Corticosteroids are used to treat the acute complications of chimeric antigen receptor (CAR) T-cell therapy. Strati et al examine the impact of corticosteroids on patient outcomes following CAR T-cell therapy for large B-cell lymphoma. Use of higher doses of steroids is associated with shorter progression-free survival at 10 months and decreased overall survival.
Key Points
Higher cumulative dose of corticosteroids is associated with early progression after CAR-T therapy in large B-cell lymphoma.
Higher cumulative dose and prolonged, early corticosteroid use is associated with shorter overall survival after CAR-T therapy.
Visual Abstract
Abstract
Corticosteroids are commonly used for the management of severe toxicities associated with chimeric antigen receptor (CAR) T-cell therapy. However, it remains unclear whether their dose, duration, and timing may affect clinical efficacy. Here, we determined the impact of corticosteroids on clinical outcomes in patients with relapsed or refractory large B-cell lymphoma treated with standard of care anti-CD19 CAR T-cell therapy. Among 100 patients evaluated, 60 (60%) received corticosteroids for management of CAR T-cell therapy–associated toxicities. The median cumulative dexamethasone-equivalent dose was 186 mg (range, 8-1803) and the median duration of corticosteroid treatment was 9 days (range, 1-30). Corticosteroid treatment was started between days 0 and 7 in 45 (75%) patients and beyond day 7 in 15 (25%). After a median follow-up of 10 months (95% confidence interval, 8-12 months), use of higher cumulative dose of corticosteroids was associated with significantly shorter progression-free survival. More importantly, higher cumulative dose of corticosteroids, and prolonged and early use after CAR T-cell infusion were associated with significantly shorter overall survival. These results suggest that corticosteroids should be used at the lowest dose and for the shortest duration and their initiation should be delayed whenever clinically feasible while managing CAR T-cell therapy–associated toxicities.
Introduction
Despite the high response rates achieved with chimeric antigen receptor (CAR) T-cell therapies in patients with relapsed or refractory large B-cell lymphoma (LBCL), long-term durability is observed in <40% of patients.1-3 Although tumor and T-cell intrinsic factors have been identified as potential mechanisms associated with resistance to CAR T-cell therapy,4,5 the role of interventions used for management of toxicities, such as corticosteroids, has not been adequately studied. In the pivotal Safety and Efficacy of KTE-C19 in Adults With Refractory Aggressive Non-Hodgkin Lymphoma trial that evaluated axicabtagene ciloleucel (axi-cel), corticosteroids were used in 27% of patients for the management of severe cytokine release syndrome (CRS) and/or neurological toxicity, also referred to as immune effector cell–associated neurotoxicity syndrome (ICANS).6 Outside of clinical trials, corticosteroids were used in up to 55% of patients for the management of toxicities arising after axi-cel infusion.7,8 Corticosteroids likely mitigate toxicities by inhibiting the proliferation and/or inflammatory cytokine production from CAR T cells and other immune cells.9-11 However, the magnitude of CAR T-cell expansion during the first month after infusion has been shown to be associated with response and durability.6,12,13 Hence, the use of corticosteroids for toxicity management raises concern about whether it could affect antitumor efficacy, and remains a clinically relevant and yet unanswered question.
Study design
This retrospective study was approved by the institutional review board of MD Anderson Cancer Center, and conducted in accordance with institutional guidelines and the principles of the Declaration of Helsinki. All patients with relapsed or refractory LBCL treated with standard of care axi-cel8 at MD Anderson Cancer Center between January 2018 and May 2019 were included. The data cutoff for follow-up was 31 December 2019. CRS and ICANS were prospectively graded and managed according to the CAR-T-cell-therapy-associated toxicity guidelines.14 Response status was determined by Lugano 2014 classification.15 Details on corticosteroid use, correlative analyses, and statistical methods are provided in supplemental Methods and supplemental Table 1, available on the Blood Web site.
