Abstract
The accumulation of reactive oxygen species (ROS) is a widespread defence mechanism in higher plants against pathogen attack and sometimes is the cause of cell death that facilitates attack by necrotrophic pathogens. Plant pathogens use superoxide dismutase (SOD) to scavenge ROS derived from their own metabolism or generated from host defence. The significance and roles of SODs in the vascular plant pathogen Verticillium dahliae are unclear. Our previous study showed a significant upregulation of Cu/Zn‐SOD1 (VdSOD1) in cotton tissues following V. dahliae infection, suggesting that it may play a role in pathogen virulence. Here, we constructed VdSOD1 deletion mutants (ΔSOD1) and investigated its function in scavenging ROS and promoting pathogen virulence. ΔSOD1 had normal growth and conidiation but exhibited significantly higher sensitivity to the intracellular ROS generator menadione. Despite lacking a signal peptide, assays in vitro by western blot and in vivo by confocal microscopy revealed that secretion of VdSOD1 is dependent on the Golgi reassembly stacking protein (VdGRASP). Both menadione‐treated ΔSOD1 and cotton roots infected with ΔSOD1 accumulated more and less H2O2 than with the wildtype strain. The absence of a functioning VdSOD1 significantly reduced symptom severity and pathogen colonization in both cotton and Nicotiana benthamiana. VdSOD1 is nonessential for growth or viability of V. dahliae, but is involved in the detoxification of both intracellular ROS and host‐generated extracellular ROS, and contributes significantly to virulence in V. dahliae.
Keywords: reactive oxygen species, ROS detoxification, superoxide dismutase, unconventional secretion, Verticillium dahliae, virulence
Verticillium dahliae Cu/Zn superoxide dismutase detoxifies self‐ and host‐generated ROS to maximize virulence.
1. INTRODUCTION
Plant‐pathogenic microbes produce reactive oxygen species (ROS) as by‐products of normal metabolism, but the burden of ROS is amplified when these microbes encounter ROS from host defences. The deleterious effects of excess ROS are well documented in the literature, including its damage to macromolecules such as the DNA, proteins, and lipids of pathogens (Apel & Hirt, 2004). ROS also act as signalling molecules that trigger a variety of downstream plant defence responses, including synthesis of pathogenesis‐related proteins and phytoalexins, and modifications to the cell wall (Segal & Wilson, 2018). Furthermore, ROS in the host cells immediately affected by the pathogen can lead to a programmed cell death type mechanism commonly referred to as the hypersensitive response (HR), an extreme measure to limit pathogen spread following successful infection (Lamb & Dixon, 1997).
To scrub excess ROS in host cells generated during infection, pathogens have evolved a complex scavenging system, including nonenzymatic (small molecule) and enzymatic scavenging systems, such as superoxide dismutases (SODs), catalases, and peroxidases (Broxton & Culotta, 2016). Cu/Zn‐SOD1 is an important member of the SOD gene family harbouring cofactor Cu2+ and Zn2+ (Zelko et al., 2002). Functional analyses of fungal Cu/Zn‐SOD1s, in Botrytis cinerea, Sclerotinia sclerotiorum, and Fusarium graminearum, have elucidated their roles in the detoxification of ROS and pathogenesis (Rolke et al., 2004; Xu & Chen, 2013; Yao et al., 2016). However, the deletion of Cu/Zn‐SOD1 in Claviceps purpurea and Aspergillus fumigatus did not alter the virulence of these pathogens (Lambou et al., 2010; Moore et al., 2002). These contrasting results suggest that Cu/Zn‐SOD1 serve divergent functions in different microbes.
Secreted proteins of fungal pathogens typically harbour an amino‐terminal signal peptide, which mediates secretion via the endoplasmic reticulum (ER)‐Golgi pathway.
Cu/Zn‐SOD1 is generally not known to possess a signal peptide and localizes in the cytoplasm to contribute to cytosolic SOD activities (Briones‐Martin‐del‐Campo et al., 2015). Previous studies have found that Cu/Zn‐SOD1 is a metalloenzyme that catalyses superoxide radicals () into less toxic ROS products (H2O2) (Sheng et al., 2014). However, cytoplasmic localization cannot offer a reasonable explanation for the involvement of Cu/Zn‐SOD1 in the detoxification of extracellular ROS produced by the host (Lopez‐Cruz et al., 2017). Increasing evidence suggests that the Cu/Zn‐SOD1 may also be localized to extracellular space. For instance, S. sclerotiorum SsSOD1 detoxifies extracellular ROS during host–pathogen interactions and is probably secreted through alternative secretion pathways, although clear evidence for this currently does not exist (Xu & Chen, 2013). In Saccharomyces cerevisiae, a signal‐peptide‐lacking Cu/Zn‐SOD1 has been shown to be secreted unconventionally through the ER‐Golgi independent secretory pathway. This unconventional secretion pathway depends on the Golgi reassembly stacking proteins (GRASP), which initially were thought to be required for the stacking of Golgi cisternae but were subsequently shown to play a role in molecular secretion (Kinseth et al., 2007). Whether GRASP is involved in signal‐peptide‐lacking protein secretion in filamentous fungi is currently unexplored.
Verticillium dahliae is a destructive phytopathogenic fungus that causes Verticillium wilt on more than 200 plant species worldwide, including many economically important crops (Inderbitzin & Subbarao, 2014; Klosterman et al., 2009). Recently, numerous proteins involved in pathogenicity have been identified in V. dahliae (de Jonge et al., 2012; Liu et al., 2013; Qin et al., 2018). Furthermore, pathogen‐secreted proteins such as VdNLP1 and VdNLP2 (Zhou et al., 2012), PevD1 (Wang et al., 2012), VdEG1/3 and VdCUT11 (Gui et al., 2017, 2018) and VdSCP7 (Zhang et al., 2017) have been demonstrated to induce host ROS accumulation. In addition, the generation of H2O2 was detected in cotton roots following infection by V. dahliae (Xie et al., 2013). A histone‐fold protein VdDpb4 was shown to play an important role in maintaining a more accessible chromatin landscape, while its interacting protein, VdIsw2, an ATP‐dependent chromatin‐remodelling factor, played an antagonistic role in balancing chromatin structure in response to ROS (Wang et al., 2020). This chromatin remodelling facilitates the DNA damage repair in response to plant ROS stress. However, the role of V. dahliae SODs in scavenging excess ROS and pathogenicity has not been elucidated.
In our previous study, the exoproteome of V. dahliae in liquid culture medium containing powdered cotton tissue contained an abundance of upregulated proteins. Among the 271 pathogenesis‐related proteins that were induced, a Cu/Zn‐SOD1 (VdSOD1) without a signal‐peptide was upregulated 3.95‐fold (Chen et al., 2016), suggesting that it may be functional during V. dahliae–host interactions. Therefore, the main objectives of the current study were to (a) determine the role of VdSOD1 in detoxifying intracellular ROS produced by V. dahliae itself as well as host‐generated ROS during plant infection, (b) explore whether signal‐peptide‐lacking VdSOD1 is secreted extracellularly and the characteristics of the secretory pathway, and (c) determine the role of VdSOD1 in the virulence of V. dahliae.
2. RESULTS
2.1. VdSOD1 encodes a signal‐peptide‐lacking Cu‐Zn superoxide dismutase
Proteomic analysis of the V. dahliae Vd991 extracellular proteins revealed a putative superoxide dismutase VdSOD1 (VEDA_03436, Gene‐ID in VdLs.17 genome: VDAG_02630) (Chen et al., 2018; Klosterman et al., 2011). The 963 bp full‐length VdSOD1 gene contains a 465 bp open reading frame (ORF), including three introns of 323, 86, and 89 bp (Figures 1a and S1). The ORF encodes a polypeptide of 154 amino acids with a predicted molecular weight of 15.85 kDa and a predicted isoelectric point of 6.41. The amino acid alignment of VdSOD1 with other fungal Cu/Zn‐SOD1s displayed high homology between fungi, with 83% identity to Fusarium oxysporum FoSOD1 and 70% to S. cerevisiae Cu/Zn‐SOD1 (Figure 1b). VdSOD1 has two typical copper/zinc superoxide dismutase signatures and Cu/Zn‐binding residues (with H47, H49, H64, and H121 for Cu2+ binding, and H64, H72, H81, and D84 for Zn2+ binding), two cysteines (C58 and C147) for disulphide bond formation, and an active site (R144) for substrate introduction, indicating VdSOD1 is a typical Cu/Zn‐SOD (Figure 1b). Sequence analysis with SignalP v. 5.0 predicted no signal peptide in the N‐terminus of VdSOD1 (Figure S2). However, VdSOD1 was predicted to be a nonconventional secreted protein by SecretomeP v. 2.0 with SecP scores of 0.87, which is above the threshold of 0.5 (Figure S2). Moreover, VdSOD1 contains a short, conserved peptide (amino acid: GAPSDEDR) (Figure 1b), which is responsible for unconventional secretion of SOD1 in S. cerevisiae (Cruz‐Garcia et al., 2017). These results suggested that VdSOD1 most probably encodes a signal‐peptide‐lacking Cu/Zn‐SOD that is probably secreted through an unconventional secretion pathway.
FIGURE 1.
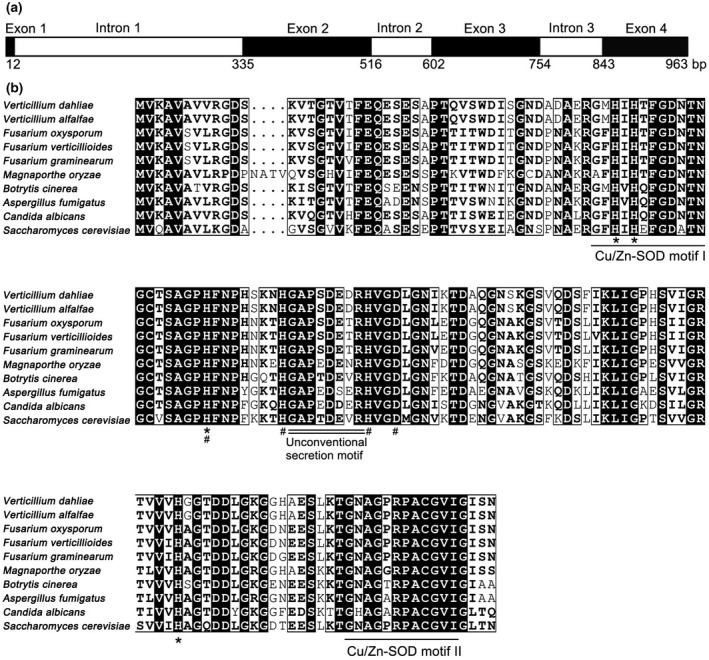
Bioinformatic analysis of VdSOD1. (a) Structure of VdSOD1. Exons are black boxes and introns are white boxes. (b) Alignment of VdSOD1 and homologs from other fungi. Highly conserved residues are marked by black boxes. The Cu/Zn‐SOD motifs are underlined, Cu2+ and Zn2+ binding sites are marked by asterisks and hashtags, respectively. The unconventional secretion motif is double‐underlined. GenBank accession number of aligned sequences are Verticillium dahliae (VEDA_03436), Verticillium alfalfae (XP_003007974.1), Fusarium oxysporum (EGU80493.1), Fusarium verticillioides (EWG38836.1), Fusarium graminearum (CEF77131.1), Magnaporthe oryzae (XP_003721165.1), Botrytis cinerea (XP_001560530.1), Aspergillus fumigatus (Q9Y8D9.3), Candida albicans (EEQ45246.1), and Saccharomyces cerevisiae (NP_012638.1)
2.2. VdSOD1 is dispensable for normal vegetative growth and conidia production
To study the function of VdSOD1, gene knockout mutants were constructed by replacing the coding sequence in the wildtype strain with a hygromycin resistance cassette via homologous recombination. Deletion of the VdSOD1 ORF was confirmed by PCR in all transformants analysed and two of them (ΔSOD1‐1 and ΔSOD1‐2) were arbitrarily selected for further characterization (Figure S3a). Complemented strains (EC‐1 and EC‐2) were generated by reintroducing VdSOD1 with its native promoter and a neomycin resistance gene in ΔSOD1 (Figure S3b), respectively. The radial growth of the ΔSOD1 strains on potato dextrose agar (PDA) was similar to that of the wildtype strain (Figure 2a). In addition, the number of conidia produced by the two VdSOD1‐deletion strains on PDA were similar to that for the wildtype strain (Figure 2c). To test different carbon source utilization capabilities of ∆SOD1, growth rate was monitored on media with sucrose, pectin, cellulose, and xylan as the carbon sources. Wildtype strain Vd991 grew well on all carbon sources tested. Growth rates of mutants were comparable with the wildtype strain on sucrose‐, starch‐, and pectin‐containing media (Figure 2a,b). However, the growth of the ∆SOD1 mutants was restricted on cellulose‐containing media, with a colony diameter that was 84% of that of the wildtype strain (Figure 2a,b). These results indicate that VdSOD1 disruption did not alter vegetative growth and conidial production under normal growth conditions, though it reduced the cellulose utilization capacity of the mutant.
