Abstract
Aim
Before the introduction of new biomaterials for prolapse surgery, animal studies on the host response are required. Unfortunately, large variation in study design hampers obtaining an overview of the safety and efficacy, and translation to clinical practice. Our aim is to systematically review the literature on all outcome measures describing the host response in animal studies assessing the biocompatibility of urogynecologic surgical mesh implants for prolapse surgery. Furthermore, by meta‐analysis, we aim to assess the effect of implantation and compare this to control animals receiving sham surgery or native tissue repair.
Methods
We performed a systematic search from inception to August 2020. Since this is an explorative study we included original, controlled, and noncontrolled animal studies describing any host response to the implant. Quantitative outcome measures reported ≥10 times in ≥2 articles were eligible for meta‐analysis.
Results
Fifty articles were included in the qualitative synthesis and 36 articles were eligible for meta‐analysis. In total, 154 outcome measures were defined and classified into (1) histomorphology, (2) biomechanics and, (3) macroscopic morphology. Animals with vaginal implants demonstrated significantly increased M1 and M2 macrophages, MMP‐2, neovascularization, TNF‐α, and stiffness, and lower vaginal contractility compared to control animals.
Conclusion
The host response significantly differs in animals after vaginal mesh implantation compared to control animals, both pro‐ and anti‐inflammatory. However, we observed a paucity in the uniformity of reported outcomes. For future animal studies, we propose the development of a core outcome set, which ideally predicts the host response in women.
Keywords: biomaterials, foreign‐body reaction, in vivo, pelvic floor, pelvic organ prolapse
1. INTRODUCTION
Pelvic floor disorders such as urinary incontinence and pelvic organ prolapse (POP) affect many women, with the incidence increasing up to 50% with age.1 Unfortunately, long‐term results of native tissue repair (NTR) are far from optimal and reoperation rates for recurrent prolapse symptoms are as high as 17%–29%.2, 3 High‐failure rates of NTR might result from the use of the patient's own—already defective—connective tissue to restore the support system. Surgical meshes were introduced to improve the outcome of POP surgery by providing durable mechanical support. However, synthetic implants might cause a persistent chronic and uncontrolled inflammatory response and this may result in fibrosis and complications as implant exposure and pain.4 This has led to an evolvement to lightweight, monofilament and macroporous implants which induce a milder host response.5 Nonetheless, for years the FDA is warning about potential risks of pelvic floor implants and in 2019 the FDA ordered manufacturers of transvaginal meshes to stop selling their devices because of insufficient effectiveness and non‐reassuring safety of the mesh.6, 7
Until today, researchers are pursuing to develop a pelvic floor implant that gives lasting restoration of the anatomy but causes minimal side effects. As opposed to the earlier, before the introduction of new biomaterials for pelvic floor surgery, animal studies on the host response have become a requirement to assess the safety and efficacy in preparation of clinical studies.8 The host response is the reaction of the body to the presence of a material and begins immediately upon implantation, but will last a lifetime and it is decisive in determining the success in the long term. It is defined by the response to tissue injury during implantation and the response evoked by the biomaterial itself.9 The host response towards these implants is essential for the development of new load‐bearing tissue, but if being uncontrolled it can cause adverse events.4 The host response is not a single well‐defined outcome measure but consists of a sequence of host reactions including tissue injury, acute inflammation, chronic inflammation, and wound healing, along with a myriad of different cell types and mediators.9 Ideally, we would like to know which animal models and outcome measures predict the host response and subsequent success of implants in women. However, various animal models have been used and various outcome measures have been reported. The large variation in study design results in difficulties to aggregate, interpret, and generalize the results and challenges translation to clinical practice. Therefore, we first aim to give an overview of all outcome measures describing the host response in animal studies assessing urogynecologic surgical mesh implants for prolapse surgery. In addition, using meta‐analysis we aim to quantitatively assess the effect of mesh implantation compared to NTR or sham surgery and investigate the influence of certain characteristics, such as species and type of implant. Finally, we aim to give insight into differences in the host response between different implantation sites in a second meta‐analysis. The overall objective of this systematic review is to eventually improve the interpretation of in vivo studies and give researchers considerations for future study design.
2. MATERIALS AND METHODS
This systematic review is adherent to the Preferred Reporting Items for Systematic Reviews and Meta‐Analysis (PRISMA) statement.10 The protocol was registered on the international prospective register of systematic reviews (PROSPERO) on August 9, 2019, under registration number CRD42019142850. Detailed methodological information can be found in Supplementary file 1.
2.1. Literature search
A medical information specialist (Jacqueline Limpens) performed a systematic search in Ovid MEDLINE and Ovid EMBASE from inception onwards (with the last update on August 14, 2020) using controlled terms (i.e,. MeSH‐terms in MEDLINE) as well as free text terms for (1) POP, pelvic floor, or vaginal reconstruction and (2) various implant terms combined with (3) an animal search filter (Supplementary file 2). Conference abstracts were excluded in EMBASE. No further restrictions were applied. We crosschecked reference lists and the citing articles of included papers and relevant reviews for additional relevant studies using Web of Science. The records retrieved were imported and de‐duplicated in EndNote X9.
