ABSTRACT
Two-component signaling systems (TCSs) are comprised of a sensory histidine kinase and a response regulator protein. In response to environmental changes, sensor kinases directly phosphorylate their cognate response regulator to affect gene expression. Bacteria typically express multiple TCSs that are insulated from one another and regulate distinct physiological processes. There are examples of cross-regulation between TCSs, but this phenomenon remains relatively unexplored. We have identified regulatory links between the ChvG-ChvI (ChvGI) and NtrY-NtrX (NtrYX) TCSs, which control important and often overlapping processes in alphaproteobacteria, including maintenance of the cell envelope. Deletion of chvG and chvI in Caulobacter crescentus limited growth in defined medium, and a selection for genetic suppressors of this growth phenotype uncovered interactions among chvGI, ntrYX, and ntrZ, which encodes a previously uncharacterized periplasmic protein. Significant overlap in the experimentally defined ChvI and NtrX transcriptional regulons provided support for the observed genetic connections between ntrYX and chvGI. Moreover, we present evidence that the growth defect of strains lacking chvGI is influenced by the phosphorylation state of NtrX and, to some extent, by levels of the TonB-dependent receptor ChvT. Measurements of NtrX phosphorylation in vivo indicated that NtrZ is an upstream regulator of NtrY and that NtrY primarily functions as an NtrX phosphatase. We propose a model in which NtrZ functions in the periplasm to inhibit NtrY phosphatase activity; regulation of phosphorylated NtrX levels by NtrZ and NtrY provides a mechanism to modulate and balance expression of the NtrX and ChvI regulons under different growth conditions.
IMPORTANCE TCSs enable bacteria to regulate gene expression in response to physiochemical changes in their environment. The ChvGI and NtrYX TCSs regulate diverse pathways associated with pathogenesis, growth, and cell envelope function in many alphaproteobacteria. We used Caulobacter crescentus as a model to investigate regulatory connections between ChvGI and NtrYX. Our work defined the ChvI transcriptional regulon in C. crescentus and revealed a genetic interaction between ChvGI and NtrYX, whereby modulation of NtrYX signaling affects the survival of cells lacking ChvGI. In addition, we identified NtrZ as a periplasmic inhibitor of NtrY phosphatase activity in vivo. Our work establishes C. crescentus as an excellent model to investigate multilevel regulatory connections between ChvGI and NtrYX in alphaproteobacteria.
KEYWORDS: Caulobacter crescentus, ChvG, ChvI, ChvT, NtrX, NtrY, cell envelope, histidine kinase, response regulator, two-component regulatory systems
INTRODUCTION
Bacteria employ two-component signaling systems (TCSs) to respond to environmental cues and maintain cellular homeostasis (1). TCS sensory modules consist of two core components, a sensory histidine kinase (HK) and a response regulator (RR). In response to a signal(s), the HK undergoes autophosphorylation on a conserved histidine residue and then passes the phosphoryl group to a conserved aspartate on the receiver (REC) domain of the RR (1). HKs may also act as phosphatases, dephosphorylating phosphorylated RRs (1). RR phosphorylation generally alters the activity of effector domains that change gene expression. TCSs are modular, and the output of a particular RR may vary between different organisms (2–5). TCSs that regulate host interactions in pathogens and symbionts are often conserved in related free-living organisms and enable responses to similar physiochemical cues present in the environment (2, 6–8).
Although typical TCSs rely on a single HK and RR pair, many systems incorporate additional proteins, such as activators or inhibitors, to form more complex signaling networks (9–17). Historically, most TCSs have been considered to be insular systems, but in many bacteria, cross-regulation between HKs and RRs may integrate multiple environmental cues (9, 11, 14, 18–20). Even when HK and RR pairs are well insulated, TCSs can interact at the transcriptional level (20, 21). For example, one TCS may regulate the expression of other TCS genes, or multiple TCSs may influence transcription of the same downstream gene (22–24).
ChvG-ChvI (ChvGI) and NtrY-NtrX (NtrYX) are conserved alphaproteobacterial TCSs that often regulate similar physiological processes, raising the possibility that they work together in a coordinated fashion (25, 26). The ChvG HK and ChvI RR were originally identified as pleiotropic regulators in the plant pathogen Agrobacterium tumefaciens, affecting virulence, detergent tolerance, and pH sensitivity (8, 27). Subsequent work has linked ChvGI to host interaction, cell motility, acid sensing, and exopolysaccharide production in a variety of Alphaproteobacteria (6, 7, 28–32). In most characterized systems, the periplasmic protein ExoR binds to ChvG and inhibits its kinase activity (15, 16, 33). Acidic pH activates the ChvGI system by triggering rapid proteolysis of ExoR (34). However, not all organisms with ChvGI, including Caulobacter crescentus, encode an ortholog of ExoR. These bacteria must regulate ChvG kinase activity by a different mechanism.
Like ChvGI, NtrYX (consisting of the NtrY HK and NtrX RR) is conserved in many Alphaproteobacteria, including multiple pathogens and symbionts (35–39). Although early studies concluded that NtrYX regulates nitrogen metabolism, recent work suggests that, in certain alphaproteobacteria, it also controls exopolysaccharide biosynthesis, cell motility, and cell envelope composition (25, 35, 39–44). Given that these processes are also regulated by ChvGI, it is conceivable that ChvGI and NtrYX act coordinately. However, no work to date has identified a substantial genetic interaction between these TCSs (25, 26).
C. crescentus, a free-living alphaproteobacterium found in freshwater and soil environments, encodes both the ChvGI and NtrYX systems (45, 46). C. crescentus ChvGI activates transcription of the small regulatory RNA chvR, which posttranscriptionally represses the TonB-dependent receptor gene chvT (6). Examination of reporters of chvR transcription indicated that ChvGI is activated by growth in defined medium, acidic pH, DNA damage, growth at stationary phase, and cell envelope stress (6, 47). However, aside from chvR, genes regulated by ChvGI have not been defined. C. crescentus NtrYX is less well characterized than ChvGI, but a recent study established that NtrX is phosphorylated in stationary phase in defined medium as a result of acidification (48). In addition, NtrX appears to play a core role in regulating C. crescentus physiology, as ntrX is essential for growth in complex medium and ΔntrX cells grow more slowly than wild-type (WT) cells in defined medium (49).
In this study, we initially took a reverse genetic approach to characterize the role of ChvGI in regulating C. crescentus physiology. Deletion of chvG and chvI caused a distinctive growth defect in defined medium. By exploiting this defect, we identified striking genetic interactions between chvGI and ntrY, ntrX, and ntrZ (a previously uncharacterized gene). Epistasis analysis provided evidence that unphosphorylated NtrX is detrimental to cells lacking chvG or chvI. We defined the ChvI transcriptional regulon and discovered that it overlaps significantly with genes regulated by NtrX. In addition, we found that NtrZ promotes NtrX phosphorylation in vivo, likely by inhibiting NtrY phosphatase activity. We conclude that ChvGI and NtrYX interact at multiple transcriptional levels, working both in concert and in opposition to regulate C. crescentus growth in defined medium.
RESULTS
Loss of the ChvGI system limits growth in defined medium.
To investigate the physiological role of ChvGI in C. crescentus, we generated strains with in-frame deletions of chvG or chvI and examined their growth. The deletion strains grew normally in complex medium (peptone-yeast extract [PYE]), but they displayed a distinctive growth defect in defined medium (M2 minimal salts medium with xylose as carbon source [M2X]) (Fig. 1A and B; see Fig. S1 in the supplemental material). Although overnight cultures of each strain inoculated from PYE agar plates grew to similar densities in M2X, strains lacking chvG or chvI exhibited reduced growth capacity after dilution, only reaching a terminal optical density at 660 nm (OD660) of ∼0.1 (Fig. 1A). This lower cell density correlated with fewer CFU (Fig. 1B). Ectopic overexpression of chvG (chvG++) or chvI (chvI++) from a xylose-inducible promoter (xylose is the sole carbon source in M2X medium) fully rescued growth of ΔchvG or ΔchvI strains, respectively, under these conditions (Fig. 1A and B).
FIG 1.
Loss of chvG or chvI limits culture density in defined M2X medium. (A) Growth curves, measured by optical density (OD660), of WT, ΔchvG, and ΔchvI strains bearing empty vector (EV) or genetic rescue plasmid (++) integrated at the xylose locus. Primary M2X cultures, inoculated from PYE plates, all grow to high density (left). However, upon back dilution to an OD660 of 0.025, ΔchvG and ΔchvI EV strains saturate at a significantly lower OD (right). Points represent averages from three biological replicates ± SD. ****, P < 0.0001, one-way ANOVA followed by Dunnett’s posttest comparison to WT EV at 24 h. (B) Growth curves, measured by CFU, corresponding to the cultures in panel A. Points represent averages from three biological replicates ± SD. ***, P < 0.0005; one-way analysis of variance (ANOVA) followed by Dunnett’s posttest comparison to WT EV at 24 h. (C) Growth of cultures inoculated from PYE agar plates at different starting densities, measured by CFU. ΔchvI cells carrying EV or genetic rescue plasmid (++) were suspended in M2X medium and immediately diluted to an OD660 of 0.001 or 0.0001. Points represent averages from three biological replicates ± SD. *, P < 0.05; one-way ANOVA followed by Dunnett’s posttest comparison to ΔchvI EV; 10−4 dilution at 30.5 h.
To evaluate if the growth defect was related to the number of cell divisions in defined medium, we resuspended cells in M2X from PYE agar plates and diluted cultures to several low starting densities. ΔchvI EV cultures started at an OD660 of 0.001 and 0.0001 saturated at similar CFU values (∼107 CFU/ml) that were significantly lower than those of ΔchvI++ cultures (∼109 CFU/ml) (Fig. 1C). Thus, ΔchvI mutants that initiate from low density are not limited in the number of times they can divide in M2X medium but, rather, reach a defined carrying capacity. ΔchvI cultures started at higher densities (OD660 of 0.05 and 0.1) and grew to ∼109 CFU/ml, indicating that a high starting density enables primary overnight cultures to saturate at higher density (Fig. S2A).
Washing ΔchvI cells once or twice with M2X before dilution had no effect on the number of CFU at 30.5 h, suggesting that trace contaminating PYE components do not contribute to growth in M2X primary cultures (Fig. S2B). Moreover, cell density alone did not determine the ability of ΔchvI cultures to grow in M2X, as denser back dilutions from ΔchvI overnight cultures did not reach higher viable cell counts (Fig. S2C). Together, our results suggest that the diminished growth capacity of ΔchvI or ΔchvG cells in M2X medium is a function of the time since resuspension from PYE plates and growth phase.
ChvI phosphorylation is critical for growth in defined medium.
Given the similarity between the phenotypes displayed by ΔchvI and ΔchvG strains, we predicted that phosphorylation of ChvI might be important for growth in M2X medium. To test this hypothesis, we generated strains harboring chvI alleles encoding changes to the conserved sites of phosphorylation (D52A, D52N, and D52E). Growth of strains carrying the nonphosphorylatable alleles chvI(D52A) and chvI(D52N) was similar to ΔchvI cells in M2X (Fig. 2A and Fig. S3A). In contrast, chvI(D52E) cultures reached higher densities than ΔchvI cultures, suggesting that ChvI(D52E) is active, albeit not to the same level as phosphorylated ChvI (Fig. 2A and Fig. S3A). Substitutions of the phosphorylatable aspartate with glutamate often act as phosphomimetic mutations and constitutively activate RRs (50–53). Thus, this result supports a critical role for ChvI phosphorylation during growth in M2X medium.
