Abstract
Previous work has shown that poorer mitochondrial function is associated with age-related perceived fatigability. However, whether glucose oxidation and anaerobic metabolism are intermediate factors underlying this association remains unclear. We examined the total cross-sectional association between mitochondrial function and perceived fatigability in 554 adults aged 22–99 years. Mitochondrial function was assessed by skeletal muscle oxidative capacity (kPCr) using 31P magnetic resonance spectroscopy. Perceived fatigability was measured by rating of perceived exertion after a 5-minute (0.67 m/s) treadmill walk. The intermediate role of glucose oxidation (measured by the rate of change of respiratory exchange ratio [RER change rate] during the 5-minute treadmill walk) and anaerobic metabolism (measured by ventilatory threshold [VeT] during a maximal treadmill test) was evaluated by examining their cross-sectional associations with kPCr and perceived exertion. For each 0.01/s lower kPCr, perceived fatigability was 0.47 points higher (p = .002). A 0.01/s lower kPCr was also associated with 8.3 L/min lower VeT (p < .001). Lower VeT was associated with higher fatigability at lower levels of kPCr but not at higher kPCr levels (β for interaction = 0.017, p = .002). kPCr and RER change rate were not significantly associated (p = .341), but a 0.01/min higher RER change rate was associated with 0.12-point higher fatigability (p = .001). Poorer mitochondrial function potentially contributes to higher perceived fatigability through higher glucose oxidation and higher anaerobic metabolism. Future studies to further explore the longitudinal mechanisms between these metabolic changes and fatigability are warranted.
Keywords: Fatigue, Metabolism, Mitochondria, Respiratory exchange ratio, Ventilatory threshold
Background
Recent studies have found that diminished energy availability, resulting from rising energetic costs and lower fitness capacity, contribute to age-related declines in physical function (1–3) and physical activity (4,5), and greater perceived fatigability (6,7). The decrease in energy availability may be related to impaired oxidative capacity of skeletal muscle mitochondria, the organelles that provide energy for movement. Mitochondrial content and ATP production capacity have been shown to decline with age (8,9), and while evidence linking mitochondrial function and physical function (9–11), physical activity level (12), and perceived fatigability (13) has begun to emerge, much remains unknown.
Perceived fatigability, defined as perceived exertion after performing a standardized activity, is believed to be an early marker of changes in physical function (14,15), and physical activity (16) among older adults. Deficits in energy production have been hypothesized to be related to fatigability (17). Santanasto et al. found a cross-sectional association between muscle oxidative capacity and perceived fatigability using a small sample of older adults (N = 30) (13). However, only age and sex were adjusted for in their analyses, excluding other factors associated with perceived fatigability, such as body composition (18).
Two physiologic mechanisms, substrate utilization and anaerobic metabolism, may be intermediate factors underlying the association between mitochondrial function and perceived fatigability. Glucose may be the preferred substrate for oxidation as mitochondrial function declines with aging because glucose oxidation produces more ATP per O2 molecule than fatty acid oxidation (19,20). Anaerobic metabolism may also be needed to close the gap between energy demands and aerobic ATP production by mitochondria (17,19,21). These 2 factors may, in turn, impact fatigability. Depletion of glycogen in the muscle is one cause of fatigue during exercise (19). The lactic acid produced by anaerobic metabolism has also been linked to muscle (19,22) and general fatigue in older adults (19,23). Older adults tend to have a higher respiratory exchange ratio (RER), indicating greater glucose utilization, than younger adults at all intensities of graded treadmill exercise (24,25). Anaerobic threshold (AT) is a measure of the exercise intensity where anaerobic metabolism begins to contribute substantially to ATP production (26). Studies using lactate threshold (LT) and ventilatory threshold (VeT), 2 common estimates of AT (26), have shown that LT (27) and VeT (28,29) are lower at older ages. These results suggest that older adults may utilize anaerobically produced energy earlier during an activity bout than younger adults. However, how the age-associated differences in substrate utilization and anaerobic metabolism are related to mitochondrial function and perceived fatigability remains unclear.
The present study aims to examine (i) the cross-sectional association between skeletal muscle oxidative capacity, a measure that strongly correlates with mitochondrial function (30), and fatigability in a large sample across a wide age range, and (ii) the cross-sectional associations between: (a) muscle oxidative capacity and substrate utilization and anaerobic metabolism, and (b) substrate utilization and anaerobic metabolism and fatigability. We hypothesize that those with poorer skeletal muscle oxidative capacity will have higher fatigability, and that this association will be partly explained by substrate utilization and anaerobic metabolism.
Method
Study Population
A total of 683 participants from the Baltimore Longitudinal Study of Aging (BLSA) aged 22–99 years completed both the perceived fatigability tests and in vivo phosphorus-31 magnetic resonance spectroscopy (31P-MRS) measurement of muscle oxidative capacity between March 2013 and June 2019. The BLSA is a study of normative human aging established in 1958 and continuously enrolling participants. The procedures for participant enrollment and the inclusion criteria have been described elsewhere (31). Briefly, all participants must be free of major chronic conditions and cognitive impairment at the time of enrollment. Participants are followed at different intervals depending on their age: every 4 years for age <60, every 2 years for age 60–79, and every year for age ≥80.
Among the 683 participants, 115 people had missing intracellular pH values during 31P-MRS measurement and were excluded from analyses. Though they had kPCr values, we could not ascertain if the pH dropped below 6.8, and thus could not assess intracellular muscle acidification. Another 14 participants had missing values for lean mass and fat mass. The remaining 554 participants constituted the analytical sample for the analysis for Aim 1. Comparison of participant characteristics between participants excluded due to missing pH values and the included participants are summarized in Supplementary Table S1. Of the 554 participants, 509 completed RER measures during a slow walking test, and 481 had measures of VeT during a maximal treadmill test in the same visit (Supplementary Figure S1). These groups constituted the analytical samples for Aim 2.
