Abstract
Regenerative engineering is defined as the convergence of the disciplines of advanced material science, stem cell science, physics, developmental biology and clinical translation for the regeneration of complex tissues and organ systems. It is an expansion of tissue engineering, which was first developed as a method of repair and restoration of human tissue. In the past three decades, advances in regenerative engineering have made it possible to treat a variety of clinical challenges by utilizing cutting-edge technology currently available to harness the body’s healing and regenerative abilities. The emergence of new information in developmental biology, stem cell science, advanced material science and nanotechnology have provided promising concepts and approaches to regenerate complex tissues and structures.
Keywords: : 3D bioprinting, advanced material science, biomaterials, developmental biology, nanotechnology, regenerative engineering, stem cell science
The ability of the human body to heal itself has fascinated clinicians and scientists since the inception of medicine. In the past three decades, the emergence of tissue engineering has fostered that fascination as physicians and scientists have dedicated their time and resources to constructing biological substitutes that can act as native tissue [1]. Tissue engineering was first proposed in 1987 by bioengineer Dr. YC Fung at the National Science Foundation’s Director for Engineering, Bioengineering and Research to Aid the Handicapped Program meeting [2]. The field was quickly endorsed by many other investigators due to its unique approach and potential to treat a variety of clinical challenges. Tissue engineering was initially defined as an interdisciplinary field which applies principles of engineering and life sciences toward the production of a biological substitute that can restore, maintain or improve tissue function [3]. However, Laurencin et al. further defined it as ‘the application of biological, chemical and engineering principles toward the repair, restoration or regeneration of living tissue using biomaterials, cells and factors alone or in combination’ [2]. Although this approach of tissue engineering has been able to regenerate single targeted tissues such as skin, bone and cartilage, the translation of these concepts into clinical therapeutics and products has not been widespread across the globe despite being established as proof-of-concept approaches [4,5].
While tissue engineering has demonstrated that the regeneration of a single lineage of tissue is possible, most organ systems comprises a multitude of lineages, all of which would require simultaneous regeneration. An approach that is distinct yet similar to tissue engineering has emerged recently to overcome the challenges of regenerating complex tissue systems. Regenerative engineering has been defined as the integration of material science and tissue engineering with stem and developmental cell biology [5,6]. This trans-disciplinary approach utilizes cutting-edge technology in order to harness the body’s healing and regenerative abilities. Distinct from tissue engineering, which focuses on the repair of tissue, regenerative engineering focuses more on the convergence of multiple disciplines including biology, material science, physical, chemical and engineering sciences to regenerate native tissues [6]. Although it may be particularly challenging to integrate a multitude of fields, each with a breadth of information, the concept of regenerative engineering has provided clinicians and researchers with opportunities to investigate novel and innovative therapeutics. In addition, it provides a unique opportunity to move toward the regeneration of complex tissues and organ systems instead of individual tissue repair. In this review paper, we will highlight advances in regenerative engineering technology within the last three decades, current limitations and future directions of the field.
Regenerative engineering
Regenerative engineering utilizes the convergence of the disciplines of advanced material science, stem cell science, physics, developmental biology and clinical translation for the regeneration of complex tissues and organ systems [2,6]. An overview of regenerative engineering and its components are shown in Figure 1. While tissue engineering sought to encourage the integration of the fields of engineering, science and medicine, regenerative engineering is an expansion of this approach to create an interdisciplinary team of scientists, engineers, physicists and clinicians for integrated strategies to treating various diseases [2,7]. As a result, recent advances have occurred within the field of biomaterials-based tissue engineering. A deeper understanding of adult and embryonic stem (ES) cells has also led to novel protocols to create induced pluripotent stem cells [8]. As a result, stem cell science has become incorporated into the protocols of numerous labs across the globe. Nanotechnology has also emerged as a tool for regenerative engineering, as advances in biomaterials allow the manipulation of biological processes at a nano-regime level [2]. Studies have demonstrated and produced a deeper understanding of mechanisms of developmental biology such as the role of the blastema in regeneration and wound repair [2,5]. These areas have evolved from concepts and ideas into practical tools for scientists and have become an integral part of many in vivo and in vitro studies that provide insight toward solving current grand clinical challenges.
Figure 1. . Overview of regenerative engineering.
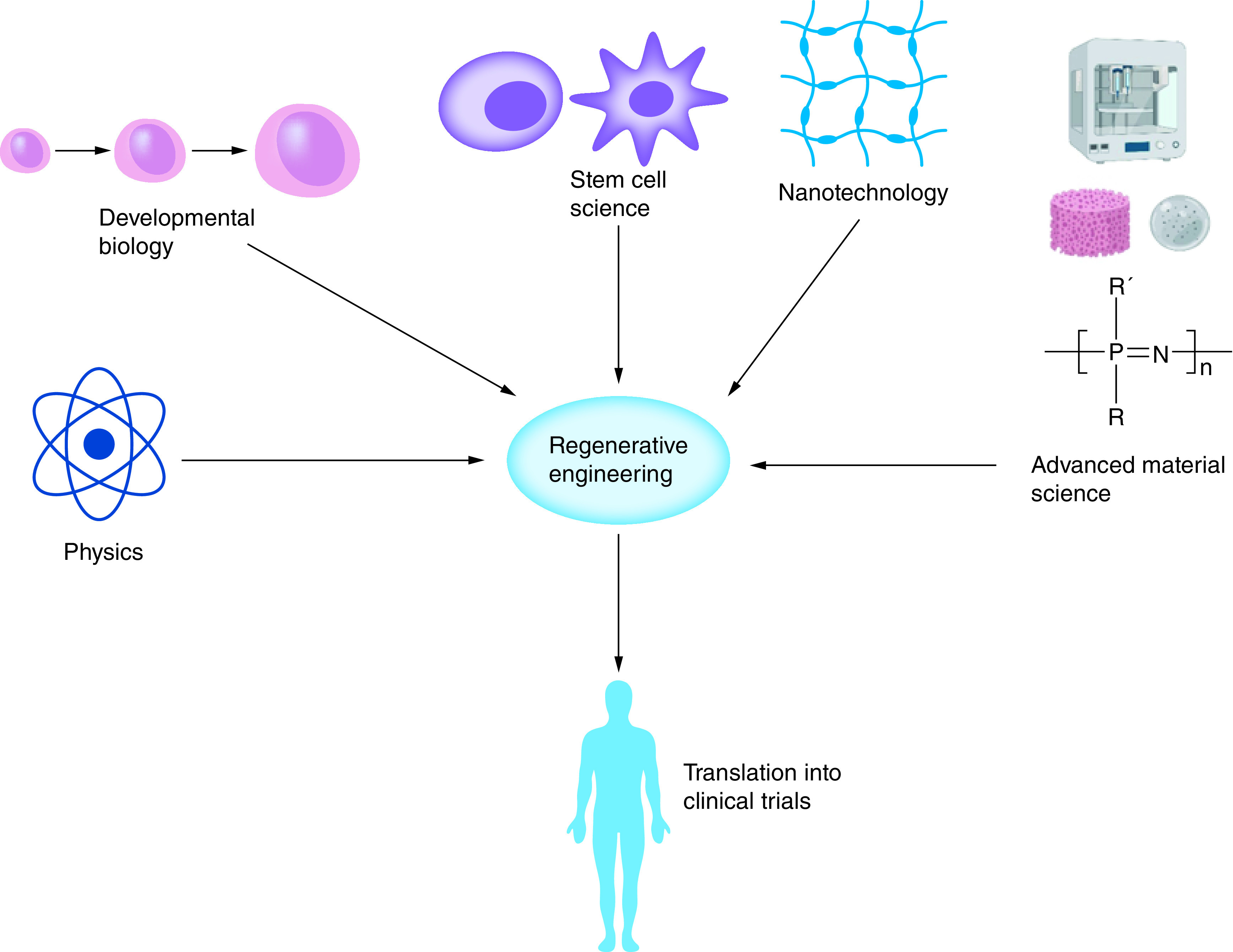
Regenerative engineering utilizes the convergence of the disciplines of advanced material science, stem cell science, physics, developmental biology and clinical translation for the regeneration of complex tissues and organ systems.
Developmental biology
A sophisticated and advanced understanding of developmental biology is a fundamental principle of regenerating complex tissues and structures within the human body. Although the regenerative capabilities of cells within the human body vary by organ system, remarkable advances in regeneration have been presented in the last three decades. For example, the regeneration of a complex structure such as an entire limb has remained a clinical wonder. Through convergence approaches in regenerative engineering and with advancements in developmental biology, it may be possible to achieve limb regeneration [5]. Urodele amphibians have the ability to grow a severed limb at any point in their lifetime due to the phenomena known as ‘Epimorphic regeneration’ [9,10]. Other animals that have served as models for the study of regeneration include the salamander and more recently, the axolotl (Ambystoma mexicanum). Advances in genomics in combination with transgenesis have been optimized using axolotls and as a result, the accessory limb model has been developed, which details a gain-of-function model for the regeneration of ectopic limbs [11]. While humans do not possess the same genetic composition as amphibians, identification of the genes, growth factors and cell signals involved in the regenerative process can be incorporated into biomedical applications to create dynamic and novel regenerative therapeutics.
