Abstract
Objective:
To assess the relationship between short- and longer-term retention in outpatient substance use disorder (SUD) treatment and pharmacotherapy for comorbid attention-deficit hyperactivity disorder (ADHD).
Methods:
This is a retrospective cohort study conducted in a single addiction psychiatry clinic. Electronic health record data were queried for clinical ADHD diagnosis, ADHD pharmacotherapy, treatment duration, demographic variables, comorbid psychiatric and SUD diagnoses, and buprenorphine therapy. Individuals with ADHD (n = 203) were grouped by ADHD pharmacotherapy status (171 receiving medication compared to 32 receiving none). Kaplan-Meier and Cox proportional hazards regression analyses were performed and assessed for significance.
Results:
ADHD was clinically diagnosed in 9.4% of outpatients and was associated with younger age, comorbid cocaine use, and private insurance (p < 0.001). Individuals receiving no ADHD pharmacotherapy were younger than those receiving medication (p = 0.003). Compared to those receiving no ADHD medication, ADHD pharmacotherapy was associated with greater long-term retention, with apparent group half-lives of 9 months and 36 months, respectively (p < 0.001). Individuals receiving no ADHD medication had a 4.9-fold increased likelihood of attrition within 90 days (p = 0.041). Regression analysis showed only ADHD pharmacotherapy to be significantly associated with treatment retention (HR 0.59, 0.40 – 0.86 95%-CI, p = 0.008).
Conclusion:
ADHD pharmacotherapy is robustly associated with improved short- and longer-term retention in outpatient SUD treatment. The retrospective, non-randomized naturalistic study design limits causal inference. Further studies addressing unmeasured covariates and associated risks of treatment in adults with ADHD and SUD are necessary.
Keywords: Attention-deficit hyperactivity disorder (ADHD), addiction, substance use disorder (SUD), stimulant, retention, drop-out
Introduction
Treatment retention is a critical outcome for individuals with substance use disorders (SUD).1 Overall retention rates in SUD treatment are similar to general psychiatric and medical treatment.2 However, the consequences of early drop-out are particularly problematic for the SUD population, risking relapse, medical and socioeconomic sequelae, overdose, and death. Some interventions for SUD have shown promise in improving retention, including contingency management, community reinforcement, motivational interviewing approaches3–6 and opioid agonist therapies.7–10
Non-SUD psychopathology further complicates SUD treatment retention.11–14 Among the most common comorbidities, attention-deficit hyperactivity disorder (ADHD) co-occurs with SUD. ADHD is a highly-heritable neurodevelopmental disorder characterized by inattention, distractibility, impulsivity, and hyperactivity. ADHD adversely affects individuals’ functional outcomes across multiple dimensions, including academic achievement, work performance, relationship stability, legal-system involvement, health-system utilization, and accidental death.15 ADHD is common and chronic, occuring in 4–5% of adults in the general population.15–17 Among treatment-seeking individuals with SUD, the prevalence of ADHD is 19–27%18--highlighting the importance of screening in SUD treatment settings.
In co-occurring SUD and ADHD, individuals experience earlier-onset substance use, longer duration of active SUD, more frequent and heavier use patterns, more difficulty achieving remission, and lower retention when compared to those without ADHD.19–21 Effective ADHD pharmacotherapy offers an opportunity to reduce ADHD symptom burden and potentially increase retention in addiction treatment.20,22,23
Pharmacotherapy is a highly effective treatment modality for ADHD, with large pooled effect sizes in both pediatric and adult populations.24,25 Pharmacotherapy improves both ADHD symptoms and associated functional outcomes both short and longer term.26 Among individuals with comorbid SUD, ADHD pharmacotherapy has demonstrated variable efficacy, though effect sizes are attenuated. In the most recent randomized clinical trials, relatively higher-dose stimulant treatment reduced ADHD symptoms and improved substance use outcomes.23,27,28 While helpful, the randomized trials of ADHD pharmacotherapy in SUD are typically limited to 12-weeks, with only 1 trial evaluating outcomes out to 24 weeks.23,27 Hence, longer-term outcomes in this group, particularly in naturalistic treatment settings, are desperately needed.
To this end, we studied the relationship between pharmacotherapy for ADHD and retention in outpatient SUD treatment. Based on the literature, we hypothesized that ADHD pharmacotherapy would improve retention compared to those with ADHD not receiving medication for this comorbidity.
Methods
Study setting and design
This retrospective observational study examined electronic health record (EHR) data from individuals admitted to a single outpatient addiction psychiatry clinic at an urban academic medical center in New England. Data were obtained from a 5.5-year period: July 14, 2014 to January 15, 2020. This period was chosen to reflect all available electronic patient data from the clinic prior to study initiation; the start date marks the clinic’s transition to a new EHR system. The clinic offered low-barrier engagement through walk-in clinic hours and rapid initiation of bridging pharmacotherapy as early as the first visit. Individual, group, and couples/family psychotherapy in multiple evidence-based modalities, as well as recovery coaching, were offered. A thrice-weekly intensive outpatient program (IOP) was also available. Case management services and care coordination with primary and specialty medical care was available to clinic providers.
