Abstract
Recent decades have seen a dramatic rise in the prevalence of obesity. While genetic factors can influence obesity, environmental factors and lifestyle may play important roles as well. Sleep can be regarded as one of these factors. This study aimed to examine sleep duration, as a potential risk factor for obesity in an Iranian population. In this cross-sectional study, the Fasa PERSIAN cohort study data was used and 10,136 subjects aged 35–70 were entered. Anthropometrics indices have been measured and the total body fat percentage (BFP) was obtained by Bio-Impedance Analysis. Also, physical activity and dietary intake have been recorded. Sleep duration was obtained and individuals categorized into two groups of “< 8” and “≥ 8” h of sleep. The mean age and sleep duration of the participants were 48.63 ± 9.57 years and 6.92 ± 1.62 h in the total population, respectively. All of the anthropometric indices were significantly higher in the “< 8 h of sleep” group than in the “≥ 8 h of sleep” group. Regarding BFP and fat mass index (FMI) the same results was seen (p-value < 0.05). Body mass index (BMI), Waist and hip circumferences (WC, HC), and waist-to-height ratio (WHtR) were in a significant negative association with night time sleep (p-value < 0.001), while these associations with daytime napping were positive (p-value < 0.001). After multi-variable adjusting, BMI, WC, HC, WHtR, and wrist circumference showed significant negative associations with 24-h sleep duration (p-value < 0.05). This study established the association between nocturnal, daytime napping, 24-h sleep duration and obesity parameters. Daytime napping was positively associated with obesity parameters and short 24-h sleep duration was associated with higher risk of overweight/obesity. These results indicate that insufficient sleep can be a screening indicator for an unhealthy lifestyle and poor health outcomes.
Subject terms: Epidemiology, Obesity
Introduction
A significant increase has occurred in the prevalence of obesity in recent decades, and the World Health Organization (WHO) has named obesity as a global epidemic1. Obesity is associated with serious consequences such as the increased risk of diabetes, cardiovascular disease, arthritis, and cancer2. Although obesity can be affected by genetic factors, environmental factors, and lifestyle can also play significant roles. Sleep can be considered as one of the factors which may affect obesity3.
Chronic sleep deprivation has increased dramatically in the last half-century, as the average night-time sleep has decreased by 1.5 to 2 h. Today, more than 30% of employed US civilian adults sleep less than 6 h overnights4. Sleep can also change the overall energy balance in the body, increasing energy intake levels. Previous studies have shown that both acute sleep deprivation and chronic partial sleep deprivation can decrease serum leptin levels that leads to obesity5. As a strong appetite stimulant, ghrelin is higher in people who sleep less. Besides, short sleep duration may lead to obesity due to increased eating time6. Sleep deprivation can also cause fatigue in individuals, which may decrease physical activity levels7,8 and change in the energy intake and consumption (such as a physical activity)3. According to the aforementioned mechanisms and relationships, sleep deprivation may lead to weight gain and obesity.
Several previous study in different regions suggested the possible association of sleep duration and obesity in Japan9, Taiwan10 and the US11,12. But the factors that influence an individual's sleep length can differ across cultures and countries13,14. The relationship between sleep characteristics and health risks can be altered as a result of these variations. For example, the sleep duration of adults in the U.S. decreased more compared to adults in Finland in the past decade15. Similar studies in Iranian populations have yet to be conducted, and it would be particularly interesting to see whether the previous results could be replicated in a large Iranian community. Because many studies have shown inconsistent findings, there is still a knowledge gap due to ambiguity and a lack of research into the relationship between sleep duration and obesity. Also, this study would be the first study to investigate the relationship between sleep and obesity in an Iranian population using information related to anthropometric/body composition data.
Given the importance of adequate sleep-in individuals' physical and mental health, as well as the above-mentioned obesity-induced problems, this study aimed to examine sleep duration, as a potential risk factor for obesity, in the target population under controlling of the sleep/obesity factors.
Methods
Population
This sub-analysis used the data provided by the Fasa Cohort Study16. The Fasa Cohort Study is a part of the longitudinal PERSIAN Cohort Study designed to assess the risk factors for developing non-communicable diseases among the residents of a rural area, known as Sheshdeh (located in Fasa, Iran) with a total population of 41,000. The target population consisted of the residents of Sheshdeh in the 35–70 years-old age group (11,097 individuals). The data were collected from 2015 to 2016, and the participants were asked to sign a written informed consent form. In total, 986 individuals were excluded because of incomplete sleep and anthropometric information, history of chronic diseases such as hypothyroidism, hyperthyroidism, renal failure, cancer or sleep apnea disorder, consumption of medicines resulting in weight gain, and regular consumption of hypnotic sedative drugs. A total of 10,111 individuals (5539 women and 4572 men) were finally enrolled.
Demographic data and anthropometrics measurement
Demographic data including age and gender were recorded in the questionnaire, which also cardiovascular diseases (CVD) history such as coronary heart diseases, myocardial infarction, and stroke. Each subject followed the protocol mentioned in reference 16. The participants’ heights and weights were measured and recorded by a trained person using a stadiometer with an accuracy of 0.1 cm (SECA 222 Stadiometer, Germany) and a calibrated digital scale with an accuracy of 0.1 kg (SECA 888 Digital Scale, Germany), respectively. The participants’ wrist (WrC), hip (HC), and waist circumference (WC) were measured using a special tape with an accuracy of 0.1 cm immediately after heights and weights measurement. Wrc was measured by asking subjects to keep their wrist anterior surface up; the tape measure's superior border was positioned just distal to the radial and ulnar bone prominences. WC was measured in the thinnest part of the waist between the tenth rib and the iliac crest. The maximum HC was also measured in the standing position.
All measurements were done without any clothes in bare foot condition. Also, waist to hip (WHR), and waist to height ratios (WHtR) were calculated by dividing the waist to hip and height in cm respectively.
Socioeconomic status index
A principal component analysis (PCA) method was gen to generate a socioeconomic status (SES) index of respondents in the PERSIAN Cohort Study17. The dataset's available data on infrastructure facilities (source of drinking water, sanitation facility), housing condition (e.g., number of rooms, type of home ownership), and ownership of a variety of durable assets (e.g., dishwasher, vehicle, television), as well as education level, were used to create the SES variable for each participant.
Body composition
Bioelectric impedance analysis (BIA) was used for measuring body composition using the Tanita BC-418 MA Segmental Body Composition Analyzer (Tanita, Japan). This is a single-frequency BIA device with eight polar electrodes and a single-point load cell weighing system in the scale platform that can provide separate body mass readings for various body segments including the right arm, left arm, trunk, right leg, and left leg. The impedance across the subject's tissues is determined with receiver electrodes after a predefined signal is passed through injector electrodes. All measurements are performed at 50 kHz with a steady current of 0.8 mA sine wave. FM percent is calculated using an algorithm that takes into account impedance, age, and height. The amount of fat mass, fat-free mass, and fat percentage in hands, trunk, legs, and the total body were reported separately. Also, the fat mass index (FMI) was calculated by dividing fat mass by the square of height in meters18. Among all individuals in the Fasa PERSIAN Cohort study, only 4661 subjects had the BIA body composition data which all of them was used in this study.
