ABSTRACT
N6-methyladenosine (m6A) has emerged as a crucial epitranscriptomic mark which regulates a broad spectrum of physiological processes including stem cell differentiation. m6A-binding YTHDF proteins have recently been proposed to mediate differentiation of leukemia cell in a redundant manner. However, whether these proteins play semblable roles in pluripotent stem cell remain largely unknown. Here, we showed the differential functions of YTHDF1 and YTHDF3 in controlling the differentiation of embryonic stem cells (ESCs). Depletion of YTHDF3 in ESCs resulted in loss of pluripotency with accelerated expressions of marker genes involved in formation of three germ layers. Phenotypic and transcriptomic analyses revealed that loss of YTHDF1 led to dramatic impairment of cardiomyocytes (CMs) differentiation, accompanied by downregulated CM-specific genes. While, knockdown of YTHDF3 accelerated differentiation through facilitating the expressions of CM-specific gene. Notably, YTHDF3 appears to modulate cellular differentiation partially through suppression of YTHDF1, supporting the distinguishable but interrelated roles of YTHDF1 and YTHDF3 in cell fate determination.
KEYWORDS: YTHDF, embryonic stem cell, cardiomyocyte, differentiation, N6-methyladenosine
Introduction
N6-methyladenosine (m6A) is an abundant mRNA internal modification which regulates gene expressions at multiple layers [1,2]. Functional identification of methyltransferases and demethylases suggests that the reversible m6A plays important roles in diverse physiological processes, especially in cellular differentiation [3–10]. Depletion of core methyltransferase METTL3 greatly impaired embryonic stem cell (ESC) differentiation potential by promoting the stability of m6A-containing pluripotency mRNAs [11,12]. By contrast, another study revealed that knockdown of METTL3 or METTL14 reduced self-renewal capability through decreasing m6A levels and stabilizing mRNAs of developmental regulators [13]. In support of the negative role of m6A in differentiation, dysfunction of METTL3 has been shown to inhibit proliferation and promoted myeloid differentiation of human hematopoietic stem/progenitor cells (HSPCs) [14]. In addition to messenger RNA, METTL5-mediated methylation on 18S ribosomal RNA may participate in modulation of ESC differentiation through translational regulation [15], highlighting the crucial but intricate roles of m6A in pluripotency and cell fate decision.
YT521-B homology domain-containing family (YTHDF) proteins are primary m6A ‘readers’ which exhibit multifarious effects on gene expressions by recognizing cytoplasmic m6A-containing transcripts [16–18]. YTHDF2 has been implicated to facilitate RNA decay through direct recruitment of the CCR4-NOT deadenylase complex [19,20], while YTHDF1 and YTHDF3 promote translation of m6A-containing transcripts [21–23]. Intriguingly, YTHDF paralogs have been recently proposed to behave redundantly to regulate mRNA degradation and differentiation of acute myeloid leukemia (AML) cells [24]. However, the functional roles of YTHDFs in pluripotent stem cells (PSCs) remain largely unknown. A latest study showed that depletion of YTHDF2 led to stabilization of neural-specific transcripts, resulting in loss of pluripotency [25]. These findings prompted us to ask whether other YTHDF proteins play semblable roles in the regulation of PSC fate determination. In this study, we characterized the functions of YTHDF1 and YTHDF3 in ESC-derived cardiomyocytes (CMs) differentiation. Through phenotypic and transcriptomic analysis of Ythdf knockdown cells, we found that YTHDF1 and YTHDF3 play opposite roles in CMs differentiation through inversely regulating expressions of lineage-specific genes. Specifically, loss of YTHDF1 dramatically impaired CMs differentiation with decreased CM-specific genes, while depletion of YTHDF3 facilitated in vitro cardiac myogenesis through promoting the expressions of CM-specific gene. Intriguingly, we found that YTHDF3 inhibited cellular differentiation partially through suppression of YTHDF1, reflecting interrelated regulation of cell fate commitment by YTHDF1 and YTHDF3.
Materials and methods
Mouse embryonic stem cell culture and differentiation
Mouse J-1 ESCs were purchased from the Cell Bank of Chinese Academy of Sciences. Mycoplasma testing was performed routinely and cells tested negative were used in further experiments. These cells were grown on 0.2% gelatin-coated dishes in Knock-out DMEM (Gibco) containing 15% KnockOut Serum Replacement (Gibco), 1% non essential amino acids (Gibco), 1% L-Glutamine (Gibco), 1% Pen-Strep (Life technologies), 0.1 mM β-mercaptoethanol (Genview) and 1000 U/ml ESGRO Recombinant Mouse LIF Protein (Millipore). For embryoid body (EB) formation, suspension of each cell line at a concentration of 10 × 104cells per milliliter in differentiating medium (Knock-out DMEM supplemented with 15% FBS (Gibco), 1% non essential amino acids, 1% L-Glutamine, 1% Pen-Strep, 0.1 mM β-mercaptoethanol) was deposited in 20 μl hanging drops in Petri dishes. After 2 days, the suspension-cultured cells were plated onto 0.2% gelatin-coated dishes in cardiomyocyte differentiation media (CMD) containing DMEM (Gibco), 15% FBS (Gibco), 1% Pen-Strep, 1% non essential amino acids (Gibco), 1% L-Glutamine (Gibco), 0.1 mM β-mercaptoethanol and 1 mM Ascorbic Acid (Sigma) for continued differentiation. Cell culture media was changed every other day and contracting patches of cells were collected at indicated time points.
Generation of shRNA-mediated knockdown of Ythdfs in mESCs
The shRNA targeting sequences were cloned into pRSI9-U6-(sh)-UbiC-TagRFP-2A-Puro (Cellecta, CA). Lentiviral particles were packaged using Lenti-X 293 T cells. Virus-containing supernatants were collected at 48 hr after transfection and filtered to eliminate cell debris. mESCs were infected by the lentivirus for 48 hr and stable mESC knockdown lines were generated using puromycin selection (0.5 µg/ml). shRNA targeting sequences are listed below:
Ythdf1 (target sequence 1): 5′-GGACATTGGTACTTGGGATAA-3′;
Ythdf1 (target sequence 2): 5′-GCACACAACCTCTATCTTTGA-3′;
Ythdf3 (target sequence 1): 5′-GGTACTTGAAACTTCCTTTAA-3′;
Ythdf3 (target sequence 2): 5′-GGTGGGCTTCACCAATTAATG-3′;
Scramble target sequence: 5′-AACAGTCGCGTTTGCGACTGG-3′.
