Key Words: animal models, chronic compression, latency, somatosensory evoked potentials, spinal cord injury, time–frequency analysis, time–frequency components, translational study
Abstract
Somatosensory evoked potentials (SEPs) have been widely used to assess neurological function in clinical practice. A good understanding of the association between SEP signals and neurological function is helpful for precise diagnosis of impairment location. Previous studies on SEPs have been reported in animal models. However, few studies have reported the relationships between SEP waveforms in animals and those in humans. In this study, we collected normal SEP waveforms and decomposed them into specific time–frequency components (TFCs). Our results showed three stable TFC distribution regions in intact goats and rats and in humans. After we induced spinal cord injury in the animal models, a greater number of small TFC distribution regions were observed in the injured goat and rat groups than in the normal group. Moreover, there were significant correlations (P < 0.05) and linear relationships between the main SEP TFCs of the human group and those of the goat and rat groups. A stable TFC distribution of SEP components was observed in the human, goat and rat groups, and the TFC distribution modes were similar between the three groups. Results in various animal models in this study could be translated to future clinical studies based on SEP TFC analysis. Human studies were approved by the Institutional Review Board of the University of Hong Kong/Hospital Authority Hong Kong West Cluster (approval No. UM 05-312 T/975) on December 5, 2005. Rat experiments were approved by the Committee on the Use of Live Animals in Teaching and Research of Li Ka Shing Faculty of Medicine of the University of Hong Kong (approval No. CULART 2912-12) on January 28, 2013. Goat experiments were approved by the Animal Ethics Committee of Affiliated Hospital of Guangdong Medical University (approval No. GDY2002132) on March 5, 2018.
Chinese Library Classification No. R444; R338.8; R745.4
Introduction
Somatosensory evoked potentials (SEPs) have been widely used in clinical practice to evaluate the integrity of somatosensory pathways (MacDonald et al., 2019; Muzyka and Estephan, 2019; Markand, 2020). In clinical settings, SEPs are used as a tool for intraoperative neurological monitoring (MacDonald et al., 2019), functional testing (Feng et al., 2020), and differential diagnosis (Melachuri et al., 2019). To support the appropriate use of SEPs in clinical practice, it is critical to have a good understanding of the association between SEP signals and neurological function. Any potential associations should be supported by findings from animal experiments, especially in areas of research in which it is difficult to confirm pathological conditions in humans.
Many animal models have been used to investigate SEPs and to verify the importance of SEP changes observed in humans (Allison and Hume, 1981; Tator, 2006; Ma et al., 2016; Jiang et al., 2017). Rats are the most widely used animals in experimental studies, and such studies have reported that somatosensory information from the periphery is transmitted to similar anatomical levels of the spinal cord in both rats and humans (Li et al., 2017; Sharif-Alhoseini et al., 2017; Krisa et al., 2018; Cheng et al., 2019). Among larger animals, the spinal cord of cats is similar in diameter and length to that of humans (Krisa et al., 2018). Comparisons of the electrophysiological functional outcomes of rats and cats show that the decline and duration of recovery differ after surgery in these two animals (Khan et al., 1999). Dogs have been used to construct contusion models of spinal cord injury (Allen, 1911) and are considered to be an intermediate between rodents and humans. They offer the advantages of convenient examination of neurological features and the collection of detailed pathophysiological information (Nardone et al., 2017). The cervical spine of goats is as upright as that in humans, with the anterior/posterior and left/right diameters of the thoracic spinal canals reported to be much larger (goats: 8.2 mm and 9.5 mm, respectively) than those in rodents (rodents: about 2.3 mm and 4.5 mm, respectively) and similar to those in humans (humans: 16.2 mm and 14.7 mm, respectively) (Panjabi et al., 1991; Cao et al., 2014).
Previous studies have shown that the components of SEP waveforms in monkeys and rats correspond closely to various anatomical structures along the somatosensory pathways (Peterson et al., 1995; Cheng et al., 2019; MacDonald et al., 2019). Compared with traditional time domain-based SEP identification and measurement methods, time–frequency analysis provides earlier and more sensitive information regarding the nervous system (Hu et al., 2001, 2003, 2011a). Previous studies have used a high-resolution algorithm, known as matching pursuit, to analyze SEPs, with the SEP signals decomposed into a series of time–frequency components (TFCs) to identify specific injury locations in the spinal cord of rats (Zhang et al., 2009; Wang et al., 2017). The main TFCs have generally been used to assess spinal cord integrity, whereas small TFCs contain information about neurological deficits (Wang et al., 2015, 2017). However, there remains a lack of detailed time–frequency results of SEPs from larger animals.
Moreover, it is difficult to translate SEP results from animals to humans because of differences in anatomical structures (Arezzo et al., 1979). Even within the same animal, the methods used to evaluate spinal cord function are not standardized, so it is difficult to compare studies or to apply findings to clinical practice. So far, no systematic analysis of the relationship between the SEPs from different animals and those from humans has been conducted. Comparative studies would be useful to reveal the characteristics of SEPs recorded from different animals and to investigate correlations in SEPs across different animals (Blight, 1991).
The purpose of this study was to explore the time–frequency characteristics of SEP waveforms, and to identify relationships of TFC distribution patterns between humans and different animals. We chose two representative animals, the rat and the goat, as small and large animal models, respectively. In the experiments presented here, SEPs were collected from humans, rats and goats, and the TFCs of the SEPs were analyzed to elucidate the electrophysiological correlations between SEPs in animals and humans.