2.2. Study selection
Titles and abstracts were independently screened by two reviewers (Kim W. J. Verhorstert and Brita S. Kortz) in Early Review Organizing Software (www.eros-systemtic-review.org) using the following exclusion criteria (1) no primary article, (2) no animal experiment, (3) no implant indicated for POP and (4) no vaginal implantation. Only original, controlled, or noncontrolled studies were eligible when animals received a vaginal implant, concomitant abdominal or subcutaneous implantation was permitted. In the second round of screening in Rayyan,11 full texts were screened using the same exclusion criteria as described above and additionally (5) no outcome measure describing host response. Since this is an explorative study, no further restrictions were made and the host response included any reaction to the implant, both direct (e.g., histological) and indirect outcomes (e.g., macroscopic observations) of the host response. Although strictly speaking NTR or sham surgery would not provoke a host response since there is no insertion of a foreign body, we also evaluated these outcome measures in control animals to make a comparison to animals with implants possible. Any discrepancies between the two reviewers were resolved by discussion, where necessary, a senior reviewer (Zeliha Guler) was consulted.
2.3. Study characteristics
From all included articles we extracted bibliographical information (author, year) and various study design and animal model characteristics (species, intervention, type of implant(s), method of insertion, and duration of follow‐up (Supplementary file 3)). Next, all outcome measures reported describing the host response were extracted. Due to the wide variety in outcome measures, we classified them into three major groups (1) histomorphology (including histology, immunohistochemistry, and biochemistry), (2) biomechanics (active and passive), and (3) macroscopic morphology, and registered whether the outcome was reported qualitative or quantitative.
2.4. Extraction outcome data
Outcome data were extracted in duplicate by two independent reviewers (Kim W. J. Verhorstert and Aksel N. Gudde). The frequency of all reported outcome measures was calculated. The effect of mesh surgery on macroscopic morphology could be analyzed without a control group, since macroscopic morphological changes (e.g., exposures) only occur in implanted animals. Other outcome measures needed to have an appropriate control group to be eligible for meta‐analysis (sham surgery or NTR), to ensure possible interpretation of the results (e.g., histological scoring). If a study reported data from several experimental groups, this was considered as separate comparisons. In case of missing data, the authors were contacted. When medians and interquartile ranges were reported, these were converted to means and standard deviations (SD) as reported by Wan et al.12
2.5. Quality assessment
We performed a risk of bias assessment for all studies with an appropriate control group using the SYRCLE Risk of Bias tool.13 Because reporting of experimental details on animals, methods, and materials is often very poor, we added two items on reporting: reporting of any measure of randomization and blinding, to overcome the problem of judging too many items as “unclear risk of bias.”14 The quality of all included articles was independently scored by two reviewers (Kim W. J. Verhorstert and Brita S. Kortz), any discrepancies were solved by discussion.
2.6. Meta‐analysis
Meta‐analysis was performed in Comprehensive Meta‐Analysis (CMA) software (version 3.0). Quantitative outcome measures reported ≥10 times in ≥2 articles were eligible for meta‐analysis. If the same group was used for multiple comparisons, the number of animals was divided by the number of comparisons. In case the animal number was not an integer, it was rounded to the nearest whole number. In some groups, the SD was 0 and we inferred the SD based on similar other groups within the same study. If a study reported dichotomous outcomes, but all other studies reported continuous data (e.g., contraction) these results were excluded for meta‐analysis.
Depending on the type of data, results were reported as odds ratio (OR) and Hedges g (histomorphology and biomechanics) or event rate and mean (macroscopic morphology), all with their 95% confidence intervals (CIs). We used the random effects model, which takes into account the precision of individual studies and the variation between studies and weights each study accordingly. I 2 was used to determine the level of between‐study heterogeneity. In the first meta‐analysis, we compared animals with vaginal implants with control animals, and in the second meta‐analysis, we compared vaginal implants with abdominal implants.
Predefined subgroup analyses were planned for species, type of implant, time point, and method of implantation (transvaginal or transabdominal) and were only conducted in case ≥3 independent comparisons were available from ≥3 articles. We expected the variance to be comparable within the subgroups; therefore, a common among‐study variance across subgroups was assumed. The p‐value was adjusted according to the Bonferroni method to account for multiple testing (p × number of comparisons).
Sensitivity analyses were conducted to determine the robustness of our findings (additional methodological information on subgroup and sensitivity analyses: Supplementary file 1). No assessment for publication bias has been performed due to the limited number of comparisons per outcome measure.
3. RESULTS
3.1. Literature search results
The search identified 399 unique references (Figure 1), of which 290 could be excluded based on title and abstract. Out of the remaining 109 articles (27%), full texts were retrieved to assess eligibility. Eventually, 50 articles could be included in the qualitative synthesis and 36 articles were eligible for meta‐analysis (Supplementary file 4).
Figure 1.
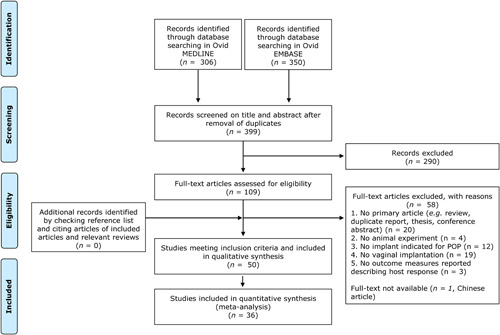
PRISMA flowchart of search and screening process
3.2. Study characteristics and outcome measures
Rabbits (31%) were the most used species, followed by rats (29%), sheep (24%), macaques (14%), and dogs (2%). Regarding the type of implant, polypropylene implants (39%) and polypropylene hybrid implants (28%) were mostly used. The timing of outcome assessment was generally more than 28 days (66%) and in 71% the method of implantation was transvaginal (Figure 2).
Figure 2.
