ABSTRACT
Work-related asthma (WRA) is highly prevalent in the adult population. WRA includes occupational asthma (OA), which is asthma caused by workplace exposures, and work-exacerbated asthma (WEA), also known as work-aggravated asthma, which is preexisting or concurrent asthma worsened by workplace conditions. In adults, the estimated prevalence of OA is 16.0%, whereas that of WEA is 21.5%. An increasing number of chemicals used in industrial production, households, and services are associated with the incidence of adult-onset asthma attributable to exposure to chemicals. This review article summarizes the different types of WRA and describes diagnostic procedures, treatment, prevention, and approaches to patient management. It is not always easy to distinguish between OA and WEA. It is important to establish a diagnosis (of sensitizer-/irritant-induced OA or WEA) in order to prevent worsening of symptoms, as well as to prevent other workers from being exposed, by providing early treatment and counseling on social security and work-related issues.
Keywords: Asthma, occupational/diagnosis; Asthma, occupational/prevention & control; Asthma, occupational/therapy; Allergens; Bronchial provocation tests
RESUMO
A asma relacionada ao trabalho (ART) é um acometimento com elevada prevalência na população adulta. A ART inclui a asma ocupacional (AO), desencadeada pela exposição a um agente presente em um determinado ambiente de trabalho, e a asma agravada ou exacerbada pelo trabalho (AA/ET), que acomete indivíduos com antecedentes de asma ou que iniciaram um quadro de asma concomitante, mas sem relação causal com o ambiente de trabalho. Estima-se que 16,0% e 21,5% da asma no adulto sejam AO e AA/ET, respectivamente. O elevado e crescente número de substâncias químicas usadas na produção industrial, no uso domiciliar ou em serviços é responsável pela incidência de asma associada à exposição a agentes químicos na vida adulta. Este artigo de revisão descreve os principais tipos de ART, os procedimentos para seu diagnóstico, tratamento e prevenção e as condutas frente ao diagnóstico. Nem sempre é fácil a distinção entre AO e AA/ET. A importância do diagnóstico (AO ou AA/ET e asma induzida por sensibilizantes ou irritantes) tem relação com a adoção de medidas de prevenção para evitar que novos indivíduos sejam expostos e que os acometidos apresentem agravamento da doença, utilizando tratamento precoce e fornecendo orientação sobre aspectos previdenciários e trabalhistas.
Descritores: Asma ocupacional/diagnóstico, Asma ocupacional/prevenção e controle, Asma ocupacional/terapia, Alérgenos, Testes de provocação brônquica
INTRODUCTION
Asthma ranks second among the most prevalent chronic respiratory diseases worldwide.1 In 2017, the prevalence of asthma was estimated at 273 million cases (3.6% of the world population) and the incidence of asthma was estimated at 43 million cases.1 , 2 Asthma is the second leading cause of death from a chronic respiratory disease worldwide, with an estimated 500,000 deaths in 20173 and a mortality rate of 6.48/100,000 population.1 Of the aforementioned deaths, approximately 7% were work-related.4
Work-related asthma (WRA) is asthma that is caused by exposures at work (occupational asthma [OA]) or that is exacerbated by exposures at work (work-exacerbated asthma [WEA], also known as work-aggravated asthma). OA is defined as asthma symptoms accompanied by reversible airflow obstruction or bronchial hyperresponsiveness caused by conditions attributable to the occupational environment rather than to stimuli encountered outside the workplace.5 , 6 OA can be caused by sensitizers or irritants.6 Sensitizer-induced OA is more common than irritant-induced OA, accounting for approximately 90% of cases.7 Sensitizer-induced OA is characterized by the onset of symptoms after a latency period (i.e., symptoms occurring months or years after exposure). An earlier onset is associated with a higher level of exposure, as well as with the sensitizing agent and individual characteristics.8 Sensitizing agents can be of high molecular weight (> 5 kDa),9 , 10 mostly proteins, with an IgE-mediated immune mechanism, or low molecular weight, with a mechanism that has yet to be elucidated in most cases.6 OA without latency (also known as nonimmunologic asthma) is asthma induced by irritants6 and accounts for 5-18% of cases, its prevalence varying across studies and environments.7 Irritant-induced asthma occurs after a single exposure or multiple exposures to high concentrations of an irritant.11 However, it has been suggested that chronic exposure to irritants at low concentrations is also associated with the development of irritant-induced asthma.12 - 14 Reactive airway dysfunction syndrome is the first and best known description of irritant-induced asthma, the onset of which is less than 24 h after exposure to high levels of an irritant, with symptoms, functional changes, or both lasting three months or more.11 , 12 WEA is defined as preexisting or concurrent asthma that is worsened by workplace conditions, the former being asthma with onset before entering the worksite of interest and the latter being asthma with onset while employed in the worksite of interest but not due to exposures in that worksite. In this case, the workplace is related to worsening or exacerbation of previously controlled asthma.15
WRA is a common and preventable occupational disease that causes limitations in work and activities of daily living, has unfavorable socioeconomic outcomes, and affects working-age individuals, requiring attention from physicians, researchers, and health care providers. When WRA goes unrecognized and untreated, it can progress to severe asthma, difficult-to-control asthma, or both.14 In a multicenter retrospective study conducted in Europe, 16.2% of OA patients met criteria for severe asthma,16 whereas, in non-WRA patients, the reported prevalence of severe asthma was approximately 5%.17 Factors associated with severe OA include persistent exposure to the causative agent at work, a longer duration of disease, a lower level of education, a diagnosis of childhood asthma, and expectoration.16
The studies included in the present review article were retrieved from the MEDLINE (PubMed) database by the following search terms (and Boolean operators): (work-related asthma) OR (occupational asthma) OR (work-aggravated asthma) OR (work-exacerbated asthma) OR (irritant-induced asthma). The search included peer-reviewed articles published between January 1, 2000 and October 31, 2020 and written in English, Portuguese, Spanish, French, or Italian. Article inclusion was determined by the authors of the present study, and the articles were not systematically reviewed. We included original articles, review articles, consensus statements, and articles published before the year 2000 and addressing the topics discussed herein.
