planned to run for 12 months.
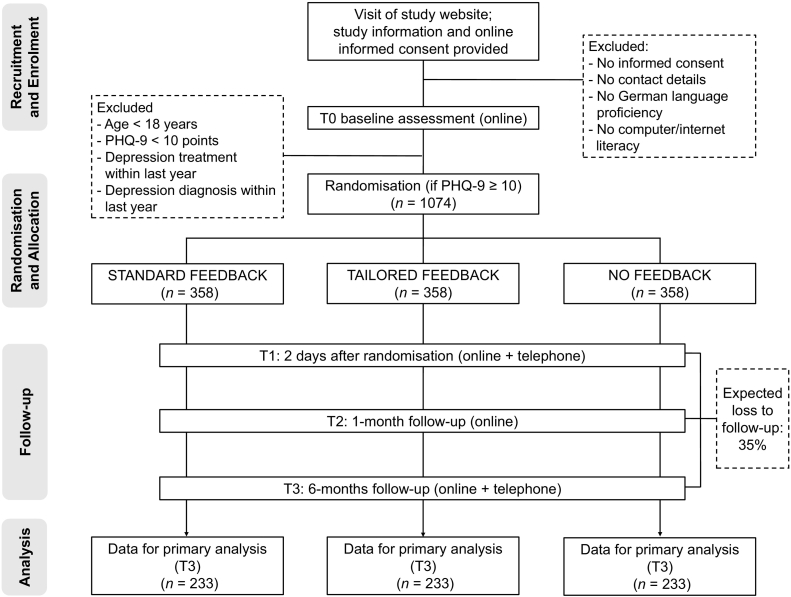
All recruitment ways will lead to the open access study website (https://www.discover-studie.de), which is designed in responsive design to ensure optimal usability for all types of devices (e.g. mobile devices, tablets). The website contains detailed information on the study, data safety procedures, the study team, and contact information. Interested applicants will be asked to provide online informed consent and thereafter to complete the T0 assessment. All participants indicating an elevated suicide risk (PHQ-9 suicide item ≥2 points) will be shown a screen with urgent advice to seek help and relevant information on available help services (e.g. general practitioner, local psychiatric emergency units, and the national emergency number). After having completed the survey, all eligible participants will be randomised and will be directly provided with feedback on their depression screening result (STANDARD and TAILORED FEEDBACK) or a ‘thank you’-note (NO FEEDBACK). They will be contacted and reminded via email on the online follow-up assessments (T1–T3) and via telephone for supplemental clinical interviews (T1 and T3). Whereas the T0 assessment will not be financially rewarded, for each complete follow-up assessment participants immediately receive a compensation of five euro as a voucher (i.e. 3 × five euro vouchers in total). Fig. 1 provides a detailed overview of the study flow.
Fig. 1.
Flow chart of the DISCOVER trial according to the SPIRIT 2010 statement.
Note. PHQ-9 = Patient-Health-Questionnaire-9.
All procedures involved in the study are consistent with generally accepted standards of ethical practice such as the Declaration of Helsinki and have been approved by the Ethics Committee of the Hamburg Medical Association in July 2019 (reference number: PV7039). The trial was registered at ClinicalTrials.gov in November 2020 (identifier: NCT04633096).
2.4. Randomisation and blinding
Randomisation will be based on a computer-generated randomisation sequence (1:1:1 allocation ratio), which was conducted by an independent researcher of the Department of Medical Biometry and Epidemiology and is not accessible to any other study team member. The sequence consists of permuted blocks of randomly arranged sizes (6, 9, and 12) and is stratified by baseline depression severity (moderate: PHQ-9 ≥ 10–14 points; severe: PHQ-9 ≥ 15 points) to guarantee equity of sample sizes across study arms and severity levels. Allocation will be performed by a computerised system, ensuring allocation concealment. Individuals who participate multiple times will be automatically allocated to the same study arm as before. This process is ensured by a privacy-preserving record linkage service which identifies double entries based on personal data and the IP address (Mainzelliste; Rohde et al., 2021).
Participants will know their allocation due to the nature of the intervention but will be kept unaware of trial hypotheses to minimise expectancy bias. The research staff assessing outcomes in the telephone interviews will be blind to the allocation at any time. Steps to control for blindness include the following: after every interview, assessors are (a) instructed to document if participants have disclosed their randomisation status and (b) asked to guess the study arm. After study closure, this guess will be compared with the actual status and Cohen's kappa will be computed to identify whether hit rates differ from what can be expected from chance.
2.5. Sample size
Based on the results of the preceding DEPSCREEN-INFO trial (Löwe et al., 2016), the study is powered to detect a small mean difference (Cohen's f = 0.118) in the primary outcome (depression severity) in any pairwise comparison between all three study arms. The calculation is based on a global one-way ANCOVA adjusted for baseline depression severity, with an alpha of 0.05 (two-sided) and a power of 80%. It results in a needed sample size of n = 233 participants per group (PASS, 2008). To allow for an estimated drop out of 35% (c.f. Christensen et al., 2009), 358 participants per group will be recruited (1074 in total).
2.6. Study arms
After completing the PHQ-9 depression screening questionnaire at T0, all eligible participants who score 10 points or higher will be directly randomised into one of the three study arms. Independent of the study arm, all participants will be provided with a ‘thank you’-note and information on further follow-up procedures.
2.6.1. No feedback
This study arm serves as a passive control condition. The participants will not get any feedback on their screening result.
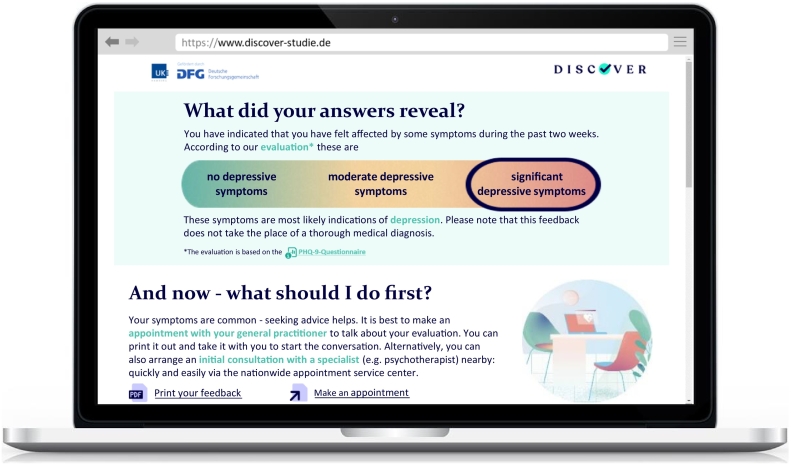
2.6.2. Standard feedback
Participants in this study arm will receive standardised feedback comprising the following four sections: (a) the depression screening result, (b) a note to seek diagnostic consultation by a health professional, (c) brief general information on depression, and (d) information on depression treatment (based on the German National Clinical Practice Guideline for Unipolar Depression; DGPPN et al., 2015). In line with the Common-Sense Model of Self-Regulation (Leventhal et al. (2003), Leventhal et al. (2016)), the feedback content is designed to trigger adaptive illness beliefs such as an adequate illness identity, a coherent understanding of the condition, and optimistic control expectations. These, in turn, should guide patient engagement in functional health behaviour such as help-seeking and depression care.