Muscle Oxidative Capacity
Muscle oxidative capacity was captured using a measure of post-exercise recovery of phosphocreatine (PCr) concentration in the vastus lateralis muscle of the left thigh from 31P-MRS using a 3-T Achieva MR scanner (Philips Healthcare, Andover, MA) with a 10-cm 31P-tuned surface coil (PulseTeq, Surrey, UK). Participants were instructed to perform a ballistic knee extension exercise. A total of 75 pulse-acquire 31P-MRS spectra were obtained with 6-second temporal resolution for 60 seconds before, approximately 30 seconds during, and 360 seconds after exercise (9,12). The spectra were processed using jMRUI (version 5.2) and quantified using a nonlinear least square algorithm (AMARES) (32–34).
The rate constant of PCr recovery, kPCr, was calculated by fitting the time-dependent changes in PCr peak area using the following monoexponential function:
where PCr0 was the PCr signal amplitude at the end of the exercise or the beginning of the recovery, ∆PCr = (PCrbaseline – PCr0) was the decrease in PCr observed during in-magnetic exercise from the pre-exercise level, PCrbaseline, to the end-exercise level, PCr0, and kPCr (/s) was the PCr recovery rate constant. kPCr has been shown to be positively and linearly dependent on maximum muscle oxidative capacity (35,36).
To standardize the measure of oxidative function across participants, exercise duration was optimized by avoiding intracellular muscle acidification, defined as pH below 6.8. Acidosis slows PCr recovery (35) and the PCr recovery cannot be described by a single rate constant from the monoexponential equation above (37). The pH value was derived from the chemical shift of Pi relative to PCr (38).
Perceived Fatigability
Perceived fatigability was measured using the Borg rating of perceived exertion (RPE) scale (range 6–20) administered immediately following a treadmill walk for 5 minutes at 1.5 mph (0.67 m/s) and 0% grade. Word anchors were provided next to odd numerals to orient the participants. Score 7 was labeled with “very, very light,” 9 “very light,” 11 “fairly light,” 13 “somewhat light,” and so on. A higher score indicates more exertion and higher perceived fatigability (39).
Respiratory Exchange Ratio Change Rate
Respiratory exchange ratio was measured using indirect calorimetry (Medical Graphics Corp., St. Paul, MN) during the same 5-minute treadmill walk used to assess perceived fatigability. The system was warmed up for at least 20 minutes before testing and calibrated using reference gases of known concentrations and a 3.0-L syringe for flow (3). Expired air was collected through a stretch neoprene facemask and a pneumotach, and was analyzed breath-by-breath for oxygen (O2) and carbon dioxide (CO2) content using a standard laboratory metabolic cart (Medgraphics Ultima CCM). Readings from the first 2 minutes of the test were discarded to allow the participant to adjust to the workload. For the remaining 3 minutes, RER was calculated as the ratio between the volume of CO2 produced and the volume of O2 consumed every 30 seconds. A linear line was fitted for 7 RER readings for each person, and the slope of the line was used to define the RER change rate per minute.
Ventilatory Threshold
Ventilatory threshold was measured during a graded maximal treadmill test using a modified Balke protocol. Speeds were commenced at 3.5 miles/h (1.6 m/s) for men and 3.0 miles/h (1.4 m/s) for women. Grade was increased by 3% every 2 minutes until voluntary exhaustion. For more aerobically fit participants, speeds were increased 1–3 times by 0.5 miles/h (40). Expired air was collected using the same standard laboratory metabolic cart (Medgraphics Ultima CCM) described above. Ventilatory threshold (L/min) was defined as the point where the increase in ventilatory volume exceeded the increase in O2 uptake, as calculated by the Medgraphics software.
Covariates
Baseline age and sex, race, and chronic conditions were self-reported during interviewer-administered interviews. Chronic conditions included history of: cardiovascular disease (myocardial infarction, congestive heart failure, angina pectoris, bypass surgery or [balloon] angioplasty, or peripheral arterial disease), hypertension (diagnosis of hypertension and taking antihypertensive medications), diabetes (diagnosis of diabetes and current medication for diabetes), cancer (non-skin [squamous or basal cell] cancer), and stroke (stroke or transient ischemic attack). Fat mass and lean mass were obtained from dual-energy x-ray absorptiometry (DEXA) (Model DPX-L Lunar Radiation, Madison, WI) (18). The end-exercise PCr depletion relative to the pre-exercise PCr level (% PCr depletion) was also included in all models as kPCr is dependent on the end-exercise level of PCr (41).
Statistical Analysis
Baseline characteristics were summarized using medians and interquartile ranges (IQRs) for continuous and count variables, and frequencies and percentages for categorical characteristics. Scatterplots and locally weighted scatterplot smoothing (LOWESS) were used to explore the univariate association between perceived exertion, kPCr, RER change rate, and VeT and age.
Multivariable linear regression was used to examine the total associations between kPCr and perceived exertion with perceived exertion as the dependent variable (Aim 1 analysis) and associations among kPCr, RER change rate (substrate utilization), VeT (anaerobic metabolism), and perceived exertion (Aim 2 analysis). Two sets of models were fitted for the Aim 2 analysis: (i) RER change rate or VeT as the dependent variable and kPCr as the independent variable, and (ii) perceived exertion as the dependent variable and RER change rate or VeT as the independent variable. The (ii) models also included kPCr as a covariate, and its interaction with RER change rate and VeT was explored. The interactions were visualized using margins plots. The associations between RER change rate/VeT and perceived exertion were estimated at levels of kPCr from 0.01/s with 0.005/s increments. The associations between kPCr and perceived exertion were estimated at levels of RER change rate (from −0.10/min with 0.05/min increments) or VeT (from 10 L/min with 20 L/min increments). All models were adjusted for baseline age, sex, race, fat and lean mass, number of chronic conditions, and % PCr depletion.