FIGURE 2.
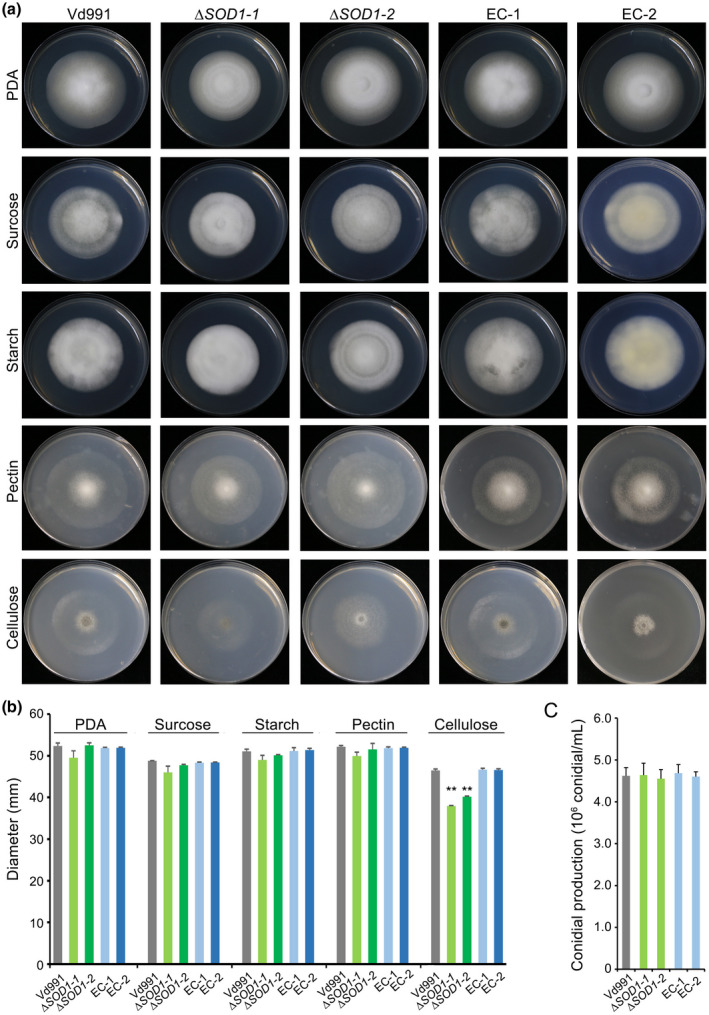
Growth rate, carbon source utilization, and conidia production by VdSOD1 deletion strains. (a) Radial growth of VdSOD1 deletion strains, complemented strains (EC), and wildtype strain Vd991 on potato dextrose agar (PDA) and Czapek medium supplemented with carbon sources sucrose, starch, pectin, or cellulose, respectively, after incubation at 25 °C for 12 days. A 2 μl conidial suspension (5 × 106 conidia/ml) was placed in the centre of the plates as inoculum. (b) Colony diameters on different growth media after 12 days’ incubation. Means and standard deviations from three biological replicates are shown. Double asterisks indicate significant differences at p < 0.01. (c) Conidia production after 7 days’ incubation on PDA. Conidia counts were from 5‐mm‐diameter PDA plugs from the colony edge. Error bars represent the standard deviations of the mean from three independent experiments
2.3. VdSOD1 contributes to scavenging of intracellular superoxide radicals in Verticillium dahliae
Considering that VdSOD1 encodes a Cu/Zn‐SOD without a conventional signal peptide, we evaluated the sensitivity of ΔSOD1 to menadione, an intracellular superoxide‐generating agent (Kawamura et al., 2006). Strains were grown on PDA plates supplemented with various concentrations of menadione. The colony diameter of the wildtype strain on PDA supplemented with 10 and 15 μM menadione was not significantly suppressed and was akin to growth on PDA without menadione at 9 days. In contrast, growth of ∆SOD1 in the same treatments was significantly inhibited, with colony diameters reduced by as much as 35% and 84%, respectively, of those mutants grown without menadione (Figure 3a–d). The growth of ΔSOD1 strains was completely inhibited at 20 μM menadione, whereas the wildtype strain still grew normally (Figure 3a–d). All complemented strains recovered their tolerance to menadione (Figure 3a–d). The total SOD activity of mycelial protein extracts of ΔSOD1 on complete medium (CM) amended with 10 μM menadione was approximately 31% less than that of the wildtype strain and this difference was statistically significant (Figure 3e). In addition, the sensitivity of VdSOD1 to H2O2 was determined in vitro. Deletion of VdSOD1 negated the H2O2 sensitivity such that the colony diameters of the mutant strains were similar on PDA supplemented with 5 and 7 μl H2O2. However, the colony diameters of the wildtype strain and complementary transformants were significantly reduced on media supplemented with 5–7 μl H2O2 (Figure S4). Together, these results demonstrate that VdSOD1 has intracellular SOD activity and contributes to scavenging of intracellular superoxide radicals.
FIGURE 3.
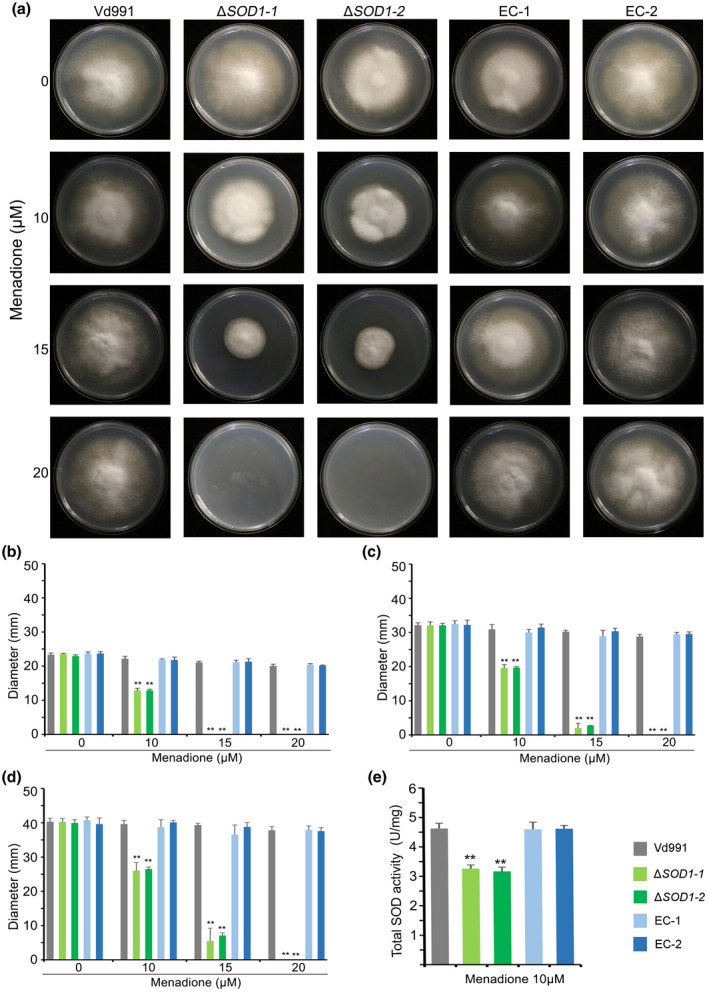
Impact of intracellular superoxide generator menadione on growth rate and superoxide dismutase (SOD) activity. (a) The wildtype strain Vd991, VdSOD1 deletion strains, and the complemented (EC) strains were cultured on potato dextrose agar (PDA) supplemented with menadione at specified concentrations for 9 days. (b–d) Colony diameters on PDA plates containing different concentration of menadione following 5 days (b), 7 days (c), and 9 days (d) of incubation. Means and standard deviations of the mean from three biological replicates are shown. Asterisks (**) denote significant differences (p < 0.01) according to Student's t test. (e) The total SOD activity of mycelial protein extract of the wildtype strain, ΔSOD1 strains, and complemented strains were detected using the nitroblue tetrazolium (NBT) reduction method. All strains were stressed with 10 μM menadione for 24 hr. Means and error bars (represent standard deviation) are from three independent experiments. Asterisks (**) mean significant differences (p < 0.01) according to Student's t test
2.4. VdSOD1 is secreted in V. dahliae
To investigate whether VdSOD1 is secreted as predicted by SecretomeP v. 2.0, a V. dahliae strain (WT::OESOD1) expressing a hemagglutinin (HA)‐tagged VdSOD1 under the constitutive promoter TrpC in a wildtype strain background was constructed. As expected, the expression level of VdSOD1 in WT::OESOD1 was higher than that of the wildtype strain (5.42 ± 0.57‐fold) (Figure S5). Western blotting detected the presence of the VdSOD1‐HA fusion protein. VdSOD1‐HA was detected in both the fungal tissue and the culture filtrate of the WT::OESOD1, and was especially enriched in the culture filtrate owing to the abundance of constitutive protein, glyceraldehyde‐3‐phosphate dehydrogenase (VdGAPDH, a cytoplasmic protein involved in glycolysis, VEDA_05255) (Figure 4a). Moreover, the high accumulation of VdSOD1 in the culture filtrate was not caused by cell disruption because of the abundance of GAPDH detected in the culture filtrate (Figure 4a). These results therefore suggest that VdSOD1 can be secreted into culture medium in vitro.
FIGURE 4.