This project was undertaken as a Quality Improvement Initiative and as such, was not formally supervised by the Institutional Review Board per their policies.
Participants
Diagnosis of ADHD was made clinically, applying DSM 5 criteria in the course of routine practice across all phases of care within the academic medical center. ADHD diagnoses made outside the addiction psychiatry clinic were re-assessed by clinic staff.
All patients referred or presenting newly to the addiction psychiatry clinic underwent intake assessments performed by licensed, terminal-degree psychology or social work staff using a semi-structured clinical interview with standardized brief screening for major categories of comorbid psychopathology. No standardized ADHD screening or symptom rating tool was required. Historical and new diagnoses were further reviewed and re-assessed by unstructured clinical interview with subsequent clinical staff providing psychotherapeutic and pharmacologic treatment. All clinic providers have subspecialty training and/or discipline-specific certification in substance use and related disorders; all are licensed, terminal-degree providers within their respective fields.
Individuals who received an ADHD diagnosis were identified by billing ICD-10 code F90*. Individuals receiving ADHD pharmacotherapy were compared to those who were prescribed no ADHD medications.
Data source, variables, and measurement
EHR data was queried for clinical ADHD diagnosis, prescription of ADHD pharmacotherapy (including amphetamine, lisdexamfetamine, methylphenidate, guanfacine, atomoxetine, modafinil, and bupropion products), date of first ADHD pharmacotherapy prescription, and dates of admission and discharge from the clinic.
Demographic variables including age, sex-assigned-at-birth, race, and insurer (private, public, or uninsured) were obtained. Buprenorphine treatment (with date of first prescription), SUD diagnoses (alcohol, benzodiazepine, opioid, cannabis, cocaine, and other stimulant use disorders), and major psychiatric diagnoses (bipolar, depression, anxiety, and post-traumatic stress disorders) were analyzed as covariates.
The main outcome was duration of treatment, measured as time from admission to discharge or end of the study period. In this study, no distinction was made between treatment drop-out and discharge; individuals not returning to treatment for >3 months with no future-scheduled appointment were considered discharged from clinic. For individuals discharged, raw duration was reduced by 90 days to account for the artifactual 3-month period from last attended visit to the date of administrative discharge recorded by the EHR.
Statistical methods
Descriptive statistics were computed for each group, including mean ages (with standard deviation), percent female, percent white, and percent privately insured. Groups were compared across these variables by two-tailed t-test for age and chi-squared test for goodness of fit for the remaining categorical variables.
Kaplan-Meier curves were generated for each group, graphing retention over 2 years from date of admission. Curves for each group were compared and assessed for significance by log-rank testing, with 95%-confidence intervals (95%-CI) generated and plotted. Apparent half-lives were estimated as the duration of treatment at which 50% of the participants had dropped out of treatment.
Cox proportional hazards regression analysis was performed for identified covariates. In order to satisfy the proportional hazards assumption, ADHD and buprenorphine pharmacotherapy were modeled as time-dependent covariates and the analysis was stratified by age and race. The time-dependent covariates account for unexposed time in treatment.
To further address potential bias due to non-randomized pre-exposure retention, additional subgroup survival analyses were performed using only data from individuals receiving ADHD pharmacotherapy within 90 days of admission.
Results
Participants
Overall, there were 2,163 individuals admitted to outpatient SUD treatment during the study period, of which 203 received an ADHD diagnosis code, yielding a 9.4% prevalence of clinically diagnosed ADHD. No cases were excluded from further analyses: 171 individuals with ADHD received ADHD pharmacotherapy; 41 received their first prescription prior to admission, 44 within 90 days of admission, and 86 more than 90 days after admission. Among the ADHD pharmacotherapy group, 63% received non-stimulants and 82% stimulants (not mutually exclusive). Overall, 67% of stimulants were extended-release or pro-drug formulations, and in those receiving amphetamine formulations (n = 105), 97% were adherent by toxicology screen. Thirty-two patients with ADHD received no ADHD pharmacotherapy.
Descriptive data
Individuals receiving a clinical ADHD diagnosis were significantly younger (38 +/− 11 vs. 45 +/− 14 years, p < 0.001), more privately insured (64% vs. 44%, p < 0.001), and more likely to have cocaine use disorder (31% vs. 12%, p < 0.001) than those without ADHD; these groups did not differ by sex-assigned-at-birth or race (Table 1).
Table 1.
