Abstract
Small-diameter vascular grafts perform poorly as arterial bypasses. We developed a cell-free, resorbable graft intended to remodel in situ into a living vessel. The graft consisted of a soft electrospun PGS core, a thin pPGS coating, and a reinforcing electrospun PCL sheath. The PGS core contained 4.37±1.95 μm fibers and had a porosity of 66.4±3.2%, giving it large pores to encourage cellular infiltration and pro-healing macrophage polarization. Similarly, the PCL sheath contained 6.63±0.89 μm fibers and had a porosity of 80.5±2.1%. Graft compliance was 1.5±1.0%/100 mmHg. Comparing uniaxial stress-strain curves with the measured compliance suggested that the stress achieved at arterial pressure would be 13-fold lower than the yield stress of the graft. Grafts were thus implanted as 7 cm carotid interpositions in two (2) sheep. Sheep were maintained on DAPT and followed with duplex US. One graft ruptured at 13 d. The second animal was euthanized with a dilated graft at 15 d. Histology showed near-total degradation of the PGS core and an excessive inflammatory response within the PCL sheath. Little neotissue had formed within the graft wall or lumen, but the graft had become surrounded by fibroblast-rich and vascularized connective tissue that may have been partially load-bearing. Because PCL is commonly and successfully used in small and large animal vascular graft designs, this mechanical destabilization was unexpected. We speculate that the inflammatory response instigated by the rapidly degrading PGS core intensified degradation and damage in the nearby PCL sheath, and that the large pores of the sheath enabled inflammatory cells to permeate and attack the entire bulk of the material, speeding weakening. Future work will examine whether slower-degrading or less cell-permeable materials would produce less aggressive inflammation in large animals.
Introduction
The ultimate limb-sparing treatment for peripheral arterial occlusion is implantation of a bypass graft.1 Approximately 460,000 such grafts are implanted each year in the U.S. alone.2 When the vessels concerned have diameters above ~6 mm, surgeons can create bypass grafts either from transplanted autologous vessels or from commercially available prosthetics. However, at smaller diameters, prosthetic grafts occlude so frequently by stenosis and thrombosis as to preclude their use.1 Even vein grafts eventually stenose under stress from the arterial environment.3 Furthermore, autograft harvest necessitates donor site morbidity and adds operative time.
Since the early 1960s, researchers have been studying tissue-engineered grafts as possible peripheral bypass conduits. The most successful designs have utilized autologous cell seeding4, extended in vitro culture4,5, and/or decellularization.5 These long and complex fabrication steps have hampered production scale-up. An alternative strategy is to use a biodegradable synthetic graft. It is typically simpler and less expensive to produce a synthetic material, and such grafts are available off the shelf.6 After implantation, the graft is remodeled by host cells into an artery-like conduit (“neoartery”).
We previously described implants of a resorbable synthetic graft in the rat infrarenal abdominal aorta.7 The bulk of the graft was a tube of foamy poly(glycerol sebacate) (PGS), a fast-degrading and soft elastomer. The PGS core was reinforced with a thin layer of nanofibrous poly(ε-caprolactone) (PCL), a stiff, ductile, and slow-degrading material. The graft did not contain any biologic components except for an adsorbed heparin coating. Within 3 mo., these grafts remodeled into neoarteries expressing contractile smooth muscle markers and containing 70% of the native amount of elastin. By 1 yr., the grafts had developed some vasoactivity and adventitial nerve ingrowth.8 Because we also saw elastin expression in vascular smooth muscle cells cultured on PGS scaffolds in vitro, we hypothesized that the soft PGS core sent regenerative mechanobiological cues to infiltrating cells,9,10 and the core’s rapid degradation removed the triggers for a chronic inflammatory response.7
Larger-diameter foamy PGS cores can be constructed. However, the process is skill- and labor-intensive, and the scaffolds tear at relatively low strains.11 Electrospinning PGS offers a higher-yield way to a tougher scaffold.12 Still, electrospinning is often criticized as poorly reproducible, and bench-scale setups typically have low throughput. For example, it takes approximately 1.5 h to electrospin one 5 mm diameter × 7 cm long PGS graft core using the parameters described in Jeffries et al., and the process requires close, trained supervision.12 Previously described electrospun PGS grafts also showed unexpectedly slow degradation in vivo,13 possibly due to over-crosslinking, residual polyvinyl alcohol (PVA) content, and/or inadequate cell infiltration into the dense scaffold.
The nanofibrous PCL sheath described previously7 is also difficult to scale to larger diameters. Those nanofibers were collected onto a salt-hardened PGS core rotating at a fixed distance in front of a collecting plate. Some fibers land on the core directly, and some are drawn from the collecting plate onto the rotating core under tension. This tension causes the core to buckle if it is not thoroughly reinforced by salt crystals (unpublished data). It also makes it difficult to track the progress of electrospinning, because newly wound fibers can depress the ones beneath, meaning sheath mass is increasing while graft diameter is not.
This report describes the design, fabrication, and characterization of electrospun PGS grafts that survived implant in a large animal model of peripheral arterial bypass. Though these grafts dilated, the new fabrication process allows for facile and rapid prototype iterations. Additionally, we hope the mechanical and histological characterization presented here will help other investigators design more robust grafts for the large animal arterial circulation and ask more nuanced questions about material interaction effects.
Materials and Methods
Graft Fabrication
Materials.
PGS prepolymer (pPGS) was synthesized in-house as described by Wang et al.14 or kindly given by the Secant Group (RG-100). PVA (G-Polymer OKS-8070P) was generously provided by Soarus. PCL (cat. no. 440744) was purchased from MilliporeSigma. Sodium hyaluronate (cat. no. 251770050) was purchased from Acros Organics. 1,1,1,3,3,3-hexafluoro-2-propanol (HFIP, cat. no. 003409) was purchased from Oakwood Chemical. 2,2,2-trifluoroethanol (TFE, cat. no. T0435) was purchased from TCI. Acetone (cat. no. 329000000CSGF) was purchased from Pharmco-Aaper. Ethanol (cat. no. 89125-172) was purchased from VWR. All fabrication was performed in an ISO 7 cleanroom maintaining a temperature of 20–22°C and a humidity of 40–50% RH.
PGS Core Fabrication.
pPGS/PVA electrospinning was adapted from Jeffries et al.12 [Fig. IV–1(A)] pPGS (house) was dissolved with PVA and HFIP at 4.1% wt. PVA, 5.0% wt. pPGS, and 90.9% wt. HFIP (0.16 g polymer : 1 mL solvent). The solution was pumped at 100 μL/min from a 22G needle charged to +14 kV. The spinneret needle was flanked by two 18 AWG stainless steel wire segments. These focusing wires formed the two vertical edges of a 12 cm × 12 cm square with the spinneret needle tip at its center. [Fig. IV–1(B)] The focusing wires and spinneret were equipotential. Fibers were collected on a grounded stainless steel mandrel (D = 4.76 mm, L = 38 cm) positioned 45 cm from the spinneret and rotating at 500 rpm. The mandrel had been pre-coated in 1% (g/mL) sodium hyaluronate and dried. A 5 cm long 22G needle, serving as a guiding electrode, was positioned another 45 cm from the mandrel and charged to −14 kV. The spinneret assembly and guiding electrode were continuously slewed parallel to the mandrel at 5 mm/s over an 18 cm range. After every 1.5 mL of solution dispensed, the polarities of the spinneret assembly and guiding electrode were reversed.
Figure IV–1.
