Abstract
Background
Since the emergence of the COVID-19 pandemic in late 2019, various public health and social measures (PHSMs) have been used to suppress and mitigate the spread of SARS-CoV-2. With mass vaccination programmes against COVID-19 being rolled out in many countries in early 2021, we aimed to evaluate to what extent travel restrictions and other PHSMs can be relaxed without exacerbating the local and global spread of COVID-19.
Methods
We adapted an existing age-structured susceptible-infectious-removed model of SARS-CoV-2 transmission dynamics that can be parameterised with country-specific age demographics and contact patterns to simulate the effect of vaccination and PHSM relaxation on transmission. We varied assumptions by age-specific susceptibility and infectiousness, vaccine uptake, contact patterns, and age structures. We used Hong Kong as a case study and assumed that, before vaccination, the population is completely susceptible to SARS-CoV-2 infection. We applied our model to 304 jurisdictions (27 countries and 277 sub-national administrative regions from eight countries). We assumed that PHSMs have suppressed the effective reproductive number (Re) to fall between 1·0 and 9·0 locally before the commencement of vaccination programmes. We evaluated the levels of PHSMs that should be maintained during the roll-out of COVID-19 vaccination to avoid a large local outbreak of COVID-19, with different assumptions about vaccine efficacy, vaccination coverage, and travel restrictions. We assumed that the maximum capacity of the health system, in terms of daily hospital admissions, is 0·005% of the population size.
Findings
At vaccine efficacy of 0·80 in reducing susceptibility to SARS-CoV-2 infection, 0·50 in reducing SARS-CoV-2 infectivity, and 0·95 in reducing symptomatic COVID-19 diseases, vaccination coverage would have to be 100% for all individuals aged 30 or older to avoid an outbreak, when relaxing PHSMs, that would overload the local health-care system, assuming a pre-vaccination Re of 2·5. Testing and quarantine of at least 5 days would have to be maintained for inbound travellers to minimise the risk of reintroducing a local outbreak until high vaccination coverages are attained locally and overseas in most countries.
Interpretation
Gradual relaxation of PHSMs should be carefully planned during the roll-out of vaccination programmes, and easing of travel restrictions weighed against risk of reintroducing outbreaks, to avoid overwhelming health systems and minimise deaths related to COVID-19.
Funding
Health and Medical Research Fund and the General Research Fund.
Introduction
Since the emergence of the COVID-19 pandemic in late 2019, various public health and social measures (PHSMs) have been implemented globally to suppress and mitigate the spread of SARS-CoV-2, including mask wearing, travel restrictions, intensive test-trace-and-isolate, physical distancing, school closure, curfews, and targeted or nationwide lockdowns. Although these PHSMs are essential to control the spread of the pandemic, many PHSMs carry a high economic and social cost. The gross domestic product of the world declined by 3·5% in 2020, according to the International Monetary Fund's World Economic Outlook released in January, 2021.1 With mass vaccination programmes against COVID-19 being rolled out in a number of countries in early 2021, many countries are hoping that regional mass vaccinations will help to contain further spread of SARS-CoV-2, such that stringent travel restriction and other PHSMs can be relaxed to resume economic growth.
However, policy making related to the relaxation of PHSMs is extremely challenging. Although the first-generation vaccines are effective in reducing symptomatic or severe COVID-19 diseases, one key uncertainty is the degree to which they can prevent onward SARS-CoV-2 transmission, particularly against the emerging variants of concern,2, 3 and thus critical vaccination coverage remains uncertain. When relaxing local PHSMs, policy makers need to consider the effectiveness and supply of vaccines, rate and age distribution of vaccine uptake, the prevalence of infections (including variants), and the capacity of health systems. Relaxation of travel restrictions further depends on travel volume and COVID-19 prevalence among travellers. It is, therefore, critical to provide an impact assessment of relaxing PHSMs as vaccination programmes are being rolled out, with consideration of key uncertainties about vaccines, to strike a balance between reopening societies and minimising the risk of substantial resurgence and health system overload.4
Research in context.
Evidence before this study
We searched PubMed and preprint archives (BioRxiv, MedRxiv, and Research Square) for articles published in English between database inception and June 15, 2021, using the keywords “coronavirus”, “COVID-19”, “SARS-CoV-2”, “vaccin*”, “vaccine”, “control measures”, “non-pharmaceutical intervention”, “public health and social measure”, “international travel”, “travel restriction”, and “relaxation”. We selected articles that contained information about the control measures against COVID-19 outbreak. Our search returned 51 studies, nine of which were relevant to this topic. Previous studies have focused on control by non-pharmaceutical interventions, the optimal allocation of vaccines, risk assessment of local relaxation of control measures, or relaxation of travel restrictions. To our knowledge, there is not a combined risk assessment of relaxation of local controls and international travel restrictions.
Added value of this study
We adapted an existing age-structured susceptible-infectious-removed model of SARS-CoV-2 transmission dynamics to assess the risks of relaxing public health and social measures (PHSMs) during the roll-out of vaccination programmes. Given that the reproductive number is 5−7 for predominating SARS-CoV-2 variants of concern and that efficacy against infection is lower than 80% for nearly all existing COVID-19 vaccines, PHSMs cannot be completely lifted without resulting in substantial epidemic resurgence, even with high vaccination coverage across all age groups. If PHSMs are relaxed, testing and quarantine would have to be maintained for inbound travellers to suppress the risk of reseeding local outbreaks until high vaccination coverages have been attained locally.
Implications of all the available evidence
Gradual relaxation of PHSMs should be carefully planned during the roll-out of vaccination programmes to minimise admissions to hospital and deaths related to COVID-19. Complete relaxation of all PHSMs would require a very high coverage locally even with the most efficacious vaccines. This might be difficult to achieve soon, given the uncertainties around the vaccine efficacies at reducing transmission, emergence of variants of concern with increased transmissibility and potential immune escapes, and the current shortage in vaccine supply.