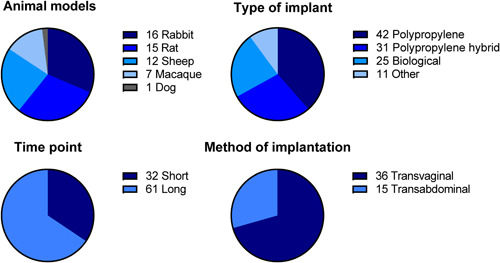
Descriptive characteristics of all included articles. Some articles included multiple animal models, type of implants, time points, or methods of implantations; therefore, the total number exceeds the 50 articles
Out of the 50 included articles in this systematic review, only 20 articles (40%) used a sham surgery or NTR control. In total, 154 unique outcome measures describing the host response were identified (Table 1A). Of these outcome measures, 101/154 (66%) were quantitative and assessed against an appropriate control group (Table 1B). However, only 17 of these outcomes were eligible for our meta‐analysis based on the frequency of reporting: apoptosis, elastin, M1‐macrophages, M2‐macrophages, matrix‐metalloproteinase 2 (MMP‐2), neovascularization, smooth muscle, tumor necrosis factor‐alpha (TNF‐α), total collagen, contractility, stiffness, contraction, degradation, erosion, exposure, extrusion, and implant retrieval.
Table 1A.
All qualitative and quantitative outcome measures reported in the included studies, categorized in histomorphology, biomechanics, and macroscopic morphology
| Histomorphology | Biomechanics | Macroscopic morphology | |
|---|---|---|---|
|
Apoptosis Arginase Calcification Cell proliferation Cellular infiltration Cellular/collagen ratio Collagen alignment Collagen composition Collagen degradation Collagen density Collagen, total Collagen I Collagen III Collagen III/I Collagen, immature/disorganized Collagen, mature/organized Collagenase activity Collagen organization Connective tissue Degeneration Delineation of layers Disruption ECM gene expression, COL1a ECM gene expression, COL3a ECM gene expression, ELN ECM gene expression, FBN5 Elastin Elastin degradation Eosinophils Epithelial thickness Epithelial trapping Epithelization Epitheloid cells Fibrin Fibroblastic proliferation Fibroblasts Fibrocytes Foreign body giant cells Foreign body reaction GAG Granulocytes Hyperplastic tissue IFN‐y IL‐1 IL‐4 IL‐6 IL‐10 IL10+IL4/TNF‐α+IL12 IL‐12 IL‐β Immune response Inflammation Integration |
Leukocytes Lymphocytes Lymphocytes, B Lymphocytes, T M1 macrophages M2 macrophages M2/M1 ratio Macrophages Mast cells Mesh integration MMP1 MMP2 MMP8 MMP9 MMP13 Monocytes Mononuclear cells Muscle penetration Myocytes Myofibroblasts Neovascularization Nerve density, adrenergic Nerve density, cholinergic Nerve density, peripheral Nerve growth factor Neuronal network Neutrophilic cells NO synthetase Plasma cells Polymorphonuclear cells Rejection Smooth muscle Smooth muscle bundle size Smooth muscle organization Smooth muscle thickness Smoothelin Sub‐epitelium Surface between epithelium and implant T‐cells, CD4 T‐cells, CD8 Th1/Th2 ratio Th‐1‐cells Th‐2‐cells Tissue ingrowth Tissular colonization TNF‐α Tropoelastin Tropoelastin degradation Ulceration Vaginal thickness |
Break strength Comfort zone length Contractility Elastic modulus Electrical field stimulation Energy absorbed Final elongation percentage against force Leak point pressure Length at break point Maximum elongation Nerve mediated contraction Receptor‐mediated contraction Stiffness Tensile strength Tissue mesh detachment strength Ultimate load Strain or load at failure Voiding interval Voiding pressure Voiding volume |
Abscess, deep Abscess, subcutaneous Adhesions Angiogenesis Color Contraction Degradation Dehiscence Ellipticity Encapsulation Erosion Exposure Exposure suture Extrusion Fibrosis Fluid collection Folding Formation of tissue bands Hematoma Incorporation Induration Infection, local Infection, systemic Palpability of material POP‐Q assessment Prominence Retrieval of implant Separation Support Thickness (mesh‐tissue complex) Topology |
Table 1B.
All quantitative outcome measures reported in the studies with appropriate control group, categorized in histomorphology, biomechanics, and macroscopic morphology
| Histomorphology | Biomechanics | Macroscopic morphology | |
|---|---|---|---|
|
Apoptosis Cell proliferation Cellular/collagen ratio Collagen degradation Collagen density Collagenase activity Collagen, total Collagen, immature/disorganized Collagen, mature/organized Collagen I Collagen III Collagen III/I Connective tissue Disruption ECM gene expression, COL1a ECM gene expression, COL3a ECM gene expression, ELN ECM gene expression, FBN5 Elastin Elastin degradation Fibroblastic proliferation Fibroblasts Foreign body giant cells Foreign body reaction GAG Granulocytes IL‐1 IL‐4 IL‐10 IL10+IL4/TNF‐α+IL12 IL‐12 Inflammation Leukocytes |
Lymphocytes Lymphocytes, B Lymphocytes, T Macrophages M1 macrophages M2 macrophages M2/M1 ratio Mast cells MMP1 MMP2 MMP8 MMP9 MMP13 Muscle penetration Myocytes Myofibroblasts Neovascularization Nerve density, adrenergic Nerve density, cholinergic Nerve density, peripheral Nerve growth factor Neuronal network Polymorphonuclear cells Smooth muscle bundle size Smooth muscle thickness Smoothelin Sub‐epithelium thickness T‐cells, CD4 T‐cells, CD8 Th‐1‐cells Th‐2‐cells TNF‐α Tropoelastin Tropoelastin degradation |
Comfort zone length Contractility Electrical field stimulation Energy absorbed Final elongation percentage against force Leak point pressure Length at break point Maximum elongation Nerve mediated contraction Receptor‐mediated contraction Stiffness Tensile strength Ultimate load Voiding interval Voiding pressure Voiding volume |
Abscess, deep Abscess, subcutaneous Adhesions Contraction Degradation Dehiscence Ellipticity Exposure Exposure suture Fluid collection Folding Incorporation Infection, local POP‐Q assessment Prominence Separation Thickness mesh‐tissue complex Topology |
3.3. Quality assessment
In general, the majority of items assessed in the risk of bias analysis showed an unclear risk of bias due to insufficient reporting of essential methodological details (Figure 3). Although 63% of the articles stated any form of randomization, in none of the included references the allocation sequence was adequately described. Blinding at any level was described in 42% of the articles, however, the allocation was adequately concealed in only 21% and none of the studies reported blinding of research staff during the course of the experiments (performance bias). Regarding blinding of the outcome assessor, for histomorphology, this was adequately performed and reported in only 20%, but not reported in most other cases.
