Abstract
Background
We studied lab-confirmed COVID-19 infection (LCCI) testing, incidence, and severity.
Methods
We included all Manitoba residents and limited our severity analysis to LCCI patients. We calculated testing, incidence and vaccination rates between March 8, 2020 and June 1, 2021. We estimated the association between patient characteristics and testing (rate ratio [RR]; Poisson regression), including the reason for testing (screening, symptomatic, contact/outbreak asymptomatic), incidence (hazard ratio [HR]; Cox regression), and severity (prevalence ratio [PR], Cox regression).
Findings
The overall testing rate during the second/third wave was 570/1,000 person-years, with an LCCI rate of 50/1,000 person-years. The secondary attack rate during the second/third wave was 16%. Across regions, young children (<10) had the lowest positivity for symptomatic testing, the highest positivity for asymptomatic testing, and the highest risk of LCCI as asymptomatic contact. People in the lowest income quintile had the highest risk of LCCI, 1.3-6x the hazard of those in the highest income quintile. Long-term care (LTC) residents were particularly affected in the second wave with HRs>10 for asymptomatic residents.
Interpretation
Although the severity of LCCI in children was low, they have a high risk of asymptomatic positivity. The groups most vulnerable to LCCI, who should remain a focus of public health, were residents of Manitoba's North, LTC facilities, and low-income neighbourhoods.
Funding
Canada Research Chair Program
Keywords: Coronavirus disease 2019, COVID-19, testing, incidence, super-spreaders, lab-confirmed COVID-19 infection
Research in context.
Evidence before this study: Although risk factors of COVID-19 incidence and severity have been reported, many studies are limited to specific populations and sometimes lack information on testing. Limited quantitative information is available on different testing patterns between symptomatic and asymptomatic patients.
Added value of this study: We examine testing, including reason for testing, incidence, spreading, and severity for the complete population of a single jurisdiction. This allows us to examine these risks without selection bias and account for testing intensity to examine incidence rates and assess severity in all LCCI patients. We show that people vulnerable to COVID-19 (the marginalized residents of Manitoba's predominantly indigenous North, long-term care facilities, and low-income neighbourhoods) are at a separately increased risk in every aspect of COVID-19: by different testing patterns, increased incidence, and increased risk of severe outcomes.
Implications of all the available evidence: Because these groups are at risk in multiple ways, COVID-19 vaccination should keep focusing on maximizing vaccine uptake in these marginalized groups and COVID-19 surveillance should remain focused on these groups as well.
Alt-text: Unlabelled box
1. Introduction
Knowledge of COVID-19 risk factors and disease burden has greatly improved since it was first detected in late 2019. Although some studies include a large population, [1] many study reports remain based on select groups that may not be generalizable to an entire population. [2] Because of limited syndromic surveillance and asymptomatic testing, much of our knowledge is based on studying symptomatic patients seeking medical care including viral testing. Incidence rates are hard to interpret without understanding testing patterns, especially given heterogeneity in testing intensity based on ethnicity and socio-economic disadvantage. [3,4] In this report, we describe the patterns and descriptors of COVID-19 laboratory testing and incidence to provide an epidemiological description of COVID-19 for the entire population of a single jurisdiction (Manitoba, Canada, a province with a large [18%] indigenous population) using the clinical and administrative registries and database of its sole health insurance provider, Manitoba Health (MH). The study was approved by the University of Manitoba Research Ethics Board and MH's Health Information Privacy Committee.
2. Methods
2.1. Data sources
MH is the publicly funded health insurance agency providing comprehensive health insurance to the province's 1.3 million residents. Coverage is universal, with no eligibility distinction based on age or income, and participation rates are very high (>99%). Insured services include hospital, physician, laboratory and preventive services. MH maintains several centralized, clinical, public health, and administrative electronic databases that are linkable using a unique personal health identification number (PHIN). The completeness and accuracy of these databases are well established. [5]
2.2. Study cohort and case definitions
The study cohort consisted of all persons included in the MH Population Registry (MHPR, which tracks addresses and vital and registration status of all insured persons) between March 8, 2020 (the week of Manitoba's first confirmed case) and June 1, 2021 (the latest date for which data was available).
The tested cohort included those in the study cohort who had a record of COVID-19 testing in the MH's COVID-19 Laboratory Results Database (LRD), which tracks all SARS-CoV-2 tests conducted in Manitoba, and includes information on testing dates, reasons (screening, contact/outbreak investigation, symptoms, or unknown, based on a free-text field) and results. In Manitoba, all testing is done using SARS-CoV-2 reverse-transcription PCR (RT-PCR) performed on nasopharyngeal or endotracheal specimens. [6]
The cases cohort included all patients in the study cohort who met the laboratory-confirmed COVID-19 infection (LCCI) case definition (see below) as recorded in the Public Health Information Management System (PHIMS) which is used by MH for COVID-19 surveillance and contact tracing and for monitoring patient status (active, resolved, died) and hospital/ICU admissions. Under the Manitoba Public Health Act, clinicians must report all cases and deaths due to COVID-19 and laboratories must report positive SARS-CoV-2 tests. Using national case definitions, a confirmed case was defined as a person with LCCI using a validated NAAT (e.g. RT-PCR or nucleic acid sequencing) assay. The diagnosis date was defined as the earliest of the specimen collection date, specimen receipt date, or testing date. Contacts were identified during public health case investigation and included in PHIMS. We obtained vaccination status from a province-wide registry that contains records of all COVID-19 vaccinations.
2.3. Covariates
We obtained information on gender, neighbourhood household income, and health region of residence (Southern, Northern and the rest of Manitoba) from the MHPR. We used previously validated algorithms [7] to identify pre-existing chronic diseases at the start of the study period using the Hospital Abstracts Database (HAD) and the Medical Services Database (MSD) which record all hospitalisation, including admissions and day surgeries, and physician services provided in hospitals, physician offices, and outpatient departments across the province. [5] We measured prescription drug use during the 90 days before each wave using the Drug Program Information Network (DPIN) database, which records all prescription drugs dispensed to Manitoba residents, including most personal care home residents. [8] Residence in a long-term care (LTC) facility was determined using MH's LTC utilization database.
2.4. Statistical analysis
To account for the epidemic phase, we divided the study period into 4 periods based on incidence rates: March 8-April 11, 2020 (first wave); April 12-September 30, 2020 (summer period); October 1, 2020-February 28, 2021 (second wave); March 1, 2021-June 1, 2021 (third wave). We also combined periods, e.g., the second and third wave, for some analyses. Follow-up time started at the start of each period and ended at the earliest of LCCI diagnosis, date of loss to follow-up (e.g., end of MH coverage), or the end of the period. We constructed weekly time-series by calculating testing and incidence rates during each week (Sunday-Saturday), period, and stratum. We used generalized estimating equation Poisson regression to estimate the rate ratios (RRs) and 95% confidence intervals (95% CIs) of the association between patient characteristics and testing. We used Cox proportional hazard models to estimate the hazard ratios (HRs) and 95% CIs of the association between patient characteristics and LCCI risk. For LCCI patients, we used Cox regression with constant time at risk and robust variance [9] to estimate the prevalence ratios and 95% CIs of the association between patient characteristics and LCCI severity.
We calculated the effective reproduction number (Rt) using a Bayesian time-series probabilistic model. [10] We calculated the average/overall secondary attack rate (SAR) as the number of positive contacts divided by the total number of contacts. We used logistic regression to calculate odds ratios (ORs) and 95% CIs of the association between patient characteristics and the risk of “super-spreading”, defined as a case who had ≥4 secondary cases (the 99th percentile of the Poisson distribution [11] of the average second wave Rt; ≥6 secondary cases for the peak Rt).
3. Results
3.1. Epidemic timeline
The first LCCI case in Manitoba was diagnosed on March 8, 2020, heralding a brief and mild first wave, possibly the result of strict public health measures including school closures (Figure 1, Supplementary Table 1). Public health restrictions began to ease in early May 2020 and the summer period had little activity. This period was characterized by sporadic cases mostly affecting essential service workers such as truck drivers and several localized outbreaks involving communal living communities in rural regions (Supplementary Figure 1, Supplementary Table 1).