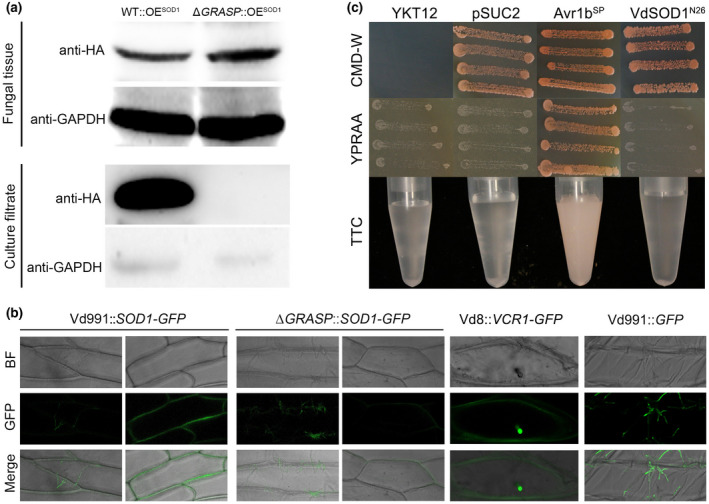
VdSOD1 can be secreted to extracellular spaces and the putative mechanism of secretion. (a) In vitro assay to demonstrate VdSOD1 secretion into culture filtrates is dependent on VdGRASP. Proteins extracted from WT::OESOD1 and ΔGRASP::OESOD1 tissue and culture filtrate were analysed using western blotting with anti‐hemagglutinin (HA) or anti‐glyceraldehyde‐3‐phosphate dehydrogenase (GAPDH) antibodies. Total proteins of fungal tissues and culture supernatants on the membrane were determined by abundance of VdGAPDH. (b) In vivo assay to show VdSOD1 can be secreted extracellularly relying on VdGRASP. Verticillium dahliae strains Vd991::VdSOD1‐GFP, ΔGRASP::VdSOD1‐GFP, Vd8::VCR1‐GFP, and Vd991::GFP were used to infect onion epidermal cells (the latter two strains were used as controls), respectively. Images were taken at 72 hr postinoculation (hpi) using confocal laser scanning microscopy to perform a subcellular localization assay. Bar, 50 μm. (c) Validation of the nonsecretion function of the 26 amino acid N‐terminal peptide of VdSOD1 by a yeast signal trap assay. Fusion of the 26 amino acid N‐terminal peptide of VdSOD1 (VdSOD1N26) in‐frame with signal‐peptide‐lacking yeast invertase could not enable YTK12::pSUC2‐VdSOD1N26 to grow on YPRAA medium. YTK12::pSUC2‐VdSOD1N26 cannot change TTC to red formazan in the formazan assay. The known functional signal peptide Avr1b was used as a positive control
To further confirm if VdSOD1 is secreted into the extracellular spaces and its possible involvement in host–pathogen interactions, onion cells were inoculated with the conidia of V. dahliae strains expressing green fluorescent protein (GFP) fusion proteins under a TrpC promoter. In this experiment, the onion epidermal cells inoculated with Vd991::GFP, a strain expressing free GFP, showed only green fluorescence in the fungal mycelium, but no fluorescence in the onion epidermal cells (Figure 4b). VdVCR1 is a known effector secreted by V. dahliae during infection (authors' unpublished data) and is located in the plant nucleus. VdVCR1 was clearly translocated into the onion cell nucleus (Figure 4b) under the same incubation conditions as with VdVCR1‐GFP overexpressing strain Vd8::VCR1‐GFP. In contrast, after 2 days of incubation with Vd991::SOD1‐GFP, the wildtype strain expressing VdSOD1‐GFP, the fluorescence was observed both in the fungal mycelium and the extracellular space around the onion epidermal cells (Figure 4b). Together, these results strongly suggested that in addition to localizing in the cell, VdSOD1 is also an extracellular secreted protein that is translocated into the host apoplastic spaces by V. dahliae.
2.5. Secretion of signal‐peptide‐lacking protein VdSOD1 is dependent on the Golgi reassembly stacking protein VdGRASP
To understand how VdSOD1 is secreted into the extracellular spaces, the secretory activity of the N‐terminal 26 amino acids of VdSOD1 (where signal peptides are generally found) was investigated with a yeast trap system. Yeast cells need secreted invertase to grow on media with raffinose as the sole carbon source. The 26 amino acids of the N‐terminus of VdSOD1 were fused to the vector pSUC2, which carries a signal‐peptide‐lacking invertase gene, to generate pSUC2‐VdSOD1N26. The recombined plasmid was then transformed into the invertase‐negative yeast strain YTK12 to create YTK12::pSUC2‐VdSOD1N26. In the CMD−W medium with sucrose as the sole carbon source, YTK12::pSUC2‐VdSOD1N26 grew well (Figure 4c). However, in YPRAA with raffinose as the sole carbon source, YTK12::pSUC2‐VdSOD1N26 did not grow (Figure 4c), indicating that the invertase was not successfully secreted by YTK12::pSUC2‐VdSOD1N26. Invertase enzymatic activity in YTK12::pSUC2‐VdSOD1N26 was also not detected by triphenyltetrazolium chloride discolouration assay (Figure 4c). As predicted by SignalP v. 5.0, the results demonstrate that the 26 amino acid N‐terminal peptide of VdSOD1 is not a functional signal peptide that mediates the secretion of invertase in the yeast strain.
In S. cerevisiae, the secretion of SOD1 was shown to be dependent on Golgi reassembly stacking proteins (GRASPs) (Cruz‐Garcia et al., 2017). To test whether GRASP in V. dahliae (VdGRASP, VEDA_09322, Gene‐ID in VdLs.17 genome: VDAG_03042) is involved in the extracellular secretion of VdSOD1, the secretion ability of VdSOD1 was tested in ΔGRASP::OESOD1, a strain overexpressing VdSOD1‐HA in the VdGRASP deletion mutant. The expression level of VdSOD1 in ΔGRASP::OESOD1 was similar to the overexpression transformant under the wildtype strain background (4.45 ± 0.17 fold) (Figure S5). Western blot analysis showed that the VdSOD1‐HA signal from the fungal tissue of ΔGRASP::OESOD1 was similar to or higher than that of WT::OESOD1, suggesting the accumulation of VdSOD1 in the intracellular space is not significantly affected by the deletion of VdGRASP in V. dahliae (Figure 4a). However, no visible VdSOD1‐HA signal was observed in the supernatant derived from the ΔGRASP::OESOD1, suggesting that the extracellular release of VdSOD1‐HA in vitro is dependent on VdGRASP (Figure 4a). Moreover, the onion cells inoculated with conidia of ΔGRASP::VdSOD1‐GFP, a strain expressing VdSOD1‐GFP under the TrpC promoter in ΔGRASP, showed similar green fluorescence in fungal mycelium but very weak extracellular fluorescence around the onion epidermal cells compared with those of Vd991::VdSOD1‐GFP (Figure 4b). This suggests that most of the secretion of VdSOD1‐GFP in planta is impaired in the ΔGRASP background. Together these results suggest that the secretion of Cu‐Zn superoxide dismutase VdSOD1 is independent of the signal peptide, but is dependent on the Golgi reassembly stacking proteins.
2.6. VdSOD1 has the ability to scavenge endogenous and exogenous superoxide radicals
It is known that VdSOD1 deletion strains are sensitive to menadione stress that can induce endogenous superoxide radicals (Figure 3), which suggests that VdSOD1 probably contributes to the metabolism of endogenous superoxide radicals to ROS products during its retention in the cytoplasm before its secretion into the extracellular space. We therefore analysed the homeostasis of superoxide radicals and ROS products mediated by VdSOD1 activity. With the ROS indicating reagent H2DCFDA, the fluorescence signals stimulated by ROS products were similar among the strains with or without VdSOD1 gene on the PDA medium. However, under the menadione stress to generate superoxide radicals, the wildtype and complementary strains, but not the ΔSOD1 strains, showed strong fluorescence, suggesting the accumulation of ROS products catalysed from superoxide radicals by VdSOD1 (Figure 5a). These results further confirmed the ability of VdSOD1 to scavenge the endogenous superoxide radicals to the less toxic ROS products under conditions of stress.
FIGURE 5.
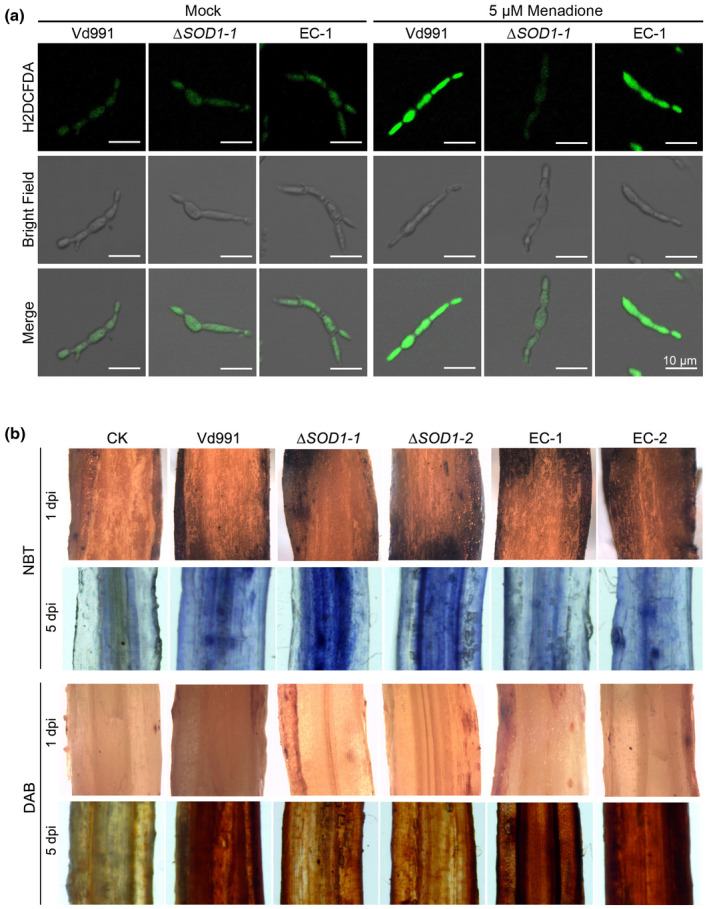
Accumulation of superoxide under menadione treatment and in cotton roots inoculated with Vd991 and ΔSOD1. (a) Hyphae from wildtype strain Vd991, VdSOD1 deletion strain, and the complemented (EC) strain were grown in liquid complete medium for 4 days and treated with 5 μM menadione for 24 hr. Strains not treated with menadione were included as untreated control. H2DCFDA was used as a fluorescent dye to visualize the production of H2O2. (b) Cotton roots inoculated with the wildtype strain, ΔSOD1, and complemented strains at 1 and 5 days postinoculation. The production of and H2O2 was visualized by p‐nitroblue tetrazolium (NBT) and 3,3′‐diaminobenzidine (DAB) staining, respectively
Considering that VdSOD1 is an unconventionally secreted protein, we further determined whether VdSOD1 scavenges extracellular superoxide radicals generated by the host during infection. Thus, we investigated loss‐of‐function of VdSOD1 on ROS change in cotton roots by analysing the accumulation of and H2O2 using p‐nitroblue tetrazolium (NBT) and 3,3′‐diaminobenzidine (DAB) staining, respectively. In the early stages of infection (1 day postinoculation [dpi]), cotton roots showed very few blue spots following NBT staining and reddish‐brown spots by DAB staining among all transformants; most probably few hyphae can enter into the root tissue in the initial infection status (Figure 5b). In contrast, at 5 dpi, cotton roots inoculated with the wildtype strain showed blue spots following NBT staining, which is an insoluble formazan compound formed from the reaction of NBT with , and reddish‐brown spots by DAB staining, produced by the precipitation of DAB oxidization by H2O2, indicating superoxide radicals were generated by the host during infection (Figure 5b). This contrasted with the mutant ΔSOD1 strain that showed increased accumulation of and decreased accumulation of H2O2, and was recovered in the complemented strains (EC‐1/2) in which VdSOD1 was reintroduced (Figure 5b). This pattern can be explained by the fact that the reduction of to H2O2 was delayed, most probably due to the absence of VdSOD1 in the apoplast. Together these results demonstrate that VdSOD1 plays a role in scavenging endogenous and exogenous superoxide radicals produced by the environmental stress and host defence, respectively.
