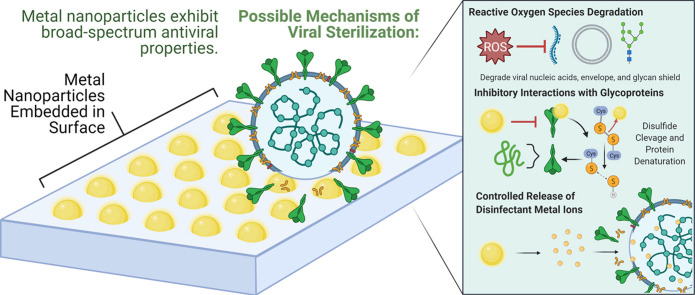
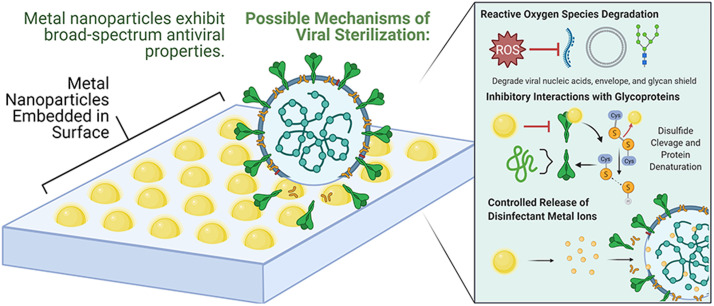
Graphical Abstract
2010 MSC: 00-01, 99-00
Keywords: Nanoparticles, Surface modifications, Virus inhibition
Abstract
Nanoparticles provide new opportunities in merging therapeutics and new materials, with current research efforts just beginning to scratch the surface of their diverse benefits and potential applications. One such application, the use of inorganic nanoparticles in antiseptic coatings to prevent pathogen transmission and infection, has seen promising developments. Notably, the high reactive surface area to volume ratio and unique chemical properties of metal-based nanoparticles enables their potent inactivation of viruses. Nanoparticles exert their virucidal action through mechanisms including inhibition of virus-cell receptor binding, reactive oxygen species oxidation and destructive displacement bonding with key viral structures. The prevention of viral outbreaks is one of the foremost challenges to medical science today, emphasizing the importance of research efforts to develop nanoparticles for preventative antiviral applications. In this review, the use of nanoparticles to inactivate other viruses, such as influenza, HIV-1, or norovirus, among others, will be discussed to extrapolate broad-spectrum antiviral mechanisms that could also inhibit SARS-CoV-2 pathogenesis. This review analyzes the published literature to highlight the current state of knowledge regarding the efficacy of metal-based nanoparticles and other antiviral materials for biomedical, sterile polymer, and surface coating applications.
Introduction
Human civilization has contended with disease outbreaks throughout recorded history, from the Antonine Plague of 250 to the 1720s Bubonic Plague, and the 1920 Spanish flu that are still infamous in our collective memory. For the first time, humanity has the ability to meaningfully use nanotechnology to combat a major infectious disease, namely the COVID-19 pandemic caused by SARS-CoV-2. SARS-CoV-2 has spread to millions of people worldwide through a novel mechanism of binding to ACE-2 receptors in the human body using its spike protein [1]. This structure not only identifies receptors on potential host cells but also facilitates fusion between the viral envelope and the host cell membrane, allowing the viral life cycle to continue unregulated [2].
Nanotechnology has proven valuable in both understanding and combating COVID-19 with applications including nanopore sequencing for rapid diagnosis and lipid nanoparticle carriers for delivering mRNA vaccines [3], [4], [5], [6]. Beyond conventional diagnostic techniques, nanomaterials also enable accurate biosensors that can detect the presence of a virus with only a microlitre of human serum [3], [7], [8], [9]. For example, the immune colloidal gold technique, an immunoassay based on labeling an antibody with plasmonic gold nanostructures, can enable an amplified fluorescent signal once a viral antigen is captured for imaging of antibody binding [9]; a chromic sensor with functionalized gold nanoparticles will show red-blue color change if the target virus is present [8]; or functionalized graphene electrodes able to accurately detect signs of COVID-19 infection through conductance changes caused by nucleocapsid protein antigen, immunoglobulins, and C-reactive protein in human serum [7]. Such technologies provide rapid and scalable point of care detection of viral infection.
Although effective pathogen detection has been made possible by nanomaterials, there remains the significant challenge of preventing infections. Transmission through contact surfaces such as doorknobs, packaging, and handrails is responsible for many preventable and nosocomial infections that cause extensive loss of life and healthcare expenditures as a pathway by which infectious pathogens spread between people [10]. Contributing to SARS-CoV-2's potent transmissibility is its ability to survive for long periods on common touch surfaces before infecting a host through hand-face contact [11], particularly in cold environments [12], [13], [14]. Therefore, targeting the virus before infection and in the early stages of its pathogenesis through self-sterilizing surfaces is an attractive strategy since essential viral components such as the spike proteins are likely to be extracellular and, therefore, relatively accessible to degradation. Though there is some evidence that this may not the most virulent pathway to infection by SARS-CoV-2 [15], [16], disinfection is the primary method of inhibiting virulent spread against viruses with no viable vaccine. Likewise, nanoparticle-based disinfection could be useful against SARS-CoV-2 in areas where vaccination rollout is challenging, or to augment vaccination efforts.
Given the prevalence and utility of plastic-based products in modern society, actively integrating nanostructures into polymers that self-sterilize against pathogens can provide a route to slow the transmission of viral infections. Face masks are another essential tool in slowing the transmission of viruses such as SARS-CoV-2, and polyimide thin films with nanoporous membranes and carbon-based nanomaterials can act as protective barriers against viruses in personal protective equipment (PPE) and air filters [3]. The surface chemistry of a polymer nanofibre filter can be fine tuned for optimal protection against a specific virus [17]. However, incorrect use and disposal of PPE may put the wearer in contact with virions trapped in the fabric, increasing their risk of infection. Therefore, the antiviral properties of nanoparticles also hold potential for equipping respiratory PPE to self-sterilize against viruses such as SARS-CoV-2 and influenza [18]. Long-lasting and sprayable antiviral materials are also valuable in keeping human environments and protective equipment clean. This can include broad-spectrum nanogels or nanoparticles that mimic cellular heparan sulfate to adhere viral glycoproteins and block their interaction with host cell receptors [19], [20]. In addition to preventing viral entry, certain nanomaterials such as zinc also inhibit viral RNA polymerase and thus viral replication through metal ion release [21], [22], [23].
There are a number of potential nanoparticles that could interfere with viral infections, particularly in preventing attachment and compromising integrity, including titanium dioxide, zinc oxide, iron oxide, silver, copper, and gold. The virucidal mechanisms exhibited by these nanomaterials include but are not limited to the inhibition of binding receptors, oxidation damage to biomolecules from reactive oxygen species (ROS), and bonding that disables key viral structures [24], [25], [26]. Compared to narrow-target antibiotic and antiviral drugs, metal-based nanoparticles have a diverse array of inactivation mechanisms, making it difficult for bacteria and viruses to develop resistance as they would have to acquire multiple protective mutations simultaneously [27]. Particularly photodynamic and photocatalytic materials, which can achieve efficient, broad-spectrum and long-acting killing of pathogens, offer a potential route to sterilizing surfaces on exposure to light. Furthermore, cytotoxicity is a core aspect that needs to be considered for nanoparticles that come in contact with biological systems as excessive cytotoxicity will affect not only pathogens but also human cells. Given the limited time span for direct research on SARS-CoV-2 and its rapidly mutating variants, the use of nanoparticles to inactivate other viruses, such as influenza, HIV-1, swine flu, herpes simplex, or norovirus, will be discussed to extrapolate broad-spectrum antiviral mechanisms that could also inhibit SARS-CoV-2 pathogenesis. This review analyzes the published literature to highlight the current state of knowledge regarding the efficacy of metal-based nanoparticles for biomedical, sterile polymer, and surface coating applications, and proposes areas of potential development specifically against SARS-CoV-2 pathogenesis.
The antiviral properties of metal and metal-based nanoparticles
Metal and metal-based nanoparticles (NPs) are at the forefront of nanomaterials research due to their high specific surface area and unique chemical properties that make them candidates for applications from disease therapeutics to antiviral surface coatings. Notably, the high surface area to volume ratio of nanoparticles enables efficient antiviral activity with relatively small amounts of metal. Further, their size enables ease of integration into medicines, polymers, and various surfaces [27]. Metals including copper, silver and gold exhibit the oligodynamic effect with well established biocidal characteristics that continue to persist on the nanoscale [28], [29], [30]. These metals and several of their oxides have been widely studied to elucidate their antiviral properties and mechanisms. Across the literature, the primary methods observed by which metal nanoparticles inhibit viruses include:[27], [30], [25], [26], [31], [32], [33], [34].
-
•
Binding or disrupting viral surface structures (e.g. spike glycoproteins) to prevent attachment and entry into host cells.
-
•
Production of metal ions and reactive oxygen species (ROS) through chemical pathways that adhere to and degrade viral components such as the glycan shield, lipid envelope, protein capsid, and nucleic acids.
-
•
Direct interaction with viral surfaces, proteins, and genetic material through the diffusion of nanoparticles and metal ions to damage viral integrity and inhibit functions such as protein synthesis and genome replication.
-
•
Cleavage of disulfide bonds between cysteine amino acid residues to denature and disable viral glycoproteins.
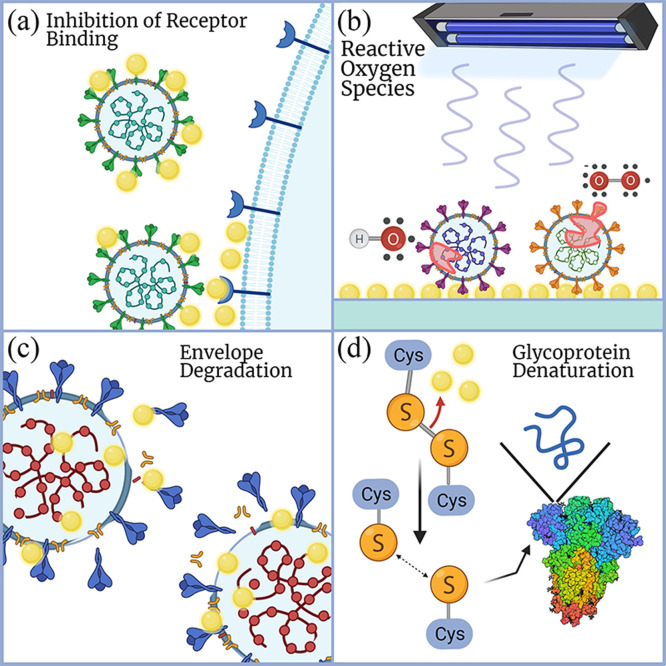
These are summarized in Fig. 1. The particular mechanisms by which specific nanoparticles act on different viruses are highly variable, dependent both on the nanoparticle reactivity and virus structures. The subsequent sections will explore each type of metal based nanoparticle and provide a snapshot of established research on their antiviral properties.
Fig. 1.
Schematic of possible antiviral mechanisms of metal and metal-based nanoparticles. (a) Nanoparticles engage in complex bond formation and electrostatic interactions with viral surface proteins to inhibit virus-host cell attachment and entry. (b) Nanoparticles undergo pathways including UV photocatalysis to generate reactive oxygen species that readily react with and damage viral biomolecules. (c) Nanoparticles and produced metal ions directly interact with and diffuse through the viral envelope or capsid to compromise its structure. (d) Nanoparticles undergo reactions that break disulfide bonds within viral glycoproteins, denaturing them to reduce infectivity by hindering viral binding of target receptors on host cells.
Titanium dioxide (TiO2) nanoparticles
Titanium dioxide has desirable properties, including low toxicity to humans and excellent UV-activated viral inhibition [35]. Its antiseptic mechanism is primarily the photocatalytic production of reactive oxygen species (ROS) such as hydroxyl radicals (OH), neutral hydroxide ions, and superoxide (O) when exposed to oxygen, moisture, and UVA photons [36], [24], [37]. ROS with unpaired electrons are highly unstable and rapidly react with biomolecules in reactions that exchange electrons. These reactions cause oxidative damage through alterations in the structure of polysaccharides, proteins, lipids, and nucleic acids, making ROS cytotoxic to a wide variety of organisms [38], [39], [40].
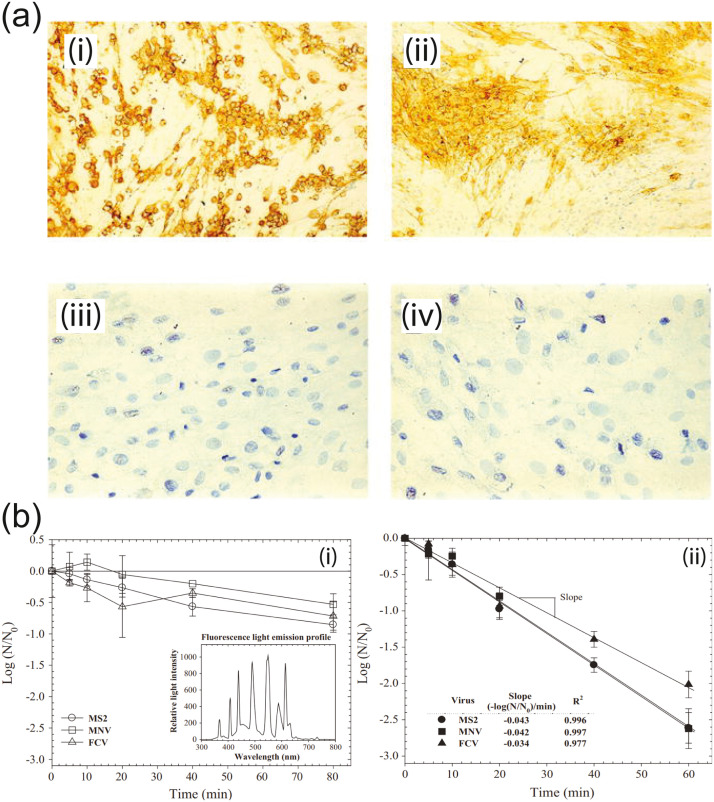
The evidence supporting TiO2 as a active antiviral coating material has been shown by a number of groups, including against human norovirus and several norovirus surrogates (bacteriophage MS2, murine norovirus (MNV), and feline calicivirus (FCV) [24]), human influenza A (A/PR8/H1N1) [37], and herpes simplex virus 1(HSV-1) [41]. Hajkova et al. conducted a study on HSV-1 using TiO2 thin films glass substrates prepared by plasma-enhanced chemical vapor deposition and compared against unmodified glass samples. Viral solutions of 109-1010 of virus particles per mL were deposited on the coated and blank glass samples and illuminated for 6 h with a UV-A lamp. Afterwards, the viral suspensions were used to infect cell cultures with a 24 h incubation period. The reduction in viral infectivity was evaluated using a glycoprotein gC monoclonal antibody, which turns brown in the presence of intact HSV-1. As shown in Fig. 2, both cell cultures infected with virus samples from blank glass whether dark or UV illuminated showed active viruses (A, B) [41]. However, the viral samples exposed to TiO2 films under UV light were inactivated and not able to infect or replicate as evidenced by the absence of brown coloration and glycoprotein gC. The antiviral mechanism is also proposed to be the destruction of virions by hydroxyl radicals and superoxide anions (free OH- and O) produced through TiO2 photocatalysis [41], similar to that observed in bacteria. The specific antiseptic mechanisms of ROS in bacteria include peroxidation of lipid membranes, carbonylation of proteins, and degradation of genetic material [38], [42], [39]. Park et al. [24] proposed that similar mechanisms may apply to viruses when exposed to Ti containing compounds, including damage to the viral membrane, protein capsid, and RNA, supporting the mechanisms suggested by Hajkova et al. [24], [41].
