Graphical abstract

Keywords: COVID-19, History of epidemics, Aerosol disease transmission, Coronavirus, Anthropocene record
Abstract
Ongoing uncertainty over the relative importance of aerosol transmission of COVID-19 is in part rooted in the history of medical science and our understanding of how epidemic diseases can spread through human populations. Ancient Greek medical theory held that such illnesses are transmitted by airborne pathogenic emanations containing particulate matter (“miasmata”). Notable Roman and medieval scholars such as Varro, Ibn al-Khatib and Fracastoro developed these ideas, combining them with early germ theory and the concept of contagion. A widely held but vaguely defined belief in toxic miasmatic mists as a dominant causative agent in disease propagation was overtaken by the science of 19th century microbiology and epidemiology, especially in the study of cholera, which was proven to be mainly transmitted by contaminated water. Airborne disease transmission came to be viewed as burdened by a dubious historical reputation and difficult to demonstrate convincingly. A breakthrough came with the classic mid-20th century work of Wells, Riley and Mills who proved how expiratory aerosols (their “droplet nuclei”) could transport still-infectious tuberculosis bacteria through ventilation systems. The topic of aerosol transmission of pathogenic respiratory diseases assumed a new dimension with the mid-late 20th century “Great Acceleration” of an increasingly hypermobile human population repeatedly infected by different strains of zoonotic viruses, and has taken centre stage this century in response to outbreaks of new respiratory infections that include coronaviruses. From a geoscience perspective, the consequences of pandemic-status diseases such as COVID-19, produced by viral pathogens utilising aerosols to infect a human population currently approaching 8 billion, are far-reaching and unprecedented. The obvious and sudden impacts on for example waste plastic production, water and air quality and atmospheric chemistry are accelerating human awareness of current environmental challenges. As such, the “anthropause” lockdown enforced by COVID-19 may come to be seen as a harbinger of change great enough to be preserved in the Anthropocene stratal record.
1. Introduction
The COVID-19 public health emergency has refocused attention on the relative importance of aerosol transmission in the spread of human epidemic diseases. Initial emphasis by influential health organisations on the role of fomite and near-range droplet transmission of SARS-CoV-2 has triggered a robust counter-response from the aerosol scientist community (e.g. Fennelly, 2020, Jimenez, 2020, Morawska and Cao, 2020, Morawska and Milton, 2020, Prather et al., 2020, Tang et al., 2020, Tang et al., 2021a,b; Bourouiba, 2021, Greenhalgh et al., 2021, Morawska et al., 2021, Randall et al., 2021). This response has increasingly insisted that many COVID-19 infections are caused by the inhalation of airborne virus-bearing particles rather than via fomite or gravity-driven droplet contact, and that future health and urban planning policies need seriously to concentrate on indoor air quality issues.
The unwillingness of some members of the medical community to accept that aerosols commonly act as an important disease vector is nothing new. Forty-one years ago, an infected man travelling home from Pakistan initiated a nosocomial outbreak of smallpox in the German town of Meschede. Once the patient had been correctly diagnosed he was transferred to a smallpox isolation unit, but by then it was too late: some days later, new cases of the viral disease appeared quite unexpectedly on all three floors of the hospital (Fig. 1 a). None of these new smallpox cases had registered direct contact with the index patient, and transmission via fomites was examined in detail but rejected as highly unlikely. The only remaining route of transmission considered reasonable was airborne spread of a virus-containing aerosol, a possibility against which all of the investigators were initially prejudiced (Gelfand and Posch, 1971). Despite their misgivings, the investigators nevertheless finally concluded that their detailed epidemiological studies have clearly indicated that 17 of the cases were infected by virus particles disseminated by air over a considerable distance within a single hospital building (Wehrle et al., 1970). This conclusion has not only stood the test of time (Fenner et al., 1988, Tellier et al., 2019) but one overview study this century has argued that the weight of evidence suggests that fine particle aerosols were the most frequent and effective mode of smallpox transmission (Milton, 2012).
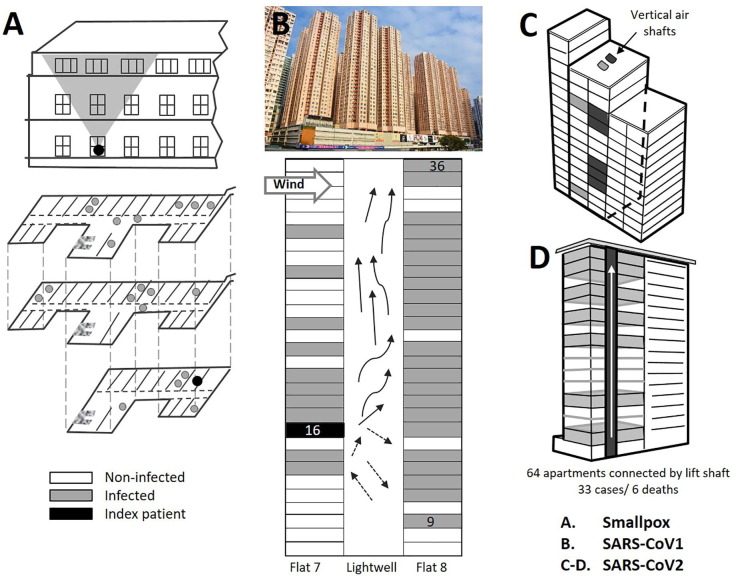
Fig. 1.
Examples of transmission clusters of viral epidemic diseases in buildings. A) Meschede Hospital, Germany 1970 smallpox outbreak, attributed to virus-bearing particles moving on air currents from index patient to infect others on all three hospital floors (Wehrle et al., 1970, Gelfand and Posch, 1971). B) Amoy Gardens residential apartments, Hong Kong 2003 outbreak of SARS-CoV-1 where infection was attributed to virus-bearing particles escaping through floor drains from the sewage-system into bathrooms and then driven as aerosols by ventilation fans into light wells serving the tower block (Yu et al., 2004;Li et al., 2004b;McKinney et al., 2006, Lee, 2012, Ng, 2020; photo: WMwiki, CC By Creativecommons.org/licenses/by/3.0> via Wikimedia Commons. https://commons.wikimedia.org/wiki/File:Amoy_gardens_2017.jpg). C) Apartment building in Seoul, South Korea in 2020 SARS-CoV-2 outbreak where movement of virus-bearing particles through vertical air ducts serving bathrooms was implicated in COVID-19 transmission (Hwang et al., 2021). D) Apartment building in Bilbao, Spain in 2021 SARS-CoV-2 outbreak where the viral transmission mode remains unclear (Galloway et al., 2021). https://english.elpais.com/society/2021-02-15/architecture-of-an-outbreak-the-spanish-apartment-building-hijacked-by-the-coronavirus.html.
The arguments in favour of aerosol transmission of COVID-19 are primarily based on case studies of outbreak clusters in indoor microenvironments such as restaurants (Kwon et al., 2020, Lu et al., 2020, Li et al., 2021), cruise ships (Almilaji and Thomas, 2020), buses (Luo et al., 2020, Shen et al., 2020), choir practices (Charlotte, 2020, Hamner et al., 2020, Miller et al., 2020), fitness centres (Jang et al., 2020), meat processing plants (Guenther et al., 2020), call centres (Park et al., 2020), department stores (Jiang et al., 2021) and apartment blocks (Huang et al., 2021, Hwang et al., 2021; Fig. 1). In addition, there are supportive data from sampling campaigns, especially in hospitals (e.g. Guo et al., 2020, Lednicky et al., 2020, Liu et al., 2020, Nissen et al., 2020), epidemiological studies, modelling and data reviews (e.g. Adam et al., 2020, Endo, 2020, Jayaweera et al., 2020, Morgenstern, 2020, Zhang et al., 2020, Bazant and Bush, 2021, Cao et al., 2021, Dillon and Dillon, 2021, Eichler et al., 2021). There are also publications on aerobiological experiments (e.g. Fears et al., 2020, Nielsen and Liu, 2020, Stadnytskyi, 2020, van Doremalen et al., 2020, Shao et al., 2021) that include successfully spreading the disease to uninfected animals via ventilation systems (Kutter et al., 2021, Sia et al., 2020, Richard et al., 2020). Taken together, the weight of evidence from this extensive body of recently published scientific work, filtered through media reporting, appears to be shifting public opinion towards embracing the importance of aerosol transmission. Thus, a stereotypic public space “superspreading event” propagating COVID-19 has come to be viewed under our current understanding as driven by short and longer range aerosol transmission from infected but symptom-free individuals with high viral loading socializing without facemasks in crowded places, especially in indoor environments with inadequate ventilation.
It is notoriously difficult, however, to demonstrate absolute proof of disease transmission via aerosols. In a prescient paper by Pan, Lednicky and Wu, published in the December 2019 issue of the Journal of Applied Microbiology the same month SARS-CoV-2 was officially recognized in Wuhan, the authors comment: …accurate determination of the presence of airborne viruses is challenging. This shortcoming limits our ability to evaluate the actual threat arising from inhalation or other relevant contact with aerosolized viruses (Pan et al., 2019). Even more technically challenging is the demonstration that pathogens detected in airborne particles or ventilation systems are still viable (Brown et al., 2015). Thus many of the studies sampling for SARS-CoV-2 in potential viral “hotspots” have only demonstrated the presence of viral traces (e.g. Ong et al., 2020, Zhou et al., 2020, Hadei et al., 2021, Moreno et al., 2021), with very few using sampling methodologies capable of proving the presence of viable virions (e.g. Lednicky et al., 2020, Santarpia et al., 2020, Lednicky et al., 2021). A continuing problem here is that current air sampling technologies are more aggressive than simple human exhalation and therefore potentially capable of damaging a captured virion sufficiently to prevent it growing in a culture (Tang et al., 2021a). However, following the traditional aphorism “absence of evidence is not evidence of absence”, the lack of proof for viable virions does not prove they are not there (Greenhalgh et al., 2021). Given the data published so far on COVID-19 patterns of propagation, the precautionary principle demands that we focus our attention on facemasks and ventilation in indoor environments.
As the evidence for aerosol involvement with COVID-19 propagation has grown stronger, so the case for emphasizing fomite and droplet transmission has weakened, despite continued reluctance expressed in some medical circles. By July 2020 the World Health Organisation, faced with the data on COVID-19 clusters in restaurants, choir practices and fitness centres, agreed that short range aerosol transmission…. cannot be ruled out but insisted that droplet and fomite transmission could also explain human-to-human transmission within these clusters (WHO, 2020). As more evidence accumulated there has been increasing emphasis on the importance of indoor ventilation and avoidance of breathing stale air (WHO, 2021a), but why such initial reticence? In this paper, we attempt to answer this question by approaching the subject from an historical perspective, tracing how our understanding of epidemic disease transmission developed and how that history has influenced current attitudes. Finally, going one step further, given the exceptional stimulus of COVID-19 and its environmental impacts, we overview our modern understanding of aerosol disease transmission and briefly explore how our responses as a 21st century society experiencing pandemic events may be enough to leave a mark on the Anthropocene stratal record.
2. From miasmata to microbiology
When a single disease affects a great number of individuals at the same time, we must attribute this to the most common cause, to that which we all use the most; and this is what we breathe (from the Hippocratic Treatise by Polybus (c.400BCE) The Nature of Man; Jouanna, 2012). The rational foundations underlying our scientific understanding of epidemic diseases and their causes are to be found in the Hippocratic Corpus, a collection of early Ancient Greek medical writings dating back to the 4th and 5th century BCE. From these Hippocratic doctrines evolved the idea that pestilential diseases, rather than having been sent by the gods using toxic atmospheric emanations as punishment for wrongdoing, are caused by inhaling vaporous “miasmata” in the air and lack a supernatural origin (Parker, 1983). The idea evolved into miasma theory, which became widely accepted by medical practitioners throughout much of the Old World. The theory postulated the presence of pathogenic evil smelling mists derived from decaying organic matter and full of tiny particles, the pestilential seeds of the Greco-Roman medical practitioner Galen (Galen, 165–175, Jouanna, 2012). In a famous quote, the Roman scholar Varro thought this bad air had an organic origin because certain tiny animals grow, which the eyes cannot detect and which pass through the air and can enter the body through the mouth and nose to cause serious diseases (Varro, 36BC, Jarcho, 1976).
Medieval medicine continued to champion miasma theory as a key explanation for the spread of epidemics, although tempered by lessons learnt from the reality of successive waves of bubonic plague. It became clear to some of those with direct experience of the plague that the disease was best dealt with less by worrying about bad air and more by avoiding physical contact, imposing quarantines, and using isolation hospitals. Conflict between authorities entrenched in their miasmatic traditions and those emphasizing the dangers of direct contagion is famously demonstrated by the fate of the 14th century Andalusian scholar Ibn al-Khatib who served as vizier to the Nasrid court in Granada. Arguing, along with others, that bubonic plague could be brought into a city by infected persons arriving by boat and spread between people and on clothing, al-Khatib insisted that isolation protected people from contagion (Ober and Aloush, 1982). His demands that orthodox teachings based on beliefs in divine will and miasmatic toxic vapours should be modified to encompass the evidence for disease transmission by close contact were perceived to be heretical and resulted in his murder in 1374 (Stearns, 2009, Hopley, 2010).
Moving from Medieval to early Modern times, another well-documented contribution to our understanding of epidemic disease was that by Girolamo Fracastoro. Building on the ideas of some of his predecessors Fracastoro in 1546 postulated that such epidemic illnesses spread via different types of seed-like “seminaria” or germs that could be transmitted by direct contact, on fomites (his “fomes”: such as contaminated clothing and surfaces), or through the air (e.g. Garrido, 2016). The clarity of his thinking is demonstrated by its direct relevance to the current fomite-droplet-aerosol debate on the transmission of COVID-19 over 475 years later. However, although certainly influential, the ideas of Fracastoro on contagious diseases (the contagium animatum) did not fundamentally shift the continuing dominance of Greco-Roman miasmatic theory with regard to the explanation of epidemic diseases in European medical science. As William H Welch, one of the founders of the John Hopkins School of Hygiene and Public Health, was to observe in 1925 hypotheses born before their time are often sterile (Welch, 1925, Ackerknecht, 2009). As a geological aside, Fracastoro was similarly ahead of his time in his logical conclusions that fossil shells had once belonged to living organisms and could not be explained by a Great Flood. Charles Lyell later lamented The clear and philosophical views of Fracastoro were disregarded, and the talent and argumentative powers of the learned were doomed for three centuries to be wasted in the discussion of these two simple and preliminary questions: first, whether fossil remains had ever belonged to living creatures; and, secondly, whether, if this be admitted, all the phenomena could be explained by the deluge of Noah (Lyell, 1830).