Materials and Methods
Three normal SEP signal datasets were recorded from 15 human participants undergoing surgical correction for scoliosis, and from 15 adult female Sprague–Dawley rats (weighing 250–330 g, aged 3–4 months, specific-pathogen-free level), and 15 adult female goats (weighing 15–20 kg, aged 3–6 months, clean level). All experimental animals were in a normal state with intact neurological function. Additionally, animals were randomly selected to form two injury groups, one comprising 7 goats and the other 12 rats. Animals in the injury groups received chronic compression at the C4 level of the spinal cord.
The procedures of rat and goat experiments were approved by the Committee on the Use of Live Animals in Teaching and Research of Li Ka Shing Faculty of Medicine of the University of Hong Kong (approval No. CULART 2912-12 on January 28, 2013) and the Animal Ethics Committee of Affiliated Hospital of Guangdong Medical University (approval No. GDY2002132 on March 5, 2018). All animal experiments were designed and reported in accordance with the Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines. The human experiments conformed to the ethical guidelines of the 1975 Declaration of Helsinki.
To collect SEPs from human participants, 15 patients (9 female and 6 male; 20 ± 7.1 years old) with idiopathic scoliosis undergoing a spinal correction operation in Queen Mary Hospital from October 2006 to May 2007 were recruited voluntarily, and all were neurologically normal with a clinical diagnosis. All patients received general anesthesia during the operation (propofol, 2–4 mg/kg and fentanyl, 3–5 µg/kg). SEP signals were recorded before any surgical incision was made to the patients. Informed consent was obtained from every patient and clinical ethical approval was awarded by the Institutional Review Board of the University of Hong Kong/Hospital Authority Hong Kong West Cluster (approval No. UM 05-312 T/975) on December 5, 2005.
Spinal cord compression in rats was performed by implanting a water-absorbing material (3 mm × 1 mm × 1 mm; Shenzhen Fulin, Shenzhen, China) (Hu et al., 2011b; Long et al., 2014; Cui et al., 2019). This material was carefully inserted into the right posterolateral side of the spinal canal at the C4 level, after which it expanded with a maximal expansion of 700% within 24 hours to provide compression. Spinal cord compression in goats was performed by posterior balloon (Edwards Lifesciences, Irvine, CA, USA) injection into the C4 intervertebral space (Cao et al., 2014; Jiang et al., 2017). The balloon was expanded slowly by percutaneous valve injection of water to produce cord compression.
Neurological function was evaluated using the Basso, Beattie, and Bresnahan (BBB) locomotor rating scale (Basso et al., 1995) before and after injury. The BBB scale ranges from complete neurological function loss (score 0) to normal neurological function (score 21). All animals were tested before surgery, and all received the maximum BBB score without any exception. After the compression injury, the BBB scale measured a slight decrease in neurological function, with an average BBB score of about 16.
SEP recordings were performed under general anesthesia in all animals, with anesthesia induced by intravenous injection of pentobarbital sodium (Sigma, St. Louis, MO, USA) in goats (40 mg/kg) and intraperitoneal injection of pentobarbital sodium in rats (60 mg/kg). After being anesthetized, animals were placed in a prone position over a warmed blanket to maintain their skin temperature above 32°C. SEPs were collected from humans under similar stimulation and recording conditions to those reported in previous studies (Hu et al., 2008; Cui et al., 2015). To elicit SEPs, the median nerve at the wrist was stimulated with a constant current stimulus using needle electrodes, using a current intensity that produced detectable twitching. SEP signals were recorded using surface electrodes placed at comparable locations in the animals and in humans, except that in humans, Fz was used as a reference, whereas in the animals Fpz was used (based on the 10–20 international system of EEG electrode placement). All signals were recorded at a sampling rate of 5 kHz using an evoked potential recording system (YRKJ-A2004; Zhuhai Yiruikeji, Zhuhai, China), amplified 10,000 times using a 20–2000 Hz bandpass filter, and automatic artifact rejection was performed. The sweep time of the SEP recording was 100 ms. In total, 1000 trials were recorded for each raw SEP.
SEP signal analysis
Raw SEP signals from each person or animal were averaged. Then, a time–frequency analysis was performed on each averaged SEP. In the time domain, latency and amplitude were considered to be the typical parameters of the SEP waveform (MacDonald et al., 2019). In the time-frequency domain, the matching pursuit method was used to calculate the time–frequency distribution of SEPs and extract the TFCs, which can decompose SEP into several time-frequency components. Three parameters were measured: peak time (measured relative to the onset of stimulation), peak frequency, and peak power (measured as the signal energy at the peak location) (Cui et al., 2019). The most distinct peak with high energy was generally defined as the main TFC, and peaks with lower energy were defined as sub-TFCs.
In this study, all signal processing routines used for the analysis were developed in MATLAB (version R2016a; MathWorks, Natick, MA, USA).
Statistical analysis
Data are presented as the mean ± standard deviation (SD). For each component, we compared the peak time, peak frequency, and peak power between two species. Correlations between the three groups with intact spinal cord function were determined using the Pearson correlation coefficient, which was calculated using the SPSS software package (version 22; IBM Corp., Armonk, NY, USA). Similarly, correlations were analyzed between the groups before and after spinal cord injury in the rat and goat groups. Linear regression analysis was used to analyze the linear relationships between the human and animal groups; it yielded the parameter R2 between 0 and 1, which represents the fitting performance of the regression model. R2 values closer to 1 indicate a better degree of fit. Statistical significance was set at a threshold of α = 0.05 using a bilateral (two-tailed) test. P < 0.05 and an absolute correlation coefficient R ≥ 0.50 were considered to indicate a significant linear correlation.
The regression of the human group relative to each animal group was expressed as:
y = ax + b,
where y is the value of the characteristic parameter in the human group, x is the corresponding value in each animal group, a and b are the coefficient of a polynomial that fit the data x and y.