To make the coefficients more interpretable, variables were centered and rescaled as needed. kPCr was centered at approximately the minimum value in the sample, 0.01/s, and then multiplied by 100, making a 1-unit difference in the rescaled variable equivalent to 0.01/s difference in kPCr on the original scale. Respiratory exchange ratio change rate was multiplied by 100, making a 1-unit difference in the rescaled variable equivalent to 0.01 difference in the rate of change in RER per minute. VeT was centered at approximately the minimum value in the sample, 10 L/min, without scaling. Covariates were also centered: baseline age at 65 years, fat mass at 25 kg, and lean mass at 45 kg.
All analyses were performed using Stata IC 15.1 (StataCorp, College Station, TX), and statistical significance was determined by an alpha level of .05.
Results
Characteristics of the participants are summarized in Table 1. The median age was 70 years (IQR: 60–80). Men constituted 43.5% of the sample. About 66.2% of the participants were of white race. The study population had few comorbidities with hypertension and cancer among the most prevalent conditions. Most participants (80%) had low fatigability (RPE ≤ 9) (39). Median kPCr, median RER change rate, and median VeT were 2.0/s, 0.01/min, and 58.3 L/min, respectively.
Table 1.
Baseline Characteristics of the Participants (N = 554)
| Characteristic | Median (IQR) or n (%) |
|---|---|
| Age (y) | 70.0 (60.0, 80.0) |
| Sex, male | 241 (43.5%) |
| Race, white | 367 (66.2%) |
| Lean mass (kg) | 43.5 (37.9, 53.8) |
| Fat mass (kg) | 24.7 (18.9, 31.8) |
| Chronic conditions | 1.0 (0.0, 2.0) |
| Cardiovascular diseasea | 44 (7.9%) |
| Hypertensionb | 219 (39.5%) |
| Diabetesc | 87 (15.7%) |
| Cancerd | 151 (27.3%) |
| Strokee | 22 (4.0%) |
| Perceived fatigability (RPE) | 7.0 (7.0, 9.0) |
| kPCr (/100 s) | 2.0 (1.7, 2.4) |
| RER change rate (/min)f | 0.01 (0.00, 0.02) |
| VeT (L/min)g | 57.3 (45.1, 76.4) |
Notes: IQR = interquartile range; RPE = rating of perceived exertion.
aIncluding myocardial infarction, congestive heart failure, angina pectoris, bypass surgery or (balloon) angioplasty, or peripheral arterial disease. bIncluding diagnosis of hypertension and taking antihypertensive medications. cIncluding diagnosis of diabetes and current medication for diabetes. dNon-skin (squamous or basal cell) cancer. eStroke or transient ischemic attack. fRER change rate = slope of respiratory exchange ratio from second to fifth minute during slow 5-min treadmill walk. gVeT = ventilatory threshold during maximal treadmill test.
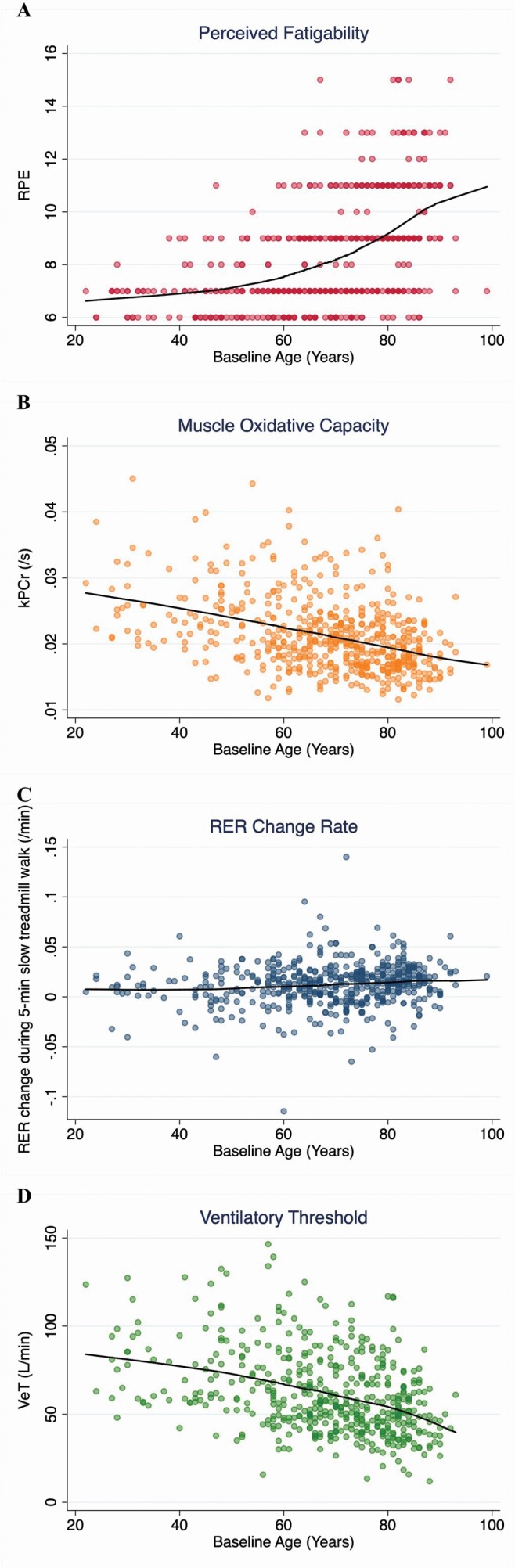
The unadjusted relationships of perceived exertion, muscle oxidative capacity, RER change rate, and VeT with age are shown in Figure 1. Older participants had higher perceived exertion, lower muscle oxidative capacity, faster switch from fatty acid to glucose oxidation during walking, and earlier utilization of anaerobic energy.
Figure 1.
Univariate scatterplot and LOWESS plot depicting the associations between (A) perceived fatigability (RPE), (B) muscle oxidative capacity (kPCr), (C) respiratory exchange ratio change rate (RER change rate), (D) ventilatory threshold (VeT), and age.