By the mid-19th century, before the key discoveries of Pasteur, Koch and other microbiologists, the medical world still lacked evidence for a unifying theory of infectious disease. Given the economic opportunities of an increasingly populated and globalised society, politicised argument between “contagionists” favouring quarantine restrictions to control epidemics and those free-marketeers who championed freedom of movement had reached a peak. Yellow fever epidemics (caused by a virus later proven to be transmitted via mosquitos) such as that in Philadelphia in 1793 in particular had convinced a majority that quarantine laws should be relaxed (Ackerknecht, 2009). Amid this confusion, a key driving force for change and clarity was scientific study of a series of cholera pandemics (Table 1 ), the first originating in the Indian sub-continent in 1817 and spreading from the Ganges Delta across Asia and as far west as the eastern Mediterranean (Barau, 1992). Subsequent waves of 19th century cholera pandemics included Western Europe in their reach (Fig. 2 ), and in 1854 medical breakthroughs in the identification of the cholera bacillus Vibrio cholerae, its epidemiological propagation and possible sources were made separately in Italy, Spain, and Great Britain. In Italy Filippo Pacini isolated and described the bacterium in samples taken directly from cholera patients during an outbreak in Florence, publishing his work in December 1854 (Pacini, 1854; Fig. 3 ). In Spain, where over 236,000 people are reported to have died from the outbreak (Kohn, 2008), Joaquín Balcells also discovered the bacterium. In late August, when the epidemic was at its peak in Barcelona, Balcells described live bacteria in a pure water sample that had been left close to the bed of a cholera patient (Fig. 3). His microscopic observations revealed hundreds of vibrios endowed with an astonishing mobility; their movement angular like lightning (Balcells, 1854). Balcells deduced that his water sample had been contaminated by airborne microorganisms carried in exhalations (exhalaciones mórbidas) from the cholera patient (Corbella i Corbella, 1989).
Table 1.
Notable documented outbreaks of human bacterial and viral epidemic diseases, estimated mortality, and growth in World population to the present day level approaching 8 billion. Most of the epidemic diseases listed are zoonotic in origin, especially those newly emerging in recent times. Increasingly rapid rise in human population becomes evident in the 18th century and especially characterises the Anthropocenic “Great Acceleration” from the mid-20th century onwards. Aerosol transmission of diseases such as tuberculosis, smallpox, measles, chickenpox, influenza and COVID-19 has been scientifically demonstrated and is likely to be commonplace, highlighting the importance of controls on indoor air quality (see text for details).
| Date |
Diseases Bacterial infections include bubonic plague, tuberculosis, cholera, typhus, enteric fever, diphtheria, leprosy, leptospirosis. Viral infections include smallpox, influenza, measles, yellow fever, chickenpox, poliomyelitis, Lassa fever, Zika fever, HIV, Ebola, Marburg, SARS, MERS. |
Estimated mortality | Estimated global human population36 (billion): bold type for years listed in column 1 |
|---|---|---|---|
| 1332BCE-1888CE |
|
Demonstrably accurate mortality records for most pre-20th century outbreaks unavailable Estimates commonly vary depending on sources Epidemics recorded as causing outstanding numbers (millions) of deaths before the 20thC include those involving the pathogenic diseases smallpox, bubonic plague, influenza, measles, tuberculosis, cholera and haemorrhagic fevers of uncertain diagnosis such as “cocoliztli” |
0.1 in 1332BCE 0.16 in 429BCE 0.2 in 165CE 0.21 in 541 0.23 in 735 0.3 in 1013 0.39 in 1346 0.4 in 1426 0.45 in 1492 0.5 in 1534 0.51 in 1545 0.6 in 1699 0.7 in 1726 0.8 in 1747 0.9 in 1774 1.0 in 1803 1.07 in 1817 1.1 in 1824 1.2 in 1841 1.24 in 1846 1.3 in 1855 1.4 in 1871 1.5 in 1884 |
| 1889-92 | Influenza A (possibly subtype H3N8) or coronavirus OC43 (“Russian flu” 17): First pandemic spread by highly connected rapid global transportation (rail and shipping) | “1 million” | 1.55 in 1889 1.57 in 1892 |
| 1911 | Measles18: Remote island “virgin soil” epidemic on Rotuma | 334 (13% of population) | 1.76 in 1911 |
| 1916 | Poliomyelitis19: United States | 7130 | 1.81 in 1916 |
| 1918-20 | Influenza A subtype H1N1 pandemic (“Spanish flu”) 20: Many fatalities due to secondary bacterial infections causing pneumonia. | 25-100 million Estimates vary |
1.83 in 1918 1.86 in 1920 |
| 1918-22 | Typhus21: Russia during and after WW1 & revolution | “2-3 million” | 1.89 in 1922 |
| 1947 | Cholera22: Egypt | 10,277 | 2.41 in 1947 |
| 1948-52 | Poliomyelitis23: United States | c. 9000 | 2.53 in 1952 |
| 1957-58 | Influenza A subtype H2N2 pandemic (“Asian flu”) 24 | >1 million | 2.86 in 1957 |
| 1960-62 | Yellow Fever25: Ethiopia | 30,000 | 3.02 in 1960 |
| 1968-70 | Influenza A subtype H3N2 pandemic (“Hong Kong flu”) 24 | >750,000 | 3.53 in 1968 |
| 1972-73 | Influenza A subtype H3N2 pandemic (“London flu”) 24 | unclear | 3.83 in 1972 |
| 1977-78 | Influenza A subtype H1N1 pandemic (“Russian flu”) 24 | 700,000 (?) | 4.21 in 1977 |
| 1981-present | HIV/AIDS pandemic27 | >35 million | 4.52 in 1981 |
| 1996 | Meningococcal meningitis28: Nigeria | >11,000 | 5.82 in 1996 |
| 2002-04 |
Severe acute respiratory syndrome coronavirus (SARS-CoV-1)29 First pandemic of the 21stC30 |
774 | 6.28 in 2002 6.44 in 2004 |
| 2009-10 | Influenza A virus subtype H1N1 pandemic (“swine flu”) 31 | >250,000 | 6.85 in 2009 |
| 2010-19 | Cholera32: Haiti | >10,000 | 6.93 in 2010 |
| 2012-present | Middle East Respiratory Syndrome coronavirus (MERS-CoV)33 | >900 | 7.10 in 2012 |
| 2013-16 | Ebola34: Western Africa | >11,000 | 7.18 in 2013 |
| 2019-2021 | Severe acute respiratory syndrome coronavirus (SARS-CoV-2)35 | >3.8 million | 7.67 in 2019 7.9 June 2021 |
1Norrie, 2014. 2Cunha, 2004, Littman, 2009. 3Haas, 2006; Sabbatani and Fiorino, 2009; Sáez Geoffroy and Parra Díaz, 2020. 4Farris, 1995, Suzuki, 2011, Fenner et al., 1988. 5Frith, 2012, Mordechai et al., 2019. 6Frith, 2012, DeWitte, 2014. 7Frith, 2012, Echenberg, 2002. 8Lina, 2008. 9Nunn and Qian, 2010. 10Acuna-Soto et al., 2004, Vågene et al., 2018. 11Shanks, 2016. 12Olsen, 1999, Conlon, 2007. 13 Brinker, 1938; Furuse et al., 2010. 14Chippaux and Chippaux, 2018. 15Snow, 2002, Huber, 2020. 16Bates and Stead, 1993. 17Vijgen et al., 2005, Taubenberger et al., 2007, Valleron et al., 2010, Kempińska-Mirosławska and Woźniak-Kosek, 2013, Ewing, 2019. 18Shanks et al., 2011. 19Emrich and Richter, 2020. 20Johnson and Mueller, 2002, Kilbourne, 2006, Morens et al., 2010, Taubenberger and Morens, 2010. 21Patterson, 1993. 22Shousha, 1948. 23Ochmann and Roser, 2017. 24 See Spanish flu refs. 25Lilay et al., 2017. 26Jester et al., 2020. 27Sharp and Hahn, 2011. 28Mohammed et al., 2000. 29Ksiazek et al., 2003. 30LeDuc and Barry, 2004. 31Dawood et al., 2012. 32Orata et al., 2014. 33Zhang et al., 2021. 34Jacob et al., 2020. 35Dong et al., 2020. 36https://ourworldindata.org/.
Fig. 2.

Cholera tramples the victor and the vanquished both: Title of a 1831 sketch by the famous illustrator Robert Seymour during the second 19th century cholera pandemic which spread west from Russia during the Polish Russian War 1830–31. Note the depiction of a black miasmatic cloud accompanying the figure of death: prevailing miasma theory did not adequately explain cholera transmission and there was much debate and speculation at the time amongst the medical and scientific community. Image in the collection of The London Library of Medicine, London. (Wikipedia Commons).
Fig. 3.
Hypotheses born before their time are often sterile. Both Filippo Pacini and Joachín Balcells separately observed the cholera bacillus in 1854 during the third 19th century cholera pandemic but the subject received little attention until the rediscovery of the microrganism by Robert Koch in 1884. Top left: one of a series of publications on cholera by Pacini (Source: The Historical Medical Library of The College of Physicians of Philadelphia. Pacini F. Du Choléra Asiatique. Tr. Janssens E. Brussels: Librairie Médicale de. H. Manceaux, 1865); lower left: Pacini’s 1854 microscope slide of the cholera bacillus (Wikipedia Commons); top right: the Spanish publication in which Balcells reported his experimental observations; lower right: translation of the front page editorial announcing Balcells’ discoveries.
Meanwhile, London in the same month of August 1854 was experiencing its worst cholera epidemic on record and, as in Florence and Barcelona, miasma theory continued to dominate medical thinking. The legendary epidemiological work by John Snow in systematically demonstrating that cholera infection in London was linked more to contaminated water rather than air had a dramatic impact (Snow, 1855, Parkes, 2013). It helped stimulate renovation of the entire city sewage system, transforming the capital into one of the healthier modern cities at the time. It also brought into disrepute those in the medical establishment with traditional “miasmatic” explanations who had refused to accept and had even strongly denigrated Snow’s conclusions. Echoes of the shock produced by this change in the scientific medical landscape may be traced even into this century. In 2013, for example, The Lancet published an extraordinary correction after an unduly prolonged period of reflection to their original dismissive obituary of Snow, who died suddenly in 1858, in which no mention of cholera is made (Hempel, 2013). The then editor of The Lancet had indeed been an outspoken critic of Snow but his views were shared by most medical men at the time: miasma, or the stench from decaying vegetable and animal matter, was widely held responsible for epidemic disease (Hempel, 2013). Now such traditional arguments based on miasmatic “filth theory” had been overturned, and in some medical circles the concept of airborne disease transmission became synonymous with a lack of proof, erroneous thinking, alarmism and the pre-scientific past.
3. Tuberculosis: The proof of aerosol transmission
The path to proving that aerosol transmission of human diseases can be important lay in the study of tuberculosis (TB), an ancient respiratory infection that has accompanied humans since at least Late Pleistocene times (Cambau and Drancourt, 2014, Barbaris et al., 2017). By the 19th century, TB had become one of the primary causes of death by epidemic disease in Europe. After many years of debate, it was finally demonstrated to be capable of transmission between mammalian species (Villemin, 1865, Villemin, 1868) and caused by the bacterium Mycobacterium tuberculosis (Koch, 1882, Sakula, 1982). The disease is now recognized to be propagated typically via exhalatory bioaerosols that are subsequently inhaled into the deep lung where they initiate an infection of alveolar macrophages (Nardell, 2016). This recognition of a primarily airborne pathology is founded upon painstaking mid-20th century experimental work centred on the three outstanding figures of William F. Wells, Richard L. Riley and Cretyl I. Mills (née Crumb) (Fig. 4 ). By the time these experiments had been performed and their key results written up by Riley (the Principle Investigator for the project), Wells was dying of cancer and Mills had herself contracted TB (Riley et al., 1959, Riley et al., 1962, Riley, 2001).
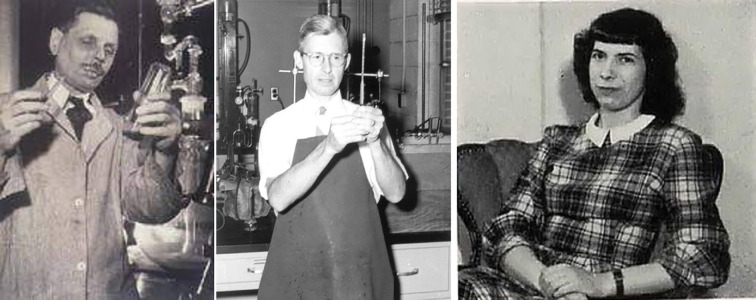
Fig. 4.
From left to right, William Firth Wells (1886–1963), Richard L. Riley (1911–2001), Cretyl Inez Mills (née Crumb) (1919–1990). These three scientists were key to proving that tuberculosis can be transmitted via aerosols (see text for details). Kind permission to reproduce the Wells and Riley photos has been given by Professor Edward Nardell who was given them by the Riley family after 2001. Nardell writes of Cretyl Mills: Riley told me that she was writing the history of their classic aerosol studies when she died but her manuscript's location is unknown. Riley (also) told me that she deserved a lot of credit for keeping track of a huge amount of data that those studies generated.
Wells had received his public health training in the wake of the influential late 19th century work of Carl Flügge on infective exhalatory particles. Potentially infective “Flügge droplets”, demonstrated by Flügge as released by breathing, talking, sneezing, coughing or vomiting, were generally thought capable of transmitting disease only in close proximity to the patient (although not, perhaps, by Flügge himself: see Randall et al., 2021). In his influential book Sources and Modes of Infection (1910), Charles Chapin wrote: Bacteriology teaches that former ideas in regard to the manner in which diseases may be airborne are entirely erroneous; that most diseases are not likely to be dust-borne, and they are spray-borne for only 2 or 3 feet…. although he did add Tuberculosis is more likely to be air-borne than is any other disease (Chapin, 1912). In the same chapter Chapin went on to argue that to emphasise airborne infection is most mischievous because it discourages attempts to minimize infection by close contact: It is impossible, as I know from experience, to teach people to avoid contact infection while they are firmly convinced that the air is the chief vehicle of infection. Such remarks express a genuine medical concern that insisting on longer-range aerosol transmission without clear proof makes the challenge of patient care and hygiene much more difficult. The same point is cogently placed into modern context over 100 years later in a commentary written just months before the emergence of SARS-CoV-2: Although short-range large-droplet transmission is possible for most respiratory infectious agents, deciding on whether the same agent is also airborne has a potentially huge impact on the types (and costs) of infection control interventions that are required (Tellier et al., 2019).
