Abstract
Aims
CONCERT-HF is an NHLBI-sponsored, double-blind, placebo-controlled, Phase II trial designed to determine whether treatment with autologous bone marrow-derived mesenchymal stromal cells (MSCs) and c-kit positive cardiac cells (CPCs), given alone or in combination, is feasible, safe, and beneficial in patients with heart failure (HF) caused by ischaemic cardiomyopathy.
Methods and results
Patients were randomized (1:1:1:1) to transendocardial injection of MSCs combined with CPCs, MSCs alone, CPCs alone, or placebo, and followed for 12 months. Seven centres enrolled 125 participants with left ventricular ejection fraction of 28.6 ± 6.1% and scar size 19.4 ± 5.8%, in New York Heart Association class II or III. The proportion of major adverse cardiac events (MACE) was significantly decreased by CPCs alone (−22% vs. placebo, P = 0.043). Quality of life (Minnesota Living with Heart Failure Questionnaire score) was significantly improved by MSCs alone (P = 0.050) and MSCs + CPCs (P = 0.023) vs. placebo. Left ventricular ejection fraction, left ventricular volumes, scar size, 6-min walking distance, and peak oxygen consumption did not differ significantly among groups.
Conclusions
This is the first multicentre trial assessing CPCs and a combination of two cell types from different tissues in HF patients. The results show that treatment is safe and feasible. Even with maximal guideline-directed therapy, both CPCs and MSCs were associated with improved clinical outcomes (MACE and quality of life, respectively) in ischaemic HF without affecting left ventricular function or structure, suggesting possible systemic or paracrine cellular mechanisms. Combining MSCs with CPCs was associated with improvement in both these outcomes. These results suggest potential important beneficial effects of CPCs and MSCs and support further investigation in HF patients.
Keywords: Cell-based therapy, Clinical trial, Heart failure
Graphical Abstract
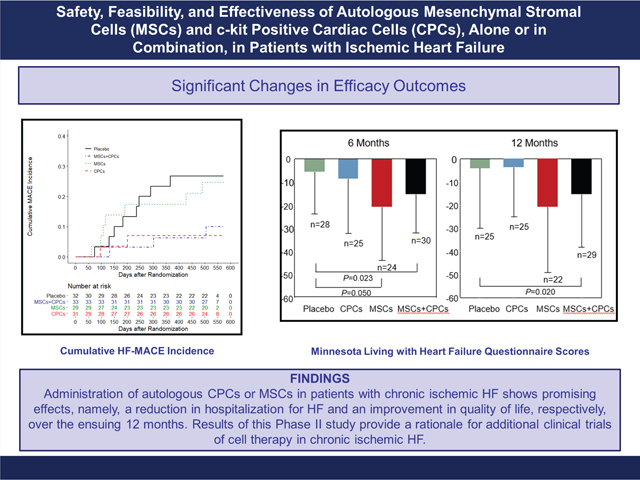
Cardiovascular Cell Therapy Research Network: the CONCERT-HF trial. CONCERT-HF is the first multicentre trial assessing c-kit positive cardiac cells (CPCs) and a combination of two cell types from different tissues in heart failure (HF) patients. Administration of autologous CPCs or mesenchymal stromal cells (MSCs) in patients with chronic ischaemic HF shows promising effects, namely, a reduction in hospitalization for HF and an improvement in quality of life, respectively, over the ensuing 12 months. Results of this Phase II study provide a rationale for additional clinical trials of cell therapy in chronic ischaemic HF.
Introduction
The prognosis of heart failure (HF) caused by chronic ischaemic cardiomyopathy (coronary artery disease and prior myocardial infarction), hereby referred to as ‘ischaemic HF’, remains poor.1 Cell therapy is a potentially useful approach to treating ischaemic HF, with several Phase I and II clinical trials yielding encouraging results.2–6 The field of cell therapy has drawn extraordinary controversy since its inception approximately 20 years ago. Although transplanted cells do not regenerate cardiomyocytes,7–13 pre-clinical studies have consistently shown that they improve cardiac performance.8–22 Accordingly, this study was designed and executed to address, using rigorous methods, whether or not cell therapy improves cardiac structure and function in patients with ischaemic HF and whether or not, regardless of the outcome in cardiac function, patients experience clinical benefit.
Bone marrow (BM) mesenchymal stromal cells (MSCs) are one of the most promising cell types being considered for patients with ischaemic HF.3,5,6 Studies in animal models of ischaemic HF have demonstrated that transendocardial injection of MSCs improves left ventricular (LV) function and reduces scar size.14–19 Clinical trials of MSCs in patients with chronic ischaemic HF have shown safety and potential therapeutic efficacy.2–4,14,23–29 MSCs do not differentiate into cardiac cells but secrete factors that exert antifibrotic, anti-apoptotic, anti-inflammatory, and pro-angiogenic actions.3,7
Another promising cell type for the treatment of ischaemic HF is c-kit positive cardiac cells (CPCs).3,8,9,13 Lineage tracing studies in vivo have shown that CPCs can differentiate into endothelial cells and, rarely, other cell types.30 Similar to MSCs, CPCs have been shown to act by releasing paracrine signals, not by differentiating into cardiac cells.7–13 In pre-clinical models of ischaemic HF, numerous studies from independent laboratories in various species have consistently shown that administration of CPCs improves LV function.8–13,20–22 However, the therapeutic efficacy of CPCs in humans with ischaemic HF has not been evaluated in double-blind, randomized, multicentre trials using cell products manufactured according to Good Manufacturing Practice (GMP) standards.
Experimental evidence suggests that combining different cell types may be therapeutically advantageous,20,21,31,32 possibly because of the complementary effects of the secretomes from different sources. Specifically, in pre-clinical models of ischaemic HF, there is evidence for a beneficial interaction between MSCs and CPCs that results in additive therapeutic effects, greater than those of either cell alone.14,20,21,32 To our knowledge, combinations of two different cell types have not been investigated in patients with ischaemic HF.
CONCERT-HF (Combination Of meseNchymal and c-kit+ Cardiac stEm cells as Regenerative Therapy for Heart Failure) (ClinicalTrials.gov Identifier: NCT02501811) was funded by the National Heart, Lung, and Blood Institute (NHLBI) to evaluate the feasibility, safety, and efficacy of MSCs and CPCs, alone and in combination, in patients with chronic ischaemic HF.33 Specifically, CONCERT-HF addresses the following questions: Is combined treatment with autologous MSCs and CPCs feasible and safe in patients with ischaemic HF? Do MSCs and CPCs, given alone or in combination, reduce major adverse cardiac events (MACE), improve quality of life, augment functional capacity, alleviate LV dysfunction, and/or reduce scar size? Is either cell type more effective than the other? Is the combination of MSCs and CPCs superior to MSCs alone or CPCs alone?
Methods
The design and methodology of CONCERT-HF have been described in detail33 and are summarized briefly below.
Design
In consultation with the NHLBI’s Gene and Cell Therapy Data and Safety Monitoring Board (DSMB) and the Food and Drug Administration (FDA), the CONCERT-HF trial was implemented in two stages. Stage 1 was performed to assess the feasibility and safety of the study procedures and to provide initial insights into the potential bioactivity of the study product; this information was used to inform the larger Stage 2 placebo-controlled trial. The results of Stage 1 are presented in the online supplementary Appendix S1. The remainder of this manuscript describes the results of Stage 2.
Screening and eligibility
A complete list of inclusion and exclusion criteria is provided in online supplementary Table S1. Briefly, for enrolment, participants had to have (i) a ‘detectable’ scar by magnetic resonance imaging (MRI), defined as ≥5% of LV volume with any subendocardial involvement; (ii) an ejection fraction ≤40% by MRI; (iii) maximally tolerated, guideline-driven medical therapy for HF at stable doses for ≥1 month prior to consent; and (iv) New York Heart Association (NYHA) class I, II, or III HF symptoms.
Study protocol
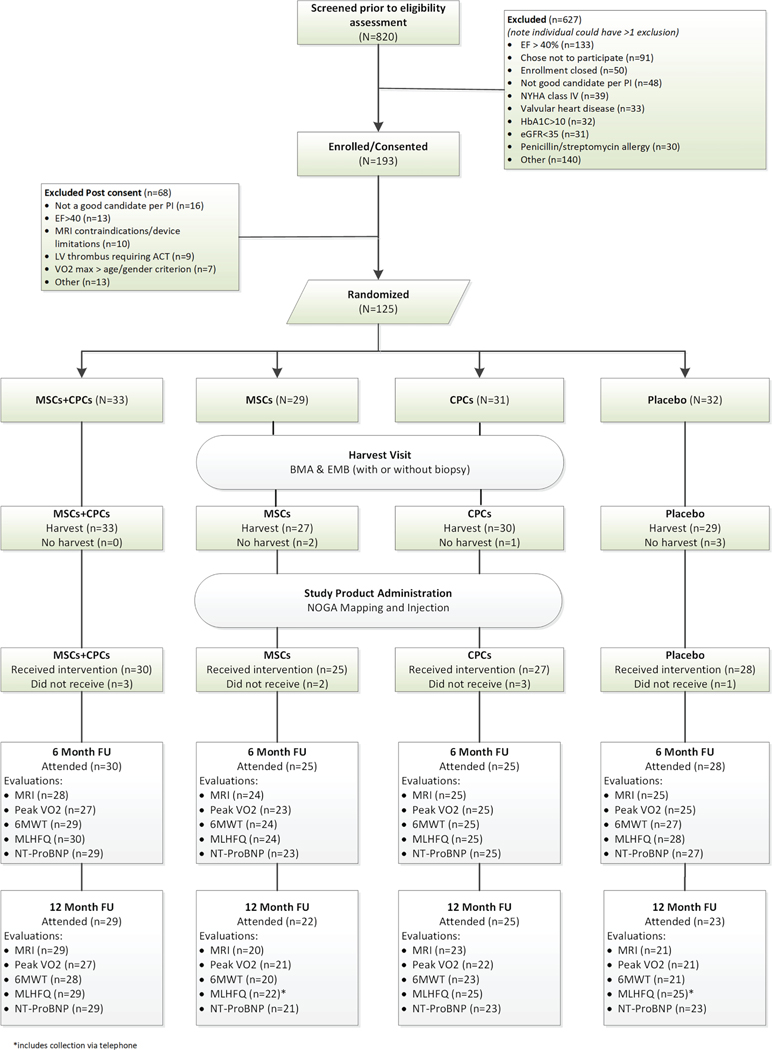
The protocol complies with the Declaration of Helsinki and was reviewed and approved by local institutional review boards at each of the seven recruiting centres and the data coordinating centre. All participants provided written informed consent prior to study procedures. After baseline testing, 125 participants were randomized (1:1:1:1) to receive (i) a combination of MSCs and CPCs, (ii) MSCs only, (iii) CPCs only, or (iv) placebo (Figure 1). All participants underwent bone marrow aspiration (BMA) and right heart catheterization (RHC). The RHC included endomyocardial biopsy (EMB) only for participants randomized to the MSCs + CPCs and CPCs alone groups; a ‘sham biopsy’ was performed in the other two groups.