Sleep duration
Using the first two questions Pittsburgh Sleep Quality questionnaire19 (When do you usually go to bed at night? “___” and When do you usually wake up in the morning? “___”) the trained person collected information about the participants’ sleep habits on the workdays. Moreover, sleep latency has been questioned from subjects and subsided from time in bed of subjects for having a better estimation of sleep duration. The same items were questioned about the weekends and sleep duration was calculated in hours by subtracting wake-up time from bedtime in both workdays and weekends. A mean of night sleep duration per day was calculated according to the participants answers. Also, regarding the napping time, the duration of the daytime napping was asked in hour and min in both workdays and weekends and an average of napping time was calculated. Finally, by adding average night sleep duration and average napping time, sleep duration per 24 h was calculated. According to the National Sleep Foundation, sleeping less than 8 h is considered insufficient20.
Food frequency questionnaire (FFQ)
Nutritional information was measured using the modified FFQ, which examines the eating habits of individuals over a year. The participants were asked to report their food consumption program on a daily, weekly, monthly, or annual basis over the last year. The consumed foods were converted from household measures to grams. This modified FFQ included food items which was designed based on the information provided by experienced nutritionists familiar with the local diet of Iran. For the most part, the USDA food composition table (FCT) was used (USDA, Release 11, 1994). The Iranian food composition table was consulted for some products such as bread, vetch, pepper green, wild plum, mint, sweet canned cherry, and sour cherry21. This population-specific FFQ validation has been studied previously22. Finally, the total energy intake per day was calculated and reported.
Physical activity (PA)
The International Physical Activity Questionnaire (IPAQ) was used to calculate physical activity levels. This 20-item questionnaire can measure routine physical activities of rural Iranians. The amount of each activity in hours and minutes was determined; the MET-value of each activity was multiplied by its duration, and total MET score was calculated.
Statistics
All variables are reported as mean ± standard deviation, number (percentage). For comparison between two groups, the independent-samples t-test was used and for categorical variables, the chi-square test was performed. In 3 linear regression models, we performed our final analysis to minimize the effect of confounding variables including age, psychical activity, and energy intake. In model 1 without any variables, in model 2 with age, cardiovascular diseases(CVD) history23, socio-economic index, in model 3 with age, CVD history, socio-economic index and physical activity (MET score), dietary intake (energy intake Kcal/day) we adjusted our results. For calculating the odds ratio (OR) and 95% Confidence Interval (CI), logistic regression was used. Categorization of anthropometric and body composition variables to binary outcomes was done using previously published cut-off points24–26 and above adjustment method. In another linear regression analysis night and daytime sleep duration were regressed on anthropometrics and body fat indices. Also, our analysis was stratified by gender due to physiological different and different patterns of sleep. A significance level of p-value < 0.05 was considered, and all analyses were performed using IBM SPSS Statistics, version 23 (IBM Corp., Armonk, N.Y., USA). For forest plot graphs, Prism version 8.00 (GraphPad Software, La Jolla, California, USA) was used.
Ethical statement
The study protocol was following the Helsinki Declaration and confirmed by the Ethics Committee of Fasa University of Medical Sciences (Approval Code: IR.FUMS.REC.1398.009). The participants were informed about the research objectives and the written informed consent was obtained from the subjects before starting the survey.
Results
In a total of 10,111 subjects, the mean age of the participants was 48.61 ± 9.61 years for men and 48.64 ± 9.54 years for women; there was no significant difference between men and women in this regard (p = 0.841). The mean duration of sleep for men and women was 6.82 ± 1.65 and 6.97 ± 1.59 h, respectively, with a significant difference (p < 0.001). The participants were divided into two groups of “< 8 h of sleep” and “≥ 8 h of sleep” which included 6817 persons (67.5%) and 3294 persons (32.5%), respectively. The means of age in the “< 8 h of sleep” group were significantly higher in both genders. Moreover, SES of subjects with under 8 h of sleep was significantly higher than the other group.
In both genders, anthropometric indices, including BMI, WC, HC, WrC, Waist to hip ratio (WHR), and Waist to height ratio (WHtR) were significantly higher in the “< 8 h of sleep” group (p < 0.05). Table 1 presents the mean ± SD of demographic variables, anthropometric indices and body composition data according to gender. Moreover, Table S1 represent the means of anthropometric and body composition data by a different strata of sleep duration. Mostly, subjects with 4–6 h of sleep duration had the highest mean of anthropometric value among other groups.