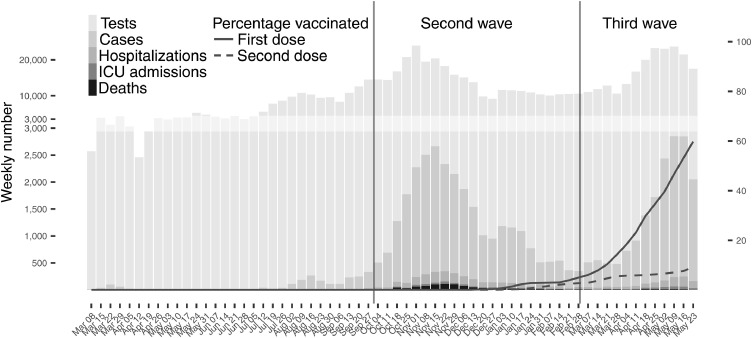
Figure 1.
Bar graph: Weekly (Sunday-Saturday) number of COVID-19 laboratory tests and laboratory-confirmed COVID-19 infection cases with the ultimate severity of these diagnosed cases (hospitalized, admitted to ICU, or death);
Line graphs: Vaccine uptake in ≥12 year-olds of the first dose (solid line) and second dose (dashed line).
The second wave began in October 2020 amid widespread community transmission after schools and other places reopened in September, reaching a peak in mid-November before beginning to decline after the implementation of stringent public health measures (Figure 1). The third wave began in March 2021, while Manitoba's vaccine campaign was ramping up, and reached its apex towards the middle of May 2021 amidst increasingly stringent public health measures. The Rt hovered around one, in line with periods of increasing and decreasing incidence (Supplementary Figure 1).
During the second and third wave, testing and incidence rates were similar (Supplementary Table 2), and higher than earlier periods (Supplementary Table 1). The overall testing rate during the second/third wave was around 570/1,000 person-years, with an LCCI rate of around 50/1,000 person-years (Table 1). A lower proportion of cases were hospitalised or died in each subsequent wave (Supplementary Table 3).
Table 1.
Testing and incidence rates (per 1,000 person-years) and test positivity (95% confidence interval) according to certain socio-economic and clinical characteristics during the seconda and thirdb waves
| Testing rate | Incidence rate | Test positivity (%) | |
|---|---|---|---|
| Overall | 564 (563-566) | 49.1 (48.6-49.6) | 8.7 (8.6-8.8) |
| Age | |||
| <10 | 535 (531-539) | 43.3 (42.1-44.5) | 8.1 (7.9-8.3) |
| 10-17 | 495 (490-499) | 54.1 (52.6-55.7) | 10.9 (10.6-11.3) |
| 18-29 | 659 (655-663) | 65.3 (64.0-66.6) | 9.9 (9.7-10.1) |
| 30-49 | 645 (642-649) | 53.0 (52.1-54.0) | 8.2 (8.1-8.4) |
| 50-64 | 493 (489-496) | 42.2 (41.2-43.2) | 8.6 (8.4-8.8) |
| ≥65 | 497 (493-500) | 36.4 (35.5-37.4) | 7.3 (7.1-7.5) |
| Gender | |||
| Male | 518 (516-520) | 48.7 (48.1-49.4) | 9.4 (9.3-9.5) |
| Female | 611 (608-613) | 49.5 (48.8-50.1) | 8.1 (8.0-8.2) |
| Income quintile | |||
| Q1 (lowest) | 664 (661-668) | 79.8 (78.5-81.1) | 12.0 (11.8-12.2) |
| Q2 | 572 (569-576) | 50.2 (49.1-51.2) | 8.8 (8.6-8.9) |
| Q3 | 524 (521-527) | 39.0 (38.1-40.0) | 7.4 (7.3-7.6) |
| Q4 | 523 (520-527) | 41.1 (40.2-42.1) | 7.9 (7.7-8.0) |
| Q5 (highest) | 521 (517-524) | 33.1 (32.3-34.0) | 6.4 (6.2-6.5) |
| Unknown | 663 (655-672) | 64.3 (61.7-67.0) | 9.7 (9.3-10.1) |
| Regional health authority of residence | |||
| Interlake-Eastern | 543 (538-548) | 35.6 (34.4-36.9) | 6.6 (6.3-6.8) |
| Northern | 1,008 (999-1,017) | 140.6 (137.3-143.9) | 13.9 (13.6-14.3) |
| Southern | 442 (438-445) | 41.0 (40.0-42.1) | 9.3 (9.0-9.5) |
| Prairie Mountain | 450 (446-454) | 24.3 (23.5-25.3) | 5.4 (5.2-5.6) |
| Winnipeg | 580 (578-583) | 49.8 (49.2-50.4) | 8.6 (8.5-8.7) |
| Public Trustee / In CFS care | 974 (943-1,006) | 117.7 (107.3-129.1) | 12.1 (11.0-13.3) |
| In long-term care - second wave | 2,384 (2,327-2,442) | 394.9 (372.1-419.0) | 16.6 (15.6-17.6) |
| In long-term care - third wave | 975 (926-1,027) | 11.6 (7.2-18.7) | 1.2 (0.7-1.9) |
| Any chronic disease | 716 (712-720) | 59.4 (58.3-60.6) | 8.3 (8.1-8.5) |
| Immunosuppressed | 747 (739-755) | 46.0 (44.0-48.0) | 6.2 (5.9-6.4) |
| Use of ARBs/ACEIsc | 596 (591-600) | 50.2 (49.0-51.5) | 8.4 (8.2-8.6) |
| Use of proton-pump inhibitors | 856 (850-862) | 62.9 (61.4-64.5) | 7.4 (7.2-7.5) |
| COVID-19 vaccination status | |||
| Unvaccinated | 558 (556-559) | 50.8 (50.3-51.2) | 9.1 (9.0-9.2) |
| ≥14 days after first dose | 649 (641-656) | 29.0 (27.3-30.7) | 4.5 (4.2-4.7) |
| ≥7 days after second dose | 697 (685-711) | 10.0 (8.5-11.7) | 1.4 (1.2-1.7) |
| No. of physician visits in 2019 | |||
| 0 | 367 (364-370) | 45.2 (44.2-46.2) | 12.3 (12.1-12.6) |
| 1-3 | 513 (510-516) | 49.8 (49.0-50.7) | 9.7 (9.5-9.9) |
| 4-8 | 573 (570-576) | 46.9 (46.0-47.7) | 8.2 (8.0-8.3) |
| ≥9 | 738 (735-742) | 53.1 (52.2-54.0) | 7.2 (7.1-7.3) |
| No. of hospitalizations in 2019 | |||
| 0 | 542 (541-544) | 47.7 (47.3-48.2) | 8.8 (8.7-8.9) |
| 1 | 827 (818-835) | 65.0 (62.7-67.4) | 7.9 (7.6-8.2) |
| ≥2 | 1,303 (1,280-1,325) | 97.5 (91.6-103.9) | 7.5 (7.0-8.0) |
a The second wave is from October 1, 2020 to Feb 28, 2021. b The third wave is from March 1, 2021 to June 1, 2021. c ARBs/ACEIs: Angiotensin receptor blockers and angiotensin converting enzyme inhibitors.
3.2. Descriptors of testing frequency and COVID-19 outcomes
In the first wave, females, 30-49 year-olds and higher income persons were more likely to be tested than other age groups; likely because testing was targeted at symptomatic travellers and their contacts (Supplementary Figure 2-4, Supplementary Table 1, 4-5). [12] Throughout the pandemic, LTC residents, the chronically ill, and residents of Northern Manitoba were more likely to be tested (Table 1), reflecting government emphasis on protecting high-risk persons. As expected, testing, incidence, and positivity was higher for symptomatic persons than those screened asymptomatically (Table 2), yet asymptomatic contacts had even higher positivity rates (Supplementary Table 6-9). Although LTC residents had above-average LCCI incidence in the second wave, which is why they were prioritized for vaccination, LTC incidence rates were much lower in the third wave. Across regions, young children (<10) had the lowest positivity for symptomatic testing and the highest positivity for asymptomatic testing (Table 2). Testing, incidence and positivity rates were lower the higher the income regardless of reasons for testing, except in the Southern region, where rates were similar between income quintiles. These patterns were similar for the second and third waves individually (Supplementary Table 6-9)
Table 2.