Figure 1.
CONCERT-HF CONSORT diagram. CONSORT diagram shows patients screened, enrolled, and treated in the CONCERT-HF trial, as well as number of patients that completed follow-up for each endpoint, along with reasons for non-completion. 6MWT, 6-min walk test; ACT, anticoagulation therapy; BMA, bone marrow aspiration; CPC, c-kit positive cardiac cell; EF, ejection fraction; eGFR, estimated glomerular filtration rate; EMB, endomyocardial biopsy; FU, follow-up; HbA1C, glycated haemoglobin; LV, left ventricular; MLHFQ, Minnesota Living with Heart Failure Questionnaire; MRI, magnetic resonance imaging; MSC, mesenchymal stromal cell; NT-proBNP, N-terminal pro-brain natriuretic peptide; NYHA, New York Heart Association; VO2, oxygen consumption.
Study products were manufactured by the central cell manufacturing facility at the University of Miami Interdisciplinary Stem Cell Institute. Manufacturing methods, including cell expansion, cryopreservation, shipment, and release testing, have been described.33 Detailed characterization of the cell products is presented in online supplementary Tables S2 and S3.
Approximately 14 weeks after BMA and RHC, participants underwent transendocardial study product injection (SPI) using the NOGA® XP Mapping and Navigation System (Biologic Delivery Systems, Johnson and Johnson). The target doses were 150 × 106 MSCs and 5 × 106 CPCs. Participants were followed at 1 day, 1 week, and 1, 3, 6, and 12 months after SPI.
Study endpoints
Study endpoints were measures of safety, feasibility, and efficacy. Safety outcomes were HF-related-MACE (HF-MACE) (all-cause death, hospitalization for worsening HF, and HF exacerbation not requiring hospitalization)34,35 and other significant clinical events, as well as all events at least grade 2 in severity. Efficacy endpoints included clinical outcomes (HF-MACE), quality of life [Minnesota Living with Heart Failure Questionnaire (MLHFQ) score], MRI measures of LV function and structure, measures of functional capacity [peak oxygen consumption (VO2), 6-min walking distance], and biomarkers [N-terminal pro-brain natriuretic peptide (NT-proBNP)]. All clinical events were reviewed by independent adjudicators (authors DA and CL), blinded to treatment. MRI and treadmill exercise (peak VO2) data were evaluated by Core Labs at Johns Hopkins University and the Massachusetts General Hospital, respectively.
Statistical analysis
All statistical analyses were conducted by authors DL and BRD using SAS version 9.4 (SAS institute Inc.) and R version 3.2.2 (R Core Team, 2015). The principal analyses were based on an intention-to-treat of the four randomized therapies. There were six possible pairwise comparisons between treatments: (i) MSCs + CPCs vs. MSCs, (ii) MSCs + CPCs vs. CPCs, (iii) MSCs + CPCs vs. placebo, (iv) MSCs vs. placebo, (v) CPCs vs. placebo, and (vi) MSCs vs. CPCs. Descriptive statistics for baseline characteristics are provided. Continuous variables were evaluated for normality using Shapiro–Wilk test and histograms. Non-normally distributed variables were log (e) transformed. Fisher’s exact test for categorical variables and two sample Student’s t-tests for continuous variables were used to evaluate differences of changes of efficacy variables between treatment groups. Secondary as-treated analyses were also conducted (online supplementary Appedix S1). Safety data were analysed by therapy group using Fisher’s exact test between baseline and (i) 6 months and (ii) 12 months. Feasibility of study procedures was evaluated as the number and percentage of participants for the event or procedure.
Cumulative incidence of HF-MACE was analysed using Kaplan–Meier curves, log-rank tests, and Cox regression. For each of the two prospectively declared follow-up durations (6 and 12 months), the change in each efficacy measure of interest was also compared using ANCOVA analyses adjusting for baseline values. Repeated-measures linear regression models (SAS Proc Mixed) were used to address trajectories (upward or downward trends) over time within each of the treatment groups in the randomized and total cohorts. Also, a time by treatment interaction was included to assess if there were treatment differences in slopes over time. All available data were used to construct likelihood function assuming data are missing at random. Partial information is included for participants with missing observations. No adjustments for multiplicity were made in this Phase II trial for reasons expounded elsewhere.33,36
Sample sizes were selected to ensure sufficient power to detect meaningful changes in endpoint measures. Sample size calculations were based on the variability encountered in previous trials in similar patient populations, including TIME,37 LateTIME,38 and SWISS-AMI.39 According to these estimates, using two sample Student’s t-test, CONCERT-HF needed a sample size of n = 144 assuming a 20% attrition rate, 2-sided alpha = 0.05, and power of 90% to detect LV ejection fraction (LVEF) change differences and infarct size changes (n = 36 per group).
CONCERT-HF was designed to enrol 144 participants. After 125 participants were randomized, recruitment was paused by the NHLBI based on a recommendation from the DSMB following their consideration of concerns raised about CPCs.40 Subsequently, the DSMB reviewed a report from an independent panel appointed by the NHLBI to evaluate cell production protocols and records and a report of an analysis of interim data to assess power for the various efficacy endpoints. Neither the DSMB nor the NHLBI raised any concerns regarding cell production, characteristics of cell products, or the conduct of the trial. The DSMB then recommended that patients who had undergone bone marrow harvest and/or EMB should undergo cell transplantation and complete the study protocol. However, because the observed variability of MRI-derived measures was less than expected, recruitment of additional participants was considered unlikely to meaningfully improve power. It is important to note that the DSMB did not stop the trial for futility but rather they reviewed the sample size calculations, and in light of our low variability in MRI endpoints, determined that power was likely sufficient to meet the trial objectives at the sample size at that time (n =125). The NHLBI then made the decision to halt enrolment at 125 participants.
Results
Study population
Between November 2016 and November 2018, 820 individuals were evaluated for enrolment, 125 of whom were randomized to the four treatment groups (Figure 1). Reasons for exclusion are given in Figure 1. Baseline characteristics are summarized in Table 1. The cohort was principally male (93%), 10% non-white and 16% Hispanic, with a mean age of 62.5 years. The average LVEF at baseline (MRI) was 28.6 ± 6.1% and scar size 19.4 ± 5.8% of the left ventricle; 80% of patients were in NYHA class II and 15% in NYHA class III. All participants were on maximally tolerated, guideline-directed therapy.
Table 1.
