Abstract
Background
Acute promyelocytic leukemia (APL) is one of the most life‐threatening hematological emergencies and requires a prompt correct diagnosis by cytomorphology and flow cytometry (FCM) with later confirmation by cytogenetics/molecular genetics. However, nucleophosmin 1 muted acute myeloid leukemia (NPM1+ AML) can mimic APL, especially the hypogranular variant of APL. Our study aimed to develop a novel, Radar plot‐based FCM strategy to distinguish APLs and NPM1+ AMLs quickly and accurately.
Method
Diagnostic samples from 52 APL and 32 NPM1+ AMLs patients were analyzed by a 3‐tube panel of 10‐color FCM. Radar plots combining all markers were constructed for each tube. Percentages of positive leukemic cells and mean fluorescence intensity were calculated for all the markers.
Results
APL showed significantly higher expression of CD64, CD2, and CD13, whereas more leukemic cells were positive for CD11b, CD11c, CD15, CD36, and HLA‐DR in NPM1+ AMLs. Radar plots featured CD2 expression, a lack of a monocytic component, lack of expression of HLA‐DR and CD15, and a lack of a prominent CD11c+ population as recurring characteristics of APL. The presence of blasts with low SSC, presence of at least some monocytes, some expression of HLA‐DR and/or CD15, and a prominent CD11c population were recurrent characteristics of NPM1+ AMLs. Radar plot analysis could confidently separate all hypergranular APL cases from any NPM1+ AML and in 90% of cases between variant APL and blastic NPM1+ AML.
Conclusion
Radar plots can potentially add to differential diagnostics as they exhibit characteristic patterns distinguishing APL and different types of NPM1+ AMLs.
Keywords: acute promyelocytic leukemia, flow cytometry, mean fluorescence intensity, NPM1 mutated acute myeloid leukemia, radar plot
1. INTRODUCTION
Acute promyelocytic leukemia (APL) with PML‐RARα is a well‐characterized distinct subtype of acute myeloid leukemia (AML) with frequent occurrence of disseminated coagulopathy, which makes their timely diagnosis critical in management of patients (Sanz et al., 2019). The characteristic cytomorphology suggests the possibility of APL with following reflex fluorescence in situ hybridization (FISH) or molecular testing for PML‐RARα for definitive confirmation of diagnosis (Arber et al., 2016). Three morphological variants: classic/hypergranular (APLc), variant/hypogranular (APLv), and the rare hyperbasophilic subtype have been described (Arber et al., 2016; Bain & Bene, 2019; WHO classification of tumours of haematopoietic and lymphoid tissue, 2017). Flow cytometry (FCM) is widely used as an important ancillary diagnostic method to confirm the lineage and/or maturation of leukemia and it contributes to correct definition of the disease entity (Porwit & Béné, 2018). There have been various studies describing the immunophenotypic features of APLs by FCM, stressing lack of CD34, HLA‐DR and CD11b expression together with a strong expression of myeloperoxidase by leukemic promyelocytes (Di Noto et al., 2007; Dong et al., 2011; Hrusak & Porwit‐MacDonald, 2002; Orfao et al., 1999; Paietta, 2003; Zhou et al., 2012). Immunophenotyping results in APLc and APLv were compared and related to results of molecular studies and to clinical outcomes (Foley et al., 2001; Gorczyca, 2012; Lin et al., 2004). However, some other subtypes of AML may mimic immunophenotypic features of APL being CD34‐negative and HLA‐DR‐negative. Notably, leukemic blasts are often CD34‐negative in nucleophosmin 1 mutated (NPM1+) AML that accounts for about one‐third of adult AML (Arber et al., 2016; Falini et al., 2007; Porwit & Béné, 2018). This may create confusion, especially if immunophenotyping is performed in a different laboratory than that evaluating the bone marrow (BM) cytomorphology. NPM1+ AMLs are heterogeneous due to its wide morphologic and mutational spectrum (Falini et al., 2007; Haferlach et al., 2009; Metzeler et al., 2016). Liu et al. (2013) evaluated the immunophenotypic features of different subtypes of NPM1+ AMLs depending upon the myeloid or monocytic differentiation of leukemia. Only a few studies associating immunophenotypic features with different subtypes of NPM1+ AMLs and FLT3 or IDH1/2 mutations have been published (Angelini et al., 2015; Mason et al., 2018).
The classical form of APL is usually easy to differentiate from NPM1+ AML by FCM due to characteristic high side scatter (SSC) (Macedo et al., 1995). However, detailed information concerning how to distinguish between the APLv and NPM1+ AMLs with a dominant immature myeloid (blastic) component (bNPM1+ AML) by FCM is scarce (Ferrari et al., 2012).
The purpose of this study was to create an easy way to distinguish APLs from NPM1+ AMLs by creating the Radar Plots (RPs) in order to integrate the characteristic features of each subtype and to facilitate quick and accurate identification of APL and NPM1+ AML related patterns. We have previously described normal BM patterns using a comprehensive 3‐tube 10‐color monoclonal antibody (Mab) panel (Jafari et al., 2018; Porwit & Rajab, 2015). Here, we found that RPs exhibit characteristic patterns in APL distinguishing APLc vs APLv subtypes as well as in two main subtypes of NPM1+ AMLs making quick differentiation between APLv and bNPM1+ AML possible.
2. MATERIALS AND METHODS
2.1. Sample preparation
Samples were analyzed within 24 h from collection. Samples were washed twice using phosphate‐buffered saline (PBS) to minimize nonspecific binding of antibodies (2 × 5 min, 540 g) and 100 μl (5 × 105 to 5 × 106 cells) were used per tube. Cells were incubated with the optimized Mab cocktails (Porwit & Rajab, 2015) (Table S1) for 15 min in the dark at room temperature and then lysed using VersaLyse® [Beckman Coulter, Miami, FL, (BC)]. After washing, samples were resuspended in IOTest 3 Fixative® (BC) and PBS. At least 105 events per tube were acquired on the Navios™ Flow cytometer (BC).
2.2. Instrument setup
Instrument setup, compensation, quality control, and validation process for Navios® (BC) were described in detail in the ICCS e‐newsletter 2014, vol. 5 no. 2 (http://www.cytometry.org/public/newsletters/eICCS-5-2/article2.php) and in previous publications (Lacombe et al., 2016; Rajab & Porwit, 2015). A brief description is provided in “Flow cytometry analysis” in Supporting Information.
2.3. Basic analysis
All analyses of the list‐mode files were carried on using the Kaluza software v.2.1 (BC). For basic analysis (Figure S1), the leukemic cell population was gated using bivariate dot plot CD33 versus side scatter (SSC). The percentage of leukemic cells expressing each of the markers was calculated on the histograms and used for comparisons between various categories of leukemia. Twenty percent of positive cells cut‐off was used to consider a particular case positive for the specific marker. The mean fluorescence intensity of the positive markers was also calculated on the histograms (Figure S1).