The association between kPCr and perceived exertion is presented in Table 2. In models adjusted for demographics, body composition, comorbidities, and % PCr depletion, a 0.01/s slower recovery rate in kPCr was associated with 0.486-point higher perceived exertion (p = .002). In the multivariable linear regression models of RER change rate, kPCr was not associated with RER change rate (p = .341) (Table 3, row [i]), but a 0.01/min higher RER change rate was associated with 0.123-point higher perceived exertion (p = .001) after adjusting for kPCr, demographics, body composition, comorbidities, and % PCr depletion (Table 3, row [ii]). No interaction was observed between RER change rate and kPCr on their associations with perceived exertion (p = .177). In the VeT models, a 0.01/s lower kPCr was associated with a 8.344 L/min lower VeT (p < .001) (Table 3, row [iii]). Further, VeT had a significant interaction with kPCr in the association with perceived exertion (β for interaction = 0.017, p = .002) (Table 3, row [iv]). This interaction indicates that a 1 L/min lower VeT was associated with a 0.044-point higher perceived exertion at the minimum value of kPCr (0.01/s) after adjusting for covariates (p < .001), but the association was weaker at higher kPCr levels. Similarly, a 0.01/s lower kPCr was associated with 1.265-point higher perceived exertion (p = .001) at the minimum VeT of 10 L/min, and the association was attenuated at higher VeT levels.
Table 2.
Linear Regression Model for the Association Between Muscle Oxidative Capacity (kPCr) and Perceived Fatigability (RPE; N = 554)
| β | 95% CI | p-Value | |
|---|---|---|---|
| kPCra | −0.486 | (−0.791, −0.182) | .002 |
| Age (years) | 0.055 | (0.042, 0.067) | <.001 |
| PCr depletion during exercise (%) | −0.011 | (−0.025, 0.005) | .157 |
| Sex | |||
| Male | 0.352 | (−0.232, 0.935) | .237 |
| Race | |||
| White | −0.227 | (−0.548, 0.093) | .164 |
| Fat mass (kg) | 0.032 | (0.015, 0.048) | <.001 |
| Lean mass (kg) | −0.033 | (−0.062, −0.003) | .030 |
| Count of chronic conditionsb | −0.042 | (−0.202, 0.119) | .612 |
| Intercept | 8.944 | (8.294, 9.593) | <.001 |
Notes: RPE = rating of perceived exertion.
aRepresents 0.01/s difference in kPCr. bIncluding cardiovascular disease (myocardial infarction, congestive heart failure, angina pectoris, bypass surgery or [balloon] angioplasty, or peripheral arterial disease), hypertension (diagnosis of hypertension and taking antihypertensive medications), diabetes (diagnosis of diabetes and current medication for diabetes), cancer (non-skin [squamous or basal cell] cancer), and stroke (stroke or transient ischemic attack).
Table 3.
Linear Regression Models for the Association Between Muscle Oxidative Capacity (kPCr) and Respiratory Exchange Ratio Change Rate (RER change rate)/Ventilatory Threshold (VeT) and Between RER Change Rate/VeT and Perceived Fatigability (RPE)
| Model Including Interaction With kPCr | Model Excluding Interaction With kPCr | |||||
|---|---|---|---|---|---|---|
| β | 95% CI | p-Value | β | 95% CI | p-Value | |
| Models for RER change rate (n = 509) | ||||||
| (i) RER change ratea as dependent variable | ||||||
| kPCr | −0.002 | (−0.006, 0.002) | .341 | −0.002 | (−0.006, 0.002) | .341 |
| (ii) Perceived fatigability as dependent variablea | ||||||
| RER change rate | 0.240 | (0.055, 0.426) | .011 | 0.123 | (0.051, 0.195) | .001 |
| kPCr | −0.368 | (−0.733, −0.004) | .048 | −0.488 | (−0.809, −0.168) | .003 |
| RER change rate × kPCr | −0.107 | (−0.262, 0.048) | .177 | − | − | − |
| Models for VeT (n = 481) | ||||||
| (iii) VeT as dependent variablea | ||||||
| kPCr | 8.344 | (5.622, 11.067) | <.001 | |||
| (iv) Perceived fatigability as dependent variablea | ||||||
| VeT | −0.044 | (−0.061, −0.027) | <.001 | |||
| kPCr | −1.265 | (−1.978, −0.553) | .001 | |||
| VeT × kPCr | 0.017 | (0.006, 0.027) | .002 |
Notes: aModels adjusted for age, sex, race, fat mass, lean mass, count of comorbidities, and % PCr depletion. bRepresents 0.01/min difference in change rate of respiratory exchange ratio from second to fifth minute during slow 5-minute treadmill walk.