Cell proliferation and Alkaline phosphatase staining assays
mESCs were seeded at a density of 5,000 cells/well in 48-well plates. Cell proliferation was monitored every day for up to 6 days using Cell Counting Kit-8 (Beyotime). For Alkaline phosphatase staining assay, 8000 mESCs were seeded in 35 mm dish and cultured for 6 days. Cells were rinsed with PBS once and stained using BCIP/NBT Alkaline Phosphatase Color Development Kit (Beyotime). Cells were visualized by a Nikon Ts2-FL microscope.
Immunofluorescence
mESCs are plated onto gelatin-coated cover slips and grown for at least 5 days prior to fixation. Cells were fixed in 4% paraformaldehyde for 20 min at room temperature. After permeabilizing in 0.1% Triton X-100 for 10 min at room temperature, the cover slips were blocked with1% bovine serum albumin (BSA) for 30 minutes. Cells were stained with MYH primary antibody (Proteintech 22,281-1-AP) overnight at 4°C, followed by incubation with Alexa Fluor 488 anti-rabbit secondary antibody for 1 hr at room temperature. The nuclei were stained with Hoechst (1:2000 dilution) for 5 min. Cover slips were mounted onto slides and visualized using a Nikon Ts2-FL fluorescent microscope.
Western blot
Proteins were separated on SDS-PAGE and transferred to PVDF Membranes (Millipore). The membranes were blocked in TBS buffer containing 5% non-fat milk and 0.1% Tween-20 for 1 hr, followed by incubation with primary antibodies overnight at 4°C. The next day after incubation with horseradish peroxidase-coupled secondary antibody at room temperature for 1 hr, the immunoblots were visualized by using the enhanced chemiluminescence (TANON SCIENCE & TECHONLOGY). Antibodies used in the experiments are listed as below: anti-YTHDF1 (Proteintech 17479-1-AP), anti- YTHDF3 (Proteintech 25537-1-AP).
RNA decay assay
To determine mRNA stability, cells were treated with actinomycin D (MCE) at a final concentration of 5 μM. Each sample was harvested at 0 and 4 h after actinomycin D treatment. The cells were collected and synthesized luciferase RNA spike -in control was added proportional to the total cell numbers. The RNA transcript levels of interest were detected by qPCR.
Real-time quantitative PCR
Total RNA was isolated by TRIzol reagent (Invitrogen) and reverse transcription was performed using HiScript III 1st Strand cDNA Synthesis Kit plus gDNA wiper (Vazyme Biotech). Real-time PCR analysis was conducted with ChamQ SYBR qPCR Master Mix (Vazyme Biotech) and carried on a QuantStudio 3 Real-Time PCR System (Applied Biosystems). Primers for amplifying each target were listed in Table S1.
RNA-Seq library construction and sequencing
The RNA-seq library preparation and sequencing were performed at the Annoroad Gene Technology (Beijing, China). Generally, RNA was purified with TRIzol reagent (Invitrogen). Poly(A)+ RNA was then isolated and used for RNA-seq libraries preparation. Two biological replicates were prepared for each sample. The libraries were sequenced on an Illumina HiSeq system using the 2 × 150-bp paired-end read mode.
RNA-Seq data analysis
RNA-seq reads data was trimmed using Trim Galore (Babraham Bioinformatics) (parameters: Trim_galore -q 25 – stringency 5 – length 30 -e 0.1 – paired R1.fa.gz R2.fa.gz – gzip), and aligned to mouse genome version GRCm38 reference sequence using the HISAT2 (Pertea et al., 2016) with the default parameters. Transcript assembly using StringTie was done for HISAT2 aligned reads (parameters: stringtie -e -B). The R package DESeq2 was used to estimate significance of differential expression between RNA samples. Gene expression changes were considered significant if passed padj ≤0.05 and |log2foldchange| ≥ 1 thresholds. Gene ontology (GO) enrichment analysis was performed in DAVID (http://david.ncifcrf.gov), and the top GO categories were selected according to FDR values. Raw and processed sequencing data for RNA-seq were deposited in the NCBI Gene Expression Omnibus (GEO).
Comparisons of m6A abundance and gene expression
MeRIP-seq data for ESC, EB and CM obtained from GEO (GSE61995, GSE131296) were used for comparison analysis in this study. To determine whether the Ythdf paralog affect the m6A-modifed gene expression, we compared the gene abundance between Control and Ythdf paralog knockdown cells.
Results
Depletion of YTHDF3 results in loss of pluripotency and induction of genes involved in formation of three germ layers
YTHDF2 has been shown to regulate the stabilization of neural-specific transcripts and pluripotent state of PSCs [25]. To assess the roles of YTHDF1 and YTHDF3 in pluripotency, we generated mouse ESC lines with knockdown of either YTHDF1 or YTHDF3. As shown in Fig. 1A,B, the protein levels of YTHDF were specifically attenuated in respective knockdown cells. Quantitative RT-PCR (qRT-PCR) analysis confirmed the decreased mRNA levels of Ythdf in each ESC lines (Fig. S1A and S1B). Interestingly, the expression of Ythdf1 was mildly but significantly upregulated in Ythdf3-knockdown cells. While, Ythdf3 harbored comparable expression levels between Ythdf1-knockdown cells and control cells transfected with scramble shRNA. Morphologically, Ythdf1-knockdown ESCs organized as tightly packed colonies (Fig. 1C, upper panel), accompanied by increased expressions of pluripotent genes (Dppa3 and Klf2) compared with control ESCs (Fig. 1E), indicating improved self-renewal. Consistent with this property, Ythdf1-knock down cells displayed saturated alkaline phosphatase (AP)-stained colonies but slightly decreased proliferation (Fig. 1C, lower panel, 1 F and Fig. S2A). While, depletion of YTHDF3 resulted in loss of tightly packed colonies and dramatic decrease in the expressions of Dppa3 and Klf2 (Fig. 1D, upper panel and 1E). In line with the differentiation-like phenotype, Ythdf3-knockdown ESCs showed light-colored AP-positive colonies but increased cell growth (Fig. 1D, lower panel, 1 F and Fig. S2A). Remarkably, expressions of Nanog, Pou5f1 and Sox2 were not influenced by depletion of Ythdf in ESCs (Fig. 1E). Together, these results suggest that YTHDF1 and YTHDF3 differentially regulate pluripotency likely independent of core transcriptional regulatory circuitry.