2.4. Radar plot analysis
Radar plot is a graphical method used to capture multidimensional data in a single plot available in the Kaluza software (BC). Different cell subsets and their patterns of maturation in normal BM can be shown in an integrated fashion on RPs (Jafari et al., 2018). In this study, RPs were constructed to combine and display characteristic immunophenotype of leukemic cells as well as of the maturing monocytic and granulocytic components. RPs for panel AML 1, 2, and 3 were optimized to declutter the cell subsets and to highlight markers of immaturity (Jafari et al., 2018). The process of optimization of RP required the subjective transformation of the axes to separate different maturing subsets (granulocytes and monocytes) as well as to separate and highlight markers of interest (Figures 1 and 3). To achieve this goal, we assigned specific color precedence in each tube to feature distinctive markers. Two RPs associated with the AML1 panel feature maturing granulocytic and monocytic component as well as the presence (or absence) of CD34 and CD117 expression on leukemic cells. The RP of AML2 panel highlights HLA‐DR expression on the leukemic cells. The RP of AML3 panel highlights CD15, CD2, and CD11c expression on the leukemic cells. The same optimized RPs were used to analyze all studied samples (Figure 2).
FIGURE 1.
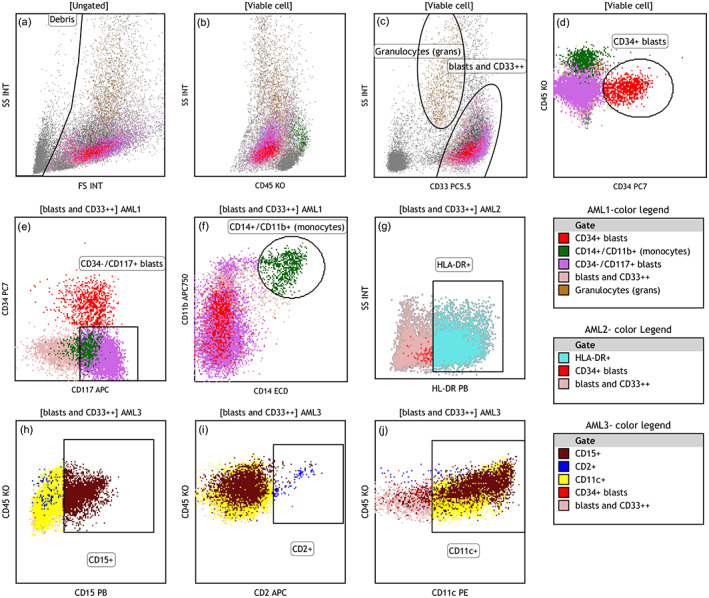
Clustering and color‐coding strategy for Radar Plot analysis exemplified using a case of blastic NPM1+ AML: (a) debris and erythrocyte aggregates were excluded. (b) “viable cell” gate on SSC/CD45 plot with CD34+ blasts shown as red dots (see d) and monocytes as green dots (see f) (c) granulocytes were separated by intermediate CD33 expression and high SSC (brown dots); other CD33+ myeloid cells were gated as “blasts and CD33++” and painte pale pink. Later the pink color was superseded by other distinctive markers in different colors. (d) CD34+ cell cluster (red dots) in AML1 panel (e) CD34−/CD117+ cell cluster (bright pink) in AML1 panel. (f) CD11b+/CD14+ monocytes were painted as green dots in AML1 panel, dim CD117 (bright pink) is seen on a fraction of monocytes (g) HLA‐DR positive leukemic cells (cyan dots) seen in AML2 panel. (h–j) expression of CD15 and CD11c is seen on leukemic cells (maroon and yellow dots, respectively) while CD2 was negative (dark blue dots). Color legends are arranged based on descending color precedence in each panel with intent to highligh distinctive markers [Color figure can be viewed at wileyonlinelibrary.com]
FIGURE 2.
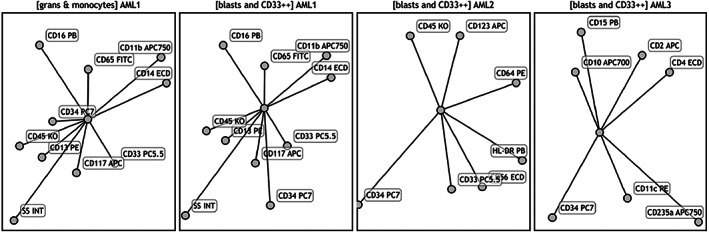
Radar plots used for analysis of all studied acute promyelocytic leukemia and NPM1+ acute myeloid leukemia cases
2.4.1. Clustering and color‐coding strategy
The sequential gating strategy was used as illustrated in Figure 1, 2:
Debris was excluded on SSC/FSC plot,
Mature granulocytes were separated on SSC/CD33 dot plot based on high SSC and intermediate CD33 expression and color‐coded (brown).
On the same dot plot, the remaining myeloid cells were gated as “blasts (CD45dim/SSClow) and CD33+ + .” This gate included all CD33 positive cells with low SSC and cells with bright CD33 expression with intermediate‐high scatter. This cluster was given a background color (light pink), which was superseded later with colors associated with other markers such as CD34 or CD117 (see below).
In the panel AML1 (Table S1), monocytes with bright CD14 and 11b expression were separated from “blasts and CD33+ + ” gate and color‐coded (green).
Expression of CD34 and CD117 on blasts cells were assessed and if present, the clusters were color‐coded individually (red and bright pink, respectively).
In the panel AML2 (Table S1), expression of HLA‐DR was assessed, and color‐coded (cyan).
In the panel AML3 (Table S1), expression of CD2 (dark blue), CD11c (yellow), and CD15 (maroon) were assessed and color‐coded individually.
The unique features that were helpful to differentiate between APL and NPM1 AML using RPs were identified as follows: SSC characteristics, presence of a monocytic cluster, expression of CD34, HLA‐DR, CD2, CD11c, and CD15 (exemplified in Figure 3 showing RPs in a case of bNPM1+ AML).
FIGURE 3.