We aimed to use a model-informed approach to assess the outcomes of relaxing PHSMs during the roll-out of vaccination programmes. We focus on age-based vaccination strategies, as adopted by most countries. We have two hypotheses. First, given the reproductive number of 5−7 for predominating variants of concern globally and that effectiveness against infection is lower than 80% for all existing COVID-19 vaccines, we hypothesise that PHSMs cannot be completely lifted without resulting in epidemic resurgence that might overload the health system, even if vaccine uptake is high across all age groups. Second, we hypothesise that testing and quarantine would need to be maintained for inbound travellers to suppress the risk of reseeding local outbreaks and overloading health systems, unless vaccination coverage is high in both the regions of origin and destination.
Methods
Study design
We adapted an existing age-structured susceptible-infectious-removed model of SARS-CoV-2 transmission dynamics that can be parameterised with country-specific age demographics and contact patterns to simulate the effect of vaccination and PHSM relaxation on transmission. We varied assumptions by age-specific susceptibility and infectiousness, vaccine uptake, contact patterns, and age structures (appendix pp 2–4, 10–16). The appendix contains full details (pp 2–4, 8, 9, 10–16).5, 6 We used Hong Kong as a case study and assume that, before vaccination, the population is completely susceptible to SARS-CoV-2 infection. Serological studies have shown that, even in countries worst hit by the first global wave of COVID-19 pandemic, pre-vaccination seroprevalence at the population level (thus the estimated cumulative infection attack rates) was lower than 20%.7, 8, 9 In our sensitivity analysis, we assumed that 20% of the population had been infected before vaccination and thus had complete protection against SARS-CoV-2 re-infection (appendix p 16).
We assumed that children younger than 10 years are 50% less susceptible to infection than adults (aged ≥20 years), and that adolescents aged 10–19 years are 20% less susceptible to infection than adults; we assumed that both groups would be as infectious as adults if infected.10
As a sensitivity analysis, we also simulated COVID-19 transmission in 27 countries and 277 sub-national administrative regions from another eight countries in the model parameterised with age demographics and social contact patterns synthesised by Mistry and colleagues.11
Efficacies of the first-generation COVID-19 vaccines
To date, eight COVID-19 vaccines have received emergency use approval in selected countries. Phase 3 trial results and real-world evidence show moderate to high vaccine efficacies in preventing virologically confirmed symptomatic disease, as well as reducing infectiousness or the probability of onward transmission.12, 13, 14, 15 Further real-world evidence shows that vaccination reduced 78% of PCR-positive infections among air passengers in Qatar.16 Here, we account for the uncertainty in vaccine efficacy by considering σm ∈(0·50, 0·60, 0·70, 0·80), which denotes efficacy in reducing susceptibility to infection; σt ∈(0·30, 0·40, 0·50), which denotes efficacy in reducing infectiousness if infected; and σs ∈(0·80, 0·90, 0·95), which denotes efficacy in reducing symptomatic diseases and admissions to hospital.
Modelling the effects of PHSM relaxation and large-scale vaccination
Previous studies have shown that prioritising the oldest age groups for vaccination leads to the greatest reduction in COVID-19-related mortality.17, 18 In reality, age-based (and risk-based) vaccine delivery strategies have indeed been implemented by most jurisdictions, including Hong Kong, the UK, and the USA.18 Hence, we assumed that vaccines are allocated first to the oldest age group (ie, ≥80 years) and then to progressively younger age groups by 10-year age bands. We considered two scenarios for vaccine uptake: (1) each eligible age group would achieve the corresponding age-specific uptake in the UK as of June 6, 2021,19 and the uptake of individuals younger than 30 years would be the same as that of those aged 30–39 years if the former are eligible; (2) the uptake is 100% for all eligible individuals.
We assumed that (1) PHSMs would be relaxed such that the effective reproductive number, R e, was between 1·0 and 9·0 before the vaccination programme starts at time t v (ie, R e was the effective reproductive number in the absence of vaccination, considering the emergence of variants of concern with increased transmissibility); and (2) the effects of vaccination (characterised by σm, σt, and σs) are realised instantaneously at time t v. We then identified combinations of R e and vaccine coverage that would prevent subsequent epidemic waves from overloading the health-care system.
We assumed that the maximum capacity of the health system (in terms of daily hospital admissions) is 0·005% of the population size, which is on par with the peak number of COVID-19-related admissions to hospital that the National Health Service has managed in the UK (3565 on April 1, 2020, and 4579 on Jan 12, 2021).19 Other parameter values used in the simulations are shown in the appendix (pp 8–9).
Relaxation of international travel restrictions
We anticipate that many jurisdictions will soon begin to relax their measures for blocking international importation of infections due to mounting and overwhelming social, economic, and political pressure. Assuming that all travellers are from foreign origins, we considered a hypothetical jurisdiction that imposes the following control measures: (1) all inbound travellers will be tested upon arrival, regardless of their vaccination status, and (2) test-positive travellers will be isolated immediately, whereas test-negative travellers will be quarantined for 1–14 days and then might be tested again upon release. We estimated the expected force of infection (FOI) that inbound travellers would exert on the destination across a wide range of scenarios. Full details are given in the appendix (pp 4–5).
We used these analytics to develop a risk-assessment and decision tool to help plan and operationalise the relaxation of travel restriction measures, accounting for the COVID-19 prevalence in, and travel volume from, all potential jurisdictions of origin into Hong Kong. Assumptions about PCR test sensitivity (pp 17–18) and algorithmic details (pp 4–7) are given in the appendix. The components of this tool are summarised as follows.
(1) To compile a list of eligible origins, we created a screening criterion that requires vaccine uptake among travellers from each eligible origin to be large enough such that the expected COVID-19 prevalence among travellers is below the local COVID-19 prevalence at the destination, or a prespecified risk tolerance level if COVID-19 has been nearly eliminated at the destination. We excluded origins that failed to meet this criterion—ie, because reliable prevalence estimates at the origins are not available or prevalence is so high that even 100% vaccine uptake among travellers would not suffice.
(2) To specify the travel volume and corresponding minimum vaccine coverage among travellers from each eligible origin, we created a decision criterion that is designed to keep the expected FOI exerted by infected inbound travellers on the destination below a prespecified threshold (appendix pp 5–6).