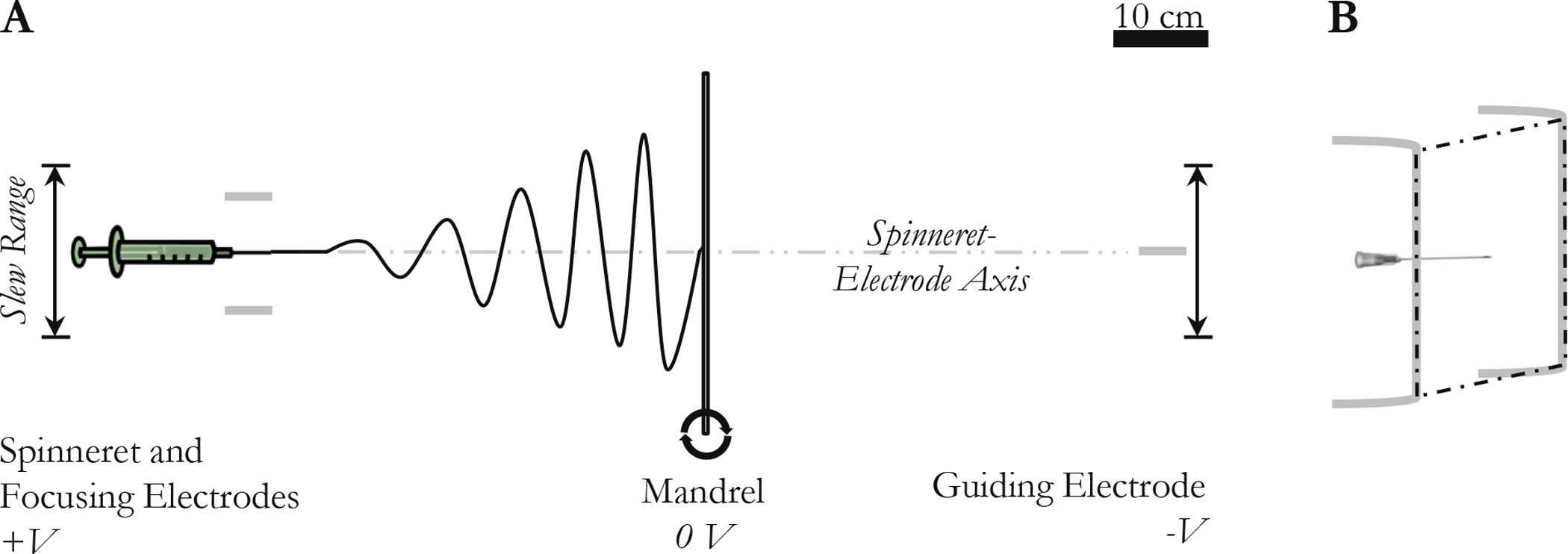
PGS electrospinning schematics. (A) Scale diagram of electrospinning system viewed from the top. The spinneret and the guiding electrode move in parallel so that the spinneret-electrode axis is always perpendicular to the mandrel. (B) The spinneret and focusing electrodes viewed from the top right. The tip of the needle and the vertical portions of the focusing electrodes are coplanar.
Electrospinning continued until the core wall thickness reached 750 μm. Electrospun cores were dried at least overnight in vacuum before curing for 48 h at 120°C and >29 inHg vacuum. During curing, core wall thicknesses shrank to approximately 500 μm. Cured cores were stored in vacuum until sheath application.
pPGS Coating Electrospraying.
pPGS (RG-100) was dissolved in acetone and ethanol at 25% wt. pPGS, 21% wt. acetone, and 54% wt. ethanol. The solution was pumped at 20 μL/min from a 22G needle charged to +17 kV. The spinneret was again flanked by the equipotential focusing wires. The spray produced was collected onto a cured PGS/PVA core, which was positioned 10 cm from the spinneret. The core was grounded and rotating at 5000 rpm. The spinneret assembly was continuously slewed parallel to the mandrel at 5 mm/s over an 18 cm range. 96 μL of solution was dispensed per core. Cores continued to rotate for 1 h afterwards to dry the coating, then were dried stationary in vacuum at least overnight.
PCL Sheath Electrospinning.
PCL dissolved at 15% wt. in TFE was pumped from a 22G spinneret at 200 μL/min. The spinneret was again flanked by the focusing wires and charged to −11 kV. Fibers were collected on a coated PGS/PVA core, which was positioned 20 cm from the spinneret horizontally (along the axis of the electrospinning jet) and 8 cm below it. The core was grounded and rotating at 300 rpm. The spinneret assembly was continuously slewed parallel to the mandrel at 5 mm/s over an 18 cm range. Electrospinning continued until the sheath wall thickness reached 375 μm.
Purification and Sterilization.
Grafts were submerged in water overnight, then removed from their mandrels. Grafts were washed for at least 7.5 h in room temperature water and overnight in 37°C water. Finally, they were lyophilized and sterilized by room temperature ethylene oxide (Andersen AN71i, 12 h cycle with 36 h aeration). Grafts to be implanted were immersed in 4°C heparinized saline (100 USP/mL) the night prior.
Graft Characterization
Gross Morphology.
5 mm long graft rings (n = 5) were imaged on a dissecting microscope (Nikon SMZ 745T). Dimensions were measured both wet and lyophilized.
Gravimetric Analysis.
5 mm long graft rings (n = 5) were delaminated and lyophilized. The dimensions of the separated cores and sheaths were measured on a dissecting microscope (Nikon SMZ 745T). Samples were then massed on a microbalance (Mettler Toledo XP26). Porosity (ϕ) was calculated according to:
where m is the mass, B is the envelope volume, and v is the bulk material specific volume. The specific volume of the core material was taken to be a 90:10 mass-weighted average of the specific volumes of PGS (0.885 mL/g)15 and PVA (0.737 mL/g): namely, 0.870 mL/g. The specific volume of the sheath material was taken to be that of PCL, which the manufacturer reported as 0.873 mL/g.
Scanning Electron Microscopy.
If sheath cross-sections were to be imaged, wetted graft segments were frozen for at least 3 min on dry ice or in liquid nitrogen and shattered with a chilled razor. If not, graft segments were simply cut wet. Lyophilized graft segments were sputter-coated with 18 nm of gold/palladium and imaged at 5 kV and 9 mm working distance on a TESCAN MIRA3 scanning electron microscope (SEM). Wall thickness measurements were taken from 150X images of five graft cross-sections (n = 5). Fiber orientation and diameter were measured from 1000X en face images using the DiameterJ v1.018 plugin for FIJI running ImageJ 1.51w (NIH).16,17 Three (3) 1000X images were analyzed from each of five (5) graft segments for a total of fifteen (15) images.
Residual PVA Quantification.
Residual PVA within the PGS cores was quantified via the heat of PVA crystallization. 4–9 mg samples of graft cores (n = 3) were thermally scanned in a Q2000 differential scanning calorimeter (DSC, TA Instruments). Samples were heated to 220°C, cooled to −10°C, and heated again to 220°C at 5°C/min. The area under the crystallization peak near 145°C was measured using the manufacturer’s software. [Fig. SIV–1(A)] Scans of pure PGS and PVA had revealed that PVA had a crystallization point there, but the PGS curve was flat. The measured heats of crystallization were compared to a calibration curve constructed using films of varying PGS:PVA mass ratios. [Fig. SIV–1(B)] Films had been cast from HFIP, cured at 120°C and 29 inHg vacuum for 48 h, and stored in desiccant until use.
Uniaxial Tensile Mechanics.
Delaminated sheaths (n = 9) or cores (n = 5) were cut into rectangles and wetted. Sheaths were assumed to be 0.32 mm thick and were cut to a width of 2 mm. Cores were assumed to be 0.54 mm thick and were cut to a width of 4 mm. Specimens were mounted into a uniaxial tensile testing system with a 50 N load cell (Instron 5943) such that the gauge length was 10 mm. Specimens were preconditioned for 10 cycles between 0 and 5% strain. Specimens were then extended further until the load reached zero, and the gauge length was reset. Finally, specimens were strained to failure. The strain rate was 5%/s. Incremental elastic moduli were fit over the 1–5% strain range in the failure cycle. The sheath yield points were defined using the incremental modulus and a 1% strain offset. Moduli were compared with a Kruskal-Wallis test followed by Wilcoxon rank-sum tests with a Bonferroni correction.
