Abstract
Classic hypothalamic-pituitary-end-organ feedback loops – the hypothalamic-pituitary-adrenal axis (HPAA), hypothalamic-pituitary-thyroidal axis (HPTA), and hypothalamic-pituitary-gonadal axis (HPGA) – are associated with the neuroendocrine and immune systems in major depressive disorder (MDD). Female patients with MDD present with evident neuroendocrine and immunological changes. Glucocorticoid, thyroid hormone, and reproductive steroid levels fluctuate with menstrual cycles, which might lead to glucocorticoid receptor resistance, impairment of triiodothyronine conversion, and sex hormone secretion disorders. In this review, we summarize the independent and interactive functions of these three axes in female MDD patients. The similar molecular structure of steroids implies an interrelationship between the hypothalamic-pituitary-end-organ axes and the competitive inhibitory effects at the receptor level, especially when considering the HPAA and HPGA.
Keywords: Major depressive disorder, Neurosecretory systems, Sex steroid hormones
Introduction
Although the monoamine hypothesis of MDD is well-accepted, precisely how antidepressants come into effect remains unclear [1]. There is increasing evidence that chronic stress derives from abnormalities in the biological processes of the human internal environment (especially the endocrine and immune systems) [2], which might raise the morbidity of MDD [3]. Postmortem and animal studies have revealed neuropathological changes in depressed patients and animals, such as monoaminergic system down-regulation, neurogenesis impairment, abnormal activity of the central nervous system (CNS), and synaptic dysfunction [4].
Proinflammatory cytokines can impair neurogenesis by affecting monoaminergic systems. Regional abnormalities found in the CNS, as well as neurodegeneration, have been reproduced in cell and animal models of depression [5]. In addition, strong evidence supports the hypothesis that inflammation and depression are closely related [6], and that it also affects the role of glucocorticoids in depression [7]. Under endogenous and exogenous stress, cytokines (such as nuclear factor-kappa and protein kinase A), can result in glucocorticoid receptor (GR) resistance [8] (impairing function of the HPAA and its reactivity to cortisol) and the induction of MDD via the neuroendocrine pathway [8, 9]. Fig. 1 shows a schematic diagram of these three classic feedback loops (HPAA, HPTA, and HPGA) in the human neuroendocrine and neuroimmune systems [10]. It is worth noting that the most common diseases of these neuroendocrine systems present with obvious sex differences [11–13] (Table 1). Clinical evidence has demonstrated that MDD is almost twice as common in females, with a first peak in prevalence in the second and third decades of life, with a second peak in the fifth decade [14]. The two onset peaks of female MDD are around the ages when neuroendocrine levels fluctuate greatly [15, 16].
Fig. 1.
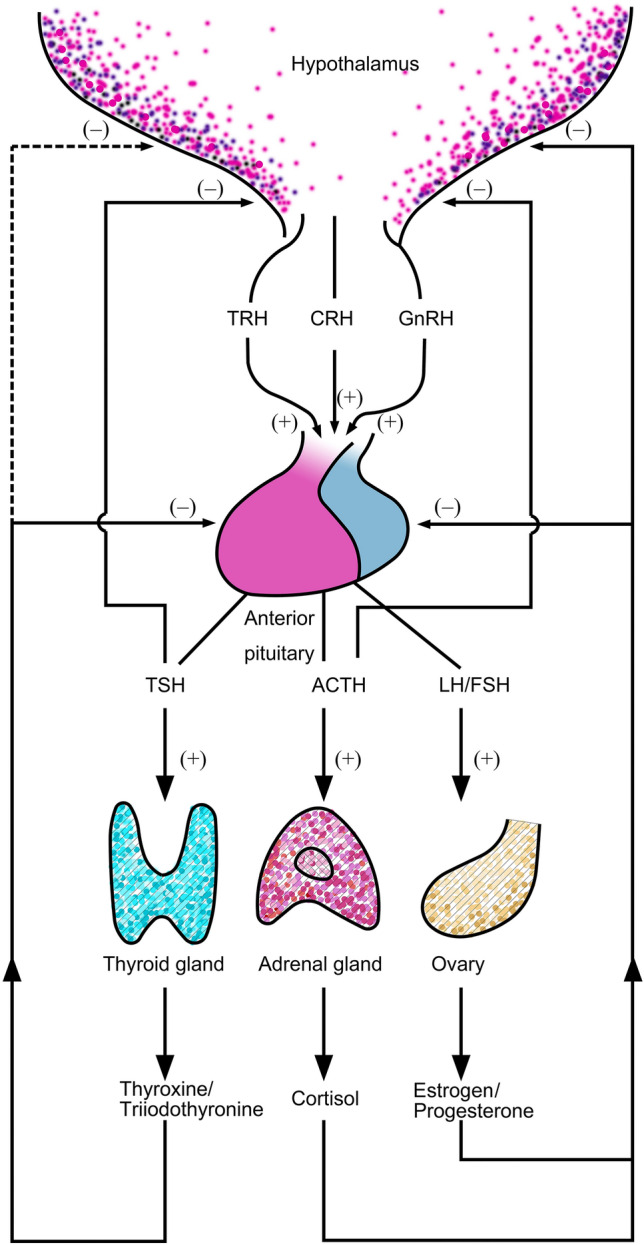
Schematic of three functional hypothalamic-pituitary-end-organ axes of females, generating the interactive mechanism among inflammatory cytokines and these axes. The dashed line represents an uncertain feedback mechanism. A special case, estrogen has a positive feedback effect on the pituitary gland during the follicular phase and a negative feedback effect during the luteal phase. TRH, thyrotropin-releasing hormone; CRH, corticotropin-releasing hormone; GnRH, gonadotrophin-releasing hormone; TSH, thyroid stimulating hormone; ACTH, adrenocorticotropic hormone; LH, luteinizing hormone; FSH, follicle-stimulating hormone.
Table 1.
Gender ratios in common hypothalamic-pituitary-glandular axis disorders and depressive disorders
| Disease | Gender ratio (male:female) |
|---|---|
| Hyperthyroidism | 1:(4–6) |
| Hypothyroidism | 1:4 |
| Cushing disease | 1:3 |
| Addison's disease | 1:(2–3) |
| Depressive disorders | 1:2 |
Hypothalamic-Pituitary-Adrenal Axis and MDD
HPAA, Chronic Stress, and MDD
The HPAA participates in a wide range of biological functions, primarily those related to the stress response. Cortisol, produced by the adrenal cortex, has a potent effect on carbohydrate metabolism. The human body shows physical and psychological adaptive reactions to potential threats via cortisol activation [17]. In MDD patients, chronic stress may result in abnormalities of HPAA homeostasis and cause relevant effects [18]. MDD individuals present with increased volumes of pituitary and adrenal glands, as well as an up-regulation of cortisol function [19]. Long-term psychological stress could give rise to the suppression of cortisol, resulting in diminished sensitivity of the stress response [20].
In addition to increasing hormone concentrations, inhibition of GR function has also been found to accompany chronic stress in MDD patients [21]. GRs are widely distributed in the hippocampus; they respond to intracellular signaling by second messengers, and regulate HPAA feedback. The most widely accepted theory is that in MDD patients, glucocorticoid resistance leads to impairments in HPAA function. Dexamethasone inhibition and corticotropin-releasing hormone inhibition tests have shown that negative feedback is more likely to be inhibited [22]. GR resistance is seen as an inadequate in vivo response of glucocorticoid target genes to dexamethasone. In chronic stress states, the quantity of GRs and the affinity of glucocorticoids to them are changeable [23]; consequently, glucocorticoid resistance can arise. Similar to like insulin and insulin resistance, it has long been debated which comes first: increases in cortisol levels or GR resistance?
