Abstract
p16INK4a (p16) inhibits the vital G1 to S phase transition during cell cycle progression through the p16/cyclin D1/CDK4/retinoblastoma(Rb)/E2F1 pathway. Hyperoxia can suppress the G1/S checkpoint and induce more lung fibroblasts (LFs) to transition from the G1 phase to the S phase and undergo cell proliferation. The present study investigated the rate of p16 gene promoter methylation and the protein expression levels of p16, cyclin D1, CDK4, Rb and E2F1 in LFs from the lungs of rats exposed to hyperoxia and normoxia on postnatal days 3, 7 and 14. In the hyperoxia-exposed group, the methylation rate was 50 and 80% on days 7 and 14, respectively. Cyclin D1 and CDK4 overexpression was associated with p16 loss and Rb inactivation by phosphorylation. Rb phosphorylation induced E2F1 release in the G1 phase, which promoted cell proliferation. No methylation was observed in the normoxia-exposed group. These observations suggested that p16 loss may stimulate aberrant LF proliferation via the p16/cyclin D1/CDK4/Rb/E2F1 pathway.
Keywords: hyperoxia, lung fibroblasts, p16/CDK4/Rb/E2F1, cell proliferation
Introduction
Premature infants with respiratory disorders usually require inhalation of high levels of oxygen. Prolonged exposure to high levels of oxygen may lead to lung injury, which often results in widespread lung fibrosis (1). The main manifestation of lung fibrosis is the uncontrolled proliferation of lung fibroblasts (LFs) that are deposited in the pulmonary interstitial space or replace the normal lung epithelium (2). The hyperplasia and replacement of fibrous tissue after tissue injury are noteworthy pathophysiological processes of tissue repair. Discovering how to effectively prevent LF proliferation in order to alleviate lung fibrosis without affecting tissue repair is the key to curing. To address this problem, the mechanism that regulates the cell cycle during LF proliferation needs to be clarified.
A cell divides into two identical cells through the precisely controlled G1, S, G2 and M phases of the cell cycle. During cell cycle progression, cells that pass the G1/S checkpoint of the cycle, characterized by the initiation of DNA synthesis, are committed to dividing into two complete cells (3). The p16INK4a (p16)/cyclin D/CDK4/retinoblastoma-associated protein (Rb)/E2F1 pathway is recognized as a key pathway in the regulation of cell proliferation, for which E2F1 is a notable downstream effector. Deregulated E2F1 activity due to the aberrance of the upstream components in this pathway, such as inactivation of p16, confers a growth advantage to cells (4-7). During the G1 phase of the normal cell cycle, Rb is inactivated by sequential phosphorylation events that are mediated by various cyclin-dependent kinases (CDKs), leading to the release of E2F transcription factors, the activation of numerous genes (such as p19ARF and p53 et al) and cell cycle progression (8). p16 is a cyclin-dependent kinase inhibitor that positively regulates the expression of cyclin D1 at the post-transcriptional level. p16 and cyclin D1 competitively bind to CDK4 to prevent cells from entering the corresponding phase of the cell cycle and, therefore, inhibit its protein kinase activity. This inhibition suppresses the CDK4-mediated phosphorylation of the Rb protein. When phosphorylated (p)-Rb binds to E2F1 to form a complex, the transcriptional activation of E2F1 is inhibited, thereby preventing the cell from transitioning from the G1 to the S phase and inhibiting cell proliferation (6,9). Therefore, together, p16, CDK4, Rb and E2F1 constitute a feedback loop that directly regulates the cell proliferation cycle. Once the p16 gene is mutated, transduction through the loop is unable to proceed.
Our previous study found that LFs isolated from rats exposed to hyperoxia in vivo showed significantly greater proliferation compared with those from rats exposed to normoxia. In addition, using flow cytometry, it was revealed that the percentage of LFs in the S and G2/M phases increased, and the proportion of LFs in the G0/G1 phase decreased simultaneously (10). In another study, we confirmed that 5-aza-2'-deoxycytidine, a DNA methylation inhibitor, inhibited hyperoxia-induced LF proliferation by restoring p16 expression (11). However, the underlying mechanism has not been investigated and the results from previous studies are incomplete. In the present study, the protein expression of p16, cyclin D1, CDK4, Rb and E2F1 was measured in LFs from rats exposed to hyperoxia or normoxia to further understand the occurrence and development of the aberrant LF proliferation induced by hyperoxia.
