Abstract
Objectives
Accelerated intermittent theta burst stimulation (aiTBS) is a promising treatment option for depressed patients. However, there is a large interindividual variability in clinical effectiveness and individual biomarkers to guide treatment outcome are needed.
Materials and Methods
Here, the relation between cortical thickness and clinical response (17‐item Hamilton Depression Rating Scale) was studied using anatomical MRI data of 50 depressed patients who were included in a randomized, sham‐controlled, double‐blinded, cross‐over aiTBS design (NCT01832805).
Results
Baseline cortical thickness in the right caudal part of the anterior cingulate cortex (cACC) was significantly correlated with direct clinical responses in the subgroup who received active aiTBS during the first stimulation week. No correlations were found between baseline cortical thickness and delayed clinical effectiveness. In this particular region, longitudinal changes in cortical thickness were significantly correlated with clinical effectiveness. Furthermore, direct changes in cortical thickness in the right cACC showed predictive potential of delayed clinical responses.
Conclusion
Cortical thickness within the right cACC might be an important biomarker to predict clinical responses to aiTBS. Additional studies are warranted to substantiate the specific biomarker potential of these parts of the ACC.
Keywords: Brain stimulation/TMS/DBS/VNS, biological markers, depression, neuroimaging, neurostimulation
INTRODUCTION
Major depressive disorder (MDD) is one of the most prevalent psychiatric disorders affecting more than 264 million people worldwide (1). Despite the availability of multiple therapeutic interventions, such as psychotherapy and pharmacotherapy, approximately one‐third of the subjects will not respond to these available interventions and will develop treatment resistant depression (TRD).
Repetitive transcranial magnetic stimulation (rTMS) is a noninvasive brain stimulation technique that is approved for the treatment of TRD by the U.S. food and drug administration (FDA). The most standard stimulation protocol for TRD treatment contains daily high‐frequency (>10 Hz, presumably excitatory) stimulation sessions targeting the left dorsolateral prefrontal cortex (DLPFC) with a figure‐of‐eight shaped coil for a period of four to six weeks (2, 3). Less time‐consuming accelerated rTMS protocols, that is, applying multiple stimulation sessions a day over a shorter period of time, are under investigation but not FDA approved yet (4). A particular rTMS design, intermittent theta burst stimulation (iTBS (5)) administered to the left DLPFC has shown promising therapeutic outcome for depression patients and mostly showed to be beneficial in terms of duration and it can be applied at a lower stimulation intensity (6). In our laboratory, an accelerated iTBS (aiTBS) protocol, in which five daily iTBS sessions were administered on four consecutive days, was investigated as treatment for TRD patients (7). Two weeks after the end of the randomized double‐blind, sham‐controlled, stimulation design, a response rate of 38% was observed, indicating delayed clinical effects. Of these responders, 30% were in remission.
Notwithstanding, the overall response rates of any rTMS treatment protocol are still rather modest. The high interindividual heterogeneity of clinical effectiveness underscores the pressing need for biomarkers to predict treatment outcome. Previous studies showed promising results using measures derived from resting‐state functional MRI (rs‐fMRI) (8, 9, 10) or diffusion weighted MRI (dMRI) (11). However, these biomarkers require extensive imaging protocols that are not always available in clinical settings, need specialized data processing, and are costly. Therefore, simpler biomarkers, for example, cortical thickness (CT) measures, as derived from anatomical MRI data, could be more feasible. Indeed, cortical thickness has shown to be a sensitive biomarker for a wide variety of neuropsychiatric diseases, including MDD (12, 13, 14). Moreover, Boes et al. (15) showed that baseline (i.e., before stimulation) thickness is related to clinical response to a four to six weeks rTMS protocol in MDD patients targeting the left DLPFC. Specifically, it was shown that smaller baseline cortical thickness in the left rostral anterior cingulate cortex (rACC) could predict better clinical response, that is, greater improvement of depressive symptoms. Furthermore, thickness of the rACC was shown to increase in responding patients.
In this aiTBS study including MDD patients documented to be resistant to at least one psychotropic intervention, we retrospectively investigated whether cortical thickness could be related to clinical response. In line with the previous findings from Boes et al. (15), it was hypothesized that especially the cortical thickness in parts of the cingulate cortex would play a pivotal role in the treatment efficacy of aiTBS. Different parts of the cingulate cortex are involved in different functions. More specifically, the anterior part (ACC) belongs to the limbic system and is involved in the regulation of cognitive and emotional processing (16). For example, greater functional activity was found in the rostral ACC in meditators compared to healthy controls, which might reflect stronger processing of distracting events (17). Functional connectivity between the subgenual part of the ACC and the stimulation site in the left DLPFC (8, 18) and indirect structural connections between the DLPFC and the caudal part of the ACC (11) were previously related to the effectiveness of rTMS treatment for depression.
MATERIALS AND METHODS
This registered RCT (http://clinicaltrials.gov/show/NCT01832805) was approved by the ethics committee of Ghent University Hospital and was conducted in line with the declaration of Helsinki. Written informed consent was obtained from all patients prior to participation.
Inclusion
For this RCT, 50 right‐handed patients with TRD (mean age 42 years, SD 12 years) were recruited from the Ghent University Hospital. MDD was diagnosed using the structured diagnostic psychiatric Mini‐International Neuropsychiatric Interview (MINI (19)). Patients were only included if treatment resistance was at least stage I (i.e., nonresponse to at least one trial with serotonin‐reuptake inhibitors or noradrenaline/serotonin‐reuptake inhibitors). More detailed information can be found in Duprat et al. (7).
Study Design
The overall design of this randomized, sham‐controlled, double‐blinded, cross‐over trial is depicted in Fig. 1. In short, prior to the start of the study, patients underwent a two‐week wash‐out period followed by a two‐week antidepressant free period. Patients were randomized to receive first sham aiTBS followed by active aiTBS (arm A in Fig. 1) or the other way around (arm B in Fig. 1). On the first day (T1), a 3 T Siemens TrioTim scanner (Erlangen, Germany) was used to record anatomical MRI data (MPRAGE, TR = 2530 msec, TE = 2.58 msec, FA = 7°, FOV = 220 × 220 mm2, resolution = 0.9 × 0.9 × 0.9 mm3, 176 slices). On the 8th day (T2) and the 15th day (T3), the MRI acquisition protocol was repeated.
Figure 1.
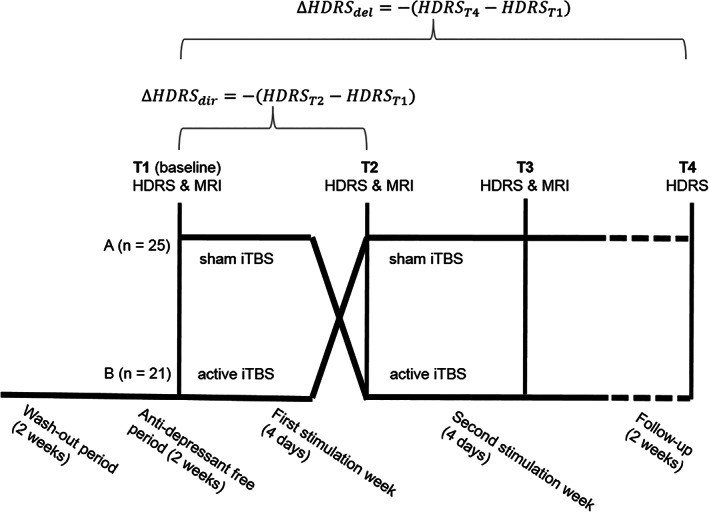
Overall design of this randomized, sham‐controlled, double‐blinded, cross‐over trial. Adapted from Duprat et al. (7).