Figure 1.
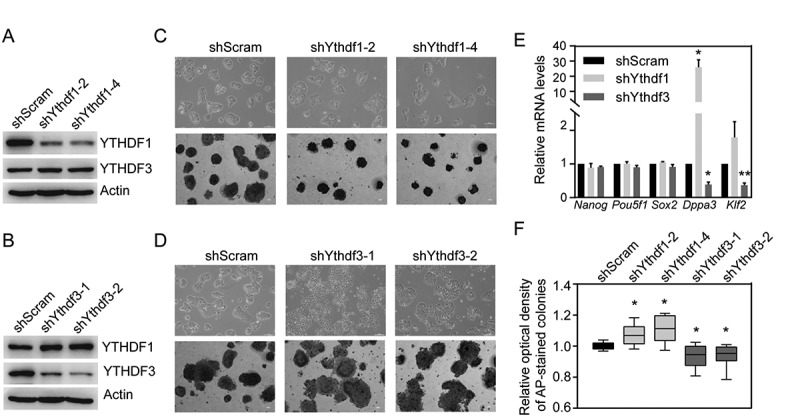
Depletion of YTHDF3 results in loss of pluripotency
(A) Western blotting showing knockdown efficiency of YTHDF1 in mouse ESCs. (B) Western blotting showing knockdown efficiency of YTHDF3 in mouse ESCs. (C) Representative image showing morphology (upper panel) and alkaline phosphatase (AP) staining (lower panel) of Ythdf1-knockdown ESC colonies in comparable with control cells (shScram). (D) Representative image showing morphology (upper panel) and AP staining (lower panel) of Ythdf3-knockdown ESC colonies in comparable with control cells (shScram). (E) Detection of pluripotency genes in Ythdf1- and Ythdf3-knockdown ESCs by qRT-PCR. Error bars, mean ± s.e.m.; *P < 0.05, **P < 0.01, unpaired two tailed t-test; n= 4 independent experiments. (F) Quantitative analysis of AP stained colonies formed by Ythdf1- and Ythdf3- knockdown ESCs. Error bars, mean ± s.e.m.; *P < 0.05, unpaired two tailed t-test; n= 3 independent experiments.
To gain insights into the molecular basis of ESC phenotypes affected by depletion of either YTHDF1 or YTHDF3, we performed RNA-sequencing (RNA-seq) analysis of control and Ythdf-knockdown cells. Supporting the role of YTHDFs in self-renewal, gene ontology (GO) analysis of differentially expressed genes (DEGs) in Ythdf1- and Ythdf3-knockdown ESCs revealed an enrichment of genes associated with cellular developmental process (GO:0048869) and cell differentiation (GO:0030154) (Fig. 2A). A close inspection of DEGs in Ythdf3-knockdown cells showed that a number of genes involved in formation of three germ layers, were upregulated, such as Gata4, Gata6, Sox7 (endoderm); Gbx2, Sox1 (ectoderm); Wnt8a, Tcf15 (mesoderm) (Fig. 2B), consistent with the loss-of-pluripotency phenotype in ESCs depleting YTHDF3. As an independent validation, qRT-PCR analysis showed that the representative genes for germ layers, including Sox7, Gata4 and Brachyury, were induced upon loss of YTHDF3 (Fig. 2C). Collectively, these results suggest that YTHDF1 and YTHDF3 play distinct roles in the regulation of stem cell pluripotency.
Figure 2.
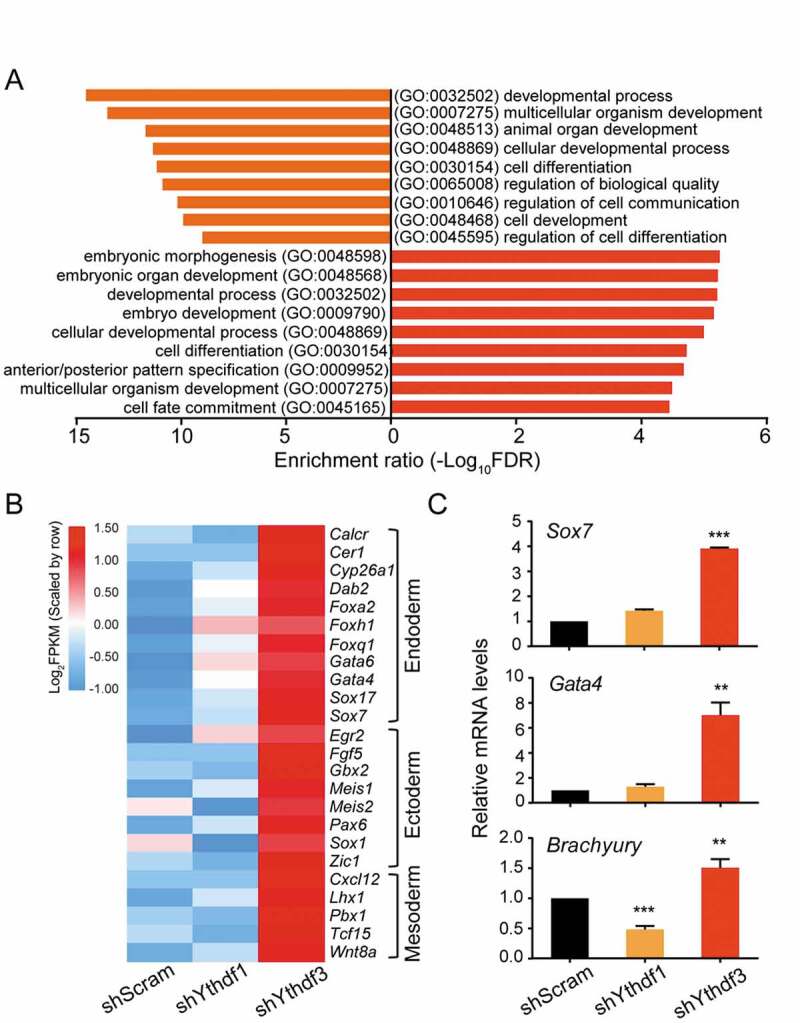
Depletion of YTHDF3 results in the induction of genes involved in formation of three germ layers
(A) GO analysis of DEGs in Ythdf1- (orange) and Ythdf3-knockdown (red) ESCs, compared with control cells. (B) Heat map showing expression of genes responsive for formation of three germ layers, endoderm, ectoderm and mesoderm, in Ythdf1- and Ythdf3-knockdown ESCs. (C) Detection of gene expression for endoderm and mesoderm by qRT-PCR. Error bars, mean ± s.e.m.; **P < 0.01, ***P < 0.001, unpaired two tailed t-test; n= 4 independent experiments.
