Abstract
Corticotropin-releasing hormone is a critical component of the hypothalamic–pituitary–adrenal axis, which plays a major role in the body’s immune response to stress. Mast cells are both sensors and effectors in the interaction between the nervous and immune systems. As first responders to stress, mast cells can initiate, amplify and prolong neuroimmune responses upon activation. Corticotropin-releasing hormone plays a pivotal role in triggering stress responses and related diseases by acting on its receptors in mast cells. Corticotropin-releasing hormone can stimulate mast cell activation, influence the activation of immune cells by peripheral nerves and modulate neuroimmune interactions. The latest evidence shows that the release of corticotropin-releasing hormone induces the degranulation of mast cells under stress conditions, leading to disruption of the blood-brain barrier, which plays an important role in neurological diseases, such as Alzheimer’s disease, Parkinson’s disease, multiple sclerosis, autism spectrum disorder and amyotrophic lateral sclerosis. Recent studies suggest that stress increases intestinal permeability and disrupts the blood-brain barrier through corticotropin-releasing hormone-mediated activation of mast cells, providing new insight into the complex interplay between the brain and gastrointestinal tract. The neuroimmune target of mast cells is the site at which the corticotropin-releasing hormone directly participates in the inflammatory responses of nerve terminals. In this review, we focus on the neuroimmune connections between corticotropin-releasing hormone and mast cells, with the aim of providing novel potential therapeutic targets for inflammatory, autoimmune and nervous system diseases.
Key Words: blood-brain barrier, corticotropin-releasing hormone, gastrointestinal tract, inflammatory, mast cells, neuroimmune, neurological disorders
Introduction
Corticotropin-releasing hormone (CRH) is a primary hypothalamic activator of the hypothalamic–pituitary–adrenal (HPA) axis, playing a key role in the stress response via the regulation of the neuroimmune and neuroendocrine systems (Inda et al., 2017; Russell and Lightman, 2019; Leistner and Menke, 2020; Theoharides, 2020a). Various neurotransmitters/neuromodulators, neuropeptides and inflammatory cytokines control the release of hypothalamic CRH from the hypothalamus, depending on stress responses that modulate neuroendocrine and neuroimmune functions (Dedic et al., 2018; Naama et al., 2020; Theoharides, 2020a). The HPA axis strongly influences neuroimmune and inflammatory processes through the release of CRH. The secretion of CRH in the brain exerts pro-inflammatory effects through mast cell (MC) activation, leading to inflammation and neurological diseases (Kempuraj et al., 2017a). Stress activates the HPA axis via the release of CRH, thereby modulating the central nervous system (CNS) immune components that mainly affect immune cells, such as MCs (Kempuraj et al., 2020a; Pondeljak and Lugović-Mihić, 2020). MCs are effector cells that connect the nervous, circulatory and immune systems (Krystel-Whittemore et al., 2015; Stakenborg et al., 2020). MCs act as sentinels of the human body and play a pivotal role in the defense mechanism against foreign pathogens and stress responses such as allergy. MCs are critical for allergic reactions and are also directly involved in innate and acquired immunity (Yamanishi and Karasuyama, 2016; González-de-Olano and Álvarez-Twose, 2018). Strong evidence indicates that CRH plays a very important role in the activation of MCs, which secrete numerous cytokines, chemokines, proteases and vasoactive compounds associated with inflammatory-related diseases (Esposito et al., 2002; Theoharides, 2020a). CRH-mediated MC activation affects gut–blood barrier and blood–brain barrier (BBB) permeability via acute psychological stress (Esposito et al., 2002; Vanuytsel et al., 2014). Acute psychological stresses activate the HPA axis via the release of CRH and significantly increase the production of glucocorticoids, resulting in the downregulation of immune responses (Herman et al., 2016; Li et al., 2019; Juruena et al., 2020). A growing body of evidence demonstrates that MCs participate in the neuroendocrine signaling pathway by producing proopiomelanocortins in response to receptor-mediated stimulation by CRH, known as a critical neuroendocrine mediator of stress responses (Harvima and Nilsson, 2012; Theoharides, 2020b; Valent et al., 2020).
MCs are prototypical neuroimmunoendocrine cells that regulate bidirectional communication between the nervous system and the immune system. Brain-resident MCs and CRH neurons regulate BBB permeability during the stress response (Esposito et al., 2002; Kritas et al., 2014a; Füzesi et al., 2016). However, further study is required to address how brain-resident MCs and CRH regulate BBB permeability. Over the past few years, significant advances in our understanding of the underlying molecular mechanisms has allowed us to focus on the effects of CRH and its receptors on MC activation in inflammatory and neurological diseases.
In this review, we discuss the current state of knowledge on the effects of CRH on MC activation in neuroimmune connections in inflammatory, autoimmune and neurological disorders. Moreover, we briefly address how CRH induces central and peripheral inflammation by promoting MC activation in inflammatory and neurological disorders. In this review, we describe the neuroimmune two-way information exchange between MCs and CRH, which will provide a new perspective for the treatment of inflammatory, autoimmune and neurological diseases. CRH is the initiating hormone for the HPA axis to adapt to the stress response. MCs are immune cells related to allergy, asthma and irritable bowel syndrome. Recent studies have advanced our understanding of these cells—they suggest that MCs play a dual role as sensor and effector in the neuroimmune networks. Under normal circumstances, MCs resident in the brain can secrete factors such as CRH, histamine and tryptase to regulate BBB permeability and maintain brain homeostasis. When intracranial or extra cranial homeostasis is perturbed, the stressor can activate the HPA axis, stimulate the secretion of CRH in the hypothalamic paraventricular nucleus (PVN) and activate intracranial MCs through the CRH receptor (CRH-R), causing the cells to degranulate. By causing the permeability of the BBB to increase, allowing peripheral inflammatory factors and cells to enter the brain, histamine and related mediators released by MCs can activate glial cells and participate in the occurrence and development of central inflammation-related disorders, such as Alzheimer’s disease (AD), amyotrophic lateral sclerosis (ALS), multiple sclerosis (MS) and Parkinson’s disease (PD), as well as perioperative cognitive dysfunction.
Search Strategy
We searched for articles published from the year 1955 to 2020 using Google Scholar, PubMed, Web of Science and Publons to comprehensively summarize the bidirectional neuroimmune communications between MCs and CRH. Keywords used for the literature search included “MCs”, “CRH and its receptors”, “HPA”, “stress”, “Urocortin”, “neurodegenerative disease”, “blood-brain barrier (BBB)”, “neuroimmune”, “MCs activation”, “acute or chronic stress”, “neuroinflammatory processes”, “neurological diseases” “inflammatory cytokines and neuropeptides”, “immunity”, “peripheral inflammation and central inflammation”, “glial cells”, “skin inflammatory diseases”, “Irritable bowel syndrome (IBS)”, “Postoperative cognitive dysfunction (POCD)”, “Alzheimer’s disease (AD)”, “Parkinson’s disease (PD)”, “autism Spectrum Disorder (ASD)”, “multiple Sclerosis (MS)”, “amyotrophic lateral sclerosis (ALS)”, “traumatic brain injury (TBI)”and “rheumatoid arthritis”.
Corticotropin-Releasing Hormone Family
Hypothalamic CRH was first reported in 1955 (Saffran and Schally, 1955) and identified in the ovine hypothalamus in 1981 (Vale et al., 1981). CRH is a neuropeptide hormone of 41 amino acids that is a critical activator of the HPA axis, which is required for stress adaptation. CRH is released from the hypothalamus and stimulates adrenocorticotropic hormone secretion from the anterior pituitary. CRH is one of the central mediators of the excitation of the HPA axis (Herman et al., 2016), and it plays a key role in the neuroendocrine regulation of the stress response. CRH in the hypothalamus is primarily controlled by parvocellular neurons, mainly located in the hypothalamic PVN. CRH neurons that project to the median eminence are found mostly in the PVN, and regulate the release of adrenocorticotropic hormone in the anterior pituitary through the portal system, which in turn stimulates the synthesis and secretion of glucocorticoids (Aguilera and Liu, 2012), which regulate immune function, glucose metabolism and cognitive function. External and internal stress responses are modulated by feedback mechanisms, whereby glucocorticoids act to reduce drive (brainstem) and promote synaptic inhibition by limbic structures (hippocampus).
Glucocorticoids also rapidly inhibit CRH neuronal activity via membrane glucocorticoid receptors in the PVN (Strehl et al., 2019). Recent studies have shown that CRH and its receptors are widely distributed in the body and participate in many critical physiological functions, including organ and visceral sensation, cardiovascular activity, emotional response, learning, mood and memory (Linthorst et al., 1997; Weninger et al., 1999; Kellner et al., 2003; Abelson et al., 2010; Liu et al., 2015). There are two main mammalian peptides in the CRH family—CRH and urocortin (UCN). The past decade has witnessed extensive research and an increasingly deep understanding of the central and peripheral expression and regulation of the CRH and UCN signaling pathways, as well as their role in health and diseases.
Corticotropin-Releasing Hormone
CRH is highly conserved among mammals, including humans, primates and rodents. The CRH precursor comprises 196 amino acid residues, and the mature peptide is located in the C-terminal domain of the precursor (Chrousos and Zoumakis, 2017; Dedic et al., 2018). A processing motif (Arg-X-Arg-Arg) is present at the N-terminus of CRH, while the dipeptide Gly-Lys is located in the C-terminal domain of the precursor (Vale et al., 1981). After processing by endopeptidase, the C-terminal Lys is cleaved off by an exopeptidase, and the C-terminus of the CRH precursor is amidated, with glycine as an amidation donor. CRH mRNA is widely distributed in the brain, particularly in the PVN, cerebral cortex, amygdala, hippocampus and Barrington’s nucleus (Merali et al., 2004; Peng et al., 2017). CRH is also expressed in other peripheral tissues, such as the digestive tract and placenta. CRH sequence homologies are comparatively high (76–95%) between human/rat/mouse and other species (Stenzel-Poore et al., 1992). The human CRH gene, located at 8q13, consists of two exons and one intron (Malagoli et al., 2004). It has been reported that CRH peptides of all species are composed of 41 amino acid residues (C-terminally amidated peptide). CRH plays a major role in controlling the basal and stress-induced activation of the pituitary–adrenal axis, thereby regulating androgen and glucocorticoid secretion. The release of CRH in response to acute stress is necessary for the survival of the organism (Li-Tempel et al., 2019), but CRH released as a result of exposure to chronic stress may influence mood and emotional behavioral and negatively affect physiological homeostasis.
Urocortin
UCN is a member of the CRH family of peptides with high similarity to CRH in amino acid sequence. In the brain, UCN suppresses food intake mediated by CRH-Rs and also participates in the regulation of emotions, anxiety, cognitive memory and body temperature, and shows neuroprotective activity (Skelton et al., 2000; Heinrichs and Koob, 2004). UCN can be classified into the following three subtypes: UCN1, UCN2 and UCN3. UCN is expressed both in the brain and peripheral organs (Pan and Kastin, 2008). Circulating UCN may reach the brain from the peripheral organs by a unique mechanism. UCN1, UCN2 and UCN3 differentially interact with the BBB (Kastin and Akerstrom, 2002). UCN1 contains 40 amino acids, with a structure similar to CRH. UCN1 is expressed in the accessory nucleus of the oculomotor nerve in the mammalian brain, as well as the heart, vascular cells and endometrium, but generally, it is more widely expressed in peripheral tissues (Fukuda et al., 2005). UCN2 is composed of 38 amino acids expressed in the parvocellular and magnocellular parts of the PVN in the hypothalamus, as well as the locus coeruleus, the ventral horn of the spinal cord, supraoptic nucleus and cranial nerve motor nuclei (trigeminal, facial and hypoglossal nuclei). Its sequence has 34% homology with CRH in humans and rats. UCN2 is also found in peripheral tissues, especially in the skin and skeletal muscle tissues (Reyes et al., 2001). UCN3 peptide is expressed in the PVN, ventromedial hypothalamus, amygdala, lateral septum, the dorsal raphe nucleus, basomedial nucleus of the stria terminalis, and the area postrema. The correlation between UCN3 and CRH is relatively weak, and there is almost no overlap in regional expression (Deussing et al., 2010).
Corticotropin-Releasing Hormone Receptors and Their Functions
Three subtypes of CRH-Rs have been identified—CRH-R1, CRH-R2 and CRH-R3. All three belong to the family of seven-transmembrane-domain G protein-coupled receptors that activate adenylate cyclase (Merali et al., 2004; Grammatopoulos and Ourailidou, 2017). CRH-R1 and CRH-R2 have been identified in mammals, amphibians, birds and teleosts (Cardoso et al., 2016). In mammals, CRH-R2 has three splice isoforms—CRH-R2α, CRH-R2β and CRH-R2γ (Catalano et al., 2003) CRH-R3 has only been found in catfish (Deussing and Chen, 2018). CRH-R1 is abundantly expressed in the brain, particularly in anterior pituitary corticotropes and lactotropes, but its expression is limited in the peripheral system. CRH-R1 is an essential neuroendocrine receptor for CRH-mediated adrenocorticotropic hormone release in the pituitary in response to stress. Single-nucleotide polymorphisms in the CRH-R1 gene are associated with a significantly increased risk of neurodegenerative diseases (Weber et al., 2016; Ketchesin et al., 2017).
CRH may activate MCs via CRH-1, leading to the release of histamine, resulting in increased vascular permeability and vasodilation (Theoharides et al., 1998). The expression of CRH-R2 in the brain is relatively limited, but its peripheral expression is relatively high, especially in cardiovascular and skeletal muscle (Sheng et al., 2012). CRHR2α and CRHR2γ are highly expressed in the brain, while CRHR2β is more predominantly localized in the heart, thymus and spleen. The functional significance of CRHR2γ in the brain remains unclear. Functional studies and experimental knockout mouse models show that CRH-R1 might mediate the CRH-induced response of the HPA axis to stress, whereas CRH-R2 modulates the stress response and behaviors (Klenerova et al., 2018). CRH-R1 and CRH-R2 have differential binding affinities for ligands and different expression patterns in the brain and periphery. Although CRH-R1 and CRH-R2 share 70% amino acid sequence homology, they have different pharmacological effects owing to lower similarity in the N-terminal domain (Kuenzel et al., 2013). The binding affinity of CRH and UCN1 for CRH-R1 is higher than that of UCN2 and UCN3 for CRH-R2. CRH-R3 has strong sequence homology to CRH-R1. In most cell types, activation of CRH-R1 or CRH-R2 mediated by CRH results in the activation of the Gs-adenylyl cyclase pathway and increased cyclic adenosine monophosphate levels; however, recent evidence suggests that different tissues and cell types express various G proteins, including Gq, Gi and Go, that are associated with the receptor, thereby resulting in the activation of various kinase pathways, including protein kinase C, mitogen-activated protein kinase and Akt (Hillhouse and Grammatopoulos, 2001; Kovalovsky et al., 2002; Grammatopoulos, 2007). After CRH-R1/2 knockout mice were injected with CRH, there was no significant change in adrenocorticotropic hormone release, indicating that the receptor may not be involved in the activation of the HPA axis (Fenzl et al., 2011; Klenerova et al., 2018). Thus, its characteristics and effects need further study. CRH-R has many subtypes with differential tissue distribution, and therefore, it can activate a variety of signal transduction pathways and produce various biological effects.
Regulatory Effect of Corticotropin-Releasing Hormone on Mast Cell-Mediated Neuroinflammatory Processes
MCs are a multifunctional immune cell. They originate from CD34+ /CD117+ bone marrow-derived cells and differentiate into different mature MCs under the influence of different environments. MCs are generally distributed along blood vessels or body surfaces and express many cell surface receptors, and accordingly, their differentiation, migration and activation are easily affected by the external environment. Therefore, MCs are regarded as sentinels of the human body and play an important role in the defense against the outside world and the stress response such as allergy (Li et al., 2017). MCs abundantly express CRH-R1 and CRH-R2 on their surface and are highly sensitive to CRH (Ayyadurai et al., 2017). The expression levels of CRH and its receptors are increased in patients with mastocytosis (Theoharides et al., 2014). It has been reported that stress is the main risk factor for the occurrence and aggravation of MC-related diseases, such as allergy, asthma and irritable bowel syndrome (Priftis et al., 2009; Andrae et al., 2013; Heffner et al., 2014). MCs are activated by the CRH signal, which leads to degranulation and the release of various inflammatory factors such as interleukin (IL)-6 and tumor necrosis factor-α (TNF-α) (Theoharides et al., 2012). Although it is known that MCs are highly activated under stress conditions, the cellular mechanisms by which stress regulates MC function and pathophysiology are still unclear. The human leukemic mast cell (HMC-1) line and human umbilical cord blood-derived MCs both express CRH and the CRH-R1α, 1β, 1c, 1e and 1f isoforms (Cao et al., 2005). CRH activates the HPA axis in the stress response, possibly through MC activation. CRH-mediated MC activation increases cyclic adenosine monophosphate levels and induces the secretion of vascular endothelial growth factor without tryptase, histamine, TNF-α, IL-6 or IL-8 release (Theoharides, 2020a).
Interestingly, human MCs synthesize and secrete a large amount of CRH, suggesting this neuroendocrine peptide may regulate MCs through CRH and its receptors in an autocrine manner (Zouboulis et al., 2002; Ayyadurai et al., 2017). CRH-mediated MC activation regulates the autocrine signaling pathway under inflammatory conditions; however, its receptors remain to be identified. MCs communicate functionally with glial cells in the brain and degranulate in response to physical and psychological stress. Studies have shown that degranulation of brain MCs modulates HPA responses via centrally released histamine and CRH (Matsumoto et al., 2001; Elenkov et al., 2005).
In the model of MC-dependent IgE-mediated passive systemic anaphylaxis, treatment with antalarmin, an antagonist of CRH-R1, can alleviate the symptoms caused by MC degranulation and hypothermia. When CRH-R1 on the surface of MCs is knocked out, serum histamine content, body temperature and clinical score recover compared with the control group, and the degranulation of MCs and intestinal permeability changes are also inhibited (Ayyadurai et al., 2017). These studies suggest that CRH-R1 regulates MC activation mediated by CRH. Accumulating evidence indicates that CRH can induce MCs to secrete vascular endothelial growth factor by increasing cAMP, which can be blocked by antalarmin, while astressin 2B, an antagonist of CRH-R2, has no effect (Cao et al., 2006; Boucher et al., 2010). These results reveal that the CRH-R1 signal plays a key role in MC degranulation. However, the regulatory effect of CRH-R2 on MCs remains unclear. It has been shown that CRH-R1 and CRH-R2 are expressed on the surface of human cord blood MCs cultured with stem cell factor and IL-6 for 10 weeks (Papadopoulou et al., 2005; Asadi et al., 2012). Only CRH-R2 is expressed when IL-4 is added in the last 3 weeks. Further analysis showed that adding IL-1 or lipopolysaccharides within 6 hours only increased CRH-R2 gene expression. CRH could not induce human cord blood MCs to secrete IL-6, but could induce human cord blood MCs to release IL-4. These results suggest that CRH-R2 is upregulated when MCs are stimulated by inflammation (Papadopoulou et al., 2005). However, there are also experimental results contrary to the above studies; namely, the loss of CRH-R2 function has been shown to aggravate MC degranulation and promote IgE-mediated anaphylaxis and psychological stress-induced intestinal barrier dysfunction. CRH-R2 on the surface of MCs inhibits degranulation induced by various stimuli by blocking calcium-triggered calcium release channels (Vicario et al., 2010; D’Costa et al., 2019), indicating that CRH-R2 can also negatively regulate MC activation. These varied findings suggest that, at the single-cell level, the effect of CRH-R activation on MCs is modulated by multiple signaling pathways.