OCCUPATIONS AND EXPOSURES ASSOCIATED WITH WRA
Given that millions of people are exposed to sensitizers and irritants at work and at home, public health surveillance is required in order to identify and prevent such exposures, as well as for the diagnosis and early treatment of those who develop asthma. Approximately 600 agents have been related to OA, 400 of which involve sensitizing mechanisms.18 Asthma symptoms can be worsened by defined agents (any substance that acts through known or unknown immune-mediated mechanisms or as an airway irritant) and environmental conditions such as low temperatures and air pollution in the workplace. In 2016, 24.0% of men and 13.4% of women worldwide were estimated to be exposed to asthma-causing agents in the workplace.19
Sensitizers
In a recent multicenter study conducted in Europe and involving a cohort of 635 workers,20 a total of 8 high- or low-molecular-weight agents were found to account for more than 70% of all OA cases: flour, isocyanates, persulfates, metals, latex, wood, quaternary ammonium compounds, and acrylates. Chart 1 shows the most prevalent sensitizers, by molecular weight (high or low), as well as commonly associated occupations/workplaces and exposures.20 - 24 High-molecular-weight agents consist of proteins of animal or vegetable origin. Low-molecular-weight agents include organic and inorganic compounds that act as haptens, some of which have IgE-mediated immune mechanisms, including platinum salts (industrial catalysts), trimellitic and phthalic anhydrides (found in paints and sealants), persulfates (henna hair dye), reactive dyes, and, more rarely, diisocyanates. Low-molecular-weight sensitizers without an IgE-mediated mechanism include diisocyanates (found in paints and varnishes, as well as in polyurethane production), acrylates (glues and adhesives), wood compounds, such as plicatic acid (red cedar), metals (chromium, nickel, and cobalt), and glutaraldehyde.
Chart 1. Most common sensitizers, by molecular weight (high or low), as well as commonly associated occupations/workplaces and exposures.
| Sensitizer | Occupation/Workplace/Exposure |
|---|---|
| High molecular weight (≥ 5 kDa) | |
| Flours and grains | bakers, food industry |
| Latex | health care workers; producers and frequent users of latex products |
| Enzymes | manufacture and use of detergents; pharmaceutical and food industries |
| Plant-derived products | agricultural workers |
| Animals and animal-derived products | laboratories, veterinaries, agricultural workers |
| Fungi | offices, schools, cleaners |
| Low molecular weight (< 5 kDa) | |
| Diisocyanates | polyurethane production; foam production; automotive paint and polyurethane varnish; plastics industry |
| Persulfates | hairdressers |
| Metals (chrome, nickel, cobalt, zinc, platinum salts) | metal coatings (galvanization), welders, pharmaceutical industry, and refineries |
| Quaternary ammonium compounds | cleaners |
| Acrylates | dentists, adhesive resins, synthetic fabrics, printer inks, plastics industry, manicurists |
| Wood dust | carpenters |
| Cleaning products: chloride, ammonia, glutaraldehyde | cleaners and health care workers |
| Drugs (e.g., antibiotics) | pharmaceutical industry |
| Phthalic and trimellitic anhydrides | epoxy resin manufacture, spray paint workers |
Irritants
Causative agents of irritant-induced OA include ammonia, cement dust, chloride, cleaning products, diesel exhaust, tobacco smoke, isocyanates, fire smoke, sulfur dioxide, mixed agents in swine confinement facilities, and welding fumes (Chart 2).12 , 13 , 24 - 26
Chart 2. Occupations/workplaces and agents/mixed agents associated with irritant-induced occupational asthma.
| Occupation/workplace | Agents/mixed agents |
|---|---|
| Cleaners and health care workers | chloride, ammonia, disinfectants, hydrochloric acid, organic solvents |
| Aluminum smelting | fluorides, sulfur dioxide, aluminum oxide |
| Pulp and paper mills | sulfur dioxide |
| Swine and dairy production | aerosols from endotoxins and organic dusts; manure gases |
| Dark-room environment | acetic acid |
| Welding | nitrogen oxides, fluorides, ozone, metals |
| Biocides | ethylene oxide, formalin, insecticides (organophosphates, organochlorines, and carbamates) |
| Construction work | spray paints, cement dust, calcium oxide (lime), floor sealant (aromatic hydrocarbons) |
| Firefighters, first responders, security agents | smoke (fires), fumes released by chemical spills |
| Mechanics, highway workers, and railroad workers | diesel exhaust, organic solvents |
Exposure to dust, environmental tobacco smoke, air pollution, stressful activities, temperature variations, and physical exertion are also associated with WEA.15 Occupations and exposures associated with WRA vary across studies. In a study conducted in Canada27 and involving workers claiming compensation for asthma, 39% had WEA; of those, most (67%) had irritant-induced asthma, and the most common irritants were paints, solvents, calcium oxide, acids, ammonia, cigarette smoke, glutaraldehyde, and welding fumes.
In recent decades, several studies conducted in industrialized countries have shown an increased risk of developing asthma in cleaners.28 , 29 Among cleaners in northern Europe, a significant relationship was found between the number of years worked as a cleaner and the risk of developing asthma.30 In a study analyzing 3,634 cases of WRA in the state of Michigan, USA, exposure to cleaning products was found to have increased from 5% to 20%, cleaning products having become the agents most commonly associated with asthma in the last 30 years.31 In a study involving 394 patients diagnosed with OA in the city of São Paulo, Brazil, women were found to be most commonly exposed to cleaning products (38.5%) and fumes released in the manufacture of plastics (18.5%), whereas men were found to be most commonly exposed to isocyanates (24.8%) and metal fumes (18.9%).32 Among the most widely used cleaning products, the most common sensitizers are quaternary ammonium compounds, amines, and flavoring agents, whereas the most common irritants are sodium hypochlorite, hydrochloric acid, and alkaline agents (ammonia and caustic soda).28 , 29
In summary, the occupations/workplaces/exposures most commonly associated with WRA are painting, cleaning, carpentry, beauty salons, health services, and the food industry (exposure to flours, animal proteins, and condiments). However, it should be noted that it is no longer enough to know the occupation of affected individuals in order to understand exposures and work environments; there is a need to know the workplaces and agents that can induce or exacerbate asthma symptoms.