Neurogenesis, in which synaptic function plays an important role, can be decreased by long-term systemic cortisol exposure in MDD [24]. Relevant studies support the glucocorticoid theory of MDD, in which chronic stress states induce HPAA hyperactivity and excessive secretion of cortisol. This would aggravate the neuropathological process of MDD [18]. This hypothesis provides a new theoretical basis to explore MDD pharmacotherapy; perhaps there is potential in glucocorticoid agonists and antagonists as new antidepressants targeting GR function and cortisol levels. Furthermore, this could also point to studying HPAA dysfunctions as new biomarkers of MDD.
HPAA, Immune Inflammation, and MDD
Inflammatory cytokines can increase the levels of cortisol by both direct and indirect pathways [25]. This results in dysregulation of the HPAA by increasing the release of corticotropin-releasing hormone and adrenocorticotropic hormone (ACTH). In addition, inflammatory cytokines regulate GR sensitivity to cortisol indirectly. From another perspective, the HPAA can, in return, influence the immune system [26]; this may inhibit the secretion and release of pro-inflammatory factors via blocking of transcription factor activity [27]. However, physiologically overdemanding use of glucocorticoids in the clinic inhibits the release of cytokines, thereby exerting anti-inflammatory and immunosuppressant effects. Increases in glucocorticoid-generating signals allow for activation of the neuroendocrine and sympathetic nervous systems, while chronic stress may lead to glucocorticoid resistance. On the other hand, opposite views suggest that glucocorticoids promote inflammation in certain circumstances, depending on environmental and temporal factors. In addition, that cortisol has a negative correlation with both pro-inflammatory factors [interleukin-1β (IL-1β) and tumor necrosis factor α], and anti-inflammatory factors (IL-4, IL-5, IL-10, and IL-13) simultaneously [28] is worth discussing.
The association between stress and immune enhancement is regulated by glucocorticoid resistance. Stressed individuals present with higher glucocorticoid resistance than non-stressed individuals. Glucocorticoid resistance is strongly linked to pro-inflammatory cytokines; enhanced inflammatory responses occur most in glucocorticoid-resistant individuals, second only to in those exposed to acute stressors, and both are linked to chronic stress and MDD [9]. According to the glucocorticoid resistance hypothesis, high concentrations of pro-inflammatory cytokines and cortisol only occur in glucocorticoid-resistant MDD patients. It has been reported that increases in glucocorticoid resistance and pro-inflammatory cytokines are more common than an increase in cortisol alone, and 85% of cases that have increases in inflammation also present with glucocorticoid resistance [10]. In a meta-analysis, glucocorticoid resistance and inflammation levels had a positive correlation, with effect size 0.38 (P = 0.06; 95% CI, 0.14–0.62) [10]. Therefore, we should perhaps focus on glucocorticoids and their signaling rather than on cortisol levels alone. The glucocorticoid resistance hypothesis provides a reasonable explanation for synchronized elevations in cortisol and pro-inflammatory cytokines.
Another hypothesis is that glucocorticoids do not only have anti-inflammatory effects in MDD. Whether they are pro-inflammatory or anti-inflammatory mainly depends on the exact environment of individuals; a pro-inflammatory response requires special settings, such as large-dose glucocorticoid therapy [29]. The reaction time of glucocorticoids depends on the severity of inflammatory injury, which is also in line with evolution. When faced with a threatening event, it is necessary to utilize immunosuppressive and glucocorticoid-elevating effects to energize for either facing an attack or escape (fight or flight). However, recovery from trauma takes time. While glucocorticoid levels normalize, immune function needs to be enhanced [29]. Therefore, stress responses and the resulting elevated glucocorticoid levels are warning signs of central and peripheral inflammation. The microglia are important CNS cells that work against neuroendocrine warning signals; they react strongly to immune stimulation by generating pro-inflammatory cytokines and down-regulating glucocorticoid inhibition. Increases in this activity can result from an increased density of microglia in the hippocampus as a result of chronic stress, inducing immune-promoting states and impairing glucocorticoid inhibition [30]. Microglia inhibitors are able to reduce hypothalamic IL-1β levels during stress responses, which contribute to pro-inflammatory effects [31].
Due to MDD-related factors, glucocorticoids have some indirect interactions with inflammation. For example, in states of chronic stress, glucocorticoids can affect the sympathetic nervous system [32], which can influence the immune response [33]. Such an interaction would be another bridge that connects the HPAA and the immune system. Clinical use of adrenergic receptor inhibitors such as propranolol reduces the unstable psychological symptoms caused by stress [33]. Therefore, it is difficult to dispute the role of glucocorticoids in the development of MDD, and more research is needed in this area. Besides, mineralocorticoids (MRs), secreted by the adrenal glomerular zone, maintain the balance of water and electrolytes in the human body. The renin-angiotensin-aldosterone system mainly regulates blood pressure, causing vascular smooth muscle contraction as well as the retention of water and sodium [34]. Previous studies have suggested that MRs are also important stress modulators, which influence stress-induced HPAA up-regulation, the appraisal of stress, and fearful memories [35]. Evidence has demonstrated that genetic variations in GRs and MRs are able to predict cognitive function in MDD, where GR genetic variation is associated with changes in attention and work-related memory, and MR genetic variation with changes in verbal memory [36].
As noted above, up-regulated levels of glucocorticoid play a driving role in inflammation, and over-activation of HPAA may be a key factor. Despite these increases in cortisol levels, the critical issue remains the decrease of glucocorticoid signaling. This hypothesis may explain some complex and perhaps more debated results of MDD treatment, including the efficacy of glucocorticoid antagonist treatment. Furthermore, GR resistance has been shown to be more predictive of depressive outcomes than hypercortisolemia [37]. Notwithstanding, it remains a challenge to study the effects of hypercortisolemia and GR resistance separately [38].
HPAA, Sex Differences and MDD
There are sex differences related to HPAA in MDD patients [39]. Young et al. have reported that depressed women manifest more disorderly secretion of ACTH. This study indicated that hormonal rhythm abnormalities do occur in depressed female patients and are associated with accentuated ACTH feedforward drive [40]. A recent study found that, after in vivo glucocorticoid stimulation, the higher GR sensitivity of healthy women was absent in depressed female patients. This suggested that sex-related differences in the regulation of HPAA may contribute to the vulnerability of females to the development of depression [41]. In the acute phase of stress, females appear to have a stronger positive correlation between cortisol levels and cognitive representation than males [42]. Serum cortisol levels are significantly higher in female patients [39]. Moreover, in first-episode MDD patients, the cortisol levels of women are higher than those in relapsed patients and healthy controls [43]. A study of chronic stress and the HPAA found that individuals who had suffered maltreatment in childhood also presented with sex differences in HPAA function: the cortisol activation response in females was significantly slower than in males [44].
Hypothalamic-Pituitary-Thyroid Axis and MDD
Thyroid Hormones and MDD
Thyroid hormones are important for metabolism and the growth and development of the nervous system. Lack of thyroid hormone in childhood may cause permanent brain damage, and can affect brain function in adulthood as well. Much clinical evidence has demonstrated that aberrant levels of thyroid hormone are associated with cognitive and emotional dysfunction. There are clearly overlapping symptoms between hypothyroidism and depression, such as fatigue, weight change, and depressive mood [45]. After release into the blood, thyrotropin (thyroid stimulating hormone, TSH) binds to the TSH receptor in the thyroid gland, resulting in the secretion of triiodothyronine (T3) and thyroxine (T4). Negative feedback in the HPTA results in elevated thyroid hormones, which inhibit pituitary gland release of TSH.
