Abstract
Background:
Intracranial atherosclerotic stenosis (ICAS) is one of the most common causes of stroke worldwide and confers a high risk of stroke recurrence in spite of aggressive management of risk factors.
Objective:
To identify the role of risk factors and risk of vascular events in people with asymptomatic ICAS for improved risk stratification.
Methods:
Stroke-free participants in the Northern Manhattan Study, prospectively followed since 1993, underwent a brain magnetic resonance angiogram from 2003-2008. We rated stenosis in 11 brain arteries as: 0=no stenosis, 1=<50% or luminal irregularities, 2=50-69%, 3=>70% stenosis or flow gap. We ascertained vascular events during the post MRI period. We used proportional odds regression to quantify the association of pre-MRI exposures, and we built proportional hazard adjusted models to identify the risk of events in the post-MRI period.
Results:
The included sample included 1211 NOMAS participants (mean age 71 ± 9 years, 59% women, 65% Hispanic, 45% had any stenosis). Older age (OR 1.02 per year, 95%CI 1.01-1.04), hypertension duration (OR 1.01 per year, 95%CI 1.00-1.02), higher number of glucose-lowering drugs (OR 1.64 per each medication, 95% CI 1.24-2.15), and HDL (OR 0.96 per mg/dl, 95%CI 0.92-0.99) were associated with ICAS. The highest event risk was noted among participants with ICAS > 70% (5.5% annual risk of vascular events, HR 2.1, 95% CI 1.4-3.2 compared with those with no ICAS).
Conclusion:
ICAS is an imaging marker of established atherosclerotic disease in stroke-free individuals, and incidental diagnosis of ICAS should trigger a thorough assessment of vascular health.
Keywords: Intracranial atherosclerosis, arterial stenosis, vascular risk, risk factor, cerebrovascular disease
CONDENSED ABSTRACT
It is uncertain whether asymptomatic intracranial atherosclerotic stenosis (ICAS) confers the same increased vascular risk than ICAS in people with stroke. In 1211 Northern Manhattan Study stroke-free participants (mean age 71 ± 9, 59% women, 65% Hispanic) we rated stenosis as: 0=no stenosis, 1=<50%, 2=50-69%, 3=>70% stenosis. We evaluated the risk of vascular events during post MRA follow up. The highest event risk was noted among participants with ICAS > 70% (5.5% annual risk of events, adjusted HR 2.1, 95% CI 1.4-3.2 compared with those with no ICAS). Incidental diagnosis of ICAS should trigger a thorough assessment of vascular health.
INTRODUCTION
Intracranial atherosclerotic stenosis (ICAS) is one of the most common causes of stroke worldwide.(1-3) The proportion of strokes caused by ICAS varies depending on the intensity of the diagnostic workup as well as on the underlying demographics of the populations studied. For example, in a population-based racially and ethnically diverse urban cohort in New York, 8% of all ischemic strokes were caused by ICAS, the risk being higher for non-Hispanic Blacks and Hispanics.(1,4) In hospital-based samples and in samples from Asia, the proportion of ICAS-related stroke is even greater (12-46%).(2,5-7) Risk factors for ICAS and ICAS-related stroke include older age,(7,8) higher systolic blood pressure,(8) dyslipidemia,(8-10) diabetes mellitus(10,11) and limited physical activity(12). In addition, post-hoc data from clinical trials have demonstrated that achieving strict risk factor control can decrease the risk of recurrent vascular events among people with ICAS, including stroke and other non-cerebral vascular events such as myocardial infarction.(12) The systemic benefit of vascular risk factor control may relate to the almost certain co-existence between ICAS and systemic atherosclerosis, including coronary atherosclerosis.(13-16).
Nonetheless, even with aggressive medical therapy, the annual rate of stroke recurrence remains >10% in ICAS cases with stenosis >70% (17)and up to 20% among those with occlusions and 3 or more vascular risk factors.(7) The high rate of recurrent vascular events in patients with stroke caused by ICAS underscores the need for a greater focus on primary prevention and targeted interventions among stroke-free individuals at the highest risk of ICAS-related stroke and vascular events. In this context, we leveraged longitudinal data acquired through decades of follow up in the Northern Manhattan Study (NOMAS) to test the hypothesis that the presence of asymptomatic ICAS may help identify stroke-free individuals at a high risk of stroke and vascular events. We also aimed to provide observational data supporting the premise that midlife-life exposure and degree of control of modifiable vascular risk factors are high priority targets for primary prevention of vascular disease.
Methods
Sample description
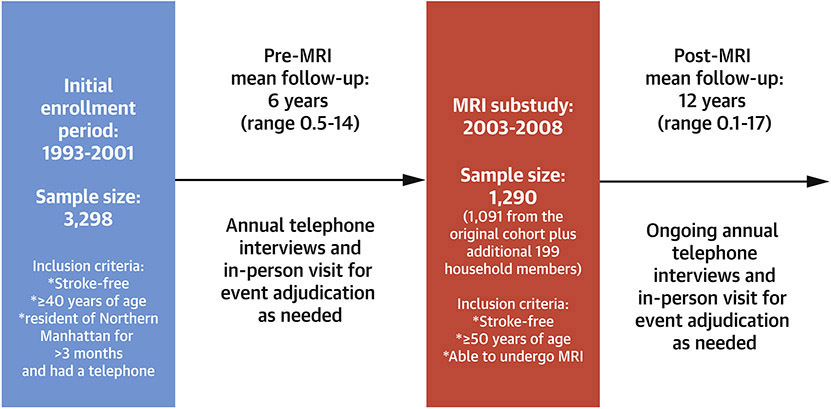
The northern Manhattan Study (NOMAS) is an ongoing urban, population-based, racially and ethnically diverse epidemiological study that began following its 3,298 participants in 1993 (Figure 1). Participants were identified via random selection among people living in northern Manhattan who had a telephone at home, were 40 years or older and were stroke-free (self-reported). The methods of this study have been previously described.(18) In 2003-2008, surviving NOMAS participants were invited to undergo a brain MRI if they remained stroke-free, were 50 years or older and had no contra-indications. Between 2006 and 2008, an additional 199 household members of the original NOMAS participants were invited to enroll in the MRI substudy, to supplement the cohort, thus bringing the total enrollment to 1290 participants. Participants were followed annually by telephone using a structured interview since 1993. Among the MRI cohort, only three (0.38%) subjects were lost to follow-up and 11 (1.4%) withdrew from active participation. Participants signed written informed consent and the study was approved by the IRBs at Columbia and the University of Miami.