Fig. 2.
(a) Effect of UV-irradiated TiO2 Film on the proliferation of HSV-1 in cell culture (i) control 1: Infected with virus from black glass sample placed in dark; (ii) control 2: Infected with virus from blank glass with UV illumination; (iii, iv) Infected with virus exposed to UV illuminated TiO2 film. Reproduced with permission from Wiley from Ref. [41] Copyright © 2007 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim (b) Inactivation kinetics of bacteriophage MS2, murine norovirus, and feline calicivirus deposited on a dry surface coated with (i) TiO2 and (ii) F-TiO2 (UVA Intensity = 10 μW cm−2, Temp = 24 °C). Reprinted from Ref. [24] with permission from Elsevier.
However, regular TiO2 is activated by only UVA photons (λ < 387 nm), which can limit its applicability indoors with predominantly visible-range lighting. Nakano et al. showed that the intensity and duration of direct UVA illumination has a substantial effect on the virucidal activity of TiO2 against H1N1 as measured by viral titer assay according to 50% tissue culture infective dose (TCID50) in Madin-Darby canine kidney (MDCK) cells [37]. This dose is the concentration of viral fluid that infects 50% of the cells, in infectious unit per mL. They showed that TiO2 coated glass had highly increased activity as illumination increased from 0.001 to 1.0 mW cm−2. At typical indoor lighting intensities (~ 0.01 mW cm−2), some activity was observed, but required nearly 24 h to decrease the viral titer below the detection limit [37]. Park et al. examine a potential solution to this challenge through the use of fluorinated TiO2 which can more efficiently utilize fluorescent lighting for photocatalytic disinfection [24]. Specifically, they analyzed the use of nanoparticle films of TiO2 and F-TiO2 in UVA-activated antiviral surface coatings against various norovirus surrogates (human norovirus, MS2, MNV, and FCV). They prepared TiO2 films by mixing TiO2 NPs in a suspension of polyethylene glycol before spreading the mixture on a glass plate, drying at room temperature, and finally baking at 450 °C for 30 min to bind the film. A surface-fluorinated TiO2 sample was also prepared by soaking a TiO2 coated plate in a 30 mM pH 3.5 sodium fluoride solution for 30 min. Viruses samples measuring 25 μL were deposited onto both pristine TiO2 and F-TiO2 surfaces and exposed to 10 μW/cm2 fluorescent lighting. Virus inactivation was subsequently measured by plaque assay or PCR RNA copy quantification [24].
Park et al. found that the fluorinated TiO2 surface inactivated MS2 phage 4 times faster than regular TiO2. Further, fluorinated TiO2 reduced the infectivity of the norovirus surrogates by 2.4 log10 PFU/mL on average, two orders of magnitude higher than pristine TiO2. The greater photocatalytic efficiency of F-TiO2 was used to explain these results under fluorescent lighting, which is composed of less than 5% UVA. Park et al. suggest that surface fluorination of TiO2 reduces UV reflectance while producing additional free OH- and O that can damage viruses [24]. This virucidal action was long-lived and potent, with MS2 infectivity reduced to undetectable levels after 12 h under common office lighting [24]. The retention of antiviral properties under widespread indoor lighting conditions makes F-TiO2 nanoparticle coatings promising for sterile surface applications and the prevention of virus transmission. These studies show that the photodegradation mechanism of TiO2 can inactivate both non-enveloped viruses such as noroviruses and enveloped viruses such as HSV-1 [24], [41]. Notably, SARS-CoV-2 is an enveloped virus like HSV-1 with a similar fundamental lipid bilayer exterior which may be vulnerable to TiO2 surface coatings.
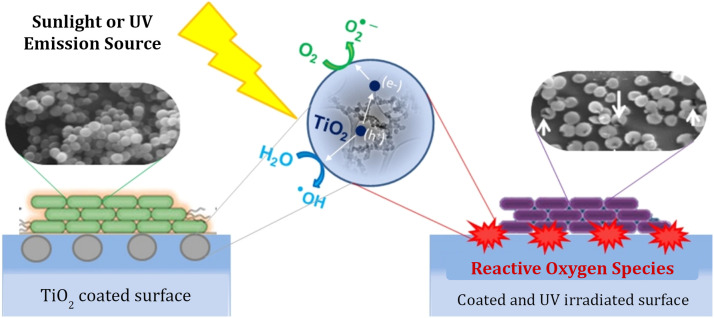
With regard to the optimal substrate for TiO2 coatings, Jalvo et al. found that TiO2 driven photodegradation of bacteria occurs at a rate 50 times higher on porous surfaces such as glass microfibre filters, compared to smooth glass slides [43]. The surface coatings employed in this study used 5 nm anatase TiO2 NPs synthesized by sol-gel with 11.5 mL of titanium tetraisopropoxide stirred with an acidic aqueous solution with a 140:1 water to nitric acid proportion that was aged and dialyzed. The nanoparticle suspension was spread over the glass slides and enriched into the glass filters by impregnation. Notably, the total amount of TiO2 required to coat the porous filters was much lower per unit area while achieving a superior overall dispersion as the glass fibers created a structure of TiO2 microsized sheets. The nanoparticle-based TiO2 coatings were analyzed using the methylene blue 365 nm UV-A photodegradation test against strains of Staphylococcus aureus and Pseudomonas putida. Following 2 h of exposure to visible/near UV light (11.2 W m−2 in the 290–400 nm range) on the porous glass filter, almost all bacterial cells (99.9%) became inactivated, showing membrane degradation from the generated intracellular ROS ( Fig. 3) [43]. As shown in other studies, ROS generation is applicable to viral inactivation as well [24], [41]. The result from this study suggests that porous surfaces may increase photoactive surface area, enabling more efficient TiO2 photocatalysis with less material and UV irradiation.
Fig. 3.
Diagram of TiO2 photocatalysis. Antimicrobial action occurs through the production of ROS on exposure to specific UV wavelengths. SEM images illustrate Staphylococcus aureus samples before and after exposure to TiO2 surface with arrows indicating damage to bacterial membranes.
Reprinted from Ref. [43], with permission from Elsevier.
TiO2 nanoparticles have also shown promising results in preventing infection of virus-inoculated cells. One study conducted by Akhtar et al. produced TiO2 NPs with an average diameter of 8 nm using a sonochemical method developed by Srivastava et al. [44] and evaluated the NPs inhibitory efficacy against Newcastle disease virus (NDV), one of the most virulent poultry viruses [45]. When NDV inoculated chicken embryos were treated with varying concentrations of TiO2 NPs, no hemagglutination was observed in NP concentrations from 6.25 μg/mL to 100 μg/mL, indicating complete viral inactivation after 96 h. The suggested mechanism of action is direct NP-virus interactions that damage the viral envelope’s lipids and deform the glycoprotein spikes, making the virus unable to attach and initiate infection [45]. This secondary mechanism is also supported by Mazurkova et al.,[46] who demonstrated in their study that TiO2 NPs still exhibited antiviral activity against influenza even when not UV illuminated. Based on their observations, it is suggested that TiO2 NPs can also achieve viral inactivation by penetrating and disintegrating the viral envelope [46].
Takeaway and applicability to SARS-CoV-2
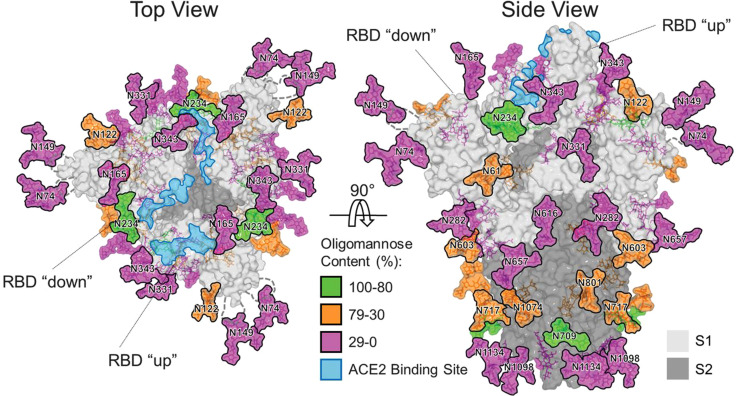
The photodegradation mechanism of TiO2 in the presence of UV wavelengths and moisture is suited to inactivating SARS-CoV-2 given that the virus spreads as aerosolized aqueous droplets that settle on surfaces [11]. A TiO2 nanoparticle-based surface coating could use the moisture in the droplets as a reagent to start the production of ROS through photocatalysis reactions, even under standard indoor lighting. Given that SARS-CoV-2 is an enveloped virus, with a lipid bilayer exterior and surface glycoproteins (see Fig. 4a), the interactions with nanoparticles and their produced ROS with the SARS-CoV-2 viral membranes may compromise surface proteins such as the spike glycoprotein, which are rooted in and depend on an intact membrane for fusion with host cells [47].
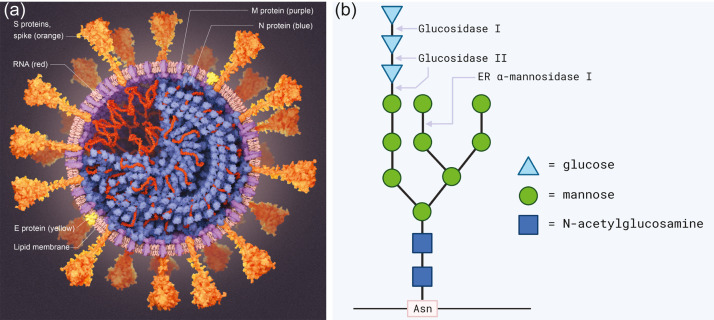
Fig. 4.
(a) Diagram of SARS-CoV-2, the virus that causes COVID-19. An enveloped virus with a lipid membrane featuring a “corona” of spike (S) proteins. Reproduced with permission from Scientific American from Ref. [48]. (b) The N-linked core oligosaccharide. N-linked glycans make up a part of SARS-CoV-2 spike proteins start as "core oligosaccharides" that have the structure shown. These are bound to the polypeptide chain through an N-glycosidic bond with the side chain of an asparagine.
SARS-CoV-2 inhibition would then be achieved through the oxidative damage to its base proteins, lipids, and nucleic acids by reactive free radicals. Furthermore, a study by Yasuda et al. found that ROS (in their case from copper ions) were able to alter and cleave the oligosaccharides of glycoproteins in a hepatitis rat model [49]. Notably, they found that cation reactions with hydrogen peroxide can readily generate hydroxyl radicals that degrade N-linked glycosidic linkages between oligosaccharides and proteins. This interaction reduces the glycosylation of glycoproteins and alters biological function. In SARS-CoV-2, the spike protein uses extensive glycosylation acquired from the human cell that produced it to shield vulnerable epitopes from immune antibodies [50] (see Fig. 4).
This glycan shield plays a crucial role in SARS-CoV-2 infectivity and helps facilitate immune evasion so the virus can successfully reach host cells in the body. Therefore, a significant ROS-mediated reduction in the viral spike protein’s glycosylation before exposure to the host could make the virus less infectious and more easily destroyed by the host’s immune response. Preemptive modification to SARS-CoV-2 oligosaccharide moieties could be achieved through a surface coating possibly based on TiO2 as its photocatalytic reaction also generates hydrogen peroxide and hydroxyl radicals. A combination of TiO2 and Cu in a composite coating may be worth further research as well to evaluate if SARS-CoV-2 inactivation can be achieved through ROS-mediated degradation of the spike protein glycan shield. The idea that TiO2 nanoparticles themselves can directly interact and physically damage SARS-CoV-2 virions is also substantiated by the studies of Akhtar et al. and Mazurkova et al. [45], [46]. They demonstrated that TiO2 NPs cause membrane destruction to inactivate of influenza and Newcastle disease virus; an action which may be transferable to SARS-CoV-2, given that all are enveloped RNA viruses with similar structures. Preliminary research [51] seems to support this possibility.
The virucidal action of TiO2 nanoparticles against human norovirus and norovirus surrogates, in addition to enveloped viruses, is another particularly promising development. Noroviruses possess high genetic variability and a rapid mutation rate that makes vaccine development highly challenging while limiting the duration of immunity for recovered persons. In addition, the low infectious dose of 18 viral particles or less [52], an alcohol sanitizer-resistant non-enveloped structure [53], [54], and ability to survive for up to several weeks on surfaces [55], results in potent transmissibility [56]. Therefore, enteric viruses are similar to SARS-CoV-2 with respect to their environmental stability, ability to survive for prolonged durations on cross-contaminated surfaces [11], and rapid mutation. Thus, rapid disinfecting methodologies that can stem the spread of both enveloped and non-enveloped virus domains in vulnerable environments such as hospitals and restaurants would be a major breakthrough.
Zinc oxide (ZnO)
The use of zinc oxide as an antiseptic may trace as far back as 4000 years to ancient Egypt when it was used in ointments to treat infected hair follicles in the form of boils and carbuncles [57]. Modern science has confirmed that ZnO and its nanoparticles (ZnO NPs) possess antibacterial properties against an extensive range of microorganisms, including Escherichia coli, Pseudomonas aeruginos, Klebsiella pneumonia, Pseudomonas vulgaris, and Campylobacter jejuni [58], [59]. At the same time, zinc oxide, even in nanoparticle form, is considered safe for human contact, as it is utilized in sunscreens, and even consumed as a supplement in zinc fortified foods [60], [61]. This is particularly because the toxic effects of ZnO NPs, bulk ZnO, and Zn2+ are similar, unlike many other systems [62]. The toxicity of ZnO to pathogens and minimal impact on human cells has led to applications of nanoscale ZnO for lining food packaging and plastics to confer antimicrobial activity against foodborne and infectious pathogens [63]. In regards to food packaging, zinc oxide has been successfully integrated with petroleum-based and biodegradable PHBV polymers to improve food shelf-life and safety, using methods such as melt mixing, electrospinning or solvent casting [64]. Zinc oxide NPs are a leading virucidal candidate for therapeutic and surface coating applications given their unique attributes of low cytotoxicity to human cells, ROS-generating photocatalysis, and proven effectiveness at damaging lipid membranes through diffusion and accumulation.