Compliance.
Compliance testing was adapted from ANSI standards.18 25 mm long graft segments (n = 5) were tied down onto appropriately sized cannulae. The cannulae were connected to a closed system containing a pressure sensor (PM-4, Living Systems), and the distance between the cannulae was fixed at the unpressurized axial length of the graft. The system was submerged in water and pressurized over the 80–120 mmHg human (and ovine) arterial pressure range.19
Graft diameter during pressurization was monitored by laser micrometer (LS-7601, Keyence). Analog outputs from both the pressure sensor and the micrometer were sampled by a data acquisition system (PowerLab, AD Instruments). Compliance (%/100 mmHg) was calculated as:
where D is the inner diameter of the graft at the indicated pressure, calculated by assuming incompressibility.
Suture Retention Load.
Suture retention load testing was adapted from ANSI standards.18 Grafts (n = 5) were cut straight across and mounted wet in a uniaxial tensile testing system with a 50 N load cell (Instron 5943) such that a 5 mm long tube protruded from the clamp. 6–0 suture (Ethicon 8709H) was passed through one wall of the tube 2 mm from the free edge. The suture was pulled out at 120 mm/min. Because failure typically damaged the entire circumference, each sample was only tested once.
Water Permeability.
Water permeability testing was adapted from ANSI standards.18 Swatches of graft material (n = 3) were secured over the bottom of a 22 cm L × 0.79 cm ID cylindrical tube. The tube was held upright and filled to the brim with water. The water leaking through the graft material over 90 s was captured and massed. The tube was refilled continuously during the test to keep the hydrostatic pressure on the material constant. Leakage rates were compared with a Kruskal-Wallis test followed by Wilcoxon rank-sum tests with a Bonferroni correction.
Ovine Carotid Interposition
Implantation.
All animal experimentation was approved by the Cornell University IACUC. Implant was performed by a practicing vascular surgeon (E. T.) Two 18 mo. old Finn ewes weighing 45 and 47 kg were purchased from a local farm. Sheep were premedicated with 325 mg/d oral aspirin and 75 mg/d oral clopidogrel beginning 5 d ahead of graft implant. A transdermal patch delivering 100 μg/h of fentanyl for 3 d was placed the night prior to implant. The Cornell University Hospital for Animals induced and maintained a surgical plane of anesthesia. Approximately 8 cm of the right common carotid artery was surgically exposed. Branches were tied with 2–0 silk (Ethicon A305). 100 U/kg heparin was administered, and the artery was clamped and transected. 7 cm grafts were placed as interpositions using 6–0 nonabsorbable sutures (Ethicon 8709H) and a running anastomotic technique. Vasospasm presented, so the native arterial stubs were spatulated. Patency was confirmed intraoperatively by palpation of the distal carotid and/or by the pressure reading in an arterial catheter placed in the ipsilateral ear. Layers were closed with absorbable 2–0 suture (Ethicon Y943H). Sheep were administered 1 mg/kg oral meloxicam once daily and 22,000 IU/kg intramuscular procaine penicillin G twice daily from the day of implant (day 0) through the end of day 3. Aspirin and clopidogrel administration continued to the study endpoint.
Imaging.
CT angiograms were performed immediately after implant (Aquilion Large-Bore 16-slice CT, Toshiba). 2 mL/kg Isovue-300 was injected intravenously at 5 mL/s. Slices were acquired in 1 mm increments along the neck. After thresholding and 3D reconstruction within the manufacturer’s software, the diameters of the graft and contralateral carotid were measured in FIJI/ImageJ.
B-mode and color Doppler ultrasound exams were performed by the Cornell University Hospital for Animals using an EPIQ 5G system with an L12-5 transducer (Philips). Exams were done on days 8 and 15. Graft diameters were measured in FIJI/ImageJ.
Sacrifice and Explant.
After administration of 100 U/kg heparin, sheep were euthanized by barbiturate overdose. Grafts were explanted immediately.
Neoartery Characterization
Explants and/or unimplanted graft segments were fixed in 2% paraformaldehyde for 2 h, soaked in three changes of 30% sucrose over 24 h, then frozen in optimal cutting temperature compound and sectioned at 6 μm. Hematoxylin and eosin (H&E) staining was performed in-house; all other stains were completed at the New York Animal Health Diagnostic Center. Immunohistochemistry (IHC) utilized primary antibodies against the monocyte/macrophage marker ionized calcium binding adapter molecule-1 (IBA1, WAKO cat. no. 019–19741), vascular smooth muscle cell/myofibroblast marker smooth muscle actin (SMA, Leica cat. no. PA0943), and platelet/vascular endothelial cell marker von Willebrand factor (vWF, Dako cat. no. A0082) with species-appropriate secondary antibodies. Slides were imaged on a Nikon Ti2 Eclipse microscope. IHC controls included unimplanted graft material, native artery, and positive ovine tissue (spleen or native artery). Slides were examined by a board-certified veterinary pathologist (K. K.)
Statistics
Values, including error bars, are given as mean ± SD unless otherwise stated. Statistical analysis was done in MS Excel or MATLAB.
Results
Graft Characterization
Overall, graft fabrication was more efficient and reproducible than previous processes. Whereas the PGS electrospinning process described by Jeffries et al.12 required approximately 3 h of labor to produce 1 cm3 of PGS core, this new process required approximately 1 h. Furthermore, of the 48 PGS cores electrospun under this new process, 43 (90%) required no more than basic unskilled maintenance to maintain system stability (i.e., no more intervention than switching the voltage polarities at the intervals instructed). By contrast, prior processes required practiced, close, and constant adjustments to maintain system stability.12,13 pPGS electrospraying and PCL sheath electrospinning together required approximately 30 min additional skilled labor per cm3 of PGS core volume. All other steps (curing, washing, etc.) were routine and unskilled.
Grafts presented a bilayered, porous structure. [Fig. IV–2(A–C)] The PGS core was composed of 4.37±1.95 μm fibers running roughly circumferentially. [Fig. IV–2(E, G)] As reported in Jeffries et al.,12 leaching out the PVA gave the fibers a rough surface texture. [Fig. IV–2(E)] The fibers partially fused on the lumenal surface where they had contacted the hyaluronate-coated mandrel. [Fig. IV–2(D)] The porosity of the dry core was 66.4±3.2%. [Fig. IV–2(H)] Heat of crystallization analysis indicated that 10.5±1.2% of the core mass was PVA that had not been removed by washing. [Fig. IV–2(H)] Since PVA is highly hydrophilic, the core swelled slightly when wet. Therefore, the wet core and sheath dimensions matched well, but the dry layers sometimes pulled apart to leave a gap, as is visible on SEM. [Fig. IV–2(B–C)] The PCL sheath was composed of smooth, lightly fused 6.63±0.89 μm fibers with a net axial orientation. [Fig. IV–2(F–G)] The sheath porosity was 80.5±2.1%. [Fig. IV–2(H)]
Figure IV–2.