Nevertheless, despite the orthodoxy of the day and as Chapin had himself implied, tuberculosis was to provide the test case. Wells, working with his wife Mildred Weeks Wells (e.g. Wells and Wells, 1936a, Wells and Wells, 1936b), knew this and applied himself on a mission to convince unbelievers (Riley, 2001) to demonstrate beyond reasonable doubt that TB could indeed be transmitted via aerosols. His persistent line of enquiry was based on the argument that particles exhaled from infected patients can remain airborne long enough for viable pathogens, in this case Mycobacterium tuberculosis, to be carried considerable distances away from the emission source. He and his colleague Riley envisaged rapid evaporative diminution of exhaled droplets to smaller-sized particles, which they termed “droplet nuclei” (Wells, 1934, Wells, 1955, Wells and Riley, 1937, Wells et al., 1948). The idea was that these “droplet nuclei” could spread the disease as aerosols, and it was upon this premise that the classic Baltimore Veterans Hospital experiment was constructed (Riley et al., 1959, Riley et al., 1962, Riley, 1974, Riley, 2001).
Having demonstrated that TB can be spread by aerosol inhalation between rabbits (Wells et al., 1948), the primary objective became to investigate if aerosols released by human TB patients could move through a ventilation system into an animal chamber and infect guinea pigs with the same disease. The study took place in a specially prepared hospital annex of six rooms all connected via a ventilation system to a penthouse directly above and containing live animals (Riley, 2001). Initial experiments demonstrated that TB bacilli atomized into the ventilation system could infect rabbits in the penthouse. This was followed by a 2-year study during which infectious human TB patients were transferred to the annex and their exhalations passed through the ventilation shaft to 150 guinea pigs housed in the penthouse. The resulting publication (Riley et al., 1959) demonstrated that on average three guinea pigs per month had become infected with TB over the 2-year period, but it was criticized for not absolutely eliminating all other possible infection routes. In dogged response, the research team conducted a second 2-year experiment, adding another unit of 150 guinea pigs connected to the same ventilation system but treating their air with ultraviolet radiation, a proven technique that the Wellses had already shown to be effective in deactivating pathogenic bioaerosols (Wells et al., 1942, Reed, 2010). At the end of this second experiment, the same infection rate was found in the original guinea pig house whereas none of the 150 animals inhaling UV-treated air developed TB (Riley et al., 1962, Riley, 2001). This decisive demonstration that TB can be transmitted via aerosols carried the added bonus, due largely to the meticulous record keeping of Mills, of discovering that some TB patients were much more infectious than others (Sultan et al., 1960): in modern COVID-19 parlance they were “superspreaders”.
4. Respiratory bioaerosols and viral pathogens
Much more is known today about human respiratory aerosols than 50 years ago, not least because of the increasing sensitivity of measuring instruments such as optical particle counters and sizers which demonstrate that most such particles range in size from just several hundred nanometres to a few microns (e.g. Fairchild and Stamper, 1987, Papeneni and Rosenthal, 1997, Fabian et al., 2008). Thus, in terms of number concentrations exhaled aerosols will be mostly too small to carry many, if any, airborne bacterial pathogens. Mycobacterium tuberculosis for example typically measures 2–4 µm in length and has been observed to be present in exhalatory particles less than 5 µm in size (Fenelly et al., 2012).
Individual viral pathogens, in contrast to bacteria, typically lie in the size range of 30–150 nm, categorizing them in aerosol science as “ultrafine to quasi-ultrafine particles”. They are much smaller than most aerosol sizes emitted by normal tidal breathing (e.g. Morawska et al., 2008, Johnson et al., 2011, Fabian et al., 2008). A key mechanism for their expulsion from the infected host involves particles of respiratory tract lining fluid being generated deep in the lung by the breakup of liquid films during repeated reopening of airway capillaries (Johnson and Morawska, 2009, Almstrand et al., 2010, Haslbeck et al., 2010, Schwarz et al., 2010, Bake et al., 2019). Exhalation of such particles will present a threat of airborne disease transmission if they carry virions capable of maintaining viability long enough to infect a new host (e.g. Jones and Brosseau, 2015, Lv et al., 2021). For a discussion on infection mechanisms and controversies over “droplets” and “aerosols” see Randall et al. (2021), but suffice to state here that there is by now a wealth of evidence that infectious exhalatory bioaerosols can be transmitted through the air for considerable distances: they are the “Modern Miasmas” of our time (Mubareka et al., 2019).
Studies on viable virus-bearing aerosols, here shortened to “viraerosols”, known to be capable of spreading epidemic diseases highlight those carrying measles (e.g. Riley et al., 1978), chickenpox (e.g. Gustafson et al., 1982), now-eradicated smallpox (Wehrle et al., 1970), and influenza (e.g. Cowling et al., 2013). These pathogens have produced massive loss of human life but information on their transmission modes can be equivocal. The relative importance of smallpox viraerosols remains unclear because the mode of smallpox transmission was never conclusively established (Milton, 2012), despite this disease having been one of the great epidemic killers. In contrast, other viruses have been more convincingly demonstrated to be commonly spread by airborne particles. It has been proposed, for example, that around 50% of influenza A virus transmissions occur via aerosols (Cowling et al., 2013), whereas measles appears to be the most “aerosol-adapted” of them all and is recognized as a classic human-specific airborne disease with an unusually high infection attack rate (e.g. Wallinga et al., 2005). When these pathogenic viruses enter unprotected human societies, such as happened in the 15-16th century American continent (Nunn and Qian, 2010) or in 18th century Australia (Dowling, 1998), the resulting epidemics can decimate entire populations. Even in societies normally more adapted to these pathogens, the appearance of a new viral strain capable of aerosol transmission in dense, mobile human populations can produce dramatic spikes in mortality, as evidenced by the H1N1 influenza A event of 1918–20 (Fig. 5 ) and the ongoing COVID-19 pandemic.
Fig. 5.
The similarities in messaging around the COVID-19 pandemic are striking (Randall et al., 2021). North American health posters and newspaper headlines during the 1918–20 Influenza A H1N1 pandemic. Although emphasis by the health authorities was based on disease transmission by near-range “Flügge droplets”, note a clear awareness of the importance of good ventilation (sources: Library of Congress https://www.loc.gov/item/rbpe.24101900/; Tuberculosis and the Cincinatti Irish by Jessica Heskett: https://libapps.libraries.uc.edu/exhibits/irish-cincinnati/cincinnati-irish-births-and-deaths/tuberculosis-and-the-cincinnati-irish/; newspapers in Chicago, Wikipedia Commons).
5. Human coronaviruses
The first coronavirus to be described was that causing avian infectious bronchitis (AIB) in chickens (Schalk and Hawn, 1931), an acute, highly contagious disease that is commonly transmitted via airborne exhalatory and faecal particles. Airborne transmission is via aerosol and occurs readily between birds kept at a distance of over 1.5 m (Ignjatovic and Sapats, 2000). Although normally associated with relatively close contact transmission, one study implicated prevailing nighttime winds as capable of spreading the disease between farms separated by over 1 km (Cumming, 1969). The morphological similarity between the AIB virus and previously uncharacterized human “common cold” respiratory viruses was revealed by the electron microscopy work of June Almeida in 1967 (Almeida and Tyrrell, 1967; Fig. 6 ) and the generic group subsequently named coronavirus (Almeida et al., 1968, Wildy, 1971).
Fig. 6.

Virologist June Almeida (née Dalziel Hart), the first person to observe and describe human coronaviruses. The photo shows her in the 1960s using a Philips EM300 electron microscope while working at St Thomas's Hospital Medical School, London. Reproduced by kind permission of Joyce Almeida. See https://www.whatisbiotechnology.org/index.php/people/summary/Almeida.
At present 7 coronaviruses are known to infect humans (HCoV-OC229E, HCoV-NL63, HCoV-OC43, HCoV-HKU1, SARS-CoV-1, SARS-CoV-2 and MERS-CoV). The first four of these coronaviruses are well established in the human population, typically causing relatively mild upper respiratory tract infections and only rarely leading to critical illness (Patrick et al., 2006, Dijkman and van der Hoek, 2009). Although now relegated to “common cold” status, regularly infecting and re-infecting humans in seasonal epidemics, their original appearance as a new respiratory coronavirus strain would have likely caused widespread serious illness until brought under control by group immunity. HCoV-NL63 and HCoV-OC229E for example are thought to have emerged from bats to infect humans possibly in the 13-15th and 18th centuries respectively (Huynh et al., 2012, Corman et al., 2015, King, 2020). In this context, HCoV-OC43 has been speculated to be linked to a late 19th century outbreak of bovine respiratory disease and synchronous 1889–1890 “Russian flu” pandemic in humans (Vijgen et al., 2005; Table 1). More recent and therefore much better documented newly emergent human coronavirus respiratory infections (SARS and MERS) have this century joined the list of human diseases with pandemic potential (Table 1).
In November 2002, reports began to emerge of atypical pneumonia cases occurring in Guangdong Province, China. The first well-publicised outbreak of this new disease, termed severe acute respiratory syndrome (SARS) and later traced to a zoonotic coronavirus reservoir of bats in Yunnan Province (Wang et al., 2018), was brought from Guangdong to Hong Kong by a Chinese medical professor in February 2003 (Peiris et al., 2004). In the resulting outbreaks, while most cases were thought to be passed on during close contact with infected individuals, there are clear indications of some contribution by aerosol transmissions. In the largest nosocomial outbreak, for example, which centred on Ward 6A of the Prince of Wales Hospital Hong Kong in March 2003, the ventilation system was identified as likely having helped to spread the disease (Li et al., 2004a, Li et al., 2004b, Yu et al., 2005). Another well-studied Hong Kong disease cluster was that occurring in the Amoy Gardens residential tower block complex where 187 SARS cases were reported (Fig. 1). In this case, the spread of the disease was identified as due to viraerosols escaping through floor drains from the sewage-system into bathrooms and then driven by ventilation fans into light wells serving the tower block (Li et al., 2004a, Li et al., 2004b, Yu et al., 2004, McKinney et al., 2006). A third case study from the Hong Kong 2003 epidemic also implicated a contribution from viraerosol transmission in reporting data from a flight to Beijing when a SARS- symptomatic individual spread the disease to an estimated 22 other passengers (Olsen et al., 2003).
A decade after the appearance of SARS-CoV-1, Middle East Respiratory Syndrome (MERS) was recognized as the second 21st century emergent zoonotic coronavirus respiratory disease with pandemic potential (de Groot et al., 2013). The disease in humans probably originated from bats infecting dromedary camels, with subsequent human-camel contagion likely and human–human transmission confirmed (e.g. Zumla et al., 2015). Discussions as to how the illness is passed to and between humans have centred on the familiar “Fracastoro tripartite” of fomite versus close contact versus longer range aerosol transmission, usually with emphasis on direct contact with camels and nosocomial close contact between people (WHO, 2021b). One modelling study has suggested that longer-range aerosol transmission from superspreader hospital patients best fits the data available from a major nosocomial outbreak in the Republic of Korea (Xiao et al., 2017). So far, however, the disease does not appear to be especially efficient in human-to-human transmission, and it has been eclipsed by the emergence of SARS-CoV-2 in 2019 and the resulting COVID-19 pandemic. Nevertheless, unlike SARS-CoV-1, the MERS coronavirus problem is far from having been eliminated, and it continues to expand its reach across the Middle East and parts of Africa and Asia. With a confirmed case fatality rate of over 30%, much higher than the SARS pathogens, MERS-CoV currently presents an imminent pandemic threat through genetic mutation or recombination with other human coronaviruses (Zhang et al., 2021).
6. Pandemic preservation potential in the geological record
The sudden appearance of a rapidly spreading acute disease has a dramatic effect on the target population and can result in long-term societal and environmental impacts. On a local level, there are many documented cases of devastating epidemics affecting isolated communities when they became exposed to the global pathogen pool. A classic example, referred to as a “benchmark in Pacific history” (Cliff and Haggett, 1985), was the arrival of the measles virus to Fiji in 1875 (Table 1). The resulting epidemic killed over 20% of the population, wiped out the leadership, and encouraged foreign worker immigration that changed the agro-economic and political future of the island (Shanks, 2016). The same pathogenic process, although magnified to continental scale during the Columbian Exchange and involving a diversity of diseases, reduced the late 15th–16th century indigenous population of the Americas by an estimated 80%–95% (Nunn and Qian, 2010). It has been hypothesised that carbon sequestration due to the regeneration of natural vegetation after this catastrophic loss of farming communities was enough to explain a minor (7–10 ppm) transient drop in atmospheric CO2 levels (e.g. Lewis and Maslin, 2015, Koch et al., 2019). This idea is not proven and alternative explanations exist for the CO2 decline (Zalasiewicz et al., 2015, Zalasiewicz et al., 2021, Rubino et al., 2016), but the hypothesis does usefully serve to highlight the possibility of atmospheric chemistry being altered by the consequences of anthropogenic epidemic disease. This in turn leads to the concept of disease-driven environmental change being of such a magnitude that it has a realistic possibility of being preserved in the geological record.
The precipitate loss of tens of millions of people by epidemic disease was to be repeated in the 1918 H1N1 influenza A “Spanish flu” pandemic (Table 1), by which time the world human population had grown to>1.5 billion, over three times that when Columbus sailed to the Americas (Table 1). In contrast to the Columbian Exchange epidemics, which involved different diseases spreading through the American continent over the decades after 1492, the 1918 influenza event was caused by just one pathogen and took place during four successive waves in only two years. A warning of such a possibility had been issued nearly 20 years earlier by another flu-like pandemic (the “Russian Flu”: either influenza A or the coronavirus OC43) which is reported to have killed around 1 million people and was the first to spread rapidly around the world via modern transport infrastructure (Table 1). The likely importance of aerosol transmission of the influenza A virus has been indicated by several studies (e.g. see review by Tellier, 2009), and emphasizes the dangers of highly infectious new strains of these respiratory pathogens in crowded indoor environments. Repeated outbreaks of new influenza A strains in the 20th century produced pandemics in 1957–8 (H2N2), 1968–9 (H3N2), and 1977–9 (H1N1) (Michaelis et al., 2009), as world human population ballooned to exceed 6 billion (Table 1). This new disease pattern has accompanied a time of unprecedented human hypermobility, energy use, technological development and environmental impact on the biosphere, a collective phenomenon that has come to be termed the “Great Acceleration” of Anthropocene time (e.g. Waters et al., 2016, Syvitski et al., 2020, Zalasiewicz et al., 2021).