Figure 3.
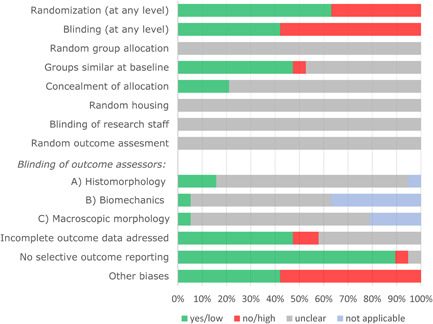
Quality assessment of studies with an appropriate control group. For “randomization” and “blinding” a “yes” score indicates “reported,” and a “no” score indicates “unreported.” For other item a “yes” score indicates low risk of bias; a “no” score indicates high risk of bias; and a “?” score indicates unknown risk of bias
3.4. Meta‐analysis
3.4.1. Histomorphology and biomechanics in controlled animal studies
Animals with vaginal implants demonstrated a significant increase in M1‐macrophages (Hedges g = 1.85 [0.83–2.88]), M2‐macrophages (Hedges g = 2.74 [1.83–3.65]), MMP‐2 (Hedges g = 2.80 [1.82–3.78]), neovascularization (Hedges g = 1.17 [0.84–1.50]), and TNF‐α (Hedges g = 0.83 [0.11–1.56]) compared to control animals (Table 2A). Furthermore, animals with vaginal implants had significantly lower tissue contractility (Hedges g = −0.55 [−0.97 to −0.13]) and higher stiffness values (Hedges g = 0.68 [0.20–1.17]) compared to control animals. For apoptosis, elastin, smooth muscle, and total collagen amount, no significant differences were observed. From all outcome measures, subgroup analyses could be performed for neovascularization, total collagen, contractility, and stiffness. Subgroup analyses revealed no significant differences in neovascularization between polypropylene and polypropylene hybrid implants, in total collagen between polypropylene hybrid and biological implants or in stiffness between ewes and macaques or transvaginal and transabdominal implantations. However, vaginal contractility was significantly more decreased after transabdominal implantation (Hedges g = −1.27 [−1.77 to −0.77]) compared to transvaginal implantation (Hedges g = 0.05 [−0.41 to 0.50]; p < 0.01). Other predefined subgroups were too small for meaningful analyses (Supplementary file 5).
Table 2A.
Histomorphologic and biomechanical outcomes meta‐analysis
| Outcome measure | Hedges g | 95% CI (p‐value for subgroups) | I 2 | No. of comparisons | No. of articles | No. of animals | Unit |
|---|---|---|---|---|---|---|---|
| Apoptosis | 0.02 | −0.44 to 0.48 | 11.4% | 10 | 4 | 106 | % apoptotic cells |
| Elastin | 0.18 | −0.67 to 1.02 | 71.5% | 12 | 4 | 117 | Concentration, normalized values, % dry weight or % positive cells |
| M1 macrophages | 1.85 | 0.83 to 2.88a | 76.8% | 13 | 3 | 106 | Histologic scoring or % positive cells |
| M2 macrophages | 2.74 | 1.83 to 3.65a | 64.4% | 13 | 3 | 106 | Histologic scoring or % positive cells |
| MMP‐2 | 2.80 | 1.82 to 3.78a | 74.9% | 16 | 4 | 150 | Density/relative expression or normalized values |
|
Neovascularization – PP vs. PP hybrid |
1.17 1.08 vs. 1.96 |
0.84 to 1.50a NS |
7.5% | 22 | 5 | 176 | Histologic scoring or density/relative expression |
| Smooth muscle | 0.20 | −0.42 to 0.82 | 63.2% | 15 | 5 | 147 | Histologic scoring or smooth muscle thickness |
| TNF‐α | 0.83 | 0.11 to 1.56a | 68.5% | 13 | 3 | 117 | Concentration or density/relative expression |
|
Total collagen – PP hybrid vs. biological |
0.17 0.61 vs. −0.25 |
−0.62 to 0.95 NS |
67.8% | 12 | 4 | 117 | Histologic scoring or % dry weight |
|
Contractility – TA vs. TV |
−0.55 −1.27 vs. 0.05 |
−0.97 to −0.13a p < 0.01 |
34.3% | 17 | 6 | 156 | mN, mN/mm3, or mN/g |
|
Stiffness – Ewe vs. Macaque – TA vs. TV |
0.68 1.06 vs. 0.14 0.35 vs. 0.88 |
0.20 to 1.17a NS NS |
52.0% | 20 | 6 | 173 | N/mm |
Note: Only subgroup analysis is shown which meets the requirements for subgroup analysis: ≥3 comparisons from ≥3 articles. See Supplementary file 4 for all subgroups per outcome measure, including CI of the above subgroup analysis.
