Abstract
Introduction
Total mesorectal excision (TME), chemotherapy (CT), and radiotherapy (RT) are usually integrated into the comprehensive treatment of stage II/III rectal cancer (RC). Neoadjuvant radiotherapy (nRT) has become the standard treatment for stage II/III RC patients to help reduce the size of a tumor or kill cancer cells that have spread. Adjuvant RT is delivered after the resection to destroy remaining cancer cells and used mainly in stage II/III RC patients who have not received preoperative radiotherapy, such as those who suffered from a bowel obstruction before surgery. It is controversial whether radiotherapy can improve the survival of stage II/III RC patients. An increasing number of studies have reported that rectal cancer exhibited mismatched biology, epidemiology, and therapeutic response to current treatment strategy in different age groups. It is necessary to investigate whether radiotherapy exhibits disparate effects in different age groups of patients with stage II/III RC.
Methods
Data from the Surveillance, Epidemiology, and End Results (SEER) Program was extracted to identify stage II/III RC diagnosed in the periods of 2004–2016. The statistical methods included Pearson’s chi-square test, log-rank test, Cox regression model, and propensity score matching.
Results
Neoadjuvant radiotherapy (nRT) cannot improve the prognosis, and postoperative RT may even reduce the survival time for early onset stage II/III RC. Postoperative RT was not able to improve the overall survival (OS), while nRT may provide limited survival improvement for middle-aged stage II/III RC patients. In addition, radiotherapy can significantly improve the prognosis for elderly stage II/III RC.
Conclusions
This study indicated the inconsistent survival effect of radiotherapy on stage II/III rectal cancer patients in different age groups. Hence, we formulated a novel flow chart of radiotherapy decision-making based on age in stage II/III RC patients.
Keywords: radiotherapy; age; overall survival; SEER (the Surveillance, Epidemiology, and End Results) database; rectal cancer
Introduction
Colorectal cancer (CRC) is ranked in the top third malignancy in males and the second in females (1) and includes approximately 30–50% rectal cancer (RC) (2). Total mesorectal excision (TME), chemotherapy (CT) and radiotherapy (RT) are usually integrated into the comprehensive treatment of stage II/III RC.
RT, which directly delivers ionizing radiation to the target area including the primary tumor and regional lymph nodes, may cause genetic damage, such as irradiation-induced DNA double-strand breaks, and can ultimately lead to apoptosis (3). Neoadjuvant radiotherapy (nRT) has become the standard treatment for stage II/III RC patients to help reduce the size of a tumor or kill cancer cells that have spread. Adjuvant RT is delivered after the resection to destroy remaining cancer cells and used mainly in stage II/III RC patients who have not received preoperative radiotherapy, such as those who suffered from a bowel obstruction before surgery. Prior clinical studies demonstrated that nRT could provide benefits to solid malignancies by inducing tumor downstaging and reducing local recurrence (4–6). However, it is controversial whether radiotherapy, including nRT and adjuvant RT, can improve the survival of stage II/III RC patients. A meta-analysis indicated that the survival benefits from radiotherapy failed to reach statistical significance in patients with rectal cancer (7). Nonetheless, another clinical research study illustrated that radiotherapy was able to prolong survival of rectal cancer patients (8).
An increasing number of studies have reported that rectal cancer exhibited mismatched biology, epidemiology, and therapeutic response to current treatment strategy in different age groups (9). Early onset rectal cancer patients are correlated with more unfavorable phenotypes and more aggressive biological behaviors (10). Meanwhile, as the incidence of rectal cancer in elderly patients has decreased, an inverse trend has been monitored in adults younger than age 50 (early onset RC) (11). In addition, younger RC patients tend to present with advanced disease, with more than 60% of those with early onset RC diagnosed with lymph nodes and even distant metastasis (12). Furthermore, a previous study found that young individuals failed to obtain equivalent survival benefits from chemotherapy compared to elderly patients with colorectal cancer (13). It is necessary to investigate whether radiotherapy exhibits disparate effects in different age groups of patients with stage II/III RC.
To address this gap in our knowledge and to verify the hypothesis that the survival effect of radiotherapy in stage II/III RC patients may be inconsistent among different age groups, we aimed to analyze overall survival by dividing stage II/III RC into early onset, middle-aged, and elderly cohorts. This work queried a large national database, specifically the Surveillance, Epidemiology, and End Results (SEER) linked database, for patients with rectal cancer who underwent proctectomy treatment to compare outcomes in cohorts defined by adherence to radiotherapy and age.
Materials And Methods
Patient Screening
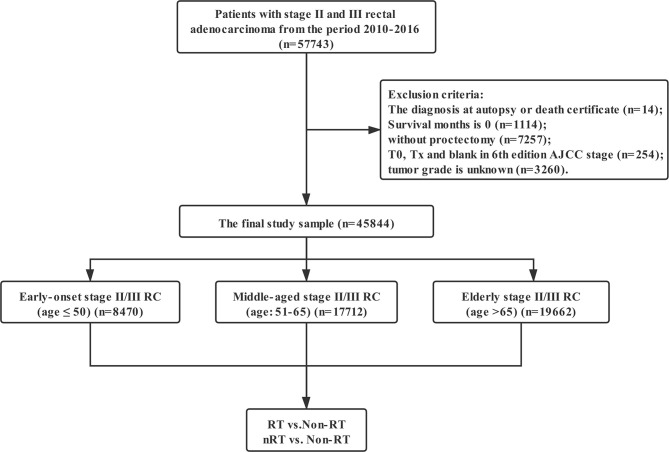
Data in this retrospective analysis were extracted from the SEER database, which collects data on cancer cases from various locations and sources throughout the United States. SEER is supported by the Surveillance Research Program (SRP) in NCI’s Division of Cancer Control and Population Sciences (DCCPS). The target population was limited to patients with stage II and III rectal adenocarcinoma (ICD-O-3: 8140, 8141, 8144, 8201, 8210, 8211, 8213, 8245, 8255, 8260, 8261, 8262, 8263, 8310, 8323, 8480, 8481, 8490) diagnosed in the periods of 2004–2016, 57,743 patients in total. Exclusion criteria: the diagnosis at autopsy or death certificate (n = 14); Survival months is 0 (n = 1,114); without proctectomy (n = 7,257); T0, Tx, and blank in 6th edition AJCC stage (n = 254); tumor grade is unknown (n = 3,260). The final study sample contained 45,844 patients (Figure 1).
