Abstract
Rationale & Objective
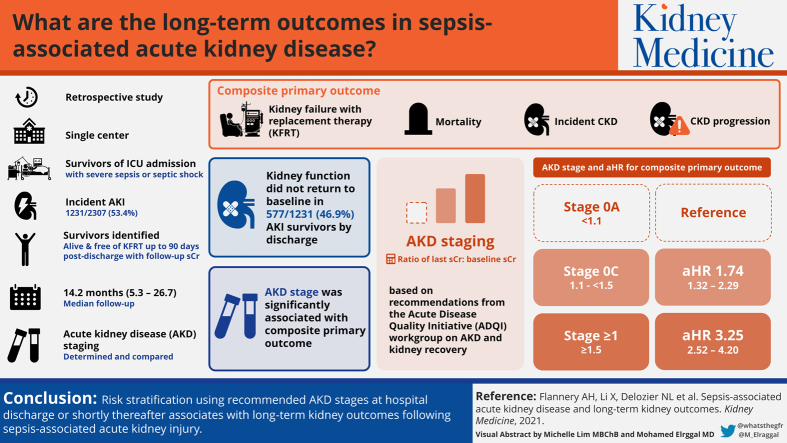
Sepsis-associated acute kidney injury often leads to acute kidney disease (AKD), predisposing patients to long-term complications such as chronic kidney disease (CKD), kidney failure with replacement therapy (KFRT), or mortality. Risk stratification of patients with AKD represents an opportunity to assist with prognostication of long-term kidney complications.
Study Design
Single-center retrospective cohort.
Setting & Participants
6,290 critically ill patients admitted to the intensive care unit with severe sepsis or septic shock. Patients were separated into cohorts based on incident acute kidney injury or not, and survivors identified who were alive and free of KFRT up to 90 days.
Predictors
AKD stage (0A, 0C, or ≥1) using the last serum creatinine concentration available by discharge or up to 90 days postdischarge.
Outcome
Time to development of incident CKD, progression of CKD, KFRT, or death.
Analytical Approach
Multivariable Cox proportional hazards models.
Results
Patients surviving kidney injury associated with sepsis often fail to return to baseline kidney function by discharge: 577/1,231 (46.9%) with stage 0C or 1 or greater AKD. AKD stage was significantly associated with the composite primary outcome. Stages 0C AKD and 1 or greater AKD were significantly and progressively associated with the primary outcome when compared with stage 0A AKD (adjusted HR [aHR], 1.74; 95% CI, 1.32-2.29, and aHR, 3.25; 95% CI, 2.52-4.20, respectively). Additionally, stage 1 or greater AKD conferred higher risk above stage 0C AKD (aHR, 1.87; 95% CI, 1.44-2.43). CKD incidence or progression and KFRT, more so than mortality, occurred with greater frequency in higher stages of AKD.
Limitations
Retrospective design, single center, exclusion of patients with KFRT within 90 days of discharge, potential ascertainment bias, and inability to subclassify above AKD stage 1.
Conclusions
Risk stratification using recommended AKD stages at hospital discharge or shortly thereafter associates with the development of long-term kidney outcomes following sepsis-associated acute kidney injury.
Index Words: Acute kidney disease, acute kidney injury, sepsis, long-term outcomes, chronic kidney disease, end-stage renal disease
Graphical abstract
Plain-Language Summary.
Sepsis is a common cause of kidney injury in hospitalized patients that often does not resolve within days of injury, which may place patients at risk for long-term kidney complications. We sought to assess how the degree of remaining kidney injury at or shortly after hospital discharge relates to a patient’s risk for long-term kidney outcomes, including new or progressive chronic kidney disease, dialysis, or death. Our results showed that a commonly used classification system for this remaining kidney injury can accurately predict long-term kidney outcomes. These results can help kidney professionals better identify a patient’s risk for long-term kidney complications after sepsis and ensure that they receive the most appropriate attention and care following hospital discharge.
Editorial, p. 475
Sepsis is the most commonly identified risk factor for acute kidney injury (AKI), present in 26% to 50% of all AKI cases.1, 2, 3, 4 Compared with nonseptic AKI, sepsis-associated AKI presents with more severe AKI and increasing risk for mortality.1 Although AKI complicates the short-term management of patients with sepsis, it also places the patient at risk for long-term complications, such as the development of chronic kidney disease (CKD), kidney failure with replacement therapy (KFRT), and short- and long-term mortality.5, 6, 7 Sepsis-associated AKI has high potential to lead to acute kidney disease (AKD), or persistently reduced kidney function for at least 7 but less than 90 days.8, 9, 10
Despite the prevalence of sepsis-associated AKI, long-term follow-up examination of kidney function in this patient population remains understudied. Given the large number of patients with sepsis-associated AKI cared for annually and many who will develop AKD, risk stratification of these patients either at hospital discharge or shortly thereafter will be critical to aid in prognostication, as well as to target interventions that reduce post-AKI morbidity and the development of kidney-11, 12, 13 and non–kidney-related complications such as cardiovascular disease.14, 15, 16
Before using risk stratification into AKD treatment studies, further research is required on the link between sepsis-associated AKD and the development of kidney complications, including incident or progression of CKD, development of KFRT, or death. This was the impetus for the current study on long-term outcomes of survivors with sepsis-associated AKI. We hypothesized that sepsis-associated AKD associates in a graded manner according to AKD staging with long-term kidney outcomes in this susceptible population.