The feedback intervention was developed in a multistage process. First, the underlying feedback version used in the preceding DEPSCREEN-INFO trial was subjected to re-evaluation and updating in several focus groups, involving patient representatives with depressive disorder (Seeralan et al., 2020). Based on the results of this qualitative study, needs and preferences of the target group could be assessed and implemented, resulting in the feedback version used in the currently running GET.FEEDBACK.GP trial (Kohlmann et al., 2020; see Supplemental Fig. I). For the use in DISCOVER, a digital art/graphic agency (Wood Agency, Hamburg) further adapted the feedback material to the possibilities of internet-based presentation. Namely, the present version is extended by (animated) graphic elements, adaptively available further information on specific contents, direct links to referenced health or social services (e.g. online therapies, self-help groups), and the possibility to download the feedback form as a pdf-file that includes the active links from the website. Throughout the process, the selection of content, design, and language was aligned to the current evidence on patients' needs in technology-based mental health interventions (e.g. Bakker et al., 2016; Hadjistavropoulos et al., 2018; Rozbroj et al., 2014; Torous et al., 2018).
Fig. 2 depicts an excerpt of the feedback screen as displayed in the desktop version (see Supplemental Fig. II, for the complete version). For smaller devices such as tablets and smartphones, the content is displayed in responsive design (i.e. the design automatically adapts to the size and type of the output device).
Fig. 2.
Standard feedback: First screen as displayed on the DISCOVER study website (English translation).
2.6.3. Tailored feedback
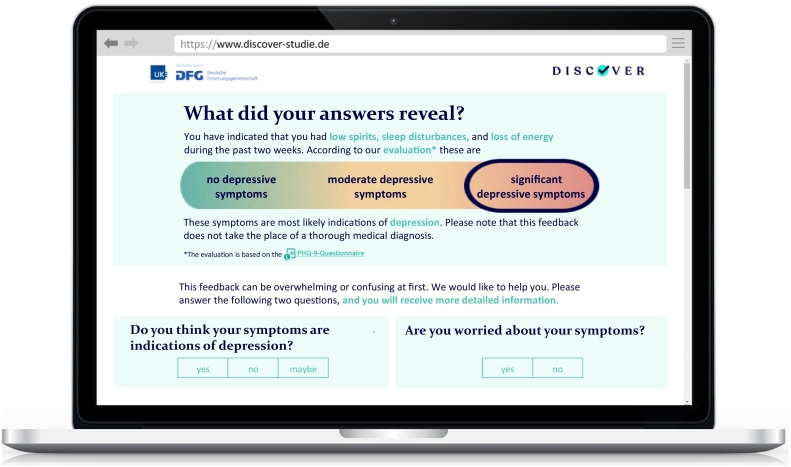
In order to trigger more salience, the content of the STANDARD FEEDBACK version is tailored to participants' characteristics as follows: First, the presentation of the screening result is framed according to participants' individual symptom profiles (e.g., ‘You have indicated that you had low spirits, sleep disturbances, and loss of energy during the past two weeks.’, see Fig. 3). Second, the note to seek further diagnostic consultation is matched to participants' specialist preferences (general practitioner vs. mental health professional). Third, the information on depression is tailored to participants' symptom profiles (e.g. ‘Typical symptoms of depression are for example low spirits and sleep disturbances.’) and their symptom causal attributions (e.g. ‘Triggers are for example stress with the partner, negative thinking patterns, or a physical illness.’). Lastly, treatment options and help seeking advices are adapted to participants' health insurance providers and local residency in Germany (e.g. by providing links to self-help groups located nearby or to online therapies which are covered by the participant's health insurance provider).
Fig. 3.
Tailored feedback: First screen as displayed on the DISCOVER study website (English translation).
Additionally, directly after being provided with the screening result, participants are asked the following two questions: ‘Do you think your symptoms are indications of depression?’ and ‘Do you worry about your symptoms?’ (see Fig. 3). According to participants' answers, the following three feedback sections are arranged in a differing order. If participants indicate assigning their symptoms to depression and worrying about them, the information on depression treatment is prefixed to the general information on depression, resulting in the following order: (b) note to seek diagnostic consultation, (c) information on depression treatment, (d) information on depression. If participants do not think that their symptoms relate to depression and/or do not worry about them, the information on depression is prefixed to the other sections, leading to the following order: (b) information on depression, (c) note to seek diagnostic consultation, (d) treatment information. Further, dependent on the combination of answers, the information on depression and the note to seek diagnostic consultation are phrased differently and are extended by information on depression prevalence and negative consequences of depression, both tailored to participants' risk profile (e.g. ‘Depression is common, and particularly people with diabetes are often affected.’, and ‘In the long term, depressive symptoms have negative consequences – for example they can worsen the course of diabetes.’). Examples for the resulting feedback versions for all combinations of answers can be found in Supplemental Fig. III.
2.7. Outcomes
The primary outcome of the study will be self-reported depression severity (Patient Health Questionnaire-9) 6 months after screening. Secondary outcomes are guideline-based depression care (i.e. proportion of individuals treated according to the German depression guideline), depression-related help-seeking behaviour (i.e. proportion of individuals seeking formal/informal help), health-related quality of life, anxiety severity, somatic symptom severity, and adverse events, all at 6 months after screening, as well as depression severity and intervention acceptance, both at 1 month after screening. Further, 6 months after screening a health economic evaluation will be conducted based on direct costs (healthcare utilisation), indirect costs (productivity loss), and health-related quality of life. Corresponding measures are described in Section 2.8.