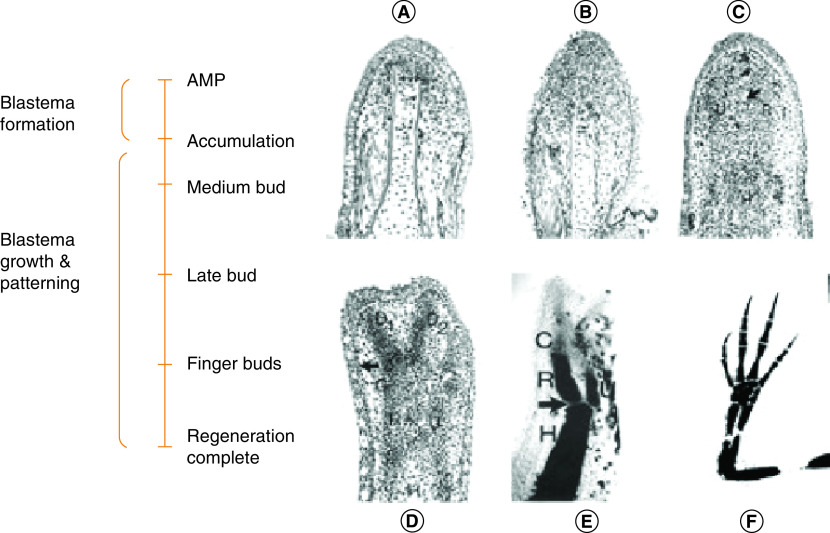
Epimorphic regeneration occurs through the formation of a blastema, a collection of progenitor cells that contain intrinsic morphogenetic cues. Once stimulated by amputation, these cells are capable of orchestrating a biological process to model the growth and pattern of the cells within the regenerated structure [9]. Figure 2 illustrates the phases and stages of blastema formation [12]. Once an injury has occurred, the surface of the limb is rapidly covered with epidermal cells to form a wound epidermis. This wound epidermis quickly thickens to form the apical epithelial cap, which coordinates the cellular processes involved in regeneration. A class of proteases in the extracellular matrix (ECM) called MMPs present growth factors to modulate this activity and to support proliferation of cells in the amputated limb. Under the apical epithelial cap, a heterogeneous population of cells forms a cluster of dedifferentiated cells and forms the blastema [13]. The heterogenicity of the cell population within the blastema results in a complex and diverse gene profile and a unique ECM. The key signaling pathways in the blastema which cue the regeneration process are still under investigation. However, studies have demonstrated that some of the pathways that cause embryonic limb formation such as FGF and wnt-β catenin are key factors [14,15]. Similarly, retinoic acid and Shh signaling pathways have been observed to play a role [16,17].
Figure 2. . Blastema formation.
Longitudinal sections of phases observed in forelimb regeneration in a urodele larva (Ambystoma maculatum) after amputation (AMP) through the mid-stylopodium of the forelimb. (A) First phase – accumulation blastema or early bud formation by a collection of undifferentiated progenitor cells; (B) second phase – medium bud formation; (C) third phase – late bud formation with an arrow pointing to a blood vessel and prominent apical ectodermal ridge; (D) fourth phase – notch displaying anlagen of anterior two digits (D1, D2), distal humerus, and R and U. The arrow indicates re-forming of a basement membrane. (E) Fifth phase – two-fingerbud whole mount stained with methylene blue showing the H, R, U and C. The arrow points to the elbow joint. (F) Fully regenerated limb mount stained with methylene blue.
Adapted with permission from [12] under the terms of the Creative Commons Attribution License (CC-BY).
AMP: Amputation; C: Carpal region; H: Humerus; R: Radius; U: Ulna.
These new insights in developmental biology have provided us with information as to which soluble factors are key in the mechanism for the regenerative process in urodele amphibians, salamanders and axolotls. The information from urodele amphibians, salamanders and axolotls on regeneration, from a cellular level, may provide hints and guide the direction of future regenerative studies. Incorporation of these new developments offers a wide range of modifications to enhance outcomes of tissue engineering and design novel translational strategies to regenerate complex tissues and structures [5].
Stem cell science
In recent decades, experimental studies and clinical applications demonstrate that stem cell therapy is an ideal approach for regenerative engineering and medicine [18]. Stem cells are undifferentiated cells that continue to divide, self-renew and differentiate into various cell types [41]. The basis for regeneration is provided by stem cells, which are present in almost every organ in the body. Stem cells allow the body to naturally maintain homeostasis by replacing aged and damaged cells with healthy cells. Stem cells are classified based on their source. For instance, ES cells are derived from early-stage embryos, neural stem cells (NSCs) are derived from the brain and adult stem cells are tissue-specific and can be isolated from almost any organ in the body. Figure 3 illustrates how stem cells are a derivative from multiple sources in the body and the vast number of stem cells that have been identified and characterized for various applications.
Figure 3. . Overview of various stem cells in the body.
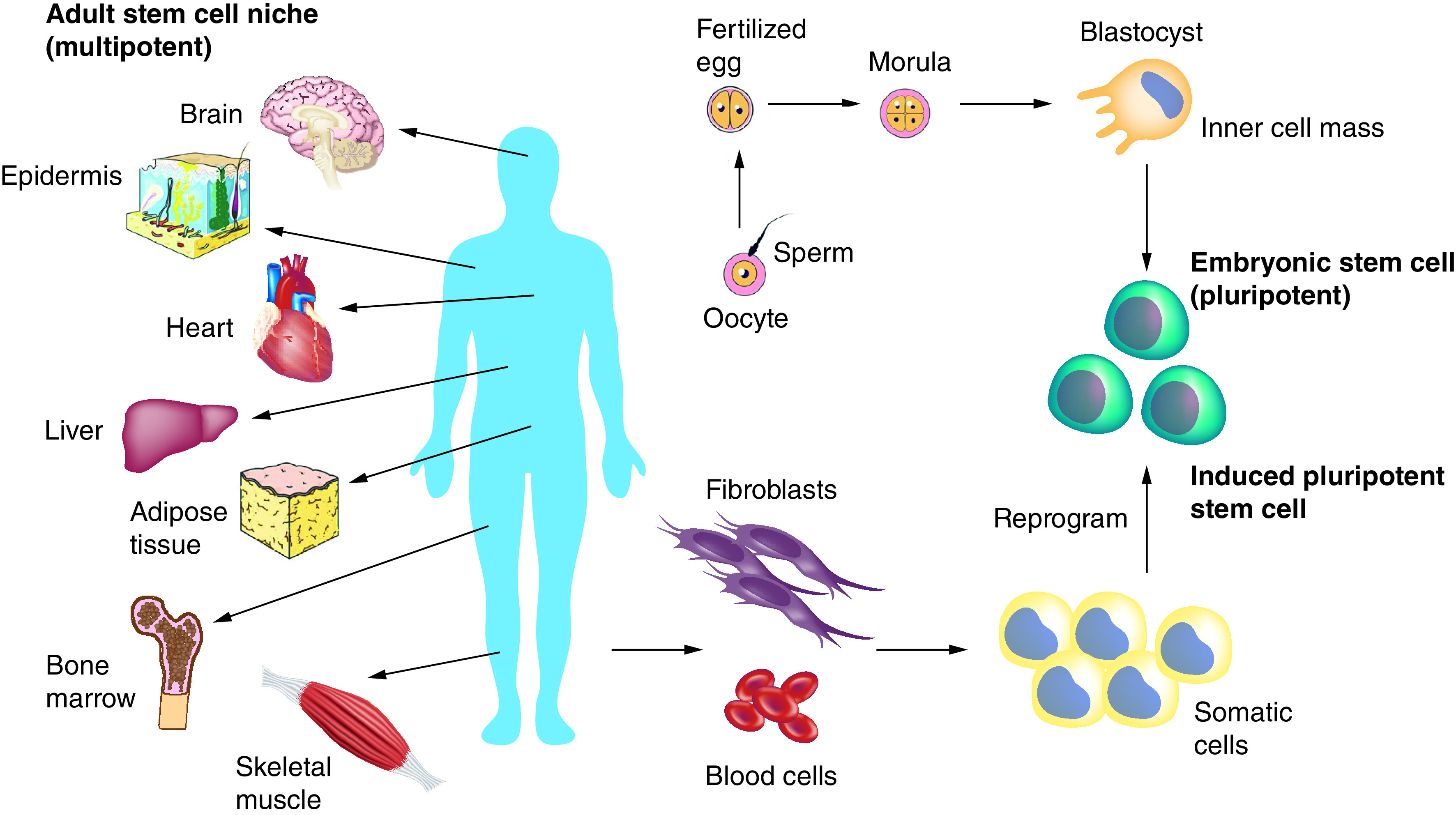
Embryonic stem cells can be derived from embryos from eggs fertilized in vitro. Adult stem cells (multipotent) are undifferentiated cells which are found among specialized cells in specific areas of adult tissues, referred to as the ‘stem cell niche’. Induced pluripotent stem cells can be derived from adult cells by genetic reprogramming to produce a pluripotent state. Pluripotent cells such as embryonic stem cells and induced pluripotent stem cells can be used to give rise to all cell types of the body and multipotent (adult stem cells) cells give rise to all cell types of a particular tissue or organ.
Adapted with permission from [97] under the terms of the Creative Commons Attribution License (CC-BY).
In recent years, stem cell-based regenerative medicine has evolved, shown in Figure 4. Stem cells are promising for regenerative medicine because of their ability to self-renew, proliferate and differentiate into numerous cell lineages. Studies have demonstrated regeneration of hematopoietic, neural and mesenchymal tissues as examples of healthy and functional stem cell (e.g., pluripotent and multipotent) differentiation. For example, NSCs have been transplanted into Huntington disease [20,21], Parkinson disease (PD) [22] and stroke experimental models, and functional improvement was reported. Research findings of a pilot study in PD in three 5-year-old female cynomolgus monkeys included the following: human neural progenitor cells (hNPCs) survived and produced GDNF in all animals 3 months post-surgery, hNPCs remained in the areas of injection as observed by GDNF immunostaining and GDNF ‘halo’ expression was observed diffusing from the center of the graft out into the surrounding area [22]. They also discovered an increase TH- and VMAT2-positive fibers in areas of GDNF delivery in two of the three animals. The two animals with TH- and VMAT2-positive fibers also showed reductions in their clinical rating scores. It was concluded that hNPCs releasing GDNF may be a possible alternative for intracerebral trophic factor delivery in PD [22]. Research has shown significant progress toward ES cells to differentiate into linage-specific precursors that can be used in applications for regenerative medicine [6]. For example, ES cells have the potential to treat degenerative disease such as PD, Huntington disease and amyotrophic lateral sclerosis. Both, mouse and human ES cells have successfully differentiated into spinal motor neurons that can make synaptic connections with muscle cells in vitro [21,23,24]. It was reported that ES cell-derived glial cells survived and had functional improvement after transplantation [25].
Figure 4. . Stem cell-based regenerative medicine.