2.7. VdSOD1 is required for full virulence in V. dahliae Vd991
To investigate the potential roles of VdSOD1 during the initial colonization of V. dahliae, the penetration abilities of ΔSOD1 were examined by incubation of the wildtype, mutant, and complemented strains on cellophane membranes laid on the minimal medium. After 4 days, hyphae of the wildtype strain had penetrated the cellophane membrane and colony growth on the medium was observed when the cellophane membrane was removed (Figure S6). Similar to the wildtype strain, ΔSOD1 also maintained the ability to penetrate the cellophane membrane (Figure S6), indicating that VdSOD1 may not affect the penetration ability of V. dahliae. Furthermore, gene expression analysis showed that the function of hyphopodium‐related genes, including VdCrz1, VdLcc, VdMde, VdMFS, VdRhom, and VdCSIN1 (Sun et al., 2019; Zhao et al., 2016), were not deficient in the VdSOD1 deletion strains (Figure 6a), which suggests that the ROS interference mediated by VdSOD1 is not involved in the hyphopodium formation during penetration. Furthermore, to assess the role of VdSOD1 in the virulence of V. dahliae, the susceptible cotton Gossypium hirsutum 'Junmian No.1' was inoculated with two independent VdSOD1 deletion mutants (ΔSOD1‐1 and ΔSOD 1 ‐ 2) along with the wildtype strain Vd991 and complemented strains using a root‐dip method. As expected, cotton inoculated with the wildtype strain showed typical Verticillium wilt symptoms including wilting, necrosis, and vascular discolouration. In contrast, plants inoculated with ΔSOD1 displayed significantly lower disease severity (Figure 6b). Quantification of fungal biomass by quantitative PCR (qPCR) confirmed that the ΔSOD1 biomass in inoculated plants was approximately 65% less than that in plants inoculated with the wildtype strain (Figure S7a). Reintroduction of VdSOD1 into the deletion mutants restored the virulence (Figure 6a), and the fungal biomass in inoculated cotton plants was comparable to that caused by the wildtype strain (Figure S7a). Similarly, ΔSOD1‐inoculated Nicotiana benthamiana plants displayed attenuated Verticillium wilt symptoms (Figure 6c) and the fungal biomass was approximately 52% less than in plants inoculated with the wildtype strain 21 days after inoculation (Figure S7b). Both virulence and biomass were restored in complemented strains (Figures 6b and S7b). These results suggest that VdSOD1 contributes to full virulence on susceptible plants by scavenging excess superoxide radicals derived from host defence during infection.
FIGURE 6.
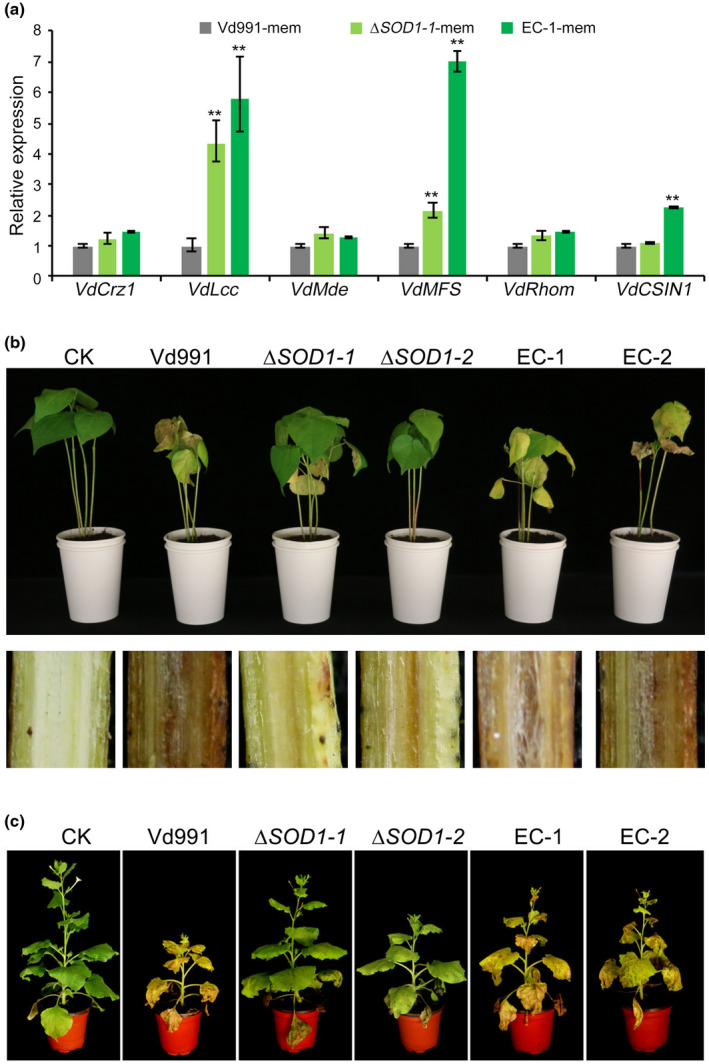
VdSOD1 is required for full virulence of Verticillium dahliae. (a) Quantitative reverse transcription PCR (RT‐qPCR) was used to detect the transcript levels of genes involved in the hyphopodium differentiation in the tested strains. The wildtype, mutants, and complemented strains were grown on minimal medium plates covered with the cellophane membrane (mem) for 2 days for hyphopodium induction. Error bars represent standard deviations (n = 3). Asterisks (*) indicate significant differences (p < 0.05) according to Student's t test. (b) Phenotypes of cotton seedlings inoculated with the wildtype strain, VdSOD1 gene deletion strains, and complemented (EC) strains (top). The discolouration in the longitudinal sections of the inoculated shoots is shown at the bottom. Plants treated with sterile water (Mock) were used as controls. Two‐week‐old seedlings of susceptible cotton (Gossypium hirsutum 'Junmian No. 1') were inoculated with indicated strains. Verticillium wilt symptoms were photographed 3 weeks after inoculation. (c) Phenotypes of Nicotiana benthamiana plants inoculated with the wildtype strain, the gene deletion mutants of VdSOD1, and corresponding complementation strains. Four‐week‐old seedlings of N. benthamiana plants were inoculated with the indicated strains. Verticillium wilt symptoms were photographed 3 weeks after inoculation
3. DISCUSSION
Although Cu/Zn‐SOD1 has been known to play important roles in ROS scavenging for a long time, not many studies have focused on the role of these genes in plant–pathogen interactions. In this study, a Cu/Zn SOD (VdSOD1) gene in V. dahliae was identified, and its ROS‐scavenging ability and enzyme activity were investigated intracellularly under oxidative stress. More importantly, we characterized the unconventional secretion trait of the signal‐peptide‐lacking VdSOD1 and investigated the role it plays in scavenging extracellular ROS during infection. Finally, the virulence function of VdSOD1 was demonstrated on two different hosts.
Because Cu/Zn‐SOD1 does not have a signal peptide, its distribution has been attributed to the cytoplasm (Yao et al., 2016), nucleus (Chang et al., 1988), mitochondria (Sturtz et al., 2001), and peroxisomes (Keller et al., 1991). The intracellular scavenging ability and intracellular SOD activity of Cu/Zn‐SOD1 has been intensively studied in several fungi. For example, SOD1‐deletion mutants in Cryptococcus neoformans var. gattii (Briones‐Martin‐del‐Campo et al., 2015) and F. graminearum (Yao et al., 2016) were extremely sensitive to menadione, while the loss‐of‐function of SOD1 in B. cinerea (Rolke et al., 2004) and S. sclerotiorum (Xu & Chen, 2013) showed higher sensitivity to the ROS‐generating compound paraquat. However, C. purpurea CpSOD1 is an exception, with no essential role in facilitating the fungus overcoming the oxidative stress imposed by paraquat (Moore et al., 2002). Intracellular SOD activity of Cu/Zn‐SOD1 has further been demonstrated in F. graminearum and B. cinerea. In this study, we found that ΔSOD1 showed significant sensitivity to menadione and its SOD activity was partially inactivated (Figure 3), and deletion of VdSOD1 rendered the ability to catalyse superoxide radicals into ROS products to be lost, consistent with previously reported results in other filamentous fungi. Interestingly, the deletion of VdSOD1 negated the H2O2 sensitivity compared to the wildtype strain and complementary transformants (Figure S4). VdSOD1 catalyses the disproportionation of superoxide to H2O2 and O2 (Sheng et al., 2014) and this probably generates superoxide under H2O2 stress, which results in increased H2O2 locally to aggravate the harmful effects of exogenous H2O2 in the wildtype strain, but not in the VdSOD1 deletion mutants. Another possibility is that VdSOD1 presents a negative feedback effect under high concentrations of H2O2, resulting in accumulation of harmful superoxide that curtails the growth of the wildtype strain and complementary transformants on media but not deletion mutants.
Cytoplasmic SOD1 is secreted unconventionally (Cruz‐Garcia et al., 2017), and the secreted SOD1 remains enzymatically active in S. cerevisiae (Cruz‐Garcia et al., 2020). A short stretch of eight amino acids of SOD1, which is absent in signal peptide‐containing extracellular SOD (EC‐SOD), is responsible for its unconventional secretion (Cruz‐Garcia et al., 2017). Despite extensive studies of secretory characteristics of SOD1 in S. cerevisiae, few studies have addressed the extracellular distribution of SOD1 in plant‐pathogenic fungi, with the exception of CpSOD1, detected in the culture filtrate of C. purpurea by western blot analysis (Moore et al., 2002). To date, the scavenging of extracellular ROS in planta has only been functionally characterized with BcSOD1 from B. cinerea (Lopez‐Cruz et al., 2017) and SsSOD1 from S. sclerotiorum (Xu & Chen, 2013) on SOD1 deletion strain‐inoculated host leaves and detecting the accumulating by NBT staining. In this study, sequence analysis suggested that VdSOD1 in V. dahliae contains an unconventional secretion peptide composed of eight amino acids (Figure 1). The presence of VdSOD1 in culture supernatants was detected by western blot, and its apoplastic localization in planta was observed by confocal microscopy, providing two lines of evidence to consider VdSOD1 as an unconventionally secreted protein (Figure 4). Not surprisingly, VdSOD1 detoxifies or neutralizes extracellular , creating an ROS imbalance in infected cotton roots. This is perhaps the first study to determine both the excretion and extracellular catalytic function of SOD1 simultaneously in a fungal plant pathogen.
Elucidating the roles of unconventionally secreted proteins in the virulence of phytopathogens is an emerging theme in plant–microbe interactions (Miura & Ueda, 2018). Powdery mildew signal‐peptide‐lacking effectors AVRa10 and AVRk1 are functional inside the host cell and enhance successful infection of susceptible host plant cells (Ridout et al., 2006). Ustilago maydis peroxisome protein, sterol carrier protein 2 (Scp2), was detected in the apoplastic fluid of infected maize and might play a role in the inhibition of competitors in the apoplast (Krombach et al., 2018). Our previous work has shown that the V. dahliae Vd991 exoproteome possesses 99 proteins without a signal peptide, implying many unconventional secreted proteins might play important roles during host–pathogen interactions (Chen et al., 2016; Wang et al., 2018). However, only one signal‐peptide‐lacking effector VdIsc1, which can translocate to its host cells and suppress salicylate‐mediated innate immunity in planta, has been reported (Liu et al., 2014). In this study, we provided yet another compelling example of an unconventionally secreted protein with an important virulence function. This study furthers the understanding of the molecular mechanisms of nonclassically secreted proteins involved in infection and interaction with V. dahliae hosts. It also reminds us of the need to redefine current extracellular protein prediction workflows that are biased towards classical signal peptide prediction.
In S. cerevisiae, the extracellular export of leaderless proteins relies on a cup‐shaped double membrane‐bounded compartment called the compartment for unconventional protein secretion (CUPS) (Cruz‐Garcia et al., 2014). GRASPs play important roles in the formation and maturation of CUPS, although the precise molecular mechanism of GRASP proteins in this process remains unclear (Cruz‐Garcia et al., 2018; Duran et al., 2010). However, the mechanism of unconventional secretion has remained undetermined in fungal plant pathogens. Here, we determined that the secretion of VdSOD1 relied on VdGRASP, suggesting that its excretion may also depend on CUPS, just as in S. cerevisiae SOD1 (Figure 4). The physiological significance of why VdSOD1 follows an unconventional secretory pathway is still unknown. We can speculate that the conventional ER‐Golgi secretion system may be limited to the duration of pathogen infection (Krishnan & Askew, 2014) or that the oxidative environment in the lumen of the ER and the Golgi apparatus might cause misfolding of VdSOD1 (Nickel & Rabouille, 2009). Also, the unconventional secretory pathway allows VdSOD1 to be biologically functional both inside and outside of the cells (Dimou & Nickel, 2018), possibly indicating a pathogen strategy to prioritize SOD regulation depending on the conditions it encounters and host availability. Interestingly, plants also release signal‐peptide‐lacking proteins into the apoplast in response to pathogen attack (Agrawal et al., 2010). In contrast to the yeasts, plants develop a different single membrane‐bound ultrastructure called the exocyst‐positive organelle (EXPO) for unconventional secretion (Wang et al., 2010). Sequence analysis of all available plant genomes revealed that plants lack a bona fide GRASP homolog (Zhao et al., 2017), hinting that this unique class of GRASPs may represent a possible target for treating infection caused by phytopathogenic fungus.
