Abstract
Objectives
The available literature on pulmonary disease in pediatric IBD is limited. We evaluated the prevalence of pulmonary manifestations in pediatric IBD and its association with disease severity.
Methods
Patients completed the St. George’s Respiratory Questionnaire (SGRQ), a self-reported measure of quality of life in patients with pulmonary disease. Chart review provided demographic information, Pediatric Crohn’s Disease Activity Index (PCDAI) and Pediatric Ulcerative Colitis Activity Index (PUCAI) scores. Regression models were utilized to evaluate associations between SGRQ score and clinical risk factors.
Results
The prevalence of pulmonary manifestations was 9.62% (95% CI: 5.48 to 15.36%). PCDAI scores in Crohn’s disease (CD) patients with pulmonary symptoms were significantly higher (SGRQ mean 10.71 ± 10.94). SGRQ score was also higher in patients with indeterminate colitis (8.64, 95% CI: 0.72 – 16.57, p=0.03), when compared with CD.
Conclusions
Additional investigations including pulmonary function tests and imaging could provide further insight into this issue.
Keywords: inflammatory bowel disease, Crohn’s disease, ulcerative colitis, extra-intestinal manifestation, St. George’s Respiratory Questionnaire, Pediatric Crohn’s Disease Activity Index, Pediatric Ulcerative Colitis Activity Index
Introduction
More than 80,000 children in United States are diagnosed with of inflammatory bowel disease (IBD), which is comprised of Crohn’s disease (CD), ulcerative colitis (UC), and indeterminate colitis (IC) 1. Extra-intestinal manifestations (EIM) including oral ulcers, joint pain, anemia and skin involvement are often present in IBD patients, and pediatric gastroenterologists routinely inquire about EIM during follow up visits. Approximately 29% of pediatric IBD patients develop an EIM within a follow-up period of up to 15 years, and one fourth of all patients experience at least one EIM within two to three years of diagnosis. 2,3 Similar to the adult population, children with IBD have a variety of EIM including anemia (40.5% in UC and 69% in CD) and musculoskeletal abnormalities (24–25% in UC and 8–41% in CD)4. A recent study showed that the brain is a possible site of EIM5. A three-year prospective study also showed that more than one third of 49 pediatric patients with CD had oral lesions 6, 7. Though pulmonary symptoms have been described in both adult and pediatric IBD, the exact prevalence of pulmonary manifestations in IBD is largely unknown. The literature on pulmonary symptoms in pediatric IBD is limited to mainly case series, case reports, and a few small studies10–12. Some of the common pulmonary symptoms reported in IBD patients include productive or nonproductive cough, wheezing, shortness of breath, exercise intolerance, hemoptysis, and chest pain8,9. We believe that pulmonary manifestations may be poorly recognized and under reported. Patients may have latent or subclinical pulmonary involvement rendering diagnosis difficult.
The goal of this study was to determine the prevalence of pulmonary manifestations in our urban pediatric IBD population. We also sought to understand whether an association exists between pulmonary symptoms and IBD disease severity.
Materials and Methods
This study was approved by the Weill Cornell Medicine Institutional Review Board. The study took place in the outpatient pediatric gastroenterology clinic or outpatient infusion suite between February 2017 and August 2018. Informed consent and assent were obtained from parents and patients, respectively (unless the patient was ≥18 years of age in which case consent was obtained directly from the patient). A total of 197 patients were approached for the study; 28 declined. Of the 169 patients who consented, four patients withdrew from the study, two did not complete the questionnaire, and four were excluded because a disease activity index was not recorded for the visit. Ultimately, 159 patients were included. Inclusion criteria were patients with IBD between 12–22 years of age who had a disease activity score recorded in the chart from the day of the visit. Exclusion criteria were patients outside the desired age range, those who did not provide consent, and lack of a recorded disease activity score.
The St. George’s Respiratory Questionnaire (SGRQ), a self-reported measure of quality of life in patients with pulmonary disease, was administered to the patients during an office visit. The SGRQ addresses frequency of respiratory symptoms such as cough, wheeze, sputum production and exertional dyspnea, disturbances to physical activity, and impairment of psychosocial functioning over the prior 3 months. SGRQ ≥ 25 is suggestive of respiratory problems13.
Chart review of the electronic medical record provided demographic information including sex, ethnicity, diagnosis, current age, age at diagnosis/duration of disease, surgical history, and current medications. The REDCap™ database was used for data collection.