Methods
Study Design
This was a single-center retrospective cohort study of critically ill patients admitted to the intensive care unit (ICU) of an urban tertiary-care academic medical center. Adult patients (aged ≥18 years) admitted to the ICU from May 2007 to June 2012 with severe sepsis or septic shock were identified using International Classification of Disease, Ninth Revision, Clinical Modification (ICD-9-CM) codes and previously published criteria.17 Additionally, patients were identified as having sepsis-associated AKI based on the serum creatinine component of the KDIGO (Kidney Disease: Improving Global Outcomes) criteria.9 The highest serum creatinine value during ICU admission was used to adjudicate KDIGO AKI staging. The most recent serum creatinine measurement within 3 months of the index ICU admission was considered the baseline and used to calculate estimated glomerular filtration rate (eGFR) using the Modification of Diet in Renal Disease (MDRD) Study equation.18 Patients surviving the ICU admission alive and free of kidney replacement therapy (KRT) up to 90 days following hospital discharge were evaluated for long-term outcomes. Patients with baseline eGFRs < 15 mL/min/1.73 m2, with KFRT before admission, or follow-up time less than 90 days after discharge were excluded.
The study was approved by the Institutional Review Board (#7044) with a waiver of informed consent due to deidentification of patient information.
Study Outcomes and Definitions
Survivors of ICU admission alive and free of KRT up to 90 days following hospital discharge were evaluated for recovery of their sepsis-associated AKI, as assessed by AKD staging, which was the independent variable of interest for this study. If patients did not have any documented serum creatinine measurements within 90 days following hospital discharge, their discharge serum creatinine value was used to determine sepsis-associated AKI recovery. AKD staging was based on the ratio of the last serum creatinine value (hospital discharge or within 90 days from discharge) divided by the baseline serum creatinine value and categorized as follows based on AKD staging recommendations from the Acute Disease Quality Initiative work group on AKD and kidney recovery: less than 1.1 stage 0A AKD, 1.1 or greater to less than 1.5 stage 0C AKD, and 1.5 or greater stage 1 or greater AKD.8
The primary composite outcome was CKD incidence, progression of CKD, KFRT, or death. Incident CKD was defined as baseline eGFR > 60 mL/min/1.73 m2, but last eGFR < 60 mL/min/1.73 m2, and ≥25% reduction of last eGFR from baseline eGFR.19 Progressive CKD was defined as baseline eGFR < 60 mL/min/1.73 m2 and last eGFR < 15 mL/min/1.73 m2 or ≥50% reduction of last eGFR from baseline eGFR.19 The average of the last 2 eGFRs was used when available after 90 days following hospital discharge.
Baseline demographics and comorbid conditions were collected using ICD-9-CM codes. eGFR < 60 mL/min/1.73 mL/min/1.73 m2 was used for defining CKD at baseline.20 Anemia was defined as admission hematocrit < 39% for men or <36% for women. Hospital billing codes were used to identify drug exposure, red blood cell transfusion, and mechanical ventilation during the ICU admission. Severity of illness was quantified using the Sepsis-Related Organ Failure Assessment (SOFA)21 and Acute Physiology and Chronic Health Evaluation II (APACHE II)22 scores within the first day of ICU admission.
Statistical Analysis
Comparisons for categorical variables were made using χ2 test. For continuous variables, 1-way analysis of variance was used for Gaussian and Kruskal-Wallis for non-Gaussian distributed data. Baseline serum creatinine data were randomly missing in the cohort and therefore multiple imputation was conducted using SAS, version 9.4 Proc MI (SAS Institute, Inc), fully conditional specification, with 50 imputed data sets. Variables included for imputation and analyzed were age, sex, race, hypertension, and diabetes.
For the primary outcome of CKD incidence, progression of CKD, KFRT, or death, a Cox proportional hazards model was constructed using AKD status at hospital discharge (or within 90 days from discharge if available) as the primary independent variable. Potential confounding variables selected for the Cox model included demographic data (age, sex, and race); comorbid conditions (diabetes, anemia, and baseline eGFR); SOFA score as an indicator of critical illness (SOFA was categorized by a median of 4); and AKI characteristics (KDIGO severity classification). Baseline eGFR was log transformed for analysis. The selected final model was based on the significance of results (P < 0.10) through backward selection. APACHE II was not included in the multivariable model because of collinearity with the SOFA score. The final model was presented with adjusted hazard ratios (aHRs) and 95% CIs. Times to events are displayed in adjusted survival curves. Statistical analysis was undertaken in SAS, version 9.4, and GraphPad Prism 6.0 (GraphPad Software). P < 0.05 was considered statistically significant. No adjustments were made for multiple comparisons.
Several sensitivity analyses were conducted, including: (1) modeling the composite of incident or progressive CKD, KFRT, and death in patients with and without CKD at baseline, (2) using a doubling of serum creatinine concentration23,24 rather than our primary definitions for incident or progressive CKD, (3) by analyzing outcomes for incident/progressive CKD and KFRT/death separately, and (4) by modeling the composite outcome in only patients with measured baseline serum creatinine concentrations (without multiple imputation).