Baseline characteristics of randomized patients
| MSCs + CPCs (n = 33) | MSCs (n = 29) | CPCs (n = 31) | Placebo (n = 32) | |
|---|---|---|---|---|
| Demographics | ||||
| Age (years) | 61.0 ± 11.1 | 61.7 ± 6.7 | 64.2 ± 8.1 | 63.1 ± 8.8 |
| Female sex | 2 (6.06) | 2 (6.90) | 4 (12.90) | 1 (3.13) |
| Race | ||||
| White | 30 (90.91) | 27 (93.10) | 28 (90.32) | 28 (87.50) |
| Black | 3 (9.09) | 0 (0.00) | 0 (0.00) | 2 (6.25) |
| Other | 0 (0.00) | 2 (6.90) | 3 (9.68) | 2 (6.25) |
| Hispanic | 5 (15.15) | 5 (18.52) | 6 (19.35) | 4 (12.50) |
| Physical findings | ||||
| Height (in.) | 69.8 ± 3.1 | 69.1 ± 3.6 | 69.2 ± 3.0 | 69.5 ± 2.7 |
| Weight (lbs) | 216.1 ± 29.4 | 206.5 ± 43.8 | 200.3 ± 38.7 | 206.8 ± 37.9 |
| Body mass index (kg/m2) | 31.2 ± 3.5 | 30.4± 5.4 | 29.4 ± 5.0 | 30.0 ± 4.4 |
| Heart rate (bpm) | 68.4 ± 7.2 | 69.4± 9.1 | 69.7 ± 9.1 | 68.3 ± 11.3 |
| SBP (mmHg) | 118.4 ± 18.2 | 114.0 ± 11 .3 | 117.3 ± 17.9 | 117.2 ± 17.6 |
| DBP (mmHg) | 70.2 ± 9.3 | 70.1 ± 9.0 | 68.3 ± 10.8 | 67.7 ± 11.2 |
| Risk factor history | ||||
| Diabetes | 7 (21.21) | 10 (35.71) | 10 (32.26) | 12 (37.50) |
| Hypertension | 29 (87.88) | 23 (82.14) | 26 (83.87) | 27 (84.38) |
| Smoking | ||||
| Previous | 16 (48.48) | 16 (55.17) | 22 (70.97) | 20 (62.50) |
| Current | 3 (9.09) | 2 (6.90) | 2 (6.45) | 2 (6.25) |
| Heart failure history | ||||
| Hospitalization for HF | 11 (33.33) | 8 (27.59) | 9 (29.03) | 13 (40.63) |
| Emergency department visit for HF | 6 (18.18) | 4 (13.79) | 8 (25.81) | 9 (28.13) |
| Ongoing ischaemia | 9 (27.27) | 5 (17.24) | 4 (12.90) | 7 (21.88) |
| NYHA class | ||||
| I | 1 (3.03) | 1 (3.45) | 3 (9.68) | 1 (3.13) |
| II | 24 (72.73) | 22 (75.86) | 26 (83.87) | 28 (87.50) |
| III | 8 (24.24) | 6 (20.69) | 2 (6.45) | 3 (9.38) |
| Device status | ||||
| Device present | 28 (84.85) | 24 (82.76) | 28 (90.32) | 21 (65.63) |
| ICD | 21 (63.64) | 16 (55.17) | 21 (67.74) | 15 (46.88) |
| Biventricular Pacing and ICD | 7 (21.21) | 8 (27.59) | 7 (22.58) | 6 (18.75) |
| Cardiovascular history | ||||
| Angina (in last 6 months) | 13 (39.39) | 7 (24.14) | 5 (16.13) | 10 (31.25) |
| Canadian classification | ||||
| Class I | 8 (61.54) | 3 (42.86) | 3 (60.00) | 4 (40.00) |
| Class II | 5 (38.46) | 3 (42.86) | 2 (40.00) | 6 (60.00) |
| Class III | 0 (0.00) | 1 (14.29) | 0 (0.00) | 0 (0.00) |
| Class IV | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| MI | ||||
| Number of MIs | 1.6 ± 1.0 | 1 .9 ± 1 .7 | 1 .9 ± 1 .2 | 1.7 ± 1.0 |
| Most recent, STEMI | 7 (70.00) | 14 (66.67) | 10 (55.56) | 9 (69.23) |
| Most recent, anterior | 14 (87.50) | 17 (80.95) | 14 (82.35) | 16 (76.19) |
| PCI | 27 (81.82) | 24 (82.76) | 27 (87.10) | 28 (87.50) |
| CABG | 17 (51.52) | 14 (48.28) | 16 (51.61) | 15 (46.88) |
| Multi-vessel disease | 25 (75.76) | 22 (75.86) | 28 (90.32) | 30 (93.75) |
| Left main disease | 19 (82.61) | 20 (74.07) | 20 (71.43) | 17 (62.96) |
| Proximal LAD involvement | 7 (36.84) | 13 (65.00) | 12 (60.00) | 12 (70.59) |
| Atrial fibrillation | 11 (33.33) | 8 (27.59) | 11 (35.48) | 10 (31.25) |
| Sustained ventricular arrhythmia | 12 (37.50) | 5 (17.24) | 4 (12.90) | 7 (21.88) |
| Valvular heart disease | 22 (66.67) | 17 (58.62) | 26 (83.87) | 24 (75.00) |
| Valvular repair | 1 (3.03) | 0 (0.00) | 3 (9.68) | 3 (9.38) |
| Valvular replacement | 0 (0.00) | 0 (0.00) | 1 (3.23) | 0 (0.00) |
| Peripheral vascular disease | 1 (3.03) | 2 (6.90) | 3 (9.68) | 5 (15.63) |
| Cerebrovascular history | ||||
| Asymptomatic carotid disease | 3 (9.38) | 1 (3.57) | 2 (6.45) | 4 (12.90) |
| Transient ischaemic attack | 0 (0.00) | 4 (13.79) | 4 (12.90) | 2 (6.25) |
| Ischaemic stroke | 1 (3.03) | 6 (20.69) | 2 (6.45) | 3 (9.38) |
| Haemorrhagic stroke | 1 (3.03) | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Comorbidity history | ||||
| Thyroid disease | 4 (12.50) | 2 (6.90) | 9 (29.03) | 9 (28.13) |
| Liver disease | 0 (0.00) | 0 (0.00) | 0 (0.00) | 1 (3.13) |
| Autoimmune disorder | 1 (3.03) | 3 (10.34) | 2 (6.45) | 2 (6.25) |
| History of malignancy | 2 (6.06) | 2 (6.90) | 1 (3.23) | 5 (15.63) |
| Medications | ||||
| Aspirin | 30 (90.91) | 25 (86.21) | 25 (80.65) | 27 (84.38) |
| Antiplatelet agents (non-aspirin) | 20 (60.61) | 19 (65.52) | 12 (38.71) | 15 (46.88) |
| β-blockers | 32 (96.97) | 26 (89.66) | 29 (93.55) | 31 (96.88) |
| ACE inhibitors | 16 (48.48) | 10 (34.48) | 18 (58.06) | 8 (25.00) |
| Angiotensin II receptor blockers | 8 (24.24) | 5 (17.24) | 6 (19.35) | 9 (28.13) |
| ARNI | 8 (24.24) | 11 (37.93) | 7 (22.58) | 13 (40.63) |
| Ivabradine | 0 (0.00) | 1 (3.45) | 0 (0.00) | 0 (0.00) |
| Aldosterone antagonists | 18 (54.55) | 20 (68.97) | 20 (64.52) | 22 (68.75) |
| Calcium channel blockers | 1 (3.03) | 1 (3.45) | 1 (3.23) | 1 (3.13) |
| Hydralazine | 0 (0.00) | 2 (6.90) | 2 (6.45) | 0 (0.00) |
| Nitrates | 14 (42.42) | 15 (51.72) | 12 (38.71) | 12 (37.50) |
| Statins | 28 (84.85) | 26 (89.66) | 27 (87.10) | 28 (87.50) |
| Diuretics | 18 (54.55) | 19 (65.52) | 19 (61.29) | 24 (75.00) |
| Anticoagulants | 8 (24.24) | 7 (24.14) | 10 (32.26) | 10 (31.25) |
| Non-insulin | 6 (18.18) | 8 (27.59) | 6 (19.35) | 11 (34.38) |
| Insulin | 3 (9.09) | 4 (13.79) | 4 (12.90) | 6 (18.75) |
| Antiarrhythmics | 14 (42.42) | 8 (27.59) | 12 (38.71) | 14 (43.75) |
| PCSK9 Inhibitors | 2 (6.06) | 1 (3.45) | 2 (6.45) | 0 (0.00) |
Values are presented as mean ± standard deviation or n (%); denominators are of those reporting.
ACE, angiotensin-converting enzyme; ARNI, angiotensin receptor–neprilysin inhibitor; CABG, coronary artery bypass graft; CPC, c-kit positive cardiac cell; DBP, diastolic blood pressure; HF, heart failure; ICD, implantable cardioverter-defibrillator; LAD, left anterior descending coronary artery; MI, myocardial infarction; MSC, mesenchymal stromal cell; NYHA, New York Heart Association; PCI, percutaneous coronary intervention; PCSK9, proprotein convertase subtilisin/kexin type 9; SBP, systolic blood pressure; STEMI, ST-elevation myocardial infarction.
Safety and feasibility
As shown in online supplementary Table S4, there were no significant differences across the treatment groups with respect to the incidence of adverse events in the 12 months after treatment.
Of the 125 randomized participants, 119 underwent harvest procedures [BMA, RHC (with or without EMB), or both]; of these, 110 received SPI (Figure 1). Six randomized participants did not undergo harvest procedures due to: death prior to procedure (n =1), patient withdrawal (n = 4), and one patient changed his/her mind about treatment but continued in follow-up. All participants (n =119) had a successful BMA; all participants randomized to the CPCs only or MSCs + CPCs groups (n = 64) had successful EMB except in one case in which EMB could not be obtained due to ventricular tachycardia. After the harvest visit, an additional nine randomized participants did not receive SPI due to: death prior to scheduled procedure (n = 2), LV assist device placement (n = 2), episodes of ventricular tachycardia (n = 2), and procedures that were cancelled by the interventionalist (n = 3).
To maintain study blinding, if a patient was randomized to MSCs + CPCs and did not meet the minimum dose of CPCs, he/she received MSCs alone and conversely, if a patient did not meet the minimum dose of MSCs, he/she received CPCs alone; in the circumstance where both products failed to meet release criteria, the patient received placebo. If a patient in either the MSCs or CPCs alone groups did not meet the minimum dose, he/she received placebo.33 Of the 110 participants scheduled to receive study product, 90 (82%) received their assigned product and 20 (18%) an alternate product as described above. Of the 55 MSC products and 57 CPC products prepared, 25% and 16%, respectively, failed release criteria due to failure of CPCs to grow (4 CPC products), insufficient cell counts (9 MSC and 1 CPC product), and insufficient viability (4 MSC and 2 CPC products) at the clinical site. Of the 110 patients who received SPI, 4 received <15 injections; 2 because of insufficient product volume and 2 because of safety concerns (patient’s anatomy, pericardial effusion). The average MSC and CPC dose cell count of administered products was 108 ± 28 × 106 cells and 4.3 ± 1.2 × 106 cells, respectively.