Table 1.
The comparison of anthropometric and body composition data between sleep groups according to gender.
| Variables | Male | Female | ||||
|---|---|---|---|---|---|---|
| Sleep duration | p-value | Sleep duration | p-value | |||
| Under 8 h | 8 h and more | Under 8 h | 8 h and more | |||
| Sample size | 3163 | 1409 | 3651 | 1888 | ||
| Age (year) | 48.8 ± 9.5 | 48.1 ± 9.9 | 0.024 | 49.2 ± 9.4 | 47.6 ± 9.7 | < 0.001 |
| Socio-economic index | 0.724 ± 2.499 | 0.17 ± 2.266 | < 0.001 | − 0.363 ± 1.716 | − 0.646 ± 1.571 | < 0.001 |
| MET score | 45.46 ± 14.53 | 44.61 ± 13.85 | 0.067 | 39.15 ± 6.65 | 37.04 ± 6.64 | < 0.001 |
| Energy (kcal/day) | 3627.6 ± 1246.9 | 3594.3 ± 1216.6 | 0.401 | 2800.7 ± 900 | 2837.6 ± 908.5 | 0.149 |
| BMI (kg/m2) | 24.42 ± 4.45 | 23.69 ± 4.26 | < 0.001 | 27.05 ± 4.83 | 26.49 ± 4.75 | < 0.001 |
| WC (cm) | 89.97 ± 11.31 | 88.49 ± 10.89 | < 0.001 | 96.68 ± 11.45 | 95.15 ± 11.50 | < 0.001 |
| HC (cm) | 97.89 ± 7.72 | 96.74 ± 7.52 | < 0.001 | 101.56 ± 9.41 | 100.71 ± 9.42 | 0.001 |
| WrC (cm) | 17.34 ± 1.24 | 17.14 ± 1.20 | < 0.001 | 16.30 ± 1.27 | 16.20 ± 1.25 | 0.004 |
| WHR | 0.917 ± 0.65 | 0.912 ± 0.62 | 0.040 | 0.95 ± 0.061 | 0.94 ± 0.062 | < 0.001 |
| WHtR | 0.53 ± 0.66 | 0.52 ± 0.64 | < 0.001 | 0.62 ± 0.074 | 0.61 ± 0.075 | < 0.001 |
| Sample size | 1528 | 627 | 1676 | 830 | ||
|---|---|---|---|---|---|---|
| Arms | ||||||
| Fat mass (kg) | 1.47 ± 0.78 | 1.35 ± 0.70 | 0.001 | 2.60 ± 1.24 | 2.47 ± 1.20 | 0.009 |
| Fat-free mass (kg) | 6.21 ± 1.17 | 6.01 ± 1.06 | < 0.001 | 4.31 ± 0.60 | 4.28 ± 0.62 | 0.145 |
| Fat mass (%) | 36.31 ± 11.52 | 34.73 ± 11.60 | 0.004 | 71.35 ± 17.71 | 69.21 ± 17.79 | 0.005 |
| Legs | ||||||
| Fat mass (kg) | 4.13 ± 2.07 | 3.81 ± 1.99 | 0.001 | 9.88 ± 2.82 | 9.57 ± 2.75 | 0.008 |
| Fat-free mass (kg) | 18.79 ± 2.85 | 18.39 ± 2.57 | 0.003 | 14.11 ± 1.81 | 13.99 ± 1.76 | 0.136 |
| Fat mass (%) | 34.36 ± 11.53 | 32.57 ± 12.01 | 0.001 | 81.00 ± 9.89 | 79.81 ± 9.85 | 0.005 |
| Trunk | ||||||
| Fat mass (kg) | 9.09 ± 4.73 | 8.38 ± 4.47 | 0.001 | 10.97 ± 4.52 | 10.39 ± 4.35 | 0.002 |
| Fat-free mass (kg) | 30.22 ± 4.00 | 29.48 ± 3.81 | < 0.001 | 24.27 ± 2.56 | 24.14 ± 2.58 | 0.227 |
| Fat mass (%) | 21.90 ± 8.38 | 20.93 ± 8.43 | 0.015 | 29.92 ± 8.11 | 28.87 ± 8.22 | 0.002 |
| Total | ||||||
| Fat mass (kg) | 14.66 ± 7.50 | 13.50 ± 7.06 | 0.001 | 23.33 ± 8.43 | 22.40 ± 4.84 | 0.003 |
| Fat-free mass (kg) | 55.23 ± 7.82 | 53.89 ± 7.20 | < 0.001 | 42.68 ± 4.84 | 42.40 ± 4.84 | 0.166 |
| Fat mass (%) | 19.91 ± 7.14 | 18.98 ± 7.19 | 0.006 | 34.37 ± 6.80 | 33.50 ± 6.86 | 0.003 |
| FMI (kg/m2) | 5.12 ± 2.57 | 4.72 ± 2.46 | 0.001 | 9.67 ± 3.37 | 9.25 ± 3.32 | 0.004 |
BMI body mass index, WC waist circumferences, HC hip circumferences, WrC wrist circumferences, WHR waist to hip ratio, WHtR waist to height ratio, FMI fat mass index.
Bold values are less than 0.05 and are statistically significant.
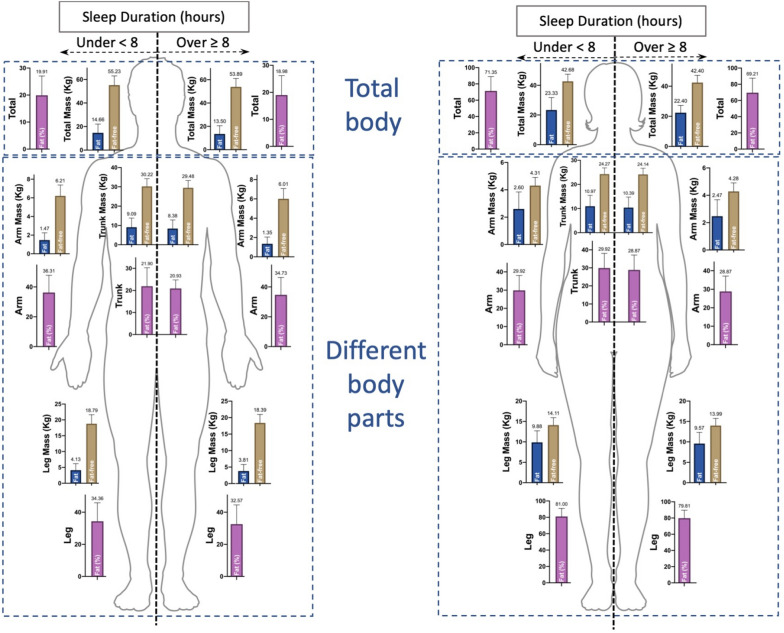
Body composition indices of arms, legs, trunk, and the whole body in the two sleep groups are compared in Table 1. All these indices were higher in the insufficient sleep group. In both genders, the amount of fat (kg and percentage) was significantly higher in the insufficient sleep group (p < 0.01). There was a significant difference between men in the two groups in terms of the amount of fat-free mass (p < 0.001); however, no significant difference was observed in women in this respect (p > 0.05). Besides, the FMI was significantly higher in men and women in the " < 8 h of sleep" group than in those in the " ≥ 8 h of sleep" group (p < 0.01). Figure 1 shows a schematic representation of the body composition data of the results of Table 1.
Figure 1.
The schematic graphs of body fat composition data in total and different body parts in both gender.
In Table 2, the linear association of the anthropometric data and fat mass index with night and day sleep hour duration as continuous variables in both genders is reported. All of the standardized beta coefficient of the association of each anthropometric/body composition variable and night sleep duration was negative, while nap time coefficients were positive. The highest positive coefficients were related to BMI, HC, WC and nap time (0.111, p-value < 0.001) in males and BMI and nap time (0.111, p-value < 0.001) in females. Also, the highest negative coefficients were related BMI and night sleep (− 0.074, p-value < 0.001) in male, WHtR and night sleep (− 0.067, p-value < 0.001) in females. Also, the linear, quadratic and cubic association of anthropometric and body composition data with sleep hours according to gender has been reported in Table S2. BMI and HC have shown slight negative significant beta coefficients in the quadratic model in both genders. In the cubic models, WC and WHtR were in significant associations with sleep duration in both genders (p-value < 0.05). However, some of the beta coefficients such as WHtR in both genders were too small (b = 0.001).
Table 2.
Linear regression model of anthropometric data, body fat percentage, and fat mass index with night and daytime sleep hour duration as continuous variables in both gender.