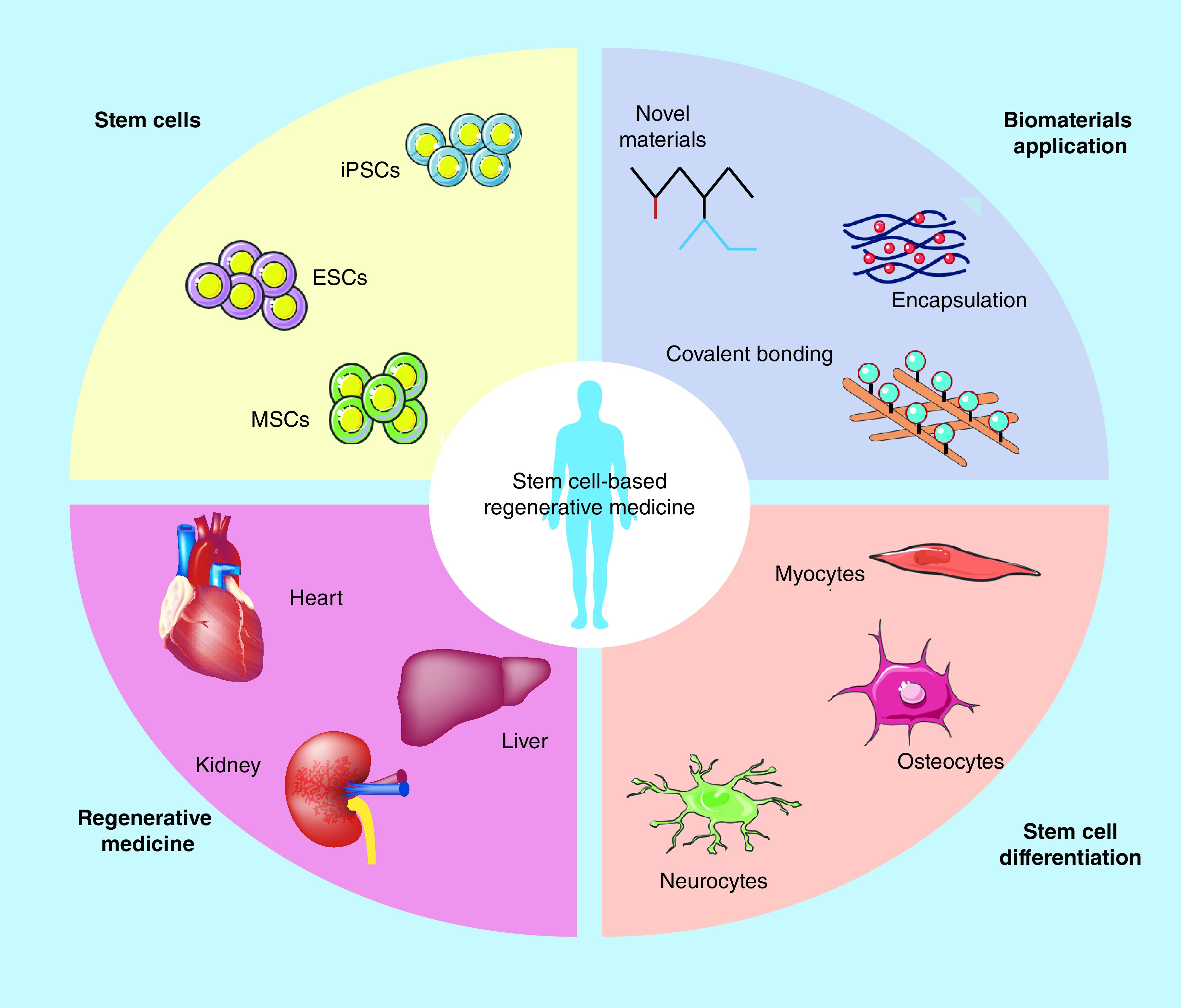
Due to the ability to self-renewal and differentiate into multiple lineages, stem cells show great promise in regenerative medicine. Different types of stem cells are identified, such as induced pluripotent stem cells, embryonic stem cells and mesenchymal stem cells, which can differentiate into linages such as neurocytes, osteocytes and myocytes. Stem cells with biomaterials have been used for degenerative diseases, such as heart, kidney and liver failures.
ESC: Embryonic stem cell; iPSC: Induced pluripotent stem cell; MSC: Mesenchymal stem cell.
Adapted with permission from [18].
Mesenchymal stem cells (MSCs), a multipotent cell type, have been shown to differentiate into adipocytes, bone, cartilage, muscle, skin and tendon. Furthermore, preclinical models have used MSCs for tissue engineering of bone, cartilage, muscle, marrow, stroma, tendon, fat and other connective tissues [26]. The use of MSCs has potential benefits for clinical applications that include the production of MSCs from self-renewing human ES cells and ES cell-derived MSCs can be genetically modified by gene targeting on human ES cells [6]. MSCs have also been recently investigated in combination of FGF-8, which has demonstrated an essential role in limb development and blastema outgrowth. FGF-8 as found to induce a robust proliferation and upregulation of chondrogenic and myofiber differentiation as well as suppressed adipogenic and tenogenic differentiation in adipose derived stem cells (ADSCs) [27].
Regenerative medicine using ADSCs has also recently shown promise approach as a treatment strategy for severe hair loss. Nakajima et al. were been able to assemble ADSCs into hair follicle germ (HFG)-like aggregates for the regeneration of de novo hair. During cell culture, ADSCs were suspended with human dermal papilla cells and murine embryonic epithelial cells which formed aggregates of HFGs with localized ADSCs. Once this was established in customized lab-made chip devices, HFGs containing ADSCS were transplanted into nude mice which efficiently generated hair shafts when compared with HFGs without ADSCs [28].
Another promising area of stem cell science that has recently developed is the use of the stem cell secretome as cell-free alternative. The secretome is defined as a set of biological factors and molecules that are secreted into the ECM. This highly dynamic composition allows for customized therapeutic effects according to the intended application [29]. Delivery strategies are currently being explored and developed to harness the therapeutic benefits of the secretome in an effort to overcome the limitations associated with cell-based therapies.
There are several limitations that should be considered when utilizing stem cells for regenerative medicine. Difficult of obtaining NSCs from humans and the ability of ES to differentiate into any cell type possibly forming malignant tumors after transplantation in immunodeficient mice [6]. Other concerns include, increased cell survival, integration of transplanted cells into the host environment and immuno-rejection. Investigating and determining if material science and bioengineering can provide stem cells with an environment that mimics the healthy environment and modulates an immune response is a key component to complex tissue regeneration. Bioethical debates have also emerged, as concerns for destruction of human embryos to derive stem cells and the creation of human-animal hybrids have grown in the last two decades [30]. In addition, there is controversy and conflicting information regarding clinical safety and efficacy of cell-based therapy which has raised public concerns regarding consumer accessibility and regulation enforcement. As a result, ethical tensions related to clinical translation and regulatory policies are now the topic of global discussion.
Two regulatory issues loom large in shaping the environment for stem cell-based interventions and their value in healthcare; approval standards and long-term follow-up after approval. Authorities such as the US FDA, along with intellectual property and data exclusivity policies that protect research investments create strong financial incentives for scientists to develop high quality evidence of safety and efficacy. However, once these treatments receive approval, post-approval studies confirming the efficacy or testing safety are often delayed and/or of low quality due to diminished incentives provided by the authorities. Some countries, such as Japan have established registries for post market safety monitoring stem cell intervention. However, similar guidelines are not always established [31]. A recent classification system that stratifies interventions based on supporting evidence and risk of harm has emerged to present the scientific community with a systemic strategy for assessment of cell-based therapy. The classifications of cutting edge, bleeding edge and off the edge interventions have been outlined in conjunction with the FDA’s framework for cellular therapy in order to guide the development of future regulations and policies [32].
Advanced material science & biomaterials
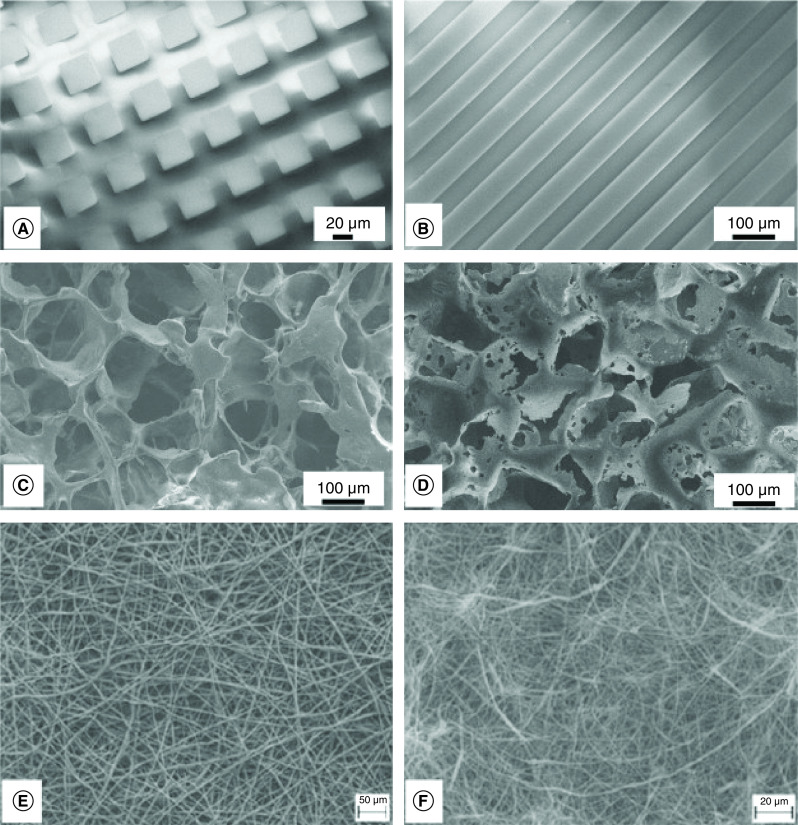
Biomaterials can be defined as any material intended to create an interface with a biological system to evaluate, treat, augment repair or replace any tissue, organ or function in the body. Biomaterials must also be deemed biocompatible for use in the desired environment [33]. A major goal of biomaterials is to provide favorable microenvironments which mimic the physiological niche of the tissue it is incorporated in as well as special organization of cells and tissue within the ECM. Hence, topography, chemical and physical properties of biomaterials are critical parameters for directing cell fate. Various designs of biomaterials are illustrated in Figure 5 [34]. The synergism of biomaterials and stem cells science is also developing rapidly indicating that a precise, physiological condition, could drive stem-cell fate both in vitro and in vivo once the desired microenvironment and tissue organization is achieved.
Figure 5. . Scanning electron miscroscopy of biomaterial designs.
Surface topography of biomaterials: squares (A), grooves (B), biodegradable polymeric scaffolds (C & D), polymeric and composite electrospun mats (E & F).
Adapted from [34] with permission from Elsevier.