Over the first two decades of this century outbreaks of influenza A have continued and have been joined by the appearance of no less than three new human respiratory zoonotic coronaviruses with pandemic potential (Table 1). Whereas SARS-CoV-1 was brought under control, and MERS-CoV has so far been of limited spread, SARS-CoV-2 rapidly fulfilled its pandemic threat. This latest pandemic is unlike any other in history. The SARS-CoV-2 pathogen entered an unprotected urban-based human population approaching 8 billion individuals (Table 1), many of whom were accustomed to traveling on what by the beginning of 2020 amounted to almost 40 million airline flights annually. Lessons regarding the likelihood of aerosol transmission of “flu-like” respiratory viruses had not been well learnt, and as a result the respiratory disease COVID-19 claimed over 4 million lives worldwide by august 2021 and will kill many more before controlled by mass immunisation.
Among the various immediate environmental impacts of the ongoing COVID-19 pandemic, many are associated with waste generation and disposal. Some of these have been positive, such as the sudden cleansing effect on highly polluted rivers such as the Ganges (Ganga) during the 2020 industrial and touristic shutdown (Shukla et al., 2021). Other impacts in contrast are clearly detrimental, such as the sudden dramatic increase in use of personal protective equipment. It can be calculated for example that over 1.5 trillion facemasks are currently adding to waste plastic worldwide, accompanied by their associated toxic additives such as organophosphate esters and heavy metals (e.g. Prata et al., 2020, Fernández-Arribas et al., 2021, Sullivan et al., 2021). While it is conceivable that an abrupt change in plastic waste output on this scale could be registered in the sedimentary record (Zalasiewicz et al., 2016), most immediate effects will be short-lived and probably quickly overtaken by the attempted re-establishment of the economic status-quo.
Potentially more promising candidates for fossilising the memory of the COVID-19 crisis in deep time may derive from our societal response to changes in atmospheric chemistry. During the 2020 pandemic lockdown “anthropause” (Rutz et al., 2020) many urban populations experienced noticeable changes in air quality. Levels of NO2 for example fell by as much as 30%–50% in cities such as Delhi, Madrid and Wuhan, mainly because of road traffic decline (e.g. Querol et al., 2021, Shi et al., 2021). Large areas of the world recorded similarly significant reductions in ambient PM2.5 (Bonardi et al., 2021), and CO2 emissions from fossil fuel combustion fell by an estimate 2.6GtCO2 in 2020, around 7% below that of 2019 (Friedlingstein et al., 2020, Le Quéré et al., 2021). Although such a drop in anthropogenic greenhouse gas emissions is unprecedented, it is not in itself a signal of significant change. It translates to a slowing of atmospheric CO2 growth by less than 1 ppm in 2020, and the rebound is already in place (Monroe, 2021). However, there are signs that institutional policies in response to the COVID-19 crisis and a “new normal” environmental awareness in a globally networked population are stimulating an accelerating effort to tackle climate change (e.g. World Economic Forum: Boccaletti, 2020). In this context 2020–2021 may become to be seen as a pivotal moment, galvanised by the experience of COVID-19, that will hold the key to “bending the Keeling Curve” enough to change Anthropocene history.
7. Concluding remarks
The term Anthropocene in geoscience describes an Earth system state in which human activities have become predominant drivers of modifications in the stratigraphic record and has been recommended to start in the mid-20th century (Zalasiewicz et al., 2021). It reflects the transforming reality of our technologically accelerated, globalized world in which we are responsible for an ongoing Great Extinction event during an experiment on wildlife habitat invasion, zoonotic disease patterns, oceanic ecosystems, atmospheric chemistry and climate. As a species, we are increasingly conscious of this, and thus increasingly capable of taking economic and political decisions that will affect future environmental outcomes more positively than has so far been the case. A recent European Parliament report observes: The COVID-19 pandemic is an example of the inextricable links between human health and the ecosystem health (EU, 2021). Thus, although the impact of the COVID-19 crisis on the atmospheric CO2 Keeling Curve for 2020 may appear minor, this does not mean that nothing has changed. The entire human race is suddenly much more aware of the importance of good air quality, whether it being indoors, well ventilated and viraerosol-free, or outdoors and uncontaminated by fossil fuel combustion emissions. Just as there is ever-increasing pressure to act on climate change and urban air pollution, there are already strong calls for a fresh start in the way we think about indoor air, ventilation, and minimising the spread of infectious diseases (e.g. Morawska et al., 2021, World Health Organization, 2021a). Enhanced societal awareness of airborne particle issues has been aroused as never before by our chastening “anthropause for thought” experience of COVID-19, and is being aided by a better appreciation of modern bioaerosol science won from a spirited burst of open access publications (e.g. Jimenez, 2020, Bourouiba, 2021, Burridge et al., 2021, Tang et al., 2021b, and many others). This is an opportunity for serious progress in air quality issues. Our reaction to the current pandemic and its airborne transmission is likely to have consequences because of its universal nature: to re-quote the Hippocratic Treatise of Polybus, written around 2,400 years ago, it involves that which we all use the most; and this is what we breathe.
Declaration of Competing Interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We wish to thank Edward Nardell for his help and encouragement regarding the photographic record of the Wells, Riley and Mills scientific trio, and Joyce Almeida for granting permission to use the photograph of her mother June Almeida. This study was supported by the Spanish Research Council (CSIC, Project COVID-19 CSIC 202030E226) and the Generalitat de Catalunya (SGR41). IDAEA-CSIC is a Severo Ochoa Centre of Research Excellence (Spanish Ministry of Science and Innovation, Project CEX2018-000794-S).
References
- Ackerknecht E.H. Anticontagionism between 1821 and 1867: The Fielding H. Garrison Lecture. Int. J. Epidemiology. 2009;38(1):7–21. doi: 10.1093/ije/dyn254. [DOI] [PubMed] [Google Scholar]
- Acuna-Soto R., Stahle D.W., Therrell M.D., Griffin R.D., Cleaveland M.K. When half of the population died: the epidemic of hemorrhagic fevers of 1576 in Mexico. FEMS Microbiol. Lett. 2004;240:1–5. doi: 10.1016/j.femsle.2004.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adam D.C., Wu P., Wong J.Y., Lau E.H.Y., Tsang T.K., Cauchemez S., Leung G.M., Cowling B.J. Clustering and superspreading potential of SARS-CoV-2 infections in Hong Kong. Nat. Med. 2020;26(11):1714–1719. doi: 10.1038/s41591-020-1092-0. [DOI] [PubMed] [Google Scholar]
- Almeida J.D., Berry D.M., Cunningham C.H., Hamre D., Hofstad M.S., Mallucci L., McIntosh K., Tyrrell D.A. Coronaviruses. Nature. 1968;220(5168):650. [Google Scholar]
- Almeida J.D., Tyrrell D.A. The morphology of three previously uncharacterized human respiratory viruses that grow in organ culture. J. Gen. Virol. 1967;1(2):175–182. doi: 10.1099/0022-1317-1-2-175. [DOI] [PubMed] [Google Scholar]
- Almilaji, O., Thomas, P., 2020. Air recirculation role in the infection with COVID-19, lessons learned from Diamond Princess cruise ship. medRxiv 2020.07.08.20148775.
- Almstrand A.C., Bake B., Ljungstrom E., Larsson P., Bredberg A., Mirgorodskaya E., Olin A.C. Effect of airway opening on production of exhaled particles. J. Appl. Physiol. 2010;108:584–588. doi: 10.1152/japplphysiol.00873.2009. [DOI] [PubMed] [Google Scholar]
- Bake B., Larsson P., Ljungkvist G., Ljungström E., Olin A.C. Exhaled particles and small airways. Resp. Res. 2019;20:8. doi: 10.1186/s12931-019-0970-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balcells P.J. Observaciones microscópicas sobre los miasmas coléricos. El Restaurador Farmacéutico. 1854;10:122–123. [Google Scholar]
- Barau, D., 1992. History of Cholera. In: Barau, D. and Greenough III, W.B. 1992. Springer, Cholera.358p. ISBN 978-1-4757-9688-9.
- Barbaris I., Bragazzi N., Galluzzo L., Martini M. The history of tuberculosis: From the first historical records to the isolation of Koch's bacillus. J. Preventive Med. Hyg. 2017;58(1):9–12. [PMC free article] [PubMed] [Google Scholar]
- Bates J.H., Stead W.W. The history of tuberculosis as a global epidemic. Med. Clin. North Am. 1993;77(6):1205–1217. doi: 10.1016/s0025-7125(16)30188-2. PMID: 8231408. [DOI] [PubMed] [Google Scholar]
- Bazant, M.Z., Bush J.W.M., 2021. A guideline to limit indoor airborne transmission of COVID-19. Proceedings of the National Academy of Sciences 118 (17), e2018995118; DOI: 10.1073/pnas.2018995118. [DOI] [PMC free article] [PubMed]
- Boccaletti, G., 2020. How the institutional response to COVID-19 can prepare us for climate change. World Economic Forum, SDG 13 Climate Action. https://www.weforum.org/agenda/2020/12/climate-change-covid-19-response/.
- Bonardi, J.P., Gallea, Q., Kalanoski, D., Lalive, R., Madhok, R., Noack, F., Rohner, D., Sonno, T., 2021. Saving the world from your couch: The heterogeneous medium-run benefits of COVID-19 lockdowns on air pollution. Env. Res. Lett. https://doi.org/10.1088/1748-9326/abee4d.
- Bourouiba L. The fluid dynamics of disease transmission. Annual Review of Fluid Mechanics. 2021;53:473–508. doi: 10.1146/annurev-fluid-060220-113712. [DOI] [Google Scholar]
- Brown, J.R., Tang, J.W., Pankhurst, L., Klein, N., Gant, V., Lai, K.M., McCauley, J., Breuer, J., 2015. Influenza virus survival in aerosols and estimates of viable virus loss resulting from aerosolization and air-sampling. J. Hosp.l Infec. 91, 278–281. ISSN 0195-6701. https://doi.org/10.1016/j.jhin.2015.08.004. [DOI] [PubMed]
- Burridge, H., Bhagat, R., Stettler, M., Kumar, P., De Mel, I., Demis, P., Hart, A., Johnson-Llambias, Y., King, M., Klymenko, O., McMillan, A., Morawiecki, P., Pennington, T., Short, M., Sykes, D., Trinh, P., Wilson, S., Wong, C., Wragg, H., Davies, W., Iddon, C., Woods, A., Mingotti, N., Bhamidipati, N., Woodward, H., Beggs, C., Davies, H., Fitzgerald, S., Pain, C., Linden, P., 2021. The ventilation of buildings and other mitigating measures for COVID-19: a focus on wintertime. Proc. R. Soc. A. 4772020085520200855. doi.org/10.1098/rspa.2020.0855.
- Cambau, E., Drancourt, M., 2014. Steps towards the discovery of Mycobacterium tuberculosis by Robert Koch, 1882. Clin. Microbiol. Infect. 20, 196–201. ISSN 1198-743X. https://doi.org/10.1111/1469-0691.12555. [DOI] [PubMed]
- Cao Y., Shao L., Jones T., Oliveira M.L.S., Ge S., Feng X., Silva L.F.O., BéruBé K. Multiple relationships between aerosol and COVID-19: A framework for global studies. Gondwana Res. 2021;93:243–251. doi: 10.1016/j.gr.2021.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapin C.V. John Wiley & Sons; New York. Web: 1912. The Sources and Modes of Infection. [Google Scholar]
- Charlotte N. High Rate of SARS-CoV-2 Transmission Due to Choir Practice in France at the Beginning of the COVID-19 Pandemic. J of Voice. 2020 doi: 10.1016/j.jvoice.2020.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chippaux J.P., Chippaux A. Yellow fever in Africa and the Americas: a historical and epidemiological perspective. J. Venom. Anim. Toxins Incl. Trop. Dis. 2018;24:20. doi: 10.1186/s40409-018-0162-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cliff A.D., Haggett P. The Australian National University; Canberra: 1985. The spread of measles in Fiji and the Pacific: Spatial components in the transmission of epidemic waves through island communities. Department of Human Geography Publication HG I 18, Research School of Pacific Studies; p. 110 pp.. [Google Scholar]
- Conlon, JM., 2007. The historical impact of epidemic typhus. http://phthiraptera.info/sites/phthiraptera.info/files/61235.pdf.