Theoharides (2020a) have suggested that CRH activates brain-resident immune cells (such as MCs, microglia and astrocytes) through CRH-Rs, and that these cells release numerous neuroinflammatory and neurotoxic factors that affect BBB permeability, thereby contributing to chronic neuroinflammation and neurodegenerative diseases. It is well recognized that MCs act as the first responders in the brain, and can initiate, amplify and modulate various nervous and immune responses upon activation. Furthermore, degranulated MCs secrete numerous mediators that trigger neuroinflammatory processes linked to several neurological disorders as well as neurovascular dysfunction. Data from clinical studies demonstrate that neurovascular dysfunction disrupts BBB permeability and is associated with neuroinflammation and cognitive decline caused by chronic stress (Najjar et al., 2013; Sweeney et al., 2018). However, further study is required to clarify the effects of stress on the neurovascular system in the brain and the relationship to CRH-mediated MC activation. MS and brain metastases may increase BBB permeability in response to acute stress that leads to the release of CRH and the activation of brain MCs, which in turn secrete vascular endothelial growth factor, IL-6 and IL-8 (Theoharides and Konstantinidou, 2007). These results indicate that acute or chronic stress may increase BBB permeability via CRH and MCs. A few studies have reported that MCs and microglia express large amounts of CRH and its receptors, and that these cells are activated by CRH released from the HPA system, which in turn has autocrine effects and modulates neuroinflammatory processes (Kritas et al., 2014a; Zhang et al., 2016d). CRH and its receptors activate MCs and microglia that release inflammatory factors (such as TNF, IL-6, IL-8, IL-33, IL-1, IL-18, nitric oxide and proteases), leading to the complication of neurological disorders via complex crosstalk between CRH, MCs and microglia (Tsilioni et al., 2016). Studies have shown that MCs are activated during acute or chronic stress through CRH released by sensory nerves, and may be involved in the disruption of neurodevelopment and the pathogenesis of neuropsychiatric disorders (Petra et al., 2015; Godoy et al., 2018). However, the mechanisms underlying the neurodevelopmental perturbation and the link with MCs and CRH remain unknown.
Surgical stress responses are connected with crosstalk interactions within the nerve–endocrine–immune network (Finnerty et al., 2013; Manley et al., 2018). Several studies reported that stress is the leading risk factor for the occurrence and aggravation of MC-related diseases, such as allergies and asthma (Krystel-Whittemore et al., 2015; Bischoff, 2016). MCs in the brain are widely distributed in the hypothalamus, especially near the nerve endings containing CRH, which can be activated by surgical stress (Traina, 2017). The activation of MCs in the rat brain can be observed in a model of acute stress caused by immobilization stress, and CRH antiserum pretreatment can block this effect. Furthermore, injection of the MC stabilizer sodium cromoglycate in the hypothalamus can reduce BBB damage caused by CRH (Jin et al., 2007; Ocak et al., 2019). In addition, stress only causes BBB destruction in the brain of wild-type mice, but has no effect on the BBB in MC knockout mice (Theoharides and Konstantinidou, 2007).
Theoharides and Konstantinidou (2007) pointed out that the increased permeability of the BBB under acute stress depends on the interaction of CRH and MCs. Kempurajet al. (2017b) suggested that CRH might influence MC activation and their release of inflammatory mediators, resulting in peripheral and central inflammation (Figure 1). However, further study is required to investigate whether peripheral surgical trauma stress promotes the release of CRH in the hypothalamus and activates MCs in the brain, resulting in increased permeability of the BBB and neuroinflammation.
Figure 1.
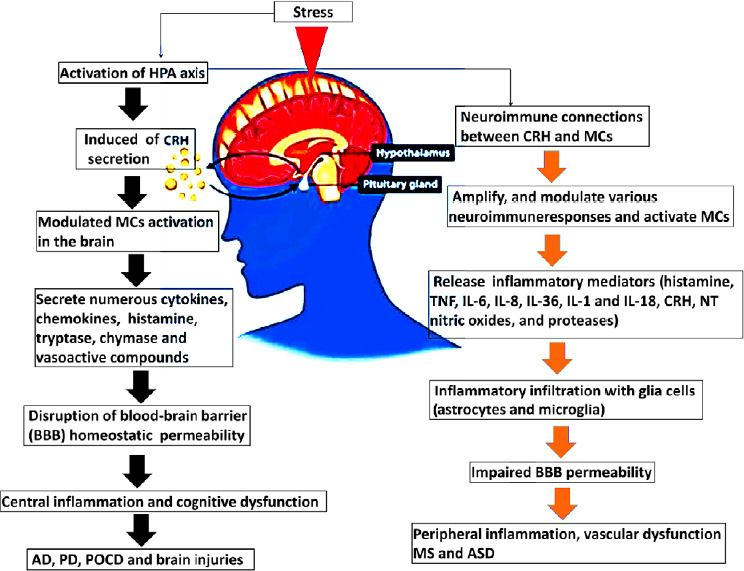
The neuroimmune connections between CRH and MCs associated with central and peripheral inflammation result in neurological disorders.
The schematic diagram shows the effect of stress-induced neuroimmune interactions between CRH and MCs and the link to neurological diseases. Stress conditions activate the HPA axis to induce neuroimmune communication between CRH and MCs to release numerous inflammatory mediators from MCs upon their activation, which in turn disrupts the BBB, leading to neuroinflammatory processes associated with neurological diseases. AD: Alzheimer’s disease; ASD: autism spectrum disorder; BBB: blood–brain barrier; CRH: corticotropin-releasing hormone; HPA: hypothalamic-pituitary-adrenal; IL: interleukin; MC: mast cell; MS: multiple sclerosis; NT: neurotensin; PD: Parkinson disease; POCD: postoperative cognitive dysfunction; TNF: tumor necrosis factor.
MCs are an immune cell of bone marrow origin that regulate neuroinflammation and, upon activation, secrete many inflammatory mediators in systemic and CNS inflammatory conditions (Dong et al., 2014; Hendriksen et al., 2017; Kempuraj et al., 2019). MCs are critical immune regulators and act as sentinels of brain immunity because of their unique ability to regulate the stress response via the HPA axis (Galli et al., 2008; Theoharides, 2020a). Matsumoto et al. (2001) first reported that brain-resident MCs are an immune gate to the HPA responses via histamine and CRH release. Emerging evidence demonstrates that acute and chronic stress has inflammatory effects that are mediated through MC activation (Traina, 2019; Wang et al., 2020).
Stress conditions are known to suppress the immune responses and worsen neuroinflammatory conditions. Stress and environmental stimuli can trigger MC degranulation, which may induce glial cell activation that results in abnormal synaptic pruning and dysfunctional neuronal connectivity in the CNS (Boscolo et al., 2008; Paolicelli and Ferretti, 2017; Theoharides et al., 2019). We previously reported that activation of brain-resident MCs and microglia by a combination of stress and environmental triggers may disrupt neuronal connectivity in the amygdala and hippocampus, thereby altering BBB homeostasis linked to the neuroinflammatory response in the CNS (Zhang et al., 2016e). Several lines of experimental evidence indicate that stress, acting through the HPA axis or the sympathetic nervous system, stimulates peripheral nerves to release CRH and other neuropeptides that bind to receptors on the MCs, causing them to activate (Conti et al., 2018; Pondeljak and Lugović-Mihić, 2020). Bidirectional interaction between stress and MCs enhance the stress response in the CNS and stimulate the release of inflammatory cytokines and neuropeptides, leading to neuroinflammation and the disruption of the BBB that results in the pathophysiology of many neuroinflammatory conditions, such as AD, PD, MS and autism spectrum disorder (ASD) (Figure 1) (Hendriksen et al., 2017; Jones et al., 2019). Chronic stress directly induces MC degranulation, which releases many inflammatory mediators, such as CRH, UCN, histamine, tryptase, vascular endothelial growth factor, neurotensin, IL-8 and TNF-α, that may disrupt the BBB, leading to neuroinflammation and neurodegeneration (Kempuraj et al., 2017a; Skaper et al., 2017). However, the mechanisms of the interaction between MCs and CRH and its receptors associated with the neuroinflammatory conditions remain unknown.
Numerous studies have demonstrated that bidirectional communication between stress and neuroinflammation is linked with MC degranulation (Theoharides et al., 2019). MCs play a critical role in the mechanisms of brain injury and damage caused by chronic stress. Psychological and physiological stress may lead to an increase in the expression, distribution and activity of MCs in the brain (Joshi et al., 2019; Theoharides, 2020a). Stress and inflammatory cytokines activate the HPA axis, which may influence the expression of CRH and its receptors, vascular permeability and MC activation (Fries, 2020; Hoppe et al., 2020; Stakenborg et al., 2020). CRH activates glial cells and MCs via CRH-Rs and releases neuroinflammatory mediators in the CNS in an autocrine and paracrine manner in stress and neuroinflammation (Karagkouni et al., 2013). MCs are closely localized with CRH neurons in the median eminence and are linked with CRH and its receptors (Cao et al., 2005). CRH is widely expressed in the basal ganglia, neocortex, amygdala and hippocampus in the brain. Activated MCs produce and secrete CRH and its receptors, which can activate microglia to release neuroinflammatory mediators that may disrupt BBB hemostasis leading to neuroinflammation (Rozniecki et al., 2010; Karagkouni et al., 2013; Kritas et al., 2014b). However, additional work is required to clarify whether CRH and its receptors mediate pro-inflammatory activity in the brain through MCs (Figure 1).
Crosstalk between the Blood–Brain Barrier, Mast Cells and Corticotropin-Releasing Hormone in Brain Inflammation
The BBB maintains microenvironmental homeostasis in the CNS for brain functional integrity (Chow and Gu, 2015). Stress, environment and lifestyle may independently and/or synergistically influence brain inflammation, causing BBB dysfunction and pericyte degeneration, resulting in neuronal death (Zhao et al., 2015b). The intact BBB can effectively block the entry into the brain of harmful substances in the circulation, thereby maintaining the homeostasis of the CNS environment (Li et al., 2017). The BBB is an essential gateway for peripheral inflammatory factors and immune cells (MCs and glial cells) to invade the brain parenchyma. Larochelle et al. (2011) found that the activation of peripheral immune cells can lead to the destruction of the BBB. Leukocytes can then migrate into the brain through the BBB, further increasing its permeability and inducing more white blood cells to infiltrate into the CNS (Chow and Gu, 2015; Keaney and Campbell, 2015). Therefore, the change in BBB permeability is an essential condition for peripheral trauma to induce acute central inflammation. Our team previously reported that activated MCs contribute to CNS inflammation and cognitive dysfunction by promoting BBB disruption (Zhang et al., 2016c). Therefore, based on our knowledge, we propose that interactions between MCs and the BBB could constitute a new and unique therapeutic target for neurological disorders.
The MC is a multifunctional effector cell of the body’s innate immune system. It is mainly located in the brain around the hypothalamus and the BBB. Under normal conditions, MCs in the brain secrete histamine, tryptase and other mediators through degranulation to regulate sensation, mood and cognition (Kempuraj et al., 2017a; Li et al., 2017). When peripheral inflammation occurs, the MCs in the brain immediately respond to the immune signal in the blood and quickly degranulate, releasing mediators that trigger the central inflammatory response. Because of its rapid response and signal transmission capabilities, the MC is involved in various CNS diseases such as AD, PD, ASD, stroke and cerebral ischemia-reperfusion injury (Theoharides and Konstantinidou, 2007; Galli et al., 2008; Skaper et al., 2018). In follow-up animal experiments, our team further found that surgery for tibial fractures in rats can cause an increase in MCs in the brain, increased BBB permeability, microglial activation and neuronal apoptosis, leading to increased central inflammation and a decline in learning and memory functions. Pretreatment with injection of an MC stabilizer into the lateral ventricle can prevent these changes (Zhang et al., 2016e). We showed that MC degranulation increases BBB permeability in the brain after peripheral surgery and the occurrence of acute central inflammation. Therefore, we speculate that the release of factors such as histamine and tryptase by degranulating MCs induce the destruction of the BBB and aggravate the central inflammatory response (Figure 2).
Figure 2.
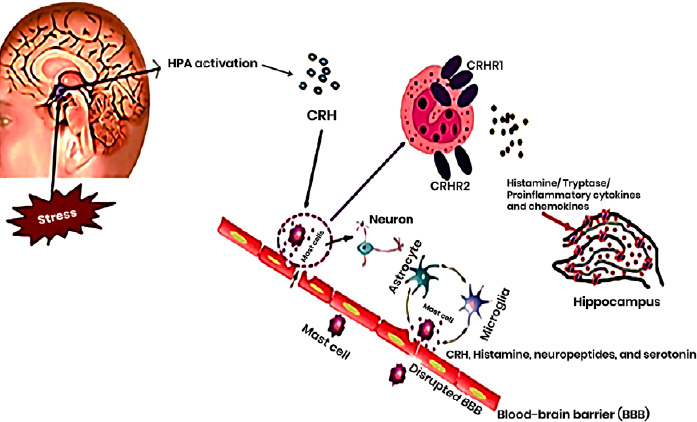
The interactions between CRH and MCs that may affect the BBB under acute and chronic stress.
Acute and chronic stress trigger HPA axis activation to induce the release of CRH, resulting in MC activation and their secretion of histamine, tryptase and proinflammatory cytokines in the hippocampus that affect the BBB and central inflammation. Two CRH receptors are localized on the surface of MCs, and these receptors have different biological effects during stress-induced MC activation. The MCs interact with astrocytes and microglia, which release CRH, histamine, neuropeptides and serotonin, leading to disruption of the BBB. BBB: Blood–brain barrier; CRH: corticotropin-releasing hormone; CRHR: corticotropin-releasing hormone receptor; HPA: hypothalamic–pituitary–adrenal; MC: mast cell.
It has been reported that CRH and its receptors are expressed on the surface of MCs (Cao et al., 2005; Alysandratos et al., 2012). Many issues need to be addressed regarding the neuroimmune connections among the BBB, MCs and CRH in central inflammation, including the following:
A. It is necessary to verify and explore the effect and mechanisms of the interaction between CRH and MCs on the BBB.
B. What is the difference between the biological effects of different CRH-Rs on MCs? How do they regulate the BBB and central inflammation?
C. Given the effects of CRH and MCs in the brain on the BBB, the role and mechanisms of the different CRH-Rs need to be urgently investigated.
The Effects of Corticotropin-Releasing Hormone on Mast Cells: Implication for Inflammatory, Autoimmune and Neurological Disorders
Skin inflammatory diseases
The skin is the largest organ of the human body and is always in contact with the external environment. Skin MCs act as functional sensors of emotional and environmental stress, and can be directly activated by CRH released under stress and related neuropeptides. CRH and its receptors may activate MCs that stimulate pro-inflammatory actions to result in the pathogenesis of various skin disorders that are triggered by acute stress, such as atopic dermatitis, psoriasis, eczema and urticaria (Suárez et al., 2012; Căruntu et al., 2014; Chen and Lyga, 2014; Siiskonen and Harvima, 2019; Figure 3). MC-related atopic dermatitis and psoriasis are triggered by CRH/CRH-R-mediated MC activation, causing the production and release of numerous cytokines and chemokines (Nedoszytko et al., 2014). Psoriasis is triggered by acute and chronic stress and is associated with increased serum CRH-mediated MC activation, but decreased CRH-R1 gene expression, in the lesioned skin, possibly because of downregulation (Vasiadi et al., 2012; Tampa et al., 2018). MC activation and accumulation are critical to the pathogenesis of psoriasis and are involved in keratinocyte proliferation and inflammation. CRH increases the incidence rate of skin allergy and other skin diseases by activating MCs near sensory nerve terminals (Gupta and Harvima, 2018). Recent studies show that many genes that are expressed in the CNS are also expressed in the epidermis of animals, indicating an actual “skin brain” (Martins et al., 2020). The skin has also been shown to have an equivalent to the HPA axis (Holland, 2003).
Figure 3.
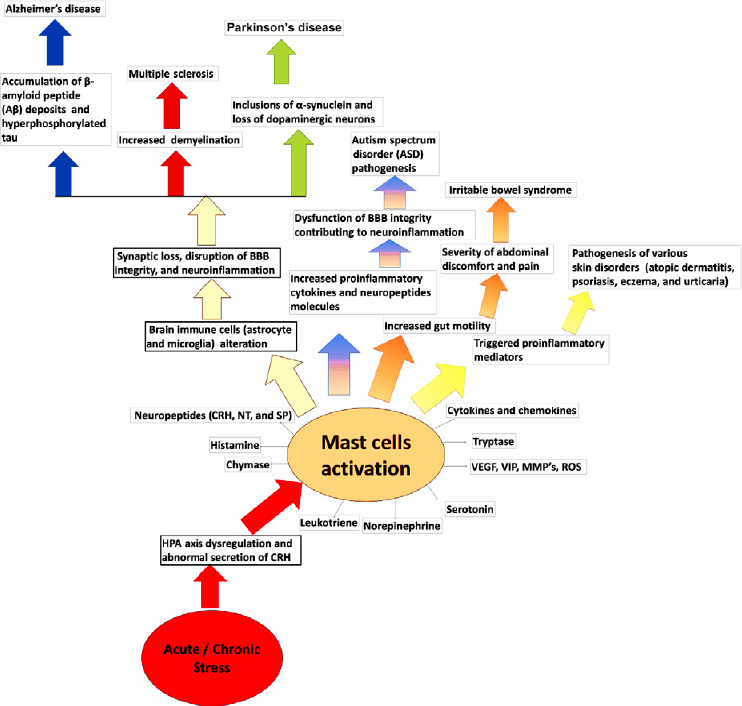
Schematic diagram showing the acute and chronic stress-associated neuroimmune connections between CRH-mediated MC activation in inflammatory and neurological diseases.
Acute and chronic stress dysregulate the HPA axis via the release of CHR in the brain. The secretions of CRH in the brain trigger MCs activation by releasing various preformed and newly synthesized inflammatory mediators. Activated MCs also trigger other neuroimmune cells activation, including astrocytes, microglia, which decrease the synaptic plasticity, disrupt BBB integrity mediate neuroinflammation associated with AD, PD, MS, and ASD. Degranulations of MCs increase gut motility related to the severity of abdominal discomfort and pain in IBS patients. Activate MCs stimulate pro-inflammatory actions to result in the pathogenesis of various skin disorders such as atopic dermatitis, psoriasis, eczema, and urticaria. BBB: Blood–brain barrier; CRH: corticotropin-releasing hormone; MC: mast cell; MMP: matrix metallopeptidase; NT: neurotensin; ROS: reactive oxygen species; SP: substance P; VEGF: vascular endothelial growth factor; VIP: vasoactive intestinal peptide.
Skin stress reactions, such as to ultraviolet radiation, can promote the release of CRH, leading to the degranulation of skin MCs, resulting in increased local vascular permeability (Slominski et al., 1998; Pisarchik and Slominski, 2002). Acute stress can also exacerbate delayed hypersensitivity, and chronic contact dermatitis of the skin in rats directly depends on CRH and its receptors, which are related to the upregulation of vascular permeability (Lytinas et al., 2003). CRH/CRH-R-mediated MC activation also causes peripheral blood vessel dilation and blush. Intraepithelial administration of CRH and UCN can activate CRH-R1 on the surface of mouse and human skin MCs to promote MC activation and increase skin vascular permeability. However, this does not occur in MC-deficient mice or in animals treated with the CRH-R1 antagonist antalarmin, indicating that CRH functions through MCs in skin lesions (Singh et al., 1999).