Results
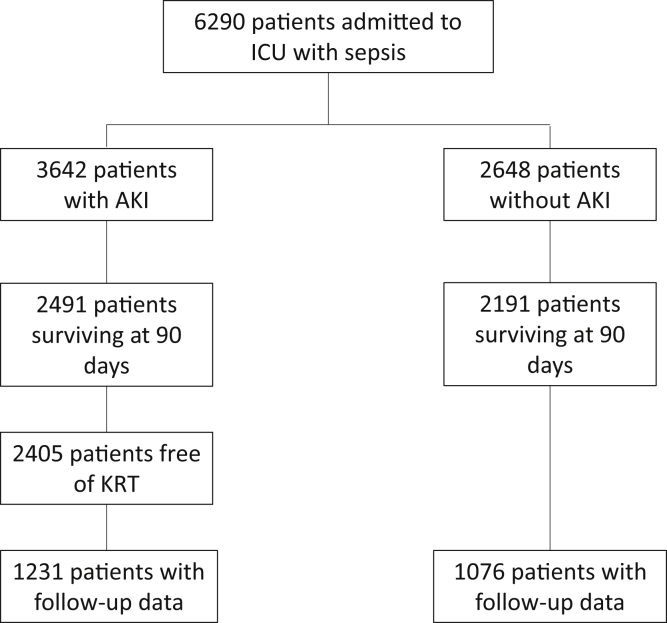
Of 6,290 patients admitted to the ICU with sepsis, 1,076 patients without AKI and 1,231 with AKI were ultimately included in the study (Fig 1). Complete data on baseline serum creatinine measurements were available for 1,177 of the 2,307 patients (51%), for which multiple imputation was used to estimate missing values (Table S1). Most serum creatinine values used for AKD staging were from the last day of hospitalization, with <10% representing a serum creatinine measured in the 90 days following discharge (Table S2). When the missing baseline serum creatinine data were imputed, of the 1,231 patients with AKI, 654 (53.1%) were classified as stage 0A AKD; 326 (26.5%), as stage 0C AKD; and 251 (20.4%), as stage 1 or greater AKD (Table 1). The population was mostly Black and middle-aged, with frequent comorbid conditions such as hypertension and diabetes. The acuity of illness was moderate as evidenced from the APACHE II and SOFA scores, with approximately one-third of patients requiring mechanical ventilation.
Figure 1.
Flow diagram of patient inclusion into the cohort. Abbreviations: AKI, acute kidney injury; ICU, intensive care unit; KRT, kidney replacement therapy.
Table 1.
Clinical Characteristics of Study Cohort
| No AKI (n =1,076) | Stage 0A AKD (n=654) | Stage 0C AKD (n=326) | Stage ≥1 AKD (n=251) | |
|---|---|---|---|---|
| Demographics | ||||
| Age, ya | 63.1 ± 16.6 | 63.9 ± 15.6 | 64.9 ± 15.2 | 59.7 ± 15.9 |
| Men | 560 (52.0%) | 362 (55.4%) | 157 (48.2%) | 130 (51.8%) |
| Race | ||||
| Black | 489 (45.6%) | 295 (45.6%) | 171 (52.9%) | 131 (52.4%) |
| White | 356 (33.2%) | 229 (35.4%) | 104 (32.2%) | 73 (29.2%) |
| Other | 228 (21.3%) | 123 (19.0%) | 48 (14.9%) | 46 (18.4%) |
| Chronic conditions | ||||
| Baseline Scr, mg/dLa,b | 1.3 ± 0.5 | 1.4 ± 0.5 | 1.3 ± 0.5 | 1.3 ± 0.5 |
| Baseline eGFR, mL/min/1.73 m2a | ||||
| ≥90 | 155 (14.4%) | 74 (11.3%) | 64 (19.6%) | 56 (22.3%) |
| <90 and ≥60 | 358 (33.3%) | 215 (32.9%) | 120 (36.8%) | 78 (31.1%) |
| <60 and ≥45 | 303 (28.2%) | 210 (32.1%) | 72 (22.1%) | 75 (29.9%) |
| <45 and ≥30 | 207 (19.2%) | 121 (18.5%) | 59 (18.1%) | 33 (13.2%) |
| <30 and ≥15 | 53 (4.9%) | 34 (5.2%) | 11 (3.4%) | 9 (3.6%) |
| Diabetes | 246 (22.9%) | 163 (24.9%) | 86 (26.4%) | 51 (20.3%) |
| Hypertensiona | 503 (46.8%) | 295 (45.1%) | 143 (43.9%) | 83 (33.1%) |
| Systolic heart failure | 46 (4.3%) | 29 (4.4%) | 13 (4.0%) | 8 (3.2%) |
| Anemiaa | 861 (82.4%) | 524 (81.6%) | 283 (88.7%) | 214 (86.3%) |
| Drug exposure | ||||
| Diuretica | 482 (44.8%) | 282 (43.1%) | 167 (51.2%) | 127 (50.6%) |
| Statin | 363 (33.7%) | 219 (33.5%) | 126 (38.7%) | 79 (31.5%) |
| Iodine contrasta | 348 (32.3%) | 138 (21.1%) | 68 (20.9%) | 45 (17.9%) |
| Aminoglycoside | 104 (9.7%) | 50 (7.7%) | 27 (8.3%) | 19 (7.6%) |
| Critical indicators | ||||
| Oliguria 72 ha | 425 (40.4%) | 273 (42.6%) | 145 (45.7%) | 130 (53.9%) |
| CFB 72 h, La | 1.1 [−1.6 to 4.5] | 2.7 [−0.6 to 6.7] | 1.2 [−1.2 to 5.4] | 1.3 [−2.0 to 5.3] |
| LOS, d | 11 [7 to 20] | 12 [6 to 21] | 12 [6 to 19] | 13 [7 to 22] |
| Pressor or inotropea | 257 (23.9%) | 212 (32.4%) | 94 (28.8%) | 68 (27.1%) |
| Mechanical ventilation | 378 (35.1%) | 239 (36.5%) | 107 (32.8%) | 84 (33.5%) |
| Blood transfusion | 34 (3.2%) | 24 (3.7%) | 10 (3.1%) | 3 (1.2%) |
| APACHE II scorea | 10 [7 to 14] | 12 [9 to 17] | 12 [8 to 17] | 11 [7 to 16] |
| SOFA scorea | 3 [1 to 5] | 4 [2 to 7] | 4 [2 to 7] | 4 [2 to 7] |
| Dipstick albuminuria > 30 mg/dLa | 333 (31.0%) | 341 (52.1%) | 155 (47.6%) | 136 (54.2%) |
| AKI characteristics | ||||
| AKI severity (KDIGO criteria)a | ||||
| Stage 1 | — | 396 (60.6%) | 193 (59.2%) | 56 (22.3%) |
| Stage 2 | 100 (15.3%) | 62 (19.0%) | 49 (19.5%) | |
| Stage 3 | 158 (24.2%) | 71 (21.8%) | 146 (58.2%) | |
| Hospital dialysisa | — | 60 (9.2%) | 20 (6.1%) | 82 (32.7%) |
| Peak Scr, mg/dLa | 1.2 [0.9 to 1.4] | 2.2 [1.7 to 3.2] | 2.2 [1.6 to 3.0] | 3.6 [2.0 to 6.3] |
| Discharge Scr, mg/dL a | 1.0 [0.8 to 1.2] | 1.1 [0.9 to 1.3] | 1.4 [1.1 to 1.9] | 2.6 [1.7 to 4.2] |
Note: Values expressed as mean ± standard deviation, number (percent), or median [25th to 75th percentile].