- Corbella i Corbella, J. El Còlera a Catalunya abans de L’Obra del Doctor Ferran. Tre. Soc. Cat. Biol. 1989;40:77–90. [Google Scholar]
- Corman V.M., Baldwin H., Fumie A., Melim R., Annan A., Owusu M., Nkrumah E., Maganga G., Oppong S., Adu-Sarkodie Y., Vallo P., Ribeiro L., Leroy E., Thiel V., van der Hoek L., Poon L., Tschapka M., Drosten C., Drexler J. Evidence for an ancestral association of human coronavirus 229E with bats. J. Virol. 2015;89(23):11858–11870. doi: 10.1128/JVI.01755-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowling B., Ip D., Fang V., Suntarattiwong P., Olsen S., Levy J., Uyeki T., Leung G., Peiris J., Chotpitayasunondh T., Nishiura H., Simmerman J. Aerosol transmission is an important mode of influenza A virus spread. Nat. Commun. 2013;4:1935. doi: 10.1038/ncomms2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cumming R.B. Studies on Australian infectious bronchitis virus. IV. Apparent farm-to-farm airborne transmission of infectious bronchitis virus. Avian Dis. 1969;14:191–195. [PubMed] [Google Scholar]
- Cunha B.A. The cause of the plague of Athens: plague, typhoid, typhus, smallpox, or measles? Infect. Dis. Clin. North Am. 2004;18(1):29–43. doi: 10.1016/S0891-5520(03)00100-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawood F.S., Iuliano A.D., Reed C., Meltzer M.I., Shay D.K., Cheng P.Y., Bandaranayake D., Breiman R.F., Brooks W.A., Buchy P., Feikin D.R., Fowler K.B., Gordon A., Hien N.T., Horby P., Huang Q.S., Katz M.A., Krishnan A., Lal R., Montgomery J.M., Mølbak K., Pebody R., Presanis A.M., Razuri H., Steens A., Tinoco Y.O., Wallinga J., Yu H., Vong S., Bresee J., Widdowson M.A. Estimated global mortality associated with the first 12 months of 2009 pandemic influenza A H1N1 virus circulation: a modelling study. Lancet Infect. Dis. 2012;12(9):687–695. doi: 10.1016/S1473-3099(12)70121-4. [DOI] [PubMed] [Google Scholar]
- de Groot, R.J., Baker, S.C., Baric, R.S., Brown, C.S., Drosten, C., Enjuanes, L., Fouchier, R.A., Galiano, M., Gorbalenya, A.E., Memish, Z.A., Perlman, S., Poon, L.L., Snijder, E.J., Stephens, G.M., Woo, P.C., Zaki, A.M., Zambon, M., Ziebuhr, J., 2013. Middle East respiratory syndrome coronavirus (MERS-CoV): announcement of the Coronavirus Study Group. J. Virol. 87(14), 7790–7792. https://doi.org/10.1128/JVI.01244-13. [DOI] [PMC free article] [PubMed]
- DeWitte, S.N., 2014, Mortality Risk and Survival in the Aftermath of the Medieval Black Death. PLOS May 7, 2014. https://doi.org/10.1371/journal.pone.0096513. [DOI] [PMC free article] [PubMed]
- Dijkman R., van der Hoek L. Human coronaviruses 229E and NL63: close yet still so far. J. Formos. Med. Assoc. 2009;108(4):270–279. doi: 10.1016/S0929-6646(09)60066-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon C., Dillon M.B. Multiscale Airborne Infectious Disease Transmission. Appl. Environ. Microbiol. 2021;87:4. doi: 10.1128/AEM.02314-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong E., Du H., Gardner L. An interactive web-based dashboard to track COVID-19 in real time. The Lancet. 2020;20:533–534. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling, P.J., 1998. 'A great deal of sickness': Introduced diseases among the aboriginal people of colonial southeast Australia, 1788—1900. Ph.D. thesis, The Australian National University (Australia), 370pp. ISBN 0599374888.
- Echenberg, M., 2002. Pestis Redux: The Initial Years of the Third Bubonic Plague Pandemic, 1894-1901. J. World Hist. 13, 429–449. JSTOR, www.jstor.org/stable/20078978. [DOI] [PubMed]
- Eichler N., Thornley C., Swadi T., Devine T., McElnay C., Sherwood J., Brunton C., Williamson F., Freeman J., Berger S., Ren X., Storey M., de Ligt J., Geoghegan J. Transmission of severe acute respiratory syndrome coronavirus 2 during border quarantine and air travel, New Zealand (Aotearoa) Emerg. Infect. Dis. 2021;27(5):1274–1278. doi: 10.3201/eid2705.210514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emrich J.S., Richter C. Polio: Part 1-Understanding and treating a perplexing disease. J. Immunol. 2020 https://www.aai.org/About/History/History-Articles-Keep-for-Hierarchy/Polio-Part-I%E2%80%94Understanding-and-Treating-a-Perplex [Google Scholar]
- Endo, A., Centre for the Mathematical Modelling of Infectious Diseases COVID-19 Working Group, Abbott, S., et al., 2020. Estimating the overdispersion in COVID-19 transmission using outbreak sizes outside China. Welcome Open Res. 5, 67. https://doi.org/10.12688/wellcomeopenres.15842.3. [DOI] [PMC free article] [PubMed]
- Ewing E.T. La Grippe or Russian influenza: Mortality statistics during the 1890 Epidemic in Indiana. Influenza Other Resp. 2019;13(3):279–287. doi: 10.1111/irv.12632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabian P., McDevitt J.J., DeHaan W.H., Fung R.O.P., Cowling B.J., Chan K., Leung G., Milton D. Influenza Virus in Human Exhaled Breath: An Observational Study. PLoS ONE. 2008;3(7) doi: 10.1371/journal.pone.0002691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairchild C.I., Stamper J.F. Particle concentration in exhaled breath. Am Ind Hyg Assoc J. 1987;48:948–949. doi: 10.1080/15298668791385868. [DOI] [PubMed] [Google Scholar]
- Farris W.W. Harvard University Press; Cambridge: 1995. Population, Disease, and Land in Early Japan. [Google Scholar]
- Fears A.C., Klimstra W.B., Duprex P., Hartman A., Weaver S.C., Plante K., Mirchandani D., Plante J.A., Aguilar P.V., Fernández D., Nalca A., Totura A., Dyer D., Kearney B., Lackemeyer M., Bohannon J.K., Johnson R., Garry R.F., Reed D.S., Roy C.J. Persistence of Severe Acute Respiratory Syndrome Coronavirus 2 in Aerosol Suspensions. Emerg. Infect. Dis. 2020;26(9):2168–2171. doi: 10.3201/eid2609.201806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fennelly K.P. Particle sizes of infectious aerosols: implications for infection control. Lancet Respir. Med. 2020;8(9):914–924. doi: 10.1016/S2213-2600(20)30323-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fennelly K.P., Jones-Lope Z.E.C., Ayakaka I., Kim S., Menyha H., Kirenga B., Muchwa C., Joloba M., Dryden-Peterson S., Reilly N., Okwera A., Elliott A.M., Smith P.G., Mugerwa R.D., Eisenach K.D., Ellner J.J. Variability of infectious aerosols produced during coughing by patients with pulmonary tuberculosis. Am. J. Respir. Crit. Care. Med. 2012;186:450–457. doi: 10.1164/rccm.201203-0444OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenner F., Henderson D.A., Arita I., Jezek Z., Ladnyi I.D. WHO; Geneva: 1988. Smallpox and its eradication; p. 1460p. [Google Scholar]
- Fernández-Arribas J., Moreno T., Bartrolí R., Eljarrat E. COVID-19 face masks: A new source of human and environmental exposure to organophosphate esters. Environ. Int. 2021;154 doi: 10.1016/j.envint.2021.106654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedlingstein P., O'Sullivan M., Jones M., Andrew R., Hauck J., Olsen A., Peters G., Peters W., Pongratz J., Sitch S., Le Quéré C., Canadell J., Ciais P., Jackson R., Alin S., Aragão L., Arneth A., Arora V., Bates N., Becker M., Benoit-Cattin A., Bittig H., Bopp L., Bultan S., Chandra N., Chevallier F., Chini L., Evans W., Florentie L., Forster P., Gasser T., Gehlen M., Gilfillan D., Gkritzalis T., Gregor L., Gruber N., Harris I., Hartung K., Haverd V., Houghton R., Ilyina T., Jain A., Joetzjer E., Kadono K., Kato E., Kitidi V., Korsbakken J., Landschützer P., Lefèvre N., Lenton A., Lienert S., Liu Z., Lombardozzi M., Marland G., Metzl N., Munro D., Nabel J., Nakaoka S., Niwa Y., O'Brien K., Ono T., Palmer P., Pierrot D., Poulter B., Resplandy L., Robertson E., Rödenbeck C., Schwinger J., Séférian R., Skjelvan I., Smith A., Sutton A., Tanhua T., Tans P., Tian H., Tilbrook B., van der Werf G., Vuichard N., Walker A., Wanninkhof R., Watson A., Willis D., Wiltshire A., Yuan W., Yue X., Zaehle S. Global carbon budget 2020. Earth Syst. Sci. Data. 2020;12:3269–3340. [Google Scholar]
- Frith J. The history of plague - Part 1. The three great pandemics. J. Milit. Veter. Health. 2012;20:11. [Google Scholar]
- Furuse Y., Suzuki A., Oshitani H. Origin of measles virus: divergence from rinderpest virus between the 11th and 12th centuries. Virol. J. 2010;7:52. doi: 10.1186/1743-422X-7-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galen., 165–175 CE. On Differences between Fevers 1, ch. 6, 7.289,4–291,11 K.
- Galloway, H., Gorospe, P., Linde, P., Catalán, N., 2021. Architecture of an outbreak: the Spanish apartment building hijacked by the coronavirus. El País, 15 February 2021. https://english.elpais.com/society/2021-02-15/architecture-of-an-outbreak-the-spanish-apartment-building-hijacked-by-the-coronavirus.html.
- Garrido R.J.H. El problema de la transmisión a distancia de las enfermedades contagiosas en el De contagione de Girolamo Fracastoro. Ludus Vitalis. 2016;24:75–100. [Google Scholar]
- Gelfand H.M., Posch J. The recent outbreak of smallpox in Meschede. West Germany. Am. J. Epidemiol. 1971;93(4):234–340. doi: 10.1093/oxfordjournals.aje.a121251. PMID: 5550338. [DOI] [PubMed] [Google Scholar]
- Greenhalgh T., Jimenez J., Prather K., Tufekci Z., Fisman D., Schooley T. Ten scientific reasons in support of airborne transmission of SARS-CoV-2. The Lancet. 2021 doi: 10.1016/S0140-6736(21)00869-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guenther T., Czech-Sioli M., Indenbirken D., Robitailles A., Tenhaken P., Exner M., Ottinger M., Fischer N., Grundhoff A., Brinkmann M. Investigation of a superspreading event preceding the largest meat processing plant-related SARS-Coronavirus 2 outbreak in Germany. EMBO Mol. Med. 2020;12 doi: 10.2139/ssrn.3654517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Z.D., Wang Z.Y., Zhang S.F., Li X., Li L., Li C., Cui Y., Fu R., Dong Y.Z., Chi X.Y., Zhang M.Y., Liu K., Cao C., Liu B., Zhang K., Gao Y., Lu B., Chen W. Aerosol and surface distribution of severe acute respiratory syndrome coronavirus 2 in hospital wards, Wuhan, China, 2020. Emerg. Infect. Dis. 2020;26(7):1583–1591. doi: 10.3201/eid2607.200885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafson T.L., Lavely G.B., Brawner E.R., Hutcheson R.H., Wright P.F., Schaffner W. An outbreak of airborne nosocomial varicella. Pediatrics. 1982;70:550–555. [PubMed] [Google Scholar]
- Haas C. La peste antonine [The Antonine plague] Bull. Acad. Natl. Med. 2006;190(4–5):1093–1098. [PubMed] [Google Scholar]
- Hadei M., Mohebbi S.R., Hopke P.K., Shahsavani A., Bazzazpour S., Alipour M., Jafari A.J., Bandpey A.M., Zali A., Yarahmadi M., Farhadi M., Rahmatinia M., Hasanzadeh V., Nazari S.S.H., Asadzadeh-Aghdaei H., Tanhaei M., Zali M.R., Kermani M., Vaziri M.H., Chobineh H. Presence of SARS-CoV-2 in the air of public places and transportation. Atmos. Pollut. Res. 2021;12(3):302–306. doi: 10.1016/j.apr.2020.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamner, L., Dubbel, P., Capron, I., Ross, A., Jordan, A., Lee, J., Lynn, J., Ball, A., Narwal, S., Russell, S., Patrick, S., Leibrand, H., 2020. High SARS-CoV-2 Attack Rate Following Exposure at a Choir Practice — Skagit County, Washington. MMWR Morb. Mortal Wkly Rep. 69, 606–610. DOI: http://dx.doi.org/10.15585/mmwr.mm6919e6. [DOI] [PubMed]
- Haslbeck K., Schwarz K., Hohlfeld J.M., Seume J., Koch W. Submicron droplet formation in the human lung. J. Aerosol. Sci. 2010;41:429–438. [Google Scholar]
- Hempel S. John Snow. The Lancet. 2013;381(9874):1269–1270. doi: 10.1016/S0140. [DOI] [PubMed] [Google Scholar]
- Hopley, R., 2010. Contagion in Islamic lands: Responses from Medieval Andalusia and North Africa. J. Early Modern Cultural Studies 10, 45–64.
- Huang J., Jones P., Zhang A., Shan Hou S., Hang J., Spengler D. Outdoor Airborne Transmission of Coronavirus Among Apartments in High-Density Cities. Front. Built Environ. 2021;7 doi: 10.3389/fbuil.2021.666923. [DOI] [Google Scholar]
- Huber V. Pandemics and the politics of difference: Rewriting the history of internationalism through nineteenth-century cholera. J. Global Hist. 2020;15(3):394–407. doi: 10.1017/S1740022820000236. [DOI] [Google Scholar]
- Huynh J., Li S., Yount B., Smith A., Sturges L., Olsen J.C., Nagel J., Johnson J.B., Agnihothram S., Gates J.E., Frieman M.B., Baric R.S., Donaldson E.F. Evidence Supporting a Zoonotic Origin of Human Coronavirus Strain NL63. J. Virol. 2012;86 (23:12816–12825. doi: 10.1128/JVI.00906-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang S.E., Chang J.H., Oh B., Heo J. Possible aerosol transmission of COVID-19 associated with an outbreak in an apartment in Seoul. South Korea. Int. J. Infect. Dis. 2021;16 doi: 10.1016/j.ijid.2020.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignjatovic J., Sapats S. Avian infectious bronchitis virus. Rev. Sci. Tech. Off. Int. Epiz. 2000;19(2):493–508. doi: 10.20506/rst.19.2.1228. [DOI] [PubMed] [Google Scholar]
- Jacob S.T., Crozier I., Fischer W.A., Hewlett A., Kraft C., de La Vega M.A., Soka M., Wahl V., Griffiths A., Bollinger L., Kuhn J. Ebola virus disease. Nat. Rev. Dis. Primers. 2020;6:13. doi: 10.1038/s41572-020-0147-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang S., Han S.H., Rhee J.-Y. Cluster of Coronavirus Disease Associated with Fitness Dance Classes, South Korea. Emerg. Infect. Dis. 2020;26:1917–1920. doi: 10.3201/eid2608.200633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarcho S. Medical and nonmedical comments on Cato and Varro, with historical observations on the concept of infection. Trans. Coll. Phys. Phila. 1976;43:372–378. [PubMed] [Google Scholar]
- Jayaweera M., Perera H., Gunawardana B., Manatunge J. Transmission of COVID-19 virus by droplets and aerosols: A critical review on the unresolved dichotomy. Environ. Res. 2020;188 doi: 10.1016/j.envres.2020.109819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jester B.J., Uyeki T.M., Jernigan D.B. Fifty Years of Influenza A(H3N2) Following the Pandemic of 1968. Am. J. Public Health. 2020;110(5):669–676. doi: 10.2105/AJPH.2019.305557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang G., Wang C., Song Lu., Wang X., Zhou Y., Fei C., Liu H. Aerosol transmission, an indispensable route of COVID-19 spread: case study of a department-store cluster. Front. Env. Sci. Eng. 2021;15 doi: 10.1007/s11783-021-1386-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez J.L. COVID-19 Data Dives: Why Arguments Against SARS-CoV-2 Aerosol Transmission Don’t Hold Water. Medscape. 2020 https://www.medscape.com/viewarticle/934837?src=uc_mscpedt&faf=1#vp_1 [Google Scholar]
- Johnson, G.R., Morawska, L., Ristovski, Z., Hargreaves, M., Mengersen, K., Chao, C., Wan, M., Li, Y., Xie, X., Katoshevski, D., Corbett, S., 2011. Modality of human expired aerosol size distributions. J. Aerosol Sci. 42, 839–851. ISSN 0021-8502. [DOI] [PMC free article] [PubMed]
- Johnson G.R., Morawska L. The Mechanism of Breath Aerosol Formation. J. Aerosol Med. Pulm. D. 2009;22(3):229–237. doi: 10.1089/jamp.2008.0720. [DOI] [PubMed] [Google Scholar]
- Johnson N.P., Mueller J. Updating the accounts: global mortality of the 1918–1920 “Spanish” influenza pandemic. Bull. Hist. Med. 2002;76(1):105–115. doi: 10.1353/bhm.2002.0022. [DOI] [PubMed] [Google Scholar]
- Jones R.M., Brosseau L.M. Aerosol Transmission of Infectious Disease. J. Occup. Environ. Med. / American College of. Occup. Environ. Med. 2015;57(5) doi: 10.1097/JOM.0000000000000448. [DOI] [PubMed] [Google Scholar]
- Jouanna, J., 2012. Air, Miasma and Contagion in the Time of Hippocrates and the Survival of Miasmas in Post-Hippocratic Medicine (Rufus of Ephesus, Galen and Palladius), In: Jouanna, Jacques, and Neil Allies(Eds.), Greek Medicine from Hippocrates to Galen: Selected Papers. JSTOR, Brill, p. 127. www.jstor.org/stable/10.1163/j.ctt1w76vxr.