Irritable bowel syndrome
CRH is a crucial mediator of the stress response in irritable bowel syndrome (IBS) pathogenesis, which is a disorder of bidirectional interaction between the gut and brain. It has been proposed that IBS pathogenesis is linked with the HPA axis and autonomic imbalances in the prefrontal–amygdala circuitry. Studies have shown that exogenous CRH administration produces IBS-like symptoms, including anxiety behaviors, perturbed gut homeostasis, increased colonic motility, hyperalgesia to colorectal distention, watery stool/diarrhea, increased colonic mucosal permeability and autonomic responses, as well as altered brain activity (Kano et al., 2017). Studies show that colon mucosal CRH content in IBS patients is significantly higher compared with the control group (Santos et al., 2008). When CRH or UCN are injected intraperitoneally or intravenously in rats, the long-term collective explosive peak potential of the ileocecum and proximal colon and the transit time of the colon are shortened. The administration of alpha-helical CRH, a non-selective CRH-R antagonist, significantly stimulates the rectum and results in higher motility indices of the colon in IBS patients compared with the control group (Sagami et al., 2004). Numerous studies have identified CRH and UCN as well as CRH-R1 and CRH-R2 in the colonic mucosa of IBS patients (Taché et al., 2004; Sato et al., 2012; Komuro et al., 2016). However, the pathological mechanisms underlying the CRH-mediated adrenocorticotropic hormonal responses induced by MC degranulation in the IBS remain unclear.
MCs are widely distributed in the gastrointestinal tract. It has been reported that MCs in the intestinal mucosa are upregulated, along with increased gut motility in IBS patients and increased MC degranulation, which are related to the severity of abdominal discomfort and pain. Therefore, MCs play a key role in the pathogenesis of IBS (Ohman and Simrén, 2010; Figure 3). CRH and its receptors are widely expressed on MCs in the gastrointestinal tract. The high content of CRH and its receptors is positively correlated with the number of intestinal MCs in IBS mice, suggesting that MCs may be one of the major cells producing intestinal CRH and its receptors (Lee and Lee, 2016; Zhang et al., 2016b). Descending colon activity in IBS patients is significantly higher after intravenous CRH injection than in the healthy control group, and CRH promotes the secretion of colonic mucus and the release of MC proteases (Vicario et al., 2010). Castgaliuolo et al. (1998) found that injecting CRH into the peritoneal cavity and the ventricles of MC-deficient mice did not stimulate colonic electrical activity, suggesting that the biological function of CRH and its receptors in the intestinal tissue is related to MCs. The stress response increases the permeability of the colonic mucosa in mice. This effect can be antagonized by CRH-R antagonists and the MC stabilizer cromolyn sodium, indicating that CRH and MCs are jointly involved in stress-induced intestinal barrier dysfunction (Santos et al., 2008; Wallon and Söderholm, 2009).
Surgical stress and postoperative cognitive dysfunction
In recent years, the stress response has attracted increasing attention from researchers. Scientists have put forward stress theory and the concept of systemic adaptive syndrome for the physiological changes after stress. Common stressors include tension, fear, cold and surgical trauma, as the most common stressor in the scope of surgery, as its intensity and effect on the body are far higher than that of common stressors (Arora et al., 2010; Finnerty et al., 2013). Surgical stress can lead to an increase in BBB permeability, and inflammatory signals enter the CNS through the damaged BBB, causing irreversible damage. Our team previously showed that the degranulation of MCs in the brain plays an essential role in the increase in BBB permeability after peripheral surgery (Zhang et al., 2016e). However, it remains unclear how surgery causes MCs in the brain to become activated and degranulate.
During the stress of surgery, the hypothalamic PVN receives information about the CNS and excites the HPA axis. CRH is considered to be the triggering hormone of the HPA axis. MCs in the brain are widely distributed in the hypothalamus, especially near the nerve endings containing CRH, which can be activated by surgical stress. It has been shown that acute stress can activate MCs in the rat brain, and this can be blocked by CRH antiserum pretreatment (Eutamene et al., 2003). The BBB injury induced by CRH can be alleviated by pre-injecting the MC stabilizer sodium cromoglycate into the hypothalamus (Nelissen et al., 2013). Stress only causes BBB damage in brain areas where MCs are present and has no effect on the BBB in MC knockout mice (Esposito et al., 2002). Although little is known about how CRH regulates MCs in surgical stress, MCs can potentially deliver neuroprotective agents released from their storage granules (Dong et al., 2014). We believe that surgical stress leads to a large amount of CRH release, probably by activating nearby MCs. We propose that CRH/CRH-R-mediated MC activation in the brain is associated with inflammatory responses, and the development of neuroinflammation is associated with postoperative cognitive dysfunction and brain damage.
AD
AD is the leading cause of dementia in the aging population worldwide. AD is characterized by the accumulation of β-amyloid peptide (Aβ) deposits and neurofibrillary tangles within the brain along with the hyperphosphorylated and the cleaved form of the microtubule-associated protein tau, which cause synaptic dysfunction and cognitive impairment (Lane et al., 2018; Soria Lopez et al., 2019). Accumulation of Aβ is one of the critical triggers of pro-inflammatory responses leading to neuroinflammation and neurodegeneration in AD (Kinney et al., 2018). Aβ modulates synaptic plasticity, synaptogenesis, axonal growth and guidance, oxidative stress, learning and memory (Parihar and Brewer, 2010; Cuestas Torres and Cardenas, 2020). In addition, changes in neuroinflammatory signaling occur in AD pathology, as evidenced by activated MCs and glial cells (Skaper et al., 2014b).
The HPA axis induces MC degranulation under chronic stress, releasing excessive pro-inflammatory mediators that cause synaptic loss, disruption of BBB integrity and neuroinflammation in brain regions affected by AD (Galli et al., 2008; Du and Pang, 2015; Justice, 2018; Kempuraj et al., 2019; Figure 3). Dysregulation of the HPA axis in AD is accompanied by other clinical alterations, including memory impairment and synaptic plasticity deficits. Accumulating evidence illustrates that MC-derived inflammatory mediators may accelerate AD pathogenesis associated with neuroinflammation in the CNS (Machado et al., 2014; Chelombitko et al., 2016; Shaik-Dasthagirisaheb and Conti, 2016). Because MCs play a key role in neuroinflammation, chronic stress and psychiatric disorders, it is essential to study its roles in the pathogenesis of AD. However, the mechanisms by which chronic stress-mediated MC activation and HPA axis changes contribute to the pathogenesis of AD remain unclear. The early stages of AD are associated with perturbation of the HPA axis and high levels of CRH in biofluids in patients (Popp et al., 2015; Peña-Bautista et al., 2019). CRH may also work with other neuropeptides, including neurotensin, which enhances MC activation, leading to the release of many inflammatory mediators under acute stress (Alysandratos et al., 2012). CRH and its receptor CRH-R1 mediate stress-induced MC degranulation and the secretion of neuropeptides that activate glial cells in neurodegenerative diseases such as AD (Kempuraj et al., 2004, 2019; Ayyadurai et al., 2017).
It has been reported that CRH and norepinephrine levels are significantly upregulated in the CSF of AD patients, compared with control subjects (Wang et al., 2018). Similarly, a significant correlation between brain glucose metabolism and CRH levels has been observed in AD (Mosconi et al., 2008), suggesting a bidirectional relationship between the HPA axis and AD progression. The degranulation of MCs induced by CRH under stress could cause dysfunction of the HPA axis, producing other immune cell alterations such as in microglia. CRH and its receptors also play a pivotal role in the development of AD pathology (Machado et al., 2014). Accumulating evidence demonstrates that CRH and its receptors modulate γ-secretase activity, increasing Aβ production (Kempuraj et al., 2004, 2019; Mosconi et al., 2008; Park et al., 2015; Popp et al., 2015; Chelombitko et al., 2016; Shaik-Dasthagirisaheb and Conti, 2016; Ayyadurai et al., 2017; Futch et al., 2017; Wang et al., 2018; Peña-Bautista et al., 2019; Vandael and Gounko, 2019). CRHR1 knockout mice exhibit blocked phosphorylation of tau, but not CRHR2 knockout mice, suggesting a specific role for CRHR1-mediated signaling in AD (Rissman et al., 2007).
CRHR1 signaling pathways show that women are more vulnerable to AD than men (Futch et al., 2017; Vuppaladhadiam et al., 2020). The CRHR1 antagonist R121919 has been shown to decrease stress-mediated neuroinflammation, prevent cognitive damage and reduce Aβ deposition in the AD mouse model (Vuppaladhadiam et al., 2020). Similarly, another study reported that administering a CRHR1 antagonist reduced the expression levels of phosphorylated tau (Rissman et al., 2007; Zhang et al., 2016a). Therefore, antagonism of CRHR1 could be a new therapeutic strategy for AD.
PD
PD is a chronic and progressive movement disorder characterized by motor symptoms (such as resting tremor, rigidity, postural instability and bradykinesia) and numerous non-motor disturbances. The pathological hallmarks of PD are intraneuronal and intra-axonal inclusions of α-synuclein (Lewy bodies and Lewy neurites) and the degeneration of dopaminergic neurons in the substantia nigra and other brain regions (Tan et al., 2020). Recent studies show that inflammatory responses by MCs and glial cells, T cell infiltration and increased expression levels of inflammatory mediators in the MCs and neuronal cells are the most prominent features of PD (Kempuraj et al., 2017b, 2018; Selvakumar et al., 2018). Neuroinflammation is another key factor in PD pathogenesis and may further induce progressive loss of dopaminergic neurons (Kempuraj et al., 2016b; Tan et al., 2020). The activation of MCs releases many pro-inflammatory mediators responsible for neurodegeneration in the pathogenesis of PD (Kempuraj et al., 2016a; Sugama et al., 2016). However, the etiology of PD pathogenesis remains unclear.
De Souza and colleagues (De Souza et al., 1987) reported that the expression levels of CRH were decreased in the cerebral cortex in PD. UCN and CRH have been proposed as cytoprotectants via CRH-R1 (Pedersen et al., 2002; Facci et al., 2003). UCN was found to arrest the development of Parkinsonian-like features in the 6-hyroxydopamine-lesioned hemiparkinsonian rat model (Abuirmeileh et al., 2007). UCN, acting via the CRH-R1 receptor, has shown anti-inflammatory activity in the periphery (Gonzalez-Rey et al., 2006). UCN significantly reduces the development of PD-like pathology (Abuirmeileh et al., 2008; Whitton, 2010). CRH and UCN reduce weight gain in a rodent PD model, but only CRH produced consistent effects with improved sympathetic activity (Oki and Sasano, 2004). It has been reported that UCN acts via the CRH-R1 receptor and can maintain nigrostriatal function in the neuroinflammatory model of PD (Abuirmeileh et al., 2007, 2009). These findings suggest that UCN-mediated neuroprotection is a potential therapeutic strategy for PD.
Although PD is mainly an idiopathic disease, it can still have a hereditary or an environmental origin, and both can be related to early life stress (Dauer and Przedborski, 2003; Lupien et al., 2009; Tsang and Chung, 2009; Hemmerle et al., 2012). Chronic stress activates the HPA axis and may trigger the dopaminergic neuron loss in the substantia nigra in PD (Mpofana et al., 2016). CRH-R1 receptors expressed in dopaminergic neurons may have an anxiolytic effect depending on the brain region (Refojo et al., 2011). MCs are surrounded by microglia and astrocytes that can become activated in PD, releasing inflammatory cytokines, CRH, neurotensin and neuromodulators that trigger bidirectional communication able to damage dopaminergic neurons (Kempuraj et al., 2018; Figure 3). However, additional studies are needed to elucidate the molecular and cellular communication mechanisms between MCs, CRH neurons and glial cells in PD pathogenesis.
ASD
ASD is a complex neurodevelopmental disorder characterized by impaired communication, obsessive behaviors and language problems (Ousley and Cermak, 2014; Muotri, 2018; Theoharides et al., 2019; Marrus and Constantino, 2020). This disorder also includes repetitive and limited patterns of behavior. The incidence of ASD is increasing worldwide, with the most recent prevalence studies indicating that they will be present in 1 in 40 children by 2025 (Leigh and Du, 2015). ASD has neither a distinct pathogenesis nor an effective treatment, yet evidence continues to mount demonstrating immune abnormalities and autoimmune or brain inflammation in specific brain regions as well as neurodevelopmental perturbations.
Neuroinflammation linked to neuroimmune dysfunction has been well established in ASD as an essential factor in its development and maintenance (Onore et al., 2012; Rossignol and Frye, 2012; Estes and McAllister, 2015). Emerging evidence illustrates that neuroinflammation plays a key role in the pathogenesis of ASD and may result from the activation of MCs (Cao et al., 2005; Skaper et al., 2014a; Girolamo et al., 2017; Siniscalco et al., 2018; Theoharides et al., 2019). Brain-resident MC activation by psychological stress or environmental stimuli may trigger the release of pro-inflammatory cytokines and neuropeptides (CRH, neurotensin) that could disrupt BBB integrity and cause allergies in specific brain regions, thus contributing to neuroinflammation in ASD pathogenesis (Siniscalco et al., 2018; Theoharides et al., 2019; Figure 3). Pro-inflammatory cytokines and neuropeptides (CRH, neurotensin) are significantly increased in children with ASD compared with non-ASD controls (Molloy et al., 2006; Tsilioni et al., 2014; Prata et al., 2019). However, further study is needed to clarify whether hyperactive MCs induce the release of inflammatory mediators via CRH neurons and HPA axis activation and directly regulate neuroinflammatory processes in the pathogenesis of ASD.
ILs are involved in neuroimmune modulation and have been studied for their roles in inflammatory states and neurodevelopmental disorders (Xu et al., 2015; Siniscalco et al., 2018). IL-6 can be selectively released from MCs upon activation (Kandere-Grzybowska et al., 2003; Theoharides et al., 2007). In addition, IL-6 expression levels are upregulated in the brain of ASD patients (Li et al., 2009; Saghazadeh et al., 2019). Furthermore, the expression levels of serum IL-6 are linked to the autistic phenotype in mice (Dahlgren et al., 2006; Smith et al., 2007). Interestingly, patients with mastocytosis show increased proliferation and activation of MCs and upregulated serum levels of IL-6 (Brockow et al., 2005). Additionally, postnatal mice exhibit increased expression levels of IL-6 and IL-17 and altered immune regulation associated with ASD-related behaviors (Hsiao et al., 2012). Tsilioni et al. (2019) reported that IL-37 is significantly increased along with the pro-inflammatory cytokine IL-18 and its receptors in the amygdala and dorsolateral prefrontal cortex of children with ASD. Therefore, we propose IL inflammatory proteins as potential therapeutic targets for ASD.
Stress is linked to the onset and worsening of ASD. This link may involve brain MCs activated by CRH from the hypothalamus, thereby contributing to neuroinflammation (Theoharides, 2020a). MCs widely express CRH, and its receptors lead to selective production of vascular endothelial growth factor (Cao et al., 2005). MCs are closely linked to CRH nerve endings in the median eminence of the PVN (Rozniecki et al., 1999). It has been reported that UCN1 induces activation of cultured human MCs (Singh et al., 1999). Acute stress results in the secretion of CRH, which activates the HPA axis, leading to stimulation of MCs and increased vascular permeability and disruption of BBB integrity associated with neuroinflammation in ASD pathogenesis (Theoharides et al., 1998; Crompton et al., 2003; Lytinas et al., 2003; Ribatti, 2015; Joshi et al., 2019; Welcome and Mastorakis, 2020). Investigating the interaction between MCs and CRH neurons in the amygdala could uncover novel therapeutic targets for ASD.
MS
MS is a neurological autoimmune disorder causing severe non-traumatic disability in young adults. The pathological hallmarks of MS are inflammatory demyelinating lesions and neurodegeneration within the CNS (Oh et al., 2018; Reich et al., 2018; Wasko et al., 2020). The mechanisms of MS involve disruption of the innate and adaptive immune systems, leading to the inflammatory infiltration of immune cells (composed of MCs, T and B cells, dendritic cells and natural killer cells) into the brain and spinal cord through an increasingly leaky BBB (Hemmer et al., 2015; Baecher-Allan et al., 2018). MCs may promote demyelination at the early stage because MCs interact with other immune cells (astrocytes and microglia) to mediate neuroinflammation (Elieh-Ali-Komi and Cao, 2017; Pinke et al., 2020; Zusso et al., 2020).
Chronic stress, CRH-mediated MC activation and BBB dysfunction may participate in the pathogenetic progression of MS (Theoharides et al., 2012; Figure 3). MCs are tightly associated with CRH-positive neurons in the median eminence and widely express CRH and its receptors (Theoharides et al., 1995). Hyperactivation of hypothalamic MCs can stimulate the HPA axis through histamine and CRH, which regulate hypothalamic function and can also increase CRH/CRH-R mRNA expression in the hypothalamus (Bugajski et al., 1995; Kjaer et al., 1998; Ayyadurai et al., 2017). It has been reported that human MCs can produce and release large amounts of CRH, as well as IL-1 and IL-6, which are critical activators of the HPA axis (Bethin et al., 2000; Cao et al., 2005). MC-derived mediators such as neuropeptides (CRH, neurotensin and SP) may increase BBB permeability (Tsilioni et al., 2016). CRH and MCs regulate BBB permeability, and possibly, neurological inflammatory disorder exacerbated by chronic stress. Benou et al. (2005) reported that CRH-deficient mice are resistant to experimental autoimmune encephalomyelitis and show decreased clinical disability and brain infiltration of immune cells. This suggests that CRH contributes to immune cell activation and the peripheral inflammatory response in experimental autoimmune encephalomyelitis. It is hypothesized that the subsequent chronic activation of MCs may activate the HPA axis that contributes to MS pathogenesis. Further studies are needed to elucidate the neuroimmune connections between MC-mediated BBB disruption, HPA axis activation and tissue damage in MS.
ALS
ALS—also known as motor neuron disease or Lou Gehrig’s disease—is an idiopathic, fatal neurodegenerative disease that is characterized by the progressive degeneration of motor neurons with heterogeneity in the age and site of disease onset. The pathophysiological mechanisms involved in the motor neuron degeneration underlying ALS are multifactorial and complex processes with interactions between genetic and environmental factors (Brown and Al-Chalabi, 2017). Mutations in numerous genes have been linked to ALS, including TARDBP (also known as TDP-43; TAR DNA-binding protein) (Sreedharan et al., 2008), SOD1 (copper/zinc ion-binding superoxide dismutase) (Zetterström et al., 2007), FUS (fused in sarcoma) (Vance et al., 2009), OPTN (optineurin) (Maruyama et al., 2010) and ANG (angiogenin, ribonuclease, RNase A family, 5) (Greenway et al., 2006). Transgenic mice expressing these mutations exhibit neuroinflammation and immune dysregulation, which lead to microglial and MC activation and reactive astrocytosis, immune cell dysfunction and the release of proinflammatory mediators from peripheral lymphocytes, monocytes and macrophages (Malyshev and Malyshev, 2015; Zhao et al., 2015a, 2017; Liu and Wang, 2017). ALS is characterized by significantly increased spinal and brain inflammation, particularly mediated by microglia and MCs (Clemente, 2012).