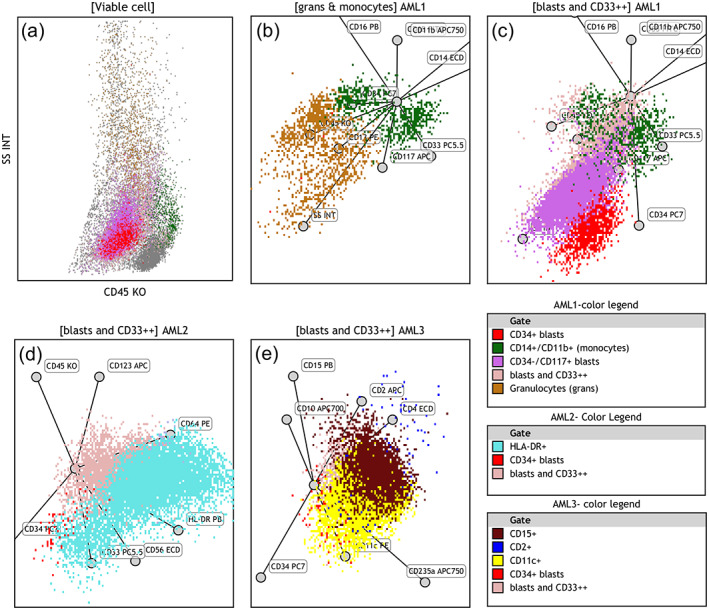
Radar plot analysis of leukemia exemplified using a case of blastic NPM1+ AML: SSC/CD45 plot and four constructed RPs show features of an NPM1+ AML case: (a) Low SSC blast population (CD117+ and partly CD34+) with intermixed granulocytes and monocytes on SSC/CD45 plot. (b) First RP of AML1 panel presenting maturing granulocytic cells (brown dots) and CD11b+/ CD14+ monocytic cells (green dots), blasts are excluded. (c) Second RP of AML1 panel presents CD117+ blasts (bright pink dots) with partial CD34 expression (red dots). (d) RP of AML2 panel highlights partial HLA‐DR expression on leukemic cells (cyan dots). (e) RP of AML3 panel features aberrant expression of CD15 (maroon dots) and CD11c (yellow dots) on blast population. Axes of RPs are arranged as shown in Figure 2 Color legends are arranged based on descending color precedence in each panel with intent to highlight distinctive markers. AML, acute myeloid leukemia; RP, radar plot [Color figure can be viewed at wileyonlinelibrary.com]
2.5. Molecular studies
Molecular analyses for PML‐RARα, ΝPΜ1, and FLT3 mutations were performed as described in Molecular studies in Supporting Information).
2.6. Statistics
Statistical analysis was performed using IBM SPSS Statistics for Windows Software, Version 23.0 (IBM Corp, Armonk, NY). Distribution of the results within subsets of studied leukemias was evaluated using the Kolmogorov–Smirnov test. Results were analyzed using the two‐tailed Student's t test for variables with normal distribution, the Mann–Whitney nonparametric test for those with non‐normal distribution, and the Chi‐square with the Fisher exact test. Due to non‐normal distribution in most markers, we used the non‐parametric Kruskall‐Wallis test for independent samples with post hoc pairwise multiple comparisons for all comparisons between more than two subsets of studied leukemias. p values <.05 were considered as associated with statistical significance.
3. RESULTS
Our cohort included consecutive samples from 52 patients with APL and 32 samples of NPM1+ AML. APL patients had a median age of 54 years (range 21–83, M:F ratio of 1.2). NPM1+ AML patients had a median age of 58 years (range 20–82, M:F ratio of 0.6). By cytomorphology, there were 33 (63%) APLc and 19 (37%) APLv cases. Two APL patients had previous chemotherapy and/or radiotherapy, thus, a therapy‐related myeloid neoplasm (t‐APL) diagnosis was rendered (WHO classification of tumours of haematopoietic and lymphoid tissue, 2017). One of the t‐APLs was APLc and one was APLv. Molecular studies revealed BCR1 PML‐RARα transcripts in 20 APLc and 9 APLv, BCR2 transcripts in 2 APLc, and BCR3 transcripts in 11 APLc and 10 APLv. Both t‐APLs had BCR3 transcripts. Fourteen of 32 NPM1+ AMLs (44%) were positive for FLT3 mutations (Table S2) with no difference between male and female patients.
3.1. Basic characteristics of APL and NPM1+ AML
3.1.1. Immunophenotypic characteristics of APL cases
Results of conventional immunophenotyping in APL cases are given in detail in Tables 1 and S3. Consistent with hypergranular versus hypogranular morphological characteristics, SSC was significantly higher in APLc than APLv (p < .001). CD13, CD33, and CD45 were positive in all APLs. CD19, CD14, CD16, and CD36 were negative in all cases. CD117 was positive in 94% of cases. When the frequency of positive cells was compared, CD34 and CD2 were more often found in variant APLs (p = .003 and p = .025, respectively). Using the 20% cut‐off, CD11c expression was noted in 50% of APLs, CD123 in 40%, and CD56 in 17% with no significant difference between APLc and APLv. Using the 20% cut‐off, HLA‐DR was found to be positive in 2 cases (4%) and CD11b in 3 cases (5%) of APLs. None of the APLv showed expression of CD15 and only two APLc were positive. CD7 was found only in 3 APLc and one APLv. Immunophenotyping results in therapy‐related APLs were similar as in de novo cases.
TABLE 1.
Significant differences in marker expression between total cohorts of acute promyelocytic leukemia and NPM1+ acute myeloid leukemia
| Marker | APL mean % positive cells in CD33+/++ gate (range) | APL no. of positive cases (%) | NPM1+ AML mean % positive cells in CD33+/++ gate (range) | NPM1+ AML no. of positive cases* (%) | p value for % positive cells in CD33+/++ gate | p value for MFI |
|---|---|---|---|---|---|---|
| CD2 | 26% (0–86%) | 22 (42.3) | <1 | 0 | .001 | NA |
| CD11b | 5% (0–65%) | 3 (5.8) | 27% (1–95%) | 14 (44) | <.001 | NS |
| CD11c | 36% (0–97%) | 26 (50) | 72% (3–96%) | 29 (91) | <.001 | <.001 |
| CD13 | 97% (67–100%) | 52 (100) | 69% (2–100%) | 29 (91) | <.001 | <.001 |
| CD14 | <1 | 0 | 11% (0–93%) | 5 (16) | <.001 | NA |
| CD15 | 1% (0–30%) | 2 (3.8) | 19.5% (0–74%) | 12 (37) | <.001 | <.001 |
| CD36 | <1 | 0 | 27% (0–95%) | 15 (47) | <.001 | NA |
| CD64 | 95% (71–100%) | 52 (100) | 55% (0–99%) | 26 (81.3) | <.001 | .034 |
| CD123 | 25% (0–88%) | 17 (33%) | 37% (0–96%) | 20 (63) | .034 | .015 |
| HLA‐DR | 21% and 43% | 2 (3.9) | 37% (0–88%) | 20 (63) | <.001 | NS |
Note: APL n = 52, NPM1 AML n = 32.
Abbreviations: AML, acute myeloid leukemia; APL, acute promyelocytic leukemia; MFI, mean fluorescence intensity; NA, not available; NS, not significant, Mann–Whitney test.
Immunophenotypic characteristics and PML‐RARα transcripts
There was no significant correlation between PML‐RARα transcript type and hyper‐ versus hypogranular APL cytomorphology. We did not find any significant difference between PML‐RARα transcript type and expression of any specific marker.