2.8. Data collection and measures
Data collection will be scheduled at baseline (before randomisation: T0; 2 days after randomisation: T1), and at 1-month (T2) and 6-months follow-up (T3). Assessments will comprise online self-report questionnaires (T0–T3) as well as clinical telephone interviews (T1 and T3 only). The baseline assessment is split into T0 and T1 two days later for two reasons: (a) to reduce potential recall effects from the PHQ-9 assessment at T0 to subsequent clinical interviews, and (b) to minimise participant burden and promote survey completion at T0. The latter is justified by the fact that only retrospective measures that are unlikely to be immediately influenced by the intervention (e.g. healthcare utilisation in the past 6 months) are assessed subsequently. To promote retention, email invitations to the online surveys will include information highlighting the importance of follow-up assessments and email reminders will be sent to participants at regular intervals if their surveys stay incomplete (up to 5, 7, and 10 reminders at T1, T2 and T3, respectively). All procedures will be managed computerised.
All measures will be entered into electronic data capture systems. The system for self-report data is implemented in the study website and shows one questionnaire (desktop version) or one question (smartphone version) per screen. It checks for completeness of questionnaires before submitting, allows participants to change their answers, and uses adaptive questioning to reduce the complexity of questionnaires, if applicable. In order to potentially identify invalid entries, all online surveys will comprise the following two questions as validity checks: (a) ‘Have you answered the questions for yourself?’ and (b) ‘Have you answered the questions seriously?’
Table 1 shows an overview of all measures and corresponding assessment time points.
Table 1.
Measures and assessment time points.
| Measures | T0 | T1 | T2 | T3 |
|---|---|---|---|---|
| Primary outcome | ||||
| Depression severity, PHQ-9 | x | x | xa | |
| Secondary outcomes/process measures | ||||
| Guideline-based depression care (e.g. depression diagnosis, psychotherapy, medication) | x | |||
| Depression-related help-seeking behaviour (e.g. seeking information about depression) | x | |||
| Anxiety severity, GAD-7 | x | x | ||
| Somatic symptom severity, SSS-8 | x | x | ||
| Health-related quality of life, EQ-5D-5L | x | x | ||
| Healthcare utilisation and productivity loss, CSSRI | x | x | ||
| Intervention acceptance, USE | x | x | ||
| Illness beliefs, Brief IPQ | x | x | x | |
| Intervention adherence | x | xb | ||
| Critical life events | xb | |||
| Depression diagnosis, SCID | xb | xb | ||
| Adverse events | xb | |||
| Website use | x | x | x | x |
| Characteristics | ||||
| Sociodemographic data | x | |||
| Medical data | x | |||
| Risk factors for depression onset | x |
Note. T0 = before randomisation; T1 = 2 days after randomisation; T2 = 1-month follow-up, T3 = 6-months follow-up; PHQ-9 = Patient Health Questionnaire-9; CSSRI = Client Sociodemographic and Service Receipt Inventory; EQ-5D-5L = EuroQol-5D 5-L; GAD-7 = Generalized Anxiety Disorder-7; SSS-8 = Somatic Symptom Scale-8; SCID = Structured Clinical Interview for DSM-5 Disorders; USE = Usefulness Scale for Patient Information Material; Brief IPQ = Brief Illness Perception Questionnaire.
Primary outcome.
Measures assessed via telephone interview.
2.8.1. Depression severity
Depression severity will be assessed by the German version of the Patient Health Questionnaire-9 (PHQ-9; Kroenke et al., 2001; Löwe et al. (2004a), Löwe et al. (2004b)). The PHQ-9 consists of 9 items covering all major depression symptom criteria as stated in the DSM-5. Each item refers to the past two weeks and is scored on a 4-point Likert scale (0–3), resulting in a total score ranging from 0 to 27. The PHQ-9 is among the most frequently used and best validated self-report depression questionnaires: it has good psychometric properties, is sensitive to change and responsive to treatment (Kroenke et al., 2001; Löwe et al., 2004b). When delivered online, it has shown to have a good inter-format reliability to the paper version (Erbe et al., 2016). With regard to depression screening, the PHQ-9 (cut-off of 10 points) is recommended as the most suitable instrument compared with others in a recent meta-analysis (Miller et al., 2021), showing high sensitivity (0.88) and specificity (0.85; Levis et al., 2019). Further, the PHQ-9 is recommended for depression screening also by national clinical expert associations such as the US Preventive Services Task Force (Siu et al., 2016) and the German National Clinical Practice Guideline for Unipolar Depression (DGPPN et al., 2015).
2.8.2. Guideline-based depression care and depression-related help-seeking behaviour
In absence of a standardised measure for evaluating depression-related health behaviour and depression care according to the German national guideline, these will be assessed via a self-developed questionnaire. The questionnaire comprises guideline-based depression care (e.g. depression diagnosis by a health professional, psychotherapy, medication), formal help-seeking (e.g. contacting any health professional), and informal help-seeking (e.g. seeking information, doing exercise), as well as the perceived helpfulness, respectively. Items are developed based on recommendations of the German National Clinical Practice Guideline for Unipolar Depression (DGPPN et al., 2015) and extended by questions in an open format. For formal help-seeking and depression care, the time point (in months after the intervention) and specific characteristics (e.g. type of professional contacted) will be assessed. In a similar version, these questions have been successfully tested in the preceding DEPSCREEN-INFO trial (Löwe et al., 2016).
2.8.3. Anxiety severity
Anxiety severity during the past two weeks will be assessed with the 7-item Generalized Anxiety Disorder Scale (GAD-7; Spitzer et al., 2006), which is widely used for this purpose and well validated in its German version (Löwe et al., 2008).
2.8.4. Somatic symptom severity
The Somatic Symptom Scale-8 (SSS-8; Gierk et al., 2014) will be used to assess somatic symptom severity. The questionnaire consists of 8 items that reflect common somatic symptoms in primary care and refer to the past two weeks. It has good psychometric properties and is sensitive to change (Gierk et al., 2017).
2.8.5. Health-related quality of life
The widely used 5-level version of the EuroQol-5D (EQ-5D-5L; Herdman et al., 2011) will be used to assess health-related quality of life. The generic questionnaire comprises 5 items relating to the following dimensions: mobility, self-care, usual activities, pain/discomfort, and anxiety/depression. Furthermore, a visual analogue scale records overall perceived health status. The instrument is widely used and responsive to treatment (Sobocki et al., 2007). Preference-based utilities derived from the EQ-5D-5L (Ludwig et al., 2018) will be used to calculate quality-adjusted life-years (QALYs) for the health economic evaluation. This approach is evaluated suitable for this purpose in the field of depression (Lamers et al., 2006; Sapin et al., 2004).