Emerging evidence now points to a fundamental role of neuroinflammatory mechanisms in pathophysiology onset and progression, with the interaction of MCs and microglia playing a key role in neurodegenerative diseases such as ALS (Trias et al., 2017). Neuroinflammatory disorders are typically associated with dysregulation of immune cells (MCs, microglia) in the nervous system (Ransohoff et al., 2015). Numerous studies demonstrate that chronic stress is an integral part of neuroinflammatory processes, which may accelerate the risk of developing neurodegenerative diseases including ALS (Glass et al., 2010; Zhang et al., 2019). The ability of MCs to respond to many diverse molecules, especially CRH during stress and neuroinflammatory processes, suggests a role in the pathogenesis of stress-related neurological disorders such as ALS (Jones et al., 2019). CRH acts as a neuropeptide and major regulator of the stress response. Dysregulation of protein levels of CRH is involved in the pathogenesis of ALS (Vandael and Gounko, 2019), but little is known of the underlying mechanisms. The neuroinflammatory processes involving MC–CRH interactions in ALS pathogenesis have yet to be elucidated.
Traumatic brain injury
Traumatic brain injury (TBI) is a form of acquired brain injury where a sudden trauma causes damage to the brain, resulting in tissue destruction and distortion (Garvin and Mangat, 2017; Stein et al., 2017b). Secondary injuries from TBI cause changes in neuroimmune cell performance, depolarization, excitotoxicity, the formation of edema, BBB disruption and imbalance of calcium homeostasis (Fehily and Fitzgerald, 2017; Galgano et al., 2017). TBI has been linked with long-term cognitive deficits including impaired memory and attention, changes in neuroimmune functional activity, and emotional instability, resulting in trauma-induced neurological disorders (Rabinowitz and Levin, 2014). Recently, research into TBI has mainly focused on stress, disruption of the BBB, cerebral blood flow and neuroinflammatory activities (Pavlova et al., 2018). Neuroinflammation associated with TBI involves the activation of neuroimmune cells (MCs, microglia and astrocytes) and the subsequent release of inflammatory mediators within the brain (Barrett et al., 2020).
In secondary injury in TBI, MCs directly communicate with glial cells and release neuroinflammatory mediators, leading to neurological diseases (Simon et al., 2017). Chronic stress conditions influence MC activation via CRH and other neuropeptides to release numerous neuroinflammatory mediators such as IL-1β, TNF-α, IL-6, C–C motif chemokine ligand 2, IL-8, tryptase, histamine, reactive oxygen species, CRH and matrix metallopeptidases (Liezmann et al., 2012). These neurotoxic inflammatory mediators lead to activation of microglia, which release additional pro-inflammatory mediators, resulting in neurological diseases and neuronal death. TBI also disrupts BBB permeability and recruits other neuroimmune cells such as MCs, microglia, astrocytes and T cells into the brain (Chodobski et al., 2011). Activated neuroimmune cells release neuroinflammatory mediators at the site of brain injury and accelerate neuroinflammatory reactions (Kempuraj et al., 2020b). Moreover, MCs also increase the expression of CRH in the brain. TBI induces neuroinflammation, which in turn causes neurological diseases via neuroimmune cells.
Rheumatoid arthritis
Rheumatoid arthritis (RA), a chronic long term inflammatory autoimmune disorder, is characterized by inflammation of the synovial membrane associated with various immune cells, including B cells, T cells, NK cells and MCs (Smolen et al., 2016). MCs are the tissue-resident innate immune cells that contribute to RA pathogenesis (Min et al., 2020); however, the mechanisms of MC involvement in RA remain unclear. Synovium from RA patients has a significantly increased population of MCs compared with healthy controls (Crisp et al., 1984; Pitzalis et al., 2013) and shows increased MC infiltration in the end stage of the disease (Rivellese et al., 2018). Rivellese et al. (2015) and Rossini et al. (2016) have suggested that synovial MCs are associated with the severity of RA disorder, including synovial inflammation, lympho-myeloid infiltrate, systemic inflammation, autoantibodies and disease progression. Activated synovial MCs release several endogenous toll-like receptor ligands, pro-inflammatory cytokines and chemokines, including TNF-α, IL-1beta and IL-1Ra (Min et al., 2020). The induction of osteoporosis can be mediated directly by MC-derived proteases (Ragipoglu et al., 2020). Lee et al. (2002) found that MC-deficient mice were resistant to the development or progression of RA. These studies suggest that MCs may act as a complex cellular link with inflammatory signaling pathways in RA. The number of MCs are increased 5- to 24-fold in human RA compared with healthy controls (Nigrovic and Lee, 2007). Furthermore, degranulated MC numbers increase more than 3-fold in multiple RA animal models (Shin et al., 2006; Kambayashi et al., 2009). Activated MCs produce cytokines and proteases that are involved in the pathogenesis of RA, particularly IL-1β, IL-17, TNF and tryptase (Hueber et al., 2010). Tryptase directly triggers synovial fibroblasts by interacting with the protease-activated receptor 2, resulting in degraded cartilage and bone (Shin et al., 2009; Sawamukai et al., 2010). The neuropeptide substance P induces MC activation through Mas-related X2 expression in RA synovium. MCs accumulate in the synovial fluids and joint tissue of patients with RA and release pro-inflammatory cytokines and chemokines (Xu and Chen, 2015). The degranulation of MCs via the IgG immune complex receptor FcγRIII may accelerate the initiation of joint inflammation by the production and release of IL-1 (Lee et al., 2013). Synovial fibroblasts in RA that modulate MCs containing proteases, tryptases and chymase may be considered a novel potential therapeutic target.
CRH is locally secreted in synovial tissue and fluids of patients with osteoarthritis and RA (Crofford et al., 1993). Stress induces the HPA axis response through CRH and its receptors and is differentially modulated by CRH gene promoter polymorphisms in RA patients (Zhou and Fang, 2018). HPA axis activation is induced by both arginine vasopressin and CRH in acute mono-arthritis (Nishimura et al., 2020). The HPA axis secretes CRH which initiates an immune and inflammatory cascade of events leading to the release of cortisone (Herman et al., 2016). Studies have reported that CRH and its receptors are identified on MCs from RA joints as well as UCN (Baerwald et al., 2000; Gonzalez-Gay et al., 2003). Furthermore, other studies have shown that activated MCs express mRNAs for CRH and its receptors in RA patients (Huang et al., 2002). Therefore, it has been postulated that degranulated MCs releasing CRH may be a major contributor to RA pathogenesis.
The Link between MCs and Stress in Neurological Disorders
Stress triggers HPA axis activation through the release of CRH and vasopressin from the PVN of the hypothalamus in the brain, leading to enhanced MC activation (Kempuraj et al., 2017a). Stress conditions activate MCs through CRH, neurotensin and substance P to release inflammatory mediators that lead to perturbed BBB permeability and the recruitment of peripheral immune and inflammatory cells into the brain, thereby contributing to neuroinflammation and leading to neurological disorders (Kempuraj et al., 2017a, 2019; Theoharides, 2020a). Acute stress directly increases BBB permeability through brain-resident MCs and microglial activation and CRH release (Esposito et al., 2001). MC activation plays a critical role in the pathogenesis of neurological disorders. Social stress exposure leads to long-term increases in the recruitment of macrophages, activated MCs and microglia in the brain (Bryan and Rudd, 2015; Stein et al., 2017a). Social stress also accelerates activation of these cell types, resulting in increased expression of pro-inflammatory cytokines. Pro-inflammatory cytokines are pleiotropic molecules that play an important role in the pathogenesis of neurological diseases and communicate with the CNS via direct interaction with the BBB or via the neuroinflammatory signaling pathway by way of the vagus nerve (Kim et al., 2016; Sinagra et al., 2020). Stress induces the release of pro-inflammatory cytokines, which leads to chronic neuroinflammation, and ultimately contributes to the pathology of depression. Inflammatory cytokines and their neuroimmune signaling pathways, via complex cytokine networks, perturb the HPA and hippocampal glucocorticoid receptors by activating microglia that are closely associated with depression, thereby leading to structural and functional changes in the brain, including impaired spatial memory, neuronal loss and altered neuroplasticity (Pace and Miller, 2009). Crosstalk between inflammatory cytokines and neural circuits via neuroimmune cells can affect behavioral responses such as depression. Increasing evidence suggests that pro-inflammatory cytokines (i.e., IL-1β, IL-6 and TNF-α) induce the activation of microglia and astrocytes to cause neuroinflammation, leading to oxidative imbalance and stress, which are correlated with depression and subsequent neuronal death in neurological diseases (Pace et al., 2007).
Long-term psychological stress triggers neuroimmune cell activation (MCs, microglia) (Walker et al., 2013; Lehmann et al., 2016; Kempuraj et al., 2020a). These activated neuroimmune cells secrete numerous inflammatory mediators under stress, and together with MCs and microglial activation, could be a major contributor to the pathogenesis of neurological diseases. Psychological stress worsens anxiety and depression, induces oxidative stress, enhances immune responses and induces the activation of MCs and microglia, resulting in neuroinflammation (Grippo and Scotti, 2013; Calcia et al., 2016; Lehmann et al., 2018; Kempuraj et al., 2020a). Inflammatory pathways are linked to MCs and stress, leading to chronic depression in mastocytosis patients (Georgin-Lavialle et al., 2016). A single exposure to social stress may promote increased neuroinflammatory processes in response to spontaneous stress exposure (Audet et al., 2011). Stress induces or worsens migraines through activation of MCs that release inflammatory cytokines and chemokines (Maleki et al., 2012; Theoharides et al., 2012; Theoharides, 2020a). Therefore, we postulate that the interaction between stress and MCs may be a major contributor to neurological disorders.
Conclusion and Future Prospects
MCs are multifunctional neuroimmune cells that participate in the stress response. Under normal conditions, brain-resident MCs secrete CRH, histamine, tryptase and other mediators that regulate BBB permeability. When peripheral inflammation occurs, the MCs in the brain immediately respond to the neuroimmune signal in the blood and quickly degranulate via CRH and its receptors, releasing neuroinflammatory mediators and acting as the initiator and catalyst of the inflammatory response in the CNS. Acute or chronic stress can activate the HPA axis within seconds and increase the secretion of CRH and other neuropeptides from the PVN of the hypothalamus, thereby activating MCs, which in turn disrupt the BBB, leading to neuroinflammation in the brain. Accumulating evidence suggests that bidirectional neuroimmune interactions between MCs, CRH and BBB tight junctions result in neuronal dysfunction, neuroinflammation and neurological diseases. However, a deeper understanding of these complex neuroimmune networks is needed to clarify their role in inflammatory and neurological disorders. Further study is required to elucidate the cellular and molecular mechanisms underlying the neuroimmune connections between CRH and MCs to uncover new therapeutic targets for inflammatory and neurological disorders.
Footnotes
Conflicts of interest:The authors declare that there are no conflicts of interest regarding this review.
Financial support:The work was supported by the National Natural Science Foundation of China, Nos. 81671387 (to YNQ), 81701375 (to HQD); and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), China.
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Funding:The work was supported by the National Natural Science Foundation of China, Nos. 81671387 (to YNQ), 81701375 (to HQD); and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), China.
C-Editor: Zhao M; S-Editors: Yu J, Li CH; L-Editors: Patel B, Yu J, Song LP; T-Editor: Jia Y
References
- 1.Abelson JL, Khan S, Young EA, Liberzon I. Cognitive modulation of endocrine responses to CRH stimulation in healthy subjects. Psychoneuroendocrinology. 2010;35:451–459. doi: 10.1016/j.psyneuen.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abuirmeileh A, Harkavyi A, Lever R, Biggs CS, Whitton PS. Urocortin, a CRF-like peptide, restores key indicators of damage in the substantia nigra in a neuroinflammatory model of Parkinson’s disease. J Neuroinflammation. 2007;4:19. doi: 10.1186/1742-2094-4-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abuirmeileh A, Harkavyi A, Kingsbury A, Lever R, Whitton PS. The CRF-like peptide urocortin produces a long-lasting recovery in rats made hemiparkinsonian by 6-hydroxydopamine or lipopolysaccharide. J Neurol Sci. 2008;271:131–136. doi: 10.1016/j.jns.2008.04.016. [DOI] [PubMed] [Google Scholar]
- 4.Abuirmeileh A, Harkavyi A, Kingsbury A, Lever R, Whitton PS. The CRF-like peptide urocortin greatly attenuates loss of extracellular striatal dopamine in rat models of Parkinson’s disease by activating CRF(1) receptors. Eur J Pharmacol. 2009;604:45–50. doi: 10.1016/j.ejphar.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 5.Aguilera G, Liu Y. The molecular physiology of CRH neurons. Front Neuroendocrinol. 2012;33:67–84. doi: 10.1016/j.yfrne.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alysandratos KD, Asadi S, Angelidou A, Zhang B, Sismanopoulos N, Yang H, Critchfield A, Theoharides TC. Neurotensin and CRH interactions augment human mast cell activation. PLoS One. 2012;7:e48934. doi: 10.1371/journal.pone.0048934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andrae DA, Patrick DL, Drossman DA, Covington PS. Evaluation of the Irritable Bowel Syndrome Quality of Life (IBS-QOL) questionnaire in diarrheal-predominant irritable bowel syndrome patients. Health Qual Life Outcomes. 2013;11:208. doi: 10.1186/1477-7525-11-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arora S, Sevdalis N, Nestel D, Woloshynowych M, Darzi A, Kneebone R. The impact of stress on surgical performance: a systematic review of the literature. Surgery. 2010;147:318–330. doi: 10.1016/j.surg.2009.10.007. 330e1-6. [DOI] [PubMed] [Google Scholar]
- 9.Asadi S, Alysandratos KD, Angelidou A, Miniati A, Sismanopoulos N, Vasiadi M, Zhang B, Kalogeromitros D, Theoharides TC. Substance P (SP) induces expression of functional corticotropin-releasing hormone receptor-1 (CRHR-1) in human mast cells. J Invest Dermatol. 2012;132:324–329. doi: 10.1038/jid.2011.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Audet MC, Jacobson-Pick S, Wann BP, Anisman H. Social defeat promotes specific cytokine variations within the prefrontal cortex upon subsequent aggressive or endotoxin challenges. Brain Behav Immun. 2011;25:1197–1205. doi: 10.1016/j.bbi.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 11.Ayyadurai S, Gibson AJ, D’Costa S, Overman EL, Sommerville LJ, Poopal AC, Mackey E, Li Y, Moeser AJ. Frontline science: corticotropin-releasing stress-induced pathophysiology. J Leukoc Biol. 2017;102:1299–1312. doi: 10.1189/jlb.2HI0317-088RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baecher-Allan C, Kaskow BJ, Weiner HL. Multiple sclerosis: Mechanisms and immunotherapy. Neuron. 2018;97:742–768. doi: 10.1016/j.neuron.2018.01.021. [DOI] [PubMed] [Google Scholar]
- 13.Baerwald CG, Mok CC, Tickly M, Lau CS, Wordsworth BP, Ollier B, Panayi GS, Lanchbury JS. Corticotropin releasing hormone (CRH) promoter polymorphisms in various ethnic groups of patients with rheumatoid arthritis. Z Rheumatol. 2000;59:29–34. doi: 10.1007/s003930050002. [DOI] [PubMed] [Google Scholar]
- 14.Barrett JP, Henry RJ, Shirey KA, Doran SJ, Makarevich OD, Ritzel RM, Meadows VA, Vogel SN, Faden AI, Stoica BA, Loane DJ. Interferon-β plays a detrimental role in experimental traumatic brain injury by enhancing neuroinflammation that drives chronic neurodegeneration. J Neurosci. 2020;40:2357–2370. doi: 10.1523/JNEUROSCI.2516-19.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Benou C, Wang Y, Imitola J, VanVlerken L, Chandras C, Karalis KP, Khoury SJ. Corticotropin-releasing hormone contributes to the peripheral inflammatory response in experimental autoimmune encephalomyelitis. J Immunol. 2005;174:5407–5413. doi: 10.4049/jimmunol.174.9.5407. [DOI] [PubMed] [Google Scholar]
- 16.Bethin KE, Vogt SK, Muglia LJ. Interleukin-6 is an essential, corticotropin-releasing hormone-independent stimulator of the adrenal axis during immune system activation. Proc Natl Acad Sci U S A. 2000;97:9317–9322. doi: 10.1073/pnas.97.16.9317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bischoff SC. Mast cells in gastrointestinal disorders. Eur J Pharmacol. 2016;778:139–145. doi: 10.1016/j.ejphar.2016.02.018. [DOI] [PubMed] [Google Scholar]
- 18.Boscolo P, Youinou P, Theoharides TC, Cerulli G, Conti P. Environmental and occupational stress and autoimmunity. Autoimmun Rev. 2008;7:340–343. doi: 10.1016/j.autrev.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 19.Boucher W, Kempuraj D, Michaelian M, Theoharides TC. Corticotropin-releasing hormone-receptor 2 is required for acute stress-induced bladder vascular permeability and release of vascular endothelial growth factor. BJU Int. 2010;106:1394–1399. doi: 10.1111/j.1464-410X.2010.09237.x. [DOI] [PubMed] [Google Scholar]