Testing and incidence rates (per 1,000 person-years) and test positivity rate (95% confidence interval) according to certain socio-economic and clinical characteristics during the seconda and thirdb waves by region and reason for testing
| Northern |
Southern |
Rest of Manitoba |
||||
|---|---|---|---|---|---|---|
| Screening | Symptomatic | Screening | Symptomatic | Screening | Symptomatic | |
| Testing rates | ||||||
| Overall | 247 (243-252) | 317 (312-322) | 96 (94-98) | 241 (239-244) | 148 (147-149) | 243 (242-244) |
| Age | ||||||
| <10 | 121 (115-128) | 212 (203-221) | 58 (55-61) | 237 (231-243) | 124 (122-127) | 307 (303-311) |
| 10-17 | 169 (159-179) | 271 (259-284) | 72 (68-76) | 209 (203-216) | 135 (132-137) | 231 (228-235) |
| 18-29 | 304 (293-316) | 379 (366-392) | 111 (106-115) | 273 (266-280) | 185 (183-188) | 291 (288-294) |
| 30-49 | 332 (322-343) | 377 (366-388) | 115 (111-118) | 280 (274-285) | 179 (178-181) | 290 (288-293) |
| 50-64 | 308 (296-321) | 332 (319-345) | 92 (88-96) | 206 (201-212) | 128 (126-130) | 195 (193-197) |
| ≥65 | 240 (225-256) | 323 (305-341) | 122 (118-128) | 221 (214-228) | 113 (111-115) | 142 (140-144) |
| Income quintile | ||||||
| Q1 (lowest) | 358 (348-368) | 334 (324-344) | 90 (86-94) | 214 (208-220) | 165 (163-167) | 238 (235-241) |
| Q2 | 258 (250-266) | 287 (279-296) | 108 (104-113) | 242 (236-249) | 149 (147-151) | 243 (241-246) |
| Q3 | 234 (218-252) | 332 (312-353) | 91 (87-95) | 240 (234-247) | 143 (141-145) | 238 (235-240) |
| Q4 | 180 (171-189) | 297 (285-309) | 90 (87-93) | 227 (222-232) | 142 (140-144) | 248 (246-251) |
| Q5 (highest) | 148 (141-156) | 349 (338-361) | 98 (94-101) | 267 (261-273) | 128 (126-130) | 246 (244-249) |
| Unknown | 220 (144-338) | 399 (290-548) | 110 (103-117) | 282 (271-293) | 217 (211-223) | 260 (254-266) |
| In long-term care - second wave | 802 (579-1,112) | 423 (270-664) | 608 (532-695) | 446 (382-522) | 994 (955-1,035) | 434 (409-462) |
| In long-term care - third wave | 328 (164-655) | 246 (110-547) | 317 (246-408) | 153 (106-221) | 419 (384-456) | 175 (153-200) |
| COVID-19 vaccination status | ||||||
| Unvaccinated | 238 (233-242) | 312 (307-317) | 94 (92-96) | 242 (239-244) | 146 (145-147) | 247 (246-248) |
| ≥14 days after first dose | 369 (346-393) | 406 (382-431) | 127 (117-137) | 236 (223-251) | 170 (166-175) | 188 (184-193) |
| ≥7 days after second dose | 350 (321-382) | 320 (292-351) | 155 (137-176) | 220 (198-244) | 203 (195-210) | 176 (169-183) |
| Incidence rates | ||||||
| Overall | 16.7 (15.6-17.8) | 40.9 (39.2-42.7) | 6.2 (5.8-6.6) | 27.5 (26.6-28.4) | 9.1 (8.8-9.3) | 23.4 (23.0-23.7) |
| Age | ||||||
| <10 | 14.0 (11.8-16.5) | 27.5 (24.5-31.0) | 5.0 (4.2-6.0) | 8.6 (7.5-9.9) | 12.0 (11.3-12.8) | 13.7 (12.9-14.5) |
| 10-17 | 17.0 (14.2-20.3) | 35.6 (31.4-40.3) | 6.6 (5.4-7.9) | 20.7 (18.7-23.0) | 10.7 (10.0-11.5) | 23.6 (22.5-24.8) |
| 18-29 | 20.8 (18.0-23.9) | 54.1 (49.5-59.1) | 7.0 (6.0-8.2) | 34.6 (32.2-37.1) | 10.9 (10.3-11.5) | 33.4 (32.4-34.5) |
| 30-49 | 19.2 (16.9-21.9) | 44.1 (40.5-48.1) | 6.7 (5.9-7.6) | 32.1 (30.3-34.0) | 9.0 (8.6-9.4) | 27.7 (27.0-28.4) |
| 50-64 | 14.8 (12.2-17.8) | 42.4 (37.9-47.3) | 6.0 (5.1-7.1) | 30.7 (28.5-33.0) | 7.0 (6.6-7.5) | 21.2 (20.4-21.9) |
| ≥65 | 11.9 (8.9-15.9) | 42.2 (36.1-49.3) | 5.8 (4.8-7.0) | 37.0 (34.3-39.9) | 7.1 (6.6-7.6) | 16.4 (15.7-17.1) |
| Income quintile | ||||||
| Q1 (lowest) | 26.9 (24.2-29.8) | 55.9 (52.1-60.1) | 6.1 (5.2-7.3) | 29.6 (27.4-31.9) | 12.4 (11.8-13.0) | 33.9 (32.9-34.8) |
| Q2 | 17.6 (15.6-19.8) | 32.4 (29.7-35.5) | 6.4 (5.4-7.6) | 28.2 (26.1-30.6) | 9.4 (8.9-9.9) | 24.3 (23.6-25.2) |
| Q3 | 18.7 (14.4-24.2) | 43.5 (36.7-51.5) | 6.2 (5.2-7.3) | 29.1 (27.0-31.4) | 7.9 (7.4-8.3) | 20.3 (19.6-21.0) |
| Q4 | 10.0 (8.1-12.4) | 38.4 (34.4-42.9) | 7.0 (6.1-7.9) | 30.3 (28.6-32.2) | 7.8 (7.3-8.3) | 20.2 (19.4-20.9) |
| Q5 (highest) | 7.0 (5.5-8.8) | 35.2 (31.8-39.0) | 4.8 (4.1-5.7) | 22.1 (20.4-23.8) | 5.5 (5.2-6.0) | 17.1 (16.4-17.8) |
| Unknown | 10.5 (1.5-74.5) | 42.0 (15.8-111.9) | 6.9 (5.4-8.9) | 22.5 (19.6-25.9) | 21.5 (19.8-23.3) | 30.1 (28.1-32.3) |
| In long-term care - second wave | 0.0 (0.0-84.6) | 22.3 (3.1-158.2) | 96.6 (69.0-135.2) | 42.6 (25.7-70.7) | 151.8 (136.9-168.3) | 103.7 (91.6-117.5) |
| In long-term care - third wave | 0.0 (0.0-151.7) | 0.0 (0.0-151.7) | 5.3 (0.7-37.5) | 21.1 (7.9-56.3) | 2.4 (0.8-7.4) | 2.4 (0.8-7.4) |
| COVID-19 vaccination status | ||||||
| Unvaccinated | 17.8 (16.6-19.1) | 43.2 (41.3-45.1) | 6.3 (5.9-6.8) | 28.3 (27.4-29.2) | 9.3 (9.1-9.5) | 24.2 (23.8-24.6) |
| ≥14 days after first dose | 4.3 (2.4-7.8) | 18.0 (13.4-24.0) | 3.5 (2.2-5.7) | 13.7 (10.8-17.5) | 6.5 (5.7-7.4) | 14.0 (12.8-15.3) |
| ≥7 days after second dose | 2.1 (0.7-6.6) | 9.9 (5.8-16.6) | 0.0 (0.0-2.3) | 3.7 (1.7-8.3) | 2.0 (1.4-2.9) | 5.2 (4.1-6.6) |
| Test positivity | ||||||
| Overall | 6.7 (6.3-7.2) | 12.9 (12.4-13.5) | 6.4 (6.0-6.9) | 11.4 (11.0-11.8) | 6.1 (6.0-6.3) | 9.6 (9.5-9.8) |
| Age | ||||||
| <10 | 11.5 (9.8-13.6) | 13.0 (11.5-14.6) | 8.7 (7.2-10.4) | 3.6 (3.2-4.2) | 9.7 (9.1-10.3) | 4.5 (4.2-4.7) |
| 10-17 | 10.1 (8.4-12.1) | 13.1 (11.6-14.8) | 9.1 (7.5-11.0) | 9.9 (8.9-11.0) | 8.0 (7.4-8.6) | 10.2 (9.7-10.7) |