The range of biomaterials has expanded from the traditional pool of biodegradable and nondegradable polymers and ceramics to advanced materials with varying functions and properties. For instance, polymers (i.e., polyphosphazenes and hydrogels) can now be designed with a range of mechanical and chemical properties specific to the tissue or application [2]. The ability of these synthetic polymers to serve as therapeutic devices such as temporary prostheses, 3D porous structures such as scaffolds and as a means of controlled/sustained drug delivery have allowed them to become key components in regenerative engineering studies [35].
Polyphosphazenes have become popular synthetic polymers due to their chemical versatility and functionality. A polyphosphazene is an inorganic–organic hybrid polymer with a backbone consisting of alternating phosphorus and nitrogen atoms and is considered the most chemically diverse and flexible synthetically derived class of inorganic polymers [36,37]. Integration of a hydrolytically labile side group such as glucosyl, glycerol and amino acid esters form a subclass known as biodegradable polymers, which are commonly used as biomaterials in regenerative engineering and controlled drug delivery. As a result of their extraordinary design flexibility, tunable properties and neutral bioactivity, polyphopsphazenes polymers have been incorporated into several biomedical applications, emerging as the next generation of biomaterials [38,39]. Recently, a new class of polyphosphazenes which features a backbone comprised thiophene-based side groups have been synthesized and chemically oxidized to optimize their optoelectrical potential. The overall results showed that chemical oxidation of polyphosphazenes offers more adaptability and has the potential for use in polymeric semiconductors and organic electrons, thus amplifying the chemical versatility that the class of polyphosphazenes possess [40]. Other examples of biodegradable synthetic polymers that can be used as biomaterials include poly(a-esters), polyurethanes, poly(ester amides), poly(ortho esters), polyanhydrides, poly(propylene fumarate), pseudo poly(amino acids), poly(alkyl cyanoacrylates) and polyphosphoesters [35,41]. The use of these polymers to create 3D scaffolds allows for matrices on which cells can proliferate, migrate, produce an ECM and form a functional tissues [42].
Utilization of advances in material science has shown promising results in vitro and in vivo. Early studies demonstrated how a novel class of polymers with mechanical properties similar to cancellous bone could be used in weight bearing areas of the body for orthopedic-related implants and applications. Poly(anhydride-co-imde) based on poly(trimellitylimidoglycine-co-1,6-bis[carboxyphenoxy]hexane) and poly(pyromellitylimidoalanine-co-1,6-bis[carboxyphenoxy]hexame) were investigated in the tibia of rats where a defect was produced. Results showed tissue compatibility as well as slowed erosion of matrices, maintenance of shape and endosteal bone growth as early as day 3 after the defect [43]. Another study investigated biodegradable blends of poly([ethyl alanato][p-phenyl phenoxy] phosphazene) (PNEAPhPh) and poly(lactic acid-glycolic acid) (PLAGA) to evaluate osteocompatibility in vitro. Primary rat osteoblasts displayed increase in the phenotypic expression and mineralized matrix synthesis when combined with a blend of 50% PNEA and 50% PhPh [44].
Lv et al. developed a biodegradable PLAGA mixed scaffold for use in tissue engineering in a high aspect ratio vessel rotating bioreactor system. The scaffold consisted of lighter than water and heavier than water microsphere composites with mechanical parameters for bone tissue engineering applications to create a 3D PLAGA/nano-hydroxyapatite mixed scaffold [45]. In addition, evaluation of human mesenchymal stem cells in the presence of the PLAGA/nano-hydroxyapatite mixed scaffolds revealed increased proliferation, differentiation and mineralization when compared with PLAGA scaffolds, indicating that mixed scaffolds are promising candidates for bone tissue engineering [45]. With an increasing use of bone grafts for large bone defects as a result of trauma, neoplasm and infection, regenerative bone grafts can be advantageous in their lack of antigenicity, high availability and flexibility of properties based on the desired application [41].
Traditionally used for drug delivery, microspheres are becoming a fundamental part of scaffolds for regenerative engineering due to their ability to provide a porous network and a physiochemical gradient with spatiotemporal and controlled release of growth factors, drugs and signaling molecules [46]. Another attractive biomaterial that has been used in regenerative engineering applications is poly(ethylene glycol) (PEG)-based hydrogels. The mechanical properties of PEG-based hydrogels can be manipulated and engineered into tissue mimetic three dimensional scaffolds that can support cell growth [47]. Hydrogels also possess the ability to encapsulate cells, adjust to span a range of stiffnesses relevant to soft tissue and control presentation of bioactive ligands [48]. The accumulation of these characteristics has allowed hydrogels to be used in the regeneration of tissues with complex compositions and architectures and expand our knowledge of cell-matrix interactions at a nanoscale level. More specifically, PEGDA hydrogels have been investigated in numerous studies to regenerate a wide range of tissue such as cartilage, bone, liver tissue, cardiac tissue and blood vessels [49,50,51,52,53,54,55,56,57].
Hydrogels can also be developed from tissues such as amnion membrane (AM), obtained from placental tissue. AM has been shown to suppress the expression of inflammatory cytokines such as IL-1α and IL-β as well as MMP through inhibitors. A recent in vitro study aimed to investigate the synergistic effects of an amnion-derived hydrogel with ADSCs on inflamed chondrocytes stimulated with IL-β chondrocytes. Results demonstrated that AM hydrogels supported cell viability, proliferation and stemness by inhibition of the catabolic responses of IL-β and the Wnt/β-catenin signaling pathway [58]. It was concluded that AM hydrogels can serve as a potential carrier for ADSCs in the treatment of osteoarthritis. Studies such as these, which incorporation multiple disciplines of regenerative engineering also emphasize the importance of convergence in addressing clinical challenges.
Although synthetic polymers have been widely used as scaffolding materials in tissue engineering, several natural polymers have also shown promising results. The natural biopolymer chitosan is a linear copolymer of β (1 → 4)-linked glucosamine and N-acetyl-d-glucosamine that can be prepared by the deacetylation of chitin. Due to its excellent biocompatibility and biodegradability, chitosan has been explored for use in a variety of biomedical applications such as wound healing, bone and cartilage regeneration, as well as in the fabrication of 3D microspheres [59]. Natural polymers themselves have numerous advantages such as natural abundance and ease of isolation. They can undergo enzymatic and/or hydrolytic degradation in biological environments without the production of toxic byproducts. Polysaccharides, which are polymers derived from plants and animal proteins such as cellulose, hyaluronic acid, chondroitin sulfate, alginates and heparin may also be used as biomaterials for drug delivery and regenerative engineering [60].
Nanomaterials & nanotechnology
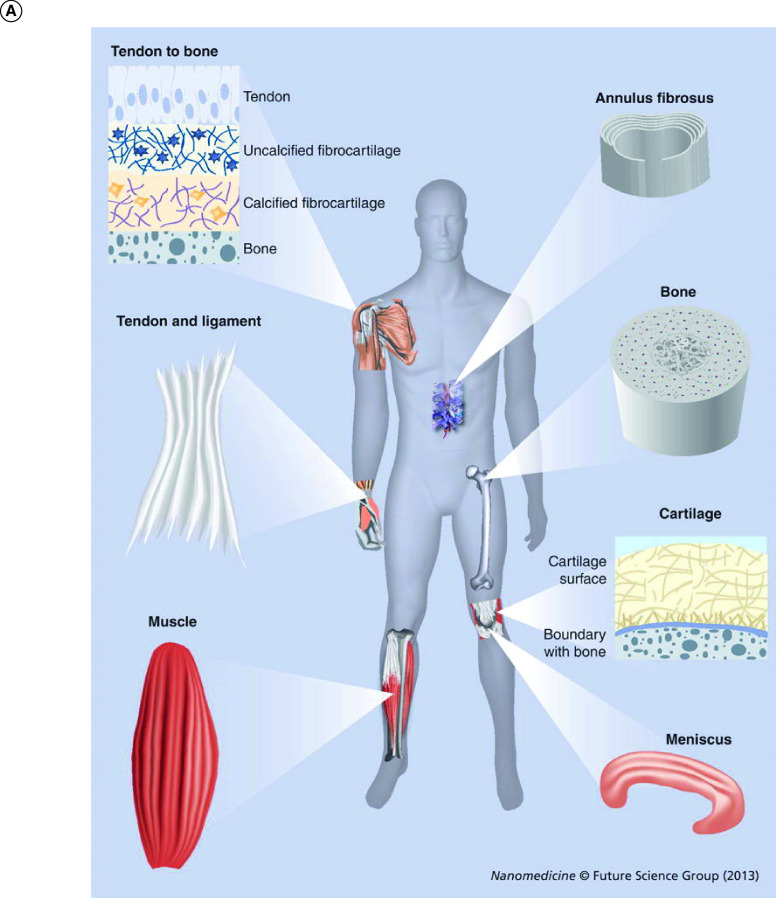
Biomaterials are imperative in optimizing culture conditions (e.g., in vitro and in vivo conditions) for stem cell therapy and regenerative engineering. Nanotechnology allows for the fabrication of nanomaterials (e.g., nanofiber scaffolds) that create positive parameters such as physiological conditions to promote regeneration of healthy tissues. In addition, the use of nanotechnology allows the tuning of the biochemical and mechanical microenvironment toward cell delivery and tissue regeneration [61,62]. Nanomaterials should also mimic the ECM of a healthy tissue environment. Nanofibers are measured on the nanoscale (1–100 nm) and are widely used in a variety of regenerative applications due to the ease of their fabrication techniques (e.g., electrospinning and self-assembly, temperature-induced phase separation and melt blowing). There are also several advantages of using nanofibers including the high reactivity, high porosity, pore-size distribution, enhanced specificity and high surface-to-volume ratio [61,63]. Nanofiber scaffolds have been developed and used for several tissue systems including cardiovascular and skin, but not limited to orthopedic tissue repair and regeneration shown in Figure 6A [64]. These structures and fibrous architectures can be recreated by scaffolds made by electrospinning [64].
Figure 6. . Overview nanofiber tissues and scaffolds.
