Abstract
Aims
To evaluate calculated total plasma osmolality as a marker of outcome prediction, fluid and metabolic balance, thrombotic risk in severe COVID-19 patients.
Methods
Retrospective data of RT-PCR confirmed hospitalized severe COVID-19 patients (total: n = 175 patients, including diabetic subset: n = 102) were analyzed. Clinically applicable cut-offs were derived using receiver operating characteristic (ROC) curve analysis for calculated total osmolality, eGFR, and D-dimer, and their correlations were studied.
Results
Among 175 severe COVID-19 patients, a significant association with mortality was seen with respect to calculated total osmolality (p < 0.001), eGFR (p < 0.001), and D-dimer (p < 0.001). In the total cohort, applicable cut-offs based on ROC curve in predicting outcome were, for total osmolality 299 mosm/kg (area under the curve (AUC)-0.773, odds ratio (OR)-1.09), eGFR 61.5 ml/min/m2 (AUC-0.789, OR-0.96), D-dimer 5.13 (AUC-0.814, OR-2.65) respectively. In diabetic subset, the cut-offs for total osmolality were 298 mosm/kg (AUC-0.794, OR-1.12), eGFR 44.9 ml/min/m2 (AUC-0.774, OR-0.96) and D-dimer 1.59 (AUC-0.769, OR-1.52) respectively.
Conclusions
Applicable cut-offs for calculated total plasma osmolality, eGFR, and D-dimer predicts clinical outcome in severe COVID-19 with and without diabetes. Correlation studies validated calculated total osmolality as a marker of the combined effect of fluid and metabolic imbalance, compromised renal function and hypercoagulability.
Keywords: Calculated total osmolality, eGFR, D-dimer, Severe COVID-19, Diabetes mellitus
1. Introduction
A major contemporary challenge to healthcare settings across the globe is to reduce the morbidity and mortality of severe COVID-19 patients, especially those with co-morbid conditions. Associated diabetes mellitus, which is the commonest metabolic disorder, markedly enhances the risk of severity [1]. Therefore, effective control of the metabolic state through early detection of high-risk patients holds the key to the management.
Severe COVID-19 is a consequence of infective, immunoreactive, inflammatory, vascular disorder resulting in severe pneumonia and multi-organ dysfunction. The pathophysiologic perspective of severe COVID-19 illness is characterized by a gamut of metabolic, osmolar, and thrombo-inflammatory phenomena resulting in the criticality of the outcome.
Several studies have focused on early identification biomarkers, which can serve as prognosticators of the outcome and in the early intensification of the treatment [2,3]. Metabolic changes such as hyperglycemia, hyperlipidemia, low HDL levels, and dyselectrolytemia are the common alterations seen in setting of severe COVID-19, and their statistically derived applicable cut-offs have been reported as predictive tools in determining the clinical outcome [4].
Disorders of metabolism can result in disturbances in osmolality of the body fluids and are often associated with adverse clinical outcomes. In intensive care setting, critically ill patients with acute coronary syndrome, cerebrovascular accidents, pulmonary embolism, the changes in serum osmolality have been linked to poor outcomes [5]. Similarly, during hyperglycemic emergencies, hyperosmolality is associated with increased mortality [6].
In hospitalized severe COVID-19 patients, hyperglycemia often prevails because of various reasons, and as a consequence electrolyte, and osmolality changes can occur. Delayed identification of these abnormalities and inadequate fluid management often worsens the osmolality, influencing the recovery of the patients. The concentrations of various solutes in body fluid such as glucose, urea, sodium, potassium, and chloride and the water content determine the serum osmolality and fluid balance [7].
We aimed to identify the spectrum of osmolar changes amongst severe COVID-19 patients admitted to our hospital in this retrospective study. The objective was to evaluate the predictive potential of calculated total plasma osmolality at hospital admission, as derived from simple biochemical measurements (plasma glucose, blood urea, and sodium levels) in determining the clinical outcome. We also attempted to validate its role through correlation studies with eGFR and d-dimer as an indicator of underlying pathophysiology and a simple guiding tool for management.
2. Materials and methods
After appropriate Institutional ethics committee clearance, we analyzed retrospective data of the hospitalized patients with RT-PCR confirmed severe COVID-19 from October and November 2020 were included in our study at King George Hospital, Visakhapatnam, Andhra Pradesh, India.
Inclusion criteria: RT-PCR confirmed severe COVID-19 patients were defined as either having:
-
1)
Severe pneumonia (RR > 30/min, respiratory distress with Spo2 < 90% at room air, < 94% O2 supplementation)
-
2)
Acute respiratory distress syndrome (ARDS)
-
3)
Septic shock
Exclusion Criteria: Patients with haematological malignancies, immunodeficiency states, and those on renal replacement therapy were excluded from the study.
The clinical and laboratory admission data of 175 patients were extracted from the medical case records, which included 102 diabetic patients. Those patients with a documented history of diabetes mellitus prior to admission were included in diabetic subset.