Several studies have endeavored to associate MDD with subclinical hypothyroidism, and attempts to use thyroid hormones as antidepressants have achieved clinical efficacy [46]. Moreover, it has been shown that T3 shortens the time to onset of tricyclic antidepressants [47]; a review of the guidelines of the British Association of Psychopharmacology found that MDD patients who had no response to first-line antidepressants could achieve treatment efficacy after the addition of highly bioactive T3 as a potentiator. The antidepressant effect of T3 augmentation of selective serotonin reuptake inhibitors is correlated with significant changes in the bioenergetic metabolism of the brain [48]. Similarly, T4 (with a relatively lower bioactivity) also has a potential synergistic effect on treatment-resistant MDD [49]. In a large-sample MDD treatment study with 1,410 MDD patients and 204 thyroid dysfunction patients, 60.64% of patients received combination therapy or synergistic therapy. Among all the risk factors, the regression coefficient of thyroid dysfunction and MDD was 0.74 (odd ratio = 2.1) [50]. A relationship has been reported between hypothyroidism and hyperlipidemia and other cardiovascular risk factors [51], as well as the aggravation of depression and anxiety, but there are also studies presenting no correlation between them [52, 53]. TSH showed a delayed response to thyrotropin-releasing hormone (TRH) only in several MDD patients who were in a depressive episode [52]. Furthermore, some studies have demonstrated that age and TSH are not factors associated with MDD, but that MDD severity might only be related to stress from life events [54]. Elucidation of the exact relationship between MDD and the HPTA requires further studies with larger sample sizes.
HPTA, Sex Differences, and MDD
From another perspective, thyroid-related diseases in women have an incidence 5–20 times greater than in men [55]. At certain physiological periods such as pregnancy, HPTA function becomes altered. The increase of estrogen during this period results in increases in thyroxine-binding globulin (TBG) as high as 150% [56]. TBG concentration may be a sensitive indicator of estrogen level, and emotional instability in postpartum MDD is also likely to be associated with gonadal and thyroid hormone concentrations [57]. Recent studies have established models in pregnant and non-pregnant women, suggesting that pregnancy factors result in decreases in TSH and free T4 (FT4) levels. However, a negative correlation between TSH and FT4 induced by the negative feedback of the physiological axis could not be established in the third trimester of pregnancy [58, 59], which is considered to be precisely the highest-risk period for female MDD. As such, decreases in T4 and FT4 levels are associated with perinatal depression [60]. Significant physiological fluctuations of thyroid hormones in prenatal and postpartum subjects provide new perspectives to study the neuroendocrine aspects of MDD.
The perimenopausal period is also significant, in which the HPTA also presents changes [55]. Morbidity of thyroid-related diseases often increases with age, including that from autoimmune thyroiditis, hypothyroidism, nodular goiter, and thyroid tumors. They are more common in older women, presenting with symptoms such as anxiety, palpitations, sweating, sudden changes in weight, and insomnia. Both thyroid and ovarian dysfunctions are able to cause these symptoms.
Hypothalamic-Pituitary-Gonadal Axis and MDD
HPGA and Reproductive Depression
Correlation studies of female sexual dysfunction and the HPGA in MDD are very limited due to the complexity involved, and females often have significant physiological fluctuations of hormone levels during the menstrual cycle. Previous studies have shown that a sudden decrease of reproductive hormones may result in an abnormal state of mood [61]. This axis is also called the hypothalamic-pituitary-ovarian axis (HPOA) in women. Sexual dysfunction can have adverse effects on the quality of life, including psychological, biological, and sociological [62]. Some factors of sexual dysfunction have been identified, such as age, metabolic syndromes, diabetes, drug effects, mental health, behavioral disorders, intrauterine devices, and the adrenal and thyroid diseases described above, among others [63–67].
The prevalence of MDD in women is almost double that in men [68]. It is undetermined if this phenomenon results from environmental, social, or hormonal factors [69]. The impact of immune exposure on depression can be assessed by decreases in gonadal hormones during specific situations [68], such as in premenstrual syndrome (PMS), premenstrual dysphoric disorder (PMDD), and postpartum depression (PPD). The menopausal transition in perimenopausal depression (PMD) is associated with profound changes in reproductive hormones [70].
Before menstruation, many women may experience irritability or other psychological and behavioral changes, and these symptoms may remit suddenly after menstruation. PMS includes a series of mental and physical symptoms that occur in the premenstrual period in women of childbearing age, and symptoms generally cease with or after menstruation. About 50–80% of females experience mild PMS, while 20% of them require pharmacological therapy [71, 72]. The clinical definition of PMS is that 7–14 days before menstruation, patients experience mental symptoms including irritability, mental lethargy, inattention, and stress, as well as symptoms of physical discomfort such as insomnia, headache, fatigue, weakness, breast pain, diarrhea, edema, and paresthesia during the luteal phase. Some of these severe symptoms are analogous to that found in the International Classification of Diseases (10th revision) criteria of MDD with the characteristic of periodic episodes. PMS is commonly found in women 30 to 40 years old; the typical course begins at the start of the luteal phase (around 1 week before menstruation) when symptoms gradually worsen and become most severe 2–3 days before menstruation, then suddenly disappear with menstruation.
There are numerous theories attempting to explain the high prevalence of MDD in women, particularly from psychosocial and biological aspects. Among the biological factors, gonadal hormones play an important role. It has been found that estrogen and progesterone can affect the circadian rhythm [73], neurotransmission, and neuroendocrine function in mood disorders. Certain states can bring about dramatic fluctuations in gonadal hormone levels, which influence the HPGA feedback loop in PPD [74] and PMD [75].
These subtypes of depression have been categorized under reproductive depression, being derived from fluctuations in endocrine hormones rather than by primary classification under antidepressant treatment response; reproductive depression includes cyclic depression, PPD, and PMD. Interestingly, severe PMDD cases occurring before pregnancy in patients with reproductive depression is rare, but with age, perinatal period depression gradually increases and recurs frequently weeks or months after delivery; such outcomes often evolve into premenstrual depression [76]. In cases of ineffective MDD treatment, recurrence of MDD in female patients is characterized by treatment resistance to multiple antidepressants. A retrospective study found that in refractory female MDD patients, symptoms disappear several years before the last pregnancy [77]. Reproductive depression can be alleviated or cease following the end of menopause. Estrogen has no therapeutic effect on senile MDD patients with or without adjunctive progestogen [78], however it can improve vasomotor symptoms, sleep quality, and pain and dryness during sexual intercourse caused by vaginal atrophy. The pathological basis of reproductive depression is unclear; it is presumably related to neurotransmitters, neuroendocrine function, genetic factors, psychosocial factors, and the interactions between them.
Sex differences also play a role in the lifetime prevalence of MDD. Due to accelerated gonadal differentiation and development after puberty, females with MDD may suffer more changeable symptoms following the menstrual cycle. The clinical manifestations and signs of MDD in females differ from those in males of the same age, including irritability, anxiety, and physical complaints [79]. Atypical MDD symptoms, such as excessive appetite, weight gain, hypersomnia, and atypical reactions to drugs, are also more common in women [80]. A recent study has summarized evidence supporting the importance of sex in modulating responses to rapid-acting antidepressant treatment [81].
Therefore, hormones play a vital role in the emotional symptoms of women. Use of hormonal contraception is associated with subsequent use of antidepressants and a first diagnosis of depression, suggesting that depression is a potential adverse effect of hormonal contraceptive use [82]. Female bipolar disorder patients have a high risk of postpartum depression [83]. Reproductive depression is characterized by periodicity, thus it is commonly misdiagnosed as bipolar disorder [83]; these misdiagnosed patients might receive mood stabilizers, antidepressants, or modified electroconvulsive therapy [84]. According to preliminary statistics, 67% of female patients with bipolar disorder develop PPD [85]; associations might include family history, genetic susceptibility, and other precipitating factors. Another viewpoint is that, with profound reproductive hormonal up-regulation during pregnancy and lactation, long-time cyclic depression can result from the rapid withdrawal of gonadal steroids, not remitting until the new menstrual cycle is established. Sex hormones interact with the neurotransmission of serotonin and gamma-aminobutyric acid (GABA) [86], both of which have close connections with mood disorders.