| Dependent variable | Independent variable | Male | Female | ||||||
|---|---|---|---|---|---|---|---|---|---|
| B | SE (B) | Beta | p-value | B | SE (B) | Beta | p-value | ||
| BMI (kg/m2) | (Constant) | 25.093 | 0.284 | < 0.001 | 27.298 | 0.292 | < 0.001 | ||
| Night sleep | − 0.003 | 0.001 | − 0.074 | < 0.001 | − 0.002 | 0.001 | − 0.043 | 0.001 | |
| Daytime sleep | 0.009 | 0.001 | 0.111 | < 0.001 | 0.010 | 0.001 | 0.108 | < 0.001 | |
| WC (cm) | (Constant) | 91.008 | 0.721 | < 0.001 | 98.293 | 0.698 | < 0.001 | ||
| Night sleep | − 0.007 | 0.002 | − 0.057 | < 0.001 | − 0.007 | 0.002 | − 0.057 | < 0.001 | |
| Daytime sleep | 0.023 | 0.003 | 0.111 | < 0.001 | 0.016 | 0.003 | 0.072 | < 0.001 | |
| HC (cm) | (Constant) | 98.817 | 0.494 | < 0.001 | 101.728 | 0.572 | < 0.001 | ||
| Night sleep | − 0.005 | 0.001 | − 0.066 | < 0.001 | − 0.003 | 0.001 | − 0.032 | 0.018 | |
| Daytime sleep | 0.015 | 0.002 | 0.111 | < 0.001 | 0.018 | 0.002 | 0.098 | < 0.001 | |
| WrC (cm) | (Constant) | 17.532 | 0.080 | < 0.001 | 16.264 | 0.077 | < 0.001 | ||
| Night sleep | − 0.001 | 0.000 | − 0.068 | < 0.001 | 0.000 | 0.000 | − 0.015 | 0.251 | |
| Daytime sleep | 0.002 | 0.000 | 0.083 | < 0.001 | 0.002 | 0.000 | 0.082 | < 0.001 | |
| WHR | (Constant) | 0.919 | 0.004 | < 0.001 | 0.966 | 0.004 | < 0.001 | ||
| Night sleep | 0.000 | 0.000 | − 0.027 | 0.068 | 0.000 | 0.000 | − 0.060 | < 0.001 | |
| Daytime sleep | 0.000 | 0.000 | 0.071 | < 0.001 | 0.000 | 0.000 | − 0.008 | 0.571 | |
| WHtR | (Constant) | 0.539 | 0.004 | < 0.001 | 0.636 | 0.005 | < 0.001 | ||
| Night sleep | 0.000 | 0.000 | − 0.056 | < 0.001 | − 0.000 | 0.000 | − 0.067 | < 0.001 | |
| Daytime sleep | 0.000 | 0.000 | 0.099 | < 0.001 | 0.000 | 0.000 | 0.061 | < 0.001 | |
| Body fat (%) | (Constant) | 51.589 | 0.843 | < 0.001 | 48.371 | 0.760 | < 0.001 | ||
| Night sleep | − 0.005 | 0.002 | − 0.051 | 0.018 | − 0.001 | 0.002 | − 0.010 | 0.603 | |
| Day sleep | 0.010 | 0.003 | 0.059 | 0.005 | 0.011 | 0.003 | 0.068 | 0.001 | |
| FMI (kg/m2) | (Constant) | 6.699 | 0.303 | < 0.001 | 8.347 | 0.334 | < 0.001 | ||
| Night sleep | 0.000 | 0.001 | − 0.009 | 0.675 | − 0.001 | 0.001 | − 0.020 | 0.326 | |
| Daytime sleep | − 0.002 | 0.001 | − 0.028 | 0.196 | 0.001 | 0.001 | 0.017 | 0.413 | |
Beta: Standardized regression coefficients (calculated as bi × SXi/SY). BMI body mass index, WC waist circumferences, HC hip circumferences, WrC wrist circumferences, WHR waist to hip ratio, WHtR waist to height ratio, FMI fat mass index.
Bold values are less than 0.05 and are statistically significant.
The relationships of sleep duration with obesity parameters are shown in Table 3. In both genders, all variables of anthropometrics and body composition such as body fat percentage and FMI were inversely correlated with hours of sleep, and all had a statistically significant relationship except for the variables WHR and FMI in men, and WHR, BFP, and FMI in women. Among the results of the model 3, the highest significant beta coefficient among anthropometrics and body composition variables were related to BMI (b = − 0.059) in men and WHtR (b = − 0.064) in women. In men, BFP was the second place with (b = − 0.056). In women, HC and WC both with (b = − 0.062) were the second and third places.
Table 3.
The linear regression models of anthropometric data, body fat percentage and fat mass index with 24-h sleep duration as a continuous variable in both gender.
| Model | Dependent variable | Independent variable: | Male | Female | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Unstandardized coefficients | Standardized coefficients | p-value | Unstandardized coefficients | Standardized coefficients | p-value | |||||
| Sleep duration | B | Std. error | Beta | B | Std. error | Beta | ||||
| Model 1 | BMI (kg/m2) | − 0.205 | 0.04 | − 0.077 | < 0.001 | − 0.102 | 0.041 | − 0.034 | 0.012 | |
| Model 2 | − 0.166 | 0.039 | − 0.062 | < 0.001 | − 0.102 | 0.041 | − 0.034 | 0.012 | ||
| Model 3 | − 0.157 | 0.038 | − 0.059 | < 0.001 | − 0.182 | 0.041 | − 0.060 | < 0.001 | ||
| Model 1 | WC (cm) | − 0.406 | 0.100 | − 0.060 | < 0.001 | − 0.369 | 0.097 | − 0.051 | < 0.001 | |
| Model 2 | − 0.283 | 0.098 | − 0.042 | 0.004 | − 0.250 | 0.098 | − 0.035 | 0.010 | ||
| Model 3 | − 0.263 | 0.097 | − 0.039 | 0.007 | − 0.446 | 0.099 | − 0.062 | < 0.001 | ||
| Model 1 | HC (cm) | − 0.316 | 0.069 | − 0.068 | < 0.001 | − 0.136 | 0.080 | − 0.023 | 0.088 | |
| Model 2 | − 0.258 | 0.068 | − 0.055 | < 0.001 | − 0.221 | 0.079 | − 0.037 | 0.005 | ||
| Model 3 | − 0.239 | 0.067 | − 0.051 | < 0.001 | − 0.368 | 0.080 | − 0.062 | < 0.001 | ||
| Model 1 | WrC (cm) | − 0.052 | 0.011 | − 0.070 | < 0.001 | − 0.007 | 0.011 | − 0.008 | 0.545 | |
| Model 2 | − 0.042 | 0.011 | − 0.057 | < 0.001 | − 0.008 | 0.011 | − 0.010 | 0.444 | ||
| Model 3 | − 0.038 | 0.011 | − 0.051 | < 0.001 | − 0.023 | 0.011 | − 0.029 | 0.035 | ||
| Model 1 | WHR | − 0.001 | 0.001 | − 0.028 | 0.054 | − 0.002 | 0.001 | − 0.061 | < 0.001 | |
| Model 2 | 0.000 | 0.001 | − 0.010 | 0.479 | 0.000 | 0.000 | − 0.010 | 0.456 | ||
| Model 3 | 0.000 | 0.001 | − 0.009 | 0.512 | − 0.001 | 0.001 | − 0.024 | 0.065 | ||
| Model 1 | WHtR | − 0.002 | 0.001 | − 0.058 | < 0.001 | − 0.003 | 0.001 | − 0.062 | < 0.001 | |
| Model 2 | − 0.002 | 0.001 | − 0.040 | 0.005 | − 0.002 | 0.001 | − 0.037 | 0.006 | ||
| Model 3 | − 0.002 | 0.001 | − 0.039 | 0.006 | − 0.003 | 0.001 | − 0.064 | < 0.001 | ||
| Model 1 | Body fat (%) | − 0.287 | 0.118 | − 0.052 | 0.016 | − 0.017 | 0.107 | − 0.003 | 0.876 | |
| Model 2 | − 0.308 | 0.119 | − 0.056 | 0.009 | − 0.145 | 0.108 | − 0.027 | 0.181 | ||
| Model 3 | − 0.310 | 0.119 | − 0.056 | 0.009 | − 0.161 | 0.112 | − 0.030 | 0.148 | ||
| Model 1 | FMI (kg/m2) | − 0.017 | 0.043 | − 0.008 | 0.692 | − 0.042 | 0.047 | − 0.018 | 0.369 | |
| Model 2 | − 0.005 | 0.042 | − 0.003 | 0.903 | 0.01 | 0.047 | 0.004 | 0.833 | ||
| Model 3 | − 0.006 | 0.043 | − 0.003 | 0.889 | − 0.035 | 0.049 | − 0.015 | 0.474 | ||
Standardized regression coefficients calculated as bi × SXi/SY. BMI body mass index, WC waist circumferences, HC hip circumferences, WrC wrist circumferences, WHR waist to hip ratio, WHtR waist to height ratio, FMI fat mass index. Model 1: unadjusted, Model 2: Adjusted with age, CVD history, socio-economic index, Model 3: adjusted with age, CVD history, socio-economic index and physical activity (MET score), dietary intake (energy intake Kcal/day).