FIG 2.
The chvI(D52E) allele is active and induces filamentation in M2X medium when overexpressed. (A) Optical density (OD660) of cultures 24 h after back dilution to an OD660 of 0.025 in M2X medium (average ± SD, n ≥ 4). chvI mutant strains were generated by restoring the ΔchvI allele with point mutant alleles through allelic exchange. Similarly, restored WT (ΔchvI::chvI) was generated by replacement of the ΔchvI locus with WT chvI. *, P < 0.05; **, P < 0.01; ****, P < 0.0001; one-way ANOVA followed by Dunnett’s posttest comparison to ΔchvI. (B) Optical density (OD660) of strains carrying empty vector (EV) or overexpression plasmids (++) 24 h after back dilution to an OD660 of 0.025 in M2X medium (average ± SD, n = 3). ****, P < 0.0001, one-way ANOVA followed by Dunnett’s posttest comparison to ΔchvG EV. (C) Phase-contrast micrographs of primary overnight cultures grown in M2X medium. On the right, ΔchvI and ΔchvG strains carry empty vector (EV) or overexpression plasmids (++). Scale bars, 5 μm.
To further examine the importance of ChvI phosphorylation, we overexpressed chvG and chvI alleles from a xylose-inducible promoter in knockout backgrounds. Although overexpression of chvG rescued growth of ΔchvG cells (Fig. 1B), overexpression of chvG(H309A), a catalytic-histidine mutant, did not, indicating that phosphorylation of ChvG, and by extension, ChvI, is important for growth in M2X medium (Fig. 2B). Overexpression of chvI(D52E) only partially rescued growth of ΔchvI and ΔchvG strains in M2X, and these strains grew similarly to the strain encoding chvI(D52E) at the native locus (Fig. 2A and B and Fig. S3B). However, in contrast to native expression of chvI(D52E), overexpression of this allele resulted in cell filamentation in M2X but not PYE, consistent with a cell division defect (Fig. 2C and Fig. S3C). Thus, overexpression of phosphomimetic chvI(D52E) appears to interfere with cell division and, perhaps as a result, only partially rescues growth in M2X medium.
Mutations in ntrY, ntrX, and a gene of unknown function rescue the growth defect of ΔchvI and ΔchvG strains.
To better understand why ChvGI is critical for growth in defined medium, we employed a selection strategy to isolate second-site mutations that alleviate the growth defect of ΔchvG and ΔchvI cells. ΔchvG and ΔchvI M2X overnight cultures were back diluted and grown until they reached high density, presumably due to proliferation of suppressor strains (Fig. 3A). We then isolated single colonies with WT-like growth in M2X and used whole-genome sequencing to identify any acquired mutations (Fig. 3B).
FIG 3.
Mutations in the NtrYX TCS and a gene of unknown function suppress the growth defect of ΔchvG and ΔchvI strains in M2X medium. (A) Schematic representation of the suppressor selection protocol. Primary overnight cultures in M2X medium were back diluted to an OD660 of 0.025 and grown until cultures grew to high turbidity. Single colonies were isolated for confirmation and sequencing. (B) Growth curves, measured by optical density (OD660), of WT, ΔchvI, and suppressor strains isolated from either the ΔchvI strain (ΔIS#) or the ΔchvG strain (ΔGS#) upon back dilution in M2X medium. Points represent averages from three biological replicates ± SD. ****, P < 0.0001, one-way ANOVA followed by Dunnett’s posttest comparison to ΔchvI at 24 h. (C) Whole-genome sequencing results of each suppressor strain. The suppressor strain, parental strain, and identified polymorphism(s) are indicated. Mutations in ntrY, ntrX, and ntrZ (CCNA_03863) are in bold. Domain structures of NtrY, NtrX, and NtrZ are diagrammed with domains in blue, signal peptides in green, transmembrane helices in yellow, and identified mutations in red.
In multiple independent strains, we identified nonsynonymous substitutions in the genes encoding the HK NtrY and its cognate RR NtrX (Fig. 3C). The ntrY mutations (L70H and A123V) are located in predicted transmembrane helices, which are involved in transmitting information from periplasmic sensing domains to the kinase domain (Fig. 3C) (54–56). We identified only one ntrX allele, A19V, which lies in helix 1 (α1) of the receiver domain (Fig. 3C). α1 is involved in the interaction interface between the HK dimerization and histidine phosphotransfer (DHp) domain and the RR REC domain (57, 58). We also note that the ntrX(A19V) strain was isolated on PYE plates and appeared to grow normally in M2X, suggesting that this is not a complete loss-of-function allele (49).
In addition to mutations in ntrY and ntrX, we identified three mutants with nonsynonymous substitutions in a gene encoding a putative periplasmic protein of unknown function (CCNA_03863, here referred to as ntrZ) (Fig. 3C). The mutations identified (Y92C and I99N) are both located outside the predicted signal sequence (Fig. 3C). Therefore, mutations in the NtrYX TCS and NtrZ appear to affect the growth of ΔchvI and ΔchvG cells in M2X medium.
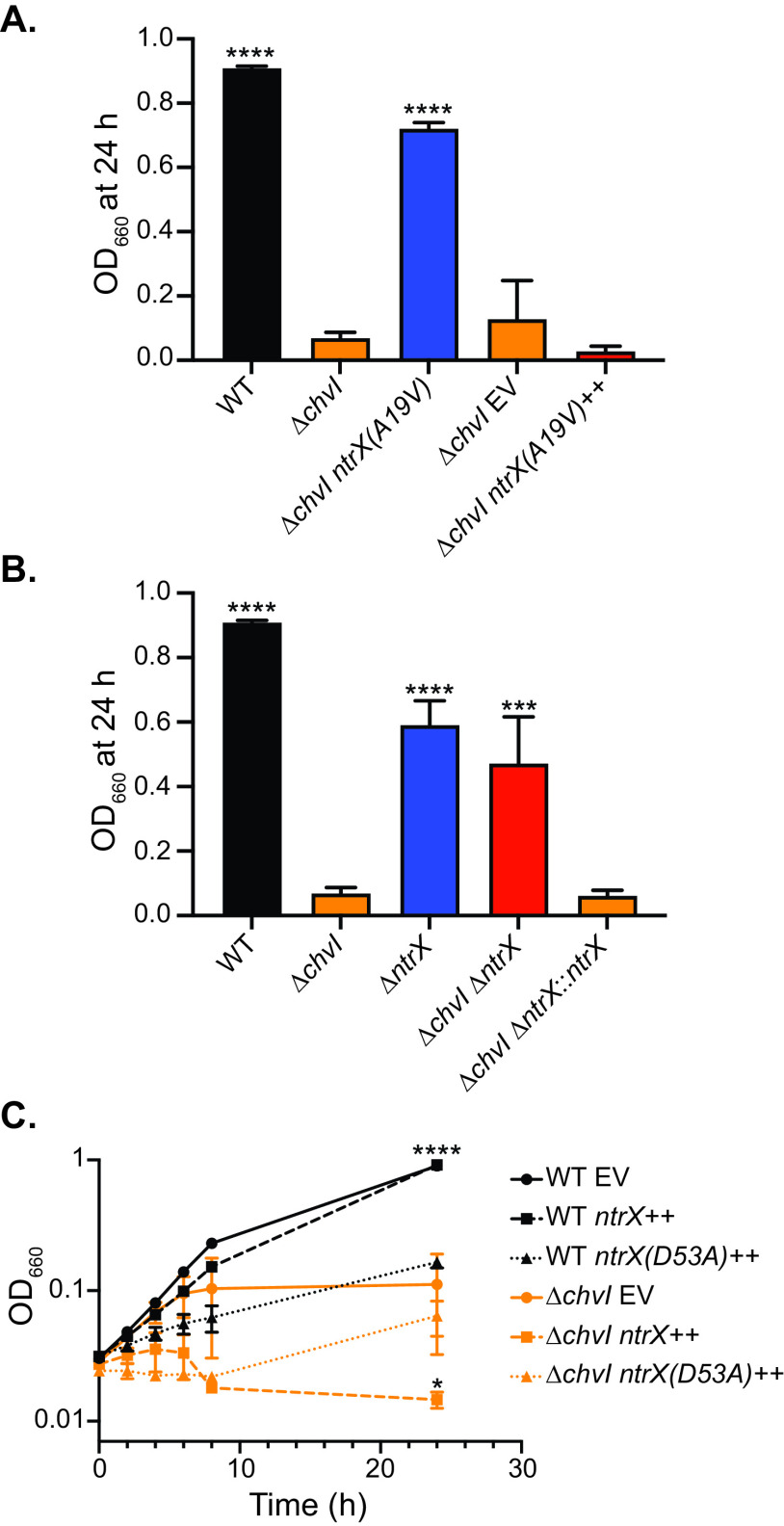
Overexpression or deletion of ntrX modulates the ΔchvI growth phenotype.
Several studies have noted similar phenotypes of chvGI and ntrYX mutants (25, 26, 41); however, to our knowledge, none have established a genetic link between these TCSs. To better understand the connections between ChvGI and NtrYX in C. crescentus, we first characterized the genetic relationship between chvI and ntrX. Replacement of ntrX with the ntrX(A19V) allele restored growth of ΔchvI cells in M2X (Fig. 4A), ruling out other background mutations as causal for suppression of the ΔchvI phenotype. However, overexpression of ntrX(A19V) in the presence of the native ntrX allele did not rescue growth of ΔchvI cells (Fig. 4A). This result indicates that the ntrX(A19V) allele is recessive and suggests that the presence of WT NtrX contributes to the growth phenotype of ΔchvI cells.
FIG 4.
Nonphosphorylatable NtrX limits growth of cells in M2X medium. (A) Optical density (OD660) of cultures 24 h after back dilution to an OD660 of 0.025 in M2X medium (average ± SD, n = 3). The ntrX(A19V) allele was introduced by allele replacement for comparison with WT and ΔchvI or by overexpression (++) for comparison with an empty vector (EV) strain. ****, P < 0.0001; one-way ANOVA followed by Dunnett’s posttest comparison to ΔchvI. (B) Optical density (OD660) of cultures 24 h after back dilution to an OD660 of 0.025 in M2X medium (average ± SD, n = 3). Restored ΔchvI (ΔchvI ΔntrX::ntrX) was generated by knocking ntrX into the native ntrX locus in ΔchvI ΔntrX cells. ***, P < 0.0005; ****, P < 0.0001; one-way ANOVA followed by Dunnett’s posttest comparison to ΔchvI. (C) Growth curves, measured by optical density (OD660), of strains upon back dilution in M2X medium. WT and ΔchvI strains bear empty vector (EV) or ntrX overexpression vectors (++). Points represent averages from three biological replicates ± SD. *, P < 0.05; ****, P < 0.0001; one-way ANOVA followed by Dunnett’s posttest comparison to ΔchvI EV at 24 h.