Results
Time domain analysis of SEP waveforms among humans, rats and goats
As shown in Figure 1, the SEP waveforms recorded from corresponding locations on the scalp of the animal and human groups were similar between the groups. The P1 parameters of SEP waveforms were present with different latencies; the peak latencies were a few milliseconds later in the human group (human: 19.47 ± 0.96 ms) than in the animal groups (rat: 11.17 ± 0.84 ms, R = 0.931, P < 0.001; goat: 11.80 ± 1.15 ms, R = 0.983, P < 0.001; Pearson’s correlation analysis).
Figure 1.
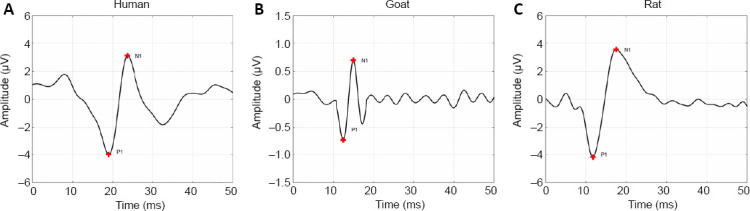
Typical SEP waveforms of human, goat and rat.
(A–C) SEP waveforms from a human (A), goat (B), and rat (C). The dominant SEP waveforms were simplified with recognizable N1 and P1. These SEPs had different latencies. SEP: Somatosensory evoked potential.
Time–frequency domain analysis of SEP among humans, rats and goats
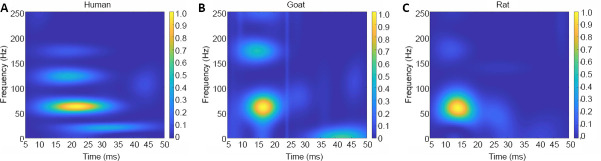
The distribution of the SEP TFCs was similar in the human and the normal goat and rat groups. There was a clear main component with high peak power in each group, which was located in an area with unique time and frequency ranges (Figure 2). However, these main components occurred at different peak times and peak frequencies between the groups (Figure 2A–C and Table 1). Additionally, there were some small sub-TFCs with relatively low peak power, which could not be seen clearly because of signal overlap.
Figure 2.
SEP time–frequency components of human, goat and rat groups.
(A–C) Time–frequency component distributions from the human (A), goat (B), and rat (C) groups. The scales of the heat-map were used to express the peak power values of SEP TFCs, where hot color represents high peak power. These SEP TFCs were located in different time and frequency regions. SEP: Somatosensory evoked potential; TFC: time–frequency component.
Table 1.
Parameter values of SEP TFCs of normal human, rat and goat groups
| Parameter | Peak time (ms) | Peak frequency (Hz) | Peak power |
|---|---|---|---|
| Main TFC | |||
| Human | 23.58±4.50 | 62.67±18.11 | 19.67±7.70 |
| Goat | 17.90±3.43 | 75.33±11.54 | 2.74±1.46 |
| Rat | 15.30±2.93 | 53.73±5.84 | 29.65±11.63 |
| Sub-TFC S1 | |||
| Human | 22.51±2.70 | 135.13±27.57 | 6.23±4.30 |
| Goat | 26.18±4.00 | 177.13±35.03 | 2.55±2.86 |
| Rat | 12.75±1.80 | 166.07±32.58 | 5.09±3.32 |
| Sub-TFC S2 | |||
| Human | 38.06±4.70 | 69.20±24.62 | 6.14±5.21 |
| Goat | 40.73±3.36 | 150.07±32.84 | 1.24±0.65 |
| Rat | 24.05±2.18 | 92.93±31.55 | 2.30±1.01 |
Data are expressed as the mean ± SD (n = 15/species). SEP: Somatosensory evoked potential; TFC: time–frequency component.
The distribution patterns of the main and sub-TFCs were then compared between the groups in detail. Further analyses revealed that the distribution patterns of the main TFCs in the human and the normal goat and rat groups showed different stable regions (marked “M” in Figure 3). The main TFCs of the human group were located within a time-frequency region of 20–30 ms and 40–70 Hz. In the normal goat group, the main TFC distribution was concentrated within 15–25 ms and 60–100 Hz, demonstrating a slightly shorter peak time and a higher frequency than the human group (Figure 3A). In the normal rat group, the main TFC distribution was clustered within 10–18 ms and 40–70 Hz, which indicates that the main SEP TFCs of the normal rat group had a similar frequency pattern to the human group, but an earlier peak latency (Figure 3B).
Figure 3.
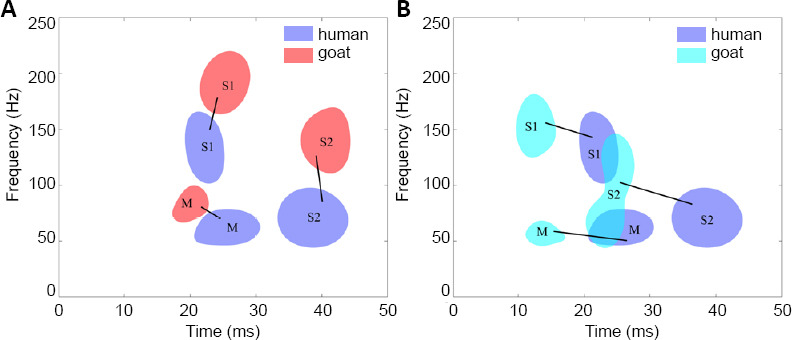
Distribution patterns of SEP TFCs from normal humans, rat and goat groups.
(A) Distribution difference between the human and goat groups. (B) Distribution difference between the human and rat groups. The distribution regions of the main components were marked as “M”, and the sub-TFCs were marked as “S1” and “S2”. The distribution patterns of the components in the rat and goat groups were similar to that in the human group, and the corresponding distribution regions of TFCs that appeared in both the human group and each animal group were matched with black lines. SEP: Somatosensory evoked potential; TFC: time–frequency component.