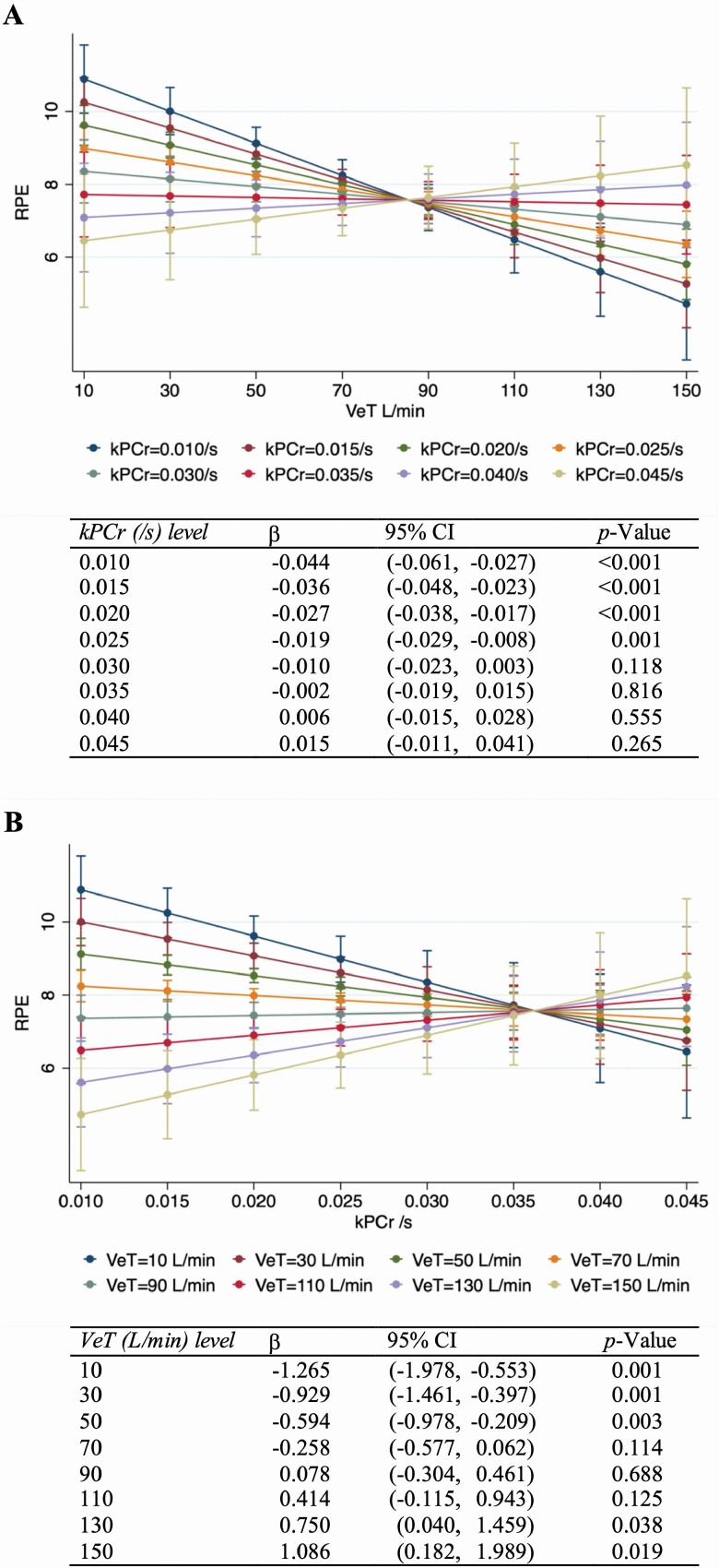
The margins plots of the kPCr-VeT interaction are presented in Figure 2. The estimated coefficients of associations between VeT and perceived exertion at different levels of kPCr and between kPCr and perceived exertion at different levels of VeT are presented at the bottom of each figure. The associations between VeT and perceived exertion were significant when kPCr was ≤0.025/s (Figure 2A), and the associations between kPCr and perceived exertion were significant when VeT was ≤50 L/min (Figure 2B). When VeT was >110 L/min, higher kPCr appeared to be associated with higher perceived exertion. To test again the positive kPCr–fatigability association seen at the highest levels of VeT, we fitted a linear regression model with VeT categorized at <50, 50 to <100, and ≥100 L/min. The VeT categories were modeled as ordinal in a multivariable linear model (Supplementary Tables S2). The associations between kPCr and perceived exertion were significantly different across the 3 VeT categories (β for interaction = 0.630, p = .007) (Supplementary Table S2). However, kPCr was not significantly associated with fatigability within the 2 higher VeT categories (p = .853 and .756, respectively) (Supplementary Table S3).
Figure 2.
Margins plot of associations (A) between ventilatory threshold (VeT) and perceived fatigability (RPE) by levels of muscle oxidative capacity (kPCr), and (B) between kPCr and RPE by levels of VeT.
Discussion
Our study showed mitochondrial function was inversely associated with perceived fatigability. Poorer mitochondrial function was also associated with earlier utilization of anaerobic metabolism, but not with higher glucose oxidation during slow walking. We also found that higher glucose utilization and earlier utilization of anaerobic metabolism were both associated with higher perceived fatigability independent of mitochondrial function. The association between anaerobic metabolism and fatigability were most robust at lower levels of mitochondrial function.
Our finding on the association between muscle oxidative capacity and perceived fatigability replicates the results previously reported by Santanasto et al. (13) in a larger sample across a wider age range with more extensive covariate adjustment. These results reinforce that mitochondrial function is an independent, physiological factor that contributes to higher perceived fatigability in older adults, even after accounting for body composition and chronic conditions. Further, our study elaborated on these findings by investigating the associations between mitochondrial function and substrate utilization and anaerobic metabolism.
Although we found no association between mitochondrial function and substrate utilization, one study in patients with mitochondrial disease found that RER and total carbohydrate oxidation were higher in patients than in healthy controls during cycling exercise of the same absolute workload (20). Another study found that muscle oxidative capacity was the sole predictor of basal RER among patients with type 2 diabetes (42). The lack of association in our study may be due to the low intensity of the 5-minute treadmill walk. The switch from fatty acid to glucose is minimal at such a low workload, so the difference in RER change rate cannot capture the difference in mitochondrial function.
The association between mitochondrial function and anaerobic metabolism observed in this study has also been shown in patients with Huntington’s disease and mitochondrial disease. Ciammola et al. found that, compared to healthy controls, Huntington’s disease patients were more likely to have mitochondrial abnormalities and lower VeTs (43). In a case report, Bendahan et al. treated 2 patients with impairment in complexes I and IV of mitochondria with coenzyme Q10 (CoQ) and found that muscle oxidative capacity was improved and VeT was shifted to higher workload after the treatment (44).
Contrary to the association between RER change rate and perceived fatigability found in our sample, Barbosa et al. found no correlation between RER level at the last minute of a 6-min fast-paced walk test and perceived fatigability at the completion of the test among 48 community-dwelling older women (7). The discrepancy between their finding and ours may have come from their use of RER level at the last minute of the test instead of RER change rate during the test. Participants are likely to achieve high exercise intensities towards the end of a fast-paced walk and RER values close to 1.0. Therefore, participants who reported different fatigability likely had similarly high RER levels and thus a correlation could not be observed.