To examine this notion further, we tested the effect of deleting ntrX in the ΔchvI strain. ΔntrX cells had a slight growth defect in M2X but were clearly distinct from ΔchvI cells (Fig. 4B). Deletion of ntrX in a ΔchvI background rescued growth, indicating that the presence of ntrX is indeed detrimental to ΔchvI cells (Fig. 4B). Suppression of the ΔchvI growth phenotype was not due to a second-site mutation, as restoration of the ntrX locus (ΔchvI ΔntrX::ntrX) restored the growth defect in M2X medium (Fig. 4B).
Given that the presence of ntrX limits growth of ΔchvI cells in M2X, we next tested whether overexpression of ntrX affects growth of WT cells. Overexpression of ntrX only moderately slowed growth of WT cells. However, overexpression of the nonphosphorylatable ntrX(D53A) allele dramatically impaired the growth of WT cells in M2X, similar to the defect observed in ΔchvI cells (Fig. 4C). Overexpression of either ntrX or ntrX(D53A) exacerbated the growth defect of ΔchvI cells (Fig. 4C). Together, these results support a model in which unphosphorylated NtrX limits growth capacity in M2X medium, especially in ΔchvI cells.
NtrZ is a predicted periplasmic protein that functions upstream of NtrY to regulate levels of phosphorylated NtrX.
We next examined the nature of the ntrY and ntrZ suppressor mutations. Overexpression of the ntrY and ntrZ alleles identified in our suppressor strains restored growth of ΔchvI cells in M2X (Fig. 5A). In addition, overexpression of WT ntrY, but not WT ntrZ, significantly suppressed the ΔchvI growth defect (Fig. 5A). We conclude that the ntrY and ntrZ mutations are dominant, likely gain-of-function, alleles. As deletion of ntrX rescued the growth of ΔchvI cells, overexpression of ntrY or ntrZ mutant alleles likely promotes phosphorylation and/or sequestration of NtrX. We attempted, but failed, to construct ΔchvI ΔntrY and ΔchvI ΔntrZ double-mutant strains, suggesting that deletion of either ntrY or ntrZ is synthetically lethal with chvI deletion. This inability to isolate either double mutant also hinted that NtrY and NtrZ function in the same pathway. To test this possibility, we evaluated the phenotype of ntrY and ntrZ deletions in M2X medium. However, the growth of both single deletions and the ΔntrY ΔntrZ double deletion was indistinguishable from WT, thus preventing epistasis analysis (Fig. 5B).
FIG 5.
NtrZ functions upstream of NtrY as a phosphatase inhibitor. (A) Optical density (OD660) of strains bearing empty vector (EV) or ntrZ and ntrY overexpression vectors (++) 24 h after back dilution to an OD660 of 0.025 in M2X medium (average ± SD, n = 3). ****, P < 0.0001; one-way ANOVA followed by Dunnett’s posttest comparison to ΔchvI EV. (B) Growth curves, measured by optical density (OD660), of WT and knockout strains upon back dilution in M2X medium. Points are averages from three biological replicates ± SD. ****, P < 0.0001, one-way ANOVA followed by Dunnett’s posttest comparison to WT at 24 h. (C) Growth curves, measured by CFU, for WT and mutant strains grown in M2X medium. Points are averages from three biological replicates ± SD. ***, P = 0.0005; one-way ANOVA followed by Dunnett’s posttest comparison to ΔntrZ at 48 h. (D) Cell density, measured by CFU, for ΔntrZ and ΔntrY strains bearing empty vector (EV) or overexpression vectors (++) at 48 h growth in M2X medium. Points are averages from three biological replicates ± SD. **, P < 0.01, one-way ANOVA followed by Dunnett’s posttest comparison to ΔntrZ EV at 48 h. (E, Left) Anti-HA Western blotting of lysates analyzed by Phos-tag gel electrophoresis. Each strain encodes ntrX-HA at the native ntrX locus. Phosphorylated (NtrX∼P) and unphosphorylated (NtrX) NtrX-HA are indicated. (E, Right) Quantification of the percentage of NtrX that is phosphorylated (% NtrX∼P). Points are averages from four biological replicates ± SD. *, P < 0.05; **, P < 0.01; ***, P < 0.001; one-way ANOVA followed by Dunnett’s posttest comparison to WT.
Phosphorylation of NtrX is likely important for stationary-phase survival (48), and thus, we hypothesized that deletion of the ntrY HK, and perhaps also ntrZ, might lead to a stationary-phase defect. Indeed, we observed significantly lower CFU in both ΔntrY and ΔntrZ cultures than WT cultures after 48 h of growth in M2X (Fig. 5C). Notably, ΔntrY ΔntrZ cultures behaved similarly to the single mutants, suggesting that NtrY and NtrZ indeed function in the same pathway.
To place ntrY and ntrZ relative to one another, we evaluated the ability of ntrY and ntrZ alleles to rescue stationary-phase survival in the deletion strains. Both ΔntrY and ΔntrZ cells were fully rescued by ectopic overexpression of each respective WT allele (Fig. 5D). Overexpression of ntrY(L70H), but not ntrY, also fully rescued the phenotype of ΔntrZ cells (Fig. 5D). In contrast, neither WT ntrZ nor ntrZ(I99N) rescued ΔntrY cells (Fig. 5D). These data provide evidence that NtrZ functions upstream of NtrY, potentially as a kinase activator and/or phosphatase inhibitor.
We next examined the phosphorylation state of NtrX directly using Phos-tag gel electrophoresis. To detect NtrX by Western blotting, we constructed strains carrying C-terminally hemagglutinin (HA)-tagged ntrX (ntrX-HA) encoded at the native ntrX locus. Phos-tag analysis of WT lysates at stationary phase revealed distinct bands for phosphorylated (NtrX∼P) and unphosphorylated (NtrX) NtrX-HA (Fig. 5E). Deletion of ntrZ ablated NtrX∼P, consistent with a role for NtrZ in promoting NtrX phosphorylation (Fig. 5E). Surprisingly, ΔntrY cells displayed higher levels of NtrX phosphorylation than WT cells, suggesting that NtrY primarily acts as a phosphatase in vivo and is not required for NtrX phosphorylation. NtrZ indeed acts upstream of NtrY, as deletion of ntrZ in the ΔntrY background did not affect NtrX phosphorylation (Fig. 5E). We conclude that NtrZ promotes NtrX phosphorylation by inhibiting NtrY phosphatase activity.
Our characterization of the ntrY, ntrX, and ntrZ mutants suggested that phosphorylation of NtrX may rescue the growth of ΔchvI or ΔchvG cells. NtrX is phosphorylated under acidic conditions, such as those encountered during the stationary phase in M2G or M2X medium (48). Therefore, we tested whether the pH of M2X affected the growth of ΔchvI cultures. For both WT and ΔchvI strains, primary overnight cultures diluted in M2X at pH 7.0 reached similar CFU at 8 h as those diluted in standard M2X (pH 7.2) (Fig. 1B; Fig. S4). WT cultures were relatively unaffected by growth in M2X at pH 6.0 but had significantly fewer CFU in M2X at pH 5.5 versus pH 7.0 (Fig. S4C). In contrast, ΔchvI cells were markedly less fit in M2X at pH 6.0 than at pH 7.0 and displayed an intermediate growth yield at pH 5.5 relative to M2X at either pH 6.0 or pH 7.0 (Fig. S4C). These results are consistent both with suppression of the ΔchvI growth defect by NtrX phosphorylation and an important role for ChvGI in acid stress responses (6).
ChvI and NtrX regulate transcription of an overlapping set of genes.
Although ChvGI is known to regulate the expression of chvR, the complete ChvI transcriptional regulon is not known. To examine the regulatory link between ntrX and chvI in greater detail, we performed a transcriptome deep sequencing (RNA-seq) experiment to comprehensively define ChvI-dependent gene regulation. As ΔchvI cells grow poorly in M2X medium, we exploited overexpression of the phosphomimetic chvI(D52E) allele to assess ChvI-dependent transcription in PYE medium. Excluding the internal chvI control, we identified 162 genes with >1.5-fold change in ΔchvI chvI(D52E)++ cells compared to ΔchvI EV cells (Fig. 6A and Table S1). Of those, 140 were upregulated and 22 were downregulated, indicating that ChvI primarily serves as a transcriptional activator. Consistent with previous work, chvR was upregulated, while chvT was downregulated by overexpression of chvI(D52E) (6, 47). In addition, expression of both chvG and hprK was enhanced in cells expressing chvI(D52E), pointing to positive autoregulation of the chvIG-hprK operon. We also observed regulation of multiple genes involved in envelope maintenance, metabolism, protein quality control, and transport (Fig. S5A). ChvI-dependent genes included multiple genes encoding proteases/peptidases (CCNA_01341, CCNA_02846, CCNA_01955, CCNA_02721, mmpA, CCNA_02594, CCNA_01121, and CCNA_01202), peptidyl-prolyl and disulfide isomerases (CCNA_02889, CCNA_01654, CCNA_01759, CCNA_01653, CCNA_00378, and CCNA_00379), members of the β-barrel assembly machine (BAM) complex (bamA, bamB, bamD, bamE, and bamF), members of the Tol-Pal complex (tolB, ybgF, and tolQ), and lipopolysaccharide biosynthesis genes (CCNA_01497, CCNA_03454, CCNA_01496, and lpxC). Moreover, nearly 40% of ChvI regulon genes encoded hypothetical proteins or proteins of unknown function (Fig. S5A). MEME analysis of the top 35 upregulated operons identified a putative ChvI binding motif, with GCC direct repeats 11 nucleotides (nt) apart, that closely resembles the recently characterized binding motif of Sinorhizobium meliloti ChvI (Fig. S5B) (5).
FIG 6.
The ChvI and NtrX regulons overlap significantly. (A) Heat map of log2(fold change) for genes in the ChvI regulon (fold change > 1.5, false-discovery rate [FDR] P < 0.05) defined by RNA-seq (ΔchvI chvI(D52E)++ versus ΔchvI EV). Log2(fold change) is also shown for a microarray data set comparing RNA levels in WT and ΔntrX cells (49); gray cells indicate no data. The expression of upregulated genes (blue) is activated by ChvI or NtrX, whereas the expression of downregulated genes (yellow) is repressed by ChvI or NtrX. The genes most strongly regulated in the ChvI regulon are annotated (expression of those in bold is investigated further in panels B and C). (B) lacZ transcriptional reporter activity for a subset of genes in the ChvI regulon in WT and ΔchvI strains in M2X medium (average ± SD, n = 3). ****, P < 0.0001, one-way ANOVA followed by Šídák’s posttest comparison for indicated pairs. (C) lacZ transcriptional reporter activity for the same genes as in panel B in WT and ΔntrX strains in M2X medium (average ± SD, n = 4). **, P < 0.01; ****, P < 0.0001; one-way ANOVA followed by Šídák’s posttest comparison for indicated pairs.