Descriptive statistics of individuals admitted to clinic over the 5.5-year study period with and without ADHD. Individuals with clinically-diagnosed ADHD were significantly younger, more likely to have cocaine use disorder, and more likely to be privately insured than individuals without an ADHD diagnosis. Age is represented as the population mean in years +/− standard deviation; p-values reflect t-test or chi-squared statistical significance.
| All admitted individuals | ADHD | No ADHD | p-value | |
|---|---|---|---|---|
| n | 2163 | 203 | 1960 | - |
| Age | 44 +/−14 | 38 +/−11 | 45 +/−14 | p <0.001 |
| Female | 37% | 43% | 37% | p = 0.64 |
| White | 87% | 89% | 86% | p = 0.92 |
| Private insurer | 46% | 64% | 44% | p <0.001 |
| Cocaine use disorder | 14% | 31% | 12% | p <0.001 |
Individuals with ADHD receiving pharmacotherapy were significantly older than those receiving no pharmacotherapy (38 +/− 11 vs 32 +/− 9 years, mean +/− standard deviation, p = 0.003); these groups did not differ by sex-assigned-at-birth, race, insurer, or cocaine use disorder (Table 2).
Table 2.
Descriptive statistics of individuals with ADHD who received ADHD pharmacotherapy or no ADHD-targeted medication. Individuals receiving ADHD pharmacotherapy were significantly older than those receiving no ADHD-targeted medication. Age is represented as the population mean in years +/− standard deviation; p-values reflect t-test or chi-squared statistical significance.
| No ADHD medication | ADHD pharmacotherapy | p-value | |
|---|---|---|---|
| n | 32 | 171 | |
| Age | 32 +/− 9 | 38 +/− 11 | p = 0.003* |
| Female | 44% | 42% | p = 0.999 |
| White | 81% | 91% | p = 0.576 |
| Private insurer | 66% | 64% | p = 0.999 |
| Cocaine use disorder | 31% | 30% | p = 0.999 |
Treatment retention
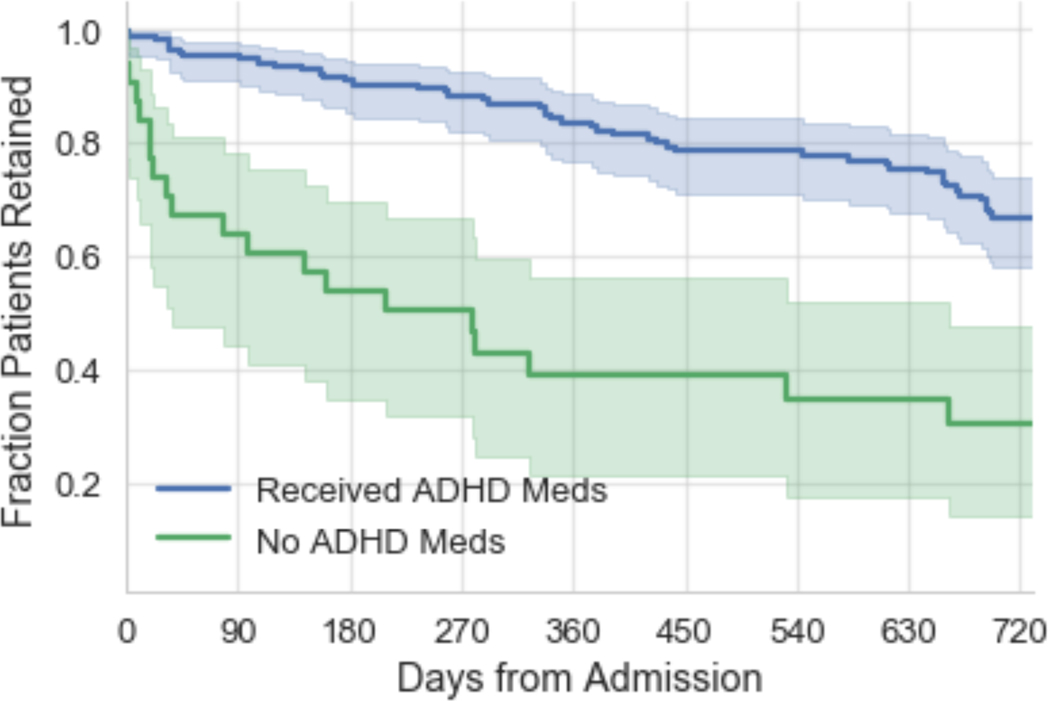
In the Kaplan-Meier retention analyses, the ADHD pharmacotherapy group had significantly greater retention than those receiving no ADHD medication (p < 0.001). The apparent half-life in treatment was 36 months for the ADHD pharmacotherapy group, with only 5% attrition at 90 days. For those not receiving ADHD pharmacotherapy, the apparent group half-life was 9 months, with 35% attrition at 90 days (Figure 1).
Figure 1.
Kaplan-Meier retention curves: ADHD pharmacotherapy vs. no ADHD medication. Vertical axis depicts proportion of patients retained in treatment. Horizontal axis depicts days in treatment after admission. Shaded area around each curve represents the 95%-CI.
This significant difference persisted when limiting the ADHD pharmacotherapy group to only those receiving ADHD medication within 90 days of admission (n = 84; 11% attrition at 90 days, p = 0.041 by chi-squared test; apparent group half-life 21 months, p = 0.025 by log-rank test). Compared to this early pharmacotherapy group, individuals receiving no ADHD medication had an almost 5-fold increased risk of attrition within 90 days (OR 4.92).