On days 2–5 (week 1) and days 9–12 (week 2), active or sham aiTBS was applied depending on the randomization order. A Magstim Rapid2 Plus1 magnetic stimulator (Magstim Company Limited, Wales, UK) connected to an active or sham figure‐of‐eight shaped coil (Magstim 70 mm double air film [sham] coil) was used to apply the active and sham stimulation, respectively. One iTBS session consisted of 54 trains containing ten bursts of three stimuli at 50 Hz, repeated every 200 msec, alternated by six seconds rest. According to the accelerated protocol, five daily stimulation sessions were given with 15 min rest in between on four consecutive days.
Depression severity was assessed with the Hamilton Depression Rating Scale (HDRS (20)) directly after randomization (baseline, T1), after the first stimulation week (T2), at the end of the second stimulation week (T3) and two weeks following the end of stimulation during follow‐up (T4).
Baseline Cortical Thickness vs. Clinical Effectiveness of aiTBS
Data Driven Whole Brain Analysis
Freesurfer 6.0 (http://surfer.nmr.mgh.harvard.edu) was used to perform cortical reconstruction of all baseline (T1) MPRAGE data (21). Using the optimized recon‐all command, automatic brain parcellation was performed including the delineation of the white and pial surface such that cortical thickness could be defined over all vertices. Freesurfer parcellation of the white and pial surfaces for each patient was reviewed manually. For this surface group analysis, a smoothing kernel of 15 mm (FWHM) was used to map the individual data to the fsaverage template (in line with Boes et al. (15)).
Because of the cross‐over design of the study, a distinction was made between the prediction of the direct clinical effects, defined as ΔHDRSdir (= −(HDRST2‐HDRST1)) and the delayed clinical effects (ΔHDRSdel = −(HDRST4‐HDRST1)). A general linear model fitting method, as implemented in query, design, estimate, contrast (QDEC), was used to study the correlations between cortical thickness in every vertex and the clinical effects, thereby correcting for the baseline (T1) HDRS scores. Additionally, correction for order (i.e., sham or active aiTBS) was performed for the prediction of the direct effects. A statistical threshold of p < 0.001 uncorrected was selected for the analysis (15). Therefore, this analysis is presumed to be exploratory.
To study the order effects in more detail, two separate analyses were performed including only the subset of subjects receiving sham or active aiTBS during the first week, respectively. Additionally, a sub‐analysis was performed on the subjects who received active aiTBS after sham stimulation (arm A, T2 vs. T3). To compensate for the loss of statistical power, statistical threshold was set to p < 0.01.
Significant ROI Analysis
Significant ROIs were defined as the vertices in the brain with p < 0.001 extended with the neighboring vertices with p < 0.01 (for the separate order effects these thresholds were set to p < 0.01 with neighboring vertices p < 0.05). These were studied in more detail on the individual level by extracting average statistical information representing the total region of interest (ROI). To that aim, the group level ROIs were mapped to the individual data and the cortical thickness of every ROI was computed as the average of the vertices within the ROI.
Additionally, we investigated the power of the baseline cortical thickness values of these ROIs to predict the direct and delayed clinical responder rates (defined as >50% decrease in HDRS scores) by means of ROC curves.
Effects of aiTBS on Cortical Thickness vs. Clinical Effectiveness
Data Driven Whole Brain Analysis
The longitudinal processing stream of Freesurfer was used to optimize detection of changes in cortical thickness of the same individual across two time points (22). Since no MRI data were available at our long‐term follow‐up (T4), this analysis was restricted to the direct clinical effects and hence the anatomical MRI data of T1 and T2 were used. Similar as for the previous analysis (i.e., baseline cortical thickness versus clinical effectiveness), a general linear model fitting method, as implemented in QDEC, was used to study the correlations between percentage change in cortical thickness () in every vertex and the clinical effects, thereby correcting for the baseline (T1) HDRS scores and for the order (i.e., sham or active aiTBS). A similar smoothing kernel and the same statistical thresholds were used to study significant ROIs in more detail.
Significant ROI Analysis
Significantly ROIs were defined according to the same method as described for the first analysis of this study (i.e., “Baseline cortical thickness versus clinical effectiveness of aiTBS”) and analyzed on an individual level accordingly. Additionally, ROC curves were computed for the prediction of the delayed clinical effects, based on the direct effects of aiTBS on cortical thickness, using the subgroup of patients receiving active aiTBS during the first stimulation week.
Assessment of Confounding Factors
Previous work showed that cortical thickness could be related to age and gender (23, 24, 25, 26). Therefore, age and gender were assessed as a potential confounding factors. Additionally, also the relation between the duration of the depression episodes and the stage of the depression and thickness were investigated. To that aim, correlations were computed between the continuous variables (i.e., age and duration of depression episodes) and the cortical thickness or the direct change in thickness in all the ROIs that showed significant results in the two analyses. For the discrete variables (i.e., gender and stage of the depression), two‐tailed t‐tests were performed between the thickness values of the different subgroups.
RESULTS
Biomarker Potential: Can Baseline Cortical Thickness Predict the Clinical Response to aiTBS?
Data of 46 patients were used to study the potential of baseline cortical thickness values to predict direct and delayed clinical responses (one subject retrospectively received an alternative diagnosis and three patients dropped out before completing the study). Of these 46 subjects, 25 received sham stimulation during the first week (arm A) and 21 received active aiTBS (arm B). At T2 two subjects responded to stimulation, that is, showed a decrease in HDRS score of at least 50%. After follow‐up, at T4, 18 patients showed clinical response. The results of the sub‐analysis including the patients who received active aiTBS after sham stimulation can be found in Supporting Information A.
Prediction of Direct Clinical Effects
Figure 2 shows an overview of the significant ROIs after correlating baseline cortical thickness values with ΔHDRSdir values.
Figure 2.
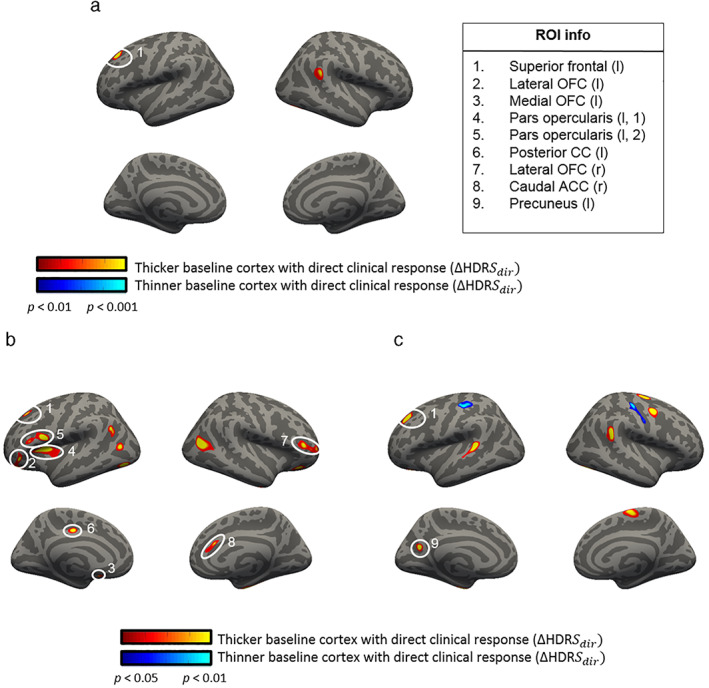
Correlations between baseline cortical thickness (CT) and direct changes in HDRS. Significant correlations between the baseline (T1) cortical thickness and ΔHDRSdir. Figure a includes all subjects (n = 46, order corrected, dof = 40), whereas in figure b and c, results are split for the subjects receiving active aiTBS (n = 21, dof = 18) or sham aiTBS (n = 25, dof = 22), respectively. Note the different statistical thresholds to compensate for the loss of power when studying the subgroups. [Color figure can be viewed at wileyonlinelibrary.com]
In Fig. 2a, a significant ROI was found in the left superior prefrontal area (see Table 1 for details). This positive correlation was found independent of the order of the stimulation sessions, that is, if the patients received active or sham aiTBS during the first week of stimulation. This is also depicted in Fig. 3, where the average baseline cortical thickness in the prefrontal ROI is plotted against the clinical responses for all subjects. Positive correlations suggest that higher baseline cortical thickness values are correlated with larger clinical responses. The ROC curve is shown in Supporting Information (A).