As our regulon was defined by overexpression of a phosphomimetic ChvI mutant, we sought to validate our data set under more physiological conditions. We constructed transcriptional β-galactosidase reporters for six selected regulon genes, from nstA (2-fold activation) to CCNA_03987 (128-fold activation). Strains carrying these transcriptional reporters were initially grown in PYE medium, followed by back dilution and 4 h of growth in M2X medium. Consistent with previous reports, the reporter for the chvR promoter (PchvR) was expressed in M2X in a chvI-dependent manner (Fig. 6B) (6, 47). The remaining reporters, apart from PnstA, which was expressed at low levels, also exhibited clear chvI dependence, supporting our RNA-seq results (Fig. 6B). Unlike the other reporters, PchvI was only 2-fold lower in ΔchvI cells than WT cells, indicating that additional factors promote expression of the chvIG-hprK operon.
We next compared our ChvI regulon with a previously published NtrX regulon that was determined using DNA microarrays (49). This experiment measured relative gene expression between C. crescentus WT (strain NA1000) and ΔntrX cells during exponential growth in M2G medium, a condition where NtrX is expected to be largely unphosphorylated (48, 49). Surprisingly, a large fraction of the genes regulated by ChvI were also represented in the genes regulated by NtrX (Fig. 6A). That is, many of the genes upregulated by ChvI also appeared to be upregulated by NtrX (and likewise for downregulated genes). Using cutoffs of 1.5-fold change for the ChvI RNA-seq data and 2.5-fold change for the NtrX microarray data, we established that the ChvI regulon is significantly enriched for NtrX-dependent genes (6.31-fold enrichment, P = 8.99 × 10−19, hypergeometric test). To confirm this overlap, we evaluated the effect of deleting ntrX on our ChvI regulon reporters. Strains carrying the transcriptional reporters were grown to log phase (OD660 of ∼0.1 to 0.2) in M2X medium before assaying β-galactosidase activity. Four of the six reporters exhibited ntrX dependence, including two genes (CCNA_00889 and chvR) not evaluated in the NtrX microarray experiment (Fig. 6C). We note that transcription from the chvI promoter (PchvI) was activated by both ntrX and chvI, raising the possibility that NtrX indirectly affects ChvI-dependent genes via upregulation of chvIG-hprK.
Given the oppositional nature of ChvI and NtrX during growth in M2X medium, we were surprised to see such a high degree of similarity in the genes they regulate. However, a small subset of genes exhibited opposite regulation by ChvI and NtrX, which might therefore account for suppression of the ΔchvI growth defect by deletion of ntrX (Fig. S6A). We overexpressed (for those genes upregulated by ChvI) or knocked out (for those genes downregulated by ChvI) each of these genes in ΔchvI cells and tested their growth capacity in M2X medium. Only one candidate, chvT, had any effect on ΔchvI cells. Specifically, deletion of chvT partially rescued growth of ΔchvI cells in M2X (Fig. 7A and Fig. S6B). Differential regulation of chvT expression by ChvI and NtrX may therefore contribute to the growth defect observed in M2X medium.
FIG 7.
Deletion of chvT partially restores growth of ΔchvI cells in M2X medium. (A) Optical density (OD660) of WT, ΔchvT, and ΔchvI ΔchvT strains, with empty vector (EV) or chvT overexpression vector (++), 24 h after back dilution to an OD660 of 0.025 in M2X medium (average ± SD, n = 4). ***, P < 0.0005; ****, P < 0.0001; one-way ANOVA followed by Dunnett’s posttest comparison to ΔchvI EV. (B) Proposed model for the regulatory interactions between ChvGI and NtrYXZ. ChvG (orange) is activated by a variety of cellular conditions (dashed arrow) and phosphorylates ChvI (blue). Phosphorylated ChvI regulates genes involved in envelope maintenance, transport, metabolism, and cell division (blue oval). In addition, ChvI represses expression of chvT. NtrY (purple) is repressed by NtrZ under acidic conditions in defined medium (dashed arrow), reducing dephosphorylation of NtrX (red). Unphosphorylated NtrX regulates much of the ChvI regulon, likely via upregulation of the chvIG-hprK operon (gray arrow). In addition, unphosphorylated NtrX upregulates chvT and regulates expression of genes involved in nitrogen metabolism and transport (red oval). Phosphorylated ChvI and unphosphorylated NtrX oppose each other in regulating growth in defined medium, partially via differential regulation of chvT. The transcriptional role of phosphorylated NtrX and source of phosphorylation (question marks) are not yet known.
DISCUSSION
The importance of the C. crescentus ChvGI system for growth in defined medium.
To our knowledge, the only reported physiological phenotype for chvGI mutants in C. crescentus is sensitivity to the antibiotic vancomycin (47). However, previous work indicated that ChvGI might be important for growth in defined medium (6). In fact, chvGI mutants in other alphaproteobacteria are sensitive to nutritional conditions, although most, with the exception of Brucella abortus, exhibit particularly poor growth in complex media (4, 8, 27, 59). Deletion of chvG or chvI in C. crescentus caused a distinctive growth defect in M2X medium. ΔchvI cultures do grow in M2X upon inoculation from PYE plates, suggesting that the physiological state of the cell in PYE agar is initially amenable to growth in defined medium (Fig. 1). However, this tolerance is limited by time, with only higher inocula able to reach high cell density (Fig. 1; see Fig. S2 in the supplemental material). Why, then, can primary overnight cultures persist at high density until back dilution in fresh M2X? One possibility is that the low pH of M2X at stationary phase preserves ΔchvI and ΔchvG cells, potentially by triggering phosphorylation of NtrX (Fig. 7B) (48). This model is supported by the observation that ΔchvI cells reach higher CFU when back diluted in M2X at pH 5.5 versus pH 6.0 (Fig. S4C). As ∼20% of the NtrX pool is phosphorylated in M2G medium at pH 6.0 versus ∼40% at pH 5.5 (by Phos-tag analysis), the pH 6 to 5.5 transition would significantly change the level of unphosphorylated NtrX (48). However, as ΔchvI and ΔchvG cells also appear to be sensitive to acidic pH, additional factors are likely at play (Fig. S4C). For example, several extracytoplasmic function (ECF) sigma factors are involved in resistance to stationary-phase stress and might play a protective role in ΔchvI and ΔchvG cells (60–62).
A recent study failed to identify chvG or chvI as being important for fitness in M2X medium (63). However, in this work, M2X cultures were inoculated from PYE starter cultures at high enough density to reach saturation in 5 doublings. Thus, these experiments likely mimicked our primary overnight cultures, obscuring detection of fitness defects for chvGI mutants.
The ChvI transcriptional regulon in C. crescentus.
Although ExoR or ChvI transcriptional regulons have been defined in several alphaproteobacteria (4, 5, 26, 64), the C. crescentus ChvI regulon was unknown prior to this work. We employed RNA-seq to detect direct and indirect transcriptional targets of ChvI in C. crescentus (Fig. 6; Table S1). Our ChvI regulon contained several classes of genes noted in other alphaproteobacteria, including those encoding outer membrane proteins and transporters, metabolic enzymes, lipopolysaccharide biosynthesis enzymes, and stress response proteins (4, 5, 26, 64). We note, in particular, that the C. crescentus ChvI regulon contained a large number of genes encoding proteins involved in envelope maintenance, including nearly the entire BAM complex, the envelope integrity protein EipA, members of the Tol-Pal complex, and a variety of chaperones and proteases (Fig. 6 and Table S1) (65–68). The idea that ChvGI is involved in envelope integrity is consistent with previous observations that ChvGI is activated by envelope stress and confers resistance to antibiotics targeting the cell wall (47). However, ∼40% of genes regulated by ChvI are annotated generically or as hypotheticals, and thus, more work will be required to characterize the pathways downstream of the ChvGI system. In A. tumefaciens, S. meliloti, and C. crescentus, ChvGI appears to suppress cell motility and/or chemotaxis (5, 15, 20, 26, 69, 70). However, unlike in A. tumefaciens and S. meliloti, C. crescentus ChvI does not regulate any obvious flagellar or chemotaxis genes, suggesting that effects on motility may be due to posttranscriptional regulation and/or alterations in cell cycle progression (5, 26, 71–73).
Overexpression of the phosphomimetic chvI(D52E) allele induced cell filamentation in M2X, implicating ChvI in regulating cell division and cytokinesis in particular. ChvI upregulates several genes related to cell division, including zauP, members of the Tol-Pal complex, smc, and nstA (68, 74–77). We note that overexpression of a proteolytically stable mutant form of nstA induces cell filamentation, and both zauP and the Tol-Pal complex are involved in regulating cytokinesis (74, 77). Perhaps overinduction of these regulon genes, in combination with the cellular state in M2X medium, interferes with proper cell division.
Genetic interactions between ChvGI and NtrYX.
The severe growth defect of ΔchvG and ΔchvI cells in M2X medium allowed us to uncover the first known genetic interaction between chvGI and ntrYX (Fig. 3). Importantly, deletion of ntrX suppressed the growth defect of ΔchvI cells, whereas overexpression of nonphosphorylatable ntrX(D53A) was deleterious for growth in both ΔchvI and WT cells (Fig. 4). These results suggest that the activity of unphosphorylated NtrX is particularly detrimental in cells lacking chvI. Although unphosphorylated response regulators are often assumed to be inactive, multiple RRs are known to affect transcription in their unphosphorylated states (78–82). Thus, we propose that phosphorylated ChvI and unphosphorylated NtrX oppose each other to regulate growth in defined medium (Fig. 7B). We note that a past study examining connections between ChvI and NtrX in S. meliloti did not test whether perturbations in NtrYX signaling might affect ΔchvI phenotypes (25). We predict that a similar ChvI-NtrX relationship may be conserved in other alphaproteobacteria.
As gain-of-function mutations in ntrY and ntrZ suppress the growth defect of ΔchvI cells, we also propose that phosphorylation of NtrX relieves its detrimental activity, possibly by changing its transcriptional regulon. Although the global transcriptional effects of NtrX phosphorylation have yet to be characterized, in vitro phosphorylation of B. abortus NtrX does induce conformational changes and alters, but does not weaken, binding to the ntrYX promoter (83). Interestingly, we were unable to construct ΔchvI ΔntrY and ΔchvI ΔntrZ strains, suggesting that these gene deletion combinations are synthetically lethal in PYE. As ntrX is also essential in PYE (20), the balance between unphosphorylated and phosphorylated NtrX may be important for growth in both complex and defined media.
To identify downstream genes that mediate the oppositional relationship between ChvI and NtrX, we compared their transcriptional regulons (Fig. 6 and Table S1). The ChvI regulon strongly overlapped with that of NtrX, as 80% of the top 30 ChvI-activated genes are also activated by NtrX (per our >1.5-fold cutoff). In contrast, only 20% of the top 30 NtrX-activated genes exhibit >1.5-fold ChvI dependence (49). Given that chvI, chvG, and hprK are upregulated by NtrX, it may be the case that NtrX simply activates expression of the chvIG-hprK operon, thereby altering transcription of genes in the ChvI regulon (Fig. 7B, gray arrow). However, further work is required to define the mechanism by which NtrX affects the ChvI regulon, as it may also indirectly affect ChvGI signaling or directly regulate expression of regulon genes. The overlap we observed between the ChvI and NtrX regulons may be restricted to C. crescentus and close relatives, as chvG and chvI are not transcriptionally regulated by NtrYX in Rhodobacter sphaeroides (40). However, ChvGI and NtrYX may still be transcriptionally linked in more distantly related alphaproteobacterial species, as ntrX is part of the A. tumefaciens ExoR regulon (26).