In subgroup analysis of those receiving stimulants within 90 days of admission, longer-term retention remained significantly improved out to 21 months follow-up, with an identical and near-significant improvement in 90-day attrition (n = 66; 11% attrition at 90 days, p = 0.074 by chi-squared test; apparent group half-life 19 months, p = 0.043). The group receiving only non-stimulant ADHD medication within 90 days did not differ from those receiving no medication, though small sample size limits interpretation (n = 11; 20% attrition at 90 days, p = 0.917 by chi-squared test; apparent group half-life 6 months, p = 0.947).
Cox proportional hazards regression analysis showed ADHD pharmacotherapy is a significant predictor of longer treatment duration in a model including all measured covariates (HR 0.59, 0.40 – 0.86 95%-CI, p = 0.008). No other measured covariate was a significant predictor of retention in this model, including buprenorphine therapy (HR 0.97, 0.59 – 1.60, p = 0.902; Supplemental Table 1).
Discussion
The main outcome of this retrospective cohort study supports our hypothesis, showing a robust association between ADHD pharmacotherapy and improved retention in both short and longer-term SUD treatment. This finding primarily reflects the use of stimulants for ADHD with a small sample size limiting assessment of the non-stimulants. While these data are limited by the retrospective, non-randomized naturalistic study design, our findings support previous calls for early diagnosis and pharmacologic treatment of ADHD in adults with SUD.22,23,29,30
To our knowledge, this is the first study assessing longer-term retention in outpatient SUD treatment among individuals receiving pharmacotherapy for ADHD. Remarkably, our observed retention half-life of 21–36 months in the treated group is comparable to treatment retention for adults with ADHD in general psychiatric settings. In these studies, 50% of adults with ADHD treated with stimulants remain on stimulant therapy with improved functional outcomes at 2- to 6-year follow-up.31–33 In contrast, a recent Cochrane review of prescribed amphetamines for adult ADHD found no improvement in retention compared to placebo (RR 1.06, 95%-CI 0.99–1.13); however, this meta-analysis reported the supporting evidence as low quality, found high overall retention rates (75–85%), and included only randomized controlled trials with an average follow-up of 5 weeks.34
Our observed rates of attrition in the group receiving no ADHD medication are comparable to a long-term follow-up study of 23 individuals with ADHD and SUD who were also not treated with ADHD pharmacotherapy. This study from an abstinence-based therapeutic community reported a 35% attrition rate at 60 days with a median 9-month duration of treatment.20 Longer-term outcomes of individuals retained in SUD treatment <90 days are equivalent to those who drop-out immediately following intake or medically-supervised withdrawal; in contrast, longer treatment duration is associated with improvement across multiple outcomes, including substance use, employment, and legal system involvement.7,35–37
Of the randomized controlled trials testing ADHD pharmacotherapy in individuals with SUD, most report only short-term retention (~90 days) ranging from 45 to 85% with few of these studies noting significant improvements compared to placebo.23,27 This variability in retention most likely reflects differences in concurrent treatment (e.g. methadone therapy enhancing retention),38 ADHD medication studied, specific SUD studied (including nicotine, alcohol, opioid, cocaine, and amphetamine use disorders), and setting (e.g. lower retention in the formerly-incarcerated group).27 While limited by our sample size, we found lower retention with non-stimulants compared to stimulants, consistent with previous work comparing atomoxetine and stimulants in adults with SUD.39 It is notable that the naturalistic design of our study addresses real-world treatment retention within a clinic utilizing flexible, individualized treatment plans, differing from retention observed in less-flexible, protocol-driven interventions in randomized controlled trials.21
We identified one study examining the impact of pharmacotherapy for ADHD on longer-term retention: a randomized controlled trial of high-dose extended-release methylphenidate evaluating retention at 6 months.27 Konstenius and colleagues reported a reduced risk of drop-out after prison release among those receiving medication comparable to our results (HR 0.38, 0.17 – 0.65 95%-CI in their study; HR 0.59, 0.40 – 0.86 95%-CI in our study), although absolute retention rates were lower in this formerly-incarcerated population.27
Prior studies have reported retention similar to non-ADHD peers among individuals with ADHD on opioid agonist treatment for comorbid opioid use disorder,38,40 though very few individuals on buprenorphine or methadone receive ADHD pharmacotherapy (3.5–4.6% in a large Norwegian study).29 Although limited by a small sample size, our regression analysis did not find buprenorphine therapy to be a significant predictor of retention in this group. In contrast, prior analyses of data from this clinic that included individuals without ADHD have shown buprenorphine to be a significant predictor of retention for the overall clinic population (V.R., unpublished data, 2018). Interestingly, our study reports an effect of ADHD pharmacotherapy on longer-term SUD treatment retention that is comparable to that of buprenorphine and methadone in opioid use disorder, where treatment half-lives are 6–12 months.7–10
We found that 1 in 10 adults in our sample were given a clinical diagnosis of ADHD. Given the internationally replicated prevalence of 19–27% in SUD treatment settings,18 our observed rate of ADHD is less than half of what is expected when using standardized diagnostic instruments. Since more flagrant symptoms may be more easily recognized, the likelihood of clinical ADHD diagnosis in our sample may positively correlate with ADHD symptom severity, leading to a sample enriched for greater ADHD symptom burden. If this is the case, our finding of a robust response to ADHD pharmacotherapy would align with prior evidence for more severe ADHD symptoms predicting response to stimulant treatment41. Clinicians caring for individuals with comorbid ADHD and addiction face significant diagnostic and therapeutic challenges. ADHD-spectrum symptoms are easily misattributed to SUD, and even when ADHD is diagnosed, delaying initiation of pharmacotherapy may be well-intentioned, especially early-on in a treatment episode.30 Particularly when considering stimulant therapy, the risk of prescribing a controlled substance with potential for non-prescribed use to an individual with addiction is often felt to outweigh the potential benefit of ADHD-symptom remission.