Figure 1: Timeline of the Northern Manhattan Study longitudinal follow up.
The Northern Manhattan Study (NOMAS) cohort has been followed on average for 18 years with annual interviews and vascular event adjudication by a multidisciplinary team. From 2003-2008, stroke-free participants were invited to undergo a brain MRI.
Covariates adjudication
Age, sex, race/ethnicity and years of education were obtained at the time of baseline enrollment by self-report. Enrollment visits occurred in person in the Columbia University Irving Medical Center, or in the participant’s home. During this visit, participants underwent a structured questionnaire for their baseline characteristics and past medical history. Participants had blood sampled to evaluate fasting glucose and a lipid profile (at baseline enrollment and at the time of MRI). Prevalent hypertension, diabetes and hypercholesterolemia were captured at the enrollment visit by a combination of direct measures of risk factors, self-reported diagnosis or self-reported medication use to treat any of these risk factors. For hypertension, we used a cutoff of ≥ 140/90 mmHg averaged from at least two separate measures of brachial blood pressure by trained research personnel; for diabetes a cutoff of fasting glucose ≥126 mg/dl, and for hypercholesterolemia a cutoff of total cholesterol of ≥ 240 mg/dl. Participants with prevalent risk factors were asked for the duration of their diagnoses. We noted the number and class of all medications used by the participants and from this survey, we derived the number of medications used to treat a given risk factor. For smoking, we asked the duration of smoking as well as number of packs per year smoked. We defined established care under primary care doctor (PMD) at the time of MRI if participants self-reported seeing their PMD at least 80% of their scheduled visits at the time of MRI or in the pre-MRI period.
Brain MRI and ICAS assessment
Imaging was performed on a 1.5-T MRI system (Philips Medical Systems, Best, Netherlands) at the Columbia University Irving Medical Center following a standardized protocol. We used 3D time of flight MRA with the following parameters: FOV of 15 cm, 1 mm effective slice thickness, acquisition matrix interpolated to 256x228 matrix, Flip angle of 25 degrees, TR/TE 20 and 2.7 ms, respectively. Each major intracranial large artery was visually inspected to decide whether further diameter measurements were indicated. If arterial stenosis was identified, we measured the narrowest lumen area to define stenosis and select the immediate preceding segment with normal lumen (or the next normal appearing lumen if the stenosis was at the arterial origin).(19) Stenosis of each artery was ascertained as: 0=no stenosis, 1=< 50% (or luminal irregularities), 2=50-69%, 3=>70% stenosis or flow gap. These categories are consistent with frequent clinical cutoffs for ICAS used in the literature.(8,17,19) A neurologist and a vascular neurologist rated the MRAs independently, blinded to the participant’s identity and to each other’s reads. The intra-class correlation coefficient for the ICAS ordinal scale was >0.90 for single and average measures. Categorizing the stenosis into >50% stenosis yielded a κ=0.93. For the analysis, a consensus variable between the two reads was used and the vascular neurologist read took precedence if a discrepancy of more than one point existed.
Post-MRI death and vascular event adjudication
Participants in the study were screened annually with standardized telephone interviews and/or in-person visits for a pre-defined outcome. The outcomes included vascular death, myocardial infarction, ischemic stroke, cardioembolic stroke, intracranial artery disease stroke (which combined intracranial small and large artery disease strokes), and any vascular events (defined as a composite of vascular death, any stroke or myocardial infarction (MI), as described before).(20) We combined intracranial small and large artery disease into a single outcome based on epidemiological and genetic evidence suggestive of the overlapping nature of these two stroke phenotypes.(21-23) We did not consider cryptogenic strokes as a separate category in this study, but cryptogenic strokes were included in the “ischemic stroke” category. Briefly, death and vascular events were adjudicated by NOMAS investigators using review of medical records from our institution (where the majority of our participants obtain medical care) or review of medical records from outside institutions (which included records from foreign hospitals if needed). Vascular death was attributed when the cause of death was MI, stroke, heart failure, pulmonary embolus or cardiac arrhythmia. Two study vascular neurologists (blinded to the study MRA) adjudicated stroke subtypes independently. A study cardiologist using the criteria from the Cardiac Arrhythmia Suppression Trial and the Lipid Research Clinics Coronary Primary Prevention Trial adjudicated myocardial infarction.(24,25) For the purpose of this manuscript, we use follow-up data collected up to July 2020.
Statistical analyses
Descriptive statistics were used to calculate the demographic characteristics and vascular risk factors of the study participants. Chi-squared and student-t test were used to compare the characteristics of the MRI cohort with available MRA versus those with no MRA available. The first part of the analyses focused on risk factors for asymptomatic ICAS at the time of MRA and we used data collected up to the time of MRI (Figure 1). For this part of the analysis, we carried out proportional odds models with ICAS ordinal variable as the dependent variable to obtain the odds ratios (OR) and their 95% confidence intervals (CI). We used the four categories of stenosis as the main exposure: 0) no stenosis; 1) luminal irregularities with <50% stenosis in at least one artery; 2) 50-69% stenosis in at least one artery; and 3) ≥ 70% stenosis or flow gap in at least one artery. We utilized risk factors in various forms, including prevalent risk factors with or without addition of direct measures of intensity and control of such risk factors.
The second part of the analysis focused on the post-MRI vascular event outcomes. We obtained the crude incidence rate of first-occurring vascular events per 1,000 person-years using four categories of ICAS severity. We calculated cumulative incidence for events other than deah using fine & gray regression to account for competing risk of death using the %pshreg SAS macro.(26) To calculate the adjusted risk of events by category of stenosis, we used adjusted Cox proportional hazards regression models to estimate hazard ratios (HR) and their 95% confidence intervals (CI) with robust sandwich error variance.(27) To evaluate the survival bias because of any death, we modelled the adjusted risk of events using Fine & Gray regression using the same covariates. The statistical analyses were carried out with SAS software, version 9.4 (SAS Institute Inc., Cary, NC).
