Abstract
Objective
Asthma often coexists with gastro-oesophageal reflux disease (GERD). The effect of proton pump inhibitors (PPIs) treatment on asthma concomitant with GERD was inconsistent. This study aimed to assess whether PPIs treatment improved morning peak expiratory flow (mPEF) in asthma patients with GERD.
Data sources
PubMed, MEDLINE, EMBASE, Web of Science, Cochrane Library and ClinicalTrials.gov; hand searching for reference lists; contacted with authors if necessary.
Study selection
All eligible trials were randomised clinical trials comparing PPIs with placebo in asthma patients accompanying with GERD.
Results
Fourteen randomised clinical trials (2182 participants) were included. Overall, PPIs versus placebo did not affect mPEF in patients with asthma having GERD (weighted mean difference 8.68 L/min, 95% CI −2.02 to 19.37, p=0.11). Trial sequential analysis (TSA) further confirmed this finding (TSA adjusted 95% CI −1.03 to 22.25). Subgroups analyses based on the percentage of patients with symptomatic GERD≥95%, treatment duration >12 weeks also found no statistically significant benefit on mPEF. Similarly, analyses of secondary outcomes (evening PEF, forced expiratory volume in 1 s, asthma symptoms score, asthma quality of life score and episodes of asthma exacerbation) did not show significant difference between PPIs and placebo.
Conclusion
In this meta-analysis, PPIs therapy did not show a statistically significant improvement on mPEF in asthma patients having GERD, neither in subgroup with symptomatic GERD nor in subgroup with treatment duration >12 weeks. This analysis does not support a recommendation for PPIs therapy as empirical treatment in asthma patients with GERD.
PROSPERO registration number
CRD42020177330.
Keywords: asthma, oesophageal disease, therapeutics
Strengths and limitations of this study.
This systematic review strictly followed the methodology recommendations of the Cochrane Handbook, together with a comprehensive literature search.
This study was carried out in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement and its study protocol was registered on PROSPERO.
We conducted trial sequential analysis in our outcomes as well as their subgroups analysis.
The current study performed a cumulative meta-analysis in all the data.
Some of the unreported raw data were still unavailable after making extensive efforts to obtain.
Introduction
Asthma is a common chronic respiratory disease affecting approximately 300 million people worldwide.1 2 Gastro-oesophageal reflux disease (GERD) develops when the reflux of gastric contents causes irritating symptoms or complications, or both.3 GERD was considered as a trigger factor for asthma. Symptoms and/or diagnosis of GERD presented in 30%–90% of patients with asthma.4–6 Association between asthma and GERD has been extensively described elsewhere.7 8 However, evidence of the causal link between asthma and GERD remains controversial. Some studies have shown that asthma may facilitate the development of GERD by the various mechanisms.7 8
Proton pump inhibitors (PPIs) were regarded as the cornerstone of antacid therapy and have been proved effective in empiric treatment of GERD.9 Given that GERD may be a risk factor for asthma, many randomised controlled trials (RCTs) were performed to identify the efficacy of different types of PPIs in the asthma patients with GERD.10–23 However, the efficacy of PPIs for the patients with asthma accompanying with GERD has been inconsistent. Previous meta-analyses have pooled the results of PPIs on asthma outcomes in children and adults, but all of them included a small sample size.24–26 The most recent systematic review examined the efficacy of PPIs treatment for the adults with asthma. However, the review only involved morning peak expiratory flow (mPEF) in subgroup of asthmatic patients diagnosed with GERD, and failed to identify the clinical characteristics of this subgroup population.27
Thus, we did a systematic review and meta-analyses to compare the effects PPIs versus placebo on asthma outcomes in the patients with GERD. Trial sequential analysis (TSA) was performed to quantify the meta-analysis monitoring boundaries and required information size (RIS) for primary outcome. Asthma outcomes included mPEF (primary outcome), evening peak expiratory flow (ePEF), forced expiratory volume in 1 s (FEV1), asthma symptoms score, asthma quality of life, episodes of asthma exacerbation.
Method and analysis
The systematic review and meta-analyses were carried out in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement. The protocol has been registered with International Prospective Register of Systematic Reviews (PROSPERO).
Eligibility criteria
Types of study
All randomised clinical trials of PPIs in the patients with asthma and GERD were included. The eligible randomised trials were required to report at least one clinical asthma outcome of interest.
Types of participants
Participants with asthma and GERD were eligible for inclusion. There were no restrictions regarding age, gender and ethnicity. Asthma was diagnosed according to doctor’s diagnosis, reported ongoing asthma-related symptoms, evidence of objective measures of lung function. GERD diagnosis based on doctors’ diagnosis, reported clinical symptoms of GERD and objective documentation.
Types of intervention and control
Trials comparing beneficial and harmful effects of PPIs with those of placebo were eligible. This review was restricted to studies with treatment duration of at least 4 weeks.27 No restrictions were imposed on drug dosage and types of PPIs which contained omeprazole, lansoprazole, pantoprazole, esomeprazole and rabeprazole. We excluded the trials that focused on the intervention with combination of PPIs and other antacids or gastrointestinal motility regulators.
Outcome measures
This review evaluated the following outcomes: mPEF, ePEF and FEV1, which were commonly used as evidence of variable expiratory airflow obstruction. Other outcomes included asthma symptoms score (validated questionnaires of all types), asthma quality of life (validated instruments of all types), episodes of asthma exacerbation and adverse events.
Information sources and search
A systematic search for evidence on the efficacy of PPIs on patients with asthma was performed through electronic databases, citation search based on reference lists and hand searching of main relevant journals. We did a search in PubMed, EMBASE, Web of Science, Cochrane Library and ClinicalTrials.gov dating from inception to 18 March 2020. No restrictions were imposed on language, publication date, publication type or publication status. The search terms and search strategies for all databases were described in online supplement 1.
bmjopen-2020-043860supp002.pdf (749KB, pdf)
Study selection
Two reviewers (ZZ and YL) independently screened titles and abstracts according to the eligibility criteria in an unblinded, standardised manner. Reviews, letters, editorials, case studies, non-human studies, study protocols, non-English-language abstract were excluded during this process. The assessments of eligible full-text articles were carried out independently by two reviewers (ZZ and YL). Disagreements between reviewers were resolved by consensus or referred to a third reviewer (JG) for resolution.
Data extraction
Two independent reviewers (ZZ and YL) extracted data from each eligible study by using a predesigned extraction form. Discrepancies were resolved by consensus or by involvement of a third author (JG). Items of characteristics of included studies were described in online supplement 1. We contacted the corresponding authors for outcomes data if required.
Risk of bias in individual studies
Two independent reviewers (ZZ and YL) evaluated risk of bias according to version 5.1.0 of Cochrane Handbook for Systematic Review of Interventions. An agreement was reached by discussion or by consultation with a third review author (JG). The domains of evaluation for all the outcomes were selection bias, performance bias, detection bias, attrition bias, reporting bias and other bias. Each potential source of bias was considered as either ‘high risk’, ‘low risk’ or ‘unclear risk’.