EPIDEMIOLOGY AND RISK FACTORS
In an analysis of epidemiological studies, the reported prevalence of adult-onset asthma attributable to occupational exposures was 16%.33 The prevalence of WEA varies across studies depending on the definition of WEA and the diagnostic criteria. It is estimated that 21.5% of adults with asthma experience exacerbation or worsening of symptoms because of exposures in the workplace.15 The overall number of deaths from OA in 2017 was approximately 34,000. The number of disability-adjusted life years for OA in 2017 was 1.910 million,4 disability-adjusted life years being the sum of the years of life lost to premature death and years lived with disability.
Factors associated with an increased risk of developing asthma include the type of exposure (the risk of developing asthma ranging from 5% for exposure to isocyanates to 50% for exposure to platinum salts), the level of exposure (a higher level of exposure translating to a higher risk of developing asthma), smoking (the risk of developing asthma being well defined for exposures such as platinum salts and anhydrides), a history of bronchial hyperresponsiveness, and presence of rhinitis/atopy (the risk of developing asthma being well defined for exposure to high-molecular-weight agents).6 , 7 , 33 , 34
DIAGNOSIS
A diagnosis of WRA should be considered in adults who develop asthma or experience exacerbation of preexisting asthma.5 Several definitions of and diagnostic criteria for WRA have been proposed.5 , 10 , 15 The proposed diagnostic criteria are summarized in Figure 1 and include the following: A) a diagnosis of asthma; B) onset or worsening/exacerbation of symptoms after entering the worksite of interest; and C) an association between asthma symptoms and work. Additional criteria include the following: 1) workplace exposure to an agent known to cause asthma; 2) workplace exposure to an agent or conditions known to cause asthma exacerbation; 3) worsening of FEV1, PEF, or a combination of the two, or hyperresponsiveness after nonspecific bronchial challenge testing during periods of work in comparison with periods away from work; 4) positive response to specific bronchial challenge testing; and 5) onset of asthma symptoms after exposure to irritants.
Figure 1. Algorithm for the diagnosis of work-related asthma. OA: occupational asthma; and WEA: work-exacerbated asthma.
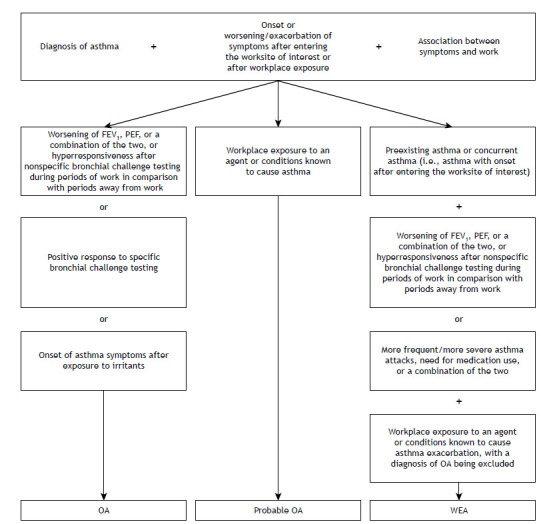
A diagnosis of OA is made if A+B+C are met, together with 3, 4, or 5. A diagnosis of probable OA is made if A+B+C are met, together with 1. A diagnosis of WEA is made if A+B+C are met, together with a diagnosis of preexisting asthma or concurrent asthma (i.e., asthma with onset after entering the worksite of interest) and 2+3 or more frequent/more severe asthma attacks, need for medication use, or a combination of the two, with a diagnosis of OA being excluded. According to the European Academy of Allergy and Clinical Immunology,12 irritant-induced OA is diagnosed in the absence of a history of asthma, being classified as follows: (i) acute (i.e., definite irritant-induced OA)-asthma that develops within a few hours after a single exposure to very high levels of irritants; (ii) subacute (i.e., probable irritant-induced OA)-asthma that develops within a few days or weeks after multiple high-level exposures to irritants; and (iii) chronic (i.e., possible irritant-induced OA)-asthma resulting from chronic exposure to moderate levels of irritants (i.e., with a latency period).
Given that each year new chemicals are produced and made available for use, the possibility of new exposures should always be considered.