2.8.6. Healthcare utilisation and productivity loss
Healthcare utilisation and productivity loss will be assessed with an adapted version of the Client Sociodemographic and Service Receipt Inventory (CSSRI; Chisholm et al., 2000). It registers the use of healthcare services (e.g. hospital stays, health professional contacts), medication (e.g. type of drug, dosage level), and work loss days (e.g. hospital days, absenteeism) during the past 6 months.
2.8.7. Depression diagnosis
To validate the suspected diagnosis of depression indicated by the PHQ-9 depression screening, the depression related modules of the Structured Clinical Interview for DSM-5 Disorders (SCID-5-CV; Beesdo-Baum et al., 2019) will be conducted. The SCID enables a reliable, valid and efficient assessment of depressive disorders according to DSM-5 criteria. Interviews will be conducted via telephone, which has demonstrated high inter-rater reliability when compared to face-to-face interviews (Crippa et al., 2008). To ensure validity and reliability, the assessors (BSc or MSc Psychology) will undergo a standardised training and will be supervised by an experienced psychotherapist (PhD).
2.8.8. Intervention acceptance
The Usefulness Scale for Patient Information Material (Holzel et al., 2015) will be used to assess the acceptance of the feedback intervention. The original instrument consists of 9 items assessing cognitive, emotional and behavioural aspects of usefulness and has excellent psychometric properties. For the present study, one item was added to assess whether the feedback information appeared trustworthy. To assess the acceptance of depression screening, directly after filling in the PHQ-9 the following dichotomous items will be added: ‘Answering these questions… (a) bothered/did not bother me, (b) was easy/complicated, (c) was too/was not too time-consuming’, (d) ‘Answering these questions on the internet is a problem/no problem’, (e) ‘Answering these questions at a general practitioner would be a problem/no problem’, and (f) ‘In a similar life situation, would you answer these questions again on the internet?’. Moreover, in the telephone interviews (T3) participants will be asked two open questions regarding the perceived helpfulness of the feedback (‘Did you find the feedback helpful (why/why not)?’) and the perceived helpfulness of internet-based depression screening (‘Do you think an internet-based questionnaire such as the one used in the DISCOVER study is helpful to improve early detection of psychological distress (why/why not?)?’).
2.8.9. Illness beliefs
Illness beliefs regarding depressive symptoms will be measured with a modified version of the well validated Brief Illness Perception questionnaire (Brief IPQ, Broadbent et al., 2006). The Brief IPQ is based on the Common-Sense Model of Self-Regulation (Leventhal et al., 2016) and covers causal, cognitive and emotional representations of an illness (identity, coherence, causes, consequences, timeline, personal and treatment control, and worry). As the target population of non-diagnosed individuals might not associate their symptoms with an ‘illness’, this term will be replaced with ‘symptoms’ throughout the questionnaire. The item assessing illness identity will be replaced by the dichotomous questions ‘Can you imagine suffering from depression?’ (T1 and T3) and ‘Can you imagine having suffered from depression within the last six months?’ (T3 only) as well as the open question ‘In your own words – how would you describe your mental health in the last six months? Do you think you suffered from depression?’ (T3 telephone interview). Further, the open question for the causal representations will be complemented by a listing of potential causes of depressive symptoms adopted from the Beliefs about Depression Questionnaire (Lynch et al., 2011).
2.8.10. Adverse events
To estimate possible unintended adverse events of the feedback intervention, at T3 participants will be asked about the occurrence of any negative event that is attributed to the trial with an open question.
2.8.11. Critical life events
Three open questions assessing relevant positive and negative critical life events will be asked at T3: ‘Within the last six months, … (a) Did you experience life events that positively influenced your mood?, (b) Did you experience life events that negatively influenced your mood?, (c) and What has been particularly helpful to you in times when you have been feeling bad?’.
2.8.12. Intervention adherence
Intervention adherence will be assessed by the item ‘Please indicate to what extent you have read the feedback with the corresponding information.’ and the following response options: ‘100%’, ‘90%’, ‘75%’, ‘50%’, ‘25%’, ‘10%’, and ‘0%’. To complement this self-report data, the system will also track technical data on feedback use (e.g. time spent on the screen, documents downloaded).
2.8.13. Website use
In order to obtain additional measures for acceptability and usability of the applications as well as to monitor and potentially improve processes during the trial (e.g. recruitment success, problems with usability), technical data on website (including questionnaire) use will be recorded by the system (e.g. hits per page, usage time).
2.8.14. Characteristics
Participant characteristics recorded at T0 will include sociodemographic data (e.g. age, gender, education, family status, rural/urban area living, local residency, health insurance provider), risk factors for depression onset (e.g. chronic somatic comorbidities, pregnancy, alcohol and nicotine consumption), and medical data (diagnosis of and treatments for depression).
2.9. Data storage and management
To ensure participants' data safety, study data and personal data will be stored in separate data bases. Security of data transmission from data capture software to data bases is guaranteed by a TLS-encrypted connection. For the duration of the study, a University-hosted pseudomisation service (Mainzelliste; Lablans et al., 2015) will enable the temporary connection of personal with study data, which is necessary for the follow-up assessments. Compliance of these procedures with the security requirements enforced by the European General Data Protection Regulation as well as German law is ensured. Constant monitoring and backups of data as well as password-restricted access will be ensured by an external IT company (Timo Stolz, Berlin).
In accordance with the German Research Foundation guidelines for the handling of research data, the de-identified data will be saved for at least 10 years (i.e. analysable data set, protocol, statistical analysis plan and statistical programming code). Data sharing will follow the FAIR Data Principles (Findable, Accessible, Interoperable and Reusable) to maximise transparency and scientific reproducibility. The data management plan will (a) ensure long-term accessibility, (b) deliver a comprehensive, reliable view of data and (c) provide a future-proof solution for international healthcare interoperability.
2.10. Data analysis
Data analysis will be conducted by an independent statistician from the Department of Medical Biometry and Epidemiology who will be blind to the research hypotheses. All pre-specified analyses will be conducted according to the intention-to-treat (ITT) principle, i.e. including all participants randomised. In addition to the following description, planned analyses will be specified in accordance with the current statistical recommendations of the European Medicine Agency in a statistical analysis plan that will be signed by the principal investigator and the responsible statistician before breaking the blinding.
A multilevel model incorporating the participants as random terms will be applied to the repeated measures in the same participant, including the factor group and the baseline value for adjustment. The primary analysis will be performed within the framework of this model as an ANCOVA of the PHQ-9 change scores (T0 to T3-difference), with subsequent pairwise comparisons of interventions by test of the corresponding contrasts. Each test will be performed at a two-sided level of alpha = 0.05. This closed testing principle will ensure a family-wise error level of 5%. The multilevel modelling approach limits the bias when handling missing data even in the case of not missing at random (NMAR). However, alternative missing data mechanisms will be applied as a sensitivity check to examine the stability of the results. No subgroup analyses are pre-specified.