Our results on anaerobic metabolism and perceived fatigability agree with some previous findings in smaller human samples (23), and in animal models (45). In a group of 27 older adults, basal glycolysis measured by extracellular acidification rate (ECAR) correlated with perceived fatigability measured by the Pittsburgh Fatigability Scale (PFS) (23). In a model using rats, hindlimb suspension was observed to increase glycogen utilization and lactate accumulation in type I fibers of soleus and gastrocnemius muscles and fatigability during contractile activity (45). However, Santanasto et al. found no difference in blood lactate level, another indicator of anaerobic metabolism, immediately after a similar 5-minute treadmill walk between people with high fatigability (RPE ≥ 10) and low fatigability (RPE ≤ 9) (13). The lack of difference could have been the effect of a small sample size and dichotomization of perceived fatigability.
The interaction between mitochondrial function and anaerobic metabolism in our results suggests that aerobic metabolism contributes to perceived fatigability only when mitochondrial function is below a certain threshold. When mitochondrial function is healthy, 2 people who have different AT seldom approach their ATs during their usual daily activities, and anaerobic metabolism barely contributes to meeting the energy demand by those activities. Hence, the difference in AT does not reflect a difference in perceived fatigability. In contrast, people with impaired mitochondrial function may rely partially on anaerobic metabolism for low-intensity daily activities. For them, any difference in the ATs will present a difference in anaerobic energy produced during activity and in the resultant lactate which may lead to greater perception of fatigability (19,23).
Collectively from these findings, we speculate that one pathway to perceived fatigability is through poor or diminished mitochondrial function which is partially mediated by higher anaerobic metabolism and glucose utilization. Though our study did not examine the temporality between mitochondrial function and anaerobic metabolism/glucose utilization, there is evidence that treatment targeting mitochondrial function improves VeT (44). Therefore, it is reasonable to speculate that mitochondrial function change precedes change in anaerobic metabolism. Similarly, mitochondrial impairment is likely to occur before the change in glucose utilization, as demonstrated by higher RER in patients affected by mitochondrial diseases (20). Other studies have suggested that metabolite accumulation resulting from anaerobic energy production and substrate depletion due to preferential consumption of glucose could then in turn trigger signals to the brain and result in the sensation of fatigue (19,46). Future longitudinal follow-up to verify these temporal associations is warranted.
This study has limitations. First, VeT is not a direct measure of anaerobic energy contribution or of blood lactate levels during the walking test where perceived fatigability is measured. VeT can only be measured during graded maximal exercise by its definition. Second, reverse causality is still possible for the associations between mitochondrial function and RER/VeT and between RER/VeT and perceived fatigability with only cross-sectional data in the analyses, though we did not find support for it in the literature.
Despite the limitations, our study provides insight into the links between physiologic aging (muscle oxidative capacity) and perceived fatigability (a proposed phenotypic measure of aging). Specifically, our study suggests that substrate utilization and anaerobic energy may lie in the pathways between mitochondrial function and perceived fatigability. Future studies should further investigate the underlying mechanisms of the associations between mitochondrial function/anaerobic metabolism/glucose utilization and perceived fatigability by, for example, assessing the dynamic relations of these metabolic measures with neural/brain activities and the perception of fatigue during physical activity. Understanding the underlying mechanisms of age-related fatigability from the peripheral metabolism to the central nervous system may provide new avenues for treating and reducing fatigue/fatigability and thereby diminish future physical function decline.
Supplementary Material
Acknowledgment
Data used in the analyses were obtained from the Baltimore Longitudinal Study of Aging, an Intramural Research Program of the National Institute on Aging.
Funding
This work was supported by grants R21AG053198 and P30AG021334 from the National Institute on Aging. F.L. was supported by U01AG057545. J.A.S. was supported by R21AG053198, P30AG021334, U01AG0057545, and R01AG061786. A.A.W. was supported by U01AG0057545, R01AG061786, P30AG021334, and P30AG059298.
Conflict of Interest
E.M.S., L.F., and J.A.S. currently serve on the editorial board for the Journal of Gerontology: Medical Sciences. F.L., A.A.W., and N.D.K. have no conflicts to disclose.