The majority of overlapping genes in the ChvI and NtrX regulons cannot account for the detrimental effect of unphosphorylated NtrX on ΔchvI cells. Therefore, we focused on eight genes that exhibited opposing transcriptional regulation by ChvI and NtrX (Fig. S6). Given that NtrX largely reinforces the ChvGI TCS, these oppositional effects may reflect direct transcriptional regulation by NtrX or the effects of unique NtrX regulon genes. Only deletion of chvT improved the growth of ΔchvI cells, indicating that suppression of chvT RNA levels by ChvI may be important for growth in M2X medium (Fig. 7). chvT is linked to diverse phenotypes in C. crescentus, including survival in stationary phase, sensitivity to cell wall-targeting antibiotics, and sensitivity to bacteriocins (47, 84, 85). However, the molecule(s) transported by ChvT remains undefined. High ChvT levels may contribute to defects in transport in defined medium and/or alter membrane integrity, impacting the viability of cells lacking ChvGI (47). It is clear, though, that altered chvT expression cannot fully explain the growth deficiency of ΔchvI cells in M2X medium. This growth defect may result from the collective action of several genes, and therefore, manipulation of individual candidates may not rescue growth in M2X. Moreover, 15 genes in the ChvI regulon are absent from the NtrX microarray data set, raising the possibility that one or more are differentially regulated by ChvI and NtrX (Fig. 6; Table S1). ChvI and NtrX might also interact more indirectly, as each regulates unique subsets of genes that may affect growth in M2X medium.
On the role of NtrZ.
NtrYX is associated with a wide range of physiological responses, from nitrogen metabolism to redox sensing and cell envelope maintenance (25, 35, 39, 40). Despite these phenotypic observations, little is known about NtrY activity in vivo and the regulation of NtrY via its periplasmic domain. Our work reveals a surprising phosphatase pathway involving the previously uncharacterized protein NtrZ. Phos-tag analysis of NtrX phosphorylation in vivo clearly demonstrates that NtrY is dispensable for NtrX phosphorylation and suggests that NtrY primarily acts as an NtrX phosphatase (Fig. 5). In addition, NtrZ appears to inhibit NtrY phosphatase activity, as deletion of ntrZ abolishes NtrX phosphorylation only when ntrY is present (Fig. 5). Thus, we propose that NtrZ inhibits NtrY phosphatase activity, stabilizing the pool of phosphorylated NtrX (Fig. 7). In the future, we are interested in determining whether NtrZ physically interacts with the NtrY periplasmic domain or affects NtrY activity indirectly. Several known periplasmic or membrane-bound TCS regulators directly interact with HK periplasmic domains (33, 86–88). Although our model does not exclude the possibility that NtrY phosphorylates NtrX under certain conditions, there is clearly another source of NtrX phosphorylation in C. crescentus. An additional HK may phosphorylate NtrX, although the most likely candidate, NtrB, does not phosphorylate NtrX in vitro (49). Alternatively, some metabolic intermediates, such as aspartyl-phosphate or carbamoyl-phosphate, can serve as phosphodonors for RRs in vivo and in vitro (1, 89–92).
Interestingly, we initially placed ntrZ upstream of ntrY by examining the similar stationary-phase survival phenotypes of ΔntrY and ΔntrZ cells (Fig. 5). Given our Phos-tag results, these phenotypes suggest that both phosphorylated and unphosphorylated NtrX play important roles in stationary-phase survival. This idea is consistent with the fact that NtrX phosphorylation increases upon entry into stationary phase but eventually decreases (48).
Fernández et al. reported that NtrX phosphorylation is triggered by acidic pH in defined medium (48). Therefore, we hypothesize that inhibition of NtrY by NtrZ may be enhanced by acidic pH. Thus, NtrZ and ExoR potentially share a regulatory theme in which their activities toward their respective HKs are affected by low pH (16, 33, 34). However, it remains to be seen if, as with ExoR, proteolysis plays a role in controlling NtrZ-dependent regulation of NtrY (16). Given the results of our suppressor selection, it would appear that mutations in NtrZ or in the transmembrane helices of NtrY may bypass regulation by acidic pH. In an alignment of 100 NtrZ homologs, ranging from 41% to 100% sequence identity, the Y92 position is conserved as an aromatic residue (95% Y), while I99 is conserved as an aliphatic hydrophobic residue (51% I, common L and V substitutions), suggesting that these residues are important for NtrZ function. Further work is required to determine how mutation of Y92 or I99 may alter interaction with the NtrY periplasmic domain or affect a different aspect of NtrZ function. An alignment of the noncytoplasmic portion of 250 NtrY sequences, ranging from 37% to 100% identity, reveals that the L70 position is largely conserved as an aliphatic hydrophobic residue (94% L), whereas A123 is conserved as a hydrophobic residue (93% A), albeit with occasional bulky aromatic substitutions. It is unsurprising, then, that the L70H and A123V substitutions appear to affect NtrY function. In fact, several studies have identified transmembrane mutations that alter HK kinase and phosphatase activities (93–95).
Unlike the periplasmic kinase regulator ExoR, which contains Sel1-like repeats typically involved in protein-protein interactions, NtrZ does not contain any conserved domains or motifs (107). Moreover, NtrZ appears restricted to the order Caulobacterales, albeit with a few distant homologs in other alphaproteobacteria. Notably, neither B. abortus, S. meliloti, nor A. tumefaciens contains NtrZ homologs, and conservation of the noncytoplasmic region of NtrY is quite low between C. crescentus and these organisms (24 to 27% identity). However, L70 is largely conserved (L or V), and A123 is entirely conserved between these NtrY homologs. Thus, the L70H and A123V substitutions may have similar effects on NtrY activity in these organisms, and further analyses of these mutants may shed light on a conserved NtrY activity switch. Moreover, it will be interesting to see if NtrY also primarily acts as a phosphatase and/or is regulated by periplasmic effectors in S. meliloti, A. tumefaciens, and B. abortus.
MATERIALS AND METHODS
Strains and plasmids.
All plasmids were constructed using standard molecular biology techniques. See Table S2 in the supplemental material for strain, plasmid, and primer information. Plasmids for generating in-frame deletions and allele replacements were generated by cloning homologous upstream and downstream regions into pNPTS138. Transcriptional reporter plasmids were generated by cloning 400 to 500 bp upstream of the open reading frame (ORF) into pRKlac290. For overexpression strains, ORFs were inserted into pMT585, a plasmid for xylose-inducible expression that integrates at the xylX locus. Plasmids were transformed into C. crescentus CB15 strain by electroporation or triparental mating. In-frame deletion and allele replacement strains were generated by a double recombination strategy involving sacB counterselection on PYE plates supplemented with 3% sucrose (96). For ΔntrX knockout strains, counterselection was carried out on M2X plus 0.5% sucrose plates due to their growth defect on PYE. ΔntrX and ΔchvI ΔntrX strains were grown only on M2 medium. Construction of ΔchvI ΔntrY and ΔchvI ΔntrZ mutants was attempted using both PYE and M2X counterselection methods. All C. crescentus strains were grown at 30°C. For strains carrying pRKlac290 plasmids, oxytetracycline was added to 1 μg/ml in liquid and 2 μg/ml in PYE agar or 1 μg/ml in M2X agar.
M2X medium contained 6.1 mM Na2HPO4, 3.9 mM KH2PO4, 9.3 mM NH4Cl, 0.25 mM CaCl2, 0.5 mM MgSO4, 1:1,000 100× ferrous sulfate chelate solution (Sigma), and 0.15% xylose. PYE medium contained 0.2% peptone, 0.1% yeast extract, 0.5 mM CaCl2, and 1 mM MgSO4.
Measurement of growth in M2X medium.
Primary M2X cultures were inoculated from plates to an approximate density of OD660 of 0.02 to 0.10 and grown overnight. Overnight cultures were back diluted to an OD660 of 0.025, and OD660 was recorded at the indicated times. To enumerate CFU, samples were taken at the indicated time points, and 10-fold serial dilutions were plated on PYE agar. For pH experiments, M2X medium was adjusted to the indicated pH using HCl.
For experiments with controlled starting densities, cells were resuspended in M2X medium directly from PYE plates. These resuspensions were then diluted to the indicated OD660, and the titers of the cultures were determined for CFU. In washing experiments, 1 ml resuspended cells were spun down at 8,000 × g for 3 min and resuspended in 1 ml fresh M2X medium once (1×) or twice (2×) before dilution. Plotting and statistical analyses were carried out using Prism (GraphPad).
Microscopy.
Samples of ΔchvG and ΔchvI cells were taken from overnight M2X or PYE plus 0.15% xylose cultures and imaged with a DMI6000 B (Leica) microscope in phase contrast using a HC PL APO 63×/1.4 numeric aperture (NA) oil Ph3 CS2 objective. Images were captured using an Orca-R2 C10600 digital camera (Hamamatsu) controlled by Leica Application Suite X (Leica). Images were processed using Fiji (97, 98).
Suppressor screen.
Overnight M2X cultures of ΔchvG and ΔchvI cells were back diluted to an OD660 of 0.025 in M2X medium. Cultures initially saturated at OD660 of ∼0.1 before growing to higher density (OD660, 0.5 to 0.8) after 2 to 3 days growth. From each culture, single colonies were isolated on PYE plates. Isolated strains were then tested for suppression by M2X growth curves. The origin of each suppressor strain is as follows: culture 1, ΔIS1; culture 2, ΔIS2 and ΔIS3; culture 3, ΔGS1; and culture 4, ΔGS2 and ΔGS3. Genomic DNA was isolated from 1 ml of overnight culture grown in PYE medium, using guanidinium thiocyanate (99). Sequencing was performed by the Microbial Genome Sequencing Center (Pittsburgh, PA) using a single-library preparation method based upon the Illumina Nextera kit. Sequences were aligned to the C. crescentus NA1000 reference genome (GenBank accession number CP001340) using breseq (100).
Phos-tag gel electrophoresis and Western blotting.
Two-milliliter M2X cultures of strains containing ntrX-HA at the native ntrX locus were grown overnight and back diluted to an OD660 of 0.01. Samples were collected after 22 h (0.25 ml · OD660; i.e., volume [ml] = 0.25/OD660 of culture) and frozen at −80°C. Samples were thawed, resuspended in 2.5 × SDS loading buffer (125 mM Tris [pH 6.8], 25% glycerol, 5% SDS, 5 mM dithiothreitol [DTT], and 0.01% bromophenol blue) containing 1:50 benzonase (Sigma), and immediately loaded onto Phos-tag gels.
Phos-tag electrophoresis was performed as described (48) using 8% acrylamide gels copolymerized with 35 μM Phos-tag acrylamide (Nard) and 150 μM ZnCl2. Proteins were transferred to polyvinylidene difluoride (PVDF; Bio-Rad) in a wet-transfer apparatus. Membranes were probed with monoclonal HA-tag antibody (1:2,000 dilution; Invitrogen; 2-2.2.14), incubated with anti-mouse IgG-horseradish peroxidase (IgG-HRP) conjugate (1:5,000 dilution; Invitrogen), and developed with ProSignal Pico spray (Prometheus). Blots were imaged using a Bio-Rad ChemiDoc MP Imager. Bands were quantified using Fiji (97, 98).