Reliable evidence that supports the theory of sex-related depression has been summarized as the following [84]: (1) cyclic depression; (2) premenstrual symptoms that have already occurred in puberty; (3) a stable mental state before pregnancy; (4) depressive syndrome within the postpartum period; (5) significant mood relief during intervals between episodes of depression; (6) several years of recurrent depression without manic episodes; (7) coexistence of periodic physical complaints with depressive symptoms such as breast pain, bloating and headache; and (8) emotional symptoms associated with oral contraceptives.
Treatment of MDD with Sex Hormones
It is worth discussing whether these symptoms are induced by abnormalities of hormone levels or by abnormal responses to normal levels. This has also caused controversy in the fields of psychiatry and endocrinology as to whether such changes mean individual or intergroup differences. It is noteworthy that the US FDA approved the first drug for treatment of PPD in 2019 [87]. Allopregnanolone (also named tetrahydroprogesterone, THP) is a derivative of progesterone, which is the most potent of the progesterone metabolites. The potential role of allopregnanolone is that it could act as a safe and effective antidepressant therapy by its function of modulating GABAA function, which can correct postpartum dysregulation of the GABAA receptor and rebalance the activity of the GABAA and N-methyl-D-aspartate receptors [88]. Besides, progesterone modulates the concentration of tryptophan hydroxylase through the expression of 5α-reductase and 3α-hydroxysteroid dehydrogenase. It also contributes to suppression of neuronal excitability, providing neuroprotection, and promoting neurogenesis and neural restoration. However, there are negative effects such as anxiety, and aggression. Such contradictory results have dose-symptom curve dependence on THP, and also takes on individual differences. THP have biphasic effects, in that low concentrations increase an adverse, anxiogenic effect whereas higher concentrations decrease this effect and show beneficial, calming properties [89]. In gonadal steroid-withdrawn postpartum female rats, inescapable stress leads to a loss of hippocampal spine synapses, which is associated with poor escape performance [90]. THP can also increase the concentration of brain-derived neurotrophic factor, promoting hippocampal neuronal regeneration and improving depressive symptoms [91].
Correlations Between Female Hypothalamic-Pituitary-Glandular Axis (HPTA, HPAA, HPGA) Hormone Levels
Indeed, it is still hard to study the effects of hormones on MDD separately, especially when the gender variable is considered, so analyzing the correlation between hormones in these axes of females is needed.
In MDD, corticosteroids may interact with T3 and T4, and depressed mood is often correlated with hypothyroidism. Although the thyroid function of patients with MDD might show no abnormality, a relative hypothyroidism can exist in the CNS. At the downstream glandular level, T4 can be converted into T3 via type II 5'-deiodinase. In patients with MDD, cortisol may inhibit this enzyme, but conversely enhance the process of reverse T3 production [92]. At the midstream pituitary level, HPAA activation is accompanied by chronic elevation of cortisol which impairs the release of TSH by TRH, resulting in declining thyroid function as well [93].
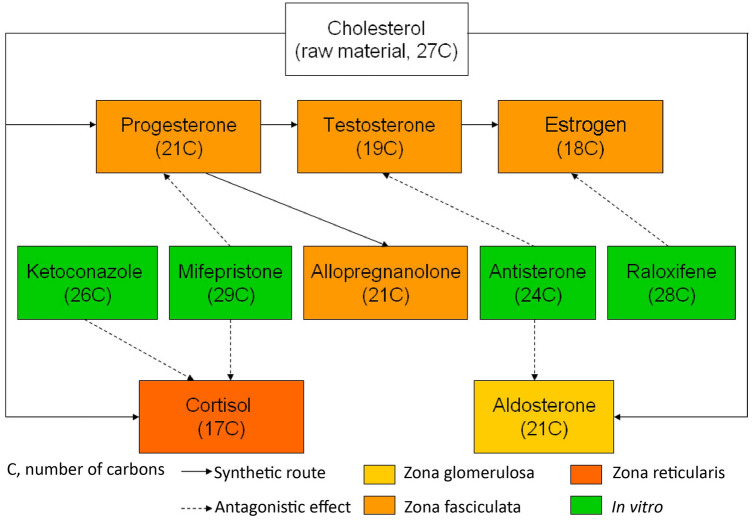
After binding to the intracellular progesterone receptor (PR), progesterone changes its configuration and form a dimer with then released heat shock protein (HSP), subsequently binding to the relevant hormone response factor in DNA. After the downstream transcription activator factor is activated, proteins are synthesized via mRNA and later on generate hormone-related biological effects. Mifepristone has a higher PR affinity than progesterone, and the mifepristone-receptor-HSP complex is more stable and shows a stronger affinity for nuclear proteins; however, mifepristone cannot act on DNA. It can competitively block the biological activity of progesterone, achieving its anti-progesterone effect. This drug also possesses another characteristic, which is its competitive action on GRs. Hence, mifepristone is a dual antagonist of the GR and PR. Such a similarity demonstrates possible associations between the HPG and HPA axes. The antidepressant effects of allopregnanolone are not only brought about by positively modulating the GABAA receptor, but also negatively modulating HPAA activity. Chronic stress in MDD down-regulates the expression of the rate-limiting enzyme 5α-reductase in the synthesis of THP. The decrease in THP levels results in decreased regulation of the HPAA, causing an increased level of cortisol and weakening nerve regeneration [94]. In addition, spironolactone, an aldosterone antagonist, has diuretic and antihypertensive effects. However, its side-effects include male breast development, which suggests that it may have anti-androgenic effects, leading to an imbalance between estrogen and androgens. This phenomenon might be due to the similar structural chemistry of steroid hormones (Fig. 2).
Fig. 2.
Steroid hormones: number of carbon atoms, locations and pathways of synthesis, and antagonistic interactions (including some produced in vitro).
Hypercortisolism resulting from HPAA imbalance is closely connected to affective disorders. Recent studies have found that mood disorders can be treated effectively with GR antagonists, among which mifepristone is the most commonly used. It is able to treat psychotic depression by GR antagonism [95]. The efficacy of mifepristone indicates that an imbalance in glucocorticoid homeostasis may exist in MDD patients. Effective antidepressant treatment can restore normal glucocorticoid levels. The finding of relationships between antidepressant action and HPAA regulation leads to the conclusion that the disruption of HPAA may be a contributing factor to depression, rather than other biological abnormalities [96]. At present, mifepristone is another potential new antidepressant drug undergoing clinical trials. Early in 2006, the efficacy of mifepristone in patients with depression was reported, especially in those with psychotic symptoms [97]. Previous studies also indicated that mifepristone could alleviate the stress response in depressed patients by inhibiting the excessive activation of GR, thereby improving cognitive function and alleviating MDD symptoms [98].
Studies have reported a therapeutic effect of estrogen on postpartum and menopausal depression, and sequential hormonal therapy with estrogen and progesterone can improve nervousness, irritability, and depressive symptoms, especially with low doses of estrogen [99]. In clinical practice, it is difficult to acknowledge a link between the improvement of symptoms and changing levels of estrogen and progesterone, because progesterone cannot carry out its biological role in the absence of estrogen, similar to the permissive action of glucocorticoids. Nonetheless, observations made during single estrogen replacement therapy in PMD patients suggest that estrogen does not show correlated effects [78], while the estrogen receptor antagonist raloxifene does not seem to cause any depressive adverse reactions [100]. All the evidence suggests that hormones and mood are not simply linearly correlated, but have a more extensive and complicated relationship.