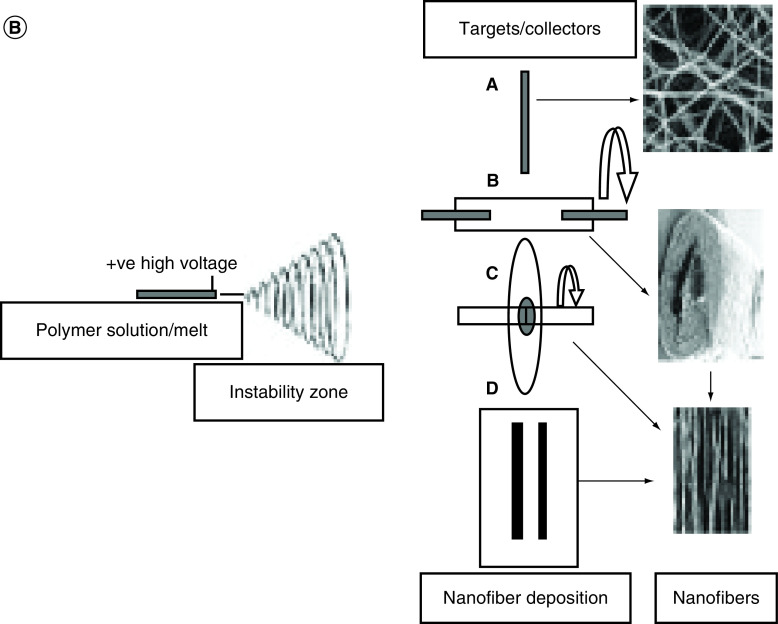
(A) Features of collagen nanofiber organizations in different musculoskeletal tissues. In tendon and ligaments, nanofibers of collagen are aligned uniaxially with a wavy appearance. Collagen fibers can have graded organizations (from uniaxially aligned to random) at tendon-to-bone insertion sites while in the meniscus and annulus fibrosus, collagen fibers are aligned circumferentially. Collagen fibers are arranged in thin-layered arrays in cartilage. Reproduced with permission from [64]. (B) Schematics of electrospinning setup. A high electric potential of a few kV is applied to the pendent polymer solution/melts which then ejects a polymer jet from the charged solution. This polymer jet then undergoes a series of bending and stretching instabilities that results in ultra-thin fibers. Nanofibers can be aligned based on the type of collector/target. A: Stationary collectors such as grounded conductive substrates produce random nanofibers. B: Rotating drum, and C: wheel-like bobbin or metal frame are rotating targets that fabricate tubular nanofiber scaffolds and aligns nanofibers. D: A split electrode with two conductive substrates separated by a void gap can result in the deposition of aligned nanofibers.
Reproduced with permission from [63].
Electrospinning is a nanofiber fabrication technique that utilizes the process of applying high electric potentials of few kV magnitudes to a droplet of polymer solution demonstrated in Figure 6B. Electrospun nanofibers scaffolds have a high range of adaptability and have been used in tissue engineering as they can mimic the structural organization of musculoskeletal tissues such as bone, cartilage, muscle and tendon [2,64]. Various studies have reported significant results in cellular behavior in response to nano-topographical features namely ridges, pores, fibers and groves [63]. Nanofibers improved cellular adhesion [65], proliferation [66], morphology [67], endocytotic activity [66] and gene expression [65]. In some cases, researchers combined natural polymers and proteins with synthetic polymers altering surface properties and improving cell behavior [35,36,41,68,69]. Polymeric nanofiber matrices can also act as carriers for bioactive agents including antibiotics, antimicrobial, antifungal, proteins (enzymes, DNA, etc.), anticancer and other valued drugs [70]. Fiber diameter and orientation also have a significant effect on cellular response. Changes in cellular behavior may result from changes in surface properties such as hydrophilicity, roughness, porosity and pore diameter [63]. Surface property changes can lead to adsorption of more ECM proteins such as vitronectin and fibronectin resulting in improved cell adhesion and proliferation. In addition, higher porosity and wide pore diameters when combining micro-nanofibers showed encouraging cell infiltration into 3D constructs [63]. All of these properties need to be considered while constructing nanofiber-based grafts for tissue-specific regeneration.
A variety of nanostructures have also been investigated for managing a multitude of aspects of bone malignancies. Magnetic/paramagnetic particles, quantum dots, fluorescent nanoparticles may be useful with for tumor imaging. Lipid nanoparticles, liposomes, micelles, dendrimers, nanocrystals and carbon nanotubes have been investigated as possible agents of drug deliver for tumor cells. Silver nanoparticles may be advantageous for the induction of apoptosis of cancerous cells and nanobodies, antibodies consisting only of a heavy chain, may be a favorable substitution for monoclonal antibodies in the treatment of cancerous cells [71]. Nanostructure scaffolds such as a potent 3D SiO2-CaO-P2O5 glasses (bioglass)/gelatin (gel), gelatin/hydroxyapatite (Gel/HA) and a gelatin/bioactive glass nanocomposite have also shown promising results as treatment for bone lesions as a result of tumor resection [71]. Researchers have also been able to extract and synthesize significant MgO/CaO nanostructures from the chemical composition of trimmed-off human finger and toenails. The fabrication of these Mg/CaO nanostructures were shown to have superior functionality in terms of antimicrobial activity, water remediation, dietary requirements and vitality. The ability to reorganize human waste remnants into valuable nanostructures may hold a great environmental impact and future studies should be conducted to determine the applicability of these nanostructure [72].
Recent research and technological advances have allowed electrospinning technology to transform from lab scale nanofiber mats to industrial application in the production of advanced products such as high strength composite materials, wound dressings, electronic device making, drug delivery, food packaging and membrane filtration [73]. Increased strength of nanofiber mats and improved control of size, shape, diameter, fiber length, orientation, porosity and fiber alignments have allowed for technological advancements and the emergence of large scale production that employs this technology [73].
Multifunctional wound dressings with the antibacterial properties and the ability to promote hemostasis have been explored using electrospinning technology. Incorporation of antibacterial materials into polymers have been fabricated by five methods; blending the polymer solution before electrospinning, fabricating core/shell structure through coaxial electrospinning, encapsulating the active agent before mixing with electrospinning solution, post-treatment of the fiber after electrospinning to convert a precursor to its active form or attaching the fiber surface with an active agent [74]. Hemostatic components can be blended with nanofibrous mats with large specific surface area and high porosity to promote hemostasis in a similar manner. Studies involving electrospinning to promote hemostasis have involved the fabrication of berberine loaded electrospun poly-(ε-caprolactone), fiber blends of chitosan, gelatin and shape memory polyurethane with an average diameter of around 300 nm and subsequent post-treatment with a silver nitrate solution, gelatin-blended chitosan nanofiber mats and a biodegradable tri-layered barrier which a PLGA/PLA-b-PEG electrospun layer was sandwiched between layers of a carbomethyl chitosan sponge [74].
Advantages of electrospun nanofibers for use in food packaging technology includes the relative ease of encapsulating agents such as nutraceuticals and small pore size which can act as a physical barrier against bacterial contamination [73]. The use of composite packing PLA with impregnation of thymol in blend with electrospun poly(vinyl alcohol)-cellulose nanocrystal nanofibers has also been advantageous for films with greater mechanical and thermal properties for severe environmental conditions [75]. Water and air filtration is dependent on pore size and filtration and the development of nanofiber technology propose a feasible route to design membranes suitable for filtration applications. In a recent study, electrospun nanofibers using a biopolymer-based composite precursor was able to simultaneously inactivate bacterial with concurrent filtration, by design of a poly(vinyl alchohol-co-ethylene) nanofibrous membrane with post-treatment of chitosan activation and graphene oxide composite solution through a simple suspension coating technique [76].
Tissue-specific approaches
As an interdisciplinary field, regenerative engineering has provided insight and progress toward tissue-specific regeneration. It is imperative to design and fabricate suitable scaffold matrices for use in specific tissue regeneration since the scaffold matrix comes directly into contact with cells providing structural support and guidance for tissue development [77]. For example, a mechanically superior matrix that enhanced the osteointegration and regeneration of anterior cruciate ligament (ACL) tissue in a rabbit model has been developed [78]. The rate of regeneration was accelerated using the bioengineered ACL matrix supplemented with bone marrow aspirate concentrate and growth factors (BMP-2, FGF-2 and FGF-8) and; increasing matrix strength retention [59,79]. This study illustrated the beneficial effect bioactive factors and polyethylene terephthalate incorporation have on ACL regeneration and signal a favorable step toward the clinical translation of a functional bioengineered ACL matrix [78]. Further studies have been performed to improve the cell adhesion properties of the bioengineering ACL matrix through the physical adsorption of fibronectin and air plasma treatment to improve the clinical efficacy of the matrix in combination with stem cell therapies [80].
The impacts of b-FGF-loaded electrospun PLGA nanofiber for rotator cuff tear have also been investigated [81]. Biochemical and histological results showed that electrospun nanofibrous matrices aid in cell attachment and proliferation and bFGF-loaded PLGA has better effect on tendon-bone healing [81]. These promising results and other studies for rotator cuff repair and regeneration are summarized in a detailed review [82]. Regeneration of healthy tissue to replace damaged or injured tissue is at the attention of many research groups to bridge the gap in current clinical challenges [82,83,84].
Additive manufacturing/3D bioprinting
Escalating cases of organ shortage and donor scarcity worldwide has driven research efforts in the field of regenerative engineering and tissue engineering toward the production of biological substitutes that can function in the same capacity as the original tissue. Additive manufacturing or 3D bioprinting is a ground breaking, innovative technology designed to fabricate the biological constructions with hierarchical architecture similar to the native counterpart [85]. By developing living functional tissue, 3D bioprinting has the potential to address an urgent need in tissue replacement and organ transplantation. The process of 3D printing refers to the printing and patterning of cells or other biological entities directly on a substrate or tissue culture dish which can be dispensed in biocompatible materials to form a desired 3D functional structure. These biomaterials are considered as ‘bioinks’ as they may consist of cells, base structure material or other prerequisites to generate functional tissue [85].
Tissue constructs of various origins have been successfully bioprinted using several approaches. Ng et al. reported the use of polyelectrolyte gelatin-chitosan hydrogels for 3D bioprinting of the skin. The inherent antimicrobial and hemostatic properties of chitosan were advantageous for use in wound healing applications [86]. Shi et al. was able to develop stable living skin constructs with >90% cell viability upon the inclusion of gelatin methacrylamide and collagen doped with tyrosinase into the scaffold by extrusion bioprinting [87]. A fully personalized contracting patch of cardiac tissue was developed by Noor et al. by developing a personalized hydrogel from processing of the ECM obtained via biopsy of fatty tissue/omentum from patients. This personalized hydrogel was combined with the patient’s cells and used as a bioink [88]. The vascularized cardiac patches fully matched the anatomical, cellular, biochemical and immunological properties of the patient which could provide a major breakthrough in transplant science [85].