| 18-29 | 6.8 (5.9-7.9) | 14.3 (13.1-15.6) | 6.3 (5.4-7.4) | 12.7 (11.8-13.6) | 5.9 (5.5-6.2) | 11.5 (11.1-11.8) |
| 30-49 | 5.8 (5.1-6.6) | 11.7 (10.8-12.8) | 5.8 (5.1-6.6) | 11.5 (10.8-12.2) | 5.0 (4.8-5.2) | 9.5 (9.3-9.8) |
| 50-64 | 4.8 (4.0-5.8) | 12.8 (11.4-14.3) | 6.5 (5.5-7.7) | 14.9 (13.8-16.0) | 5.5 (5.2-5.8) | 10.8 (10.5-11.2) |
| ≥65 | 5.0 (3.7-6.6) | 13.1 (11.2-15.3) | 4.8 (4.0-5.8) | 16.7 (15.5-18.0) | 6.3 (5.9-6.7) | 11.6 (11.1-12.1) |
| Income quintile | ||||||
| Q1 (lowest) | 7.5 (6.8-8.3) | 16.8 (15.6-18.0) | 6.8 (5.8-8.1) | 13.8 (12.8-14.9) | 7.5 (7.2-7.9) | 14.2 (13.8-14.6) |
| Q2 | 6.8 (6.0-7.7) | 11.3 (10.3-12.3) | 5.9 (5.0-7.0) | 11.7 (10.8-12.6) | 6.3 (6.0-6.7) | 10.0 (9.7-10.3) |
| Q3 | 8.0 (6.2-10.3) | 13.1 (11.1-15.5) | 6.8 (5.8-8.0) | 12.1 (11.2-13.1) | 5.5 (5.2-5.8) | 8.5 (8.2-8.8) |
| Q4 | 5.6 (4.5-6.9) | 12.9 (11.6-14.4) | 7.7 (6.8-8.7) | 13.4 (12.6-14.2) | 5.5 (5.2-5.8) | 8.1 (7.8-8.4) |
| Q5 (highest) | 4.7 (3.7-5.9) | 10.1 (9.1-11.2) | 4.9 (4.2-5.8) | 8.3 (7.7-8.9) | 4.3 (4.0-4.7) | 6.9 (6.7-7.2) |
| Unknown | 4.8 (0.7-33.8) | 10.5 (4.0-28.0) | 6.3 (4.9-8.1) | 8.0 (7.0-9.2) | 9.9 (9.1-10.7) | 11.6 (10.8-12.4) |
| In long-term care - second wave | 0.0 (0.0-10.5) | 5.3 (0.7-37.4) | 15.9 (11.4-22.2) | 9.6 (5.8-15.8) | 15.3 (13.8-16.9) | 23.9 (21.1-27.1) |
| In long-term care - third wave | 0.0 (0.0-46.1) | 0.0 (0.0-61.5) | 1.7 (0.2-11.8) | 13.8 (5.2-36.8) | 0.6 (0.2-1.8) | 1.4 (0.4-4.2) |
| COVID-19 vaccination status | ||||||
| Unvaccinated | 7.5 (7.0-8.0) | 13.8 (13.2-14.5) | 6.7 (6.3-7.2) | 11.7 (11.3-12.1) | 6.4 (6.2-6.6) | 9.8 (9.6-9.9) |
| ≥14 days after first dose | 1.2 (0.6-2.1) | 4.4 (3.3-5.9) | 2.8 (1.7-4.5) | 5.8 (4.6-7.4) | 3.8 (3.3-4.4) | 7.4 (6.8-8.1) |
| ≥7 days after second dose | 0.6 (0.2-1.9) | 3.1 (1.8-5.2) | 0.0 (0.0-1.5) | 1.7 (0.8-3.8) | 1.0 (0.7-1.5) | 3.0 (2.3-3.8) |
a The second wave is from October 1, 2020 to Feb 28, 2021. b The third wave is from March 1, 2021 to June 1, 2021.
In the second/third wave, males and younger persons had a higher relative risk of LCCI (Table 3). Children <10 had a higher relative risk of being positive after asymptomatic screening in the major urban areas, HR=1.33 (1.23-1.44), but a lower risk of having symptomatic LCCI there, HR=0.53 (0.49-0.56). These children's relative risk was particularly high (HR>1.8 outside of the South) when they were screened as an asymptomatic contact (Supplementary Table 10). People in the lowest income quintile persistently had a higher HR of LCCI, but the size of the effect differed by region and reason for testing, the HR of the highest vs lowest income quintile varied between 0.27 (0.21-0.36) for asymptomatic Northerners (0.18, 0.16-0.20, when a contact) to 0.76 (0.60-0.96) for asymptomatic Southerners. LTC residents were particularly affected in the second wave with HRs>10 for asymptomatic residents, but less so in the third wave. The chronically ill, those with higher healthcare utilization in the previous year, and patients using proton-pump inhibitors also had a HR>1 for LCCI, most likely due to increased testing (Table 3).
Table 3.
Adjusteda associations between certain socio-economic and clinical characteristics and COVID-19 testing and incidence during the secondb and thirdc waves by region and reason for testing
| Northern |
Southern |
Rest of Manitoba |
||||
|---|---|---|---|---|---|---|
| Screening | Symptomatic | Screening | Symptomatic | Screening | Symptomatic | |
| Testing (rate ratio) | ||||||
| Age | ||||||
| <10 | 0.40 (0.37-0.43) | 0.70 (0.66-0.74) | 0.55 (0.52-0.59) | 1.01 (0.97-1.04) | 0.72 (0.71-0.74) | 1.17 (1.15-1.19) |
| 10-17 | 0.56 (0.52-0.60) | 0.89 (0.84-0.95) | 0.70 (0.65-0.75) | 0.91 (0.87-0.94) | 0.81 (0.79-0.83) | 0.93 (0.91-0.94) |
| 18-29 | 0.99 (0.94-1.05) | 1.16 (1.11-1.22) | 1.03 (0.98-1.09) | 1.11 (1.07-1.14) | 1.10 (1.08-1.12) | 1.13 (1.12-1.15) |
| 30-49 | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| 50-64 | 0.80 (0.75-0.84) | 0.72 (0.69-0.76) | 0.72 (0.68-0.76) | 0.61 (0.59-0.63) | 0.65 (0.63-0.66) | 0.58 (0.57-0.59) |
| ≥65 | 0.55 (0.51-0.59) | 0.60 (0.56-0.65) | 0.76 (0.72-0.81) | 0.53 (0.50-0.55) | 0.43 (0.42-0.44) | 0.34 (0.34-0.35) |
| Female | 1.12 (1.08-1.17) | 1.13 (1.09-1.17) | 1.06 (1.02-1.09) | 1.09 (1.07-1.12) | 0.96 (0.95-0.97) | 1.14 (1.13-1.15) |
| Income quintile | ||||||
| Q1 (lowest) | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| Q2 | 0.70 (0.67-0.73) | 0.86 (0.82-0.89) | 1.16 (1.09-1.23) | 1.11 (1.07-1.16) | 0.91 (0.89-0.93) | 1.04 (1.02-1.06) |
| Q3 | 0.62 (0.57-0.67) | 1.01 (0.94-1.08) | 0.99 (0.94-1.06) | 1.09 (1.05-1.14) | 0.88 (0.87-0.90) | 1.04 (1.02-1.05) |
| Q4 | 0.47 (0.44-0.50) | 0.90 (0.86-0.95) | 1.00 (0.95-1.06) | 1.05 (1.01-1.09) | 0.88 (0.86-0.90) | 1.08 (1.07-1.10) |
| Q5 (highest) | 0.38 (0.36-0.40) | 1.05 (1.00-1.10) | 1.07 (1.01-1.14) | 1.20 (1.16-1.24) | 0.80 (0.78-0.82) | 1.08 (1.06-1.10) |
| Unknown | 0.34 (0.20-0.58) | 1.23 (0.87-1.76) | 1.19 (1.10-1.29) | 1.25 (1.19-1.32) | 1.05 (1.02-1.08) | 1.04 (1.01-1.07) |
| In long-term care-second wave | 3.40 (2.21-5.23) | 0.86 (0.52-1.43) | 4.47 (3.83-5.21) | 1.68 (1.42-1.99) | 8.21 (7.79-8.66) | 2.33 (2.18-2.49) |
