Abstract
Background
A pangenome is the collection of all genes found in a set of related genomes. For microbes, these genomes are often different strains of the same species, and the pangenome offers a means to compare gene content variation with differences in phenotypes, ecology, and phylogenetic relatedness. Though most frequently applied to bacteria, there is growing interest in adapting pangenome analysis to bacteriophages. However, working with phage genomes presents new challenges. First, most phage families are under-sampled, and homologous genes in related viruses can be difficult to identify. Second, homing endonucleases and intron-like sequences may be present, resulting in fragmented gene calls. Each of these issues can reduce the accuracy of standard pangenome analysis tools.
Methods
We developed an R pipeline called Rephine.r that takes as input the gene clusters produced by an initial pangenomics workflow. Rephine.r then proceeds in two primary steps. First, it identifies three common causes of fragmented gene calls: (1) indels creating early stop codons and new start codons; (2) interruption by a selfish genetic element; and (3) splitting at the ends of the reported genome. Fragmented genes are then fused to create new sequence alignments. In tandem, Rephine.r searches for distant homologs separated into different gene families using Hidden Markov Models. Significant hits are used to merge families into larger clusters. A final round of fragment identification is then run, and results may be used to infer single-copy core genomes and phylogenetic trees.
Results
We applied Rephine.r to three well-studied phage groups: the Tevenvirinae (e.g., T4), the Studiervirinae (e.g., T7), and the Pbunaviruses (e.g., PB1). In each case, Rephine.r recovered additional members of the single-copy core genome and increased the overall bootstrap support of the phylogeny. The Rephine.r pipeline is provided through GitHub (https://www.github.com/coevoeco/Rephine.r) as a single script for automated analysis and with utility functions to assist in building single-copy core genomes and predicting the sources of fragmented genes.
Keywords: Bacteriophage, Gene clustering, Fragmented genes, Pangenome
Introduction
A pangenome is the collection of all genes found in a set of related genomes (Tettelin et al., 2005; Vernikos et al., 2015). These genomes might be different strains of the same species or taken from the same genus or higher taxonomic level. Pangenomes are useful, because they allow one to compare gene content variation to differences in phenotypes, ecology, and evolutionary history. For instance, by mapping gene content of potential pathogens onto a phylogeny and contrasting clade-specific genes with differences in reported strain virulence, the pangenome can help reveal how these genes relate to pathogenicity while placing them in an evolutionary context (e.g., Hurtado et al., 2018; Wyres et al., 2019). Pangenomes have also been used to describe which functions are conserved among members of bacterial taxa in different environments (e.g., Zhang & Sievert, 2014).
Pangenome analysis is most commonly applied to bacteria. Due to the explosion of data from metagenomes and microbiome studies, many bacterial taxa are well-sampled and can be associated with large sets of ecological or health-related metadata. Additionally, multiple software packages are available that facilitate automated inference of bacterial pangenomes, such as Anvi’o (Eren et al., 2021) and Roary (Page et al., 2015).
A typical pangenome analysis pipeline starts with two main steps: gene prediction and gene clustering. Often, workflows also include subsequent steps for function prediction, sequence alignment, and core gene identification. The accuracy of the two primary steps of inferring a pangenome is paramount. If a gene caller ignores an open reading frame (ORF) or inaccurately returns the end position of the ORF, genes may be truncated or merged. Errors in clustering—the process of placing related sequences into gene families—can include grouping unrelated genes or failing to place homologs in the same cluster. Together, these errors in gene calling and clustering may significantly impact identification of the “single-copy core genome” (SCG). The SCG is commonly used as the basis for phylogenetic inference, and excluding genes can mean missing important sequence variation and building less informative trees.
There is growing interest in applying pangenomic and phylogenomic workflows to bacteriophages (e.g., Edwards et al., 2019; Bellas et al., 2020). Just as the deluge of metagenomic data has expanded bacterial comparative genomics, thousands of phage genomes are now published every year (Roux et al., 2019; Dion, Oechslin & Moineau, 2020). Because no single gene is conserved among all phage genomes, gene content profiles and gene sharing networks have become standard tools in virus taxonomy for identifying and comparing related viruses (Bolduc et al., 2017; Shapiro & Putonti, 2018). In the process, pangenomics has become an intrinsic component of phage bioinformatics.
Many of the potential sources of error for bacterial pangenome analysis are amplified when studying phages. First, phages are under-sampled despite regular publication of new genomes and identification of prophages within bacterial genomes (Dion, Oechslin & Moineau, 2020). Isolation, even of better-sampled groups through dedicated programs like SEA-PHAGES continues to discover novel viruses with genes lacking obvious homology to any known sequence (Pope et al., 2015). As a result, we often try to compare virus genomes that are more distantly related than expected for most pangenomic workflows. This can make it difficult to recognize homologs between phage genomes that have low sequence identity. Further, many phages include intron-like sequences and homing endonucleases (Belfort, 1990; Stoddard, 2005). These selfish genetic elements interrupt genes and cause fragmented gene calls during annotation. Thus, the two main tasks of a pangenome analysis—gene identification and gene clustering—are more error-prone with phages than with bacteria.