3.1.2. Immunophenotypic characteristics of NPM1+ AML cases
Since there are no defined cytomorphological subsets of NPM1+ AML (WHO classification of tumours of haematopoietic and lymphoid tissue, 2017), we have divided NPM1+ AML cases into three categories depending on scatter characteristics and lineage involvement. Blastic NPM1+ AMLs (bNPM1+, n = 17) were defined as those with a dominant immature component and only small or no monocytic components. Myelomonocytic NPM1+ AMLs (n = 12) were cases with a small blastic component and prominent monocytic component. Three NPM1+ AMLs were purely monocytic. Myelomonocytic and monocytic NPM1+ AMLs were considered as one group (mNPM1+ AML) in further analysis.
Results of conventional immunophenotyping in NPM1+ AML cases are given in detail Tables 1 and S4. CD11c, CD13, CD33, and CD38 were positive in most cases while CD2 was negative in most NPM1+ AMLs. CD11b was more often expressed in mNPM1+ AMLs (p = .04) and CD34 slightly more often seen in bNPM1+ AMLs. CD117 was found to be positive in all bNPM1+ AML as compared to 80% mNPM1+ AML (p = .004). CD36 was seen slightly more often in mNPM1 than in bNPM1 AML (p = .041). mNPM1+ AML was characterized by stronger CD13 expression than bNPM1+ AML (p = .02).
Immunophenotypic markers in NPM1+ AMLs with and without FLT3 mutations
There was no correlation between the presence of any FLT3 mutation and immunophenotypic category of NPM1+ AML. The percentages of CD34, CD117, and CD123 positive leukemic cells were significantly higher in FLT3 mutated NPM1+ AMLs than in unmutated ones (Table S5). Cases with no FLT3 mutation had a slightly higher percentage of CD64+ cells (p = .04). There was no significant correlation between any other marker expression and FLT3 mutational status.
3.1.3. Differential diagnosis between APL and NPM1+ AML
The results of the comparison between the total cohorts of APL and NPM1+ AML are given in Table 1. A higher frequency of leukemic cells positive for CD2, CD13, and CD64 was found in APLs than NPM1+ AMLs (p = .001). CD11b, CD11c, CD14, CD15, CD36, HLA‐DR, and CD38 were significantly more positive in NPM1+ AMLs than in APLs (p < .001, for all).
We have also compared the two subtypes of APLs with two subtypes of NPM1+ AML separately (Table 2). Focusing on the comparison between APLv and bNPM1+ AML that are the most difficult to differentiate by immunophenotype, we found that leukemic cells were significantly more positive for CD4, CD11c, CD15, CD36, and CD123 in bNPM1+ AML (Table 2).
TABLE 2.
Differences in frequency of positive leukemic cells between studied subtypes of acute promyelocytic leukemia and NPM1+ acute myeloid leukemiaa
| Marker | p value bNPM1 versus APLc | p value bNMP1+ versus APLv | p value mNMP1 versus APLc | p value mNPM1 versus APLv |
|---|---|---|---|---|
| CD2 | .003 | NS | <.001 | .015 |
| CD4 | .005 | <.001 | .017 | .001 |
| CD11b | NS | NS | <.001 | <.001 |
| CD11c | .001 | .009 | <.001 | .006 |
| CD13 | <.001 | <.001 | .001 | <.001 |
| CD14 | .007 | .016 | <.001 | <.001 |
| CD15 | <.001 | <.001 | <.001 | <.001 |
| CD34 | NS | NS | .012 | NS |
| CD36 | NS | .003 | NS | <.001 |
| CD56 | NS | .029 | NS | .042 |
| CD64 | <.001 | <.001 | .011 | <.001 |
| CD71 | NS | <.001 | .042 | <.001 |
| CD117 | NS | NS | .002 | .002 |
| CD123 | NS | .005 | NS | NS |
| HLA‐DR | NS | <.001 | .002 | <.001 |
Note: CD7, CD10, CD19, CD38, CD65 NS for all.
Kruskall‐Wallis test for independent samples.
3.2. Radar plot analysis of APL cases
We have identified recurring characteristics in APL cases and displayed those in four radar plots (Figure 4). The following features: the absence of monocytes, the lack of HLA‐DR and CD15 expression, and a lack of a prominent CD11c+ population were common in both classic and variant APLs. Three other characteristics, seen in 70% of cases classified by cytomorphology as APLc and easily identified on RPs, included: high SSC, lack of CD34 expression and CD2 expression (Figure 4, upper row). Seventy four percent of cases classified by cytomorphology as APLv presented with intermediate SSC and at least some expression of CD34 and CD2 (Figure 4, middle row). Thirty percent of cases defined by cytomorphology as APLc presented with RP features overlapping with APLv, including intermediate SSC and partial expression of CD34 and CD2 (Figure 4, lower row). Thus, the immunophenotypic characteristics by RP correlated with cytomorphology in approximately 70% of APL cases.
FIGURE 4.
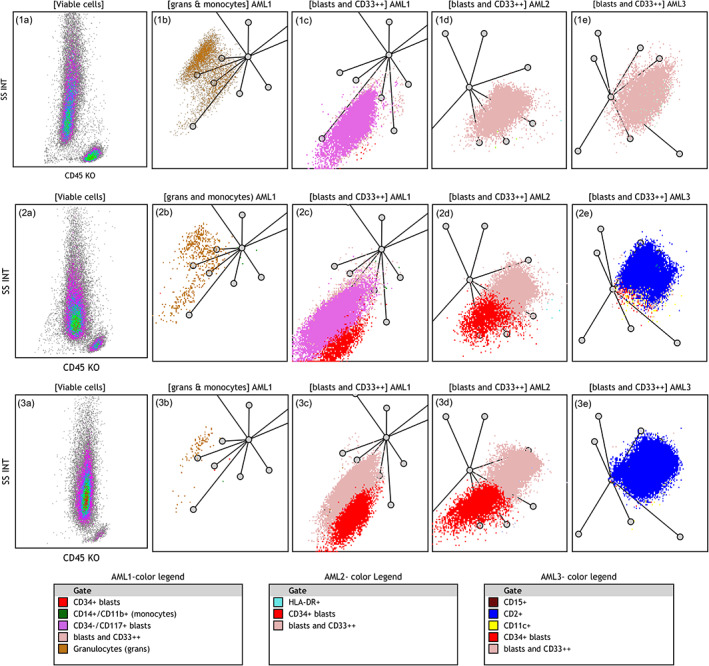