Statistical analysis
The weighted mean difference /standardised mean difference (SMD) and 95% CIs were calculated for continuous outcomes. The relative risk with 95% CIs was calculated for dichotomous outcomes. Predefined subgroup analysis was undertaken in accordance with patients aged 18 years and older or patients younger than 18 years, the percentage of subjects with symptomatic GERD≥95%, treatment duration (≤12 weeks vs >12 weeks) and types of PPIs (omeprazole, pantoprazole, lansoprazole, esomeprazole). Given the anticipated variability among patient characteristic and study design, a random effects model with 95% CIs was used in the forest plots (RevMan V.5.3). Statistical heterogeneity was quantified using I2 statistic, with I2 cut-off value of 25%, 50% and 75% to quantify low, moderate and high thresholds, respectively. We adopted cumulative meta-analysis in all the data and conducted sensitivity analysis and Egger’s test to identify data stability and publication bias, respectively (StataSE V.12.0). TSA (V.0.9.5.10 Beta) was performed in mPEF and ePEF to quantify meta-analysis monitoring boundaries and RIS using parameters of mean difference of mPEF=20 L/min, estimate variance from the meta-analysis of PEF data, α at 0.05, power of 80%, and I2 value of 0%.
Patient and public involvement
There was no patient or public involvement in this study.
Results
Study selection and characteristics
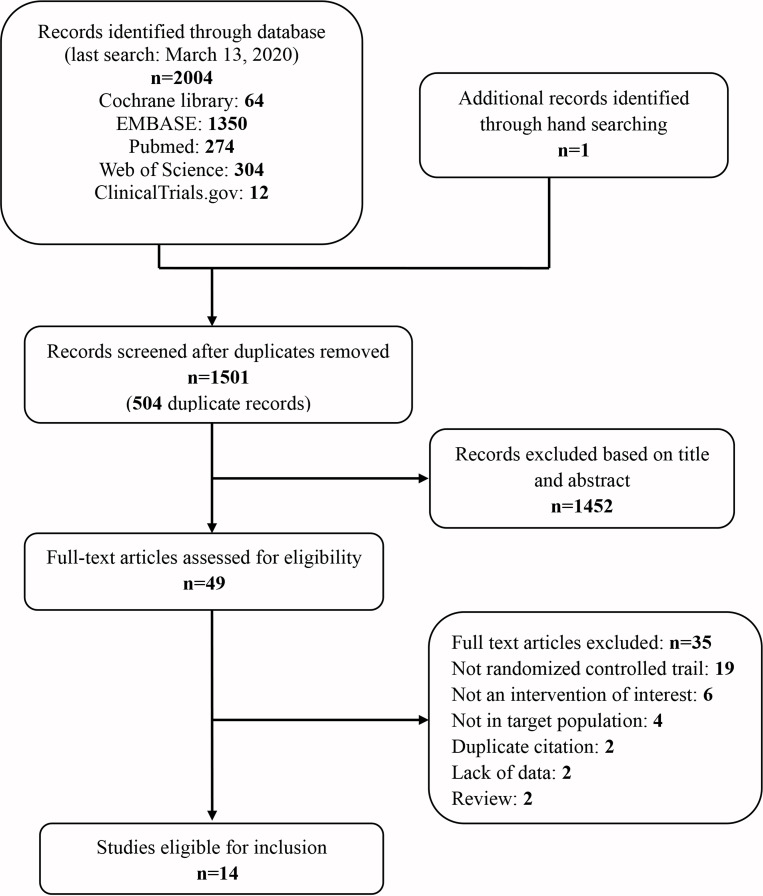
The search strategy yielded 2005 abstracts, of which 49 abstracts were retrieved and under full-articles assessment for eligible articles. All studies conducted lasted for more than 4 weeks. Of these trials, 14 RCTs were included, 6 of which were cross-over studies,10–12 14 15 20 and 8 were of a parallel design.13 16–19 21–23 The flow diagram for study inclusion is described in figure 1. Table 1 and online supplemental table 1 summarise the characteristics of the included studies (2182 participants) and the characteristics of the subjects, respectively. Of the 14 eligible trials, 12 included subjects aged ≥18 years, while only 2 aimed at patients aged <18 years (ranged from 6 to 17 years old).17 23 Mild to severe asthmatics were included. The severity of GERD was reported inconsistently among the trials. Symptoms of heartburn, regurgitation and dysphagia were the common presentations of GERD reported in most studies. The percentage of the subjects with symptomatic GERD was greater than 95% in eight studies, of which six studies reported 100%.10 11 14 17 20 22
Figure 1.
Flow diagram of identification of eligible studies for inclusion.
Table 1.
Summary of participants characteristics of included studies
| Trials | Mean (SD or range) age (years) | Male, n (%) | Severity of asthma | Severity of GERD | Complications of GERD | Symptomatic GERD (%) | Association between asthma and GERD reported | |
| Ford et al10 | 63 (50–80) | 5 (50%) | Mean PEFR before and after terbutaline use (SD), 1/min: 253 (83) and 308 (±94) | Number per grade of esophagitis: grade I (n=1), grade II (n=2), grade III (n=4); Barrett’s oesophagus (n=2) | Heartburn, regurgitation, lack of proportion | 100% | No | |
| Meier et al11 | 49 (34–63) | 9 (60%) | Not stated; inclusion criteria: reversibility of FEV1 and/ or PEF after bronchodilator use:>15% | Number per grade of oesophageal inflammation: grade I (n=1), grade II (n=4), grade III (n=8), grade IV (n=2); hiatal hernia n=10; Barrett’s oesophagus and peptic stricture n=10 | Not specified | 100% | Yes | |
| Teichtahl et al12 | 46 (12) | 12 960%) | Not stated, inclusion criteria: reversibility of FEV1 >15%; diurnal variation of PEF:>20% | GERD symptoms in all | Not specified | 95% | No | |
| Boeree et al13 | 51 (10) | 17 (47.2%) | Mean FEV1 %, pred (SD): Int 66(20); cont 75(23); mPEF mean (SD): omeprazole group 329 (91); placebo group 321 (109) | Increased gastro-oesophageal reflux reported in all | Dysphagia Int n=2/1/0, Cont n=3/0/0; heartburn Int n=9/0/0, Cont n=9/3/0; regurgitation Int n=3/0/0, Cont n=4/3/0 | 50% | No | |