Results and discussion
Of 100 patients with relapsed or refractory LBCL treated with standard of care axi-cel, 60 (60%) received corticosteroids within 30 days after axi-cel infusion. Compared with patients who did not receive corticosteroids, they were more likely to have had a diagnosis of diffuse large B-cell lymphoma/high-grade B-cell lymphoma and a European Cooperative Oncology Group performance status >0. Other baseline characteristics were not significantly different when comparing the 2 groups (Table 1).
Table 1.
Baseline characteristics before initiation of conditioning therapy and association with use of corticosteroids
| Median [range] | ||||
|---|---|---|---|---|
| Total (N = 100) | No corticosteroids (n = 40) | Corticosteroids (n = 60) | P | |
| DLBCL/HGBCL, N (%) | 77 (77) | 26 (65) | 51 (85) | .03 |
| Age, y | 60 [18-85] | 61 [28-74] | 60 [18-85] | .91 |
| Male, N (%) | 74 (74) | 33 (83) | 41 (68) | .16 |
| ECOG performance status >0, N (%) | 69 (69) | 23 (58) | 46 (77) | .05 |
| Ann Arbor stage III-IV, N (%) | 84 (84) | 35 (88) | 49 (82) | .58 |
| Bone marrow involvement, N (%) | 22 (22) | 11 (28) | 11 (18) | .33 |
| IPI score 3-4, N (%) | 55 (55) | 22 (55) | 33 (55) | 1 |
| Absolute neutrophil count, 109/L | 2.8 [0-20] | 2.8 [0-19.7] | 2.9 [0.4-17.5] | .96 |
| Absolute lymphocyte count, 109/L | 0.6 [0-2] | 0.7 [0-2.2] | 0.5 [0-1.7] | .45 |
| Absolute monocyte count, 109/L | 0.5 [0.02-2] | 0.6 [0.1-1.9] | 0.4 [0.1-2] | .15 |
| Hemoglobin, g/dL | 10 [5-16] | 10.8 [7.3-16.5] | 10.1 [5.4-15.9] | .06 |
| Platelet count, 109/L | 141 [9-391] | 136 [9-370] | 143 [9-391] | .87 |
| C-reactive protein, mg/L | 33 [0.3-284] | 13.2 [0.7-114] | 37 [0.3-284] | .43 |
| Ferritin, mg/L | 812 [13-38,964] | 684 [36-2658] | 850 [13-38,964] | .65 |
| Lactate dehydrogenase > ULN, N (%) | 74 (74) | 26 (65) | 48 (80) | .11 |
| Creatinine clearance, mL/min | 84 [35-152] | 86 [36-135] | 84 [35-152] | .81 |
| Previous therapies, no. | 4 [2-15] | 5 [2-15] | 4 [2-11] | .07 |
| Refractory disease, N (%) | 89 (89) | 37 (93) | 52 (87) | .52 |
| Previous autologous SCT, N (%) | 29 (29) | 13 (33) | 16 (27) | .65 |
| Previous CAR T therapy, N (%) | 5 (5) | 4 (10) | 1 (2) | .15 |
| Prior CNS lymphoma, N (%) | 8 (8) | 1 (3) | 7 (12) | .14 |
Only 1 patient had a previous allogeneic SCT. DLBCL/HGBCL was compared with transformed follicular lymphoma and/or primary mediastinal B-cell lymphoma. Bold indicates statistically significant (P ≤ .05).
CAR, chimeric antigen receptor; CNS, central nervous system; DLBCL, diffuse large B-cell lymphoma; ECOG, European Cooperative Oncology Group; HGBCL, high grade B-cell lymphoma; IPI, international prognostic index; SCT, stem cell transplant; ULN, upper limit of normal.
Because a central memory phenotype (CD8+CCR7+CD27+) in CAR T-infusion product has been shown to be associated with better outcome and an exhaustion phenotype (CD8+LAG3+TIM3+) with worse outcome,4 we performed single cell RNA-sequencing of CAR T-infusion products in a subgroup of 24 patients, 15 (62.5%) of whom received corticosteroids. We did not observe any significant differences in the distribution of these 2 phenotypes based on corticosteroid dose, duration, and timing (supplemental Figure 1).