Table 1.
Detailed Information About the ROI in the Left Superior Prefrontal Cortex Showing Significant Correlation Between the Baseline CT and ΔHDRSdir.
| Group level results (Fig. 2a) | Individual level results (Fig. 3) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| ROI | Nr vertices p < 0.001 | Nr vertices in ROI (p < 0.01) | Min t‐value (vertex number) | X | Y | Z | Correlations and p‐values | ||
| All patients (n = 46) | Sham subgroup (n = 25) | Active subgroup (n = 21) | |||||||
| Superior prefrontal cortex (l) | 218 | 529 | −4.1 (152,289) | −19.4 | 29.2 | 32.9 | 0.35, 0.02 | 0.37, 0.07 | 0.45, 0.04 |
Figure 3.
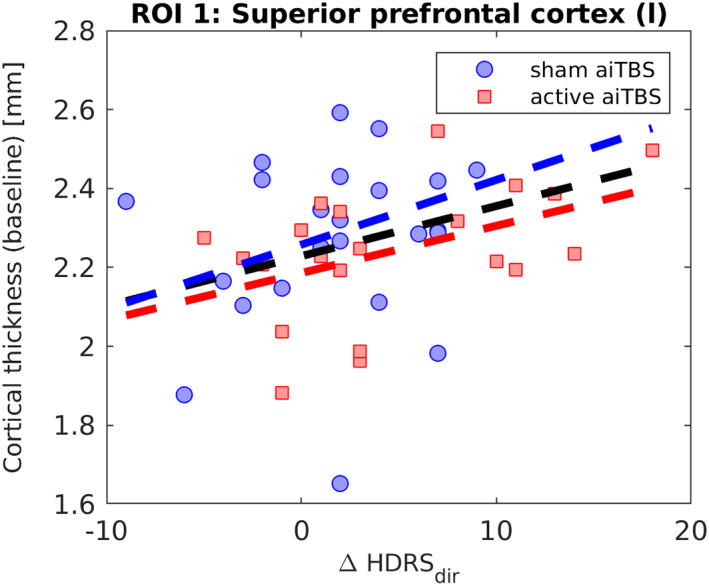
Overview of the baseline (T1) cortical thickness vs. ΔHDRSdir in the ROI in the left superior prefrontal cortex. [Color figure can be viewed at wileyonlinelibrary.com]
Of notable interest, the significant cluster in the left prefrontal area was found to be close to the subjects' stimulation position in the left DLPFC. Therefore, an additional analysis was performed based on the subject‐specific stimulation position. Figure 4a shows the individual stimulation positions (i.e., center of the stimulation coil, in red). The actual brain region that is affected extends beyond this region. To provide additional insight in the size of the affected brain region, a simulation of the TMS‐induced electric field was added to the figure. For this simulation, an average brain was used, and the stimulation coil was positioned at the mean coil position in our subjects (MNI ‐38 19 54 mm, shown in yellow). The peak of the significant ROI (represented in green in the figure) shows overlap with the average TMS‐induced electric field distribution.
Figure 4.
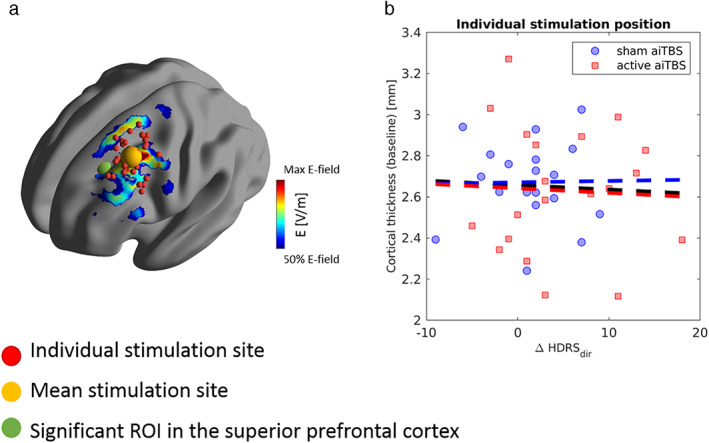
Significant ROI in the left dorsolateral prefrontal cortex vs. individual stimulation positions. (a) Shows the position of the significant ROI in the left superior prefrontal cortex (green) with respect to the individual stimulation sites (red, mean shown in yellow). The TMS‐induced electric field distribution is shown (coil placement at the mean coil position) to provide additional insight in the spread of the TMS effects. In (b), there is no significant correlation between the baseline cortical thickness in the individual stimulation site and the direct clinical response to aiTBS. Note that because information on the individual coil positioning was not available for all subjects, this analysis was performed on a subset of 41 patients. [Color figure can be viewed at wileyonlinelibrary.com]
However, as can be seen in Fig. 4b, no significant correlations were found between the baseline cortical thickness values in the ROIs derived from the subject‐specific stimulation position and the direct clinical response (for details on methods see Supporting Information C). The overall correlation coefficient was −0.05 (p = 0.77) when all patients were included, and 0.02 (p = 0.94) and − 0.05 (p = 0.85) for the subgroup of patients receiving sham and active stimulation during the first week, respectively.
As can be seen in Fig. 1b,c, additional significant ROIs are found when separating the data into subsets of patients who received active (b) and sham (c) aiTBS during the first week. Specifically, significant ROIs were found in the lateral orbitofrontal cortex (OFC) bilaterally, left medial OFC, two different regions in the left pars opercularis, the right caudal ACC (cACC), left posterior CC in the subgroup of patients who received active aiTBS in the first week. The left precuneus showed a significant correlation between baseline cortical thickness and ΔHDRSdir in the subgroup of patients who received sham stimulation first. Detailed information about these ROIs is presented in Table 2. In all these ROIs, a positive correlation was found between the baseline cortical thickness and the direct clinical responses, that is, higher baseline thickness is related to more clinical improvement (Fig. 5, the corresponding ROC‐curves can be found in Supporting Information B).
Table 2.