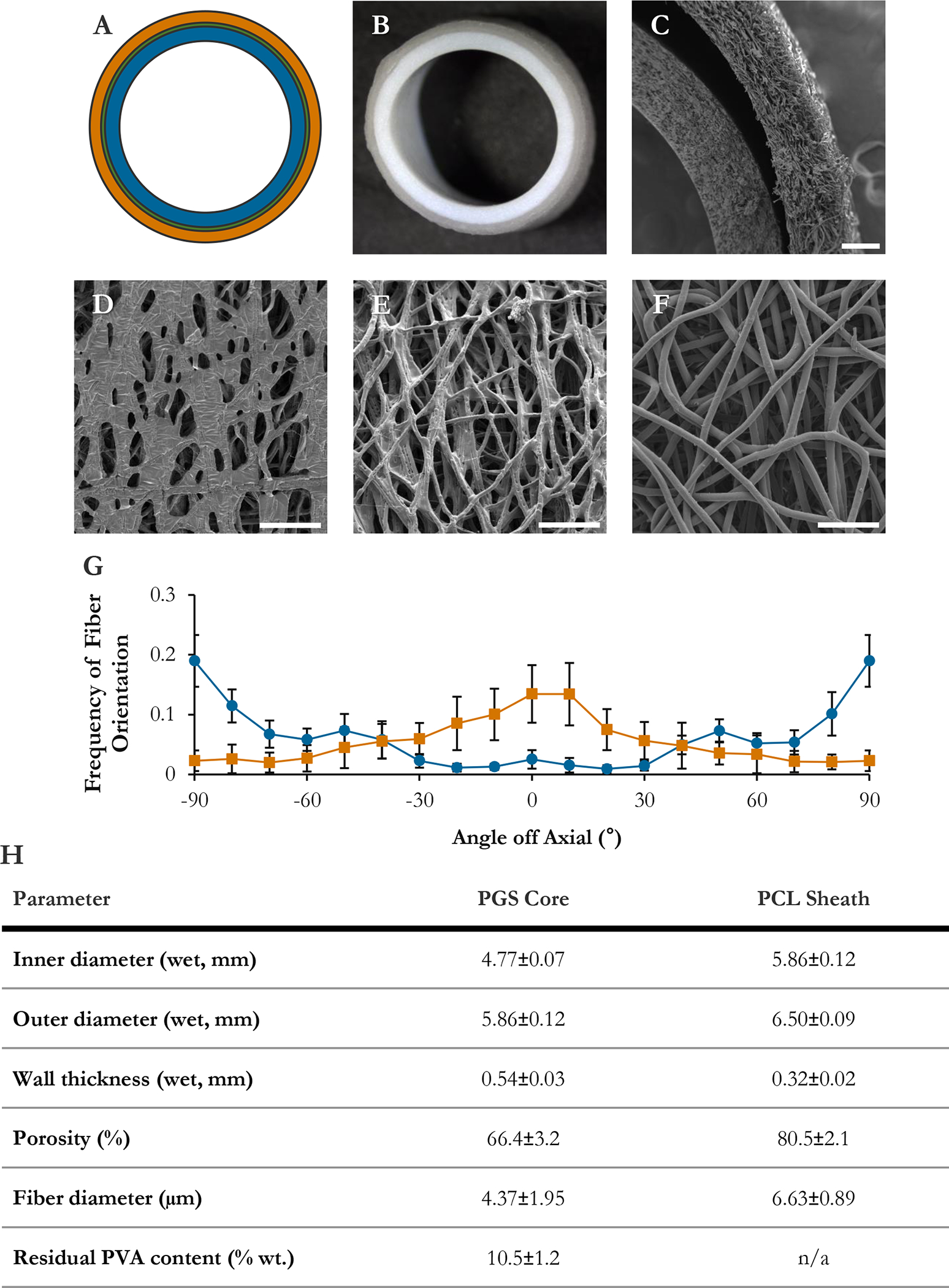
Graft morphology. (A) Scale schematic of graft design. Blue: PGS core. Green: pPGS coating (thin). Orange: PCL sheath. (B) Macroscopic image of wet graft cross-section. (C) Graft wall in transverse. (D) Lumen of PGS core. (E) Ablumen of PGS core. (F) Ablumen of PCL sheath. (G) Fiber orientation distributions in core (blue, circles) and sheath (orange, squares), binned in 10° increments. (H) Graft physical parameters. Bars are 200 μm (C) and 50 μm (D, E, F).
Tensile testing indicated that the PCL sheath was far stronger than the PGS core. The core had extremely low moduli (Eθ = 0.083±0.018 MPa, EZ = 0.044±0.017 MPa) and extended linearly to failure at high strain (UTSθ = 1.13±0.16 MPa at 344.3±23.0% strain, UTSZ = 0.253±0.04 MPa at 216.8±22.0% strain). [Fig. IV–3(A,C)] The sheath was much stiffer (Eθ = 6.11±0.43 MPa, EZ = 19.2±2.9 MPa). [Fig. IV–3(A,C)] It showed a pronounced ductile failure mode. [Fig. IV–3(B)] The circumferential yield stress was 0.59±0.07 MPa at 11±1% strain, and the axial yield stress was 1.25±0.08 MPa at 8±1% strain. In accord with the fiber orientation distributions measured on SEM, the PCL sheath was stronger in the axial direction, and the PGS core trended stronger in the circumferential.
Figure IV–3.
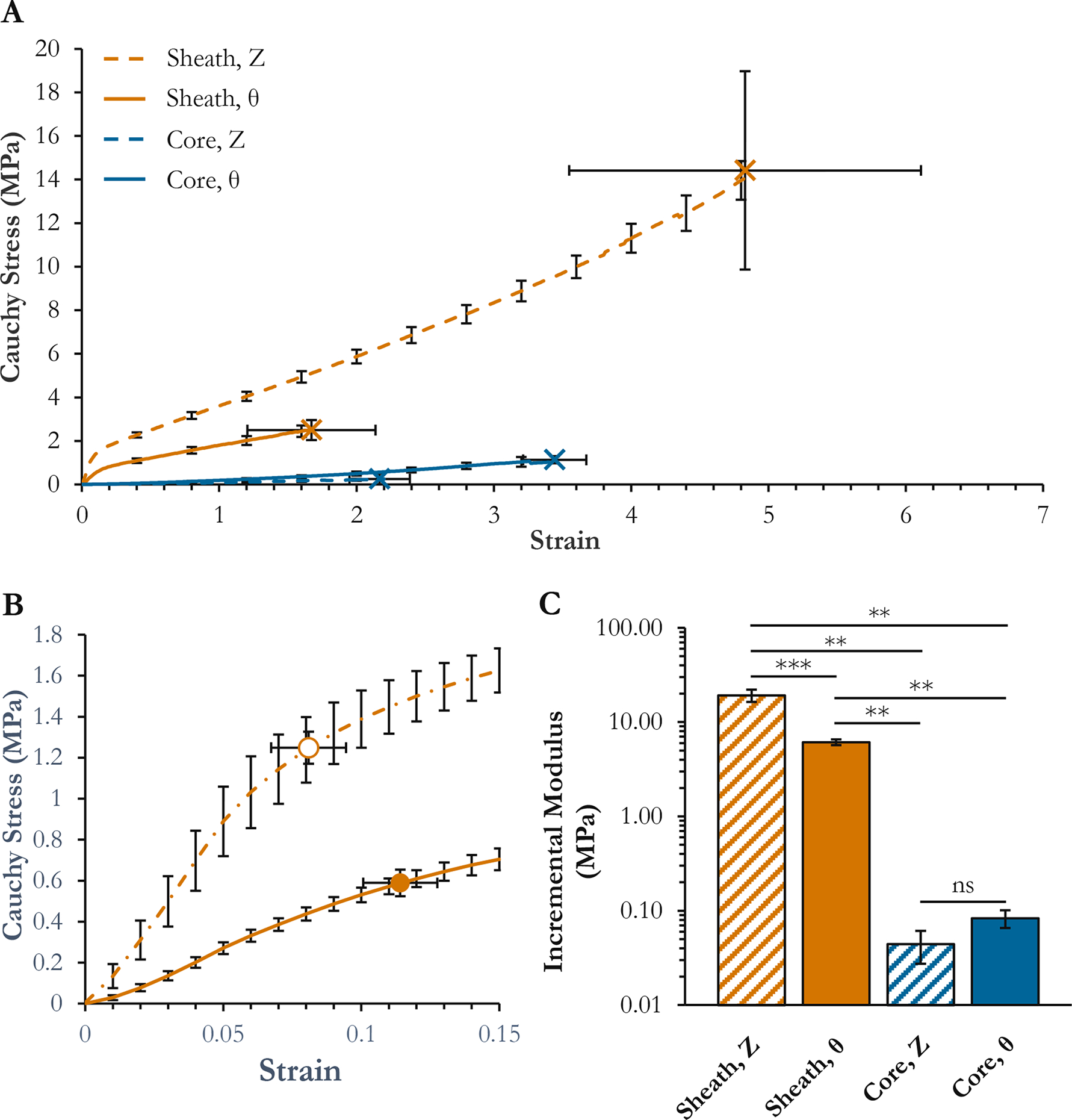
Graft mechanics as assessed by uniaxial tensile strain-to-failure. (A) Strain-to-failure curves. X’s with error bars in both directions are break points. (B) Inset of PCL strain-to-failure curves over the 0–15% strain range. Circles with error bars in both directions are yield points. (C) Incremental moduli fit over the 1–5% strain regions of each curve. ***Significant at p < 0.001. **Significant at p < 0.01. ns: not significant.