Overall, 9 (9%) patients had grade ≥3 CRS and 41 (41%) grade ≥3 ICANS; the latter was associated with higher cumulative dose of corticosteroids (P < .001). The indication for use of corticosteroids was represented by ICANS in 37 (37%) patients, CRS in 7 (7%), and both in 16 (16%). Among the 60 patients who received corticosteroids, the median cumulative dexamethasone-equivalent dose was 186 mg (range, 8-1803); the median duration of corticosteroid use was 9 days (range, 1-30), and the reason for prolonged use (≥10 days, in 26 patients) was persistent or recurrent ICANS. Forty-five (75%) of 60 patients received corticosteroids during the first 7 days after axi-cel infusion and 15 (25%) beyond day 7.
Ninety-six patients were evaluable for efficacy, and complete response was observed in 55 (57%). After a median follow-up of 10 months (95% confidence interval [CI], 8-12 months), median progression-free survival (PFS) was 8 months (95% CI, 3-13) and 54 (54%) patients progressed and/or died. CD19 status was assessed by flow cytometry in 21 patients at progression, and CD19− relapse rate (29%) was similar in patients with or without corticosteroids use.
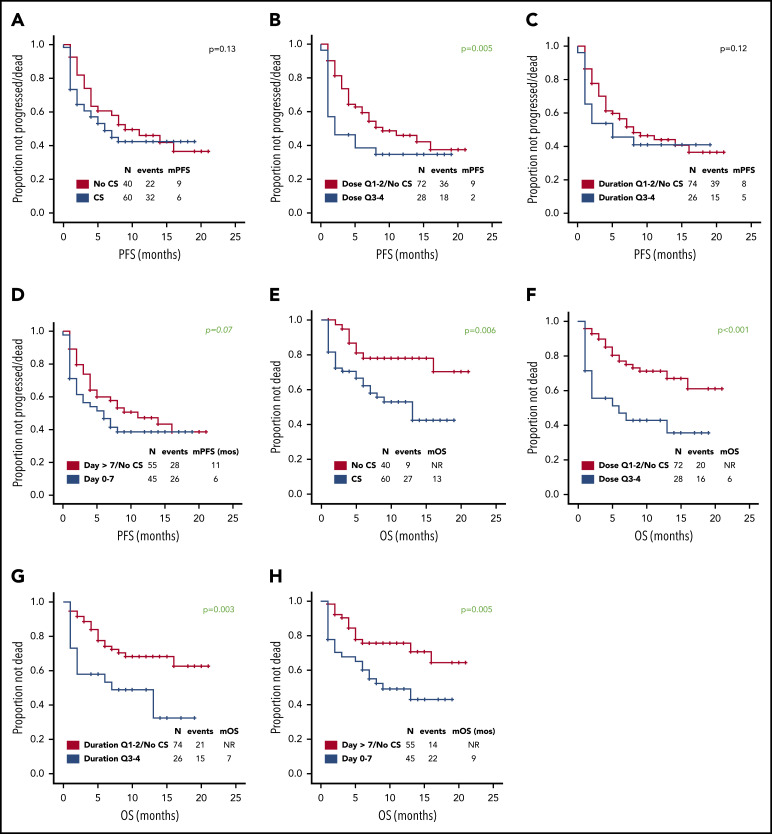
Use of higher cumulative dose of corticosteroids associated with shorter PFS and a trend toward inferior PFS was observed with early use, whereas the use of corticosteroids or duration did not appear to impact PFS (Figure 1A-D). Baseline factors significantly associated with shorter PFS on univariate analysis were advanced stage, high international prognostic index, and elevated lactate dehydrogenase (LDH) (supplemental Table 2); on multivariate analysis, only elevated LDH maintained its association with PFS (hazard ratio, 0.3; 95% CI, 0.1-0.8; P = .01). The association between use of higher cumulative dose of corticosteroids and shorter PFS was maintained when limiting the analysis to the 74 patients with elevated baseline LDH level, which was used as a measure of higher tumor burden (P = .002). No significant difference in relapse rate was observed between the first and last 50 patients (46% vs 48%, P = 1) consistent with lack of time-dependent variation in corticosteroid use or dosing. Of interest, 73 (73%) patients needed tocilizumab for the management of CRS, but no difference in PFS was observed based on tocilizumab use (11 months vs 9 months, P = .60). No patient received siltuximab.