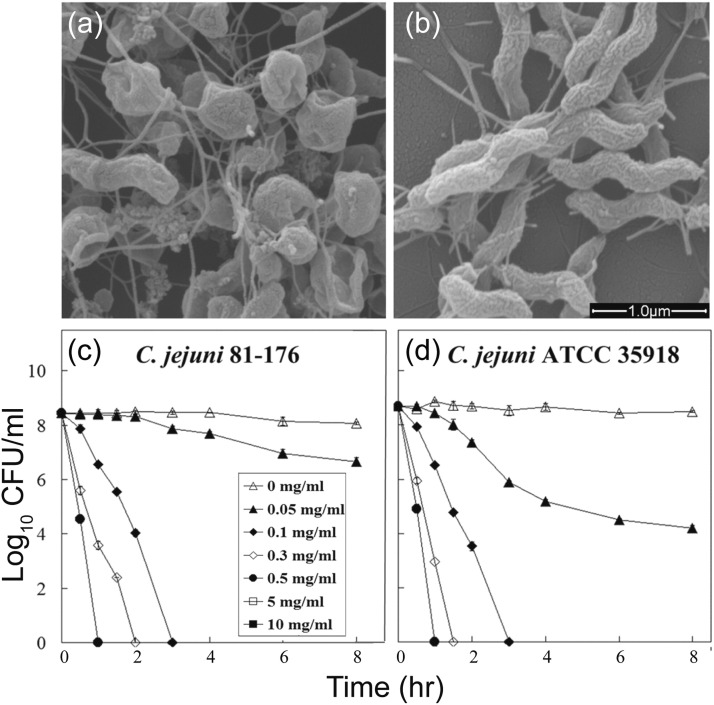
The unique chemical properties of ZnO NPs compared to their bulk form, as with most NP systems, results from their high specific surface area, leading to greater activity against pathogens. Against bacteria, ZnO NPs can act through a mechanism of destructive diffusion through the cell membrane, which causes lysis [59]. For instance, Xie et al. used SEM imaging to study the impact of 0.5 mg/L ZnO NPs on Campylobacter jejuni [63]. They found that the bacteria changed from their usual spiral shape to a coccoid morphology with instances of membrane penetration and rupture ( Fig. 5) [63]. The images suggest that ZnO NPs accumulate on and compromise the lipid membrane to inactivate the bacteria. From across the literature, ZnO NP biocidal activity is generally observed to improve with decreasing particle size, affording greater effective surface area [65].
Fig. 5.
(a) SEM images of Campylobacter jejuni cells treated with 0.5 mg/mL of zinc oxide nanoparticles for 12 h under microaerobic conditions. (b) untreated cells as a control. (c, d) Antibacterial activities of ZnO nanoparticles against C. jejuni. Freshly grown bacterial cultures (108–109 CFU/mL) were treated with a range of concentrations of ZnO nanoparticles. Culturable cell numbers were determined at the time intervals after treatment shown on the figure. The values for CFU/mL are the means of 12 replicates. Error bars indicate standard deviations of the means.
Adapted from Ref. [63] with permission from the American Society for Microbiology.
Furthermore, ZnO has a similar band gap to TiO2 and can also act as an effective, low-cost photocatalyst in the presence of water and sunlight or artificial UV [66]. ZnO NPs have been reported by groups such as Premanathan et al. and Ong et al. to undergo UV photocatalysis pathways that generate excess ROS such as superoxide, hydroxyl radicals, and hydrogen peroxide [66], [67]. As seen in TiO2 photodegradation, these ROS can cause oxidative stress and lipid peroxidation that damages biological membranes and may induce apoptosis [24], [41], [67]. Zinc ions have also been demonstrated to exhibit virucidal effects on viruses through protease inhibition and disruption of viral RNA polymerase [68]. Inactivation by ZnO NPs, like other metal nanoparticles, benefits from increasing exposure time and concentration.
As transmission through contact surfaces is a challenging infectious pathway [10], there have been efforts to combat this by developing long-lasting coatings and sprays that target viruses and bacteria while remaining safe for people. One method of creating a stable, biocompatible coating is the use of hydrogel polymers mixed with ZnO NPs, as reported by Schwartz et al. [69]. The coating developed is an antimicrobial composite hydrogel formed by mixing poly(N-isopropylacrylamide) with zinc oxide nanoparticles. SEM imaging of the resultant film showed an even distribution of ZnO NPs that was highly effective in entirely disabling E. coli at a low zinc oxide concentration of 1.33 mM. The composite coating was also found to be safely non-toxic during seven days of exposure to mammalian NIH/3T3 cells. The researchers propose that the demonstrated coating could be employed for self-sterilizing biomedical devices and hospital surfaces.
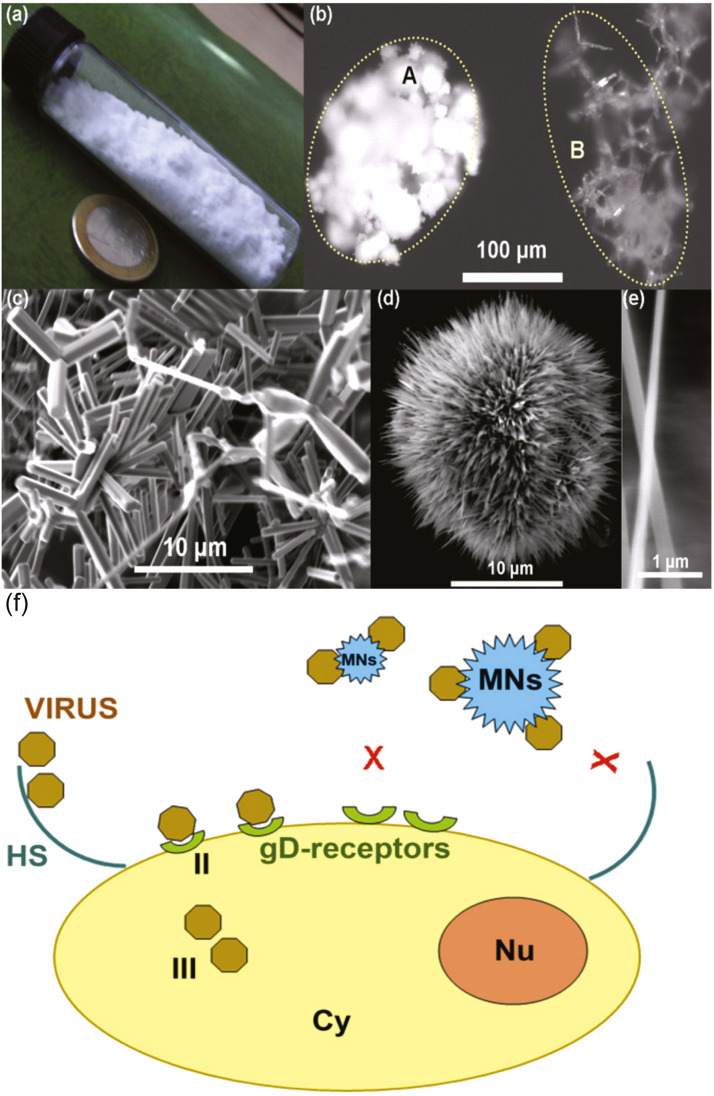
Although studies focused on the antiviral influence of zinc oxide nanoparticles are far less prevalent in the literature than those examining its antibacterial properties, there are a few notable studies. Mishra et al. have researched zinc oxide as an antiviral agent through a micro-nano structure (MNS) morphology that targets the attachment phase of herpes simplex virus type-1 (HSV-1) pathogenesis [70]. HSV-1 possesses positively charged envelope glycoproteins that electrostatically bind to negatively charged heparan sulfate (HS) areas on the cell surface to induce filopodia-like structures that enable viral entry [71]. The researchers targeted this entry mechanism using ZnO MNSs, which resemble sea urchins with nanoscopic spikes, mimicking the HS structures on the cell surface that the HSV-1 normally targets with its glycoprotein spikes. The partial negative charge of the MNSs causes electrostatic binding to HSV-1 virions, which trap them on the surface of the MNSs and prevent the virus from reaching any host cell receptors ( Fig. 6) [70]. The inhibitory effect was further enhanced when ZnO MNSs were pretreated with UV-irradiation, which creates additional oxygen vacancies and negative charge centers on the MNS surface, leading to higher attraction and binding to HSV-1 virions [72]. The ZnO nanostructures were able to effectively prevent infection of zebrafish embryos and human corneal fibroblasts by HSV-1. Thus, ZnO MNSs proved powerful at inactivating the virus with low toxicity to host cells at concentrations up to 500 μg/mL. The study of Wu et al. [72] supports the potential for ZnO nanotechnology as a biocompatible prophylactic agent that can prevent infection of enveloped viruses such as herpes simplex through applications such as topical creams or surface coatings; it may be possible to adapt this approach to mimic other viral targets, potentially including SARS-CoV-2, so that it can be used against a broader range of pathogens.
Fig. 6.
ZnO micro-nanostructures (MNSs). (a) Synthesis of the ZnO material can be done in large quantities, please note the 23 mm diameter coin. (b) Microscopic image, comparison between a standard powder (A) and the material synthesized here (B). (c) Electron micrograph showing the complex geometries. (d) The powder contains a larger quantity of filopodia like structures, which have (e) spikes down to the nanoscale. (f) Model for the ZnO-MNSs based HSV-1 inhibition. Interaction of HSV with ZnO-MNSs bearing nanospikes results in HSV-1 trapping that prevents early phases of virus-cell interactions and viral entry.
Adapted from Ref. [70], with permission from Elsevier.
In a recent study, El-Megharbel et al. examined the antiviral activity of ZnO NPs against SARS-CoV-2 directly and found potent inhibition at a low nanoparticle concentration (IC50 of 526 ng/mL) [73]. The ZnO NPs were characterized using a suite of spectroscopic tools and found to have a diameter of 40–60 nm as determined by TEM. The antiviral activity and IC50 were determined by infecting Vero-E6 cells with SARS-CoV-2 and then overlaying cell culture with different concentrations of ZnO NPs. Following 72 h incubation, the cells were stained to evaluate the viral inhibition and determine which concentration reduced the cytopathic effect by 50% relative to the control (IC50). The low concentration IC50 suggests substantial impairment of SARS-CoV-2 pathogenesis. However, MTT assay found that ZnO NPs were moderately cytotoxic to Vero cells with a 292.2 ng/mL concentration resulting in 50% cell death. The proposed mechanism is that ZnO NPs produce Zn2+ ions and varied reactive oxygen species that can damage proteins, lipid membranes, and nucleic acids to cause both SARS-CoV-2 inactivation and cell apoptosis [73].
Another approach uses polymer functionalization of ZnO NPs to provide greater stability, lower toxicity, and controlled release antiviral action. Ghaffari et al. [74] and Tavakoli et al. [75] explored this option for H1N1 influenza virus, and for herpes simplex virus type 1, respectively. By examining the antiviral effect of zinc oxide nanoparticles both with and without polyethylene glycol (PEG) surface functionalization on infected cell lines, Ghaffari et al. found that the highest non-toxic concentration for PEGylated ZnO-NPs was 200 μg/mL, resulting in an H1N1 inhibition rate of 94.6% while the non-PEGylated ZnO-NPs were only non-toxic up to 75 μg/mL which achieved 52.2% inhibition for H1N1 influenza-infected Madin-Darby canine kidney (MDCK)-SIAT1 cells [74]. For the same concentration (200 μg/mL), Tavakoli et al. saw an inhibition rate of approximately 92% with HSV-1-infected Vero cells [75]. Time-of-addition assay found that PEGylated ZnO NPs were most effective when added 1 h post-infection, which suggests that the nanoparticles influence early stages of viral pathogenesis and may interfere with the initiation of infection or subsequent viral genome replication [75], though confirmation requires quantitative protein expression measurements. PEGylation of the nanoparticles was achieved by mechanical ball milling, which also created smaller nanoparticles. This decreased size is also a possible mechanism of increased antiviral activity since the smaller NPs are expected to be more reactive with a greater surface area to volume ratio and greater facilitated diffusion [74]. Overall, coating the surface of ZnO NPs with polyethylene glycol can enhance antiviral efficacy and lower in vitro cytotoxicity.
Takeaway and applicability to SARS-CoV-2
Given that enveloped viruses such as SARS-CoV-2 also have a lipid bilayer outer membrane, nanoparticles could disrupt and permeate the membrane in a similar manner to the bacterial inactivation mechanism [47], [76], and in fact this is what El-Megharbel et al. observed [73]. Amphiphilic soap is well known to effectively destroy SARS-CoV-2 virions by dissolving its lipid membrane, which supports the possibility that nanoparticles such as ZnO may achieve viral inactivation by targeting the same structures [11]. The ZnO micro-nano structure morphology electrostatically targeting virions suggested by Mishra et al. [70], [72] developed for HSV may be adapted to resemble other viral targets such as that of SARS-CoV-2. Furthermore, with similar photocatalytic action, ZnO may share inactivation mechanisms with TiO2, including the cleavage of oligosaccharides from the glycan shield by hydroxyl radicals and ROS-mediated degradation of viral integrity [49]. However, due to the high cytotoxicity of ZnO-NPs observed by El-Megharbel et al. [73], such nanoparticles would be best suited to use outside the body such as in disinfectants and coatings where the antiviral action can be leveraged with minimal risk of contact with live human cells.
Iron oxide nanoparticles
Iron oxide nanoparticles (IO-NPs) are a prominent nanomaterial used in biomedical applications ranging from cancer nanotherapy to biosensors to contrast agents for imaging, owing to their high biocompatibility and magnetic properties [77], [78], [79], [80]. Notably, IO-NPs can be modulated under an external magnetic field and used for drug targeting or to induce local hyperthermia for tumor treatment [81]. Their proven safety in anemia treatment has led to US Food and Drug Administration (FDA) and various European Union agency approvals [82], which combined with demonstrated antibacterial [83] and antiviral activity against influenza subtypes [84], [85], rotavirus [86], and Dengue virus [87], may make IO-NPs valuable tools in preventing the spread of viral infections [88], [89].
Qin et al. evaluated the efficacy of large (~ 200 nm) iron oxide (magnetite, Fe3O4) nanoparticles against influenza A, and found broad-spectrum antiviral activity against 12 viral subtypes (H1-H12) through a range of in vitro and in vivo tests [84]. Pre-exposure of influenza A virus to a concentration of 4 mg/mL iron oxide nanoparticles for 2 h efficiently inhibited virus-induced cell death in MDCK cells and mouse models showed a 100% survival rate 14 days post-infection [84]. Similarly, Kumar et al. [85] saw significant antiviral potency against H1N1 influenza plaque formation in a dose and time dependent manner for small, 10–15 nm diameter, nanoparticles, where an 8-fold reduction in viral RNA transcripts was observed using RT-PCR, for in vitro treatment of the virus with IO-NPs compared to untreated control.