Graft functionality metrics were deemed sufficient for implant. Given the stiff sheath, graft compliance was unsurprisingly low. The grafts had a compliance of 1.5±1.0%/100 mmHg over the normal human arterial pressure range of 80–120 mmHg. [Fig. IV–4(A)] This translated into a systolic circumferential strain of 0.85% at the core-sheath interface, which was approximately thirteen (13) times lower than the circumferential yield strain of the sheath. For context, Konig et al. reported the compliance of human internal mammary arteries (IMA) at 11.5±3.9%/100 mmHg.20 The suture retention strength of the graft was similar to that of human IMA: 165±17 gf vs. 138±50 gf, respectively.20 [Fig. IV–4(B)] Finally, the coating and the sheath were both found to somewhat reduce transmural leakage. [Fig. IV–4(C)] But the difference was not significant, at least with the limited sample size, lowered pressure, and low viscosity fluid (water) tested.
Figure IV–4.
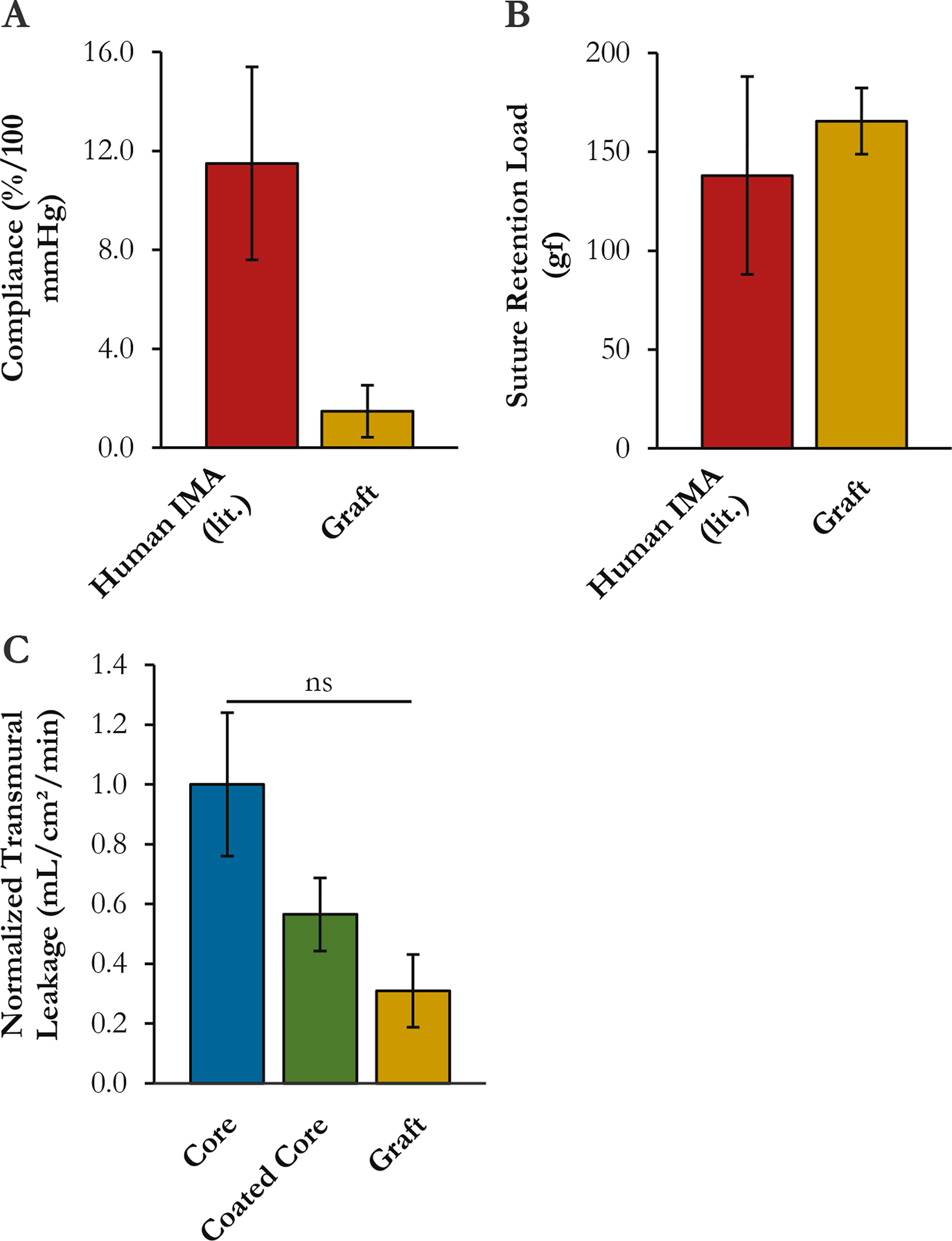
Graft functional properties. (A) Compliance. (B) Suture retention load. (C) Leakage rate at constant pressure. For context, reference values for human IMA from Konig et al.207 are shown. No statistical comparisons to reference values were performed. ns: not significant.
Arterial Interposition
Grafts held suture and handled well as interpositions. The graft visibly reddened upon unclamping, indicating full wetting of the wall with blood. [Fig. IV–5(A)] Hemostasis was achieved with light pressure and, in one animal, use of a GelFoam hemostatic sponge (removed prior to closure). Postoperative CT indicated both grafts were patent, though they were undersized by 10 and 23% relative to the contralateral carotids, and the anastomoses were complicated by vasospasm. [Fig. IV–5(B)] Ultrasound exams at 8 d indicated both grafts were patent with turbulent flow, and had dilated and/or eroded to inner diameters of 4.9±1.1 and 5.6±0.3 mm. [Fig. IV–5(C)] The graft that had been more dilated at 8 d ruptured at 13 d. Necropsy confirmed that both anastomoses were intact; the graft had split along its length. An ultrasound exam on the remaining graft at 15 d showed an inner diameter of 9.4±0.1 mm. [Fig. IV–5(D)] The second animal was immediately euthanized.
Figure IV–5.