Transcriptome deep sequencing.
Two milliliters of PYE medium were inoculated with ΔchvI EV (ΔchvI xylX::pMT585) and ΔchvI chvI(D52E)++ [ΔchvI xylX::pMT585-chvI(D52E)] cells and grown overnight. Cultures were diluted to an OD660 of 0.001 in 2 ml fresh PYE and grown for 22.5 h. Cultures were diluted to an OD660 of 0.075 in 5 ml PYE plus 0.15% xylose and grown for 3.5 h before TRIzol extraction and RNA isolation, as described previously (101). RNA-seq libraries were prepared using an Illumina TruSeq stranded RNA kit and sequenced on an Illumina NextSeq 500 instrument at the University of Chicago Functional Genomics Facility. Sequencing data were analyzed using CLC Genomics Workbench 20 (Qiagen) by mapping reads to the C. crescentus NA1000 genome (84). Motif searching was carried out using MEME (102). Heatmaps were generated using Java TreeView3, and hypergeometric analysis was performed using phyper in R (103, 104).
β-Galactosidase assays.
For assays of WT versus ΔchvI strains, cells were grown overnight in 2 ml PYE medium. Then, 500 μl of each culture was centrifuged at 8,000 × g for 3 min and resuspended in 500 μl M2X medium. Resuspended cultures were used to inoculate 2 ml M2X medium at a starting OD660 of 0.075. Cultures were grown for 4 h before assaying β-galactosidase activity. For assays of WT versus ΔntrX strains, cells were grown overnight in 2 ml M2X medium. Overnight cultures were diluted in M2X medium such that they would reach OD660 of 0.1 to 0.2 after 23.5 h of growth. β-Galactosidase assays were performed as previously described using 200 μl of M2X culture plus 100 μl sterile PYE medium as an emulsifier (105). Plotting and statistical analyses were carried out using Prism.
Data availability.
RNA-seq data are available in the NCBI’s Gene Expression Omnibus (GEO) database (106) under the GEP series accession number GSE168965.
ACKNOWLEDGMENTS
We thank Clare Kirkpatrick, Régis Hallez, Alex Quintero, and members of the Crosson laboratory for helpful discussion and Jen Mach and plant editors for constructive feedback on the manuscript.
Research in this publication was supported by the National Institute of General Medical Sciences of the National Institutes of Health (NIH) under award numbers F32 GM128283 (to B.J.S.) and R35 GM131762 (to S.C.).
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
We declare no conflict of interest.
Footnotes
Supplemental material is available online only.
Contributor Information
Sean Crosson, Email: crosson4@msu.edu.
Ann M. Stock, Rutgers University-Robert Wood Johnson Medical School
REFERENCES
- 1.Stock AM, Robinson VL, Goudreau PN. 2000. Two-component signal transduction. Annu Rev Biochem 69:183–215. 10.1146/annurev.biochem.69.1.183. [DOI] [PubMed] [Google Scholar]
- 2.Crosson S, McGrath PT, Stephens C, McAdams HH, Shapiro L. 2005. Conserved modular design of an oxygen sensory/signaling network with species-specific output. Proc Natl Acad Sci U S A 102:8018–8023. 10.1073/pnas.0503022102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.David M, Daveran ML, Batut J, Dedieu A, Domergue O, Ghai J, Hertig C, Boistard P, Kahn D. 1988. Cascade regulation of nif gene expression in Rhizobium meliloti. Cell 54:671–683. 10.1016/S0092-8674(88)80012-6. [DOI] [PubMed] [Google Scholar]
- 4.Viadas C, Rodríguez MC, Sangari FJ, Gorvel JP, García-Lobo JM, López-Goñi I. 2010. Transcriptome analysis of the Brucella abortus BvrR/BvrS two-component regulatory system. PLoS One 5:e10216. 10.1371/journal.pone.0010216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ratib NR, Sabio EY, Mendoza C, Barnett MJ, Clover SB, Ortega JA, Cruz Dela FM, Balderas D, White H, Long SR, Chen EJ. 2018. Genome‐wide identification of genes directly regulated by ChvI and a consensus sequence for ChvI binding in Sinorhizobium meliloti. Mol Microbiol 110:596–615. 10.1111/mmi.14119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fröhlich KS, Förstner KU, Gitai Z. 2018. Post-transcriptional gene regulation by an Hfq-independent small RNA in Caulobacter crescentus. Nucleic Acids Res 66:325. 10.1093/nar/gky765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Landa AS, Cerdá JP, Grilló MJ, Moreno E, Moriyón I, Blasco JM, Gorvel JP, Goñi IL. 1998. A two‐component regulatory system playing a critical role in plant pathogens and endosymbionts is present in Brucella abortus and controls cell invasion and virulence. Mol Microbiol 29:125–138. 10.1046/j.1365-2958.1998.00913.x. [DOI] [PubMed] [Google Scholar]
- 8.Charles TC, Nester EW. 1993. A chromosomally encoded two-component sensory transduction system is required for virulence of Agrobacterium tumefaciens. J Bacteriol 175:6614–6625. 10.1128/JB.175.20.6614-6625.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gottschlich L, Geiser P, Bortfeld-Miller M, Field CM, Vorholt JA. 2019. Complex general stress response regulation in Sphingomonas melonis Fr1 revealed by transcriptional analyses. Sci Rep 9:9404. 10.1038/s41598-019-45788-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaczmarczyk A, Hochstrasser R, Vorholt JA, Francez-Charlot A. 2014. Complex two-component signaling regulates the general stress response in Alphaproteobacteria. Proc Natl Acad Sci U S A 111:E5196–E5204. 10.1073/pnas.1410095111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lori C, Kaczmarczyk A, de Jong I, Jenal U. 2018. A single-domain response regulator functions as an integrating hub to coordinate general stress response and development in Alphaproteobacteria. mBio 9:e00809-18. 10.1128/mBio.00809-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Francis VI, Porter SL. 2019. Multikinase networks: two-component signaling networks integrating multiple stimuli. Annu Rev Microbiol 73:199–223. 10.1146/annurev-micro-020518-115846. [DOI] [PubMed] [Google Scholar]
- 13.Howell A, Dubrac S, Noone D, Varughese KI, Devine K. 2006. Interactions between the YycFG and PhoPR two‐component systems in Bacillus subtilis: the PhoR kinase phosphorylates the non‐cognate YycF response regulator upon phosphate limitation. Mol Microbiol 59:1199–1215. 10.1111/j.1365-2958.2005.05017.x. [DOI] [PubMed] [Google Scholar]
- 14.Perego M, Hanstein C, Welsh KM, Djavakhishvili T, Glaser P, Hoch JA. 1994. Multiple protein-aspartate phosphatases provide a mechanism for the integration of diverse signals in the control of development in B. subtilis. Cell 79:1047–1055. 10.1016/0092-8674(94)90035-3. [DOI] [PubMed] [Google Scholar]
- 15.Wells DH, Chen EJ, Fisher RF, Long SR. 2007. ExoR is genetically coupled to the ExoS-ChvI two-component system and located in the periplasm of Sinorhizobium meliloti. Mol Microbiol 64:647–664. 10.1111/j.1365-2958.2007.05680.x. [DOI] [PubMed] [Google Scholar]
- 16.Lu H-Y, Luo L, Yang M-H, Cheng H-P. 2012. Sinorhizobium meliloti ExoR is the target of periplasmic proteolysis. J Bacteriol 194:4029–4040. 10.1128/JB.00313-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stein BJ, Fiebig A, Crosson S. 2020. Feedback control of a two-component signaling system by an Fe-S-binding receiver domain. mBio 11:e03383-19. 10.1128/mBio.03383-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Noriega CE, Lin H-Y, Chen L-L, Williams SB, Stewart V. 2010. Asymmetric cross-regulation between the nitrate-responsive NarX-NarL and NarQ-NarP two-component regulatory systems from Escherichia coli K-12. Mol Microbiol 75:394–412. 10.1111/j.1365-2958.2009.06987.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller MB, Skorupski K, Lenz DH, Taylor RK, Bassler BL. 2002. Parallel quorum sensing systems converge to regulate virulence in Vibrio cholerae. Cell 110:303–314. 10.1016/S0092-8674(02)00829-2. [DOI] [PubMed] [Google Scholar]
- 20.Skerker JM, Prasol MS, Perchuk BS, Biondi EG, Laub MT. 2005. Two-component signal transduction pathways regulating growth and cell cycle progression in a bacterium: a system-level analysis. PLoS Biol 3:e334. 10.1371/journal.pbio.0030334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bijlsma JJE, Groisman EA. 2003. Making informed decisions: regulatory interactions between two-component systems. Trends Microbiol 11:359–366. 10.1016/S0966-842X(03)00176-8. [DOI] [PubMed] [Google Scholar]
- 22.Birkey SM, Liu W, Zhang X, Duggan MF, Hulett FM. 1998. Pho signal transduction network reveals direct transcriptional regulation of one two-component system by another two-component regulator: Bacillus subtilis PhoP directly regulates production of ResD. Mol Microbiol 30:943–953. 10.1046/j.1365-2958.1998.01122.x. [DOI] [PubMed] [Google Scholar]
- 23.Mouslim C, Groisman EA. 2003. Control of the Salmonella ugd gene by three two-component regulatory systems. Mol Microbiol 47:335–344. 10.1046/j.1365-2958.2003.03318.x. [DOI] [PubMed] [Google Scholar]
- 24.Huang J, Carney BF, Denny TP, Weissinger AK, Schell MA. 1995. A complex network regulates expression of eps and other virulence genes of Pseudomonas solanacearum. J Bacteriol 177:1259–1267. 10.1128/jb.177.5.1259-1267.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang D, Xue H, Wang Y, Yin R, Xie F, Luo L. 2013. The Sinorhizobium meliloti ntrX gene is involved in succinoglycan production, motility, and symbiotic nodulation on alfalfa. Appl Environ Microbiol 79:7150–7159. 10.1128/AEM.02225-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heckel BC, Tomlinson AD, Morton ER, Choi JH, Fuqua C. 2014. Agrobacterium tumefaciens ExoR controls acid response genes and impacts exopolysaccharide synthesis, horizontal gene transfer, and virulence gene expression. J Bacteriol 196:3221–3233. 10.1128/JB.01751-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mantis NJ, Winans SC. 1993. The chromosomal response regulatory gene chvI of Agrobacterium tumefaciens complements an Escherichia coli phoB mutation and is required for virulence. J Bacteriol 175:6626–6636. 10.1128/JB.175.20.6626-6636.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheng HP, Walker GC. 1998. Succinoglycan production by Rhizobium meliloti is regulated through the ExoS-ChvI two-component regulatory system. J Bacteriol 180:20–26. 10.1128/JB.180.1.20-26.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guzman-Verri C, Manterola L, Sola-Landa A, Parra A, Cloeckaert A, Garin J, Gorvel JP, Moriyon I, Moreno E, Lopez-Goni I. 2002. The two-component system BvrR/BvrS essential for Brucella abortus virulence regulates the expression of outer membrane proteins with counterparts in members of the Rhizobiaceae. Proc Natl Acad Sci U S A 99:12375–12380. 