Figure 1.
The flow diagram.
Statistical Analysis
Intergroup comparisons were analyzed using Pearson’s chi-square test. Log-rank test was used to compare overall survival (OS) between different groups. A hazard ratio (HR) and a 95% confidence interval (CI) were evaluated by a multivariate Cox proportional hazards regression model. All variables were included directly in the Cox regression model for multivariate analysis. In order to eliminate the influence of other variables, we conducted a propensity score matching (PSM). Statistical analyses were performed with IBM SPSS statistics trial ver. 25.0 (IBM, Armonk, NY, USA). All reported p-values lower than 0.05 were considered significant.
Results
Patients Characteristics
The characteristics of 45,844 patients with locally advanced rectal cancer enrolled from the SEER database are summarized in Table 1. The total population included 8,470 patients with early onset stage II/III RC (age ≤ 50), 17,712 cases of middle-aged stage II/III RC (age: 51–65), and 19,662 elderly patients with stage II/III RC (age > 65). The three groups exhibited significant differences regarding clinicopathological factors. The ratio of T3–4 in the elderly stage II/III RC patients was significantly higher than that in the other two cohorts (p < 0.001). The proportion of stage II/III RC patients with metastatic lymph nodes was the highest among the early onset group (5,658, 66.80%), followed by middle-aged (10,708, 60.46%) and older adults (10,435, 53.07%) (p < 0.001). Moreover, stage II/III RC treatment decision-making and execution seem to be affected by age. Elderly stage II/III RC patients tend to give up chemotherapy (non-CT: 44.12%) and radiotherapy (non-RT: 53.38%) as well as received proctectomy with RNE <12 (34.97%) compared to early onset (non-CT: 12.66%; non-RT: 28.85%; RNE < 12: 24.88%), and middle-aged patients (non-CT: 19.00%; non-RT: 33.17%; RNE < 12: 30.49%).
Table 1.
Characteristics of stage II/III rectal cancer.
| Characteristics | Total (n = 45,844) | Early onset stage II/III RC (n = 8,470) | Middle-aged stage II/III RC (n = 17,712) | Elderly stage II/III RC (n = 19,662) | p-value | ||||
|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | N | % | ||
| Gender | 0.004 | ||||||||
| Female | 18,905 | 41.2% | 3,685 | 43.5% | 6,691 | 37.8% | 8529 | 43.4% | |
| Male | 26,939 | 58.8% | 4,785 | 56.5% | 11,021 | 62.2% | 11133 | 56.6% | |
| Marital status | <0.001 | ||||||||
| Married | 26,541 | 57.9% | 5,175 | 61.1% | 10,838 | 61.2% | 10528 | 53.5% | |
| Unmarried/NOS | 19,303 | 42.1% | 3,295 | 38.9% | 6,874 | 38.8% | 9134 | 46.5% | |
| Race | <0.001 | ||||||||
| White | 37,052 | 80.8% | 6,668 | 78.7% | 14,095 | 79.6% | 16289 | 82.8% | |
| Non-white | 8,792 | 19.2% | 1,802 | 21.3% | 3,617 | 20.4% | 3373 | 17.2% | |
| Pathologic grade | 0.816 | ||||||||
| Grade I/II | 38,411 | 83.8% | 7,006 | 82.7% | 15,008 | 84.7% | 16397 | 83.4% | |
| Grade III/IV | 7,433 | 16.2% | 1,464 | 17.3% | 2,704 | 15.3% | 3265 | 16.6% | |
| Histologic type | 0.110 | ||||||||
| Adenocarcinomas | 42,518 | 92.7% | 7,839 | 92.6% | 16,526 | 93.3% | 18153 | 92.3% | |
| MCC/SRCC | 3,326 | 7.3% | 631 | 7.4% | 1,186 | 6.7% | 1509 | 7.7% | |
| T staging | <0.001 | ||||||||
| T1–2 | 4,874 | 10.6% | 985 | 11.6% | 2,026 | 11.4% | 1863 | 9.5% | |
| T3–4 | 40,970 | 89.4% | 7,485 | 88.4% | 15,686 | 88.6% | 17799 | 90.5% | |
| N staging | <0.001 | ||||||||
| N0 | 19,043 | 41.5% | 2,812 | 33.2% | 7,004 | 39.5% | 9227 | 46.9% | |
| N+ | 26,801 | 58.5% | 5,658 | 66.8% | 10,708 | 60.5% | 10435 | 53.1% | |
| Radiotherapy | <0.001 | ||||||||
| Non-RT | 18,814 | 41.0% | 2,444 | 28.8% | 5,875 | 33.2% | 10495 | 53.4% | |
| RT | 8,149 | 17.8% | 1,626 | 19.2% | 3,510 | 19.8% | 3013 | 15.3% | |
| nRT | 18,881 | 41.2% | 4,400 | 52.0% | 8,327 | 47.0% | 6154 | 31.3% | |
| Chemotherapy | <0.001 | ||||||||
| No | 13,112 | 28.6% | 1,072 | 12.7% | 3,366 | 19.0% | 8674 | 44.1% | |
| Yes | 32,732 | 71.4% | 7,398 | 87.3% | 14,346 | 81.0% | 10988 | 55.9% | |
| RNE | <0.001 | ||||||||
| <12 | 14,382 | 31.4% | 2,107 | 24.9% | 5,400 | 30.5% | 6875 | 35.0% | |
| ≥12 | 31,145 | 67.9% | 6,294 | 74.3% | 12,200 | 68.9% | 12651 | 64.3% | |
| NOS | 317 | 0.7% | 69 | 0.8% | 112 | 0.6% | 136 | 0.7% | |
| CEA | <0.001 | ||||||||
| Negative | 16,429 | 35.8% | 3,389 | 40.0% | 6,594 | 37.2% | 6446 | 32.8% | |
| Positive | 12,413 | 27.1% | 2,205 | 26.0% | 4,914 | 27.8% | 5294 | 26.9% | |
| NOS | 17,002 | 37.1% | 2,876 | 34.0% | 6,204 | 35.0% | 7922 | 40.3% | |
| Tumor size (cm) | <0.001 | ||||||||
| ≤5cm | 27,656 | 60.3% | 4,858 | 57.4% | 10,610 | 59.9% | 12188 | 62.0% | |
| >5cm | 13,287 | 29.0% | 2,562 | 30.2% | 5,013 | 28.3% | 5712 | 29.0% | |
| NOS | 4,901 | 10.7% | 1,050 | 12.4% | 2,089 | 11.8% | 1762 | 9.0% | |
MCC, mucinous cell carcinoma; SRCC, signet ring cell carcinoma; RNE, regional nodes examined; nRT: neoradiotherapy; RT, radiotherapy (not neoadjuvant); NOS, not otherwise specified.