Moreover, distribution patterns of sub-TFCs from the human group were compared with those from the normal animal groups. The sub-TFC distributions in the human group were concentrated in two regions (S1: 19–25 ms, 100–165 Hz; S2: 32–45 ms, 40–100 Hz; marked “S1” and “S2” in Figure 3). Similar to the human group, two corresponding stable sub-TFC distribution regions were also observed around the human regions in the normal goat and rat groups. As shown in Figure 3A, the corresponding sub-TFC distribution regions in the goat group were located within S1 (20–30 ms, 160–220 Hz) and S2 (35–45 ms, 110–165 Hz). Compared with the human group, the sub-TFCs in the goat group were concentrated within a similar time range, but at a higher frequency. As shown in Figure 3B, the corresponding sub-TFCs in the rat group were located within S1 (10–17 ms, 135–185 Hz) and S2 (20–27 ms, 40–150 Hz). These results indicated that the two stable regions of sub-TFCs in the normal goat group and the human group were closer than those in the normal rat group and the human group.
The distribution patterns of SEP TFCs in the spinal cord injury animals showed a greater number of TFC distribution regions than those in the normal rat and goat groups (Figure 4). Both the corresponding main TFCs (marked “M” in Figure 4) and the sub-TFCs observed before injury (marked “S1” and “S2” in Figure 4) were still present after spinal cord injury in both animal groups. Although the time range of sub-TFCs S1 and S2 was longer and the frequency range changed after injury, these SEP distributions before and after showed a significant correlation (Table 2). Furthermore, additional small sub-TFCs appeared in both goat and rat injury groups (marked “S3”, “S4”, and “S5” in Figure 4), which may indicate the spinal cord injury.
Figure 4.
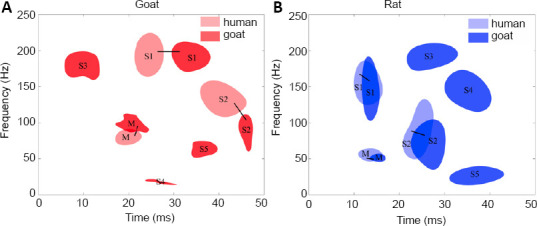
TFC distribution patterns comparison between normal and spinal injury model goat (A) and rat (B) groups.
The distribution regions of the main components were marked as “M”, the region of sub-TFC that appeared in both the normal and injury groups were marked as “S1” and “S2”, and the other small sub-TFCs in the injury group only were marked as “S3”, “S4” and “S5”. In both goats and rats, the distribution patterns of the components in the injury groups were similar to those of the normal groups, and the corresponding distribution regions of TFCs that appeared in both the normal groups and injury groups were connected with black lines. SEP: Somatosensory evoked potential; TFC: time–frequency component.
Table 2.
Pearson correlation analysis of SEP characteristics between normal and injury rats and goats
| Parameter | Goat | Rat |
|---|---|---|
| Main TFC | ||
| Peak time | R = 0.905* | R = 0.903*** |
| P = 0.005 | P < 0.001 | |
| Peak frequency | R = 0.963*** | R = 0.897*** |
| P < 0.001 | P < 0.001 | |
| Peak power | R = 0.896* | R = 0.698* |
| P = 0.006 | P = 0.012 | |
| Sub-TFC S1 | ||
| Peak time | R = 0.925* | R = 0.975*** |
| P = 0.003 | P < 0.001 | |
| Peak frequency | R = 0.961* | R = 0.923*** |
| P = 0.001 | P < 0.001 | |
| Peak power | R = 0.909* | R = 0.722* |
| P = 0.005 | P = 0.008 | |
| Sub-TFC S2 | ||
| Peak time | R = 0.976*** | R = 0.941*** |
| P < 0.001 | P < 0.001 | |
| Peak frequency | R = 0.944* | R = 0.943*** |
| P = 0.001 | P < 0.001 | |
| Peak power | R = 0.792* | R = 0.838* |
| P < 0.034 | P = 0.001 |
*P < 0.05, ***P < 0.001. TFC: Time–frequency component.
Inter-group relationship analysis of SEP characteristics in normal animal and human groups
Inter-group relationships were analyzed (Table 3) and the relationships between these potentials in each group were similar. The results showed a significant correlation (R > 0.828, P < 0.05) between the parameters describing SEP characteristics (peak time, peak frequency, and peak power) in the animal and human groups.
Table 3.
Pearson correlation analysis of SEP characteristics between human and normal animal groups
| Parameter | Human vs. Goat | Human vs. Rat |
|---|---|---|
| Main TFC | ||
| Peak time | R = 0.929*** | R = 0.965*** |
| P < 0.001 | P < 0.001 | |
| Peak frequency | R = 0.873*** | R = 0.962* |
| P < 0.001 | P = 0.001 | |
| Peak power | R = 0.950*** | R = 0.924*** |
| P < 0.001 | P < 0.001 | |
| Sub-TFC S1 | ||
| Peak time | R = 0.961*** | R = 0.955*** |
| P < 0.001 | P < 0.001 | |
| Peak frequency | R = 0.934*** | R = 0.879*** |
| P < 0.001 | P < 0.001 | |
| Peak power | R = 0.910*** | R = 0.906*** |
| P < 0.001 | P < 0.001 | |
| Sub-TFC S2 | ||
| Peak time | R = 0.988*** | R = 0.942*** |
| P < 0.001 | P < 0.001 | |
| Peak frequency | R = 0.962*** | R = 0.971*** |
| P < 0.001 | P < 0.001 | |
| Peak power | R = 0.828*** | R = 0.910*** |
| P < 0.001 | P < 0.001 |
*P < 0.05, ***P < 0.001. TFC: Time–frequency component.