Materials and methods
Animals and oxygen exposure
The present study was approved by The China Medical University Animal Research Committee (Shenyang, China; approval no. 2017PS140K). Healthy Sprague-Dawley rats (n=40; female:male, 4:1) obtained from The Center of Animal Experiments, China Medical University, were used for this experiment. The higher the concentration of oxygen inhaled, the more obvious the changes in lung fibrosis. A fraction of inspired oxygen (FiO2) >85% (the lethal oxygen concentration) is frequently employed in experimental studies (12-14). In the present study, the newborn rats (<12-h old) were randomly divided into the normoxia (FiO2, 21%) or hyperoxia (FiO2, 90±2%) groups. The hyperoxia group was monitored twice daily (MT-01 Gas Monitor). CO2 was absorbed by soda lime to maintain a concentration of <0.5%. The average birth weight of the rats was 5.12±0.26 g in the hyperoxia group and 4.98±0.19 g in the normoxia group. No statistically significant difference was observed. Within 12 h of birth, the newborn rats (with the mother rats) were housed in standard cages placed inside 425-l capacity plexiglass isolation chambers that received humidified O2 or room air (30l/min) at ambient pressure for up to 14 days. Each rat was fed at 20-26˚C and 12 h light/dark cycle. The cages were opened for 0.5 h every day to add standard laboratory food and water and change the padding. To avoid oxygen toxicity, the mother rats were rotated between the normoxia and hyperoxia environments every day.
Histological examination
On postnatal days 3, 7 and 14, 5 pup rats in each group were sacrificed with an intraperitoneal injection of pentobarbital sodium (100 mg/kg) and were exsanguinated by aortic transection. Right lung tissue samples were washed with PBS, placed in 4% paraformaldehyde at room temperature for 24 h, then serially dehydrated with increasing concentrations of ethanol before being embedded in paraffin. The paraffin-embedded lung tissue samples were cut into 4-µm sections, stained with hematoxylin and eosin (H&E; for 5 min) and Masson's trichrome staining (Weigert's iron hematoxylin solution for 3 min, Ponceau xylidine and fuchsin S solution for 30 min) at room temperature, examined using light microscopy and assessed for the presence of intra-alveolar edema, inflammatory cell infiltration and fibrosis.
Primary culture of LFs
The rats in both the hyperoxia and normoxia groups were euthanized on postnatal days 3, 7 and 14. The left lungs were quickly removed from the chest, minced into 1-mm3 pieces and digested in 0.2% trypsin and 0.016% deoxyribonuclease I in minimum essential medium (MEM; Beijing Solarbio Science & Technology Co., Ltd.) for 2-4 h at 37˚C in a shaking water bath. Next, 10% FBS (Clark Bioscience) was added to the filtered cell suspensions to stop further digestion. The harvested cells were obtained by centrifugation at 400 x g for 3 min at 4˚C, and then cultured in air and 5% CO2 at 37˚C in MEM with 10% FBS, 10,000 U/ml penicillin and 10,000 µg/ml streptomycin for 72 h. The LF purity was confirmed at >95% by immunocytochemical staining for vimentin. Briefly, cultured LFs were fixed in 4% paraformaldehyde for 30 min at 37˚C. Next, a primary antibody against vimentin (1:100; cat. no. sc-5565; Santa Cruz Biotechnology, Inc.) was added to the cells and incubated overnight at 4˚C. Then, cells were stained with goat anti-rat secondary antibody (1:100; Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd.) for 30 min at 37˚C. When passaged, the cells were seeded into 150-cm2 culture flasks at a density of 2.5x105 or 5x105 cells/flask. All the experiments were performed using fibroblasts from passage number two.
MTT assay
A 3-(4,5-dimethy-lthiazol-2-yl)-2,5-diphenyltetrazolium (MTT) assay was performed for cell proliferation. Cells were seeded into 96-well plates at a density of 5x104 cells per well and cultured at 37˚C in air and 5% CO2 for 24 h. Next, 20 µl of 5 mg/ml MTT (Sigma-Aldrich; Merck KGaA) was added into each well and the cells were cultured for an additional 4 h at 37˚C. After culture, the supernatant was discarded and the resulting formazan crystals were solubilized by adding 150 µl of DMSO to each well. The optical density level was measured at a wavelength of 570 nm. Experiments were performed in quintuplicate.
Methylation-specific polymerase chain reaction (MSP)
LFs from the hyperoxia and normoxia groups were collected at 120 h post-incubation in MEM with 10% FBS. A ZR-96 Quick-gDNA™ kit (Zymo Research Corp.) was used to extract the DNA as recommended by the manufacturer. An EZ DNA Methylation-Gold™ kit (Zymo Research Corp.) was used to perform the bisulfite modification of the genomic DNA according to the manufacturer's instructions. PCR amplification was performed using p16 promoter gene fragment-specific primers for methylated or unmethylated DNA (Sangon Biotech Co., Ltd.). The primers used to amplify unmethylated p16 were as follows: Sense, 5'-TTTTTGGTGTTAAAGGGTGGTGTACT-3' and antisense, 5'-CACAAAAACCCTCACTCACAACAA-3', which yielded a 132-bp fragment. The primers used to amplify methylated p16 were as follows: Sense, 5'-GTGTTAAAGGGCGGCGTAGC-3' and antisense, 5'-AAAACCCTCACTCGCGACGA-3', which yielded a PCR product of 122 bp. PCR was performed under the following conditions: 95˚C for 4 min, followed by 25 cycles of 94˚C for 25 sec, 62˚C for 25 sec and 72˚C for 30 sec, and a final extension at 72˚C for 5 min. CpGenome universal methylated DNA (EMD Millipore; Merck KGaA) was used as the control for methylated DNA. The PCR-amplified products were separated by electrophoresis on a 2% agarose gel and visualized using ethidium bromide staining for 20 min under ultraviolet light. Images were then captured and the DNA methylation of p16 was determined by MSP as previously described (15).