An imbalance in the HPAA may be caused by the accumulation of menstrual cycle-related disorders. When adapting to pressure, critical stressors correlated to menstrual cycles constitute a series of repeated stimuli. If these stimuli persist, repeated injury to the psychological coping mechanism can occur, inducing HPAA dysregulation. The cortisol synthesis inhibitor ketoconazole promotes hippocampal function in MDD patients; it was unexpectedly found that this drug improves the prognosis of MDD [98]. No studies have shown a significant correlation between cortisol levels and the menstrual cycle [101, 102]. There is also a lack of evidence for differences in cortisol dysregulation between patients with PMS and PMDD, and healthy controls [103]; the reason might be that various points were not elaborated systematically. In current models, the relationship between female periodic depressive symptoms and HPAA function may depend on particular critical stressors and the extent of disease prognosis. This connection might have been established during sensitive periods of human development. Long-term data on the stability of recurrence is required.
Perspectives
With respect to molecular structure, sex hormones and glucocorticoids both belong to the neurosteroid class, in that they are more similar in structure than with thyroid hormones. Their differences include the number of carbon atoms, benzene ring stability, and mirror image isomerism (Fig. 3). Affected by the concentration of these hormones, non-specific receptors may produce different biological effects; these effects could be more closely related than previously thought, and this may better explain the high morbidity of neuroendocrine diseases in women. Through the perspective of new drug developments based on the hormone levels and hormonal changes of these three axes, individual differences in female MDD patients become obvious. Phenotypic differences among individuals might be determined by genetic factors, but the main factors are supposed to consist of the relevant hormone receptors and environment-related epigenetic factors. The molecular structure of hormones is consistent across individuals, but differences can arise in receptors and hormone-receptor-protein complexes, leading to incongruence among biological factors of depression. Different responses to these hormones should be the core element of pathological theory and research.
Fig. 3.
Downstream hormones of the HPA, HPG, and HPT axes and the similarity of cholesterol to its derivatives in the HPAA and HPGA. HPA, hypothalamic-pituitary-adrenal; HPG, hypothalamic-pituitary-gonadal; HPT, hypothalamic-pituitary-thyroidal.
Acknowledgements
We thank Yueyin Pan for image processing. This review was supported by the National Key Research and Development Program of China (2016YFC1307100, 2016YFC1307105), the National Natural Science Foundation of China (81771465, 81930033), the National Key Technologies R&D Program of China (2012BAI01B04), Shanghai Key Project of Science and Technology (2018SHZDZX05), the Sanming Project of Medicine in Shenzheng (SZSM201612006), Shanghai Key Medicine Specialties Program (ZK2019A06), Shanghai Clinical Research Center for Mental Health (SCRC-MH, 19MC1911100), the Special Project for Clinical Research in Health Industry of Shanghai Municipal Health Commission (20204Y0025), the Innovative Research Team of High-level Local Universities in Shanghai, and the National Health System “Good Doctor” Construction Project of Yangpu District of Shanghai Municipality (2020–2023).
Conflict of interest
The authors declare that they have no conflicts of interest.
Footnotes
Yuncheng Zhu and Xiaohui Wu contributed equally to this work.
Contributor Information
Fang Wang, Email: wangfangpsychiatry@163.com.
Yiru Fang, Email: yirufang@aliyun.com.
References
- 1.Lee EH, Han PL. Reciprocal interactions across and within multiple levels of monoamine and cortico-limbic systems in stress-induced depression: A systematic review. Neurosci Biobehav Rev. 2019;101:13–31. doi: 10.1016/j.neubiorev.2019.03.014. [DOI] [PubMed] [Google Scholar]
- 2.Liang X, Zhu Y, Fang Y. COVID-19 and post-traumatic stress disorder: A vicious circle involving immunosuppression. CNS Neurosci Ther. 2020;26:876–878. doi: 10.1111/cns.13431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohen S, Janicki-Deverts D, Miller GE. Psychological stress and disease. JAMA. 2007;298:1685–1687. doi: 10.1001/jama.298.14.1685. [DOI] [PubMed] [Google Scholar]
- 4.Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009;65:732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zunszain PA, Hepgul N, Pariante CM. Inflammation and depression. Curr Top Behav Neurosci. 2013;14:135–151. doi: 10.1007/7854_2012_211. [DOI] [PubMed] [Google Scholar]
- 6.Wang F, Jin J, Wang J, He R, Li K, Hu X, et al. Association between olfactory function and inhibition of emotional competing distractors in major depressive disorder. Sci Rep. 2020;10:6322. doi: 10.1038/s41598-020-63416-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pace TW, Miller AH. Cytokines and glucocorticoid receptor signaling. Relevance to major depression. Ann N Y Acad Sci 2009, 1179: 86–105. [DOI] [PMC free article] [PubMed]
- 9.Jeon SW, Kim YK. The role of neuroinflammation and neurovascular dysfunction in major depressive disorder. J Inflamm Res. 2018;11:179–192. doi: 10.2147/JIR.S141033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Horowitz MA, Zunszain PA. Neuroimmune and neuroendocrine abnormalities in depression: two sides of the same coin. Ann N Y Acad Sci. 2015;1351:68–79. doi: 10.1111/nyas.12781. [DOI] [PubMed] [Google Scholar]
- 11.Bale TL. Neuroendocrine and immune influences on the CNS: it's a matter of sex. Neuron. 2009;64:13–16. doi: 10.1016/j.neuron.2009.09.036. [DOI] [PubMed] [Google Scholar]
- 12.Zhu Y, Ji H, Tao L, Cai Q, Wang F, Ji W, et al. Functional status of hypothalamic–pituitary–thyroid and hypothalamic–pituitary–adrenal axes in hospitalized schizophrenics in Shanghai. Front Psychiatry. 2020;11:65. doi: 10.3389/fpsyt.2020.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Han Y, Ji H, Liu L, Zhu Y, Jiang X. The relationship of functional status of cortisol, testosterone, and parameters of metabolic syndrome in male schizophrenics. Biomed Res Int. 2020;2020:9124520. doi: 10.1155/2020/9124520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Malhi GS, Mann JJ. Depression. Lancet. 2018;392:2299–2312. doi: 10.1016/S0140-6736(18)31948-2. [DOI] [PubMed] [Google Scholar]
- 15.Chen X, Zheng X, Ding Z, Su Y, Wang S, Cui B, et al. Relationship of gender and age on thyroid hormone parameters in a large Chinese population. Arch Endocrinol Metab. 2020;64:52–58. doi: 10.20945/2359-3997000000179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Honour JW. Biochemistry of the menopause. Ann Clin Biochem. 2018;55:18–33. doi: 10.1177/0004563217739930. [DOI] [PubMed] [Google Scholar]
- 17.Kudielka BM, Buske-Kirschbaum A, Hellhammer DH, Kirschbaum C. HPA axis responses to laboratory psychosocial stress in healthy elderly adults, younger adults, and children: impact of age and gender. Psychoneuroendocrinology. 2004;29:83–98. doi: 10.1016/S0306-4530(02)00146-4. [DOI] [PubMed] [Google Scholar]