The Effect of Radiotherapy on Early Onset Stage II/III RC
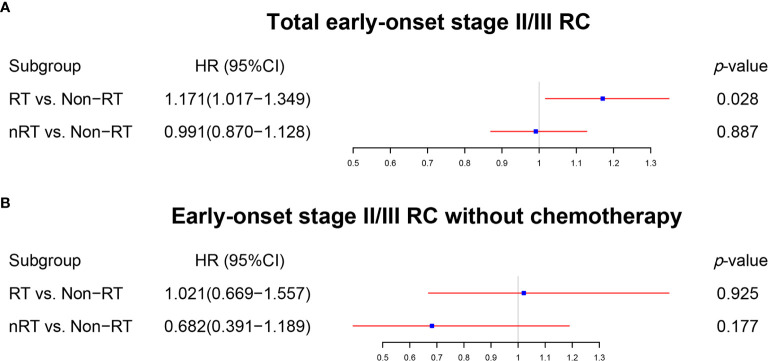
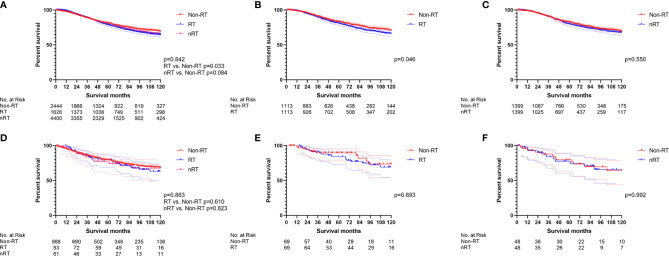
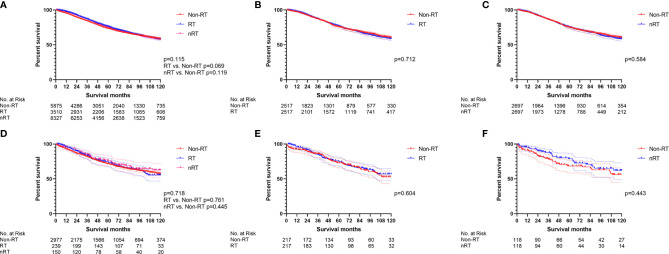
The survival effect of radiotherapy on early onset stage II/III RC was analyzed first. The multivariate Cox regression models (Figure 2A, Table S1) displayed that nRT was not able to improve OS (p = 0.887), while RT played a risk factor (p = 0.028, HR = 1.171) in early onset stage II/III RC patients. Similar results were obtained by the survival analysis before PSM (Figure 3A; RT vs. non-RT: p = 0.033; nRT vs. non-RT: p = 0.084). PSM was then used to eliminate the influence of other variables (Table S2). Unfortunately, early onset stage II/III RC patients with radiotherapy developed worse OS compared to those without radiotherapy (Figure 3B, p = 0.046). In addition, there was no significant survival difference between nRT and non-RT (Figure 3C, p = 0.550). Afterward, for further analysis, patients without chemotherapy were extracted to eliminate the impact of chemotherapy used. Both the multivariate Cox regression analysis (Figure 2B, Table S1) and the survival analysis before PSM (Figure 3D) indicated that radiotherapy, including nRT and RT, cannot significantly affect the OS of early onset stage II/III RC patients. The survival curves after PSM (Table S2) confirmed the previous results (Figure 3E: RT vs. non-RT, p = 0.693; Figure 3F: nRT vs. non-RT, p = 0.992). Collectively, nRT cannot improve the prognosis, and RT may even reduce the survival time for early onset stage II/III RC.
Figure 2.
The forest plot was used to show the results of the multivariable Cox regression in early onset stage II/III RC. (A) RT vs. non-RT and nRT vs. non-RT in total early onset stage II/III RC patients. (B) RT vs. non-RT and nRT vs. non-RT in early onset stage II/III RC patients without chemotherapy. (The results were extracted from Table S1).
Figure 3.
The survival curves were used to demonstrate the effect of radiotherapy in early onset stage II/III RC patients. (A) The total early onset stage II/III RC patients before PSM. (B) RT vs. non-RT in all early onset stage II/III RC patients after PSM. (C) nRT vs. non-RT in all early onset stage II/III RC patients after PSM. (D) The early onset stage II/III RC patients without chemotherapy before PSM. (E) RT vs. non-RT in early onset stage II/III RC patients without chemotherapy after PSM. (F) nRT vs. non-RT in early onset stage II/III RC patients without chemotherapy after PSM. (The results of PSM are summarized in Table S2).