Abbreviations: CI, confidence interval; I 2, heterogeneity; MMP‐2, matrix metalloproteinase‐2; N, number; NS, nonsignificant; PP, polypropylene; TA, transabdominal; TNF‐α, tumor necrosis factor‐alpha; TV, transvaginal; vs., versus.
Significant difference between animals with vaginal implants and control animals.
3.4.2. Macroscopic morphology in all animals with implants
Overall, there was 32.7% contraction [27.8–37.7] and subgroup analysis showed no significant differences in contraction between polypropylene and polypropylene hybrid implants (Table 2B). In one article, a group showed “too much contraction to measure,” and provided no absolute data.15 Since leaving this data out would provide an underestimation, it was decided to use the highest mean contraction percentage and SD from all included interventions (61.2 ± 17.3). During a sensitivity analysis, this data was left out and the overall contraction was still 31.8% [26.9–36.7].
Table 2B.
Macroscopic morphology outcomes meta‐analysis
| Outcome measure | Event rate/mean | 95% CI | I 2 | No. of comparisons | No. of articles | No. of animals |
|---|---|---|---|---|---|---|
|
Contraction ‐ PP vs. PP hybrid Sensitivity analysis |
32.7%a 30.3% vs. 32.1% 31.8% |
27.8 to 37.7 NS 26.9 to 36.7 |
90.1% | 29 | 8 | 174 |
| Degradation | 40.1%b | 23.9 to 58.8 | 36.5% | 15 | 5 | 68 |
|
Erosion ‐ Short vs. long |
11.6%b 12.1% vs. 11.1% |
6.8 to 19.0 NS |
0% | 24 | 4 | 95 |
|
Exposure ‐ Ewe vs. rabbit ‐ Rabbit vs. rat ‐ Ewe vs. rat ‐ PP vs. PP hybrid ‐ PP vs. biological ‐ PP vs. other ‐ PP hybrid vs. biological ‐ PP hybrid vs. other ‐ biological vs. other ‐ Short vs. long ‐ TA vs. TV |
20.1%b 25.5% vs. 23.0% 23.0% vs. 10.5% 25.5% vs. 10.5% 24.2% vs. 19.3% 24.2% vs. 14.3% 24.2% vs. 16.1% 19.3% vs. 14.3% 19.3% vs. 16.1% 14.3% vs. 16.1% 17.1% vs. 21.2% 16.8% vs. 20.4% |
16.8 to 24.0 NS p = 0.03 p < 0.01 NS NS NS NS NS NS NS NS |
0% | 114 | 25 | 584 |
| Extrusion | 26.0%b | 13.9 to 43.3 | 27.3% | 13 | 3 | 65 |
|
Implant retrieval ‐ PP vs. biological |
75.3%b 85.3% vs. 70.4% |
65.0 to 83.3 NS |
13.6% | 17 | 5 | 133 |
Note: Only subgroup analysis is shown which meets the requirements for subgroup analysis: ≥3 comparisons from ≥3 articles. See Supplementary file 4 for all subgroups per outcome measure, including CI of the above subgroup analysis.
Abbreviations: CI, confidence interval; I 2, heterogeneity; N, number; NS, nonsignificant; PP, polypropylene; TA, transabdominal; TV, transvaginal; vs., versus.
Mean value.
Event rate.
The erosion rate was 11.6% [6.8–19.0] and subgroup analysis revealed no significant differences in erosion rates between short or long follow‐up, nor after a sensitivity analysis changing the definitions of follow‐up (as described in Supplementary file 1). While the overall vaginal exposure rate was 20.1% [16.8–24.0], vaginal exposures were less common in rats (10.5% [6.9–15.5]) compared to ewes (25.5% [19.6–32.3]; p < 0.01) and rabbits (23.0% [16.7–30.8]; p = 0.03).
3.4.3. Comparison of vaginal and abdominal wall implantations
In the second meta‐analysis, we compared vaginal and abdominal wall implantations in the same animal, five studies reported the results of contraction on both sites (11 independent comparisons, 72 vaginal and 83 abdominal implants). Contraction in vaginally implanted animals was significantly higher than abdominally implanted animals (Hedges g = 2.16 [1.66–2.67], I 2 = 40%), even after a sensitivity analysis leaving “too much contraction to measure” data out (Hedges g = 2.04 [1.56–2.52], I 2 = 30%) as described above. Seven articles reported exposures of both vaginal and abdominal implants (23 independent comparisons, 163 vaginal and 187 abdominal implants), and exposures were significantly more common in the vagina compared to the abdominal wall (OR = 3.44 [1.61–7.35], I 2 = 0%).