YTHDF1 and YTHDF3 are downregulated during cardiac differentiation
Given the high enrichment of YTHDF-associated DEGs in differentiation (Fig. 2A), we initially evaluated the expression of YTHDFs during ESCs differentiation towards cardiomyocytes (CMs). As expected, a pluripotent and early cardiac marker, Nanog and Nkx2.5, were downregulated and upregulated upon CMs differentiation, respectively (Fig. 3B). Intriguingly, the protein levels of YTHDF were gradually attenuated (Fig. 3A), while the abundance of Ythdf transcripts were slightly increased at day 2 and decreased thereafter (Fig. 3B). Consistent with these results, gene expressions of both Ythdfs were obviously reduced in the absence of leukemia inhibitory factor (LIF) (Fig. 3C), which is essential for supporting proliferation and stem cell maintenance. Supporting the potential functions of YTHDFs in the regulation of cell fate determination, both Ythdfs harbors much higher expressions in pluripotent ESCs than differentiated MEF cells (Fig. 3D). These observations indicated that YTHDF1 and YTHDF3 might play certain roles in regulating commitment to the cardiac lineage.
Figure 3.
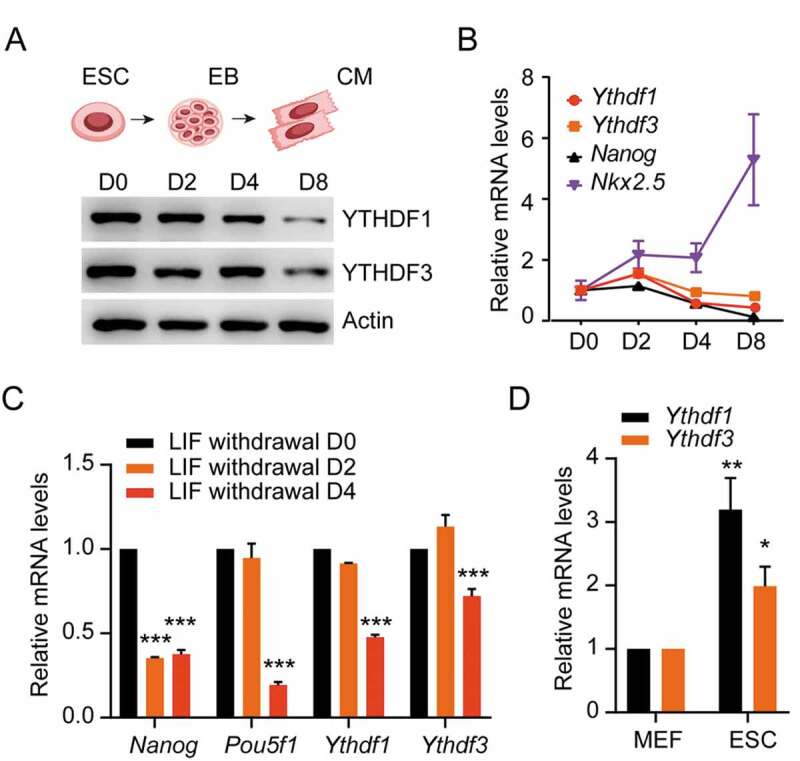
YTHDF1 and YTHDF3 are downregulated during cardiac differentiation
(A) Detection of YTHDF1 and YTHDF3 protein levels during CMs differentiation by Western blot. (B) Detection of Ythdf1 and Ythdf3 mRNA levels during CMs differentiation by qRT-PCR. (C) Detection of YTHDF1 and YTHDF3 transcript abundance in the presence and absence of leukemia inhibitory factor (LIF) treatment by qRT-PCR. (D) Detection of YTHDF1 and YTHDF3 transcript abundance in ESC and MEF cells by qRT-PCR. Error bars, mean ± s.e.m.; *P < 0.05, **P < 0.01, ***P < 0.001, unpaired two tailed t-test; n= 4 independent experiments.
YTHDF1 and YTHDF3 play differential roles in ESC-derived cardiac differentiation
We observed above that YTHDF1 depletion in ESCs resulted in condensed AP-positive colonies, accompanied with increased expressions of pluripotent genes Dppa3 and Klf2. Whereas lack of YTHDF3 caused abrogated expressions of Dppa3 and Klf2, and the activation of genes responsible for generating three germ layers. These observations suggest that YTHDF1 and YTHDF3 may positively and negatively influence the differentiation process, respectively. To test this hypothesis, we examined the capacity of Ythdf-knockdown ESCs for directed differentiation towards CMs. Compared with control ESCs, Ythdf1-knockdown cells generated relatively small embryonic bodies (EBs), while Ythdf3-knockdown cells produced EBs with irregular shape (Fig. 4A and Fig. S2B). Remarkably, in the presence of ascorbic acid, Ythdf3-depleted EBs are able to produce ~ 80% beating CMs at day 6, only ~16% of control EBs generated beating CMs. While, no beating CMs were observed for Ythdf1-knockdown EBs (Fig. 4B). In parallel, CM structural protein MYH6 expressed at relatively high levels and low levels in Ythdf3- and Ythdf1-knockdown CMs, respectively (Fig. 4D). At day 10, although both control and Ythdf3-knockdown EBs generated ~ 95% beating CMs, significantly lower beating rate (~18%) were still observed for Ythdf1-depleted EBs (Fig. 4B). The phenotypic differences of Ythdf-knockdown CMs were more pronounced by removal of ascorbic acid from differentiation medium (Fig. 4C). Consistent with the beating properties, pluripotency genes (Nanog, Sox2, etc.) remained at high levels in Ythdf1-knockdown CMs (Fig. 4E), while CMs-specific genes (Actc1, Myh7, etc.) were dramatically upregulated and downregulated in Ythdf3- and Ythdf1-knockdown CMs, respectively (Fig. 4F). Collectively, these results indicated that YTHDF1 and YTHDF3 play contrasting roles in the regulation of ESC-derived CMs differentiation programme.