In non-SUD populations, pharmacotherapy for ADHD is highly effective, improving both neurobehavioral symptoms and functional outcomes.24,25 Stimulants consistently demonstrate greater clinical efficacy than non-stimulants,24,25 but stimulant prescribing is complicated by clinicians’ valid concern for non-prescribed use and diversion.42–44 While early stimulant therapy for ADHD is known to reduce the risk of progression to SUD,45,46 the relative risks and benefits posed by stimulant therapy for individuals who have already developed SUD is less-well defined.
Among individuals with comorbid SUD, high-dose stimulant treatment has been shown to reduce ADHD-symptom burden and improve substance use outcomes in randomized controlled trials.23 Coupling the outcomes of these trials with our current data suggests that ADHD pharmacotherapy offers an opportunity to reduce ADHD symptom burden and increase early engagement and longer-term retention in SUD treatment.
Limitations
The current study has several methodological limitations. Our data are from a single site with low racial diversity, a relatively small sample size, and a wide-range of available services and treatment modalities, limiting generalizability to other settings and populations. Our clinically-diagnosed sample likely represents <50% of individuals who would meet diagnostic criteria for ADHD using standardized instruments,18 and the unexpectedly elevated rate of privately-insured individuals in our sample suggests this undiagnosed group may differ across other social determinants of health. Our findings may not generalize to the undiagnosed group.
The retrospective observational study design precludes causal inference. Unmeasured covariates or reverse causation may plausibly explain the observed association. Additional covariates not measured here should be explored in future analyses, including prescribed doses of ADHD medication, other concurrent pharmacotherapy (e.g., benzodiazepines), medical comorbidity, housing stability, additional social determinants of health, and clinician assessment of global functioning. Further, the finding of a demographic variable (age) significantly differing between groups receiving and not receiving ADHD pharmacotherapy suggests non-random bias within prescribing clinicians. This prescriber bias may be fruitfully explored in future analyses and may be controlled-for in subsequent prospective, randomized trials, if feasible.
This study did not distinguish treatment drop-out from clinic discharge after successful course. Our EHR data does not capture prescriptions written outside the affiliated integrated healthcare system, and this analysis only measured medication adherence among those receiving amphetamine formulations.
In considering how to apply the findings of this study in clinical practice, it is important to recognize that risk and number-needed-to-harm associated with ADHD pharmacotherapy are not well-defined in this population. Future studies should assess adverse SUD outcomes (e.g. overdose, death), medical adverse events and sequelae, non-prescribed medication use and diversion, and number-needed-to-harm to inform safe clinical practice.
Conclusions
Despite these limitations, this retrospective cohort study suggests that treating ADHD pharmacologically is associated with less early attrition and greater likelihood of longer-term retention in outpatient SUD treatment, with the potential to improve functional outcomes. These results warrant replication and testing using study designs capable of addressing the above-mentioned limitations. Careful attention to risk-mitigating components of an outpatient treatment frame may allow for safe, individualized trials of ADHD pharmacotherapy in individuals seeking SUD treatment.
Supplementary Material
Supplemental Table 1. Covariates included in regression analysis predicting treatment duration. Hazard ratios (HR), 95%-CI, and p-values are reported for each measured covariate. Of measured covariates, ADHD medication alone significantly affects duration of treatment. * indicates significance at alpha = 0.05.
Acknowledgments
Potential conflicts of interest: Dr. Timothy Wilens receives or has received grant support from the fol-lowing sources: NIH(NIDA). Dr. Timothy Wilens is or has been a consultant for Arbor, Otsuka, Iron-shore, KemPharm and Vallon. Dr. Timothy Wilens has published books: Straight Talk About Psychiatric Medications for Kids (Guilford Press); and co/edited books ADHD in Adults and Children (Cambridge University Press), Massachusetts General Hospital Comprehensive Clinical Psychiatry (Elsevier) and Massachusetts General Hospital Psychopharmacology and Neurotherapeutics (Elsevier). Dr. Wilens is co/owner of a copyrighted diagnostic questionnaire (Before School Functioning Questionnaire). Dr. Wilens has a licensing agreement with Ironshore (BSFQ Questionnaire). Dr. Wilens is Chief, Division of Child and Adolescent Psychiatry and (Co) Director of the Center for Addiction Medicine at Massachusetts General Hospital. He serves as a clinical consultant to the US National Football League (ERM As-sociates), U.S. Minor/Major League Baseball; Gavin Foundation and Bay Cove Human Services.