Results
Of 1290 NOMAS participants in the MRI study, we included 1211 in these analyses (mean age 71 ± 9 years, 59% women, 65% Hispanic). Participants without MRA were older and more likely to be women, Hispanic, diabetic or smokers (table 1).
Table 1:
Characteristics of the Northern Manhattan Study MRI cohort (N=1290)
| Available MRA N=1211 |
No MRA N=79 |
P value | ||
|---|---|---|---|---|
| Age (mean±SD, in years) | 71 ± 9 | 73 ± 10 | 0.04 | |
| Men (%) | 41 | 18 | <0.001 | |
| Ethnicity (%) | ||||
| NH white | 15 | 6 | 0.02 | |
| NH black | 18 | 13 | ||
| Hispanic | 65 | 78 | ||
| Insured (%) | 85 | 82 | 0.50 | |
| High School completed (%) | 47 | 24 | <0.001 | |
| Hypertension (%) | 78 | 83 | 0.24 | |
| Diabetes (%) | 25 | 41 | 0.001 | |
| Hypercholesterolemia (%) | 90 | 90 | 0.98 | |
| Current Smoking (%) | 11 | 19 | 0.03 | |
Abbreviations: NH, non-Hispanic; SD, standard deviation, N, number.
The prevalence of any stenosis in any assessed intracranial large artery was 45% (37% had at least one artery with < 50% stenoses or luminal irregularities, 3% had at least one artery with 50-69% stenosis, and 5% had at least one artery with ≥ 70% stenosis or flow gap).
Pre-MRI risk factor exposure:
From the time of enrollment to the brain MRI, NOMAS participants were followed on average six years (Figure 1). Using a simple model with risk factor prevalence expressed categorically, older age (OR 1.05 per year, 95% CI 1.03-1.06), hypertension (OR 1.74, 95% CI 1.22-2.49), diabetes (OR 1.60, 95% CI 1.21-2.13) and dyslipidemia (OR 2.03, 95% CI 1.19-3.46) were associated with ICAS prevalence and incremental severity (Table 2). Further adjustment for measures of risk factor severity and chronicity revealed that older age (OR 1.02 per year, 95% CI 1.01-1.04), longer duration of hypertension (OR 1.01 per year, 95% CI 1.00-1.02), higher number of glucose-lowering drugs (OR 1.64 per each medication, 95% CI 1.24-2.15), and HDL (OR 0.96 per mg/dl, 95% CI 0.92-0.99) were associated with ICAS prevalence and severity.. Notably, there were no racial or ethnic differences in ICAS score (Table 2).
Table 2:
Pre-MRI vascular risk factor exposure relationship with asymptomatic intracranial large artery stenosis (ordinal categories).
| Model 1 | Model 2 | Model 3 | ||
|---|---|---|---|---|
| Odds ratio, 95% confidence intervals | ||||
| Age (in years) at MRI | 1.05, 1.03-1.06s | 1.05, 1.03-1.06 | 1.02, 1.01-1.04 | |
| Male sex | 1.08, 0.84-1.40 | 1.18, 0.91-1.53 | 0.87, 0.67-1.14 | |
| Ethnicity | ||||
| NH white | Reference group | Reference group | Reference group | |
| NH black | 1.11, 0.75-1.68 | 1.03, 0.68-1.58 | 1.00, 0.67-1.451 | |
| Hispanic | 1.03, 0.69-1.52 | 0.89, 0.60-1.34 | 1.04, 0.72-1.52 | |
| High school completed (yes/no) | 0.85, 0.62-1.15 | 0.86, 0.63-1.18 | 1.12, 0.84-1.49 | |
| Visits to PMD during pre MRI follow up period | 1.06, 0.55-2.04 | 1.00, 0.51-1.96 | 0.77, 0.38-1.57 | |
| Hypertension (yes/no) at MRI | 1.74, 1.22-2.49 | |||
| SBP (per each 5 mmHg) | 1.03, 0.99-1.07 | |||
| DBP (per each 5 mmHg) | 0.98, 0.91-1.06 | |||
| Number of antihypertensives at MRI | 1.03, 0.88-1.22 | |||
| Hypertension duration (in years) at MRI | 1.01, 1.001-1.025 | |||
| Diabetes(yes/no) at MRI | 1.60, 1.21-2.13 | |||
| Fasting glucose (per each 5 mg/dL) | 1.00, 0.98-1.01 | |||
| Number of glucose-lowering drugs at MRI | 1.64, 1.24-2.15 | |||
| Diabetes duration (in years) | 0.99, 0.96-1.02 | |||
| Hypercholesterolemia (yes/no) at MRI | 2.03, 1.19-3.46 | |||
| LDL (per each 5 mg/dL) | 1.01, 1.00-1.03 | |||
| HDL (per each 5 mg/dL) | 0.96, 0.92-0.99 | |||
| Triglycerides (mg/dL) | 1.01, 0.99-1.02 | |||
| Number of cholesterol-lowering drugs at MRI | 1.02, 0.81-1.34 | |||
| Smoking (yes/no) at MRI | 1.21, 0.81-1.82 | |||
| Smoking duration (in years) | 1.01, 1.00-1.01 | |||
| Packs per day (pear each 5) | 0.95, 0.76-1.19 | |||
Statistical note: Model included all variables with valid beta estimate in each column.
Abbreviations: SD, standard deviation; NH, non-Hispanic; PMD, primary care doctor; SBP, systolic blood pressure; DBP, diastolic blood pressure; mg, milligrams; dL, deciliter; LDL, low density lipoprotein; HDL, high density lipoprotein.