10.1073/pnas.192439399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yao SY, Luo L, Har KJ, Becker A, Ruberg S, Yu GQ, Zhu JB, Cheng HP. 2004. Sinorhizobium meliloti ExoR and ExoS proteins regulate both succinoglycan and flagellum production. J Bacteriol 186:6042–6049. 10.1128/JB.186.18.6042-6049.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Foreman DL, Vanderlinde EM, Bay DC, Yost CK. 2010. Characterization of a gene family of outer membrane proteins (ropB) in Rhizobium leguminosarum bv. viciae VF39SM and the role of the sensor kinase ChvG in their regulation. J Bacteriol 192:975–983. 10.1128/JB.01140-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Quebatte M, Dehio M, Tropel D, Basler A, Toller I, Raddatz G, Engel P, Huser S, Schein H, Lindroos HL, Andersson SGE, Dehio C. 2010. The BatR/BatS two-component regulatory system controls the adaptive response of Bartonella henselae during human endothelial cell infection. J Bacteriol 192:3352–3367. 10.1128/JB.01676-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen EJ, Sabio EA, Long SR. 2008. The periplasmic regulator ExoR inhibits ExoS/ChvI two-component signalling in Sinorhizobium meliloti. Mol Microbiol 69:1290–1303. 10.1111/j.1365-2958.2008.06362.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu C-F, Lin J-S, Shaw G-C, Lai E-M. 2012. Acid-induced type VI secretion system is regulated by ExoR-ChvG/ChvI signaling cascade in Agrobacterium tumefaciens. PLoS Pathog 8:e1002938. 10.1371/journal.ppat.1002938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pawlowski K, Klosse U, de Bruijn FJ. 1991. Characterization of a novel Azorhizobium caulinodans ORS571 two-component regulatory system, NtrY/NtrX, involved in nitrogen fixation and metabolism. Mol Gen Genet 231:124–138. 10.1007/BF00293830. [DOI] [PubMed] [Google Scholar]
- 36.Ishida ML, Assumpção MC, Machado HB, Benelli EM, Souza EM, Pedrosa FO. 2002. Identification and characterization of the two-component NtrY/NtrX regulatory system in Azospirillum brasilense. Braz J Med Biol Res 35:651–661. 10.1590/S0100-879X2002000600004. [DOI] [PubMed] [Google Scholar]
- 37.Cheng Z, Lin M, Rikihisa Y. 2014. Ehrlichia chaffeensis proliferation begins with NtrY/NtrX and PutA/GlnA upregulation and CtrA degradation induced by proline and glutamine uptake. mBio 5:e02141-14. 10.1128/mBio.02141-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Atack JM, Srikhanta YN, Djoko KY, Welch JP, Hasri NHM, Steichen CT, Hoven RNV, Grimmond SM, Othman DSMP, Kappler U, Apicella MA, Jennings MP, Edwards JL, McEwan AG. 2013. Characterization of an ntrX mutant of Neisseria gonorrhoeae reveals a response regulator that controls expression of respiratory enzymes in oxidase-positive proteobacteria. J Bacteriol 195:2632–2641. 10.1128/JB.02062-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.del Carmen Carrica M, Fernández I, Martí MA, Paris G, Goldbaum FA. 2012. The NtrY/X two‐component system of Brucella spp. acts as a redox sensor and regulates the expression of nitrogen respiration enzymes. Mol Microbiol 85:39–50. 10.1111/j.1365-2958.2012.08095.x. [DOI] [PubMed] [Google Scholar]
- 40.Lemmer KC, Alberge F, Myers KS, Dohnalkova AC, Schaub RE, Lenz JD, Imam S, Dillard JP, Noguera DR, Donohue TJ. 2020. The NtrYX two-component system regulates the bacterial cell envelope. mBio 11:e00957-20. 10.1128/mBio.00957-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Calatrava-Morales N, Nogales J, Ameztoy K, van Steenbergen B, Soto MJ. 2017. The NtrY/NtrX system of Sinorhizobium meliloti GR4 regulates motility, EPS I production, and nitrogen metabolism but is dispensable for symbiotic nitrogen fixation. Mol Plant Microbe Interact 30:566–577. 10.1094/MPMI-01-17-0021-R. [DOI] [PubMed] [Google Scholar]
- 42.Bonato P, Alves LR, Osaki JH, Rigo LU, Pedrosa FO, Souza EM, Zhang N, Schumacher J, Buck M, Wassem R, Chubatsu LS. 2016. The NtrY–NtrX two‐component system is involved in controlling nitrate assimilation in Herbaspirillum seropedicae strain SmR1. FEBS J 283:3919–3930. 10.1111/febs.13897. [DOI] [PubMed] [Google Scholar]
- 43.Urtecho G, Campbell DE, Hershey DM, Hussain FA, Whitaker RJ, O’Toole GA. 2020. Discovering the molecular determinants of Phaeobacter inhibens susceptibility to Phaeobacter phage MD18. mSphere 5:666. 10.1128/mSphere.00898-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Drepper T, Wiethaus J, Giaourakis D, Groà S, Schubert B, Vogt M, Wiencek Y, McEwan AG, Masepohl B. 2006. Cross-talk towards the response regulator NtrC controlling nitrogen metabolism in Rhodobacter capsulatus. FEMS Microbiol Lett 258:250–256. 10.1111/j.1574-6968.2006.00228.x. [DOI] [PubMed] [Google Scholar]
- 45.Poindexter JS. 1964. Biological properties and classification of the Caulobacter group. Bacteriol Rev 28:231–295. 10.1128/MMBR.28.3.231-295.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wilhelm RC. 2018. Following the terrestrial tracks of Caulobacter - redefining the ecology of a reputed aquatic oligotroph. ISME J 12:3025–3037. 10.1038/s41396-018-0257-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vallet S-U, Hansen LH, Bistrup FC, Laursen SA, Chapalay JB, Chambon M, Turcatti G, Viollier PH, Kirkpatrick CL. 2020. Loss of bacterial cell pole stabilization in Caulobacter crescentus sensitizes to outer membrane stress and peptidoglycan-directed antibiotics. mBio 11:e00538-20. 10.1128/mBio.00538-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fernández I, Sycz G, Goldbaum FA, del Carmen Carrica M. 2018. Acidic pH triggers the phosphorylation of the response regulator NtrX in alphaproteobacteria. PLoS One 13:e0194486. 10.1371/journal.pone.0194486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Capra EJ, Perchuk BS, Skerker JM, Laub MT. 2012. Adaptive mutations that prevent crosstalk enable the expansion of paralogous signaling protein families. Cell 150:222–232. 10.1016/j.cell.2012.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Karl KE, David WS, Sydney K. 1993. Glutamate at the site of phosphorylation of nitrogen-regulatory protein NTRC mimics aspartyl-phosphate and activates the protein. J Mol Bio 232:67–78. 10.1006/jmbi.1993.1370. [DOI] [PubMed] [Google Scholar]
- 51.Moore JB, Shiau SP, Reitzer LJ. 1993. Alterations of highly conserved residues in the regulatory domain of nitrogen regulator I (NtrC) of Escherichia coli. J Bacteriol 175:2692–2701. 10.1128/jb.175.9.2692-2701.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gupte G, Woodward C, Stout V. 1997. Isolation and characterization of rcsB mutations that affect colanic acid capsule synthesis in Escherichia coli K-12. J Bacteriol 179:4328–4335. 10.1128/JB.179.13.4328-4335.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lan CY, Igo MM. 1998. Differential expression of the OmpF and OmpC porin proteins in Escherichia coli K-12 depends upon the level of active OmpR. J Bacteriol 180:171–174. 10.1128/JB.180.1.171-174.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gushchin I, Gordeliy V. 2018. Transmembrane signal transduction in two‐component systems: piston, scissoring, or helical rotation? Bioessays 40:1700197. 10.1002/bies.201700197. [DOI] [PubMed] [Google Scholar]
- 55.Gushchin I, Melnikov I, Polovinkin V, Ishchenko A, Yuzhakova A, Buslaev P, Bourenkov G, Grudinin S, Round E, Balandin T, Borshchevskiy V, Willbold D, Leonard G, Büldt G, Popov A, Gordeliy V. 2017. Mechanism of transmembrane signaling by sensor histidine kinases. Science 356:eaah6345. 10.1126/science.aah6345. [DOI] [PubMed] [Google Scholar]
- 56.Molnar KS, Bonomi M, Pellarin R, Clinthorne GD, Gonzalez G, Goldberg SD, Goulian M, Sali A, DeGrado WF. 2014. Cys-scanning disulfide crosslinking and Bayesian modeling probe the transmembrane signaling mechanism of the histidine kinase, PhoQ. Structure 22:1239–1251. 10.1016/j.str.2014.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Skerker JM, Perchuk BS, Siryaporn A, Lubin EA, Ashenberg O, Goulian M, Laub MT. 2008. Rewiring the specificity of two-component signal transduction systems. Cell 133:1043–1054. 10.1016/j.cell.2008.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yamada S, Sugimoto H, Kobayashi M, Ohno A, Nakamura H, Shiro Y. 2009. Structure of PAS-linked histidine kinase and the response regulator complex. Structure 17:1333–1344. 10.1016/j.str.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 59.Bélanger L, Dimmick KA, Fleming JS, Charles TC. 2009. Null mutations in Sinorhizobium meliloti exoS and chvI demonstrate the importance of this two‐component regulatory system for symbiosis. Mol Microbiol 74:1223–1237. 10.1111/j.1365-2958.2009.06931.x. [DOI] [PubMed] [Google Scholar]
- 60.Lourenço RF, Kohler C, Gomes SL. 2011. A two‐component system, an anti‐sigma factor and two paralogous ECF sigma factors are involved in the control of general stress response in Caulobacter crescentus. Mol Microbiol 80:1598–1612. 10.1111/j.1365-2958.2011.07668.x. [DOI] [PubMed] [Google Scholar]
- 61.Alvarez-Martinez CE, Lourenço RF, Baldini RL, Laub MT, Gomes SL. 2007. The ECF sigma factor σ Tis involved in osmotic and oxidative stress responses in Caulobacter crescentus. Mol Microbiol 66:1240–1255. 10.1111/j.1365-2958.2007.06005.x. [DOI] [PubMed] [Google Scholar]
- 62.Alvarez-Martinez CE, Baldini RL, Gomes SL. 2006. A Caulobacter crescentus extracytoplasmic function sigma factor mediating the response to oxidative stress in stationary phase. J Bacteriol 188:1835–1846. 10.1128/JB.188.5.1835-1846.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hentchel KL, Ruiz LMR, Curtis PD, Fiebig A, Coleman ML, Crosson S. 2019. Genome-scale fitness profile of Caulobacter crescentus grown in natural freshwater. ISME J 13:523–536. 10.1038/s41396-018-0295-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen EJ, Fisher RF, Perovich VM, Sabio EA, Long SR. 2009. Identification of direct transcriptional target genes of ExoS/ChvI two-component signaling in Sinorhizobium meliloti. J Bacteriol 191:6833–6842. 10.1128/JB.00734-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Herrou J, Willett JW, Fiebig A, Varesio LM, Czyż DM, Cheng JX, Ultee E, Briegel A, Bigelow L, Babnigg G, Kim Y, Crosson S. 2019. Periplasmic protein EipA determines envelope stress resistance and virulence in Brucella abortus. Mol Microbiol 111:637–661. 10.1111/mmi.14178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hagan CL, Silhavy TJ, Kahne D. 2011. β-barrel membrane protein assembly by the Bam complex. Annu Rev Biochem 80:189–210. 10.1146/annurev-biochem-061408-144611. [DOI] [PubMed] [Google Scholar]