Figure 4.
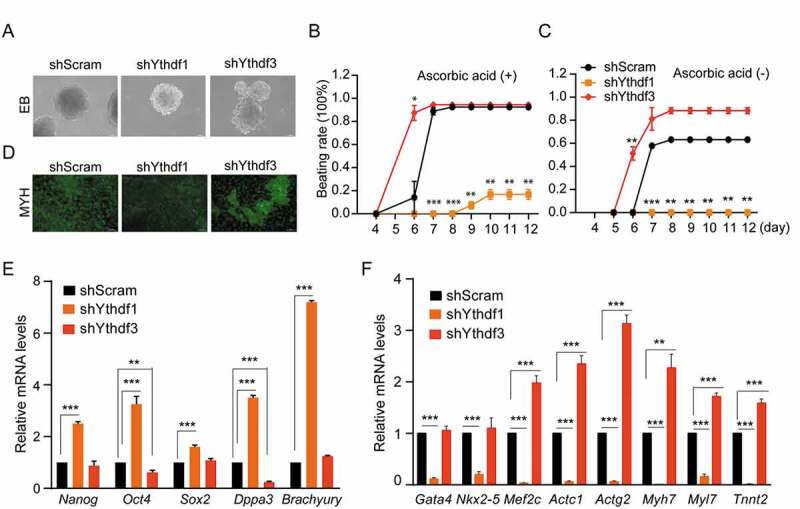
YTHDF1 and YTHDF3 play divergent roles in ESC-derived cardiac differentiation
(A) Representative morphologic images of Ythdf1- and Ythdf3-knockdown embryoid bodies (EBs) compared with shScram EBs. (B) Beating rate of cardiomyocytes during differentiation in Ythdf-knockdown and shScram cells in the presence of ascorbic acid. (C) Beating rate of cardiomyocytes during differentiation in Ythdf-knockdown and shScram cells in the absence of ascorbic acid. Error bars, mean ± s.e.m.; *P< 0.05, **P < 0.01, ***P < 0.001, unpaired two tailed t-test; n= 2 independent experiments, 20 EBs were quantified for each experiment. (D) Representative images of CMs stained by anti-MYH antibody. (E) Detection of pluripotency genes in Ythdf1- and Ythdf3-knockdown cardiomyocytes by qRT-PCR. (F) Detection of mRNA levels of CM marker genes at day 6 in Ythdf-knockdown and shScram cells. Error bars, mean ± s.e.m.; **P < 0.01, ***P < 0.001, unpaired two tailed t-test; n= 4 independent experiments.
YTHDF1 and YTHDF3 oppositely regulate cardiac gene expressions
To further validate the indispensability of YTHDFs in the process of CMs differentiation, we sought to monitor the global gene expression profiles in control and Ythdf-knockdown cells (ESC, EB and CMs). Measurement of transcript abundance from independent biological samples are highly correlated (Fig. S3), indicating the reproducibility of two gene expression datasets. As shown in Fig. 5A, the number of DEGs upon knockdown of YTHDF1 and YTHDF3 were found to mainly differ in ESC and CM. Particularly, more upregulated genes were captured in Ythdf3-knockdown CMs (554) than Ythdf1-knockdown CMs (367) (Fig. S4A). DEGs upon either YTHDF depletion are overrepresented in molecular functions involved in DNA binding and regulation of transcription (Fig. S4C), but enriched in distinct biological pathways. DEGs in Ythdf3-knockdown CMs are linked to the formation of endoderm, ectoderm and mesoderm (Fig. S4B), validating the involvement of YTHDF3 in the induction of three-germ layer formation and further differentiation. Directed differentiation of CMs from ESCs are generally accompanied with deactivation of pluripotent genes and simultaneous induction of cardiac-specific genes. A close inspection of significantly upregulated genes in CMs versus ESC revealed that induction of these genes were slowed down in Ythdf1-knockdown CMs, but were further accelerated in Ythdf3-knockdown CMs (Fig. 5B). Additionally, compared with control cells, a large number of cardiac genes displayed opposite regulatory pattern between Ythdf1- and Ythdf3-knockdown CMs in two biological differentiation replicates (Fig. 5C, D), which may reflect differential roles of YTHDFs in CMs differentiation.
Figure 5.
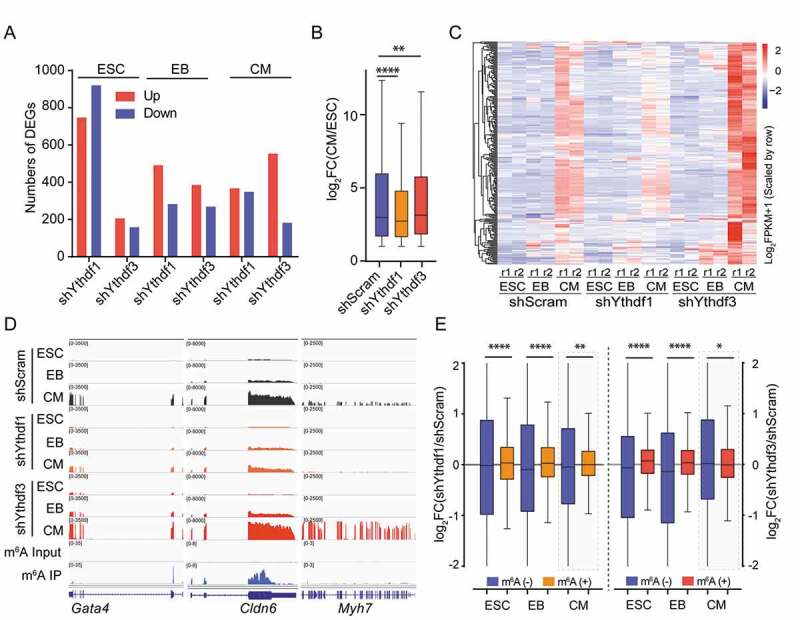
YTHDF1 and YTHDF3 conversely regulate the expression of cardiac genes
(A) Number of differentially expressed genes captured in Ythdf-knockdown and shScram cells at day 0 (ESC), day 2 (EB) and day 8 (CM). (B) Box plot showing the fold changes (CM/ESC) of RNA-seq FPKM in upregulated genes (fold change > 2) upon differentiation in shScram and Ythdf-depleted cells. Two-tailed Mann-Whitney test, P **<0.01, P ****<0.0001, n = 2. (C) Heat map showing RNA-seq FPKM of CM-specific genes in shScram and Ythdf-knockdown cells at indicated differentiation stages (n = 238). (D) Genome browser view of representative genes showing RNA-seq and m6A-seq read density in shScram and Ythdf-knockdown cells. (E) Box plot showing m6A-containg RNAs bearing significantly increased abundance upon depletion of YTHDF1 or YTHDF3 in ESC and EB rather than CM. Two-tailed Mann-Whitney test, P *<0.05, P **<0.01, P ****<0.0001, n = 2.