DIAGNOSTIC PROCEDURES
Occupational history and evaluation of the causative agent
In the early stages of WRA, patients report experiencing asthma symptoms or worsening of asthma symptoms at work, the symptoms resolving or improving when patients are away from work (on weekends/vacation). The symptoms tend to worsen with continued exposure, and it takes longer for any noticeable improvement to occur when patients are away from work. Late asthmatic reactions to the allergen, leading to worsening of symptoms at the end of the day or after work hours, can make it difficult to evaluate WRA, whereas exposure to irritants and sensitizers in the home can worsen asthma outside the workplace.7 The clinical history is more reliable to exclude than to confirm OA. In a study of asthma patients, clinical history had a negative predicted value of 83%, whereas a history suggestive of OA had a positive predictive value of 63%.35 Therefore, medication use, emergency department visits, and hospitalizations are useful objective parameters to quantify the course of the disease.5 Identification of the causative agent also supports a diagnosis of WRA and makes it possible to minimize future exposures and prevent new cases among exposed workers, as well as to identify new agents.36 , 37 If the clinical history, the occupational history, and the presence of a causative agent in the workplace are suggestive of WRA, timely further investigation is needed, preferably while the patient is still employed and exposed to the causative agent, for an objective assessment of asthma to establish its relationship with the work environment and adopt measures to protect workers.5
Objective measurements
Serial PEF measurements
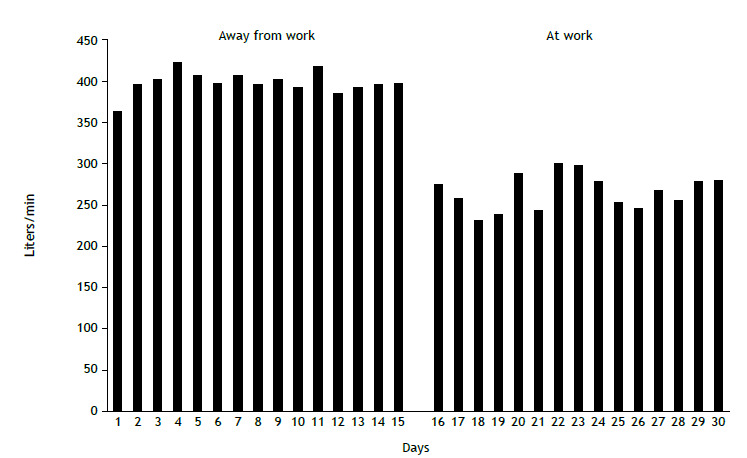
Serial measurements of PEF (Figure 2) have a good level of evidence for the diagnosis of WRA. Good-quality measurements can be obtained with appropriate training and patient instructions. Although false or inaccurate measurements cannot be ruled out, serial PEF measurements provide the simplest and least expensive method to assess patient response to inhaled agents or work environment conditions, being recommended worldwide.5 , 10 Serial PEF measurements should be obtained at least four times a day, no more than 2-3 h apart, during the course of two weeks at work and two weeks away from work. Patients perform three maximal inspiratory maneuvers followed by a maximal forced expiratory maneuver. The best of triplicate recordings made at each time point is used for comparative analysis.5 , 10 Serial PEF measurements are highly sensitive and specific for the diagnosis of OA.5 Although visual interpretation of measurements is the most commonly used method of analysis, statistical methods can be used, such as comparison of daily means between periods at work and away from work.37 , 38 In addition, PEF records can be interpreted by Oasys-2 freeware (Occupational Asthma System; Oasys Research Group, UK), developed to aid in the diagnosis of WRA through analysis of PEF records. The developers of Oasys-2 recommend that PEF records be analyzed for periods of work-rest-work (two periods of days at work, separated by a period of days away from work) and rest-work-rest (two periods of days away from work, separated by a period of days at work), with measurements obtained at least four times a day.39 , 40
Figure 2. Daily means of serial PEF measurements: periods away from work and periods of work. The patient is a 40-year-old man who had been a carpenter for approximately 21 years. He presented with a 3-year history of progressive rhinitis, dry cough, wheezing, and dyspnea, with attacks. He reported multiple emergency department visits, as well as symptom improvement during periods away from work and with the use of bronchodilators.
Spirometry and nonspecific bronchial challenge testing
Spirometry and nonspecific bronchial challenge testing play an essential role in the diagnosis of asthma and can aid in confirming the impact of work exposures on lung function. Although it is possible to compare FEV1 measurements before and after a work shift, they are less accurate and more difficult to perform than PEF measurements. In contrast, serial measurements of bronchial hyperresponsiveness to methacholine or histamine are useful diagnostic tools for the investigation of WRA when performed during periods of work and periods away from work.5 These measurements are based on the hypothesis that hyperresponsiveness is greater when workers are exposed to the causative or exacerbating agent than when they are not (for at least two weeks).5 , 10 , 41 The result is considered significant when more than twice the concentration of the inhaled agent is needed to cause a drop of 20% or more in FEV1 during a period away from work in comparison with a period of work.34 Serial measurements of bronchial hyperresponsiveness are moderately sensitive and specific for the diagnosis of OA (48-67% and 54-78%, respectively) and can aid in the diagnosis when PEF measurements are inconclusive or when patients are unable to perform the maneuvers/recordings correctly.37 , 38 Normal results in workers away from exposure are not sufficient to exclude the diagnosis.42 In symptomatic workers exposed to the suspected causative agent, a negative result practically rules out the diagnosis of WRA, although the seasonal use of products in work environments should be investigated.41 Exacerbating factors such as cold air and exercise are less sensitive to nonspecific bronchial challenge testing, exercise testing and exposure to temperature variations therefore being more appropriate, although the latter is more feasible in the workplace than in health care facilities.43
Specific bronchial challenge testing
Specific bronchial challenge testing is considered the gold standard for the diagnosis of OA; however, it is difficult to standardize and must be performed in specialized centers, as well as requiring specific test kits, challenge chambers equipped with dosimeters, and a 24-hour hospitalization. Because it is not widely available,37 its usefulness is limited.44 Despite its high sensitivity and specificity, false-negative results can occur when individuals are exposed to the wrong agent, when the concentration of the exposure is inadequate, or when individuals are away from exposure.37 Therefore, specific bronchial challenge testing is recommended when other forms of investigation are unavailable or when the diagnosis remains uncertain.45
Immunological tests
Immunological tests such as skin prick tests and specific IgE measurements in blood samples can aid in the diagnosis of sensitizer-induced OA by demonstrating sensitization to an occupational agent. However, the presence of sensitization alone is not sufficient to establish a causal relationship between sensitization and asthma.46 In addition, standardized tests are currently available for only a few of the more than 400 known allergens, and the lack of standardization limits the validity of the results.46 Skin prick tests for common aeroallergens (house dust, mites, pollens) can be performed to determine the presence of atopy, which is associated with an increased risk of sensitization to high-molecular-weight agents.37 However, workers should not be denied a job on the basis of the results of skin prick tests performed during pre-employment medical examination.