Abbreviations: AKD, acute kidney disease; AKI, acute kidney injury; APACHE II, Acute Physiology and Chronic Health Evaluation II score; CFB, cumulative fluid balance; eGFR, estimated glomerular filtration rate; iodine contrast, only if intravenous or intra-arterial; LOS, length of hospital stay; oliguria 72 h, urine output < 500 mL/d in the first 72 hours of intensive care unit admission; Scr, serum creatinine; SOFA, Sequential Organ Failure Assessment score; KDIGO, Kidney Disease Improving Global Outcomes.
P < 0.05 for comparison across all 4 groups.
Includes imputed missing baseline Scr value.
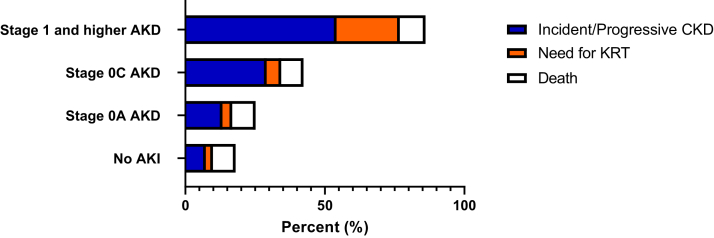
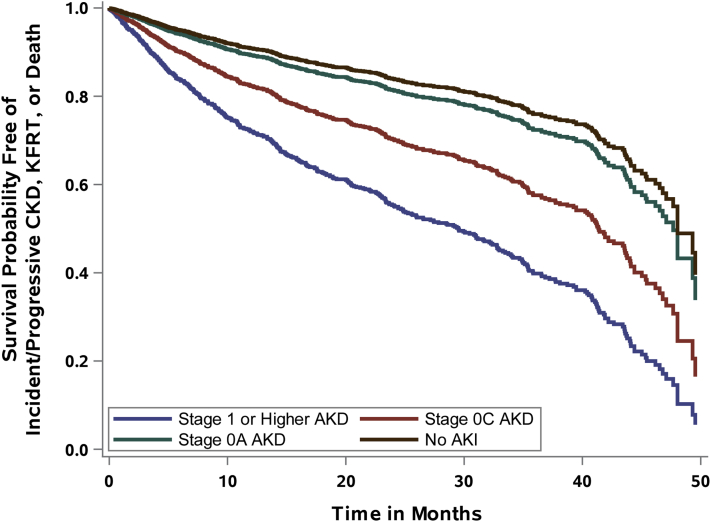
Patients were followed up for a median of 14.2 (IQR, 5.3-26.7) months. Most had at least 2 serum creatinine measurements during follow-up after 90 days postdischarge for CKD adjudication (Table S3). The specific outcomes of incident or progressive CKD, KFRT, and death are shown across AKD stages and the no-AKI group in Fig 2 and Table S4. Incident CKD, progressive CKD, and need for KRT were all significantly different and progressively occurred more frequently across the different AKD stages assessed; death was not. The adjusted survival curves for time to development of the primary composite outcome are shown in Figs 3 (entire cohort) and S1 (limited to patients with measured baseline serum creatinine).
Figure 2.
Long-term kidney outcomes based on presence of acute kidney injury (AKI) and acute kidney disease (AKD) stage. Abbreviations: CKD, chronic kidney disease; KRT, kidney replacement therapy.
Figure 3.
Adjusted survival curve for the composite outcome of incident or progressive chronic kidney disease (CKD), kidney failure with replacement therapy (KFRT), or death. Abbreviations: AKD, acute kidney disease; AKI, acute kidney injury.
In the analysis of the primary outcome, AKD stage at hospital discharge or within 90 days was significantly associated with the composite of CKD incidence, progression, KFRT, or death, with worsening AKD stage associated with a progressively greater aHR (Table 2). Compared with stage 0A AKD, stages 0C (aHR, 1.74; 95% CI, 1.32-2.29) and 1 or greater AKD (aHR, 3.25; 95% CI, 2.52-4.20) were both significantly associated with the outcome of CKD incidence, progression, KFRT, or death following 90 days postdischarge. Additionally, stage 1 or greater AKD carried risk above that of stage 0C AKD (aHR, 1.87; 95% CI, 1.44-2.43).