The SOD1‐deletion mutants in many plant pathogens showed no significant differences in growth and sporulation relative to the wildtype strains, with CpSOD1 in C. purpurea (plays a role in the normal growth of the fungus) and BbSOD1 in B eauveria bassiana (essential for conidial germination) being the exceptions (Li et al., 2015; Moore et al., 2002). In our study, the ΔSOD1 strain in V. dahliae maintained growth and sporulation in complete media such as PDA (Figure 2), indicating VdSOD1 is not essential for normal growth. The loss of VdSOD1, however, resulted in decreased growth rate on the cellulose medium (Figure 2). Fungal GH61 glycosyl hydrolases internally cleave cellulose molecules via oxidation (Jagadeeswaran et al., 2018; Quinlan et al., 2011), which suggests that fungi probably activate the process of oxidation during cellulose utilization in the cell. Whether the changed oxidative environment caused by VdSOD1 deletion results in the dysfunction of GH61 when V. dahliae is grown on cellulose medium requires further investigation.
Cu/Zn‐SODs are important virulence factors in many pathogenic fungi, including the animal and human pathogens Candida albicans (Frohner et al., 2009) and C. neoformans (Narasipura et al., 2003), and phytopathogenic fungi, such as B. cinerea, S. sclerotiorum, and F. graminearum. However, CpSOD1 in C. purpurea and AfSOD1 in A. fumigatus (Lambou et al., 2010) are not required for virulence. These studies demonstrate the functional diversity and differentiation of SOD1 in pathogenic fungi. In our study, the pathogenic role of VdSOD1 in cotton and N. benthamiana was determined. The alleviated disease symptoms and reduced fungal biomass in host plants infected by ΔSOD1 strains suggest that the VdSOD1 is required for the virulence of V. dahliae (Figure 6). Penetration assay showed loss of VdSOD1 did not disrupt the ability of the mutant strain to penetrate cellophane membrane (Figure S6), and the expression levels of genes involved in the hyphopodium formation remained intact and functional following the deletion of VdSOD1 in V. dahliae (Figure 6a). Thus, ΔSOD1 probably maintained normal hyphopodium formation and the ability to colonize host tissue. The reduced virulence of the VdSOD1 deletion mutant was probably due to its inability to scavenge excess ROS during infection and not due to its altered penetration ability. First, the impaired intracellular superoxide detoxification mechanism in ΔSOD1 may lead to the inability of the pathogen to manage fungal metabolic to promote survival within the host. Second, VdSOD1 plays a role in scavenging extracellular ROS by catalysing host‐derived into less toxic H2O2 (Figure 5b). According to the classical studies of the invasion progress of V. dahliae on the host plant, the invasion hyphae of V. dahliae cross into epidermal cells in about 3 days and can be observed within the xylem vessels by 5 dpi (Fradin & Thomma, 2006; Zhao et al., 2014). In this study, we detected ROS in vivo at 5 dpi and found that VdSOD1 deletion led to increased at infection sites. Thus, VdSOD1 may help the pathogen survive the host‐derived ROS. In addition, ROS is a key signalling molecule that triggers a variety of responses in the host. The imbalance in ROS at the infection site, with increasing and decreasing H2O2, may also impair plant defence responses (Lehmann et al., 2015).
In conclusion, signal‐peptide‐lacking VdSOD1 is a dual function superoxide dismutase. Its presence in fungal tissue contributes to scavenging superoxide radicals intracellularly. VdSOD1 can also be secreted unconventionally depending on the Golgi reassembly stacking protein, catalysing into H2O2 during pathogen–host interactions. It is not critical for normal growth and sporulation of the pathogen, but VdSOD1 is necessary for full virulence of V. dahliae on susceptible hosts.
4. EXPERIMENTAL PROCEDURES
4.1. Growth of microbials and plants
The V. dahliae wildtype strain Vd991 was cultured on PDA (potato 200 g/L, glucose 20 g/L, agar 15 g/L) or in liquid Czapek‐Dox medium (NaNO3 3 g/L, MgSO4.7H2O 0.5 g/L, KCl 0.5 g/L, FeSO4.7H2O 0.01 g/L, K2HPO4 1 g/L, sucrose 30 g/L) at 25 °C. Minimal medium (NaNO3 6 g/L, KCl 0.52 g/L, MgSO4.7H2O 0.152 g/L, KH2PO4 1.52 g/L, vitamin B1 0.01 g/L, glucose 10 g/L, 1,000 × trace element 1 ml/L, agar 15 g/L, pH 6.5) was used for penetration assays. Agrobacterium tumefaciens AGL‐1 was used for fungal transformations in YEB at 28 °C. Escherichia coli TOP10 was used to propagate all plasmids in Luria‐Bertani broth (LB) with constant shaking at 250 rpm at 37 °C. Cotton (G. hirsutum 'Junmian No. 1') was grown at 28 °C for 2 weeks (until development of the first euphylla) for pathogenicity assays. N. benthamiana plants were grown at 25 °C for 4 weeks for pathogenicity assays. Both cotton Junmian No.1 and N. benthamiana were grown with a 14‐hr light/10‐hr dark photoperiod in a greenhouse at the temperatures noted above.
4.2. Gene cloning and bioinformatics analysis
The VdSOD1 gene was amplified from genomic DNA and cDNA samples of Vd991 based on the VdSOD1 sequence in the V. dahliae genome database (Chen et al., 2018). The physicochemical properties of the protein sequence of VdSOD1 were determined by the ProtParam tool of ExPASy (http://www.expasy.org). The protein domains and active sites were predicted by searching NCBI’s conserved domain database. VdSOD1 homologs were retrieved using BLASTP with nonredundant databases of NCBI. Clustal X2 was used for multiple sequence alignment of SOD1 orthologs in other fungi (Larkin et al., 2007). The signal peptide analyses were performed using SignalP v. 5.0 (Almagro Armenteros et al., 2019). Unconventionally secreted protein prediction was performed using SecretomeP v. 2.0 (Bendtsen et al., 2004).
4.3. Construction of VdSOD1 deletion mutants and complemented strains
Gene knockout plasmids were generated based on previously described methods with modifications (Liu et al., 2013). Briefly, 1,211 bp of the upstream sequence and 1,219 bp of the downstream sequence of VdSOD1 were amplified from V. dahliae Vd991 genomic DNA with primers VdSOD1‐Up‐F/R and VdSOD1‐Down‐F/R, respectively. The hygromycin phosphotransferase gene cassette, which includes the TrpC‐promoter, hygromycin phosphotransferase gene, and Nos‐terminator, was amplified from the pUC‐Hyg vector with primers HYG‐F/R. The above three amplicons were fused into a single fragment via fusion PCR. Subsequently, a nested PCR was carried out with primers VdSOD1‐Nest‐F/R containing 24‐bp overlap adjoining adapters to allow the assembly of DNA fragments by homologous recombination. Then the internal amplicon was recombined with a HindIII/XbaI‐linearized pGKO2‐Gateway vector to generate the gene knockout plasmid using a standard homologous recombination reaction (Khang et al., 2005). All primers used for vector construction are listed in Table S1. The complementation fragments, containing the VdSOD1 wildtype coding sequences with c.1 kb native promoters and c.0.5 kb downstream terminator regions, were amplified from V. dahliae Vd991 genomic DNA with primers VdSOD1‐C‐F/R and fused into binary vector pCOM, which carried the geneticin resistance marker to generate a complementation plasmid by homologous recombination (Zhou et al., 2013). A. tumefaciens‐mediated transformation of V. dahliae was performed as described previously (Mullins et al., 2001). Gene knockout mutants were selected on PDA with 200 μg/ml cefotaxime, 50 μg/ml hygromycin, and 200 μg/ml 5‐fluoro‐2′‐deoxyuridine, while complemented strains were selected in the presence of 200 μg/ml cefotaxime and 50 μg/ml geneticin. Single‐spore isolations were performed for all transformants. Transformants were screened by PCR verification with primers HYG‐Test‐F/R (to amplify a fragment of hygromycin resistant cassette) and primers VdSOD1‐Test‐F/R (to amplify a fragment of VdSOD1 coding sequence), respectively.
4.4. Generation of strains overexpressing HA‐ or GFP‐tagged VdSOD1
For overexpression of the VdSOD1‐HA fusion protein under the constitutive TrpC promoter, the VdSOD1‐HA fusion from cDNA of Vd991with primers VdSOD1‐HA‐F and VdSOD1‐HA‐R and recombined with a SacI/XbaI‐linearized pCOM‐TrpC (Zhou et al., 2013), which contained the TrpC promoter and TrpC terminator. The recombined plasmid was transformed into the wildtype strain and ∆GRASP to create WT::OESOD1 and ΔGRASP::OESOD1, respectively. For overexpression of the VdSOD1‐GFP fusion protein, the VdSOD1 ORF was amplified with the primers VdSOD1‐GFP‐F and VdSOD1‐GFP‐R using cDNA of Vd991 as template, and cloned into the KpnI sites of pCOM‐GFP (integrating the GFP ORF into pCOM‐TrpC) (Li et al., 2019), using the same recombination method. The recombined plasmid was transformed into Vd991 and ∆VdGRASP to create Vd991::VdSOD1‐GFP and ∆GRASP::VdSOD1‐GFP, respectively. Transformants were selected in the presence of geneticin (50 μg/ml). After single spore isolations, PCR verification was done with primers Geneticin‐F/R to amplify a fragment of the geneticin‐resistant cassette. All V. dahliae strains used in this study are listed in Table S2.
Quantitative reverse transcription‐PCR (RT‐qPCR) was performed to identify the VdSOD1 expression levels of the wild type and the VdSOD1 overexpression strains described above, using the primers qVdSOD1‐F/R. Relative gene expression levels were calculated using the 2−ΔΔ C t method (Livak & Schmittgen, 2001) with V. dahliae elongation factor 1‐α (EF‐1α) as an internal control. All primers are listed in Table S1.
4.5. Growth and conidiogenesis assays
For the radial growth rate assay, 2 μl of conidial suspension with a concentration of 5 × 106 conidia/ml of the wildtype strain, ΔSOD1strains, or complemented strains was placed in the centre of PDA or Czapek‐Dox agar plates and incubated at 25 °C for 12 days. Different carbon sources, including sucrose (30 g/L), pectin (10 g/L), sodium carboxymethyl cellulose (10 g/L), and starch (15 g/L), were tested by incorporating them individually into Czapek–Dox agar. Mycelial growth rates were also measured on PDA plates amended with menadione (0, 10, 15, and 20 μM) and H2O2 (5 and 7 μl per 50 ml of PDA). Following 5, 7, and 9 days of incubation with menadione and 9 days of incubation with H2O2, the colony diameters were measured on all plates.
To assess conidial production by ΔSOD1 relative to the wildtype strain, agar plugs were collected using a 5‐mm diameter cork borer from the edge of a 7‐day‐old PDA culture. Each plug was shaken in 1 ml of sterile water, and the number of conidia quantified using a haemocytometer. Experiments were conducted in triplicate for each strain.