Characteristic features of APL cases on radar plots: The upper row shows typical features of a classic APL case: (1a) Leukemic cells with high SSC. (1b) First RP in AML1 panel highlights lack of monocytes (absence of the green cluster). (1c) Second RP in AML1 panel shows CD117+ leukemic cells (the bright pink cluster) and lack of CD34 expression (absence of the red cluster). (1d) RP in AML2 panel shows lack of HLA‐DR expression (absence of the cyan cluster). (1e) RP in AML3 panel highlights lack of CD2 expression on leukemic cells (absence of the dark blue cluster). The middle row shows characteristic features of a variant APL case: (2a) Intermediate SSC. (2b) Lack of monocytes. (2c) CD117+ (the bright pink cluster) and partial CD34 expression in the blast population (the red cluster). (2d) Lack of HLA‐DR expression. (2e) CD2 expression on leukemic cells (the dark blue cluster). The lower row shows a case of APL diagnosed as classic APL by morphology but with some immunophenotype features of vAPL. (3a) Intermediate SSC. (3b) Very few maturing granulocytes and lack of monocytes (3c) Partial CD34 expression (red) but negative CD117 on blast cells (the pale pink cluster). (3d) Lack of HLA‐DR expression. (3e) Partial CD2 expression on leukemic cells (the dark blue cluster). Axes of RPs are arranged as shown in Figure 2. Color legends are arranged based on descending color precedence in each panel with intent to highlight distinctive markers. AML, acute myeloid leukemia; APL, acute promyelocytic leukemia; RP, radar plot [Color figure can be viewed at wileyonlinelibrary.com]
3.2.1. Radar plot analysis of NPM1+ AML cases
The RP characteristics of NPM1+ AML, which were useful for differentiation from APL, included the presence of blasts with low SSC, presence of at least some monocytic cluster or pronounced monocytic differentiation, at least some expression of HLA‐DR, CD15, or a prominent CD11c population. Eighty five percent (27/32) of the cases in our cohort presented three or more of these features. Figure 5 shows three patterns found in NPM1+ AML. The upper row shows a case with purely monocytic differentiation. The blast population was negative for CD34 and CD117, expressed HLA‐DR, CD11c, and showed partial expression of CD15. The middle row shows a typical case of bNPM1+ AML with a dominant population of blasts with low SSC and variable expression of CD34, CD117, HLA‐DR, and CD11c but without a prominent monocytic component. The lower row shows a typical pattern in a case with myelomonocytic differentiation. The blast population shows low SSC with variable CD34, CD117, HLA‐DR, and CD11c expression, with no overt CD15 expression and an easily defined monocytic component. However, in 3 cases of bNPM1+ AML, the main differentiating feature against APLv was low scatter (supported by a minor CD11c+ population in one case). The final classification by FCM was less certain in these cases (exemplified in Figure 6). Five cases of APLv (26%) with no CD2 expression could still be distinguished from NPM1+ AML based on intermediate scatter and lack of monocytic component. Thus, RP analysis of our 3‐tube 10‐color panel could confidently separate all APLc cases from any NPM1+ AML and in 90% of cases between APLv and bNPM1+ AML.
FIGURE 5.
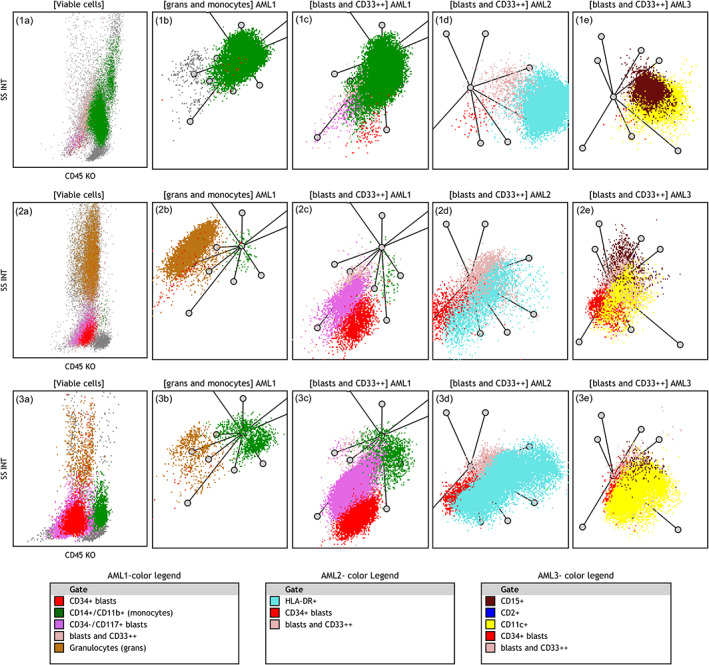
Characteristics features of NPM1+ AML cases on radar plots: The distinctive features include low SSC, monocytic differentiation or prominent monocyte component, complete or partial expression of HLA‐DR, CD11c and CD15 on blast population. Three different patterns of NPM1+ AML are shown. The upper row shows a case with purely monocytic differentiation (1a–1c) CD11b+/CD14+ monocytic cells (seen as green dots) on SSC/CD45 and in two AML1 panel RPs. Only minimal CD34+ and CD117+ populations are seen (red and bright pink dots). (1d) HLA‐DR expression on leukemic cells (cyan dots). (1e) Leukemic cells express CD15 (maroon dots) and CD11c+ (yellow dots). The middle row shows a case of blastic NPM1+ AML with almost no monocytic component: (2a) Low SSC/CD45dim blasts merging with maturing granulocytes. (2b) Granulocytes (brown dots) with minimal monocyte population (green dots). (2c) CD117 and partial CD34 expression (bright pink and red clusters). (2d) Only partial HLA‐DR expression in blast population (cyan dots). (2e) Low CD15 expression (maroon dots) and partial CD11c expression (yellow dots). The lower row shows a case of myelomonocytic NPM1+ AML: (3a–c) Low SSC, CD117+ and partly CD34+ blasts (pink/red dots) and a distinct monocytic population (green dots). (3d) Uniform expression of HLA‐DR (the cyan cluster). (3e) Uniform expression of CD11c (the yellow cluster but no CD15 or CD2 expression). Axes of RPs are arranged as shown in Figure 2. Color legends are arranged based on descending color precedence in each panel with intent to highlight distinctive markers. AML, acute myeloid leukemia; RP, radar plot [Color figure can be viewed at wileyonlinelibrary.com]
FIGURE 6.