The data of the biochemical parameters at admission such as blood sugar, urea, creatinine, measured sodium, and D-dimer were noted. Corrected sodium was derived in relation to the blood sugar using a correction factor of 2.3 [8].
Calculated total osmolality derived from Worthley formula: Plasma osmolality = 2 [Na+] + glucose (mg/dL)/18 + BUN (mg/dL)/2.8 [9]. Effective osmolality was calculated using the formula = 2 [Na+] + glucose (mg/dL)/18. eGFR was derived from the CKD-EPI equation.
All these parameters were correlated with mortality. Their prognostic value as simple, cost-effective tools for determining the outcome of the entire cohort and the diabetic subset was analyzed.
To strengthen the validation of total calculated plasma osmolality as an indicator of the underlying pathophysiology and a guiding tool for management, correlation studies were done with eGFR and D-dimer.
2.1. Statistical analysis
Continuous variables were expressed as appropriate means ± standard deviations. Categorical variables were summarized as frequencies and percentages. For categorical variables, Chi-square tests, and for continuous variables, independent T-tests were done.
Clinically applicable cut-offs of total plasma osmolality, eGFR, and D-dimer were derived using receiver operator curve (ROC) analysis, and odds ratio (OR) of mortality above the cut-off were calculated. In addition, the correlation of total plasma osmolality with eGFR and D-dimer were analyzed using the Pearson correlation coefficient.
Similar analyses were performed among the diabetic subset. P value < 0.05 was recognized as statistically significant. Statistical analysis was performed using SPSS v21.0 software.
3. Results
3.1. Age and mortality
A significant association was observed in both the total cohort and diabetic subset with respect to age and mortality (p < 0.001). The total cohort comprised 175 patients (mean age 54 years), including a diabetic subset of 102 patients (mean age 58.4 years). Among those who expired (n = 68), the mean age was 61 years, and among those who recovered (n = 107), it was 49 years in the total cohort. The mean age in diabetic subset among the deceased (n = 46) was 61.9 years, and among those who recovered, it was 55 years (n = 56). (Table 1 ).
Table 1.
Demographic, biochemical parameters and their association with mortality in total cohort and diabetic subset.
| Variable | Total cohort (n = 175) |
Diabetic Subset (n = 102) |
||
|---|---|---|---|---|
| Mean | P∗ value | Mean | P∗ value | |
| Age (in years) | 61.04 | <0.001 | 61.9 | <0.001 |
| Sex (males) | 70.28% | 0.543 | 70.58% | 0.817 |
| Calculated total osmolality (mosm/kg) | 305.47 | <0.001 | 307 | <0.001 |
| Effective osmolality(mosm/kg) | 293.4 | 0.001 | 295.14 | <0.001 |
| Measured Sodium (meq/lit) | 138.5 | 0.611 | 137 | 0.32 |
| Corrected Sodium (meq/lit) | 140 | 0.003 | 140 | 0.035 |
| Admission RBS (random blood sugar) (mg/dl) | 238 | <0.001 | 296 | <0.001 |
| eGFR (estimated glomerular filtration rate) (ml/min/1.73m2) | 48.01 | <0.001 | 43.6 | <0.001 |
| D-dimer (mg/L) | 2.28 | <0.001 | 2.38 | 0.014 |
∗p-value of association between parameters and mortality.
3.2. Calculated total osmolality vs mortality
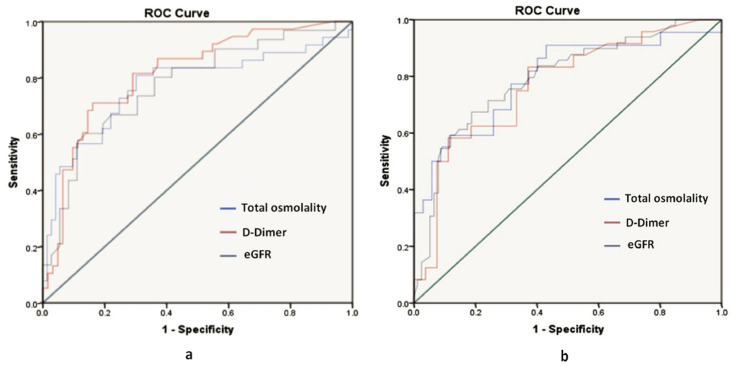
The mean calculated total osmolality was 298.3 mosm/kg and 301 mosm/kg in total cohort and diabetic subset, respectively. Among those who expired, the mean total osmolality was 305.4 mosm/in total cohort and 307 mosm/kg in diabetic subset. There was a significant association with mortality in both total cohort and diabetic subset (p value < 0.01) (Table 1). ROC curve analysis showed an AUC of 0.773.
An applicable threshold for total osmolality at 299 mosmol/kg predicted mortality with a sensitivity of 81% and specificity of 69.9% in total cohort with an odds ratio of 1.09(CI:1.05–1.14) (Table 2 , Fig. 1 a). And in diabetic subset, AUC was 0.794, at an applicable threshold of 298mosm/kg, it predicted mortality with a sensitivity of 90% and specificity of 57%with an odds ratio of 1.12 (CI: 1.04–1.19) (Table 2, Fig. 1b).