- Kempińska-Mirosławska B., Woźniak-Kosek A. The influenza epidemic of 1889–90 in selected European cities–a picture based on the reports of two Poznań daily newspapers from the second half of the nineteenth century. Med. Sci. Monit. 2013;19:1131–1141. doi: 10.12659/MSM.889469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilbourne E.D. Influenza pandemics of the 20th century. Emerg. Infect. Dis. 2006;12(1):9–14. doi: 10.3201/eid1201.051254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King A. An uncommon cold. New Sci. 2020;246(3280):32–35. doi: 10.1016/S0262-4079(20)30862-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch R. Die Äetiologie der Tuberkulose. Berliner klinische Wochenschrift. 1882;15:221–230. [Google Scholar]
- Koch A., Brierley C., Maslin M.M., Lewis S.L. Earth system impacts of the European arrival and Great Dying in the Americas after 1492. Quaternary Sci. Rev. 2019;207:13–36. doi: 10.1016/j.quascirev.2018.12.004. [DOI] [Google Scholar]
- Kohn G.C. Infobase Publishing; New York: 2008. Encyclopedia of Plague and Pestilence: from Ancient Times to the Present; p. 369. [Google Scholar]
- Ksiazek T.G., Erdman D., Goldsmith C.S., Zaki S.R., Peret T., Emery S., Tong S., Urbani C., Comer J.A., Lim W., Rollin P.E., Dowell S.F., Ling A.E., Humphrey C.D., Shieh W.J., Guarner J., Paddock C.D., Rota P., Fields B., DeRisi J., Yang J.Y., Cox N., Hughes J.M., LeDuc J.W., Bellini W.J., Anderson L.J., SARS Working Group A novel coronavirus associated with severe acute respiratory syndrome. N. Engl. J. Med. 2003;348(20):1953–1966. doi: 10.1056/NEJMoa030781. [DOI] [PubMed] [Google Scholar]
- Kutter J.S., de Meulder D., Bestebroer T.M., Lexmond P., Mulders A., Richard M., Fouchier R., Herfst S. SARS-CoV and SARS-CoV-2 are transmitted through the air between ferrets over more than one meter distance. Nat. Commun. 2021;12:1653. doi: 10.1038/s41467-021-21918-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon K.S., Park J.I., Park Y.J., Jung D.M., Ryu K.W., Lee J.H. Evidence of Long-Distance Droplet Transmission of SARS-CoV-2 by Direct Air Flow in a Restaurant in Korea. J. Korean. Med. Sci. 2020;35(46) doi: 10.3346/jkms.2020.35.e415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Quéré C., Peters G.P., Friedlingstein P., Andrew R., Canadell J., Davis S., Jackson R., Jones M. Fossil CO2 emissions in the post-COVID-19 era. Nat. Clim. Change. 2021;11:197–199. doi: 10.1038/s41558-021-01001-0. [DOI] [Google Scholar]
- Lednicky J.A., Lauzardo M., Fan Z.H., Jutla A., Tilly T.B., Gangwar M., Usmani M., Shankar S.N., Mohamed K., Eiguren-Fernandez A., Stephenson C.J., Alam M.M., Elbadry M.A., Loeb J.C., Subramaniam K., Waltzek T.B., Cherabuddi K., Morris J.G., Jr., Wu C.Y. Viable SARS-CoV-2 in the air of a hospital room with COVID-19 patients. Int. J. Infect. Dis. 2020;100:476–482. doi: 10.1016/j.ijid.2020.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lednicky J.A., Lauzardo M., Alam Md.M., Elbadry Ma.ha.A., Stephenson Caroline J., Gibson Julia C., Morris J.G. Isolation of SARS-CoV-2 from the air in a car driven by a COVID patient with mild illness. Int. J. Infect. Dis. ISSN. 2021;1201–9712 doi: 10.1016/j.ijid.2021.04.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDuc J.W., Barry M.A. SARS, the First Pandemic of the 21st Century. Emerg. Infect. Dis. 2004;10(11) doi: 10.3201/eid1011.040797_02. [DOI] [Google Scholar]
- Lee J.H.W. Mixing of Multiple Buoyant Jets. J. Hydraul. Eng. 2012;138:12. [Google Scholar]
- Lewis S., Maslin M. Defining the Anthropocene. Nature. 2015;519:171–180. doi: 10.1038/nature14258. [DOI] [PubMed] [Google Scholar]
- Li Y., Duan S., Yu I.T.S., Wong T.W. Multi-zone modelling of probable SARS virus transmission by airflow between flats in Block E, Amoy Gardens. Indoor Air. 2004;15:96–111. doi: 10.1111/j.1600-0668.2004.00318.x. [DOI] [PubMed] [Google Scholar]
- Li Y., Huang X., Yu I.S., Wong T.W., Qian H. Role of air distribution in SARS transmission during the largest nosocomial outbreak in Hong Kong. Indoor Air. 2004;15:83–95. doi: 10.1111/j.1600-0668.2004.00317.x. [DOI] [PubMed] [Google Scholar]
- Li Y., Qian H., Hang J., Chen X., Cheng P., Ling H., Wang S., Liang P., Li J., Xiao S., Wei J., Liu L., Cowling B.J., Kang M. Probable airborne transmission of SARS-CoV-2 in a poorly ventilated restaurant. Build Environ. 2021;196 doi: 10.1016/j.buildenv.2021.107788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilay A., Asamene N., Bekele A., Mengesha M., Wendabeku M., Tareke I., Girmay A., Wuletaw Y., Adossa A., Ba Y., Sall A., Jima D., Mengesha D. Reemergence of yellow fever in Ethiopia after 50 years, 2013: epidemiological and entomological investigations. BMC Infect. Dis. 2017;17(1):343. doi: 10.1186/s12879-017-2435-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lina, B., 2008. History of Influenza Pandemics. In: Raoult, D., Drancourt, M. (Eds), Paleomicrobiology. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-540-75855-6_12.
- Littman R.J. The Plague of Athens: Epidemiology and Paleopathology. MT Sinai J. Med. 2009;76:456–467. doi: 10.1002/msj.20137. [DOI] [PubMed] [Google Scholar]
- Liu Y., Ning Z., Chen Y., Guo M., Liu Y., Gali N.K., Sun L., Duan Y., Cai J., Westerdahl D., Liu X., Xu K., Ho K.F., Kan H., Fu Q., Lan K. Aerodynamic analysis of SARS-CoV-2 in two Wuhan hospitals. Nature. 2020;582:557–560. doi: 10.1038/s41586-020-2271-3. [DOI] [PubMed] [Google Scholar]
- Lu J., Gu J., Li K., Xu C., Su W., Lai Z., Zhou D., Yu C., Xu B., Yang Z. COVID-19 Outbreak Associated with Air Conditioning in Restaurant, Guangzhou. China. Emerg. Infect. Dis. 2020;26(7):1628–1631. doi: 10.3201/eid2607.200764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo, K., Lei, Z., Hai, Z., Xiao, S., Rui, J., Yang, H., Jing, X., Wang, H., Xie, Z., Luo, P., Li, W., Li, Q., Tan, H., Xu, Z., Yang, Y., Hu, S., Chen, T., 2020. Transmission of SARS-CoV-2 in Public Transportation Vehicles: A Case Study in Hunan Province, China. Open Forum Infect. Dis. doi:10.1093/ofid/ofaa430. [DOI] [PMC free article] [PubMed]
- Lv J., Gao J., Wu B., Yao M., Yang Y., Chai T., Li N. Aerosol Transmission of Coronavirus and Influenza Virus of Animal Origin. Front. Veterinary Sci. 2021;8:109. doi: 10.3389/fvets.2021.572012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyell, C. C., 1830. Principles of Geology, Book 1, pp 20-21. Project Gutenberg EBook.
- McKinney K., Yu Yang G., Lewis T. Environmental Transmission of SARS at Amoy Gardens. J. Environ. Health. 2006;68:26–30. [PubMed] [Google Scholar]
- Michaelis M., Doerr H.W., Cinatl J. Novel swine-origin influenza A virus in humans: another pandemic knocking at the door. Med. Microbiol. Immunol. 2009;198:175–183. doi: 10.1007/s00430-009-0118-5. [DOI] [PubMed] [Google Scholar]
- Miller S.L., Nazaroff W.W., Jimenez J.L., Boerstra A., Buonanno G., Dancer S.J., Kurnitsk J., Marr L.C., Morawska L., Noakes C. Transmission of SARS-CoV-2 by inhalation of respiratory aerosol in the Skagit Valley Chorale superspreading event. Indoor Air. 2020 doi: 10.1111/ina.12751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milton D.K. What was the primary mode of smallpox transmission? Implications for biodefense. Front. Cell Infect. Microbiol. 2012;2:150. doi: 10.3389/fcimb.2012.00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammed I., Nasidi A., Alkali A.S., Garbati M.A., Ajayi-Obe E.K., Audu K.A., Usman A., Abdullahi S. A severe epidemic of meningococcal meningitis in Nigeria, 1996. Trans. R. Soc. Trop. Med. Hyg. 2000;94(3):265–270. doi: 10.1016/s0035-9203(00)90316-x. [DOI] [PubMed] [Google Scholar]
- Monroe R. Why COVID didn’t have a bigger effect on CO2 emissions? The Blog. The Keeling Curve. Scripps Institution of Oceanography CO2. Program. 2021 [Google Scholar]
- Morawska L., Cao J. Airborne transmission of SARS-CoV-2: The world should face the reality. Environ. Int. 2020;139 doi: 10.1016/j.envint.2020.105730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morawska L., Milton D.K. It Is Time to Address Airborne Transmission of Coronavirus Disease 2019 (COVID-19) Clin. Infect. Dis. 2020;71(9):2311–2313. doi: 10.1093/cid/ciaa939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morawska L., Allen J., Bahnfleth W., Bluyssen P., Boerstra A., Buonanno G., Cao J., Dancer S.J., Floto A., Franchimon F., Greenhalgh T., Haworth C., Hogeling J., Isaxon C., Jimenez J.L., Kurnitski J., Li Y., Loomans M., Marks G., Marr L.C., Mazzarella L., Melikov A.K., Miller S., Milton D.K., Nazaroff W., Nielsen P.V., Noakes C., Peccia J., Prather K., Querol X., Sekhar C., Seppänen O., Tanabe S.I., Tang J.W., Tellier R., Tham K.W., Wargocki P., Wierzbicka A., Yao M. A paradigm shift to combat indoor respiratory infection. Science. 2021;372:689–691. doi: 10.1126/science.abg2025. [DOI] [PubMed] [Google Scholar]
- Morawska, L., Johnson, G.R., Ristovski, Z., Hargreaves, M. Mengersen, K.L., Chao, C., Wan, MP., Li, Y., Xie, X., Katoshevski, D., 2008. Droplets expelled during human expiratory activities and their origin. Proceedings 11th International Conference on Indoor Air Quality and Climate Paper - 1023, Copenhagen, Denmark.
- Mordechai L., Eisenberg M., Newfield T.P., Izdebski A., Kay J.E., Poinar H. The Justinianic Plague: An inconsequential pandemic? Proc. Natl. Acad. Sci. USA. 2019;116(51):25546–25554. doi: 10.1073/pnas.1903797116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno T., Pintó R.M., Bosch A., Moreno N., Alastuey A., Minguillón M.C., Anfruns-Estrada E., Guix S., Fuentes C., Buonanno G., Stabile L., Morawska L., Querol X. Tracing surface and airborne SARS-CoV-2 RNA inside public buses and subway trains. Environ. Int. 2021;147 doi: 10.1016/j.envint.2020.106326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morens D.M., Taubenberger J.K., Harvey H.A., Memoli M.J. The 1918 influenza pandemic: lessons for 2009 and the future. Crit. Care Med. 2010;38(4 Suppl):e10–e20. doi: 10.1097/CCM.0b013e3181ceb25b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgenstern, J., 2020. COVID-19 is spread by aerosols: an evidence review. First10EM blog. https://first10em.com/covid-19-is-spread-by-aerosols-an-evidence-review/.