(3) We continuously monitored the validity of the tool (eg, on a daily basis) to ensure that the underlying assumptions are valid (eg, consistency between the expected and detected number of infections among inbound travellers), given the uncertainty and dynamics of disease transmission. A circuit breaker should be used to suspend travel from origins that show higher-than-expected exportation of infections, and their eligibility and traveller requirements should be updated accordingly (appendix pp 6–7)
Ethical approval was not required for data used in the analysis. All data are publicly available. We used MATLAB (version 2021a; MathWorks, MA, USA) for modelling and data analysis.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
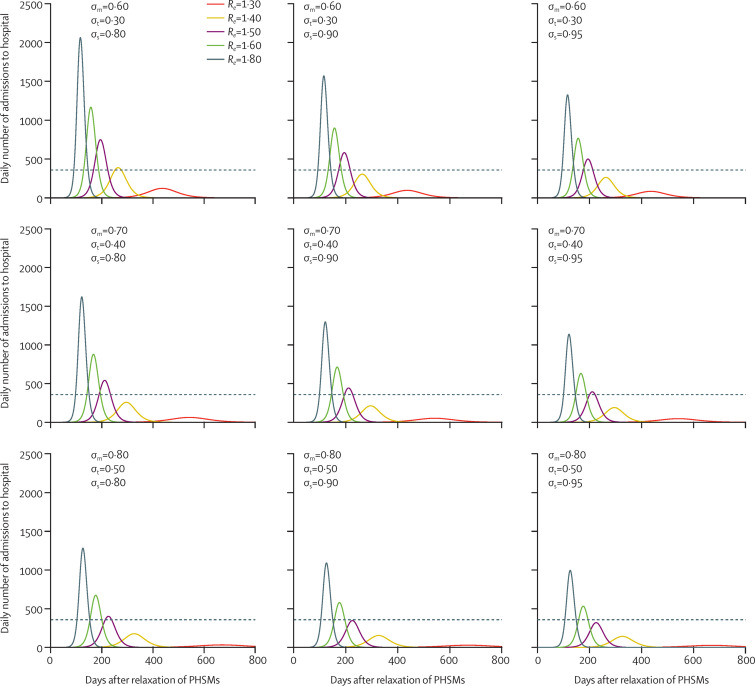
We first used Hong Kong as a case study to illustrate the course of the COVID-19 epidemic after PHSM relaxation and vaccination, assuming all individuals aged 50 years or older have been fully vaccinated (population coverage 36%). As expected, COVID-19-related admissions to hospital and deaths in subsequent waves of infection decreased with increasing vaccine efficacies (varied as σm, σt, and σs; figure 1 ). However, even under our most optimistic assumption—which assumed σm=0·80, σt=0·50, and σs=0·95 and is similar to the empirical efficacies of the approved mRNA vaccines such as BNT162b2—modest relaxation of PHSMs (with R e of 1·6–1·8) would result in epidemics that overload the local health system (figure 1). This conclusion remains valid across a wide range of contact patterns (appendix p 10) and for all 304 jurisdictions that we considered (27 countries and 277 sub-national administrative regions from another eight countries; appendix pp 13–16). These findings suggest that, when vaccination coverage is not high (eg, when only individuals aged ≥50 years are fully vaccinated), relaxation of PHSMs is not feasible without overloading the health-care system for all plausible ranges of σm, σt, and σs. As such, PHSM relaxation with R e above 1·6−1·8 should only be considered if high vaccination coverage can be attained with high confidence. For example, if all individuals aged 30 years or older have been fully vaccinated with σm=0·80, σt= 0·50, and σs=0·95 so that population coverage is 74%, then PHSMs can be relaxed to R e=2·5−3·5 without overloading the health-care system (appendix pp 11–12). We emphasise that R e was the effective reproductive number in the absence of vaccination; R e=2·5–3·5 corresponded to the basic reproductive number of the SARS-CoV-2 strain that seeded the pandemic in 2020.20
Figure 1.
Estimated daily number of admissions to hospital in Hong Kong following relaxation of PHSMs after all individuals aged ≥50 years have been fully vaccinated
Re is the effective reproductive number after relaxation of PHSMs in the absence of vaccination. In all the scenarios we assume all individuals aged ≥50 years have been fully vaccinated when PHSMs are relaxed. We assume the vaccine efficacy is σm in reducing the susceptibility to SARS-CoV-2 infection, σt in reducing the infectivity of SARS-CoV-2, and σs in reducing symptomatic COVID-19 diseases among those infected. The epidemics are seeded with one introduction event immediately after PHSMs are relaxed. The dashed line shows the maximum number of daily COVID-19-related admissions to hospital that the local health system could manage, which is assumed to be 0·005% of the total population. PHSMs=public health and social measures.
If the vaccine is only modestly effective in blocking infection (ie, σm below 0·80 and σt below 0·50), complete relaxation of local PHSMs without overloading the health-care system either requires a very high vaccination coverage or is outright infeasible (appendix pp 11–12). For example, if σm=0·50, σt=0·30, and σs= 0·80, the vaccination coverage would have to be 90–100% across all age groups to avoid overloading the health-care system when R e=2·5−3·5 (ie, when PHSMs completely relaxed). As of July, 2021, the variants of concern with increased transmissibility (eg, alpha [B.1.1.7] and delta [B.1.617.2]) are already prevalent in most parts of the world, and are estimated to be 50–70% (alpha) and 150–250% (delta) more transmissible than the original strain identified in December, 2020.20, 21, 22 If the epidemic is seeded or dominated by the newly emerged variants that are 50−250% more transmissible,20, 21, 22 complete relaxation of PHSM would result in an R e of 5−7 in the absence of vaccination; in this scenario, vaccination coverage would need to be 90−100% to avoid overloading the health-care system even under our most optimistic assumptions of σm=0·80, σt= 0·50, and σs=0·95 (appendix pp 11–12).
Given that vaccine uptake is unlikely to be 100% as we have optimistically assumed here (eg, due to vaccine hesitancy and refusal), lenient relaxation of PHSMs will probably result in resurgence of cases and carry the risk of overloading the health-care system, especially in the context of mutant variants.