Drs. Kristopher A. Kast and Vinod Rao report no financial or other relationship relevant to the subject of this article.
Contributor Information
Kristopher A. Kast, Department of Psychiatry and Behavioral Sciences, Vanderbilt University Medical Center, Nashville, TN, USA.
Vinod Rao, Department of Psychiatry, Massachusetts General Hospital, Boston, MA, USA.
Timothy E. Wilens, Department of Psychiatry, Massachusetts General Hospital, Boston, MA, USA.
References
- 1.Onken LS, Blaine JD, Boren JJ. Beyond the Therapeutic Alliance: Keeping the Drug-Dependent Individual in Treatment. Rockville, MD: (5600 Fishers Lane, Rockville, 20857):; 1997. http://hdl.handle.net/2027/mdp.39015050792830 [Google Scholar]
- 2.Stark MJ. Dropping out of substance abuse treatment: A clinically oriented review. Clin Psychol Rev. 1992;12(1):93–116. doi: 10.1016/0272-7358(92)90092-M [DOI] [Google Scholar]
- 3.Petry NM. Contingency management: what it is and why psychiatrists should want to use it. The Psychiatrist. 2011;35(5):161–163. doi: 10.1192/pb.bp.110.031831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Higgins ST, Budney AJ, Bickel WK, Foerg FE, Donham R, Badger GJ. Incentives Improve Outcome in Outpatient Behavioral Treatment of Cocaine Dependence. Arch Gen Psychiatry. 1994;51(7):568–576. doi: 10.1001/archpsyc.1994.03950070060011 [DOI] [PubMed] [Google Scholar]
- 5.Carroll KM, Ball SA, Nich C, et al. Motivational interviewing to improve treatment engagement and outcome in individuals seeking treatment for substance abuse: a multisite effectiveness study. Drug Alcohol Depend. 2006;81(3):301–312. doi: 10.1016/j.drugalcdep.2005.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meyers RJ, Roozen HG, Smith JE. The Community Reinforcement Approach. Alcohol Res Health. 2011;33(4):380–388. [PMC free article] [PubMed] [Google Scholar]
- 7.Simpson DD, Joe GW, Broome KM, Hiller ML, Knight K, Rowan-Szal GA. Program diversity and treatment retention rates in the Drug Abuse Treatment Outcome Study (DATOS). Psychol Addict Behav. 1997;11(4):279–293. doi: 10.1037/0893-164X.11.4.279 [DOI] [Google Scholar]
- 8.Kelly SM, O’Grady KE, Mitchell SG, Brown BS, Schwartz RP. Predictors of methadone treatment retention from a multi-site study: A survival analysis. Drug Alcohol Depend. 2011;117(2–3):170–175. doi: 10.1016/j.drugalcdep.2011.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee JD, Nunes EV, Novo P, et al. Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): a multicentre, open-label, randomised controlled trial. Lancet Lond Engl. 2018;391(10118):309–318. doi: 10.1016/S0140-6736(17)32812-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lander LR, Zheng W, Hustead JD, et al. Long-term treatment retention in West Virginia’s comprehensive opioid addiction treatment (COAT) program. J Neurol Sci. 2020;411:116712. doi: 10.1016/j.jns.2020.116712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown PJ, Stout RL, Mueller T. Substance use disorder and posttraumatic stress disorder comorbidity: Addiction and psychiatric treatment rates. Psychol Addict Behav. 1999;13(2):115–122. doi: 10.1037/0893-164X.13.2.115 [DOI] [Google Scholar]
- 12.Cacciola JS, Alterman AI, Rutherford MJ, McKay JR, Mulvaney FD. The relationship of psychiatric comorbidity to treatment outcomes in methadone maintained patients. Drug Alcohol Depend. 2001;61(3):271–280. doi: 10.1016/S0376-8716(00)00148-4 [DOI] [PubMed] [Google Scholar]
- 13.Kelly TM, Daley DC, Douaihy AB. Treatment of Substance Abusing Patients with Comorbid Psychiatric Disorders. Addict Behav. 2012;37(1):11–24. doi: 10.1016/j.addbeh.2011.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mangrum LF, Spence RT, Lopez M. Integrated versus parallel treatment of co-occurring psychiatric and substance use disorders. J Subst Abuse Treat. 2006;30(1):79–84. doi: 10.1016/j.jsat.2005.10.004 [DOI] [PubMed] [Google Scholar]
- 15.Levin FR, Mariani JJ. Co-occurring substance use disorder and attention deficit hyperactivity disorder. In: The ASAM Principles of Addiction Medicine. 6th ed. Wolters Kluwer; 2019. [Google Scholar]
- 16.Faraone SV, Sergeant J, Gillberg C, Biederman J. The worldwide prevalence of ADHD: is it an American condition? World Psychiatry. Published online 2003:10. [PMC free article] [PubMed] [Google Scholar]