| Levin et al14 | 57 (35–72) | 6 (67%) | Mean FEV1 (range): 1.9 (1.0–2.9); mean PEFR (range), L/min: mPEF 376 (283–488), ePEF 381 (286–468) | 24-hour pH monitoring, mean % time with pH <4 (range): total: 24.4 (4.7–64.0), supine: 17.6 (0–39.8), upright: 23.8 (5.6–74.4) | Not specified | 100% | No | |
| Kiljander et al15 | 49 (21–75) | 18 (35%) | Mean PEF (range) L/min, 455 (250–700); FEV1 % of predicted (range), 81 (31–114) | Median % time pH <4 (75%–25% quartiles) : total 9.0 (14.7–5.0), upright 10.1 (15.1–6.9), supine: 4.0 (15.7–0.8) | Not specified | 65% | No | |
| Littner et al16 | 47 (12) | 66 (31.9%) | Moderate-to-severe persistent asthma | Mean severity score (SD): overall reflux symptoms: Int 1.66 (0.69), Cont 1.70 (0.65)* | Patients with symptoms (%): heartburn Int 97%, Cont 95%; regurgitation Int 80%, Cont 80%; dysphagia: Int 32%, Cont 47% | Int 96.1%±8.0%, Cont 97.3%±5.2% | No | |
| Størdal et al17 | 10.2 (9.2), 11.3 (11.0) | 29 (76.3%) | GINA classification of asthma severity (step 1/2/3/4): Int 4/8/7/0, Cont 3/6/10/0 | Reflux index, mean (%, SD): Int 8.8 (4.0), Cont 9.7 (5.1); reflux index ≥10% (n): Int n=5, Cont n=6 | Not specified | 100% | No | |
| Kiljander et al18 | GERD+/NOC+ (Kiljander-1) | 46.3 | 80 (36.5%) | FEV1, % pred: Int 67.3%, Cont 66.2%; Morning PEF, % pred: Int 73.0%, Cont 73.0% | Abnormal 24 hours oesophageal pH in all | Mean number heartburn symptoms/day: (night-time) Int 0.42, Cont 0.44; (daytime) Int 0.68, Cont 0.71 | Not stated | Yes |
| GERD+/NOC− (Kiljander-2) |
44.3 | 94 (26.9%) | FEV1, % pred: Int 65.5%, Cont 67.4%; mPEF, % pred: Int 68.7%, Cont 69.2% | Abnormal 24 hours oesophageal pH in all | Mean number heartburn symptoms/day: (night-time) Int 0.46, Cont 0.47; (daytime) Int 0.68, Cont 0.62 | |||
| dos Santos et al19 | Int 40 (12), Cont 45 (12) | 9 (22.0%) | Mean FEV1 % predicted (SD): Int 61.6 (19), Cont 60.4 (19); mean diurnal PEF (SD): Int 317 (13), Cont 264 (86) | Mean GERD symptoms score (SD): Int 12.9 (9), Cont 11.4 (7) | Not specified | 80% | No | |
| Susanto et al20 | Int 42.69 (11.11), Cont 37.88 (11.01) | 9 (28.1%) | Moderate persistent asthma; mean FEV1 % prediction (SD): Int 72.9 (6.7), Cont 71.2 (7.7); mean PEFR, L/min (SD): Int 258.8 (33.2), Cont 269.5 (76.4) | One or more typical GERD symptoms in all patients with histopathological esophagitis (%): 87.5% | Heartburn: Int 68%. Cont 87%; atypical chest pain: Int 81.3%, Cont 75%, regurgitation: Int 100%, Cont 100%, dysphagia: Int 12.5%, Cont 25%, water brash: Int 37.5%, Cont 37.5% | 100% | No | |
| Mastronarde et al21 | (>18) | Not stated | Persistent and poorly controlled asthma | PH monitoring positive in all | Not specified | 0% | No | |
| Kiljander et al22 | 45 (19–70) | 233 (24.3%) | Moderate-to-severe asthma | Moderate severity | Heartburn, acid regurgitation dyspepsia | 100% | No | |
| Holbrook et al23 | (6–17) | Not stated | Poorly controlled asthma | Abnormal 24 hours oesophageal pH in all | Not specified | 0% | No | |
*An investigator-assessed scale was used, as follows: 0, none; 1, mild; 2, moderate and 3, severe.
Cont, control; FEV1, forced expiratory volume in 1 s; GERD, gastro-oesophageal reflux disease; GINA, Global Initiative for Asthma; Int, intervention; mPEF, morning peak expiratory flow; NOC, nocturnal respiratory symptoms; PEFR, peak expiratory flow; PPI, proton pump inhibitor; pred, predicted; PUD, peptic ulcer disease.;
bmjopen-2020-043860supp001.pdf (45KB, pdf)
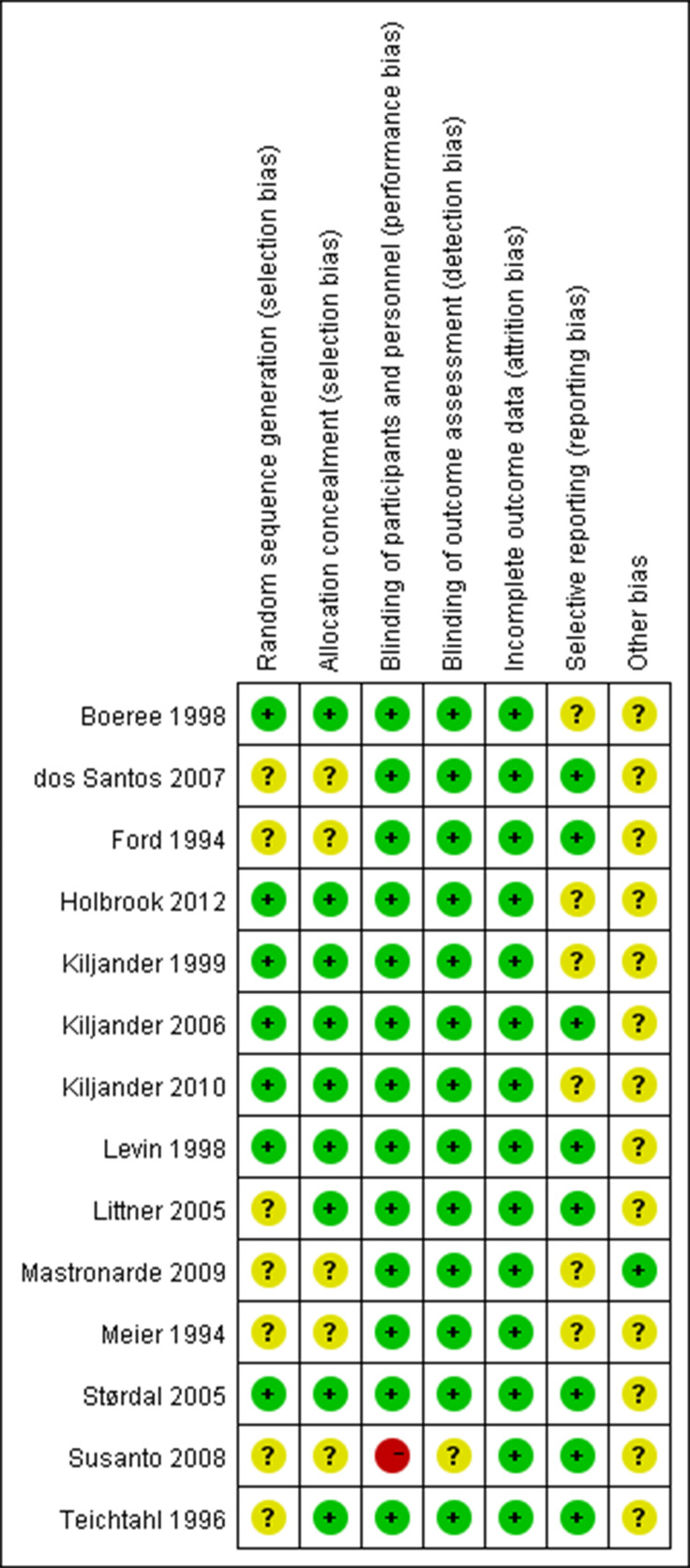
Risk of bias within studies
Each study was assessed in accordance with the Cochrane risk of bias tool (figure 2).28 Double-blinding method was adopted in all studies except one trial which used a single-blinding fashion.20 Three trials were supported by pharmaceutical companies.16 18 22
Figure 2.
Risk of bias summary displaying review authors’ judgements about each risk of bias item for each included study.