Western blotting
Western blotting was performed as previously described (7). The following specific primary antibodies were used: Anti-cyclin D1 (cat. no. SC-450; 1:500), anti-CDK6 (cat. no. SC-7961; 1:500), anti-p16 (cat. no. sc-74400; 1:400), anti-Rb (cat. no. sc-102; 1:500) and anti-p-Rb (cat. nos. ser-795 and sc-21875; 1:200) obtained from Santa Cruz Biotechnology, Inc. An anti-CDK4 antibody (cat. no. 12790S; 1:1,000) was obtained from Cell Signaling Technology, Inc. A β-actin antibody was obtained (cat. no. A-3853 Sigma-Aldrich; Merck KGaA) and used at a 1:1,000 dilution. The proteins in the membrane were detected using an enhanced chemiluminescence system (ECL Advance; Amersham Biosciences; Cytiva) and images were captured and analyzed using a BioRad ChemiDoc XRS system (BioRad Laboratories, Inc.).
Statistical analysis
The values are expressed as the mean ± standard deviation. SPSS software (v17.0; SPSS, Inc.) was used for the statistical analyses. The statistical significance of the differences was analyzed using an unpaired two-tailed t-test for comparison between two groups. P<0.05 was considered to indicate a statistically significant difference.
Results
Effect of hyperoxia on lung histology and morphometrics
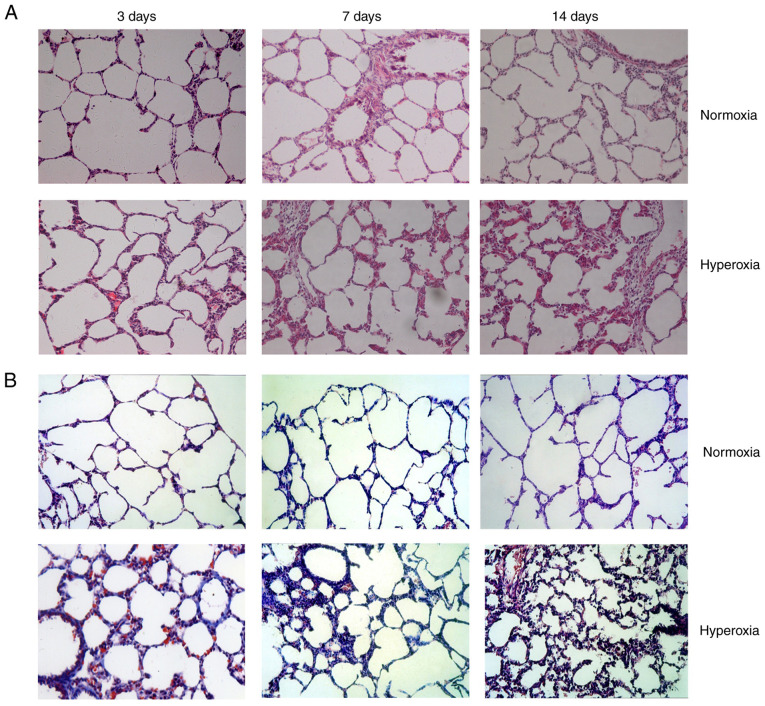
Normal structure and alveolarization were observed in the normoxia group on postnatal days 3, 7 and 14. No obvious differences in pathology were observed between the groups on postnatal day 3. Hyperoxia treatment induced the development of a disordered lung tissue structure, alveolar collapse, obvious alveolar wall thickening and numerous blue-stained stripes and flakes, indicating collagen deposition, and these changes were present on postnatal day 7 and increased over time through postnatal day 14 (Fig. 1).
Figure 1.
Histological examination of normoxia- and hyperoxia-exposed lung tissues. (A) Hematoxylin-eosin and (B) Masson's trichrome staining (magnification, x200).
Effect of hyperoxia on cell proliferation
As seen in Fig. 2, the LFs isolated from the rats exposed to hyperoxia in vivo showed significantly greater proliferation compared with the LFs isolated from normoxia-exposed animals on postnatal day 7 and 14 (P<0.05 and P<0.01, respectively).
Figure 2.