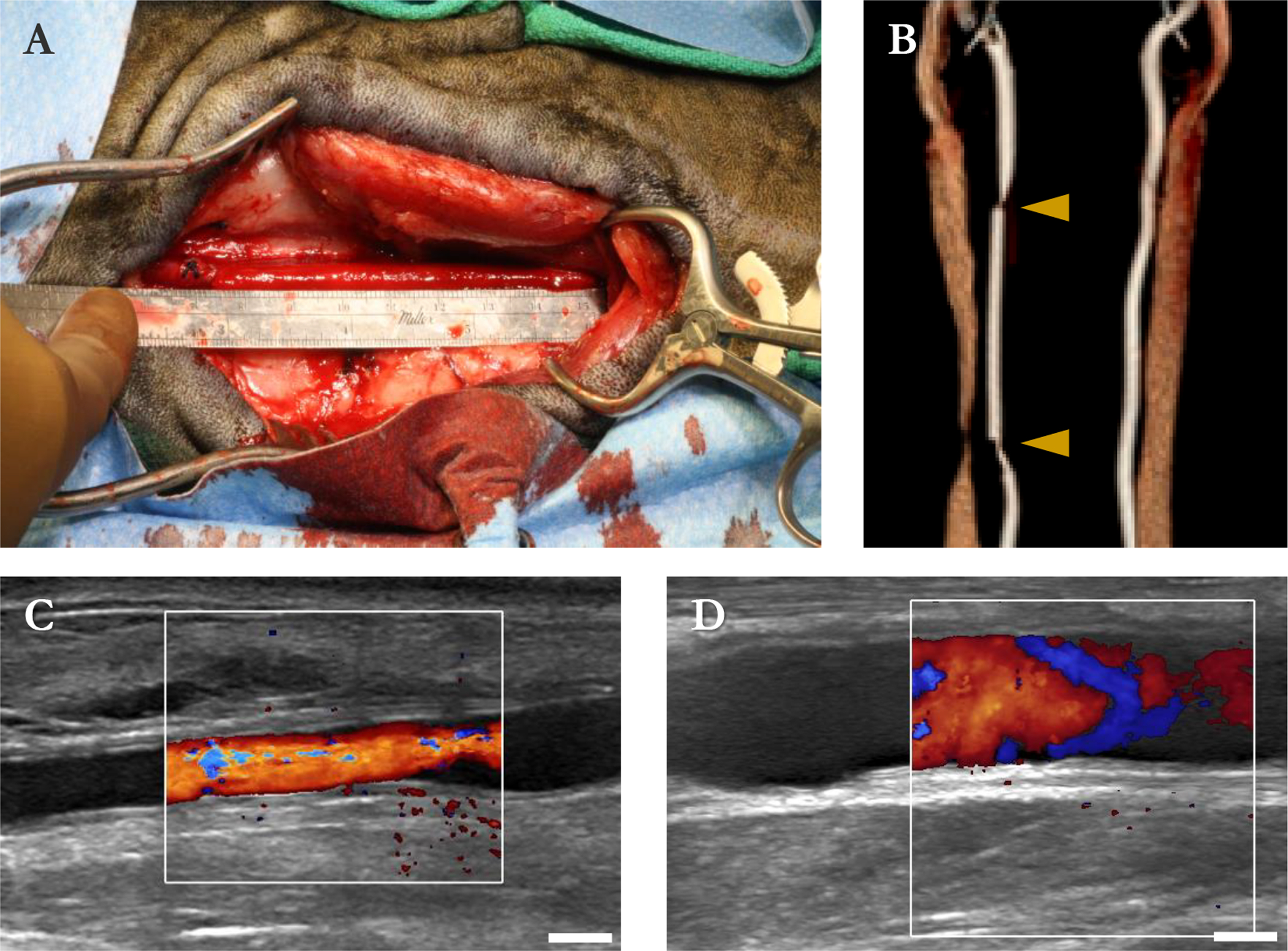
Sheep carotid interposition. (A) Graft immediately after unclamping and hemostasis. (B) CT angiogram of graft and contralateral carotid immediately after implant. Arrowheads: anastomoses. (C) B-mode and color Doppler image of graft at 8 d. (D) Same graft at 15 d. Bars are 5 mm (C, D).
Neotissue Characterization
The graft explanted at 15 d was fixed and stained. Histologic sections demonstrate layers of connective tissue, acute and chronic foreign-body inflammation, and necrosis arranged in coats typical of vascular grafts. PCL fibers were visible, but PGS was undetectable.
The inner coat was 40–400 μm thick with a lumen covered by a thick layer of amorphous, brightly eosinophilic necrosis and fibrin embedded with degenerate and viable neutrophils, monocyte-macrophages, and fewer eosinophils. [Fig. IV–6(A–C)] This largely acellular material was vWF+. [Fig. SIV–2(A)] At the interface of the inner coat with the graft material, there was a variable layer of connective tissue, SMA-fibroblasts, and variable inflammation. [Fig. IV–6(A–C), Fig. SIV–2(B–C)] Some collagen had formed in this area. [Fig. SIV–2(D)] The endothelial layer was absent except at the anastomosis sites.
Figure IV–6.
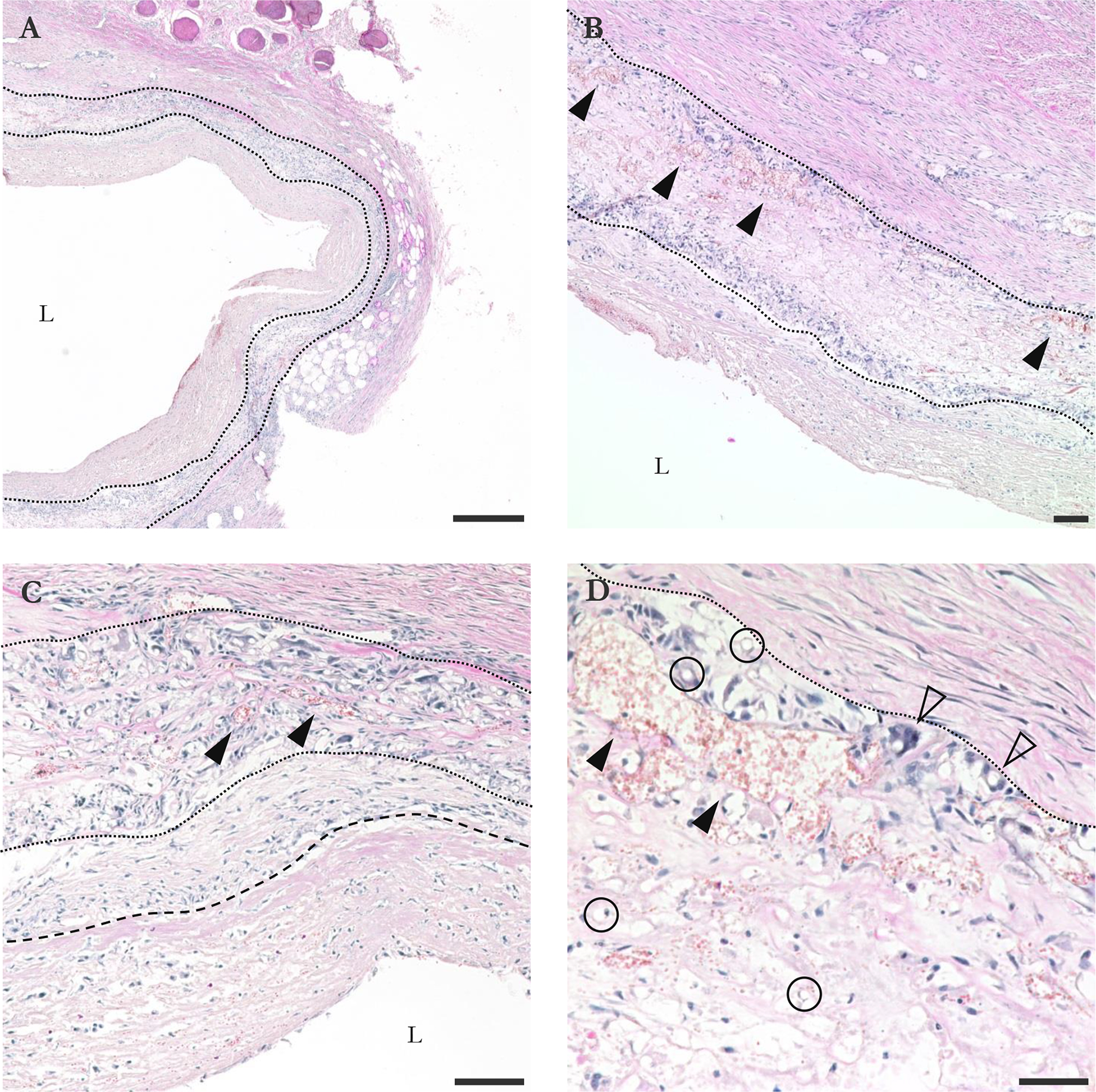
Transverse H&E sections of graft explanted at 15 d. (A) 4X view. Tears in inner and outer capsule are sectioning artifacts; tissue was delicate. Graft material is bounded by dotted lines. (B) 10X view. Solid arrowheads point out some of many capillaries within the graft wall. (C) 20X view. Transition between fibroblast-enriched area and less cellular area of inner capsule marked by dashed line. (D) 40X view of graft-outer capsule interface. Circles show some of many fibers, more easily identified in transverse. Hollow arrowheads show some of many multinucleated monocytes/macrophages. L indicates lumen. Bars are 500 (A), 100 (B, C), and 50 μm (D).