- 17.Barkley RA, Fischer M, Smallish L, Fletcher K. The persistence of attention-deficit/hyperactivity disorder into young adulthood as a function of reporting source and definition of disorder. J Abnorm Psychol. 2002;111(2):279–289. doi: 10.1037/0021-843X.111.2.279 [DOI] [PubMed] [Google Scholar]
- 18.van Emmerik-van Oortmerssen K, van de Glind G, van den Brink W, et al. Prevalence of attention-deficit hyperactivity disorder in substance use disorder patients: a meta-analysis and meta-regression analysis. Drug Alcohol Depend. 2012;122(1–2):11–19. doi: 10.1016/j.drugalcdep.2011.12.007 [DOI] [PubMed] [Google Scholar]
- 19.Wilens TE, Biederman J, Mick E. Does ADHD Affect the Course of Substance Abuse?: Findings From a Sample of Adults With and Without ADHD. Am J Addict. 1998;7(2):156–163. doi: 10.1111/j.1521-0391.1998.tb00330.x [DOI] [PubMed] [Google Scholar]
- 20.Levin FR, Evans SM, Vosburg SK, Horton T, Brooks D, Ng J. Impact of attention-deficit hyperactivity disorder and other psychopathology on treatment retention among cocaine abusers in a therapeutic community. Addict Behav. 2004;29(9):1875–1882. doi: 10.1016/j.addbeh.2004.03.041 [DOI] [PubMed] [Google Scholar]
- 21.Carroll KM, Rounsaville BJ. History and significance of childhood attention deficit disorder in treatment-seeking cocaine abusers. Compr Psychiatry. 1993;34(2):75–82. doi: 10.1016/0010-440X(93)90050-E [DOI] [PubMed] [Google Scholar]
- 22.Crunelle CL, van den Brink W, Moggi F, et al. International Consensus Statement on Screening, Diagnosis and Treatment of Substance Use Disorder Patients with Comorbid Attention Deficit/Hyperactivity Disorder. Eur Addict Res. 2018;24(1):43–51. doi: 10.1159/000487767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carpentier P-J, Levin FR. Pharmacological Treatment of ADHD in Addicted Patients: What Does the Literature Tell Us? Harv Rev Psychiatry. 2017;25(2):50–64. doi: 10.1097/HRP.0000000000000122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Faraone SV. Using Meta-analysis to Compare the Efficacy of Medications for Attention-Deficit/Hyperactivity Disorder in Youths. Pharm Ther. 2009;34(12):678–694. [PMC free article] [PubMed] [Google Scholar]
- 25.Faraone SV, Glatt SJ. A comparison of the efficacy of medications for adult attention-deficit/hyperactivity disorder using meta-analysis of effect sizes. J Clin Psychiatry. 2010;71(6):754–763. doi: 10.4088/JCP.08m04902pur [DOI] [PubMed] [Google Scholar]
- 26.Boland H, DiSalvo M, Fried R, Woodworth KY, Wilens T, Faraone SV, Biederman J. A Literature Review and Meta-Analysis on the Effects of ADHD Medications on Functional Outcomes. Journal of Psychiatric Research 123 (April 2020): 21–30. 10.1016/j.jpsychires.2020.01.006. [DOI] [PubMed] [Google Scholar]
- 27.Konstenius M, Jayaram-Lindström N, Guterstam J, Beck O, Philips B, Franck J. Methylphenidate for attention deficit hyperactivity disorder and drug relapse in criminal offenders with substance dependence: a 24-week randomized placebo-controlled trial. Addict Abingdon Engl. 2014;109(3):440–449. doi: 10.1111/add.12369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levin FR, Mariani JJ, Specker S, et al. Extended-Release Mixed Amphetamine Salts vs Placebo for Comorbid Adult Attention-Deficit/Hyperactivity Disorder and Cocaine Use Disorder. JAMA Psychiatry. 2015;72(6):593–602. doi: 10.1001/jamapsychiatry.2015.41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vold JH, Aas C, Skurtveit S, et al. Dispensation of attention deficit hyperactivity disorder (ADHD) medications in patients receiving opioid agonist therapy; a national prospective cohort study in Norway from 2015 to 2017. BMC Psychiatry. 2020;20(1):119. doi: 10.1186/s12888-020-02526-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mariani JJ, Levin FR. Treatment Strategies for Co-Occurring ADHD and Substance Use Disorders. Am J Addict. 2007;16(s1):45–56. doi: 10.1080/10550490601082783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bijlenga D, Kulcu S, van Gellecum T, Eryigit Z, Kooij JJS. Persistence and Adherence to Psychostimulants, and Psychological Well-Being Up to 3 Years After Specialized Treatment of Adult Attention-Deficit/Hyperactivity Disorder: A Naturalistic Follow-Up Study. J Clin Psychopharmacol. 2017;37(6):689–696. doi: 10.1097/JCP.0000000000000787 [DOI] [PubMed] [Google Scholar]