Here, we describe a pipeline implemented in R, Rephine.r, for identifying and correcting common errors in the initial gene clusters and gene calls returned by pangenomic workflows. Given the results from a traditional pangenome analysis, Rephine.r: (1) merges gene clusters using Hidden Markov Models (HMMs) and (2) identifies fragmented gene calls to avoid the overprediction of paralogs and to improve sequence alignments. Each of the steps in Rephine.r can also be run separately for individual use cases that require only cluster merging or defragmentation. We demonstrate the value of Rephine.r using three phage taxa: the Tevenvirinae (e.g., T4), the Studiervirinae (e.g., T7), and the Pbunaviruses (e.g., PB1). These virus groups represent a range of genome sizes and sampling depth, and each has at least 30 members with a RefSeq assembly. We show that correcting errors in gene cluster and gene fragmentation increases the size of the SCG in each case and enables inference of better-supported phylogenies. The tool is available through GitHub as a command line R script (https://www.github.com/coevoeco/Rephine.r) and includes utility scripts for returning the single-copy core genes and classifying the causes of gene fragmentation events.
Materials & Methods
Overview of the pipeline
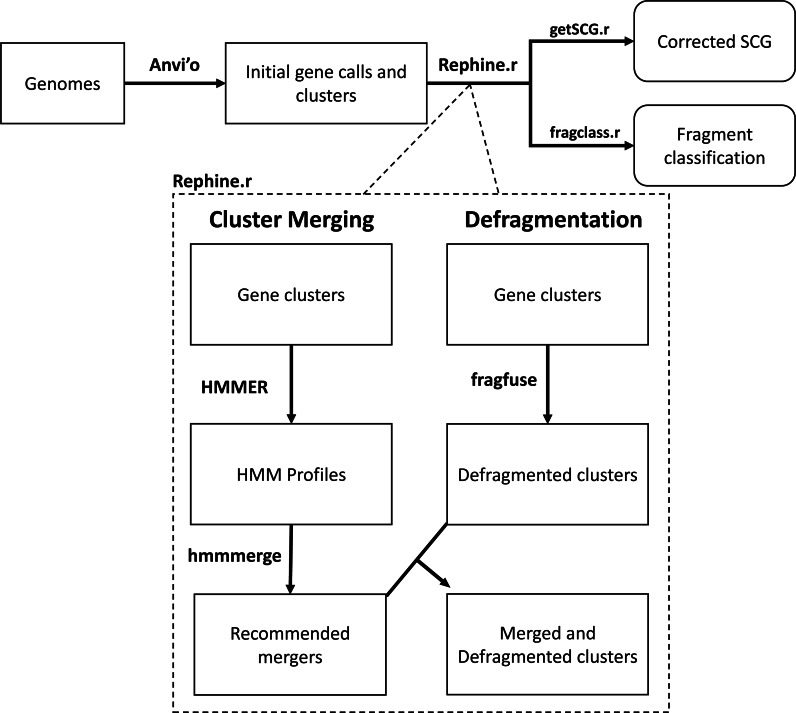
The Rephine.r pipeline (summarized in Fig. 1) assumes the researcher has already completed a workflow for predicting gene clusters in a pangenome, such as the combination of blastp (Altschul et al., 1990) and MCL (Enright, Van Dongen & Ouzounis, 2002) implemented by Anvi’o (Eren et al., 2021) and other programs (e.g., vConTACT Bolduc et al., 2017 and Roary Page et al., 2015). In what follows, we use Anvi’o as the basis for initial pangenomes, as Anvi’o is both a popular tool for bacterial pangenomes and includes several useful commands for facilitating our corrections. Future updates will expand Rephine.r’s compatibility with other tools.
Figure 1. Flowchart of the Rephine.r pipeline.
Following initial gene clustering Rephine.r pipeline: (1) identifies and merges gene clusters containing distantly related homologs using HMMs, and (2) identifies fragmented gene calls that can be fused for the purpose of SCG inference and generating phylogenies. By default, Rephine.r will first run the cluster merging and defragmentation steps in tandem, produce a set of new clusters that combine the results of these corrections, and will then run a second round of defragmentation to identify any new cases that emerge due to the prior steps. Command line options are also offered for users that wish to run the HMM merging or fragment fusion steps individually. In addition to the main pipeline, we include two complementary scripts: getSCG.r returns the single-copy core genes and a concatenated alignment file for phylogenetics; fragclass.r categorizes the likely events that led to fragmented gene calls.
Merging gene families with HMMs
Gene clustering based on sequence similarity relies on threshold criteria for defining when two sequences are related and for clustering related sequences into groups. In Anvi’o, the default identity heuristic is defined by the “minbit” score, the ratio of the BLAST bit score between two sequences and the minimum bit score from blasting each sequence against itself. This metric generally performs well, and for bacteria, where homologs are typically over 50% identical, it is especially successful. For phages, however, this approach can miss more distant homologs. Even using a 35% amino acid identity threshold (Cresawn et al., 2011; Shapiro & Putonti, 2018), we may miss cases that only appear related when viewing alignments or comparing phage genes by structure or synteny. Unfortunately, it is not as simple as specifying a lower minbit threshold, since doing so will also increase the number of unrelated genes that are clustered together erroneously.