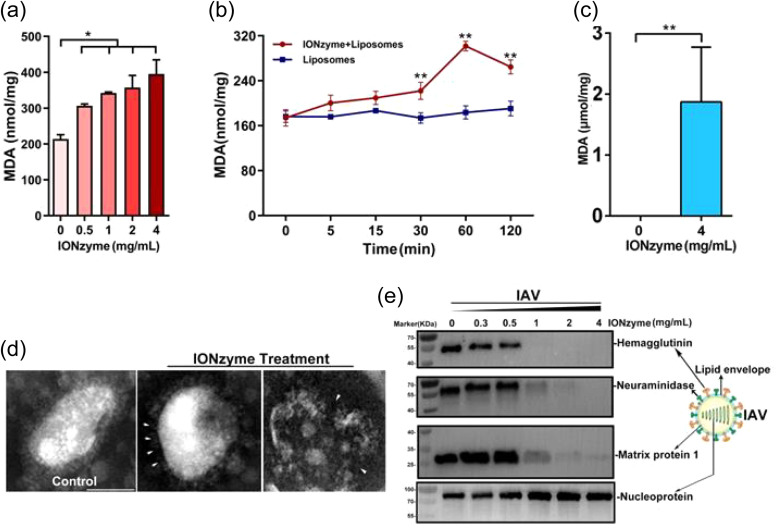
The broad spectrum efficacy against influenza subtypes was made possible by targeting the viral envelope, which is a highly conserved component across enveloped viruses [84]. The main mechanism observed by Qin et al. for influenza sub-type viruses was lipid peroxidation of the viral envelope and damage to neighboring proteins that compromised virion integrity and infectivity [84]. In this way, the nanoparticles exert lipoxidase enzyme-like activity and the nanozyme action enables effective catalysis of viral lipid structures. Destruction of the viral envelope was confirmed through transmission electron microscopy and degradation of influenza glycoproteins, hemagglutinin and neuraminidase was found through Western blot ( Fig. 7). For smaller particles, Kumar et al. proposed that the small size of these IO-NPs enabled preferential reaction and binding with the sulfur-bearing residues (-SH groups) of influenza proteins to prevent virus-host cell binding, inhibiting viral proteins and replication [85].
Fig. 7.
IONzymes compromise the lipid envelope and neighboring proteins of IAVs through lipid peroxidation. (a, b) The level of lipid peroxidation (MDA detection) when liposomes were treated by IONzymes, (c) or when IAVs (H5N1 SY strain) were treated by IONzymes. (d) TEM image of IAVs treated by IONzymes (4 mg/mL). The left image: untreated IAVs. The asterisk: the destroyed lipid envelope of virion. Scale bar: 50 μm. (E) Western blot analysis of hemagglutinin, neuraminidase, matrix protein 1, and nucleoprotein protein of IAVs treated by IONzymes. Adapted from Ref. [84], under CC BY 4.0.
Furthermore, it has been found that coating Fe3O4 nanoparticles with polymers such as polyvinyl pyrrolidone (PVP) [90], polyethylene glycol (PEG) [90] or glycine [85] can enhance their anti-influenza activity, stability, and safety. MDCK cell viability studies for PVP and PEG coating using MTT assay showed that polymer-coated magnetite nanoparticles exerted less cellular toxicity at all concentrations compared to uncoated magnetite nanoparticles after 72 h of exposure [90]. With functionalization with glycine, Kumar et al. [85] found that IO-NPs were also not significantly cytotoxic at the effective concentration of 2 pg/mL for Monkey African green kidney (MA104) cells. The improved safety may be a result of the polymer-coating reducing nanoparticle-cell interactions or membrane alterations yielding higher stability [90], [85].
Coated particles have an added effect, where they not only retain their antiviral efficacy, but are seen to even exceed that of pristine NPs. In cytopathic assay, Kumar et al. [90] observed a 30 μg/mL concentration of pristine magnetite NPs inhibited influenza viral plaque formation by 30% whereas the PEG- and PVP-coated magnetite NPs achieved 45% and 55% plaque inhibition, respectively. TEM imaging showed that polymer-coated magnetite NPs also entered the cytoplasm of infected cells where they bound with the viral nucleic acids before replication. A significant reduction in influenza RNA was confirmed by RT-PCR [90]. The enhanced antiviral efficacy from polymer coating may be caused by the deprotonation of the magnetite nanoparticle surface which confers a negative charge. Kumar et al. found that negatively charged nanoparticles strongly impede the H1N1 virus due to electrostatic interactions between the nanoparticles and viral structures such as the lipid membrane, glycoproteins and RNA. NP binding interactions can compromise viral viability, prevent cellular entry, or impair replication intracellularly [90].
Due to their relatively low cytotoxicity and approved use in vivo in iron replacement therapies and as contrast enhancement reagents for magnetic resonance imaging [82], IO-NPs have great potential in infection prevention and control in healthcare settings. IO-NPs can be integrated into PPE such as masks and gloves, as well as patient-contact materials such as bedsheets and pillow covers [84]. The use of sterilizing nanoparticles could inactivate viruses within fabrics and limit the spread of infections among healthcare providers and patients [88]. In fact, Qin et al. showed that when integrated into a facemask, iron oxide nanoparticles provided increased protection against 3 epidemic influenza A subtypes: H1N1, H5N1, and H7N9 [84]. The use of IO-NPs in disinfection is also supported by prior research exploring their use for water treatment. When embedded in fiberfill, Fe2O3 NPs were found to disinfect MS2 phage and rotavirus in solution [86]. This effect could be enhanced, and extended to bacteria such as E. Coli, by producing hybrid metal compounds, such as Ag-modified Fe2O3 nanoparticles [91], embedded in the fiberfill. Through a mechanism of electrostatic adsorption to the high surface area nanoparticles [86], the capsids of rotavirus virions were structurally degraded through interaction with the hematite, as observed in TEM microscopy. In aqueous solutions, hematite acquires a surface charge from the dissociation of surface hydroxyl groups that remove viruses from water by electrostatic interactions and the formation of surface complexes [92]. Such a mechanism, which can be manipulated using polymer functionalization or environmental conditions to modify the surface charge, makes IO-NPs particularly attractive as a surface coating.
Iron oxide nanoparticles thus show potent inhibition of viruses and biocompatibility that may be enhanced with polymer surface modification or hybridization with other particles [91]. Further studies can help determine practical applications, better elucidate methods of mitigating cytotoxicity risk, and examine efficacy against other viruses including SARS-CoV-2 [85], [90].
Takeaway and applicability to SARS-CoV-2
In summary, iron oxide nanoparticles exhibit a broad range of antiviral mechanisms including ROS generation, lipid peroxidation, and binding to viral surface proteins to impair attachment to host cells. The lipid membrane envelope of SARS-CoV-2 renders it vulnerable to lipid peroxidation while its integral membrane proteins can be inhibited by ROS and nanoparticle binding. By targeting the viral envelope, which is a highly conserved component across enveloped viruses [84], or its spike proteins, iron oxide could also be effective against other enveloped viruses such as coronaviruses. Recently, Abo-zeid et al. performed a detailed theoretical molecular docking study showing the specific interactions of IO-NPs with the viral glycoproteins of SARS-CoV-2 [88]. They evaluated the binding affinity of Fe3O4 (magnetite) and Fe2O3 (hematite) nanoparticles to SARS-CoV-2 spike protein receptor binding domain (S1-RBD) and hepatitis C virus (HCV) E1 and E2 glycoproteins. Fe3O4 NPs were found to form more stable complexes and preferentially bind with S1-RBD compared to Fe2O3 NPs. In contrast, Fe2O3 NPs had stronger interactions than Fe3O4 for HCV E1 and E2 glycoproteins. The binding of IO-NPs to viral glycoproteins involves the formation of hydrogen bonds to amino acids in the glycoprotein. For instance, the interaction of Fe3O4 with S1-RBD involved the formation of four hydrogen bonds, with a total intermolecular energy of 11.40 Kcal/mol. The authors propose that nanoparticle binding induces irreversible conformational changes to the glycoproteins that prevents viral binding and entry into host cells. For SARS-CoV-2, IO-NPs may inhibit the spike protein receptor binding domain from attaching to host cell ACE-2 receptors, thus stopping the initiation of cellular infection. Further, it is proposed that IO-NPs could initiate inactivating oxidative damage to viral lipid envelopes through the generation of reactive oxygen species [88]. This mechanism of oxidative inactivation is supported by studies with other metal nanoparticles and by Qin et al. where lipid peroxidation of the influenza lipid envelope destroyed virions [84]. Antibacterial and synergistic antiviral activity can also be achieved when IO-NPs are utilized alongside other metal nanoparticles such as Ag-NPs, which demonstrates potential for use in general disinfectants and coatings. Iron oxide nanoparticles also exhibit good biocompatibility and the existing FDA approval means iron oxide nanoparticles could be repurposed with relative ease and haste into antiviral nanomaterials for safeguarding human environments and PPE.
Silver-based nanoparticles
Silver is a noble metal with an extensive history of medical uses in many cultures and a myriad of applications in current biomedical technology. Often, these applications utilize the unique microbicidal activity of Ag, which makes it an excellent sterilizing agent in wound care products, surgical devices, disinfecting filters and for protecting contact surfaces. In Japan, for example, silver thiosulfate in silica gel microspheres is often integrated into plastics from children’s toys to toilet seats for long-term microbicidal properties [93]. Silver derivatives such as AgNO3 have also been used for decades in eye drops, and topical burn creams where they provide silver ions that inhibit pathogens and prevent infection [94]. With advances in nanotechnology, pure silver nanoparticles (AgNPs) with high efficiency and chemical activity are now available for antiseptic uses. The advent of AgNPs has enabled new strategies to overcome challenges facing modern medicine, including antibiotic-resistant bacteria and epidemic viruses.
Dung et al. recently applied 14 nm AgNPs in antiseptic applications against African swine fever virus (ASFV) [95]. With no vaccine available against ASFV, disinfection is the primary method of inhibiting its virulent spread among domestic pig populations. Likewise, nanoparticle-based disinfection could be useful against SARS-CoV-2 in areas with barriers to vaccination rollout , or to augment vaccination efforts. To ensure viral inhibition without cell cytotoxicity, the researchers exposed porcine alveolar macrophage (PAM) cells to various concentrations of AgNPs and assessed cell death. The highest non-toxic concentration to PAM cells was found to be 0.78 ppm AgNPs. At this concentration, AgNPs showed potent antiviral activity and completely inhibited all viral titers (infectious unit per mL) of less than or equal to 103 50% hemadsorbing doses (≤103 HAD5 0) from infecting PAM cells. They suggest that AgNPs can bind to and disable areas of ASFV’s membrane proteins, such as the binding domains of glycoproteins, thereby preventing viral penetration into host cells. Dung et al. have also shown the biocidal activity of AgNPs against a range of microbes, including Salmonella enterica, E. Coli, Vibrio cholerae, and Coliform bacteria and fungi [96], [95]. Smaller particles, ~5 nm with a narrow size distribution formed by reverse micelle techniques, were able to 100% inhibit bacteria cultures at a concentration of 3 ppm and fungi at 15 ppm after 30 min of exposure [96], showing the effectiveness of AgNPs across a broad range of pathogens.
The binding of AgNPs to inactivate membrane proteins is also supported by research into other enveloped viruses such as hepatitis B [31] and H1N1 influenza A [97]. Lu et al. found that AgNPs of sizes 10 nm and 50 nm are inhibitors of hepatitis B virus (HBV) replication [31]. Using a real-time PCR method [98] to compare the HBV DNA content from AgNP treated and untreated hepatoblastoma cells, they quantified dose-dependent viral inactivation after 48 h of incubation. 10 nm AgNPs showed 38% inhibition at 5 μM and 80% viral inhibition at 50 μM, while 50 nm AgNPs showed 53% and 92% inhibition at the same concentrations, respectively. They propose that there is a high binding affinity of nanoparticles for HBV DNA and extracellular virions. This direct binding, which was determined using a UV–vis absorption titration assay, could inhibit nucleotide replication through DNA damage and disable virions by membrane degradation [31]. Regarding toxicity, this study also found that larger nanoparticles (800 nm) presented significantly higher cytotoxicity in cell culture compared to the 10 and 50 nm NPs. Similarly Xiang et al. showed that Ag NPs with a range of sizes between 5 nm and 20 nm can provide enhanced protection against influenza virus infection without the risk of cell toxicity, at an optimal concentration [97].
The targeting of viral glycoproteins particularly through the disulfide bonds is explored by Lara et al. in their study examining the efficacy of AgNPs against HIV-1 [25]. They found that silver nanoparticles attach to and disrupt cysteine-cysteine disulfide bond regions on the CD4 binding domain of the HIV-1 glycoprotein gp120 [25]. The carboxyl half of the gp120 CD4 binding domain has two disulfide bonds that represent vulnerable targets with which silver ions can form complexes [99]. Specifically, silver ions can bind sulfhydryl groups on cysteine residues to disrupt disulfide bonds. The result would be denaturation due to changes to the protein fold of gp120, which inactivates it and blocks the virus from fusing with target cells through the CD4 receptor [94]. Kim et al. also observed such a mechanism in Ag NPs targeting influenza A virus [100]. A study by Siriwardana et al. supports the ability of silver nanoparticles to bind and cleave disulfide bonds [101]. Using surface-enhanced Raman spectroscopic (SERS) measurements, they find that cysteine binds to silver nanoparticles initially through its carboxylate group before forming a thiolate. This interaction eventually forms an S—Ag bond that cleaves the S-S disulfide bond [101]. This disruption of disulfide bonds would fundamentally change the protein fold of a glycoprotein and may inhibit its function in attaching to target receptors. An inactivating mechanism targeting disulfide bonds in glycoproteins would be highly relevant to combating SARS-CoV-2, which also relies on its spike glycoprotein to bind to ACE-2 receptors.
Galdiero et al. provides a review of the literature on AgNPs as antiviral agents upto 2011, summarizing that for enveloped viruses, AgNPs inhibit the copy process of viral nucleotides in infected cells, which consequently prevents viral replication. This is a result of silver cations (Ag+) generated by the AgNPs or surface binding to electron donor groups such as sulfur, oxygen, and nitrogen that are found as thiols or phosphates on viral glycoproteins, enzyme factors and genetic structures. When proteins and enzymes form complexes with silver, their protein folding changes, which can cause denaturation that incapacitates essential viral functions such as RNA replication and vesicle formation for cell entry. Fig. 8 illustrates a typical viral life cycle and several antiviral mechanisms of Ag nanoparticles [27].
Fig. 8.
Schematic model of a virus infecting an eukaryotic cell and a snapshot of the antiviral mechanisms of Ag nanoparticles. Reproduced from Ref. [27] under CC BY 4.0.