Post-MRI follow up
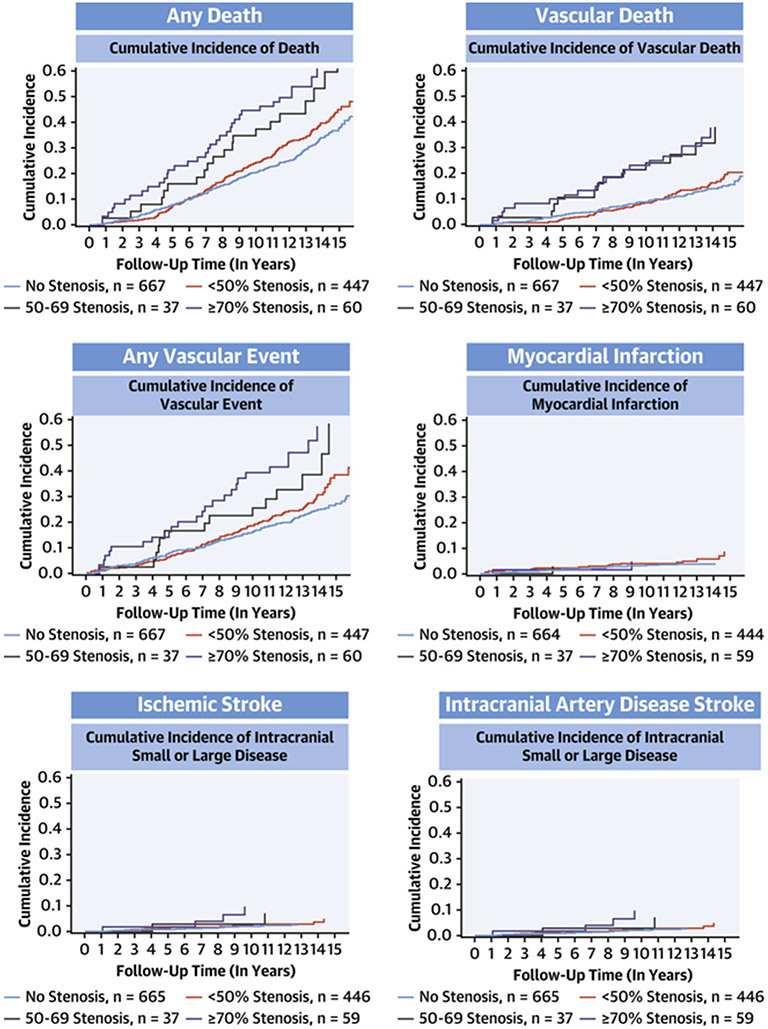
From the time of MRI, participants were followed for 12 years on average (Figure 1). Overall, the incidence of vascular events was higher with greater stenosis severity (Table 3, Central Illustration); the highest risk of vascular events was with ICAS => 70% (5.5% annual risk of any vascular event). The risk of events increased linearly during the time of follow-up after the brain MRI. The risk of vascular events with greater stenosis severity (Table 4). After adjusting for demographics, vascular risk factors, use of antiplatelets or anticoagulation, and established primary medical doctor care, the risk of intracranial artery disease stroke sand any vascular events remained significant among participants with ≥ 70% stenosis. Of the incident strokes initially classified as small artery disease, 80% occurred in participants that had evidence of ICAS (any degree) at their baseline MRI.
Table 3:
Incidence rate of events per 1000 person-years of follow up by asymptomatic intracranial large artery stenosis category in the Northern Manhattan Study cohort (N=1211).
| Incidence rate per 1000 person-year, 95% confidence interval | ||||||
|---|---|---|---|---|---|---|
| No stenosis N=667 |
<50% stenosis N=447 |
50-70% stenosis N=37 |
>70% stenosis or flow gap N=70 |
|||
| Death, N=454 | 28, 25-33 |
32, 27-36 |
53, 34-82 |
63, 46-88 |
||
| Vascular death, N=200 | 12, 9-14 |
14, 11-17 |
32, 18-56 |
35, 23-54 |
||
| Myocardial infarction, N=61 | 4, 2-5 |
6, 0.5-19 |
3, 0.5-19 |
8, 3-21 |
||
| Stroke, N=122 | 6, 4-8 |
11, 9-14 |
16, 7-35 |
17, 9-33 |
||
| Ischemic stroke, N=104 | 5, 3-7 |
9, 7-36 |
16, 7-36 |
15, 7-30 |
||
| Cardioembolic, N=39 | 2, 1-4 |
3, 2-4 |
8, 3-25 |
5, 2-17 |
||
| Intracranial (small or large) artery disease, N=36 | 1, 0.5-2 |
4, 2-6 |
5, 1-21 |
7, 3-19 |
||
| Small artery disease N=25 | 1, 0.5-2 |
3, 2-5 |
3, 0.5-19 |
2, 0.5-13 |
||
| Intracranial atherosclerosis, N=11 | 0.3, 0.1-1 |
0.8, 0.3-2 |
3, 0.5-18 |
5, 2-17 |
||
| Cryptogenic, N=19 | 1, 0.5-2 |
2, 1-3 |
- | - | ||
| Any vascular event, N=324 | 19, 16-22 |
27, 24-32 |
38, 22-64 |
55, 38-80 |
||
Central illustration: Cumulative incidence of event outcomes by category of stenosis.
The unadjusted cumulative incidence of event outcomes is higher in people with asymptomatic intracranial large artery stenosis compared with those with no stenosis. The highest risk of events is noted among those with >70% stenosis.
Table 4:
Risk of vascular events per intracranial large artery stenosis category in adjusted models.