Despite 81% of patients having cardiac devices (most of which were MRI non-conditional), MRI scans were safely performed in 96% and 95% of participants at the 6- and 12-month visits, respectively. LV volumes were obtained in 98% of the scans both at 6 and 12 months; measurements of scar size and viable mass were obtained in 82% and 78% of scans, respectively. Primary reasons for the inability to assess these variables were device artefacts (creating low signal-to-noise ratio), breathing or motion artefacts, and technologist error during image acquisition.
Efficacy
Heart failure-related major adverse cardiac events
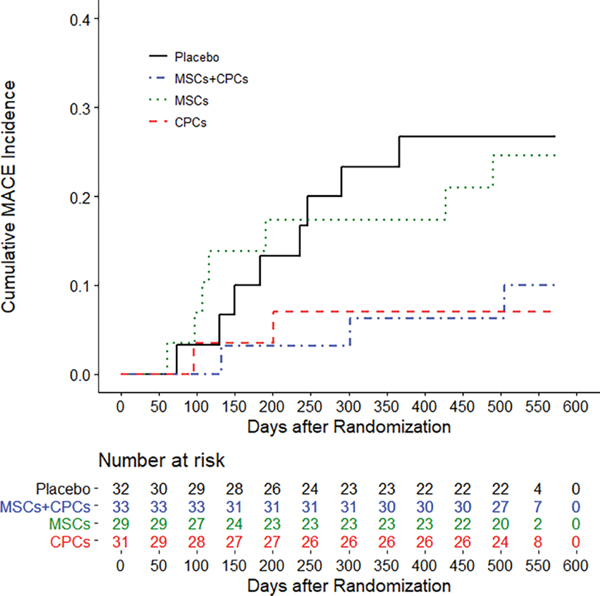
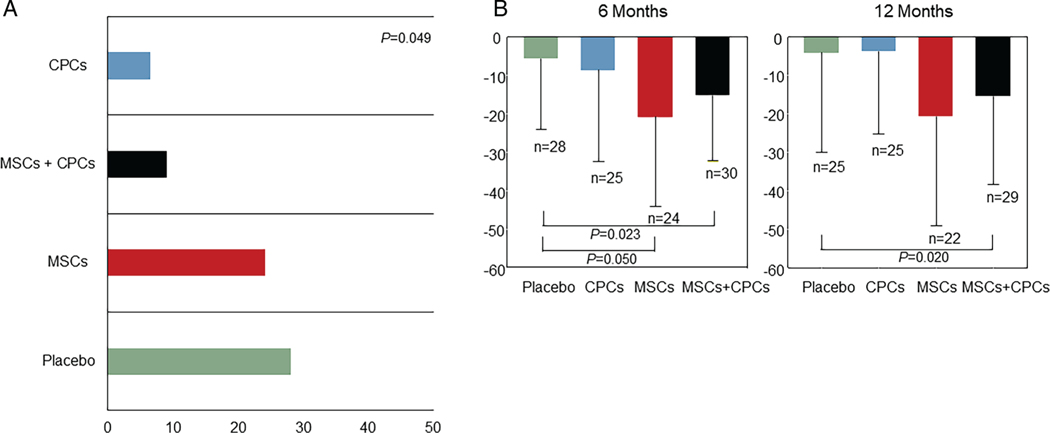
The number of patients with HF-MACE is presented in Figure 2 and Table 2. The proportion of HF-MACE was significantly different across the groups (P = 0.049) (Figure 3A); it was highest in the placebo group (28.1%) and lowest in the CPCs alone group (6.5%; P = 0.043 vs. placebo). In the MSCs + CPCs group, HF-MACE occurred in 9.1% of patients (P = 0.061 vs. placebo). The differences in HF-MACE were driven primarily by hospitalization for HF, which was reduced from 21.9% of patients in the placebo group to 3.2% in the CPCs alone group (P = 0.053 vs. placebo) and 6.1% in the MSCs + CPCs group (P = 0.082 vs. placebo) (Table 2, Figure 3A). Cox regression analysis of the time to event demonstrated that, compared with the placebo group, the hazard ratio (HR) was significantly lower in the CPCs alone group [HR 0.200 (95% confidence interval-CI 0.043–0.934), P = 0.041] and in the MSCs + CPCs group [HR 0.256 (95% CI 0.069–0.934), P = 0.043] (Figure 2). Adjusted analyses (including baseline covariates of gender, smoking status, presence of a cardiac device, and NYHA class) were consistent with the unadjusted ones. Additionally, baseline characteristics for the reduced sample size showed balance across the treatment groups. Other cardiovascular clinical events were not significantly different among groups (Table 2).
Figure 2.
Heart failure-related major adverse cardiac events (MACE)–time to event. Cumulative (Kaplan–Meier) incidence of heart failure-related MACE. Events occurring between randomization and 30 days past the 12-month follow-up visit were adjudicated as endpoints. Some participants had extended 12-month visit windows due to the COVID-19 pandemic. Log rank P = 0.0364 is based on the entire follow-up of all participants. CPC, c-kit positive cardiac cell; MSC, mesenchymal stromal cell.
Table 2.
Patients with heart failure-related major adverse cardiac events and other significant clinical events by treatment group
| Post-randomozation (n = 125) | MSCs + CPCs (n = 33) | MSCs (n = 29) | CPCs (n = 31) | Placebo (n = 32) | P-value | |
|---|---|---|---|---|---|---|
| Patients with HF-MACE and other significant clinical events* | 43 (34.4) | 10 (30.3) | 9 (31) | 11 (35.5) | 13 (40.6) | 0.828 |
| Patients with HF-MACE | 21 (16 . 8) | 3 (9.1) | 7 (24.1) | 2 (6.5) | 9 (28.1) | 0.049 |
| Death | 11 (8.8) | 2 (6.1) | 3 (10.3) | 2 (6.5) | 4 (12.5) | 0.767 |
| Hospitalization for worsening HF | 14 (11.2) | 2 (6.1) | 4 (13.8) | 1 (3.2) | 7 (21.9) | 0.092 |
| Exacerbation of HF (non-hospitalization) | 2 (1.6) | 0 (0) | 1 (3.4) | 0 (0) | 1 (3.1) | 0.48 |
| Patients with other significant clinical events | 29 (23.2) | 8 (24.2) | 3 (10.3) | 11 (35.5) | 7 (21.9) | 0.148 |
| Non-fatal stroke | 2 (1.6) | 1 (3) | 0 (0) | 0 (0) | 1 (3.1) | 1 |
| Non-fatal MI | 4 (3.2) | 0 (0) | 1 (3.4) | 2 (6.5) | 1 (3.1) | 0.505 |
| Coronary artery revascularization | 7 (5.6) | 2 (6.1) | 1 (3.4) | 2 (6.5) | 2 (6.2) | 1 |
| Ventricular tachycardia/fibrillation | 19 (15.2) | 5 (15.2) | 3 (10.3) | 8 (25.8) | 3 (9.4) | 0.289 |
| Pericardial tamponade | 2 (1.6) | 1 (3) | 0 (0) | 1 (3.2) | 0 (0) | 0.864 |
| Patients with HF-MACE and other significant clinical events – SAEs | 37 (29.6) | 8 (24.2) | 9 (31) | 8 (25.8) | 12 (37.5) | 0.668 |
Values are presented as n (%).
Denominators are per column.
CPC, c-kit positive cardiac cell; HF, heart failure; MACE, major adverse cardiac event; MI, myocardial infarction; MSC, mesenchymal stromal cell; SAE, serious adverse event.
Categories of HF-MACE and patients with other significant clinical events are not mutually exclusive.
Figure 3.
Clinical outcome findings. (A) Proportion of first heart failure-related major adverse cardiac events (HF-MACE) by treatment group. Percentage of participants experiencing HF-MACE (first event) during the trial displayed by treatment group. (B) Quality of life results at baseline and follow-up by treatment group. Change in Minnesota Living with Heart Failure Questionnaire scores from baseline to 6 months and from baseline to 12 months by treatment group. Data are mean± standard deviation. CPC, c-kit positive cardiac cell; MSC, mesenchymal stromal cell.
Because 15 of the 125 randomized patients were not treated and 20 received a treatment different from that to which they had been randomized due to product release failure (vide supra), we also performed an as-treated analysis of HF-MACE in which patient allocation to a group was based upon the treatment actually received (online supplementary Table S5). In this analysis, the 15 non-treated patients constitute a fifth group. The as-treated analysis showed that the proportion of HF-MACE differed significantly among the five groups (P = 0.027), being highest in the placebo and non-treated patients (24.4% and 33.3%, respectively). In the group that received CPCs alone, only 3.6% of patients experienced a HF-MACE (P = 0.022 vs. placebo). In the group that received MSCs + CPCs, HF-MACE occurred in 5% of patients (P = 0.084 vs. placebo). Again, these differences reflected primarily the rate of hospitalization for HF, which differed significantly among the five groups (P = 0.046) and was highest in placebo and non-treated patients (19.5% and 20%, respectively). In contrast, none of the 28 patients who received CPCs alone were hospitalized for HF (P = 0.018 vs. placebo). The rate of HF hospitalization was numerically lower in patients who received MSCs alone and MSCs + CPCs (9.5% and 5%, respectively), but these differences were not statistically significant vs. placebo.