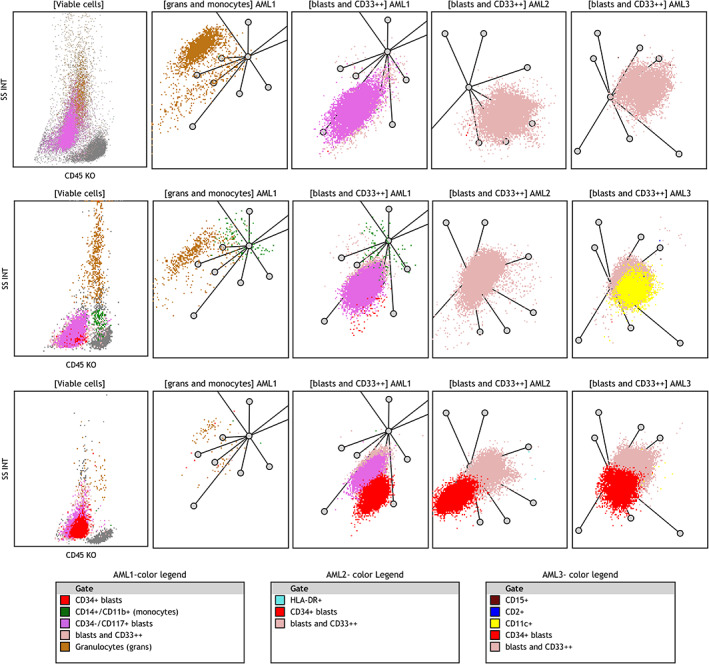
Examples of rare cases where differentiation between vAPL and NPM‐1+ AML was more difficult on RPs. Upper row illustrates a case of variant APL and middle and lower rows show two cases of blastic NPM1+ AML. These three cases show similar features with minor differences and did not show all distinctive features associated with each entity. The APL‐variant case in the upper row shows intermediate SSC on SSC/CD45 plot and lack of monocytes as APL‐related features. The NPM1+ AML case in the middle row shows low SSC and expression of CD11c on leukemic cells (yellow dots in AML3 ‐RP) as a characteristic feature. The NPM1+ AML case in the lower row shows low SSC and a partial expression of CD34 on leukemic cells (the red cluster). Axes of RPs are arranged as shown in Figure 2. Color legends are arranged based on descending color precedence in each panel with intent to highlight distinctive markers. AML, acute myeloid leukemia; APL, acute promyelocytic leukemia; RP, radar plot [Color figure can be viewed at wileyonlinelibrary.com]
4. DISCUSSION
In clinical practice, blood and BM cytology alone is sufficient to make the preliminary diagnosis of most APLc, but the cytological diagnosis of APLv may be challenging (Bain & Bene, 2019). FCM results usually are available before FISH and molecular results, so it is helpful to utilize FCM findings in the preliminary leukemia diagnosis. NPM1+ AML can mimic the hypogranular variant of APL by both cytology and immunophenotype. Our goal was to develop a novel method for quick evaluation of FCM data, which would help to differentiate between these two subtypes of leukemia.
Frequently described immunophenotypic features of APL are: positivity for CD117, strong expression of CD33 and myeloperoxidase, and lack of CD34, HLA‐DR, CD15, CD11b, and CD11c on leukemic cells (Orfao et al., 1999; Paietta, 2003). However, the ATRA treatment may upregulate the expression of integrins, seen in diagnostic samples collected after treatment was started (Di Noto et al., 1994). This phenomenon may explain the presence of small CD11c positive populations noted in some of our APL cases. In agreement with previous reports, we found an association between CD34 and CD2 expression and APLv. However, we did not see any significant difference between cases carrying various transcripts.
Based on primary immunohistochemical studies, NPM1 mutated AML has been described as a CD34+ and HLA‐DR negative leukemia with frequent monocytic differentiation (Falini et al., 2008). Later FCM studies confirmed this general association. However, a fraction of NPM1+ AML cases (20–28%) shows CD34 and/or HLA‐DR expression in at least a subpopulation of blasts (Angelini et al., 2015; Martelli et al., 2010; Zhu et al., 2013). CD34+ cells belong to the leukemic clone even when present at low frequency (<10%) (Dang et al., 2013). In some studies, the presence of FLT3 mutation was associated with CD34, CD7, HLA‐DR, and CD123 expression (Angelini et al., 2015; Martelli et al., 2010). Positivity for CD34, CD7, and HLA‐DR was also related to a worse prognosis (Dang et al., 2013). In agreement with the previous publications, we have found two distinct immunophenotypic groups of NPM1+ AML: one with blastic characteristics and one showing various levels of monocytic differentiation. CD117, CD34, and CD123 were significantly more often positive in FLT3 mutated NPM1+ AMLs.
In our previous studies, we have applied RP analysis to normal BM samples to capture the multidimensionality of FCM data (Jafari et al., 2018; Violidaki et al., 2020). We have displayed various cell subsets in clusters on RPs and were able to illustrate the maturation patterns in both myelomonocytic and erythroid lineages. The strength of RP analysis lies in visualizing all markers applied in one multicolor combination of antibodies in one multidimensional plot. In the present study, combinations of the phenotypic characteristics of each leukemia case were visualized on multivariate RPs created for three panels (AML 1–3), which were applied at diagnosis. These characteristics included markers of immaturity (CD34 and CD117), and the specific immunophenotype (positivity for HLA‐DR, CD15, CD2, and a prominent CD11c cluster). Also, we recognized that the maturing BM component varies in APL and NMP1+ AMLs. Therefore, we integrated the maturing granulocytes and monocytes present in the sample into the RPs. The presence of a monocytic cluster was a characteristic feature in most NMP1+ AMLs.
Using constructed RPs, all APLc and most of APLv could be differentiated easily from NPM1+ AML. Rare cases of blastic NPM1+ AML with no clear monocytic differentiation, expression of CD34, lack of HLA‐DR, and lack of CD15, could still be differentiated from APLv based on low scatter. Similarly, rare APLv cases that lacked expression of CD2 and showed low expression of CD11c and CD15, had intermediate scatter characteristics that differed from the bNPM1+ AML.
One previous study described a unique localization of APL on RPs applying a four‐tube 8‐color FCM panel including cytoplasmic myeloperoxidase. A limited number of APL (n = 8) and NPM1+ AML samples were compared (n = 12) (Karai et al., 2019). The authors defined a characteristic location of APL on created RPs using merged files for hypergranular (n = 6) and hypogranular (n = 2) APLs. NPM1+ AMLs were usually located in different areas with some overlap. We have chosen to analyze each case individually, and we have incorporated color coding to visualize distinctive markers on RPs and to use them to distinguish these two entities.
In conclusion, we have shown that radar plots enable us to display integrated FCM characteristics for APL and NPM1+ AML. RP analysis may facilitate the appropriate selection of cases for further investigation by FISH, molecular analysis, and immunofluorescence staining with the anti‐PML antibody (Falini et al., 1997).
Supporting information
Appendix S1: Supplementary Information
Suppl. Figure 1Example of basic analysis of marker expression in panel AML 2. After dead cells were excluded the gate was set on CD33+ /+ + cells independent of scatter (upper panel left). Upper middle panel shows backgating of the CD33+ /+ + gate on SSC/CD45 plot. Percentages of leukemic cells positive for various markers were analysed using histograms with the linear gate set using the level of autofluorescence and/or negative populations present in the sample as cut‐off on the left. A case of hypergranular APL is shown in A, a hypogranular APL in B and a NPM‐1+ AML with myelomonocytic pattern in C
Gupta M, Jafari K, Rajab A, et al. Radar plots facilitate differential diagnosis of acute promyelocytic leukemia and NPM1+ acute myeloid leukemia by flow cytometry. Cytometry. 2021;100:409–420. 10.1002/cyto.b.21979
Monali Gupta and Katayoon Jafari contributed equally to this study.