Figure 1.
Prognostic impact of corticosteroid use on PFS and OS. Association between use of (A,E) corticosteroids, (B,F) cumulative corticosteroid dose, (C,G) duration, and (D,H) timing and (A-D) PFS and (E-H) OS in patients with relapsed or refractory large B-cell lymphoma treated with axi-cel. No significant difference in complete response rate was observed based on corticosteroid use, dose, duration, and timing. The association between use of higher cumulative dose of corticosteroids and shorter PFS was maintained when limiting the analysis to the 43 patients with high-grade ICANS (P = .05). The association between higher cumulative dose (P = .04) and shorter OS was maintained when limiting the analysis to the 43 patients with high-grade ICANS. Only 2 patients died of infectious complications. Quartiles for cumulative dexamethasone-equivalent dose: first quartile (Q1), 8-116 mg; second quartile (Q2), 118-186 mg; third quartile (Q3), 195-390 mg; and fourth quartile (Q4), 440-1083 mg. Quartiles for duration of corticosteroid use: Q1, 1-6 days; Q2, 7-9 days; Q3, 10-14 days; and Q4, 15-30 days. CS, corticosteroid; m, median. Analyzing the data by treating LDH as a continuous variable did not show any significant differences in the LDH levels based on corticosteroid use (P = .21), dose (P = .22), duration (P = .09), or timing (P = .07).
At most recent follow-up, 36 patients died, 28 of progressive lymphoma. Median overall survival (OS) has not been reached. Median OS was significantly shorter among patients who received any dose of corticosteroids, higher cumulative dose of corticosteroids, prolonged corticosteroid use (for ≥10 days), or early use (within 7 days) (Figure 1E-H). Baseline factors significantly associated with shorter OS on univariate analysis were high international prognostic index and elevated LDH (supplemental Table 3). On multivariate analysis, only elevated LDH maintained its association (hazard ratio, 0.2; 95% CI, 0.1-0.6; P = .01). The association between higher cumulative dose (P < .001), prolonged (P = .008), and early use of corticosteroids (P = .01) and shorter OS was maintained when limiting the analysis to the 74 patients with elevated baseline LDH.
In the pivotal cohorts (cohorts 1 and 2) of the Safety and Efficacy of KTE-C19 in Adults With Refractory Aggressive Non-Hodgkin Lymphoma registration trial, use of corticosteroids was mostly reserved for the management of high-grade CRS and ICANS, and did not appear to affect clinical response or durability.6 Recent multicenter reports on the use of axi-cel in the standard of care setting, as well as our report here, suggest that corticosteroid use likely has become more liberal, with initiation at lower grades of CRS or ICANS.7,16,17 Whether corticosteroids may affect CAR T-cell efficacy when used at lower grades has not been adequately studied. We observed that the use of corticosteroids within the first 30 days after CAR T-cell infusion is associated with shorter OS. Specifically, higher cumulative dose and prolonged and early use of corticosteroids were associated with shorter PFS and/or OS. Our results suggest that corticosteroids have prognostic impact, independent of baseline clinical variables including tumor burden as well as CAR-T product characteristics.8,18,19 In a recent study, high-grade ICANS was associated with shorter PFS and OS.20 It is possible that this association may be secondary to corticosteroid use in these patients. Although our analysis showed that corticosteroid dose, duration, and timing did not affect CAR T-cell amplification (supplemental Figure 2), additional investigations are required to assess their impact on the cytotoxic activity, cytokine production, and motility of CAR T cells.21 Until we understand the underlying mechanism, it may be prudent to use corticosteroids sparingly, focusing on delaying their initiation and limiting their cumulative dose and duration, as clinically appropriate. Moreover, evaluation of alternative interventions that could minimize the need for corticosteroids to mitigate CAR T-cell–associated toxicities is warranted.22
Supplementary Material
The online version of this article contains a data supplement.