For the health-economic evaluation, the cost-effectiveness of the feedback interventions compared to no feedback will be determined. For this, incremental cost-effectiveness ratios (ICER) will be calculated as the difference in mean costs divided by the difference in mean QALYs between each of the two intervention groups and the control group. Net benefit regressions will be conducted to determine the uncertainty of the point estimates and to adjust for potential baseline differences and confounders (Briggs et al., 2002). To show the intervention's probability of being cost-effective at different willingness-to-pay margins in comparison to each of the two comparators, cost-effectiveness acceptability curves will be derived.
3. Discussion
The high prevalence of undetected major depression underscores the relevance of new approaches that ideally target both its early detection and resolution. With the DISCOVER RCT, we address this by testing the efficacy of automated feedback after internet-based depression screening.
The primary outcome of the trial will be depression severity six months after screening. Based on the results of our preceding trial (Löwe et al., 2016), we expect the feedback intervention to have a small effect on depression severity. Further, we expect the tailored feedback version to amplify the effect of the standard version to a small extent. Although being small in magnitude, this effect size is clinically relevant as it intents to address a so far un-diagnosed population that, until now, falls outside the scope of any form of depression care. Therefore, we believe that the small effect at the individual level leads to a substantial effect at the larger population level.
Whereas our preceding RCTs DEPSCREEN-INFO and GET.FEEDBACK.GP investigate(d) feedback after depression screening in patients with coronary heart disease (Löwe et al., 2016) and in primary care (Kohlmann et al., 2020), the internet-based format of DISCOVER allows for a wider reach and may also attract people who are reluctant to seek traditional health services, but use the internet for mental health information (c.f. Berger et al., 2005). Addressing this large population of affected individuals outside of the medical system, the results of DISCOVER will expand on those of our preceding trials.
Furthermore, the DISCOVER RCT will allow for a deeper understanding of the early detection and resolution processes. So far, it is unclear how exactly informing patients about their screening result translates into improved depression severity (Löwe et al., 2016). Also, there appears to be a lack of knowledge on how to get undetected individuals into treatment. The comprehensive examination of process variables such as illness beliefs and depression-related help-seeking behaviour could be a contribution in this regard. Depending on the ultimately reached recruitment rate and the resulting power, also process focussed analyses could be conducted. Results regarding the underlying processes of feedback after depression screening could improve the refinement and development of further feedback as well as other interventions targeting patient engagement in early depression detection.
With regard to practical implication, the brevity of the feedback intervention makes it suitable, when further validated, for widespread implementation in different contexts: potential modes of dissemination could target for example mental health-related websites (e.g. of health insurances, doctors' practices), but also social media (e.g. forums on mental health topics) or websites of community institutions with a high reach (e.g. universities). Taking into account these aspects, the internet-based feedback intervention could be a worthwhile contribution to improving early detection and resolution of depression.
3.1. Strengths and limitations
Testing the feedback intervention in an internet-based trial involves possible limitations, which we try to overcome using the following approaches. First, the trial relies on self-selection of participants, and internet-savvy individuals and/or those interested in mental health might be overrepresented. To minimise this potential bias, we will monitor sample characteristics during recruitment and will adapt strategies appropriately (e.g. by targeted advertisement and by involving a population wide survey panel). Second, drop-out in internet-based interventions can be moderately to high (Melville et al., 2010), which can lead to reduced power of analyses. We will approach this problem in different ways. To promote retention, the importance of follow-ups will be highlighted in all study instructions and participants will receive automated email reminders. Furthermore, to handle inevitable drop-out, we anticipated a drop-out rate of 35% in the sample size calculation and will further analyse data on an ITT basis using adequate mechanisms for handling missing data. Third, part of the intervention effect might be due to the feedback intervention increasing individuals' awareness of their symptoms. It cannot be ruled out that the questionnaires and interviews at baseline might trigger a similar process. Due to randomisation this effect should occur in all three study arms. However, as it could be confounded with the intervention effect, this might lead to the resulting efficacy being underestimated as compared to real-life conditions. Lastly, some researchers argue that depression screening by self-report questionnaires might pose the risk of over-diagnosis of depression, which again might lead to over-treatment (Thombs et al., 2014). To account for this, we investigate possible over-treatment due to our intervention by verifying suspected depression diagnosis with a gold standard clinical interview (SCID) and by recording participants' healthcare use six months after the intervention.
Several strengths of the DISCOVER trial should be highlighted as well. First, the feedback intervention is a result of an elaborated multistage development process, which combined strengths and perspectives of different domains: clinical, research, and IT/graphic design expertise, empirical evidence, and first-hand patients' needs and preferences (Seeralan et al., 2020). Second, the selection of a broad range of further outcomes (depression care and help-seeking behaviour, additional clinical outcomes, intervention acceptance, illness beliefs, adverse events, and the health-economic evaluation) allows for a comprehensive trial evaluation. Third, DISCOVER extensively exploits the potential of technology-based trial design - for example, by (a) automated randomisation and allocation to ensure standardisation of trial conduction, (b) interactively tailoring the feedback intervention to participant characteristics to increase its suitability, (c) automated management of assessments and reminders to improve retention, (d) impeding double or ‘fake’ entries by several security checks, and (e) well-designed online questionnaire administration (e.g. adaptive questioning) to minimise participant burden. Lastly, the assessment of technical data on participants' website and questionnaire use allows for a thorough investigation of user behaviour, which could contribute to the evidence on optimal clinical trial design of internet-based interventions in the future.
3.2. Conclusion
Taken together, the DISCOVER RCT is well designed to yield comprehensive information on how automated feedback after internet-based screening could improve early detection and resolution of depression. If the results show that automated feedback after internet-based depression screening can reduce depression severity, the intervention could be easily and widely disseminated. The trial could further contribute to an understanding of the help-seeking behaviour processes initiated after internet-based depression screening with automated feedback, which could inform further research and practical implementation. Therefore, the results of the DISCOVER RCT will show whether, and if so, how automated feedback after internet-based screening can improve the early detection and resolution of undetected depression.
The following are the supplementary data related to this article.
GFGP feedback.
Standard feedback.
Tailored feedback final.