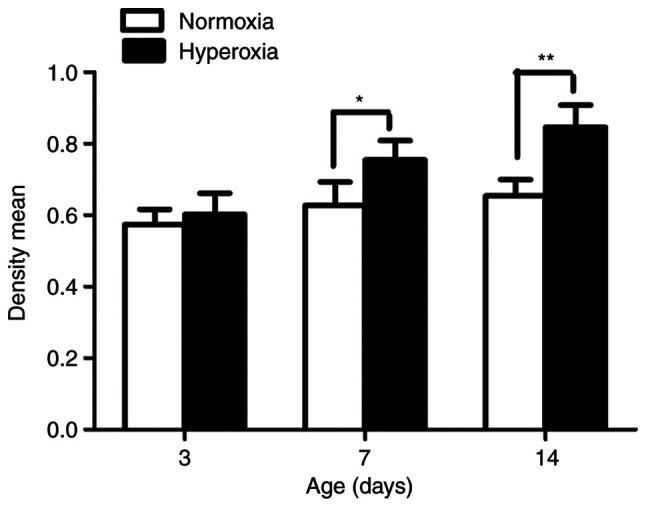
Effect of hyperoxia on cell proliferation on postnatal days 3, 7 and 14. n=5 for each experimental group. *P<0.05 and **P<0.01. OD, optical density.
Effect of hyperoxia on p16 methylation rate and p16 protein expression
No methylation was observed in any of the normoxia-exposed groups (postnatal days 3, 7 and 14). In the hyperoxia-exposed group, the rate of p16 promoter methylation on day 7 was 50% (n=10; the complete methylation rate was 30% and the partial methylation rate was 20%). The rate of p16 promoter methylation on day 14 was 80% (n=10; the complete methylation rate was 70% and the partial methylation rate was 10%). No methylation was observed on day 3 in the hyperoxia-exposed group (Fig. 3).
Figure 3.
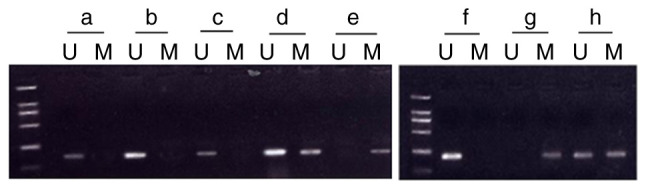
Methylation state of the p16 gene in lung fibroblasts. (a) Normoxia and (b) hyperoxia on postnatal day 3. (c) Normoxia and (d and e) hyperoxia on postnatal day 7. (f) Normoxia and (g and h) hyperoxia on postnatal day 14. U, unmethylated; M, methylated.
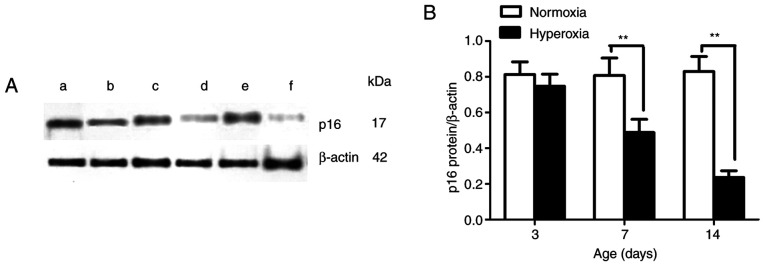
The newborn rats that were exposed to hyperoxia displayed lower protein expression levels of p16 in LFs on postnatal days 7 and 14 compared with the newborn rats that were exposed to normoxia (P<0.01; Fig. 4). No statistically significant differences were found in the LFs in the two groups on day 3. These results suggest that the hyperoxia-induced p16 gene promoter hypermethylation in the LFs may have suppressed or blocked the protein expression of p16.
Figure 4.
Effect of hyperoxia on p16 expression. (A) Representative protein bands for p16 and loading control β-actin. (a) Normoxia and (b) hyperoxia on postnatal day 3. (c) Normoxia and (d) hyperoxia on postnatal day 7. (e) Normoxia and (f) hyperoxia on postnatal day 14. (B) p16 protein expression in lung fibroblasts. n=5 for each experiment group. **P<0.01. p16, p16INK4a.
Effect of hyperoxia on cyclin D1, CDK4 and CDK6 protein expression
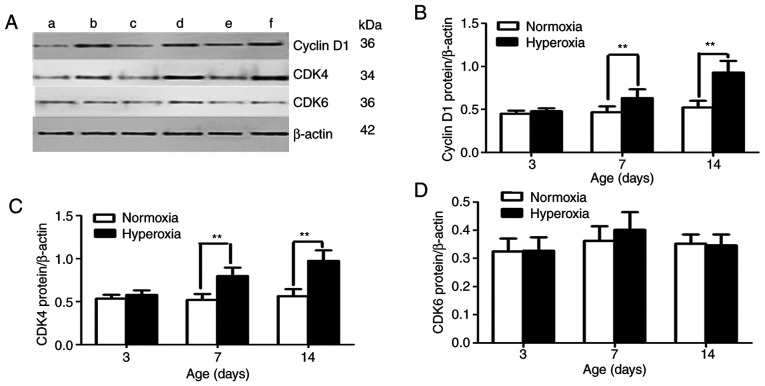
By controlling cell proliferation in the G1 phase, p16 inhibits the ability of the cyclin D/CDK4 complex to phosphorylate p-Rb. Therefore, p16 loss is correlated with cyclin D1 upregulation. Protein levels of cyclin D1 and CDK4 were examined in cultured lung cells in the present study. On postnatal day 3, no statistically significant difference was observed between the cyclin D1 and CDK4 protein expression levels in the LFs in the two groups (Fig. 5). The cyclin D1 and CDK4 protein expression levels were significantly greater in the hyperoxia-exposed group compared with those in the normoxia-exposed group on postnatal days 7 and 14 (all P<0.01; Fig. 5B and C). However, no statistically significant difference was found in CDK6 protein expression between the two groups at the different time points (P>0.05; Fig. 5D).