Other endpoints
Compared with placebo, at 6 months the MLHFQ score was improved in the MSCs alone group (−15.09; ANCOVA adjusted for baseline P = 0.050) and MSCs + CPCs group (−9.64; ANCOVA adjusted for baseline P = 0.023) (Table 3, Figure 3B, online supplementary Table S6). Similarly, at 12 months the MLHFQ score was improved in the MSCs + CPCs group vs. placebo (−11.35; ANCOVA adjusted for baseline P = 0.020) (Table 3, Figure 3B, online supplementary Table S7).
Table 3.
Primary endpoints by domain at baseline and follow-up
| MSCs + CPCs | MSCs | CPCS | Placebo | ||
|---|---|---|---|---|---|
| LVEF (%) | |||||
| Baseline | [33] 29.21 (6.69) | [26] 29.26 (5.91 ) | [28] 26.31 (4.89) | [27] 29.66 (6.18) | |
| 6 months | [28] 29.29 (5.79) | [24] 29.43 (7.00) | [25] 27.21 (6.01) | [26] 29.18 (4.72) | |
| 12 months | [29] 29.91 (6.74) | [20] 31.12 (7.06) | [23] 26.96 (5.12) | [21] 29.35 (5.88) | |
| Global circumferential strain (%) | |||||
| Baseline | [26] −8.71 (2.50) | [17] −8.63 (3.23) | [22] −8.30 (2.88) | [16] −9.03 (3.73) | |
| 6 months | [26] −9.06 (3.34) | [17] −8.15 (2.57) | [22] −7.74 (2.96) | [16] −9.13 (2.29) | |
| 12 months | [26] −8.47 (2.78) | [17] −9.14 (3.12) | [22] −7.85 (2.54) | [16] −8.08 (3.46) | |
| Longitudinal strain (%) | |||||
| Baseline | [26] −10.18 (2.85) | [18] −10.00 (2.42) | [22] −9.47 (2.51) | [17] −9.58 (2.93) | |
| 6 months | [26] −10.68 (3.16) | [18] −10.51 (2.82) | [22] −10.22 (2.69) | [17] −10.49 (2.97) | |
| 12 months | [26] −10.13 (2.78) | [18] −10.77 (3.27) | [22] −9.92 (2.68) | [17] −10.32 (3.16) | |
| LVEDVI (mL/m2) | |||||
| Baseline | [33] 129.16 (32.44) | [26] 127.72 (34.36) | [28] 133.97 (27.75) | [27] 124.97 (29.83) | |
| 6 months | [28] 127.11 (30.36) | [24] 128.92 (33.69) | [25] 132.19 (22.20) | [26] 129.74 (29.76) | |
| 12 months | [29] 128.34 (33.74) | [20] 122.28 (34.15) | [23] 136.38 (26.22) | [21] 131.70 (28.26) | |
| LVESVI (mL/m2) | |||||
| Baseline | [33] 92.61 (29.71) | [26] 91.73 (30.89) | [28] 99.29 (24.30) | [27] 89.03 (27.25) | |
| 6 months | [28] 90.72 (26.45) | [24] 92.59 (31.04) | [25] 96.67 (20.35) | [26] 92.59 (25.57) | |
| 12 months | [29] 90.95 (29.45) | [20] 85.52 (30.57) | [23] 100.30 (24.39) | [21] 93.48 (23.74) | |
| Sphericity (mL) | |||||
| Baseline | [33] 0.56 (0.10) | [26] 0.56 (0.09) | [28] 0.57 (0.10) | [27] 0.55 (0.18) | |
| 6 months | [26] 0.58 (0.10) | [24] 0.55 (0.08) | [25] 0.58 (0.09) | [26] 0.57 (0.14) | |
| 12 months | [28] 0.58 (0.12) | [20] 0.54 (0.09) | [23] 0.59 (0.10) | [21] 0.55 (0.09) | |
| Scar size (%) | |||||
| Baseline | [28] 18.05 (7.01) | [23] 19.91 (5.86) | [27] 19.42 (4.73) | [27] 20.31 (5.50) | |
| 6 months | [23] 8.72 (6.62) | [21] 19.36 (6.55) | [23] 17.83 (4.94) | [22] 20.94 (5.01) | |
| 12 months | [23] 18.34 (6.27) | [17] 19.59 (5.79) | [20] 1.03 (6.17) | [18] 20.87 (5.42) | |
| Scar tissue mass (g) | |||||
| Baseline | [28] 30.54 (11.47) | [23] 30.42 (11.87) | [27] 32.81 (9.56) | [27] 33.20 (11.02) | |
| 6 months | [23] 31.78 (13.02) | [21] 29.60 (11.90) | [23] 30.04 (9.42) | [22] 34.64 (10.34) | |
| 12 months | [23] 32.09 (12.58) | [17] 29.69 (10.84) | [20] 32.29 (12.54) | [18] 33.61 (9.91) | |
| Peak VO2 (mL/kg/min) | |||||
| Baseline | [33] 16.37 (5.05) | [29] 17.39 (5.18) | [31] 15.62 (4.32) | [32] 15.60 (4.68) | |
| 6 months | [28] 15.74 (4.56) | [23] 16.84 (4.33) | [25] 16.57 (5.76) | [26] 16.55 (4.89) | |
| 12 months | [27] 15.78 (5.13) | [21] 18.02 (4.47) | [22] 16.28 (6.08) | [21] 16.54 (4.79) | |
| 6-min walk test (m) | |||||
| Baseline | [33] 366.11 (79.07) | [29] 369.34 (88.64) | [31] 378.81 (87.61) | [32] 367.50 (85.60) | |
| 6 months | [29] 390.31 (90.46) | [24] 390.75 (102.81) | [25] 379.88 (93.55) | [27] 376.78 (94.32) | |
| 12 months | [28] 397.07 (87.66) | [20] 400.38 (98.55) | [23] 391.65 (102.56) | [21] 384.88 (101.69) | |
| MLHFQ | |||||
| Baseline | [33] 43.72 (24.18) | [29] 46.59 (24.10) | [31] 29.47 (22.67) | [32] 39.00 (25.06) | |
| 6 months | [30] 25.54 (19.09) | [24] 29.93 (18.37) | [25] 21.69 (18.47) | [28] 34.25 (23.23) | |
| 12 months | [29] 25.35 (15.77) | [22] 30.02 (19.67) | [25] 25.68 (19.02) | [25] 36.55 (21.13) | |
| NT-proBNP (pg/mL) | |||||
| Baseline | [32] 1386.49 (4534.52) | [28] 665.65 (869.71 ) | [31] 801.78 (808.39) | [32] 856.72 (1364.72) | |
| 6 months | [30] 720.71 (851.24) | [24] 568.20 (592.43) | [25] 1084.04 (1070.04) | [27] 1388.43 (2356.32) | |
| 12 months | [29] 699.94 (755.80) | [21] 580.81 (756.89) | [23] 915.15 (757.94) | [23] 1072.32 (2161.64) |
Values are presented as [n] mean (standard deviation).
CPC, c-kit positive cardiac cell; LVEDVI, left ventricular end-diastolic volume index; LVEF, left ventricular ejection fraction; LVESVI, left ventricular end-systolic volume index; MLHFQ, Minnesota Living with Heart Failure Questionnaire; MSC, mesenchymal stromal cell; NT-proBNP, N-terminal pro-brain natriuretic peptide; VO2, oxygen consumption.
There were no significant differences between treated and placebo groups with respect to LVEF, LV end-systolic and end-diastolic volume index, global circumferential strain, longitudinal strain, sphericity index, scar size, peak VO2, 6-min walking distance, or NT-proBNP, either at 6 or 12 months (Table 3).
Using repeated-measures regression modelling, we conducted a trend analysis of the above-mentioned variables to compare trajectories (changes over 6 months) among the groups (online supplementary Tables S8 and S9). This analysis demonstrated that in the MSCs alone group the rate of decrease in MLHFQ score (i.e. negative regression equation slope) was greater compared to the placebo group (zero slope, P = 0.037) and to the CPCs alone group (positive slope, P = 0.031) (online supplementary Table S8). Trajectories did not differ significantly for other variables; however, the as-treated analyses were consistent with this finding.
As-treated analysis of efficacy endpoints at 6 months (online supplementary Table S10) and at 12 months (online supplementary Table S11) were consistent with the intention-to-treat findings.
Discussion
CONCERT-HF is the first multicentre, randomized, double-blind, placebo-controlled trial to assess a combination of two cell types from different tissues in patients with chronic ischaemic HF. The salient results are summarized as follows: (i) transendocardial injection of autologous MSCs and CPCs, alone or in combination, is safe and feasible; (ii) despite background maximal guideline-directed therapy, both MSCs and CPCs had measurable effects over 12 months, albeit in different ways; (iii) specifically, CPCs were associated with a reduction in the incidence of HF-MACE compared to placebo by 80% (HR 0.2), which was driven primarily by a reduction in hospitalization for HF; (iv) a similar outcome, i.e. a 70% decrease in HF-MACE, was observed when CPCs were combined with MSCs although this effect was not statistically significant; (v) MSCs, either alone or in combination with CPCs, were associated with improved MLHFQ score, reflecting improved quality of life, at both 6 and 12 months; (vi) these seemingly beneficial effects of CPCs and MSCs on clinical outcome were not associated with changes in LV function or structure, suggesting possible systemic or paracrine cellular mechanisms; (vii) combination therapy with MSCs and CPCs was associated with the best clinical outcomes with respect to both HF-MACE and quality of life. Taken together, the results of CONCERT-HF identify important beneficial effects in patients with chronic ischaemic HF following administration of CPCs and MSCs. Further research will be needed to elucidate the mechanism(s) underlying these effects (e.g. possible anti-inflammatory and antifibrotic actions and/or improvement in endothelial function).