Table 2.
Cox Proportional Hazards Model for the Primary Outcome of CKD Incidence, Progression, Kidney Failure With Replacement Therapy, or Death
| Patient Characteristics (N = 1,231) | aHRa | 95% CI | aHRb | 95% CI |
|---|---|---|---|---|
| AKD stage at hospital discharge or within 90 d | ||||
| Stage 0C vs 0A | 1.76 | 1.33-2.32 | 1.74 | 1.32-2.29 |
| Stage ≥1 vs 0A | 3.01 | 2.29-3.96 | 3.25 | 2.52-4.20 |
| Stage ≥1 vs 0C | 1.72 | 1.30-2.26 | 1.87 | 1.44-2.43 |
| Age, 10-y | 1.09 | 1.01-1.17 | 1.09 | 1.01-1.17 |
| Man vs woman | 0.96 | 0.77-1.19 | — | — |
| Black vs other | 1.23 | 0.99-1.52 | 1.23 | 1.00-1.53 |
| Anemia | 1.69 | 1.18-2.42 | 1.69 | 1.18-2.41 |
| Diabetes | 1.00 | 0.78-1.28 | — | — |
| Log-transformed baseline eGFR | 0.87 | 0.67-1.14 | — | — |
| AKI severity | ||||
| AKI severity KDIGO 2-3 vs 1 | 1.37 | 0.99-1.90 | — | — |
| AKI severity KDIGO 3D vs 1 | 1.16 | 0.90-1.48 | — | — |
| SOFA score ≥ 4 vs <4 | 1.36 | 1.09-1.70 | 1.41 | 1.14-1.76 |
Abbreviations: aHR, adjusted hazard ratio; AKD, acute kidney disease; AKI, acute kidney injury; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; KDIGO, Kidney Disease: Improving Global Outcomes; SOFA, Sequential Organ Failure Assessment.
Initial model.
Final model after backward selection.
In sensitivity analysis in which the cohort was stratified by CKD status at baseline, similar findings were observed in the overall cohort with the exception that in patients without CKD at baseline, there was no difference in aHRs between stage 0C and 0A AKD (Table S5). The adjusted survival curves for patients with and without CKD are shown in Figs S2 and S3. When doubling of serum creatinine concentration was used in the composite outcome rather than our primary definitions of incident or progressive CKD, similar outcomes were observed (Table S6). A similarly strong and graded association according to severity of AKD remained for AKD status when the outcomes of incident/progressive CKD (Table S7) or KRT/death (Table S8) were analyzed separately in the overall cohort. The composite primary outcome analysis was repeated for the cohort of patients who had measured (nonimputed) baseline serum creatinine values (n=623) and the progressive risks for stages 0C and 1 or greater were confirmed when compared with stage 0A (Table S9). The additional risk of stage 0C versus 0A AKD appeared to be least robust in sensitivity analysis, without statistical significance in Tables S5, S6, and S8. Otherwise, AKD staging was confirmed to be progressively and strongly associated with worse long-term kidney outcomes.
Discussion
We evaluated long-term kidney-related outcomes of critically ill septic patients with and without AKI who survived and were KFRT-free up to 90 days postdischarge. A large proportion of survivors of sepsis-associated AKI, excluding those with KFRT up to 90 days postdischarge, failed to return to baseline kidney function as evidenced by 577/1,231 (46.9%) with stage 0C or 1 or greater AKD. Using AKD staging according to the Acute Disease Quality Initiative AKD consensus report, primarily at the time of hospital discharge, we demonstrated progressive risk for incident or progressive CKD, development of KFRT, or death as the AKD stage increased from 0A to 0C to 1 or greater AKD.8 Our results show that the proposed 0A, 0C, and 1 or greater staging appropriately risk stratifies patients at the time of discharge or within 90 days of discharge in terms of their risk for developing long-term kidney-related complications, including incident or progressive CKD, KFRT, or death. Importantly, this staging system associates with CKD and dialysis-related outcomes more than death.