Figure 5.
Effect of hyperoxia on cyclin D1 and CDK4 expression. (A) Representative protein bands for cyclin D1, CDK4 and the β-actin loading control for western blotting. (a) Normoxia and (b) hyperoxia on postnatal day 3. (c) Normoxia and (d) hyperoxia on postnatal day 7. (e) Normoxia and (f) hyperoxia on postnatal day 14. (B) Cyclin D1, (C) CDK4 and (D) CDK6 protein expression in lung fibroblasts. n=5 for each experiment group. **P<0.01.
Effect of hyperoxia on Rb, p-Rb and E2F1 protein expression
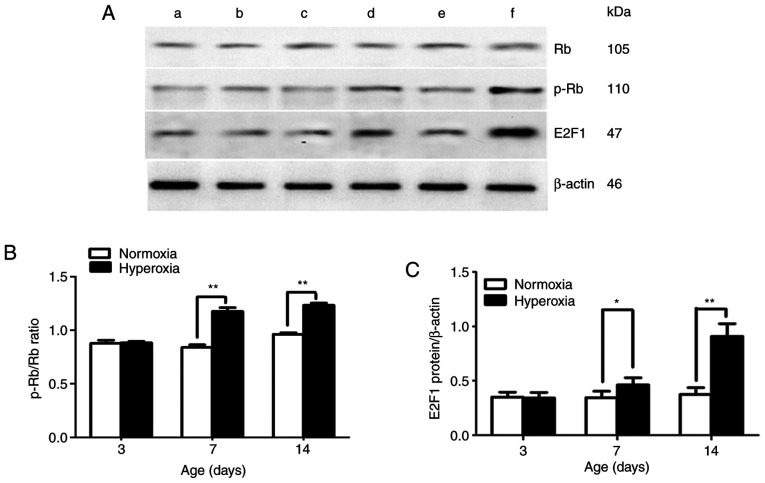
In the present study, no statistically significant difference was found in Rb protein expression between the two groups at the different time points (days 3, 7 and 14). p-Rb/Rb ratio and E2F1 protein expression were significantly greater in the hyperoxia-exposed group compared with that in the normoxia-exposed group on postnatal days 7 and 14 (P<0.05 and P<0.01, respectively; Fig. 6B and C).
Figure 6.
Effect of hyperoxia on Rb, p-Rb and E2F1 expression. (A) Representative protein bands for Rb, p-Rb, E2F1 and the β-actin loading control. (a) Normoxia and (b) hyperoxia on postnatal day 3. (c) Normoxia and (d) hyperoxia on postnatal day 7. (e) Normoxia and (f) hyperoxia on postnatal day 14. (B) p-Rb/Rb ratio in LFs. (C) E2F1 protein expression in lung fibroblasts. n=5 for each experiment group. *P<0.05 and **P<0.01. p-, phosphorylated; Rb, retinoblastoma-associated protein.
Discussion
Excessive LF proliferation is a key process in pulmonary fibrosis, and abnormal regulation of the cell cycle at the G1/S phase is necessary for hyperoxia-induced LF overproliferation (10). Our previous study found that exposure to hyperoxia weakened the G1/S checkpoint, enabling more LFs to pass this checkpoint and progress from the G1 phase to the S and G2/M phases to complete DNA replication; this phenomenon promotes eventual LF division and proliferation (10). In the transition from the G1 phase to the S phase, the activity of cyclin D1-CDK4/6 plays a decisive role. Once activated, the Rb protein is phosphorylated by cyclin D1-CDK4 to become p-Rb, which releases sequestered E2F transcription factors, thereby promoting the transcription of genes required for cell cycle progression (16). Cell cycle progression is under the control of CDK inhibitors, such as p16, that competitively bind to CDK4/6 kinases and prevent them from binding to their regulator cyclin D1; this blocks Rb protein phosphorylation, which sequesters the E2F transcription factors that control the transcription of S-phase genes (17). Therefore, the p16/cyclin D/CDK4/Rb/E2F pathway is a critical regulator of the important G1 phase to S phase transition of the cell cycle (18-20).