4. DISCUSSION
This systematic review identified 154 unique outcome measures in 50 articles describing the host response in animal experimental research assessing the biocompatibility of urogynecologic surgical mesh implants. Outcome measures investigated were classified as histomorphologic outcome measures (n = 103) including histology and biochemistry, or macroscopic morphologic (n = 31) or biomechanical (n = 20) outcomes. Meta‐analysis could only include 11% of the outcomes due to the infrequent and qualitative nature of outcome reporting. We, therefore, conclude that animal studies on host response after vaginal mesh surgery are highly heterogeneous, confirming previous observations in narrative reviews.16, 17
Our meta‐analysis revealed significant differences, both pro‐ and anti‐inflammatory, in host response after vaginal mesh implantation compared to control animals. During a controlled host response, the formation of new tissue and subsequent wound healing is expressed by an increase in neovascularization and this was significantly higher in implanted animals. M1 and M2 macrophages, MMP‐2 and TNF‐α were also significantly increased after vaginal mesh implantation compared to control animals. TNF‐α is a pro‐inflammatory cytokine, can be secreted by macrophages and plays together with other cytokines an important role in the early inflammatory response.18 MMP‐2 is a proteolytic enzyme capable of degrading and digesting components within the extracellular matrix and important proteins like collagen and elastin.19, 20 In vaginal tissue of women with mesh complications, TNF‐α, MMP‐2, and pro‐inflammatory M1 macrophages have shown to be significantly higher compared to tissue of women without a mesh,21 as an expression of impaired wound healing. Although in these women macrophages were predominantly of the pro‐inflammatory M1 phenotype, as seen in our meta‐analysis of animal studies, also anti‐inflammatory M2 macrophages were significantly increased,21 presumably as an expression of constructive remodeling.22
Regarding the biomechanical outcomes, we observed a significant increase in vaginal stiffness and a decrease in vaginal contractility, indicating the possible negative effect of mesh implantation on the vaginal wall functionality. Whereas a certain degree of stiffness is required for load‐bearing capacity, too high stiffness can cause an impairment in the normal functioning of the vagina.23, 24 Furthermore, while during a controlled host response the contractile function of the vagina is maintained, vaginal contractility can be altered in the presence of implants due to fibrosis, or a decreased collagen and elastin content and is an expression of smooth muscle functioning.23, 25
Meta‐analysis showed an overall exposure rate of 20.1%, but the incidence of vaginal exposures differed hugely among studies. While in many studies no exposures were observed, in others over half of the animals developed a vaginal exposure. Subsequently, subgroup analysis revealed that exposures were significantly more common in ewes and rabbits compared to rats. Larger implants were used in sheep and rabbits and these cause a larger mesh burden and have shown to be a risk factor for vaginal exposures26 which is in line with observations in women.27, 28 Although the implants in rats were smaller, the rat also has a smaller vagina, but this could also have led to an underestimation in the observation of exposures due to the limited view. Exposure rates in women are lower, approximately around 12%.29 The higher rate in animals, could be explained for various reasons, such as the different vaginal environment, the experience of the surgeon in the technique, and possibly the use of more experimental types of implants in animal studies.
When comparing vaginal and abdominal implantations, we observed significantly more exposures and a higher contraction rate in the vagina compared to the abdominal wall. The vagina has a different microflora and increased vascularization compared to the abdominal wall, which may cause differences in the local host response.30
4.1. Strengths and limitations
To our knowledge, this is the first systematic review on all outcome measures describing the host response in animal experimental research on urogynecologic surgical mesh implants and assessing the effect of mesh implantation by meta‐analysis. Further strengths of this review are the broad search, and ensuring methodological quality by a collaboration with the SYstematic Review Center for Laboratory animal Experimentation (SYRCLE). Although we observed a moderate level of between‐study heterogeneity for most outcome measures, exploring this heterogeneity is one of the added values and might help to inform the design of future studies. Unfortunately, our planned subgroup analyses contained often too few comparisons to conduct meaningful analyses (Supplementary file 5). Nevertheless, to account for anticipated heterogeneity, we used a random rather than fixed‐effects meta‐analysis.
However, this review has some limitations. Since the etiology of mesh complications is a multifactorial process in women,31 certain outcomes may not solely be the result of the host response elicited by the implant. This is one of the limitations of the translation of these animal studies to clinical practice. Furthermore, the large variety in outcome measures and the lack of an appropriate control group in the majority of included articles, hampered meta‐analysis of possibly relevant outcomes (e.g., infection, inflammation, or fibroblastic proliferation). However, we observed a trend over the years towards more studies including a sham surgery or NTR group (Supplementary file 6). In addition, the risk of bias could not be estimated for the majority of the studies due to the lack of reporting certain essential methodological details. Although this is common for animal studies, it may influence the results and conclusions drawn. This review also suffers from indirectness issues32 since most animals in this systematic review did not have clinical signs of prolapse and were not postmenopausal, as most human patients are, and this may have an effect on the local host response and wound healing.33 However, increased TNF‐α, MMP‐2, and M1‐ and M2‐macrophages after mesh implantation were seen both in women21 as the animals in this review. Finally, the estimated effects in this review may be inflated as a consequence of publication bias. Unfortunately, we could not assess publication bias due to too limited number of studies.
4.2. Implications
We suggest for future studies to include an appropriate control group and focus on reporting all important methodological details using guidelines.34, 35 Items such as randomization, sample size calculation, and blinding are key aspects in ensuring rigorous and reproducible animal research. Furthermore, we suggest developing a core outcome set of quantitative outcome measures including direct (e.g., histology) and indirect (e.g., macroscopic observations and biomechanics) representatives for the host response. The host response should be evaluated in the short and long term. In our opinion, the direct outcome measures of the host response should include cell types and mediators of acute (e.g., neutrophils) and chronic inflammation (e.g. macrophages), and subsequent wound healing with granulation tissue development (e.g., neovascularization, fibroblasts, and connective tissue formation), the foreign body reaction (e.g., foreign body giant cells) and fibrous capsule formation (e.g., collagen and elastin deposition). Based on our systematic review and previous studies, animal experiments demonstrating (1) non or limited macroscopic changes such as exposure or contraction, (2) an improved tissue regeneration indicated by an M2 response and neovascularization, and (3) improved tissue biomechanics without fibrotic tissue formation which may be represented by a change in tissue stiffness by the contribution of collagen, elastin and MMP activity without or limited decline in contractility; may demonstrate the minimal requirements to assess the performance of the urogynecologic surgical mesh implants. However, this core outcome set should ideally be designed and scaled up/down by experts in the field, including (uro)gynecologists, animal ethics, and scientists with expertise in animal experimental research, histology, biochemistry, and/or biomechanics. Besides as demonstrated by meta‐analysis, the host response in the vagina differs from the abdomen. For this reason, we believe that future in vivo studies on new biomaterials for POP should mainly focus on vaginal implantations. Yet for some specific research questions, a smaller animal model might be more suitable. Most challenging, but of high relevance and necessity to study, these outcome measures should predict the host response in women and the possible development of local adverse events. Although no single animal model is able to mimic all human aspects, using this core set of outcome measures improves interpretation, aggregation, and translation of results and probably makes animal experimental research more effective.