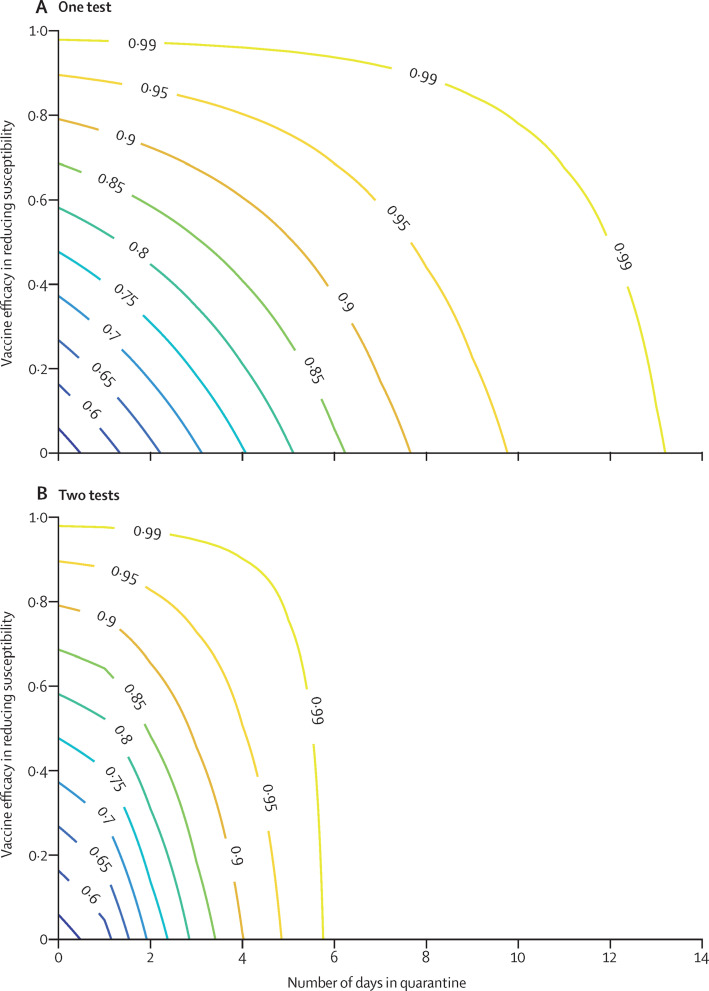
Next, we estimated the extent to which international travel restrictions could be relaxed safely. Let X (σm , q, s) be the expected FOI that an inbound traveller would impose on the destination if they are: (1) fully vaccinated and their susceptibility to infection is reduced by σm (σm=0 if unvaccinated; we ignored the effect of σt and σs to avoid underestimating the expected FOI among fully vaccinated individuals); (2) quarantined for q days; and (3) tested s times (upon arrival only if s=1 and an additional test upon quarantine release if s=2). Figure 2 shows Y(σm,q,s)=1 – X(σm,q,s) / X(0,0,0) which is the reduction in expected FOI from an inbound traveller.
Figure 2.
Reduction in the expected FOI an inbound traveller exerts on the destination as a function of vaccine efficacy in reducing susceptibility to infection and quarantine duration
(A) Travellers are tested upon arrival only. (B) Travellers are tested upon both arrival and release from quarantine. The values embedded in the curves are the reduction in the expected FOI an inbound traveller exerts on the destination. FOI=force of infection.
Under the assumption that an inbound traveller could be infected at any time before arrival with equal probability, we estimate that testing inbound travellers upon arrival without quarantining them would only reduce their expected FOI by 60−65% (appendix pp 17–18). This estimation is largely consistent with observed data in Hong Kong, which indicated that 25−35% of imported infections were not identified by the first RT-PCR test upon arrival at the airport.23 Our estimate of 60–65% is also consistent with the results from a previous modelling study.24
In the worst-case scenario wherein infected travellers are infected right before arrival, testing upon arrival without quarantine would have no effect on their FOI because their viral load upon arrival would be below the detection threshold. Fully vaccinated inbound travellers would have their expected FOI lowered by σm due to the vaccine reducing their susceptibility to infection. With a moderate σm of 50−60%, 5-day quarantine (ie, 4 full days), and two PCR tests, the expected FOI of fully vaccinated inbound travellers would be reduced by 92−96% (figure 2B). Extending the quarantine to at least 7 days, with two PCR tests, would reduce the expected FOI of both fully vaccinated and unvaccinated imported infections by 99%.
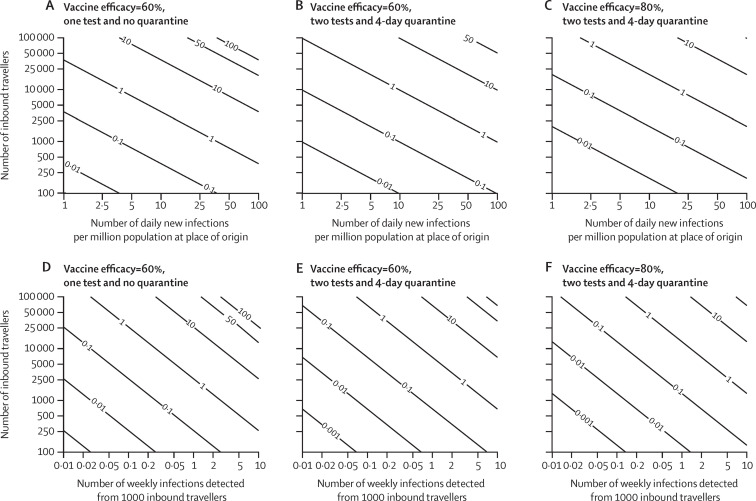
Suppose travel control measures at the destination aim to maintain the expected weekly number of undetected infections among inbound travellers from a given origin below Z. Suppose all travellers from the origin location have been fully vaccinated. Figure 3 shows how the travel volume quota for this origin depends on the risk threshold Z, the infection incidence at the origin, and the quarantine and testing requirements for travellers from this origin. For example, if Z=1, the daily incidence at the origin is 2 cases per million and all travellers are required to be tested twice and quarantine for 5 days, then the weekly number of inbound travellers from this origin should not exceed 50 000 travellers if σm=0·60 or 100 000 travellers if σm=0·80. If travellers are only required to be tested upon arrival without quarantine, then the corresponding travel quota is reduced to 20 000 travellers if σm=0·60 or 50 000 travellers if σm=0·80. When multiple origins are considered simultaneously, the risk threshold for each origin should be set lower accordingly so that the number of inbound travellers remains within an overall threshold set for all foreign places of origin.