Detailed Information About the ROIs.
| Group level results (Fig. 2b,c) | Individual level results (Fig. 5) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| ROI | Nr vertices p < 0.01 | Nr vertices in ROI (p < 0.05) | Min t‐value (vertex number) | X | Y | Z | Correlations and p‐values | ||
| Sham subgroup (n = 25) | Active subgroup (n = 21) | All patients (n = 46) | |||||||
| Lateral OFC (l) | 81 | 770 | −2.522 (14,908) | −24.5 | 35.1 | −9.4 | −0.26, 0.19 | 0.71, <0.01 | 0.06, 0.65 |
| Medial OFC (l) | 4 | 77 | −2.051 (49,397) | −6.5 | 14.6 | −12.8 | −0.04, 0.84 | 0.49, 0.02 | 0.15, 0.33 |
| Pars opercularis (l,1) | 514 | 1554 | −2.982 (98,541) | −32.9 | 11.4 | 11.9 | −0.31, 0.13 | 0.57, <0.01 | 0.06, 0.68 |
| Pars opercularis (l,2) | 246 | 952 | −2.816 (23,956) | −44.9 | 6.6 | 14.2 | −0.02, 0.93 | 0.74, <0.01 | 0.40, <0.01 |
| Posterior CC (l) | 91 | 315 | −2.498 (10,547) | −8.1 | −15.0 | 36.2 | 0.12, 0.56 | 0.67, <0.01 | 0.33, 0.03 |
| Lateral OFC (r) | 82 | 407 | −2.353 (67,779) | 31.5 | 29.8 | −10.6 | −0.51, <0.01 | 0.54, 0.01 | −0.01, 0.94 |
| Caudal ACC (r) | 2 | 306 | −2.008 (16,032) | 6.0 | 26.0 | 20.3 | 0.07, 0.74 | 0.51, 0.02 | 0.28, 0.06 |
| Precuneus (l) | 108 | 361 | −2.589 (117,982) | −15.1 | −57.3 | 22.4 | 0.57, <0.01 | 0.25, 0.27 | 0.35, 0.02 |
Figure 5.
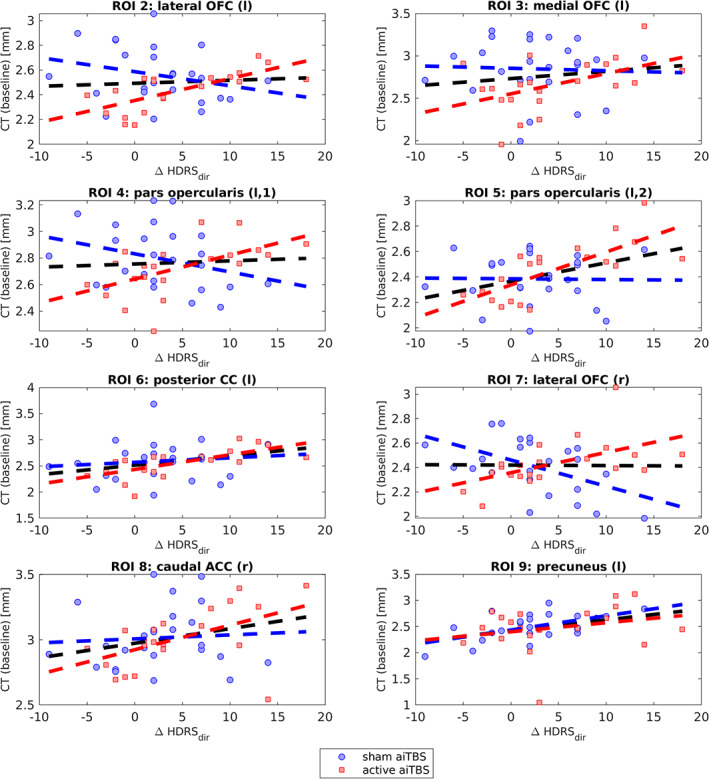
Significant ROIs when splitting the analysis in subgroups who received active (n = 21) or sham (n = 25) aiTBS during the first stimulation week. [Color figure can be viewed at wileyonlinelibrary.com]
Prediction of Delayed Clinical Effects
No significant correlations were found between baseline cortical thickness and the delayed clinical response, ΔHDRSdel.
Mechanism of Action of aiTBS: Can Longitudinal Changes in Cortical Thickness Be Linked to Clinical Effectiveness?
Anatomical MRI data of T1 and T2 were used to study the relation between longitudinal changes in cortical thickness and the clinical responses. Because of insufficient MRI settings for one subject (incomplete FOV), this analysis was performed using 45 datasets.
Figure 6 shows an overview of the significant ROIs when correlating ΔCT values with ΔHDRSdir values.
Figure 6.
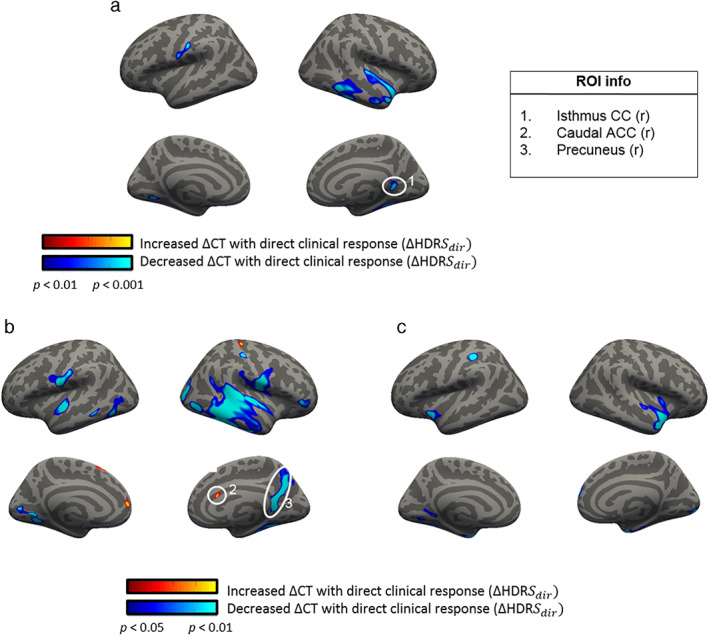
Significant correlations between ΔCT and ΔHDRSdir. Figure a includes all subjects (n = 45, order corrected, dof = 39) whereas in b and c, results are split for the subjects receiving active aiTBS (n = 21, dof = 18) or sham aiTBS (n = 24, dof = 21), respectively. Note the different statistical thresholds. [Color figure can be viewed at wileyonlinelibrary.com]
In Fig. 6a, a significant ROI was found in the right isthmus part of the CC (for details see Table 3). The relation between ΔCT and ΔHDRSdir is shown in more detail in Fig. 7.
Table 3.
Details of the ROI in the Right Isthmus Part of the Cingulate Cortex in Which Changes in CT Are Significantly Correlated With the Direct Clinical Responses to aiTBS.
| Group level results (Fig. 6a) | Individual level results (Fig. 7) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| ROI | Nr vertices p < 0.001 | Nr vertices in ROI (p < 0.01) | Min t‐value | X | Y | Z | Correlations and p‐values | ||
| Sham subgroup (n = 24) | Active subgroup (n = 21) | All subjects (n = 45) | |||||||
| Isthmus CC (r) | 184 | 707 | 3.551 (51,928) | 18.7 | −48.4 | 6.3 | −0.22, 0.30 | −0.60, <0.01 | −0.42, <0.01 |
Figure 7.
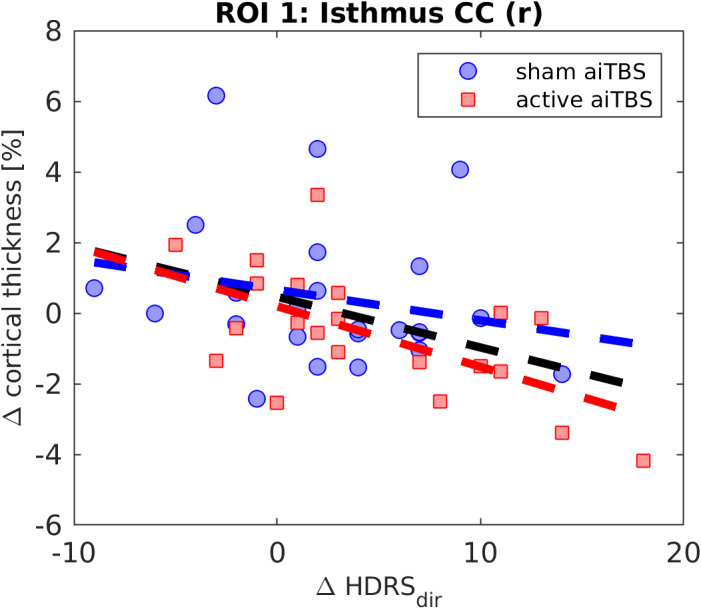
Overview of ΔCT vs. ΔHDRSdir in the ROI in the right isthmus part of the cingulate cortex (CC). [Color figure can be viewed at wileyonlinelibrary.com]
Two additional ROIs were found when studying the subgroup who received active aiTBS during the first week (Fig. 7b, for details see Table 4). Positive and negative correlations were found between ΔCT and ΔHDRSdir in the right caudal part of the ACC and in the right precuneus area respectively. As can be seen in Fig. 7b, the right precuneus ROI also comprises the right isthmus part of the CC. Figure 8 shows the relation between ΔCT and ΔHDRSdir for these two ROIs.