- Mubareka, S., Groulx, N., Savory, E., Cutts, T., Theriault, S., Scott, J., Roy, C., Turgeon, N., Bryce, E., Astrakianakis, G., Kirychuk, S., Girard, M., Zhang, C., Duchaine, C., 2019. Bioaerosols and Transmission, a Diverse and Growing Community of Practice. Front. Public Health. https://doi.org/10.3389/fpubh.2019.00023. [DOI] [PMC free article] [PubMed]
- Nardell E.A. Transmission and Institutional Infection Control of Tuberculosis. Cold Spring Harb. Perspect. Med. 2016;6(2) doi: 10.1101/cshperspect.a018192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng, E. 2020. From SARS to COVID-19 and beyond: public health lessons for buildings. https://www.buildingsandcities.org/insights/commentaries/from-sars-to-covid-19-and-beyond.html.
- Nielsen P.V., Liu L. Department of Civil Engineering, Aalborg University; DCE Technical Memorandum No: 2020. The influence of air distribution on droplet infection and airborne cross infection; p. 77. [Google Scholar]
- Nissen K., Krambrich J., Akaberi D., Hoffman T., Ling J., Lundkvist A., Svensson L., Salaneck E. Long-distance airborne dispersal of SARS-CoV-2 in COVID-19 wards. Sci. Rep. 2020;10:19589. doi: 10.1038/s41598-020-76442-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrie P. University of New South Wales; 2014. An account of diseases in the Near East during the Bronze Age – an historical view; p. 172. PhD. thesis. [Google Scholar]
- Nunn N., Qian N. The Columbian Exchange: A History of Disease, Food, and Ideas. J. Econ. Perspect. 2010;24:163–188. [Google Scholar]
- Ober W.B., Aloush N. The plague at Granada, 1348–1349: Ibn Al-Khatib and ideas of contagion. Bull. N. Y. Acad. Med. 1982;58(4):418–424. [PMC free article] [PubMed] [Google Scholar]
- Ochmann, S., Roser, M., 2017. “Polio”. Published online at OurWorldInData.org. Retrieved from: 'https://ourworldindata.org/polio'.
- Olsen J.G. In: Emerging Infections. third ed. Scheld W.M., Craig W.A., Hughes J.M., editors. John Wiley and Sons; New York: 1999. Epidemic Typhus: a Forgotten but Lingering Threat. [DOI] [Google Scholar]
- Olsen S.J., Chang H.L., Cheung T.Y., Tang A.F., Fisk T.L., Ooi S.P., Kuo H.W., Jiang D.D., Chen K.T., Lando J., Hsu K.H., Chen T.J., Dowell S.F. Transmission of the severe acute respiratory syndrome on aircraft. N. Engl. J. Med. 2003;18:2416–2422. doi: 10.1056/NEJMoa031349. [DOI] [PubMed] [Google Scholar]
- Ong S.W.X., Tan Y.K., Chia P.Y., Lee T.H., Ng O.T., Wong M.S.Y., Marimuthu K. Air, Surface Environmental, and Personal Protective Equipment Contamination by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) From a Symptomatic Patient. JAMA. 2020;323(16):1610–1612. doi: 10.1001/jama.2020.3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orata F.D., Keim P.S., Boucher Y. The 2010 cholera outbreak in Haiti: how science solved a controversy. PLoS Pathog. 2014;10(4) doi: 10.1371/journal.ppat.1003967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacini F. Osservazioni microscopiche e deduzioni patologiche sul cholera asiatico (Microscopic observations and pathological deductions on Asiatic cholera) Gazz Med. Ital. 1854;4(50):397–405. [Google Scholar]
- Pan, M., Lednicky, JA, Wu, CY., 2019. Collection, particle sizing and detection of airborne viruses. J Appl Microbiol 127(6), 1596–1611. doi: 10.1111/jam.14278. Epub 2019 Jun 26. PMID: 30974505; PMCID: PMC7167052. [DOI] [PMC free article] [PubMed]
- Papeneni R., Rosenthal F. The Size Distribution of Droplets in the Exhaled Breath of Healthy Human Subjects. J. Aerosol Med. 1997;10(2):105–116. doi: 10.1089/jam.1997.10.105. [DOI] [PubMed] [Google Scholar]
- Park S.Y., Kim Y.-M., Yi S., Lee S., Na B.J., Kim C.B., Kim J.I., Kim H.S., Kim Y.B., Park Y., Huh I.S., Kim H.K., Yoon H.J., Jang H., Kim K., Chang Y., Kim I., Lee H., Gwack J., Kim S.S., Kim M., Kweon S., Choe Y.J., Park O., Park Y.J., Jeong E.K. Coronavirus Disease Outbreak in Call Center, South Korea. Emerg. Infect. Dis. 2020;26:1666–1670. doi: 10.3201/eid2608.201274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker, R., 1983. Miasma: Pollution and Purification in Early Greek Religion. Oxford University Press. Reissued in 1996 by Clarendon Press, 216pp.
- Parkes E.A. Mode of Communication of Cholera. Int. J. Epidemiol. 2013;42(6):1543–1552. doi: 10.1093/ije/dyt193. [DOI] [PubMed] [Google Scholar]
- Parliament European. Public Health and Food Safety; Committee on the Environment: 2021. Report on the implementation of the Ambient Air Quality Directives: Directive 2004/107/EC and Directive 2008/50/EC. [Google Scholar]
- Patrick D.M., Petric M., Skowronski D.M., Guasparini R., Booth T.F., Krajden M., McGeer P., Bastien N., Gustafson L., Dubord J., Macdonald D., David S.T., Srour L.F., Parker R., Andonov A., Isaac-Renton J., Loewen N., McNabb G., McNabb A., Goh S.H., Henwick S., Astell C., Guo J.P., Drebot M., Tellier R., Plummer F., Brunham R.C. An Outbreak of Human Coronavirus OC43 Infection and Serological Cross-reactivity with SARS Coronavirus. Can. J. Infect. Dis. Med. Microbiol. 2006;17(6):330–335. doi: 10.1155/2006/152612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson, K., 1993. Typhus and its control in Russia, 1870–1940. Med.cal Hist. 37(4), 361–381. doi:10.1017/S0025727300058725. [DOI] [PMC free article] [PubMed]
- Peiris J., Guan Y., Yuen K. Severe acute respiratory syndrome. Nat. Med. 2004;10:S88–S97. doi: 10.1038/nm1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prata J.C., Silva A.L.P., Walker T.R., Duarte A.C., Rocha-Santos T. COVID-19 Pandemic Repercussions on the Use and Management of Plastics. Environ. Sci. Technol. 2020;54(13):7760–7765. doi: 10.1021/acs.est.0c02178. [DOI] [PubMed] [Google Scholar]
- Prather K.A., Wang C.C., Schooley R.T. Reducing transmission of SARS-CoV-2. Science. 2020;368(6498):1422–1424. doi: 10.1126/science.abc6197. [DOI] [PubMed] [Google Scholar]
- Querol X., Massagué J., Alastuey A., Moreno T., Gangoiti G., Mantilla E., Duéguez J., Escudero M., Monfort E., Pérez García-Pando C., Petetin H., Jorba O., Vázquez V., de la Rosa J., Campos A., Muñóz M., Monge S., Hervás M., Javato R., Cornide M. Lessons from the COVID-19 air pollution decrease in Spain: Now what? Sci. Tot. Environ. 2021;779 doi: 10.1016/j.scitotenv.2021.146380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall, K., Ewing, E., Marr, L., Jimenez, J.L., Bourouiba, L., 2021. How Did We Get Here: What Are Droplets and Aerosols and How Far Do They Go? A Historical Perspective on the Transmission of Respiratory Infectious Diseases. Available at SSRN: https://ssrn.com/abstract=3829873. [DOI] [PMC free article] [PubMed]
- Reed N.G. The history of ultraviolet germicidal irradiation for air disinfection. Public Health Rep. 2010;125(1):15–27. doi: 10.1177/003335491012500105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard M., Kok A., de Meulder D., Bestebroer T., Lamers M., Okba N., van Vlissingen M., Rockx B., Haagmans B., Koopmans M., Fouchier R., Herfst S. SARS-CoV-2 is transmitted via contact and via the air between ferrets. Nat. Commun. 2020;11(1):3496. doi: 10.1038/s41467-020-17367-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley R. Airborne infection. Am. J. Med. 1974;57:466–475. doi: 10.1016/0002-9343(74)90140-5. [DOI] [PubMed] [Google Scholar]
- Riley R.L. What Nobody Needs to Know About Airborne Infection. Am. J. Respir. Crit. Care Med. 2001;163:7–8. doi: 10.1164/ajrccm.163.1.hh11-00. [DOI] [PubMed] [Google Scholar]
- Riley R.L., Mills C.C., Nyka W., Weinstock N., Storey P.B., Sultan L.U., Riley M.C., Wells W.F. Aerial dissemination of pulmonary tuberculosis. A two-year study of contagion in a tuberculosis ward. Am. J. Epidemiol. 1959;70:185–196. doi: 10.1093/oxfordjournals.aje.a120069. [DOI] [PubMed] [Google Scholar]
- Riley R.L., Mills C.C., O’Grady F., Sultan L.U., Wittstadt F., Shivpuri D.N. Infectiousness of air from a tuberculosis ward. Ultraviolet irradiation of infected air: comparative infectiousness of different patients. Am Rev Respir Dis. 1962;85:511–525. doi: 10.1164/arrd.1962.85.4.511. [DOI] [PubMed] [Google Scholar]
- Riley E.C., Murphy G., Riley R.L. Airborne spread of measles in a suburban elementary school. Am. J. Epidemiol. 1978;107:421–432. doi: 10.1093/oxfordjournals.aje.a112560. [DOI] [PubMed] [Google Scholar]
- Rubino M., Etheridge D., Trudinger C., Allison C., Rayner P., Enting I., Mulvaney R., Steele L., Langenfelds R., Sturges W., Curran M., Smith A. Low atmospheric CO2 levels during the Little Ice Age due to cooling-induced terrestrial uptake. Nature Geosci. 2016;9:691–694. doi: 10.1038/ngeo2769. [DOI] [Google Scholar]
- Rutz C., Loretto M.C., Bates A.E., Davidson S.C., Duarte C.M., Jetz W., Johnson M., Kato A., Kays R., Mueller T., Primack R.B., Ropert-Coudert Y., Tucker M.A., Wikelski M., Cagnacci F. COVID-19 lockdown allows researchers to quantify the effects of human activity on wildlife. Nat. Ecol. Evol. 2020;4:1156–1159. doi: 10.1038/s41559-020-1237-z. [DOI] [PubMed] [Google Scholar]
- Sabbatani, S., Fiorino, S., 2009. La peste antonina e il declino dell'Impero Romano. Ruolo della guerra partica e della guerra marcomannica tra il 164 e il 182 d.c. nella diffusione del contagio [The Antonine Plague and the decline of the Roman Empire]. Infez. Med. 17(4), 261–75. [PubMed]
- Sáez Geoffroy A., Parra Díaz J. Rev. Chil. Infectol. 2020;37(4):450–455. doi: 10.4067/S0716-10182020000400450. [DOI] [PubMed] [Google Scholar]
- Sakula A. Robert Koch: centenary of the discovery of the tubercle bacillus. Thorax. 1982;4:246–251. doi: 10.1136/thx.37.4.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarpia J.L., Rivera D.N., Herrera V.L., Morwitzer M.J., Creager H.M., Santarpia G.W., Crown K., Brett-Major D., Schnaubelt E., Broadhurst M.J., Lawler J., Reid S.P., Lowe J. Aerosol and surface contamination of SARS-CoV-2 observed in quarantine and isolation care. Sci Rep. 2020;10:12732. doi: 10.1038/s41598-020-69286-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schalk A.F., Hawn M.C. An apparently new respiratory disease of baby chicks. J. Am. Vet. Med. Assoc. 1931;78:413–423. [Google Scholar]
- Schwarz K., Biller H., Windt H., Koch W., Hohlfeld J.M. Characterization of exhaled particles from the healthy human lung–a systematic analysis in relation to pulmonary function variables. J. Aerosol Med. Pulm. Drug Deliv. 2010;23:371–379. doi: 10.1089/jamp.2009.0809. [DOI] [PubMed] [Google Scholar]
- Shanks D. Pacific Island Societies Destabilised by Infectious Diseases. History. 2016;24:71–74. [Google Scholar]
- Shanks D., Lee S.E., Howard A., Brundage J.F. Extreme Mortality After First Introduction of Measles Virus to the Polynesian Island of Rotuma, 1911. Am. J. Epidemiol. 2011;173:1211–1222. doi: 10.1093/aje/kwq504. [DOI] [PubMed] [Google Scholar]
- Shao, S., Zhou, D., He, R., Li, J., Zou, S., Mallery, K., Kumar, S., Yang, S., Hong, J., 2021. Risk assessment of airborne transmission of COVID-19 by asymptomatic individuals under different practical settings. J. Aerosol Sci. 151, art. no. 105661. [DOI] [PMC free article] [PubMed]
- Sharp P.M., Hahn B.H. Origins of HIV and the AIDS pandemic. Cold Spring Harb. Perspect. Med. 2011;1(1) doi: 10.1101/cshperspect.a006841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y., Li C., Dong H., Wang Z., Martinez L., Sun Z., Handel A., Chen Z., Chen E., Ebell M.H., Wang F., Yi B., Wang H., Wang X., Wang A., Chen B., Qi Y., Liang L., Li Y., Ling F., Chen J., Xu G. Community Outbreak Investigation of SARS-CoV-2 Transmission Among Bus Riders in Eastern China. JAMA Intern. Med. 2020;180(12):1665–1671. doi: 10.1001/jamainternmed.2020.5225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Z., Song C., Liu B., Lu G., Xu J., Vu T.V., Elliott R.J.R., Li W., Bloss W.J., Harrison R.M. Abrupt but smaller than expected changes in surface air quality attributable to COVID-19 lockdowns. Sci. Adv. 2021;13:eabd6696. doi: 10.1126/sciadv.abd6696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shousha A.T. Cholera Epidemic in Egypt (1947): A Preliminary Report. Bull World Health Organ. 1948;1(2):353–381. [PMC free article] [PubMed] [Google Scholar]
- Shukla T., Sen I.S., Boral S., Sharma S. A Time-Series Record during COVID-19 Lockdown Shows the High Resilience of Dissolved Heavy Metals in the Ganga River. Environ. Sci. Technol. Lett. 2021;8(4):301–306. doi: 10.1021/acs.estlett.0c00982. [DOI] [PubMed] [Google Scholar]