Inflammatory markers
Induced sputum cell counts can aid in the diagnosis of OA. An increase in sputum eosinophils following exposure to the causative agent at work in comparison with measurements away from work (and vice versa) is indicative of OA.10 , 37 Diagnostic sensitivity and specificity increase when an increased sputum eosinophil count is associated with serial measurements of nonspecific bronchial hyperresponsiveness47 or serial PEF measurements during periods of work and periods away from work.6 , 48 Although only a few studies have examined inflammatory changes in individuals with WEA following exposure to occupational agents (predominantly sensitizers), sputum cell counts appear to be useful in differentiating between OA and WEA.49 WEA is most commonly associated with no changes in airway inflammation or with neutrophilic airway inflammation, whereas sensitizer-induced OA is most commonly associated with an eosinophilic phenotype.49 An increase in sputum neutrophils has been reported in individuals with OA caused by exposure to certain low-molecular-weight agents and irritants such as ozone, diesel exhaust particles, and endotoxins.50 Despite their diagnostic utility, induced sputum cell counts are not widely available, and approximately 20% of individuals are unable to produce sputum samples of adequate quality for analysis.50
Few studies have examined the utility of fractional exhaled nitric oxide (FeNO) in the diagnosis of OA, and the results have been inconsistent because the specificity of FeNO measurements is low in comparison with that of induced sputum cell counts.7 , 24 Increased FeNO levels might be related to exposure to occupational sensitizers, most of which are high-molecular-weight agents,51 although some are low-molecular-weight agents, such as isocyanates.52 , 53 Analysis of FeNO changes before and after exposure to a sensitizer can be a useful alternative in patients who are unable to produce adequate sputum samples or perform serial PEF measurements.7 , 24 , 37
Analysis of exhaled breath condensate (EBC) is a more recent noninvasive method for assessing airway inflammation. In a study in which workers underwent analysis of EBC before and after specific bronchial challenge testing,54 those suspected of having OA showed a decrease of 0.4 units in EBC pH during periods of work in comparison with periods away from work, with high (90%) specificity for the diagnosis of OA, despite low sensitivity. This preliminary finding suggests that analysis of EBC could be incorporated into the diagnostic workup of OA as an additional test or as an alternative to other tests.38
Differentiation between WEA and OA
OA and WEA are not mutually exclusive; an individual with OA can develop WEA, and vice versa. It can be challenging to differentiate between OA and WEA; asthma present before occupational exposure is not always sufficient to discriminate between the two.10 Immune-mediated OA can affect patients with preexisting asthma (i.e., OA superimposed on previous non-OA), with workers becoming sensitized to a specific agent (“de novo asthma”).15 In patients with sensitizer-induced OA, asthma exacerbation caused by exposure to the original causative agent is considered a recurrence of OA. However, workers with OA can also develop asthma exacerbation caused by agents in the workplace that are different from the causative agent of OA.15 Given that specific bronchial challenge testing is performed in only a few centers and for a minority of agents, the differentiation between OA and WEA in clinical practice is based on a temporal relationship between the onset of asthma symptoms and occupational exposure.55 Asthma symptoms occurring before an occupational exposure and aggravated by it are suggestive of WEA, provided that the diagnosis of asthma is confirmed by an objective measurement of lung function showing differences between values obtained during periods of work and those obtained during periods away from work.14
With regard to the differentiation between WEA and irritant-induced OA, it has been recommended that reactive airway dysfunction syndrome be considered only in individuals without preexisting asthma.25 However, it has been suggested that acute exposure to high levels of irritants can lead to recurrence of previously quiescent asthma or worsening of previously controlled asthma.22 There is debate as to whether this accidental worsening of asthma should be categorized as irritant-induced OA or a form of WEA. According to the European Academy of Allergy and Clinical Immunology,12 irritant-induced OA is asthma in complete remission (no symptoms and no medication use for at least one year) before high-level exposure to irritants, whereas WEA is asthma that is clinically active before high-level exposure to irritants.
Differential diagnosis
The differential diagnosis of WRA should include COPD, hypersensitivity pneumonitis, bronchitis, eosinophilic bronchiolitis, and vocal cord dysfunction; in addition, it should be borne in mind that these conditions can coexist with asthma, albeit not commonly.10
Although WRA is diagnosed on the basis of objective measurements to determine a causal relationship between asthma symptoms and occupational exposure, this is not always possible in clinical practice, either because the required tests are not widely available or because patients are not exposed at the time of diagnosis. In a study conducted in the city of São Paulo, Brazil, 50% of patients undergoing medical evaluation were not working, either because they had been dismissed or because they were on sick leave in most cases.32 In such cases, the diagnosis is made on the basis of careful evaluation of the clinical history and the workplace, as well as on the basis of an understanding of the toxicology of the different exposures.38 , 56 Material safety data sheets are sources of information that can help to identify workplace exposures. Occupational hygienists and experienced professionals can aid in establishing a diagnosis and (temporarily or permanently) relocating workers to unexposed areas/duties, when necessary.15 , 22
WRA PATIENT MANAGEMENT
Early diagnosis and complete removal of exposure to the causative agent are the most effective interventions for the prevention and treatment of WRA,10 , 57 preventing disease progression and limitations in work activities.58 Complete removal of exposure is the recommended intervention for patients diagnosed with sensitizer-induced OA. Depending on the severity of asthma and the extent of exacerbating factors at work, individuals experiencing WEA are often able to maintain their jobs/positions after exposure to relevant agents has been controlled/reduced, and this can reduce the socioeconomic impact of work absenteeism.34 If complete removal of exposure is not sufficient to prevent exacerbation of asthma symptoms, workers should be relocated to unexposed areas/duties.6 In a systematic review conducted in 2019,59 removal of exposure and reduction of exposure were reported to improve asthma symptoms when compared with continued exposure, although only removal of exposure was found to improve lung function.
Several studies suggest that WRA is associated with increased rates of prolonged unemployment and reduced income, primarily in workers who are completely removed from exposure; this is likely due to the need for reassignment to other, less important, jobs and the fact that such workers are often denied job opportunities on the basis of pre-employment medical examination results.58 , 59 Therefore, an accurate diagnosis is essential because of the socioeconomic impact of OA.58 Despite evidence of the importance of early diagnosis, studies suggest that mean time to diagnosis is two to four years after the onset of symptoms, and delayed diagnosis/misdiagnosis can lead to progressive worsening of symptoms.37
SOCIAL SECURITY ISSUES
A correct diagnosis of WRA is important because it has implications for formally employed workers regarding social security issues, given that diagnosed cases of WRA must be reported to the Brazilian National Institute of Social Security, even those in which a medical leave of absence is not required. Notification ensures paid medical leave (when applicable) and monthly compensation until retirement in cases in which the disease leads to a functional limitation, a job/career change, or both.60 In Brazil, workers diagnosed with occupational or work-related disease and on leave for more than 15 days are granted one-year job stability by the social security system.