- 20.Brockow K, Akin C, Huber M, Metcalfe DD. IL-6 levels predict disease variant and extent of organ involvement in patients with mastocytosis. Clin Immunol. 2005;115:216–223. doi: 10.1016/j.clim.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 21.Brown RH, Jr, Al-Chalabi A. Amyotrophic lateral sclerosis. N Engl J Med. 2017;377:1602. doi: 10.1056/NEJMc1710379. [DOI] [PubMed] [Google Scholar]
- 22.Bryan CJ, Rudd MD. Response to Stankiewicz et al. Am J Psychiatry. 2015;172:1022–1023. doi: 10.1176/appi.ajp.2015.15060774r. [DOI] [PubMed] [Google Scholar]
- 23.Bugajski AJ, Chłap Z, Gadek-Michalska A, Borycz J, Bugajski J. Degranulation andpound 48/80. Inflamm Res 44 Suppl. 1995;1:S50–51. doi: 10.1007/BF01674391. [DOI] [PubMed] [Google Scholar]
- 24.Calcia MA, Bonsall DR, Bloomfield PS, Selvaraj S, Barichello T, Howes OD. Stress and neuroinflammation: a systematic review of the effects of stress on microglia and the implications for mental illness. Psychopharmacology (Berl) 2016;233:1637–1650. doi: 10.1007/s00213-016-4218-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cao J, Boucher W, Kempuraj D, Donelan JM, Theoharides TC. Acute stress and intravesical corticotropin-releasing hormone induces mast cell dependent vascular endothelial growth factor release from mouse bladder explants. J Urol. 2006;176:1208–1213. doi: 10.1016/j.juro.2006.04.026. [DOI] [PubMed] [Google Scholar]
- 26.Cao J, Papadopoulou N, Kempuraj D, Boucher WS, Sugimoto K, Cetrulo CL, Theoharides TC. Human mast cells express corticotropin-releasing hormone (CRH) receptors and CRH leads to selective secretion of vascular endothelial growth factor. J Immunol. 2005;174:7665–7675. doi: 10.4049/jimmunol.174.12.7665. [DOI] [PubMed] [Google Scholar]
- 27.Cardoso JC, Bergqvist CA, Félix RC, Larhammar D. Corticotropin-releasing hormone family evolution: five ancestral genes remain in some lineages. J Mol Endocrinol. 2016;57:73–86. doi: 10.1530/JME-16-0051. [DOI] [PubMed] [Google Scholar]
- 28.Căruntu C, Boda D, Musat S, Căruntu A, Mandache E. Stress-induced mast cell activation in glabrous and hairy skin. Mediators Inflamm. 2014;2014:105950. doi: 10.1155/2014/105950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Castagliuolo I, Wershil BK, Karalis K, Pasha A, Nikulasson ST, Pothoulakis C. Colonic mucin release in response to immobilization stress is mast cell dependent. Am J Physiol. 1998;274:G1094–1100. doi: 10.1152/ajpgi.1998.274.6.G1094. [DOI] [PubMed] [Google Scholar]
- 30.Catalano RD, Kyriakou T, Chen J, Easton A, Hillhouse EW. Regulation of corticotropin-releasing hormone type 2 receptors by multiple promoters and alternative splicing: identification of multiple splice variants. Mol Endocrinol. 2003;17:395–410. doi: 10.1210/me.2002-0302. [DOI] [PubMed] [Google Scholar]
- 31.Chelombitko MA, Fedorov AV, Ilyinskaya OP, Zinovkin RA, Chernyak BV. Role of reactive oxygen species in mast cell degranulation. Biochemistry (Mosc) 2016;81:1564–1577. doi: 10.1134/S000629791612018X. [DOI] [PubMed] [Google Scholar]
- 32.Chen Y, Lyga J. Brain-skin connection: stress, inflammation and skin aging. Inflamm Allergy Drug Targets. 2014;13:177–190. doi: 10.2174/1871528113666140522104422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chodobski A, Zink BJ, Szmydynger-Chodobska J. Blood-brain barrier pathophysiology in traumatic brain injury. Transl Stroke Res. 2011;2:492–516. doi: 10.1007/s12975-011-0125-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chow BW, Gu C. The molecular constituents of the blood-brain barrier. Trends Neurosci. 2015;38:598–608. doi: 10.1016/j.tins.2015.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chrousos GP, Zoumakis E. Milestones in CRH research. Curr Mol Pharmacol. 2017;10:259–263. doi: 10.2174/1874467210666170109165219. [DOI] [PubMed] [Google Scholar]
- 36.Clemente S. Amyotrophic lateral sclerosis treatment with ultramicronized palmitoylethanolamide: a case report. CNS Neurol Disord Drug Targets. 2012;11:933–936. doi: 10.2174/1871527311201070933. [DOI] [PubMed] [Google Scholar]
- 37.Conti P, Caraffa A, Ronconi G, Kritas SK, Mastrangelo F, Tettamanti L, Theoharides TC. Impact of mast cells in mucosal immunity of intestinal inflammation: Inhibitory effect of IL-37. Eur J Pharmacol. 2018;818:294–299. doi: 10.1016/j.ejphar.2017.09.044. [DOI] [PubMed] [Google Scholar]
- 38.Crisp AJ, Chapman CM, Kirkham SE, Schiller AL, Krane SM. Articular mastocytosis in rheumatoid arthritis. Arthritis Rheum. 1984;27:845–851. doi: 10.1002/art.1780270802. [DOI] [PubMed] [Google Scholar]
- 39.Crofford LJ, Sano H, Karalis K, Friedman TC, Epps HR, Remmers EF, Mathern P, Chrousos GP, Wilder RL. Corticotropin-releasing hormone in synovial fluids and tissues of patients with rheumatoid arthritis and osteoarthritis. J Immunol. 1993;151:1587–1596. [PubMed] [Google Scholar]
- 40.Crompton R, Clifton VL, Bisits AT, Read MA, Smith R, Wright IM. Corticotropin-releasing hormone causes vasodilation in human skin via mast cell-dependent pathways. J Clin Endocrinol Metab. 2003;88:5427–5432. doi: 10.1210/jc.2003-030377. [DOI] [PubMed] [Google Scholar]
- 41.Cuestas Torres DM, Cardenas FP. Synaptic plasticity in Alzheimer’s disease and healthy aging. Rev Neurosci. 2020;31:245–268. doi: 10.1515/revneuro-2019-0058. [DOI] [PubMed] [Google Scholar]
- 42.D’Costa S, Ayyadurai S, Gibson AJ, Mackey E, Rajput M, Sommerville LJ, Wilson N, Li Y, Kubat E, Kumar A, Subramanian H, Bhargava A, Moeser AJ. Mast cell corticotropin-releasing factor subtype 2 suppresses mast cell degranulation and limits the severity of anaphylaxis and stress-induced intestinal permeability. J Allergy Clin Immunol. 2019;143:1865–1877e4. doi: 10.1016/j.jaci.2018.08.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dahlgren J, Samuelsson AM, Jansson T, Holmäng A. Interleukin-6 in the maternal circulation reaches the rat fetus in mid-gestation. Pediatr Res. 2006;60:147–151. doi: 10.1203/01.pdr.0000230026.74139.18. [DOI] [PubMed] [Google Scholar]
- 44.Dauer W, Przedborski S. Parkinson’s disease: mechanisms and models. Neuron. 2003;39:889–909. doi: 10.1016/s0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- 45.De Souza EB, Whitehouse PJ, Price DL, Vale WW. Abnormalities in corticotropin-releasing hormone (CRH) in Alzheimer’s disease and other human disorders. Ann N Y Acad Sci. 1987;512:237–247. doi: 10.1111/j.1749-6632.1987.tb24964.x. [DOI] [PubMed] [Google Scholar]
- 46.Dedic N, Chen A, Deussing JM. The CRF family of neuropeptides and their receptors - mediators of the central stress response. Curr Mol Pharmacol. 2018;11:4–31. doi: 10.2174/1874467210666170302104053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Deussing JM, Chen A. The corticotropin-releasing factor family: Physiology of the stress response. Physiol Rev. 2018;98:2225–2286. doi: 10.1152/physrev.00042.2017. [DOI] [PubMed] [Google Scholar]
- 48.Deussing JM, Breu J, Kühne C, Kallnik M, Bunck M, Glasl L, Yen YC, Schmidt MV, Zurmühlen R, Vogl AM, Gailus-Durner V, Fuchs H, Hölter SM, Wotjak CT, Landgraf R, de Angelis MH, Holsboer F, Wurst W. Urocortin 3 modulates social discrimination abilities via corticotropin-releasing hormone receptor type 2. J Neurosci. 2010;30:9103–9116. doi: 10.1523/JNEUROSCI.1049-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dong H, Zhang X, Qian Y. Mast cells and neuroinflammation. Med Sci Monit Basic Res. 2014;20:200–206. doi: 10.12659/MSMBR.893093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Du X, Pang TY. Is dysregulation of the HPA-axis a core pathophysiology mediating co-morbid depression in neurodegenerative diseases. Front Psychiatry. 2015;6:32. doi: 10.3389/fpsyt.2015.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Elenkov IJ, Iezzoni DG, Daly A, Harris AG, Chrousos GP. Cytokine dysregulation, inflammation and well-being. Neuroimmunomodulation. 2005;12:255–269. doi: 10.1159/000087104. [DOI] [PubMed] [Google Scholar]
- 52.Elieh-Ali-Komi D, Cao Y. Role of mast cells in the pathogenesis of multiple sclerosis and experimental autoimmune encephalomyelitis. Clin Rev Allergy Immunol. 2017;52:436–445. doi: 10.1007/s12016-016-8595-y. [DOI] [PubMed] [Google Scholar]
- 53.Esposito P, Gheorghe D, Kandere K, Pang X, Connolly R, Jacobson S, Theoharides TC. Acute stress increases permeability of the blood-brain-barrier through activation of brain mast cells. Brain Res. 2001;888:117–127. doi: 10.1016/s0006-8993(00)03026-2. [DOI] [PubMed] [Google Scholar]
- 54.Esposito P, Chandler N, Kandere K, Basu S, Jacobson S, Connolly R, Tutor D, Theoharides TC. Corticotropin-releasing hormone and brain mast cells regulate blood-brain-barrier permeability induced by acute stress. J Pharmacol Exp Ther. 2002;303:1061–1066. doi: 10.1124/jpet.102.038497. [DOI] [PubMed] [Google Scholar]
- 55.Estes ML, McAllister AK. Immune mediators in the brain and peripheral tissues in autism spectrum disorder. Nat Rev Neurosci. 2015;16:469–486. doi: 10.1038/nrn3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Eutamene H, Theodorou V, Fioramonti J, Bueno L. Acute stress modulates the histamine content of mast cells in the gastrointestinal tract through interleukin-1 and corticotropin-releasing factor release in rats. J Physiol. 2003;553:959–966. doi: 10.1113/jphysiol.2003.052274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Facci L, Stevens DA, Pangallo M, Franceschini D, Skaper SD, Strijbos PJ. Corticotropin-releasing factor (CRF) and related peptides confer neuroprotection via type 1 CRF receptors. Neuropharmacology. 2003;45:623–636. doi: 10.1016/s0028-3908(03)00211-9. [DOI] [PubMed] [Google Scholar]
- 58.Fehily B, Fitzgerald M. Repeated mild traumatic brain injury: potential mechanisms of damage. Cell Transplant. 2017;26:1131–1155. doi: 10.1177/0963689717714092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fenzl T, Romanowski CP, Flachskamm C, Deussing JM, Kimura M. Wake-promoting effects of orexin: Its independent actions against the background of an impaired corticotropinereleasing hormone receptor system. Behav Brain Res. 2011;222:43–50. doi: 10.1016/j.bbr.2011.03.026. [DOI] [PubMed] [Google Scholar]
- 60.Finnerty CC, Mabvuure NT, Ali A, Kozar RA, Herndon DN. The surgically induced stress response. JPEN J Parenter Enteral Nutr. 2013;37:21S–29S. doi: 10.1177/0148607113496117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fries GR. Hypothalamus-pituitary-adrenal axis programming by early-life stress: A role played by inflammatory and epigenetic mechanisms. In: Teixeira AL, Macedo D, Baune BT, editors. Perinatal inflammation and adult psychopathology: From preclinical models to humans. Cham: Springer International Publishing; 2020. pp. 49–61. [Google Scholar]
- 62.Fukuda T, Takahashi K, Suzuki T, Saruta M, Watanabe M, Nakata T, Sasano H. Urocortin 1, urocortin 3/stresscopin, and corticotropin-releasing factor receptors in human adrenal and its disorders. J Clin Endocrinol Metab. 2005;90:4671–4678. doi: 10.1210/jc.2005-0090. [DOI] [PubMed] [Google Scholar]
- 63.Futch HS, Croft CL, Truong VQ, Krause EG, Golde TE. Targeting psychologic stress signaling pathways in Alzheimer’s disease. Mol Neurodegener. 2017;12:49. doi: 10.1186/s13024-017-0190-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Füzesi T, Daviu N, Wamsteeker Cusulin JI, Bonin RP, Bains JS. Hypothalamic CRH neurons orchestrate complex behaviours after stress. Nat Commun. 2016;7:11937. doi: 10.1038/ncomms11937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Galgano M, Toshkezi G, Qiu X, Russell T, Chin L, Zhao LR. Traumatic brain injury: Current treatment strategies and future endeavors. Cell Transplant. 2017;26:1118–1130. doi: 10.1177/0963689717714102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Galli SJ, Grimbaldeston M, Tsai M. Immunomodulatory mast cells: negative, as well as positive, regulators of immunity. Nat Rev Immunol. 2008;8:478–486. doi: 10.1038/nri2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Garvin R, Mangat HS. Emergency neurological life support: severe traumatic brain injury. Neurocrit Care. 2017;27:159–169. doi: 10.1007/s12028-017-0461-0. [DOI] [PubMed] [Google Scholar]
- 68.Georgin-Lavialle S, Gaillard R, Moura D, Hermine O. Mastocytosis in adulthood and neuropsychiatric disorders. Transl Res. 2016;174:77–85e1. doi: 10.1016/j.trsl.2016.03.013. [DOI] [PubMed] [Google Scholar]
- 69.Girolamo F, Coppola C, Ribatti D. Immunoregulatory effect of mast cells influenced by microbes in neurodegenerative diseases. Brain Behav Immun. 2017;65:68–89. doi: 10.1016/j.bbi.2017.06.017. [DOI] [PubMed] [Google Scholar]
- 70.Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH. Mechanisms underlying inflammation in neurodegeneration. Cell. 2010;140:918–934. doi: 10.1016/j.cell.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Godoy LD, Rossignoli MT, Delfino-Pereira P, Garcia-Cairasco N, de Lima Umeoka EH. A comprehensive overview on stress neurobiology: Basic concepts and clinical implications. Front Behav Neurosci. 2018;12:127. doi: 10.3389/fnbeh.2018.00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.González-de-Olano D, Álvarez-Twose I. Mast cells as key players in allergy and inflammation. J Investig Allergol Clin Immunol. 2018;28:365–378. doi: 10.18176/jiaci.0327. [DOI] [PubMed] [Google Scholar]
- 73.Gonzalez-Gay MA, Hajeer AH, Garcia-Porrua C, Dababneh A, Amoli MM, Botana MA, Thomson W, Llorca J, Ollier WE. Corticotropin-releasing hormone promoter polymorphisms in patients with rheumatoid arthritis from northwest Spain. J Rheumatol. 2003;30:913–917. [PubMed] [Google Scholar]
- 74.Gonzalez-Rey E, Fernandez-Martin A, Chorny A, Delgado M. Therapeutic effect of urocortin and adrenomedullin in a murine model of Crohn’s disease. Gut. 2006;55:824–832. doi: 10.1136/gut.2005.084525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Grammatopoulos DK. The role of CRH receptors and their agonists in myometrial contractility and quiescence during pregnancy and labour. Front Biosci. 2007;12:561–571. doi: 10.2741/2082. [DOI] [PubMed] [Google Scholar]
- 76.Grammatopoulos DK, Ourailidou S. CRH receptor signalling: potential roles in pathophysiology. Curr Mol Pharmacol. 2017;10:296–310. doi: 10.2174/1874467210666170110125747. [DOI] [PubMed] [Google Scholar]
- 77.Greenway MJ, Andersen PM, Russ C, Ennis S, Cashman S, Donaghy C, Patterson V, Swingler R, Kieran D, Prehn J, Morrison KE, Green A, Acharya KR, Brown RH, Jr, Hardiman O. ANG mutations segregate with familial and ‘sporadic’ amyotrophic lateral sclerosis. Nat Genet. 2006;38:411–413. doi: 10.1038/ng1742. [DOI] [PubMed] [Google Scholar]
- 78.Grippo AJ, Scotti MA. Stress and neuroinflammation. Modern Trends Pharmacopsychiatry. 2013;28:20–32. doi: 10.1159/000343965. [DOI] [PubMed] [Google Scholar]
- 79.Gupta K, Harvima IT. Mast cell-neural interactions contribute to pain and itch. Immunol Rev. 2018;282:168–187. doi: 10.1111/imr.12622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Harvima IT, Nilsson G. Stress, the neuroendocrine system and mast cells: current understanding of their role in psoriasis. Expert Rev Clin Immunol. 2012;8:235–241. doi: 10.1586/eci.12.1. [DOI] [PubMed] [Google Scholar]
- 81.Heffner KL, Kiecolt-Glaser JK, Glaser R, Malarkey WB, Marshall GD. Stress and anxiety effects on positive skin test responses in young adults with allergic rhinitis. Ann Allergy Asthma Immunol. 2014;113:13–18. doi: 10.1016/j.anai.2014.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Heinrichs SC, Koob GF. Corticotropin-releasing factor in brain: a role in activation, arousal, and affect regulation. J Pharmacol Exp Ther. 2004;311:427–440. doi: 10.1124/jpet.103.052092. [DOI] [PubMed] [Google Scholar]
- 83.Hemmer B, Kerschensteiner M, Korn T. Role of the innate and adaptive immune responses in the course of multiple sclerosis. Lancet Neurol. 2015;14:406–419. doi: 10.1016/S1474-4422(14)70305-9. [DOI] [PubMed] [Google Scholar]
- 84.Hemmerle AM, Herman JP, Seroogy KB. Stress, depression and Parkinson’s disease. Exp Neurol. 2012;233:79–86. doi: 10.1016/j.expneurol.2011.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hendriksen E, van Bergeijk D, Oosting RS, Redegeld FA. Mast cells in neuroinflammation and brain disorders. Neurosci Biobehav Rev. 2017;79:119–133. doi: 10.1016/j.neubiorev.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 86.Herman JP, McKlveen JM, Ghosal S, Kopp B, Wulsin A, Makinson R, Scheimann J, Myers B. Regulation of the hypothalamic-pituitary-adrenocortical stress response. Compr Physiol. 2016;6:603–621. doi: 10.1002/cphy.c150015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hillhouse EW, Grammatopoulos DK. Control of intracellular signalling by corticotropin-releasing hormone in human myometrium. Front Horm Res. 2001;27:66–74. doi: 10.1159/000061042. [DOI] [PubMed] [Google Scholar]
- 88.Holland ND. Early central nervous system evolution: an era of skin brains. Nat Rev Neurosci. 2003;4:617–627. doi: 10.1038/nrn1175. [DOI] [PubMed] [Google Scholar]
- 89.Hoppe A, Katsoulis-Dimitriou K, Edler HJ, Dudeck J, Drube S, Dudeck A. Mast cells initiate the vascular response to contact allergens by sensing cell stress. J Allergy Clin Immunol. 2020;145:1476–1479.e3. doi: 10.1016/j.jaci.2020.01.036. [DOI] [PubMed] [Google Scholar]
- 90.Hsiao EY, McBride SW, Chow J, Mazmanian SK, Patterson PH. Modeling an autism risk factor in mice leads to permanent immune dysregulation. Proc Natl Acad Sci U S A. 2012;109:12776–12781. doi: 10.1073/pnas.1202556109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Huang M, Berry J, Kandere K, Lytinas M, Karalis K, Theoharides TC. Mast cell deficient W/W(v) mice lack stress-induced increase in serum IL-6 levels, as well as in peripheral CRH and vascular permeability, a model of rheumatoid arthritis. Int J Immunopathol Pharmacol. 2002;15:249–254. doi: 10.1177/039463200201500314. [DOI] [PubMed] [Google Scholar]