Yue et al (21) first showed that the p16 promoter was methylated in the lung tissues of hyperoxia-exposed mice, and that p16 promoter methylation increased as the duration of hyperoxia exposure increased. We previously repeated this experiment at the cellular level and reached the same conclusion, namely, that exposure to hyperoxia inhibited p16 gene expression in LFs and that high levels of p16 methylation in the promoter region may be the main cause of p16 transcriptional inactivation (11). The loss of p16 function may promote cellular proliferation and impair cell cycle arrest or senescence, allowing the survival of genetically damaged cells (22). The p16 protein acts as a cell proliferation inhibitor by competitively binding to CDK4/6 kinases, which prevents them from binding to their regulator cyclin D1 and blocks Rb protein phosphorylation, thus leading to cell cycle arrest (23). The loss of p16 during G1/S phase cell cycle regulation is closely associated with the upregulation of cyclin D1 and CDK4, which act synergistically with p16 loss to subvert the control of the G1 phase in Rb-positive normal cells (24,25).
In the present study, cyclin D1 and CDK4 were expressed at significantly higher levels in the LFs of hyperoxia-exposed rats compared with the levels in the LFs from normoxia-exposed rats on postnatal days 7 and 14. However, no statistically significant difference was observed in CDK6 protein expression between the two groups at the different time points. This finding indicated that the loss of p16 expression was an early event in abnormal LF proliferation that activated the cyclin D1-CDK4 complex. The Rb protein was phosphorylated by the cyclin D1-CDK4 complex to become p-Rb.
Rb is the link between extracellular signals and nuclear signals (26). Rb exhibits two phosphorylation states. The non-phosphorylated form of Rb is expressed in the G0/G1 phase of the cell cycle, and it is phosphorylated in the S/G2 phase, which suggests that Rb is a key regulatory gene (27). In its hypo-phosphorylated form, the Rb proteins acts as a cell cycle regulator to induce G1 arrest. Dysfunction of the proteins involved in the p16 pathway, such as p16 gene deletion and cyclin D1 upregulation, leads to Rb phosphorylation, subsequent G1/S phase transition and uncontrolled cell proliferation (28-30).
The present study first determined the overall expression level of Rb in LFs and found no significant difference between the Rb expression levels in the hyperoxia-exposed and normoxia-exposed groups. This finding showed that hyperoxia did not change the overall expression level of Rb. The Rb protein contains 16 cyclin-dependent kinase phosphorylation sites. The functional state of the Rb protein depends on its phosphorylation at these different sites. Ser-795 phosphorylation indicates that Rb has transitioned from an active non-phosphorylated state to an inactive phosphorylated state. Ser-795 is phosphorylated by cyclin D1/CDK4, which arrests the Rb-mediated cell cycle (31). The current study next examined the expression levels of p-Rb (Ser-795) in LFs and found that the p-Rb/Rb ratio was significantly higher in the hyperoxia-exposed group compared with that in the normoxia-exposed group on postnatal days 7 and 14. This finding indicated that hyperoxia did not change the overall expression levels of Rb in LFs, but did change its phosphorylation state.
Once Rb is phosphorylated, it induces the release of E2F in the late G1 phase, which in turn enhances the expression of genes that encode the regulatory proteins that are necessary for cell cycle progression (27). E2F family members play a noteworthy role during the G1/S transition in the mammalian cell cycle. The E2F family is generally divided into two groups by function: The transcriptional activators and the transcriptional repressors. Activators such as E2F1, E2F2 and E2F3a promote and help perpetuate the cell cycle, while repressors inhibit the cell cycle (32). E2F1 upregulation stimulates cellular proliferation. In numerous types of human tumors (such as breast cancer, lung tumors, et al), E2F1 overexpression accelerates the transition of cells from the G1 phase to the S phase, resulting in excessive cell proliferation, malignant transformation and tumor formation (33-35). In the present study, a high level of E2F1 expression was observed in primary cultured LFs from hyperoxia-exposed rats.
Our previous study determined that the p16 promoter methylation induced by hyperoxia plays a role in the excessive proliferation of LFs (11). The current study found that the loss of p16 expression activated the cyclin D1-CDK4 complex, and that the Rb protein was phosphorylated by cyclin D1-CDK4 to become p-Rb, after which E2F1 binding on it could be released. E2F1 upregulation accelerated the transition of cells, resulting in excessive LF proliferation. In summary, the disruption of the p16/cyclin D/CDK4/Rb/E2F1 pathway may play a key role in the aberrant LF proliferation induced by hyperoxia.
Acknowledgements
The authors would like to thank Dr Xindong Xue (Shengjing Hospital of China Medical University, Shenyang, Liaoning, China) for advice on experimental design and support of the study.
Funding Statement
Funding: No funding was received.
Availability of data and materials
All datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
SZ performed the experiments. ZC and SH collected and analyzed the data. HW designed the study and was the major contributor in writing the manuscript. All authors read and approved the final manuscript. ZC and SH confirmed the authenticity of all the raw data.