The remaining graft wall was up to 400 μm thick. [Fig. IV–6(A)] Fibers were intermingled with a mixture of multinucleated macrophages, lymphocytes and plasma cells, rare neutrophils, and connective tissue with capillaries and edema. [Fig. IV–6(B–D), Fig. SIV–2(B–D)] Inflammation was transmural but most intense at the interface of the inner and outer coats. [Fig. IV–6(B), Fig. SIV–2(C)] The variably apparent negative relief of the colorless graft fibers were often coated by multinucleated macrophages. [Fig. IV–6(D)] The connective tissue included lightly basophilic collagen, fibroblasts, and few SMA+ myofibroblasts. [Fig. IV–6(A–C), Fig. SIV–2(B–C)]
The outer coat was approximately 750 μm thick and composed of abundant spindled SMA+ myofibroblasts and fibroblasts with more darkly basophilic collagen arranged in concentric layers with lesser inflammation. [Fig. IV–6, Fig. SIV–2(B,D)] Scattered within the outer layers and extending into the surrounding connective tissue were variably sized demarcated nodular granulomas composed of multinucleated macrophages, lymphocytes, and peripheral fibrovascular tissue. [Fig. IV–6(A)]
Discussion
The dilation and rupture seen in these grafts was unexpected. PCL is common in load-bearing scaffolds precisely because it is considered strong and slow-degrading. In a 2018 review, we counted 16 publications describing animal implants of acellular vascular grafts containing PCL.6 These authors rarely reported dilation. Even in the more demanding large animal applications, PCL-based grafts were generally stable over the 1–6 mo. studied.21–23
Of course, a graft made of any material might fail if it is designed too thin for the pressure. Prior to implant, we had performed and passed all mechanical tests common in the field. (One exception: we could not test for burst pressure because the grafts leaked too much to inflate.) Uncommonly, we had also designed to avoid yield, not just rupture. This was inspired by the law of Laplace for thin-walled cylinders:
where σθ is the circumferential stress in the wall, P is the lumenal pressure, r is the average radius, and t is the wall thickness. If the graft dilates, radius will increase, and thickness will decrease. This will increase the wall stress at a given arterial pressure, which will cause the graft to dilate more in a positive feedback loop. Absent reinforcement from neotissue or special material properties that allow stiffening with strain, any plastic deformation once begun will therefore ultimately lead to rupture (though possibly after a very long time).
Because of the complexity of the viscoelastic and microstructural effects at play, we could not predict when rupture would occur, even absent the effects of degradation on material properties. We therefore decided to design conservatively. We assumed that the entire load of pressurization and any axial load would be borne by the PCL sheath, and we chose a sheath thickness such that the strain experienced under arterial pressure would be at least ten (10) times less than the yield strain measured from uniaxial tensile testing. The achieved safety factor was 13. Additionally, given ~1 N estimates of the axial loads in the sheep carotid,24 we adjusted the fiber alignment of the PCL sheath such that the axial strength was approximately twice the circumferential. An earlier implant attempt using a thinner PCL sheath and a safety factor of 5 had resulted in 3/4 graft ruptures within approximately 10 d, with 3/4 ruptures known to have occurred within minutes of animals distending their necks axially.
These thicker, stronger grafts survived longer, and only 1/2 ruptured before the planned 15 d endpoint. In fact, the level of dilation observed in the surviving animal (~100%) was far past the measured yield point of the PCL sheath, suggesting that load had partially been transferred to neotissue—presumably, the fibrotic tissue encapsulating the graft. The graft was also palpably softer and more flexible at explant, suggesting the stiff PCL sheath had lost much of its load-bearing capacity. Some level of fibrotic tissue formation is expected from any surgery and ideally would resolve with time. Resorbable graft designers might take advantage of this effect for extra structural support early in remodeling.25 But this was not sufficient to prevent dilation and rupture. The uneven thickness of the sheath on histology suggests that different portions may have stretched out more or less depending on how well they were locally supported by the outer capsule.
A second surprise was the extremely rapid degradation of the PGS core. In mice, electrospun PGS had been present up to 3 mo., perhaps because those grafts’ low pore size limited cell-mediated degradation to the lumenal surface.13 We increased the core porosity in these grafts in an attempt to encourage cell infiltration and speed remodeling. That effort apparently succeeded. Yet the lack of substantial neoarterial tissue indicates degradation now occurred faster than matrix synthesis. Possibly, the fibroblast-enriched region of the inner capsule represents the last region of PGS to degrade and the furthest along in neotissue formation. The thick lumenal layer of fibrin and necrosis with embedded neutrophils is consistent with continuation of the early phase (first 2 wks.) of the host reaction to traditional prosthetic grafts and does not appear to be involved in productive remodeling.26
We suspect these two surprising results are related. Our previous work in mice and rats used a nanofibrous and dense PCL sheath.7,8,13,27 This sheath was difficult to apply to large grafts, and furthermore, we had noticed acellular regions in the resulting neoarteries that we guessed corresponded to where cells had struggled to penetrate and remodel the tightly packed sheath material. So, we developed a new PCL electrospinning method. This technique was applicable to grafts of any size and produced a more porous structure. We hoped thereby to encourage cell infiltration and generate pro-healing phenotypes.28 Though we did not measure pore size, our PCL fiber diameter and porosity was similar to previously reported PCL grafts that polarized macrophages towards M2 phenotypes in the rat aorta.29 The overall inflammatory response did not seem to be a pro-healing one, but we did indeed see greatly improved cellular infiltration into the PCL sheath. Additionally, recently published work has highlighted inter-species differences in inflammation and remodeling of vascular grafts. When identical grafts were implanted in sheep or rats, sheep demonstrated more aggressive inflammation and faster biomaterial degradation.30
We therefore speculate that the inflammatory response initiated by the rapidly degrading PGS core spilled over into the PCL sheath. Inflammation may have been further aggravated by the ovine physiology degrading the PGS more rapidly and/or by an influx of cells from the ablumen into the porous PCL sheath. Overall, the PCL sheath accumulated damage more rapidly than was expected, resulting in graft dilation and poor neotissue formation.