| In long-term care-third wave | 1.98 (0.91-4.31) | 0.79 (0.34-1.86) | 2.85 (2.15-3.77) | 0.73 (0.50-1.06) | 3.81 (3.46-4.20) | 1.20 (1.04-1.38) |
| Any chronic disease | 1.24 (1.17-1.30) | 1.28 (1.22-1.34) | 1.15 (1.10-1.21) | 1.20 (1.16-1.24) | 1.16 (1.14-1.18) | 1.10 (1.08-1.11) |
| Immunosuppressed | 0.97 (0.89-1.06) | 1.09 (1.01-1.17) | 1.17 (1.09-1.26) | 1.15 (1.09-1.21) | 1.04 (1.01-1.06) | 1.09 (1.07-1.11) |
| Use of ARBs/ACEIsd | 1.07 (1.01-1.13) | 0.99 (0.94-1.05) | 0.96 (0.91-1.02) | 0.98 (0.94-1.02) | 0.98 (0.96-1.00) | 0.91 (0.89-0.92) |
| Use of proton-pump inhibitors | 1.35 (1.28-1.43) | 1.59 (1.52-1.67) | 1.38 (1.31-1.46) | 1.56 (1.51-1.61) | 1.23 (1.21-1.25) | 1.42 (1.40-1.44) |
| No. of physician visits in 2019 | ||||||
| 0 | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| 1-3 | 1.16 (1.11-1.22) | 1.32 (1.27-1.38) | 1.26 (1.19-1.33) | 1.60 (1.54-1.66) | 1.38 (1.35-1.41) | 1.70 (1.67-1.73) |
| 4-8 | 1.40 (1.32-1.48) | 1.47 (1.40-1.55) | 1.44 (1.35-1.52) | 2.03 (1.95-2.11) | 1.48 (1.45-1.51) | 2.14 (2.10-2.18) |
| ≥9 | 1.67 (1.55-1.79) | 1.79 (1.68-1.91) | 1.57 (1.47-1.67) | 2.62 (2.51-2.73) | 1.62 (1.59-1.66) | 2.77 (2.72-2.82) |
| No. of hospitalizations in 2019 | ||||||
| 0 | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| 1 | 0.90 (0.84-0.97) | 1.04 (0.98-1.10) | 1.09 (1.02-1.18) | 1.01 (0.96-1.05) | 0.99 (0.96-1.01) | 1.03 (1.01-1.05) |
| ≥2 | 0.88 (0.78-0.99) | 1.17 (1.06-1.29) | 1.52 (1.35-1.71) | 1.35 (1.25-1.46) | 1.20 (1.15-1.27) | 1.29 (1.25-1.35) |
| Incidence (hazard ratio) | ||||||
| Age | ||||||
| <10 | 0.86 (0.67-1.12) | 0.77 (0.65-0.92) | 0.80 (0.63-1.03) | 0.29 (0.25-0.34) | 1.33 (1.23-1.44) | 0.53 (0.49-0.56) |
| 10-17 | 1.12 (0.84-1.49) | 1.11 (0.92-1.34) | 1.12 (0.87-1.45) | 0.73 (0.64-0.84) | 1.27 (1.16-1.40) | 0.98 (0.92-1.04) |
| 18-29 | 1.03 (0.82-1.30) | 1.32 (1.14-1.52) | 1.15 (0.92-1.43) | 1.10 (1.00-1.22) | 1.23 (1.14-1.33) | 1.30 (1.25-1.36) |
| 30-49 | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| 50-64 | 0.68 (0.53-0.87) | 0.78 (0.67-0.91) | 0.87 (0.70-1.09) | 0.85 (0.77-0.94) | 0.78 (0.72-0.85) | 0.72 (0.69-0.76) |
| ≥65 | 0.49 (0.34-0.71) | 0.66 (0.54-0.81) | 0.59 (0.44-0.80) | 0.87 (0.78-0.98) | 0.49 (0.44-0.55) | 0.45 (0.43-0.48) |
| Female | 0.97 (0.85-1.12) | 1.03 (0.94-1.12) | 0.97 (0.85-1.11) | 0.97 (0.91-1.04) | 0.91 (0.87-0.96) | 0.94 (0.91-0.97) |
| Income quintile | ||||||
| Q1 (lowest) | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| Q2 | 0.68 (0.58-0.80) | 0.61 (0.55-0.69) | 0.98 (0.77-1.25) | 0.92 (0.82-1.03) | 0.79 (0.74-0.85) | 0.74 (0.71-0.78) |
| Q3 | 0.71 (0.53-0.94) | 0.81 (0.67-0.97) | 0.96 (0.76-1.22) | 0.97 (0.87-1.08) | 0.68 (0.63-0.74) | 0.64 (0.61-0.67) |
| Q4 | 0.40 (0.32-0.51) | 0.72 (0.63-0.83) | 1.12 (0.91-1.38) | 1.03 (0.94-1.14) | 0.69 (0.64-0.75) | 0.64 (0.61-0.67) |
| Q5 (highest) | 0.27 (0.21-0.36) | 0.66 (0.58-0.76) | 0.76 (0.60-0.96) | 0.73 (0.66-0.82) | 0.49 (0.45-0.54) | 0.55 (0.52-0.58) |
| Unknown | 0.62 (0.09-4.42) | 0.98 (0.36-2.69) | 0.96 (0.70-1.31) | 0.72 (0.61-0.85) | 1.06 (0.96-1.18) | 0.74 (0.68-0.80) |
| In long-term care-second wave | No cases | 0.19 (0.03-1.50) | 13.01 (8.27-20.45) | 0.57 (0.34-0.95) | 10.86 (9.24-12.77) | 2.31 (1.99-2.67) |
| In long-term care-third wave | No cases | No cases | 1.55 (0.20-11.97) | 1.01 (0.37-2.78) | 0.58 (0.19-1.84) | 0.19 (0.06-0.58) |
| Any chronic disease | 1.38 (1.11-1.70) | 1.54 (1.36-1.75) | 1.03 (0.83-1.28) | 1.20 (1.10-1.32) | 1.20 (1.11-1.29) | 1.29 (1.24-1.35) |
| Immunosuppressed | 1.02 (0.70-1.47) | 1.10 (0.89-1.36) | 0.84 (0.58-1.21) | 0.96 (0.83-1.11) | 0.85 (0.75-0.96) | 0.91 (0.85-0.97) |
| Use of ARBs/ACEIsd | 0.98 (0.76-1.25) | 1.09 (0.94-1.26) | 0.80 (0.62-1.05) | 1.07 (0.96-1.18) | 1.05 (0.96-1.15) | 1.09 (1.03-1.15) |
| Use of proton-pump inhibitors | 1.51 (1.21-1.87) | 1.54 (1.35-1.76) | 1.49 (1.19-1.86) | 1.33 (1.21-1.46) | 1.13 (1.04-1.23) | 1.24 (1.19-1.30) |
| No. of physician visits in 2019 | ||||||
| 0 | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| 1-3 | 0.87 (0.73-1.03) | 1.04 (0.93-1.17) | 1.25 (1.01-1.54) | 1.31 (1.17-1.45) | 1.23 (1.14-1.33) | 1.27 (1.20-1.34) |
| 4-8 | 0.85 (0.68-1.06) | 0.99 (0.86-1.14) | 1.25 (1.01-1.56) | 1.46 (1.31-1.63) | 1.16 (1.07-1.26) | 1.37 (1.30-1.44) |
| ≥9 | 0.87 (0.66-1.16) | 0.94 (0.79-1.12) | 1.45 (1.14-1.84) | 1.59 (1.41-1.79) | 1.21 (1.11-1.33) | 1.47 (1.39-1.56) |
| No. of hospitalizations in 2019 | ||||||
| 0 | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| 1 | 1.20 (0.92-1.56) | 1.23 (1.04-1.45) | 0.74 (0.53-1.04) | 1.03 (0.89-1.19) | 1.11 (1.00-1.23) | 1.09 (1.02-1.16) |
| ≥2 | 1.18 (0.75-1.86) | 1.73 (1.35-2.21) | 0.50 (0.25-1.03) | 1.52 (1.22-1.89) | 0.91 (0.75-1.12) | 1.40 (1.25-1.57) |
a Adjusted for age, gender, income quintile, long-term care status, chronic disease, ARB/ACEI use, proton-pump inhibitor use, immunosuppression, and number of physician visits and hospitalizations in 2019. b The second wave is from October 1, 2020 to Feb 28, 2021. c The third wave is from March 1, 2021 to June 1, 2021. d ARBs/ACEIs: Angiotensin receptor blockers and angiotensin converting enzyme inhibitors.