Given the initial gene clusters returned by Anvi’o, Rephine.r builds separate HMM profiles for each cluster using the hmmbuild function from HMMER (Eddy, 1998) and converts the concatenated HMM profiles into a database with hmmpress. The script then uses hmmscan to compare every original gene call against each HMM profile. This step is expected to be more sensitive for recognizing distant homologs than the initial blastp, as the HMM profiles make use of variation from multiple members of the same cluster. Significant hits are then defined as follows: for each original gene cluster, the “minimum self-bit” (or “selfbit”) score is recorded as the minimum of the bit scores for each of the gene calls that was initially assigned to that cluster by MCL. This selfbit score then serves as a profile-specific significance threshold. Any gene call that was originally assigned to another cluster but has a bit score greater than this value is then used to establish a putative connection between gene clusters. We also include the option of specifying an absolute minimum bit score as an additional criterion. These connections are recorded in the form of a network edgelist linking gene calls to gene clusters. Next, this edgelist is relabeled to define edges between the original gene clusters that share putative homologs. Finally, this edgelist is used to generate a network with the R (R Core Team, 2013) package igraph (Csardi et al. , 2006), and the connected components are returned with the function “components”. The result defines sets of the original gene clusters that are suitable for merging into a single, larger cluster.
Identifying fragmented gene calls
To find fragmented genes, Rephine.r first identifies every gene cluster that includes at least two sequences from the same genome. These sequences may represent true duplicates or paralogs, or they may be separate pieces of the same original sequence that have been split by one of several processes, including: a frameshift due to an indel, insertion of a selfish genetic element, or being artificially split across the ends of the genome when it was reported to GenBank. This third case may also arise as an artifact of the two other mechanisms. For any of these scenarios, the two pieces of the gene will be notable in two ways: (1) they will align with separate parts of the gene in a multiple sequence alignment, with one piece corresponding to an N-terminal fragment, and the other to the C-terminus; (2) they should have lower sequence similarity to each other than to the average comparison with other sequences in the multiple sequence alignment. Fig. 2A illustrates how a fragmented gene may appear in an alignment.
Figure 2. Fragmented gene calls can be identified from alignments.
(A) An original multiple sequence alignment where the gene from NC_041902 has been split into two fragments by an indel. (B) The corrected alignment following Rephine.r. Highlighted colors are used to indicate regions of each fragment and where they correspond within an intact homolog.
Given clusters with potential fragments, every gene call within an affected cluster is compared using blastp to every other gene call in the same cluster. For the two focal gene calls from a potential fragmented gene, the bit score from their blast alignment is compared to the mean bit score for other blast results within the gene cluster. We defined the ratio of this pairwise blast to the cluster average as the “relative bit” (or “relbit”). Mathematically, for potential fragments A and B within a gene cluster G, this is defined as:
| (1) |
where the overbar refers to the mean. The maximum of these relbit values is then used as a criterion for judging similarity between A and B. If this value is below a chosen threshold, the ORFs are considered to be sufficiently dissimilar.
Rephine.r also compares the extent of overlap within the pairwise alignment space between each potential paralog. This step is needed, because dissimilar gene fragments may still have overlaps in the alignment due to alignment errors or if the original fragmentation event was caused by a short duplication. To quantify this overlap, the “percent overlap” is calculated as the size of the ORFs’ intersection within the alignment divided by the number of unique, aligned positions between the two sequences. In mathematical terms, for a gene with potential fragments A and B, we define:
| (2) |
where the size terms are based solely on the aligned positions within the multiple sequence alignment.
Ultimately, sequence pairs with low relative bit scores (“relbit”) and low percent overlaps (“percoverlap”) are the likeliest to fit our expectations of a fragmented gene call. In practice, we implemented default parameters for these criteria of 0.25 for “relbit” and 0.25 for “percoverlap.” These choices are based on plotting values of each parameter (Fig. S1) from the test cases described below and identifying a set of points that weakly cluster together in the graph. When checked manually, each of these genes appeared to correspond to fragmented calls, whereas nearby points in the graph included potential errors. These parameters can be adjusted at the command line, and we would encourage others to visually inspect their alignments.
Once fragmented genes are identified, a new FASTA file is created in which the original pieces of the full-length gene are artificially spliced (or “fused”) into a single gene call. To preserve the original event that separated the sequences, the script inserts an “X” between the two pieces of the gene. New alignments are then made with MUSCLE (Edgar, 2004) for each affected gene cluster, with these X’s imposing a gap in the alignment (see Fig. 2B for an illustration of this step). If desired, the user can then use the additional script, getSCG.r, to return a list of the single-copy core gene clusters, along with a concatenated alignment file that is suitable for phylogenetics. The script, fragclass.r, can also be used to obtain a table summarizing predicted causes for each type of fragment based on the separation between the original gene calls.
Virus genomic data
Phages in the subfamily Studiervirinae (family Autographiviridae), the subfamily Tevenvirinae (family Myoviridae), and the genus Pbunavirus (family Myoviridae) were chosen as well-studied examples for testing Rephine.r. We downloaded all available RefSeq genomes from each of these taxa from the National Center for Biotechnology Information’s (NCBI) genome browser (as of February 2021). This data set included 145 Studierviruses, 127 Tevenviruses, and 38 Pbunaviruses (a full list of accessions is included in Table S1). The Studiervirinae (e.g., phages T3 and T7) and the Tevenvirinae (e.g., phage T4) are among the best-studied phage subfamilies and include characterized examples of introns and homing endonucleases (Chu et al., 1986; Belle, Landthaler & Shub, 2002; Bonocora & Shub, 2004; Petrov, Ratnayaka & Karam, 2010). These features made these two subfamilies ideal for testing methods for identifying distant homologs and fragmented gene calls. The Pbunaviruses were chosen due to the relatively large number of available genomes at the genus level, offering a less diverse contrast to the other phage groups.