CRediT authorship contribution statement
FS wrote the first draft of this manuscript under supervision of SK. All authors revised the draft critically for important intellectual content and contributed substantially to the conception of the study. The applicants of the DISCOVER trial are SK (principal investigator), BL, KW & H-HK. KW and AZ are the trial statisticians and contributed to the analysis aspect of the protocol. All authors gave approval of the version published.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
The authors would like to thank Marieke Volkmann and Nele Schade for extensively pilot-testing the website and the data entry systems, as well Marieke Volkmann for providing help with creating the figures for the manuscript.
Funding
This work was funded by the German Research Foundation (grant number: 424162019).
Compliance with ethical standards
The trial was approved by the Ethics Committee of the Medical Chamber Hamburg in July 2019 (reference number: PV7039).
Contributor Information
Franziska Sikorski, Email: f.sikorski@uke.de.
Sebastian Kohlmann, Email: s.kohlmann@uke.de.
References
- Andersson G. Internet-delivered psychological treatments. Annu. Rev. Clin. Psychol. 2016;12:157–179. doi: 10.1146/annurev-clinpsy-021815-093006. [DOI] [PubMed] [Google Scholar]
- Andersson G., Titov N. Advantages and limitations of internet-based interventions for common mental disorders. World Psychiatry. 2014;13(1):4–11. doi: 10.1002/wps.20083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker D., Kazantzis N., Rickwood D., Rickard N. Mental health smartphone apps: review and evidence-based recommendations for future developments. JMIR Ment. Health. 2016;3(1) doi: 10.2196/mental.4984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beesdo-Baum K., Zaudig M., Wittchen H.U., Williams Janet B.W., Karg Rhonda S., Spitzer Robert L. In: SCID-5-CV Strukturiertes Klinisches Interview für DSM-5-Störungen–Klinische Version: Deutsche Bearbeitung des Structured Clinical Interview for DSM-5 Disorders–Clinician Version. Von Michael B. First., editor. Hogrefe; 2019. [Google Scholar]
- Berger M., Wagner T.H., Baker L.C. Internet use and stigmatized illness. Soc. Sci. Med. 2005;61(8):1821–1827. doi: 10.1016/j.socscimed.2005.03.025. [DOI] [PubMed] [Google Scholar]
- Boerema A.M., Kleiboer A., Beekman A.T., van Zoonen K., Dijkshoorn H., Cuijpers P. Determinants of help-seeking behavior in depression: a cross-sectional study. BMC Psychiatry. 2016;16(1):78. doi: 10.1186/s12888-016-0790-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutron I., Altman D.G., Moher D., Schulz K.F., Ravaud P. CONSORT statement for randomized trials of nonpharmacologic treatments: a 2017 update and a CONSORT extension for nonpharmacologic trial abstracts. Ann. Intern. Med. 2017;167(1):40–47. doi: 10.7326/m17-0046. [DOI] [PubMed] [Google Scholar]
- Briggs Andrew, O’´Brien Bernie, Blackhouse Gordon. Thinking outside the box: recent advances in the analysis and presentation of uncertainty in cost-effectiveness studies. Annual Review of Public Health. 2002;23:377–401. doi: 10.1146/annurev.publhealth.23.100901.140534. [DOI] [PubMed] [Google Scholar]
- Broadbent E., Petrie K.J., Main J., Weinman J. The brief illness perception questionnaire. J. Psychosom. Res. 2006;60(6):631–637. doi: 10.1016/j.jpsychores.2005.10.020. [DOI] [PubMed] [Google Scholar]
- Busch M., Maske U., Ryl L., Schlack R., Hapke U. Prevalence of depressive symptoms and diagnosed depression among adults in Germany - results of the german health interview and examination survey for adults (DEGS1) Epidemiol. Gesundheitsberichterstattung. 2013;56(5):733–739. doi: 10.1007/s00103-013-1688-3. [DOI] [PubMed] [Google Scholar]
- Chan A.W., Tetzlaff J.M., Altman D.G., Laupacis A., Gotzsche P.C., Krleza-Jeric K., Moher D. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann. Intern. Med. 2013;158(3):200–207. doi: 10.7326/0003-4819-158-3-201302050-00583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisholm D., Knapp M.R.J., Knudsen H.C., Amaddeo F., Gaite L., Van Wijngaarden B. Client socio-demographic and service receipt inventory-european version: development of an instrument for international research. Br. J. Psychiatry Suppl. 2000;177(39):s28–s33. doi: 10.1192/bjp.177.39.s28. [DOI] [PubMed] [Google Scholar]
- Chisholm D., Sweeny K., Sheehan P., Rasmussen B., Smit F., Cuijpers P., Saxena S. Scaling-up treatment of depression and anxiety: a global return on investment analysis. Lancet Psychiatry. 2016;3(5):415–424. doi: 10.1016/S2215-0366(16)30024-4. [DOI] [PubMed] [Google Scholar]
- Christensen H., Griffiths K.M., Farrer L. Adherence in internet interventions for anxiety and depression: systematic review. J. Med. Internet Res. 2009;11(2) doi: 10.2196/jmir.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crippa J.A., de Lima Osório F., Del-Ben C.M., Filho A.S., da Silva Freitas M.C., Loureiro S.R. Comparability between telephone and face-to-face structured clinical interview for DSM-IV in assessing social anxiety disorder. Perspect. Psychiatr. Care. 2008;44(4):241–247. doi: 10.1111/j.1744-6163.2008.00183.x. [DOI] [PubMed] [Google Scholar]
- DGPPN. BÄK. KBV. AWMF . 2015. S3-Leitlinie/Nationale VersorgungsLeitlinie Unipolare Depression – Langfassung (edition 2, version 5)Retrieved from: https://www.awmf.org/leitlinien/detail/ll/nvl-005.html [Google Scholar]
- Ebert D.D., Cuijpers P., Muñoz R.F., Baumeister H. Prevention of mental health disorders using internet-and Mobile-based interventions: a narrative review and recommendations for future research. Front. Psychiatry. 2017;8 doi: 10.3389/fpsyt.2017.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenberg Christiane, Wolters Carolin, Brähler Elmar. The internet as a mental health advisor in Germany—results of a national survey. PloS one. 2013;8(11):e79206. doi: 10.1371/journal.pone.0079206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erbe D., Eichert H.C., Rietz C., Ebert D. Interformat reliability of the patient health questionnaire: validation of the computerized version of the PHQ-9. Internet Interv. 2016;5:1–4. doi: 10.1016/j.invent.2016.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eysenbach G., Group, C.-E CONSORT-EHEALTH: improving and standardizing evaluation reports of Web-based and mobile health interventions. J. Med. Internet Res. 2011;13(4) doi: 10.2196/jmir.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fichter M.M., Quadflieg N., Fischer U.C., Kohlboeck G. Twenty-five-year course and outcome in anxiety and depression in the upper bavarian longitudinal community study. Acta Psychiatr. Scand. 2010;122(1):75–85. doi: 10.1111/j.1600-0447.2009.01512.x. [DOI] [PubMed] [Google Scholar]