References
- 1.Schrack JA, Zipunnikov V, Simonsick EM, Studenski S, Ferrucci L. Rising energetic cost of walking predicts gait speed decline with aging. J Gerontol A Biol Sci Med Sci. 2016;71(7):947–953. doi: 10.1093/gerona/glw002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schrack JA, Simonsick EM, Chaves PH, Ferrucci L. The role of energetic cost in the age-related slowing of gait speed. J Am Geriatr Soc. 2012;60(10):1811–1816. doi: 10.1111/j.1532-5415.2012.04153.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schrack JA, Simonsick EM, Ferrucci L. The relationship of the energetic cost of slow walking and peak energy expenditure to gait speed in mid-to-late life. Am J Phys Med Rehabil. 2013;92(1):28–35. doi: 10.1097/PHM.0b013e3182644165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tanaka H, Desouza CA, Jones PP, Stevenson ET, Davy KP, Seals DR. Greater rate of decline in maximal aerobic capacity with age in physically active vs. sedentary healthy women. J Appl Physiol (1985). 1997;83(6):1947–1953. doi: 10.1152/jappl.1997.83.6.1947 [DOI] [PubMed] [Google Scholar]
- 5.Schrager MA, Schrack JA, Simonsick EM, Ferrucci L. Association between energy availability and physical activity in older adults. Am J Phys Med Rehabil. 2014;93(10):876–883. doi: 10.1097/PHM.0000000000000108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schrack JA, Wanigatunga AA, Zipunnikov V, Kuo PL, Simonsick EM, Ferrucci L. Longitudinal association between energy regulation and fatigability in mid-to-late life. J Gerontol A Biol Sci Med Sci. 2020. doi: 10.1093/gerona/glaa011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barbosa JF, Bruno SS, Cruz NS, de Oliveira JS, Ruaro JA, Guerra RO. Perceived fatigability and metabolic and energetic responses to 6-minute walk test in older women. Physiotherapy. 2016;102(3):294–299. doi: 10.1016/j.physio.2015.08.008 [DOI] [PubMed] [Google Scholar]
- 8.Short KR, Bigelow ML, Kahl J, et al. . Decline in skeletal muscle mitochondrial function with aging in humans. Proc Natl Acad Sci USA. 2005;102(15):5618–5623. doi: 10.1073/pnas.0501559102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choi S, Reiter DA, Shardell M, et al. . 31P Magnetic resonance spectroscopy assessment of muscle bioenergetics as a predictor of gait speed in the Baltimore Longitudinal Study of Aging. J Gerontol A Biol Sci Med Sci. 2016;71(12):1638–1645. doi: 10.1093/gerona/glw059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Santanasto AJ, Coen PM, Glynn NW, et al. . The relationship between mitochondrial function and walking performance in older adults with a wide range of physical function. Exp Gerontol. 2016;81:1–7. doi: 10.1016/j.exger.2016.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coen PM, Jubrias SA, Distefano G, et al. . Skeletal muscle mitochondrial energetics are associated with maximal aerobic capacity and walking speed in older adults. J Gerontol A Biol Sci Med Sci. 2013;68(4):447–455. doi: 10.1093/gerona/gls196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adelnia F, Urbanek J, Osawa Y, et al. . Moderate-to-vigorous physical activity is associated with higher muscle oxidative capacity in older adults. J Am Geriatr Soc. 2019;67(8):1695–1699. doi: 10.1111/jgs.15991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Santanasto AJ, Glynn NW, Jubrias SA, et al. . Skeletal muscle mitochondrial function and fatigability in older adults. J Gerontol A Biol Sci Med Sci. 2015;70(11):1379–1385. doi: 10.1093/gerona/glu134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simonsick EM, Glynn NW, Jerome GJ, Shardell M, Schrack JA, Ferrucci L. Fatigued, but not frail: perceived fatigability as a marker of impending decline in mobility-intact older adults. J Am Geriatr Soc. 2016;64(6):1287–1292. doi: 10.1111/jgs.14138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simonsick EM, Schrack JA, Santanasto AJ, Studenski SA, Ferrucci L, Glynn NW. Pittsburgh Fatigability Scale: one-page predictor of mobility decline in mobility-intact older adults. J Am Geriatr Soc. 2018;66(11):2092–2096. doi: 10.1111/jgs.15531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wanigatunga AA, Simonsick EM, Zipunnikov V, et al. . Perceived fatigability and objective physical activity in mid- to late-life. J Gerontol A Biol Sci Med Sci. 2018;73(5):630–635. doi: 10.1093/gerona/glx181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eldadah BA. Fatigue and fatigability in older adults. PM R. 2010;2(5):406–413. doi: 10.1016/j.pmrj.2010.03.022 [DOI] [PubMed] [Google Scholar]
- 18.Martinez-Amezcua P, Simonsick EM, Wanigatunga AA, et al. . Association between adiposity and perceived physical fatigability in mid- to late life. Obesity (Silver Spring). 2019;27(7):1177–1183. doi: 10.1002/oby.22500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Newsholme EA, Blomstrand E, Ekblom B. Physical and mental fatigue: metabolic mechanisms and importance of plasma amino acids. Br Med Bull. 1992;48(3):477–495. doi: 10.1093/oxfordjournals.bmb.a072558 [DOI] [PubMed] [Google Scholar]
- 20.Jeppesen TD, Orngreen MC, van Hall G, Haller RG, Vissing J. Fat metabolism during exercise in patients with mitochondrial disease. Arch Neurol. 2009;66(3):365–370. doi: 10.1001/archneurol.2009.24 [DOI] [PubMed] [Google Scholar]
- 21.Stein TP, Wade CE. Metabolic consequences of muscle disuse atrophy. J Nutr. 2005;135(7):1824S–1828S. doi: 10.1093/jn/135.7.1824S [DOI] [PubMed] [Google Scholar]
- 22.Allen DG, Lamb GD, Westerblad H. Skeletal muscle fatigue: cellular mechanisms. Physiol Rev. 2008;88(1):287–332. doi: 10.1152/physrev.00015.2007 [DOI] [PubMed] [Google Scholar]