CHALLENGES IN DEVELOPING COUNTRIES
Worldwide, WRA is underdiagnosed and ineffectively managed, with patients being inadequately compensated for disease-related losses. Epidemiological data on WRA are scarce, and its impact on low- and middle-income countries is likely underestimated.7 Therefore, there is a need to prioritize research into WRA in order to mitigate the burden of WRA on the working population, the health care system, and the social security system.61 Improved characterization of WRA can aid in the identification of high-risk industries and occupations, and, consequently, in the creation and implementation of health surveillance programs to establish preventive measures.54 For example, there is an urgent need for interventions to improve the occupational safety of cleaners, including replacing some cleaning products with less toxic products and providing training on how to prepare and use cleaning products safely.62 Social security and worker protection systems need to be improved and employers need to be held accountable to provide socioeconomic stability to workers with WRA.
Footnotes
Financial support: None.
Study carried out at the Instituto do Coração, Faculdade de Medicina, Universidade de São Paulo - FMUSP - São Paulo (SP) Brasil.
REFERENCES
- 1.GBD Chronic Respiratory Disease Collaborators Prevalence and attributable health burden of chronic respiratory diseases, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Respir Med. 2020;8(6):585–596. doi: 10.1016/S2213-2600(20)30105-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.GBD 2017 Disease and Injury Incidence and Prevalence Collaborators Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017 [published correction appears in Lancet. 2019 Jun 22;393(10190):e44] Lancet. 2018;392(10159):1789–1858. doi: 10.1016/S0140-6736(18)32279-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.GBD 2017 Causes of Death Collaborators Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980-2017: a systematic analysis for the Global Burden of Disease Study 2017 [published correction appears in Lancet. 2019 Jun 22;393(10190):e44] [published correction appears in Lancet. 2018 Nov 17;392(10160):2170] Lancet. 2018;392(10159):1736–1788. doi: 10.1016/S0140-6736(18)32203-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.GBD 2017 Risk Factor Collaborators Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017 [published correction appears in Lancet. 2019 Jan 12;393(10167):132] [published correction appears in Lancet. 2019 Jun 22;393(10190):e44] Lancet. 2018;392(10159):1923–1994. doi: 10.1016/S0140-6736(18)32225-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chan-Yeung M. Assessment of asthma in the workplace ACCP consensus statement. American College of Chest Physicians. Chest. 1995;108(4):1084–1117. doi: 10.1378/chest.108.4.1084. [DOI] [PubMed] [Google Scholar]
- 6.Mapp CE, Boschetto P, Maestrelli P, Fabbri LM. Occupational asthma. Am J Respir Crit Care Med. 2005;172(3):280–305. doi: 10.1164/rccm.200311-1575SO. [DOI] [PubMed] [Google Scholar]
- 7.Cormier M, Lemière C. Occupational asthma. Int J Tuberc Lung Dis. 2020;24(1):8–21. doi: 10.5588/ijtld.19.0301. [DOI] [PubMed] [Google Scholar]
- 8.Vandenplas O. Occupational asthma etiologies and risk factors. Allergy Asthma Immunol Res. 2011;3(3):157–167. doi: 10.4168/aair.2011.3.3.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mapp CE. Agents, old and new, causing occupational asthma. Occup Environ Med. 2001;58(5):354–290. doi: 10.1136/oem.58.5.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tarlo SM, Balmes J, Balkissoon R, Beach J, Beckett W, Bernstein D. Diagnosis and management of work-related asthma American College Of Chest Physicians Consensus Statement [published correction appears in. Chest. 2008;134(4):892–892. doi: 10.1378/chest.08-0201. [DOI] [PubMed] [Google Scholar]
- 11.Tarlo SM. Irritant-induced asthma in the workplace. Curr Allergy Asthma Rep. 2014;14(1):406–406. doi: 10.1007/s11882-013-0406-4. [DOI] [PubMed] [Google Scholar]
- 12.Vandenplas O, Wiszniewska M, Raulf M, de Blay F, Gerth van Wijk R, Moscato G, et al. EAACI position paper irritant-induced asthma. Allergy. 2014;69(9):1141–1153. doi: 10.1111/all.12448. [DOI] [PubMed] [Google Scholar]
- 13.Baur X, Bakehe P, Vellguth H. Bronchial asthma and COPD due to irritants in the workplace - an evidence-based approach. J Occup Med Toxicol. 2012;7(1):19–19. doi: 10.1186/1745-6673-7-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Friedman-Jimenez G, Harrison D, Luo H. Occupational asthma and work-exacerbated asthma. Semin Respir Crit Care Med. 2015;36(3):388–407. doi: 10.1055/s-0035-1550157. [DOI] [PubMed] [Google Scholar]
- 15.Henneberger PK, Redlich CA, Callahan DB, Harber P, Lemière C, Martin J. An official american thoracic society statement work-exacerbated asthma. Am J Respir Crit Care Med. 2011;184(3):368–378. doi: 10.1164/rccm.812011ST. [DOI] [PubMed] [Google Scholar]
- 16.Vandenplas O, Godet J, Hurdubaea L, Rifflart C, Suojalehto H, Walusiak-Skorupa J. Severe Occupational Asthma Insights From a Multicenter European Cohort. J Allergy Clin Immunol Pract. 2019;7(7):2309–2318. doi: 10.1016/j.jaip.2019.03.017. [DOI] [PubMed] [Google Scholar]
- 17.Hekking PW, Wener RR, Amelink M, Zwinderman AH, Bouvy ML, Bel EH. The prevalence of severe refractory asthma. J Allergy Clin Immunol. 2015;135(4):896–902. doi: 10.1016/j.jaci.2014.08.042. [DOI] [PubMed] [Google Scholar]