Initial pangenome workflow with Anvi’o
We built an initial pangenome for each phage group using Anvi’o v6.2 (Eren et al., 2021) following the standard pangenomics workflow (https://merenlab.org/2016/11/08/pangenomics-v2/), which uses Prodigal (Hyatt et al., 2010) for gene calling. Alternative gene callers can also be used, and these gene calls can be imported into Anvi’o as part of the anvi-gen-contigs-database program. The “–use-ncbi-blast” flag was specified for the anvi-pan-genome command. Due to the large genetic diversity of phages, we set the minbit threshold to 0.35, based on prior work (Cresawn et al., 2011; Shapiro & Putonti, 2018).
Phylogenetics
Maximum likelihood phylogenies were estimated using IQTREE v2.0.3 (Nguyen et al., 2015) with ModelFinder (Kalyaanamoorthy et al., 2017) to automate choosing the optimal substitution model for each tree. For each of the three virus groups, trees were built based on concatenated alignments for the original SCGs and again following Rephine.r using the expanded SCGs. Tree summary statistics were computed in R using the ape package (Paradis, Claude & Strimmer, 2004) and drawn using ggtree (Yu et al., 2017).
Code Availability
All code for this work is provided on GitHub (https://github.com/coevoeco/Rephine.r).The code includes a walkthrough for running Rephine.r following a standard Anvi’o workflow, as well as utility scripts, getSCG.r and fragclass.r, that provide additional output of the SCG genes and predicted causes of fragmentation events.
Results
To test the Rephine.r pipeline, we downloaded all available RefSeq genomes for the Studiervirinae, Tevenvirinae, and Pbunaviruses from NCBI. We then followed the standard pangenomic workflow for Anvi’o to facilitate initial MCL clustering based on blastp scores. Results and basic information about these taxa are summarized in Table 1. Across all Studierviruses, there were only 12 core genes, of which three were single-copy. Tevenviruses included 27 core genes (13 single-copy), and the Pbunaviruses had 28 core genes (19 single-copy).
Table 1. Summary of results of running Rephine.r for each phage group.
| Studiervirinae | Tevenvirinae | Pbunaviruses | |
|---|---|---|---|
| Number of genomes | 145 | 127 | 30 |
| Mean genome size | 39696 | 174775 | 66068 |
| Initial gene calls | 6956 | 35436 | 3540 |
| Initial gene clusters | 558 | 4067 | 195 |
| Initial core genes | 12 | 27 | 28 |
| Initial SCG size | 3 | 13 | 19 |
| New clusters after merging | 16 | 64 | 2 |
| Clusters involved in a merger | 63 | 270 | 5 |
| Biggest merger | 7 | 30 | 3 |
| Core genes after merging | 14 | 37 | 28 |
| SCG size after merging | 3 | 13 | 19 |
| Defragmented clusters | 14 | 99 | 17 |
| SCG size after fusion and merge | 8 | 22 | 26 |
| Additional fusions after merge | 1 | 7 | 1 |
| New core genes after final fusion | 0 | 0 | 0 |
| Total SCG gain | 5 | 9 | 7 |
| Mean tree support before | 77.14 | 87.24 | 63.6 |
| Mean tree support after | 90.55 | 93.44 | 69.57 |
We ran Rephine.r with default settings, which first predicts fragmented gene calls within each gene cluster. In tandem, it identifies related gene clusters using HMMs. It then combines the results from these steps to produce new merged clusters with corrections for fragmented genes. Last, it runs a second defragmentation step to identify instances where fragmented gene calls were originally split into separate gene clusters. We examined results to see how the core genome changed after each step and how the final SCG affected phylogenetic inference.
The initial HMM merging step resulted in two additional core genes for Studierviruses and 10 additional core genes for Tevenviruses but no new single-copy core genes for any of the virus groups. Notably, several mergers involved more than two gene clusters. In one case for the Tevenvirinae, 30 separate gene clusters were merged, corresponding to the phage tail fiber. Defragmenting gene calls expanded the SCG for each taxon, increasing the Studiervirinae SCG to 8 genes, the Tevenvirinae to 22 genes, and the Pbunaviruses to 26 genes (all but two of the Pbunavirus core genes). The final round of defragmentation identified additional fragmented genes but no additional core genes.
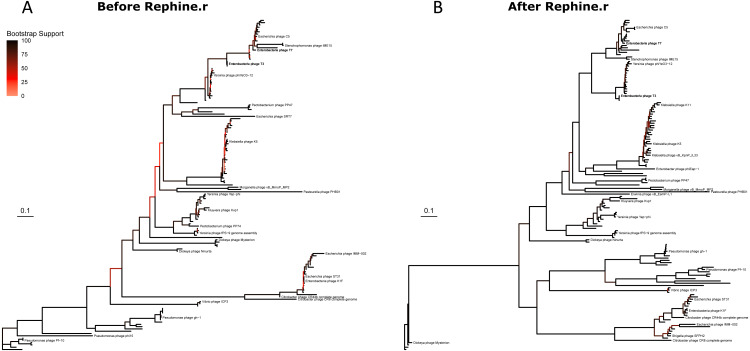
We then built phylogenies for each taxon with the original SCGs and with expanded SCGs following Rephine.r. With only three single-copy core genes, the initial Studiervirinae tree contained multiple unresolved polytomies and branches with poor support (Fig. 3A). The updated tree based on eight genes had improved overall bootstrap support and displayed greater resolution of closely related genomes (Fig. 3B). (A version of the tree with all labels is provided in Fig. S2). Trees for the Tevenvirinae and Pbunaviruses (Figs. S3 and S4) also had improved bootstrap support. In the case of the Pbunaviruses, the tree remains poorly resolved with very short branches, despite being built from the most genes, as there was insufficient variation among the viruses from this genus.