Table 2.
Area under curve (AUC) and applicable cut-offs with sensitivity, specificity, and odds ratio (OR) for mortality derived from the receiver operating characteristics curve for statistically significant variables.
| Variable | Cohort | AUC | Cut-off | Sensitivity | Specificity | Odds Ratio | CI |
|---|---|---|---|---|---|---|---|
| Calculated total osmolality (mosm/kg) | Total | 0.773 | 299 | 81 | 69.9 | 1.09 | 1.05–1.14 |
| Diabetic | 0.794 | 298 | 90 | 57 | 1.12 | 1.04–1.19 | |
| eGFR (estimated glomerular filtration rate) (ml/min/1.73m2) | Total | 0.789 | 61.5 | 67 | 81 | 0.965 | 0.95–0.97 |
| Diabetic | 0.774 | 44.9 | 60 | 86 | 0.96 | 0.94–0.98 | |
| D-dimer (mg/L) | Total | 0.814 | 0.97 | 71 | 83 | 2.65 | 1.63–4.31 |
| Diabetic | 0.769 | 1.59 | 58 | 88 | 1.52 | 1.03–2.25 |
Fig. 1.
a. Receiver operating characteristic curve analysis of total calculated osmolality (area under curve 0.773), estimated glomerular filtration rate (eGFR) (area under curve 0.789), D-dimer (area under curve 0.814) in the total cohort.
Fig. 1b. Receiver operating characteristic curve analysis of total calculated osmolality (area under curve 0.779), estimated glomerular filtration rate (eGFR) (area under curve 0.794), D-dimer (area under curve 0.769) in a diabetic subset.
3.3. Effective osmolality
The mean calculated effective osmolality was 289.9 mosm/kg and 289.17 mosm/kg in the total cohort and diabetic subset, respectively. It was 295.4 mosm/kg in total cohort and 295.14 mosm/kg in diabetic subset among those who expired. There was a significant association with mortality in both the total cohort and diabetic subset (p-value <0.01). (Table 1).
3.4. Relationship between the osmolality range and mortality
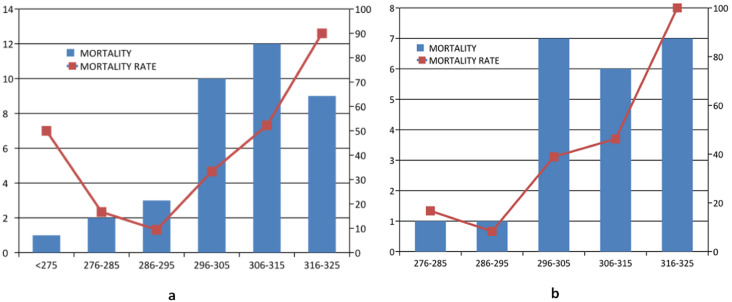
Categorizing the total calculated osmolality in total cohort into three groups, lower range osmolality (<275mosm/kg), normal range osmolality (275–295 mosm/kg) and higher range osmolality (>295mosm/kg), it was found that one among two patients in lower range group, 36 of 109 patients in normal range group, and 31 of 64 patients belonging to the higher group expired.
This pattern followed a typical J-shaped curve of mortality as is depicted. Further sub-categorization of the higher range osmolality group into 295–305, 305–315, and 315–325 mosm/kg, respectively, it was found that the mortality percentages were 33.3%, 52.2%, and 90%, respectively, implying higher mortality as the osmolality increases (Fig. 2 a).
Fig. 2.
a. J shaped curve of mortality in total osmolality
Fig. 2b. Curve representing mortality in diabetic subset.
Those patients with an osmolality of more than 295 mosm/kg had a highly statistically significant association with mortality (p < 0.001). Similar categorization of diabetic subsets basing on the osmolality range showed that 41% of patients in normal range and 51.3% in higher osmolality range expired. Further sub-categorization of the higher osmolality group into 295–305, 305–315 and 315–325 mosm/kg it was found that the mortality percentages were 38.9%, 46.2% and 100%, respectively, implying higher mortality as the osmolality increases (Fig. 2b).
3.5. Measured sodium
The mean measured sodium was 138meq/lit in total cohort, and it showed no significant association with mortality (p = 0.61). The mean measured sodium was 137meq/lit in diabetic subset, and it showed no significant association with mortality (p = 0.32). (Table 1).
3.6. Corrected sodium
The mean corrected sodium was 140meq/lit in total cohort, and it showed significant association with mortality (p = 0.025), while the mean corrected sodium was 140 meq/lit in diabetic subset, and it also showed significant association with mortality (p = 0.035) (Table 1). Fifteen patients had hyponatremia in total cohort, and 24 patients had hypernatremia. While in diabetic subset, 7 had hyponatremia, and 16 had hypernatremia.
3.7. Admission RBS
The mean admission RBS was 238.9 mg/dl and 296 mg/dl in total cohort and diabetic subset, respectively. Among those who expired, the mean admission RBS was 293 mg/dl in total cohort and 339 mg/dl in diabetic subset. There was a significant association with mortality in both the total cohort and diabetic subset (p-value <0.001). (Table 1).