As YTHDF proteins are able to regulate transcript expressions through recognizing methylated adenosines, we next evaluated how m6A-marked transcripts were affected in Ythdf-knockdown cells. As shown in Fig. 5E, compared with non-m6A RNA, depletion of either YTHDF1 or YTHDF3 significantly increased the abundance of m6A-containing RNAs in ESC and EB, which appears consistent with the role of YTHDFs in the regulation of RNA stability [24]. Surprisingly, in CMs, YTHDF1 depletion was associated with a significant increase in the abundance of m6A-containing transcripts. While, lack of YTHDF3 showed the inverse effect with a decrease in the levels of m6A RNAs. This observation is possibly due to increased abundance of non-m6A RNAs in Ythdf3-knockdown CMs in comparison with control cells, as depletion of either YTHDF1 or YTHDF3 didn’t apparently affect the levels of m6A-containing transcripts (Fig. 5E). Consistently, we noticed that a majority of CM-specific genes (65%) which simultaneously downregulated in Ythdf1-depleted cells and upregulated in Ythdf3-knockdown cells were less likely to be m6A-modified (Fig. S5A). These results suggest that the primary effect of YTHDFs on CM gene expressions is probably not exerted through RNA stability control. Indeed, we didn’t observe dramatic change of CM transcripts decay rate in Ythdf-knockdown CMs compared to control CMs (Fig. S5B).
Depletion of YTHDF1 partially reverses the differentiation phenotype of Ythdf3 knockdown ESCs
We showed above the almost opposite differentiation phenotype and cardiac gene expression between Ythdf1-knockdown and Ythdf3-knockdown cells. As depletion of YTHDF3 resulted in increased expression of YTHDF1 (Fig. 1C and Fig. S1B), we suspect that accelerated Ythdf3-knockdown CMs differentiation might be partially due to relatively high abundance of YTHDF1. Supporting this notion, overlapping upregulated genes in Ythdf3-knockdown CMs showed obviously higher fold-change than that in Ythdf1-knockdown CMs, while overlapping downregulated genes in Ythdf3-knockdown CMs bore lower fold-change than that in Ythdf1-knockdown CMs (Fig. S5C, S5D). To further explore the mutual relation of YTHDFs in CMs differentiation, we generated Ythdfs double mutant by depleting Ythdf1 in Ythdf3-knockdown cells (Fig. 6A). Apparently, depletion of YTHDF1 greatly impaired the accelerated differentiation potential in Ythdf3-knockdown cells (Fig. 6B). Consistent with the phenotype, high expression of a number of cardiac genes in Ythdf3-depleted CMs, such as Mef2c, Actc1 and Myh7, become largely reduced upon loss of YTHDF1 (Fig. 6C), supporting that YTHDF3 may modulate CMs differentiation at least partially through suppression of YTHDF1.
Figure 6.
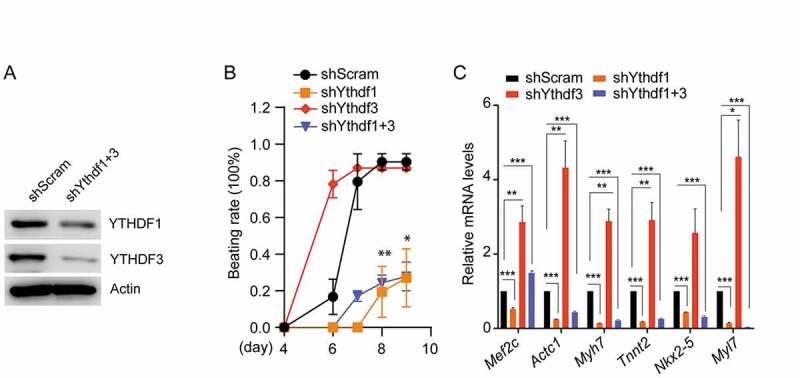
Identification of cardiac differentiation phenotypes in cells with depletion of both YTHDF1 and YTHDF3
(A) Western blotting showing double knockdown of Ythdf1 and Ythdf3 (shYthdf1 + 3) in mouse ESCs. (B) Beating rate of cardiomyocytes in double knockdown of Ythdfs cells. Error bars, mean ± s.e.m.; *P < 0.05, **P < 0.01, ***P < 0.001, unpaired two tailed t-test; n= 2 independent experiments, 20 EBs were quantified for each experiment. (C) Detection of mRNA levels of CM marker genes in double knockdown of Ythdfs cells. Error bars, mean ± s.e.m.; *P< 0.05**P < 0.01, ***P < 0.001, unpaired two tailed t-test; n= 4 independent experiments.
Discussion
Stem cells are defined by the capacity of maintaining a state of self-renewal as well as differentiating in the presence of external stimuli. Transcriptional regulation is generally considered as a master switch for rapidly turning on and off the genes during differentiation [26]. The coordinated remodeling of global gene expression is essential for achieving cell lineage specification. Through functional studies of methyltransferases and demethylases, m6A has been demonstrated to function as a posttranscriptional regulator of gene expression during proliferation and differentiation of stem cells [27]. With respect to the m6A-binding reader proteins, only the role of YTHDF2 has been extensively explored in different stem cells [28–31]. In this study, we characterized the functions of other two YTHDF proteins, YTHDF1 and YTHDF3, in the regulation of ESC pluripotency and differentiation. Distinct from the proposed redundant function of YTHDF proteins in the regulation of AML cell differentiation [24], our phenotypic and transcriptomic results revealed that YTHDF1 and YTHDF3 play a positive and negative role in guiding ESC-derived CMs differentiation, respectively.