Outcomes
Fourteen included studies investigated PPIs therapy on patients with asthma and GERD (2182 patients). Asthma outcomes were reported inconsistently among studies, leading to limitation of meta-analysis (table 2). All studies reported one or more outcomes of lung function.
Table 2.
Summary of results of proton pump inhibitors treatment on asthma outcomes
| Trials | mPEF, L/min | ePEF, L/min | FEV1, L | FEV1 %, pred | Asthma symptom score | AQLQ | Episodes of asthma exacerbation |
| Ford et al10 | – | – | NA | NA | – | NA | NA |
| Meier et al11 | NA | NA | – | NA | – | NA | NA |
| Teichtahl et al12 | – | + | NA | – | NA | NA | NA |
| Boeree et al13 | – | – | – | NA | – | NA | NA |
| Levin et al14 | + | – | – | NA | NA | + | NA |
| Kiljander et al15 | – | – | +* | NA | + | NA | NA |
| Littner et al16 | – | – | – | – | – | + | + |
| Størdal et al17 | NA | NA | – | NA | – | – | NA |
| GERD+/NOC−, Kiljander-1 2006 |
– | – | NA | – | – | – | NA |
| GERD+/NOC* Kiljander-2 2006 |
+ | + | NA | – | – | – | NA |
| dos Santos et al19 | – | – | NA | – | – | + | NA |
| Susanto et al20 | + | – | NA | NA | + | NA | NA |
| Mastronarde et al21 | – | NA | – | NA | – | – | NA |
| Kiljander et al22 | – | – | + | – | + | + | |
| Holbrook et al23 | NA | NA | – | NA | NA | – | NA |
+, significant therapy effect; –, not significant therapy effect.
*Decline during omeprazole use.
AQLQ, Asthma Quality of Life Questionnaire; ePEF, evening peak expiratory flow; FEV1, forced expiratory volume in 1 s; mPEF, morning peak expiratory flow; NA, not available; pred, predicted.
Primary outcome
Morning PEF
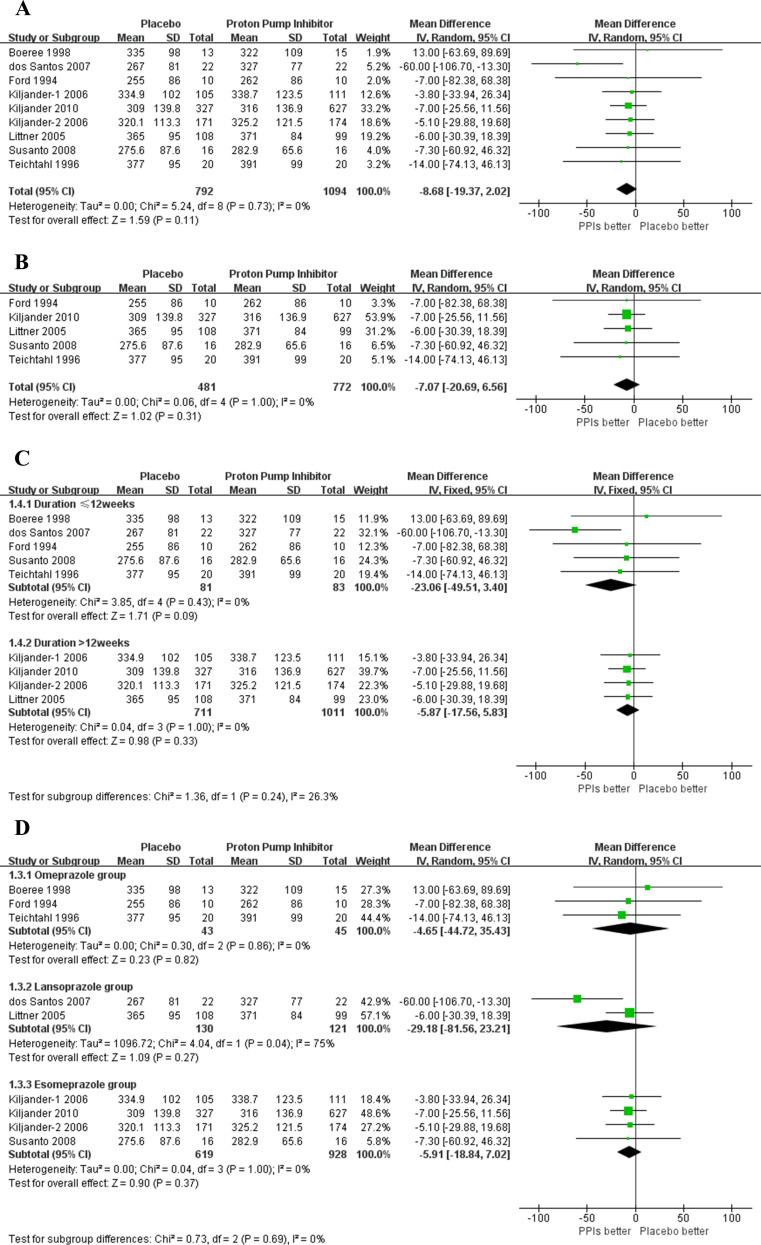
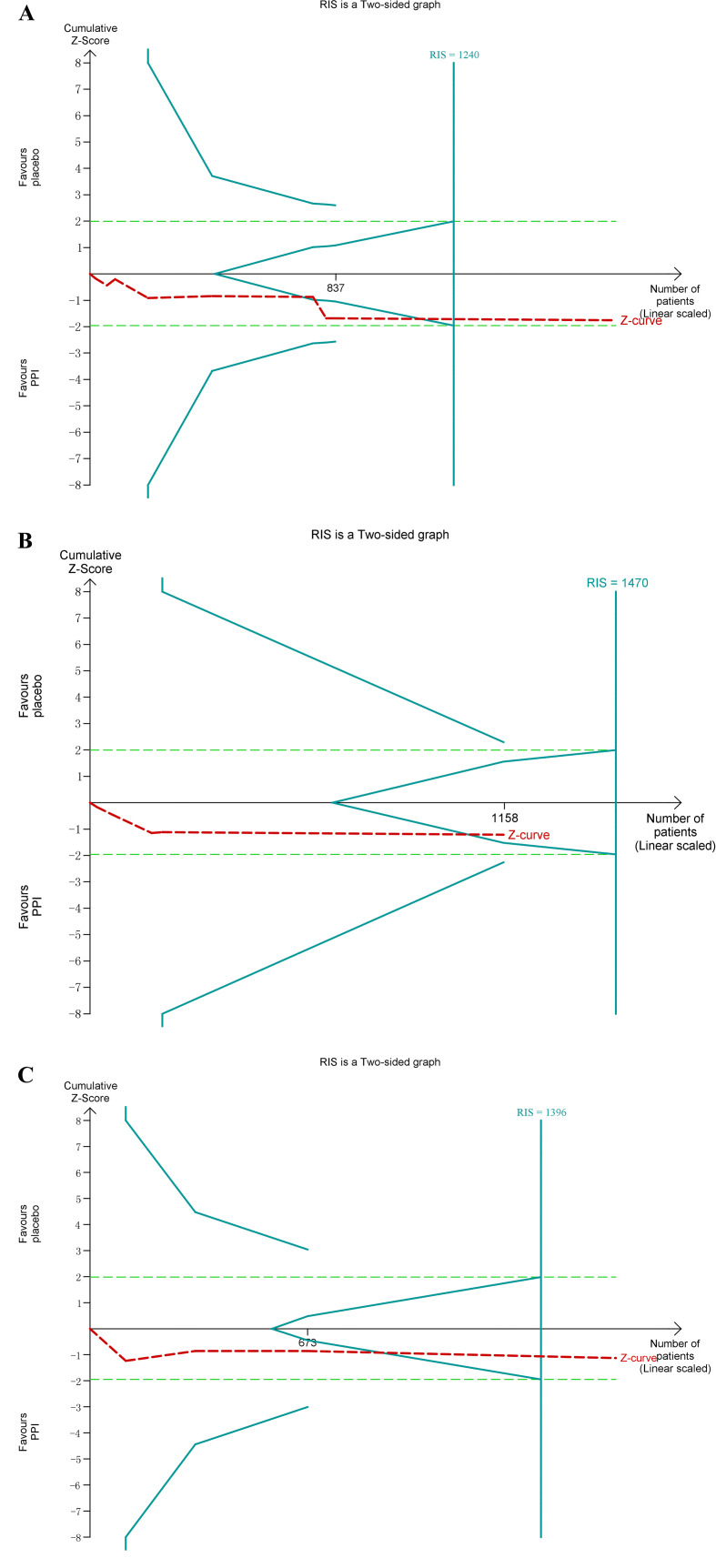
Only one of the studies with data available found a significant improvement on mPEF.19 Eight studies containing nine groups were included in meta-analysis (1886 subjects). Among the nine groups, eight showed improvement in asthma symptoms,10 12 13 16 18–20 22 but only one group did not cross the neutral (zero) line.19 The overall analysis found no statistically significant benefit on mPEF with PPIs treatment (8.68 L/min, 95% CI −2.02 to 19.37, p=0.11). Heterogeneity was absent (I2=0%; p=0.73) (figure 3A). TSA showed a heterogeneity adjusted RIS of 1240 patients without the cumulative Z curve crossing boundaries for benefit or harm (TSA adjusted 95% CI −1.03, 22.25), suggesting that PPIs may not show benefit on mPEF of the patients with asthma and GERD (figure 4A). No publication bias reported in mPEF, and the sensitivity analysis confirmed the robustness of these findings (online supplemental figure 1).
Figure 3.
(A) Forest plot for morning peak expiratory flow (mPEF). (B) Forest plot for mPEF in subgroup of the percentage of subjects with symptomatic gastro-oesophageal reflux disease ≥95%. (C) Forest plot for mPEF in subgroups of treatment duration ≤12 weeks and >12 weeks. (D) Forest plot for mPEF in subgroups of different types of proton pump inhibitors. PPIs, proton pump inhibitors.