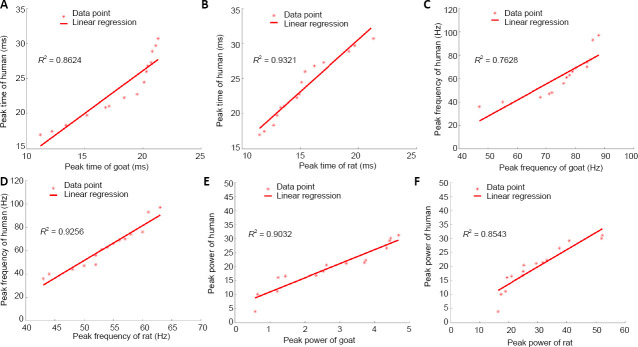
In the regression model, the R2 between the human and rat groups was higher (R2 > 0.772) than that between the human and goat groups (R2 > 0.686; Table 4). To better illustrate this, Figure 5 shows examples of the linear regression curves of the main SEP TFCs between the human and normal animal groups. According to the values of determination coefficient R2 in Figure 5, there are good linear relationships in the peak time, peak frequency, and peak power of main SEP TFCs between the human and normal animal groups. For example, in Figure 5A, R2 = 0.8624 means that the regression model can explain 86.24% of the variance of peak time in the human group, and the fitting performance is very good.
Table 4.
Linear regression of SEP characteristics between human and normal animal groups
| Parameter | Human vs. Goat | Human vs. Rat |
|---|---|---|
| Main TFC | ||
| Peak time | y = 1.2209x + 1.7252 | y = 1.4875x + 0.8216 |
| R2 = 0. 8624 | R2 = 0.9321 | |
| Peak frequency | y = 1.371x – 40.617 | y = 2.985x – 97.73 |
| R² = 0.7628 | R2 = 0.9256 | |
| Peak power | y = 5.0089x + 5.9297 | y = 0.6115x + 1.5394 |
| R2 = 0.9032 | R2 = 0.8543 | |
| Sub-TFC S1 | ||
| Peak time | y = 0.6483x + 5.5334 | y = 1.4323x + 4.2399 |
| R2 = 0.9229 | R2 = 0.9119 | |
| Peak frequency | y = 0.7347x + 5.0005 | y = 0.7437x + 11.626 |
| R2 = 0.8717 | R2 = 0.7728 | |
| Peak power | y = 1.371x + 2.734 | y = 1.1754x + 0.2508 |
| R2 = 0.8282 | R2 = 0.8202 | |
| Sub-TFC S2 | ||
| Peak time | y = 1.3813x – 18.195 | y = 2.0256x – 10.661 |
| R2 = 0.9758 | R2 = 0.8875 | |
| Peak frequency | y = 0.721x – 39.002 | y = 0.7579x – 1.237 |
| R2 = 0.9247 | R2 = 0.9431 | |
| Peak power | y = 6.6148x – 2.0369 | y = 4.6861x – 4.6497 |
| R2 = 0.6864 | R2 = 0.8277 |
TFC: Time–frequency component.
Figure 5.
Linear regression curve of main SEP TFCs between human and animals.
(A–F) Linear regressions of main SEP TFCs between peak time: human and goat groups (A), human and rat groups (B); between peak frequency: human and goat groups (C), human and rat groups (D); and between peak power: human and goat groups (E), human and rat groups (F). Data points were marked as “*”, and red lines represent the regression lines. R2 is the determination coefficient, which represents the fitting performance of the model. SEP: Somatosensory evoked potential; TFC: time–frequency component.
Discussion
SEPs are useful for assessing the integrity of somatosensory neural function (Arezzo et al., 1979), and provide an objective measurement of neurological impairment severity based on changes in latency and amplitude (Liverman et al., 2005; Hernández-Laín et al., 2011; Ye et al., 2016). Additionally, SEP recordings yield a series of waveforms associated with sequential neural activities along the somatosensory pathways. The components in this study are different from those reported in some electrophysiological papers. Previous studies in clinical electrophysiology have defined some standard components in the time-domain waveform, such as N9, N17, and others (Emori et al., 1991; Sonoo et al., 1996). In this study, the components of SEPs were decomposed in the time-frequency domain to study the neurophysiological significance of SEPs from another perspective. Time-frequency analysis can be used to decompose SEP waveforms into smaller components in the time-frequency domain. Understanding the neurophysiological meaning of these TFCs is essential to developing clinical applications, which rely on animal studies as it is unethical to validate such research in humans. This study demonstrated the existence of stable TFCs in the normal SEPs of the human and animal groups. After registering the main components in the time–frequency domain, two consistent sub-TFCs in the animal groups showed strong correlations with the TFC patterns in the human group. Additionally, the animal spinal cord injury groups showed changes in SEP sub-TFCs, which suggests that these SEP sub-TFCs may be useful in detecting neurological deficits. The results of this study could help findings from animal experiments to be translated into clinical applications.