- 92.Hueber AJ, Asquith DL, Miller AM, Reilly J, Kerr S, Leipe J, Melendez AJ, McInnes IB. Cutting edge: Mast cells express IL-17A in rheumatoid arthritis synovium. J Immunol. 2010;184:3336–3340. doi: 10.4049/jimmunol.0903566. [DOI] [PubMed] [Google Scholar]
- 93.Inda C, Armando NG, Dos Santos Claro PA, Silberstein S. Endocrinology and the brain: corticotropin-releasing hormone signaling. Endocr Connect. 2017;6:R99–120. doi: 10.1530/EC-17-0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jin Y, Silverman AJ, Vannucci SJ. Mast cell stabilization limits hypoxic-ischemic brain damage in the immature rat. Dev Neurosci. 2007;29:373–384. doi: 10.1159/000105478. [DOI] [PubMed] [Google Scholar]
- 95.Jones MK, Nair A, Gupta M. Mast cells in neurodegenerative disease. Front Cell Neurosci. 2019;13:171. doi: 10.3389/fncel.2019.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Joshi A, Page CE, Damante M, Dye CN, Haim A, Leuner B, Lenz KM. Sex differences in the effects of early life stress exposure on mast cells in the developing rat brain. Horm Behav. 2019;113:76–84. doi: 10.1016/j.yhbeh.2019.04.012. [DOI] [PubMed] [Google Scholar]
- 97.Juruena MF, Eror F, Cleare AJ, Young AH. The role of early life stress in HPA axis and anxiety. Adv Exp Med Biol. 2020;1191:141–153. doi: 10.1007/978-981-32-9705-0_9. [DOI] [PubMed] [Google Scholar]
- 98.Justice NJ. The relationship between stress and Alzheimer’s disease. Neurobiol Stress. 2018;8:127–133. doi: 10.1016/j.ynstr.2018.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kambayashi T, Allenspach EJ, Chang JT, Zou T, Shoag JE, Reiner SL, Caton AJ, Koretzky GA. Inducible MHC class II expression by mast cells supports effector and regulatory T cell activation. J Immunol. 2009;182:4686–4695. doi: 10.4049/jimmunol.0803180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kandere-Grzybowska K, Letourneau R, Kempuraj D, Donelan J, Poplawski S, Boucher W, Athanassiou A, Theoharides TC. IL-1 induces vesicular secretion of IL-6 without degranulation from human mast cells. J Immunol. 2003;171:4830–4836. doi: 10.4049/jimmunol.171.9.4830. [DOI] [PubMed] [Google Scholar]
- 101.Kano M, Muratsubaki T, Van Oudenhove L, Morishita J, Yoshizawa M, Kohno K, Yagihashi M, Tanaka Y, Mugikura S, Dupont P, Ly HG, Takase K, Kanazawa M, Fukudo S. Altered brain and gut responses to corticotropin-releasing hormone (CRH) in patients with irritable bowel syndrome. Sci Rep. 2017;7:12425. doi: 10.1038/s41598-017-09635-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Karagkouni A, Alevizos M, Theoharides TC. Effect of stress on brain inflammation and multiple sclerosis. Autoimmun Rev. 2013;12:947–953. doi: 10.1016/j.autrev.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 103.Kastin AJ, Akerstrom V. Differential interactions of urocortin/corticotropin-releasing hormone peptides with the blood-brain barrier. Neuroendocrinology. 2002;75:367–374. doi: 10.1159/000059433. [DOI] [PubMed] [Google Scholar]
- 104.Keaney J, Campbell M. The dynamic blood-brain barrier. FEBS J. 2015;282:4067–4079. doi: 10.1111/febs.13412. [DOI] [PubMed] [Google Scholar]
- 105.Kellner M, Yassouridis A, Hübner R, Baker DG, Wiedemann K. Endocrine and cardiovascular responses to corticotropin-releasing hormone in patients with posttraumatic stress disorder: a role for atrial natriuretic peptide. Neuropsychobiology. 2003;47:102–108. doi: 10.1159/000070018. [DOI] [PubMed] [Google Scholar]
- 106.Kempuraj D, Thangavel R, Fattal R, Pattani S, Yang E, Zaheer S, Santillan DA, Santillan MK, Zaheer A. Mast cells release chemokine CCL2 in response to parkinsonian toxin 1-methyl-4-phenyl-pyridinium (MPP(+)) Neurochem Res. 2016a;41:1042–1049. doi: 10.1007/s11064-015-1790-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kempuraj D, Thangavel R, Natteru PA, Selvakumar GP, Saeed D, Zahoor H, Zaheer S, Iyer SS, Zaheer A. Neuroinflammation induces neurodegeneration. J Neurol Neurosurg Spine. 2016b;1:1003. [PMC free article] [PubMed] [Google Scholar]
- 108.Kempuraj D, Selvakumar GP, Zaheer S, Thangavel R, Ahmed ME, Raikwar S, Govindarajan R, Iyer S, Zaheer A. Cross-talk between glia, neurons and mast cells in neuroinflammation associated with Parkinson’s disease. J Neuroimmune Pharmacol. 2018;13:100–112. doi: 10.1007/s11481-017-9766-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kempuraj D, Papadopoulou NG, Lytinas M, Huang M, Kandere-Grzybowska K, Madhappan B, Boucher W, Christodoulou S, Athanassiou A, Theoharides TC. Corticotropin-releasing hormone and its structurally related urocortin are synthesized and secreted by human mast cells. Endocrinology. 2004;145:43–48. doi: 10.1210/en.2003-0805. [DOI] [PubMed] [Google Scholar]
- 110.Kempuraj D, Selvakumar GP, Thangavel R, Ahmed ME, Zaheer S, Raikwar SP, Iyer SS, Bhagavan SM, Beladakere-Ramaswamy S, Zaheer A. Mast cell activation in brain injury, stress, and post-traumatic stress disorder and Alzheimer’s disease pathogenesis. Front Neurosci. 2017a;11:703. doi: 10.3389/fnins.2017.00703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kempuraj D, Mentor S, Thangavel R, Ahmed ME, Selvakumar GP, Raikwar SP, Dubova I, Zaheer S, Iyer SS, Zaheer A. Mast cells in stress, pain, blood-brain barrier, neuroinflammation and Alzheimer’s disease. Front Cell Neurosci. 2019;13:54. doi: 10.3389/fncel.2019.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kempuraj D, Thangavel R, Selvakumar GP, Zaheer S, Ahmed ME, Raikwar SP, Zahoor H, Saeed D, Natteru PA, Iyer S, Zaheer A. Brain and peripheral atypical inflammatory mediators potentiate neuroinflammation and neurodegeneration. Front Cell Neurosci. 2017b;11:216. doi: 10.3389/fncel.2017.00216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kempuraj D, Selvakumar GP, Ahmed ME, Raikwar SP, Thangavel R, Khan A, Zaheer SA, Iyer SS, Burton C, James D, Zaheer A. COVID-19, mast cells, cytokine storm, psychological stress, and neuroinflammation. Neuroscientist. 2020a;26:402–414. doi: 10.1177/1073858420941476. [DOI] [PubMed] [Google Scholar]
- 114.Kempuraj D, Ahmed ME, Selvakumar GP, Thangavel R, Dhaliwal AS, Dubova I, Mentor S, Premkumar K, Saeed D, Zahoor H, Raikwar SP, Zaheer S, Iyer SS, Zaheer A. Brain injury-mediated neuroinflammatory response and Alzheimer’s disease. Neuroscientist. 2020b;26:134–155. doi: 10.1177/1073858419848293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ketchesin KD, Stinnett GS, Seasholtz AF. Corticotropin-releasing hormone-binding protein and stress: from invertebrates to humans. Stress. 2017;20:449–464. doi: 10.1080/10253890.2017.1322575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kim YK, Na KS, Myint AM, Leonard BE. The role of pro-inflammatory cytokines in neuroinflammation, neurogenesis and the neuroendocrine system in major depression. Prog Neuropsychopharmacol Biol Psychiatry. 2016;64:277–284. doi: 10.1016/j.pnpbp.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 117.Kinney JW, Bemiller SM, Murtishaw AS, Leisgang AM, Salazar AM, Lamb BT. Inflammation as a central mechanism in Alzheimer’s disease. Alzheimers Dement (N Y) 2018;4:575–590. doi: 10.1016/j.trci.2018.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kjaer A, Larsen PJ, Knigge U, Jørgensen H, Warberg J. Neuronal histamine and expression of corticotropin-releasing hormone, vasopressin and oxytocin in the hypothalamus: relative importance of H1 and H2 receptors. Eur J Endocrinol. 1998;139:238–243. doi: 10.1530/eje.0.1390238. [DOI] [PubMed] [Google Scholar]
- 119.Klenerova V, Kvetnansky R, Hynie S. The effect of acute and repeated stress on CRH-R1 and CRH-R2 mRNA expression in pituitaries of wild type and CRH knock-out mice. Cell Mol Neurobiol. 2018;38:163–169. doi: 10.1007/s10571-017-0556-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Komuro H, Sato N, Sasaki A, Suzuki N, Kano M, Tanaka Y, Yamaguchi-Kabata Y, Kanazawa M, Warita H, Aoki M, Fukudo S. Corticotropin-releasing hormone receptor 2 gene variants in irritable bowel syndrome. PLoS One. 2016;11:e0147817. doi: 10.1371/journal.pone.0147817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kovalovsky D, Refojo D, Liberman AC, Hochbaum D, Pereda MP, Coso OA, Stalla GK, Holsboer F, Arzt E. Activation and induction of NUR77/NURR1 in corticotrophs by CRH/cAMP: involvement of calcium, protein kinase A, and MAPK pathways. Mol Endocrinol. 2002;16:1638–1651. doi: 10.1210/mend.16.7.0863. [DOI] [PubMed] [Google Scholar]
- 122.Kritas SK, Saggini A, Cerulli G, Caraffa A, Antinolfi P, Pantalone A, Rosati M, Tei M, Speziali A, Saggini R, Conti P. Corticotropin-releasing hormone, microglia and mental disorders. Int J Immunopathol Pharmacol. 2014a;27:163–167. doi: 10.1177/039463201402700203. [DOI] [PubMed] [Google Scholar]
- 123.Kritas SK, Caraffa A, Antinolfi P, Saggini A, Pantalone A, Rosati M, Tei M, Speziali A, Saggini R, Pandolfi F, Cerulli G, Conti P. Nerve growth factor interactions with mast cells. Int J Immunopathol Pharmacol. 2014b;27:15–19. doi: 10.1177/039463201402700103. [DOI] [PubMed] [Google Scholar]
- 124.Krystel-Whittemore M, Dileepan KN, Wood JG. Mast cell: A multi-functional master cell. Front Immunol. 2015;6:620. doi: 10.3389/fimmu.2015.00620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kuenzel WJ, Kang SW, Jurkevich A. Neuroendocrine regulation of stress in birds with an emphasis on vasotocin receptors (VTRs) Gen Comp Endocrinol. 2013;190:18–23. doi: 10.1016/j.ygcen.2013.02.029. [DOI] [PubMed] [Google Scholar]
- 126.Lane CA, Hardy J, Schott JM. Alzheimer’s disease. Eur J Neurol. 2018;25:59–70. doi: 10.1111/ene.13439. [DOI] [PubMed] [Google Scholar]
- 127.Larochelle C, Alvarez JI, Prat A. How do immune cells overcome the blood-brain barrier in multiple sclerosis. FEBS Lett. 2011;585:3770–3780. doi: 10.1016/j.febslet.2011.04.066. [DOI] [PubMed] [Google Scholar]
- 128.Lee DM, Friend DS, Gurish MF, Benoist C, Mathis D, Brenner MB. Mast cells: a cellular link between autoantibodies and inflammatory arthritis. Science. 2002;297:1689–1692. doi: 10.1126/science.1073176. [DOI] [PubMed] [Google Scholar]
- 129.Lee H, Kashiwakura J, Matsuda A, Watanabe Y, Sakamoto-Sasaki T, Matsumoto K, Hashimoto N, Saito S, Ohmori K, Nagaoka M, Tokuhashi Y, Ra C, Okayama Y. Activation of human synovial mast cells from rheumatoid arthritis or osteoarthritis patients in response to aggregated IgG through Fcγ receptor I and Fcγ receptor II. Arthritis Rheum. 2013;65:109–119. doi: 10.1002/art.37741. [DOI] [PubMed] [Google Scholar]
- 130.Lee KN, Lee OY. The role of mast cells in irritable bowel syndrome. Gastroenterol Res Pract. 2016;2016:2031480. doi: 10.1155/2016/2031480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lehmann ML, Cooper HA, Maric D, Herkenham M. Social defeat induces depressive-like states and microglial activation without involvement of peripheral macrophages. J Neuroinflammation. 2016;13:224. doi: 10.1186/s12974-016-0672-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lehmann ML, Weigel TK, Cooper HA, Elkahloun AG, Kigar SL, Herkenham M. Decoding microglia responses to psychosocial stress reveals blood-brain barrier breakdown that may drive stress susceptibility. Sci Rep. 2018;8:11240. doi: 10.1038/s41598-018-28737-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Leigh JP, Du J. Brief report: Forecasting the economic burden of autism in 2015 and 2025 in the United States. J Autism Dev Disord. 2015;45:4135–4139. doi: 10.1007/s10803-015-2521-7. [DOI] [PubMed] [Google Scholar]
- 134.Leistner C, Menke A. Hypothalamic-pituitary-adrenal axis and stress. Handb Clin Neurol. 2020;175:55–64. doi: 10.1016/B978-0-444-64123-6.00004-7. [DOI] [PubMed] [Google Scholar]
- 135.Li-Tempel T, Suer T, Tempel T, Larra MF, Winnikes U, Schächinger H, Meyer J, Schote AB. Promoter haplotypes of the corticotropin-releasing hormone encoding gene modulate the physiological stress response in vitro and in vivo. Stress. 2019;22:44–52. doi: 10.1080/10253890.2018.1501020. [DOI] [PubMed] [Google Scholar]
- 136.Li M, Teng H, Sun G, Zhao J, Fan M, Zhao Z, Zhou J, Zhao M. Transcriptome profiles of corticosterone-induced cytotoxicity reveals the involvement of neurite growth-related genes in depression. Psychiatry Res. 2019;276:79–86. doi: 10.1016/j.psychres.2019.04.017. [DOI] [PubMed] [Google Scholar]
- 137.Li N, Zhang X, Dong H, Hu Y, Qian Y. Bidirectional relationship of mast cells-neurovascular unit communication in neuroinflammation and its involvement in POCD. Behav Brain Res. 2017;322:60–69. doi: 10.1016/j.bbr.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 138.Li X, Chauhan A, Sheikh AM, Patil S, Chauhan V, Li XM, Ji L, Brown T, Malik M. Elevated immune response in the brain of autistic patients. J Neuroimmunol. 2009;207:111–116. doi: 10.1016/j.jneuroim.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Liezmann C, Stock D, Peters EM. Stress induced neuroendocrine-immune plasticity: A role for the spleen in peripheral inflammatory disease and inflammaging. Dermatoendocrinol. 2012;4:271–279. doi: 10.4161/derm.22023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Linthorst AC, Flachskamm C, Hopkins SJ, Hoadley ME, Labeur MS, Holsboer F, Reul JM. Long-term intracerebroventricular infusion of corticotropin-releasing hormone alters neuroendocrine, neurochemical, autonomic, behavioral, and cytokine responses to a systemic inflammatory challenge. J Neurosci. 1997;17:4448–4460. doi: 10.1523/JNEUROSCI.17-11-04448.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Liu HR, Fang XY, Wu HG, Wu LY, Li J, Weng ZJ, Guo XX, Li YG. Effects of electroacupuncture on corticotropin-releasing hormone in rats with chronic visceral hypersensitivity. World J Gastroenterol. 2015;21:7181–7190. doi: 10.3748/wjg.v21.i23.7181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Liu J, Wang F. Role of neuroinflammation in amyotrophic lateral sclerosis: Cellular mechanisms and therapeutic implications. Front Immunol. 2017;8:1005. doi: 10.3389/fimmu.2017.01005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10:434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- 144.Lytinas M, Kempuraj D, Huang M, Boucher W, Esposito P, Theoharides TC. Acute stress results in skin corticotropin-releasing hormone secretion, mast cell activation and vascular permeability, an effect mimicked by intradermal corticotropin-releasing hormone and inhibited by histamine-1 receptor antagonists. Int Arch Allergy Immunol. 2003;130:224–231. doi: 10.1159/000069516. [DOI] [PubMed] [Google Scholar]
- 145.Machado A, Herrera AJ, de Pablos RM, Espinosa-Oliva AM, Sarmiento M, Ayala A, Venero JL, Santiago M, Villarán RF, Delgado-Cortés MJ, Argüelles S, Cano J. Chronic stress as a risk factor for Alzheimer’s disease. Rev Neurosci. 2014;25:785–804. doi: 10.1515/revneuro-2014-0035. [DOI] [PubMed] [Google Scholar]
- 146.Malagoli D, Mandrioli M, Ottaviani E. ProCRH in the teleost Ameiurus nebulosus: gene cloning and role in LPS-induced stress response. Brain Behav Immun. 2004;18:451–457. doi: 10.1016/j.bbi.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 147.Maleki N, Becerra L, Borsook D. Migraine: maladaptive brain responses to stress. Headache 52 Suppl. 2012;2:102–106. doi: 10.1111/j.1526-4610.2012.02241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Malyshev I, Malyshev Y. Current concept and update of the macrophage plasticity concept: Intracellular mechanisms of reprogramming and M3 macrophage “switch” phenotype. Biomed Res Int. 2015;2015:341308. doi: 10.1155/2015/341308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Manley K, Han W, Zelin G, Lawrence DA. Crosstalk between the immune, endocrine, and nervous systems in immunotoxicology. Curr Opin Toxicol. 2018;10:37–45. [Google Scholar]
- 150.Marrus N, Constantino JN. Autism spectrum disorders. In: Benson JB, editor. Encyclopedia of infant and early childhood development. Second Edition. Oxford: Elsevier; 2020. pp. 130–138. [Google Scholar]
- 151.Martins AM, Ascenso A, Ribeiro HM, Marto J. The brain-skin connection and the pathogenesis of psoriasis: A review with a focus on the serotonergic system. Cells. 2020;9:796. doi: 10.3390/cells9040796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Maruyama H, Morino H, Ito H, Izumi Y, Kato H, Watanabe Y, Kinoshita Y, Kamada M, Nodera H, Suzuki H, Komure O, Matsuura S, Kobatake K, Morimoto N, Abe K, Suzuki N, Aoki M, Kawata A, Hirai T, Kato T, et al. Mutations of optineurin in amyotrophic lateral sclerosis. Nature. 2010;465:223–226. doi: 10.1038/nature08971. [DOI] [PubMed] [Google Scholar]
- 153.Matsumoto I, Inoue Y, Shimada T, Aikawa T. Brain mast cells act as an immune gate to the hypothalamic-pituitary-adrenal axis in dogs. J Exp Med. 2001;194:71–78. doi: 10.1084/jem.194.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Merali Z, Du L, Hrdina P, Palkovits M, Faludi G, Poulter MO, Anisman H. Dysregulation in the suicide brain: mRNA expression of corticotropin-releasing hormone receptors and GABA(A) receptor subunits in frontal cortical brain region. J Neurosci. 2004;24:1478–1485. doi: 10.1523/JNEUROSCI.4734-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Min HK, Kim KW, Lee SH, Kim HR. Roles of mast cells in rheumatoid arthritis. Korean J Intern Med. 2020;35:12–24. doi: 10.3904/kjim.2019.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Molloy CA, Morrow AL, Meinzen-Derr J, Schleifer K, Dienger K, Manning-Courtney P, Altaye M, Wills-Karp M. Elevated cytokine levels in children with autism spectrum disorder. J Neuroimmunol. 2006;172:198–205. doi: 10.1016/j.jneuroim.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 157.Mosconi L, Pupi A, De Leon MJ. Brain glucose hypometabolism and oxidative stress in preclinical Alzheimer’s disease. Ann N Y Acad Sci. 2008;1147:180–195. doi: 10.1196/annals.1427.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Mpofana T, Daniels WM, Mabandla MV. Exposure to early life stress results in epigenetic changes in neurotrophic factor gene expression in a Parkinsonian rat model. Parkinsons Dis. 2016;2016:6438783. doi: 10.1155/2016/6438783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Muotri AR. Autism spectrum disorders: challenges and perspectives. Dev Neurobiol. 2018;78:431–433. doi: 10.1002/dneu.22586. [DOI] [PubMed] [Google Scholar]