Figure 3.
The expected number of undetected infected travellers at the destination as a function of vaccine efficacy, quarantine duration, test regimens, travel volume, and infection prevalence among travellers
We assumed all inbound travellers are fully vaccinated against SARS-CoV-2. (A, D) Vaccine efficacy in reducing susceptibility is σm=60%, with one test upon arrival. (B, E) σm=60%, with one test upon arrival and one test before release from 4-day quarantine (4 full days). (C, F) σm=80%, with one test upon arrival and one test before release from 4-day quarantine (4 full days).
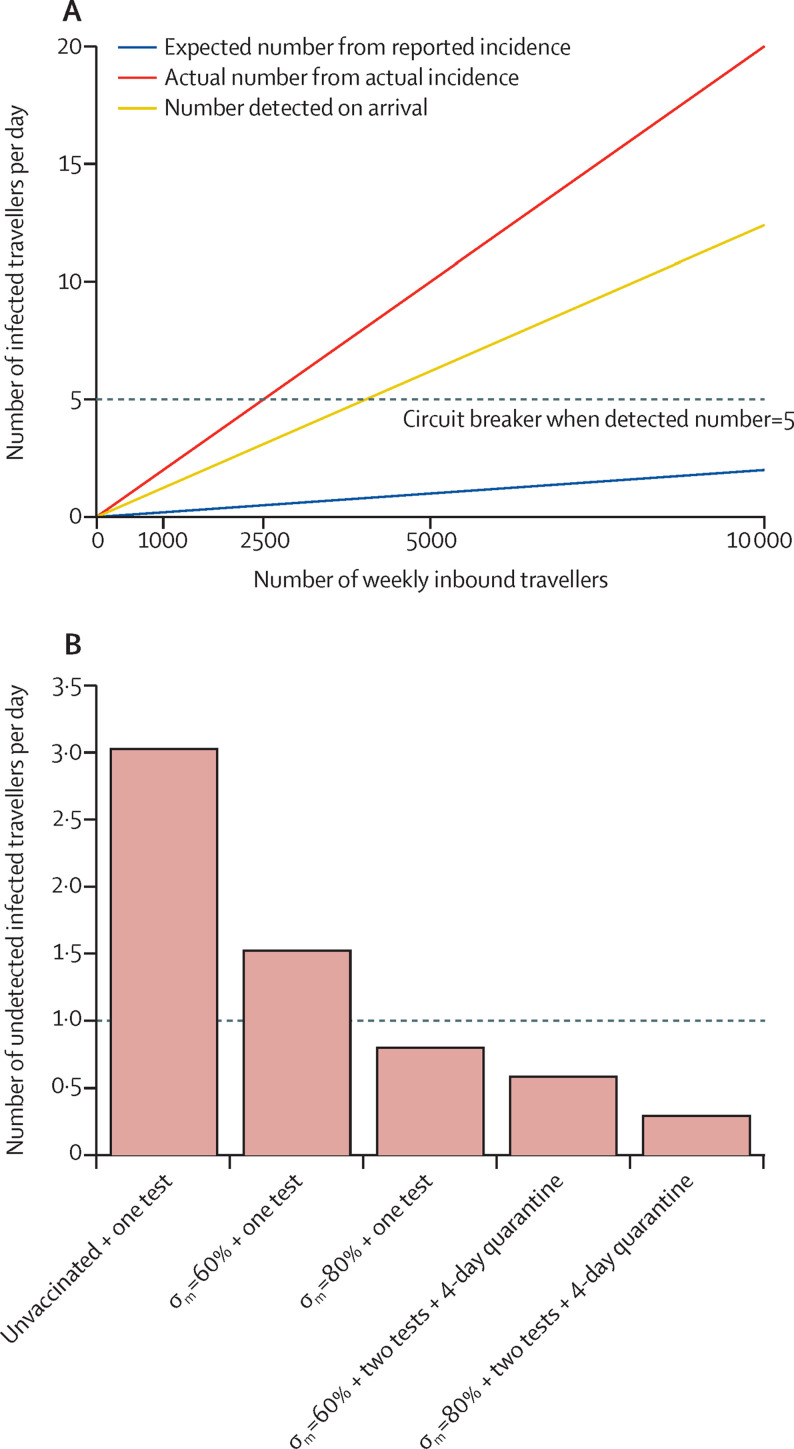
When travel restrictions are relaxed, a circuit breaker should be introduced as necessary on the basis of the reported daily incidence of the origins (figure 4 ). When the daily or weekly number of infections detected from the travellers exceeds the expected number estimated from reported incidence in the origin, travelling from the origin should be suspended, and the travel volumes from all origins should be re-evaluated, such that the total travel volume would not exceed the threshold determined by the actual incidence estimated from the detected number of infections at the border of the destination (appendix pp 5–6).
Figure 4.
An illustrative example of the circuit breaker
We assume in a hypothetical origin, the daily number of new infections is 100 per million but the reported daily number of new infections is only 10 per million (ie, the reporting rate is 10%). The actual and reported incidence is assumed to be relatively constant in the past 14 days, the vaccination coverage is unknown, and the PCR test result would be positive up to 28 days after infection. (A) The daily number of infected inbound travellers detected on arrival by the number of weekly inbound travellers. Under this scenario, if the number of weekly inbound travellers is 20 000, the expected daily number of infections detected on arrival should not exceed 1 if the daily number of new infections were really 10 per million (ie, 10 / 106 × 28 × 20 000 / 7 × 0·62=0·50), assuming 62% (range 60–65) of infections are detected at the border at the first PCR test. A circuit breaker would be triggered when the daily number of infections detected upon arrival exceeds 5, and, in this case, the actual daily number of infections among inbound travellers is 5/0·62=8·06 (red line). (B) The undetected number of infections per day by vaccine efficacy, testing frequency and quarantine duration at the point at which the circuit breaker is triggered. If all inbound travelers were fully vaccinated and tested twice after arrival, even if actual daily number of infections were 8·06 per day, the number of undetected infections per day would not exceed 1. From figure 2, the expected FOI would be reduced by 93% and 96% if an infected traveller is fully vaccinated, tested twice, and quarantined by at least 5 days (4 full days), for σm=0·60 and σm=0·80 respectively. The undetected FOI per day from this origin would be 5 / 0·62 × (1 – 0·93)=0·56 if σm=0·60 and 5 / 0·62 × (1 – 0·96)=0·32 if σm=0·80. The dashed line shows the threshold when the undetected number of infections per day is 1. σm denotes efficacy in reducing susceptibility to infection. FOI=force of infection.