Figure 3. Studiervirinae phylogeny before (A) and after (B) using Rephine.r to correct the SCG.
Bootstrap support is shown by coloring branches preceding nodes, with low support (from 0 to 70) ranging from white to red. Increasing the size of the SCG reduced the number of low-support branches.
Last, we checked the results from gene call defragmentation for known instances of introns and homing endonucleases in the Studiervirinae and Tevenvirinae. These include interruptions to DNA polymerase in members of Studiervirinae (Bonocora & Shub, 2004) and Tevenvirinae (Petrov, Ratnayaka & Karam, 2010) and thymidylate synthase in T4 (Chu et al., 1986). After running Rephine.r, we identified a single-copy core gene that corresponded to each gene of interest. In each case, inclusion in the SCG was only possible after fragment identification.
Discussion
We describe Rephine.r, a pipeline for improving results of phage pangenome analysis by merging gene clusters containing distant homologs and correcting gene calls that have been fragmented or interrupted by selfish genetic elements. Using the Tevenvirinae, Studiervirinae, and Pbunaviruses as test cases, we show how this process expands the putative SCG for each group, enabling more accurate estimates of gene conservation. For the Tevenvirinae and Studiervirinae, this also improved the quality of the phylogenies, whereas for Pbunaviruses there was still insufficient variation among the genomes to produce a reliable tree.
The present work provides a first step for expanding the usage of phylogenetics with diverse phage genomes. A key concept that we include (which we took advantage of using manual corrections previously (Shapiro & Putonti, 2020) is the use of artificially spliced sequences following the identification of interrupted genes. This type of correction is unsurprising when working with eukaryotic exons, but it is generally ignored with microbes, because we often fail to appreciate that intron-like sequences are common features of many phages. Biologically, it is uncertain how often these interrupted genes remain functional or if the separated ORFs correspond to separate functions. However, several studies report fully functional, single protein products for phage genes separated by introns (Belfort, 1990) or inteins (Kelley et al., 2016), as well as at least one case where a gene split by a homing endonuclease remains active (Friedrich et al., 2007). Though these ORFs may be interrupted by over 1,000 nucleotides, these interruptions likely correspond to a single mutational event, and the ORFs should still be treated as a single gene when reconstructing the SCG and an associated phylogeny. In both the Studiervirinae and the Tevenvirinae, our approach accurately recognized known homing endonucleases and introns, and these genes remain the most common multi-copy core genes following Rephine.r. How interrupted genes are interpreted in functional genomics studies is an important question, and these fragmented genes should be treated with additional care when reporting the functional repertoire of genomes.
It is important to note that we have focused our application of Rephine.r on test cases involving single-contig, RefSeq assemblies. In the case of draft genome assemblies comprised of multiple contigs (less common for phages under 100 kb), we expect to observe instances where a gene call is separated into different ORFs on different contigs. These errors will result in overestimating gene content and incorrect predictions of paralogous sequences. Similar issues have been noted to cause errors in the analysis of gene content evolution in eukaryotes (Denton et al., 2014). The current implementation of gene defragmentation in Rephine.r should successfully resolve many of these mistakes, and it may offer a future approach for consolidating contigs in assemblies. For instance, suppose a gene is split by a transposase that includes short palindromic repeats. These regions are difficult to assemble with short reads and may lead to one contig ending with half of the original gene, while a second contig starts with the transposase and the remainder of the gene. Scaffolding these contigs can be challenging, but by recognizing gene fragments, it may be possible to resolve the assembly.
Last, bacterial pangenome workflows typically do not account for specific issues that may arise for prophage regions, such as errors in clustering and gene fragmentation that we observe in the genomes of phage isolates. Our expectation is that these same errors will impact prophages, and future work will need to consider how these issues may impact the accuracy of bacterial pangenomes. Moreover, bacterial genes themselves can be interrupted by mobile genetic elements (including phages), and Rephine.r should offer a novel approach for identifying these events.
Conclusions
The Rephine.r pipeline offers an efficient means to identify and correct errors in phage pangenomes caused by incomplete gene clustering and fragmented gene calls. Correcting these errors, in particular for cases of genes interrupted by selfish genetic elements, increases the size of the SCG in each of our test cases. These corrections provide more genetic variation for improved phylogenetic inference and are especially useful for large, diverse phage groups where standard methods produce limited core genomes and poorly resolved phylogenies.
Supplemental Information
Red dots correspond to cases with both low overlap and low sequence identity, indicating the likeliest fragmented gene calls.
The type phages T3 and T7 are shown in bold. Bootstrap support is shown by coloring branches preceding nodes, with low support (from 0 to 70) ranging from white to red.