3.8. eGFR
The mean eGFR was 68.5 and 59.9 ml/min/m2 in total cohort and diabetic subset, respectively. Among those who expired, the mean eGFR was 48 ml/min/m2 in total cohort and 43.6 ml/min/m2 in diabetic subset. There was a significant association with mortality in both the total cohort and diabetic subset (p-value <0.001). (Table 1).
ROC curve analysis showed an AUC of 0.789, an applicable threshold for eGFR61.5 ml/min/m2 predicted mortality with a sensitivity of 67% and specificity of 81% in total cohort with an odds ratio of 0.965 (CI:0.95–0.97). (Table 2 and Fig. 1a).
And in diabetic subset, AUC was 0.774, at an optimal threshold of 44.9 ml/min/m2; it predicted mortality with a sensitivity of 60% and specificity of 86% with an odds ratio of 0.96 (CI: 0.94–0.98). (Table 2 and Fig. 1b).
3.9. D-dimer
The mean D-dimer was 1.32 mg/L and 1.61 mg/L in total cohort and diabetic subset, respectively. Among those who expired, the mean D-dimer was 2.28 mg/L in total cohort and 2.38 mg/L in diabetic subset. There was a significant association with mortality in both the total cohort and diabetic subset (p-value <0.001, p-value 0.014 respectively) (Table 1).
ROC curve analysis showed an AUC of 0.814, an applicable threshold for D-dimer 0.97 predicted mortality with a sensitivity of 71% and specificity of 83% in total cohort with an odds ratio of 2.65 (CI:1.63–4.31) (Table 2 and Fig. 1a) And in diabetic subset AUC was 0.769, at optimal threshold 1.59, it predicted mortality with a sensitivity of 58% and specificity of 88% with an odds ratio of 1.52 (CI:1.03–2.25). (Table 2 and Fig. 1b).
Correlation studies among Admission biochemical parameters such as Random Blood Sugar, eGFR and inflammatory and procoagulant protein, D-Dimer (Table 3 ).
Table 3.
Correlations between Calculated Total Osmolality with eGFR (estimated glomerular filtration rate) and D-dimer.
| Variable |
Calculated Total Osmolality in Total cohort |
Calculated Total Osmolality in Diabetic Subset |
||
|---|---|---|---|---|
| r value | P∗ value | r value | P∗ value | |
| eGFR (estimated glomerular filtration rate) | −0.502 | <0.001 | −0.403 | 0.002 |
| D-dimer | 0.374 | 0.042 | 0.131 | 0.041 |
∗p value significant when less than 0.05.
Calculated Total Osmolality: There was a significant correlation between calculated total osmolality and eGFR (p < 0.01), D-dimer (p = 0.008) in total cohort as well as in diabetic subset, GFR (p = 0.002) and D-dimer(p = 0.041).
eGFR correlation with D-Dimer: A significant independent correlation was observed between GFR with D-dimer (total p < 0.01, a diabetic subset(p = 0.021).
Admission RBS: No significant correlation was seen between admission RBS, eGFR, and D-dimer in total and diabetic subsets.
4. Discussion
Serum osmolality plays an important role in intracellular and extracellular water distribution, and its changes have been linked to adverse clinical outcomes [10]. Delayed identification of osmolar abnormalities and inadequate fluid management can worsen the clinical outcome. In severe COVID-19 patients, hyperglycemia, electrolyte, and osmolality changes often co-exist for various reasons [11].
In this retrospective study, we analyzed the prognostic potential of admission calculated total plasma osmolality in outcome prediction amongst the hospitalized severe COVID-19 patients. Correlation studies between eGFR, D-dimer, and calculated total osmolality were done in order to validate it as a pathophysiological marker and a guiding tool for the management of severe Covid-19. The diabetes subset was analyzed separately as they are vulnerable to both osmotic changes and severe COVID-19.
In our study, the total cohort comprised 175 patients, of which 102 patients constituted the diabetic subset. A significant association was observed in both groups with respect to age and mortality (p < 0.001). (Table 1).
Usually, total plasma osmolality is chiefly determined by the content of water, electrolytes, glucose, urea, and serum proteins contribute to less than 0.5%. The terms osmolality (mosm/kg) or osmolarity (mosm/L) are often interchangeable in clinical practice, as there is only a trivial difference between them [12]. Worthley's equation for measuring total osmolality is widely used clinically, though the freezing point depression method is the gold standard [13]. The term osmolality is being used henceforth in this paper. Perturbations in osmolality resulting in intracellular dehydration or edema contribute to adverse outcomes in critical patients [14,15].
Hyperosmolality is associated with increased mortality in various clinical settings [[16], [17], [18]]. In severe COVID-19, increased osmolality was related to mortality in a single study [19]. Our study provided a detailed analysis of the relation between osmolality, renal function, pro-coagulation status, and clinical outcome. A significant association between mean calculated total plasma osmolality and mortality was seen (p value < 0.01) (Table 1). A mean value of 305.4 mosm/kg in the total cohort and 307 mosm/kg in the diabetic subset was observed among those who expired.