- 160.Naama AG, El-Bakry A, Rasha E, Ahmed R. Maternal sodium valproate exposure alters neuroendocrine cytokines and oxido-inflammatory axes in neonatal albino rats. Endocr Metab Immune Disord Drug Targets. 2020 doi: 10.2174/1871530320999200918120617. doi: 102174/1871530320999200918120617. [DOI] [PubMed] [Google Scholar]
- 161.Najjar S, Pearlman DM, Devinsky O, Najjar A, Zagzag D. Neurovascular unit dysfunction with blood-brain barrier hyperpermeability contributes to major depressive disorder: a review of clinical and experimental evidence. J Neuroinflammation. 2013;10:142. doi: 10.1186/1742-2094-10-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Nedoszytko B, Sokołowska-Wojdyło M, Ruckemann-Dziurdzińska K, Roszkiewicz J, Nowicki RJ. Chemokines and cytokines network in the pathogenesis of the inflammatory skin diseases: atopic dermatitis, psoriasis and skin mastocytosis. Postepy Dermatol Alergol. 2014;31:84–91. doi: 10.5114/pdia.2014.40920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Nelissen S, Lemmens E, Geurts N, Kramer P, Maurer M, Hendriks J, Hendrix S. The role of mast cells in neuroinflammation. Acta Neuropathol. 2013;125:637–650. doi: 10.1007/s00401-013-1092-y. [DOI] [PubMed] [Google Scholar]
- 164.Nigrovic PA, Lee DM. Synovial mast cells: role in acute and chronic arthritis. Immunol Rev. 2007;217:19–37. doi: 10.1111/j.1600-065X.2007.00506.x. [DOI] [PubMed] [Google Scholar]
- 165.Nishimura H, Kawasaki M, Matsuura T, Suzuki H, Motojima Y, Baba K, Ohnishi H, Yamanaka Y, Fujitani T, Yoshimura M, Maruyama T, Ueno H, Sonoda S, Nishimura K, Tanaka K, Sanada K, Onaka T, Ueta Y, Sakai A. Acute mono-arthritis activates the neurohypophysial system and hypothalamo-pituitary adrenal axis in rats. Front Endocrinol (Lausanne) 2020;11:43. doi: 10.3389/fendo.2020.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ocak U, Ocak PE, Wang A, Zhang JH, Boling W, Wu P, Mo J, Zhang T, Huang L. Targeting mast cell as a neuroprotective strategy. Brain Inj. 2019;33:723–733. doi: 10.1080/02699052.2018.1556807. [DOI] [PubMed] [Google Scholar]
- 167.Oh J, Vidal-Jordana A, Montalban X. Multiple sclerosis: clinical aspects. Curr Opin Neurol. 2018;31:752–759. doi: 10.1097/WCO.0000000000000622. [DOI] [PubMed] [Google Scholar]
- 168.Ohman L, Simrén M. Pathogenesis of IBS: role of inflammation, immunity and neuroimmune interactions. Nat Rev Gastroenterol Hepatol. 2010;7:163–173. doi: 10.1038/nrgastro.2010.4. [DOI] [PubMed] [Google Scholar]
- 169.Oki Y, Sasano H. Localization and physiological roles of urocortin. Peptides. 2004;25:1745–1749. doi: 10.1016/j.peptides.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 170.Onore C, Careaga M, Ashwood P. The role of immune dysfunction in the pathophysiology of autism. Brain Behav Immun. 2012;26:383–392. doi: 10.1016/j.bbi.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Ousley O, Cermak T. Autism spectrum disorder: Defining dimensions and subgroups. Curr Dev Disord Rep. 2014;1:20–28. doi: 10.1007/s40474-013-0003-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Pace TW, Miller AH. Cytokines and glucocorticoid receptor signaling. Relevance to major depression. Ann N Y Acad Sci. 2009;1179:86–105. doi: 10.1111/j.1749-6632.2009.04984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Pace TW, Hu F, Miller AH. Cytokine-effects on glucocorticoid receptor function: relevance to glucocorticoid resistance and the pathophysiology and treatment of major depression. Brain Behav Immun. 2007;21:9–19. doi: 10.1016/j.bbi.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Pan W, Kastin AJ. Urocortin and the brain. Prog Neurobiol. 2008;84:148–156. doi: 10.1016/j.pneurobio.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Paolicelli RC, Ferretti MT. Function and dysfunction of microglia during brain development: Consequences for synapses and neural circuits. Front Synaptic Neurosci. 2017;9:9. doi: 10.3389/fnsyn.2017.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Papadopoulou NG, Oleson L, Kempuraj D, Donelan J, Cetrulo CL, Theoharides TC. Regulation of corticotropin-releasing hormone receptor-2 expression in human cord blood-derived cultured mast cells. J Mol Endocrinol. 2005;35:R1–8. doi: 10.1677/jme.1.01833. [DOI] [PubMed] [Google Scholar]
- 177.Parihar MS, Brewer GJ. Amyloid-β as a modulator of synaptic plasticity. J Alzheimers Dis. 2010;22:741–763. doi: 10.3233/JAD-2010-101020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Park HJ, Ran Y, Jung JI, Holmes O, Price AR, Smithson L, Ceballos-Diaz C, Han C, Wolfe MS, Daaka Y, Ryabinin AE, Kim SH, Hauger RL, Golde TE, Felsenstein KM. The stress response neuropeptide CRF increases amyloid-β production by regulating γ-secretase activity. EMBO J. 2015;34:1674–1686. doi: 10.15252/embj.201488795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Pavlova V, Filipova E, Uzunova K, Kalinov K, Vekov T. Pioglitazone therapy and fractures: systematic review and meta- analysis. Endocr Metab Immune Disord Drug Targets. 2018;18:502–507. doi: 10.2174/1871530318666180423121833. [DOI] [PubMed] [Google Scholar]
- 180.Pedersen WA, Wan R, Zhang P, Mattson MP. Urocortin, but not urocortin II, protects cultured hippocampal neurons from oxidative and excitotoxic cell death via corticotropin-releasing hormone receptor type I. J Neurosci. 2002;22:404–412. doi: 10.1523/JNEUROSCI.22-02-00404.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Peña-Bautista C, Baquero M, Ferrer I, Hervás D, Vento M, García-Blanco A, Cháfer-Pericás C. Neuropsychological assessment and cortisol levels in biofluids from early Alzheimer’s disease patients. Exp Gerontol. 2019;123:10–16. doi: 10.1016/j.exger.2019.05.007. [DOI] [PubMed] [Google Scholar]
- 182.Peng J, Long B, Yuan J, Peng X, Ni H, Li X, Gong H, Luo Q, Li A. A quantitative analysis of the distribution of CRH neurons in whole mouse brain. Front Neuroanat. 2017;11:63. doi: 10.3389/fnana.2017.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Petra AI, Panagiotidou S, Hatziagelaki E, Stewart JM, Conti P, Theoharides TC. Gut-microbiota-brain axis and its effect on neuropsychiatric disorders with suspected immune dysregulation. Clin Ther. 2015;37:984–995. doi: 10.1016/j.clinthera.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Pinke KH, Zorzella-Pezavento SFG, de Campos Fraga-Silva TF, Mimura LAN, de Oliveira LRC, Ishikawa LLW, Fernandes AAH, Lara VS, Sartori A. Calming down mast cells with ketotifen: A potential strategy for multiple sclerosis therapy. Neurotherapeutics. 2020;17:218–234. doi: 10.1007/s13311-019-00775-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Pisarchik A, Slominski A. Corticotropin releasing factor receptor type 1: molecular cloning and investigation of alternative splicing in the hamster skin. J Invest Dermatol. 2002;118:1065–1072. doi: 10.1046/j.1523-1747.2002.01770.x. [DOI] [PubMed] [Google Scholar]
- 186.Pitzalis C, Kelly S, Humby F. New learnings on the pathophysiology of RA from synovial biopsies. Curr Opin Rheumatol. 2013;25:334–344. doi: 10.1097/BOR.0b013e32835fd8eb. [DOI] [PubMed] [Google Scholar]
- 187.Pondeljak N, Lugović-Mihić L. Stress-induced interaction of skin immune cells, hormones, and neurotransmitters. Clin Ther. 2020;42:757–770. doi: 10.1016/j.clinthera.2020.03.008. [DOI] [PubMed] [Google Scholar]
- 188.Popp J, Wolfsgruber S, Heuser I, Peters O, Hüll M, Schröder J, Möller HJ, Lewczuk P, Schneider A, Jahn H, Luckhaus C, Perneczky R, Frölich L, Wagner M, Maier W, Wiltfang J, Kornhuber J, Jessen F. Cerebrospinal fluid cortisol and clinical disease progression in MCI and dementia of Alzheimer’s type. Neurobiol Aging. 2015;36:601–607. doi: 10.1016/j.neurobiolaging.2014.10.031. [DOI] [PubMed] [Google Scholar]
- 189.Prata J, Machado AS, von Doellinger O, Almeida MI, Barbosa MA, Coelho R, Santos SG. The contribution of inflammation to autism spectrum disorders: recent clinical evidence. Methods Mol Biol. 2019;2011:493–510. doi: 10.1007/978-1-4939-9554-7_29. [DOI] [PubMed] [Google Scholar]
- 190.Priftis KN, Papadimitriou A, Nicolaidou P, Chrousos GP. Dysregulation of the stress response in asthmatic children. Allergy. 2009;64:18–31. doi: 10.1111/j.1398-9995.2008.01948.x. [DOI] [PubMed] [Google Scholar]
- 191.Rabinowitz AR, Levin HS. Cognitive sequelae of traumatic brain injury. Psychiatr Clin North Am. 2014;37:1–11. doi: 10.1016/j.psc.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Ragipoglu D, Dudeck A, Haffner-Luntzer M, Voss M, Kroner J, Ignatius A, Fischer V. The role of mast cells in bone metabolism and bone disorders. Front Immunol. 2020;11:163. doi: 10.3389/fimmu.2020.00163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Ransohoff RM, Schafer D, Vincent A, Blachère NE, Bar-Or A. Neuroinflammation: Ways in which the immune system affects the brain. Neurotherapeutics. 2015;12:896–909. doi: 10.1007/s13311-015-0385-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Refojo D, Schweizer M, Kuehne C, Ehrenberg S, Thoeringer C, Vogl AM, Dedic N, Schumacher M, von Wolff G, Avrabos C, Touma C, Engblom D, Schütz G, Nave KA, Eder M, Wotjak CT, Sillaber I, Holsboer F, Wurst W, Deussing JM. Glutamatergic and dopaminergic neurons mediate anxiogenic and anxiolytic effects of CRHR1. Science. 2011;333:1903–1907. doi: 10.1126/science.1202107. [DOI] [PubMed] [Google Scholar]
- 195.Reich DS, Lucchinetti CF, Calabresi PA. Multiple sclerosis. N Engl J Med. 2018;378:169–180. doi: 10.1056/NEJMra1401483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Reyes TM, Lewis K, Perrin MH, Kunitake KS, Vaughan J, Arias CA, Hogenesch JB, Gulyas J, Rivier J, Vale WW, Sawchenko PE. Urocortin II: a member of the corticotropin-releasing factor (CRF) neuropeptide family that is selectively bound by type 2 CRF receptors. Proc Natl Acad Sci U S A. 2001;98:2843–2848. doi: 10.1073/pnas.051626398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Ribatti D. The crucial role of mast cells in blood-brain barrier alterations. Exp Cell Res. 2015;338:119–125. doi: 10.1016/j.yexcr.2015.05.013. [DOI] [PubMed] [Google Scholar]
- 198.Rissman RA, Lee KF, Vale W, Sawchenko PE. Corticotropin-releasing factor receptors differentially regulate stress-induced tau phosphorylation. J Neurosci. 2007;27:6552–6562. doi: 10.1523/JNEUROSCI.5173-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Rivellese F, Suurmond J, Habets K, Dorjée AL, Ramamoorthi N, Townsend MJ, de Paulis A, Marone G, Huizinga TW, Pitzalis C, Toes RE. Ability of interleukin-33- and immune complex-triggered activation of human mast cells to down-regulate monocyte-mediated immune responses. Arthritis Rheumatol. 2015;67:2343–2353. doi: 10.1002/art.39192. [DOI] [PubMed] [Google Scholar]
- 200.Rivellese F, Mauro D, Nerviani A, Pagani S, Fossati-Jimack L, Messemaker T, Kurreeman FAS, Toes REM, Ramming A, Rauber S, Schett G, Jones GW, Jones SA, Rossi FW, de Paulis A, Marone G, El Shikh MEM, Humby F, Pitzalis C. Mast cells in early rheumatoid arthritis associate with disease severity and support B cell autoantibody production. Ann Rheum Dis. 2018;77:1773–1781. doi: 10.1136/annrheumdis-2018-213418. [DOI] [PubMed] [Google Scholar]
- 201.Rossignol DA, Frye RE. A review of research trends in physiological abnormalities in autism spectrum disorders: immune dysregulation, inflammation, oxidative stress, mitochondrial dysfunction and environmental toxicant exposures. Mol Psychiatry. 2012;17:389–401. doi: 10.1038/mp.2011.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Rossini M, Viapiana O, Zanoni G, Tripi G, Idolazzi L, Fracassi E, Adami G, Adami S, Gatti D. Serum levels of tryptase suggest that mast cells might have an antiinflammatory role in rheumatoid arthritis: comment on the article by Rivellese et al. Arthritis Rheumatol. 2016;68:769. doi: 10.1002/art.39506. [DOI] [PubMed] [Google Scholar]
- 203.Rozniecki JJ, Dimitriadou V, Lambracht-Hall M, Pang X, Theoharides TC. Morphological and functional demonstration of rat dura mater mast cell-neuron interactions in vitro and in vivo. Brain Res. 1999;849:1–15. doi: 10.1016/s0006-8993(99)01855-7. [DOI] [PubMed] [Google Scholar]
- 204.Rozniecki JJ, Sahagian GG, Kempuraj D, Tao K, Jocobson S, Zhang B, Theoharides TC. Brain metastases of mouse mammary adenocarcinoma is increased by acute stress. Brain Res. 2010;1366:204–210. doi: 10.1016/j.brainres.2010.09.085. [DOI] [PubMed] [Google Scholar]
- 205.Russell G, Lightman S. The human stress response. Nat Rev Endocrinol. 2019;15:525–534. doi: 10.1038/s41574-019-0228-0. [DOI] [PubMed] [Google Scholar]
- 206.Saffran M, Schally AV. The release of corticotrophin by anterior pituitary tissue in vitro. Can J Biochem Physiol. 1955;33:408–415. [PubMed] [Google Scholar]
- 207.Sagami Y, Shimada Y, Tayama J, Nomura T, Satake M, Endo Y, Shoji T, Karahashi K, Hongo M, Fukudo S. Effect of a corticotropin releasing hormone receptor antagonist on colonic sensory and motor function in patients with irritable bowel syndrome. Gut. 2004;53:958–964. doi: 10.1136/gut.2003.018911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Saghazadeh A, Ataeinia B, Keynejad K, Abdolalizadeh A, Hirbod-Mobarakeh A, Rezaei N. A meta-analysis of pro-inflammatory cytokines in autism spectrum disorders: Effects of age, gender, and latitude. J Psychiatr Res. 2019;115:90–102. doi: 10.1016/j.jpsychires.2019.05.019. [DOI] [PubMed] [Google Scholar]
- 209.Santos J, Yates D, Guilarte M, Vicario M, Alonso C, Perdue MH. Stress neuropeptides evoke epithelial responses via mast cell activation in the rat colon. Psychoneuroendocrinology. 2008;33:1248–1256. doi: 10.1016/j.psyneuen.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 210.Sato N, Suzuki N, Sasaki A, Aizawa E, Obayashi T, Kanazawa M, Mizuno T, Kano M, Aoki M, Fukudo S. Corticotropin-releasing hormone receptor 1 gene variants in irritable bowel syndrome. PLoS One. 2012;7:e42450. doi: 10.1371/journal.pone.0042450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Sawamukai N, Yukawa S, Saito K, Nakayamada S, Kambayashi T, Tanaka Y. Mast cell-derived tryptase inhibits apoptosis of human rheumatoid synovial fibroblasts via rho-mediated signaling. Arthritis Rheum. 2010;62:952–959. doi: 10.1002/art.27331. [DOI] [PubMed] [Google Scholar]
- 212.Selvakumar GP, Iyer SS, Kempuraj D, Raju M, Thangavel R, Saeed D, Ahmed ME, Zahoor H, Raikwar SP, Zaheer S, Zaheer A. Glia maturation factor dependent inhibition of mitochondrial pgc-1α triggers oxidative stress-mediated apoptosis in N27 rat dopaminergic neuronal cells. Mol Neurobiol. 2018;55:7132–7152. doi: 10.1007/s12035-018-0882-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Shaik-Dasthagirisaheb YB, Conti P. The role of mast cells in Alzheimer’s disease. Adv Clin Exp Med. 2016;25:781–787. doi: 10.17219/acem/61914. [DOI] [PubMed] [Google Scholar]
- 214.Sheng H, Xu Y, Chen Y, Zhang Y, Xu X, He C, Ni X. CRH-R1 and CRH-R2 differentially modulate dendritic outgrowth of hippocampal neurons. Endocrine. 2012;41:458–464. doi: 10.1007/s12020-012-9603-5. [DOI] [PubMed] [Google Scholar]
- 215.Shin K, Gurish MF, Friend DS, Pemberton AD, Thornton EM, Miller HR, Lee DM. Lymphocyte-independent connective tissue mast cells populate murine synovium. Arthritis Rheum. 2006;54:2863–2871. doi: 10.1002/art.22058. [DOI] [PubMed] [Google Scholar]
- 216.Shin K, Nigrovic PA, Crish J, Boilard E, McNeil HP, Larabee KS, Adachi R, Gurish MF, Gobezie R, Stevens RL, Lee DM. Mast cells contribute to autoimmune inflammatory arthritis via their tryptase/heparin complexes. J Immunol. 2009;182:647–656. doi: 10.4049/jimmunol.182.1.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Siiskonen H, Harvima I. Mast cells and sensory nerves contribute to neurogenic inflammation and pruritus in chronic skin inflammation. Front Cell Neurosci. 2019;13:422. doi: 10.3389/fncel.2019.00422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Simon DW, McGeachy MJ, Bayır H, Clark RS, Loane DJ, Kochanek PM. The far-reaching scope of neuroinflammation after traumatic brain injury. Nat Rev Neurol. 2017;13:171–191. doi: 10.1038/nrneurol.2017.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Sinagra E, Utzeri E, Morreale GC, Fabbri C, Pace F, Anderloni A. Microbiota-gut-brain axis and its affect inflammatory bowel disease: Pathophysiological concepts and insights for clinicians. World J Clin Cases. 2020;8:1013–1025. doi: 10.12998/wjcc.v8.i6.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Singh LK, Boucher W, Pang X, Letourneau R, Seretakis D, Green M, Theoharides TC. Potent mast cell degranulation and vascular permeability triggered by urocortin through activation of corticotropin-releasing hormone receptors. J Pharmacol Exp Ther. 1999;288:1349–1356. [PubMed] [Google Scholar]
- 221.Siniscalco D, Schultz S, Brigida AL, Antonucci N. Inflammation and neuro-immune dysregulations in autism spectrum disorders. Pharmaceuticals (Basel) 2018;11:56. doi: 10.3390/ph11020056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Skaper SD, Facci L, Giusti P. Neuroinflammation, microglia and mast cells in the pathophysiology of neurocognitive disorders: a review. CNS Neurol Disord Drug Targets. 2014a;13:1654–1666. doi: 10.2174/1871527313666141130224206. [DOI] [PubMed] [Google Scholar]
- 223.Skaper SD, Facci L, Giusti P. Mast cells, glia and neuroinflammation: partners in crime. Immunology. 2014b;141:314–327. doi: 10.1111/imm.12170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Skaper SD, Facci L, Zusso M, Giusti P. Neuroinflammation, mast cells, and glia: Dangerous liaisons. Neuroscientist. 2017;23:478–498. doi: 10.1177/1073858416687249. [DOI] [PubMed] [Google Scholar]