The improvement in clinical outcomes (HF-MACE and quality of life) without improvement in LV function or reduction in scar size may seem counterintuitive, but is consistent with a growing body of literature indicating that in patients with chronic ischaemic HF, cell therapy can effect significant clinical changes without concomitant changes in LVEF.4,26,28,29 For example, in the ixCELL-DCM trial, transendocardial injection of BM MSCs and macrophages (ixmyelocel-T) reduced MACE by 37% despite no significant changes in LVEF or LV volumes.4 Similarly, TAC-HFT demonstrated that transendocardial injection of autologous BM MSCs improved quality of life (measured by the MLHFQ score) but did not affect LV volumes or LVEF, although it decreased scar size.26 Based on Phase II results,28 MACE are the primary endpoint of the DREAM-HF trial, a Phase III study of MSCs in HF.41 Preliminary reports indicate that BM MSCs reduced MACE in DREAM-HF.42 The mechanism whereby MSCs and CPCs may improve clinical outcomes without improving LV function or reducing scar size requires further investigation. Potential mechanisms include the paracrine actions of these cells via the secretion of anti-inflammatory, immunomodulatory, antifibrotic, antiapoptotic, and proangiogenic factors3,7,41 or improvement in endothelial function,41 all of which would be expected to be beneficial in HF, where chronic low-grade inflammation, progressive deposition of interstitial collagen, increased apoptosis, reduced vasculogenesis, and endothelial dysfunction are thought to play an important role in the progression of the disease.41,43,44
CONCERT-HF is the first clinical trial of c-kit+ CPCs produced by a cell manufacturing facility according to GMP under strict quality control standards. The marked reduction in HF-MACE observed in patients treated with CPCs (either alone or together with MSCs) is encouraging and provides a rationale for continued investigation of these cells. As reviewed elsewhere,13 despite scientific misconduct in one laboratory,40 at least 50 studies by more than 25 independent groups in various animal models of ischaemic cardiomyopathy have shown improvement in LV function and/or structure after administration of CPCs, providing robust evidence for therapeutic efficacy at the pre-clinical level.
Although the combination of MSCs and CPCs did not confirm pre-clinical studies showing additive effects with respect to LV function or structure,20,32 it was associated with the best overall results in terms of clinical outcomes, i.e. HF-MACE and quality of life (as measured by the MLHFQ score). The effects of CPCs and MSCs appear to be complementary. CPCs alone were associated with reduced HF-MACE but not with improved quality of life, whereas MSCs alone were associated with increased quality of life but not with a reduction in HF-MACE. The combination of MSCs and CPCs was associated not only with reduced HF-MACE (as shown by the HR), but also with improved quality of life (Graphical Abstract). Furthermore, patients receiving this combination had improved MLHFQ score both at 6 and 12 months, whereas those receiving MSCs alone improved only at 6 months (Figure 3B). This report provides initial evidence that combining two different cell products results in a better outcome than either product alone. The present findings provide a rationale for further studies of combinatorial cell therapy.
CONCERT-HF demonstrates the safety and feasibility of MRI in patients with MRI-conditional and non-conditional cardiac devices, a finding that may have broad applications that transcend the field of cell therapy. Although 81% of patients had a cardiac device present at baseline (MRI non-conditional in most cases), we were able to obtain measurements of LV volumes in 98% of the scans at both 6 and 12 months and measurements of scar size and viable mass in ∼80% of the scans at both time points. So far, most HF trials have not utilized MRI in patients with cardiac devices. The fact that MRI was used safely and effectively in the vast majority of these patients in CONCERT-HF should promote widespread adoption of MRI in future clinical trials of HF.
CONCERT-HF has a number of limitations. First, due to the complexity and cost of the study, the groups are relatively small. As discussed above, the number of patients enrolled was sufficient to achieve adequate power for MRI-based measurements. Second, of the 125 patients randomized, 15 did not receive SPI and 20 received a treatment different from that to which they had been randomized, reflecting the complexity of the protocol and the challenges associated with cell expansion and shipment. However, the results of the as-treated analysis were similar to those of the intention-to-treat analysis. The experience of CONCERT-HF will be useful for future studies. Third, since multiple endpoints spanning several domains (MACE, quality of life, cardiac function and structure, functional capacity, biomarkers) were examined with no adjustment for multiple comparisons, the findings are not conclusive but, rather, hypothesis generating for future trials. The reason for this design was the exploratory nature of the study, as the effects of the combination of CPCs + MSCs, have never been tested before. As discussed previously,36 the role of a Phase II study is to broadly survey the possible benefits of the study product. Phase II studies do not definitely confirm benefit but rather identify its first signal, providing a rationale for larger confirmatory Phase III trials.36 In CONCERT-HF, the exploration of multiple endpoints in several key domains provides a wealth of information on candidate outcomes for further study. While many of these endpoints were not significant, we highlight the promising ones that are perhaps worth further investigation. The apparent reduction in HF-MACE in patients randomized to CPCs alone or CPCs + MSCs is corroborated by the results of the as-treated analysis (online supplementary Table S5); if this reduction is confirmed in Phase III trials, it would be a significant advance. In conclusion, the CONCERT-HF trial suggests that a single administration of autologous CPCs or MSCs in patients with chronic ischaemic HF shows promising effects, namely, reduction in hospitalization for HF and improved quality of life, respectively, over the ensuing 12 months. The most promising overall results with respect to these outcomes were observed when MSCs were combined with CPCs. Further research will be needed to elucidate the mechanism(s) underlying these salubrious effects, because they were not accompanied by improved LV function or reduced scar size. Nevertheless, the results of this Phase II study provide a rationale for additional clinical trials of cell therapy in chronic ischaemic HF.
Supplementary Material
Acknowledgements
We wish to dedicate this manuscript to the memory of Dr. James T. Willerson, who provided guidance and was a source of inspiration to the Network for 13 years. Also, we thank the NHLBI Data and Safety Monitoring Board for their review and guidance of the CONCERT-HF trial. We wish to acknowledge our dear friend and colleague, the late Omaima Malik, for her integral role in cell manufacturing.
The Cardiovascular Cell Therapy Research Network would like to extend its appreciation to the research coordinators and staff who made this trial possible: Jennifer Chambers, James Chen, Nichole Piece, Stephanie Wagner, Jacob Izquierdo, Jennifer Parenti, Sarah Long, Mohammed Mohammed, Eileen Handberg, Nicole Bostick, Dana Leach, Jane Fox, Jacob Jenson, Patti Mitchell, JoAnne Goldman, Jairo Tovar, Russel Saltzman, Mayra Vidro, Lina Caceres, Vela Karakeshishyan, Darcy DiFede, Fouzia Khan, Banu Priya, Teri Strickland, Annie Thorpe, Heidi Wilson, Julie Caswell, Shari Stidham, and Sibi Mathew.
Funding
This work was supported by National Institutes of Health [HL087318, HL113530, HL113460, HL087394, HL113457, HL087366, HL087365].
All investigators received funding from the NIH National Heart, Lung, and Blood Institute (NHLBI) for conduct of the CONCERT-HF trial through the Cardiovascular Cell Therapy Research Network (CCTRN). R.F.E. is a staff member of the National Heart, Lung, and Blood Institute (NHLBI), the source of funding for the CONCERT-HF trial. The views expressed in this article are those of the authors and do not necessarily represent the views of the NHLBI, National Institutes of Health, or the United States Department of Health and Human Services. I.H.S. is currently a staff member of the National Institute of Diabetes and Digestive, and Kidney Diseases (NIDDK). The views expressed in this article are those of the authors and do not necessarily represent the views of the NIDDK, National Institutes of Health, or the United States Department of Health and Human Services. J.M.H. is a scientific co-founder and holds equity in Vestion, Inc. Vestion did not fund or participate in this study. Dr. Hare’s role in Vestion has been disclosed to the University of Miami and to the steering committee of the CCTRN, and appropriate management plans have been instituted. T.D.H. has served as a consultant for Biosense Webster. C.J.P. has served as a consultant for XyloCor, Caladrius, Imbria, and Biocardia; and has grants with Adelphi Values, Brigham and Women’s Hospital, Department of Defense, Gilead Sciences, Inc., McJunkin Foundation, Mesoblast, and Sanofi US. E.C.P. has served as a consultant for Mesoblast. D.A.T. is co-founder of Stem Cell Security. P.C.Y. has served as a consultant for Terumo.
Footnotes
Data availability
The data underlying this article will be available in BioLINCC (Biologic Specimen and Data Repository Information Coordinating Center at www.biolincc.nhlbi.nih.gov in 2021.
Supplementary Information
Additional supporting information may be found online in the Supporting Information section at the end of the article.
Conflict of interest:
All other authors have nothing to disclose.