The Effect of Radiotherapy on Middle-Aged Patient With Stage II/III RC
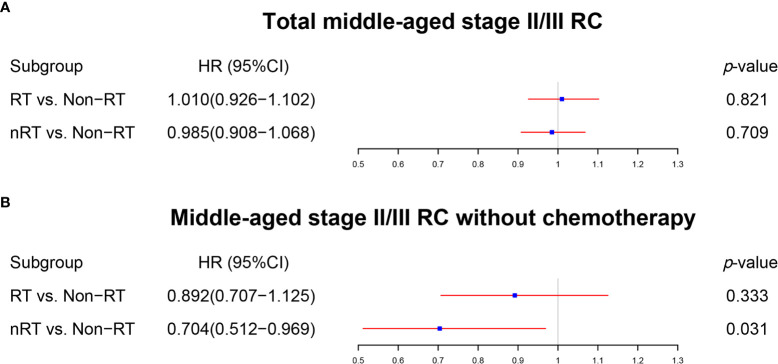
The same methods were applied to analyze middle-aged patients with stage II/III RC. The multivariate Cox regression analysis (Figure 4A, Table S3) and the survival analysis before PSM (Figure 5A) illustrated that both RT and nRT failed to prolong OS of middle-aged patients with stage II/III RC, which was further confirmed by survival analysis after PSM (Table S4 ; Figure 5B: RT vs. non-RT, p = 0.712; Figure 5C: nRT vs. non-RT, p = 0.584). Interestingly, nRT can be used as a prognostic factor in the multivariate Cox regression model analyzing middle-aged stage II/III RC patients without chemotherapy (Figure 4B, Table S3). However, the survival curves did not show the survival advantage of radiotherapy for middle-aged stage II/III RC patients (before PSM: Figure 5D, p = 0.718; RT vs. non-RT, p = 0.761; nRT vs. non-RT, p = 0.445) (after PSM: Table S4 ; Figure 5E, RT vs. non-RT, p = 0.604; Figure 5F, nRT vs. non-RT, p = 0.443). Aggregately, RT was not able to improve the OS, while nRT may provide limited survival improvement for middle-aged stage II/III RC patients.
Figure 4.
The forest plot was used to show the results of the multivariable Cox regression in middle-aged stage II/III RC patients. (A) RT vs. non-RT and nRT vs. non-RT in total middle-aged stage II/III RC patients. (B) RT vs. non-RT and nRT vs. non-RT in middle-aged stage II/III RC patients without chemotherapy. (The results were extracted from Table S3).
Figure 5.
The survival curves were applied to display the effect of radiotherapy in middle-aged stage II/III RC patients. All survival comparisons failed to reach statistical differences. (A) The total middle-aged stage II/III RC patients before PSM. (B) RT vs. non-RT in all middle-aged stage II/III RC patients after PSM. (C) nRT vs. non-RT in all middle-aged stage II/III RC patients after PSM. (D) Middle-aged stage II/III RC patients without chemotherapy before PSM. (E) RT vs. non-RT in middle-aged stage II/III RC patients without chemotherapy after PSM. (F) nRT vs. non-RT in middle-aged stage II/III RC patients without chemotherapy after PSM. (The results of PSM are summarized in Table S4).
The Effect of Radiotherapy on Elderly Stage II/III RC Patients
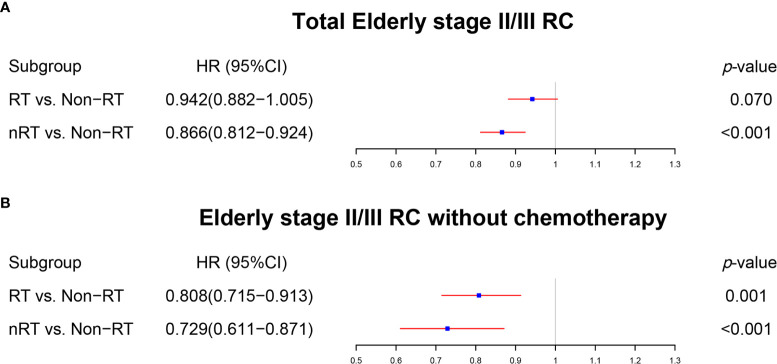
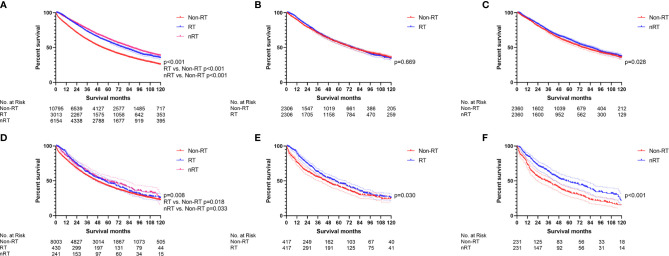
Radiotherapy played a key role in the treatment of over-65 stage II/III RC patients. Although they failed to obtain significant benefit from RT (p = 0.070), over-65 stage II/III RC patients received superior survival from nRT (p < 0.001) in the multivariate Cox regression model (Figure 6A, Table S5). The survival curves without PSM demonstrated that both RT and nRT provided survival benefit to elderly stage II/III RC patients (Figure 7A, p < 0.001; RT vs. non-RT, p < 0.001; nRT vs. non-RT, p < 0.001). However, the survival curves with PSM (Table S6) showed indistinguishable survival between RT and non-RT groups in elderly stage II/III RC patients (Figure 7B, p = 0.669). Nevertheless, nRT can still provide significant survival benefits to elderly patients with stage II/III RC after PSM (Figure 7C, p = 0.028). Among the elderly stage II/III RC patients, 8,647 who did not receive chemotherapy were used for further analysis. Intriguingly, both of the multivariate Cox regression analysis (Figure 6B, Table S5) and the survival analysis before PSM (Figure 7D) indicated that radiotherapy, including nRT and RT, can prolong OS of elderly stage II/III RC patients, which was verified by the survival curves using data after PSM (Table S6) (Figure 7E: RT vs. non-RT, p = 0.030; Figure 7F: nRT vs. non-RT, p < 0.001). To sum up, radiotherapy can significantly improve the prognosis of over-65 stage II/III RC patients.
Figure 6.
The forest plot was used to show the results of the multivariable Cox regression in elderly stage II/III RC. (A) RT vs. non-RT and nRT vs. non-RT in total elderly stage II/III RC patients. (B) RT vs. non-RT and nRT vs. non-RT in elderly stage II/III RC patients without chemotherapy. (The results were extracted from Table S5).