Table 4.
Additional Information of the ROIs.
| Group level results (Fig. 6b) | Individual level results (Fig. 8) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| ROI | Nr vertices p < 0.01 | Nr vertices in ROI (p < 0.05) | Min t‐value | X | Y | Z | Correlations and p‐values | ||
| Sham subgroup (n = 25) | Active subgroup (n = 21) | All subjects (n = 45) | |||||||
| Caudal ACC (r) | 1 | 109 | −2.012 (25,900) | 7.1 | 20.2 | 18.6 | −0.13, 0.52 | 0.34, 0.12 | 0.03, 0.86 |
| Precuneus (r) | 1404 | 3585 | 3.043 (130,097) | 6.0 | −58.8 | 23.6 | −0.01, 0.95 | −0.53, 0.01 | −0.30, 0.05 |
Figure 8.
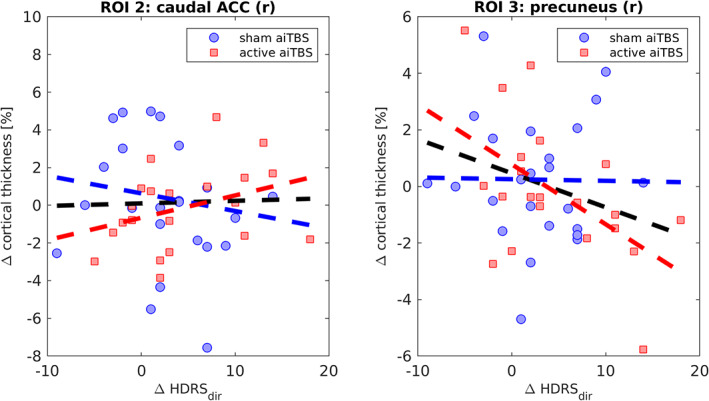
Relation between ΔCT and ΔHDRSdir for the two ROIs that showed significant (p < 0.01) correlations: right caudal part of the ACC and the right precuneus. [Color figure can be viewed at wileyonlinelibrary.com]
Of note, no significant changes in cortical thickness at the stimulation site were found before and after aiTBS (see Supporting Information C1 for more detailed information).
The direct changes in cortical thickness (T2 vs. T1) showed predictive potential for the delayed clinical effects, that is, the AUC of the ROC curve is 0.76, in the right caudal ACC ROI in the subgroup of patients who received active aiTBS during the first week (see Fig. 9, n = 21, eight responders at T4).
Figure 9.
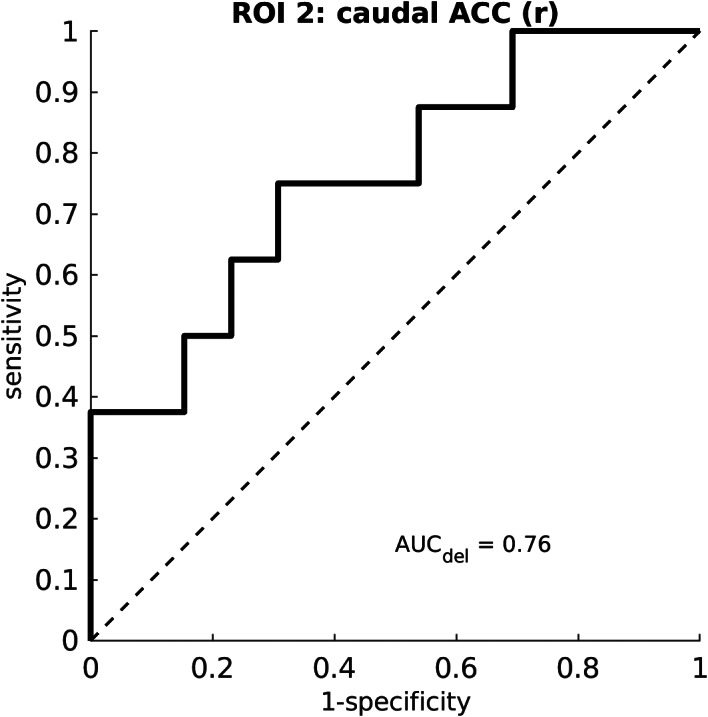
ROC curve of the predictive value of the direct changes in cortical thickness in the right caudal part of the cingulate cortex for the delayed clinical responders. The figure is based on the subgroup of patients who received active aiTBS during the first week of stimulation (n = 21, of which eight responded at T4).
Confounding Factors
For all the significant ROIs that were reported in this study, no significant correlations were found between the baseline cortical thickness and the duration of the depression, neither were statistical differences found between baseline cortical thickness in males vs. females, or when the different depression stages were compared. Correlations between age and baseline thickness were significant in the pars opercularis, left precuneus, and the left posterior cingulate cortex. Changes in cortical thickness in the right caudal ACC ROI after the first week of stimulation were statistically different for patient from stage I and II. A full overview of this assessment can be found in Supporting Information D.
DISCUSSION
This study investigated the relation between cortical thickness and the clinical response to left dorsolateral prefrontal aiTBS. Baseline cortical thickness values were correlated with direct changes in HDRS, measured after four days of stimulation, and delayed changes, measured during follow‐up two weeks after the end of the cross‐over design. Baseline cortical thickness in the left superior prefrontal gyrus was positively correlated with the direct clinical response, both for the subjects receiving sham and active aiTBS. This positive correlation suggests that TRD patients with “relatively” thicker left prefrontal cortical areas may show better clinical responses, irrespective of the type of treatment. Accelerated stimulation protocols indeed tend to yield positive clinical changes not different between active and sham stimulation (7).
Even though the link between cortical thickness and the responses to brain stimulation treatment has been reported before (15, 27, 28), there is currently no full understanding of the potential underlying physiology. rTMS is thought to induce neuroplastic effects, that is, the reorganization of the brain in response to stimulation (29). Variation in cortical thickness is an example of potential neuroplastic effects and could reflect differences in glial cell density (30). Variation in cortical thickness might affect the distribution of the rTMS‐induced electric field within the brain and thereby influence the effectiveness of the stimulation (27). As reviewed by Sackeim (28) similar as for electroconvulsive therapy (ECT), it may be that volumetric changes after rTMS are determined by multiple processes. Future studies would benefit from recording structural MRI data at multiple time points to retrieve information about the temporal changes in cortical thickness after aiTBS. Additionally, small animal studies could help to gain more insight in the neuroplastic effects of rTMS, for example, by counting neuronal cells in cortical regions that are expected to be affected by this type of neurostimulation.