- Sia S.F., Yan L.M., Chin A.W.H., Fung K., Choy K.T., Wong A.Y.L., Kaewpreedee P., Perera R.A.P.M., Poon L.L.M., Nicholls J.M., Peiris M., Yen H.L. Pathogenesis and transmission of SARS-CoV-2 in golden hamsters. Nature. 2020;583(7818):834–838. doi: 10.1038/s41586-020-2342-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow J. John Churchill, New Burlington Street, England; London: 1855. On the Mode of Communication of Cholera. [Google Scholar]
- Snow S.J. Commentary: Sutherland, Snow and water: the transmission of cholera in the nineteenth century. Int. Epid. Ass. 2002;31:908–911. doi: 10.1093/ije/31.5.908. [DOI] [PubMed] [Google Scholar]
- Stadnytskyi, V., Bax., CE, Bax, A., Anfinrud, P., 2020. The airborne lifetime of small speech droplets and their potential importance in SARS-CoV-2 transmission. Proc. Natl. Acad. Sci. USA 2, 117(22): 11875–11877. doi: 10.1073/pnas.2006874117. [DOI] [PMC free article] [PubMed]
- Stearns J. New Directions in the Study of Religious Responses to the Black Death. Hist. Compass. 2009;7:1363–1375. doi: 10.1111/j.1478-0542.2009.00634.x. [DOI] [Google Scholar]
- Sullivan G.L., Delgado-Gallardo J., Watson T.M., Sarp S. An investigation into the leaching of micro and nano particles and chemical pollutants from disposable face masks - linked to the COVID-19 pandemic. Water Res. 2021;196 doi: 10.1016/j.watres.2021.117033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultan L., Nyka W., Mills C., O'Grady F., Wells W., Riley R.L. Tuberculosis disseminators. A study of the variability of aerial infectivity of tuberculous patients. Am. Rev. Respir. Dis. 1960;82:358–369. doi: 10.1164/arrd.1960.82.3.358. [DOI] [PubMed] [Google Scholar]
- Suzuki A. Smallpox and the epidemiological heritage of modern Japan: towards a total history. Med. Hist. 2011;55(3):313–318. doi: 10.1017/s0025727300005329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syvitski J., Waters C.N., Day J., Milliman J., Summerhayes C., Steffen W., Zalasiewicz J., Cearreta A., Gałuszka A., Hajdas I., Head M., Leinfelder R., McNeill J., Poirier C., Rose N., Shotyk W., Wagreich M., Williams M. Extraordinary human energy consumption and resultant geological impacts beginning around 1950 CE initiated the proposed Anthropocene Epoch. Commun. Earth Environ. 2020;1:32. doi: 10.1038/s43247-020-00029-y. [DOI] [Google Scholar]
- Tang J.W., Bahnfleth W.P., Bluyssen P.M., Buonanno G., Jimenez J.L., Kurnitski J., Li Y., Miller S., Sekhar C., Morawska L., Marr L.C., Melikov A.K., Nazaroff W.W., Nielsen P.V., Tellier R., Wargocki P., Dancer S.J. Dismantling myths on the airborne transmission of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) J. Hosp. Infect. 2021;110:89–96. doi: 10.1016/j.jhin.2020.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, S., Mao, Y., Jones, RM., Tan, Q., Ji, JS., Li, N., Shen, J., Lv, Y., Pan, L., Ding, P., Wang, X., Youbin W., MacIntyre, R., Shi, X., 2020. Aerosol transmission of SARS-CoV-2? Evidence, prevention and control. Environ. Int. 144, 106039, ISSN 0160-4120. https://doi.org/10.1016/j.envint.2020.106039. [DOI] [PMC free article] [PubMed]
- Tang J.W., Marr L.C., Li Y., Dancer S.J. Covid-19 has redefined airborne transmission. BMJ. 2021;373 doi: 10.1136/bmj.n913. [DOI] [PubMed] [Google Scholar]
- Taubenberger J.K., Morens D.M. Influenza: the once and future pandemic. Public Health Rep. 2010;125(Suppl 3):16–26. [PMC free article] [PubMed] [Google Scholar]
- Taubenberger J.K., Morens D.M., Fauci A.S. The next influenza pandemic: can it be predicted? JAMA. 2007;297(18):2025–2027. doi: 10.1001/jama.297.18.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tellier R. Aerosol transmission of influenza A virus: a review of new studies. J. R. Soc. Interface. 2009;6:S783–S790. doi: 10.1098/rsif.2009.0302.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tellier R., Li Y., Cowling B.J., Tang J. Recognition of aerosol transmission of infectious agents: a commentary. BMC Infect. Dis. 2019;19:101. doi: 10.1186/s12879-019-3707-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vågene, Å.J., Herbig, A., Campana, M., Robles García, N., Warinner, C., Sabin, S., Spyrou, MA., Andrades Valtueña, A., Huson, D., Tuross, N., Bos, KI., Krause, J., 2018. Salmonella Enterica Genomes from Victims of a Major Sixteenth-Century Epidemic in Mexico. Nat. Ecol. Evol. 2, 520–528. Nature, Springer Nature Limited, https://www.nature.com/articles/s41559-017-0446-6. [DOI] [PubMed]
- Valleron A.J., Cori A., Valtat S., Meurisse S., Carrat F., Boëlle P.Y. Transmissibility and geographic spread of the 1889 influenza pandemic. Proc. Natl. Acad. Sci. USA. 2010;107(19):8778–8781. doi: 10.1073/pnas.1000886107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Doremalen, N., Bushmaker, T., Morris, D.H., Holbrook, M.G., Gamble, A., Williamson, B.N., Tamin, A., Harcourt, JL., Thornburg, NJ., Gerber, SI., Lloyd-Smith, JO., de Wit, E., Munster, VJ., 2020. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N. Engl. J. Med. 16, 382(16): 1564-1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed]
- Varro, M.T., 36BC. De Re Rusticae (On Agriculture).
- Vijgen L., Keyaerts E., Moës E., Thoelen I., Wollants E., Lemey P., Vandamme A.-M., Van Ranst M. Complete Genomic Sequence of Human Coronavirus OC43: Molecular Clock Analysis Suggests a Relatively Recent Zoonotic Coronavirus Transmission Event. J. Virol. 2005;79(3):1595–1604. doi: 10.1128/JVI.79.3.1595-1604.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villemin J.A. Cause et nature de la tuberculose. Bull Acad Med. 1865;37:211–216. [Google Scholar]
- Villemin, J.A., 1868. Etudes sur la tuberculose: preuves rationnelles et expérimentales de sa spécificité et de son inoculabilité, J.-B. Baillière, Paris.
- Wallinga J., Heijne J.C.M., Kretzschmar M. A Measles Epidemic Threshold in a Highly Vaccinated Population. PLoS Med. 2005;2(11) doi: 10.1371/journal.pmed.0020316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, N., Li, S-Y, Yang, X-L., Huang, H-M., Zhang, Y-J, Guo, H., Luo, C-M., Miller, M., Zhu, G., Chmura, AA., Hagan, E., Zhou, J-H., Zhang, Y-Z., Wang, LF., Daszak, P., Shi, Z-L., 2018. Serological Evidence of Bat SARS-Related Coronavirus Infection in Humans, China. Virol. Sin. 33(1),108–111.https://doi.org/10.1007/s12250-018-0012-7. [DOI] [PMC free article] [PubMed]
- Waters C.N., Zalasiewicz J., Summerhayes C., Barnosky A.D., Poirier C., Gałuszka A., Cearreta A., Edgeworth M., Ellis E.C., Ellis M., Jeandel C., Leinfelder R., McNeill J.R., Richter Dd., Steffen W., Syvitski J., Vidas D., Wagreich M., Williams M., Zhisheng A., Grinevald J., Odada E., Oreskes N., Wolfe A.P. The Anthropocene is functionally and stratigraphically distinct from the Holocene. Science. 2016;351, 6269:aad2622. doi: 10.1126/science.aad2622. [DOI] [PubMed] [Google Scholar]
- Wehrle P.F., Posch J., Richter K.H., Henderson D.A. An airborne outbreak of smallpox in a German hospital and its significance with respect to other recent outbreaks in Europe. Bull. World Health Organ. 1970;43(5):669–679. [PMC free article] [PubMed] [Google Scholar]
- Welch W.H. Public Health in Theory and Practice. New Haven, Yale University Press. 1925;1925:27. [Google Scholar]
- Wells W.F. On Airborne Infection, Study II. Droplets and Droplet Nuclei. Am. J. Hyg. 1934;20:611–618. [Google Scholar]
- Wells W.F. Harvard University Press, Cambridge; An Ecological Study of Droplet Infections: 1955. Airborne Contagion and Air Hygiene; p. 423. [Google Scholar]
- Wells W.F., Riley E.C. Droplet nuclei transmission. J Indust Hygiene and Toxicology. 1937;19:513–561. [Google Scholar]
- Wells W.F., Wells M.W. Air-Borne Infection. JAMA. 1936;107:1698–1703. doi: 10.1001/JAMA.1936.02770470016004. [DOI] [Google Scholar]
- Wells W.F., Wells M.W. Air-Borne Infection: Sanitary Control. JAMA. 1936;107(22):1805–1809. doi: 10.1001/jama.1936.02770480037010. [DOI] [Google Scholar]
- Wells W.F., Wells M.W., Wilder T.S. The environmental control of epidemic contagion I: an epidemiologic study of radiant disinfection of air in day schools. Am. J. Hyg. 1942;35:97–101. [Google Scholar]
- Wells W.F., Ratcliffe H.L., Crumb C. On the mechanics of droplet nuclei infection; quantitative experimental air-borne tuberculosis in rabbits. Am. J. Hyg. 1948;47(1):11–28. doi: 10.1093/oxfordjournals.aje.a119179. [DOI] [PubMed] [Google Scholar]
- Wildy P. Classification and nomenclature of viruses. First report of the International Committee on Nomenclature of Viruses. Monographs in Virol. 1971;5:27–73. [Google Scholar]
- World Health Organisation, 2020. Transmission of SARS-CoV-2: implications for infection prevention precautions. Scientific brief, 9 July 2020. WHO/2019-nCoV/Sci_Brief/Transmission_modes/2020.3
- World Health Organisation, 2021b. Middle East respiratory syndrome coronavirus (MERS-CoV). http://www.emro.who.int/health-topics/mers-cov/latest-updates.html.
- World Health Organization, 2021a. Roadmap to improve and ensure good indoor ventilation in the context of COVID-19. Licence: CC BY-NC-SA 3.0 IGO.
- Xiao S., Li Y., Sung M., Wei J., Yang Z. A study of the probable transmission routes of MERS-CoV during the first hospital outbreak in the Republic of Korea. Indoor Air. 2017;28:51–63. doi: 10.1111/ina.12430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu I.T.S., Li Y., Wong T.W., Tam W., Chan A.T., Lee J.H.W., Leung D.Y.C., Ho T. Evidence of Airborne Transmission of the Severe Acute Respiratory Syndrome Virus. N. Engl. J. Med. 2004;350:1731–1739. doi: 10.1056/NEJMoa032867. [DOI] [PubMed] [Google Scholar]
- Yu I.T.S., Wong T.W., Chiu Y.L., Lee N., Li Y. Temporal-Spatial Analysis of Severe Acute Respiratory Syndrome among Hospital Inpatients. Clin. Infect. Dis. 2005;40(9):1237–1243. doi: 10.1086/428735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalasiewicz J., Waters C.N., Barnosky A.D., Cearreta A., Edgeworth M., Ellis E., Gałuszka A., Gibbard P., Grinevald J., Hajdas I., Ivar do Sul J., Jeandel C., Leinfelder R., McNeill J.R., Poirier C., Revkin A., deB Richter D., Steffen W., Summerhayes C., Syvitski J., Vidas D., Wagreich M., Williams M., Wolfe A. Colonization of the Americas, ‘Little Ice Age’ climate, and bomb-produced carbon: Their role in defining the Anthropocene. Anthropocene Rev. 2015;2(2):117–127. doi: 10.1177/2053019615587056. [DOI] [Google Scholar]
- Zalasiewicz J., Waters C.N., Ivar do Sul J., Corcoran P.L., Barnosky A.D., Cearreta A., Edgeworth M., Galuszka A., Jeandel C., Leinfelder R., McNeill J.R., Steffen W., Summerhayes C., Wagreich M., Williams M., Wolfe A.P., Yonan Y. The geological cycle of plastics and their use as a stratigraphic indicator of the Anthropocene. Anthropocene. 2016;13:4–17. [Google Scholar]
- Zalasiewicz J., Waters C.N., Ellis E., Head M., Vidas D., Steffen W., Thomas J., Horn E., Summerhayes C., Leinfelder R., Mcneill J., Gałuszka A., Williams M., Barnosky A., Richter D., Gibbard P., Syvitski J., Jeandel C., Cearreta A., Zinke J. The Anthropocene: Comparing Its Meaning in Geology (Chronostratigraphy) with Conceptual Approaches Arising in Other Disciplines. Earth's Future. 2021;9:1–25. doi: 10.1029/2020EF001896. [DOI] [Google Scholar]
- Zhang R., Li Y., Zhang A.L., Wang Y., Molina M.J. Identifying airborne transmission as the dominant route for the spread of COVID-19. Proc. Natl. Acad. Sci. USA. 2020;117(26):14857–14863. doi: 10.1073/pnas.2009637117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang A.R., Shi W.Q., Liu K., Li X.L., Liu M.J., Zhang W.H., Zhao G.P., Chen J.J., Zhang X.A., Miao D., Ma W., Liu W., Yang Y., Fang L.Q. Epidemiology and evolution of Middle East respiratory syndrome coronavirus, 2012–2020. Infect. Dis. Poverty. 2021;10:66. doi: 10.1186/s40249-021-00853-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J., Otter J.A., Price J.R., Cimpeanu C., Garcia D.M., Kinross J., Boshier P.R., Mason S., Bolt F., Holmes A.H., Barclay W.S. Investigating SARS-CoV-2 surface and air contamination in an acute healthcare setting during the peak of the COVID-19 pandemic in London. Clin. Infect. Dis. 2020;ciaa905 doi: 10.1093/cid/ciaa905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zumla A., Hui D.S., Perlman S. Middle East respiratory syndrome. Lancet. 2015;386:995–1007. doi: 10.1016/S0140-6736(15)60454-8. [DOI] [PMC free article] [PubMed] [Google Scholar]