Figure 7.
The survival curves were utilized to indicate the effect of radiotherapy in elderly stage II/III RC patients. (A) The total elderly stage II/III RC patients before PSM. (B) RT vs. non-RT in all elderly stage II/III RC patients after PSM. (C) nRT vs. non-RT in all elderly stage II/III RC patients after PSM. (D) The total elderly stage II/III RC patients without chemotherapy before PSM. (E) RT vs. non-RT in elderly stage II/III RC patients without chemotherapy after PSM. (F) nRT vs. non-RT in elderly stage II/III RC patients without chemotherapy after PSM. (The results of PSM are summarized in Table S6).
Discussion
This study focused on a possible important causality between radiotherapy and the age of stage II/III RC patients. To the best of our knowledge, this study was the first research to specifically investigate the survival effect of radiotherapy on different ages of patients with stage II/III RC. Numerous tumors exhibit differences in molecular background, biological behavior, etiology as well as therapeutic response among various age groups (9, 14–18). A recent research study focusing on young breast cancer patients found that chemotherapy was an insignificant prognostic factor in the multivariable analysis with Cox regression for overall survival and cancer-specific survival (19), which is obviously inconsistent with most studies without age grouping. Moreover, young individuals failed to obtain equivalent survival benefits from chemotherapy compared to over-65 patients with colorectal cancer (13). This evidence drove us to explore the differentiated impact of radiotherapy in various age groups of stage II/III RC patients. The results of this study using a large national database indicated the inconsistent survival effect of radiotherapy on stage II/III rectal cancer patients in different age groups, which imply that age should be used as a deciding factor for radiotherapy for stage II/III RC patients.
Use of radiotherapy in the treatment of stage II/III RC patients continues to evolve (20). The sphincter preservation is the main issue affecting the quality of life faced by rectal cancer patients. Therefore, we planned to discuss radiotherapy-decision-making according to survival prolongation combined with sphincter preservation for patients with stage II/III RC. The CAO/ARO/AIO-94 trial indicated that neoadjuvant chemoradiotherapy (nCRT) was associated with a significant reduction in local recurrence and treatment-associated toxicity compared to postoperative chemoradiotherapy (CRT) (5, 21). Meanwhile, preoperative chemoradiotherapy demonstrated increased rates of pathological complete response (pCR) and improved local disease recurrence rates relative to chemotherapy (22) or radiotherapy alone (6, 23, 24). Hence, neoadjuvant chemoradiotherapy (nCRT) is still the first choice for those mid-low rectal cancer patients with influence on the sphincter preservation. In fact, radiotherapy cannot play the best role without chemotherapy, which is considered as a sensitizer for radiotherapy (25). The results from patients who received radiotherapy without chemotherapy as an unconventional treatment can only be considered as secondary evidence. Therefore, the multivariate Cox regression model analyzing middle-aged stage II/III RC patients without chemotherapy could be an inferior evidence to suggest that nRT can be used as an alternative option for middle-aged stage II/III RC patients who cannot tolerate CT.
Phase III FOWARC trial demonstrated a non-significant difference in survival outcomes between neoadjuvant chemotherapy (nCT) and nCRT (26). In fact, nCT alone, which is able to spare patients the morbidities associated with radiation, should herein be recommended as a first-line treatment for stage II/III RC patients without influencing the sphincter preservation, which is also supported by the National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines (20). Nevertheless, postoperative radiotherapy should be avoided for early onset and middle-aged stage II/III RC patients, regardless if they received neoadjuvant treatment or not, due to the ineffective or even harmful effects of RT for them.
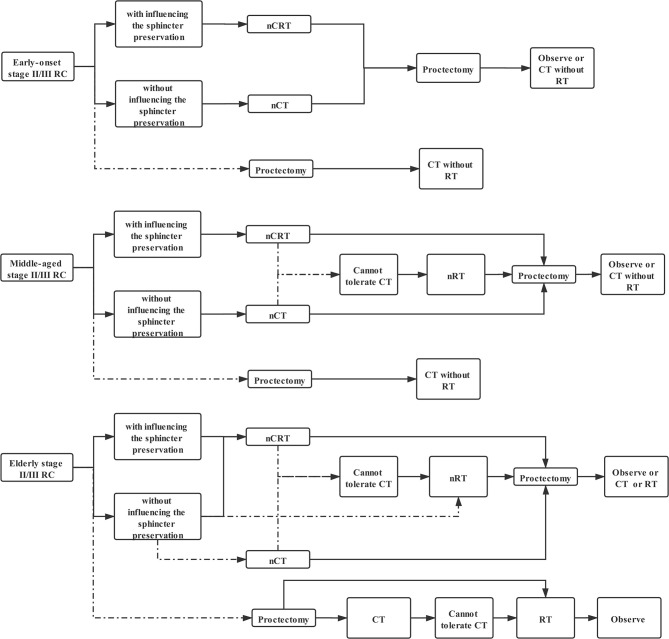
Clinicians should pay more attention to the application of radiotherapy in over-65 stage II/III RC patients. In fact, the results of the Phase III FOWARC trial, which excluded patients older than 75, cannot be fully applied to elderly stage II/III RC (26). It is necessary to update radiotherapy strategies suitable for elderly individuals. First of all, the evidence that nRT significantly improved the prognosis of elderly stage II/III RC in the analysis including patients with chemotherapy supported that nCRT should take precedence over nCT in all elderly stage II/III RC patients. In addition, the analysis excluding patients with chemotherapy certified that nRT alone was able to prolong OS of elderly individuals with stage II/III RC, which can be used as reliable evidence to sustain that nRT was an alternative option for those who cannot tolerate chemotherapy as well as a preferred choice for some frail elderly patients without influencing the sphincter preservation. Moreover, postoperative radiotherapy can only be recommended to elderly stage II/III RC patients. However, elderly stage II/III RC patients should cautiously receive postoperative combined radiotherapy and chemotherapy, which may be too aggressive for older adults, since RT failed to provide survival benefit compared with non-RT in the analysis including those patients with chemotherapy, while RT was able to improve OS when the analysis excluded elderly patients with chemotherapy. Collectively, we can summarize a novel flow chart of radiotherapy decision-making based on age (Figure 8).