Bold values are less than 0.05 and are statistically significant.
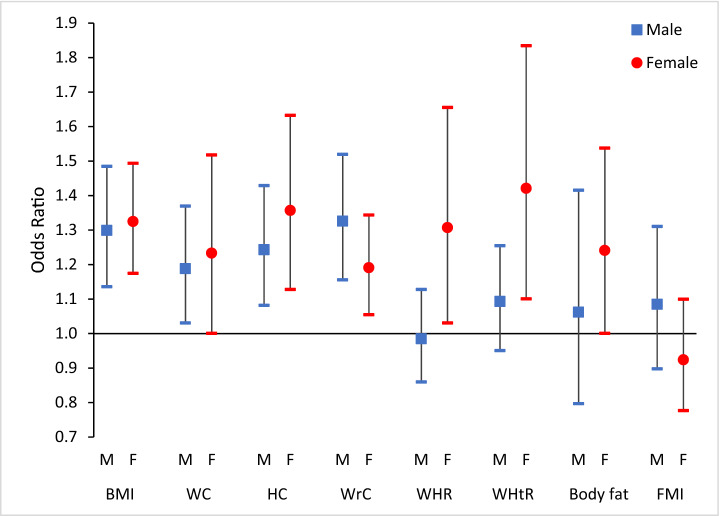
Figure 2 shows the odds ratios in a binary logistic model between sleep and anthropometric data, body fat percentage, and fat mass index, all as binary outcomes in both genders. BMI, WC, HC, and WrC in males showed significant ORs in the final multi-variable adjusted model. In females, in addition to the above-mentioned variables, WHR, WHtR, and BFP had significant ORs in the third model. Highest OR in males was 1.326 (1.156–1.520, p-value < 0.001) which was related to WrC and in females it was 1.421 (1.101–1.835, p-value < 0.05) which was related to WHtR. ORs was higher in female than the male with one exception which was WrC, and also significant levels in males were stronger. Table S3 in the supplementary file represents all the ORs in 3 models of binary logistic between sleep and anthropometric data, body fat percentage, and fat mass index in both genders. Moreover, Table S4 representing the intercorrelation coefficients between anthropometric and body composition data.
Figure 2.
The odds ratios of multivariate model of binary logistic regression between sleep and anthropometric data, body fat percentage and fat mass index as binary outcomes in both genders. BMI = Body Mass Index, WC = Waist Circumferences, HC = Hip Circumferences, WrC = Wrist Circumferences, WHR = Waist to Hip Ratio, WHtR = Waist to Height Ratio, FMI = Fat Mass Index. Multivariate model: adjusted with age, physical activity and dietary intake. Sleep considered as a binary variable in the model (Sleep hours < 8 = 1).
Discussion
Main findings
The main findings of the present study were: a) All of the anthropometric indices were significantly higher in the “< 8 h of sleep” group than in the “≥ 8 h of sleep” group. Regarding BFP and FMI the same results was seen. b) BMI, WC, HC, and WHtR were in a significant negative association with night time sleep, while these associations with nap time sleep duration were positive in both genders. c) After adjusting for age, CVD history, socio-economic index and physical activity, dietary intake, BMI, WC, HC, WrC and WHtR showed significant negative associations with 24-h sleep duration in both genders. To the best of our knowledge, this is the first study to investigate the relationship between sleep and obesity in an Iranian population using information related to different body parts’ fat percentage adjusted by the numerous obesity/sleep-related variables.
Sleep duration and physical activity
Chronic partial sleep deprivation leads to fatigue, which in turn reduces physical activity levels. In this study, subjects with under 8 h of sleep reported higher MET score which was only significant in women. Previous studies have shown that shorter sleep duration is associated with increased television viewing and decreased participation in sports27. Short sleepers spend more time watching TV, and the number of hours spent watching TV is associated with the amount of weight gained28. A report from the UK shows increased bedtime TV viewing among the target group, which is associated with reduced sleep duration29. Accelerometers were used in another study, which results showed that short sleepers significantly spend more time on low-mobility activities than those who sleep a minimum of 8 h a day. These results remained significant after adjusting for BMI, indicating that the relationship between inactivity and sleep duration is independent of obesity6. Although these studies have been conducted on adolescents and children, similar results are also expected to be obtained for adults. Similar to our findings, in a study conducted on adults in the United States, it was found that short-term sleep loss reduces spontaneous daytime activity, leading to lower intensities of physical activity under free-living conditions30. The results of some studies are not in line with the present results. For example, in an interventional study, two groups of volunteers slept for 5.5 and 8.5 h per day over 14 days, and the results showed an insignificant difference between two groups in daily physical activity levels31.
Sleep duration and food intake habits
Repeated sleep restrictions can disrupt the amount, distribution, and composition of human food intake. In general, we have more time to eat when we sleep less. In this study, nine main categories of foods including cereals, dairy, vegetables, fruits, salt, oil, beans, meat, and sugar were examined. Based on the results, the total intake of calories per day for did not show any significant difference in both genders. Another study showed that short sleepers are less likely to have a good diet compared with those who sleep more than 8 h a day. Garaulet et al. found that people with an insufficient amount of sleep eat fewer fruits, vegetables, fish, milk, cereals, and breakfast, but more unhealthy foods such as pizza, hamburgers, and pasta compared to others6. Although no significant difference was observed between the two sleep groups in terms of energy intake, studies have suggested that people are more likely to eat food, especially snacks, when they sleep less31,32.
Daytime napping duration and anthropometric data
The association of daytime napping with obesity has been envaulted in several previous studies. Chen et al. studied 1267 Chinese adults and reported that the frequency of daytime napping was negatively associated with BMI and short nocturnal sleep duration was associated with higher risk of overweight/obesity33. In our study, BMI had the highest significant positive association among other anthropometric data with daytime sleep duration. It has been also suggested that obesity was associated with the incidence and persistence of daytime sleepiness, while weight loss was associated with its remission34. There are also other studies which was in a line with our findings35,36. Another study in the US reported that nappers have higher values of WHR in comparison to non-nappers37. Also, in our study WHR showed a positive association with daytime sleep duration in men. Moreover, longer napping time has been showed that is associated with type 2 diabetes38,39, CVD40, and non-alcoholic fatty liver disease41.