These data indicate that AKD status at discharge is an important clinical parameter of long-term kidney outcomes and may serve as a measurable indictor of impaired/maladaptive kidney repair from sepsis-associated AKI and contributor to the development of CKD and KFRT.8,25,26 Accordingly, the hospital stay may be an important window of time to restore kidney function following sepsis-associated AKI. A reduction in AKD stage from 1 or greater to 0C, or 0C to 0A, appears to reduce the long-term risk for kidney complications significantly. Additionally, these data also portray a poor trajectory for patients with sepsis-associated AKI who survive hospitalization and are free of KRT at discharge, even those whose serum creatinine values return to baseline by hospital discharge (stage 0A AKD). Strategies that alter this trajectory could carry important health care implications for the development of long-term sequalae of sepsis-associated AKI. Importantly, even patients without sepsis-associated AKI appeared to carry significant risks following discharge. This highlights the potential importance of subclinical sepsis-associated AKI, which requires specific injury biomarkers for detection, as well as the importance of long-term sequelae of sepsis.27,28
Although early nephrologist follow-up after an episode of severe AKI is associated with reduced all-cause mortality,29 the number of patients who are seen by a nephrologist within 1 year of AKI diagnosis is estimated at only 20%.30 A recent KDIGO conference proposed that non-nephrology providers be involved in post-AKI care depending on patient risk factors such as KDIGO stage, recovery status, and comorbid conditions.31 The National Institutes of Health has recently acknowledged the gap in post-AKI care and announced the Caring for Outpatients After Acute Kidney Injury (COPE-AKI) consortium.32
Previous literature has shown the poor long-term outcomes of patients with sepsis-associated AKI but has primarily focused on risk assessment and stratification in the ICU. Lopes et al33 found that compared with patients without prior AKI, patients with sepsis-associated AKI were at greater risk for 2-year mortality. Similarly, Kim et al34 evaluated patients at 1 year following septic shock–associated AKI and found that KDIGO stage significantly, and progressively from stages 1-3, was associated with all-cause mortality within 1 year. In contrast, KDIGO stage was not associated with the development of CKD.34 This is consistent with our model development in which KDIGO severity stage was not a significant predictor of the composite outcome and was removed from the final model. The implications of this finding are that KDIGO severity classification does not capture level of kidney recovery post-AKI, which represents a surrogate of the multimodal kidney repair pathways after AKI, and therefore the newly proposed AKD classification8 appears to be a more critical factor for risk stratification of long-term kidney-related outcomes after sepsis-associated AKI, as shown in our study. This concept is further supported by findings from another large cohort study demonstrating that patient phenotypes of recovery from AKI (examples include early sustained reversal and lack of reversal among others) are associated with poor clinical outcomes, including ICU mortality and mortality up to 1 year of follow-up.35 Our findings build on the importance of recovery of kidney function in sepsis-associated AKI and to our knowledge represent the first epidemiologic study of long-term outcomes in sepsis-associated AKI as assessed by the newly proposed AKD stage classification.8 Given the high prevalence of AKD observed in survivors of sepsis-associated AKI in our study and prior work,10 the term sepsis-associated AKD appears worthy of its own classification given the impact on survivors of sepsis. Our study estimates of AKD may be on the higher side given that a considerable proportion of the studied population were Black and with underlying CKD, both clinical factors that were previously identified to be strongly associated with sepsis-associated AKD.10 Our sensitivity analyses suggested that AKD classifications are more distinctly linked with long-term kidney outcomes in patients with CKD than in patients without CKD.
Our study has several strengths, including the large sample size, use of consistent and recommended staging terminology regarding AKD status, and follow-up beyond 1 year of discharge, which allow it to build on prior literature.
Some limitations are also noteworthy to discuss, including the retrospective single-center design and the reliance on ICD-9-CM coding for patient identification. Based on the design of the study, by excluding patients who received KRT within 90 days of discharge, we may have excluded patients on a worse recovery trajectory from the analysis and thus our estimates, given that most patients’ AKD status was determined at discharge, may underestimate the risk for AKD status because these patients were excluded. The 90-day window for AKD classification was originally selected for the examination of AKD resolution following discharge. Fewer patients than desired had postdischarge serum creatinine values during this 90-day window following discharge. Although we were able to use discharge serum creatinine values in most cases, we recognize that AKD classification at discharge may not adequately represent a patient’s AKD stage within a 90-day window from discharge in some cases. Due to persistent KRT potentially influencing the postdischarge serum creatinine to baseline serum creatinine ratio to classify AKD stage, patients who received KRT within 90 days of hospital discharge were excluded. Due to the retrospective nature of data acquisition, not all patients discharged were able to contribute follow-up data and patients may not have been able to be followed up for the same amount of time, particularly patients with sepsis-associated AKI during the last few months of the study period. This may have introduced ascertainment bias into the study. Because we used only the last 2 serum creatinine measurements available during the follow-up period to adjudicate CKD, the intensity of follow-up may have served as a possible confounder. Additionally, the most commonly assessed outcome of the composite end point (CKD) was based on eGFR. The use of serum creatinine concentrations in these equations compared with other biomarkers such as cystatin C,36 as well as the inclusion of race, have come under intense scrutiny in recent years, including creation of a National Kidney Foundation/American Society of Nephrology task force on reassessing the inclusion of race in these equations.37 Future work in this area will benefit from advances in eGFR consensus and refinement. We also did not subclassify AKD higher than stage 1 (stage ≥ 1 was used) due to the relatively smaller number of patients in this category compared with others, which limits our ability to make conclusions about the risk stratification of stage 1 versus 2 versus 3 AKD.8 Finally, although we have attempted to adjust for relevant covariates with our modeling, residual confounding may nonetheless remain.
In conclusion, in critically ill patients surviving sepsis-associated AKI and free of KRT within 90 days of discharge, AKD status at discharge is strongly and progressively associated with the development of long-term kidney events, particularly CKD and KFRT. AKD stage may therefore be an important risk stratification tool for post-AKI care in patients surviving sepsis-associated AKI.
Article Information
Authors’ Full Names and Academic Degrees
Alexander H. Flannery, PharmD, FCCM, BCCCP, BCPS, Xilong Li, PhD, MBA, Natalie L. Delozier, PharmD, Robert D. Toto, MD, Orson W. Moe, MD, Jerry Yee, MD, and Javier A. Neyra, MD, MSCS.
Authors’ Contributions
Research idea and study design: AHF, JY, JAN; data acquisition: XL, JAN; data analysis/interpretation: AHF, NLD, XL, JAN; statistical analysis: XL; supervision or mentorship: RDT, OWM, JAN. AHF and XL contributed equally to this work and should be considered coprimary authors. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Support
This work was supported in part by a Henry Ford Hospital Resident Research Grant (to Dr Neyra), the University of Texas Southwestern Medical Center O’Brien Kidney Research Core Center (P30 DK079328-06), and the National Center for Advancing Translational Sciences (UL1TR001105). The content is solely the responsibility of the authors and does not represent the official views of the Henry Ford Hospital, National Institutes of Health (NIH), or University of Texas Southwestern. Dr Flannery is supported by KidneyCure/American Society of Nephrology Pre-Doctoral Fellowship and grant support from La Jolla Pharmaceutical Company. Dr Neyra is currently supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases (R56 DK126930 and P30 DK079337) and National Heart, Lung, and Blood Institute (R01 HL148448-01 and R21 HL145424-01A1). Dr Toto is supported by the NIH (R21- HL145424, UH3- DK114870). Dr Moe is supported by the NIH (R01-DK091392, R01-DK092461, the George O’Brien Kidney Research Center P30-DK-07938), the Charles and Jane Pak Center Endowed Professor Collaborative Research Support, and Innovative Research Support. The funders of this work had no role in study design; collection, analysis, and interpretation of the data; writing the report; or the decision to submit the report for publication.