Funding information Lund University Medical Faculty Foundation
REFERENCES
- Angelini, D. F., Ottone, T., Guerrera, G., Lavorgna, S., Cittadini, M., Buccisano, F., de Bardi, M., Gargano, F., Maurillo, L., Divona, M., Noguera, N. I., Consalvo, M. I., Borsellino, G., Bernardi, G., Amadori, S., Venditti, A., Battistini, L., & Lo‐Coco, F. (2015). A leukemia‐associated CD34/CD123/CD25/CD99+ immunophenotype identifies FLT3‐mutated clones in acute myeloid leukemia. Clinical Cancer Research, 21, 3977–3985. [DOI] [PubMed] [Google Scholar]
- Arber, D. A., Orazi, A., Hasserjian, R., Thiele, J., Borowitz, M. J., Le Beau, M. M., Bloomfield, C. D., Cazzola, M., & Vardiman, J. W. (2016). The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood, 127, 2391–2405. [DOI] [PubMed] [Google Scholar]
- Bain, B. J., & Bene, M. C. (2019). Morphological and immunophenotypic clues to the WHO categories of acute myeloid leukaemia. Acta Haematologica, 141, 232–244. [DOI] [PubMed] [Google Scholar]
- Dang, H., Chen, Y., Kamel‐Reid, S., Brandwein, J., & Chang, H. (2013). CD34 expression predicts an adverse outcome in patients with NPM1‐positive acute myeloid leukemia. Human Pathology, 44, 2038–2046. [DOI] [PubMed] [Google Scholar]
- Di Noto, R., Mirabelli, P., & Del Vecchio, L. (2007). Flow cytometry analysis of acute promyelocytic leukemia: The power of 'surface hematology'. Leukemia, 21, 4–8. [DOI] [PubMed] [Google Scholar]
- Di Noto, R., Schiavone, E. M., Ferrara, F., Manzo, C., Lo Pardo, C., & Del Vecchio, L. (1994). Expression and ATRA‐driven modulation of adhesion molecules in acute promyelocytic leukemia. Leukemia, 8, 1900–1905. [PubMed] [Google Scholar]
- Dong, H. Y., Kung, J. X., Bhardwaj, V., & McGill, J. (2011). Flow cytometry rapidly identifies all acute promyelocytic leukemias with high specificity independent of underlying cytogenetic abnormalities. American Journal of Clinical Pathology, 135, 76–84. [DOI] [PubMed] [Google Scholar]
- Falini, B., Flenghi, L., Fagioli, M., Coco, F. L., Cordone, I., Diverio, D., Pasqualucci, L., Biondi, A., Riganelli, D., Orleth, A., Liso, A., Martelli, M. F., Pelicci, P. G., & Pileri, S. (1997). Immunocytochemical diagnosis of acute promyelocytic leukemia (M3) with the monoclonal antibody PG‐M3 (anti‐PML). Blood, 90, 4046–4053. [PubMed] [Google Scholar]
- Falini, B., Mecucci, C., Saglio, G., Lo Coco, F., Diverio, D., Brown, P., Pane, F., Mancini, M., Martelli, M. P., Pileri, S., Haferlach, T., Haferlach, C., & Schnittger, S. (2008). NPM1 mutations and cytoplasmic nucleophosmin are mutually exclusive of recurrent genetic abnormalities: A comparative analysis of 2562 patients with acute myeloid leukemia. Haematologica, 93, 439–442. [DOI] [PubMed] [Google Scholar]
- Falini, B., Nicoletti, I., Bolli, N., Martelli, M. P., Liso, A., Gorello, P., Mandelli, F., Mecucci, C., & Martelli, M. F. (2007). Translocations and mutations involving the nucleophosmin (NPM1) gene in lymphomas and leukemias. Haematologica, 92, 519–532. [DOI] [PubMed] [Google Scholar]
- Ferrari, A., Bussaglia, E., Ubeda, J., Facchini, L., Aventin, A., Sierra, J., & Nomdedéu, J. F. (2012). Immunophenotype distinction between acute promyelocytic leukaemia and CD15‐ CD34‐ HLA‐DR‐ acute myeloid leukaemia with nucleophosmin mutations. Hematological Oncology, 30, 109–114. [DOI] [PubMed] [Google Scholar]
- Foley, R., Soamboonsrup, P., Carter, R. F., Benger, A., Meyer, R., Walker, I., Wan, Y., Patterson, W., Orzel, A., Sunisloe, L., Leber, B., & Neame, P. B. (2001). CD34‐positive acute promyelocytic leukemia is associated with leukocytosis, microgranular/hypogranular morphology, expression of CD2 and bcr3 isoform. American Journal of Hematology, 67, 34–41. [DOI] [PubMed] [Google Scholar]
- Gorczyca, W. (2012). Acute promyelocytic leukemia: Four distinct patterns by flow cytometry immunophenotyping. Polish Journal of Pathology, 63, 8–17. [PubMed] [Google Scholar]
- Haferlach, C., Mecucci, C., Schnittger, S., Kohlmann, A., Mancini, M., Cuneo, A., Testoni, N., Rege‐Cambrin, G., Santucci, A., Vignetti, M., Fazi, P., Martelli, M. P., Haferlach, T., & Falini, B. (2009). AML with mutated NPM1 carrying a normal or aberrant karyotype show overlapping biologic, pathologic, immunophenotypic, and prognostic features. Blood, 114, 3024–3032. [DOI] [PubMed] [Google Scholar]
- Hrusak, O., & Porwit‐MacDonald, A. (2002). Antigen expression patterns reflecting genotype of acute leukemias. Leukemia, 16, 1233–1258. [DOI] [PubMed] [Google Scholar]
- Jafari, K., Tierens, A., Rajab, A., Musani, R., Schuh, A., & Porwit, A. (2018). Visualization of cell composition and maturation in the bone marrow using 10‐color flow cytometry and radar plots. Cytometry Part B, Clinical Cytometry, 94, 219–229. [DOI] [PubMed] [Google Scholar]
- Karai, B., Habok, M., Remenyi, G., Rejto, L., Ujfalusi, A., Kappelmayer, J., & Hevessy, Z. (2019). A novel flow cytometric method for enhancing acute promyelocytic leukemia screening by multidimensional dot‐plots. Annals of Hematology, 98, 1413–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacombe, F., Bernal, E., Bloxham, D., Couzens, S., Porta, M. G., Johansson, U., Kern, W., Macey, M., Matthes, T., Morilla, R., Paiva, A., Palacio, C., Preijers, F., Ratei, R., Siitonen, S., Allou, K., Porwit, A., & Béné, M. C. (2016). Harmonemia: A universal strategy for flow cytometry immunophenotyping—A European LeukemiaNet WP10 study. Leukemia, 30, 1769–1772. [DOI] [PubMed] [Google Scholar]
- Lin, P., Hao, S., Medeiros, L. J., Estey, E. H., Pierce, S. A., Wang, X., Glassman, A. B., Bueso‐Ramos, C., & Huh, Y. O. (2004). Expression of CD2 in acute promyelocytic leukemia correlates with short form of PML‐RARalpha transcripts and poorer prognosis. American Journal of Clinical Pathology, 121, 402–407. [DOI] [PubMed] [Google Scholar]