| Any death |
Vascular death |
Myocardial Infarction |
Ischemic stroke |
Cardioembolic Stroke |
Intracranial small or large disease |
Stroke, myocardial infarction, or vascular death |
|
|---|---|---|---|---|---|---|---|
| Number of events |
454 | 200 | 61 | 122 | 39 | 36 | 324 |
| HR, 95% CI |
HR, 95% CI |
HR, 95% CI | HR, 95% CI | HR, 95% CI | HR, 95% CI | HR, 95% CI | |
| Cox proportional models | |||||||
| No stenosis | Referent group | ||||||
| <50% stenosis | 0.95, 0.77-1.17 |
0.90, 0.65-1.25 |
1.22, 0.71-2.09 |
1.06, 0.69-1.62 |
0.92, 0.75-1.13 |
1.40, 0.67-2.89 |
1.04, 0.81-1.32 |
| 50-70% stenosis | 1.24, 0.77-1.99 |
1.53, 0.81-2.89 |
0.49, 0.07-3.69 |
1.59, 0.65-3.90 |
1.05, 0.65-1.69 |
2.10, 0.45-9.68 |
1.09, 0.62-1.92 |
| >70% stenosis | 1.31, 0.91-1.90 |
1.60, 0.96-2.65 |
1.22, 0.40-3.56 |
1.50, 0.69-3.26 |
1.22, 0.85-1.76 |
3.60, 1.17-11.14 |
1.52, 1.003-2.31 |
| Fine & Gray competing risk models | |||||||
| No stenosis | Referent group | ||||||
| <50% stenosis | - | 0.8, 0.6-1.1 |
1.2, 0.6-2.2 |
1.0, 0.7-1.6 |
0.9, 0.5-1.9 |
1.4, 0.6-2.8 |
1.0, 0.8-1.3 |
| 50-70% stenosis | - | 1.7, 0.8-3.4 |
0.6, 0.1-5.0 |
2.1, 0.8-5.1 |
2.4, 0.6-9.3 |
2.4, 0.5-10.7 |
1.3, 0.7-2.3 |
| >70% stenosis | - | 1.4, 0.8-2.4 |
0.8, 0.2-4.1 |
1.8, 0.8-3.7 |
1.1, 0.3-3.9 |
3.9, 1.2-12.5 |
1.7, 1.1-2.5 |
Abbreviations:
Model adjusted for age, sex, ethnicity, prevalent risk factors at the time of MRI (hypertension, diabetes, dyslipidemia and smoking), self-reported medications to treat vascular risks at the time of MRI, use of oral antiplatelets or anticoagulants at the time of MRI, established PMD care at the time of MRI.
Among people with ICAS (any degree), established primary care doctor at the time of MRI was associated with lower risk of events. Older age, longer pre MRI duration of hypertension and smoking were associated with higher risk of vascular death, myocardial infarction and ischemic stroke (Table 5).
Table 5:
Role of vascular risks in post MRI vascular events in NOMAS participants with asymptomatic intracranial large artery stenosis
| Vascular death | Myocardial infarction | Ischemic stroke | ||
|---|---|---|---|---|
| HR, 95% CI | HR, 95% CI | HR, 95% CI | ||
| Age (in years) | 1.1, 1.1-1.1 | 1.0, 1.0-1.0 | 1.1, 1.1-1.1 | |
| Male sex | 1.1, 0.8-1.5 | 1.1, 0.6-2.0 | 1.2, 0.8-1.8 | |
| Ethnicity | ||||
| NH white | Reference | Reference | Reference | |
| NH black | 0.8, 0.5-1.2 | 0.8, 0.3-1.9 | 0.9, 0.5-1.7 | |
| Hispanic | 0.6, 0.4-0.8 | 0.6, 0.3-1.1 | 1.2, 0.7-2.0 | |
| PMD visits post MRI | 0.3, 0.2-0.50 | 0.2, 0.1-0.60 | 0.6, 0.2-1.5 | |
| Antiplatelets exposure (in years) post MRI | 1.0, 0.7-1.3 | 0.6, 0.3-1.2 | 1.1, 0.7-1.6 | |
| Anticoagulant exposure (in years) post MRI | 1.4, 0.6-3.0 | 2.4, 0.6-9.5 | 3.4, 1.7-7.1 | |
| Hypertension at MRI or after | 1.2, 0.8-2.0 | 1.4, 0.4-4.1 | 0.9, 0.5-1.8 | |
| Antihypertensive use at the time of MRI | 1.0, 0.7-1.5 | 1.7, 0.7-4.1 | 0.8, 0.5-1.5 | |
| Hypertension duration (in years) prior to MRI | 1.0, 1.0-1.0 | 1.0, 1.0-1.0 | 1.0, 1.0-1.0 | |
| Diabetes at MRI or after | 1.0, 0.5-1.8 | 2.1, 0.9-5.1 | 0.5, 0.2-1.0 | |
| Hypoglycemic at the time of MRI | 1.6, 0.8-3.2 | 0.6, 0.2-1.7 | 3.4, 1.5-7.8 | |
| Diabetes duration (in years) prior to MRI | 1.0, 1.0-1.0 | 1.0, 1.0-1.1 | 1.0, 0.9-1.0 | |
| Hypercholesterolemia at MRI or after | 0.6, 0.4-1.1 | 0.9, 0.3-2.8 | 1.4, 0.6-3.5 | |
| Lipid-lowering drugs at the time of MRI | 1.2, 0.9-1.7 | 1.4, 0.8-2.7 | 1.1, 0.7-1.8 | |
| Smoking at the time of MRI | 0.8, 0.5-1.5 | 0.4, 0.1-1.3 | 1.9, 0.9-4.1 | |
| Smoking duration (in years) prior to MRI | 1.0, 1.0-1.0 | 1.0, 1.0-1.0 | 1.0, 1.0-1.0 | |
Statistical note: Model adjusted for all variables listed in column one of this table. Estimates calculated with Fine and Gray regressions.
Abbreviations: SD, standard deviation; NH, non-Hispanic; PMD, primary medical doctor; SBP, systolic blood pressure; DBP, diastolic blood pressure; mg, milligrams; dL, deciliter; LDL, low density lipoprotein; HDL, high density lipoprotein.
DISCUSSION
Intracranial large artery stenosis is an important cause of stroke and a marker of systemic atherosclerosis. In this study of stroke-free individuals, we provide evidence that asymptomatic ICAS is a risk factor for cerebral and systemic vascular events and that the risk is increased with greater stenosis severity. Furthermore, longer exposure to vascular risk factors, specifically uncontrolled and/or more severe risk factors, has a direct impact on the presence of ICAS and ICAS-related vascular events. These results recapitulate the idea that the aggressive control of vascular risk factors is the cornerstone of secondary stroke prevention among people with ICAS, extending this same principle to those with asymptomatic ICAS. People with stroke caused by ICAS have more severe stroke at presentation (median national institute of health stroke scale [NIHSS] 5 in those with ICAS versus median NIHSS of 3 for other etiologies),(7) longer hospital stay, and higher risk of permanent disability.(28) Therefore, the societal benefits of aggressively controlling risk factors in people with asymptomatic ICAS to prevent their first stroke may be greater than after a stroke occurs.