Financial Disclosure
The authors declare that they have no relevant financial interests.
Peer Review
Received September 30, 2020. Evaluated by 3 external peer reviewers, with direct editorial input from the Statistical Editor, an Associate Editor, and the Editor-in-Chief. Accepted in revised form February 15, 2021.
Footnotes
Complete author and article information provided before references.
Figure S1: Adjusted Survival Curve for CKD Incidence, Progression, KFRT, or Death for Patients With Measured (nonimputed) Baseline Serum Creatinine Data
Figure S2: Adjusted Survival Curve for CKD Incidence, KFRT, or Death for Patients Without Baseline CKD
Figure S3: Adjusted Survival Curve for CKD Progression, KFRT, or Death for Patients With Baseline CKD
Table S1: Multiple Imputation Table
Table S2: Timing of Serum Creatinine Values Used for AKD Staging in Patients Surviving AKI
Table S3: Serum Creatinine Measurements Used for CKD Adjudication
Table S4: Study Outcomes by Stage of AKD for Patients Surviving AKI and for Patients Without AKI
Table S5: Cox Proportional-Hazards Model for Incident or Progressive CKD, KFRT, or Death by Baseline CKD Status
Table S6: Cox Proportional-Hazards Model for Doubling of Serum Creatinine, KFRT, or Death
Table S7: Cox Proportional Hazards Model for Incident or Progressive CKD
Table S8: Cox Proportional Hazards Model for KFRT or Death
Table S9: Cox Proportional Hazards Model for CKD Incidence, Progression, KFRT, or Death for Patients With Measured (nonimputed) Baseline Serum Creatinine Data
Supplementary Material
Figure S1-S3; Tables S1-S9.
References
- 1.Bagshaw S.M., George C., Bellomo R. Early acute kidney injury and sepsis: a multicentre evaluation. Crit Care. 2008;12(2):R47. doi: 10.1186/cc6863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bagshaw S.M., Uchino S., Bellomo R. Septic acute kidney injury in critically ill patients: clinical characteristics and outcomes. Clin J Am Soc Nephrol. 2007;2(3):431–439. doi: 10.2215/CJN.03681106. [DOI] [PubMed] [Google Scholar]
- 3.Cruz D.N., Bolgan I., Perazella M.A. North East Italian Prospective Hospital Renal Outcome Survey on Acute Kidney Injury (NEiPHROS-AKI): targeting the problem with the RIFLE criteria. Clin J Am Soc Nephrol. 2007;2(3):418–425. doi: 10.2215/CJN.03361006. [DOI] [PubMed] [Google Scholar]
- 4.Uchino S., Kellum J.A., Bellomo R. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005;294(7):813–818. doi: 10.1001/jama.294.7.813. [DOI] [PubMed] [Google Scholar]
- 5.Coca S.G., Singanamala S., Parikh C.R. Chronic kidney disease after acute kidney injury: a systematic review and meta-analysis. Kidney Int. 2012;81(5):442–448. doi: 10.1038/ki.2011.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gallagher M., Cass A., Bellomo R. Long-term survival and dialysis dependency following acute kidney injury in intensive care: extended follow-up of a randomized controlled trial. PLoS Med. 2014;11(2) doi: 10.1371/journal.pmed.1001601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wald R., Shariff S.Z., Adhikari N.K. The association between renal replacement therapy modality and long-term outcomes among critically ill adults with acute kidney injury: a retrospective cohort study∗. Crit Care Med. 2014;42(4):868–877. doi: 10.1097/CCM.0000000000000042. [DOI] [PubMed] [Google Scholar]
- 8.Chawla L.S., Bellomo R., Bihorac A. Acute kidney disease and renal recovery: consensus report of the Acute Disease Quality Initiative (ADQI) 16 Workgroup. Nat Rev Nephrol. 2017;13(4):241–257. doi: 10.1038/nrneph.2017.2. [DOI] [PubMed] [Google Scholar]
- 9.Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2:1–138. [Google Scholar]
- 10.Peerapornratana S., Priyanka P., Wang S. Sepsis-associated acute kidney disease. Kidney Int Rep. 2020;5(6):839–850. doi: 10.1016/j.ekir.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chawla L.S., Amdur R.L., Amodeo S., Kimmel P.L., Palant C.E. The severity of acute kidney injury predicts progression to chronic kidney disease. Kidney Int. 2011;79(12):1361–1369. doi: 10.1038/ki.2011.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thakar C.V., Christianson A., Himmelfarb J., Leonard A.C. Acute kidney injury episodes and chronic kidney disease risk in diabetes mellitus. Clin J Am Soc Nephrol. 2011;6(11):2567–2572. doi: 10.2215/CJN.01120211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heung M., Steffick D.E., Zivin K. Acute kidney injury recovery pattern and subsequent risk of CKD: an analysis of Veterans Health Administration data. Am J Kidney Dis. 2016;67(5):742–752. doi: 10.1053/j.ajkd.2015.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu V.C., Wu C.H., Huang T.M. Long-term risk of coronary events after AKI. J Am Soc Nephrol. 2014;25(3):595–605. doi: 10.1681/ASN.2013060610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Odutayo A., Wong C.X., Farkouh M. AKI and long-term risk for cardiovascular events and mortality. J Am Soc Nephrol. 