- 23.Braganza A, Corey CG, Santanasto AJ, et al. . Platelet bioenergetics correlate with muscle energetics and are altered in older adults. JCI Insight. 2019;5:e128248. doi: 10.1172/jci.insight.128248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mittendorfer B, Klein S. Effect of aging on glucose and lipid metabolism during endurance exercise. Int J Sport Nutr Exerc Metab. 2001;11(Suppl.):S86–S91. doi: 10.1123/ijsnem.11.s1.s86 [DOI] [PubMed] [Google Scholar]
- 25.Sial S, Coggan AR, Carroll R, Goodwin J, Klein S. Fat and carbohydrate metabolism during exercise in elderly and young subjects. Am J Physiol. 1996;271(6 Pt 1):E983–E989. doi: 10.1152/ajpendo.1996.271.6.E983 [DOI] [PubMed] [Google Scholar]
- 26.Ghosh AK. Anaerobic threshold: its concept and role in endurance sport. Malays J Med Sci. 2004;11(1):24–36. [PMC free article] [PubMed] [Google Scholar]
- 27.Marcell TJ, Hawkins SA, Tarpenning KM, Hyslop DM, Wiswell RA. Longitudinal analysis of lactate threshold in male and female master athletes. Med Sci Sports Exerc. 2003;35(5):810–817. doi: 10.1249/01.MSS.0000065002.69572.6F [DOI] [PubMed] [Google Scholar]
- 28.Cunningham DA, Nancekievill EA, Paterson DH, Donner AP, Rechnitzer PA. Ventilation threshold and aging. J Gerontol. 1985;40(6):703–707. doi: 10.1093/geronj/40.6.703 [DOI] [PubMed] [Google Scholar]
- 29.Posner JD, Gorman KM, Klein HS, Cline CJ. Ventilatory threshold: measurement and variation with age. J Appl Physiol (1985). 1987;63(4):1519–1525. doi: 10.1152/jappl.1987.63.4.1519 [DOI] [PubMed] [Google Scholar]
- 30.Lanza IR, Bhagra S, Nair KS, Port JD. Measurement of human skeletal muscle oxidative capacity by 31P-MR spectroscopy: a cross-validation with in vitro measurements. J Magn Reson Imaging. 2011;34(5):1143–1150. doi: 10.1002/jmri.22733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuo PL, Schrack JA, Shardell MD, et al. . A roadmap to build a phenotypic metric of ageing: insights from the Baltimore Longitudinal Study of Aging. J Intern Med. 2020;287:373–394. doi: 10.1111/joim.13024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Naressi A, Couturier C, Devos JM, et al. . Java-based graphical user interface for the MRUI quantitation package. MAGMA. 2001;12(2–3):141–152. doi: 10.1007/BF02668096 [DOI] [PubMed] [Google Scholar]
- 33.Naressi A, Couturier C, Castang I, de Beer R, Graveron-Demilly D. Java-based graphical user interface for MRUI, a software package for quantitation of in vivo/medical magnetic resonance spectroscopy signals. Comput Biol Med. 2001;31(4):269–286. doi: 10.1016/s0010-4825(01)00006-3 [DOI] [PubMed] [Google Scholar]
- 34.Vanhamme L, Van Huffel S, Van Hecke P, van Ormondt D. Time-domain quantification of series of biomedical magnetic resonance spectroscopy signals. J Magn Reson. 1999;140(1):120–130. doi: 10.1006/jmre.1999.1835 [DOI] [PubMed] [Google Scholar]
- 35.Paganini AT, Foley JM, Meyer RA. Linear dependence of muscle phosphocreatine kinetics on oxidative capacity. Am J Physiol. 1997;272(2 Pt 1):C501–C510. doi: 10.1152/ajpcell.1997.272.2.C501 [DOI] [PubMed] [Google Scholar]
- 36.Prompers JJ, Wessels B, Kemp GJ, Nicolay K. Mitochondria: investigation of in vivo muscle mitochondrial function by 31P magnetic resonance spectroscopy. Int J Biochem Cell Biol. 2014;50:67–72. doi: 10.1016/j.biocel.2014.02.014 [DOI] [PubMed] [Google Scholar]
- 37.McMahon S, Jenkins D. Factors affecting the rate of phosphocreatine resynthesis following intense exercise. Sports Med. 2002;32(12):761–784. doi: 10.2165/00007256-200232120-00002 [DOI] [PubMed] [Google Scholar]
- 38.Heerschap A, Houtman C, in ‘t Zandt HJ, van den Bergh AJ, Wieringa B. Introduction to in vivo 31P magnetic resonance spectroscopy of (human) skeletal muscle. Proc Nutr Soc. 1999;58(4):861–870. doi: 10.1017/s0029665199001160 [DOI] [PubMed] [Google Scholar]
- 39.Simonsick EM, Schrack JA, Glynn NW, Ferrucci L. Assessing fatigability in mobility-intact older adults. J Am Geriatr Soc. 2014;62(2):347–351. doi: 10.1111/jgs.12638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Talbot LA, Morrell CH, Metter EJ, Fleg JL. Comparison of cardiorespiratory fitness versus leisure time physical activity as predictors of coronary events in men aged < or = 65 years and > 65 years. Am J Cardiol. 2002;89(10):1187–1192. doi: 10.1016/s0002-9149(02)02302-0 [DOI] [PubMed] [Google Scholar]
- 41.Roussel M, Bendahan D, Mattei JP, Le Fur Y, Cozzone PJ. 31P magnetic resonance spectroscopy study of phosphocreatine recovery kinetics in skeletal muscle: the issue of intersubject variability. Biochim Biophys Acta. 2000;1457(1–2):18–26. doi:S0005-2728(99)00111-5[pii]. [DOI] [PubMed] [Google Scholar]
- 42.van de Weijer T, Sparks LM, Phielix E, et al. . Relationships between mitochondrial function and metabolic flexibility in type 2 diabetes mellitus. PLoS ONE. 2013;8(2):e51648. doi: 10.1371/journal.pone.0051648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ciammola A, Sassone J, Sciacco M, et al. . Low anaerobic threshold and increased skeletal muscle lactate production in subjects with Huntington’s disease. Mov Disord. 2011;26(1):130–137. doi: 10.1002/mds.23258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bendahan D, Desnuelle C, Vanuxem D, et al. . 31P NMR spectroscopy and ergometer exercise test as evidence for muscle oxidative performance improvement with coenzyme Q in mitochondrial myopathies. Neurology. 1992;42(6):1203–1208. doi: 10.1212/wnl.42.6.1203 [DOI] [PubMed] [Google Scholar]
- 45.Grichko VP, Heywood-Cooksey A, Kidd KR, Fitts RH. Substrate profile in rat soleus muscle fibers after hindlimb unloading and fatigue. J Appl Physiol (1985). 2000;88(2):473–478. doi: 10.1152/jappl.2000.88.2.473 [DOI] [PubMed] [Google Scholar]
- 46.Lambert EV, St Clair Gibson A, Noakes TD. Complex systems model of fatigue: integrative homoeostatic control of peripheral physiological systems during exercise in humans. Br J Sports Med. 2005;39(1):52–62. doi: 10.1136/bjsm.2003.011247 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.