The prevalence of any stenosis in NOMAS (45%) is similar to the prevalence reported in the Atherosclerosis Risk in Communities (ARIC) study (34%),(8,29) a comparable study to NOMAS in its design other than ARIC does not include Hispanic populations. Historically, a cutoff of 50% stenosis has been used in clinical studies of ICAS,(19) and as we showed here, there is an incremental risk of vascular events with higher degree of stenosis, thus justifying cutoff as more clinically relevant that any stenosis. Using ≥ 50% stenosis cutoff, the prevalence of ICAS is even more similar between ARIC (9%)(8) and NOMAS (8%). Compared to international studies, the NOMAS prevalence of asymptomatic ICAS ≥ 50% stenosis was higher than it was reported in Japan (6%),(30) but lower than it was reported the UK (11%),(31) Spain (9%)(32) or Hong Kong (13%)(33). These differences may relate to important differences in study design. For example, the Japanese study included healthy volunteers and reported low rates of hypertension and diabetes compared to NOMAS. The Oxford Vascular Study, the Barcelona-Asymptomatic Intracranial Atherosclerosis Study, and the Hong Kong cohorts focused on high-risk patients based on history of TIA/minor stroke or risk factor burden, which would expectedly select population at a higher risk of IICAS. Furthermore, the Barcelona-Asymptomatic Intracranial Atherosclerosis Study and the Hong Kong study use transcranial Dopplers, as oppose to structural arterial imaging, which may have underestimated the prevalence of ICAS in these high-risk populations. The cumulative evidence presented by others and us does highlight a hidden or clinically covert burden of ICAS across world populations that is not traditionally considered a therapeutic target.
Although the nature of these data prevent us from inferring a causative effect, extrapolation from other trials and the overall body of literature on the topic would support the value of aggressive control of risk factors at this stage to prevent the occurrence of a first stroke and other vascular events. For secondary stroke prevention, in the Stenting versus Aggressive Medical Therapy for Intracranial Arterial Stenosis (SAMMPRIS) trial, patients who achieved risk factor control targets had a lower risk of stroke recurrence.(12) In the Carotid Occlusion Surgery Study (COSS) trial, which included patients with unilateral carotid occlusion and hemodynamic failure, those who achieved normotension had lower risks of stroke recurrence.(34) This finding is particularly important in the setting of ICAS, as one may hypothesize that relative hypertension may be beneficial to overcome the arterial flow resistance posed by luminal stenosis. There may be a role for permissive hypertension in some patients with ICAS in the acute stroke phase, but there is less evidence that permissive hypertension has a protective long-term role in ICAS.(35) In fact, data from the Warfarin–Aspirin Symptomatic Intracranial Disease (WASID) trial showed that among people with ICAS with > 50 % stenosis, higher blood pressure is associated with increased (not decreased) risk of ischemic stroke and stroke in the territory of the stenotic vessel.(36).
Atherosclerosis is largely a cholesterol-mediated disease. Pathologically, atherosclerosis is diagnosed by the presence of atheroma (from the Greek, άθήρα [athera] or “gruel-like”).(37) The underlying assumed pathology of ICAS in most adult cases is atherosclerosis, especially in the setting of vascular risk factors. Other less common causes of ICAS include Moyamoya disease,(38) infectious arteriopathies (e.g. varicella zoster virus vasculitis),(39) focal cerebral arteriopathy in children,(40) etc. These etiologies of ICAS are not likely to be the cause of ICAS in our sample given the participants asymptomatic status, their age and vascular comorbidities. Nonetheless, it is difficult to determine the underlying etiology with certainty using MRA. Contrary to what has been reported about coronary arteries, the relationship between atherosclerotic plaque burden and luminal stenosis in brain arteries is linear for the most part.(41) Therefore, a higher degree of luminal stenosis implies a proportionally greater amount of cholesterol deposition and/or atherosclerotic plaque area. There exists robust evidence that statins decrease the risk of vascular events in primary prevention.(42) Trials focused on non-cardioembolic strokes (which includes ICAS) have demonstrated that high-dose statins reduce the risk of vascular events.(43) In a subgroup analysis from WASID that included participants with > 50% stenosis, participants with an LDL of < 70 mg/dl had a risk of recurrent stroke of 7% compared to 23% among those with LDL > 70 mg/dl.(44) In SAMMPRIS, with a trial population that included > 70% stenosis, 47% achieved an LDL target of <70 mg/dl, and those out of target had an 80% greater risk of recurrent stroke. Similarly, aggressive control of diabetes and smoking cessation are opportunities to reduce vascular risk in the general population.(45) Our study offers observational data supporting smoking cessation as a means of preventing ICAS given the association between greater number of packs per year and/or smoking duration and ICAS prevalence and ICAS-related vascular events. Similarly, the role of aggressive control of diabetes with a target glycosylated hemoglobin < 7% in general appears applicable to people with asymptomatic ICAS.(46) Finally, health care utilization defined here as established primary care doctor at the time of MRI was associated with a lower risk of vascular events in the ICAS population. Frequent visits to primary care providers may imply better risk factor control and relate to other unmeasured confounders (health literacy, health care trust, health care access and availability, etc).
The results of this study should be interpreted in the context of its limitations. For example, the reliance on self-reported use of certain medications may be less reliable and subject to recall bias. The annual intervals for our telephone follow up may not be frequent enough to capture the variability in biological variables such as blood pressure, or cholesterol levels, and therefore, underestimate other aspects of traditional risk factors that may play a role in ICAS and ICAS-events. Also, the lack of participants of Asian ancestry/ethnicities is a limitation. Despite these limitations, the overall results are consistent with the extensive and well-established body of literature supporting aggressive control of risk factor as the main means to reduce vascular risks in the general population. Strengths of this work include its population-based design, the unique ethnically diverse stroke-free population with asymptomatic ICAS, the low rate of loss to follow up, and the rigorous ascertainment of vascular outcomes and the population.