Functional analysis of YTHDFs in ESCs showed that depletion of YTHDF1 promoted the maintenance of pluripotent state with increased the expression of Dppa3 and Klf2 (Fig. 1A, 1E). By contrast, a recent study showed that YTHDF1 was required for maintenance of mouse intestinal stem cells (ISCs) in Wnt-dependent manner, as deletion of YTHDF1 resulted in a dramatic decrease in the expression of ISC markers [32,33], indicating cellular context-dependent regulation of stem cell pluoripotent state by YTHDF1. Similar as the loss-of-pluripotency phenotype in Ythdf2-knockdown ESCs [25], Ythdf3-depleted cells displayed differentiation-like morphology coupled with not only downregulated pluripotent transcripts but also elevated expressions of genes involved in the formation of endoderm, ectoderm and mesoderm (Figs. 1D, F and 2). It is worth noting that both YTHDFs regulate ESC pluripotency probably independent of core transcriptional regulatory circuitry, as expressions of Nanog, Pou5f1 and Sox2 were not influenced by depletion of Ythdf in ESC (Fig. 1E).
The opposite pluripotent state of Ythdf1- and Ythdf3-knockdown cells prompted us to hypothesize that two YTHDFs may guide cellular differentiation in a distinguishable manner. Indeed, we observed that lack of YTHDF1 significantly impaired the differentiation potential, even no beating CMs captured in Ythdf1-knockdown cells without ascorbic acid in the medium (Fig. 4B). Whereas, depletion of YTHDF3 dramatically accelerated differentiation progress with promoted expression of cardiac marker genes (Fig. 4F). In contrast to the distinct effects on ESC-derived cardiac differentiation, the Ythdf genes possessed the semblable response to differentiation signal [25] (Fig. 3). It is likely that an uniform upstream pathway guides the cellular response of YTHDFs to differentiation signal, such as LIF withdrawal. The differentiation-promoting or suppressing effects of YTHDFs could be due to the divergent gene expression pattern in Ythdf-depleted cells. Actually, this is the case. Transcriptomic analysis revealed that a subset of cardiac genes were downregulated in Ythdf1-knockdown cells but upregulated in Ythdf3-knockdown cells (Fig. 5C, D). As both YTHDF1 and YTHDF3 have been suggested to regulate expressions of m6A-containing transcripts [21–23], we evaluated the m6A-dependency of YTHDF-mediated gene expression at three differentiation stages. Loss of YTHDF1 or YTHDF3 resulted in elevated expressions of m6A transcripts in ESCs and EBs rather than CMs, compared to non-m6A RNAs (Fig. 5E), suggesting that YTHDFs may negatively control the stability of m6A-containing RNAs in a cell-state-dependent mode. In support of this, CM transcripts with or without m6A showed comparable turnover rate in Ythdf-knockdown and control cells (Fig. S5B). YTHDF1 and YTHDF3 have been identified to cooperatively enhance mRNA translation efficiency [23]. Considering YTHDF-regulated genes are mainly associated with DNA binding and transcription (Fig. S4C), we hypothesized that YTHDF1 and YTHDF3 probably promote the protein synthesis of key m6A-bearing transcription regulators in either ESCs or CMs, thereby affecting the progress of cardiac differentiation. However, the exact translation-related mechanisms need to be further investigated.
Perhaps the most surprising finding in our study is the opposite but interrelated roles of YTHDF1 and YTHDF3 involved in cell fate specification. Lack of YTHDF3 accelerated the expression of YTHDF1 and cardiac differentiation (Fig. 1B and Fig. S1B). While, depletion of YTHDF1 in Ythdf3-depleted cells obviously reduced the beating CMs and levels of cardiac transcripts (Fig. 6B, C). Although YTHDF proteins harbor conserved YTH domains which recognize m6A codes embedded in RNA (Fig. S6), non-conserved protein regions are possible to affect the selective binding and expression of target transcripts through recruiting different regulatory factors under certain cellular conditions, resulting in context-dependent dissimilar roles of YTHDFs. It will be of great interest to uncover distinguishable functions of YTHDFs during other physiological processes.
In summary, our findings showed that YTHDF1 and YTHDF3 not only play differential roles in the regulation of pluripotency, but also conversely contribute to the cardiac differentiation. Remarkably, YTHDF3 may mediate cellular differentiation partially through suppression of YTHDF1, supporting the contrasting but interrelated roles for cell fate decision.
Supplementary Material
Funding Statement
The authors wish to thank lab members for helpful discussions and reviews for constructive suggestions. This work was supported by the National Key R&D Program of China [2018YFA0902000], the National Natural Science Foundation of China [81974527, 32070868], the Natural Science Foundation of Jiangsu Province, China [BK20190533], and the China Pharmaceutical University start-up fund [3150040072].
Disclosure Statement
No potential conflicts of interest were disclosed.
Author contributions
X.-M. L. and J.Z. conceived the project and wrote the manuscript. S.W. performed most of the experiments. J.Z. conducted the majority of data analysis. X.W. and X.L. helped with cell culture and data collection. All the authors read and approved the final manuscript.
Data availability statement
The detailed methods and datasets are included in the article and its additional files. MeRIP-seq data from ESC, EB and CM used in this study are obtained from GEO (GSE61995, GSE131296). Other deep sequencing data have been submitted to the NCBI Gene Expression Omnibus (SRA-PRJNA642989, https://www.ncbi.nlm.nih.gov/sra). All biological materials used in this study are available from the corresponding author on reasonable request.
Supplementary material
Supplemental data for this article can be accessed here.