Figure 8.
The flow chart of radiotherapy decision-making based on age in stage II/III RC patients. (Solid line: preferred option; dotted line: alternative choice).
Our research also displayed, to a certain extent, that nRT was superior to postoperative RT. For instance, RT reduced the OS of patients with early onset stage II/III RC, while nRT did not; there was a significant survival difference between nRT and non-RT in the multivariate Cox regression model analyzing middle-aged stage II/III RC patients without chemotherapy; nRT provided more obvious survival benefits to elderly stage II/III RC patients compared to RT. Putative advantages of nRT, as opposed to RT given postoperatively, are related to both tumor volume and preservation of normal tissue (21, 27, 28). Irradiating tissue that is surgery-naïve and thus better oxygenated may result in increased sensitivity to RT. Furthermore, nRT can avoid the occurrence of radiation-induced injury to small bowel trapped in the pelvis by post-surgical adhesion. nRT that includes structures that will increase the likelihood that an anastomosis with healthy colon can be performed.
Mismatched biology may be responsible for the inconsistent survival benefits among different age groups. A recent research study reported that young rectal cancer patients have a higher proportion of cancer stem cells (CSCs) (29), which is considered as an important factor reflecting radiotherapy resistance in rectal cancer (30, 31). In addition, we took out stage II RC patients separately for analysis and got similar results comparing to the total stage II/III RC patients (Supplementary Files). In fact, stage II/III RC is often discussed as a whole because of consistent treatment strategies. Therefore, it is reasonable for stage II/III RC to be studied as a whole. Moreover, the results displayed that there was more RNE ≥12 in the early onset group, which implied that young patients were more likely to receive extensive treatment. Therefore, we conducted further analysis and found that these elderly stage II/III RC patients with RNE ≥12 can get survival benefit from nRT but not postoperative RT, which is similar to the results of the total elderly stage II/III RC patients, while early onset and middle-aged ones with RNE <12 cannot obtain survival benefit from radiotherapy, including nRT and postoperative RT (Supplementary Files). Overall, it is reliable that age is an important factor affecting the efficacy of radiotherapy in stage II/III RC patients.
However, the evidence effect of our study is weakened by some limitations. Firstly, despite that the PSM method was performed to reduce the confounding factors of independent features, some biases were inevitable due to the retrospective nature of this study. Secondarily, detailed information regarding chemotherapy and surgery for included patients was not recorded in the SEER database, which, to some extent, weakened the evidence effect of this study because young patients were more likely to receive multimodal treatment compared to the middle-aged and elderly RC patients. We used RNE as the priority for the assessment of the quality of surgery, which was mentioned in the previous studies (32, 33), since the SEER database failed to provide information regarding TME as well as surgical R-stage, which may be serious confounding factors with regard to the other modalities. Undoubtedly, the lack of data regarding surgical R-stage and the distance from the primary tumor to the mesorectal fascia weakened the reliability of the conclusions in this study. Furthermore, the SEER database does not provide detailed information about the distance between the tumor and the anus, which is one of the decisive factors that affect the decision-making of radiotherapy for RC. A recent research showed that the distance from the anal verge cannot affect the survival of LARC patients with radiotherapy (34). Hence, the lack of data regarding the distance between the tumor and the anus may hardly affect the scientific conclusions of this research. In addition, some recent studies indicated that chemotherapy added to radiotherapy in patients with stage II/III RC was able to reduce the risk of local recurrence but had no effect on survival (23, 24). This study failed to use local recurrence as an endpoint to discuss the decision-making of radiotherapy for rectal cancer due to the SEER database does not provide information about local recurrence. At last, detailed information regarding radiotherapy for included patients was not recorded in the SEER database. Radiotherapy for rectal cancer usually includes long-term (46–50 Gy in 23–25 fractions) and short-term (5 Gy × 5) radiotherapy. However, there is no significant survival difference between rectal cancer patients receiving long-term and short-term radiotherapy (20). This study only focused on the survival effect of radiotherapy on stage II/III RC, which to a certain extent can ignore the limitation about lacking detailed radiotherapeutic information of the SEER database. Overall, this study stated a potential correlation between radiotherapy and the age of stage II/III RC patients. However, the findings of this study still need to be further confirmed by a prospective cohort of stage II/III RC patients due to the natural limitation of the SEER database.
Conclusion
This study indicated the inconsistent survival effect of radiotherapy on stage II/III rectal cancer patients in different age groups. Hence, we formulated a novel flow chart of radiotherapy decision-making based on age in stage II/III RC patients (Figure 8). Furthermore, clinicians should pay more attention to the application of radiotherapy in elderly stage II/III RC patients.
Data Availability Statement
Publicly available datasets were analyzed in this study. This data can be found here: These data were derived from the Surveillance, Epidemiology and End Results (SEER) database (https://seer.cancer.gov/) and identified using the SEER*Stat software (Version 8.3.5) (https://seer.cancer.gov/seerstat/).
Author Contributions
FT, HP and YL conceived and designed the study. YZ, YL and WL wrote the article. LZ downloaded and screened the data from SEER database. All authors participated in analyzing the data. All authors contributed to the article andapproved the submitted version.