5. CONCLUSIONS
Animals with vaginal implants show a significant increase in M1 and M2 macrophages, MMP‐2, neovascularization, TNF‐α and stiffness, and lower vaginal contractility compared to control animals receiving sham surgery or NTR. Furthermore, implant exposure and contraction were significantly higher in the vagina as compared to the abdominal wall. However, we observed a large variety in outcome measures used in this type of research and consequently meta‐analysis could only be performed for 11% of the outcomes due to the insufficient quality and incompleteness of reported outcomes. Finally, we would like to address the urge for animal experimental research using appropriate control groups, rigorous reporting of all essential methodological details, and inclusion of standardized quantitative outcome measures representing the different phases of the host response, to eventually improve the translation of animal experimental research to clinical practice.
CONFLICT OF INTERESTS
Ms. Verhorstert reports grants from ZonMw, during the conduct of the study. Mr. Gudde has nothing to disclose. Ms. Kortz has nothing to disclose. Dr. Limpens has nothing to disclose. Dr. Roovers reports grants from ZonMw, during the conduct of the study; grants from Tepha, grants from Urogyn, grants from Coloplast, outside the submitted work. Dr. Hooijmans reports grants from ZonMw, during the conduct of the study. Dr. Guler has nothing to disclose.
AUTHOR CONTRIBUTIONS
Kim W. J. Verhorstert: Conceptualization, formal analysis (lead), funding acquisition, investigation, methodology, writing – original draft preparation, and writing – review & editing. Aksel N. Gudde: Investigation and writing – review & editing. Brita S. Kortz: Investigation and writing – review & editing. Jacqueline Limpens: Investigation and writing – review & editing. Jan‐Paul W. R. Roovers: Conceptualization, supervision, and writing – review & editing. Carlijn R. Hooijmans: Formal analysis, methodology, supervision, and writing – review & editing. Zeliha Guler: Conceptualization, funding acquisition, supervision, and writing – review & editing.
Supporting information
Supplementary Information
ACKNOWLEDGMENT
Project: “A synthesis of Evidence”, MKMD from ZonMW (Project number: 114024138) for funding of the collaboration with SYRCLE (SYstematic Review Center for Laboratory animal Experimentation).
Verhorstert KWJ, Gudde AN, Kortz BS, et al. Animal experimental research assessing urogynecologic surgical mesh implants: Outcome measures describing the host response, a systematic review and meta‐analysis. Neurourology and Urodynamics. 2021;40:1107–1119. 10.1002/nau.24677
REFERENCES
- 1.Nygaard I. Prevalence of symptomatic pelvic floor disorders in US women. J Am Med Assoc. 2008;300(11):1311‐1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Olsen AL, Smith VJ, Bergstrom JO, Colling JC, Clark AL. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol. 1997;89(4):501‐506. [DOI] [PubMed] [Google Scholar]
- 3.Denman MA, Gregory WT, Boyles SH, Smith V, Edwards SR, Clark AL. Reoperation 10 years after surgically managed pelvic organ prolapse and urinary incontinence. Am J Obstet Gynecol. 2008;198(5):555. [DOI] [PubMed] [Google Scholar]
- 4.Gigliobianco G, Roman Regueros S, Osman NI, et al. Biomaterials for pelvic floor reconstructive surgery: how can we do better? BioMed Res Int. 2015;2015:968087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kelly M, Macdougall K, Olabisi O, McGuire N. In vivo response to polypropylene following implantation in animal models: a review of biocompatibility. Int Urogynecology J. 2017;28(2):171‐180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.FDA . Urogynecologic Surgical Mesh: Update on the Safety and Effectiveness of Transvaginal Placement for Pelvic Organ Prolapse. U.S. Food and Drug Administration; 2011. [Google Scholar]
- 7.FDA . Urogynecologic Surgical Mesh Implants. U.S. Food and Drug Administration; 2019. https://www.fda.gov/medical-devices/implants-and-prosthetics/urogynecologic-surgical-mesh-implants. Accessed May 23, 2019. [Google Scholar]
- 8.Slack M, Ostergard D, Cervigni M, Deprest J. A standardized description of graft‐containing meshes and recommended steps before the introduction of medical devices for prolapse surgery. Consensus of the 2nd IUGA grafts roundtable: optimizing safety and appropriateness of graft use in transvaginal pelvic reconstructive surgery. Int Urogynecol Journal. 2012;23(suppl 1):15‐26. [DOI] [PubMed] [Google Scholar]
- 9.Badylak SF. Host response to biomaterials – the impact of host response on biomaterial selection. In: Badylak SF, ed. Host Response to Biomaterials. Elsevier; 2015:1‐36. [Google Scholar]
- 10.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. BMJ. 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ouzzani HH M, Fedorowicz Z, Elmagarmid A. Rayyan – a web and mobile app for systematic reviews. Qatar. https://rayyan.qcri.org/welcome [DOI] [PMC free article] [PubMed]