- 18.Pariante CM, Lightman SL. The HPA axis in major depression: classical theories and new developments. Trends Neurosci. 2008;31:464–468. doi: 10.1016/j.tins.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 19.Pariante CM. Risk factors for development of depression and psychosis. Glucocorticoid receptors and pituitary implications for treatment with antidepressant and glucocorticoids. Ann N Y Acad Sci 2009, 1179: 144–152. [DOI] [PMC free article] [PubMed]
- 20.Burke HM, Davis MC, Otte C, Mohr DC. Depression and cortisol responses to psychological stress: a meta-analysis. Psychoneuroendocrinology. 2005;30:846–856. doi: 10.1016/j.psyneuen.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 21.Wang SS, Mu RH, Li CF, Dong SQ, Geng D, Liu Q, et al. microRNA-124 targets glucocorticoid receptor and is involved in depression-like behaviors. Prog Neuropsychopharmacol Biol Psychiatry. 2017;79:417–425. doi: 10.1016/j.pnpbp.2017.07.024. [DOI] [PubMed] [Google Scholar]
- 22.Pariante CM, Miller AH. Glucocorticoid receptors in major depression: relevance to pathophysiology and treatment. Biol Psychiatry. 2001;49:391–404. doi: 10.1016/S0006-3223(00)01088-X. [DOI] [PubMed] [Google Scholar]
- 23.Bekhbat M, Rowson SA, Neigh GN. Checks and balances: The glucocorticoid receptor and NFkB in good times and bad. Front Neuroendocrinol. 2017;46:15–31. doi: 10.1016/j.yfrne.2017.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee DY, Kim E, Choi MH. Technical and clinical aspects of cortisol as a biochemical marker of chronic stress. Bmb Reports. 2015;48:209–216. doi: 10.5483/BMBRep.2015.48.4.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Makhija K, Karunakaran S. The role of inflammatory cytokines on the aetiopathogenesis of depression. Aust N Z J Psychiatry. 2013;47:828–839. doi: 10.1177/0004867413488220. [DOI] [PubMed] [Google Scholar]
- 26.Niu Z, Yang L, Wu X, Zhu Y, Chen J, Fang Y. The relationship between neuroimmunity and bipolar disorder: Mechanism and translational application. Neurosci Bull. 2019;35:595–607. doi: 10.1007/s12264-019-00403-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Silverman MN, Sternberg EM. Glucocorticoid regulation of inflammation and its functional correlates: from HPA axis to glucocorticoid receptor dysfunction. Ann N Y Acad Sci. 2012;1261:55–63. doi: 10.1111/j.1749-6632.2012.06633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shelton MM, Schminkey DL, Groer MW. Relationships among prenatal depression, plasma cortisol, and inflammatory cytokines. Biol Res Nurs. 2015;17:295–302. doi: 10.1177/1099800414543821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frank MG, Watkins LR, Maier SF. Stress-induced glucocorticoids as a neuroendocrine alarm signal of danger. Brain Behav Immun. 2013;33:1–6. doi: 10.1016/j.bbi.2013.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tynan RJ, Naicker S, Hinwood M, Nalivaiko E, Buller KM, Pow DV, et al. Chronic stress alters the density and morphology of microglia in a subset of stress-responsive brain regions. Brain Behav Immun. 2010;24:1058–1068. doi: 10.1016/j.bbi.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 31.Blandino P, Jr, Barnum CJ, Deak T. The involvement of norepinephrine and microglia in hypothalamic and splenic IL-1beta responses to stress. J Neuroimmunol. 2006;173:87–95. doi: 10.1016/j.jneuroim.2005.11.021. [DOI] [PubMed] [Google Scholar]
- 32.Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci. 2009;10:397–409. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walker FR, Nilsson M, Jones K. Acute and chronic stress-induced disturbances of microglial plasticity, phenotype and function. Curr Drug Targets. 2013;14:1262–1276. doi: 10.2174/13894501113149990208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alt SR, Turner JD, Klok MD, Meijer OC, Lakke EA, Derijk RH, et al. Differential expression of glucocorticoid receptor transcripts in major depressive disorder is not epigenetically programmed. Psychoneuroendocrinology. 2010;35:544–556. doi: 10.1016/j.psyneuen.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 35.ter Heegde F, De Rijk RH, Vinkers CH. The brain mineralocorticoid receptor and stress resilience. Psychoneuroendocrinology. 2015;52:92–110. doi: 10.1016/j.psyneuen.2014.10.022. [DOI] [PubMed] [Google Scholar]
- 36.Keller J, Gomez R, Williams G, Lembke A, Lazzeroni L, Murphy GM, Jr, et al. HPA axis in major depression: cortisol, clinical symptomatology and genetic variation predict cognition. Mol Psychiatry. 2017;22:527–536. doi: 10.1038/mp.2016.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Raison CL, Miller AH. When not enough is too much: the role of insufficient glucocorticoid signaling in the pathophysiology of stress-related disorders. Am J Psychiatry. 2003;160:1554–1565. doi: 10.1176/appi.ajp.160.9.1554. [DOI] [PubMed] [Google Scholar]
- 38.Anacker C, Zunszain PA, Carvalho LA, Pariante CM. The glucocorticoid receptor: pivot of depression and of antidepressant treatment? Psychoneuroendocrinology. 2011;36:415–425. doi: 10.1016/j.psyneuen.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pochigaeva K, Druzhkova T, Yakovlev A, Onufriev M, Grishkina M, Chepelev A, et al. Hair cortisol as a marker of hypothalamic-pituitary-adrenal axis activity in female patients with major depressive disorder. Metab Brain Dis. 2017;32:577–583. doi: 10.1007/s11011-017-9952-0. [DOI] [PubMed] [Google Scholar]
- 40.Young EA, Veldhuis JD. Disordered adrenocorticotropin secretion in women with major depression. J Clin Endocrinol Metab. 2006;91:1924–1928. doi: 10.1210/jc.2005-2397. [DOI] [PubMed] [Google Scholar]
- 41.Rampp C, Eichelkraut A, Best J, Czamara D, Rex-Haffner M, Uhr M, et al. Sex-related differential response to dexamethasone in endocrine and immune measures in depressed in-patients and healthy controls. J Psychiatr Res. 2018;98:107–115. doi: 10.1016/j.jpsychires.2017.12.020. [DOI] [PubMed] [Google Scholar]
- 42.Roos LE, Beauchamp KG, Giuliano R, Zalewski M, Kim HK, Fisher PA. Children's biological responsivity to acute stress predicts concurrent cognitive performance. Stress. 2018;21:347–354. doi: 10.1080/10253890.2018.1458087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wei J, Sun G, Zhao L, Yang X, Liu X, Lin D, et al. Analysis of hair cortisol level in first-episodic and recurrent female patients with depression compared to healthy controls. J Affect Disord. 2015;175:299–302. doi: 10.1016/j.jad.2015.01.023. [DOI] [PubMed] [Google Scholar]
- 44.Kaess M, Whittle S, O'Brien-Simpson L, Allen NB, Simmons JG. Childhood maltreatment, pituitary volume and adolescent hypothalamic-pituitary-adrenal axis - Evidence for a maltreatment-related attenuation. Psychoneuroendocrinology. 2018;98:39–45. doi: 10.1016/j.psyneuen.2018.08.004. [DOI] [PubMed] [Google Scholar]
- 45.Zhu Y, Wu Z, Sie O, Cai Y, Huang J, Liu H, et al. Causes of drug discontinuation in patients with major depressive disorder in China. Prog Neuropsychopharmacol Biol Psychiatry. 2020;96:109755. doi: 10.1016/j.pnpbp.2019.109755. [DOI] [PubMed] [Google Scholar]
- 46.Fischer S, Ehlert U. Hypothalamic-pituitary-thyroid (HPT) axis functioning in anxiety disorders. A systematic review. Depress Anxiety. 2018;35:98–110. doi: 10.1002/da.22692. [DOI] [PubMed] [Google Scholar]