Figure 4.
(A) Trial sequential analysis (TSA) of morning peak expiratory flow (mPEF). (B) TSA of mPEF in subgroup of the percentage of subjects with symptomatic gastro-oesophageal reflux disease ≥95%. (C) TSA of mPEF in subgroup of treatment duration >12 weeks. PPIs, proton pump inhibitors; RIS, required information size.
A subgroup was performed according to the percentage of subjects with symptomatic GERD≥95% (1253 participants). Of eight eligible studies, five reported available data for meta-analysis.10 12 16 20 22 No statistically significant effect was found for mPEF in this subgroup (7.07 L/min, 95% CI −6.56 to 20.69, p=0.31) (figure 3B). TSA showed that only 1158 (79%) of the heterogeneity adjusted RIS of 1470 patients were calculated. However, the cumulative Z curve crossed the boundaries for futility (TSA adjusted 95% CI −5.94 to 25.58) (figure 4B).
Next, we conducted subgroups analysis based on duration of PPIs treatment (duration ≤12 weeks with a population of 164 vs >12 weeks with 1722 participants). No statistically significant benefit was demonstrated in both subgroups (duration ≤12 weeks: 23.06 L/min, 95% CI −3.40 to 49.51, p=0.09, p=0.43; duration >12 weeks: 5.87 L/min, 95% CI −5.83 to 17.56, p=0.33) (figure 3C). Then we conducted TSA in the subgroup with duration >12 weeks. TSA did not alter the efficacy on mPEF with a PPIs treatment duration >12 weeks (TSA adjusted 95% CI −4.99 to 20.50) (figure 4C).
Also, three subgroups meta-analyses based on types of PPIs did not show statistically significant treatment benefit (omeprazole: 88 subjects, 4.65 L/min, 95% CI −35.43 to 44.72, p=0.82; lansoprazole: 251 subjects, 29.18 L/min, 95% CI −23.21 to 81.56, p=0.27; esomeprazole: 1547 subjects, 5.91 L/min, 95% CI −7.02 to 18.84, p=0.37) on mPEF (figure 3D).
We carried out a cumulative meta-analysis of the effect of PPIs on the mPEF and its subgroups analysis based on the data of publication. However, the effect of PPIs remained unchanged (online supplemental figure 2).
Secondary outcomes
Evening PEF
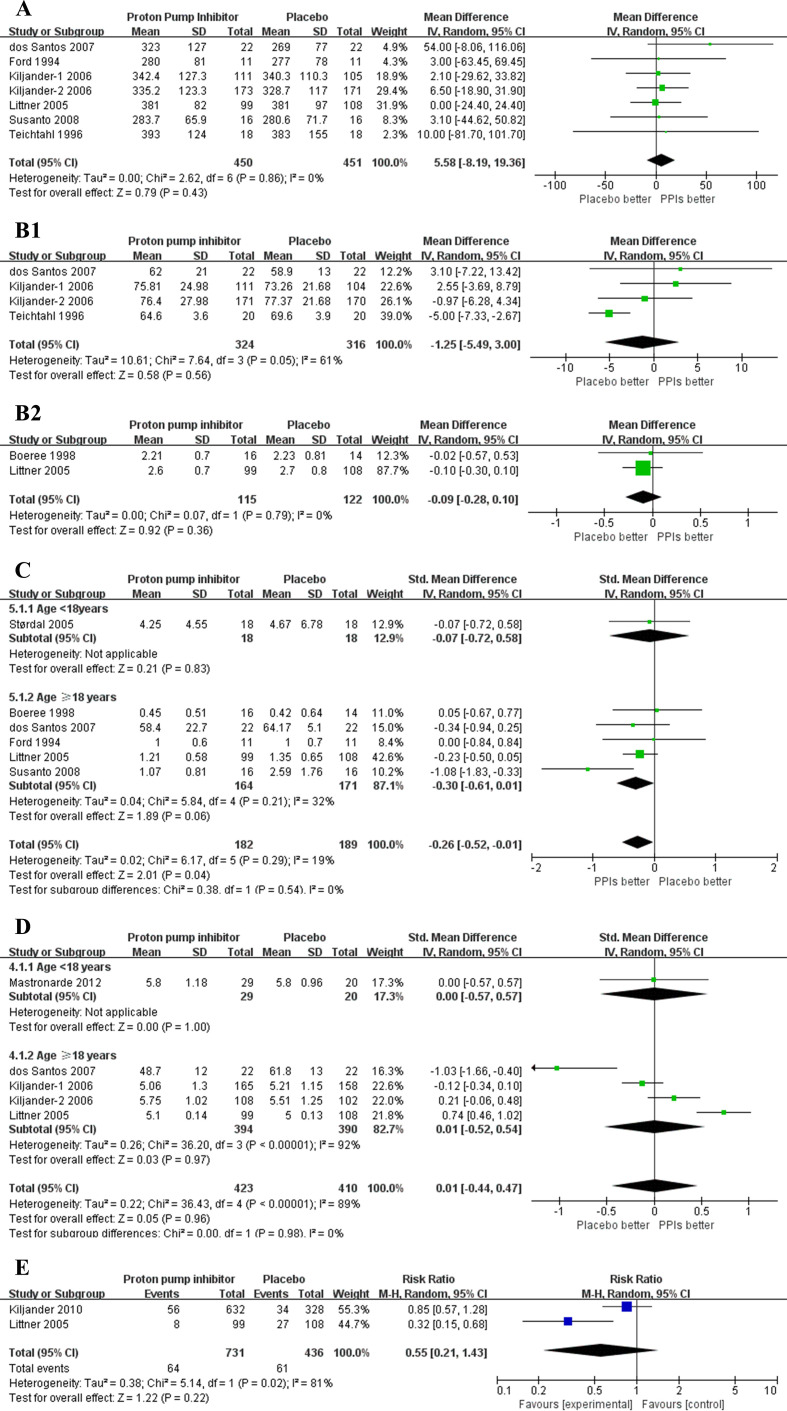
Ten trials reported ePEF of the subjects with asthma and GERD, of which two trials demonstrated statistically significant improvement on ePEF.12 18 Of these 10 trials, 6 studies provided information and were included in the meta-analyses (901 participants).10 12 16 18–20 Meta-analysis did not show statistically significant effect on ePEF (5.58 L/min; 95% CI −8.19 to 19.36, p=0.43) (figure 5A). TSA showed that the cumulative Z curve crossed boundaries for futility, suggesting no statistically significant improvement on ePEF with PPIs therapy (TSA adjusted 95% CI −6.87 to 25.35). No publication bias reported in ePEF, and the sensitivity analysis showed solid results (online supplemental figure 3A).