The prevalence of respiratory symptoms in our cohort was calculated based on the SGRQ. Disease activity scores, including the Pediatric Crohn’s Disease Activity Index (PCDAI) and the Pediatric Ulcerative Colitis Activity Index (PDCAI), were calculated as per usual protocol on the day of the office visit and Wilcoxon rank-sum tests were used to test the difference of PCDAI score and PUCAI score among children with and without respiratory problems based on the SGRQ. Linear regression on SGRQ score was used to assess the relationship between SGRQ score and other parameters including age at diagnosis, sex, ethnicity, surgery history, corticosteroid use, and classification of IBD. All p-values were two-sided with statistical significance evaluated at the 0.05 alpha level. Ninety-five percent confidence intervals (95% CI) were calculated to assess the precision of the obtained estimates. All analyses were performed in R (Version 3.3.2) and SAS Version 9.4 (SAS Institute, Inc., Cary, NC).
Results:
One hundred and fifty-nine patients were included in the study. Of these, 121 patients had CD, 29 had UC, and 9 had IC. The median age at diagnosis was 13 years (range 12–20 years) and the median age of patients at the time of the study was 17 years. Seventy-eight patients were male and 11 patients were on corticosteroids at the time of the study.
Of the 159 patients, 15 (9%) patients had a SGRQ score of ≥25, suggesting a prevalence of pulmonary symptoms in the cohort to be 9.62% (95% CI: 5.48 to 15.36). Regression results showed that compared to patients with CD, SGRQ score was significantly higher in patients with IC (8.64, 95% CI: 0.72 – 16.57, p=0.03). A significant association was also found between SGRQ score and a history of surgery with SGRQ score being low in patients with a history of surgery (−4.02, 95% CI: −7.94 - −0.11, p=0.04). There was no significant association seen between SGRQ score and other factors, including age at diagnosis, sex, ethnicity, and use of steroids (Table 2).
Table 2.
Association between total SGRQ scores and IBD- age, sex, ethnicity, surgery history, and IBD diagnosis.
| Variable | Odds ratio (95% CI) | P-value |
|---|---|---|
|
| ||
| Univariate linear regression on SGRQ: | ||
| Hispanic (Non-Hispanic as reference) | −0.59 (−7.33, 6.15) | 0.8628 |
| Other (Non-Hispanic as reference) | −3.48 (−7.45, 0.49) | 0.0854 |
| Surgery: Yes | −4.02 (−7.94, −0.11) | 0.0442 |
| Sex: female | 3.33 (−0.25, 6.91) | 0.0684 |
| Age at diagnosis | 0.29 (−0.24, 0.82) | 0.2816 |
| UC (CD as reference) | −3.09 (−7.67, 1.48) | 0.1831 |
| IC (CD as reference) | 8.64 (0.72, 16.57) | 0.0327 |
| Steroids | 4.15 (−2.23, 10.53) | 0.2006 |
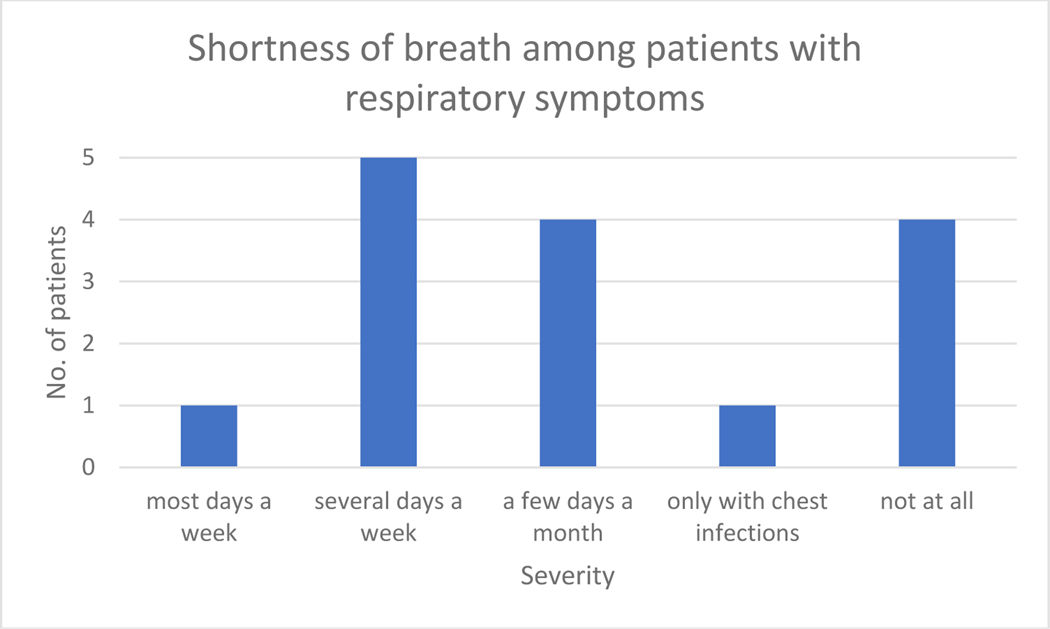
Out of the 15 patients who had an SGRQ score of ≥25, 10 (66%) patients reported shortness of breath, six patients (40%) reported difficulty breathing on several days a week or few days a month (Figure 3), seven (46%) reported a cough (Figure 1), six (40%) reported sputum production on most or several days a week (Figure 2), and four (3%) experienced wheezing a few days a month (Figure 4).