The type phage PB1 is shown in bold. Bootstrap support is shown by coloring branches preceding nodes, with low support (from 0 to 70) ranging from white to red. Note: an outlier genome ( NC_009015) was dropped from the tree to enable visualiza
The type phage T4 is shown in bold. Bootstrap support is shown by coloring branches preceding nodes, with low support (from 0 to 70) ranging from white to red.
Acknowledgments
We are grateful to the members of the Putonti Lab for feedback on this work.
Funding Statement
This work was supported by the National Science Foundation (1661357 to Catherine Putonti). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Jason W Shapiro conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft.
Catherine Putonti conceived and designed the experiments, authored or reviewed drafts of the paper, and approved the final draft.
Data Availability
The following information was supplied regarding data availability:
All raw data is available at figshare: https://doi.org/10.6084/m9.figshare.14485389.v1.
The code is available at GitHub: https://github.com/coevoeco/Rephine.r.
References
- Altschul et al. (1990).Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. Journal of Molecular Biology. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Belfort (1990).Belfort M. Phage T4 introns: self-splicing and mobility. Annual Review of Genetics. 1990;24:363–385. doi: 10.1146/annurev.ge.24.120190.002051. [DOI] [PubMed] [Google Scholar]
- Bellas et al. (2020).Bellas CM, Schroeder DC, Edwards A, Barker G, Anesio AM. Flexible genes establish widespread bacteriophage pan-genomes in cryoconite hole ecosystems. Nature Communications. 2020;11:4403. doi: 10.1038/s41467-020-18236-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belle, Landthaler & Shub (2002).Belle A, Landthaler M, Shub DA. Intronless homing: site-specific endonuclease SegF of bacteriophage T4 mediates localized marker exclusion analogous to homing endonucleases of group I introns. Genes & Development. 2002;16:351–362. doi: 10.1101/gad.960302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolduc et al. (2017).Bolduc B, Jang HB, Doulcier G, You Z-Q, Roux S, Sullivan MB. vConTACT: an iVirus tool to classify double-stranded DNA viruses that infect Archaea and Bacteria. PeerJ. 2017;5:e3243. doi: 10.7717/peerj.3243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonocora & Shub (2004).Bonocora RP, Shub DA. A self-splicing group I intron in DNA polymerase genes of T7-like bacteriophages. Journal of Bacteriology. 2004;186:8153–8155. doi: 10.1128/JB.186.23.8153-8155.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu et al. (1986).Chu FK, Maley GF, West DK, Belfort M, Maley F. Characterization of the intron in the phage T4 thymidylate synthase gene and evidence for its self-excision from the primary transcript. Cell. 1986;45:157–166. doi: 10.1016/0092-8674(86)90379-X. [DOI] [PubMed] [Google Scholar]
- Cresawn et al. (2011).Cresawn SG, Bogel M, Day N, Jacobs-Sera D, Hendrix RW, Hatfull GF. Phamerator: a bioinformatic tool for comparative bacteriophage genomics. BMC Bioinformatics. 2011;12:395. doi: 10.1186/1471-2105-12-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csardi et al. (2006).Csardi G, Nepusz T, et al. The igraph software package for complex network research. InterJournal, Complex Systems. 2006;1695:1–9. [Google Scholar]
- Denton et al. (2014).Denton JF, Lugo-Martinez J, Tucker AE, Schrider DR, Warren WC, Hahn MW. Extensive error in the number of genes inferred from draft genome assemblies. PLOS Computational Biology. 2014;10:e1003998. doi: 10.1371/journal.pcbi.1003998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dion, Oechslin & Moineau (2020).Dion MB, Oechslin F, Moineau S. Phage diversity, genomics and phylogeny. Nature Reviews Microbiology. 2020;18:125–138. doi: 10.1038/s41579-019-0311-5. [DOI] [PubMed] [Google Scholar]
- Eddy (1998).Eddy SR. Profile hidden Markov models. Bioinformatics. 1998;14:755–763. doi: 10.1093/bioinformatics/14.9.755. [DOI] [PubMed] [Google Scholar]