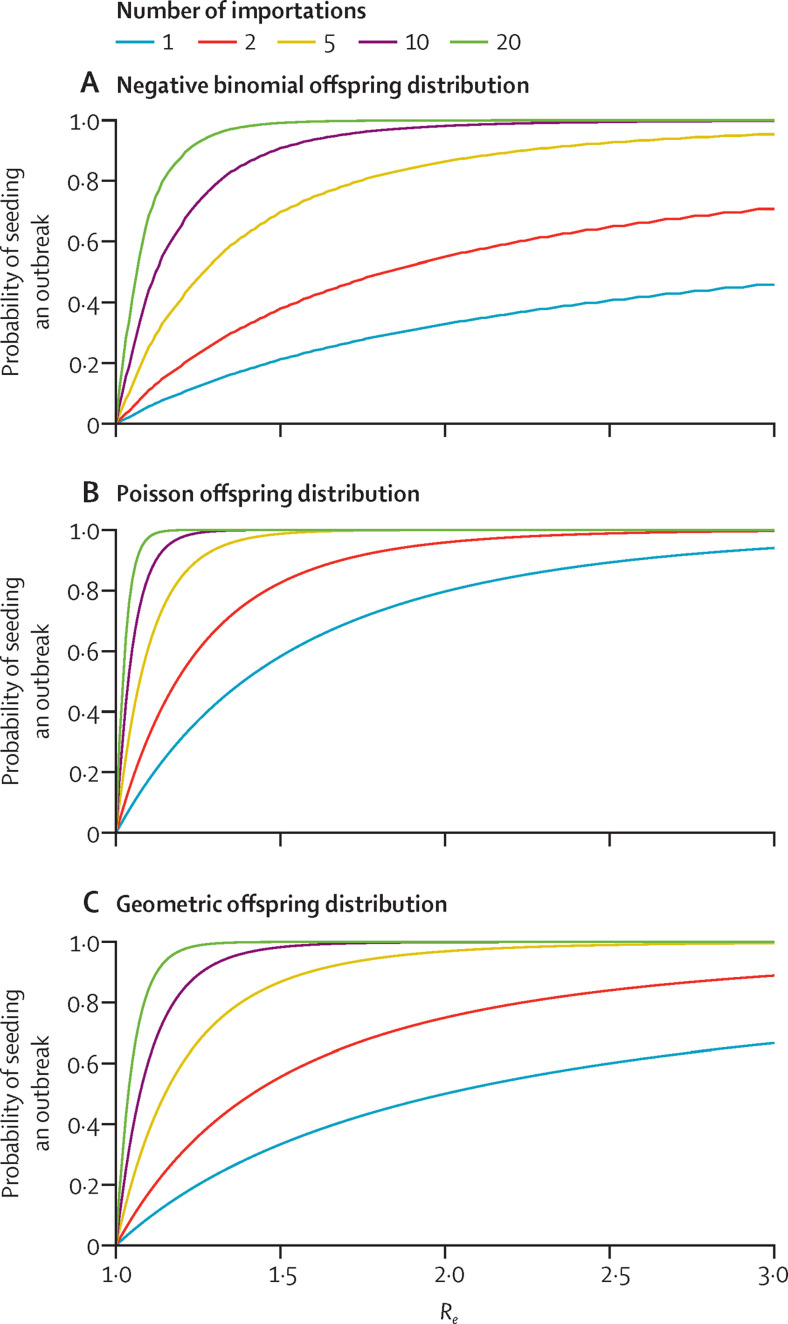
Early and premature relaxation of PHSMs, before sufficient population immunity is attained in the destination location, would lead to a pronounced subsequent wave of infection if imported infections undetected from the relaxation of travel restrictions seeded an established local outbreak (figure 5 ). At pre-vaccination R e=2·5, the probability of seeding a local outbreak is essentially 100% even if the number of imported infections is fewer than five.
Figure 5.
Probability of seeding a local COVID-19 outbreak in a completely susceptible destination as a function of the number of missed or undetected infections (coloured lines) among inbound travellers and the Re
The offspring distribution is assumed to be negative binomial with k=0·43 (A),25 Poisson (B), or geometric (C). Re=effective reproductive number.
Discussion
The findings of our modelling analysis suggest that effective vaccines that provide some level of transmission blocking offer a means of adjusting PHSMs without reintroducing a large wave of COVID-19-related admissions to hospital and deaths. However, complete relaxation of all PHSMs would require a very high coverage locally even with the most efficacious vaccines. This scenario might be difficult to achieve soon, given the uncertainties around how efficacious the vaccines are at reducing transmission, emergence of variants of concern with increased transmissibility and potential immune escapes, and the current shortage in vaccine supply globally.