Figure 3:
Symptoms of shortness of breath in patients with SGRQ score of ≥25.
Figure 1:
Symptoms of cough in patients with SGRQ score of ≥25.
Figure 2.
Symptoms of sputum production in patients with SGRQ score of ≥25.
Figure 4:
Symptoms of wheezing in patients with SGRQ score of ≥25.
Of the 159 patients in the study, 125 CD patients had a calculated PCDAI score documented in the chart from the day of the visit and 33 UC and IC patients had a PUCAI score documented. PCDAI scores in CD children with pulmonary symptoms (SGRQ mean 10.71 ± 10.94, Mean PCDAI score 11.67) were significantly higher than PCDAI scores in CD children without pulmonary symptoms (SGRQ mean 3.77 ± 7.47, Mean PCDAI score 4.2, p-value = 0.01). No significant relationship was found between PUCAI scores of children with and without pulmonary symptoms (p=0.15).
Discussion
Our findings suggest that pulmonary manifestations in pediatric patients with IBD occurred in 9% of our cohort. These results differ from the prevalence found by Peradzynska et al., who found no difference in pulmonary symptom prevalence in fifty pediatric IBD patients compared to age-matched, healthy, controls32. While our prevalence was much smaller, our results showed similar symptomatology, including cough, sputum production, and shortness of breath, with a small percentage reporting wheeze.
The literature on prevalence of pulmonary symptoms in pediatric IBD is sparse and the reasons cited are multifactorial. According to a review of 135 papers on IBD and pulmonary manifestations, only two papers studied prevalence of pulmonary manifestations in IBD patients, and only one of these papers studied a pediatric cohort9. Peradzynska et al., concluded that there is no evidence that disease activity and duration correlate with lung function parameters. One reason could be that the pulmonary manifestations may appear independent of the bowel disease activity and do not typically coincide with IBD exacerbations. Instead pulmonary symptoms may manifest during remission or quiescent periods, making it challenging to link to IBD severity18. Our study showed a significant association of pulmonary symptoms to active CD in children. This is consistent with reports in the pediatric literature of increased pulmonary symptoms in CD19–21. It is worth noting that only 33 patients with PUDAI scores were assessed. A larger sample size of PUDAI patients is required to find a more definitive possible association between UC and pulmonary symptoms.
Our results showed that pulmonary symptoms are significantly increased during active disease than those in remission. Furthermore, there is a significant association between presence of pulmonary symptoms and surgical treatment of bowel disease22–24. The gut and the lung share a common embryological origin from the primitive foregut, and one could postulate that disease activity in one could have an impact on the other. However, the causes or mechanisms of the various forms of lung involvement in IBD and its subtypes and relation to surgical intervention remains poorly understood.
About 40–50% of adult IBD patients seen in gastroenterology clinics report respiratory symptoms, including wheezing, cough, sputum production, and shortness of breath 15–17. Camus et al. have reported a higher prevalence of pulmonary symptoms in adults with UC. Airway disease from the trachea to the bronchioles is associated with IBD in adults. In a retrospective study over a ten-year period, IBD was four times more prevalent among adult patients with airway disease compared with published local IBD prevalence 33. Cases of coexistence of IBD and bronchiectasis, bronchitis, tracheobronchitis, and bronchiolitis have been reported. While of these complications were found to be specific for UC or CD, the majority of cases featured abnormalities in proximal or distal conducting airways in UC9.