Lee et al. reported bioprinting of primary rat hepatocytes, human umbilical vein endothelial cell line and human lung fibroblasts with multiple nozzle-based extrusion-based bioprinting for liver tissue engineering. Infusion of collagen bioink containing cells into the canals of the poly(ε-caprolactone) framework resulted in a co-cultured 3D environment-induced heterotypic interaction among cells, thereby enhancing survival and functionality of hepatocytes [89]. This study demonstrated the potential of 3D printed constructions containing a capillary-like network for functional liver regeneration. Soft neural tissue has also been successfully bioprinted. Haring et al. conducted a series of in vitro studies which revealed HA and Pluronic F-127 bioink as biocompatible bioinks relative to the control alginate hydrogels [90].
The interdisciplinary approach of 3D bioprinting provides an opportunity for scientists from different backgrounds to collaborate and address grand clinical challenges. Although proof-of-concept examples have been developed in vivo and in vitro studies, there are many challenges that have to be addressed before clinical translation is truly achieved. There is currently no standard method for fabrication of tissues and organ systems due to the remarkable differences that exist between individuals. As a result of this variance, cells proliferation and differentiation will also differ among individuals which makes developing a standard method challenging. Lack of speed during the bioprinting process and biocompatibility of biomaterials are also pressing issues [91]. Another major challenge in 3D bioprinting is vascularization and innervation of grafts. A few studies have been successfully able to vascularize tissues in animals and humans but further studies are required to receive approval by relevant authorities such as the FDA or the EMA [91]. If these challenges are successfully addressed, 3D bioprinting has the potential to revolutionize transplant medicine by providing personalized constructs or grafts to suit the particular needs of a vulnerable population of patients.
Conclusion & future directions
Although regenerative engineering and tissue engineering aim to restore tissue and organ function through the regeneration of cellular components, many limitations still exist before clinical translation can occur. Regenerative medicine is often described as a disruptive innovation, as it can lead to sectoral transformation and displacement of incumbent companies or on the other hand, create entirely new sectors all with significant societal and economic benefits. However, there have been few studies and inquiries into the nature and location of the disruption [92]. Variability that exists among regenerative engineering products is also fairly challenging for achieving a product that has consistent efficacy [84]. Sources of variability include characteristics of the donor or cell source such as health, disease state, genetics, medication history, patient demographics, immune system and the manufacturing process with an emphasis on production and storage. The status of a patient’s immune system may impact the response of a given therapy; a response that is not always predictable as patients with the same conditions may respond in a completely different manner. In addition, products manufactured under different processing sites under different conditions may contribute to inconsistency [84]. However, by addressing sources of variability, the success of the product can be improved by mitigating these sources and preserving product safety and efficacy. Another more pressing challenge that exists in the translation of regenerative engineering therapeutics is the high cost to bring these technologies through the regulatory and approval process. With economic and political agendas to reduce the costs of healthcare in some countries, this hurdle makes the approval process even more challenging [93]. However, despite these limitations, the regeneration of a damaged tissue rather than repair or replacing it may be a key feature in advancing current therapeutics and addressing grand clinical challenges for complex tissue regeneration.
It has been proposed that although variability is inevitable, it is possible to estimate and harness the variability among patients by utilizing advances in material sciences, biomaterial science and data science. Ameer et al. 2020 proposed that the implantation of ‘smart’ medical devices may address this issue of variability. A smart regenerative system would serve as a regenerative scaffold with an integrated computing system that is capable of interacting with the host tissue, and trigger the regenerative process within an injured microenvironment [93]. This regenerative process would be monitored by the patient and doctor, with adjustments being made based on the patient’s immune response to the regenerative scaffold. In order to achieve this feat, a collaboration between the fields of regenerative engineering, data science, information systems and machine learning would be required to facilitate this controlled regeneration of tissue [93].
With the pandemic caused by the outbreak of the novel coronavirus (SARS-CoV-2), communities all across the globe have been affected. While much has been discovered about the virus and its pathogenesis, there is still much work to be done with regards to developing an appropriate treatment strategy. The variations in clinical presentation among patients complicates this, as not all people exposed to SARS-CoV-2 are symptomatic, but a certain subset has shown to progress to severe respiratory illness which can often be fatal [94]. This severe respiratory illness is thought to be caused by cytokine release syndrome mediated by leukocytes. Future trials in regenerative medicine may play a key role in providing an acute intervention as blocking IL-6 and TNF may provide benefit and protection [94]. Some clinical trials in China have shown that the use of MSCs in severe cases may be beneficial, but more investigation is required as activation of IFNγ is needed for MSCs to have an anti-inflammatory effect. This feature may be absent in severely affected patients as T-lymphocytes are not well activated by SARS-CoV-2 infection [94]. Long term pulmonary complications in patients post infection such as interstitial thickening and fibrosis have also been documented [95]. As more long-term data is attained on pulmonary complications, future regenerative engineering studies may emerge that target fibrotic or damage lung tissue and focus on the regeneration of native lung tissue.
The emergence of 3D printing and ground-breaking technological advancements will allow for patient-specific and tailor-made grafts that can be integrated into the host tissue with developing knowledge on graft vascularization and innervation [91]. In combination with improved methods of release of essential growth factors, more predictable and controlled healing and regeneration may take place. Alterations in the host environment through synthetic polymers and biomaterials to support cell growth and attachment with bioactive factors can also lower the rejection of 3D bio-printed tissue as the most desirable condition for regeneration can be engineered [91]. This may revolutionize transplant medicine as we know it.
By utilizing a top-down and bottom-up approach, regenerative engineering has shown the potential to regenerate complex tissues, such as a limb. The bottom-up approach is largely comprised cellular mechanisms combined with advancements in the field of developmental biology. The top-down approach is built on the integration of biomaterials, advanced material science and engineering to create cells with high regenerative potential and place them in an environment that is supportive of cell growth [96]. As these described convergence applications continue to advance, regenerative engineering is on the brink of creating countless possibilities to develop next-generation resolutions for tissue and organ regeneration.
Executive summary.
Introduction
Since its emergence over three decades ago, tissue engineering has demonstrated that it has the potential to restore, maintain or improve native tissue function. However, translation of these concepts into clinical therapeutics and products has not been widespread despite established proof-of-concept approaches.
While tissue engineering focuses on a single lineage of tissue, most organ systems comprised a multitude of tissues which would require simultaneous restoration.
Regenerative engineering has recently emerged as an expansion of tissue engineering to overcome the challenges of regenerating complex tissue systems by harnessing the body’s innate ability to regenerative native tissue.
Regenerative engineering
Regenerative engineering utilizes the convergence of the disciplines of advanced material science, stem cell science and developmental biology to achieve clinical translation for the regeneration of complex tissues and organ system.
As more work is being done in the field, theories and concepts have evolved into practical tools for scientists and have become an integral part of many studies aiming to solve grand clinical challenges.
Developmental biology
In order to understand the principles of regenerating complex tissues, a deep understanding of the developmental processes of each organ system is required.
Urodele amphibians, salamanders and axolotls (Ambystoma mexicanum) have served as models of the study of genes, growth factors and cell signaling pathways involved in limb regeneration due to their instinctive regenerative capacity.
Stem cell science
Embryonic stem cells, neural stem cells and mesenchymal stem cells have shown the ability to differentiate into a wide range of tissues such as adipocytes, bone, cartilage, muscle, skin, hair follicles and tendon.
The stem cell secretome, a cell free alternative, also being explored which utilizes the paracrine effect of growth factors secreted by stem cells to induce the therapeutic effect without the risk of adverse effects that may occur with stem cells.
Advanced material science & biomaterials
The range of biomaterials has expanded from the traditional pool of biodegradable and nondegradable polymers and ceramics to advanced materials with modifiable properties and functions.
Nanomaterials & nanotechnology
Developments in nanotechnology and electrospinning has allowed for the creation of nanomaterials with diverse properties that resemble the extracellular matrix of tissues such as cardiovascular, skin, cartilage and bone.
Electrospinning technology has also been explored industrially for the production of wound dressings, high strength composite materials, wound dressings, food packaging and membrane filtration.
Tissue-specific approaches
Scaffold matrices have been designed to resemble specific tissues such as a mechanically superior matrix that enhanced the osteointegration and regeneration of ACL tissue and nanofibers for rotator cuff tears.
Additive manufacturing/3D bioprinting
A 3D bioprinting has the potential to revolutionize transplant medicine by providing tissue grafts and biological substitutes that can function in the same capacity.
Tissue constructs of various origins such as skin, cardiovascular, lung, liver and soft neural tissue have been successfully developed in in vivo and in vitro studies.
Conclusion & future directions
As the described convergence applications continue to advance, regenerative engineering is on the brink of creating countless possibilities to develop next-generation resolutions for tissue and organ regeneration once clinical translation is achieved.
Footnotes
Financial & competing interests disclosure
Support was received from the Raymond and Beverly Sackler Center for Biomedical, Biological, Physical and Engineering Sciences and NIHDP1 AR068147 is gratefully acknowledged. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Berthiaume F, Maguire TJ, Yarmush ML. Tissue engineering and regenerative medicine: history, progress, and challenges. Annu. Rev. Chem. Biomol. Eng. 2, 403–430 (2011). [DOI] [PubMed] [Google Scholar]
- 2.Laurencin CT, Khan Y. Regenerative engineering. Sci. Transl. Med. 4(160), 160ed9 (2012). [DOI] [PubMed] [Google Scholar]; • Provides an overview of regenerative engineering and its origins as an expansion of tissue engineering.
- 3.Langer R, Vacanti JP. Tissue engineering. Science 260(5110), 920–926 (1993). [DOI] [PubMed] [Google Scholar]
- 4.Samadikuchaksaraei A. Scientific and industrial status of tissue engineering. African J. Biotechnol. 6(25), 2897–2909 (2007). [Google Scholar]
- 5.Laurencin CT, Nair LS. The quest toward limb regeneration: a regenerative engineering approach. Regen. Biomater. 3(2), 123–125 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Elaborates the importance of developmental biology and how investigations of growth factors and cellular mechanisms play a role in the regeneration of complex tissues such as a limb.