Ethics approval and consent to participate
The current study was approved by the Medical Ethics Committee at the China Medical University (grant no. 2017PS140K).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Chen CM. Therapy for neonatal hyperoxia-induced lung injury. Pediatr Neonatol. 2014;55:329–330. doi: 10.1016/j.pedneo.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 2.Crapo JD, Barry BE, Foscue HA, Shelburne J. Structural and biochemical changes in rat lungs occurring during exposures to lethal and adaptive dose of oxygen. Am Rev Respir Dis. 1980;122:123–143. doi: 10.1164/arrd.1980.122.1.123. [DOI] [PubMed] [Google Scholar]
- 3.Reddy GP. Cell cycle: Regulatory events in G1->S transition of mammalian cells. J Cell Biochem. 1994;54:379–386. doi: 10.1002/jcb.240540404. [DOI] [PubMed] [Google Scholar]
- 4.Al-Khalaf HH, Colak D, Al-Saif M, Al-Bakheet A, Hendrayani SF, Al-Yousef N, Kaya N, Khabar KS, Aboussekhra A. p16(INK4a) positively regulates cyclin D1 and E2F1 through negative control of AUF1. PLoS One. 2011;6(e21111) doi: 10.1371/journal.pone.0021111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vogelstein B, Kinzler KW. Cancer genes and the pathways they control. Nat Med. 2004;10:789–799. doi: 10.1038/nm1087. [DOI] [PubMed] [Google Scholar]
- 6.Haller F, Löbke C, Ruschhaupt M, Cameron S, Schulten HJ, Schwager S, von Heydebreck A, Gunawan B, Langer C, Ramadori G, et al. Loss of 9p leads to p16(INK4A) down-regulation and enables RB/E2F1-dependent cell cycle promotion in gastrointestinal stromal tumours (GISTs) J Pathol. 2008;215:253–262. doi: 10.1002/path.2352. [DOI] [PubMed] [Google Scholar]
- 7.Wu Z, Yu Q. E2F1-mediated apoptosis as a target of cancer therapy. Curr Mol Pharmacol. 2009;2:149–160. doi: 10.2174/1874467210902020149. [DOI] [PubMed] [Google Scholar]
- 8.Giacinti C, Giordano A. RB and cell cycle progression. Oncogene. 2006;25:5220–5227. doi: 10.1038/sj.onc.1209615. [DOI] [PubMed] [Google Scholar]
- 9.Tyagi E, Liu B, Li C, Liu T, Rutter J, Grossman D. Loss of p16 INK4A stimulates aberrant mitochondrial biogenesis through a CDK4/Rb-independent pathway. Oncotarget. 2017;8:55848–55862. doi: 10.18632/oncotarget.19862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao SM, Wu HM, Cao ML, Hna D. Primary culture of lung fibroblasts from hyperoxia-exposed rats and a proliferative characteristics study. Cytotechnology. 2018;70:751–760. doi: 10.1007/s10616-017-0179-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao S, Cao M, Wu H, Hu Y, Xue X. 5-aza-2'-deoxycytidine inhibits the proliferation of lung fibroblasts in neonatal rats exposed to hyperoxia. Pediatr Neonatol. 2017;58:122–127. doi: 10.1016/j.pedneo.2015.11.009. [DOI] [PubMed] [Google Scholar]
- 12.Chen X, Zhang X, Pan J. Effect of montelukast on bronchopulmonary dysplasia (BPD) and related mechanisms. Med Sci Monit. 2019;25:1886–1893. doi: 10.12659/MSM.912774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qi XJ, Ning W, Xu F, Dang HX, Fang F, Li J. Fasudil, an inhibitor of Rho-associated coiled-coil kinase, attenuates hyperoxia-induced pulmonary fibrosis in neonatal rats. Int J Clin Exp Pathol. 2015;8:12140–12150. [PMC free article] [PubMed] [Google Scholar]
- 14.Hu Y, Fu J, Xue X. Association of the proliferation of lung fibroblasts with the ERK1/2 signaling pathway in neonatal rats with hyperoxia-induced lung fibrosis. Exp Ther Med. 2019;17:701–708. doi: 10.3892/etm.2018.6999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herman JG, Graff JR, Myohanen S, Nelkin BD, Baylin SB. Methylation specific PCR: A novel PCR assay for methylation status of CpG islands. Proc Natl Acad Sci USA. 1996;93:9821–9826. doi: 10.1073/pnas.93.18.9821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang Y, Wu G, Fan H, Ye J, Liu X. Electroacupuncture promotes chondrocyte proliferation via accelerated G1/S transition in the cell cycle. Int JMol Med. 2013;31:1443–1448. doi: 10.3892/ijmm.2013.1336. [DOI] [PubMed] [Google Scholar]
- 17.Sharpless NE, DePinho RA. The INK4A/ARF locus and its two gene products. Curr Opin Genet Dev. 1999;9:22–30. doi: 10.1016/s0959-437x(99)80004-5. [DOI] [PubMed] [Google Scholar]