Additionally, Lara et al. conducted a time of addition experiment to discover that AgNPs reduced infectivity in a variety of HIV-1 strains, including those with resistance to antiretroviral drugs [25]. When compared to the antiretrovirals, which each target only a particular stage of the HIV life cycle such as entry, retrotranscription, or gene integration, AgNPs exhibited sustained antiviral activity at every stage, suggesting broader inhibitory mechanisms. Beyond targeting of gp120-CD4 in HIV-1 to prevent cell entry, they observe that AgNPs suppress the expression of TNF-α, an essential cytokine for HIV-1 replication that modulates proper RNA transcription [25], [94]. This suggests that AgNPs can target both cell entry and retrotranscription steps in the HIV-1 replication cycle, and this variety in targets decreases significantly the likelihood that AgNP resistant strains may emerge.
Huy et al. synthesized AgNPs using an electrochemical method and found that the AgNPs could also inhibit non-enveloped viruses at non-toxic, low concentrations [102]. To elaborate, they found that AgNPs at a concentration of 3.13 ppm were able to almost completely inhibit poliovirus particles, after 30 min for viral concentration of the 50% tissue culture infectious dose (1TCID50) and after 60 min for viral concentration 10TCID50. The AgNPs were found to be biocompatible and safe for human rhabdomyosarcoma (RD) cells at concentrations up to 100 ppm while being toxic to poliovirus. The researchers found reduced cytopathic effect and cell death when infected RD cells were incubated with NPs compared to non-treated controls. The high cell viability presents possible inhibitory effects of AgNPs on viral binding and reproduction. Huy et al. hypothesized that the small size of AgNPs at about 7.1 nm and their high specific surface area enabled them to react readily with and prevent viral binding to RD cells [102]. Chen et al. showed that silver nanoparticles can directly damage the protein capsid of adenovirus type 3 [103] using TEM, and it is likely that AgNPs could cause similar disruption of poliovirus proteins [102]. These studies reinforce the broad antiviral capabilities of silver nanoparticles against both enveloped and non-enveloped viruses.
In addition to pure silver nanoparticles, bulk forms and nanoparticles of silver compounds have also been found to be functional antivirals. For instance, Du et al. examined the antiviral mechanism of Ag2S nanoclusters (NCs) against coronaviruses with porcine epidemic diarrhea virus (PEDV) as a model [104]. The Ag2S NCs were produced with glutathione as a capping reagent and had an average size of 5.3 nm. After 12 h of exposure, Ag2S NCs were determined to significantly suppress PEDV titers by 3 log (99.9%) in plaque assay remaining non-cytotoxic at 46 μg/mL [104]. The researchers’ analysis of the antiviral mechanisms indicated that Ag2S NCs treatment inhibits the synthesis of viral negative-strand template RNA and blocks viral budding, preventing cell-cell transmission. They also found that Ag2S NCs stimulated the innate immune response to combat viral infection by activating the production of interferon-stimulated genes (ISGs) and the expression of proinflammatory cytokines in Vero cells [105]. The effectiveness of a silver nanomaterial in this study is notable because PEDV and SARS-CoV-2 share a very similar makeup being in the same coronavirus family with shared components such as positive-sense single-stranded RNA, spike proteins, and an envelope. The results, therefore, suggest that anti-SARS-CoV-2 agents could be developed from silver-based nanomaterials.
Though Minoshima et al. found that solid-state silver compounds such as Ag2S display little antiviral efficacy against enveloped influenza A and non-enveloped bacteriophage Qβ [106], they observed that water-soluble silver compounds, AgNO3 and Ag2O, did show significant antiviral action against influenza with exceptional degradation of neuraminidase [106]. The mechanism behind the spike protein degradation is again the breakage of disulfide bonds, which are essential for proper protein folding and function. The effect on disulfide bonds was determined using the technique developed by Koide et al. [107] where monobromobimane fluorescence of a trypsin inhibitor model protein in phosphate buffer is used to detect thiol concentration. Trypsin inhibitor protein has two disulfide bonds between thiol groups and shows less fluorescence when these bonds are broken. The largely ineffective Ag2S did not affect the thiol concentration, while the water-soluble compounds AgNO3 and Ag2O, which dissociated silver ions, showed significantly reduced fluorescence, indicating the breakage of disulfide bonds in the trypsin model protein [106]. It is suggested that silver ions denature proteins by breakage of S-S bonds according to the following reactions [108], [109]:
(R—S—S—R and R—S—H represent disulfide (S-S) bond and cysteine residues, respectively, in trypsin inhibitor protein).
The results substantiate the disulfide cleaving mechanism for the effect of pure AgNPs [101], [25]. Further, it proves that ions sourced from compound nanoparticles or bulk coatings also have the ability to break disulfide bonds in glycoproteins to disrupt their function and ability to infect cells.
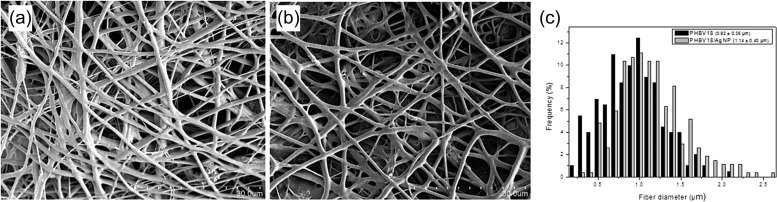
Given the prevalence and utility of plastic-based products in modern society, another approach for virus inactivation is to produce composite polymers incorporating nanoparticles that can self-sterilize against pathogens. Castro-Mayorga, et al. explored the antiviral properties of silver nanoparticles against norovirus surrogates, murine norovirus (MNV) and feline calicivirus (FCV), when integrated into a polyhydroxyalkanoate bioplastic film ( Fig. 9) [110].
Fig. 9.
SEM images of electrospun fibers, (a) without AgNP (PHBV18), (b) with AgNP (PHVB18/AgNP), and (c) size distribution of fibers.
Reprinted from Ref. [110], with permission from Elsevier.
A homogeneous distribution of 0.27 ppm AgNPs within poly (3-hydroxy butyrate-co-3-hydroxyvalerate) (PHBV) films was achieved by depositing a coating of thermally post-processed electrospun PHBV18/AgNP fiber mats over compression molded PHBV3 films [110]. The two norovirus surrogates were inoculated onto the AgNP fiber films with 100% relative humidity at 25 °C and 37 °C following an adapted ISO Standard 22196:2011 [111], [112]. Subsequently virus inactivation was measured in cell culture based on the formation of cytopathic effects, followed by the quantification of the viruses by plaque assay, at 50% tissue culture infectious dose (TCID50). After 24 h of exposure at 37 °C, FCV was inactivated entirely, and MNV infectivity decreased by 0.86 log TCID50/mL. In the same conditions, the bacteria S. enterica and Listeria monocytogenes were also completely inactivated after contact with the film. At 25 °C, viral inactivation was less potent with FCV and MNV titers only decreasing by 1.42 and 0.14 log TCID50/mL, respectively. The proposed mechanisms include AgNP driven denaturation of protein capsids and a synergic effect with nanoparticle-generated Ag+ ions that displace essential bonds in the viral structure. The low concentration integration of AgNPs into the PHBV film provided virucidal activity against norovirus surrogates with negligible alteration to optical and mechanical properties [110]. The effectiveness of silver-infused plastics against FCV demonstrated by Castro-Mayorga et al. [110] is supported by a similar study by Martínez-Abad et al. [113], who used solvent casting to integrate silver ions into polylactide plastic. They also found that after 24 h of exposure, FCV was reduced by 2 log TCID50/mL on 0.1% silver films and fully inactivated on 1% silver films [113]. Thus, the use of silver for antiviral polymers is possible with potential applications in custom packaging and contact surfaces.
Another approach to an antiviral environment was conducted by Park et al., who developed and tested micrometer-sized magnetic hybrid colloids (MHCs), which integrate AgNPs on their surfaces. They evaluated the efficacy of AgMHCs for inactivating bacteriophage ϕX174, murine norovirus (MNV), and adenovirus serotype 2 (AdV2) in solutions of tap water and surface river water [114], [115]. Ag-MHCs with 30 nm silver nanoparticles (silver content: 400 ppm) were found to be the most effective, reducing the infectivity of phage ϕX174 and MNV 2 log after exposure to 4.6 × 109 particles/mL for 1 h at 25 °C. The authors describe the primary antiviral mechanisms of the MHCs as the creation of complexes with thiol groups on viral coat proteins and ROS catalysis. In the former, the nanoparticles coating the MHC bind with viral surface structures such as the glycoproteins or capsid, which chemisorbs virions to prevent them from infecting cells. In the latter, silver is involved in aerobic reactions that generate free radicals, which in turn, causes oxidative damage to viral biomolecules [116]. Further, another study conducted by their group found that Ag-MHCs caused E. coli reductions of more than 6 log CFU/mL [115]. These results suggest that AgNP-MHCs can be applied for disinfection of bacteria and viruses in mediums such as sprays or surface coatings while minimizing environmental pollution since they can be magnetically recovered.
It is also possible to form composite material systems where both the nanoparticle and the support are active against viral bodies. Chen et al. demonstrated that graphene oxide sheets with silver nanoparticles lowered the infectivity of enveloped feline coronavirus and non-enveloped infectious bursal disease virus, with low cytotoxicity [117]. They found that silver nanoparticles conjugated with sulfur groups in viral proteins to break disulfide bonds while the negatively charged graphene oxide interacted with positively charged lipids in enveloped viruses to cause membrane rupture. Therefore, the antiviral activity of the composite was enhanced relative to each component on their own [117].
Takeaway and applicability to SARS-CoV-2
Silver was among the most studied nanoparticles in the published literature, and this is for good reason, given its exceptional virucidal and bactericidal effectiveness against a large range of viruses and potential for applications in self-sterilizing materials. Across these papers, the antiviral mechanisms of silver nanoparticles have centered around its ability to directly bind and react with chemical groups on viral lipid membranes and protein structures, as well as provide competition for the binding of the virus to the cell. Silver, in both nanoparticle and ionic form, can inactivate viruses by disrupting membranes and denaturing enzymes via reactions with sulfhydryl, amino, carboxyl, phosphate, and imidazole groups [94], [118], [119], [117]. The recurring factors that affect antiviral activity are nanoparticle size and concentration with a high concentration generally correlated with greater efficacy and an optimal size range of 5–15 nm [27], [25], [94], [110], [95], [97], [96], [102], [119]. Larger nanoparticles in the sizes of 30 nm and 50 nm were also found to be potent, but there was an observed trend of broader spectrum activity and lower cytotoxicity for smaller Ag nanoparticles, possibly due to their ability to more easily bind virions [27], [24], [31]. Analysis of the SARS-CoV-2 spike protein conducted by Shang et al. shows that it also contains disulfide bridges between cysteines that are essential to its structure and operation [120]. Therefore, the proven antiviral mechanism of silver and silver compound nanoparticles against HIV-1, influenza A, and particularly PEDV may transfer to the inactivation of SARS-CoV-2 through the disruption of bonds in its spike protein [101], [100], [25], [104]. The potential of silver nanoparticles for broad-spectrum antiseptic surface coatings is further enhanced by their bactericidal effect against burdensome multi-drug resistant bacteria such as Methicillin-resistant Streptococcus aureus (MRSA), Pseudomonas aeruginos, ampicillin-resistant E. coli O157: H7 and erythromycin-resistant S. pyogenes [25], [121].
Copper-based nanoparticles
The first recorded use of copper for its antimicrobial properties is found in the Smith Papyrus, an Egyptian medical text dated between 2600 and 2200 B.C [122]. It records how people discovered copper’s utility in sterilizing chest wounds and storing safe drinking water. In the 19th century, copper’s medical potential became more widely recognized by the observation that copper workers were far less susceptible to cholera in the 1832 and subsequent outbreaks in Paris, France [122]. Since then, scientific research into copper has enabled a more complete understanding of the mechanism for copper as a natural microbicide, and it has become an important metal with many applications such as sterile touch surfaces and medicines [122], [123], [124], [125]. In contrast to other nanoparticle materials such as gold, silver, and silica, copper is an essential trace element for regular metabolism in the human body, which makes it safe in the small dosages that might be used in Cu nanoparticle-based surface coatings [126]. Copper and several of its compounds are now promising candidates in nanomaterials research, given their versatility, availability, and relatively low toxicity. This section highlights several notable studies into the antiviral potency of copper-based nanoparticles.
Copper(I) iodide
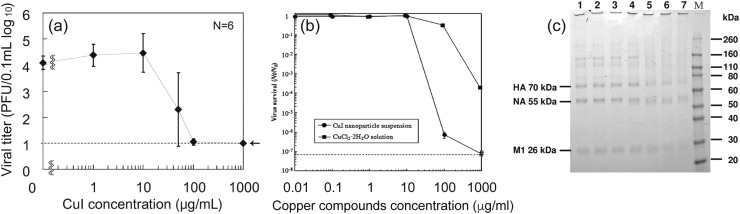
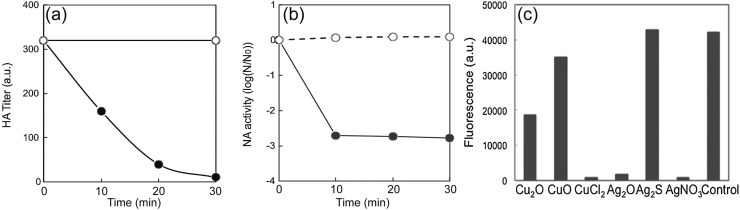
Cuprous iodide nanoparticles of various diameters have been studied for their antiviral activity against a strain of H1N1 influenza [127] and feline calicivirus (FCV) [128]. Cuprous iodide was chosen for its stability, and white color that can be easily integrated into masks, filters, and other surfaces without the black-brown discoloration associated with other copper compounds [127]. CuI of 160 nm diameter was found to present significant viral inhibition against influenza with increasing nanoparticle dose with a 50% effective concentration (EC50) of about 17 μg/mL after exposure for 60 min [127]. At CuI nanoparticle concentrations above 100 μg/mL, the viral titer declined to the lower detectable limit of plaque titration assay ( Fig. 10) [127]. The researchers also performed gel electrophoresis (SDS-PAGE) analysis on virus samples treated with different concentrations of CuI NPs. The SDS-PAGE data showed that influenza treated with at least 1 μg/mL CuI NPs (Fig. 10, Column 4) had wider protein bands with lower intensity compared to intact viruses in columns 1–3, which had defined narrow bands. The widening band trend with greater CuI concentration was consistent for the 70 kDa hemagglutinin (HA) protein, the 55 kDa neuraminidase (NA) protein, and the 26 kDa matrix (M1) protein (Fig. 10c). Therefore, the data confirm the degradation of influenza proteins, including the spike proteins, hemagglutinin and neuraminidase by the CuI nanoparticles. With regards to the inactivation of FCV, 60 min of exposure to CuI NPs reduced infectivity of FCV to Crandell-Rees feline kidney (CRFK) cells by 6 log (99.9999% reduction) when exposed to a concentration of 100 μg/mL and reduced below the lower detection threshold of plaque assay at 1000 μg/mL CuI nanoparticles (Fig. 10) [128]. Further, CuI nanoparticles were compared to a CuCl2·2H2O solution generating Cu ions alone, and the nanoparticles were found to have superior antiviral activity.