Advanced age was a major predictor of severe illness (Table 4); overall 2% of LCCI patients died (Supplementary Table 3), although death was more prevalent in older patients, 17.5% of ≥65 year-olds with LCCI died in the second wave and 6.5% in the third wave compared to around 0.5% of 30-49 year-old patients. Income also had a big impact on severity, e.g., LCCI patients had a PR of 0.48 (0.35-0.64) of ICU admission when living in high income versus low-income neighbourhoods (Table 4). Patients in LTC and patients with chronic disease also had an increased prevalence of severe outcomes, although a lot of this is explained by other factors (the PR is much closer to the null after adjustment). Residents of the North, who are relatively young on average, seem to have a lower prevalence of severe outcomes in unadjusted analyses, but this PR increases after adjustment.
Table 4.
Prevalence ratios (95% confidence interval) of the association between severe COVID-19 outcomes and certain socio-economic and clinical characteristics during the seconda and thirdb waves
| Unadjusted |
Adjustedc |
|||||
|---|---|---|---|---|---|---|
| Hospitalization | ICU admission | Death | Hospitalization | ICU admission | Death | |
| Age | ||||||
| <10 | 0.08 (0.05-0.12) | No cases | 0.05 (0.01-0.35) | 0.10 (0.07-0.16) | No cases | 0.06 (0.01-0.43) |
| 10-17 | 0.17 (0.13-0.24) | 0.06 (0.02-0.17) | No cases | 0.24 (0.18-0.33) | 0.10 (0.04-0.26) | No cases |
| 18-29 | 0.66 (0.57-0.75) | 0.31 (0.22-0.43) | 0.20 (0.10-0.42) | 0.80 (0.69-0.91) | 0.41 (0.29-0.58) | 0.23 (0.11-0.48) |
| 30-49 | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| 50-64 | 2.14 (1.93-2.39) | 2.60 (2.13-3.16) | 4.10 (2.96-5.66) | 1.58 (1.41-1.76) | 1.64 (1.33-2.02) | 3.30 (2.37-4.59) |
| ≥65 | 6.43 (5.85-7.06) | 3.42 (2.80-4.16) | 40.71 (30.83-53.75) | 4.09 (3.65-4.58) | 1.79 (1.41-2.28) | 19.51 (14.21-26.76) |
| Female | 1.03 (0.96-1.10) | 0.79 (0.68-0.92) | 1.00 (0.88-1.14) | 0.90 (0.84-0.97) | 0.72 (0.62-0.84) | 0.73 (0.64-0.83) |
| Income quintile | ||||||
| Q1 (lowest) | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| Q2 | 0.73 (0.66-0.81) | 0.68 (0.55-0.84) | 0.78 (0.63-0.96) | 0.78 (0.70-0.86) | 0.76 (0.62-0.94) | 0.72 (0.58-0.89) |
| Q3 | 0.74 (0.66-0.82) | 0.72 (0.57-0.90) | 0.93 (0.75-1.16) | 0.73 (0.66-0.82) | 0.76 (0.60-0.95) | 0.75 (0.60-0.93) |
| Q4 | 0.80 (0.72-0.89) | 0.58 (0.46-0.74) | 1.28 (1.05-1.55) | 0.74 (0.66-0.82) | 0.60 (0.48-0.77) | 0.88 (0.73-1.07) |
| Q5 (highest) | 0.58 (0.51-0.66) | 0.43 (0.32-0.57) | 0.76 (0.59-0.98) | 0.61 (0.54-0.70) | 0.48 (0.35-0.64) | 0.69 (0.54-0.89) |
| Unknown | 0.81 (0.68-0.95) | 0.63 (0.44-0.92) | 6.21 (5.20-7.40) | 0.46 (0.37-0.58) | 0.55 (0.34-0.91) | 1.05 (0.82-1.33) |
| Regional health authority of residence | ||||||
| Interlake-Eastern | 1.05 (0.92-1.21) | 1.47 (1.14-1.89) | 0.67 (0.50-0.89) | 0.97 (0.85-1.12) | 1.28 (0.99-1.65) | 0.84 (0.62-1.12) |
| Northern | 0.92 (0.83-1.02) | 0.89 (0.71-1.11) | 0.38 (0.29-0.49) | 1.05 (0.94-1.17) | 0.95 (0.76-1.21) | 0.90 (0.69-1.19) |
| Southern | 1.28 (1.16-1.42) | 0.83 (0.65-1.07) | 1.06 (0.88-1.27) | 1.07 (0.97-1.18) | 0.76 (0.59-0.97) | 0.97 (0.80-1.17) |
| Prairie Mountain | 1.09 (0.95-1.26) | 0.99 (0.72-1.36) | 0.92 (0.71-1.19) | 0.95 (0.82-1.10) | 0.82 (0.60-1.14) | 0.82 (0.63-1.07) |
| Winnipeg | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| Public Trustee / In CFS care | 2.15 (1.67-2.77) | 1.78 (1.00-3.17) | 4.95 (3.71-6.60) | 1.89 (1.35-2.66) | 1.71 (0.81-3.60) | 1.06 (0.77-1.47) |
| In long-term care-second wave | 1.09 (0.88-1.35) | 0.64 (0.34-1.19) | 15.82 (13.78-18.17) | 0.23 (0.18-0.29) | 0.20 (0.10-0.40) | 1.88 (1.55-2.28) |
| In long-term care-third wave | 5.97 (2.71-13.16) | No cases | 34.76 (12.92-93.48) | 1.01 (0.41-2.49) | No cases | 2.58 (0.62-10.74) |
| Any chronic disease | 6.10 (5.68-6.56) | 7.42 (6.33-8.71) | 12.84 (11.04-14.94) | 1.97 (1.80-2.16) | 2.49 (2.04-3.03) | 2.06 (1.72-2.46) |
| Immunosuppressed | 3.62 (3.28-4.00) | 3.77 (3.07-4.65) | 4.12 (3.48-4.88) | 1.33 (1.19-1.47) | 1.46 (1.17-1.81) | 1.33 (1.12-1.59) |
| Use of ARBs/ACEIsd | 4.21 (3.92-4.53) | 5.22 (4.49-6.07) | 3.62 (3.18-4.13) | 1.13 (1.04-1.23) | 1.40 (1.17-1.67) | 0.76 (0.67-0.88) |
| Use of proton-pump inhibitors | 4.26 (3.97-4.58) | 4.33 (3.71-5.04) | 4.95 (4.36-5.61) | 1.48 (1.36-1.60) | 1.50 (1.27-1.78) | 1.23 (1.08-1.41) |
| No. of physician visits in 2019 | ||||||
| 0 | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| 1-3 | 1.13 (0.95-1.34) | 1.16 (0.79-1.69) | 1.37 (0.87-2.17) | 1.02 (0.85-1.21) | 0.93 (0.64-1.36) | 0.91 (0.57-1.43) |
| 4-8 | 2.13 (1.82-2.49) | 2.07 (1.46-2.94) | 3.41 (2.25-5.15) | 1.12 (0.94-1.32) | 0.92 (0.64-1.33) | 0.91 (0.60-1.39) |
| ≥9 | 5.74 (4.97-6.62) | 6.13 (4.47-8.41) | 16.79 (11.44-24.65) | 1.29 (1.08-1.52) | 1.27 (0.88-1.82) | 1.15 (0.76-1.74) |
| No. of hospitalizations in 2019 | ||||||
| 0 | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| 1 | 2.76 (2.49-3.05) | 2.15 (1.71-2.71) | 3.33 (2.80-3.94) | 1.61 (1.45-1.80) | 1.23 (0.96-1.57) | 1.25 (1.04-1.49) |
| ≥2 | 6.38 (5.67-7.17) | 4.84 (3.71-6.32) | 8.12 (6.72-9.82) | 2.33 (2.05-2.66) | 1.70 (1.28-2.26) | 1.45 (1.18-1.79) |
a The second wave is from October 1, 2020 to Feb 28, 2021. b The third wave is from March 1, 2021 to June 1, 2021. c Adjusted for age, gender, income quintile, regional health authority of residence, long-term care status, chronic disease, ARB/ACEI use, proton-pump inhibitor use, immunosuppression, and number of physician visits and hospitalizations in 2019. d ARBs/ACEIs: Angiotensin receptor blockers and angiotensin converting enzyme inhibitors.