Nevertheless, the most consistent findings throughout this study showed that the right caudal part of the cingulate cortex was the anatomical region implicated in prediction and changes to aiTBS treatment. First, baseline cortical thickness in the right cACC was significantly correlated with the direct clinical response to active aiTBS. Second, longitudinal changes in cortical thickness between T1 and T2 were significantly correlated with the direct clinical responses and these changes in thickness showed predictive potential for the delayed clinical effects, measured at T4. The caudal part of the cingulate cortex has previously been linked to the response to MDD treatment (11, 31, 32). Interestingly, using the same dataset, it was shown that indirect structural connections (derived from diffusion weighted MRI data) between the stimulation site in the left DLPFC and the right cACC was significantly correlated with the direct clinical responses (11). Moreover, Tik et al. (33) showed network specific changes in functional connectivity after active but not sham rTMS applied to the left DLPFC in healthy subjects. This specific network contains the caudal part of the ACC. After active rTMS, the network showed increased functional connectivity with the ACC. The results of Tik et al. (33) confirmed the earlier findings from Fox et al. (8, 9); namely that the functional connectivity between the stimulation site in the left DLPFC and the subgenual part of the ACC are relevant for rTMS treatment efficacy for depression subjects. In another functional connectivity study, including only healthy subjects, it was also shown that individual resting‐state functional connectivity between left DLPFC and caudal ACC was linked to a larger attenuation of stress‐system sensitivity during active, but not during sham iTBS (34).
Even though the most significant peak of the caudal ACC ROI for the prediction of the direct clinical response (ROI 8, Fig. 2) is in the caudal part of the ACC, the total ROI extends within the rostral part (rACC). Specific predictive potential within the right rACC (volume measures) have been shown for the prediction of cognitive behavioral therapy for depression (35). Moreover, these findings furthermore support the observations of Boes et al. (15), who showed significant correlations between baseline cortical thickness in the rACC and the clinical responses to standard rTMS, though on the left side. The subgenual part of the rostral ACC, in particular the functional connectivity to the DLPFC, has been linked to rTMS efficacy before (8, 9). The most important difference between this study and the study by Boes et al. is the stimulation protocol. Whereas Boes et al. studied MDD patients who received standard daily rTMS treatment administered to the left DLPFC, this study describes subjects who were enrolled in an aiTBS study. Given that the mechanisms of action of TMS treatment are not fully understood (29), it is possible that there are different mechanisms involved in the responses to daily rTMS and aiTBS. As was speculated before (7, 36), the meaningful clinical effects of accelerated stimulation protocols may occur later in time, indicating delayed plasticity (37, 38).
Besides our findings for the right cACC, baseline cortical thickness was furthermore positively correlated with the direct clinical response in the lateral and medial orbitofrontal cortices, the pars opercularis, and posterior cingulate cortex. Meta‐analyses have shown that these regions have thinner cortical gray matter in MDD patients compared to healthy subjects (39). Specifically for treatment response, Järnum et al. (40) showed a thinner posterior cingulate cortex in a subgroup of nonresponders to antidepressant medication and Martinot et al. (41) showed higher metabolic activity, as measured with positron emission tomography, in the orbitofrontal cortices in responders to 10 Hz rTMS treatment for depression. The longitudinal analysis showed a significant negative correlation between ΔCT and ΔHDRS in the right temporal region, indicating that decreases in cortical thickness are linked to more effective rTMS treatment. Previous work (39) showed reduced cortical thickness in the temporal lobe in MDD patients compared to healthy volunteers. Even though normalization (in this case increase) of altered brain morphology would be expected with successful treatment, this study showed a significant (p < 0.01, uncorrected) negative correlation between the longitudinal changes in cortical thickness and clinical responses in the right temporal lobe. Although this seems a counterintuitive finding, in MDD patients antipsychotic medication was found to decrease cortical thickness (42). Additionally, Wang et al. (43) reported temporal cortical volumetric decreases on the long term after ECT, suggesting that volumetric cortical decreases are not always related to clinical worsening. One potential explanation for the rapid decrease of temporoparietal cortical thickness after aiTBS could be fast neurophysiological changes (44). For instance, prompt decreases in regional cerebral blood flow (45) and regional brain metabolism (46) in the temporal lobe were observed after effective ECT. A relationship between cortical thinning and decreased perfusion was suggested earlier (47, 48). Here, we also want to emphasize that one of the reasons of the development of accelerated rTMS paradigms was not only to yield faster response but also higher response and remission rates challenging those of ECT (35).
Correlation between baseline cortical thickness and ΔHDRSdel did not result in any significant findings. A possible explanation could be the use of the cross‐over design. The endpoint of the last HDRS measurement, T4, was exactly four weeks after the start of the experimental protocol for all patients. Depending on the randomization order, the time laps between active stimulation and follow‐up measurements was two weeks for the patients who received sham stimulation first (arm A, Fig. 1) or three weeks for the ones who received active aiTBS first (arm B, Fig. 1).
It was shown in our behavioral results (7) that subjects who received sham stimulation first also had a significant decrease in HDRS scores, indicating an important placebo effect. A limitation of our cross‐over design is that only data from T1 and T2 could be used to study the effect of active aiTBS, without including any placebo biases. Previous work (49) showed that head motion during MRI acquisition can affect measures of cortical thickness. Even though a visual quality check was performed on our data, no specific motion parameters were taken into account in this study. Especially the results of the longitudinal analysis can be affected by different motion confounds. Future studies investigating cortical thickness in relation to the response to brain stimulation will benefit from collection of subject motion information during scan acquisition. Furthermore, no accurate correction for multiple comparison testing was performed which might increase the risk of type I error. We here feel that a whole brain exploratory analysis is the right first step since the effects of our specific accelerated iTBS protocol on cortical thickness have not been investigated earlier. This first study might lead to more directed future studies, using a priori hypotheses.
CONCLUSION
This is the first study that investigates the link between cortical thickness and the response to aiTBS treatment for depression. Especially the right caudal part of the cingulate cortex could be an important neuroanatomical structure for the mechanism of action of aiTBS. Baseline cortical thickness in the right caudal ACC was significantly correlated with the direct clinical response to active aiTBS. Also, these direct changes in thickness showed predictive potential for the delayed clinical effects, as measured during follow‐up. Our findings may guide future studies, with larger datasets, to investigate the specific biomarker potential of cortical thickness to predict the clinical response to aiTBS in more detail.
Authorship Statements
Chris Baeken and Debby Klooster designed the study. Patient inclusion and data collection was performed by Romain Duprat. Data analysis was performed by Chris Baeken, Vince van Beek, and Debby Klooster, with important input from, Marie‐Anne Vanderhasselt. Interpretation of the findings were discussed with all the co‐authors. Chris Baeken and Debby Klooster prepared the manuscript draft. All authors reviewed this draft, commented on it, and approved the final version.
COMMENTS
This is an interesting piece that continues to explore the space between response to brain stimulation and changes in cortical composition. It, like others in this emerging area of the literature, describes important changes as they related to brain regions implicated in mood (and other) elements. While the precise nature of observed relationships requires further characterization, this study clearly indicates this area of inquiry is important for future study.