- Liu, Y. R., Zhu, H. H., Ruan, G. R., Qin, Y. Z., Shi, H. X., Lai, Y. Y., Chang, Y., Wang, Y. Z., Lu, D., Hao, L., Li, J. L., Li, L. D., Jiang, B., & Huang, X. J. (2013). NPM1‐mutated acute myeloid leukemia of monocytic or myeloid origin exhibit distinct immunophenotypes. Leukemia Research, 37, 737–741. [DOI] [PubMed] [Google Scholar]
- Macedo, A., Orfao, A., Gonzalez, M., Vidriales, M. B., Lopez‐Berges, M. C., Martinez, A., & Miguel, J. F. S. (1995). Immunological detection of blast cell subpopulations in acute myeloblastic leukemia at diagnosis: Implications for minimal residual disease studies. Leukemia, 9, 993–998. [PubMed] [Google Scholar]
- Martelli, M. P., Pettirossi, V., Thiede, C., Bonifacio, E., Mezzasoma, F., Cecchini, D., Pacini, R., Tabarrini, A., Ciurnelli, R., Gionfriddo, I., Manes, N., Rossi, R., Giunchi, L., Oelschlägel, U., Brunetti, L., Gemei, M., Delia, M., Specchia, G., Liso, A., … Falini, B. (2010). CD34+ cells from AML with mutated NPM1 harbor cytoplasmic mutated nucleophosmin and generate leukemia in immunocompromised mice. Blood, 116, 3907–3922. [DOI] [PubMed] [Google Scholar]
- Mason, E. F., Kuo, F. C., Hasserjian, R. P., Seegmiller, A. C., & Pozdnyakova, O. (2018). A distinct immunophenotype identifies a subset of NPM1‐mutated AML with TET2 or IDH1/2 mutations and improved outcome. American Journal of Hematology, 93, 504–510. [DOI] [PubMed] [Google Scholar]
- Metzeler, K. H., Herold, T., Rothenberg‐Thurley, M., Amler, S., Sauerland, M. C., Gorlich, D., Schneider, S., Konstandin, N. P., Dufour, A., Bräundl, K., Ksienzyk, B., Zellmeier, E., Hartmann, L., Greif, P. A., Fiegl, M., Subklewe, M., Bohlander, S. K., Krug, U., Faldum, A., … AMLCG Study Group . (2016). Spectrum and prognostic relevance of driver gene mutations in acute myeloid leukemia. Blood, 128, 686–698. [DOI] [PubMed] [Google Scholar]
- Orfao, A., Chillon, M. C., Bortoluci, A. M., Lopez‐Berges, M. C., Garcia‐Sanz, R., Gonzalez, M., Tabernero, M. D., García‐Marcos, M. A., Rasillo, A. I., Hernández‐Rivas, J., & Miguel, J. F. S. (1999). The flow cytometric pattern of CD34, CD15 and CD13 expression in acute myeloblastic leukemia is highly characteristic of the presence of PML‐RARalpha gene rearrangements. Haematologica, 84, 405–412. [PubMed] [Google Scholar]
- Paietta, E. (2003). Expression of cell‐surface antigens in acute promyelocytic leukaemia. Best Practice & Research Clinical Haematology, 16, 369–385. [DOI] [PubMed] [Google Scholar]
- Porwit, A., & Béné, M. (2018). Multiparameter flow cytometry in the diagnosis of hematological malignancies. Cambridge University Press. [Google Scholar]
- Porwit, A., & Rajab, A. (2015). Flow cytometry immunophenotyping in integrated diagnostics of patients with newly diagnosed cytopenia: One tube 10‐color 14‐antibody screening panel and 3‐tube extensive panel for detection of MDS‐related features. International Journal of Laboratory Hematology, 37(Suppl. 1), 133–143. [DOI] [PubMed] [Google Scholar]
- Rajab, A., & Porwit, A. (2015). Screening bone marrow samples for abnormal lymphoid populations and myelodysplasia‐related features with one 10‐color 14‐antibody screening tube. Cytometry Part B, Clinical Cytometry, 88, 253–260. [DOI] [PubMed] [Google Scholar]
- Sanz, M. A., Fenaux, P., Tallman, M. S., Estey, E. H., Lowenberg, B., Naoe, T., Lengfelder, E., Döhner, H., Burnett, A. K., Chen, S.‐J., Mathews, V., Iland, H., Rego, E., Kantarjian, H., Adès, L., Avvisati, G., Montesinos, P., Platzbecker, U., Ravandi, F., … Lo‐Coco, F. (2019). Management of acute promyelocytic leukemia: Updated recommendations from an expert panel of the European LeukemiaNet. Blood, 133, 1630–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Violidaki, D., Axler, O., Jafari, K., Bild, F., Nilsson, L., Mazur, J., Ehinger, M., & Porwit, A. (2020). Analysis of erythroid maturation in the nonlysed bone marrow with help of radar plots facilitates detection of flow cytometric aberrations in myelodysplastic syndromes. Cytometry. Part B, Clinical Cytometry, 98(5), 399–411. [DOI] [PubMed] [Google Scholar]
- Swerdlow, S. H., Campo, E., Harris, N. L., Jaffe, E., Pileri, S. A., Stein, H., & Pileri, S. A. (Eds.). (2017). WHO classification of tumours of haematopoietic and lymphoid tissue. IARC. [Google Scholar]
- Zhou, Y., Jorgensen, J. L., Wang, S. A., Ravandi, F., Cortes, J., Kantarjian, H. M., Medeiros, L. J., & Konoplev, S. (2012). Usefulness of CD11a and CD18 in flow cytometric immunophenotypic analysis for diagnosis of acute promyelocytic leukemia. American Journal of Clinical Pathology, 138, 744–750. [DOI] [PubMed] [Google Scholar]
- Zhu, H. H., Liu, Y. R., Jiang, H., Lu, J., Qin, Y. Z., Jiang, Q., Bao, L., Ruan, G. R., Jiang, B., & Huang, X. (2013). CD34 expression on bone marrow blasts is a novel predictor of poor prognosis independent of FlT3‐ITD in acute myeloid leukemia with the NPM1‐mutation. Leukemia Research, 37, 624–630. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supplementary Information
Suppl. Figure 1Example of basic analysis of marker expression in panel AML 2. After dead cells were excluded the gate was set on CD33+ /+ + cells independent of scatter (upper panel left). Upper middle panel shows backgating of the CD33+ /+ + gate on SSC/CD45 plot. Percentages of leukemic cells positive for various markers were analysed using histograms with the linear gate set using the level of autofluorescence and/or negative populations present in the sample as cut‐off on the left. A case of hypergranular APL is shown in A, a hypogranular APL in B and a NPM‐1+ AML with myelomonocytic pattern in C