3.3. Infectivity
The SAR increased with age of both the case and the contact. Widespread community transmission occurred during the second/third wave with an overall SAR of 16% (10% in the first wave; Supplementary Table 12), while the SAR for <18 year-olds remained relatively low. Children, lower income patients, and Northern residents were more likely to be associated with more secondary cases (Supplementary Table 13). LTC facilities were also a source of substantial super-spreading events.
4. Discussion
Similar to other jurisdictions, [13], [14], [15] Manitoba had a small first wave, which declined as strict interventions were implemented, including a ban/limit on small gatherings. [16,17] The first wave was followed by a relatively calm summer period and a larger second and third wave of infections. The increase in the SAR after the first wave may indicate that people were practicing less physical distancing and that super-spreading [18] may have been a larger problem. A household contact study in Winnipeg from the first wave showed that the majority of cases did not cause secondary cases. [12] Periods of higher percent positivity (used as a marker to implement or loosen interventions) corresponded with periods of increased incidence in Manitoba. Limiting testing to symptomatic individuals or those with a high likelihood of disease, would lead to higher positivity, as symptomatic persons and asymptomatic contacts had the highest positivity. Observed increased relative risk of LCCI was often driven by increased testing rates in the specific groups, e.g., for proton-pump inhibitors, which was also reported in a Korean study. [19] The pandemic affected different provinces in Canada differently, Manitoba had an above-average incidence rate. [20] As elsewhere in Canada, Manitoba delayed its second dose of COVID-19 vaccines in an attempt to partially vaccinate as many people as possible during the peak of the third wave.
Elementary schools remained open until May 12, 2021, for the entire second wave and a large part of the third wave. We found relatively low symptomatic positivity in young children, likely due to competing respiratory infections and the requirement of receiving a negative test to return to school without a mandatory self-isolation period after symptoms resolved. These children may have been more likely to be asymptomatic carriers given their relatively high screening positivity and their high relative risk of LCCI as an asymptomatic contact. Although the SAR was relatively low in children, as was transmission in elementary schools in Northern Italy's second wave, [21] they had relatively high odds spreading to multiple secondary cases. As in other jurisdictions, [22] younger age groups had relatively higher incidence as older age groups got vaccinated.
LTC residents were particularly at risk in Manitoba during the second wave, as they were in other jurisdictions; [23] and LTC outbreaks were common throughout Canada, [24] including Manitoba. Relative risk for LTC residents was much lower in the third wave, who were one of the first priority populations to be vaccinated. As reported extensively before, [25] we show that age, chronic disease, and LTC residence are major risk factors for severe LCCI (hospitalization, ICU admission, and death).
Although Manitoba's asymptomatic screening rates came close to symptomatic testing, it is unlikely that screening covered a representative part of the province, making it difficult to assess the proportion of the population already infected or the effect herd immunity may have had. At times, there have been significant backlogs in Manitoba's contact tracing, [26] which may have reduced its accuracy and have affected our SAR estimates. Our analysis on super-spreading only considered positive contacts, the number of reported secondary cases is likely underestimated.
Although Manitoba's North, a remote, sparsely-populated region with a large Indigenous population (∼75%) and with limited healthcare resources, was mostly spared during the first wave, it had both higher testing and higher incidence rates than the rest of Manitoba during the second wave. High positivity and low testing rates in Manitoba's South, a conservative, mostly agrarian rural region, may indicate underdiagnosis of LCCI there. Although testing was similar between income levels, especially during the second wave, persons living in low-income neighbourhoods were at increased relative risk of LCCI; we also observed an income-gradient in the SAR, with lower income corresponding to higher SAR. Residents of low-income neighbourhoods were at increased relative risk for severe outcomes after accounting for testing and LCCI. These disparities are likely due to a combination of crowded living situations, continuing essential work in proximity to many others, and barriers to healthcare, including the remoteness of the North, a lack of local healthcare infrastructure, and the systemic racism that disadvantages both Indigenous [27,28] and migrant [29] populations in Canada. It has been shown in the US that poorer and more diverse areas have higher LCCI incidence and mortality [30] and that US Hispanics had higher LCCI rates and a higher risk of LCCI in-hospital mortality. [31] In this study, we also show that secondary cases and super-spreading is more common in these areas.
4.1. Limitations
A major strength of this study is the availability of high-quality, population-based health administrative databases in Manitoba, [2,3] although the LCCI data has not been validated. Misclassification of SARS-CoV-2 status is possible, either due to false test results or due to data entry errors. Manitoba uses RT-PCR tests with ∼90% sensitivity and ∼98% specificity, [32] but we have no reason to believe differential sensitivity or specificity would affect our relative risk estimates. We cannot quantify data entry errors, but due to the seriousness of the pandemic and the use of automated systems, we think that data quality errors were uncommon. Incidence is impacted by the interventions in place; we did not assess the rolling implementation of various restrictions in this study. Approximately 8.6% of Manitoba blood donors tested positive for SARS-CoV-2 antibodies in November, [33] more than the approximately 1.3% of the population diagnosed with LCCI at that time. Although blood donors are in good health and not representative of the general population, many infections went undetected, which may have caused differential misclassification of LCCI status, especially as LCCI risk in marginalized people is likely higher than in blood donors. This study was not designed as a vaccine effectiveness study and our reported rates for vaccinated versus unvaccinated persons should not be interpreted as an estimate of the effect of the vaccine, as vaccination status is largely dependent on age and risk factors for LCCI.
5. Conclusion
The groups most vulnerable to LCCI were the marginalized residents of Manitoba's North, low-income neighbourhoods, and, in the second wave, LTC facilities. Underdiagnosis in Manitoba's South may mask the true burden of disease there. Young children remain the only group not eligible for a vaccine and, despite low prevalence of severe outcomes, are at high relative risk to be asymptomatic carriers and continue to spread the disease.
Funding
This study was not funded. Salaheddin M. Mahmud's work is supported, in part, by funding from the Canada Research Chair Program. The funders played no role in any aspect of writing the manuscript or decision to submit it for publication. None of the authors received payment to write this article.
Contributors
Christiaan H. Righolt: Conceptualization, Methodology, Formal analysis, Resources, Writing - Original Draft, Visualization, Supervision
Geng Zhang: Methodology, Software, Formal analysis, Writing - Review & Editing, Visualization
Emrah Sever: Software, Validation, Formal analysis, Writing - Review & Editing, Visualization
Krista Wilkinson: Methodology, Writing - Original Draft
Salaheddin M. Mahmud: Conceptualization, Methodology, Resources, Writing - Review & Editing, Supervision, Funding acquisition
Declaration of Interest
CHR has received an unrestricted research grant from Pfizer for an unrelated study. SMM has received unrestricted research grants from Merck, GlaxoSmithKline, Sanofi Pasteur, Pfizer and Roche-Assurex for unrelated studies. SMM has received fees as an advisory board member for GlaxoSmithKline, Merck, Pfizer, Sanofi Pasteur and Seqirus. None of the other authors have any conflicts of interest to disclose.
Data sharing statement
Data used in this article was derived from administrative health and social data as a secondary use. The data was provided under specific data sharing agreements only for approved use at the Manitoba Centre for Health Policy (MCHP). The original source data is not owned by the researchers or MCHP and as such cannot be provided to a public repository. The original data source and approval for use has been noted in the acknowledgments of the article. Where necessary, source data specific to this article or project may be reviewed at MCHP with the consent of the original data providers, along with the required privacy and ethical review bodies.
Acknowledgements
The authors acknowledge the Manitoba Centre for Health Policy for use of data contained in the Manitoba Population Research Data Repository under project # 2020-037 (HIPC # 2020/2021–04, REB # HS23859 (H2020:181)). The results and conclusions are those of the authors and no official endorsement by the Manitoba Centre for Health Policy, Manitoba Health, or other data providers is intended or should be inferred. Data used in this study are from the Manitoba Population Research Data Repository housed at the Manitoba Centre for Health Policy, University of Manitoba and were derived from data provided by Manitoba Health.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.lana.2021.100038.