Acknowledgments
This research is supported in part by The University of Texas Anderson Cancer Center Support Grant from National Institutes of Health, National Cancer Institute (P30 CA016672) and generous philanthropic contributions to the University of Texas Anderson Moon Shots Program. P. Strati’s salary is supported by the Lymphoma Research Foundation Career Development Award. M.R.G. is supported by a Scholar Award from the Leukemia and Lymphoma Society.
Footnotes
For original data, please contact the corresponding author at sneelapu@mdanderson.org.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: P. Strati and S.A. designed the study, analyzed data, and wrote the paper; L.E.F., H.J.L., S.P.I., R.N., L.J.N., S.P., M.A.R., F.S., R.E.S., M.W., C.C.P., P.K., E.J.S., and C.R.F. provided clinical care to patients and coauthored the paper; M.R.G. provided scientific support and coauthored the paper; F.F., S.B.H., C.M.C., M.C.H., N.A.J., P. Singh, H.M., S.J., and S.A. collected clinical data and coauthored the paper; L.F. and R.S. provided statistical support and coauthored the paper; and J.W. and S.S.N. designed the study, analyzed the data, provided clinical care to patients, and wrote the paper.
Conflict-of-interest disclosure: P. Strati reports research support from AstraZeneca. L.J.N. reports honoraria from Celgene, Genentech, Gilead, Janssen, Juno, Novartis, Spectrum, and TG Therapeutics and research support from Celgene, Genentech, Janssen, Karus Therapeutics, and Merck. F.S. reports honoraria from Celgene. M.R.G. reports stock ownership interest in KDAc Therapeutics and research support from Kite/Gilead and Sanofi. S.S.N. served as consultant to Kite, a Gilead Company, Merck, Bristol-Myers Squibb, Novartis, Celgene, Pfizer, Allogene Therapeutics, Cell Medica/Kuur, Incyte, Precision Biosciences, Legend Biotech, Adicet Bio, Calibr, and Unum Therapeutics; received research support from Kite, a Gilead Company, Bristol-Myers Squibb, Merck, Poseida, Cellectis, Celgene, Karus Therapeutics, Unum Therapeutics, Allogene Therapeutics, Precision Biosciences, and Acerta; received royalties from Takeda Pharmaceuticals, and has intellectual property related to cell therapy. The remaining authors declare no competing financial interests.
Correspondence: Sattva S. Neelapu, Department of Lymphoma and Myeloma, Division of Cancer Medicine, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd, Unit 429, Houston, TX 77030; e-mail: sneelapu@mdanderson.org.