An applicable threshold of 299 mosmol/kg in the total cohort and 298 mosm/kg in the diabetic subset predicted mortality with ROC curve analysis (Table 2, Fig. 1a and b).
Our observations are similar to Holtfreter et al., who demonstrated that serum osmolality at a cut-off of 298 mosm/kg had the largest predictive value for mortality, among 933 non-COVID ICU cases (AUC 0.732, sensitivity:76.4 specificity: 61.3) [20].
Shen et al. observed a ‘U' shaped relationship between osmolality and mortality among non-COVID ICU patients, especially in those with respiratory diseases suggesting higher mortality at extremes of the osmolality spectrum [10].
However, a typical J-shaped relationship between total calculated plasma osmolality and mortality rate could be documented in our retrospective study of COVID-19 among both total and diabetic subsets (Fig. 2a and b).
Our study observations establish that in severe COVID-19, Hyperosmolality prevails and is a significant mortality risk. Hyperosmolality due to increased glucose, sodium, and potassium levels have been reported as risk factors for cardiac mortality [21,22]. Redistribution of body fluids into the intravascular space increases the cardiac preload and risk of heart failure or arrhythmias [10]. Similarly, in hyperglycemic emergencies, hyperosmolality is associated with increased mortality [23].
Though hypoosmolality is the commonest disorder of fluid and electrolyte imbalance in the hospitalized patients with significant potential for mortality, only two patients had a total osmolality of less than 275 mosm/kg in our study, and one among the two expired. Hyponatremia and hypoosmolality are usually synonymous since sodium is a major osmolyte [8,24].
However, the measured sodium in our study was not statistically associated with mortality (p = 0.32).
Corrected sodium is a better indicator of clinical outcome compared to measured sodium in severe hyperglycemia [25]. A progressive increase in mortality with increasing sodium levels was seen in the total cohort (p = 0.011). Admission hyperglycemia has been suggested as a marker of mortality in patients with sepsis irrespective of the severity of presentation [26].
Liu et al. demonstrated that a mean admission fasting glucose of 133 mg/dl as an independent risk factor for developing critical illness in COVID-19 patients [27]. Whereas in our study, a statistically significant association with mortality (p < 0.001) was seen in both total and diabetic subsets at a mean admission plasma glucose (238 mg/dl and 296 mg/dl, respectively).
It is to be noted this is not a comparative study between those with and without diabetes. Only those patients on anti-diabetic medications were included in the diabetic subset, constituting a significant proportion of the total cohort. Certain limitations such as unavailability of hba1c due to resource constraints, COVID-19 related hyperglycemia due to steroid treatment, possible viral attack on beta-cell may explain the hyperglycemia in the total cohort. Widespread use of steroids was prevalent in a full-blown phase of the pandemic; many of our patients were on varied formulations and dosages of steroids for a short duration prior to admission. A clear relation between decreased renal function and adverse clinical outcomes in severe COVID-19 has been observed in our study (Table 1).
Tribulus et al. made similar observations of higher mortality among patients with an eGFR under 60 mL/min/1.73 m2 in COVID-19 [28]. Clinically applicable prognostic ROC eGFR cut-offs of 61.5 ml/min/m2 and 44.9 ml/min/m2 predicted mortality in total and diabetic groups, respectively (Fig. 1a and b and Table 2). Acute kidney injury in severe COVID-19 is a result of cytokine storm and multi-organ dysfunction; virus-induced renal cellular injury is a risk factor for in-hospital mortality [29,30].
One striking finding of our study is that the calculated total plasma osmolality correlates negatively with eGFR in the setting of severe COVID-19, indicating a relation between disturbed metabolic and osmolar milieu and compromised renal function. Associated hypertension, hypoxemia, and compromised cardiac function can worsen this critical state. Understanding and early identification of this pathophysiology help in the early institution of appropriate fluid management strategy to tackle hyperosmolality.
Keenan et al. demonstrated a higher risk of venous thromboembolism with hyperosmolality [31].
Severe COVID-19 is characterized by elevation of d-dimer and marked inflammatory and prothrombotic state. Mechanical ventilation, immobilized state, venous stasis enhance the risk, thus requiring thromboprophylaxis [32]. Usage of systemic glucocorticoids increase the risk further [33].
Increased thrombo-inflammatory proteins reduce the water content of plasma, increasing the osmolality [10] and the risk of thromboembolism [34].
D-dimer was significantly associated with mortality in our study, and predictive cut-offs were documented. (Table 2 and Fig. 1a and b). Zhang et al. predicted in-hospital mortality at a D-dimer level at admission greater than 2.0 mg/L [35].
Our study documented D-dimer negatively correlates with eGFR in severe COVID-19. Reduced elimination by the kidneys and activation of the coagulation cascade in these patients explain the higher D-dimer levels [36].