In this study, SEPs were recorded from rats, goats, and human participants to compare waveform morphology in the time–frequency domain. In previous studies, time–frequency analysis presented various components distribution in the time–frequency domain. The main peak component of the SEP in healthy humans and rats has been located within a specific stable region (Hu et al., 2011a; Wang et al., 2015). In this study, we found a stable region of main SEP TFCs in all three normal groups. The main peak TFC feature reflects the major signal passing along the somatosensory pathway, which is believed to indicate the same underlying neurophysiological response to the stimulation in both humans and other animals. Additionally, we found some other small stable TFCs. In the normal condition, each of the three groups showed two stable sub-TFCs around the main TFC regions. Overall, the characteristics of the SEP sub-TFCs in the animal groups had high similarity compared with those in the human group. After registering the main TFC in the time-frequency domain, we observed a clear relationship between the sub-TFCs between groups. Compared with SEPs of the human group, which showed a peak latency of approximately 20 ms, the SEPs of the rat and goat groups had relatively shorter latencies, which is consistent with previous reports (Hu et al., 2008; Hernández-Laín et al., 2011; Wen et al., 2014; Wang et al., 2015, 2017; Jiang et al., 2017). The differences in latency reflect inter-group differences in the length of conduction pathways and in cortical processing time, which supports the hypothesis that there is correspondence in such pathways between humans and other animals (Arezzo et al., 1981). Sensory transduction pathways differ between the groups in the present study, with the difference in peak latency related to the larger dimensions of the human intra-cortical dendritic and axonal fields. Therefore, differences in the peak time and peak frequency between the human and animal groups likely resulted from differences in the structure and length of neurological pathways in the groups studied (small animal, large animal, and human).
Peak power and peak amplitude have been reported to show large inter-subject variability (Hu et al., 2011a). Among the SEP parameters used for inter-group comparisons, the normal statistical ranges of latency, peak time, and peak frequency are more reliable than those of amplitude and peak power. In this study, we found a specific correlation between SEP parameters between the different animal groups, with linear regression analysis showing a positive correlation between the animal groups (goat and rat) and the human group with a high goodness of fit. In accordance with the regression equation, we predicted that it would be possible to estimate the human SEP signal from the animal signal results. In this way, electrophysiological findings in commonly used animal models can be translated to spinal cord diseases in human studies, and used to develop new diagnoses for clinical practice.
In the spinal cord injury groups, the number of TFC distribution regions increased and showed more diffuse patterns compared with the normal groups. The TFCs of the injury groups tended to have longer latencies and wider distribution ranges both in the time and frequency domains compared with those of the normal groups. Spinal cord compression would be expected to affect the speed of stimulation conduction and produce several smaller components related to anatomical spinal cord deficits, as has also been reported in previous studies (Hu et al., 2003, 2011a; Wang et al., 2017). Previous studies have performed the time–frequency component analysis only in rats and have not investigated other kinds of animals. After spinal cord injury, we observed some sub-TFCs (S3–S5) in both the rat and goat models. The explanation of these waveforms that appeared after spinal cord injury is outside the scope of this study. These components may be noise in the sensory cortex produced by stimulation to peripheral nerves after spinal cord nerve injury. A more detailed examination of S3–S5 is needed and will be required to ascertain whether these abnormal TFC features after spinal cord injury can be detected in patients with similar neurological deficits.
This paper mainly discussed the relationships between SEP in humans and different animal models, in effort to translate the results of animal experiments. The purpose of the SEP analysis in animals after spinal cord injury was to show that the SEP time–frequency component changes when there are pathological changes. The correlation between SEP changes and nerve injury severity, and how the findings in animal models correspond to the human pathological changes, were not within the scope of the present study and need to be investigated in future studies.
We found several stable TFCs reflecting small waveforms within the overall SEP waveform morphology. We observed changes in TFC patterns in the human, goat, and rat groups, with a consistent trend in rats and goats with spinal cord injury. These findings motivate further study to explore similar patterns in humans with similar neurological deficits. This study provides a foundation for translating SEP findings in animals to clinical applications in humans.
Footnotes
Conflicts of interest:All the authors declare that they have no competing interests.
Financial support:This work was supported by the National Natural Science Foundation of China, No. 81871768 (to YH); the Natural Science Foundation of Tianjin of China, No. 18JCYBJC29600 (to HYC); and High Level-Hospital Program, Health Commission of Guangdong Province of China, No. HKUSZH201902011 (to YH). The funders had no roles in the study design, conduction of experiment, data collection and analysis, decision to publish, or preparation of the manuscript.
Institutional review board statement:Human experiment was approved by the Institutional Review Board of the University of Hong Kong/Hospital Authority Hong Kong West Cluster (approval No. UM 05-312 T/975) on December 5, 2005; rat experiment was approved by the Committee on the Use of Live Animals in Teaching and Research of Li Ka Shing Faculty of Medicine of the University of Hong Kong (approval No. CULART 2912-12) on January 28, 2013; goat experiment was approved by the Institutional Committee of Affiliated Hospital of Guangdong Medical University (approval No. GDY2002132) on March 5, 2018.
Declaration of patient consent: The authors certify that they have obtained the consent forms from patients. In the form, patients have given their consents for the data and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published.
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement:Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Funding:This work was supported by the National Natural Science Foundation of China, No. 81871768 (to YH); the Natural Science Foundation of Tianjin of China, No. 18JCYBJC29600 (to HYC); and High Level-Hospital Program, Health Commission of Guangdong Province of China, No. HKUSZH201902011 (to YH).