- Edgar (2004).Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Research. 2004;32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards et al. (2019).Edwards RA, Vega AA, Norman HM, Ohaeri M, Levi K, Dinsdale EA, Cinek O, Aziz RK, McNair K, Barr JJ, Bibby K, Brouns SJJ, Cazares A, De Jonge PA, Desnues C, Díaz Muñoz SL, Fineran PC, Kurilshikov A, Lavigne R, Mazankova K, McCarthy DT, Nobrega FL, Reyes Muñoz A, Tapia G, Trefault N, Tyakht AV, Vinuesa P, Wagemans J, Zhernakova A, Aarestrup FM, Ahmadov G, Alassaf A, Anton J, Asangba A, Billings EK, Cantu VA, Carlton JM, Cazares D, Cho G-S, Condeff T, Cortés P, Cranfield M, Cuevas DA, Dela Iglesia R, Decewicz P, Doane MP, Dominy NJ, Dziewit L, Elwasila BM, Eren AM, Franz C, Fu J, Garcia-Aljaro C, Ghedin E, Gulino KM, Haggerty JM, Head SR, Hendriksen RS, Hill C, Hyöty H, Ilina EN, Irwin MT, Jeffries TC, Jofre J, Junge RE, Kelley ST, Khan Mirzaei M, Kowalewski M, Kumaresan D, Leigh SR, Lipson D, Lisitsyna ES, Llagostera M, Maritz JM, Marr LC, McCann A, Molshanski-Mor S, Monteiro S, Moreira-Grez B, Morris M, Mugisha L, Muniesa M, Neve H, Nguyen N-P, Nigro OD, Nilsson AS, O’Connell T, Odeh R, Oliver A, Piuri M, Prussin AJ Ii, Qimron U, Quan Z-X, Rainetova P, Ramírez-Rojas A, Raya R, Reasor K, Rice GAO, Rossi A, Santos R, Shimashita J, Stachler EN, Stene LC, Strain R, Stumpf R, Torres PJ, Twaddle A, Ugochi Ibekwe M, Villagra N, Wandro S, White B, Whiteley A, Whiteson KL, Wijmenga C, Zambrano MM, Zschach H, Dutilh BE. Global phylogeography and ancient evolution of the widespread human gut virus crAssphage. Nature Microbiology. 2019;4:1727–1736. doi: 10.1038/s41564-019-0494-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enright, Van Dongen & Ouzounis (2002).Enright AJ, Van Dongen S, Ouzounis CA. An efficient algorithm for large-scale detection of protein families. Nucleic Acids Research. 2002;30:1575–1584. doi: 10.1093/nar/30.7.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eren et al. (2021).Eren AM, Kiefl E, Shaiber A, Veseli I, Miller SE, Schechter MS, Fink I, Pan JN, Yousef M, Fogarty EC, Trigodet F, Watson AR, Esen ÖC, Moore RM, Clayssen Q, Lee MD, Kivenson V, Graham ED, Merrill BD, Karkman A, Blankenberg D, Eppley JM, Sjödin A, Scott JJ, Vázquez-Campos X, McKay LJ, McDaniel EA, Stevens SLR, Anderson RE, Fuessel J, Fernandez-Guerra A, Maignien L, Delmont TO, Willis AD. Community-led, integrated, reproducible multi-omics with anvi’o. Nature Microbiology. 2021;6:3–6. doi: 10.1038/s41564-020-00834-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich et al. (2007).Friedrich NC, Torrents E, Gibb EA, Sahlin M, Sjöberg B-M, Edgell DR. Insertion of a homing endonuclease creates a genes-in-pieces ribonucleotide reductase that retains function. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:6176–6181. doi: 10.1073/pnas.0609915104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurtado et al. (2018).Hurtado R, Carhuaricra D, Soares S, Viana MVC, Azevedo V, Maturrano L, Aburjaile F. Pan-genomic approach shows insight of genetic divergence and pathogenic-adaptation of Pasteurella multocida. Gene. 2018;670:193–206. doi: 10.1016/j.gene.2018.05.084. [DOI] [PubMed] [Google Scholar]
- Hyatt et al. (2010).Hyatt D, Chen G-L, Locascio PF, Land ML, Larimer FW, Hauser LJ. Prodigal: prokaryotic gene recognition and translation initiation site identification. BMC Bioinformatics. 2010;11:119. doi: 10.1186/1471-2105-11-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalyaanamoorthy et al. (2017).Kalyaanamoorthy S, Minh BQ, Wong TKF, Von Haeseler A, Jermiin LS. ModelFinder: fast model selection for accurate phylogenetic estimates. Nature Methods. 2017;14:587–589. doi: 10.1038/nmeth.4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley et al. (2016).Kelley DS, Lennon CW, SEA-PHAGES. Belfort M, Novikova O. Mycobacteriophages as incubators for intein dissemination and evolution. mBio. 2016;7(5):e01537–16. doi: 10.1128/mBio.01537-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen et al. (2015).Nguyen L-T, Schmidt HA, Haeseler Avon, Minh BQ. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Molecular Biology and Evolution. 2015;32:268–274. doi: 10.1093/molbev/msu300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page et al. (2015).Page AJ, Cummins CA, Hunt M, Wong VK, Reuter S, Holden MTG, Fookes M, Falush D, Keane JA, Parkhill J. Roary: rapid large-scale prokaryote pan genome analysis. Bioinformatics. 2015;31:3691–3693. doi: 10.1093/bioinformatics/btv421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradis, Claude & Strimmer (2004).Paradis E, Claude J, Strimmer K. APE: analyses of Phylogenetics and Evolution in R language. Bioinformatics. 2004;20:289–290. doi: 10.1093/bioinformatics/btg412. [DOI] [PubMed] [Google Scholar]
- Petrov, Ratnayaka & Karam (2010).Petrov VM, Ratnayaka S, Karam JD. Genetic insertions and diversification of the PolB-type DNA polymerase (gp43) of T4-related phages. Journal of Molecular Biology. 2010;395:457–474. doi: 10.1016/j.jmb.2009.10.054. [DOI] [PubMed] [Google Scholar]
- Pope et al. (2015).Pope WH, Bowman CA, Russell DA, Jacobs-Sera D, Asai DJ, Cresawn SG, Jacobs WR, Hendrix RW, Lawrence JG, Hatfull GF, Science Education Alliance Phage Hunters Advancing Genomics and Evolutionary Science, Phage Hunters Integrating Research and Education, Mycobacterial Genetics Course Whole genome comparison of a large collection of mycobacteriophages reveals a continuum of phage genetic diversity. ELife. 2015;4:e06416. doi: 10.7554/eLife.06416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team (2013).R Core Team R: A language and environment for statistical computing 2013