A significant positive correlation was seen with D-dimer in the total cohort (p = 0.008) and the diabetic subset (p = 0.041) with calculated total plasma osmolality (Table 3). Re-emphasizing the importance of calculated total plasma osmolality as a marker of altered fluid and metabolic milieu, decreased renal function, and hypercoagulability correlation studies between calculated total osmolality, eGFR, and D-dimer were done and were found to be correlating significantly in total and diabetic groups. Thus severe COVID-19 patients with high admission total calculated osmolality have significant renal dysfunction and increased risk of thrombotic events.
Steroids also contribute to the hyperosmolar state through severe hyperglycemia and hypernatremia due to sodium retention, suppression of ADH, and increased free water clearance [37].
The treating team needs to be alert about the fluid management measures in severe COVID-19 patients. Appropriate fluid resuscitation improves venous return, cardiac output, and intravascular circulation. But it is equally prudent to anticipate poor respiratory reserve and the impending cytokine storm and underlying likely cardiac dysfunction in these patients. Liberal or aggressive fluid administration can result in volume overload and ARDS. Restricted fluid resuscitation treatment pattern may be preferred over fluid removal measures following liberal administration in such a scenario [38].
In severe COVID-19 patients with marked hyperglycemia, hydration with isotonic fluid may be the initial fluid of choice to reduce serum glucose by about 70–80 mg/dl/hour by reducing the counter-regulatory hormonal surge, as is the practice in the hyperosmolar hyperglycemic state in non COVID-19 situations [39]. Hypotonic crystalloids may be employed above an effective osmolality of 320 mOsm/kg. Below this threshold, isotonic electrolyte solutions are preferred as in hyperosmolar non COVID-19 ICU admissions [40].
Our findings reinforce the clinical utility of calculated total plasma osmolality at hospital admission as an early identification marker of the above pathophysiologic changes in severe COVID-19 and a simple guiding tool for early intensive management strategies.
The retrospective nature, lack of proper documentation of steroid usage, diabetes medications prior to admission are the limitations of this study.
The clinically applicable cut-offs of calculated total plasma osmolality in severe COVID-19 could serve as a simple, widely utilizable management tool.
5. Conclusions
The raging COVID-19 pandemic prompted us to focus on economical tools for the management of severe COVID-19. Our study demonstrated that admission calculated total plasma osmolality predicts clinical outcomes in severe COVID-19 patients with and without diabetes.
Our findings validate calculated total plasma osmolality at the time of admission in severe COVID-19 as a dependable, bedside, simple indicator of the combined effect of compromised renal function, disturbed fluid homeostasis, and increased risk of thrombosis.
These key observations guide the treating clinicians to widely using this economical management tool in optimal fluid management and early intensive care in these patients.
Authors contributions and acknowledgements
Conceptualization, implementation, data review, manuscript drafting, revision, finalization – JR.
Patient data collection, statistical data analysis, references collation, interdepartmental collaboration – MR, JV and SR.
The authors would like to thank the Principal Dr PV Sudhakar MBBS MS Mch, Andhra Medical College, for his support, Dr Kiran Pamarthi MD (Asst professor, department of preventive medicine, Andhra Medical College) for statistical analysis, and Dr Srivalli Madhira MBBS MS for her invaluable insights.
All authors read and approved the final version of the manuscript.
Declaration of competing interest
The authors whose names are listed immediately below certify that they have NO affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
References
- 1.Riddle M.C., Buse J.B., Franks P.W., Knowler W.C., Ratner R.E., Selvin E., Wexler D.J., Kahn S.E. COVID-19 in people with diabetes: urgently needed lessons from early reports. Diabetes Care. 2020 Jul 1;43(7):1378–1381. doi: 10.2337/dci20-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ji P., Zhu J., Zhong Z., Li H., Pang J., Li B., Zhang J. Association of elevated inflammatory markers and severe COVID-19: a meta-analysis. Medicine. 2020 Nov 20;99(47) doi: 10.1097/MD.0000000000023315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thirumalaisamy P.V., Meyer C.G. Mild versus severe COVID-19: laborytorma markers. Int J Infect Dis. 2020;95:304–307. doi: 10.1016/j.ijid.2020.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marazuela M., Giustina A., Puig-Domingo M. Endocrine and metabolic aspects of the COVID-19 pandemic. Rev Endocr Metab Disord. 2020 Dec;21(4):495–507. doi: 10.1007/s11154-020-09569-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rohla M., Freynhofer M.K., Tentzeris I., Farhan S., Wojta J., Huber K., Weiss T.W. Plasma osmolality predicts clinical outcomes in patients with acute coronary syndrome undergoing percutaneous coronary intervention. Eur Heart J: Acute Cardiovascular Care. 2014 Mar 1;3(1):84–92. doi: 10.1177/2048872613516018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gosmanov A.R., Gosmanova E.O., Kitabchi A.E. 2000. Hyperglycemic crises: diabetic ketoacidosis and hyperglycemic hyperosmolar state. Endotext [Internet] [PubMed] [Google Scholar]