REFERENCES
- 1.Locke FL, Ghobadi A, Jacobson CA, et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1-2 trial. Lancet Oncol. 2019;20(1):31-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schuster SJ, Bishop MR, Tam CS, et al. ; JULIET Investigators . Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. 2019;380(1):45-56. [DOI] [PubMed] [Google Scholar]
- 3.Abramson JS, Palomba ML, Gordon LI, et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet. 2020;396(10254):31366-31360. [DOI] [PubMed] [Google Scholar]
- 4.Deng Q, Han G, Puebla-Osorio N, et al. Characteristics of anti-CD19 CAR T cell infusion products associated with efficacy and toxicity in patients with large B cell lymphomas. Nat Med. 2020;26(12):1878-1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Locke FL, Rossi JM, Neelapu SS, et al. Tumor burden, inflammation, and product attributes determine outcomes of axicabtagene ciloleucel in large B-cell lymphoma. Blood Adv. 2020;4(19):4898-4911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377(26):2531-2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jacobson CA, Hunter BD, Redd R, et al. Axicabtagene ciloleucel in the non-trial setting: outcomes and correlates of response, resistance, and toxicity. J Clin Oncol. 2020;38(27):3095-3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nastoupil LJ, Jain MD, Feng L, et al. Standard-of-care axicabtagene ciloleucel for relapsed or refractory large B-cell lymphoma: results from the US Lymphoma CAR T Consortium. J Clin Oncol. 2020;38(27):3119-3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paliogianni F, Ahuja SS, Balow JP, Balow JE, Boumpas DT. Novel mechanism for inhibition of human T cells by glucocorticoids. Glucocorticoids inhibit signal transduction through IL-2 receptor. J Immunol. 1993;151(8):4081-4089. [PubMed] [Google Scholar]
- 10.Lanza L, Scudeletti M, Puppo F, et al. Prednisone increases apoptosis in in vitro activated human peripheral blood T lymphocytes. Clin Exp Immunol. 1996;103(3):482-490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Franchimont D, Louis E, Dewe W, et al. Effects of dexamethasone on the profile of cytokine secretion in human whole blood cell cultures. Regul Pept. 1998;73(1):59-65. [DOI] [PubMed] [Google Scholar]
- 12.Turtle CJ, Hanafi LA, Berger C, et al. Immunotherapy of non-Hodgkin’s lymphoma with a defined ratio of CD8+ and CD4+ CD19-specific chimeric antigen receptor-modified T cells. Sci Transl Med. 2016;8(355):355ra116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kochenderfer JN, Somerville RPT, Lu T, et al. Lymphoma remissions caused by anti-CD19 chimeric antigen receptor T cells are associated with high serum interleukin-15 levels. J Clin Oncol. 2017;35(16):1803-1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Neelapu SS, Tummala S, Kebriaei P, et al. Chimeric antigen receptor T-cell therapy - assessment and management of toxicities. Nat Rev Clin Oncol. 2018;15(1):47-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheson BD, Fisher RI, Barrington SF, et al. ; United Kingdom National Cancer Research Institute . Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32(27):3059-3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nastoupil LJ, Jain MD, Spiegel JY, et al. Axicabtagene ciloleucel (axi-cel) CD19 chimeric antigen receptor (CAR) T-cell therapy for relapsed/refractory large B-cell lymphoma: real world experience [abstract]. Blood. 2018;132(suppl 1). Abstract 91. [Google Scholar]
- 17.Pasquini MC, Locke FL, Herrera AF, et al. Post-marketing use outcomes of an anti-CD19 chimeric antigen receptor (CAR) T cell therapy, axicabtagene ciloleucel (axi-Cel), for the treatment of large B cell lymphoma (LBCL) in the United States (US) [abstract]. Blood. 2019;134(suppl 1). Abstract 764. [Google Scholar]
- 18.Hay KA, Hanafi LA, Li D, et al. Kinetics and biomarkers of severe cytokine release syndrome after CD19 chimeric antigen receptor-modified T-cell therapy. Blood. 2017;130(21):2295-2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Santomasso BD, Park JH, Salloum D, et al. Clinical and biological correlates of neurotoxicity associated with CAR T-cell therapy in patients with B-cell acute lymphoblastic leukemia. Cancer Discov. 2018;8(8):958-971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Strati P, Nastoupil LJ, Westin J, et al. Clinical and radiologic correlates of neurotoxicity after axicabtagene ciloleucel in large B-cell lymphoma. Blood Adv. 2020;4(16):3943-3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liadi I, Singh H, Romain G, et al. Individual motile CD4(+) T cells can participate in efficient multikilling through conjugation to multiple tumor cells. Cancer Immunol Res. 2015;3(5):473-482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Strati P, Ahmed S, Kebriaei P, et al. Clinical efficacy of anakinra to mitigate CAR T-cell therapy-associated toxicity in large B-cell lymphoma. Blood Adv. 2020;4(13):3123-3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.