C-Editor: Zhao M; S-Editors: Yu J, Li CH; L-Editors: McCollum L, Yu J, Song LP; T-Editor: Jia Y
References
- 1.Allen AR. Surgery of experimental lesion of spinal cord equivalent to crush injury of fracture dislocation of spinal column: a preliminary report. J Am Med Assoc. 1911;LVII:878–880. [Google Scholar]
- 2.Allison T, Hume AL. A comparative analysis of short-latency somatosensory evoked potentials in man, monkey, cat, and rat. Exp Neurol. 1981;72:592–611. doi: 10.1016/0014-4886(81)90008-x. [DOI] [PubMed] [Google Scholar]
- 3.Arezzo J, Legatt AD, Vaughan HG., Jr Topography and intracranial sources of somatosensory evoked potentials in the monkey. I Early components. Electroencephalogr Clin Neurophysiol. 1979;46:155–172. doi: 10.1016/0013-4694(79)90065-8. [DOI] [PubMed] [Google Scholar]
- 4.Arezzo JC, Vaughan HG, Jr, Legatt AD. Topography and intracranial sources of somatosensory evoked potentials in the monkey. II Cortical components. Electroencephalogr Clin Neurophysiol. 1981;51:1–18. doi: 10.1016/0013-4694(81)91505-4. [DOI] [PubMed] [Google Scholar]
- 5.Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- 6.Blight AR. Morphometric analysis of a model of spinal cord injury in guinea pigs, with behavioral evidence of delayed secondary pathology. J Neurol Sci. 1991;103:156–171. doi: 10.1016/0022-510x(91)90159-5. [DOI] [PubMed] [Google Scholar]
- 7.Cao P, Zheng Y, Zheng T, Sun C, Lu J, Rickett T, Shi R. A model of acute compressive spinal cord injury with a minimally invasive balloon in goats. J Neurol Sci. 2014;337:97–103. doi: 10.1016/j.jns.2013.11.024. [DOI] [PubMed] [Google Scholar]
- 8.Cheng XH, Zhang L, Fu J. Somatosensory evoked potential changes and decompression timing for spinal cord function recovery and evoked potentials in rats with spinal cord injury. Brain Res Bull. 2019;146:7–11. doi: 10.1016/j.brainresbull.2018.12.003. [DOI] [PubMed] [Google Scholar]
- 9.Cui H, Wang Y, Xie X, Xu S, Hu Y. Single Trial Extraction of Somatosensory Evoked Potentials for Monitoring Spinal Cord Injury: An Animal Study. J Med Imaging Health Inform. 2015;5:385–390. [Google Scholar]
- 10.Cui H, Li H, Li G, Kang C, Yao X, Feng S, Hu Y. Utility of trial-to-trial latency variability of somatosensory evoked potentials for diagnosis of spinal cord demyelination. J Neurotrauma. 2019;36:3356–3362. doi: 10.1089/neu.2018.6293. [DOI] [PubMed] [Google Scholar]
- 11.Emori T, Yamada T, Seki Y, Yasuhara A, Ando K, Honda Y, Leis AA, Vachatimanont P. Recovery functions of fast frequency potentials in the initial negative wave of median SEP. Electroencephalogr Clin Neurophysiol. 1991;78:116–123. doi: 10.1016/0013-4694(91)90111-g. [DOI] [PubMed] [Google Scholar]
- 12.Feng X, Hu Y, Ma X. Progression prediction of mild cervical spondylotic myelopathy by somatosensory-evoked potentials. Spine (Phila Pa 1976) 2020;45:E560–567. doi: 10.1097/BRS.0000000000003348. [DOI] [PubMed] [Google Scholar]
- 13.Hernández-Laín A, Piedras MJ, Cavada C. Functional evaluation of paraplegic monkeys (Macaca mulatta) over fourteen months post-lesion. Neurosci Res. 2011;69:144–153. doi: 10.1016/j.neures.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 14.Hu Y, Liu H, Luk KD. Time-frequency analysis of somatosensory evoked potentials for intraoperative spinal cord monitoring. J Clin Neurophysiol. 2011a;28:504–511. doi: 10.1097/WNP.0b013e318231c15c. [DOI] [PubMed] [Google Scholar]
- 15.Hu Y, Luk KD, Lu WW, Leong JC. Application of time-frequency analysis to somatosensory evoked potential for intraoperative spinal cord monitoring. J Neurol Neurosurg Psychiatry. 2003;74:82–87. doi: 10.1136/jnnp.74.1.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu Y, Luk KD, Lu WW, Holmes A, Leong JC. Prevention of spinal cord injury with time-frequency analysis of evoked potentials: an experimental study. J Neurol Neurosurg Psychiatry. 2001;71:732–740. doi: 10.1136/jnnp.71.6.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu Y, Ding Y, Ruan D, Wong YW, Cheung KM, Luk KD. Prognostic value of somatosensory-evoked potentials in the surgical management of cervical spondylotic myelopathy. Spine (Phila Pa 1976) 2008;33:E305–310. doi: 10.1097/BRS.0b013e31816f6c8e. [DOI] [PubMed] [Google Scholar]
- 18.Hu Y, Wen CY, Li TH, Cheung MM, Wu EX, Luk KD. Somatosensory-evoked potentials as an indicator for the extent of ultrastructural damage of the spinal cord after chronic compressive injuries in a rat model. Clin Neurophysiol. 2011b;122:1440–1447. doi: 10.1016/j.clinph.2010.12.051. [DOI] [PubMed] [Google Scholar]
- 19.Jiang H, Wang J, Xu B, Yang H, Zhu Q. A model of acute central cervical spinal cord injury syndrome combined with chronic injury in goats. Eur Spine J. 2017;26:56–63. doi: 10.1007/s00586-016-4573-6. [DOI] [PubMed] [Google Scholar]
- 20.Khan T, Havey RM, Sayers ST, Patwardhan A, King WW. Animal models of spinal cord contusion injuries. Lab Anim Sci. 1999;49:161–172. [PubMed] [Google Scholar]