Appendix. Supplementary materials
References
- 1.Riley S, Ainslie KEC, Eales O, et al. Resurgence of SARS-CoV-2: Detection by community viral surveillance. Science. 2021;372:990–995. doi: 10.1126/science.abf0874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Griffith GJ, Morris TT, Tudball MJ, et al. Collider bias undermines our understanding of COVID-19 disease risk and severity. Nat Commun. 2020;11:5749. doi: 10.1038/s41467-020-19478-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lieberman-Cribbin W, Tuminello S, Flores RM, Taioli E. Disparities in COVID-19 Testing and Positivity in New York City. Am J Prev Med. 2020;59:326–332. doi: 10.1016/j.amepre.2020.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang L, Ma H, Yiu KCY, et al. Heterogeneity in testing, diagnosis and outcome in SARS-CoV-2 infection across outbreak settings in the Greater Toronto Area, Canada: an observational study. CMAJ Open. 2020;8:E627–E636. doi: 10.9778/cmajo.20200213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roos LL, Mustard CA, Nicol JP, et al. Registries and administrative data: organization and accuracy. Med Care. 1993;31:201–212. doi: 10.1097/00005650-199303000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Bullard J, Dust K, Funk D, et al. Predicting Infectious Severe Acute Respiratory Syndrome Coronavirus 2 From Diagnostic Samples. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa638. published online May 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lix L, Yogendran M, Burchill C, et al. Manitoba Centre for Health Policy; Winnipeg: 2006. Defining and Validating Chronic Diseases: An Administrative Data Approach. [Google Scholar]
- 8.Kozyrskyj AL, Mustard CA. Validation of an electronic, population-based prescription database. Ann Pharmacother. 1998;32:1152–1157. doi: 10.1345/aph.18117. [DOI] [PubMed] [Google Scholar]
- 9.Barros A, Hirakata V. Alternatives for logistic regression in cross-sectional studies: an empirical comparison of models that directly estimate the prevalence ratio. BMC Med Res Methodol. 2003;3:21. doi: 10.1186/1471-2288-3-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bettencourt LMA, Ribeiro RM. Real Time Bayesian Estimation of the Epidemic Potential of Emerging Infectious Diseases. PLOS ONE. 2008;3:e2185. doi: 10.1371/journal.pone.0002185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lloyd-Smith JO, Schreiber SJ, Kopp PE, Getz WM. Superspreading and the effect of individual variation on disease emergence. Nature. 2005;438:355–359. doi: 10.1038/nature04153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilkinson K, Chen X, Shaw S. Secondary attack rate of COVID-19 in household contacts in the Winnipeg Health Region. Can J Public Health. 2020 doi: 10.17269/s41997-020-00451-x. published online Nov 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xiao Y, Tang B, Wu J, Cheke RA, Tang S. Linking key intervention timing to rapid decline of the COVID-19 effective reproductive number to quantify lessons from mainland China. Int J Infect Dis. 2020;97:296–298. doi: 10.1016/j.ijid.2020.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sciré J, Nadeau SA, Vaughan T, et al. Reproductive number of the COVID-19 epidemic in Switzerland with a focus on the Cantons of Basel-Stadt and Basel-Landschaft. Swiss Med Wkly. 2020;150:w20271. doi: 10.4414/smw.2020.20271. [DOI] [PubMed] [Google Scholar]
- 15.Caicedo-Ochoa Y, DE Rebellón-Sánchez, Peñaloza-Rallón M, Cortés-Motta HF, Méndez-Fandiño YR. Effective Reproductive Number estimation for initial stage of COVID-19 pandemic in Latin American Countries. Int J Infect Dis. 2020;95:316–318. doi: 10.1016/j.ijid.2020.04.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haug N, Geyrhofer L, Londei A, et al. Ranking the effectiveness of worldwide COVID-19 government interventions. Nat Hum Behav. 2020;4:1303–1312. doi: 10.1038/s41562-020-01009-0. [DOI] [PubMed] [Google Scholar]
- 17.Li Y, Campbell H, Kulkarni D, et al. The temporal association of introducing and lifting non-pharmaceutical interventions with the time-varying reproduction number (R) of SARS-CoV-2: a modelling study across 131 countries. Lancet Infect Dis. 2021;21:193–202. doi: 10.1016/S1473-3099(20)30785-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee EC, Wada NI, Grabowski MK, Gurley ES, Lessler J. The engines of SARS-CoV-2 spread. Science. 2020;370:406–407. doi: 10.1126/science.abd8755. [DOI] [PubMed] [Google Scholar]
- 19.Lee SW, Ha EK, Yeniova AÖ, et al. Severe clinical outcomes of COVID-19 associated with proton pump inhibitors: a nationwide cohort study with propensity score matching. Gut. 2021;70:76–84. doi: 10.1136/gutjnl-2020-322248. [DOI] [PubMed] [Google Scholar]
- 20.Public Health Agency of Canada COVID-19 daily epidemiology update. aem. 2021 https://health-infobase.canada.ca/covid-19/epidemiological-summary-covid-19-cases.html?stat=num&measure=active&map=pt#a2 published online July 20. accessed July 20, 2021. [Google Scholar]
- 21.Larosa E, Djuric O, Cassinadri M, et al. Secondary transmission of COVID-19 in preschool and school settings in northern Italy after their reopening in September 2020: a population-based study. Eurosurveillance. 2020;25 doi: 10.2807/1560-7917.ES.2020.25.49.2001911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Monod M, Blenkinsop A, Xi X, et al. Age groups that sustain resurging COVID-19 epidemics in the United States. Science. 2021;371 doi: 10.1126/science.abe8372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.D'Adamo H, Yoshikawa T, Ouslander JG. Coronavirus Disease 2019 in Geriatrics and Long-Term Care: The ABCDs of COVID-19. J Am Geriatr Soc. 2020;68:912–917. doi: 10.1111/jgs.16445. [DOI] [PubMed] [Google Scholar]
- 24.Webster P. COVID-19 highlights Canada's care home crisis. The Lancet. 2021;397:183. doi: 10.1016/S0140-6736(21)00083-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mallapaty S. The coronavirus is most deadly if you are older and male — new data reveal the risks. Nature. 2020;585:16–17. doi: 10.1038/d41586-020-02483-2. [DOI] [PubMed] [Google Scholar]
- 26.Froese I. Manitoba builds its contact-tracing team while backlog lingers | CBC News. CBC. 2020:2021. https://www.cbc.ca/news/canada/manitoba/manitoba-adding-contact-tracing-team-backlog-remains-1.5813434 published online Nov 24. accessed Jan 25. [Google Scholar]
- 27.Power T, Wilson D, Best O, et al. COVID-19 and Indigenous Peoples: An imperative for action. J Clin Nurs. 2020;29:2737–2741. doi: 10.1111/jocn.15320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Richardson L, Crawford A. COVID-19 and the decolonization of Indigenous public health. CMAJ. 2020;192:E1098–E1100. doi: 10.1503/cmaj.200852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tuyisenge G, Goldenberg SM. COVID-19, structural racism, and migrant health in Canada. The Lancet. 2021:0. doi: 10.1016/S0140-6736(21)00215-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Adhikari S, Pantaleo NP, Feldman JM, Ogedegbe O, Thorpe L, Troxel AB. Assessment of Community-Level Disparities in Coronavirus Disease 2019 (COVID-19) Infections and Deaths in Large US Metropolitan Areas. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.16938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dai CL, Kornilov SA, Roper RT, et al. Characteristics and Factors Associated with COVID-19 Infection, Hospitalization, and Mortality Across Race and Ethnicity. Clin Infect Dis Off Publ Infect Dis Soc Am. 2021 doi: 10.1093/cid/ciab154. published online Feb 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mustafa Hellou M, Górska A, Mazzaferri F, et al. Nucleic acid amplification tests on respiratory samples for the diagnosis of coronavirus infections: a systematic review and meta-analysis. Clin Microbiol Infect. 2020 doi: 10.1016/j.cmi.2020.11.002. published online Nov 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Canadian Blood Services COVID-19 Seroprevalence Report #4: November 2020 Survey. Ottawa. 2021 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.