- Ghio L., Gotelli S., Marcenaro M., Amore M., Natta W. Duration of untreated illness and outcomes in unipolar depression: a systematic review and meta-analysis. J. Affect. Disord. 2014;152:45–51. doi: 10.1016/j.jad.2013.10.002. [DOI] [PubMed] [Google Scholar]
- Gierk B., Kohlmann S., Hagemann-Goebel M., Löwe B., Nestoriuc Y. Monitoring somatic symptoms in patients with mental disorders: sensitivity to change and minimal clinically important difference of the somatic symptom scale – 8 (SSS-8) Gen. Hosp. Psychiatry. 2017;48:51–55. doi: 10.1016/j.genhosppsych.2017.07.002. [DOI] [PubMed] [Google Scholar]
- Gierk B., Kohlmann S., Kroenke K., Spangenberg L., Zenger M., Brähler E., Löwe B. The somatic symptom Scale–8 (SSS-8): a brief measure of somatic symptom burden. JAMA Intern. Med. 2014;174(3):399–407. doi: 10.1001/jamainternmed.2013.12179. [DOI] [PubMed] [Google Scholar]
- Gilbody S., Sheldon T., House A. Screening and case-finding instruments for depression: a meta-analysis. CMAJ. 2008;178(8):997–1003. doi: 10.1503/cmaj.070281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjistavropoulos H.D., Faller Y.N., Klatt A., Nugent M.N., Dear B.F., Titov N. Patient perspectives on strengths and challenges of therapist-assisted internet-delivered cognitive behaviour therapy: using the patient voice to improve care. Community Ment. Health J. 2018;54(7):944–950. doi: 10.1007/s10597-018-0286-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herdman M., Gudex C., Lloyd A., Janssen M., Kind P., Parkin D., Badia X. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L) Qual. Life Res. 2011;20(10):1727–1736. doi: 10.1007/s11136-011-9903-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzel L.P., Ries Z., Dirmaier J., Zill J.M., Kriston L., Klesse C., Bermejo I. Usefulness scale for patient information material (USE) - development and psychometric properties. BMC Med. Inform. Decis. Mak. 2015;15 doi: 10.1186/s12911-015-0153-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karyotaki E., Riper H., Twisk J., Hoogendoorn A., Kleiboer A., Mira A., Littlewood E. Efficacy of self-guided internet-based cognitive behavioral therapy in the treatment of depressive symptoms: a meta-analysis of individual participant data. JAMA Psychiatry. 2017;74(4):351–359. doi: 10.1001/jamapsychiatry.2017.0044. [DOI] [PubMed] [Google Scholar]
- Kohlmann S., Lehmann M., Eisele M., Braunschneider L.E., Marx G., Zapf A., Lowe B. Depression screening using patient-targeted feedback in general practices: study protocol of the german multicentre GET.FEEDBACK.GP randomised controlled trial. BMJ Open. 2020;10(9) doi: 10.1136/bmjopen-2019-035973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke K., Spitzer R.L., Williams J.B.W. The PHQ-9. J. Gen. Intern. Med. 2001;16(9):606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lablans M., Borg A., Uckert F. A RESTful interface to pseudonymization services in modern web applications. BMC Med. Inform. Decis. Mak. 2015;15:2. doi: 10.1186/s12911-014-0123-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamers L.M., Bouwmans C.A., van Straten A., Donker M.C., Hakkaart L. Comparison of EQ-5D and SF-6D utilities in mental health patients. Health Econ. 2006;15(11):1229–1236. doi: 10.1002/hec.1125. [DOI] [PubMed] [Google Scholar]
- Leventhal H., Brissette I., Leventhal E. The common-sense model of self-regulation of health and illness. In: Cameron L.D., Leventhal H., editors. The Self-Regulation of Health and Illness Behavior. Routledge; London: 2003. pp. 42–65. [Google Scholar]
- Leventhal H., Phillips L.A., Burns E. The common-sense model of self-regulation (CSM): a dynamic framework for understanding illness self-management. J. Behav. Med. 2016;39(6):935–946. doi: 10.1007/s10865-016-9782-2. [DOI] [PubMed] [Google Scholar]
- Levesque D.A., Van Marter D.F., Schneider R.J., Bauer M.R., Goldberg D.N., Prochaska J.O., Prochaska J.M. Randomized trial of a computer-tailored intervention for patients with depression. Am. J. Health Promot. 2011;26(2):77–89. doi: 10.4278/ajhp.090123-QUAN-27. [DOI] [PubMed] [Google Scholar]
- Levis B., Benedetti A., Thombs B.D. Accuracy of patient health Questionnaire-9 (PHQ-9) for screening to detect major depression: individual participant data meta-analysis. BMJ. 2019;365 doi: 10.1136/bmj.l1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löwe B., Blankenberg S., Wegscheider K., König H.-H., Walter D., Murray A.M., Kohlmann S. Depression screening with patient-targeted feedback in cardiology: DEPSCREEN-INFO randomised clinical trial. Br. J. Psychiatry. 2016;1–8 doi: 10.1192/bjp.bp.116.184168. [DOI] [PubMed] [Google Scholar]
- Löwe B., Decker O., Müller S., Brähler E., Schellberg D., Herzog W., Herzberg P.Y. Validation and standardization of the generalized anxiety disorder screener (GAD-7) in the general population. Med. Care. 2008;46(3):266–274. doi: 10.1097/MLR.0b013e318160d093. [DOI] [PubMed] [Google Scholar]
- Löwe B., Spitzer R.L., Gräfe K., Kroenke K., Quenter A., Zipfel S., Buchholz C., Witte S., Herzog W. Comparative validity of three screening questionnaires for DSM-IV depressive disorders and physicians' diagnoses. J. Affect. Disord. 2004;78(2):131–140. doi: 10.1016/s0165-0327(02)00237-9. [DOI] [PubMed] [Google Scholar]
- Löwe B., Unützer J., Callahan C.M., Perkins A.J., Kroenke K. Monitoring depression treatment outcomes with the patient health questionnaire-9. Med. Care. 2004;42(12):1194–1201. doi: 10.1097/00005650-200412000-00006. [DOI] [PubMed] [Google Scholar]