- 21.Krisa L, Runyen M, Detloff MR. Translational challenges of rat models of upper extremity dysfunction after spinal cord injury. Top Spinal Cord Inj Rehabil. 2018;24:195–205. doi: 10.1310/sci2403-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liverman CT, Altevogt BM, Joy JE, Johnson RT. Washington, DC: The National Academies Press; 2005. Spinal cord injury: Progress, promise, and priorities. [Google Scholar]
- 23.Li X, Li G, Luk KDK, Hu Y. Neurorestoratology evidence in an animal model with cervical spondylotic myelopathy. J Neurorestoratol. 2017;5:21–29. [Google Scholar]
- 24.Long HQ, Xie WH, Chen WL, Xie WL, Xu JH, Hu Y. Value of micro-CT for monitoring spinal microvascular changes after chronic spinal cord compression. Int J Mol Sci. 2014;15:12061–12073. doi: 10.3390/ijms150712061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ma Z, Zhang YP, Liu W, Yan G, Li Y, Shields LBE, Walker M, Chen K, Huang W, Kong M, Lu Y, Brommer B, Chen X, Xu XM, Shields CB. A controlled spinal cord contusion for the rhesus macaque monkey. Exp Neurol. 2016;279:261–273. doi: 10.1016/j.expneurol.2016.02.008. [DOI] [PubMed] [Google Scholar]
- 26.MacDonald DB, Dong C, Quatrale R, Sala F, Skinner S, Soto F, Szelényi A. Recommendations of the International Society of Intraoperative Neurophysiology for intraoperative somatosensory evoked potentials. Clin Neurophysiol. 2019;130:161–179. doi: 10.1016/j.clinph.2018.10.008. [DOI] [PubMed] [Google Scholar]
- 27.Markand ON. Somatosensory evoked potentials. In: Markand ON, editor. Clinical evoked potentials: An illustrated manual. Cham: Springer International Publishing; 2020. pp. 139–207. [Google Scholar]
- 28.Melachuri SR, Kaur J, Melachuri MK, Ninaci D, Crammond DJ, Balzer JR, Thirumala PD. The diagnostic accuracy of somatosensory evoked potentials in evaluating neurological deficits during 1057 lumbar interbody fusions. J Clin Neurosci. 2019;61:78–83. doi: 10.1016/j.jocn.2018.10.140. [DOI] [PubMed] [Google Scholar]
- 29.Muzyka IM, Estephan B. Somatosensory evoked potentials. Handb Clin Neurol. 2019;160:523–540. doi: 10.1016/B978-0-444-64032-1.00035-7. [DOI] [PubMed] [Google Scholar]
- 30.Nardone R, Florea C, Höller Y, Brigo F, Versace V, Lochner P, Golaszewski S, Trinka E. Rodent, large animal and non-human primate models of spinal cord injury. Zoology (Jena) 2017;123:101–114. doi: 10.1016/j.zool.2017.06.004. [DOI] [PubMed] [Google Scholar]
- 31.Panjabi MM, Takata K, Goel V, Federico D, Oxland T, Duranceau J, Krag M Thoracic human vertebrae. Quantitative three-dimensional anatomy. Spine (Phila Pa 1976) 1991;16:888–901. doi: 10.1097/00007632-199108000-00006. [DOI] [PubMed] [Google Scholar]
- 32.Peterson NN, Schroeder CE, Arezzo JC. Neural generators of early cortical somatosensory evoked potentials in the awake monkey. Electroencephalogr Clin Neurophysiol. 1995;96:248–260. doi: 10.1016/0168-5597(95)00006-e. [DOI] [PubMed] [Google Scholar]
- 33.Sharif-Alhoseini M, Khormali M, Rezaei M, Safdarian M, Hajighadery A, Khalatbari MM, Safdarian M, Meknatkhah S, Rezvan M, Chalangari M, Derakhshan P, Rahimi-Movaghar V. Animal models of spinal cord injury: a systematic review. Spinal Cord. 2017;55:714–721. doi: 10.1038/sc.2016.187. [DOI] [PubMed] [Google Scholar]
- 34.Sonoo M, Kobayashi M, Genba-Shimizu K, Mannen T, Shimizu T. Detailed analysis of the latencies of median nerve somatosensory evoked potential components, 1: selection of the best standard parameters and the establishment of normal values. Electroencephalogr Clin Neurophysiol. 1996;100:319–331. doi: 10.1016/0168-5597(96)95035-2. [DOI] [PubMed] [Google Scholar]
- 35.Tator CH. Review of treatment trials in human spinal cord injury: issues, difficulties, and recommendations. Neurosurgery. 2006;59:957–982. doi: 10.1227/01.NEU.0000245591.16087.89. discussion 982-987. [DOI] [PubMed] [Google Scholar]
- 36.Wang Y, Li G, Luk KDK, Hu Y. Component analysis of somatosensory evoked potentials for identifying spinal cord injury location. Sci Rep. 2017;7:2351. doi: 10.1038/s41598-017-02555-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang Y, Cui H, Pu J, Luk KD, Hu Y. Time-frequency patterns of somatosensory evoked potentials in predicting the location of spinal cord injury. Neurosci Lett. 2015;603:37–41. doi: 10.1016/j.neulet.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 38.Wen CY, Cui JL, Mak KC, Luk KD, Hu Y. Diffusion tensor imaging of somatosensory tract in cervical spondylotic myelopathy and its link with electrophysiological evaluation. Spine J. 2014;14:1493–1500. doi: 10.1016/j.spinee.2013.08.052. [DOI] [PubMed] [Google Scholar]
- 39.Ye J, Ma M, Xie Z, Wang P, Tang Y, Huang L, Chen K, Gao L, Wu Y, Shen H, Zeng Y. Evaluation of the neural function of nonhuman primates with spinal cord injury using an evoked potential-based scoring system. Sci Rep. 2016;6:33243. doi: 10.1038/srep33243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang ZG, Yang JL, Chan SC, Luk KD, Hu Y. Time-frequency component analysis of somatosensory evoked potentials in rats. Biomed Eng Online. 2009;8:4. doi: 10.1186/1475-925X-8-4. [DOI] [PMC free article] [PubMed] [Google Scholar]