Fig. 10.
(a) Inactivation of influenza (105 PFU/0.1 mL) with various concentrations of CuI suspensions in phosphate-buffered saline (PBS) after exposure for 60 min. The dotted line indicates the lower detection limit. (n = 6 samples per plot). Reproduced from Ref. [127] with permission from American Society for Microbiology (b) Effect of CuI nanoparticles (circle) or Cu2+ (square) on feline calicivirus (FCV) infectivity in Crandell-Rees feline kidney (CRFK) cells. FCV cells (1.3 × 108 PFU mL−1) were subjected to various concentrations of CuI nanoparticles and CuCl2·2H2O for 60 min. Dotted line represents a detection limit. Reprinted from Ref. [128], with permission from Elsevier. (c) Degradative effects of CuI on A/H1N1pdm. The data represent SDS-PAGE gel results (CBB stain). Concentrations of CuI in PBS are the following lane 1 0 μ/mL (only PBS); lane 2 0.01 μ/mL; lane 3 0.1 μ/mL; lane 4 1 μ/mL; lane 5 10 μ/mL; lane 6 100 μ/mL; lane 7 1000 μ/mL. M indicates the molecular mass marker. HA, hemagglutinin; NA neuraminidase; M1, matrix protein. Reproduced from Ref. [127] with permission from American Society for Microbiology.
Electron spin resonance (ESR) spectroscopy was used to determine the mechanism of viral inactivation by CuI NPs [127], [128], which showed that CuI NPs dissociate into Cu+ and generate highly reactive hydroxyl radicals (OH-) in phosphate-buffered saline. Such ROS are known to cause oxidative damage to biological molecules resulting in nucleic acid mutations and random amino acid modifications [39], [40]. Production of OH- is suggested to occur through a multistep Fenton-like reaction [129], [130]:
This generation of OH- by CuI is suggested by Fujimori et al. to be responsible for the observed spike protein degradation in influenza [127]. Although not directly measured, peroxidation of the lipid membrane from Cu+ and ROS reactions are also known to contribute to viral inactivation [131], and could be a mechanism for influenza deactivation. For FCV, amino acid oxidation within the viral capsid proteins was confirmed by nano LC-MS analysis [128]. Therefore, the mechanism of inactivation for non-enveloped viruses appears to be predominately NP generated Cu+ ions reacting to produce ROSs that inflict oxidative damage to the protein capsid [127], [128].
As respiratory viruses, influenza and coronaviruses share structural characteristics such as a lipid envelope with spike glycoproteins that bind target cells. Thus, with demonstrated impairment of influenza and viral proteins, it is possible that CuI nanoparticles will exert similar antiviral effects against SARS-CoV-2 and other similar viruses.
Copper sulfide
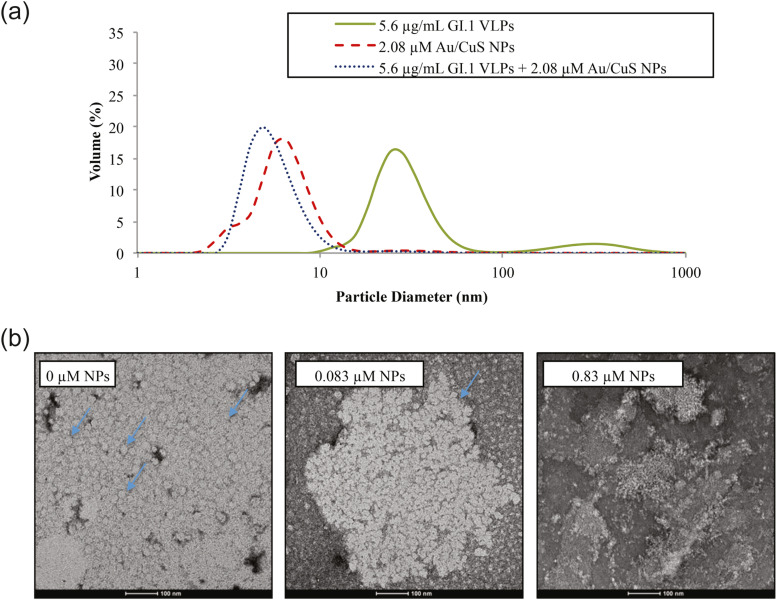
Broglie et al. authored one of the only papers investigating the antiviral potential of copper sulfide shell/gold core nanoparticles (Au@CuS NPs), specifically for inhibiting human norovirus [132]. The researchers hypothesize that nanoparticles can act as potent virucides due to their high specific surface area that enables them to directly disrupt viral membranes and denature proteins. Au@CuS NPs of 2–5 nm were mixed in tiered concentrations with 0.37 μg/mL of non-infectious norovirus-like particles (VLPs), and antiviral activity was measured using an ELISA method [133]. After 10 min of exposure, Au@CuS nanoparticles were able to inactivate about 50% of the VLPs at 0.083 μM NP concentration. At ten times higher concentration of 0.83 μM, the NPs were able to achieve 100% inactivation of the VLPs after 10 min. In addition to concentration, contact time was found to be a key factor in antiviral effectiveness, with the lower 0.083 μM NP dose also achieving 100% VLP inactivation after 60 min. Subsequent analyses with Western blot and dynamic light scattering showed a 86–95% reduction of a 32 kDa P domain protein and a decline of average particle size from the 38 nm size of VLPs before treatment to fragments of<10 nm after NP exposure, respectively ( Fig. 11a). These results suggest that Au@CuS NPs caused the breakdown of VLP proteins into smaller fragments. TEM imaging of the VLPs supported this conclusion as physical degradation and capsid rupture was observed after NP contact (Fig. 11b).
Fig. 11.
(a) DLS measured mean size of particles in treated and untreated VLP solutions. Green represents the average size of untreated VLPs. Red dashed represents the size of Au@CuS NPs. Blue dotted represents the size of viral fragments and NPs after treatment of NPs. (b) TEM images of untreated VLPs and VLPs treated with 0.083 μM and 0.83 μM Au@CuS NPs for 30 min. Arrows indicate examples of intact VLPs which are lighted colored circles and aggregated together; Treatment with 0.083 μM NPs caused VLPs to entirely break down into fragments and no intact VLP can be seen. Reproduced from Ref. [132] under CC BY 4.0.
The authors proposed mechanisms including contact binding, which compromises the VLP capsid integrity and diffusion of NPs or Cu2+ ions across the viral capsid. In the latter case, the NPs and Cu2+ are proposed to cause RNA and protein damage through chemical reactions that induce ROS formation. The central role of ROS in viral inactivation by nanoparticles is consistent with other Cu-based NP studies [127], [128], [134], [135]. Though the nanoparticles in the Broglie et al. study were synthesized by a two-step method by first growing gold NPs using a seeded growth method and then coating these cores with CuS nanoshell [136], they suggest that the CuS shell is most likely the active component and that the gold core could be substituted or even pure CuS nanoparticles could be used instead with similar effectiveness [132].
Copper oxides
A wide variety of studies have examined the anti-septic properties of copper oxides with several mechanisms proposed for the viral inhibition observed:
-
•
Interference with viral replication stages such as transport into the cell nucleus or during gene replication and packaging [135].
-
•
Nanoparticles disrupt viral proteins that enable viral egress into the nuclear membrane and prevent cell enzymes from building new virions [137].
-
•
ROS generation by free copper ions released from the nanoparticles, which precipitate inactivation via oxidation for loss of envelope or capsid integrity, and degradation of the viral genome [138], with denaturation of DNA and damaged cell integrity [134].
-
•
Damage to viral proteins through the breakage of disulfide bonds, which are essential for proper protein folding and function [106].
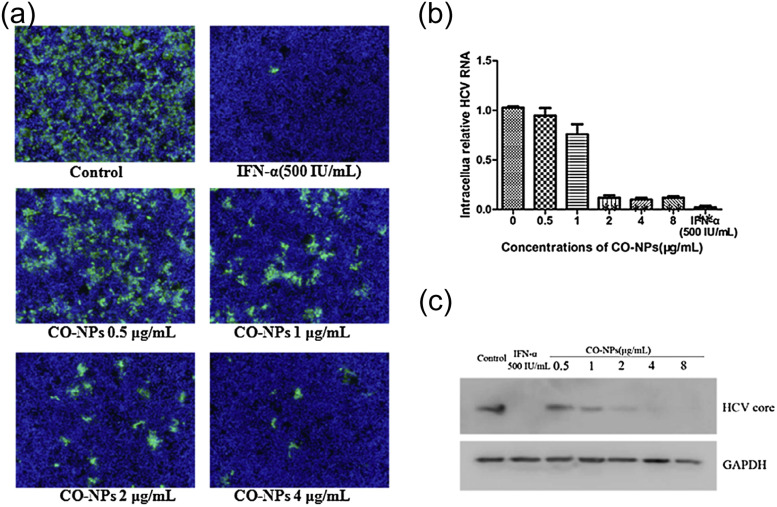
Both Cu(I) and Cu(II) oxides have been shown to be effective anti-viral agents. Hang et al. for example investigated the potential of cuprous oxide (Cu2O-NPs) nanoparticles as an inhibitor against hepatitis C virus (HCV) [139], a pathogen expected to become resistant to existing antiviral drugs due to its high mutation rate. Cu2O-NPs were studied in cell culture with Huh7.5.1 cells, and antiviral efficacy was evaluated through immunofluorescence assay, qRT-PCR count of HCV RNA, and Western blot analysis. The Cu2O-NPs with an average size of 45.4 ± 6.8 nm, as determined by TEM, were found to significantly inhibit HCV infection at a concentration as low as 2 μg/mL, while not being cytotoxic to Huh7.5.1 cells at NP concentrations of ≤16 μg/mL even when incubated for 72 h [139]. Immunofluorescence assays consistently showed fewer positive HCV-infected Huh7.5.1 cells after treatment with Cu2O-NPs compared to the untreated control ( Fig. 12a). Compared to the control, qRT-PCR also found that HCV RNA levels were reduced by 18%, 88%, and 90% following Cu2O-NP doses of 1, 2, and 4 μg/mL respectively (Fig. 12b). Finally, when HCV core protein expression was analyzed by western blot assay, the Cu2O-NP concentrations that effectively inhibited HCV RNA levels also caused reductions in viral core proteins, suggesting degradation by the NPs (Fig. 12c) [139].
Fig. 12.
Copper oxide NPs inactivate HCV. (a) Immunofluorescence images of Huh7.5.1 cells at 72 h post-infection. Cells were stained with HCV-positive serum treated with tiered concentration of copper oxide NPs (b) copper oxide NPs reduce HCV RNA in a dose-dependent manner; RNA content was determined by qRT-PCR. (c) Western blot analysis of HCV core protein expression showing significant degradation compared to GAPDH antibody loading control.
Reprinted from Ref. [139] with permission from Elsevier.
When analyzing the antiviral mechanism with a time of addition assay, the researchers found that Cu2O-NPs did not prevent the later stage HCV replication within cells but rather interfered with the early steps of the attachment and fusion of infectious virions into hepatic host cells. Therefore, the determined mechanism is that Cu2O-NPs with larger diameters than the HCV virions (>60 nm) inhibit virus-cell binding by polyvalent binding of virions [139]. Since HCV is an enveloped virus, it utilizes glycoproteins on its surface envelope to bind specific receptors on the host cell [140], [141], [142], [143]. Following attachment, the virus fuses with the host cell membrane to form a vesicle and then releases its genetic material and enzymes into the cytoplasm. Through this study, Hang et al. demonstrated that Cu2O-NPs block the HCV attachment to the host cells and inhibit the viral entry step to prevent infection. Cu2O-NPs may interact with the virion surface to result in permanent blocking of receptor binding domains on HCV envelope glycoproteins, thus inhibiting the virus’s ability to infect hepatocytes through CD81 and CLDN1 co-receptors [144]. This mechanism presents the possibility that Cu2O-NPs could bind similarly to the spike glycoprotein of SARS-CoV-2 to functionally inactivate it from infecting cells through the ACE-2 receptor. Therefore, cuprous oxide nanoparticles present a promising option for a novel antiviral agent and warrant further research for practical applications.
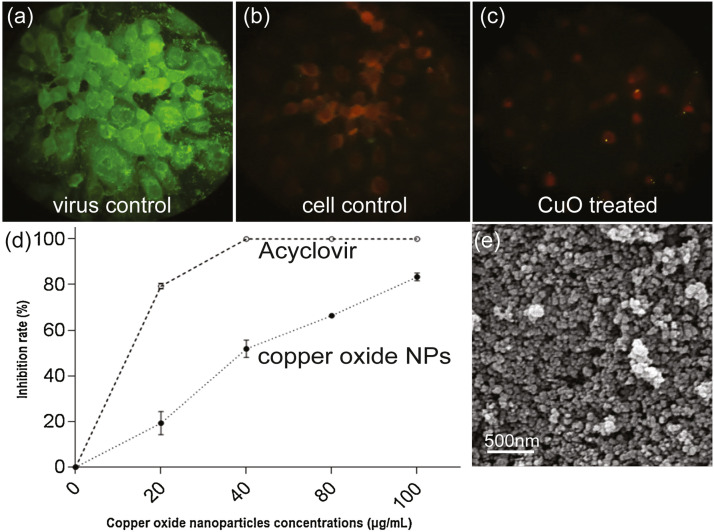
On the other hand, Tavakoli et al. examined the antiviral ability of similarly sized (40 nm) cupric oxide nanoparticles (CuO-NPs) against herpes simplex virus type 1 (HSV-1), while considering the cytotoxicity of nanoparticles to the cells themselves. TCID50 assay of infected cells treated with tiered concentrations of CuO-NPs was conducted to determine antiviral effectiveness. They found that CuO nanoparticles exhibit substantial antiviral properties with an 83.3% inhibition rate of HSV-1 at the most effective non-toxic concentration of 100 μg/mL [135].
The CuO nanoparticles were also compared to the established drug Acyclovir ( Fig. 13) [135]. Although the CuO-NPs were not as effective as the synthetic drug against HSV-1 (83.3% vs 100% at a 100 μg/mL dose), there remained substantial viral inhibition at higher NP concentrations, which supports the use of NPs in combating novel drug-resistant HSV-1 strains.
Fig. 13.
Evaluation of effect of CuO-NPs on HSV-1 antigens expression on Vero cells using the immunofluorescence assay (IFA). (a) Virus control, (b) Cell control, (c) HSV-1 infected cells treated with 100 μg/mL CuO-NPs. Intensity of fluorescence signals in HSV-1 infected cells treated with CuO-NPs was significantly decreased as compared with virus control, indicating a strong antiviral activity of CuO-NPs on the expression of HSV-1 antigens. In C, the green dots are viral antigens expressed in the different cell compartments, which were stained with the goat anti-human IgG conjugated with fluorescein isothiocyanate (FITC). (d) The impact of CuO-NPs against HSV-1 viral load, determined by real-time PCR. CuO-NPs at the concentrations of 40, 80, and 100 μg/mL led to 51.8%, 66.3%, and 83.3% inhibition rates. Compared with established Acyclovir antiviral drug. (e) FE-SEM image of CuO-NPs (magnification 70k×). Structural and morphological evaluation of CuO-NPs revealed a uniform particle size and shape distribution with nearly spherical shape with an average size of 40 nm.