Sleep duration and anthropometric data
In both genders, all anthropometric indices were higher in the “< 8 h of sleep” group which was in a line with different studies from Sweden42, Canada32, and the US. The National Health and Nutrition Examination Survey (NHANES) data showed that the highest and the lowest BMI was observed in people with the shortest and longest hours of sleep, respectively43. In both genders, FMI was significantly lower in the “≥ 8 h of sleep” group. According to previous studies, poor sleep quality was associated with increased FMI in adults, because a reduction in sleep efficiency (from 90 to 85%) would lead to an increase in FMI (from 5.3 to 6.5 kg/m2 in women and from 3.6 to 4.8 kg/m2 in men)44. The above studies confirm the present results in terms of significant increases in anthropometric indices of people with less sleep. Differences in the result of previous literature and this study may be due to differences in sampling, gender, ethnicity, age, classification method, sample size, cultural and geographical differences.
We examined 3 models to determine whether factors such as age, CVD history, socio-economic index and physical activity (MET score), dietary intake (energy intake Kcal/day) affect the relationship between sleep and obesity. In men, the beta coefficient of the multi-variable adjusted model of BMI was the highest. However, in a previous study, the correlation between BMI and sleep duration hours was − 0.070 in the total population which was higher than our results6. Beta coefficients for WC remained significant after adjusting, but it was higher in women comparing to men. The results of several articles in the UK indicate that there is a significant negative relationship between sleep duration and WC45 which was in line with our results. Regarding the HC variable, the beta coefficient in the first model of men was significant while in the female it was not significant. Interestingly, after multivariable adjustment, the beta coefficient in females became significant and higher than the males’ beta coefficient. It is noteworthy that beta coefficients of BMI, WC, WHtR, and HC were higher in females than males which indicates that shorter sleep hours may have a higher effect in increasing these indices in females. In the case of WrC, the beta coefficients after adjustment were weakened but remained significant in males while there was only a significant relationship in females just in model 3. In previously published articles, it has been declared that WrC may be a novel predictor for hypertension, cardiovascular disease46, and diabetes, and prediabetes26. Our results suggested that sleep hours may affect WrC and other anthropometric indices which should be considered in further studies as an independent factor. There was not any significant relationship between WHR and sleep hours in final model. Moreover, regarding the variable of WHtR, the relationship remained significant after multi-variable adjusting. It should be mention that the beta coefficient for WHtR in women was the highest beta coefficient among other variables in both genders. In the BFP variable in men, we observed a slight increase in beta coefficients, while the relationship remained significant. Also, the BFP’s final beta coefficient was the second rank among other indices in men while it was not significant in women.
Possible mechanisms
The association between sleep duration and obesity may be due to changes in the levels of some neuropeptides involved in appetite-regulation32,47,48. Sleep deprivation can cause neuro-hormonal disorders resulting in increased calorie intake levels48. Hormones also play a key role in this mechanism. Leptin and ghrelin are two of these hormones, which play different roles in appetite-regulation49,50. Adiponectin, which is secreted by adipose tissues, also plays a major role in the energy balance as a mediator of metabolism51,52.
Limitations
The current paper is not free of limitations this was a cross-sectional study; therefore, we cannot confidently specify the relationships of hours of sleep with obesity parameters. To suggest a cause-and-effect method, close observation must be made during the analysis of the Fasa PERSIAN cohort study at longer follow-up intervals, which can add to the importance of the research. Also, the current research was based on the individuals' self-report and therefore the data were subjective and may be prone to substantial recall bias. However, Sankai et al. have suggested that self-report sleep is valid compared to actigraphy53. In the current research, factors affecting the duration of sleep, and other quality factors, were not assessed; rather, the emphasis was focused solely on the amount of sleep. There was no recorded cause for the short period of sleep among individuals in the data; it may be the product of psychological or physical disorders, or just being tired from overwork. These variables are recommended to be considered during the follow-ups. The current study examined a rural population; a related study is suggested to be held in an urban community to determine any potential variations.
Conclusion
The present study suggests that anthropometric parameters such as BMI, WC, HC, WrC, WHtR are in inverse relationships with sleep hours. In women, anthropometric parameters such as BMI, WC, HC, and WHtR are more likely to increase with decreasing sleep hours. Finally, in the linear regression models, BFP was inversely associated with hours of sleep in men, while no significant correlation was found between this variable and hours of sleep in women. These findings suggest that a lack of sleep may be a warning sign of an unhealthy lifestyle and poor health effects.
Supplementary Information
Acknowledgements
The authors appreciate all people that patiently contributed to this study and Fasa University of medical sciences for financial supports of this work.
Abbreviations
- BMI
Body mass index
- BFP
Body fat percentage
- BIA
Bioelectric impedance analysis
- FCT
USDA food composition table
- FFQ
Food frequency questionnaire
- FMI
Fat mass index
- HC
Hip circumference
- IPAQ
International physical activity questionnaire
- MET
Metabolic equivalent of task
- PCA
Principal component analysis
- SES
Socioeconomic status
- WC
Waist circumference
- WHR
Waist to hip ratios
- WHtR
Waist to height ratios
- WrC
Wrist circumference
Author contributions
Conceptualization: R.H., M.H.Y., M.F. Methodology: M.H.Y., R.H. Software: M.H.Y. Validation: F.J., K.M. Formal analysis: M.H.Y. Investigation: M.H.Y., F.J. Resources: R.H., M.F. Data curation: F.J., K.M., M.H.Y. Original draft preparation: M.H.Y., R.H. Writing (review and editing): R.H., M.F. Visualization: R.H., M.F. Supervision: R.H. Project administration: R.H. Funding acquisition: R.H.
Funding
The authors did not receive any specific funding in this study.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-95796-9.
References
- 1.Gottlieb DJ, Punjabi NM, Newman AB, Resnick HE, Redline S, Baldwin CM, Nieto FJ. Association of sleep time with diabetes mellitus and impaired glucose tolerance. Arch. Intern. Med. 2005;165(8):863–867. doi: 10.1001/archinte.165.8.863. [DOI] [PubMed] [Google Scholar]
- 2.Must A, Jacques PF, Dallal GE, Bajema CJ, Dietz WH. Long-term morbidity and mortality of overweight adolescents: A follow-up of the Harvard growth study of 1922 to 1935. N. Engl. J. Med. 1992;327(19):1350–1355. doi: 10.1056/NEJM199211053271904. [DOI] [PubMed] [Google Scholar]
- 3.Taheri S. The link between short sleep duration and obesity: We should recommend more sleep to prevent obesity. Arch. Dis. Child. 2006;91(11):881–884. doi: 10.1136/adc.2005.093013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Foundation NS. Sleep in America poll. In Summary of Findings (2005).