The novel observations made in this study support a more aggressive primary prevention effort for vascular events in the general population. A targeted approach to identifying high-risk community dwellers that harbor asymptomatic ICAS may be desirable to test whether aggressive targets of risk factor control prevent stroke and vascular events. The data presented here support the notion that ICAS is an imaging marker of established atherosclerotic disease in stroke-free individuals, and that incidental diagnosis of ICAS should trigger thorough assessment of vascular health. Preventing a first-ever stroke at this asymptomatic stage may magnify the societal benefits of vascular prevention and decrease stroke-related disability and vascular death in our communities.
Clinical Perspectives.
Competency in Medical Knowledge: Intracranial atherosclerotic stenosis (ICAS) is associated with high rates of initial and recurrent stroke and extracranial vascular events, proportionate to the severity of stenosis.
Translational Outlook: Additional research is needed to determine whether interventions that decrease recurrent stroke among patients with ICAS are helpful in asymptomatic individuals as well.
Funding:
NIH R01 AG057709, R01 AG066162, R01 NS36286
ABBREVIATIONS
- ICAS
Intracranial atherosclerotic stenosis
- NOMAS
Northern Manhattan Study
- CI
Confidence intervals
- OR
Odds ratio
- COSS
Carotid Occlusion Surgery Study
- LDL
Low-density lipoprotein
- WASID
Warfarin–Aspirin Symptomatic Intracranial Disease
- SAMMPRIS
Stenting versus Aggressive Medical Therapy for Intracranial Arterial Stenosis
- MI
Myocardial infarction
- NIHSS
National institute of health stroke scale
Footnotes
Disclosures: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.White H, Boden-Albala B, Wang C et al. Ischemic stroke subtype incidence among whites, blacks, and Hispanics: the Northern Manhattan Study. Circulation 2005;111:1327–31. [DOI] [PubMed] [Google Scholar]
- 2.Sharma VK, Tsivgoulis G, Teoh HL, Ong BK, Chan BP. Stroke risk factors and outcomes among various Asian ethnic groups in Singapore. J Stroke Cerebrovasc Dis 2012;21:299–304. [DOI] [PubMed] [Google Scholar]
- 3.Petty GW, Brown RD Jr., Whisnant JP, Sicks JD, O'Fallon WM, Wiebers DO. Ischemic stroke subtypes: a population-based study of incidence and risk factors. Stroke 1999;30:2513–6. [DOI] [PubMed] [Google Scholar]
- 4.Sacco RL, Boden-Albala B, Gan R et al. Stroke incidence among white, black, and Hispanic residents of an urban community - The Northern Manhattan Stroke Study. American Journal of Epidemiology 1998;147:259–268. [DOI] [PubMed] [Google Scholar]
- 5.Bang OY, Saver JL, Liebeskind DS, Pineda S, Yun SW, Ovbiagele B. Impact of metabolic syndrome on distribution of cervicocephalic atherosclerosis: data from a diverse race-ethnic group. J Neurol Sci 2009;284:40–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gutierrez J, Koch S, Dong C et al. Racial and ethnic disparities in stroke subtypes: a multiethnic sample of patients with stroke. Neurol Sci 2014;35:577–82. [DOI] [PubMed] [Google Scholar]
- 7.Wang Y, Zhao X, Liu L et al. Prevalence and Outcomes of Symptomatic Intracranial Large Artery Stenoses and Occlusions in China. Stroke 2014;45:663–669. [DOI] [PubMed] [Google Scholar]
- 8.Suri MFK, Qiao Y, Ma X et al. Prevalence of Intracranial Atherosclerotic Stenosis Using High-Resolution Magnetic Resonance Angiography in the General Population. The Atherosclerosis Risk in Communities Study 2016;47:1187–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Turan TN, Makki AA, Tsappidi S et al. Risk factors associated with severity and location of intracranial arterial stenosis. Stroke 2010;41:1636–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shitara S, Fujiyoshi A, Hisamatsu T et al. Intracranial Artery Stenosis and Its Association With Conventional Risk Factors in a General Population of Japanese Men. Stroke 2019;50:2967–2969. [DOI] [PubMed] [Google Scholar]
- 11.Rincon F, Sacco RL, Kranwinkel G et al. Incidence and risk factors of intracranial atherosclerotic stroke: the Northern Manhattan Stroke Study. Cerebrovasc Dis 2009;28:65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Turan TN, Nizam A, Lynn MJ et al. Relationship between risk factor control and vascular events in the SAMMPRIS trial. Neurology 2017;88:379–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oh HG, Chung PW, Rhee EJ. Increased risk for intracranial arterial stenosis in subjects with coronary artery calcification. Stroke 2015;46:151–6. [DOI] [PubMed] [Google Scholar]
- 14.Chatzikonstantinou A, Ebert AD, Schoenberg SO, Hennerici MG, Henzler T. Atherosclerosis in intracranial, extracranial, and coronary arteries with aortic plaques in patients with ischemic stroke of undetermined etiology. Int J Neurosci 2014. [DOI] [PubMed] [Google Scholar]
- 15.Sollberg LA, McGarry PA, Moossy J, Strong JP, Tejada C, Loken AC. Severity of atherosclerosis in cerebral arteries, coronary arteries, and aortas. Ann N Y Acad Sci 1968;149:956–73. [DOI] [PubMed] [Google Scholar]
- 16.Giertsen JC. Atherosclerosis in an autopsy series. 3. Inter-relationship between atherosclerosis in the aorta, the coronary and the cerebral arteries. Acta pathologica et microbiologica Scandinavica 1965;63:391–403. [DOI] [PubMed] [Google Scholar]
- 17.Chimowitz MI, Lynn MJ, Derdeyn CP et al. Stenting versus aggressive medical therapy for intracranial arterial stenosis. N Engl J Med 2011;365:993–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sacco RL, Anand K, Lee HS et al. Homocysteine and the risk of ischemic stroke in a triethnic cohort: the NOrthern MAnhattan Study. Stroke 2004;35:2263–9. [DOI] [PubMed] [Google Scholar]