- 18.Baur X, Akdis CA, Budnik LT, Cruz MJ, Fischer A, Förster-Ruhrmann U. Immunological methods for diagnosis and monitoring of IgE-mediated allergy caused by industrial sensitizing agents (IMExAllergy) Allergy. 2019;74(10):1885–1897. doi: 10.1111/all.13809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.GBD 2016 Risk Factors Collaborators . Global, regional, and national comparative risk assessment of 84. behavioural: environmental and occupational, and metabolic risks or clusters of risks; 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vandenplas O, Godet J, Hurdubaea L, Rifflart C, Suojalehto H, Wiszniewska M, et al. Are high- and low-molecular-weight sensitizing agents associated with different clinical phenotypes of occupational asthma? Allergy. 2019;74(2):261–272. doi: 10.1111/all.13542. [DOI] [PubMed] [Google Scholar]
- 21.Baur X. A compendium of causative agents of occupational asthma. J Occup Med Toxicol. 2013;8(1):15–15. doi: 10.1186/1745-6673-8-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tarlo SM, Lemiere C. Occupational asthma. N Engl J Med. 2014;370(7):640–649. doi: 10.1056/NEJMra1301758. [DOI] [PubMed] [Google Scholar]
- 23.Maestrelli P, Henneberger PK, Tarlo S, Mason P, Boschetto P. Causes and Phenotypes of Work-Related Asthma. Int J Environ Res Public Health. 2020;17(13):4713–4713. doi: 10.3390/ijerph17134713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tiotiu AI, Novakova S, Labor M, Emelyanov A, Mihaicuta S, Novakova P. Progress in Occupational Asthma. Int J Environ Res Public Health. 2020;17(12):4553–4553. doi: 10.3390/ijerph17124553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brooks S. Irritant-induced asthma and Reactive Airways Dysfunction Syndrome (RADS) J Allergy Ther. 2014;05(03) doi: 10.4172/2155-6121.1000174. [DOI] [Google Scholar]
- 26.Kurt OK, Basaran N. Occupational Exposure to Metals and Solvents Allergy and Airway Diseases. Curr Allergy Asthma Rep. 2020;20(8):38–38. doi: 10.1007/s11882-020-00931-7. [DOI] [PubMed] [Google Scholar]
- 27.Tarlo SM, Liss G, Corey P, Broder I. A workers' compensation claim population for occupational asthma Comparison of subgroups. Chest. 1995;107(3):634–641. doi: 10.1378/chest.107.3.634. [DOI] [PubMed] [Google Scholar]
- 28.Folletti I, Siracusa A, Paolocci G. Update on asthma and cleaning agents. Curr Opin Allergy Clin Immunol. 2017;17(2):90–95. doi: 10.1097/ACI.0000000000000349. [DOI] [PubMed] [Google Scholar]
- 29.Siracusa A, de Blay F, Folletti I, Moscato G, Olivieri M, Quirce S. Asthma and exposure to cleaning products - a European Academy of Allergy and Clinical Immunology task force consensus statement. Allergy. 2013;68(12):1532–1545. doi: 10.1111/all.12279. [DOI] [PubMed] [Google Scholar]
- 30.Svanes Ø, Skorge TD, Johannessen A, Bertelsen RJ, Bràtveit M, Forsberg B. Respiratory Health in Cleaners in Northern Europe Is Susceptibility Established in Early Life?. PLoS. One. 2015;10(7):e0131959. doi: 10.1371/journal.pone.0131959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reilly MJ, Wang L, Rosenman KD. The Burden of Work-related Asthma in Michigan, 1988-2018. Ann Am Thorac Soc. 2020;17(3):284–292. doi: 10.1513/AnnalsATS.201905-401OC. [DOI] [PubMed] [Google Scholar]
- 32.Mendonça EM, Algranti E, de Freitas JB, Rosa EA, dos Santos Freire JA, de Paula Santos U, et al. Occupational asthma in the city of São Paulo, 1995-2000, with special reference to gender analysis. Am J Ind Med. 2003;43(6):611–617. doi: 10.1002/ajim.10210. [DOI] [PubMed] [Google Scholar]
- 33.Blanc PD, Annesi-Maesano I, Balmes JR, Cummings KJ, Fishwick D, Miedinger D. The Occupational Burden of Nonmalignant Respiratory Diseases An Official American Thoracic Society and European Respiratory Society Statement. Am J Respir Crit Care Med. 2019;199(11):1312–1334. doi: 10.1164/rccm.201904-0717ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fishwick D, Barber CM, Bradshaw LM, Harris-Roberts J, Francis M, Naylor S. Standards of care for occupational asthma. Thorax. 2008;63(3):240–250. doi: 10.1136/thx.2007.083444. [DOI] [PubMed] [Google Scholar]
- 35.Malo JL, Ghezzo H. L'Archevêque J.Lagier F.Perrin B.Cartier A Is the clinical history a satisfactory means of diagnosing occupational asthma . Am Rev Respir. Dis. 1991;143(3):528–532. doi: 10.1164/ajrccm/143.3.528. [DOI] [PubMed] [Google Scholar]
- 36.Tarlo SM, Liss GM, Blanc PD. How to diagnose and treat work-related asthma key messages for clinical practice from the American college of chest physicians consensus statement. Pol Arch Med Wewn. 2009;119(10):660–666. doi: 10.20452/pamw.799. [DOI] [PubMed] [Google Scholar]
- 37.Lau A, Tarlo SM. Update on the Management of Occupational Asthma and Work-Exacerbated Asthma. Allergy Asthma Immunol Res. 2019;11(2):188–200. doi: 10.4168/aair.2019.11.2.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Muñoz X, Cruz MJ, Bustamante V, Lopez-Campos JL, Barreiro E. Work-related asthma diagnosis and prognosis of immunological occupational asthma and work-exacerbated asthma. J Investig Allergol Clin Immunol. 2014;24(6):396–405. [PubMed] [Google Scholar]