- Roux et al. (2019).Roux S, Adriaenssens EM, Dutilh BE, Koonin EV, Kropinski AM, Krupovic M, Kuhn JH, Lavigne R, Brister JR, Varsani A, Amid C, Aziz RK, Bordenstein SR, Bork P, Breitbart M, Cochrane GR, Daly RA, Desnues C, Duhaime MB, Emerson JB, Enault F, Fuhrman JA, Hingamp P, Hugenholtz P, Hurwitz BL, Ivanova NN, Labonté JM, Lee K-B, Malmstrom RR, Martinez-Garcia M, Mizrachi IK, Ogata H, Páez-Espino D, Petit M-A, Putonti C, Rattei T, Reyes A, Rodriguez-Valera F, Rosario K, Schriml L, Schulz F, Steward GF, Sullivan MB, Sunagawa S, Suttle CA, Temperton B, Tringe SG, Thurber RV, Webster NS, Whiteson KL, Wilhelm SW, Wommack KE, Woyke T, Wrighton KC, Yilmaz P, Yoshida T, Young MJ, Yutin N, Allen LZ, Kyrpides NC, Eloe-Fadrosh EA. Minimum Information about an Uncultivated Virus Genome (MIUViG) Nature Biotechnology. 2019;37:29–37. doi: 10.1038/nbt.4306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro & Putonti (2018).Shapiro JW, Putonti C. Gene co-occurrence networks reflect bacteriophage ecology and evolution. mBio. 2018;9(2):e01870–17. doi: 10.1128/mBio.01870-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro & Putonti (2020).Shapiro JW, Putonti C. UP Φ phages, a new group of filamentous phages found in several members of Enterobacteriales. Virus Evolution. 2020;6:veaa030. doi: 10.1093/ve/veaa030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoddard (2005).Stoddard BL. Homing endonuclease structure and function. Quarterly Reviews of Biophysics. 2005;38:49–95. doi: 10.1017/S0033583505004063. [DOI] [PubMed] [Google Scholar]
- Tettelin et al. (2005).Tettelin H, Masignani V, Cieslewicz MJ, Donati C, Medini D, Ward NL, Angiuoli SV, Crabtree J, Jones AL, Scott Durkin A, DeBoy RT, Davidsen TM, Mora M, Scarselli M, y RosIM, Peterson JD, Hauser CR, Sundaram JP, Nelson WC, Madupu R, Brinkac LM, Dodson RJ, Rosovitz MJ, Sullivan SA, Daugherty SC, Haft DH, Selengut J, Gwinn ML, Zhou L, Zafar N, Khouri H, Radune D, Dimitrov G, Watkins K, O’Connor KJB, Smith S, Utterback TR, White O, Rubens CE, Grandi G, Madoff LC, Kasper DL, Telford JL, Wessels MR, Rappuoli R, Fraser CM. Genome analysis of multiple pathogenic isolates of Streptococcus agalactiae: implications for the microbial pan-genome. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:13950–13955. doi: 10.1073/pnas.0506758102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernikos et al. (2015).Vernikos G, Medini D, Riley DR, Tettelin H. Ten years of pan-genome analyses. Current Opinion in Microbiology. 2015;23:148–154. doi: 10.1016/j.mib.2014.11.016. [DOI] [PubMed] [Google Scholar]
- Wyres et al. (2019).Wyres KL, Wick RR, Judd LM, Froumine R, Tokolyi A, Gorrie CL, Lam MMC, Duchêne S, Jenney A, Holt KE. Distinct evolutionary dynamics of horizontal gene transfer in drug resistant and virulent clones of Klebsiella pneumoniae. PLOS Genetics. 2019;15:e1008114. doi: 10.1371/journal.pgen.1008114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu et al. (2017).Yu G, Smith DK, Zhu H, Guan Y, Lam TT. Ggtree: an r package for visualization and annotation of phylogenetic trees with their covariates and other associated data. Methods in Ecology and Evolution / British Ecological Society. 2017;8:28–36. doi: 10.1111/2041-210X.12628. [DOI] [Google Scholar]
- Zhang & Sievert (2014).Zhang Y, Sievert SM. Pan-genome analyses identify lineage- and niche-specific markers of evolution and adaptation in Epsilonproteobacteria. Frontiers in Microbiology. 2014;5:110. doi: 10.3389/fmicb.2014.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Red dots correspond to cases with both low overlap and low sequence identity, indicating the likeliest fragmented gene calls.
The type phages T3 and T7 are shown in bold. Bootstrap support is shown by coloring branches preceding nodes, with low support (from 0 to 70) ranging from white to red.
The type phage PB1 is shown in bold. Bootstrap support is shown by coloring branches preceding nodes, with low support (from 0 to 70) ranging from white to red. Note: an outlier genome ( NC_009015) was dropped from the tree to enable visualiza
The type phage T4 is shown in bold. Bootstrap support is shown by coloring branches preceding nodes, with low support (from 0 to 70) ranging from white to red.
Data Availability Statement
The following information was supplied regarding data availability:
All raw data is available at figshare: https://doi.org/10.6084/m9.figshare.14485389.v1.
The code is available at GitHub: https://github.com/coevoeco/Rephine.r.