Figure 5.
(A) Forest plot for evening peak expiratory flow. (B1) Forest plot for FEV1 % predicted. (B2) Forest plot for FEV1 (L). (C) Forest plot for asthma symptoms score. (D) Forest plot for asthma quality of life score. (E) Forest plot for episodes of asthma exacerbation. FEV1, forced expiratory volume in 1 s; PPIs, proton pump inhibitors.
No statistically significant benefit was showed on ePEF by subgroups analyses of the studies in accordance with the percentage of subjects with symptomatic GERD≥95%, length of PPIs treatment and types of PPIs (online supplemental figure 3B).
Forced expiratory volume in 1 s
Three studies with a population of 640 provided information of FEV1 % predicted,12 18 19 and only two with 237 participants provided available data of FEV1 (L),13 16 which were included in analyses, respectively. At the analysis of FEV1 % predicted, no therapy effect was found on the patients with PPIs use (−1.25%, 95% CI −4.9 to 3.00, p=0.56) (figure 5B1). Heterogeneity was substantial (I2=61%; p=0.05). The analysis of the two studies may not demonstrated a benefit on the FEV1 (L) in the patients with PPIs therapy (−0.09 L, 95% CI −0.28 to 0.10, p=0.36)(figure 5B2). No publication reported in FEV1 % predicted, the sensitivity analysis showed robust results (online supplemental figure 4).
Asthma symptoms score
Six studies reported information of asthma symptoms score and were included in meta-analysis (371 participants).10 13 16 17 19 20 Five of six trials included the patients aged older than 18 years (335 participants). The subgroup of adults showed no statistically significant effect on asthma symptoms score with PPIs treatment (SMD −0.30, 95% CI −0.61 to 0.01, p=0.06, heterogeneity I2=32%, p=0.21). However, the analysis found a small statistically significant improvement on asthma symptoms score (SMD −0.26, 95% CI −0.52 to –0.01, p=0.04), when we pooled the studies in adults and those in children. Heterogeneity was low (I2=19%, p=0.29) (figure 5C). No publication reported in asthma symptoms score, and the sensitivity analysis showed that the results were robust (online supplemental figure 5).
Asthma quality of life
Four eligible studies were included for meta-analysis (853 subjects).16 18 19 23 The result showed no overall effect on the asthma quality of life (SMD 0.01, 95% CI −0.44 to 0.47, p=0.96). Heterogeneity was substantial (I2=89%, p<0.00001) (figure 5D). No publication bias was reported in this outcome (p=0.588), but sensitivity analysis showed the results were unstable (online supplemental figure 6). Therefore, the pooled result for asthma quality of life had limited meaning.
Episodes of asthma exacerbation
Only two studies including 1167 patients provided information of episodes of asthma exacerbation and showed an improvement in this variance.16 22 However, no effect was showed in meta-analysis (relative risk 0.55, 95% CI 0.21 to 1.43, p=0.22). Heterogeneity was substantial (I2=81%, p<0.02) (figure 5E).
Cumulative meta-analysis was performed in all the data of secondary outcomes. Similarly, except a minor improvement on asthma symptoms score, it was likely that no significant effect was found on ePEF, FEV1 % predicted, asthma quality of life and episodes of asthma exacerbation with the application of PPIs (online supplemental figure 7).
Discussion
For primary outcome mPEF, we assessed eight studies including nine independent comparisons (1886 participants) and found no statistically significant improvement with PPIs treatment in patients with asthma and GERD compared with placebo. Subgroups analyses according to duration >12 weeks and the percentage of subjects with symptomatic GERD≥95%, did not demonstrated statistically significant benefit with PPIs therapy. Also, no statistically significant improvement was observed on the secondary outcomes including ePEF, FEV1, asthma symptoms, quality of life and asthma exacerbation. These results were further confirmed by the application of TSA and cumulative meta-analysis.
To enlarge sample size, our analysis not only included trials with asthma subjects having GERD diagnosis for entry criterion, but also those reported GERD subjects in subgroups analyses.18 20 To the best of our knowledge, this analysis included the largest number of participants to date describing the effect of PPIs treatment in patients with asthma accompanying with GERD. The previous meta-analysis aiming to examine the efficacy of PPIs in the adult patients with asthma, reported a subgroup analysis based on GERD diagnosis for entry criterion with seven trials (1004 patients).27 In contrast to our study, a small statistically significant improvement was reported for mPEF in this subgroup, therefore, this analysis might overestimate the benefits on mPEF and exaggerate the effect of positive improvement, because of incomplete and inadequate population inclusion. However, in line with our results, this previous review did not show benefit on in patients with asthma with PPIs treatment on ePEF, FEV1, asthma symptoms score and asthma quality of life.