Conclusion
Little is known about inflammatory and remodeling responses to bioresorbable scaffolds in large animals, primarily due to the expense and difficulty of the models. This has unfortunately left many developers of resorbable grafts in the dark about what new phenomena they may encounter as they make the jump from small to large. These results, though not traditionally positive, still provide the field several useful pieces of information. Firstly, as these grafts survived implant and several days in the ovine carotid, the mechanical properties reported here can serve as a minimum for other designs. Researchers may well find that in the absence of rapid degradation-triggered inflammation, such grafts survive; for example, PCL-based grafts that had lost 50% of their ultimate tensile strength by 2 wks. in the mouse aorta still survived to 6 mo. in the sheep carotid without dilation.23 Secondly, these results support the idea that sheep might degrade biomaterials more quickly than rodent models. Further research is needed, but biomaterials developers might wish to reduce their scaffolds’ degradation rates when making the transition to large animal models. Thirdly, these results add to the porosity debate within the field of resorbable grafts. Large pores might be good because they can produce pro-healing macrophage responses and allow for progenitor cell infiltration. But as this work shows, they may also expose more of the scaffold to cell-mediated bulk degradation and allow more inflammatory cells to infiltrate from outside. And finally, this work highlights the importance of considering normally neglected biomaterial interaction effects. Future work will slow the degradation rate of the PGS core, reduce the pore size of the PCL sheath, and/or implant PCL sheaths alone to determine the cause of the excessive inflammation.
Supplementary Material
Acknowledgments
The authors thank the following people for helpful discussions: Anne M. Robertson, Piyusha Gade, Jay D. Humphrey, Christopher K. Breuer, Mohammed El-Kurdi, Robin Gleed, and Frederick J. Schoen. The assistance of the University of Pittsburgh’s Department of Laboratory Animal Resources and Cornell University’s Hospital for Animals was critical in providing quality animal care. The Department of Vascular Surgery at the University of Pittsburgh Medical Center provided survey and ethnographic feedback on important vascular graft usability and handling features that informed this design. This work made use of the Cornell Center for Materials Research Facilities supported by the National Science Foundation under Award Number DMR-1719875. This material is based upon work supported by the National Science Foundation under grant nos. 1247842 and EEC-1359308. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
References
- 1.Gerhard-Herman MD et al. 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: A report of the American college of cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation vol. 135 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim LK et al. Trends in hospital treatments for peripheral arterial disease in the United States and association between payer status and quality of care/outcomes, 2007–2011. Catheter. Cardiovasc. Interv 86, 864–872 (2015). [DOI] [PubMed] [Google Scholar]
- 3.Hillis LD et al. 2011 ACCF/AHA guideline for coronary artery bypass graft surgery. J. Am. Coll. Cardiol 58, e123–e210 (2011). [DOI] [PubMed] [Google Scholar]
- 4.L’Heureux N, McAllister TN & de la Fuente LM Tissue-engineered blood vessel for adult arterial revascularization. N. Engl. J. Med 357, 1451–1453 (2007). [DOI] [PubMed] [Google Scholar]
- 5.Kirkton RD et al. Bioengineered human acellular vessels recellularize and evolve into living blood vessels after human implantation. Sci. Transl. Med 11, eaau6934 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stowell CET & Wang Y Quickening: Translational design of resorbable synthetic vascular grafts. Biomaterials 173, 71–86 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu W, Allen RA & Wang Y Fast-degrading elastomer enables rapid remodeling of a cell-free synthetic graft into a neoartery. Nat. Med 18, 1148–53 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Allen RA et al. Nerve regeneration and elastin formation within poly(glycerol sebacate)-based synthetic arterial grafts one-year post-implantation in a rat model. Biomaterials 35, 165–73 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crapo PM & Wang Y Physiologic compliance in engineered small-diameter arterial constructs based on an elastomeric substrate. Biomaterials 31, 1626–1635 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee K-W, Stolz DB & Wang Y Substantial expression of mature elastin in arterial constructs. Proc. Natl. Acad. Sci 108, 2705–2710 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crapo PM, Gao J & Wang Y Seamless tubular poly(glycerol sebacate) scaffolds: High-yield fabrication and potential applications. J. Biomed. Mater. Res. - Part A 86, 354–63 (2008). [DOI] [PubMed] [Google Scholar]
- 12.Jeffries EM, Allen RA, Gao J, Pesce M & Wang Y Highly elastic and suturable electrospun poly(glycerol sebacate) fibrous scaffolds. Acta Biomater. 18, 30–39 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khosravi R et al. Long-term functional efficacy of a novel electrospun poly(glycerol sebacate)-based arterial graft in mice. Ann. Biomed. Eng 44, 2402–2416 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Y, Ameer GA, Sheppard BJ & Langer R A tough biodegradable elastomer. Nat. Biotechnol 20, 602–606 (2002). [DOI] [PubMed] [Google Scholar]
- 15.Pomerantseva I et al. Degradation behavior of poly(glycerol sebacate). J. Biomed. Mater. Res. - Part A 91, 1038–1047 (2009). [DOI] [PubMed] [Google Scholar]
- 16.Hotaling NA, Bharti K, Kriel H & Simon CG DiameterJ: A validated open source nanofiber diameter measurement tool. Biomaterials 61, 327–338 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hotaling NA et al. Training to improve precision and accuracy in the measurement of fiber morphology. PLoS One 11, 1–14 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cardiovascular implants - Tubular vascular prostheses. ISO 7198:1998/2001/(R)2004. Geneva, Switzerland: ISO. [Google Scholar]
- 19.Magarey FR & Stehbens WE The blood pressure of sheep. Aust. J. Exp. Biol. Med. Sci 35, 347–352 (1957). [DOI] [PubMed] [Google Scholar]
- 20.Konig G et al. Mechanical properties of completely autologous human tissue engineered blood vessels compared to human saphenous vein and mammary artery. Biomaterials 30, 1542–1550 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ye L et al. The in vitro and in vivo biocompatibility evaluation of heparin-poly(ε-caprolactone) conjugate for vascular tissue engineering scaffolds. J. Biomed. Mater. Res. - Part A 100 A, 3251–3258 (2012). [DOI] [PubMed] [Google Scholar]
- 22.Mrowczynski W et al. Porcine carotid artery replacement with biodegradable electrospun poly-e-caprolactone vascular prosthesis. J. Vasc. Surg 59, 210–219 (2014). [DOI] [PubMed] [Google Scholar]
- 23.Fukunishi T et al. Tissue-engineered small diameter arterial vascular grafts from cell-free nanofiber PCL/chitosan scaffolds in a sheep model. PLoS One 11, 1–15 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prim DA et al. Comparative mechanics of diverse mammalian carotid arteries. PLoS One 13, 1–18 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van der Lei B et al. Regeneration of the arterial wall in microporous, compliant, biodegradable vascular grafts after implantation into the rat abdominal aorta - Ultrastructural observations. Cell Tissue Res. 242, 569–578 (1985). [DOI] [PubMed] [Google Scholar]
- 26.Muller KM & Dasbach G The pathology of vascular grafts. Curr. Top. Pathol 86, 273–306 (1994). [DOI] [PubMed] [Google Scholar]
- 27.Lee K-W et al. A biodegradable synthetic graft for small arteries matches the performance of autologous vein in rat carotid arteries. Biomaterials (2018) doi: 10.1016/j.biomaterials.2018.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Madden LR et al. Proangiogenic scaffolds as functional templates for cardiac tissue engineering. Proc. Natl. Acad. Sci 107, 15211–6 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Z et al. The effect of thick fibers and large pores of electrospun poly(ε-caprolactone) vascular grafts on macrophage polarization and arterial regeneration. Biomaterials 35, 5700–10 (2014). [DOI] [PubMed] [Google Scholar]
- 30.Fukunishi T et al. Different degradation rates of nanofiber vascular grafts in small and large animal models. J. Tissue Eng. Regen. Med (2019). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