4.6. Measurement of enzyme activity
Mycelia of the wildtype strain, ΔSOD1 strains, and complemented strains grown in complete medium (CM) liquid medium for 3 days were harvested. After reculturing in CM supplemented with 10 μM menadione for 1 day, three aliquots of 0.5 g fresh mycelia were ground in liquid nitrogen and suspended in 50 mM ice‐cold phosphate buffer (pH 7.4). After centrifugation at 16,000 × g at 4 °C for 20 min, the total SOD activity in the supernatant of each sample was determined following the NBT reduction method of Beauchamp and Fridovich (1971). One SOD unit was defined as the amount of crude enzyme that was required to inhibit the reduction of NBT by 50% and was expressed as units per mg protein (U/mg protein). Protein content was measured by the Bradford procedure (Bradford, 1976), using bovine serum albumin as a standard.
4.7. Yeast signal sequence trap system
Functional validation of the N‐terminal peptide of VdSOD1 was performed as previously described (Jacobs et al., 1997). The coding region of the 26 amino acid N‐terminal peptide of VdSOD1 was amplified with primers SP‐VdSOD1‐F and SP‐VdSOD1‐R, and fused in‐frame to the secretion‐defective invertase gene in the pSUC2 vector. The resulting plasmid, pSUC2‐VdSOD1N26, was transformed into the yeast strain YTK12 using the lithium acetate method to yield YTK12::pSUC2‐VdSOD1N26 (Table S2). Positive clones were selected by PCR using primers SP‐VdSOD1‐F and SP‐VdSOD1‐R (Table S1). Invertase secretion was assayed by plating YTK12::pSUC2‐VdSOD1N26 on YPRAA plates (1% yeast extract, 2% peptone, 2% raffinose, and 2 μg/mL antimycin A). The yeast strain YTK12 transformed with an empty pSUC2 vector or the pSUC2‐Avr1bSP vector was used as the negative and positive control, respectively (Table S2). Moreover, the invertase enzymatic activity was verified by the reduction of 2,3,5‐triphenyltetrazolium chloride (TTC) to insoluble red triphenylformazan. Briefly, the yeast strains were incubated in 5 ml of sucrose medium for 24 hr at 30 °C, and then the pellet was collected and incubated with 0.1% of the colourless dye TTC at 35 °C for 35 min. Then the invertase enzymatic activity was assayed by a colorimetric change 5 min after incubation at room temperature.
4.8. Protein extraction and western blot analyses
WT::OESOD1 and ΔGRASP:: OESOD1 were used to analyse the secretion of VdSOD1 in vitro. Strains were cultivated in a flask for 4 days at 25 °C in liquid minimal (MM) medium. Secreted proteins were prepared using the method described previously (Wang et al., 2011). Briefly, Miracloth was used to filter out mycelia from the culture solution. Each filtrate was extracted twice by adding 0.5 volumes of phenol and shaking well on ice for 10 min, followed by centrifugation at 900 × g for 10 min. Four volumes of methanol containing 100 mM ammonium acetate were added to precipitate the phenol layer at −20 ℃ for 2 hr. The precipitated protein was spun down at 900 × g for 10 min. The protein was washed twice with a methanol/ammonium acetate mixture and stored in 80% acetone at −20 ℃ before using. To prepare the cytosolic protein fractions, fungal tissues were collected by configuration and disrupted by grinding with liquid nitrogen. The total proteins were extracted with a lysis buffer (25 mM HEPES, 300 mM NaCl, 2 mM EDTA, plus a proteinase inhibitor PMSF). For western blotting, proteins were denatured with sodium dodecyl sulphate (SDS)‐containing loading buffer before separation on a 12% polyacrylamide gel. After electrophoresis, separated proteins were transferred to a polyvinylidene difluoride (PVDF) membrane for 1 hr. The blots were incubated with anti‐HA (Sigma) or anti‐GAPDH (Sigma), respectively. Signals were detected using an enhanced chemiluminescence (ECL) system according to the instructions provided by the manufacturer.
4.9. Confocal microscopy
Onion epidermal cells inoculated with Vd991::VdSOD1‐GFP and ΔGRASP::VdSOD1‐GFP were monitored for extracellular localization following the procedure previously described (Lyu et al., 2016). Briefly, to observe fluorescence, a conidial suspension (107 conidia/ml) of two strains was inoculated on the inner layer of onion epidermal cells and then incubated on a 1% water agar plate at room temperature for 3 days. Fluorescence images of the GFP fusion protein in onion epidermal cells was viewed with laser scanning confocal microscopy 200× magnification with excitation and emission wavelengths of 488 and 510 nm, respectively. Vd8::VCR1‐GFP‐treated onion epidermal cells were used as the positive control, while Vd991::GFP‐treated onion epidermal cells were used as the negative controls. To observe the green fluorescence‐labelled ROS following H2DCFDA staining, strain samples were observed by laser scanning confocal microscopy at 200× magnification with excitation and emission wavelengths at 488 and 525 nm, respectively. Confocal microscopy was performed at least twice.
4.10. Histochemical detection of superoxide
Detection of superoxide anions () and hydrogen peroxide (H2O2) in the infected roots of cotton was performed by staining with NBT or DAB (Bournonville & Diaz‐Ricci, 2011). Briefly, cotton roots inoculated with the wildtype strain, VdSOD1 deletion mutants, or complemented strains at 1 and 5 dpi were immersed in NBT staining solution (0.05% wt/vol NBT) or DAB staining solution (0.05% wt/vol DAB; Sigma). Tubes were wrapped with aluminium foil and kept overnight at room temperature. Staining solution from the test tubes was drained off. Seedlings were immersed in absolute ethanol and heated in a boiling waterbath for 10 min. Stained seedlings were photographed against a contrast background to assess superoxide accumulation. The wildtype strain Vd991, VdSOD1 deletion strains, and the complemented strains were cultured in the liquid complete medium for 4 days and stained with 50 μM H2DCFDA for 20 min. The hyphae were then washed with sterile water three times and observed under laser scanning confocal microscopy.
4.11. Pathogenicity assays
For penetration assays, sterilized cellophane membrane (Solarbio) was overlaid on minimal medium in Petri plates. Inoculum containing equal numbers of conidia of the wildtype strain, ΔSOD1 strains, and complemented strains were spread on the cellophane in separate plates and incubated for 4 days. The membranes were then removed and the plates incubated for an additional 3 days to determine if the fungus had breached the cellophane and produced colonies on the minimal medium.
Pathogenicity assays were carried on N. benthamiana and cotton seedlings using a root‐dip method as previously described (Gui et al., 2017). Briefly, 4‐week‐old N. benthamiana and 2‐week‐old cotton seedlings were gently uprooted, washed of soil, and immersed in 15 ml of 5 × 106 conidia/ml suspension from each transformant or the wildtype strain for 5 min and then replanted into new pots and maintained at 28 °C (14 hr/10 hr, day/night cycle) with three replicates. In each replicate, 12 N. benthamiana plants or six pots of cotton seedlings (five plants for each pots) were used for each transformant. Verticillium wilt disease symptoms, including wilting and vascular discolouration, were evaluated and photographed 3 weeks after inoculation. Fungal biomass was quantified 3 weeks after inoculation from roots by pooling three plants per treatment group for DNA extraction and qPCR by targeting the Verticillium elongation factor 1‐α (EF‐1α) with the cotton 18S rRNA or N. benthamiana EF‐1α as endogenous plant control (Santhanam et al., 2013).
4.12. Gene expression analysis
Expression levels of genes involved in the hyphopodium differentiation were measured after the test strains were grown on MM plates covered with cellophane membrane for 2 days to induce hyphopodium development. Total RNA was extracted from the mycelium using the Multisource Total RNA Miniprep Kit (Axygen). RT‐qPCR was performed under the following conditions: an initial 95 °C denaturation step for 10 min followed by 40 cycles of 95 °C for 15 s and 60 °C for 1 min. The transcription levels of the target genes relative to the constitutively expressed elongation factor 1‐α of Verticillium dahliae were quantified using the 2−∆∆ C t method from three independent experiments (Livak & Schmittgen, 2001). Unpaired Student's t tests were performed to determine statistical differences. Primers used for expression profiling are listed in Table S1.
CONFLICT OF INTEREST
The authors declare no competing financial or nonfinancial interests.
AUTHOR CONTRIBUTIONS
X.F.D., J.Y.C., and K.V.S. conceived the study and designed all experiments. L.T., J.J.L., D.D.Z., J.S., and N.W.Q. performed the data analysis and interpretation. L.T., J.J.L., C.M.H., D.D.Z., Y.X., and D.W. performed the experiments. L.T., J.Y.C., D.D.Z., and X.Y.Y. contributed to the writing of the manuscript. K.V.S. conceptualized the study, reviewed the data, and edited the manuscript along with D.P.G.S. and P.I. All authors have read, commented on, and approved the manuscript.
Supporting information
FIGURE S1 Full‐length sequence alignment and open reading frames (ORF) of VdSOD1
FIGURE S2 Bioinformatics prediction of secretory characteristics of VdSOD1. (a) Signal peptide prediction using SignalP 5.0 program. (b) Nonconventional secretion protein prediction by SecretomeP 2.0
FIGURE S3 Confirmation of VdSOD1 targeted deletion mutants and complementation strains in Verticillium dahliae. (a) Detection of the positive targeted VdSOD1 deletion strains by detection of the Hpt fragment. WT, wild‐type strain Vd991; KO1‐KO2, and two independent targeted deletion mutants of VdSOD1. (b) Detection of the positive targeted VdSOD1 deletion strains and complementation strains by amplifying a VdSOD1 internal fragment
FIGURE S4 The effect of H2O2 on growth rate of Verticillium dahliae. (a) The wild‐type strain Vd991, VdSOD1 deletion strains, and the complemented strains were cultured on PDA medium supplemented with H2O2 at specified concentrations for 9 days. (b) Colony diameters on potato dextrose agar plates containing different concentrations of H2O2 following 9 days of incubation. Means and standard deviations of the mean from three biological replicates are shown. Asterisks (**) denote significant differences (p < .01), according to Student’s t test
FIGURE S5 Expression levels of VdSOD1 in overexpression transformants. (a) Expression levels of VdSOD1 in wild‐type strain and WT::OESOD1 determined via quantitative reverse transcription PCR (RT‐qPCR). (b) Expression levels of VdSOD1 in ΔGRASP and ΔGRASP::OESOD1 via RT‐qPCR. The housekeeping gene elongation factor 1‐α (EF‐1α) was used as an endogenous control. Error bars represent the standard deviation of three replicate experiments and a double asterisk indicates the statistical significance (p < .01) by Student’s t test
FIGURE S6 VdSOD1 does not mediate cellophane membrane penetration in Verticillium dahliae. The wild‐type strain Vd991, VdSOD1 deletion strains, and the complemented strains were grown on the top of cellophane membranes overlaid onto minimal medium for 4 days at 25 °C (“Before” status). The cellophane membranes were then removed from the plates and incubated for an additional 3 days to determine the cellophane membrane penetration by the presence or absence of hyphal growth on medium (“After” status)
FIGURE S7 Quantification of fungal biomass in Verticillium dahliae‐infected cotton and Nicotiana benthamiana. Quantitative PCR was used to measure the fungal biomass of strains in the infected cotton (a) and N. benthamiana (b). Error bars represent standard deviations (n = 3). Asterisks (**) indicate significant differences (p < .01), according to Student’s t test
TABLE S1 Primers used in this study
TABLE S2 Strains used in this study
ACKNOWLEDGEMENTS
The authors gratefully acknowledge Dr Nikhilesh Dhar's presubmission review of this manuscript. This work was supported by the National Key Research and Development Program of China (2018YFE0112500), the National Natural Science Foundation of China (31501588, 31972228, 31970142, 31870138), the Elite Youth Program CAAS to J.Y.C., and the Fundamental Research Funds for Central Non‐profit Scientific Institution in CAAS (Y2021XK22, Y2018PT70).
Tian, L., Li, J., Huang, C., Zhang, D., Xu, Y., Yang, X., et al (2021) Cu/Zn superoxide dismutase (VdSOD1) mediates reactive oxygen species detoxification and modulates virulence in Verticillium dahliae . Molecular Plant Pathology, 22, 1092–1108. 10.1111/mpp.13099
Li Tian and Junjiao Li contributed equally.