References
- [1].Zhao BS, Roundtree IA, He C.. Post-transcriptional gene regulation by mRNA modifications. Nat Rev Mol Cell Biol. 2017;18:31–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Roundtree IA, Evans ME, Pan T, et al. Dynamic RNA modifications in gene expression regulation. Cell. 2017;169:1187–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Aguilo F, Zhang F, Sancho A, et al. Coordination of m6A mRNA methylation and gene transcription by ZFP217 regulates pluripotency and reprogramming. Cell Stem Cell. 2015;17:689–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Wang Y, Li Y, Yue M, et al. N6-methyladenosine RNA modification regulates embryonic neural stem cell self-renewal through histone modifications. Nat Neurosci. 2018;21:195–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Wen J, Lv R, Ma H, et al. Zc3h13 regulates Nuclear RNA m6A methylation and mouse embryonic stem cell self-renewal. Mol Cell. 2018;69(1028–1038.e6):1028–1038.e6. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bertero A, Brown S, Madrigal P, et al. The SMAD2/3 interactome reveals that TGFβ controls m6A mRNA methylation in pluripotency. Nature. 2018;555:256–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Wu Y, Xie L, Wang M, et al. Mettl3-mediated m 6 A RNA methylation regulates the fate of bone marrow mesenchymal stem cells and osteoporosis. Nat Commun. 2018;9. DOI: 10.1038/s41467-018-06898-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Shi H, Wei J, He C. Where, when, and how: context-dependent functions of RNA methylation writers, readers, and erasers. Mol Cell. 2019;74:640–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Su R, Dong L, Li Y, et al. Targeting FTO suppresses cancer stem cell maintenance and immune evasion. Cancer Cell. 2020;38:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Shen C, Sheng Y, Zhu AC, et al. RNA demethylase ALKBH5 selectively promotes tumorigenesis and cancer stem cell self-renewal in acute myeloid leukemia. Cell Stem Cell. 2020;27(64–80.e9):64–80.e9. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Batista PJ, Molinie B, Wang J, et al. m6A RNA modification controls cell fate transition in mammalian embryonic stem cells. Cell Stem Cell. 2014;15:707–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Mansour AA, Kol N, Hershkovitz V, et al. m6A mRNA methylation facilitates resolution of naïve pluripotency toward differentiation. Science. 2015;347:1002–1006. [DOI] [PubMed] [Google Scholar]
- [13].Wang Y, Li Y, Toth JI, et al. N6-methyladenosine modification destabilizes developmental regulators in embryonic stem cells. Nat Cell Biol. 2014;16:191–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Vu LP, Pickering BF, Cheng Y, et al. The N6-methyladenosine (m6A)-forming enzyme METTL3 controls myeloid differentiation of normal hematopoietic and leukemia cells. Nat Med. 2017;23:1369–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Ignatova VV, Stolz P, Kaiser S, et al. The rRNA m6A methyltransferase METTL5 is involved in pluripotency and developmental programs. Genes Dev. 2020;34:715–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Berlivet S, Scutenaire J, Deragon JM, et al. Readers of the m6A epitranscriptomic code. Biochim Biophys Acta. 2019;1862:329–342. [DOI] [PubMed] [Google Scholar]
- [17].Patil DP, Pickering BF, Jaffrey SR. Reading m6A in the transcriptome: m6A-binding proteins. Trends Cell Biol. 2018;28:113–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Wu B, Li L, Huang Y, et al. Readers, writers and erasers of N6-methylated adenosine modification. Curr Opin Struct Biol. 2017;47:67–76. [DOI] [PubMed] [Google Scholar]
- [19].Wang X, Lu Z, Gomez A, et al. N6-methyladenosine-dependent regulation of messenger RNA stability. Nature. 2014;505:117–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Du H, Zhao Y, He J, et al. YTHDF2 destabilizes m6A-containing RNA through direct recruitment of the CCR4-NOT deadenylase complex. Nat Commun. 2016;7. DOI: 10.1038/ncomms12626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Wang X, Zhao BS, Roundtree IA, et al. N6-methyladenosine modulates messenger RNA translation efficiency. Cell. 2015;161:1388–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Li A, Chen Y-S, Ping X-L, et al. Cytoplasmic m6A reader YTHDF3 promotes mRNA translation. Cell Res. 2017;27:444–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Shi H, Wang X, Lu Z, et al. YTHDF3 facilitates translation and decay of N6-methyladenosine-modified RNA. Cell Res. 2017;27:315–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Zaccara S, Jaffrey SR. A unified model for the function of YTHDF proteins in regulating m6A-modified mRNA. Cell. 2020;181:1582–1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Heck AM, Russo J, Wilusz J, et al. YTHDF2 destabilizes m6A-modified neural-specific RNAs to restrain differentiation in induced pluripotent stem cells. RNA. 2020;26:739–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Zhao H, Jin Y. Signaling networks in the control of pluripotency. Curr Opin Genet Dev. 2017;46:141–148. [DOI] [PubMed] [Google Scholar]
- [27].Aguilo F, Walsh MJ. The N6-Methyladenosine RNA modification in pluripotency and reprogramming. Curr Opin Genet Dev. 2017;46:77–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Zhang C, Chen Y, Sun B, et al. m6A modulates haematopoietic stem and progenitor cell specification. Nature. 2017;549:273–276. [DOI] [PubMed] [Google Scholar]
- [29].Li M, Zhao X, Wang W, et al. Ythdf2-mediated m6A mRNA clearance modulates neural development in mice. Genome Biol. 2018;19:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Wu R, Liu Y, Yao Y, et al. FTO regulates adipogenesis by controlling cell cycle progression via m6A-YTHDF2 dependent mechanism. Biochim Biophys Acta - Mol Cell Biol Lipids. 2018;1863:1323–1330. [DOI] [PubMed] [Google Scholar]
- [31].Paris J, Morgan M, Campos J, et al. Targeting the RNA m6A reader YTHDF2 selectively compromises cancer stem cells in acute myeloid leukemia. Cell Stem Cell. 2019;25:137–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Liu X, Qian S. Linking m6A to Wnt signaling. EMBO Rep. 2020;53:436–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Han B, Yan S, Wei S, et al. YTHDF1‐mediated translation amplifies Wnt‐driven intestinal stemness. EMBO Rep. 2020;21(4):e49229. . [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The detailed methods and datasets are included in the article and its additional files. MeRIP-seq data from ESC, EB and CM used in this study are obtained from GEO (GSE61995, GSE131296). Other deep sequencing data have been submitted to the NCBI Gene Expression Omnibus (SRA-PRJNA642989, https://www.ncbi.nlm.nih.gov/sra). All biological materials used in this study are available from the corresponding author on reasonable request.