Adapted from Ref. [135], with permission from Elsevier.
Regarding the difference between cupric oxide (CuO) and cuprous oxide (Cu2O) for antiviral activity, several studies have sought to compare the two copper oxidation states and determine the optimal compound. For example, Mazurkow et al. conducted zeta potential measurements of the two compounds and found that Cu2O had a higher isoelectric point of 11.0 compared to 7.4 for CuO [145]. The isoelectric point of Cu2O means it has a higher positive surface charge at around pH 7 where the researchers evaluated antiviral activity. This positive surface charge enabled Cu2O filters to better absorb and inactivate bacteriophage MS2 due to electrostatic interactions. Similar results were reached by Sunada et al. who determined that Cu2O loaded onto a glass substrate was able to reduce bacteriophage Qβ infectivity by five orders of magnitude (99.999% reduction) within 30 min while CuO coated glass was not able to achieve a significant reduction in the same time frame [146]. Further, the antiviral effect of Cu2O against bacteriophage Qβ was found to exceed that of silver ions from a silver-powdered glass substrate. The proposed mechanism is that direct contact with the surface of solid-state cuprous compounds causes more efficient denaturation of biomolecules in viruses, but the difference with CuO is not fully explained [146].
Minoshima et al. also found that Cu2O is a more effective antiviral than CuO against both enveloped influenza A and non-enveloped bacteriophage [106]. Against titers of influenza glycoproteins, neuraminidase and especially hemagglutinin, solid-state cuprous oxide (Cu2O) was able to significantly degrade the proteins within 30 min based on red blood cell and chemiluminescence assays, a finding which aligns with the anti-influenza efficacy of cuprous iodide (CuI) determined by Fujimori et al. [127]. However, cupric oxide (CuO) did not substantially affect the titers of either protein ( Fig. 14). Cu2O also exhibited rapid inactivation of bacteriophage with a 6 log (99.9999%) reduction after 30 min while cupric compounds CuO and CuCl2 only achieved 0.088 log and 0.24 log reductions, respectively. These results show significant contrast between cuprous and cupric compounds and suggest that cuprous copper (Cu(I)X) compounds are effective against both enveloped and non-enveloped viruses by targeting viral surface proteins [106].
Fig. 14.
Hemagglutinin (HA) titer and neuraminidase (NA) activity exposed to Cu2O and CuO suspensions. Effect on (a) HA titer and (b) NA activity of Cu2O (filled circles) and CuO (open circles) as determined by a hemagglutination test and chemiluminescence using the NA-Star method, respectively. Cu2O shows significantly greater degradation of the proteins. (c) Level of fluorescence, corresponding to total thiol (R-SH) from trypsin inhibitor protein, after incubation with different copper and silver compounds. Incubations were carried out for 4 h with each compound suspension (2.8 mmol). A control without compound was included.
Adapted from Ref. [106], with permission from Elsevier.
By comparing the effect of various copper compounds to those of silver, Minoshima et al. proposed that the mechanism behind the glycoprotein degradation was the breakage of disulfide bonds, which are essential for proper protein folding and function [106]. Using monobromobimane fluorescence of a trypsin inhibitor model protein in phosphate buffer to detect thiol concentration [107], they found that CuO did not affect the thiol concentration; Cu2O was able to reduce fluorescence by half, suggesting weak but still present interactions with disulfide bonds; and water-soluble CuCl2 with dissociated copper ions showed significantly reduced fluorescence, indicating the breakage of disulfide bonds in the trypsin model protein [106]. It is suggested that copper denatures proteins by cleavage of disulfide bonds similar to the mechanism for silver nanoparticles discussed previously, according to the following reactions [108], [109]:
(R—S—S—R and R—S—H represent disulfide (S-S) bond and cysteine residues, respectively, in trypsin inhibitor protein).
Therefore, copper ions sourced from nanoparticles or bulk coatings also have the ability to break disulfide bonds in glycoproteins to disrupt their function and ability to infect cells.
Due to their proven efficacy, low cytotoxicity, and relatively low cost as a coinage metal, the incorporation of copper oxide NPs into self-sterilizing structures, including biodegradable antiviral polymers and woven fibers has been a rapidly developing area. Castro-Mayorga et al. examined two electrospun PHBV polymers that were enriched with 0.1% or 0.05% CuO nanoparticles as antiviral biodegradable polymers [110], [147]. In an antiviral assay against murine norovirus [111], [112], the 0.1% and 0.05% nanoparticle films achieved 1.83 and 3.19 log TCID50/mL reductions of viral infectivity, respectively [147]. After MNV was exposed to the coated complex for a full 24 h at 25 °C, no infectious virus was detected.
Borkow et al. [148] examined the potential of copper-based nanoparticles for a self-sterilizing antiviral face mask by impregnating Cu2O NPs into a 4 layer N95 mask following established methods [149], [150]. The mask was exposed to H1N1 in a simulated breathing apparatus, and no infectious virions were recovered after 30 min, showing potent inactivation of human influenza A ( Fig. 15) [148]. The presence of Cu2O NPs within the mask fibers effectively disabled any trapped virions without altering the mask’s filtration properties or compliance of the NIOSH N95 standard. Compared to control N95 masks, the antiviral function of copper-interwoven masks was greater by five orders of magnitude [148]. Fujimori et al. also claimed to have coated non-woven fibers with CuI nanoparticles while maintaining a white color and antiviral potency, though the data was unpublished [127]. The potent antiviral action of CuI NPs against influenza and feline calicivirus suggest that they could also be similarly incorporated into masks, protective equipment and surfaces.
Fig. 15.
(a) The test mask was composed of 2 external spunbond polypropylene layers [A and D] containing 2.2% Cu2O particles (weight/weight), one internal meltblown polypropylene layer [B] containing 2% Cu2O particles (w/w) and one polyester layer [C] containing no nanoparticles. (b) SEM picture and energy dispersive x-ray spectrum analysis of external layer A (c) SEM picture and EDX of internal layer B. Reproduced from Ref. [148] under CC BY 4.0.
Takeaway and applicability to SARS-CoV-2
In summary, the oxidation state of cuprous copper compounds provides greater antiviral efficacy over cupric oxide. CuI and Cu2O compounds have been demonstrated to effectively inhibit influenza, HCV, HSV, FCV, and bacteriophages on solid-state surfaces and in solution [127], [145], [146], [106]. Copper-based nanoparticles and compounds exhibit promising antiviral ability through the entire range of antiviral mechanisms including free radical generation, disulfide bond cleavage, direct membrane interaction, and competitive inhibition of receptors [127], [128], [132], [139], [135]. In contrast to the optimal sizes for silver and zinc nanoparticles, the size range for effective copper nanoparticles against enveloped viruses in the literature (40–160 nm) was found to feature larger nanoparticles on average [127], [139], [135]. In regards to real-world applications, copper is generally considered safe for human contact, being non-irritating to the skin and, unless consumed, is highly non-cytotoxic [151]. Therefore, the element has been incorporated into antimicrobial fabrics, polymers, and self-sterilizing PPE [147], [148], [150], some of which have achieved FDA or US government Environmental Protection Agency (EPA) approval [152], [153], [154] and already exist as commercial products [155], [156].
Throughout the discussed studies, oxidative damage to biomolecules caused by ROS is a common antiviral mechanism of copper nanoparticles. The generation of ROS, including hydroxyl radicals, is facilitated by copper as a catalyst in Fenton and Haber–Weiss like reactions [127], [129]. Hydroxyl radicals will rapidly bond with biomolecules to resolve unpaired electrons resulting in lipid peroxidation, protein misformation, and genetic damage. As a lipid enveloped RNA virus with spike and capsid proteins, SARS-CoV-2 is susceptible to oxidative damage from copper nanoparticles and ROS [47], [76]. Furthermore, the copper and free radical antiviral mechanism can also specifically target the oligosaccharide glycosylation on the SARS-CoV-2 spike protein. Eguchi et al. demonstrated in their study that the free radical products of copper ions and hydrogen peroxide are able to cleave glycosidic linkages [157] in oligosaccharides monomers as well as those in glycoproteins in a hepatitis rat model [49]. Specifically, using reversed-phase high performance liquid chromatographic analysis, they found that free radicals destroyed the N-acetyl group in N-Acetylglucosamine (GlcNAc) residues. This targeting of N-linked glycosylation is notable because the glycan shield of SARS-CoV-2 is primarily N-linked with 22 sites. At these sites, many important oligosaccharides on the spike protein attach to the protein via GlcNAc at their base (Fig. 4b) including oligomannose (Man5GlcNAc2 and Man9GlcNAc2) [1]. As part of the glycan shield, oligomannose structures play a role in protecting the spike protein receptor-binding domain which SARS-CoV-2 uses to bind ACE-2 receptors (Fig. 22) [34]. If copper nanoparticles are able to generate hydroxyl radicals that target and destroy the oligomannose and other glycans by cleaving their GlcNac N-linked bonds, then the SARS-CoV-2 spike protein could be inhibited from binding to host cells and made more susceptible to antibodies [158] ( Fig. 16). Furthermore, the demonstrated ability of copper ions to break disulfide bonds is applicable to SARS-CoV-2, which has S—S bonds that maintain the proper fold of its spike protein [120], [106]. Like Ag+, copper ions interact with and form displacing bonds with disulfide and cysteine residues that could result in misfolding and denaturation of the SARS-CoV-2 spike protein [108], [109]. An inactivated spike protein would leave the virus unable to infect host cells through ACE-2. Both ROS-mediated glycosylation and disulfide breakage mechanisms of copper-based nanomaterials could prophylactically disable SARS-CoV-2. These inactivation mechanisms may explain the results seen by van Doremalen et al. [10]. Their findings revealed that copper surfaces were able to quickly disable SARS-CoV-2 with a half-life reduction in 0.774 h and a complete reduction with no viable virus detected after 4 h. The efficacy of copper exceeded that of stainless steel and polypropylene polymer [10].
Fig. 22.
The glycan shield of SARS-CoV-2 in top down and side orientations. The glycans are colored according to oligomannose content as defined by the key. ACE-2 receptor binding sites are highlighted in light blue. The S1 and S2 subunits are rendered with translucent surface representation, colored light and dark gray, respectively. The flexible loops on which the N74 and N149 glycan sites reside are represented as gray dashed lines, with glycan sites on the loops mapped at their approximate regions. Reproduced from Ref. [1], under CC BY 4.0.
Fig. 16.
Comparison of viral spike (S) protein glycan shields. From left to right, MERS-CoV S, SARS-CoV-1 S, SARS-CoV-2 S, LASV GPC, and HIV-1 Env. Site-specific N-linked glycan oligomannose qualifications are colored according to the key. Oligomannose and dense glycosylation contributes to viral immune evasion. For instance, the extreme glycosylation of the HIV Env protein protects it from antibodies and contributes to the virus’s ability to infect the immune system. Reducing SARS-CoV-2 glycosylation would alter S protein function while making it more vulnerable to recognition and neutralization in the body. Reproduced from Ref. [1], under CC BY 4.0.
The virucidal action of various Cu based nanoparticles, similar to TiO2, against human norovirus and norovirus surrogates [128], [132], [147], as well as enveloped viruses such as influenza, could be another pathway to broad spectrum disinfecting surfaces. Rapid disinfecting approaches that disable both enveloped and non-enveloped virus domains would be a major breakthrough. With EPA approval of copper surfaces for use against coronavirus [153], and incorporation of copper compounds into commercial textile products, further study of Cu-based NPs is critical to examine inactivation efficacy for SARS-CoV-2 and stability for applications in self-sterilizing surfaces.
Gold-based nanoparticles
Gold is a metal known for its stability, inert biocompatibility, and tendency to form complexes with biomolecules, all of which contribute to its antiviral properties and potential for safe sterilizing materials. As a nanoparticle, one of gold’s primary mechanisms to limit viral spread is coupling with and blocking binding receptors on viral membranes, which prevents attachment and fusion into host cells. For instance, Meléndez-Villanueva et al. observe this antiviral mechanism against measles virus (MeV) with 11 nm gold nanoparticles synthesized according to the Turkevich method [159] adapted with garlic extract (Allium sativum) as a reducing agent combined with chloroauric acid (HAuCl4) [26]. The AuNPs were characterized by surface plasmon resonance with a wavelength peak at 537 nm. In cultures of infected Vero cells, the 50% effective concentration (EC50) of AuNPs was 8.829 μg/mL, and MeV replication was actively restricted. Viral infectivity was reduced by 57.07% at the highest tested NP concentration of 10 μg/mL after 1 h. In cytotoxicity assays, AuNPS were not statistically harmful to Vero cells from tested concentrations up to 100 μg/mL. Further, PFU and qPCR tests determined that AuNPs had direct interaction with viral particles that prevented their contact with cells. Virucidal assays then measured viral load reductions of 84% after 3 h and 92% after 6 h ( Fig. 17). TEM imaging also showcased bound nanoparticles surrounding the MeV envelope (Fig. 17).
Fig. 17.
(a) Images demonstrating the virucidal effect of AuNPs, showing cellular control (CC), viral control (VC), and NP treated samples at different periods of incubation (0, 3, 6 h) (b) TEM image of a regular Measles virus. (c) TEM image of a measles virus exposed to AuNPs. Inset shows the nanoparticle that is interacting with the measles virus envelope. Reproduced from Ref. [26], under CC BY 4.0.
Time of addition studies, where the Vero cells were exposed to AuNPs at different times after MeV infection, showed that the most substantial inhibition occurred in the early stages, 15 min and 30 min after infection. The early action suggests that AuNPs act in the initial stages of the measles virus life cycle and could be inhibiting viral entry into cells. The prompt inactivation of measles viruses led the researchers to propose the mechanism of AuNPs preventing viral attachment to host cells by binding to and blocking receptors on the viral membrane. This mechanism is enabled by the garlic extract-induced positive surface charge of the AuNPs, which attracts toward the negative charge of the viral membranes where the NPs couple with and block receptors ( Fig. 18).
Fig. 18.
Schematic representation of the proposed virucidal action of gold nanoparticles reduced with Allium sativum garlic extract (AuNPs-As) on Measles virus infection. (Left) A regular viral infection starts upon virion-Vero cell receptor binding. (Right) In cells treated with AuNPs-As, the attachment step is blocked by the binding of AuNPs-As and the viral envelope which prevents infection. Reproduced from Ref. [26], under CC BY 4.0.
Overall, Meléndez-Villanueva et al. find that gold nanoparticles synthesized with garlic extract possess low cytotoxicity with an effective inhibitory mechanism against measles virus, which currently lacks an established post-infection treatment [160]. The demonstrated virucidal activity of AuNPs could make them applicable to the prevention of infections for MeV and other enveloped viruses such as SARS-CoV-2.