- 47.Cleare A, Pariante CM, Young AH, Anderson IM, Christmas D, Cowen PJ, et al. Evidence-based guidelines for treating depressive disorders with antidepressants: A revision of the 2008 British Association for Psychopharmacology guidelines. J Psychopharmacol. 2015;29:459–525. doi: 10.1177/0269881115581093. [DOI] [PubMed] [Google Scholar]
- 48.Iosifescu DV, Bolo NR, Nierenberg AA, Jensen JE, Fava M, Renshaw PF. Brain bioenergetics and response to triiodothyronine augmentation in major depressive disorder. Biol Psychiatry. 2008;63:1127–1134. doi: 10.1016/j.biopsych.2007.11.020. [DOI] [PubMed] [Google Scholar]
- 49.Tundo A, de Filippis R, Proietti L. Pharmacologic approaches to treatment resistant depression: Evidences and personal experience. World J Psychiatry. 2015;5:330–341. doi: 10.5498/wjp.v5.i3.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dold M, Bartova L, Mendlewicz J, Souery D, Serretti A, Porcelli S, et al. Clinical correlates of augmentation/combination treatment strategies in major depressive disorder. Acta Psychiatr Scand. 2018;137:401–412. doi: 10.1111/acps.12870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Martin SS, Daya N, Lutsey PL, Matsushita K, Fretz A, McEvoy JW, et al. Thyroid function, cardiovascular risk factors, and incident atherosclerotic cardiovascular disease: The atherosclerosis risk in communities (ARIC) study. J Clin Endocrinol Metab. 2017;102:3306–3315. doi: 10.1210/jc.2017-00986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hage MP, Azar ST. The link between thyroid function and depression. J Thyroid Res. 2012;2012:590648. doi: 10.1155/2012/590648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Giynas Ayhan M, Uguz F, Askin R, Gonen MS. The prevalence of depression and anxiety disorders in patients with euthyroid Hashimoto's thyroiditis: a comparative study. Gen Hosp Psychiatry. 2014;36:95–98. doi: 10.1016/j.genhosppsych.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 54.Saidi S, Iliani Jaafar SN, Daud A, Musa R, Nik Ahmad NNF. Relationship between levels of thyroid stimulating hormone, age, and gender, with symptoms of depression among patients with thyroid disorders as measured by the Depression Anxiety Stress Scale 21 (DASS-21) Enferm Clin. 2018;28(Suppl 1):180–183. doi: 10.1016/S1130-8621(18)30063-9. [DOI] [Google Scholar]
- 55.Gietka-Czernel M. The thyroid gland in postmenopausal women: physiology and diseases. Prz Menopauzalny. 2017;16:33–37. doi: 10.5114/pm.2017.68588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gaberscek S, Zaletel K. Thyroid physiology and autoimmunity in pregnancy and after delivery. Expert Rev Clin Immunol 2011, 7: 697–706; quiz 707. [DOI] [PubMed]
- 57.Pedersen C, Leserman J, Garcia N, Stansbury M, Meltzer-Brody S, Johnson J. Late pregnancy thyroid-binding globulin predicts perinatal depression. Psychoneuroendocrinology. 2016;65:84–93. doi: 10.1016/j.psyneuen.2015.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kurioka H, Takahashi K, Miyazaki K. Maternal thyroid function during pregnancy and puerperal period. Endocr J. 2005;52:587–591. doi: 10.1507/endocrj.52.587. [DOI] [PubMed] [Google Scholar]
- 59.Jonklaas J, Kahric-Janicic N, Soldin OP, Soldin SJ. Correlations of free thyroid hormones measured by tandem mass spectrometry and immunoassay with thyroid-stimulating hormone across 4 patient populations. Clin Chem. 2009;55:1380–1388. doi: 10.1373/clinchem.2008.118752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pedersen CA, Johnson JL, Silva S, Bunevicius R, Meltzer-Brody S, Hamer RM, et al. Antenatal thyroid correlates of postpartum depression. Psychoneuroendocrinology. 2007;32:235–245. doi: 10.1016/j.psyneuen.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 61.Rubinow DR, Schmidt PJ. Sex differences and the neurobiology of affective disorders. Neuropsychopharmacology. 2019;44:111–128. doi: 10.1038/s41386-018-0148-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Barut MU, Coksuer H, Sak S, Bozkurt M, Agacayak E, Hamurcu U, et al. Evaluation of sexual function in women with hypogonadotropic hypogonadism using the female sexual function index (FSFI) and the beck depression inventory (BDI) Med Sci Monit. 2018;24:5610–5618. doi: 10.12659/MSM.910304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sakinci M, Ercan CM, Olgan S, Coksuer H, Karasahin KE, Kuru O. Comparative analysis of copper intrauterine device impact on female sexual dysfunction subtypes. Taiwan J Obstet Gynecol. 2016;55:460–461. doi: 10.1016/j.tjog.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 64.Basson R, Rees P, Wang R, Montejo AL, Incrocci L. Sexual function in chronic illness. J Sex Med. 2010;7:374–388. doi: 10.1111/j.1743-6109.2009.01621.x. [DOI] [PubMed] [Google Scholar]
- 65.Berman JR, Bassuk J. Physiology and pathophysiology of female sexual function and dysfunction. World J Urol. 2002;20:111–118. doi: 10.1007/s00345-002-0281-4. [DOI] [PubMed] [Google Scholar]
- 66.Atis G, Dalkilinc A, Altuntas Y, Atis A, Caskurlu T, Ergenekon E. Sexual dysfunction in women with clinical hypothyroidism and subclinical hypothyroidism. J Sex Med. 2010;7:2583–2590. doi: 10.1111/j.1743-6109.2010.01815.x. [DOI] [PubMed] [Google Scholar]
- 67.Bhasin S, Enzlin P, Coviello A, Basson R. Sexual dysfunction in men and women with endocrine disorders. Lancet. 2007;369:597–611. doi: 10.1016/S0140-6736(07)60280-3. [DOI] [PubMed] [Google Scholar]
- 68.Goldstein JM, Hale T, Foster SL, Tobet SA, Handa RJ. Sex differences in major depression and comorbidity of cardiometabolic disorders: impact of prenatal stress and immune exposures. Neuropsychopharmacology. 2019;44:59–70. doi: 10.1038/s41386-018-0146-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Studd J. Personal view: Hormones and depression in women. Climacteric. 2015;18:3–5. doi: 10.3109/13697137.2014.918595. [DOI] [PubMed] [Google Scholar]
- 70.Santoro N. Perimenopause: From research to practice. J Womens Health (Larchmt) 2016;25:332–339. doi: 10.1089/jwh.2015.5556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Naheed B, Kuiper JH, Uthman OA, O'Mahony F, O'Brien PM. Non-contraceptive oestrogen-containing preparations for controlling symptoms of premenstrual syndrome. Cochrane Database Syst Rev 2017, 3: CD010503. [DOI] [PMC free article] [PubMed]
- 72.Biggs WS, Demuth RH. Premenstrual syndrome and premenstrual dysphoric disorder. Am Fam Physician. 2011;84:918–924. [PubMed] [Google Scholar]
- 73.Carrier J, Semba K, Deurveilher S, Drogos L, Cyr-Cronier J, Lord C, et al. Sex differences in age-related changes in the sleep-wake cycle. Front Neuroendocrinol. 2017;47:66–85. doi: 10.1016/j.yfrne.2017.07.004. [DOI] [PubMed] [Google Scholar]
- 74.Schiller CE, Meltzer-Brody S, Rubinow DR. The role of reproductive hormones in postpartum depression. CNS Spectr. 2015;20:48–59. doi: 10.1017/S1092852914000480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gordon JL, Girdler SS, Meltzer-Brody SE, Stika CS, Thurston RC, Clark CT, et al. Ovarian hormone fluctuation, neurosteroids, and HPA axis dysregulation in perimenopausal depression: a novel heuristic model. Am J Psychiatry. 2015;172:227–236. doi: 10.1176/appi.ajp.2014.14070918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Studd J, Nappi RE. Reproductive depression. Gynecol Endocrinol. 2012;28(Suppl 1):42–45. doi: 10.3109/09513590.2012.651932. [DOI] [PubMed] [Google Scholar]