Contributor Information
Krishna V. Subbarao, Email: kvsubbarao@ucdavis.edu.
Jieyin Chen, Email: chenjieyin@caas.cn.
Xiaofeng Dai, Email: daixiaofeng_caas@126.com.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Agrawal, G.K., Jwa, N.S., Lebrun, M.H., Job, D. & Rakwal, R. (2010) Plant secretome: unlocking secrets of the secreted proteins. Proteomics, 10, 799–827. [DOI] [PubMed] [Google Scholar]
- Almagro Armenteros, J.J., Tsirigos, K.D., Sønderby, C.K., Petersen, T.N., Winther, O., Brunak, S. et al. (2019) SignalP 5.0 improves signal peptide predictions using deep neural networks. Nature Biotechnology, 37, 420–423. [DOI] [PubMed] [Google Scholar]
- Apel, K. & Hirt, H. (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annual Review of Plant Biology, 55, 373–399. [DOI] [PubMed] [Google Scholar]
- Beauchamp, C. & Fridovich, I. (1971) Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Analytical Biochemistry, 44, 276–287. [DOI] [PubMed] [Google Scholar]
- Bendtsen, J.D., Jensen, L.J., Blom, N., Von Heijne, G. & Brunak, S. (2004) Feature‐based prediction of non‐classical and leaderless protein secretion. Protein Engineering, Design & Selection, 17, 349–356. [DOI] [PubMed] [Google Scholar]
- Bournonville, C.F. & Diaz‐Ricci, J.C. (2011) Quantitative determination of superoxide in plant leaves using a modified NBT staining method. Phytochemcal Analysis, 22, 268–271. [DOI] [PubMed] [Google Scholar]
- Bradford, M.M. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein‐dye binding. Analytical Biochemistry, 72, 248–254. [DOI] [PubMed] [Google Scholar]
- Briones‐Martin‐del‐Campo, M., Orta‐Zavalza, E., Cañas‐Villamar, I., Gutiérrez‐Escobedo, G., Juárez‐Cepeda, J., Robledo‐Márquez, K. et al. (2015) The superoxide dismutases of Candida glabrata protect against oxidative damage and are required for lysine biosynthesis, DNA integrity and chronological life survival. Microbiology, 161, 300–310. [DOI] [PubMed] [Google Scholar]
- Broxton, C.N. & Culotta, V.C. (2016) SOD enzymes and microbial pathogens: surviving the oxidative storm of infection. PLoS Pathogens, 12, e1005295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, L.Y., Slot, J.W., Geuze, H.J. & Crapo, J.D. (1988) Molecular immunocytochemistry of the CuZn superoxide dismutase in rat hepatocytes. Journal of Cell Biology, 107, 2169–2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, J.Y., Liu, C., Gui, Y.J., Si, K.W., Zhang, D.D., Wang, J. et al. (2018) Comparative genomics reveals cotton‐specific virulence factors in flexible genomic regions in Verticillium dahliae and evidence of horizontal gene transfer from Fusarium . New Phytologist, 217, 756–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, J.‐Y., Xiao, H.‐L., Gui, Y.‐J., Zhang, D.‐D., Li, L., Bao, Y.‐M. et al. (2016) Characterization of the Verticillium dahliae exoproteome involves in pathogenicity from cotton‐containing medium. Frontiers in Microbiology, 7, 1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz‐Garcia, D., Brouwers, N., Duran, J.M., Mora, G., Curwin, A.J. & Malhotra, V. (2017) A diacidic motif determines unconventional secretion of wild‐type and ALS‐linked mutant SOD1. Journal of Cell Biology, 216, 2691–2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz‐Garcia, D., Brouwers, N., Malhotra, V. & Curwin, A.J. (2020) Reactive oxygen species triggers unconventional secretion of antioxidants and Acb1. Journal of Cell Biology, 219, e201905028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz‐Garcia, D., Curwin, A.J., Popoff, J.F., Bruns, C., Duran, J.M. & Malhotra, V. (2014) Remodeling of secretory compartments creates CUPS during nutrient starvation. Journal of Cell Biology, 207, 695–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz‐Garcia, D., Malhotra, V. & Curwin, A.J. (2018) Unconventional protein secretion triggered by nutrient starvation. Seminars in Cell and Developmental Biology, 83, 22–28. [DOI] [PubMed] [Google Scholar]
- Dimou, E. & Nickel, W. (2018) Unconventional mechanisms of eukaryotic protein secretion. Current Biology, 28, R406–R410. [DOI] [PubMed] [Google Scholar]
- Duran, J.M., Anjard, C., Stefan, C., Loomis, W.F. & Malhotra, V. (2010) Unconventional secretion of Acb1 is mediated by autophagosomes. Journal of Cell Biology, 188, 527–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fradin, E.F. & Thomma, B.P. (2006) Physiology and molecular aspects of Verticillium wilt diseases caused by V. dahliae and V. albo‐atrum . Molecular Plant Pathology, 7, 71–86. [DOI] [PubMed] [Google Scholar]
- Frohner, I.E., Bourgeois, C., Yatsyk, K., Majer, O. & Kuchler, K. (2009) Candida albicans cell surface superoxide dismutases degrade host‐derived reactive oxygen species to escape innate immune surveillance. Molecular Microbiology, 71, 240–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gui, Y.J., Chen, J.Y., Zhang, D.D., Li, N.Y., Li, T.G., Zhang, W.Q. et al. (2017) Verticillium dahliae manipulates plant immunity by glycoside hydrolase 12 proteins in conjunction with carbohydrate‐binding module 1. Environmental Microbiology, 19, 1914–1932. [DOI] [PubMed] [Google Scholar]
- Gui, Y.J., Zhang, W.Q., Zhang, D.D., Zhou, L., Short, D.P.G., Wang, J. et al. (2018) A Verticillium dahliae extracellular cutinase modulates plant immune responses. Molecular Plant‐Microbe Interactions, 31, 260–273. [DOI] [PubMed] [Google Scholar]
- Inderbitzin, P. & Subbarao, K.V. (2014) Verticillium systematics and evolution: how confusion impedes Verticillium wilt management and how to resolve it. Phytopathology, 104, 564–574. [DOI] [PubMed] [Google Scholar]
- Jacobs, K.A., Collins‐Racie, L.A., Colbert, M., Duckett, M., Golden‐Fleet, M., Kelleher, K. et al. (1997) A genetic selection for isolating cDNAs encoding secreted proteins. Gene, 198, 289–296. [DOI] [PubMed] [Google Scholar]
- Jagadeeswaran, G., Gainey, L. & Mort, A.J. (2018) An AA9‐LPMO containing a CBM1 domain in Aspergillus nidulans is active on cellulose and cleaves cello‐oligosaccharides. AMB Express, 8, 171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jonge, R., Peter van Esse, H., Maruthachalam, K., Bolton, M.D., Santhanam, P., Saber, M.K. et al. (2012) Tomato immune receptor Ve1 recognizes effector of multiple fungal pathogens uncovered by genome and RNA sequencing. Proceedings of the National Academy of Sciences of the United States of America, 109, 5110–5115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura, F., Hirashima, N., Furuno, T. & Nakanishi, M. (2006) Effects of 2‐methyl‐1,4‐naphtoquinone (menadione) on cellular signaling in RBL‐2H3 cells. Biological and Pharmaceutical Bulletin, 29, 605–607. [DOI] [PubMed] [Google Scholar]
- Keller, G.A., Warner, T.G., Steimer, K.S. & Hallewell, R.A. (1991) Cu, Zn superoxide dismutase is a peroxisomal enzyme in human fibroblasts and hepatoma cells. Proceedings of the National Academy of Sciences of the United States of America, 88, 7381–7385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khang, C.H., Park, S.Y., Lee, Y.H. & Kang, S. (2005) A dual selection based, targeted gene replacement tool for Magnaporthe grisea and Fusarium oxysporum . Fungal Genetics and Biology, 42, 483–492. [DOI] [PubMed] [Google Scholar]
- Kinseth, M.A., Anjard, C., Fuller, D., Guizzunti, G., Loomis, W.F. & Malhotra, V. (2007) The Golgi‐associated protein GRASP is required for unconventional protein secretion during development. Cell, 130, 524–534. [DOI] [PubMed] [Google Scholar]
- Klosterman, S.J., Atallah, Z.K., Vallad, G.E. & Subbarao, K.V. (2009) Diversity, pathogenicity, and management of Verticillium species. Annual Review of Phytopathology, 47, 39–62. [DOI] [PubMed] [Google Scholar]
- Klosterman, S.J., Subbarao, K.V., Kang, S., Veronese, P., Gold, S.E., Thomma, B.P.H.J. et al. (2011) Comparative genomics yields insights into niche adaptation of plant vascular wilt pathogens. PLoS Pathogens, 7, e1002137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan, K. & Askew, D.S. (2014) The fungal UPR: a regulatory hub for virulence traits in the mold pathogen Aspergillus fumigatus . Virulence, 5, 334–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krombach, S., Reissmann, S., Kreibich, S., Bochen, F. & Kahmann, R. (2018) Virulence function of the Ustilago maydis sterol carrier protein 2. New Phytologist, 220, 553–566. [DOI] [PubMed] [Google Scholar]
- Lamb, C. & Dixon, R.A. (1997) The oxidative burst in plant disease resistance. Annual Review of Plant Physiology and Plant Molecular Biology, 48, 251–275. [DOI] [PubMed] [Google Scholar]
- Lambou, K., Lamarre, C., Beau, R., Dufour, N. & Latge, J.P. (2010) Functional analysis of the superoxide dismutase family in Aspergillus fumigatus . Molecular Microbiology, 75, 910–923. [DOI] [PubMed] [Google Scholar]
- Larkin, M.A., Blackshields, G., Brown, N.P., Chenna, R., McGettigan, P.A., McWilliam, H. et al. (2007) Clustal W and Clustal X version 2.0. Bioinformatics, 23, 2947–2948. [DOI] [PubMed] [Google Scholar]
- Lehmann, S., Serrano, M., L'Haridon, F., Tjamos, S.E. & Metraux, J.P. (2015) Reactive oxygen species and plant resistance to fungal pathogens. Phytochemistry, 112, 54–62. [DOI] [PubMed] [Google Scholar]
- Li, F., Shi, H.Q., Ying, S.H. & Feng, M.G. (2015) Distinct contributions of one Fe‐ and two Cu/Zn‐cofactored superoxide dismutases to antioxidation, UV tolerance and virulence of Beauveria bassiana . Fungal Genetics and Biology, 81, 160–171. [DOI] [PubMed] [Google Scholar]
- Li, J.J., Zhou, L., Yin, C.M., Zhang, D.D., Klosterman, S.J., Wang, B.L. et al. (2019) The Verticillium dahliae Sho1‐MAPK pathway regulates melanin biosynthesis and is required for cotton infection. Environmental Microbiology, 21, 4852–4874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, S.‐Y., Chen, J.‐Y., Wang, J.‐L., Li, L., Xiao, H.‐L., Adam, S.M. et al. (2013) Molecular characterization and functional analysis of a specific secreted protein from highly virulent defoliating Verticillium dahliae . Gene, 529, 307–316. [DOI] [PubMed] [Google Scholar]
- Liu, T., Song, T., Zhang, X., Yuan, H., Su, L., Li, W. et al. (2014) Unconventionally secreted effectors of two filamentous pathogens target plant salicylate biosynthesis. Nature Communications, 5, 4686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak, K.J. & Schmittgen, T.D. (2001) Analysis of relative gene expression data using real‐time quantitative PCR and the 2−ΔΔCT method. Methods, 25, 402–408. [DOI] [PubMed] [Google Scholar]