- 32.Bejerot S, Rydén EM, Arlinde CM. Two-year outcome of treatment with central stimulant medication in adult attention-deficit/hyperactivity disorder: a prospective study. J Clin Psychiatry. 2010;71(12):1590–1597. doi: 10.4088/JCP.09m05168pur [DOI] [PubMed] [Google Scholar]
- 33.Edvinsson D, Ekselius L. Long-Term Tolerability and Safety of Pharmacological Treatment of Adult Attention-Deficit/Hyperactivity Disorder: A 6-Year Prospective Naturalistic Study. J Clin Psychopharmacol. 2018;38(4):370–375. doi: 10.1097/JCP.0000000000000917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Castells X, Blanco-Silvente L, Cunill R. Amphetamines for attention deficit hyperactivity disorder (ADHD) in adults. Cochrane Database Syst Rev. 2018;8:CD007813. doi: 10.1002/14651858.CD007813.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gossop M, Marsden J, Stewart D, Rolfe A. Treatment retention and 1 year outcomes for residential programmes in England. Drug Alcohol Depend. 1999;57(2):89–98. doi: 10.1016/S0376-8716(99)00086-1 [DOI] [PubMed] [Google Scholar]
- 36.Simpson DD. The relation of time spent in drug abuse treatment to posttreatment outcome. Am J Psychiatry. 1979;136(11):1449–1453. doi: 10.1176/ajp.136.11.1449 [DOI] [PubMed] [Google Scholar]
- 37.Simpson DD. Treatment for Drug Abuse: Follow-up Outcomes and Length of Time Spent. Arch Gen Psychiatry. 1981;38(8):875–880. doi: 10.1001/archpsyc.1981.01780330033003 [DOI] [PubMed] [Google Scholar]
- 38.Levin FR, Evans SM, Brooks DJ, Kalbag AS, Garawi F, Nunes EV. Treatment of methadone-maintained patients with adult ADHD: double-blind comparison of methylphenidate, bupropion and placebo. Drug Alcohol Depend. 2006;81(2):137–148. doi: 10.1016/j.drugalcdep.2005.06.012 [DOI] [PubMed] [Google Scholar]
- 39.Wilens TE, Adler LA, Weiss MD, et al. Atomoxetine treatment of adults with ADHD and comorbid alcohol use disorders. Drug Alcohol Depend. 2008;96(1):145–154. doi: 10.1016/j.drugalcdep.2008.02.009 [DOI] [PubMed] [Google Scholar]
- 40.King VL, Brooner RK, Kidorf MS, Stoller KB, Mirsky AF. Attention deficit hyperactivity disorder and treatment outcome in opioid abusers entering treatment. J Nerv Ment Dis. 1999;187(8):487–495. doi: 10.1097/00005053-199908000-00005 [DOI] [PubMed] [Google Scholar]
- 41.Tamm K, Trello-Rishel K, Riggs P, et al. , Predictors of treatment response in adolescents with comorbid substance use disorder and attention deficit/hyperactivity disorder. J Subst Abuse Treat. 2013;44(2):224–230) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McCabe SE, West BT. Medical and nonmedical use of prescription stimulants: results from a national multicohort study. J Am Acad Child Adolesc Psychiatry. 2013;52(12):1272–1280. doi: 10.1016/j.jaac.2013.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McCabe SE, Veliz P, Wilens TE, Schulenberg JE. Adolescents’ Prescription Stimulant Use and Adult Functional Outcomes: A National Prospective Study. J Am Acad Child Adolesc Psychiatry. 2017;56(3):226–233.e4. doi: 10.1016/j.jaac.2016.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wilens TE, Adler LA, Adams J, et al. Misuse and Diversion of Stimulants Prescribed for ADHD: A Systematic Review of the Literature. J Am Acad Child Adolesc Psychiatry. 2008;47(1):21–31. doi: 10.1097/chi.0b013e31815a56f1 [DOI] [PubMed] [Google Scholar]
- 45.Faraone W Does Stimulant Treatment Lead to Substance Use Disorders? Published online 2003:5. [PubMed] [Google Scholar]
- 46.McCabe SE, Dickinson K, West BT, Wilens TE. Age of Onset, Duration, and Type of Medication Therapy for Attention-Deficit/Hyperactivity Disorder and Substance Use During Adolescence: A Multi-Cohort National Study. J Am Acad Child Adolesc Psychiatry. 2016;55(6):479–486. doi: 10.1016/j.jaac.2016.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1. Covariates included in regression analysis predicting treatment duration. Hazard ratios (HR), 95%-CI, and p-values are reported for each measured covariate. Of measured covariates, ADHD medication alone significantly affects duration of treatment. * indicates significance at alpha = 0.05.