- 6.Laurencin CT, Khan Y. Regenerative Engineering CRC Press/Taylor & Francis Group, FL, USA: (2013). [Google Scholar]
- 7.Sharp PA, Langer R. Promoting convergence in biomedical science. Science 333(6042), 527 (2011). [DOI] [PubMed] [Google Scholar]
- 8.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126(4), 663–676 (2006). [DOI] [PubMed] [Google Scholar]
- 9.Stoick-Cooper CL, Moon RT, Weidinger G. Advances in signaling in vertebrate regeneration as a prelude to regenerative medicine. Genes Dev. 21(11), 1292–1315 (2007). [DOI] [PubMed] [Google Scholar]
- 10.McCusker C, Gardiner DM. The axolotl model for regeneration and aging research: a mini-review. Gerontology 57(6), 565–571 (2011). [DOI] [PubMed] [Google Scholar]
- 11.Seyedhassantehrani N, Otsuka T, Singh S, Gardiner DM. The axolotl limb regeneration model as a discovery tool for engineering the stem cell niche. Curr. Stem Cell Reports 3(3), 156–163 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stocum DL. Mechanisms of urodele limb regeneration. Regeneration 4(4), 159–200 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tamura K, Ohgo S, Yokoyama H. Limb blastema cell: a stem cell for morphological regeneration. Dev. Growth Differ. 52(1), 89–99 (2009). [DOI] [PubMed] [Google Scholar]
- 14.Kawakami Y, Esteban CR, Raya Met al. Wnt/β-catenin signaling regulates vertebrate limb regeneration. Genes Dev. 20(23), 3232–3237 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferretti P, Zhang F, Santos-Ruiz L, Clarke JDW. FGF signalling and blastema growth during amphibian tail regeneration. : International Journal of Developmental Biology UPV/EHU Press, London, England, S127–S128 (2001). [Google Scholar]
- 16.Cunningham TJ, Duester G. Mechanisms of retinoic acid signalling and its roles in organ and limb development. Nat. Rev. Mol. Cell Biol. 16(2), 110–123 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singh BN, Koyano-Nakagawa N, Donaldson A, Weaver CV, Garry MG, Garry DJ. Hedgehog signaling during appendage development and regeneration. Genes (Basel) 6(2), 417–435 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao X, Cui K, Li Z. The role of biomaterials in stem cell-based regenerative medicine. Future Med. Chem. 11(14), 1779–1792 (2019). [DOI] [PubMed] [Google Scholar]
- 20.Kordower JH, Goetz CG, Freeman TB, Olanow CW. Dopaminergic transplants in patients with Parkinson's disease: neuroanatomical correlates of clinical recovery. Exp. Neurol. 144(1), 41–46 (1997). [DOI] [PubMed] [Google Scholar]
- 21.Lee ST, Chu K, Park JEet al. Intravenous administration of human neural stem cells induces functional recovery in Huntington's disease rat model. Neurosci. Res. 52(3), 243–249 (2005). [DOI] [PubMed] [Google Scholar]
- 22.Emborg ME, Ebert AD, Moirano Jeffet al. GDNF-secreting human neural progenitor cells increase tyrosine hydroxylase and VMAT2 expression in MPTP-treated cynomolgus monkeys - PubMed. Cell Transpl. 17(4), 383–395 (2008). [PubMed] [Google Scholar]
- 23.Wichterle H, Lieberam I, Porter JA, Jessell TM. Directed differentiation of embryonic stem cells into motor neurons. Cell 110(3), 385–397 (2002). [DOI] [PubMed] [Google Scholar]
- 24.Li XJ, Du ZW, Zarnowska EDet al. Specification of motoneurons from human embryonic stem cells. Nat. Biotechnol. 23(2), 215–221 (2005). [DOI] [PubMed] [Google Scholar]
- 25.Lepore AC, Rauck B, Dejea Cet al. Focal transplantation-based astrocyte replacement is neuroprotective in a model of motor neuron disease. Nat. Neurosci. 11(11), 1294–1301 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Caplan AI. Mesenchymal stem cells: cell-based reconstructive therapy in orthopedics. Tissue Eng. 11(7), 1198–1211 (2005). [DOI] [PubMed] [Google Scholar]
- 27.Otsuka T, Mengsteab PY, Laurencin CT. Control of mesenchymal cell fate via application of FGF-8b in vitro. Stem Cell Res. 51, 102155 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakajima R, Tate Y, Yan L, Kageyama T, Fukuda J. Impact of adipose-derived stem cells on engineering hair follicle germ-like tissue grafts for hair regenerative medicine. J. Biosci. Bioeng. (2021). [DOI] [PubMed] [Google Scholar]
- 29.Daneshmandi L, Shah S, Jafari Tet al. Emergence of the stem cell secretome in regenerative engineering. Trends Biotechnol. 38(12), 1373–1384 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Introduces the concept of the stem cell secretome, a promising cell-free alternative to stem cell-based therapy which offers engineering design flexibility without the risks induced by the presence of stem cells.
- 30.Sipp D, Munsie M, Sugarman J. Emerging stem cell ethics. Science 360(6395), 1275 (2018). [DOI] [PubMed] [Google Scholar]
- 31.MacPherson A, Kimmelman J. Ethical development of stem-cell-based interventions. Nat. Med. 25(7), 1037–1044 (2019). [DOI] [PubMed] [Google Scholar]
- 32.Laurencin CT, McClinton A. Regenerative cell-based therapies: cutting edge, bleeding edge, and off the edge. Regen. Eng. Transl. Med. 6(1), 78–89 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Williams DF. The Williams Dictionary of Biomaterials. Liverpool University Press, Liverpool, England: (1999). [Google Scholar]
- 34.Martino S, D'Angelo F, Armentano I, Kenny JM, Orlacchio A. Stem cell-biomaterial interactions for regenerative medicine. Biotechnol. Adv. 30(1), 338–351 (2012). [DOI] [PubMed] [Google Scholar]
- 35.Nair LS, Laurencin CT. Biodegradable polymers as biomaterials. Prog. Polym. Sci. 32(8–9), 762–798 (2007). [Google Scholar]
- 36.Nair LS, Laurencin CT. An Introduction to Biomaterials - Google Books (2nd Edition). CRC Press/Taylor & Francis Group, FL, USA: (2003). [Google Scholar]
- 37.Nukavarapu SP, Kumbar SG, Allcock HR, Laurencin CT. Polyphosphazenes for biomedical applications - Google Books. : Polyphosphazanes for Biomedical Applications. Andrianov AK (). John Wiley & Sons Inc, NJ, USA, 117–138 (2008). [Google Scholar]
- 38.Ogueri KS, Ogueri KS, Ude CC, Allcock HR, Laurencin CT. Biomedical applications of polyphosphazenes. Med. Devices Sensors 3(6), e10113 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ogueri KS, Ogueri KS, Allcock HR, Laurencin CT. Polyphosphazene polymers: the next generation of biomaterials for regenerative engineering and therapeutic drug delivery. J. Vac. Sci. Technol. B 38(3), 030801 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]; • Reviews polyphosphazenes and their uses in regenerative engineering and drug delivery.
- 40.Ogueri KS, Ogueri KS, Laurencin CT, Allcock HR. Thiophene-based polyphosphazenes with tunable optoelectronic properties. J. Polym. Sci. 58(23), 3294–3310 (2020). [Google Scholar]
- 41.Ogueri KS, Jafari T, Escobar Ivirico JL, Laurencin CT. Polymeric biomaterials for scaffold-based bone regenerative engineering. Regen. Eng. Transl. Med. 5(2), 128–154 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Laurencin CT, Nair LS. Nanotechnology and Tissue Engineering: The scaffold. CRC Press/Taylor & Francis Group, FL, USA: (2003). [Google Scholar]
- 43.Ibim SEM, Uhrich KE, Attawia Met al. Preliminary in vivo report on the osteocompatibility of poly(anhydride-co-imides) evaluated in a tibial model. Journal of Biomedical Materials Research 43(4), 374–379 (1998). [DOI] [PubMed] [Google Scholar]
- 44.Deng M, Nair LS, Nukavarapu SPet al. Miscibility and in vitro osteocompatibility of biodegradable blends of poly[(ethyl alanato) (p-phenyl phenoxy) phosphazene] and poly(lactic acid-glycolic acid). Biomaterials 29(3), 337–349 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lv Q, Nair L, Laurencin CT. Fabrication, characterization, and in vitro evaluation of poly(lactic acid glycolic acid)/nano-hydroxyapatite composite microsphere-based scaffolds for bone tissue engineering in rotating bioreactors. J. Biomed. Mater. Res. Part A 91A(3), 679–691 (2009). [DOI] [PubMed] [Google Scholar]
- 46.Gupta V, Khan Y, Berkland CJ, Laurencin CT, Detamore MS. Microsphere-based scaffolds in regenerative engineering. Annu. Rev. Biomed. Eng. 19, 135–161 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moore EM, West JL. Bioactive poly(ethylene glycol) acrylate hydrogels for regenerative engineering. Regen. Eng. Transl. Med. 5(2), 167–179 (2019). [Google Scholar]
- 48.Guan X, Avci-Adali M, Alarçin Eet al. Development of hydrogels for regenerative engineering. Biotechnol. J. 12(5), p1600394 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Elisseeff J, McIntosh W, Anseth K, Riley S, Ragan P, Langer R. Photoencapsulation of chondrocytes in poly(ethylene oxide)-based semi-interpenetrating networks. J. Biomed. Mater. Res. 51(2), 164–171 (2000). [DOI] [PubMed] [Google Scholar]
- 50.Martens PJ, Bryant SJ, Anseth KS. Tailoring the degradation of hydrogels formed from multivinyl poly(ethlene glycol) and poly(vinyl alcohol) macromers for cartilage tissue engineering. Biomacromolecules 4(2), 283–292 (2003). [DOI] [PubMed] [Google Scholar]
- 51.Sonnet C, Simpson CL, Olabisi RMet al. Rapid healing of femoral defects in rats with low dose sustained BMP2 expression from PEGDA hydrogel microspheres. J. Orthop. Res. 31(10), 1597–1604 (2013). [DOI] [PubMed] [Google Scholar]
- 52.Schweller RM, Wu ZJ, Klitzman B, West JL. Stiffness of protease sensitive and cell adhesive PEG hydrogels promotes neovascularization in vivo. Ann. Biomed. Eng. 45(6), 1387–1398 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moore EM, Suresh V, Ying G, West JL. M0 and M2 macrophages enhance vascularization of tissue engineering scaffolds. Regen. Eng. Transl. Med. 4(2), 51–61 (2018). [Google Scholar]