Funding
This study was supported by the following projects:i,the Natural Scientific Foundation of China (No.81702956); ii, the Natural Science Foundation of Hunan Province (No.2020JJ4903 and 2020JJ5920); iii, the Construction of Innovative Ability of National Clinical Research Center for Geriatric Disorders (No. 2019SK2335); iv, the Strategy-Oriented Special Project of Central South University of China (No. ZLXD2017003); v, the Colorectal Cancer Medical Seed Research Fund of Beijing Bethune Public Welfare Foundation Named “Effect and mechanism of YAP1 on EGFR resistance in K-ras wild-type metastatic colorectal cancer”.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The first author, Yuqiang Li, gratefully acknowledges financial support from China Scholarship Council.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.695640/full#supplementary-material
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA: Cancer J Clin (2018) 68(6):394–424. 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2.Bailey CE, Hu CY, You YN, Bednarski BK, Rodriguez-Bigas MA, Skibber JM, et al. Increasing Disparities in the Age-Related Incidences of Colon and Rectal Cancers in the United States, 1975-2010. JAMA Surg (2015) 150(1):17–22. 10.1001/jamasurg.2014.1756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khanna KK, Jackson SP. DNA Double-Strand Breaks: Signaling, Repair and the Cancer Connection. Nat Genet (2001) 27(3):247–54. 10.1038/85798 [DOI] [PubMed] [Google Scholar]
- 4.Bosset JF, Calais G, Mineur L, Maingon P, Stojanovic-Rundic S, Bensadoun RJ, et al. Fluorouracil-Based Adjuvant Chemotherapy After Preoperative Chemoradiotherapy in Rectal Cancer: Long-Term Results of the EORTC 22921 Randomised Study. Lancet Oncol (2014) 15(2):184–90. 10.1016/S1470-2045(13)70599-0 [DOI] [PubMed] [Google Scholar]
- 5.Sauer R, Liersch T, Merkel S, Fietkau R, Hohenberger W, Hess C, et al. Preoperative Versus Postoperative Chemoradiotherapy for Locally Advanced Rectal Cancer: Results of the German Cao/Aro/Aio-94 Randomized Phase III Trial After a Median Follow-Up of 11 Years. J Clin Oncol Off J Am Soc Clin Oncol (2012) 30(16):1926–33. 10.1200/JCO.2011.40.1836 [DOI] [PubMed] [Google Scholar]
- 6.Gérard JP, Conroy T, Bonnetain F, Bouché O, Chapet O, Closon-Dejardin MT, et al. Preoperative Radiotherapy With or Without Concurrent Fluorouracil and Leucovorin in T3-4 Rectal Cancers: Results of FFCD 9203. J Clin Oncol Off J Am Soc Clin Oncol (2006) 24(28):4620–5. 10.1200/JCO.2006.06.7629 [DOI] [PubMed] [Google Scholar]
- 7.Rahbari NN, Elbers H, Askoxylakis V, Motschall E, Bork U, Büchler MW, et al. Neoadjuvant Radiotherapy for Rectal Cancer: Meta-Analysis of Randomized Controlled Trials. Ann Surg Oncol (2013) 20(13):4169–82. 10.1245/s10434-013-3198-9 [DOI] [PubMed] [Google Scholar]
- 8.Zhao F, Wang J, Yu H, Cheng X, Li X, Zhu X, et al. Neoadjuvant Radiotherapy Improves Overall Survival for T3/4N+M0 Rectal Cancer Patients: A Population-Based Study of 20300 Patients. Radiat Oncol (Lond Engl) (2020) 15(1):49. 10.1186/s13014-020-01497-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kolarich A, George TJ, Jr., Hughes SJ, Delitto D, Allegra CJ, Hall WA, et al. Rectal Cancer Patients Younger Than 50 Years Lack a Survival Benefit From NCCN Guideline-Directed Treatment for Stage II and III Disease. Cancer (2018) 124(17):3510–9. 10.1002/cncr.31527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khan SA, Morris M, Idrees K, Gimbel MI, Rosenberg S, Zeng Z, et al. Colorectal Cancer in the Very Young: A Comparative Study of Tumor Markers, Pathology and Survival in Early Onset and Adult Onset Patients. J Pediatr Surg (2016) 51(11):1812–7. 10.1016/j.jpedsurg.2016.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fass OZ, Poels KE, Qian Y, Zhong H, Liang PS. Demographics Predict Stage III/IV Colorectal Cancer in Individuals Under Age 50. J Clin Gastroenterol (2020) 54(8):714–9. 10.1097/MCG.0000000000001374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dozois EJ, Boardman LA, Suwanthanma W, Limburg PJ, Cima RR, Bakken JL, et al. Young-Onset Colorectal Cancer in Patients With No Known Genetic Predisposition: Can We Increase Early Recognition and Improve Outcome? Medicine (2008) 87(5):259–63. 10.1097/MD.0b013e3181881354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kneuertz PJ, Chang GJ, Hu CY, Rodriguez-Bigas MA, Eng C, Vilar E, et al. Overtreatment of Young Adults With Colon Cancer: More Intense Treatments With Unmatched Survival Gains. JAMA Surg (2015) 150(5):402–9. 10.1001/jamasurg.2014.3572 [DOI] [PubMed] [Google Scholar]
- 14.Leon P, Cancel-Tassin G, Bourdon V, Buecher B, Oudard S, Brureau L, et al. Bayesian Predictive Model to Assess BRCA2 Mutational Status According to Clinical History: Early Onset, Metastatic Phenotype or Family History of Breast/Ovary Cancer. Prostate (2021) 81(6):318–25. 10.1002/pros.24109 [DOI] [PubMed] [Google Scholar]
- 15.O’Sullivan DE, Sutherland RL, Town S, Chow K, Fan J, Forbes N, et al. Risk Factors for Early-Onset Colorectal Cancer: A Systematic Review and Meta-Analysis. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc (2021) S1542-3565(21)00087-2. 10.1016/j.cgh.2021.01.037 [DOI] [PubMed] [Google Scholar]