- 7.Roumelioti M.E., Glew R.H., Khitan Z.J., Rondon-Berrios H., Argyropoulos C.P., Malhotra D., Raj D.S., Agaba E.I., Rohrscheib M., Murata G.H., Shapiro J.I. Fluid balance concepts in medicine: principles and practice. World J Nephrol. 2018 Jan 6;7(1):1. doi: 10.5527/wjn.v7.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Melmed S., Auchus R.J., GoldfineAB, Rosen C.J. 14/e. Elsevier India; 2020. (Williams textbook of endocrinology). [Google Scholar]
- 9.Worthley L.I., Guerin M., Pain R.W. For calculating osmolality, the simplest formula is the best. Anesthesia and intensive care. 1987 May;15(2):199–202. doi: 10.1177/0310057X8701500214. [DOI] [PubMed] [Google Scholar]
- 10.Shen Y., Cheng X., Ying M., Chang H.T., Zhang W. Association between serum osmolarity and mortality in patients who are critically ill: a retrospective cohort study. BMJ Open. 2017 May 1;7(5) doi: 10.1136/bmjopen-2016-015729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Muneer M., Akbar I. In: Islam M.S., editor. vol. 1307. Springer; Cham: 2020. Acute metabolic emergencies in diabetes: DKA, HHS, and EDKA. (Diabetes: from research to clinical practice. Advances in experimental medicine and biology). [DOI] [PubMed] [Google Scholar]
- 12.Rasouli M. Basic concepts and practical equations on osmolality: a biochemical approach. Clin Biochem. 2016 Aug 1;49(12):936–941. doi: 10.1016/j.clinbiochem.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 13.Lin K.Y., Chen P.C., Chen S.C., Huang Y.L., Chen H.C. Evaluation of the effects of glucose on osmolal gap using freezing point depression and vapour pressure methods. Kaohsiung J Med Sci. 2018 Jul;34(7):409–414. doi: 10.1016/j.kjms.2018.03.002. [DOI] [PubMed] [Google Scholar]
- 14.Stookey J.D. High prevalence of plasma hypertonicity among community-dwelling older adults: results from NHANES III. J Am Diet Assoc. 2005;105:1231–1239. doi: 10.1016/j.jada.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 15.El-Sharkawy A.M., Watson P., Neal K.R. Hydration and outcome in older patients admitted to hospital (The HOOP prospective cohort study) Age Ageing. 2015;44:943–947. doi: 10.1093/ageing/afv119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arévalo-Lorido J.C., Gómez J.C., Formiga F., Conde-Martel A., Carrera-Izquierdo M., Muela-Molinero A., Dávila-Ramos M.F., Serrado-Iglesias A., Manzano-Espinosa L., Montero-Pérez-Barquero M. High serum osmolarity at admission determines a worse outcome in patients with heart failure: is a new target emerging? Int J Cardiol. 2016 Oct 15;221:238–242. doi: 10.1016/j.ijcard.2016.07.084. [DOI] [PubMed] [Google Scholar]
- 17.Nag C., Das K., Ghosh M. Plasma osmolality in acute spontaneous intracerebral haemorrhage: does it influence hematoma volume and clinical outcome? J Res Med Sci. 2012;17:548–551. [PMC free article] [PubMed] [Google Scholar]
- 18.Bhalla A., Sankaralingam S., Dundas R. Influence of raised plasma osmolality on clinical outcome after acute stroke. Stroke. 2000;31:2043–2048. doi: 10.1161/01.STR.31.9.2043. [DOI] [PubMed] [Google Scholar]
- 19.Gou L., Xiang M., Ran X., Wang F., Zhang S., Li S., Dong K., Chen X., Huang Y., Meng C., Fan Q. Hyperosmolarity deserves more attention in critically ill COVID-19 patients with diabetes: a cohort-based study. Diabetes, Metab Syndrome Obes Targets Ther. 2021;14:47. doi: 10.2147/DMSO.S284148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holtfreter B., Bandt C., Kuhn S.O., Grunwald U., Lehmann C., Schütt C., Gründling M. Serum osmolality and outcome in intensive care unit patients. Acta Anaesthesiol Scand. 2006 Sep;50(8):970–977. doi: 10.1111/j.1399-6576.2006.01096.x. [DOI] [PubMed] [Google Scholar]