- 54.Tsang VL, Chen AA, Cho LMet al. Fabrication of 3D hepatic tissues by additive photopatterning of cellular hydrogels. FASEB J. 21(3), 790–801 (2007). [DOI] [PubMed] [Google Scholar]
- 55.Unal AZ, Jeffs SE, West JL. 3D co-culture with vascular cells supports long-term hepatocyte phenotype and function in vitro. Regen. Eng. Transl. Med. 4(1), 21–34 (2018). [Google Scholar]
- 56.Kraehenbuehl TP, Zammaretti P, Van der Vlies AJet al. Three-dimensional extracellular matrix-directed cardioprogenitor differentiation: systematic modulation of a synthetic cell-responsive PEG-hydrogel. Biomaterials 29(18), 2757–2766 (2008). [DOI] [PubMed] [Google Scholar]
- 57.Li Z, Guan J. Hydrogels for cardiac tissue engineering. Polymers (Basel) 3(2), 740–761 (2011). [Google Scholar]
- 58.Bhattacharjee M, Ivirico JLE, Kan HMet al. Preparation and characterization of amnion hydrogel and its synergistic effect with adipose derived stem cells towards IL1β activated chondrocytes. Sci. Rep. 10(1), 18751 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Abdel-Fattah WI, Jiang T, El-Bassyouni GET, Laurencin CT. Synthesis, characterization of chitosans and fabrication of sintered chitosan microsphere matrices for bone tissue engineering. Acta Biomater. 3(4), 503–514 (2007). [DOI] [PubMed] [Google Scholar]
- 60.Shelke NB, James R, Laurencin CT, Kumbar SG. Polysaccharide biomaterials for drug delivery and regenerative engineering. Polym. Adv. Technol. 25(5), 448–460 (2014). [Google Scholar]
- 61.Jiang T, Carbone EJ, Lo KWH, Laurencin CT. Electrospinning of polymer nanofibers for tissue regeneration. Prog. Polym. Sci. 46, 1–24 (2015). [Google Scholar]
- 62.Nada AA, James R, Shelke NBet al. A smart methodology to fabricate electrospun chitosan nanofiber matrices for regenerative engineering applications. Polym. Adv. Technol. 25(5), 507–515 (2014). [Google Scholar]
- 63.Kumbar SG, James R, Nukavarapu SP, Laurencin CT. Electrospun nanofiber scaffolds: engineering soft tissues. Biomed. Mater. 3(3), 034002 (2008). [DOI] [PubMed] [Google Scholar]
- 64.Ma B, Xie J, Jiang J, Shuler FD, Bartlett DE. Rational design of nanofiber scaffolds for orthopedic tissue repair and regeneration. Nanomedicine. 8(9), (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Glass-Brudzinski J, Perizzolo D, Brunette DM. Effects of substratum surface topography on the organization of cells and collagen fibers in collagen gel cultures. J. Biomed. Mater. Res. 61(4), 608–618 (2002). [DOI] [PubMed] [Google Scholar]
- 66.Dalby MJ, Yarwood SJ, Riehle MO, Johnstone HJH, Affrossman S, Curtis ASG. Increasing fibroblast response to materials using nanotopography: morphological and genetic measurements of cell response to 13-nm-high polymer demixed islands. Exp. Cell Res. 276(1), 1–9 (2002). [DOI] [PubMed] [Google Scholar]
- 67.Karuri NW, Liliensiek S, Teixeira AIet al. Bilogical length scale topography enhances cell-substratum adhesion of human corneal epithelial cells. J. Cell Sci. 117(15), 3153–3164 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sun Z, Nair LS, Laurencin CT. The paracrine effect of adipose-derived stem cells inhibits IL-1β-induced inflammation in chondrogenic cells through the Wnt/β-catenin signaling pathway. Regen. Eng. Transl. Med. 4(1), 35–41 (2018). [Google Scholar]
- 69.Barajaa MA, Nair LS, Laurencin CT. Bioinspired scaffold designs for regenerating musculoskeletal tissue interfaces. Regen. Eng. Transl. Med. 6(4), 451–483 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kumbar SG, Nair LS, Bhattacharyya S, Laurencin CT. Polymeric nanofibers as novel carriers for the delivery of therapeutic molecules. J. Nanosci. Nanotechnol. 6(9), 2591–2607 (2006). [DOI] [PubMed] [Google Scholar]
- 71.Moradi M, Abdolhosseini M, Zarrabi A, johari B. A review on application of nano-structures and nano-objects with high potential for managing different aspects of bone malignancies. Nano-Structures and Nano-Objects 19, 100348 (2019). [Google Scholar]
- 72.Dwivedi P, Tiwary D, Mishra PK, Chakraborty JP. MgO/CaO nanostructures fabricated from trimmed-off human finger and toe nails. Nano-Structures and Nano-Objects 22, 100485 (2020). [Google Scholar]
- 73.Aruchamy K, Mahto A, Nataraj SK. Electrospun nanofibers, nanocomposites and characterization of art: insight on establishing fibers as product. Nano-Structures and Nano-Objects 16, 45–58 (2018). [Google Scholar]; • Reviews the various applications of nanomaterials, nanofibers and electrospinning.
- 74.Liu M, Duan XP, Li YM, Yang DP, Long YZ. Electrospun nanofibers for wound healing. Mater. Sci. Eng. C 76, 1413–1423 (2017). [DOI] [PubMed] [Google Scholar]
- 75.Alvarado N, Romero J, Torres Aet al. Supercritical impregnation of thymol in poly(lactic acid) filled with electrospun poly(vinyl alcohol)-cellulose nanocrystals nanofibers: development an active food packaging material. J. Food Eng. 217, 1–10 (2018). [Google Scholar]
- 76.Liu K, Cheng P, Wang Yet al. Concurrent filtration and inactivation of bacteria using poly (vinyl alcohol-co-ethylene) nanofibrous membrane facilely modified using chitosan and graphene oxide. pubs.rsc.org
- 77.Khademhosseini A, Vacanti JP, Langer R. Progress in tissue engineering. Sci. Am. 300(5), 64–71 (2009). [DOI] [PubMed] [Google Scholar]
- 78.Mengsteab PY, Otsuka T, McClinton Aet al. Mechanically superior matrices promote osteointegration and regeneration of anterior cruciate ligament tissue in rabbits. Proc. Natl Acad. Sci. USA 117(46), 28655–28666 (2020).33144508 [Google Scholar]
- 79.Mengsteab PY, Conroy P, Badon Met al. Evaluation of a bioengineered ACL matrix's osteointegration with BMP-2 supplementation. PLoS ONE 15(1), e0227181 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yu X, Mengsteab PY, Narayanan G, Nair LS, Laurencin CT. Enhancing the surface properties of a bioengineered anterior cruciate ligament matrix for use with point-of-care stem cell therapy. Engineering 7(2), 153–161 (2020). [PMC free article] [PubMed] [Google Scholar]
- 81.Zhao S, Zhao J, Dong Set al. Biological augmentation of rotator cuff repair using bFGF-loaded electrospun poly(lactide-co-glycolide) fbrous membranes. Int. J. Nanomedicine 9(1), 2373–2385 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Washington KS, Shemshaki NS, Laurencin CT. The role of nanomaterials and biological agents on rotator cuff regeneration. Regen. Eng. Transl. Med. 6(3), 1–10 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Saveh-Shemshaki N, Nair LS, Laurencin CT. Nanofiber-based matrices for rotator cuff regenerative engineering. Acta Biomater. 94, 64–81 (2019). [DOI] [PubMed] [Google Scholar]
- 84.Beachy SH, Nair L, Laurencin C, Tsokas KA, Lundberg MS. Sources of variability in clinical translation of regenerative engineering products: insights from the National Academies Forum on Regenerative Medicine. Regen. Eng. Transl. Med. 6(1), 1–6 (2020). [Google Scholar]
- 85.Matai I, Kaur G, Seyedsalehi A, McClinton A, Laurencin CT. Progress in 3D bioprinting technology for tissue/organ regenerative engineering. Biomaterials 226, 119536 (2020). [DOI] [PubMed] [Google Scholar]; •• Provides an indepth discussion of 3D bioprinting methods and tissues/organs that have successfully bioprinted in previous studies.
- 86.Ng WL, Yeong WY, Naing MW. Polyelectrolyte gelatin-chitosan hydrogel optimized for 3D bioprinting in skin tissue engineering. Int. J. Bioprinting. 2(1), 53–62 (2016). [Google Scholar]
- 87.Shi Y, Xing TL, Zhang HBet al. Tyrosinase-doped bioink for 3D bioprinting of living skin constructs. Biomed. Mater. 13(3), 035008 (2018). [DOI] [PubMed] [Google Scholar]
- 88.Noor N, Shapira A, Edri R, Gal I, Wertheim L, Dvir T. 3D printing of personalized thick and perfusable cardiac patches and hearts. Adv. Sci. 6(11), 1900344 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lee JW, Choi YJ, Yong WJet al. Development of a 3D cell printed construct considering angiogenesis for liver tissue engineering. Biofabrication 8(1), 015007 (2016). [DOI] [PubMed] [Google Scholar]
- 90.Haring AP, Thompson EG, Tong Yet al. Process- and bio-inspired hydrogels for 3D bioprinting of soft free-standing neural and glial tissues. Biofabrication 11(2), 025009 (2019). [DOI] [PubMed] [Google Scholar]
- 91.Dzobo K, Thomford NE, Senthebane DAet al. Advances in regenerative medicine and tissue engineering: innovation and transformation of medicine. Stem Cells Int. 2018, p2495848 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Banda G, Tait J, Mittra J. Regenerative medicine as a disruptive technology: implications for manufacturing & clinical adoption. Cell Gene Ther. Insights 5(10), 1287–1303 (2019). [Google Scholar]
- 93.Ameer GA. Understanding and harnessing variability in regenerative engineering. Regen. Eng. Transl. Med. 6(4), 429–432 (2020). [Google Scholar]
- 94.Shi Y, Wang Y, Shao Cet al. COVID-19 infection: the perspectives on immune responses. Cell Death Differ. 27(5), 1451–1454 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.del Rio C, Collins LF, Malani P. Long-term health consequences of COVID-19. JAMA 324(17), 1723–1724 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Laurencin CT, Nair LS. Regenerative engineering: approaches to limb regeneration and other grand challenges. Regen. Eng. Transl. Med. 1(1–4), 1–3 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Naqvi SM, McNamara LM. Stem cell mechanobiology and the role of biomaterials in governing mechanotransduction and matrix production for tissue regeneration. Front. Bioeng. Biotechnol. 8, 597661 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]