Noah Philip, MD
Providence, RI USA
***
Repetitive transcranial magnetic stimulation is increasingly used as a treatment for major depression, and it is helpful for slightly more than half of patients that do not respond adequately to medications (1). As pointed out by Baeken and colleagues, rTMS is a treatment modality that would greatly benefit from biomarkers that can be used to personalize the treatment parameters. Biomarkers may help us predict who will respond to a standard rTMS protocol, versus who may benefit from alternative protocols or other treatment options. Similarly, having a better understanding of the biological correlates of effective treatment may inform the underlying mechanisms of rTMS. In the search for biomarkers of rTMS treatment response in depression, cortical thickness is relatively understudied compared to EEG and functional MRI. This article makes an important contribution, being the first to look at cortical thickness changes in association with accelerated intermittent theta burst rTMS. The current findings align with prior results that also implicate cortical thickness of the anterior cingulate cortex, both as a region that correlates with eventual treatment response and as a correlate with actual treatment response (2). These findings raise a number of interesting questions that will be important to explore going forward: are the observed cortical thickness changes unique to rTMS, or do they overlap with those seen with other treatment modalities? How do these changes in cortical thickness relate to alterations in functional connectivity patterns or neurotransmitter metabolites? How early are cortical thickness changes seen, and how long do they last? What cellular or subcellular processes underlie the observed changes in cortical thickness? This will be fertile ground for investigation that promises to aid in our understanding of TMS and how we can further optimize it as a therapy.
Aaron D. Boes, MD, PhD
Iowa City, IA USA
1. Boes AD, Kelly MS, Trapp NT, Stern AP, Press DZ. Noninvasive Brain Stimulation: Challenges and Opportunities for a New Clinical Specialty. Journal of Neuropsychiatry and Clinical Neurosciences. 2018;30(3):173‐179.
2. Boes AD, Uitermarkt BD, Albazron FM, et al. Rostral anterior cingulate cortex is a structural correlate of repetitive TMS treatment response in depression. Brain Stimul. May ‐ Jun 2018;11(3):575‐581.
Supporting information
Appendix S1. Supporting Information.
For more information on author guidelines, an explanation of our peer review process, and conflict of interest informed consent policies, please go to http://www.wiley.com/WileyCDA/Section/id-301854.html
Source(s) of financial support: Chris Baeken and Debby Klooster were supported by the “Rode Neuzen” funding for scientific research [grant number G0F4617N], the Ghent University Multidisciplinary Research Partnership “The integrative neuroscience of behavioral control”, and a grant BOF16/GOA/017 for a Concerted Research Action of Ghent University, Marie‐Anne Vanderhasselt is supported by grant BOFSTA2017002501 for research at Ghent University.
Conflict of Interest: All authors have nothing to disclose.
Data Availability
The data that support the findings of this study are available from the corresponding or first author upon reasonable request.
REFERENCES
- 1.WHO factsheet: Depression. https://www.who.int/news-room/fact-sheets/detail/depression.
- 2.Perera T, George M, Grammer G, Janicak P, Pascual‐leone A, Wirecki T. TMS therapy for major depressive disorder: evidence review and treatment recommendations for clinical practice. Brain Stimul 2016;9:336–346. 10.1016/j.brs.2016.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mcclintock SM, Reti IM, Carpenter LL et al. Consensus recommendations for the clinical application of repetitive Transcranial magnetic stimulation (rTMS) in the treatment of depression on behalf of both the National Network of depression centers rTMS task group and the American psychiatric Associat. J Clin Psychiatry 2018;79:1–32. 10.4088/JCP.16cs10905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holtzheimer PE, McDonald WM, Mufti M et al. Accelerated repetitive transcranial magnetic stimulation for treatment‐resistant depression. Depress Anxiety 2010;27:960–963. 10.1002/da.20731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang YZ, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta burst stimulation of the human motor cortex. Neuron 2005;45:201–206. 10.1016/j.neuron.2004.12.033. [DOI] [PubMed] [Google Scholar]
- 6.Blumberger DM, Vila‐rodriguez F, Thorpe KE et al. Effectiveness of theta burst versus high‐frequency repetitive transcranial magnetic stimulation in patients with depression ( THREE‐D ): a randomised non‐inferiority trial. Lancet 2018;391:1683–1692. 10.1016/S0140-6736(18)30295-2. [DOI] [PubMed] [Google Scholar]
- 7.Duprat R, Desmyter S, Rudi DR et al. Accelerated intermittent theta burst stimulation treatment in medication‐resistant major depression: a fast road to remission? J Affect Disord 2016;200:6–14. 10.1016/j.jad.2016.04.015. [DOI] [PubMed] [Google Scholar]
- 8.Fox MD, Buckner RL, White MP, Greicius MD, Pascual‐Leone A. Efficacy of TMS targets for depression is related to intrinsic functional connectivity with the subgenual cingulate. Biol Psychiatry 2012;72:595–603. 10.1016/j.biopsych.2012.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weigand A, Horn A, Caballero R et al. Prospective validation that subgenual connectivity predicts antidepressant efficacy of transcranial magnetic stimulation sites. Biol Psychiatry 2017;84:28–37. 10.1016/j.biopsych.2017.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cash RFH, Zalesky A, Thomson RH, Tian Y, Cocchi L, Fitzgerald PB. Subgenual functional connectivity predicts antidepressant treatment response to transcranial magnetic stimulation: independent validation and evaluation of personalization. Biol Psychiatry 2019;86:e5–e7. 10.1016/j.biopsych.2018.12.002. [DOI] [PubMed] [Google Scholar]
- 11.Klooster DCW, Vos IN, Caeyenberghs K et al. Indirect frontocingulate structural connectivity predicts clinical response to accelerated rTMS in major depressive disorder. J Psychiatry Neurosci. 2020;45:243–252. 10.1503/jpn.190088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reynolds S, Carrey N, Jaworska N, Langevin LM, Yang XR, MacMaster FP. Cortical thickness in youth with major depressive disorder. BMC Psychiatry 2014;14:83. 10.1186/1471-244X-14-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qiu L, Lui S, Kuang W et al. Regional increases of cortical thickness in untreated, first‐episode major depressive disorder. Transl Psychiatry 2014;4:e378. 10.1038/tp.2014.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wagner G, Schultz CC, Koch K, Schachtzabel C, Sauer H, Schlösser RG. Prefrontal cortical thickness in depressed patients with high‐risk for suicidal behavior. J Psychiatr Res 2012;46:1449–1455. 10.1016/j.jpsychires.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 15.Boes AD, Uitermarkt BD, Albazron FM et al. Rostral anterior cingulate cortex is a structural correlate of repetitive TMS treatment response in depression. Brain Stimul 2018;11:1–7. 10.1016/j.brs.2018.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bush G, Luu P, Posner MI, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci 2000;4:215–222. 10.1016/S1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- 17.Britta KH, Ott U, Hempel H et al. Differential engagement of anterior cingulate and adjacent medial frontal cortex in adept meditators and non‐meditators. Neurosci Lett 2007;421:16–21. 10.1016/j.neulet.2007.04.074. [DOI] [PubMed] [Google Scholar]
- 18.Baeken C, Marinazzo D, Wu G‐R et al. Accelerated HF‐rTMS in treatment‐resistant unipolar depression: insights from subgenual anterior cingulate functional connectivity. World J Biol Psychiatry 2014;15:286–297. 10.3109/15622975.2013.872295. [DOI] [PubMed] [Google Scholar]