- 12.Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hooijmans CR, Rovers MM, de Vries RB, Leenaars M, Ritskes‐Hoitinga M, Langendam MW. SYRCLE's risk of bias tool for animal studies. BMC Med Res Methodol. 2014;14:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hooijmans CR, Hlavica M, Schuler FAF, et al. Remyelination promoting therapies in multiple sclerosis animal models: a systematic review and meta‐analysis. Sci Rep. 2019;9(1):822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ozog Y, Mazza E, De Ridder D, Deprest J. Biomechanical effects of polyglecaprone fibers in a polypropylene mesh after abdominal and rectovaginal implantation in a rabbit. Int Urogynecol J Pelvic Floor Dysfunct. 2012;23(10):1397‐1402. [DOI] [PubMed] [Google Scholar]
- 16.Patel H, Ostergard DR, Sternschuss G. Polypropylene mesh and the host response. Int Urogynecol J. 2012;23(6):669‐679. [DOI] [PubMed] [Google Scholar]
- 17.Mackova K, Da Cunha M, Krofta L, Albersen M, Deprest J. The importance of developing relevant animal models to assess existing and new materials. Curr Opin Urol. 2019;29(4):400‐406. [DOI] [PubMed] [Google Scholar]
- 18.Anderson JM, Rodriguez A, Chang DT. Foreign body reaction to biomaterials. Semin Immunol. 2008;20(2):86‐100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones JA, McNally AK, Chang DT, et al. Matrix metalloproteinases and their inhibitors in the foreign body reaction on biomaterials. J Biomed Mater Res A. 2008;84(1):158‐166. [DOI] [PubMed] [Google Scholar]
- 20.Liang R, Zong W, Palcsey S, Abramowitch S, Moalli PA. Impact of prolapse meshes on the metabolism of vaginal extracellular matrix in rhesus macaque. Am J Obstet Gynecol. 2015;212(2):174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nolfi AL, Brown BN, Liang R, et al. Host response to synthetic mesh in women with mesh complications. Am J Obstet Gynecol. 2016;215(2):206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brown BN, Valentin JE, Stewart‐Akers AM, McCabe GP, Badylak SF. Macrophage phenotype and remodeling outcomes in response to biologic scaffolds with and without a cellular component. Biomaterials. 2009;30(8):1482‐1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feola A, Abramowitch S, Jallah Z, et al. Deterioration in biomechanical properties of the vagina following implantation of a high‐stiffness prolapse mesh. BJOG. 2013;120(2):224‐232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bigozzi MA, Provenzano S, Maeda F, Palma P, Riccetto C. In vivo biomechanical properties of heavy versus light weight monofilament polypropylene meshes. Does the knitting pattern matter? Neurourol Urodyn. 2017;36(1):73‐79. [DOI] [PubMed] [Google Scholar]
- 25.Liang R, Abramowitch S, Knight K, et al. Vaginal degeneration following implantation of synthetic mesh with increased stiffness. BJOG. 2013;120(2):233‐243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Manodoro S, Endo M, Uvin P, et al. Graft‐related complications and biaxial tensiometry following experimental vaginal implantation of flat mesh of variable dimensions. BJOG. 2013;120(2):244‐250. [DOI] [PubMed] [Google Scholar]
- 27.Withagen MI, Vierhout ME, Hendriks JC, Kluivers KB, Milani AL. Risk factors for exposure, pain, and dyspareunia after tension‐free vaginal mesh procedure. Obstet Gynecol. 2011;118(3):629‐636. [DOI] [PubMed] [Google Scholar]
- 28.Wu PY, Chang CH, Shen MR, Chou CY, Yang YC, Huang YF. Seeking new surgical predictors of mesh exposure after transvaginal mesh repair. Int Urogynecol J. 2016;27(10):1547‐1555. [DOI] [PubMed] [Google Scholar]
- 29.Maher C, Feiner B, Baessler K, Christmann‐Schmid C, Haya N, Marjoribanks J. Transvaginal mesh or grafts compared with native tissue repair for vaginal prolapse. Cochrane Database Syst Rev. 2016;2:Cd012079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hilger WS, Walter A, Zobitz ME, Leslie KO, Magtibay P, Cornella J. Histological and biomechanical evaluation of implanted graft materials in a rabbit vaginal and abdominal model. Am J Obstet Gynecol. 2006;195(6):1826‐1831. [DOI] [PubMed] [Google Scholar]
- 31.Liang R, Knight K, Abramowitch S, Moalli PA. Exploring the basic science of prolapse meshes. Curr Opin Obstet Gynecol. 2016;28(5):413‐419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hooijmans CR, de Vries RBM, Ritskes‐Hoitinga M, et al. Facilitating healthcare decisions by assessing the certainty in the evidence from preclinical animal studies. PLOS One. 2018;13(1):e0187271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krause M, Wheeler TL 2nd, Snyder TE, Richter HE. Local effects of vaginally administered estrogen therapy: a review. J Pelvic Med Surg. 2009;15(3):105‐114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Percie du Sert N, Hurst V, Ahluwalia A, et al. The ARRIVE guidelines 2.0: updated guidelines for reporting animal research. J Physiol. 2020;598:3793‐3801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hooijmans CR, Leenaars M, Ritskes‐Hoitinga M. A gold standard publication checklist to improve the quality of animal studies, to fully integrate the Three Rs, and to make systematic reviews more feasible. Altern Lab Anim. 2010;38(2):167‐182. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information