A study reported that the minimal patient perceivable improvement differences for PEF was 18.79 L/min.29 The minimal difference in PEF ranging from 15 to 20 L/min were summarised in a review.30 Our analysis found that the pooled mean difference for mPEF and ePEF were 7.30 and 5.58 L/min, respectively, which were far smaller than the minimal effective line, probably showing a lack of evidence to believe the efficacy of PPIs. In alignment with our study, previous meta-analysis published by Cochrane Collaboration found no statistically significant improvement on mPEF and ePEF.25 Also, a recent large three-arms RCT was consistent with our study.22
Several trials have reported that PPIs played no role in asthma patients with asymptomatic GERD, whether in children or adults.21 23 Similarly, in our subgroup meta-analysis, no statistically significant benefit appeared for mPEF in asthma patients with symptomatic GERD. This result was in keeping with a large trial including all asthma participants with symptomatic GERD.22 Our subgroup analysis for mPEF based on duration >12 weeks was conducted, suggesting that no improvement appeared with PPIs therapy. In agreement with our result, two large trials did not find improvement for mPEF with PPIs treatment for 24 or 26 weeks.16 22
Mechanistically, GERD may trigger asthma via directly damage to the respiratory tree leading to bronchoconstriction by micro-aspiration of gastric or duodenal (or both) contents.31 32 Previous studies have reported that bile acids and pepsin were found graft failure in lung transplant patients, indicating that acid materials may not be the only one of many irritants in the aspirate during gastro-oesophageal reflux.33 34
PPIs treatment significantly improved asthma symptoms and lung function in patients with exercise-triggered asthma, with asthma and nocturnal respiratory symptoms, or taking LABAs.18 35 It appeared that benefits of PPIs may be restricted to patients with certain types or status of asthma. Further studies are warranted to examine the pathophysiological mechanism to determine the causality between asthma and GERD. Notably, if the improvement for asthma conditions were delayed or required more time to present, then the overall effect may be underestimated. Thus, further RCTs should be conducted with a treatment period for more than 6 months. Previous RCTs combined omeprazole and domperidone therapy in patients with asthma and GERD, showing that combined therapy improved asthma symptoms and lung function with treatment period of 12 or 16 weeks.36 37 Therefore, the efficacy of combined therapy should be further explored. Furthermore, we hopefully expect the effect of genotype-tailored PPIs in patients with asthma and comorbid GERD.38
There are several limitations in the present study. First, we could not extract the data from all the 11 eligible trials reporting mPEF, because of the unavailable reported form (mean difference only,14 medians and quartiles15) or unavailable data in subgroup.21 However, the overall sample size of these three trials was small and we do not think these studies would make a significant difference in our meta-analysis. Second, we could not perform a subgroup according to the severity of asthma or GERD as expected, because the severity reported inconsistently and we could not sort out the disease status of each trial. Third, only two RCTs in children were eligible in the present study, making it difficult to evaluate the effect for PPIs on all outcomes in children.17 23 However, both trials reported no improvement for PPIs in all the asthma outcomes, which were in line with the overall effect in adults in our analysis.
Conclusion
Compared with placebo, PPIs therapy for asthma patients with GERD did not show statistically significant improvement in mPEF. This futility did not alter in asthma patients neither with symptomatic GERD nor with PPIs treatment for more than 12 weeks. This analysis does not support a recommendation for the empirical use of PPIs therapy in asthma patients having GERD.
Supplementary Material
Acknowledgments
The authors would like to thank Dr Xiaoling Li for her suggestions on grammar and vocabularies of this manuscript. We would also like to thank Dr Xiaorui Lyu (Department of Endocrinology, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College) for his advice on data analysis data extraction. This work was supported by the National Natural Science Foundation of China (Grant No. 81970025).
Footnotes
ZZ and YL contributed equally.
Contributors: ZZ led the meta-analysis was involved at every stage, including protocol development, screening, data extraction, quality assessment, data analysis and manuscript drafting. YL was involved in screening, data extraction, quality assessment, interpretation of results and manuscript revisions. JL facilitated manuscript revisions for important intellectual content. JG supervised this review and was involved in protocol preparation, consensus on disagreement in data extraction, quality assessment, data analysis, interpretation of results, manuscript drafting and revisions.
Funding: This work was supported by the National Natural Science Foundation of China (81970025).
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
No data are available. No additional data are available.
Ethics statements
Patient consent for publication
Not required.
References
- 1.Li X, Cao X, Guo M, et al. Trends and risk factors of mortality and disability adjusted life years for chronic respiratory diseases from 1990 to 2017: systematic analysis for the global burden of disease study 2017. BMJ 2020;368:m234. 10.1136/bmj.m234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Global Initiative for Asthma . Global strategy for asthma management and prevention, 2019. Available: www.ginasthma.org
- 3.Vakil N, van Zanten SV, Kahrilas P, et al. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol 2006;101:1900–20. 10.1111/j.1572-0241.2006.00630.x [DOI] [PubMed] [Google Scholar]
- 4.Kiljander TO, Laitinen JO. The prevalence of gastroesophageal reflux disease in adult asthmatics. Chest 2004;126:1490–4. 10.1378/chest.126.5.1490 [DOI] [PubMed] [Google Scholar]
- 5.Sandur V, Murugesh M, Banait V, et al. Prevalence of gastro-esophageal reflux disease in patients with difficult to control asthma and effect of proton pump inhibitor therapy on asthma symptoms, reflux symptoms, pulmonary function and requirement for asthma medications. J Postgrad Med 2014;60:282–6. 10.4103/0022-3859.138754 [DOI] [PubMed] [Google Scholar]
- 6.Broers C, Tack J, Pauwels A. Review article: gastro-oesophageal reflux disease in asthma and chronic obstructive pulmonary disease. Aliment Pharmacol Ther 2018;47:176–91. 10.1111/apt.14416 [DOI] [PubMed] [Google Scholar]
- 7.Harding SM. Gastroesophageal reflux and asthma: insight into the association☆☆☆★. J Allergy and Immunol 1999;104:251–9. 10.1016/S0091-6749(99)70360-X [DOI] [PubMed] [Google Scholar]
- 8.Zerbib F, Guisset O, Lamouliatte H, et al. Effects of bronchial obstruction on lower esophageal sphincter motility and gastroesophageal reflux in patients with asthma. Am J Respir Crit Care Med 2002;166:1206–11. 10.1164/rccm.200110-033OC [DOI] [PubMed] [Google Scholar]
- 9.Gyawali CP, Fass R. Management of gastroesophageal reflux disease. Gastroenterology 2018;154:302–18. 10.1053/j.gastro.2017.07.049 [DOI] [PubMed] [Google Scholar]