- Lopez‐Cruz, J., Oscar, C.S., Emma, F.C., Pilar, G.A. & Carmen, G.B. (2017) Absence of Cu‐Zn superoxide dismutase BCSOD1 reduces Botrytis cinerea virulence in Arabidopsis and tomato plants, revealing interplay among reactive oxygen species, callose and signalling pathways. Molecular Plant Pathology, 18, 16–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyu, X., Shen, C., Fu, Y., Xie, J., Jiang, D., Li, G. et al. (2016) A small secreted virulence‐related protein is essential for the necrotrophic interactions of Sclerotinia sclerotiorum with its host plants. PLoS Pathogens, 12, e1005435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura, N. & Ueda, M. (2018) Evaluation of unconventional protein secretion by Saccharomyces cerevisiae and other fungi. Cells, 7, 128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore, S., de Vries, O.M. & Tudzynski, P. (2002) The major Cu, Zn SOD of the phytopathogen Claviceps purpurea is not essential for pathogenicity. Molecular Plant Pathology, 3, 9–22. [DOI] [PubMed] [Google Scholar]
- Mullins, E.D., Chen, X., Romaine, P., Raina, R., Geiser, D.M. & Kang, S. (2001) Agrobacterium‐mediated transformation of Fusarium oxysporum: an efficient tool for insertional mutagenesis and gene transfer. Phytopathology, 91, 173–180. [DOI] [PubMed] [Google Scholar]
- Narasipura, S.D., Ault, J.G., Behr, M.J., Chaturvedi, V. & Chaturvedi, S. (2003) Characterization of Cu, Zn superoxide dismutase (SOD1) gene knock‐out mutant of Cryptococcus neoformans var. gattii: role in biology and virulence. Molecular Microbiology, 47, 1681–1694. [DOI] [PubMed] [Google Scholar]
- Nickel, W. & Rabouille, C. (2009) Mechanisms of regulated unconventional protein secretion. Nature Reviews: Molecular Cell Biology, 10, 148–155. [DOI] [PubMed] [Google Scholar]
- Qin, J., Wang, K., Sun, L., Xing, H., Wang, S., Li, L. et al. (2018) The plant‐specific transcription factors CBP60g and SARD1 are targeted by a Verticillium secretory protein VdSCP41 to modulate immunity. eLife, 7, e34902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan, R.J., Sweeney, M.D., Lo Leggio, L., Otten, H., Poulsen, J.‐C.N., Johansen, K.S. et al. (2011) Insights into the oxidative degradation of cellulose by a copper metalloenzyme that exploits biomass components. Proceedings of the National Academy of Sciences of the United States of America, 108, 15079–15084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridout, C.J., Skamnioti, P., Porritt, O., Sacristan, S., Jones, J.D.G. & Brown, J.K.M. (2006) Multiple avirulence paralogues in cereal powdery mildew fungi may contribute to parasite fitness and defeat of plant resistance. The Plant Cell, 18, 2402–2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolke, Y., Liu, S., Quidde, T., Williamson, B., Schouten, A., Weltring, K.‐M. et al. (2004) Functional analysis of H2O2‐generating systems in Botrytis cinerea: the major Cu‐Zn‐superoxide dismutase (BCSOD1) contributes to virulence on French bean, whereas a glucose oxidase (BCGOD1) is dispensable. Molecular Plant Pathology, 5, 17–27. [DOI] [PubMed] [Google Scholar]
- Santhanam, P., van Esse, H.P. , Albert, I., Faino, L., Nurnberger, T. & Thomma, B.P. (2013) Evidence for functional diversification within a fungal NEP1‐like protein family. Molecular Plant‐Microbe Interactions, 26, 278–286. [DOI] [PubMed] [Google Scholar]
- Segal, L.M. & Wilson, R.A. (2018) Reactive oxygen species metabolism and plant–fungal interactions. Fungal Genetics and Biology, 110, 1–9. [DOI] [PubMed] [Google Scholar]
- Sheng, Y., Abreu, I.A., Cabelli, D.E., Maroney, M.J., Miller, A.‐F., Teixeira, M. et al. (2014) Superoxide dismutases and superoxide reductases. Chemical Reviews, 114, 3854–3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturtz, L.A., Diekert, K., Jensen, L.T., Lill, R. & Culotta, V.C. (2001) A fraction of yeast Cu, Zn‐superoxide dismutase and its metallochaperone, CCS, localize to the intermembrane space of mitochondria. A physiological role for SOD1 in guarding against mitochondrial oxidative damage. Journal of Biological Chemistry, 276, 38084–38089. [DOI] [PubMed] [Google Scholar]
- Sun, L., Qin, J., Rong, W., Ni, H., Guo, H.‐S. & Zhang, J. (2019) Cellophane surface‐induced gene, VdCSIN1, regulates hyphopodium formation and pathogenesis via cAMP‐mediated signalling in Verticillium dahliae . Molecular Plant Pathology, 20, 323–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, B., Yang, X., Zeng, H., Liu, H., Zhou, T., Tan, B. et al. (2012) The purification and characterization of a novel hypersensitive‐like response‐inducing elicitor from Verticillium dahliae that induces resistance responses in tobacco. Applied Microbiology and Biotechnology, 93, 191–201. [DOI] [PubMed] [Google Scholar]
- Wang, J., Ding, Y., Wang, J., Hillmer, S., Miao, Y., Lo, S.W. et al. (2010) EXPO, an exocyst‐positive organelle distinct from multivesicular endosomes and autophagosomes, mediates cytosol to cell wall exocytosis in Arabidopsis and tobacco cells. The Plant Cell, 22, 4009–4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, J., Tian, L., Zhang, D.D., Short, D.P.G., Zhou, L., Song, S.S. et al. (2018) SNARE‐encoding genes VdSec22 and VdSso1 mediate protein secretion required for full virulence in Verticillium dahliae . Molecular Plant‐Microbe Interactions, 31, 651–664. [DOI] [PubMed] [Google Scholar]
- Wang, Y., Wu, J., Park, Z.Y., Kim, S.G., Rakwal, R., Agrawal, G.K. et al. (2011) Comparative secretome investigation of Magnaporthe oryzae proteins responsive to nitrogen starvation. Journal of Proteome Research, 10, 3136–3148. [DOI] [PubMed] [Google Scholar]
- Wang, S., Xu, X.M., Liu, C.H., Shang, J.Y., Gao, F. & Guo, H.S. (2020) Verticillium dahliae chromatin remodeling facilitates the DNA damage repair in response to plant ROS. PLoS Pathogens, 16, e1008481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie, C.J., Wang, C.Y., Wang, X.K. & Yang, X.Y. (2013) Proteomics‐based analysis reveals that Verticillium dahliae toxin induces cell death by modifying the synthesis of host proteins. Journal of General Plant Pathology, 79, 335–345. [Google Scholar]
- Xu, L. & Chen, W. (2013) Random T‐DNA mutagenesis identifies a Cu/Zn superoxide dismutase gene as a virulence factor of Sclerotinia sclerotiorum . Molecular Plant‐Microbe Interactions, 26, 431–441. [DOI] [PubMed] [Google Scholar]
- Yao, S.H., Guo, Y., Wang, Y.Z., Zhang, D., Xu, L. & Tang, W.H. (2016) A cytoplasmic Cu‐Zn superoxide dismutase SOD1 contributes to hyphal growth and virulence of Fusarium graminearum . Fungal Genetics and Biology, 91, 32–42. [DOI] [PubMed] [Google Scholar]
- Zelko, I.N., Mariani, T.J. & Folz, R.J. (2002) Superoxide dismutase multigene family: a comparison of the CuZn‐SOD (SOD1), Mn‐SOD (SOD2), and EC‐SOD (SOD3) gene structures, evolution, and expression. Free Radical Biology and Medicine, 33, 337–349. [DOI] [PubMed] [Google Scholar]
- Zhang, L., Ni, H., Du, X., Wang, S., Ma, X.W., Nurnberger, T. et al. (2017) The Verticillium‐specific protein VdSCP7 localizes to the plant nucleus and modulates immunity to fungal infections. New Phytologist, 215, 368–381. [DOI] [PubMed] [Google Scholar]
- Zhao, J., Li, B., Huang, X., Morelli, X. & Shi, N. (2017) Structural basis for the interaction between golgi reassembly‐stacking protein GRASP55 and Golgin45. Journal of Biological Chemistry, 292, 2956–2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, P., Zhao, Y.L., Jin, Y., Zhang, T. & Guo, H.S. (2014) Colonization process of Arabidopsis thaliana roots by a green fluorescent protein‐tagged isolate of Verticillium dahliae . Protein Cell, 5, 94–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, Y.L., Zhou, T.T. & Guo, H.S. (2016) Hyphopodium‐specific VdNoxB/ VdPls1‐dependent ROS‐Ca2+ signaling is required for plant infection by Verticillium dahliae . PLoS Pathogens, 12, e1005793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, B.J., Jia, P.S., Gao, F. & Guo, H.S. (2012) Molecular characterization and functional analysis of a necrosis‐ and ethylene‐inducing, protein‐encoding gene family from Verticillium dahliae . Molecular Plant‐Microbe Interactions, 25, 964–975. [DOI] [PubMed] [Google Scholar]
- Zhou, L., Zhao, J., Guo, W. & Zhang, T. (2013) Functional analysis of autophagy genes via Agrobacterium‐mediated transformation in the vascular wilt fungus Verticillium dahliae . Journal of Genetics and Genomics, 40, 421–431. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
FIGURE S1 Full‐length sequence alignment and open reading frames (ORF) of VdSOD1
FIGURE S2 Bioinformatics prediction of secretory characteristics of VdSOD1. (a) Signal peptide prediction using SignalP 5.0 program. (b) Nonconventional secretion protein prediction by SecretomeP 2.0
FIGURE S3 Confirmation of VdSOD1 targeted deletion mutants and complementation strains in Verticillium dahliae. (a) Detection of the positive targeted VdSOD1 deletion strains by detection of the Hpt fragment. WT, wild‐type strain Vd991; KO1‐KO2, and two independent targeted deletion mutants of VdSOD1. (b) Detection of the positive targeted VdSOD1 deletion strains and complementation strains by amplifying a VdSOD1 internal fragment
FIGURE S4 The effect of H2O2 on growth rate of Verticillium dahliae. (a) The wild‐type strain Vd991, VdSOD1 deletion strains, and the complemented strains were cultured on PDA medium supplemented with H2O2 at specified concentrations for 9 days. (b) Colony diameters on potato dextrose agar plates containing different concentrations of H2O2 following 9 days of incubation. Means and standard deviations of the mean from three biological replicates are shown. Asterisks (**) denote significant differences (p < .01), according to Student’s t test
FIGURE S5 Expression levels of VdSOD1 in overexpression transformants. (a) Expression levels of VdSOD1 in wild‐type strain and WT::OESOD1 determined via quantitative reverse transcription PCR (RT‐qPCR). (b) Expression levels of VdSOD1 in ΔGRASP and ΔGRASP::OESOD1 via RT‐qPCR. The housekeeping gene elongation factor 1‐α (EF‐1α) was used as an endogenous control. Error bars represent the standard deviation of three replicate experiments and a double asterisk indicates the statistical significance (p < .01) by Student’s t test
FIGURE S6 VdSOD1 does not mediate cellophane membrane penetration in Verticillium dahliae. The wild‐type strain Vd991, VdSOD1 deletion strains, and the complemented strains were grown on the top of cellophane membranes overlaid onto minimal medium for 4 days at 25 °C (“Before” status). The cellophane membranes were then removed from the plates and incubated for an additional 3 days to determine the cellophane membrane penetration by the presence or absence of hyphal growth on medium (“After” status)
FIGURE S7 Quantification of fungal biomass in Verticillium dahliae‐infected cotton and Nicotiana benthamiana. Quantitative PCR was used to measure the fungal biomass of strains in the infected cotton (a) and N. benthamiana (b). Error bars represent standard deviations (n = 3). Asterisks (**) indicate significant differences (p < .01), according to Student’s t test
TABLE S1 Primers used in this study
TABLE S2 Strains used in this study
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