Local control of the COVID-19 pandemic should, therefore, prioritise protecting the most vulnerable groups to reduce COVID-19 disease burden. PHSMs should be maintained to a certain level during the roll-out of vaccination programmes, such that the maximum daily admissions to hospital would not exceed the capacity of local health systems. Policy makers attempting to make adjustments to PHSMs could take reference from the experience of previous epidemic waves to identify the associations between introducing and lifting PHSMs and the time varying R e (eg, comparing R e and the COVID-19 Stringency Index).26 Public adherence to PHSMs should also be monitored by longitudinal surveys and real-time mobility data.27, 28 In the long term, with all the elderly and vulnerable individuals fully vaccinated, the COVID-19 disease burden will be substantially reduced and the impact of the virus will lessen, although it might continue to circulate and become endemic, and could still damage the health of younger patients who become infected.29
Local relaxation of PHSMs relies not only on vaccine characteristics but also vaccine uptake in the population. In practice, vaccine uptake can differ regionally and is particularly affected by socioeconomic factors. Vaccine uptake is also likely to vary over time as the perceived risks of the population vary. Given the finite supply of vaccines, it is important that uptake is prioritised among individuals who require protection against symptomatic disease, including the elderly and vulnerable groups with comorbidities. A complicated interaction is anticipated between the prevalence of COVID-19 disease, PHSM policy, PHSM adherence, and vaccine uptake; therefore, it is essential to understand the drivers of vaccine hesitancy and plan accordingly.30
Even if travel restrictions are eased to allow unobstructed travel between countries, testing and quarantine would have to be maintained for inbound travellers to minimise the risk of reintroducing a local outbreak until high vaccination coverages are attained locally and overseas in most countries. Travel restrictions can be adjusted dynamically, starting from restricting the number of inbound travellers and shortening the duration of quarantine for fully vaccinated travellers from places with low SARS-CoV-2 prevalence (appendix p 19). Restrictions can be further relaxed as vaccine coverage increases in both places of origin and destination. The duration of quarantine for vaccinated travellers might be further shortened, when rapid antibody tests are introduced and the associations are well established between antibody titres and reduction in onward transmission.
Since the case ascertainment probability might vary among different countries and regions, a circuit breaker should be introduced if an increasing number of infections are confirmed among inbound travellers. For example, assuming the risk tolerance level is 2.0 new local cases per million population for Hong Kong (appendix p 19), we estimate that the threshold proportion of vaccinated travellers for many countries in Africa is lower than 50% based on the reported incidence on April 27, 2021. However, the actual threshold proportion of vaccinated travellers from these countries should be set higher than 50%, because a recent study reported that cases of COVID-19 were under-reported in Africa and thus extra caution is required with these less relilable data.31
Our study has several limitations. First, we did not consider vaccine availability and the process of building up vaccine-induced immunity. Therefore, the scenarios we present are the most optimistic, but feasibility and constraints should be considered in practice. Second, as we discuss throughout, it is difficult to establish a mapping between R e and the specific PHSMs that should be maintained, and hence relaxation of PHSMs should be carefully planned and monitored, especially with regard to the variants of concern. The association between PHSMs and R e before the emergence of variants of concern can be estimated by linking R e from the previous waves and the COVID-19 Stringency Index,26 or inferred from mobility data,28 but more experimental and observation data are required to refine the model assumptions. Third, we expect that vaccine efficacy (σm, σt, and σs) varies with age and among risk groups. When more data of vaccine trials in specific groups are available, incorporating such heterogeneity into the future models will provide more robust estimations. Fourth, our model assumed that the Hong Kong population was completely susceptible to SARS-CoV-2 and that there was no virus circulating before the commencement of vaccination programmes as a case study. Given the high levels of virus circulation worldwide, the assumed R e should be maintained well below our estimates given in the appendix (pp 13–16) to avoid overloading the health-care system in regions with high local prevalence. More accurate estimation of the effects of vaccination programmes can be obtained by fitting the model to local epidemic situations and simulating vaccination roll-out with local data about efficacy and uptake. Furthermore, countries with lower capacity in the health-care systems should be more cautious when considering relaxation of PHSMs and travel restrictions. Fifth, we assumed the weighted SARS-CoV-2 prevalence for inbound travellers assuming each of them can be assigned an origin and applied the same vaccine efficacy (σm). The risk of imported SARS-CoV-2 infection might be underestimated if the traveller has a complex travel history with multiple stopovers as international travel reopens. Finally, we have not considered waning immunity derived from both natural infection and vaccination. Seasonal vaccination programmes might be required if immunity wanes, and SARS-CoV-2 becomes endemic in the long term.
In summary, efficacious vaccines with high uptake are essential to the control of the COVID-19 pandemic in the near future. However, the findings of our modelling analysis show that other PHSMs, such as intensive test-trace-and-isolate, moderate levels of physical distancing, and international travel restrictions, will be needed until high vaccination coverages are attained in most countries. Gradual relaxation of PHSMs should be carefully planned during the roll-out of vaccination programmes to minimise admissions to hospital and deaths related to COVID-19.
Data sharing
We collated all data for this analysis from publicly available data sources. All data included in the analyses are available in the main text or the appendix.
Declaration of interests
Acknowledgments
Acknowledgments
This work was supported by grants from the Health and Medical Research Fund (CID-HKU2 and COVID19F05) and General Research Fund (17110020), and a special grant of the InnoHK Programme from Innovation and Technology Commission of the Government of the Hong Kong Special Administrative Region. We thank Di Liu, Shirley Kwok, and Miky Wong from the School of Public Health at The University of Hong Kong (Hong Kong), and Chi-Kin Lam from the Laboratory of Data Discovery for Health (Hong Kong) for their technical support.
Editorial note: the Lancet Group takes a neutral position with respect to territorial claims in published maps and institutional affiliations.
Contributors
KL, JTW, and GML designed the study, developed the model, analysed the data, interpreted the results, and wrote the manuscript. KL and JTW accessed and verified the study data. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
KL reports grants from the Food and Health Bureau of The Government and University Grant Council of The Government of Hong Kong Special Administrative Region, during the conduct of the study. JTW and GML declare no competing interests.