- 39.Baldwin DR, Gannon P, Bright P, Newton DT, Robertson A, Venables K. Interpretation of occupational peak flow records level of agreement between expert clinicians and Oasys-2. Thorax. 2002;57(10):860–864. doi: 10.1136/thorax.57.10.860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moore VC, Jaakkola MS, Burge PS. A systematic review of serial peak expiratory flow measurements in the diagnosis of occupational asthma. Ann Resp Med. 2010;1:31–34. [Google Scholar]
- 41.Clin Exp Allergy Guidelines for the diagnosis of occupational asthma Subcommittee on 'Occupational Allergy' of the European Academy of Allergology and Clinical Immunology. Clin Exp Allergy. 1992;22(1):103–108. doi: 10.1111/j.1365-2222.1992.tb00121.x. [DOI] [PubMed] [Google Scholar]
- 42.Pralong JA, Cartier A. Review of Diagnostic Challenges in Occupational Asthma. Curr Allergy Asthma Rep. 2017;17(1):1–1. doi: 10.1007/s11882-017-0676-3. [DOI] [PubMed] [Google Scholar]
- 43.Moscato G, Pala G, Barnig C, de Blay F, Del Giacco SR, Folletti I. EAACI consensus statement for investigation of work-related asthma in non-specialized centres. Allergy. 2012;67(4):491–501. doi: 10.1111/j.1398-9995.2011.02784.x. [DOI] [PubMed] [Google Scholar]
- 44.Henneberger P, Liang X, Lemière C. A comparison of work-exacerbated asthma cases from clinical and epidemiological settings. Can Respir J. 2013;20(3):159–164. doi: 10.1155/2013/495767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tarlo SM, Cartier A. Canadian Thoracic Society Asthma Committee Work-related asthma A case-based guide. Can Respir J. 2009;16(6):e57–e61. doi: 10.1155/2009/757192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tarlo SM, Quirce S. Impact of Identification of Clinical Phenotypes in Occupational Asthma. J Allergy Clin Immunol Pract. 2020;8(10):3277–3282. doi: 10.1016/j.jaip.2020.06.003. [DOI] [PubMed] [Google Scholar]
- 47.Racine G, Castano R, Cartier A, Lemiere C. Diagnostic Accuracy of Inflammatory Markers for Diagnosing Occupational Asthma. J Allergy Clin Immunol Pract. 2017;5(5):1371–1377. doi: 10.1016/j.jaip.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 48.Girard F, Chaboillez S, Cartier A, Côté J, Hargreave FE, Labrecque M. An effective strategy for diagnosing occupational asthma use of induced sputum. Am J Respir Crit Care Med. 2004;170(8):845–850. doi: 10.1164/rccm.200403-380OC. [DOI] [PubMed] [Google Scholar]
- 49.Lemière C, Boulet LP, Chaboillez S, Forget A, Chiry S, Villeneuve H. Work-exacerbated asthma and occupational asthma do they really differ?. J Allergy Clin. Immunol. 2013;131(3):704–710. doi: 10.1016/j.jaci.2012.08.024. [DOI] [PubMed] [Google Scholar]
- 50.Malo JL, Vandenplas O. Definitions and classification of work-related asthma. Immunol Allergy Clin North Am. 2011;31(4):645–64v. doi: 10.1016/j.iac.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 51.Lemiere C, NGuyen S, Sava F, D'Alpaos V, Huaux F, Vandenplas O. Occupational asthma phenotypes identified by increased fractional exhaled nitric oxide after exposure to causal agents. J Allergy Clin Immunol. 2014;134(5):1063–1067. doi: 10.1016/j.jaci.2014.08.017. [DOI] [PubMed] [Google Scholar]
- 52.Sastre J, Costa C. del Garcia Potro M.Aguado E.Mahillo I.Fernández-Nieto M Changes in exhaled nitric oxide after inhalation challenge with occupational agents. J Investig Allergol Clin Immunol. 2013;23(6):421–427. [PubMed] [Google Scholar]
- 53.Ferrazzoni S, Scarpa MC, Guarnieri G, Corradi M, Mutti A, Maestrelli P. Exhaled nitric oxide and breath condensate ph in asthmatic reactions induced by isocyanates. Chest. 2009;136(1):155–162. doi: 10.1378/chest.08-2338. [DOI] [PubMed] [Google Scholar]
- 54.Fishwick D. Work aggravated asthma; a review of the recent evidence. Br Med Bull. 2014;110(1):77–88. doi: 10.1093/bmb/ldu004. [DOI] [PubMed] [Google Scholar]
- 55.Lemiere C. Occupational and work-exacerbated asthma similarities and differences. Expert Rev Respir Med. 2007;1(1):43–49. doi: 10.1586/17476348.1.1.43. [DOI] [PubMed] [Google Scholar]
- 56.Baur X, Aasen TB, Burge PS, Heederik D, Henneberger PK, Maestrelli P. The management of work-related asthma guidelines a broader perspective. Eur Respir Rev. 2012;21(124):125–139. doi: 10.1183/09059180.00004711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jares EJ, Baena-Cagnani CE, Gómez RM. Diagnosis of occupational asthma an update. Curr Allergy Asthma Rep. 2012;12(3):221–231. doi: 10.1007/s11882-012-0259-2. [DOI] [PubMed] [Google Scholar]
- 58.Vandenplas O, Suojalehto H, Cullinan P. Diagnosing occupational asthma. Clin Exp Allergy. 2017;47(1):6–18. doi: 10.1111/cea.12858. [DOI] [PubMed] [Google Scholar]
- 59.Henneberger PK, Patel JR, de Groene GJ, Beach J, Tarlo SM, Pal TM. Workplace interventions for treatment of occupational asthma. Cochrane Database Syst Rev. 2019;10(10):CD006308–CD006308. doi: 10.1002/14651858.CD006308.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fernandes AL, Stelmach R, Algranti E. Occupational asthma [Article in Portuguese] J Bras. Pneumol. 2006;32(2):S27–S34. doi: 10.1590/S1806-37132006000800006. [DOI] [PubMed] [Google Scholar]
- 61.Hoy R. Occupational asthma in developing countries requires further research. Int J Tuberc Lung Dis. 2015;19(4):372–372. doi: 10.5588/ijtld.15.0129. [DOI] [PubMed] [Google Scholar]
- 62.de Fátima Maçãira E, Algranti E, Medina Coeli Mendonça E, Antônio Bussacos M. Rhinitis and asthma symptoms in non-domestic cleaners from the Sao Paulo metropolitan area, Brazil. Occup Environ Med. 2007;64(7):446–453. doi: 10.1136/oem.2006.032094. [DOI] [PMC free article] [PubMed] [Google Scholar]