What is more challenging is the evaluation of pulmonary manifestations with latent presentation. Mansi et al. demonstrated that bronchial hyperresponsiveness occurs in children and adolescents with CD even in the absence of clinical, radiological, and functional evidence of airway disease15. The authors postulated that bronchial hyperresponsiveness could represent expression of subclinical airway inflammation responsible for the development of various pulmonary manifestations in CD25. Similarly, Ben-Tov et al. showed that pediatric patients with IBD have higher mean exhaled nitric-oxide levels (a marker of airway inflammation) on pulmonary function testing than controls irrespective of the severity of the intestinal disease and the spirometry values26.
While our results suggest that pulmonary symptoms are significantly associated with active disease and disease history of surgical intervention, it is still unclear whether the pulmonary manifestations were a result of the IBD itself, due to the adverse effects of medications, or both.
Several case studies of adult and pediatric CD and UC patients of have suggested that pulmonary manifestations can be a complication of various medications used to treat IBD. Parry et al. evaluated 50 patients aged 12–88 with sulfasalazine lung toxicity presenting with cough, fever, new onset dyspnea, crepitations, peripheral eosinophilia, and lung infiltrates on chest x-ray. Most patients promptly responded to discontinuation of sulfasalazine and 40 percent of patients were prescribed additional steroids27. In the case of a pediatric patient with IBD on sulfasalazine who had developed non-caseating granulomas and bronchiolitis obliterans, switching from sulfasalazine to mesalamine and 6-mercaptopurine (6-MP) resulted in improvement in pulmonary function tests28.
Mesalamines, commonly used in mild to moderate UC, have been associated with interstitial pneumonitis and eosinophilic pneumonia29–31. In Basseri et al.’s series of case studies on five adults and two children with IBD evaluated for mesalamine-induced pulmonary toxicity, all six patients on mesalamine had resolution of pulmonary symptoms after discontinuation of mesalamine8. Further studies are needed to address gaps in the areas of drug use and pulmonary symptoms in IBD.
There are limitations to our study. The first is the sample size is small. We used a convenience sample of patients returning to clinic for subsequent office visits. Secondly, our center is a tertiary care/referral center for pediatric IBD that attracts patients with severe and complicated disease. Given this, our results may not be generalizable to a pediatric IBD population with more mild disease severity. Third, while it would have been beneficial to have spirometry or bronchoscopy data in our cohort, these tests were not medically indicated and thus not performed. We did not collect patient allergy history, which could have contributed to the onset and severity of pulmonary symptoms. Additionally, there are various respiratory side-effects associated with infliximab such as cough, chest pain or shortness of breath. We had 79 patients on infliximab and the association between the drug and pulmonary symptoms were not specifically analyzed. The scope of our data also does not address whether pulmonary symptoms reported on the SGRQ are related to IBD and not a previously unrelated condition. However, this study both expands the limited literature on prevalence of pulmonary disease and pediatric IBD, and is novel as the only study in the literature that documents whether pulmonary disease is associated with higher disease activity scores. The study also involves the largest group of pediatric IBD patients reported in the literature. Further research on history of allergies, timing of pulmonary symptom onset, and timing of IBD diagnosis would be helpful for answering some of these questions.
In conclusion, our study suggests that pulmonary manifestations do occur with frequency in patients with IBD. Pediatric gastroenterologists routinely inquire about EIM such as aphthous ulcers, joint pain, and rashes but many do not ask about pulmonary symptoms. Patients and families may not recognize that pulmonary symptoms may be associated with active IBD and thus may fail to report them to their gastroenterologists. Additionally, though CD patients in our cohort who were in remission reported fewer pulmonary symptoms compared to those with active CD, latent pulmonary abnormalities may still exist. We recommend that providers consider inquiring about pulmonary symptoms including cough, wheeze, pneumonia, dyspnea, and chest pain during follow up visits, including for children in remission. Early recognition of these symptoms may facilitate timey lconsultation with pulmonary specialists and intervention when necessary.
Table 1.
Demographic information of study subjects
| Age at dx: Median (Range) | 13 [ 3 , 20 ] |
|
| |
| Age at survey completion: Median (Range) | 17 [ 12 , 22 ] |
|
| |
| Sex: N (%) | |
| Male: | 78 ( 49 ) |
| Female: | 81 ( 51 ) |
|
| |
| Diagnosis: N (%) | |
| CD: | 121 ( 76 ) |
| UC: | 29 ( 18 ) |
| IC: | 9 ( 6 ) |
|
| |
| Ethnicity: N (%) | |
| Declined | 45 ( 28 ) |
| Hispanic or Latino origin or Spanish origin | 12 ( 8 ) |
| Not Hispanic or Latino origin or Spanish origin | 95 ( 60 ) |
| Unknown | 7 ( 4 ) |
|
| |
| Use of steroids | |
| No | 143 ( 90 ) |
| Yes | 16 ( 10 ) |
Acknowledgements
The authors thank Dr. Stefan Worgall for his guidance on data analysis.