- 21.Wannamethee S.G., Shaper A.G., Lennon L., Papacosta O., Whincup P. Mild hyponatremia, hypernatremia and incident cardiovascular disease and mortality in older men: a population-based cohort study. Nutr Metabol Cardiovasc Dis. 2016 Jan 1;26(1):12–19. doi: 10.1016/j.numecd.2015.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xie J., Cui K., Hao H., Zhang Y., Lin H., Chen Z., Huang X., Cao S., Liao W., Bin J., Kitakaze M. Acute hyperglycemia suppresses left ventricular diastolic function and inhibits autophagic flux in mice under prohypertrophic stimulation. Cardiovasc Diabetol. 2016 Dec;15(1):1–3. doi: 10.1186/s12933-016-0452-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kitabchi A.E., Umpierrez G.E., Miles J.M., Fisher J.N. Hyperglycemic crises in adult patients with diabetes. Diabetes Care. 2009 Jul 1;32(7):1335–1343. doi: 10.2337/dc09-9032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Corona G., Giuliani C., Parenti G. Moderate hyponatremia is associated with increased risk of mortality: evidence from a meta-analysis. PloS One. 2013;8(12) doi: 10.1371/journal.pone.0080451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chuang C., Guo Y.W., Chen H.S. Corrected sodium levels for hyperglycemia are better predictors than measured sodium levels for clinical outcomes among patients with extreme hyperglycemia. J Chin Med Assoc. 2020 Sep;83(9):845. doi: 10.1097/JCMA.0000000000000407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Robba C., Bilotta F. Admission hyperglycemia and outcome in ICU patients with sepsis. J Thorac Dis. 2016 Jul;8(7):E581. doi: 10.21037/jtd.2016.06.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu Q., Chen H., Li J., Huang X., Lai L., Li S. Fasting blood glucose predicts the occurrence of critical illness in COVID-19 patients: a multicenter retrospective cohort study. J Infect. 2020;81(3):e20–e23. doi: 10.1016/j.jinf.2020.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Trabulus S., Karaca C., Balkan, Dincer M.T., Murt A., Ozcan S.G., Karaali R., Mete B., Bakir A., Kuskucu M.A., Altiparmak M.R. Kidney function on admission predicts in-hospital mortality in COVID-19. PloS One. 2020 Sep 3;15(9) doi: 10.1371/journal.pone.0238680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Raza A., Estepa A., Chan V., Jafar M.S. Acute renal failure in critically ill COVID-19 patients with a focus on the role of renal replacement therapy: a review of what we know so far. Cureus. 2020 Jun;12(6) doi: 10.7759/cureus.8429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun J., Zhu A., Li H., Zheng K., Zhuang Z., Chen Z., Shi Y., Zhang Z., Chen S.B., Liu X., Dai J. Isolation of infectious SARS-CoV-2 from the urine of a COVID-19 patient. Emerg Microb Infect. 2020 Jan 1;9(1):991–993. doi: 10.1080/22221751.2020.1760144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Keenan C.R., Murin S., White R.H. High risk for venous thromboembolism in diabetics with the hyperosmolar state: comparison with other acute medical illnesses. J Thromb Haemostasis. 2007 Jun;5(6):1185–1190. doi: 10.1111/j.1538-7836.2007.02553.x. [DOI] [PubMed] [Google Scholar]
- 32.Voicu S., Ketfi C., Stépanian A., Chousterman B.G., Mohamedi N., Siguret V., Mebazaa A., Mégarbane B., Bonnin P. Pathophysiological processes underlying the high prevalence of deep vein thrombosis in critically ill COVID-19 patients. Front Physiol. 2020;11:1680. doi: 10.3389/fphys.2020.608788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johannesdottir S.A., Horváth-Puhó E., Dekkers O.M., Cannegieter S.C., Jørgensen J.O., Ehrenstein V., Vandenbroucke J.P., Pedersen L., Sørensen H.T. Use of glucocorticoids and risk of venous thromboembolism: a nationwide population-based case-control study. JAMA internal medicine. 2013 May 13;173(9):743–752. doi: 10.1001/jamainternmed.2013.122. [DOI] [PubMed] [Google Scholar]
- 34.Abou-Ismail M.Y., Diamond A., Kapoor S., Arafah Y., Nayak L. The hypercoagulable state in COVID-19: incidence, pathophysiology, and management. Thromb Res. 2020 Oct 1;194:101–115. doi: 10.1016/j.thromres.2020.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang L., Yan X., Fan Q., Liu H., Liu X., Liu Z., Zhang Z. D-dimer levels on admission to predict in-hospital mortality in patients with Covid-19. J Thromb Haemostasis. 2020 Jun;18(6):1324–1329. doi: 10.1111/jth.14859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Navarrete J.E., Tong D.C., Cobb J., Rahbari-Oskoui F.F., Hosein D., Caberto S.C., Lea J.P., Franch H.A. Epidemiology of COVID-19 infection in hospitalized end-stage kidney disease patients in a predominantly african-American population. Am J Nephrol. 2021;52(3):190–198. doi: 10.1159/000514752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bähr V., Franzen N., Oelkers W., Pfeiffer A.F., Diederich S. Effect of exogenous glucocorticoid on osmotically stimulated antidiuretic hormone secretion and water reabsorption in man. Eur J Endocrinol. 2006 Dec 1;155(6):845–848. doi: 10.1530/eje.1.02299. [DOI] [PubMed] [Google Scholar]
- 38.Claure-Del Granado R., Mehta R.L. Fluid overload in the ICU: evaluation and management. BMC Nephrol. 2016 Dec;17(1):1–9. doi: 10.1186/s12882-016-0323-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Adeyinka A, Kondamudi NP. Hyperosmolar hyperglycemic nonketotic coma.
- 40.Matz R. Management of the hyperosmolar hyperglycemic syndrome. Am Fam Physician. 1999 Oct 1;60(5):1468–1476. [PubMed] [Google Scholar]