Supplementary Material
References
- 1.International Monetary Fund Policy support and vaccines expected to lift activity. 2021. https://www.imf.org/en/Publications/WEO/Issues/2021/01/26/2021-world-economic-outlook-update
- 2.Peiris M, Leung GM. What can we expect from first-generation COVID-19 vaccines? Lancet. 2020;396:1467–1469. doi: 10.1016/S0140-6736(20)31976-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garcia-Beltran WF, Lam EC, Denis KS. Multiple SARS-CoV-2 variants escape neutralization by vaccine-induced humoral immunity. Cell. 2021;184:2372–2383. doi: 10.1016/j.cell.2021.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson SC, Mulberry N, Edwards AM. How much leeway is there to relax COVID-19 control measures? Epidemics. 2021;35 doi: 10.1016/j.epidem.2021.100453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu JT, Leung K, Bushman M. Estimating clinical severity of COVID-19 from the transmission dynamics in Wuhan, China. Nat Med. 2020;26:506–510. doi: 10.1038/s41591-020-0822-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu JT, Mei S, Luo S. A global assessment of the impact of school closure in reducing COVID-19 spread. Res Square. 2020 doi: 10.21203/rs.3.rs-53593/v1. published online Aug 6. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Poustchi H, Darvishian M, Mohammadi Z. SARS-CoV-2 antibody seroprevalence in the general population and high-risk occupational groups across 18 cities in Iran: a population-based cross-sectional study. Lancet Infect Dis. 2021;21:473–481. doi: 10.1016/S1473-3099(20)30858-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bajema KL, Wiegand RE, Cuffe K. Estimated SARS-CoV-2 seroprevalence in the US as of September 2020. JAMA Intern Med. 2021;181:450–460. doi: 10.1001/jamainternmed.2020.7976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davies NG, Barnard RC, Jarvis CI. Association of tiered restrictions and a second lockdown with COVID-19 deaths and hospital admissions in England: a modelling study. Lancet Infect Dis. 2021;21:482–492. doi: 10.1016/S1473-3099(20)30984-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Monod M, Blenkinsop A, Xi X. Age groups that sustain resurging COVID-19 epidemics in the United States. Science. 2021;371 doi: 10.1126/science.abe8372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mistry D, Litvinova M, y Piontti AP. Inferring high-resolution human mixing patterns for disease modeling. Nature Comm. 2021;12:1–2. doi: 10.1038/s41467-020-20544-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Voysey M, Clemens SAC, Madhi SA. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bernal JL, Andrews N, Gower C. Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca vaccines on covid-19 related symptoms, hospital admissions, and mortality in older adults in England: test negative case-control study. BMJ. 2021;373 doi: 10.1136/bmj.n1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haas EJ, Angulo FJ, McLaughlin JM. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet. 2021;397:1819–1829. doi: 10.1016/S0140-6736(21)00947-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thompson MG, Burgess JL, Naleway A. Prevention and attenuation of COVID-19 by BNT162b2 and mRNA-1273 vaccines. medRxiv. 2021 doi: 10.1101/2021.06.01.21257987. published online June 9. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bertollini R, Chemaitelly H, Yassine HM, Al-Thani MH, Al-Khal A, Abu-Raddad LJ. Associations of vaccination and of prior infection with positive PCR test results for SARS-CoV-2 in airline passengers arriving in Qatar. JAMA. 2021;326:185–188. doi: 10.1001/jama.2021.9970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bubar KM, Reinholt K, Kissler SM. Model-informed COVID-19 vaccine prioritization strategies by age and serostatus. Science. 2021;371:916–921. doi: 10.1126/science.abe6959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moore S, Hill EM, Tildesley MJ, Dyson L, Keeling MJ. Vaccinationand non-pharmaceutical interventions for COVID-19: a mathematical modelling study. Lancet Infect Dis. 2021;21:793–802. doi: 10.1016/S1473-3099(21)00143-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.UK Government COVID-19 in the UK: healthcare in United Kingdom. 2021. https://www.england.nhs.uk/statistics/wp-content/uploads/sites/2/2021/06/COVID-19-daily-announced-vaccinations-07-June-2021.xlsx
- 20.Leung K, Shum MH, Leung GM, Lam TT, Wu JT. Earlytransmissibility assessment of the N501Y mutant strains of SARS-CoV-2 in the United Kingdom, October to November 2020. Euro Surveill. 2021;26 doi: 10.2807/1560-7917.ES.2020.26.1.2002106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davies NG, Abbott S, Barnard RC. Estimated transmissibility and impact of SARS-CoV-2 lineage B.1.1.7 in England. Science. 2021;372 doi: 10.1126/science.abg3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keeling MJ. University of Warwick: Estimating the transmission advantage for Delta variant (B.1.617.2), 3 June 2021. June 14, 2021. https://www.gov.uk/government/publications/university-of-warwick-estimating-the-transmission-advantage-for-delta-variant-b16172-3-june-2021
- 23.Centre for Health Protection, The Government of Hong Kong Special Administrative Region COVID-19 thematic website. 2020. https://www.chp.gov.hk/files/pdf/local_situation_covid19_en.pdf
- 24.Dickens BL, Koo JR, Lim JT. Determining quarantine length and testing frequency for international border opening. J Travel Med. 2021 doi: 10.1093/jtm/taab088. published online June 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adam D, Wu P, Wong J. Clustering and superspreading potential of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections in Hong Kong. Nat Med. 2020;26:1714–1719. doi: 10.1038/s41591-020-1092-0. [DOI] [PubMed] [Google Scholar]
- 26.Li Y, Campbell H, Kulkarni D. The temporal association of introducing and lifting non-pharmaceutical interventions with the time-varying reproduction number (R) of SARS-CoV-2: a modelling study across 131 countries. Lancet Infect Dis. 2021;21:193–202. doi: 10.1016/S1473-3099(20)30785-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhanwei D, Lin W, Songwei S. Pandemic fatigue impedes mitigation of COVID-19 in Hong Kong. Res Square. 2021 doi: 10.21203/rs.3.rs-591241/v1. published online June 10. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leung K, Wu JT, Leung GM. Real-time tracking and prediction of COVID-19 infection using digital proxies of population mobility and mixing. Nat Commun. 2021;12 doi: 10.1038/s41467-021-21776-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lavine JS, Bjornstad ON, Antia R. Immunological characteristics govern the transition of COVID-19 to endemicity. Science. 2021;371:741–745. doi: 10.1126/science.abe6522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Loomba S, de Figueiredo A, Piatek SJ, de Graaf K, Larson HJ. Measuring the impact of COVID-19 vaccine misinformation on vaccination intent in the UK and USA. Nat Hum Behav. 2021;5:337–348. doi: 10.1038/s41562-021-01056-1. [DOI] [PubMed] [Google Scholar]
- 31.Mwananyanda L, Gill CJ, MacLeod W. Covid-19 deaths in Africa: prospective systematic postmortem surveillance study. BMJ. 2021;372:n334. doi: 10.1136/bmj.n334. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
We collated all data for this analysis from publicly available data sources. All data included in the analyses are available in the main text or the appendix.