References
- 1.Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Delling FN, Djousse L, Elkind MS, Ferguson JF, Fornage M, Khan SS, Kissela BM, Knutson KL, Kwan TW, Lackland DT, Lewis TT, Lichtman JH, Longenecker CT, Loop MS, Lutsey PL, Martin SS, Matsushita K, Moran AE, Mussolino ME, Perak AM, Rosamond WD, Roth GA, Sampson UKA, Satou GM, Schroeder EB, Shah SH, Shay CM, Spartano NL, Stokes A, Tirschwell DL, VanWagner LB, Tsao; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics – 2020 update: a report from the American Heart Association. Circulation 2020;141:e139–e596. [DOI] [PubMed] [Google Scholar]
- 2.Mathiasen AB, Qayyum AA, Jorgensen E, Helqvist S, Fischer-Nielsen A, Kofoed KF, Haack-Sorensen M, Ekblond A, Kastrup J. Bone marrow-derived mesenchymal stromal cell treatment in patients with severe ischaemic heart failure: a randomized placebo-controlled trial (MSC-HF trial). Eur Heart J 2015;36:1744–1753. [DOI] [PubMed] [Google Scholar]
- 3.Banerjee MN, Bolli R, Hare JM. Clinical studies of cell therapy in cardiovascular medicine: recent developments and future directions. Circ Res 2018;123:266–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patel AN, Henry TD, Quyyumi AA, Schaer GL, Anderson RD, Toma C, East C, Remmers AE, Goodrich J, Desai AS, Recker D, DeMaria A; ixCELL-DCM Investigators. Ixmyelocel-T for patients with ischaemic heart failure: a prospective randomised double-blind trial. Lancet 2016;387:2412–2421. [DOI] [PubMed] [Google Scholar]
- 5.Fernández-Avilés F, Sanz-Ruiz R, Climent AM, Badimon L, Bolli R, Charron D, Fuster V, Janssens S, Kastrup J, Kim HS, Lüscher TF, Martin JF, Menasché P, Simari RD, Stone GW, Terzic A, Willerson JT, Wu JC; TACTICS (Transnational Alliance for Regenerative Therapies in Cardiovascular Syndromes) Writing Group, Authors/Task Force Members. Chairpersons, Basic Research Subcommittee, Translational Research Subcommittee, Challenges of Cardiovascular Regenerative Medicine Subcommittee, Tissue Engineering Subcommittee, Delivery, Navigation, Tracking and Assessment Subcommittee, Clinical Trials Subcommittee, Regulatory and funding strategies subcommittee, Delivery, Navigation, Tracking and Assessment Subcommittee. Global position paper on cardiovascular regenerative medicine. Eur Heart J 2017;38:2532–2546.28575280 [Google Scholar]
- 6.Mathur A, Fernández-Avilés F, Dimmeler S, Hauskeller C, Janssens S, Menasche P, Wojakowski W, Martin JF, Zeiher A; BAMI Investigators. The consensus of the Task Force of the European Society of Cardiology concerning the clinical investigation of the use of autologous adult stem cells for the treatment of acute myocardial infarction and heart failure: update 2016. Eur Heart J 2017;38:2930–2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wysoczynski M, Khan A, Bolli R. New paradigms in cell therapy: repeated dosing, intravenous delivery, immunomodulatory actions, and new cell types. Circ Res 2018;123:138–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sanganalmath SK, Bolli R. Cell therapy for heart failure: a comprehensive overview of experimental and clinical studies, current challenges, and future directions. Circ Res 2013;113:810–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keith MC, Bolli R. “String theory” of c-kit(pos) cardiac cells: a new paradigm regarding the nature of these cells that may reconcile apparently discrepant results. Circ Res 2015;116:1216–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tokita Y, Tang XL, Li Q, Wysoczynski M, Hong KU, Nakamura S, Wu WJ, Xie W, Li D, Hunt G, Ou Q, Stowers H, Bolli R. Repeated administrations of cardiac progenitor cells are markedly more effective than a single administration: a new paradigm in cell therapy. Circ Res 2016;119:635–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tang XL, Li Q, Rokosh G, Sanganalmath SK, Chen N, Ou Q, Stowers H, Hunt G, Bolli R. Long-term outcome of administration of c-kit(POS) cardiac progenitor cells after acute myocardial infarction: transplanted cells do not become cardiomyocytes, but structural and functional improvement and proliferation of endogenous cells persist for at least one year. Circ Res 2016;118:1091–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hong KU, Guo Y, Li QH, Cao P, Al-Maqtari T, Vajravelu BN, Du J, Book MJ, Zhu X, Nong Y, Bhatnagar A, Bolli R. c-kit+ cardiac stem cells alleviate post-myocardial infarction left ventricular dysfunction despite poor engraftment and negligible retention in the recipient heart. PLoS One 2014;9:e96725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bolli R, Tang XL, Guo Y, Li QH. After the storm: an objective appraisal of the efficacy of c-kit+ cardiac progenitor cells in preclinical models of heart disease. Can J Physiol Pharmacol 2021;99:129–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karantalis V, Hare JM. Use of mesenchymal stem cells for therapy of cardiac disease. Circ Res 2015;116:1413–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schuleri KH, Amado LC, Boyle AJ, Centola M, Saliaris AP, Gutman MR, Hatzistergos KE, Oskouei BN, Zimmet JM, Young RG, Heldman AW, Lardo AC, Hare JM. Early improvement in cardiac tissue perfusion due to mesenchymal stem cells. Am J Physiol Heart Circ Physiol 2008;294:H2002–H2011. [DOI] [PubMed] [Google Scholar]
- 16.Amado LC, Saliaris AP, Schuleri KH, St John M, Xie JS, Cattaneo S, Durand DJ, Fitton T, Kuang JQ, Stewart G, Lehrke S, Baumgartner WW, Martin BJ, Heldman AW, Hare JM. Cardiac repair with intramyocardial injection of allogeneic mesenchymal stem cells after myocardial infarction. Proc Natl Acad Sci U S A 2005;102:11474–11479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Amado LC, Schuleri KH, Saliaris AP, Boyle AJ, Helm R, Oskouei B, Centola M, Eneboe V, Young R, Lima JA, Lardo AC, Heldman AW, Hare JM. Multimodality noninvasive imaging demonstrates in vivo cardiac regeneration after mesenchymal stem cell therapy. J Am Coll Cardiol 2006;48:2116–2124. [DOI] [PubMed] [Google Scholar]
- 18.Schuleri KH, Feigenbaum GS, Centola M, Weiss ES, Zimmet JM, Turney J, Kellner J, Zviman MM, Hatzistergos KE, Detrick B, Conte JV, McNiece I, Steenbergen C, Lardo AC, Hare JM. Autologous mesenchymal stem cells produce reverse remodelling in chronic ischaemic cardiomyopathy. Eur Heart J 2009;30:2722–2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Quevedo HC, Hatzistergos KE, Oskouei BN, Feigenbaum GS, Rodriguez JE, Valdes D, Pattany PM, Zambrano JP, Hu Q, McNiece I, Heldman AW, Hare JM. Allogeneic mesenchymal stem cells restore cardiac function in chronic ischemic cardiomyopathy via trilineage differentiating capacity. Proc Natl Acad Sci U S A 2009;106:14022–14027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Williams AR, Hatzistergos KE, Addicott B, McCall F, Carvalho D, Suncion V, Morales AR, Da Silva J, Sussman MA, Heldman AW, Hare JM. Enhanced effect of combining human cardiac stem cells and bone marrow mesenchymal stem cells to reduce infarct size and to restore cardiac function after myocardial infarction. Circulation 2013;127:213–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bao L, Meng Q, Li Y, Deng S, Yu Z, Liu Z, Zhang L, Fan H. C-kit positive cardiac stem cells and bone marrow-derived mesenchymal stem cells synergistically enhance angiogenesis and improve cardiac function after myocardial infarction in a paracrine manner. J Card Fail 2017;23:403–415. [DOI] [PubMed] [Google Scholar]
- 22.Zwetsloot PP, Végh AM, Jansen of Lorkeers SJ, van Hout GP, Currie GL, Sena ES, Gremmels H, Buikema JW, Goumans MJ, Macleod MR, Doevendans PA, Chamuleau SA, Sluijter JP. Cardiac stem cell treatment in myocardial infarction: a systematic review and meta-analysis of preclinical studies. Circ Res 2016;118:1223–1232. [DOI] [PubMed] [Google Scholar]