- 77.Studd JW. A guide to the treatment of depression in women by estrogens. Climacteric. 2011;14:637–642. doi: 10.3109/13697137.2011.609285. [DOI] [PubMed] [Google Scholar]
- 78.Whedon JM, KizhakkeVeettil A, Rugo NA, Kieffer KA. Bioidentical estrogen for menopausal depressive symptoms: A systematic review and meta-analysis. J Womens Health (Larchmt) 2017;26:18–28. doi: 10.1089/jwh.2015.5628. [DOI] [PubMed] [Google Scholar]
- 79.Wang F, Wu X, Gao J, Li Y, Zhu Y, Fang Y. The relationship of olfactory function and clinical traits in major depressive disorder. Behav Brain Res. 2020;386:112594. doi: 10.1016/j.bbr.2020.112594. [DOI] [PubMed] [Google Scholar]
- 80.Rodgers S, Vandeleur CL, Ajdacic-Gross V, Aleksandrowicz AA, Strippoli MF, Castelao E, et al. Tracing the associations between sex, the atypical and the combined atypical-melancholic depression subtypes: A path analysis. J Affect Disord. 2016;190:807–818. doi: 10.1016/j.jad.2015.10.067. [DOI] [PubMed] [Google Scholar]
- 81.Herzog DP, Wegener G, Lieb K, Muller MB, Treccani G. Decoding the mechanism of action of rapid-acting antidepressant treatment strategies: Does gender matter? Int J Mol Sci. 2019;20:949. doi: 10.3390/ijms20040949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Skovlund CW, Morch LS, Kessing LV, Lidegaard O. Association of hormonal contraception with depression. JAMA Psychiatry. 2016;73:1154–1162. doi: 10.1001/jamapsychiatry.2016.2387. [DOI] [PubMed] [Google Scholar]
- 83.Sharma V, Al-Farayedhi M, Doobay M, Baczynski C. Should all women with postpartum depression be screened for bipolar disorder? Med Hypotheses. 2018;118:26–28. doi: 10.1016/j.mehy.2018.06.016. [DOI] [PubMed] [Google Scholar]
- 84.Studd J. Spotlight on severe premenstrual syndrome and bipolar disorder: a frequent tragic confusion. Climacteric. 2011;14:602. [PubMed] [Google Scholar]
- 85.Sharma V, Burt VK, Ritchie HL. Assessment and treatment of bipolar II postpartum depression: a review. J Affect Disord. 2010;125:18–26. doi: 10.1016/j.jad.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 86.Barth C, Villringer A, Sacher J. Sex hormones affect neurotransmitters and shape the adult female brain during hormonal transition periods. Front Neurosci. 2015;9:37. doi: 10.3389/fnins.2015.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Frieder A, Fersh M, Hainline R, Deligiannidis KM. Pharmacotherapy of postpartum depression: Current approaches and novel drug development. CNS Drugs. 2019;33:265–282. doi: 10.1007/s40263-019-00605-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Patte-Mensah C, Meyer L, Taleb O, Mensah-Nyagan AG. Potential role of allopregnanolone for a safe and effective therapy of neuropathic pain. Prog Neurobiol. 2014;113:70–78. doi: 10.1016/j.pneurobio.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 89.Andreen L, Nyberg S, Turkmen S, van Wingen G, Fernandez G, Backstrom T. Sex steroid induced negative mood may be explained by the paradoxical effect mediated by GABAA modulators. Psychoneuroendocrinology. 2009;34:1121–1132. doi: 10.1016/j.psyneuen.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 90.Baka J, Csakvari E, Huzian O, Dobos N, Siklos L, Leranth C, et al. Stress induces equivalent remodeling of hippocampal spine synapses in a simulated postpartum environment and in a female rat model of major depression. Neuroscience. 2017;343:384–397. doi: 10.1016/j.neuroscience.2016.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Schule C, Eser D, Baghai TC, Nothdurfter C, Kessler JS, Rupprecht R. Neuroactive steroids in affective disorders: target for novel antidepressant or anxiolytic drugs? Neuroscience. 2011;191:55–77. doi: 10.1016/j.neuroscience.2011.03.025. [DOI] [PubMed] [Google Scholar]
- 92.Brown SB, MacLatchy DL, Hara TJ, Eales JG. Effects of cortisol on aspects of 3,5,3'-triiodo-L-thyronine metabolism in rainbow trout (Oncorhynchus mykiss) Gen Comp Endocrinol. 1991;81:207–216. doi: 10.1016/0016-6480(91)90005-Q. [DOI] [PubMed] [Google Scholar]
- 93.Sahoo M, Subho C. Cortisol hypersecretion in unipolar major depression with melancholic and psychotic features: dopaminergic, noradrenergic and thyroid correlates. Psychoneuroendocrinology 2007, 32: 210; author reply 211–212. [DOI] [PubMed]
- 94.Evans J, Sun Y, McGregor A, Connor B. Allopregnanolone regulates neurogenesis and depressive/anxiety-like behaviour in a social isolation rodent model of chronic stress. Neuropharmacology. 2012;63:1315–1326. doi: 10.1016/j.neuropharm.2012.08.012. [DOI] [PubMed] [Google Scholar]
- 95.Block T, Petrides G, Kushner H, Kalin N, Belanoff J, Schatzberg A. Mifepristone plasma level and glucocorticoid receptor antagonism associated with response in patients with psychotic depression. J Clin Psychopharmacol. 2017;37:505–511. doi: 10.1097/JCP.0000000000000744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mason BL, Pariante CM. The effects of antidepressants on the hypothalamic-pituitary-adrenal axis. Drug News Perspect. 2006;19:603–608. doi: 10.1358/dnp.2006.19.10.1068007. [DOI] [PubMed] [Google Scholar]
- 97.DeBattista C, Belanoff J, Glass S, Khan A, Horne RL, Blasey C, et al. Mifepristone versus placebo in the treatment of psychosis in patients with psychotic major depression. Biol Psychiatry. 2006;60:1343–1349. doi: 10.1016/j.biopsych.2006.05.034. [DOI] [PubMed] [Google Scholar]
- 98.Soria V, Gonzalez-Rodriguez A, Huerta-Ramos E, Usall J, Cobo J, Bioque M, et al. Targeting hypothalamic-pituitary-adrenal axis hormones and sex steroids for improving cognition in major mood disorders and schizophrenia: a systematic review and narrative synthesis. Psychoneuroendocrinology. 2018;93:8–19. doi: 10.1016/j.psyneuen.2018.04.012. [DOI] [PubMed] [Google Scholar]
- 99.Bjorn I, Sundstrom-Poromaa I, Bixo M, Nyberg S, Backstrom G, Backstrom T. Increase of estrogen dose deteriorates mood during progestin phase in sequential hormonal therapy. J Clin Endocrinol Metab. 2003;88:2026–2030. doi: 10.1210/jc.2002-020755. [DOI] [PubMed] [Google Scholar]
- 100.Khorsand I, Kashef R, Ghazanfarpour M, Mansouri E, Dashti S, Khadivzadeh T. The beneficial and adverse effects of raloxifene in menopausal women: A mini review. J Menopausal Med. 2018;24:183–187. doi: 10.6118/jmm.2018.24.3.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hoyer J, Burmann I, Kieseler ML, Vollrath F, Hellrung L, Arelin K, et al. Menstrual cycle phase modulates emotional conflict processing in women with and without premenstrual syndrome (PMS) - a pilot study. Plos One. 2013;8:e59780. doi: 10.1371/journal.pone.0059780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wolfram M, Bellingrath S, Kudielka BM. The cortisol awakening response (CAR) across the female menstrual cycle. Psychoneuroendocrinology. 2011;36:905–912. doi: 10.1016/j.psyneuen.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 103.Kiesner J, Granger DA. A lack of consistent evidence for cortisol dysregulation in premenstrual syndrome/premenstrual dysphoric disorder. Psychoneuroendocrinology. 2016;65:149–164. doi: 10.1016/j.psyneuen.2015.12.009. [DOI] [PubMed] [Google Scholar]