- 225.Skaper SD, Facci L, Zusso M, Giusti P. An inflammation-centric view of neurological disease: Beyond the neuron. Front Cell Neurosci. 2018;12:72. doi: 10.3389/fncel.2018.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Skelton KH, Owens MJ, Nemeroff CB. The neurobiology of urocortin. Regul Pept. 2000;93:85–92. doi: 10.1016/s0167-0115(00)00180-4. [DOI] [PubMed] [Google Scholar]
- 227.Slominski A, Ermak G, Mazurkiewicz JE, Baker J, Wortsman J. Characterization of corticotropin-releasing hormone (CRH) in human skin. J Clin Endocrinol Metab. 1998;83:1020–1024. doi: 10.1210/jcem.83.3.4650. [DOI] [PubMed] [Google Scholar]
- 228.Smith SE, Li J, Garbett K, Mirnics K, Patterson PH. Maternal immune activation alters fetal brain development through interleukin-6. J Neurosci. 2007;27:10695–10702. doi: 10.1523/JNEUROSCI.2178-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. Lancet. 2016;388:2023–2038. doi: 10.1016/S0140-6736(16)30173-8. [DOI] [PubMed] [Google Scholar]
- 230.Soria Lopez JA, González HM, Léger GC. Alzheimer’s disease. Handb Clin Neurol. 2019;167:231–255. doi: 10.1016/B978-0-12-804766-8.00013-3. [DOI] [PubMed] [Google Scholar]
- 231.Sreedharan J, Blair IP, Tripathi VB, Hu X, Vance C, Rogelj B, Ackerley S, Durnall JC, Williams KL, Buratti E, Baralle F, de Belleroche J, Mitchell JD, Leigh PN, Al-Chalabi A, Miller CC, Nicholson G, Shaw CE. TDP-43 mutations in familial and sporadic amyotrophic lateral sclerosis. Science. 2008;319:1668–1672. doi: 10.1126/science.1154584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Stakenborg N, Viola MF, Boeckxstaens GE. Intestinal neuro-immune interactions: focus on macrophages, mast cells and innate lymphoid cells. Curr Opin Neurobiol. 2020;62:68–75. doi: 10.1016/j.conb.2019.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Stein DJ, Vasconcelos MF, Albrechet-Souza L, Ceresér KMM, de Almeida RMM. Microglial over-activation by social defeat stress contributes to anxiety- and depressive-like behaviors. Front Behav Neurosci. 2017a;11:207. doi: 10.3389/fnbeh.2017.00207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Stein DM, Feather CB, Napolitano LM. Traumatic brain injury advances. Crit Care Clin. 2017b;33:1–13. doi: 10.1016/j.ccc.2016.08.008. [DOI] [PubMed] [Google Scholar]
- 235.Stenzel-Poore MP, Heldwein KA, Stenzel P, Lee S, Vale WW. Characterization of the genomic corticotropin-releasing factor (CRF) gene from Xenopus laevis: two members of the CRF family exist in amphibians. Mol Endocrinol. 1992;6:1716–1724. doi: 10.1210/mend.6.10.1448118. [DOI] [PubMed] [Google Scholar]
- 236.Strehl C, Ehlers L, Gaber T, Buttgereit F. Glucocorticoids-all-rounders tackling the versatile players of the immune system. Front Immunol. 2019;10:1744. doi: 10.3389/fimmu.2019.01744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Suárez AL, Feramisco JD, Koo J, Steinhoff M. Psychoneuroimmunology of psychological stress and atopic dermatitis: pathophysiologic and therapeutic updates. Acta Derm Venereol. 2012;92:7–15. doi: 10.2340/00015555-1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Sugama S, Sekiyama K, Kodama T, Takamatsu Y, Takenouchi T, Hashimoto M, Bruno C, Kakinuma Y. Chronic restraint stress triggers dopaminergic and noradrenergic neurodegeneration: Possible role of chronic stress in the onset of Parkinson’s disease. Brain Behav Immun. 2016;51:39–46. doi: 10.1016/j.bbi.2015.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Sweeney MD, Sagare AP, Zlokovic BV. Blood-brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat Rev Neurol. 2018;14:133–150. doi: 10.1038/nrneurol.2017.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Taché Y, Martinez V, Wang L, Million M. CRF1 receptor signaling pathways are involved in stress-related alterations of colonic function and viscerosensitivity: implications for irritable bowel syndrome. Br J Pharmacol. 2004;141:1321–1330. doi: 10.1038/sj.bjp.0705760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Tampa M, Sarbu MI, Mitran MI, Mitran CI, Matei C, Georgescu SR. The pathophysiological mechanisms and the quest for biomarkers in psoriasis, a stress-related skin disease. Dis Markers. 2018;2018:5823684. doi: 10.1155/2018/5823684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Tan EK, Chao YX, West A, Chan LL, Poewe W, Jankovic J. Parkinson disease and the immune system - associations, mechanisms and therapeutics. Nat Rev Neurol. 2020;16:303–318. doi: 10.1038/s41582-020-0344-4. [DOI] [PubMed] [Google Scholar]
- 243.Theoharides TC. Effect of stress on neuroimmune processes. Clin Ther. 2020a;42:1007–1014. doi: 10.1016/j.clinthera.2020.05.002. [DOI] [PubMed] [Google Scholar]
- 244.Theoharides TC. The impact of psychological stress on mast cells. Ann Allergy Asthma Immunol. 2020b;125:388–392. doi: 10.1016/j.anai.2020.07.007. [DOI] [PubMed] [Google Scholar]
- 245.Theoharides TC, Konstantinidou AD. Corticotropin-releasing hormone and the blood-brain-barrier. Front Biosci. 2007;12:1615–1628. doi: 10.2741/2174. [DOI] [PubMed] [Google Scholar]
- 246.Theoharides TC, Kavalioti M, Tsilioni I. Mast cells, stress, fear and autism spectrum disorder. Int J Mol Sci. 2019;20:3611. doi: 10.3390/ijms20153611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Theoharides TC, Kempuraj D, Tagen M, Conti P, Kalogeromitros D. Differential release of mast cell mediators and the pathogenesis of inflammation. Immunol Rev. 2007;217:65–78. doi: 10.1111/j.1600-065X.2007.00519.x. [DOI] [PubMed] [Google Scholar]
- 248.Theoharides TC, Petra AI, Stewart JM, Tsilioni I, Panagiotidou S, Akin C. High serum corticotropin-releasing hormone (CRH) and bone marrow mast cell CRH receptor expression in a mastocytosis patient. J Allergy Clin Immunol. 2014;134:1197–1199. doi: 10.1016/j.jaci.2014.05.023. [DOI] [PubMed] [Google Scholar]
- 249.Theoharides TC, Singh LK, Boucher W, Pang X, Letourneau R, Webster E, Chrousos G. Corticotropin-releasing hormone induces skin mast cell degranulation and increased vascular permeability, a possible explanation for its proinflammatory effects. Endocrinology. 1998;139:403–413. doi: 10.1210/endo.139.1.5660. [DOI] [PubMed] [Google Scholar]
- 250.Theoharides TC, Spanos C, Pang X, Alferes L, Ligris K, Letourneau R, Rozniecki JJ, Webster E, Chrousos GP. Stress-induced intracranial mast cell degranulation: a corticotropin-releasing hormone-mediated effect. Endocrinology. 1995;136:5745–5750. doi: 10.1210/endo.136.12.7588332. [DOI] [PubMed] [Google Scholar]
- 251.Theoharides TC, Alysandratos KD, Angelidou A, Delivanis DA, Sismanopoulos N, Zhang B, Asadi S, Vasiadi M, Weng Z, Miniati A, Kalogeromitros D. Mast cells and inflammation. Biochim Biophys Acta. 2012;1822:21–33. doi: 10.1016/j.bbadis.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Traina G. Mast cells in the brain -Old cells, new target. J Integr Neurosci. 2017;16:S69–83. doi: 10.3233/JIN-170068. [DOI] [PubMed] [Google Scholar]
- 253.Traina G. Mast cells in gut and brain and their potential role as an emerging therapeutic target for neural diseases. Front Cell Neurosci. 2019;13:345. doi: 10.3389/fncel.2019.00345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Trias E, Ibarburu S, Barreto-Núñez R, Varela V, Moura IC, Dubreuil P, Hermine O, Beckman JS, Barbeito L. Evidence for mast cells contributing to neuromuscular pathology in an inherited model of ALS. JCI Insight. 2017;2:e95934. doi: 10.1172/jci.insight.95934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Tsang AH, Chung KK. Oxidative and nitrosative stress in Parkinson’s disease. Biochim Biophys Acta. 2009;1792:643–650. doi: 10.1016/j.bbadis.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 256.Tsilioni I, Russell IJ, Stewart JM, Gleason RM, Theoharides TC. Neuropeptides CRH, SP, HK-1, and inflammatory cytokines IL-6 and TNF are increased in serum of patients with fibromyalgia syndrome, implicating mast cells. J Pharmacol Exp Ther. 2016;356:664–672. doi: 10.1124/jpet.115.230060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Tsilioni I, Patel AB, Pantazopoulos H, Berretta S, Conti P, Leeman SE, Theoharides TC. IL-37 is increased in brains of children with autism spectrum disorder and inhibits human microglia stimulated by neurotensin. Proc Natl Acad Sci U S A. 2019;116:21659–21665. doi: 10.1073/pnas.1906817116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Tsilioni I, Dodman N, Petra AI, Taliou A, Francis K, Moon-Fanelli A, Shuster L, Theoharides TC. Elevated serum neurotensin and CRH levels in children with autistic spectrum disorders and tail-chasing Bull Terriers with a phenotype similar to autism. Transl Psychiatry. 2014;4:e466. doi: 10.1038/tp.2014.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981;213:1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- 260.Valent P, Akin C, Hartmann K, Nilsson G, Reiter A, Hermine O, Sotlar K, Sperr WR, Escribano L, George TI, Kluin-Nelemans HC, Ustun C, Triggiani M, Brockow K, Gotlib J, Orfao A, Kovanen PT, Hadzijusufovic E, Sadovnik I, Horny HP, et al. Mast cells as a unique hematopoietic lineage and cell system: From Paul Ehrlich’s visions to precision medicine concepts. Theranostics. 2020;10:10743–10768. doi: 10.7150/thno.46719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Vance C, Rogelj B, Hortobágyi T, De Vos KJ, Nishimura AL, Sreedharan J, Hu X, Smith B, Ruddy D, Wright P, Ganesalingam J, Williams KL, Tripathi V, Al-Saraj S, Al-Chalabi A, Leigh PN, Blair IP, Nicholson G, de Belleroche J, Gallo JM, et al. Mutations in FUS, an RNA processing protein, cause familial amyotrophic lateral sclerosis type 6. Science. 2009;323:1208–1211. doi: 10.1126/science.1165942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Vandael D, Gounko NV. Corticotropin releasing factor-binding protein (CRF-BP) as a potential new therapeutic target in Alzheimer’s disease and stress disorders. Transl Psychiatry. 2019;9:272. doi: 10.1038/s41398-019-0581-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Vanuytsel T, van Wanrooy S, Vanheel H, Vanormelingen C, Verschueren S, Houben E, Salim Rasoel S, Tόth J, Holvoet L, Farré R, Van Oudenhove L, Boeckxstaens G, Verbeke K, Tack J. Psychological stress and corticotropin-releasing hormone increase intestinal permeability in humans by a mast cell-dependent mechanism. Gut. 2014;63:1293–1299. doi: 10.1136/gutjnl-2013-305690. [DOI] [PubMed] [Google Scholar]
- 264.Vasiadi M, Therianou A, Sideri K, Smyrnioti M, Sismanopoulos N, Delivanis DA, Asadi S, Katsarou-Katsari A, Petrakopoulou T, Theoharides A, Antoniou C, Papadavid E, Stavrianeas N, Kalogeromitros D, Theoharides TC. Increased serum CRH levels with decreased skin CRHR-1 gene expression in psoriasis and atopic dermatitis. J Allergy Clin Immunol. 2012;129:1410–1413. doi: 10.1016/j.jaci.2012.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Vicario M, Guilarte M, Alonso C, Yang P, Martínez C, Ramos L, Lobo B, González A, Guilà M, Pigrau M, Saperas E, Azpiroz F, Santos J. Chronological assessment of mast cell-mediated gut dysfunction and mucosal inflammation in a rat model of chronic psychosocial stress. Brain Behav Immun. 2010;24:1166–1175. doi: 10.1016/j.bbi.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 266.Vuppaladhadiam L, Ehsan C, Akkati M, Bhargava A. Corticotropin-releasing factor family: A stress hormone-receptor system’s emerging role in mediating sex-specific signaling. Cells. 2020;9:839. doi: 10.3390/cells9040839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Walker FR, Nilsson M, Jones K. Acute and chronic stress-induced disturbances of microglial plasticity, phenotype and function. Curr Drug Targets. 2013;14:1262–1276. doi: 10.2174/13894501113149990208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Wallon C, Söderholm JD. Corticotropin-releasing hormone and mast cells in the regulation of mucosal barrier function in the human colon. Ann N Y Acad Sci. 2009;1165:206–210. doi: 10.1111/j.1749-6632.2009.04030.x. [DOI] [PubMed] [Google Scholar]
- 269.Wang F, Yang TB, Kim BS. The return of the mast cell: New roles in neuroimmune itch biology. J Invest Dermatol. 2020;140:945–951. doi: 10.1016/j.jid.2019.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Wang LY, Raskind MA, Wilkinson CW, Shofer JB, Sikkema C, Szot P, Quinn JF, Galasko DR, Peskind ER. Associations between CSF cortisol and CSF norepinephrine in cognitively normal controls and patients with amnestic MCI and AD dementia. Int J Geriatr Psychiatry. 2018;33:763–768. doi: 10.1002/gps.4856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Wasko NJ, Nichols F, Clark RB. Multiple sclerosis, the microbiome, TLR2, and the hygiene hypothesis. Autoimmun Rev. 2020;19:102430. doi: 10.1016/j.autrev.2019.102430. [DOI] [PubMed] [Google Scholar]
- 272.Weber H, Richter J, Straube B, Lueken U, Domschke K, Schartner C, Klauke B, Baumann C, Pané-Farré C, Jacob CP, Scholz CJ, Zwanzger P, Lang T, Fehm L, Jansen A, Konrad C, Fydrich T, Wittmann A, Pfleiderer B, Ströhle A, et al. Allelic variation in CRHR1 predisposes to panic disorder: evidence for biased fear processing. Mol Psychiatry. 2016;21:813–822. doi: 10.1038/mp.2015.125. [DOI] [PubMed] [Google Scholar]
- 273.Welcome MO, Mastorakis NE. Stress-induced blood brain barrier disruption: Molecular mechanisms and signaling pathways. Pharmacol Res. 2020;157:104769. doi: 10.1016/j.phrs.2020.104769. [DOI] [PubMed] [Google Scholar]
- 274.Weninger SC, Dunn AJ, Muglia LJ, Dikkes P, Miczek KA, Swiergiel AH, Berridge CW, Majzoub JA. Stress-induced behaviors require the corticotropin-releasing hormone (CRH) receptor, but not CRH. Proc Natl Acad Sci U S A. 1999;96:8283–8288. doi: 10.1073/pnas.96.14.8283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Whitton PS. Neuroinflammation and the prospects for anti-inflammatory treatment of Parkinson’s disease. Curr Opin Investig Drugs. 2010;11:788–794. [PubMed] [Google Scholar]
- 276.Xu N, Li X, Zhong Y. Inflammatory cytokines: potential biomarkers of immunologic dysfunction in autism spectrum disorders. Mediators Inflamm. 2015;2015:531518. doi: 10.1155/2015/531518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Xu Y, Chen G. Mast cell and autoimmune diseases. Mediators Inflamm. 2015;2015:246126. doi: 10.1155/2015/246126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Yamanishi Y, Karasuyama H. Basophils and mast cells in immunity and inflammation. Semin Immunopathol. 2016;38:535–537. doi: 10.1007/s00281-016-0582-0. [DOI] [PubMed] [Google Scholar]
- 279.Zetterström P, Stewart HG, Bergemalm D, Jonsson PA, Graffmo KS, Andersen PM, Brännström T, Oliveberg M, Marklund SL. Soluble misfolded subfractions of mutant superoxide dismutase-1s are enriched in spinal cords throughout life in murine ALS models. Proc Natl Acad Sci U S A. 2007;104:14157–14162. doi: 10.1073/pnas.0700477104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Zhang C, Kuo CC, Moghadam SH, Monte L, Campbell SN, Rice KC, Sawchenko PE, Masliah E, Rissman RA. Corticotropin-releasing factor receptor-1 antagonism mitigates beta amyloid pathology and cognitive and synaptic deficits in a mouse model of Alzheimer’s disease. Alzheimers Dement. 2016a;12:527–537. doi: 10.1016/j.jalz.2015.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Zhang L, Song J, Hou X. Mast cells and irritable bowel syndrome: From the bench to the bedside. J Neurogastroenterol Motil. 2016b;22:181–192. doi: 10.5056/jnm15137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Zhang P, Fan B, Yang P, Temirov J, Messing J, Kim HJ, Taylor JP. Chronic optogenetic induction of stress granules is cytotoxic and reveals the evolution of ALS-FTD pathology. Elife. 2019;8:e39578. doi: 10.7554/eLife.39578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Zhang S, Dong H, Zhang X, Li N, Sun J, Qian Y. Cerebral mast cells contribute to postoperative cognitive dysfunction by promoting blood brain barrier disruption. Behav Brain Res. 2016c;298:158–166. doi: 10.1016/j.bbr.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 284.Zhang X, Wang Y, Dong H, Xu Y, Zhang S. Induction of microglial activation by mediators released from mast cells. Cell Physiol Biochem. 2016d;38:1520–1531. doi: 10.1159/000443093. [DOI] [PubMed] [Google Scholar]
- 285.Zhang X, Dong H, Li N, Zhang S, Sun J, Zhang S, Qian Y. Activated brain mast cells contribute to postoperative cognitive dysfunction by evoking microglia activation and neuronal apoptosis. J Neuroinflammation. 2016e;13:127. doi: 10.1186/s12974-016-0592-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Zhao W, Beers DR, Bell S, Wang J, Wen S, Baloh RH, Appel SH. TDP-43 activates microglia through NF-κB and NLRP3 inflammasome. Exp Neurol. 2015a;273:24–35. doi: 10.1016/j.expneurol.2015.07.019. [DOI] [PubMed] [Google Scholar]
- 287.Zhao W, Beers DR, Hooten KG, Sieglaff DH, Zhang A, Kalyana-Sundaram S, Traini CM, Halsey WS, Hughes AM, Sathe GM, Livi GP, Fan GH, Appel SH. Characterization of gene expression phenotype in amyotrophic lateral sclerosis monocytes. JAMA Neurol. 2017;74:677–685. doi: 10.1001/jamaneurol.2017.0357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Zhao Z, Nelson AR, Betsholtz C, Zlokovic BV. Establishment and dysfunction of the blood-brain barrier. Cell. 2015b;163:1064–1078. doi: 10.1016/j.cell.2015.10.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Zhou JN, Fang H. Transcriptional regulation of corticotropin-releasing hormone gene in stress response. IBRO Rep. 2018;5:137–146. doi: 10.1016/j.ibror.2018.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Zouboulis CC, Seltmann H, Hiroi N, Chen W, Young M, Oeff M, Scherbaum WA, Orfanos CE, McCann SM, Bornstein SR. Corticotropin-releasing hormone: an autocrine hormone that promotes lipogenesis in human sebocytes. Proc Natl Acad Sci U S A. 2002;99:7148–7153. doi: 10.1073/pnas.102180999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Zusso M, Stokes L, Moro S, Giusti P. Editorial: neuroinflammation and its resolution: from molecular mechanisms to therapeutic perspectives. Front Pharmacol. 2020;11:480. doi: 10.3389/fphar.2020.00480. [DOI] [PMC free article] [PubMed] [Google Scholar]