2017;28(1):377–387. doi: 10.1681/ASN.2016010105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gammelager H., Christiansen C.F., Johansen M.B., Tønnesen E., Jespersen B., Sørensen H.T. Three-year risk of cardiovascular disease among intensive care patients with acute kidney injury: a population-based cohort study. Crit Care. 2014;18(5):492. doi: 10.1186/s13054-014-0492-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Angus D.C., Linde-Zwirble W.T., Lidicker J., Clermont G., Carcillo J., Pinsky M.R. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29(7):1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 18.Levey A.S., Bosch J.P., Lewis J.B., Greene T., Rogers N., Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130(6):461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 19.Go A.S., Parikh C.R., Ikizler T.A. The assessment, serial evaluation, and subsequent sequelae of acute kidney injury (ASSESS-AKI) study: design and methods. BMC Nephrol. 2010;11:22. doi: 10.1186/1471-2369-11-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group KDIGO 2012 clinical practice guideline for theevaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1–150. [Google Scholar]
- 21.Vincent J.L., Moreno R., Takala J. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22(7):707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 22.Knaus W.A., Draper E.A., Wagner D.P., Zimmerman J.E. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13(10):818–829. [PubMed] [Google Scholar]
- 23.Rossing P. Doubling of serum creatinine: is it sensitive and relevant? Nephrol Dial Transplant. 1998;13(2):244–246. doi: 10.1093/ndt/13.2.244. [DOI] [PubMed] [Google Scholar]
- 24.Lambers Heerspink H.J., Perkovic V., de Zeeuw D. Is doubling of serum creatinine a valid clinical ‘hard’ endpoint in clinical nephrology trials? Nephron Clin Pract. 2011;119(3):c195–c199. doi: 10.1159/000327614. discussion c199. [DOI] [PubMed] [Google Scholar]
- 25.Ferenbach D.A., Bonventre J.V. Mechanisms of maladaptive repair after AKI leading to accelerated kidney ageing and CKD. Nat Rev Nephrol. 2015;11(5):264–276. doi: 10.1038/nrneph.2015.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sato Y., Takahashi M., Yanagita M. Pathophysiology of AKI to CKD progression. Semin Nephrol. 2020;40(2):206–215. doi: 10.1016/j.semnephrol.2020.01.011. [DOI] [PubMed] [Google Scholar]
- 27.Dépret F., Hollinger A., Cariou A. Incidence and outcome of subclinical acute kidney injury using penKid in critically ill patients. Am J Respir Crit Care Med. 2020;202(6):822–829. doi: 10.1164/rccm.201910-1950OC. [DOI] [PubMed] [Google Scholar]
- 28.Prescott H.C., Osterholzer J.J., Langa K.M., Angus D.C., Iwashyna T.J. Late mortality after sepsis: propensity matched cohort study. BMJ. 2016;353:i2375. doi: 10.1136/bmj.i2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harel Z., Wald R., Bargman J.M. Nephrologist follow-up improves all-cause mortality of severe acute kidney injury survivors. Kidney Int. 2013;83(5):901–908. doi: 10.1038/ki.2012.451. [DOI] [PubMed] [Google Scholar]
- 30.Silver S.A., Siew E.D. Follow-up care in acute kidney injury: lost in transition. Adv Chronic Kidney Dis. 2017;24(4):246–252. doi: 10.1053/j.ackd.2017.05.008. [DOI] [PubMed] [Google Scholar]
- 31.Ostermann M., Bellomo R., Burdmann E.A. Controversies in acute kidney injury: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Conference. Kidney Int. 2020;98(2):294–309. doi: 10.1016/j.kint.2020.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Caring for OutPatiEnts after Acute Kidney Injury (COPE-AKI) – Clinical Centers. National Institutes of Health, Department of Health and Human Services. https://grants.nih.gov/grants/guide/rfa-files/rfa-dk-20-011.html Accessed August 22, 2020.
- 33.Lopes J.A., Fernandes P., Jorge S. Long-term risk of mortality after acute kidney injury in patients with sepsis: a contemporary analysis. BMC Nephrol. 2010;11:9. doi: 10.1186/1471-2369-11-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim J.S., Kim Y.J., Ryoo S.M. One--year progression and risk factors for the development of chronic kidney disease in septic shock patients with acute kidney injury: a single-centre retrospective cohort study. J Clin Med. 2018;7(12):554. doi: 10.3390/jcm7120554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kellum J.A., Sileanu F.E., Bihorac A., Hoste E.A., Chawla L.S. Recovery after acute kidney injury. Am J Respir Crit Care Med. 2017;195(6):784–791. doi: 10.1164/rccm.201604-0799OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shlipak M.G., Matsushita K., Ärnlöv J. Cystatin C versus creatinine in determining risk based on kidney function. N Engl J Med. 2013;369(10):932–943. doi: 10.1056/NEJMoa1214234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.National Kidney Foundation NKF/ASN Task Force on Reassessing the Inclusion of Race in Diagnosing Kidney Diseases. https://www.kidney.org/content/nkf-asn-task-force-reassessing-inclusion-race-diagnosing-kidney-diseases Accessed January 18, 2021. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1-S3; Tables S1-S9.