- Ludwig K., Graf von der Schulenburg J.M., Greiner W. German value set for the EQ-5D-5L. PharmacoEconomics. 2018;36(6):663–674. doi: 10.1007/s40273-018-0615-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch J., Moore M., Moss-Morris R., Kendrick T. Are patient beliefs important in determining adherence to treatment and outcome for depression? Development of the beliefs about depression questionnaire. J. Affect. Disord. 2011;133(1–2):29–41. doi: 10.1016/j.jad.2011.03.019. [DOI] [PubMed] [Google Scholar]
- Melville K.M., Casey L.M., Kavanagh D.J. Dropout from internet-based treatment for psychological disorders. Br. J. Clin. Psychol. 2010;49(Pt 4):455–471. doi: 10.1348/014466509x472138. [DOI] [PubMed] [Google Scholar]
- Miller P., Newby D., Walkom E., Schneider J., Li S.C., Evans T.-J. The performance and accuracy of depression screening tools capable of self-administration in primary care: a systematic review and meta-analysis. Eur. J. Psychiatry. 2021;35(1):1–18. doi: 10.1016/j.ejpsy.2020.10.002. [DOI] [Google Scholar]
- Mitchell A.J., Vaze A., Rao S. Clinical diagnosis of depression in primary care: a meta-analysis. Lancet. 2009;374(9690):609–619. doi: 10.1016/S0140-6736(09)60879-5. [DOI] [PubMed] [Google Scholar]
- Moher D., Hopewell S., Schulz K.F., Montori V., Gøtzsche P.C., Devereaux P.J., Altman D.G. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340 doi: 10.1136/bmj.c869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery P., Grant S., Mayo-Wilson E., Macdonald G., Michie S., Hopewell S., Group, o. b. o. t. C.-S Reporting randomised trials of social and psychological interventions: the CONSORT-SPI 2018 extension. Trials. 2018;19(1):407. doi: 10.1186/s13063-018-2733-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards D., Richardson T. Computer-based psychological treatments for depression: a systematic review and meta-analysis. Clin. Psychol. Rev. 2012;32(4):329–342. doi: 10.1016/j.cpr.2012.02.004. [DOI] [PubMed] [Google Scholar]
- Rohde F., Franke M., Sehili Z., Lablans M., Rahm E. Optimization of the mainzelliste software for fast privacy-preserving record linkage. J. Transl. Med. 2021;19(1):1–12. doi: 10.1186/s12967-020-02678-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozbroj T., Lyons A., Pitts M., Mitchell A., Christensen H. Assessing the applicability of E-therapies for depression, anxiety, and other mood disorders among lesbians and gay men: analysis of 24 web- and Mobile phone-based self-help interventions. J. Med. Internet Res. 2014;16(7):84–94. doi: 10.2196/jmir.3529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan G.L., Skinner C.S., Farrell D., Champion V.L. Examining the boundaries of tailoring: the utility of tailoring versus targeting mammography interventions for two distinct populations. Health Educ. Res. 2001;16(5):555–566. doi: 10.1093/her/16.5.555. [DOI] [PubMed] [Google Scholar]
- Sapin C., Fantino B., Nowicki M.L., Kind P. Usefulness of EQ-5D in assessing health status in primary care patients with major depressive disorder. Health Qual. Life Outcomes. 2004;2:20. doi: 10.1186/1477-7525-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schomerus G., Angermeyer M.C. Stigma and its impact on help-seeking for mental disorders: what do we know? Epidemiol. Psichiatr. Soc. 2008;17(1):31–37. doi: 10.1017/s1121189x00002669. [DOI] [PubMed] [Google Scholar]
- Schulz K.F., Altman D.G., Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMC Med. 2010;8(1):18. doi: 10.1186/1741-7015-8-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeralan T., Harter M., Koschnitzke C., Scholl M., Kohlmann S., Lehmann M., Brutt A.L. Patient involvement in developing a patient-targeted feedback intervention after depression screening in primary care within the randomized controlled trial GET.FEEDBACK.GP. Health Expect. 2020 doi: 10.1111/hex.13039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah R., Franks P., Jerant A., Feldman M., Duberstein P., Fernandez y Garcia E., Hinton L., Strohecker L., Kravitz R.L. The effect of targeted and tailored patient depression engagement interventions on patient-physician discussion of suicidal thoughts: a randomized control trial. J. Gen. Intern. Med. 2014;29(8):1148–1154. doi: 10.1007/s11606-014-2843-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siu A.L., Bibbins-Domingo K., Grossman D.C., Baumann L.C., Davidson K.W., Ebell M., Kemper A.R. Screening for depression in adults: US preventive services task force recommendation statement. JAMA. 2016;315(4):380–387. doi: 10.1001/jama.2015.18392. [DOI] [PubMed] [Google Scholar]
- Sobocki P., Ekman M., Agren H., Krakau I., Runeson B., Martensson B., Jonsson B. Health-related quality of life measured with EQ-5D in patients treated for depression in primary care. Value Health. 2007;10(2):153–160. doi: 10.1111/j.1524-4733.2006.00162.x. [DOI] [PubMed] [Google Scholar]
- Spitzer R.L., Kroenke K., Williams J.B., Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch. Intern. Med. 2006;166(10):1092–1097. doi: 10.1001/archinte.166.10.1092. [DOI] [PubMed] [Google Scholar]
- Thombs B.D., Ziegelstein R.C., Roseman M., Kloda L.A., Ioannidis J.P. There are no randomized controlled trials that support the United States preventive services task force guideline on screening for depression in primary care: a systematic review. BMC Med. 2014;12:13. doi: 10.1186/1741-7015-12-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torous J., Nicholas J., Larsen M.E., Firth J., Christensen H. Clinical review of user engagement with mental health smartphone apps: evidence, theory and improvements. Evid. Based Ment. Health. 2018;21(3):116–119. doi: 10.1136/eb-2018-102891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trautmann S., Beesdo-Baum K. The treatment of depression in primary care: a cross-sectional epidemiological study. Dtsch. Arztebl. Int. 2017;114(43):721. doi: 10.3238/arztebl.2017.0721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos T., Lim S.S., Abbafati C., Abbas K.M., Abbasi M., Abbasifard M., Murray C.J.L. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the global burden of disease study 2019. Lancet. 2020;396(10258):1204–1222. doi: 10.1016/S0140-6736(20)30925-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
GFGP feedback.
Standard feedback.
Tailored feedback final.