- 18.Williams RT, Barnhill LM, Kuo HH, Lin WD, Batova A, Yu AL, Diccianni MB. Chimeras of p14ARF and p16: Functional hybrids with the ability to arrest growth. PLoS One. 2014;9(e88219) doi: 10.1371/journal.pone.0088219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gil J, Peters G. Regulation of the INK4b-ARF-INK4a tumour suppressor locus: All for one or one for all. Nat Rev Mol Cell Biol. 2006;7:667–677. doi: 10.1038/nrm1987. [DOI] [PubMed] [Google Scholar]
- 20.Sharpless NE. INK4a/ARF: A multifunctional tumor suppressor locus. Mutat Res. 2005;576:22–38. doi: 10.1016/j.mrfmmm.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 21.Yue X, Fu J, Xue X, Gao H, Liu D, Zong Z, Wang W, Yuan Z. Detection of p16 promoter methylation in premature rats with chronic lung disease induced by hyperoxia. Pediatr Int. 2010;52:520–526. doi: 10.1111/j.1442-200X.2010.03089.x. [DOI] [PubMed] [Google Scholar]
- 22.Rayess H, Wang MB, Srivatsan ES. Cellular senescence and tumor suppressor gene p16. Int J Cancer. 2012;130:1715–1725. doi: 10.1002/ijc.27316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thompson JF, Scolyer RA, Kefford RF. Cutaneous melanoma. Lancet. 2005;365:687–701. doi: 10.1016/S0140-6736(05)17951-3. [DOI] [PubMed] [Google Scholar]
- 24.Myong NH. Cyclin D1 overexpression, p16 loss, and pRb inactivation play a key role in pulmonary carcinogenesis and have a prognostic implication for the long-term survival in non-small cell lung carcinoma patients. Cancer Res Treat. 2008;40:45–52. doi: 10.4143/crt.2008.40.2.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dobashi Y, Goto A, Fukayama M, Abe A, Ooi A. Overexpression of cdk4/cyclin D1, a possible mediator of apoptosis and an indicator of prognosis in human primary lung carcinoma. Int J Cancer. 2004;110:532–541. doi: 10.1002/ijc.20167. [DOI] [PubMed] [Google Scholar]
- 26.Mittnacht S. The retinoblastoma protein-from bench to bedside. Eur J Cell Biol. 2005;84:97–107. doi: 10.1016/j.ejcb.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 27.Wang X, Huang K, Xu LN. Interaction among Rb/p16, Rb/E2F1 and HDAC1 proteins in gallbladder carcinoma. J Huazhong Univ Sci Technolog Med Sci. 2009;29:729–731. doi: 10.1007/s11596-009-0611-5. [DOI] [PubMed] [Google Scholar]
- 28.Haluska FG, Tsao H, Wu H, Haluska FS, Lazar A, Goel V. Genetic alterations in signaling pathways in melanoma. Clin Cancer Res. 2006;12:2301s–2307s. doi: 10.1158/1078-0432.CCR-05-2518. [DOI] [PubMed] [Google Scholar]
- 29.Palmieri G, Capone M, Ascierto ML, Gentilcore G, Stroncek DF, Casula M, Sini MC, Palla M, Mozzillo N, Ascierto PA. Main roads to melanoma. J Transl Med. 2009;7(86) doi: 10.1186/1479-5876-7-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wikman H, Kettunen E. Regulation of the G1/S phase of the cell cycle and alterations in the RB pathway in human lung cancer. Expert Rev Anticancer Ther. 2006;6:515–530. doi: 10.1586/14737140.6.4.515. [DOI] [PubMed] [Google Scholar]
- 31.Thomas NS, Pizzey AR, Tiwari S, Williams CD, Yang J. p130, p107, and pRb are differentially regulated in proliferating cells and during cell cycle arrest by alpha-interferon. J Biol Chem. 1998;273:23659–23667. doi: 10.1074/jbc.273.37.23659. [DOI] [PubMed] [Google Scholar]
- 32.Clijsters L, Hoencamp C, Calis JJA, Marzio A, Handgraaf SM, Cuitino MC, Rosenberg BR, Leone G, Pagano M. Cyclin F controls cell-cycle transcriptional outputs by directing the degradation of the three activator E2Fs. Mol Cell. 2019;74:1264–1277.e7. doi: 10.1016/j.molcel.2019.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stanelle J, Stiewe T, Theseling CC, Peter M, Pützer BM. Gene expression changes in response to E2F1 activition. Nucleic Acides Res. 2002;30:1859–1867. doi: 10.1093/nar/30.8.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Han S, Park K, Bae BN, Kim KH, Kim HJ, Kim YD, Kim HY. E2F1expression is related with the poor survival of lymph node-positive breast cancer patients treated with fluorouracil, doxorubicin and cyclophosphamide. Breast Cancer Res Treat. 2003;82:11–16. doi: 10.1023/B:BREA.0000003843.53726.63. [DOI] [PubMed] [Google Scholar]
- 35.Eymin B, Gazzeri S, Brambilla C, Brambilla E. Distinct pattern of E2F1 expression in human lung tumours: E2F1 is upregulated in small cell lung carcinomal. Oncogene. 2001;20:1678–1687. doi: 10.1038/sj.onc.1204242. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.