- 19.Sheehan DV, Lecrubier Y, Sheehan KH et al. The Mini‐International Neuropsychiatric Interview (M.I.N.I): the development and validation of a structured diagnostic psychiatric interview for DSM‐IV and ICD‐10. J Clin Psychiatry 1998;59:22–33. [PubMed] [Google Scholar]
- 20.Hamilton M. Development of a rating scale for primary depressive illness. Br J Clin Psychol 1967;6:278–296. 10.1111/j.2044-8260.1967.tb00530.x. [DOI] [PubMed] [Google Scholar]
- 21.Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex. Neuroimage 1999;97:11050–11055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reuter M, Schmansky NJ, Rosas HD, Fischl B. Within‐subject template estimation for unbiased longitudinal image analysis. Neuroimage 2012;61:1402–1418. 10.1016/j.neuroimage.2012.02.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thambisetty M, Wan J, Carass A, An Y, Prince JL, Resnick SM. Longitudinal changes in cortical thickness associated with normal aging. Neuroimage 2010;52:1215–1223. 10.1016/j.neuroimage.2010.04.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bashir S, Perez JM, Horvath JC et al. Differential effects of motor cortical excitability and plasticity in young and old individuals: a transcranial magnetic stimulation (TMS) study. Front Aging Neurosci 2014;6:1–13. 10.3389/fnagi.2014.00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luders E, Narr KL, Thompson PM et al. Gender effects on cortical thickness and the influence of scaling. Hum Brain Mapp 2006;27:314–324. 10.1002/hbm.20187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lv B, Li J, He H et al. Gender consistency and difference in healthy adults revealed by cortical thickness. Neuroimage 2010;53:373–382. 10.1016/j.neuroimage.2010.05.020. [DOI] [PubMed] [Google Scholar]
- 27.Filmer HL, Ehrhardt SE, Shaw TB, Mattingley JB, Dux PE. The efficacy of transcranial direct current stimulation to prefrontal areas is related to underlying cortical morphology. Neuroimage 2019;196:41–48. 10.1016/j.neuroimage.2019.04.026. [DOI] [PubMed] [Google Scholar]
- 28.Sackeim HA. The impact of electroconvulsive therapy on brain grey matter volume: what does it mean? Brain Stimul 2020;13:1226‐1231:1226–1231. 10.1016/j.brs.2020.04.014. [DOI] [PubMed] [Google Scholar]
- 29.Hoogendam JM, Ramakers GMJ, Di Lazzaro V. Physiology of repetitive transcranial magnetic stimulation of the human brain. Brain Stimul 2010;3:95–118. 10.1016/j.brs.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 30.Cotter D, Mackay D, Landau S, Kerwin R, Everall I. Reduced glial cell density and neuronal size in the anterior cingulate cortex in major depressive disorder. Arch Gen Psychiatry 2001;58:545–553. [DOI] [PubMed] [Google Scholar]
- 31.Baeken C, De Raedt R, Van Hove C, Clerinx P, De Mey J, Bossuyt A. HF‐rTMS treatment in medication‐ resistant melancholic depression: results from 18FDG‐PET brain imaging. CNS Spectr 2009;14:439–448. [DOI] [PubMed] [Google Scholar]
- 32.Langguth B, Wiegand R, Kharraz A et al. Pre‐treatment anterior cingulate activity as a predictor of antidepressant response to repetitive transcranial magnetic stimulation (rTMS). Neuro Endocrinol Lett. 2007;28:633–638. [PubMed] [Google Scholar]
- 33.Tik M, Hoffmann A, Sladky R et al. Towards understanding rTMS mechanism of action: stimulation of the DLPFC causes network‐specific increases in functional connectivity. Neuroimage 2017;15:289–296. [DOI] [PubMed] [Google Scholar]
- 34.de Wandel L, Pulopulos MM, Labanauskas V, de Witte S, Vanderhasselt MA, Baeken C. Individual resting‐state frontocingular functional connectivity predicts the intermittent theta burst stimulation response to stress in healthy female volunteers. Hum Brain Mapp 2020;41:5301–5312. 10.1002/hbm.25193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Webb CA, Olson EA, Killgore WDS, Pizzagalli DA, Rauch SL, Rosso IM. Rostral anterior cingulate cortex morphology predicts treatment response to internet‐based cognitive behavioral therapy for depression. Biol Psychiatry Cogn Neurosci Neuroimaging 2018;3:255–262. 10.1016/j.bpsc.2017.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baeken C, Vanderhasselt MA, Remue J et al. Intensive HF‐rTMS treatment in refractory medication‐resistant unipolar depressed patients. J Affect Disord 2013;151:625–631. 10.1016/j.jad.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 37.Prasser J, Schecklmann M, Poeppl TB et al. Bilateral prefrontal rTMS and theta burst TMS as an add‐on treatment for depression: a randomized placebo controlled trial. World J Biol Psychiatry 2015;16:57–65. 10.3109/15622975.2014.964768. [DOI] [PubMed] [Google Scholar]
- 38.Chung SW, Rogasch NC, Hoy KE et al. Measuring brain stimulation induced changes in cortical properties using TMS‐EEG. Brain Stimul 2015;8:1010–1020. 10.1016/j.brs.2015.07.029. [DOI] [PubMed] [Google Scholar]
- 39.Schmaal L, Hibar DP, Sämann PG et al. Cortical abnormalities in adults and adolescents with major depression based on brain scans from 20 cohorts worldwide in the ENIGMA major depressive disorder working group. Mol Psychiatry 2017;22:900–909. 10.1038/mp.2016.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Järnum H, Eskildsen SF, Steffensen EG et al. Longitudinal MRI study of cortical thickness, perfusion, and metabolite levels in major depressive disorder. Acta Psychiatr Scand 2011;124:435–446. 10.1111/j.1600-0447.2011.01766.x. [DOI] [PubMed] [Google Scholar]
- 41.Martinot MLP, Martinot JL, Ringuenet D et al. Baseline brain metabolism in resistant depression and response to transcranial magnetic stimulation. Neuropsychopharmacology 2011;36:2710–2719. 10.1038/npp.2011.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Voineskos AN, Mulsant BH, Dickie EW et al. Effects of antipsychotic medication on brain structure in patients with major depressive disorder and psychotic features: Neuroimaging findings in the context of a randomized placebo‐controlled clinical trial. JAMA Psychiat 2020;77:674–683. 10.1001/jamapsychiatry.2020.0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang J, Tang Y, Curtin A et al. ECT‐induced brain plasticity correlates with positive symptom improvement in schizophrenia by voxel‐based morphometry analysis of grey matter. Brain Stimul 2019;12:319–328. 10.1016/j.brs.2018.11.006. [DOI] [PubMed] [Google Scholar]
- 44.Baeken C, Wu GR, Sackeim HA. Accelerated iTBS treatment applied to the left DLPFC in depressed patients results in a rapid volume increase in the left hippocampal dentate gyrus, not driven by brain perfusion. Brain Stimul 2020;13:1211–1217. 10.1016/j.brs.2020.05.015. [DOI] [PubMed] [Google Scholar]
- 45.Kohn Y, Freedman N, Lester H et al. 99mTc‐HMPAO SPECT study of cerebral perfusion after treatment with medication and electroconvulsive therapy in major depression. J Nucl Med. 2007;48:1273–1278. 10.2967/jnumed.106.039354. [DOI] [PubMed] [Google Scholar]
- 46.Nobler MS, Oquendo MA, Kegeles LS et al. Decreased regional brain metabolism after ECT. Am J Psychiatry 2001;158:305–308. 10.1176/appi.ajp.158.2.305. [DOI] [PubMed] [Google Scholar]
- 47.Madhyastha T, Askren MK, Boord P, Zhang J, Leverenz JB, Grabowski T. Cerebral perfusion and cortical thickness indicate cortical involvement in mild PD. Mov Disord 2015;30:1893–1900. 10.1002/mds.26128.Cerebral. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Alosco ML, Gunstad J, Jerskey BA et al. The adverse effects of reduced cerebral perfusion on cognition and brain structure in older adults with cardiovascular disease. Brain Behav 2013;3:626–636. 10.1002/brb3.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reuter M, Tisdall MD, Qureshi A, Buckner RL, van der Kouwe AJW, Fischl B. Head motion during MRI acquisition reduces gray matter volume and thickness estimates. NeuroImage 2015;15:107–115. 10.1016/j.neuroimage.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supporting Information.
Data Availability Statement
The data that support the findings of this study are available from the corresponding or first author upon reasonable request.