- 19.Chimowitz MI, Lynn MJ, Howlett-Smith H et al. Comparison of warfarin and aspirin for symptomatic intracranial arterial stenosis. The New England journal of medicine 2005;352:1305–16. [DOI] [PubMed] [Google Scholar]
- 20.Boden-Albala B, Roberts ET, Bazil C et al. Daytime Sleepiness and Risk of Stroke and Vascular Disease: Findings from the Northern Manhattan Study (NOMAS). Circulation: Cardiovascular Quality and Outcomes 2012;5:500–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khan A, Kasner SE, Lynn MJ, Chimowitz MI. Risk factors and outcome of patients with symptomatic intracranial stenosis presenting with lacunar stroke. Stroke 2012;43:1230–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holliday EG, Traylor M, Malik R et al. Genetic overlap between diagnostic subtypes of ischemic stroke. Stroke 2015;46:615–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gutierrez J, Gil-Guevara A, Ramaswamy S et al. Classification of Covert Brain Infarct Subtype and Risk of Death and Vascular Events. Stroke 2020;51:90–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Echt DS, Liebson PR, Mitchell LB et al. Mortality and morbidity in patients receiving encainide, flecainide, or placebo. The Cardiac Arrhythmia Suppression Trial. N Engl J Med 1991;324:781–8. [DOI] [PubMed] [Google Scholar]
- 25.Schaefer EJ, Lamon-Fava S, Jenner JL et al. Lipoprotein(a) levels and risk of coronary heart disease in men. The lipid Research Clinics Coronary Primary Prevention Trial. JAMA 1994;271:999–1003. [DOI] [PubMed] [Google Scholar]
- 26.Kohl M, Plischke M, Leffondré K, Heinze G. PSHREG: a SAS macro for proportional and nonproportional subdistribution hazards regression. Comput Methods Programs Biomed 2015;118:218–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.So Y, Lin G, Johnston G. Using the PHREG Procedure to Analyze Competing-Risks Data. SAS Global Forum, 2014. [Google Scholar]
- 28.Wong KS, Li H. Long-term mortality and recurrent stroke risk among Chinese stroke patients with predominant intracranial atherosclerosis. Stroke 2003;34:2361–6. [DOI] [PubMed] [Google Scholar]
- 29.Qiao Y, Guallar E, Suri FK et al. MR Imaging Measures of Intracranial Atherosclerosis in a Population-based Study. Radiology 2016;280:860–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matsui R, Nakagawa T, Takayoshi H et al. A Prospective Study of Asymptomatic Intracranial Atherosclerotic Stenosis in Neurologically Normal Volunteers in a Japanese Cohort. Frontiers in neurology 2016;7:39–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hurford R, Wolters FJ, Li L, Lau KK, Kuker W, Rothwell PM. Prognosis of Asymptomatic Intracranial Stenosis in Patients With Transient Ischemic Attack and Minor Stroke. JAMA neurology 2020;77:947–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Planas-Ballvé A, Crespo AM, Aguilar LM et al. The Barcelona-Asymptomatic Intracranial Atherosclerosis study: Subclinical intracranial atherosclerosis as predictor of long-term vascular events. Atherosclerosis 2019;282:132–136. [DOI] [PubMed] [Google Scholar]
- 33.Wong KS, Ng PW, Tang A, Liu R, Yeung V, Tomlinson B. Prevalence of asymptomatic intracranial atherosclerosis in high-risk patients. Neurology 2007;68:2035–2038. [DOI] [PubMed] [Google Scholar]
- 34.Powers WJ, Clarke WR, Grubb RL Jr. et al. Lower stroke risk with lower blood pressure in hemodynamic cerebral ischemia. Neurology 2014;82:1027–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McManus M, Liebeskind DS. Blood Pressure in Acute Ischemic Stroke. Journal of clinical neurology (Seoul, Korea) 2016;12:137–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Turan TN, Cotsonis G, Lynn MJ, Chaturvedi S, Chimowitz M, Warfarin-Aspirin Symptomatic Intracranial Disease Trial I. Relationship between blood pressure and stroke recurrence in patients with intracranial arterial stenosis. Circulation 2007;115:2969–75. [DOI] [PubMed] [Google Scholar]
- 37.Halsey CS. An etymology of Latin and Greek. Boston, USA: Ginn & Company, 1889. [Google Scholar]
- 38.Yamashita M, Oka K, Tanaka K. Histopathology of the brain vascular network in moyamoya disease. Stroke 1983;14:50–8. [DOI] [PubMed] [Google Scholar]
- 39.Gutierrez J, Ortiz G. HIV/AIDS patients with HIV vasculopathy and VZV vasculitis: a case series. Clin Neuroradiol 2011;21:145–51. [DOI] [PubMed] [Google Scholar]
- 40.Elkind MS, Hills NK, Glaser CA et al. Herpesvirus Infections and Childhood Arterial Ischemic Stroke: Results of the VIPS Study. Circulation 2016;133:732–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gutierrez J, Goldman J, Honig LS, Elkind MSV, Morgello S, Marshall RS. Determinants of cerebrovascular remodeling: Do large brain arteries accommodate stenosis? Atherosclerosis 2014;235:371–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gutierrez J, Ramirez G, Rundek T, Sacco RL. Statin Therapy in the Prevention of Recurrent Cardiovascular Events: A Sex-Based Meta-analysisStatin Therapy to Prevent Recurrent CV Events. Arch Intern Med 2012;172:909–19. [DOI] [PubMed] [Google Scholar]
- 43.Amarenco P, Bogousslavsky J, Callahan A rd et al. High-dose atorvastatin after stroke or transient ischemic attack. The New England journal of medicine 2006;355:549–559. [DOI] [PubMed] [Google Scholar]
- 44.Chaturvedi S, Turan TN, Lynn MJ et al. Risk factor status and vascular events in patients with symptomatic intracranial stenosis. Neurology 2007;69:2063–8. [DOI] [PubMed] [Google Scholar]
- 45.Duncan MS, Freiberg MS, Greevy RA Jr, Kundu S, Vasan RS, Tindle HA. Association of Smoking Cessation With Subsequent Risk of Cardiovascular Disease. JAMA 2019;322:642–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.6. Glycemic Targets: Standards of Medical Care in Diabetes—2020. Diabetes Care 2020;43:S66–S76. [DOI] [PubMed] [Google Scholar]