- 16.Burnett-Hartman AN, Lee JK, Demb J, Gupta S. An Update on the Epidemiology, Molecular Characterization, Diagnosis, and Screening Strategies for Early-Onset Colorectal Cancer. Gastroenterology (2021) 160(4):1041–9. 10.1053/j.gastro.2020.12.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holowatyj AN, Eng C, Wen W, Idrees K, Guo X. Spectrum of Somatic Cancer Gene Variations Among Adults With Appendiceal Cancer by Age at Disease Onset. JAMA Network Open (2020) 3(12):e2028644. 10.1001/jamanetworkopen.2020.28644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Campa D, Gentiluomo M, Obazee O, Ballerini A, Vodickova L, Hegyi P, et al. Genome-Wide Association Study Identifies an Early Onset Pancreatic Cancer Risk Locus. Int J Cancer (2020) 147(8):2065–74. 10.1002/ijc.33004 [DOI] [PubMed] [Google Scholar]
- 19.Sun Y, Li Y, Wu J, Tian H, Liu H, Fang Y, et al. Nomograms for Prediction of Overall and Cancer-Specific Survival in Young Breast Cancer. Breast Cancer Res Treat (2020) 184(2):597–613. 10.1007/s10549-020-05870-5 [DOI] [PubMed] [Google Scholar]
- 20.National Comprehensive Cancer Network. Clinical Practice Guidelines in Oncology (Nccn Guidelines®), Rectal Cancer, Version 6 (2020). [Google Scholar]
- 21.Sauer R, Becker H, Hohenberger W, Rödel C, Wittekind C, Fietkau R, et al. Preoperative Versus Postoperative Chemoradiotherapy for Rectal Cancer. N Engl J Med (2004) 351(17):1731–40. 10.1056/NEJMoa040694 [DOI] [PubMed] [Google Scholar]
- 22.Deng Y, Chi P, Lan P, Wang L, Chen W, Cui L, et al. Modified FOLFOX6 With or Without Radiation Versus Fluorouracil and Leucovorin With Radiation in Neoadjuvant Treatment of Locally Advanced Rectal Cancer: Initial Results of the Chinese Fowarc Multicenter, Open-Label, Randomized Three-Arm Phase III Trial. J Clin Oncol Off J Am Soc Clin Oncol (2016) 34(27):3300–7. 10.1200/JCO.2016.66.6198 [DOI] [PubMed] [Google Scholar]
- 23.De Caluwé L, Van Nieuwenhove Y, Ceelen WP. Preoperative Chemoradiation Versus Radiation Alone for Stage II and III Resectable Rectal Cancer. Cochrane Database Systematic Rev (2013) 2:Cd006041. 10.1002/14651858.CD006041.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCarthy K, Pearson K, Fulton R, Hewitt J. Pre-Operative Chemoradiation for Non-Metastatic Locally Advanced Rectal Cancer. Cochrane Database Systematic Rev (2012) 12:Cd008368. 10.1002/14651858.CD008368.pub2 [DOI] [PubMed] [Google Scholar]
- 25.Li Y, Liu W, Zhao L, Xu Y, Yan T, Yang Q, et al. The Main Bottleneck for Non-Metastatic Pancreatic Adenocarcinoma in Past Decades: A Population-Based Analysis. Med Sci Monitor Int Med J Exp Clin Res (2020) 26:e921515. 10.12659/MSM.921515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deng Y, Chi P, Lan P, Wang L, Chen W, Cui L, et al. Neoadjuvant Modified Folfox6 With or Without Radiation Versus Fluorouracil Plus Radiation for Locally Advanced Rectal Cancer: Final Results of the Chinese Fowarc Trial. J Clin Oncol Off J Am Soc Clin Oncol (2019) 37(34):3223–33. 10.1200/JCO.18.02309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wagman R, Minsky BD, Cohen AM, Guillem JG, Paty PP. Sphincter Preservation in Rectal Cancer With Preoperative Radiation Therapy and Coloanal Anastomosis: Long Term Follow-Up. Int J Radiat Oncol Biol Phys (1998) 42(1):51–7. 10.1016/S0360-3016(98)00180-1 [DOI] [PubMed] [Google Scholar]
- 28.Kachnic LA. Should Preoperative or Postoperative Therapy be Administered in the Management of Rectal Cancer? Semin Oncol (2006) 33(6 Suppl 11):S64–9. 10.1053/j.seminoncol.2006.10.018 [DOI] [PubMed] [Google Scholar]
- 29.Zhang Y, Yan L, Wu Y, Xu M, Liu X, Guan G. Worse Treatment Response to Neoadjuvant Chemoradiotherapy in Young Patients With Locally Advanced Rectal Cancer. BMC Cancer (2020) 20(1):854. 10.1186/s12885-020-07359-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sprenger T, Conradi LC, Beissbarth T, Ermert H, Homayounfar K, Middel P, et al. Enrichment of CD133-Expressing Cells in Rectal Cancers Treated With Preoperative Radiochemotherapy is an Independent Marker for Metastasis and Survival. Cancer (2013) 119(1):26–35. 10.1002/cncr.27703 [DOI] [PubMed] [Google Scholar]
- 31.Saigusa S, Tanaka K, Toiyama Y, Yokoe T, Okugawa Y, Ioue Y, et al. Correlation of CD133, OCT4, and SOX2 in Rectal Cancer and Their Association With Distant Recurrence After Chemoradiotherapy. Ann Surg Oncol (2009) 16(12):3488–98. 10.1245/s10434-009-0617-z [DOI] [PubMed] [Google Scholar]
- 32.Li Y, Zhao L, Gungor C, Tan F, Zhou Z, Li C, et al. The Main Contributor to the Upswing of Survival in Locally Advanced Colorectal Cancer: An Analysis of the SEER Database. Ther Adv Gastroenterol (2019) 12:1756284819862154. 10.1177/1756284819862154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cai Y, Cheng G, Lu X, Ju H, Zhu X. The Re-Evaluation of Optimal Lymph Node Yield in Stage II Right-Sided Colon Cancer: Is a Minimum of 12 Lymph Nodes Adequate? Int J Colorectal Dis (2020) 35(4):623–31. 10.1007/s00384-019-03483-z [DOI] [PubMed] [Google Scholar]
- 34.Liu W, Li Y, Zhu H, Pei Q, Tan F, Song X, et al. The Relationship Between Primary Gross Tumor Volume and Tumor Response of Locally Advanced Rectal Cancer: pGTV as a More Accurate Tumor Size Indicator. J Invest Surg (2019) 34(2):181–90. 10.1080/08941939.2019.1615153 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Publicly available datasets were analyzed in this study. This data can be found here: These data were derived from the Surveillance, Epidemiology and End Results (SEER) database (https://seer.cancer.gov/) and identified using the SEER*Stat software (Version 8.3.5) (https://seer.cancer.gov/seerstat/).