- 23.Hare JM, Traverse JH, Henry TD, Dib N, Strumpf RK, Schulman SP, Gerstenblith G, DeMaria AN, Denktas AE, Gammon RS, Hermiller JB Jr, Reisman MA, Schaer GL, Sherman W. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J Am Coll Cardiol 2009;54:2277–2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hare JM, Fishman JE, Gerstenblith G, Difede Velazquez DL, Zambrano JP, Suncion VY, Tracy M, Ghersin E, Johnston PV, Brinker JA, Breton E, Davis-Sproul J, Schulman IH, Byrnes J, Mendizabal AM, Lowery MH, Rouy D, Altman P, Wong Po Foo C, Ruiz P, Amador A, Da Silva J, McNiece IK, Heldman AW. Comparison of allogeneic vs autologous bone marrow-derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: the POSEIDON randomized trial. JAMA 2012;308:2369–2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karantalis V, Difede DL, Gerstenblith G, Pham S, Symes J, Zambrano JP, Fishman J, Pattany P, McNiece I, Conte J, Schulman S, Wu K, Shah A, Breton E, Davis-Sproul J, Schwarz R, Feigenbaum G, Mushtaq M, Suncion VY, Lardo AC, Borrello I, Mendizabal A, Karas TZ, Byrnes J, Lowery M, Heldman AW, Hare JM. Autologous mesenchymal stem cells produce concordant improvements in regional function, tissue perfusion, and fibrotic burden when administered to patients undergoing coronary artery bypass grafting: the Prospective Randomized Study of Mesenchymal Stem Cell Therapy in Patients Undergoing Cardiac Surgery (PROMETHEUS) trial. Circ Res 2014;114:1302–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heldman AW, DiFede DL, Fishman JE, Zambrano JP, Trachtenberg BH, Karantalis V, Mushtaq M, Williams AR, Suncion VY, McNiece IK, Ghersin E, Soto V, Lopera G, Miki R, Willens H, Hendel R, Mitrani R, Pattany P, Feigenbaum G, Oskouei B, Byrnes J, Lowery MH, Sierra J, Pujol MV, Delgado C, Gonzalez PJ, Rodriguez JE, Bagno LL, Rouy D, Altman P, Foo CW, da Silva J, Anderson E, Schwarz R, Mendizabal A, Hare JM. Transendocardial mesenchymal stem cells and mononuclear bone marrow cells for ischemic cardiomyopathy: the TAC-HFT randomized trial. JAMA 2014;311:62–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Williams AR, Trachtenberg B, Velazquez DL, McNiece I, Altman P, Rouy D, Mendizabal AM, Pattany PM, Lopera GA, Fishman J, Zambrano JP, Heldman AW, Hare JM. Intramyocardial stem cell injection in patients with ischemic cardiomyopathy: functional recovery and reverse remodeling. Circ Res 2011;108:792–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perin EC, Borow KM, Silva GV, DeMaria AN, Marroquin OC, Huang PP, Traverse JH, Krum H, Skerrett D, Zheng Y, Willerson JT, Itescu S, Henry TD. A phase II dose-escalation study of allogeneic mesenchymal precursor cells in patients with ischemic or nonischemic heart failure. Circ Res 2015;117:576–584. [DOI] [PubMed] [Google Scholar]
- 29.Henry TD, Traverse JH, Hammon BL, East CA, Bruckner B, Remmers AE, Recker D, Bull DA, Patel AN. Safety and efficacy of ixmyelocel-T: an expanded, autologous multi-cellular therapy, in dilated cardiomyopathy. Circ Res 2014;115:730–737. [DOI] [PubMed] [Google Scholar]
- 30.van Berlo JH, Kanisicak O, Maillet M, Vagnozzi RJ, Karch J, Lin SC, Middleton RC, Marbán E, Molkentin JD. C-kit+ cells minimally contribute cardiomyocytes to the heart. Nature 2014;509:337–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hatzistergos KE, Quevedo H, Oskouei BN, Hu Q, Feigenbaum GS, Margitich IS, Mazhari R, Boyle AJ, Zambrano JP, Rodriguez JE, Dulce R, Pattany PM, Valdes D, Revilla C, Heldman AW, McNiece I, Hare JM. Bone marrow mesenchymal stem cells stimulate cardiac stem cell proliferation and differentiation. Circ Res 2010;107:913–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karantalis V, Suncion-Loescher VY, Bagno L, Golpanian S, Wolf A, Sanina C, Premer C, Kanelidis AJ, McCall F, Wang B, Balkan W, Rodriguez J, Rosado M, Morales A, Hatzistergos K, Natsumeda M, Margitich I, Schulman IH, Gomes SA, Mushtaq M, DiFede DL, Fishman JE, Pattany P, Zambrano JP, Heldman AW, Hare JM. Synergistic effects of combined cell therapy for chronic ischemic cardiomyopathy. J Am Coll Cardiol 2015;66:1990–1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bolli R, Hare JM, March KL, Pepine CJ, Willerson JT, Perin EC, Yang PC, Henry TD, Traverse JH, Mitrani RD, Khan A, Schulman IH, Taylor DA, DiFede DL, Lima JA, Chugh AR, Loughran J, Vojvodic RW, Sayre SL, Bettencourt J, Cohen M, Moye L, Ebert RF, Simari RD. Rationale and design of the CONCERT-HF (Combination Of meseNchymal and c-kit(+) Cardiac stEm cells as Regenerative Therapy for Heart Failure) trial. Circ Res 2018;122:1703–1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hicks KA, Tcheng JE, Bozkurt B, Chaitman BR, Cutlip DE, Farb A, Fonarow GC, Jacobs JP, Jaff MR, Lichtman JH, Limacher MC, Mahaffey KW, Mehran R, Nissen SE, Smith EE, Targum SL. 2014 ACC/AHA key data elements and definitions for cardiovascular endpoint events in clinical trials: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Data Standards (Writing Committee to Develop Cardiovascular Endpoints Data Standards). J Am Coll Cardiol 2015;66:403–469. [DOI] [PubMed] [Google Scholar]
- 35.Hicks KA, Mahaffey KW, Mehran R, Nissen SE, Wiviott SD, Dunn B, Solomon SD, Marler JR, Teerlink JR, Farb A, Morrow DA, Targum SL, Sila CA, Thanh Hai MT, Jaff MR, Joffe HV, Cutlip DE, Desai AS, Lewis EF, Gibson CM, Landray MJ, Lincoff AM, White CJ, Brooks SS, Rosenfield K, Domanski MJ, Lansky AJ, McMurray JJV, Tcheng JE, Steinhubl SR, Burton P, Mauri L, O’Connor CM, Pfeffer MA, Hung HMJ, Stockbridge NL, Chaitman BR, Temple RJ; Standardized Data Collection for Cardiovascular Trials Initiative (SCTI). 2017 Cardiovascular and stroke endpoint definitions for clinical trials. J Am Coll Cardiol 2018;71: 1021–1034. [DOI] [PubMed] [Google Scholar]
- 36.Hare JM, Bolli R, Cooke JP, Gordon DJ, Henry TD, Perin EC, March KL, Murphy MP, Pepine CJ, Simari RD, Skarlatos SI, Traverse JH, Willerson JT, Szady AD, Taylor DA, Vojvodic RW, Yang PC, Moye LA; Cardiovascular Cell Therapy Research Network (CCTRN). Phase II clinical research design in cardiology: learning the right lessons too well: observations and recommendations from the Cardiovascular Cell Therapy Research Network (CCTRN). Circulation 2013;127: 1630–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Traverse JH, Henry TD, Pepine CJ, Willerson JT, Zhao DX, Ellis SG, Forder JR, Anderson RD, Hatzopoulos AK, Penn MS, Perin EC, Chambers J, Baran KW, Raveendran G, Lambert C, Lerman A, Simon DI, Vaughan DE, Lai D, Gee AP, Taylor DA, Cogle CR, Thomas JD, Olson RE, Bowman S, Francescon J, Geither C, Handberg E, Kappenman C, Westbrook L, Piller LB, Simpson LM, Baraniuk S, Loghin C, Aguilar D, Richman S, Zierold C, Spoon DB, Bettencourt J, Sayre SL, Vojvodic RW, Skarlatos SI, Gordon DJ, Ebert RF, Kwak M, Moye LA, Simari RD; Cardiovascular Cell Therapy Research Network (CCTRN). Effect of the use and timing of bone marrow mononuclear cell delivery on left ventricular function after acute myocardial infarction: the TIME randomized trial. JAMA 2012;308:2380–2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Traverse JH, Henry TD, Ellis SG, Pepine CJ, Willerson JT, Zhao DX, Forder JR, Byrne BJ, Hatzopoulos AK, Penn MS, Perin EC, Baran KW, Chambers J, Lambert C, Raveendran G, Simon DI, Vaughan DE, Simpson LM, Gee AP, Taylor DA, Cogle CR, Thomas JD, Silva GV, Jorgenson BC, Olson RE, Bowman S, Francescon J, Geither C, Handberg E, Smith DX, Baraniuk S, Piller LB, Loghin C, Aguilar D, Richman S, Zierold C, Bettencourt J, Sayre SL, Vojvodic RW, Skarlatos SI, Gordon DJ, Ebert RF, Kwak M, Moye LA, Simari RD; Cardiovascular Cell Therapy ResearchNetwork. Effect of intracoronary delivery of autologous bone marrow mononuclear cells 2 to 3 weeks following acute myocardial infarction on left ventricular function: the LateTIME randomized trial. JAMA 2011;306:2110–2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Surder D, Manka R, Moccetti T, Lo Cicero V, Emmert MY, Klersy C, Soncin S, Turchetto L, Radrizzani M, Zuber M, Windecker S, Moschovitis A, Buhler I, Kozerke S, Erne P, Luscher TF, Corti R. Effect of bone marrow-derived mononuclear cell treatment, early or late after acute myocardial infarction: twelve months CMR and long-term clinical results. Circ Res 2016;119:481–490. [DOI] [PubMed] [Google Scholar]
- 40.Retraction Watch. Harvard and the Brigham recommend 31 retractions for cardiac stem cell work. October 14, 2018. https://retractionwatch.com/2018/10/14/harvard-and-the-brigham-recommend-31-retractions-for-cardiac-stemcell-work/ (3 April 2021).
- 41.Borow KM, Yaroshinsky A, Greenberg B, Perin EC. Phase 3 DREAM-HF trial of mesenchymal precursor cells in chronic heart failure. Circ Res 2019;125:265–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pharmabiz.com Mesoblast announces positive results from phase 3 DREAM-HF trial of rexlemestrocel-L to treat chronic heart failure. December 16, 2020. http://www.pharmabiz.com/NewsDetails.aspx?aid=134180&sid=2 (3 April 2021).
- 43.Murphy SP, Kakkar R, McCarthy CP, Januzzi JL Jr. Inflammation in heart failure: JACC state-of-the-art review. J Am Coll Cardiol 2020;75:1324–1340. [DOI] [PubMed] [Google Scholar]
- 44.Frangogiannis NG, Kovacic JC. Extracellular matrix in ischemic heart disease, part 4/4: JACC focus seminar. J Am Coll Cardiol 2020;75:2219–2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.