- 5.Mullington J, Chan J, Van Dongen H, Szuba M, Samaras J, Price N, Meier-Ewert H, Dinges D, Mantzoros C. Sleep loss reduces diurnal rhythm amplitude of leptin in healthy men. J. Neuroendocrinol. 2003;15(9):851–854. doi: 10.1046/j.1365-2826.2003.01069.x. [DOI] [PubMed] [Google Scholar]
- 6.Garaulet M, Ortega F, Ruiz J, Rey-Lopez J, Beghin L, Manios Y, Cuenca-Garcia M, Plada M, Diethelm K, Kafatos A. Short sleep duration is associated with increased obesity markers in European adolescents: Effect of physical activity and dietary habits. The HELENA study. Int. J. Obes. 2011;35(10):1308–1317. doi: 10.1038/ijo.2011.149. [DOI] [PubMed] [Google Scholar]
- 7.Dinges DF, Pack F, Williams K, Gillen KA, Powell JW, Ott GE, Aptowicz C, Pack AI. Cumulative sleepiness, mood disturbance, and psychomotor vigilance performance decrements during a week of sleep restricted to 4–5 hours per night. Sleep. 1997;20(4):267–277. [PubMed] [Google Scholar]
- 8.Patel SR, Malhotra A, White DP, Gottlieb DJ, Hu FB. Association between reduced sleep and weight gain in women. Am. J. Epidemiol. 2006;164(10):947–954. doi: 10.1093/aje/kwj280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kagamimori S, Yamagami T, Sokejima S, Numata N, Handa K, Nanri S, Saito T, Tokui N, Yoshimura T, Yoshida K. The relationship between lifestyle, social characteristics and obesity in 3-year-old Japanese children. Child: Care, Health Dev. 1999;25(3):235–248. doi: 10.1046/j.1365-2214.1999.00127.x. [DOI] [PubMed] [Google Scholar]
- 10.Chen M-Y, Wang EK, Jeng Y-J. Adequate sleep among adolescents is positively associated with health status and health-related behaviors. BMC Public Health. 2006;6(1):59. doi: 10.1186/1471-2458-6-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Agras WS, Hammer LD, McNicholas F, Kraemer HC. Risk factors for childhood overweight: A prospective study from birth to 9.5 years. J. Pediatr. 2004;145(1):20–25. doi: 10.1016/j.jpeds.2004.03.023. [DOI] [PubMed] [Google Scholar]
- 12.Knutson KL. Sex differences in the association between sleep and body mass index in adolescents. J. Pediatr. 2005;147(6):830–834. doi: 10.1016/j.jpeds.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 13.Kronholm E, Härmä M, Hublin C, Aro AR, Partonen T. Self-reported sleep duration in Finnish general population. J. Sleep Res. 2006;15(3):276–290. doi: 10.1111/j.1365-2869.2006.00543.x. [DOI] [PubMed] [Google Scholar]
- 14.Basner M, Fomberstein KM, Razavi FM, Banks S, William JH, Rosa RR, Dinges DF. American time use survey: Sleep time and its relationship to waking activities. Sleep. 2007;30(9):1085–1095. doi: 10.1093/sleep/30.9.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kronholm E, Partonen T, Laatikainen T, Peltonen M, Härmä M, Hublin C, Kaprio J, Aro AR, Partinen M, Fogelholm M. Trends in self-reported sleep duration and insomnia-related symptoms in Finland from 1972 to 2005: A comparative review and re-analysis of Finnish population samples. J. Sleep Res. 2008;17(1):54–62. doi: 10.1111/j.1365-2869.2008.00627.x. [DOI] [PubMed] [Google Scholar]
- 16.Farjam M, Bahrami H, Bahramali E, Jamshidi J, Askari A, Zakeri H, Homayounfar R, Poustchi H, Malekzadeh R. A cohort study protocol to analyze the predisposing factors to common chronic non-communicable diseases in rural areas: Fasa cohort study. BMC Public Health. 2016;16(1):1090. doi: 10.1186/s12889-016-3760-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karyani, A. K., Rezaei, S., Matin, B. K. & Amini, S. Poor health-related quality of life in Iran: Decomposition analysis of affecting factors. Int. J. Hum. Rights Healthc. (2019).
- 18.VanItallie T, Yang M-U, Heymsfield SB, Funk RC, Boileau RA. Height-normalized indices of the body’s fat-free mass and fat mass: Potentially useful indicators of nutritional status. Am. J. Clin. Nutr. 1990;52(6):953–959. doi: 10.1093/ajcn/52.6.953. [DOI] [PubMed] [Google Scholar]
- 19.Buysse D, Reynolds C, Monk T, Berman S, Kupfer D. Pittsburgh sleep quality index (PSQI) Psychiatr. Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 20.Lund HG, Reider BD, Whiting AB, Prichard JR. Sleep patterns and predictors of disturbed sleep in a large population of college students. J. Adolesc. Health. 2010;46(2):124–132. doi: 10.1016/j.jadohealth.2009.06.016. [DOI] [PubMed] [Google Scholar]
- 21.Azar M, Sarkisian E. Food Composition Table of Iran: National Nutrition and Food Research Institute. Shaheed Beheshti University; 1980. [Google Scholar]
- 22.Malekshah AF, Kimiagar M, Saadatian-Elahi M, Pourshams A, Nouraie M, Goglani G, Hoshiarrad A, Sadatsafavi M, Golestan B, Yoonesi A, et al. Validity and reliability of a new food frequency questionnaire compared to 24 h recalls and biochemical measurements: Pilot phase of Golestan cohort study of esophageal cancer. Eur. J. Clin. Nutr. 2006;60(8):971–977. doi: 10.1038/sj.ejcn.1602407. [DOI] [PubMed] [Google Scholar]
- 23.Yazdanpanah MH, Homayounfar R, Khademi A, Zarei F, Shahidi A, Farjam M. Short sleep is associated with higher prevalence and increased predicted risk of cardiovascular diseases in an Iranian population: Fasa PERSIAN cohort study. Sci. Rep. 2020;10(1):1–13. doi: 10.1038/s41598-020-61506-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu P, Ma F, Lou H, Liu Y. The utility of fat mass index vs. body mass index and percentage of body fat in the screening of metabolic syndrome. BMC Public Health. 2013;13(1):629. doi: 10.1186/1471-2458-13-629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.World Health Organization . Waist Circumference and Waist-Hip Ratio: Report of a WHO Expert Consultation, 8–11 December 2008. World Health Organization; 2011. [Google Scholar]
- 26.Jahangiri Noudeh Y, Hadaegh F, Vatankhah N, Momenan AA, Saadat N, Khalili D, Azizi F. Wrist circumference as a novel predictor of diabetes and prediabetes: Results of cross-sectional and 8.8-year follow-up studies. J. Clin. Endocrinol. Metab. 2013;98(2):777–784. doi: 10.1210/jc.2012-2416. [DOI] [PubMed] [Google Scholar]
- 27.Von Kries R, Toschke AM, Wurmser H, Sauerwald T, Koletzko B. Reduced risk for overweight and obesity in 5-and 6-y-old children by duration of sleep—A cross-sectional study. Int. J. Obes. 2002;26(5):710. doi: 10.1038/sj.ijo.0801980. [DOI] [PubMed] [Google Scholar]
- 28.Kuriyan R, Bhat S, Thomas T, Vaz M, Kurpad AV. Television viewing and sleep are associated with overweight among urban and semi-urban South Indian children. Nutr. J. 2007;6(1):25. doi: 10.1186/1475-2891-6-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Owens J, Maxim R, McGuinn M, Nobile C, Msall M, Alario A. Television-viewing habits and sleep disturbance in school children. Pediatrics. 1999;104(3):e27–e27. doi: 10.1542/peds.104.3.e27. [DOI] [PubMed] [Google Scholar]