Funding Source: Dr. Paul Christos, Yao Lu, M.S. and Xiaoyue Ma, M.S. were partially supported by the following grant: Clinical and Translational Science Center at Weill Cornell Medical College (1-UL1-TR002384–01).
Footnotes
Dr Pillai conducted the study at WCM but currently associated with Mayo clinic, MN
References
- 1.Centers for Disease Control and Prevention. Disease of the Week. Website: http://www.cdc.gov/dotw/ibd/index.html Published May 16, 2018. Accessed June18, 2018.
- 2.Hyams JS, Markowitz J, LeLeiko NS, Mack DR, Evans JS, Pfefferkorn MD, Griffiths AM, Otley AR, Bousvaros A et al. Extraintestinal manifestations of pediatric inflammatory bowel disease and their relation to disease type and severity. J Pediatr Gastroenterol Nutr 2010;51: 140. [DOI] [PubMed] [Google Scholar]
- 3.Jose FA., Garnett EA, Vittinghoff E, Ferry GD, Winter HS, Baldassano RN, Kirschner BS, Cohen SA, Gold BD, Abramson O et al. Development of extraintestinal manifestations in pediatric patients with inflammatory bowel disease. Inflamm Bowel Dis 2009. 15: 63–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jose FA, Heyman MB. Extraintestinal manifestations of inflammatory bowel disease. J Pediatr Gastroenterol Nutr 2008;46:124–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mrakotsky C, Augustine DW et al. O-003 The Brain as Extraintestinal IBD Manifestation: Are Brain and Cognitive Differences in Pediatric Crohn’s Disease Associated with Immune Gene Expression? Inflamm Bowel Dis 2017. Feb; 23 Suppl 1:S1–S2. [Google Scholar]
- 6.Lankarani KB., Sivandzadeh GR, and Hassanpour S. Oral manifestation in inflammatory bowel disease: a review. World J Gastroenterol, 2009;19: 8571–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harty S, Fleming P, Rowland M, Crushell E, McDermott M, Drumm B, Bourke B. A prospective study of the oral manifestations of Crohn’s disease, Clin Gastroenterol Hepatol 2005;3: 886–91. [DOI] [PubMed] [Google Scholar]
- 8.Basseri B, Enayati P, Marchevsky A, Papadakis KA. Pulmonary manifestations of inflammatory bowel disease: case presentations and review. J Crohns Colitis 2010;4(4):390–397. [DOI] [PubMed] [Google Scholar]
- 9.Majewski S, and Piotrowski W. Pulmonary manifestations of inflammatory bowel disease. Arch Med Sci 2015;11: 1179–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Munck A, Murciano D, Pariente R, Cezard JP, Navarro J. Latent pulmonary function abnormalities in children with Crohn’s disease. Eur Respir J. 1995;8(3):377–380. [DOI] [PubMed] [Google Scholar]
- 11.Bentur L, Lachter J, Koren I, Ben-Izhak O, Lavy A, Bentur Y, Rosenthal E. Severe pulmonary disease in association with Crohn’s disease in a 13-year-old girl. Pediatr Pulmonol. 2000;29(2):151–154. [DOI] [PubMed] [Google Scholar]
- 12.Vadlamudi NB, Navaneethan U, Thame KA, Kelly DR, Dimmitt RA, Harris WT. Crohn’s disease with pulmonary manifestations in children: 2 case reports and review of the literature. J Crohns Colitis. 2013;7(3):e85–92. [DOI] [PubMed] [Google Scholar]
- 13.American Thoracic Society. St. George’s Respiratory Questionnaire (SGRQ). Website: https://www.thoracic.org/members/assemblies/assemblies/srn/questionaires/sgrq.php Accessed June 18, 2018.