- 67.Anwari K, Poggio S, Perry A, Gatsos X, Ramarathinam SH, Williamson NA, Noinaj N, Buchanan S, Gabriel K, Purcell AW, Jacobs-Wagner C, Lithgow T. 2010. A modular BAM complex in the outer membrane of the alpha-proteobacterium Caulobacter crescentus. PLoS One 5:e8619. 10.1371/journal.pone.0008619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yeh Y-C, Comolli LR, Downing KH, Shapiro L, McAdams HH. 2010. The Caulobacter Tol-Pal complex is essential for outer membrane integrity and the positioning of a polar localization factor. J Bacteriol 192:4847–4858. 10.1128/JB.00607-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang C, Kemp J, Da Fonseca IO, Equi RC, Sheng X, Charles TC, Sobral BWS. 2010. Sinorhizobium meliloti 1021 loss-of-function deletion mutation in chvI and its phenotypic characteristics. Mol Plant Microbe Interact 23:153–160. 10.1094/MPMI-23-2-0153. [DOI] [PubMed] [Google Scholar]
- 70.Tomlinson AD, Ramey-Hartung B, Day TW, Merritt PM, Fuqua C. 2010. Agrobacterium tumefaciens ExoR represses succinoglycan biosynthesis and is required for biofilm formation and motility. Microbiology (Reading) 156:2670–2681. 10.1099/mic.0.039032-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Aldridge P, Jenal U. 1999. Cell cycle‐dependent degradation of a flagellar motor component requires a novel‐type response regulator. Mol Microbiol 32:379–391. 10.1046/j.1365-2958.1999.01358.x. [DOI] [PubMed] [Google Scholar]
- 72.Hershey DM, Fiebig A, Crosson S. 2021. Flagellar perturbations activate adhesion through two distinct pathways in Caulobacter crescentus. mBio 12:e03266-20. 10.1128/mBio.03266-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ardissone S, Viollier PH. 2015. Interplay between flagellation and cell cycle control in Caulobacter. Curr Opin Microbiol 28:83–92. 10.1016/j.mib.2015.08.012. [DOI] [PubMed] [Google Scholar]
- 74.Woldemeskel SA, McQuillen R, Hessel AM, Xiao J, Goley ED. 2017. A conserved coiled-coil protein pair focuses the cytokinetic Z-ring in Caulobacter crescentus. Mol Microbiol 105:721–740. 10.1111/mmi.13731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schwartz MA, Shapiro L. 2011. An SMC ATPase mutant disrupts chromosome segregation in Caulobacter. Mol Microbiol 82:1359–1374. 10.1111/j.1365-2958.2011.07836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jensen RB, Shapiro L. 1999. The Caulobacter crescentus smc gene is required for cell cycle progression and chromosome segregation. Proc Natl Acad Sci U S A 96:10661–10666. 10.1073/pnas.96.19.10661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Narayanan S, Janakiraman B, Kumar L, Radhakrishnan SK. 2015. A cell cycle-controlled redox switch regulates the topoisomerase IV activity. Genes Dev 29:1175–1187. 10.1101/gad.257030.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schindel HS, Bauer CE. 2016. The RegA regulon exhibits variability in response to altered growth conditions and differs markedly between Rhodobacter species. Microb Genom 2:e000081. 10.1099/mgen.0.000081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Feldheim YS, Zusman T, Speiser Y, Segal G. 2016. The Legionella pneumophila CpxRA two-component regulatory system: new insights into CpxR's function as a dual regulator and its connection to the effectors regulatory network. Mol Microbiol 99:1059–1079. 10.1111/mmi.13290. [DOI] [PubMed] [Google Scholar]
- 80.Plate L, Marletta MA. 2013. Phosphorylation-dependent derepression by the response regulator HnoC in the Shewanella oneidensis nitric oxide signaling network. Proc Natl Acad Sci U S A 110:E4648–E4657. 10.1073/pnas.1318128110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chakraborty S, Winardhi RS, Morgan LK, Yan J, Kenney LJ. 2017. Non-canonical activation of OmpR drives acid and osmotic stress responses in single bacterial cells. Nat Comms 8:1587. 10.1038/s41467-017-02030-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ali MM, Provoost A, Mijnendonckx K, Van Houdt R, Charlier D. 2020. DNA-binding and transcription activation by unphosphorylated response regulator AgrR From Cupriavidus metallidurans involved in silver resistance. Front Microbiol 11:1635. 10.3389/fmicb.2020.01635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fernández I, Cornaciu I, Carrica MDC, Uchikawa E, Hoffmann G, Sieira R, Márquez JA, Goldbaum FA. 2017. Three-dimensional structure of full-length NtrX, an unusual member of the NtrC family of response regulators. J Mol Bio 429:1192–1212. 10.1016/j.jmb.2016.12.022. [DOI] [PubMed] [Google Scholar]
- 84.Marks ME, Castro-Rojas CM, Teiling C, Du L, Kapatral V, Walunas TL, Crosson S. 2010. The genetic basis of laboratory adaptation in Caulobacter crescentus. JB 192:3678–3688. 10.1128/JB.00255-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.García-Bayona L, Gozzi K, Laub MT. 2019. Mechanisms of resistance to the contact-dependent bacteriocin CdzC/D in Caulobacter crescentus. J Bacteriol 201:e00538-18. 10.1128/JB.00538-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hu X, Zhao J, DeGrado WF, Binns AN. 2013. Agrobacterium tumefaciens recognizes its host environment using ChvE to bind diverse plant sugars as virulence signals. Proc Natl Acad Sci U S A 110:678–683. 10.1073/pnas.1215033110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Göpel Y, Görke B. 2018. Interaction of lipoprotein QseG with sensor kinase QseE in the periplasm controls the phosphorylation state of the two-component system QseE/QseF in Escherichia coli. PLoS Genet 14:e1007547. 10.1371/journal.pgen.1007547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lippa AM, Goulian M. 2009. Feedback inhibition in the PhoQ/PhoP signaling system by a membrane peptide. PLoS Genet 5:e1000788. 10.1371/journal.pgen.1000788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lukat GS, McCleary WR, Stock AM, Stock JB. 1992. Phosphorylation of bacterial response regulator proteins by low molecular weight phospho-donors. Proc Natl Acad Sci U S A 89:718–722. 10.1073/pnas.89.2.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.McCleary WR, Stock JB. 1994. Acetyl phosphate and the activation of two-component response regulators. J Biol Chem 269:31567–31572. 10.1016/S0021-9258(18)31731-9. [DOI] [PubMed] [Google Scholar]
- 91.Kim S-B, Shin B-S, Choi S-K, Kim C-K, Park S-H. 2001. Involvement of acetyl phosphate in the in vivo activation of the response regulator ComA in Bacillus subtilis. FEMS Microbiol Lett 195:179–183. 10.1111/j.1574-6968.2001.tb10518.x. [DOI] [PubMed] [Google Scholar]
- 92.Bouché S, Klauck E, Fischer D, Lucassen M, Jung K, Hengge-Aronis R. 1998. Regulation of RssB‐dependent proteolysis in Escherichia coli: a role for acetyl phosphate in a response regulator‐controlled process. Mol Microbiol 27:787–795. 10.1046/j.1365-2958.1998.00725.x. [DOI] [PubMed] [Google Scholar]
- 93.Hsing W, Russo FD, Bernd KK, Silhavy TJ. 1998. Mutations that alter the kinase and phosphatase activities of the two-component sensor EnvZ. J Bacteriol 180:4538–4546. 10.1128/JB.180.17.4538-4546.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lemmin T, Soto CS, Clinthorne G, DeGrado WF, Peraro MD. 2013. Assembly of the transmembrane domain of E. coli PhoQ histidine kinase: implications for signal transduction from molecular simulations. PLoS Comput Biol 9:e1002878. 10.1371/journal.pcbi.1002878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Goldberg SD, Clinthorne GD, Goulian M, DeGrado WF. 2010. Transmembrane polar interactions are required for signaling in the Escherichia coli sensor kinase PhoQ. Proc Natl Acad Sci U S A 107:8141–8146. 10.1073/pnas.1003166107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ried JL, Collmer A. 1987. An nptI-sacB-sacR cartridge for constructing directed, unmarked mutations in Gram-negative bacteria by marker exchange-eviction mutagenesis. Gene 57:239–246. 10.1016/0378-1119(87)90127-2. [DOI] [PubMed] [Google Scholar]
- 97.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez J-Y, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A. 2012. Fiji: an open-source platform for biological-image analysis. Nat Methods 9:676–682. 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Schneider CA, Rasband WS, Eliceiri KW. 2012. NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9:671–675. 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pitcher DG, Saunders NA, Owen RJ. 1989. Rapid extraction of bacterial genomic DNA with guanidium thiocyanate. Lett Appl Microbiol 8:151–156. 10.1111/j.1472-765X.1989.tb00262.x. [DOI] [Google Scholar]
- 100.Deatherage DE, Barrick JE. 2014. Identification of mutations in laboratory evolved microbes from next-generation sequencing data using breseq. Methods Mol Biol 1151:165–188. 10.1007/978-1-4939-0554-6_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tien MZ, Stein BJ, Crosson S. 2018. Coherent feedforward regulation of gene expression by Caulobacter σT and GsrN during hyperosmotic stress. J Bacteriol 200:e00349-18. 10.1128/JB.00349-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bailey TL, Boden M, Buske FA, Frith M, Grant CE, Clementi L, Ren J, Li WW, Noble WS. 2009. MEME Suite: tools for motif discovery and searching. Nucleic Acids Res 37:W202–W208. 10.1093/nar/gkp335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Saldanha AJ. 2004. Java Treeview—extensible visualization of microarray data. Bioinformatics 20:3246–3248. 10.1093/bioinformatics/bth349. [DOI] [PubMed] [Google Scholar]
- 104.R Core Team. 2020. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://wwwR-project.org/ [Google Scholar]
- 105.Foreman R, Fiebig A, Crosson S. 2012. The LovK-LovR two-component system is a regulator of the general stress pathway in Caulobacter crescentus. J Bacteriol 194:3038–3049. 10.1128/JB.00182-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Edgar R, Domrachev M, Lash AE. 2002. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res 30:207–210. 10.1093/nar/30.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wiech EM, Cheng H-P, Singh SM. 2015. Molecular modeling and computational analyses suggests that the Sinorhizobium meliloti periplasmic regulator protein ExoR adopts a superhelical fold and is controlled by a unique mechanism of proteolysis. Protein Sci 24:319–327. 10.1002/pro.2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 to S6 and Tables S1 and S2. Download JB.00199-21-s0001.pdf, PDF file, 4.7 MB (4.7MB, pdf)
Data Availability Statement
RNA-seq data are available in the NCBI’s Gene Expression Omnibus (GEO) database (106) under the GEP series accession number GSE168965.