- 30.Schmid SM, Hallschmid M, Jauch-Chara K, Wilms B, Benedict C, Lehnert H, Born J, Schultes B. Short-term sleep loss decreases physical activity under free-living conditions but does not increase food intake under time-deprived laboratory conditions in healthy men. Am. J. Clin. Nutr. 2009;90(6):1476–1482. doi: 10.3945/ajcn.2009.27984. [DOI] [PubMed] [Google Scholar]
- 31.Nedeltcheva AV, Kilkus JM, Imperial J, Kasza K, Schoeller DA, Penev PD. Sleep curtailment is accompanied by increased intake of calories from snacks. Am. J. Clin. Nutr. 2009;89(1):126–133. doi: 10.3945/ajcn.2008.26574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chaput JP, Després JP, Bouchard C, Tremblay A. Short sleep duration is associated with reduced leptin levels and increased adiposity: Results from the Quebec family study. Obesity. 2007;15(1):253–261. doi: 10.1038/oby.2007.512. [DOI] [PubMed] [Google Scholar]
- 33.Chen M, Li D, Zhang X, Quan L, Xue H, Lü X, Cheng G. Effect of nocturnal sleep duration and daytime napping on overweight/obesity among adults in Chengdu city. Wei sheng yan jiu = J. Hyg. Res. 2018;47(6):918–923. [PubMed] [Google Scholar]
- 34.Fernandez-Mendoza J, Vgontzas AN, Kritikou I, Calhoun SL, Liao D, Bixler EO. Natural history of excessive daytime sleepiness: Role of obesity, weight loss, depression, and sleep propensity. Sleep. 2015;38(3):351–360. doi: 10.5665/sleep.4488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang N, Zou J, Fang S, Zhou J. Association between daytime napping and obesity in Chinese middle-aged and older adults. J. Global Health. 2020;10(2):020804. doi: 10.7189/jogh.10.020804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Patel SR, Hayes AL, Blackwell T, Evans DS, Ancoli-Israel S, Wing YK, Stone KL. The association between sleep patterns and obesity in older adults. Int. J. Obes. 2014;38(9):1159–1164. doi: 10.1038/ijo.2014.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Devine JK, Wolf JM. Integrating nap and night-time sleep into sleep patterns reveals differential links to health-relevant outcomes. J. Sleep Res. 2016;25(2):225–233. doi: 10.1111/jsr.12369. [DOI] [PubMed] [Google Scholar]
- 38.Leng Y, Cappuccio FP, Surtees PG, Luben R, Brayne C, Khaw KT. Daytime napping, sleep duration and increased 8-year risk of type 2 diabetes in a British population. Nutr. Metab. Cardiovasc. Dis. 2016;26(11):996–1003. doi: 10.1016/j.numecd.2016.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yamada T, Shojima N, Yamauchi T, Kadowaki T. J-curve relation between daytime nap duration and type 2 diabetes or metabolic syndrome: A dose-response meta-analysis. Sci. Rep. 2016;6(1):1–10. doi: 10.1038/s41598-016-0001-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yamada T, Hara K, Shojima N, Yamauchi T, Kadowaki T. Daytime napping and the risk of cardiovascular disease and all-cause mortality: A prospective study and dose-response meta-analysis. Sleep. 2015;38(12):1945–1953. doi: 10.5665/sleep.5246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peng K, Lin L, Wang Z, Ding L, Huang Y, Wang P, Xu Y, Lu J, Xu M, Bi Y, et al. Short sleep duration and longer daytime napping are associated with non-alcoholic fatty liver disease in Chinese adults. J. Diabetes. 2017;9(9):827–836. doi: 10.1111/1753-0407.12489. [DOI] [PubMed] [Google Scholar]
- 42.Björkelund C, Bondyr-Carlsson D, Lapidus L, Lissner L, Månsson J, Skoog I, Bengtsson C. Sleep disturbances in midlife unrelated to 32-year diabetes incidence: The prospective population study of women in Gothenburg. Diabetes Care. 2005;28(11):2739–2744. doi: 10.2337/diacare.28.11.2739. [DOI] [PubMed] [Google Scholar]
- 43.Grandner MA, Schopfer EA, Sands-Lincoln M, Jackson N, Malhotra A. Relationship between sleep duration and body mass index depends on age. Obesity. 2015;23(12):2491–2498. doi: 10.1002/oby.21247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kahlhöfer J, Karschin J, Breusing N, Bosy-Westphal A. Relationship between actigraphy-assessed sleep quality and fat mass in college students. Obesity. 2016;24(2):335–341. doi: 10.1002/oby.21326. [DOI] [PubMed] [Google Scholar]
- 45.Sperry SD, Scully ID, Gramzow RH, Jorgensen RS. Sleep duration and waist circumference in adults: A meta-analysis. Sleep. 2015;38(8):1269–1276. doi: 10.5665/sleep.4906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mohebi R, Mohebi A, Sheikholeslami F, Azizi F, Hadaegh F. Wrist circumference as a novel predictor of hypertension and cardiovascular disease: Results of a decade follow up in a West Asian cohort. J. Am. Soc. Hypertens. 2014;8(11):800–807. doi: 10.1016/j.jash.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 47.Van Cauter E, Knutson KL. Sleep and the epidemic of obesity in children and adults. Eur. J. Endocrinol. 2008;159(suppl 1):S59–S66. doi: 10.1530/EJE-08-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Karine S, Esra T, Plamen P. Sleep curtailment in healthy young men is assocated with decreased leptin leveles, elevated ghrelin levels, and increased hunger and appetite. Am. College Phys. 2004;141:846–850. doi: 10.7326/0003-4819-141-11-200412070-00008. [DOI] [PubMed] [Google Scholar]
- 49.Leibel RL. The role of leptin in the control of body weight. Nutr. Rev. 2002;60(suppl_10):S15–S19. doi: 10.1301/002966402320634788. [DOI] [PubMed] [Google Scholar]
- 50.Cummings DE, Foster KE. Ghrelin-leptin tango in body-weight regulation. Gastroenterology. 2003;124(5):1532–1535. doi: 10.1016/S0016-5085(03)00350-0. [DOI] [PubMed] [Google Scholar]
- 51.Tsao T-S, Lodish HF, Fruebis J. ACRP30, a new hormone controlling fat and glucose metabolism. Eur. J. Pharmacol. 2002;440(2–3):213–221. doi: 10.1016/S0014-2999(02)01430-9. [DOI] [PubMed] [Google Scholar]
- 52.Berg AH, Combs TP, Scherer PE. ACRP30/adiponectin: An adipokine regulating glucose and lipid metabolism. Trends Endocrinol. Metab. 2002;13(2):84–89. doi: 10.1016/S1043-2760(01)00524-0. [DOI] [PubMed] [Google Scholar]
- 53.Sankai T, Miyagaki T, Iso H, Shimamoto T, Iida M, Tanigaki M, Naito Y, Sato S, Kiyama M, Kitamura A. A population-based study of the proportion by type of stroke determined by computed tomography scan. [Nihon koshu eisei zasshi] Jpn. J. Public Health. 1991;38(12):901–909. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.