- 14.Asher MI, Keil U, Anderson HR, Beasley R, Crane J, Martine F, Mitchell EA, Pearce N, Sibbald B, Stewart AW. International Study of Asthma and Allergies in Childhood (ISAAC): rationale and methods. Eur Respir J. 1995;8(3):483–491. [DOI] [PubMed] [Google Scholar]
- 15.Hoffmann RM, Kruis W. Rare extraintestinal manifestations of inflammatory bowel disease. Inflamm Bowel Dis. 2004;10:140–147. [DOI] [PubMed] [Google Scholar]
- 16.Kelly MG, Frizelle FA, Thornley PT, Beckert L, Epton M, Lynch AC. Inflammatory bowel disease and the lung: is there a link between surgery and bronchiectasis? Int J Colorectal Dis. 2006;21:754–757. [DOI] [PubMed] [Google Scholar]
- 17.Mohamed-Hussein AA, Mohamed NA, Ibrahim ME. Changes in pulmonary function in patients with ulcerative colitis. Respir Med. 2007;101:977–982. [DOI] [PubMed] [Google Scholar]
- 18.Douglas JG, Respir Med 1989; 83:389–394 [DOI] [PubMed] [Google Scholar]
- 19.Fan LL, Mullen AL, Brugman SM, Inscore SC, Parks DP, White CW:Clinical spectrum of chronic interstitial lung disease in children. J Pediatr 1992, 121:867–72. [DOI] [PubMed] [Google Scholar]
- 20.Calder CJ, Lacy D, Raafat F, Weller PH, Booth IW: Crohn’s disease with pulmonary involvement in a 3 year old boy. Gut 1993,34:1636–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Puntis JW, Tarlow MJ, Rafaat F, Booth IW: Crohns disease of thelung. Arch Dis Child 1990, 65:1270–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eaton TE, Lambie N, Wells AU. Bronchiectasis following colectomy for Crohn’s disease. Thorax. 1998;53:529–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vasishta S, Wood JB, McGinty F. Ulcerative tracheobronchitis years after colectomy for ulcerative colitis. Chest. 1994;106:1279–1281. [DOI] [PubMed] [Google Scholar]
- 24.Alcázar Navarrete B, Quiles Ruiz-Rico N, González Vargas F, Cabrera Torres L. Bronchiectasis following colectomy in a patient with ulcerative colitis and factor V Leiden mutation Arch Bronconeumol. 2005;41:230–232. [DOI] [PubMed] [Google Scholar]
- 25.Mansi A, Cucchiara S, Greco L, Sarnelli P, Pisanti C, Franco MT, Santamaria F. Bronchial hyperresponsiveness in children and adolescents with Crohn’s disease. Am J Respir Crit Care Med. 2000;161(3 Pt 1):1051–1054. [DOI] [PubMed] [Google Scholar]
- 26.Gut G1, Ben-Tov A, Lahad A, Soferman R, Cohen S, Tauman R, Sivan Y. Pulmonary functions in children with inflammatory bowel disease. Eur J Gastroenterol Hepatol. 2016;28:708–713. [DOI] [PubMed] [Google Scholar]
- 27.Parry SD, Barbatzas C, Peel ET and Barton JR. Sulphasalazine and lung toxicity. Eur Respir J 2002;19: 756–64. [DOI] [PubMed] [Google Scholar]
- 28.Hamadeh MA, Atkinson Jand Smith LJ. Sulfasalazine-induced pulmonary disease. Chest 1992;101: 1033–7. [DOI] [PubMed] [Google Scholar]
- 29.Yonker L, Tse S et al. Mesalamine Induced Pneumonitis In A 15 Year Old Boy With Inflammatory Bowel Disease. ATS Conferences. 2011. [Google Scholar]
- 30.Moeser A, Pletz MW, Hagel S, Kroegel C, Stallmach A. Lung disease and ulcerative colitis--mesalazine-induced bronchiolitis obliterans with organizing pneumonia or pulmonary manifestation of inflammatory bowel disease? Z Gastroenterol 2015;53: 1091–8. [DOI] [PubMed] [Google Scholar]
- 31.Bitton A, Peppercorn MA, Hanrahan JP, Upton MP. Mesalamine-induced lung toxicity, Am J Gastroenterol, 1996;91: 1039–40. [PubMed] [Google Scholar]
- 32.Peradzyńska J, Krenke K, Lange J, et al. Low prevalence of pulmonary involvement in children with inflammatory bowel disease. Respir Med. 2012;106:1048–54. [DOI] [PubMed] [Google Scholar]
- 33.Raj AA, Birring SS, Green R, Grant A, de Caestecker J, Pavord ID. Prevalence of inflammatory bowel disease in patients with airways disease. Respir Med. 2008;102:780–785. [DOI] [PubMed] [Google Scholar]