- 10.Ford GA, Oliver PS, Prior JS, et al. Omeprazole in the treatment of asthmatics with nocturnal symptoms and gastro-oesophageal reflux: a placebo-controlled cross-over study. Postgrad Med J 1994;70:350–4. 10.1136/pgmj.70.823.350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meier JH, McNally PR, Punja M. Does omeprazole (Prilosec) improve respiratory function in asthmatics with gastroesophageal reflux? A double-blind, placebo-controlled crossover study. Dig Dis Sci 1994;39:2127–33. [DOI] [PubMed] [Google Scholar]
- 12.Teichtahl H, Kronborg IJ, Yeomans ND, et al. Adult asthma and gastro-oesophageal reflux: the effects of omeprazole therapy on asthma. Aust N Z J Med 1996;26:671–6. 10.1111/j.1445-5994.1996.tb02938.x [DOI] [PubMed] [Google Scholar]
- 13.Boeree MJ, Peters FTM, Postma DS, et al. No effects of high-dose omeprazole in patients with severe airway hyperresponsiveness and (a)symptomatic gastro-oesophageal reflux. Eur Respir J 1998;11:1070–4. 10.1183/09031936.98.11051070 [DOI] [PubMed] [Google Scholar]
- 14.Levin TR, Sperling RM, McQuaid KR. Omeprazole improves peak expiratory flow rate and quality of life in asthmatics with gastroesophageal reflux. Am J Gastroenterol 1998;93:1060–3. 10.1111/j.1572-0241.1998.329_q.x [DOI] [PubMed] [Google Scholar]
- 15.Kiljander TO, Salomaa ER, Hietanen EK. Gastroesophageal reflux in asthmatics: a double-blind, placebo-controlled crossover study with omeprazole. Chest 1999;116:1257–64. [DOI] [PubMed] [Google Scholar]
- 16.Littner MR, Leung FW, Ballard ED, et al. Effects of 24 weeks of lansoprazole therapy on asthma symptoms, exacerbations, quality of life, and pulmonary function in adult asthmatic patients with acid reflux symptoms. Chest 2005;128:1128–35. 10.1378/chest.128.3.1128 [DOI] [PubMed] [Google Scholar]
- 17.Størdal K, Johannesdottir GB, Bentsen BS, et al. Acid suppression does not change respiratory symptoms in children with asthma and gastro-oesophageal reflux disease. Arch Dis Child 2005;90:956–60. 10.1136/adc.2004.068890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kiljander TO, Harding SM, Field SK, et al. Effects of esomeprazole 40 Mg twice daily on asthma: a randomized placebo-controlled trial. Am J Respir Crit Care Med 2006;173:1091–7. 10.1164/rccm.200507-1167OC [DOI] [PubMed] [Google Scholar]
- 19.dos Santos LH, Ribeiro IOeS, Sánchez PG, et al. Evaluation of pantoprazol treatment response of patients with asthma and gastroesophageal reflux: a randomized prospective double-blind placebo-controlled study. J Bras Pneumol 2007;33:119–27. 10.1590/s1806-37132007000200004 [DOI] [PubMed] [Google Scholar]
- 20.Susanto AD, Yunus F, Wiyono WH, et al. Asthma symptoms improvement in moderate persistent asthma patients with gastroesophageal reflux disease (GERD): the role of proton-pump inhibitor. Medical Journal of Indonesia 2008;17:169–74. 10.13181/mji.v17i3.317 [DOI] [Google Scholar]
- 21.American Lung Association Asthma Clinical Research Centers, Mastronarde JG, Anthonisen NR, et al. Efficacy of esomeprazole for treatment of poorly controlled asthma. N Engl J Med 2009;360:1487–99. 10.1056/NEJMoa0806290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kiljander TO, Junghard O, Beckman O, et al. Effect of esomeprazole 40 Mg once or twice daily on asthma: a randomized, placebo-controlled study. Am J Respir Crit Care Med 2010;181:1042–8. 10.1164/rccm.200910-1537OC [DOI] [PubMed] [Google Scholar]
- 23.Writing Committee for the American Lung Association Asthma Clinical Research Centers, Holbrook JT, Wise RA, et al. Lansoprazole for children with poorly controlled asthma: a randomized controlled trial. JAMA 2012;307:373–80. 10.1001/jama.2011.2035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coughlan JL, Gibson PG, Henry RL. Medical treatment for reflux oesophagitis does not consistently improve asthma control: a systematic review. Thorax 2001;56:198–204. 10.1136/thorax.56.3.198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gibson PG, Henry RL, Coughlan JL. Gastro-Oesophageal reflux treatment for asthma in adults and children. Cochrane Database Syst Rev 2003;2:Cd001496. 10.1002/14651858.CD001496 [DOI] [PubMed] [Google Scholar]
- 26.Sopo SM, Radzik D, Calvani M. Does treatment with proton pump inhibitors for gastroesophageal reflux disease (GERD) improve asthma symptoms in children with asthma and GERD? A systematic review. J Investig Allergol Clin Immunol 2009;19:1–5. [PubMed] [Google Scholar]
- 27.Chan WW, Chiou E, Obstein KL, et al. The efficacy of proton pump inhibitors for the treatment of asthma in adults. Arch Intern Med 2011;171:620–9. 10.1001/archinternmed.2011.116 [DOI] [PubMed] [Google Scholar]
- 28.Higgins JPT, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Santanello Nc, Zhang J, Seidenberg B, et al. What are minimal important changes for asthma measures in a clinical trial? Eur Respir J 1999;14:23–7. 10.1034/j.1399-3003.1999.14a06.x [DOI] [PubMed] [Google Scholar]
- 30.Reddel HK, Taylor DR, Bateman ED. An official American thoracic Society/European respiratory Society statement: asthma control and exacerbations: standardizing endpoints for clinical asthma trials and clinical practice. Am J Respir Crit Care Med 2009;180:59–99. [DOI] [PubMed] [Google Scholar]
- 31.Hom C, Vaezi MF. Extra-esophageal manifestations of gastroesophageal reflux disease: diagnosis and treatment. Drugs 2013;73:1281–95. 10.1007/s40265-013-0101-8 [DOI] [PubMed] [Google Scholar]
- 32.Naik RD, Vaezi MF. Extra-esophageal gastroesophageal reflux disease and asthma: understanding this interplay. Expert Rev Gastroenterol Hepatol 2015;9:969–82. 10.1586/17474124.2015.1042861 [DOI] [PubMed] [Google Scholar]
- 33.Vos R, Blondeau K, Vanaudenaerde BM, et al. Airway colonization and gastric aspiration after lung transplantation: do birds of a feather flock together? J Heart Lung Transplant 2008;27:843–9. 10.1016/j.healun.2008.05.022 [DOI] [PubMed] [Google Scholar]
- 34.Griffin SM, Robertson AGN, Bredenoord AJ, et al. Aspiration and allograft injury secondary to gastroesophageal reflux occur in the immediate post-lung transplantation period (prospective clinical trial). Ann Surg 2013;258:705–12. 10.1097/SLA.0b013e3182a6589b [DOI] [PubMed] [Google Scholar]
- 35.Peterson KA, Samuelson WM, Ryujin DT, et al. The role of gastroesophageal reflux in exercise-triggered asthma: a randomized controlled trial. Dig Dis Sci 2009;54:564–71. 10.1007/s10620-008-0396-6 [DOI] [PubMed] [Google Scholar]
- 36.Sharma B, Sharma M, Daga MK, et al. Effect of omeprazole and domperidone on adult asthmatics with gastroesophageal reflux. World J Gastroenterol 2007;13:1706–10. 10.3748/wjg.v13.i11.1706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bediwy AS, Al-Biltagi M, Amer HG, et al. Combination therapy versus monotherapy for gastroesophageal reflux in children with difficult-to-treat bronchial asthma. Egypt J Chest Dis Tuberc 2014;63:33–8. 10.1016/j.ejcdt.2013.10.014 [DOI] [Google Scholar]
- 38.Tang M, Blake KV, Lima JJ, et al. Genotype tailored treatment of mild symptomatic acid reflux in children with uncontrolled asthma (GenARA): rationale and methods. Contemp Clin Trials 2019;78:27–33. 10.1016/j.cct.2019.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2020-043860supp002.pdf (749KB, pdf)
bmjopen-2020-043860supp001.pdf (45KB, pdf)
Data Availability Statement
No data are available. No additional data are available.