Functionalizing AuNPs has been shown to be effective in both reducing cytotoxicity and improving the efficacy of anti-viral activity. Cagno et al. developed an antiviral approach using non-cytotoxic gold nanoparticles coated with 1-undecanesulfonic acid [20]. These nanoparticles had long and flexible linkers that enabled them to mimic the structure of heparan sulfate proteoglycans, a highly conserved cellular target of many viruses. The nanoparticles were found to capture and exert forces of about 190 pN to irreversibly deform viruses before they could infect cells and showed high efficacy, even at low concentrations, against herpes simplex virus (HSV), human papillomavirus, respiratory syncytial virus (RSV), Dengue, and Lentivirus [20]. Papp et al. also find antiviral activity against enveloped influenza in their study examining sialic acid functionalized AuNPs of two different diameters, 2 nm and 14 nm [161]. Via hemagglutination assay, the particle size was found to be critical to antiviral effectiveness as the larger NPs were able to inhibit hemagglutination by influenza in red blood cells, while the smaller NPs did not have any effect. Additionally, when an infected cell culture was treated with 14 nm Au, the cell survival rate achieved was 60%, while the same test with 2 nm AuNPs only resulted in 5% cell survival. This substantial disparity in efficacy was attributed by the authors to multivalent effects causing the binding forces between viral surface proteins and larger AuNPs to be stronger [162]. TEM imaging was conducted of influenza virions incubated with 14 nm AuNPs, and the nanoparticles were shown to readily attach to surface structures such as the hemagglutinin spike protein (shown for larger functionalised particles in Fig. 19) [161]. The mechanism of viral inactivation is described as blocking the sialic acid receptor-binding between influenza and its target cells, which stops the fusion step of influenza pathogenesis. This paper demonstrates the importance of nanoparticle size to antiviral activity and proves that gold nanoparticles can inactivate respiratory viruses through competitive inhibition of viral fusion proteins needed for cell entry.
Fig. 19.
(a–c) Cryo-TEM images of VSV incubated with (a) 19 nm AuNP-OH, (b) 19 nm AuNP-SO4Na and (c) 52 nm AuNP-SO4Na, respectively. While the hydroxyl-functionalized gold nanoparticles did not show any visible attachment to the virus, the use of 19 nm polysulfated gold nanoparticles resulted in a multiple decoration of VSV; 52 nm polysulfated nanoparticles, on the other hand, show the formation of dense clusters consisting of virus and gold particles. Cluster formation is initiated by strong polyvalent AuNP-SO4Na/virus interaction. (d) Cryo-ET reconstruction of a 19 nm AuNP-SO4Na/VSV sample area (virions are labeled in green). Removal of the top section of the reconstructed volume affirmed the accumulation of unbound particles at the surface of the vitrified icelayer and the spatial resolution of the multiple virus decoration. The clipping plane is marked by arrows. Reproduced with permission of The Royal Society of Chemistry from Ref. [163], through CCC.
Regarding the optimal size of gold nanoparticles for antiviral effect, Vonnemann et al. found that larger ligand-functionalized gold nanoparticles (>50 nm) inhibit the vesicular stomatitis virus (VSV) up to two orders of magnitude more efficiently than smaller particles, which suggests a unique mechanism of viral inactivation. Using electron microscopy, they noted that gold nanoparticles that were equal to or larger than the virus act as efficient cross-linkers that could polyvalently bind and trap many virions. In contrast, smaller gold nanoparticles less efficiently coat the surface of individual virus particles [163] ( Fig. 20). The VSV-NP clusters formed by larger 52 nm nanoparticles have greater contact area and more interaction sites by which to attach and disable VSV virions. Although less effective, the smaller 19 nm nanoparticles were still able to reduce VSV infectivity by preventing its binding to its cellular target, namely low-density lipoprotein receptors [164]. The prominent finding from this study is that polysulfated gold nanoparticles inhibit VSV cell infection through a mechanism strongly-dependent on particle size.
Fig. 20.
Comparison of the size-dependent virus-inhibition by ligand functionalized gold nanoparticles (a) Although smaller sized gold nanoparticles decorate virions, the inhibition of virus-cell binding turned out to be inefficient. (b) Larger virus-sized gold nanoparticles induce the formation of virus-inhibitor clusters, inhibiting the virus-cell binding more efficiently. Reproduced with permission of The Royal Society of Chemistry from Ref. [163], through CCC.
Gold nanostructures of non-spherical morphologies also have unique antiviral properties and mechanisms. For example, Bawage et al. researched the antiviral action of gold nanorods (45 nm x 10 nm) against respiratory syncytial virus (RSV) [33]. The survival rate of cells infected with RSV was 82% when treated with 2.5 μg/mL of gold nanorods. Gold nanorods were suggested to inhibit viral infections primarily via stimulation of the innate cellular immune response. Porous gold nanoparticles were shown by Kim et al. [100] to decrease influenza viability by binding to disulfide bonds in hemagglutinin due to gold-thiol interactions. These interactions caused conformational changes to HA that interfered with viral entry. The survival of MDCK cells after exposure to AuNP-treated influenza (H1N1, N3N2, H9N2) improved to 96.8% compared to 33.9% survival in cells infected with non-treated virus. Carja et al. minimize cytotoxicity of AuNPs by engineering plasmonic gold and layered double hydroxide (LDH) self-assemblies (AuNPs/LDHs) [165]. AuNPs/LDHs were constructed by organizing 3 nm AuNPs on top of larger 150 nm LDH nanoparticles. The AuNP/LDH complexes showed exceptional antiviral abilities at very low concentrations and reduced the number of virions emitted from infected cells by up to 90%. The gold released from the AuNPs/LDHs composites prevented viral budding and transmission from the surface of host cells by forming a membrane sequestering layer that trapped virions. Kim et al. found that a mesoporous structure achieved through a soft-templating method [100] could improve the activity of AuNPs, allowing it to inactivate influenza A virus, regardless of genetic mutation through disulfide bond breakdown [100].
Takeaway and applicability to SARS-CoV-2
In summation, gold nanoparticles are potent antiviral agents with mechanisms that involve binding to viral surface structures to inhibit their function, similar to that observed for Ag and Cu. If gold nanoparticles are synthesized with a positive surface charge such as that demonstrated by Meléndez-Villanueva et al., then they will be electrostatically attracted to the negative residues of SARS-CoV-2 [26], [166]. The electrostatic attraction would promote greater binding of AuNPs to the viral membrane and glycoproteins, which can result in functional inactivation through receptor blockage and cluster formation, as shown by Papp et al. and Vonnemann et al. [161], [163]. Furthermore, Rai et al. have demonstrated that the virucidal potential of metal nanoparticles to a specific virus can be increased through surface modification if the viral pathogenesis and inhibitory mechanism are well defined [30]. Given established research into SARS-CoV-2 pathogenesis through spike protein- ACE-2 receptor binding and the knowledge that gold nanoparticles can bind spike proteins, the surface of AuNPs could be modified to specifically accommodate SARS-CoV-2 virions for targeted inactivation [120], [167]. However, the overall literature on AuNPs for industrial applications such as surface coatings or personal protective equipment is limited compared to other metal nanoparticles, likely due to the higher cost of gold nanoparticles limiting their practicality.
Hybrid surface coating
Given the action of various nanoparticles through different mechanisms of virus inactivation, a promising approach is to produce hybrid surfaces with more than one type of particle to increase the efficacy in self-sterilization. Hodek et al., for example, developed a hybrid surface coating containing silver, copper and zinc cations with the goal of combating viral transmission through contaminated surfaces [168]. The surface coatings were produced through radical polymerization via a sol-gel method and spread on glass slides or into the wells of polymethylmethacrylate (PMMA) plates. To test viral inactivation, the coated glass slides or PMMA plates were exposed to 10 μl droplets of several viruses such as human immunodeficiency virus type 1 (HIV-1), influenza, dengue virus, herpes simplex virus, and coxsackie virus before incubation for 5–240 min. These slides were then sampled and compared against virus samples on the equivalent uncoated control during titer determination of recovered virus [168].
Using SEM imaging to measure the coatings’ morphology, the researchers found superior adhesion to the glass slides. Consequently, the HIV-1 titer was reduced by a substantial 99.5–100% after only 20 min of exposure to the coating on glass. On PMMA plates, the inactivation was 75–100% after 20 min and 98–100% after 120 min. The inactivation of other viruses was slower on the surface coating with 240 min of exposure achieving 97% (dengue), 100% (herpes simplex) and 77% (influenza) reduction in viral titers [168]. The coating was less effective against the non-enveloped coxsackievirus with only marginal reductions after 4 h. The researchers observed that the overall properties of the hybrid surface coating depended more on the sol synthesis and curing methods than the substrate. For instance, the coatings cured at 150 °C were thinner than those cured at 90 °C, while heat polymerization created better mechanical properties than photopolymerization. Cytotoxicity is an essential consideration for surface coatings that may be used in human environments. Even though Ag+, Cu2+, and Zn2+ ions are generally more cytotoxic than their corresponding nanoparticles, the hybrid coating in this study was found to be safe with minimal viability impact on Vero and HeLa cells [168], [169]. Through repeated washings, the researchers did not find significant leaching of the metal ions suggesting that the coating stably releases ions at a rate that produces virucidal activity while being safe to human cells.
The proposed antiviral mechanism is the release of ions from the coating, including Ag+, Cu2+, and Zn2+, that react with and damage virions. As discussed previously, silver and copper ions can induce membrane lysis and bind or cleave thiol groups on proteins to compromise their function. Zinc ions have also been demonstrated to exhibit virucidal effects on viruses through protease inhibition and disruption of DNA to RNA transcription [68]. The rapid inactivation of HIV-1 by the studied surface coating suggests direct damage to the viral membrane or infectivity nullification through binding to glycoproteins. The antiviral inhibition of dengue virus is also significant because it and SARS-CoV-2 are both positive-sense single-stranded RNA viruses. Dengue virions also bind to host cell receptors through their viral envelope glycoprotein E, which contains 12 conserved cysteine residues that are connected by six disulfide bonds. Silver ions may disrupt these disulfide bonds to prevent viral endocytosis akin to the antiviral mechanism against HIV-1 described by Lara et al. [25], [94]. Copper ions may also contribute to binding disulfide bonds and cysteine residues to denature viral glycoproteins as demonstrated by Minoshima et al. [106]. Furthermore, the complete inactivation of herpes simplex virus suggests that silver may also disrupt its double-stranded DNA due to its high binding specific affinity. This mechanism was also reported by Lu et al. for silver nanoparticles against hepatitis B virus where DNA replication processes were inhibited to cease viral infectivity [31].
Finally, the small impact of the hybrid coating on the non-enveloped coxsackie virus suggests that the antiviral mechanism of metal ions is primarily driven by interactions with the viral envelope and surface structures such as glycoproteins [168]. Thus, this coating design would target the enveloped viruses, a classification which, importantly, includes SARS-CoV-2. Overall, the silver-copper-zinc hybrid coating evaluated in this study exhibits potent virucidal effect which, combined with its previously reported inhibition of MRSA bacteria, presents a promising sterile surface coating [170].
Nangmenyi et al. sought to improve antibacterial performance of iron oxide nanoparticles by conjugating a small amount of silver nanoparticles alongside the iron oxide [91]. The result was a hybrid material composed of Ag-modified Fe2O3 nanoparticles embedded in fiberglass created with aqueous hydrothermal reduction process. A 99% inactivation of 106 CFU/mL E. coli culture was observed within the first minute of contact with the Ag/Fe2O3 fiberglass. This system leveraged the strengths of both silver and iron oxide to achieve disinfection efficacy against E. coli and MS2 phage that was greater than either material alone. It should be noted that using metal nanoparticles together may also reduce the risk of pathogen resistance given the multiple inactivation mechanisms [91].
Ditta et al. also compared the antiviral activity of glass coated with thin films of titanium dioxide (TiO2), copper oxides (CuO and Cu2O), and a hybrid of copper oxides and TiO2 under UVA irradiation prepared by atmospheric chemical vapor deposition or sol-gel process [171]. Factors that affected the antiviral ability of the films included thickness to provide greater UV absorption and roughness for greater surface area. The composite coating was created by first depositing a copper oxide film of both Cu2O and CuO on a glass substrate and then a layer of TiO2. Using bacteriophage T4 as the viral model and E. Coli as the bacteria model, the CuXO/TiO2 composite coating exposed to water and UV light achieved a>9 log reduction after 80 min, which was higher than the films of only TiO2 or copper oxides [171]. The virtually complete inactivation of pathogens suggests that TiO2 photocatalysis and the reaction pathways of copper may act synergistically to disable viral suspensions. The combination of free radicals from TiO2 and solid-state contact killing from copper likely combine to provide a more efficient inactivation mechanism than either substance alone. Furthermore, copper can generate ROS as well through Fenton-like reactions that require hydrogen peroxide and superoxide as intermediates [127]. TiO2 photocatalysis with water and oxygen generates H2O2 and O and may supply these intermediates for the copper-catalyzed reactions, accelerating the production of ROS via two pathways. A combination of the two substances in a composite coating may be worth further research as well to evaluate if SARS-CoV-2 inactivation can be achieved through ROS-mediated degradation of the spike protein glycan shield. The promising result from this study supports the use of TiO2 in combination with copper oxides to enhance the effectiveness of antiviral thin films.
Another promising option for virucidal surfaces features coatings based on water-insoluble hydrophobic polycations such as N, N-dodecyl methyl-polyethylenimine (PEI). One group who has explored this method is Haldar et al., who exposed droplets of H1N1 influenza to glass slides painted with 750 kDa PEI and evaluated the viral inactivation with plaque assay in Madin-Darby canine kidney (MDCK) cells. After 30 min of exposure to the PEI slide, not a single plaque was formed in the subsequent assay, suggesting very potent antiviral activity. When the researchers retested the PEI slide with a higher initial concentration of influenza, plaques were still not observed, which indicates a reduction in viral infectivity of at least 10,000 times (4 log) [172]. To further determine the pace of viral inactivation by PEI, the experiments were redone varying the virus-slide exposure time from 1 min to 2 h. It was found that a 4 log reduction was achieved in only 5 min of viral exposure to PEI, suggesting almost instantaneous inactivation on contact. The proposed mechanism credits the many polycationic branch chains in PEI for damaging and disintegrating the viral membrane of influenza.
Hsu et al. build upon the results of Haldar et al. by applying PEI to disinfect aqueous solutions of influenza and further elucidating the antiviral mechanisms. Unlike temporary chemical disinfection methods, PEI-based coatings can render a surface antibacterial and antiviral for long time periods while being resistant to multiple washes. Expanding on previous findings, Hsu et al. applied PEI to polyethylene and polypropylene substrates in addition to glass and found that PEI thoroughly disinfected influenza solutions regardless of the surface it is on [173]. Further, PEI coatings were tested against a broader range of influenza viruses, including avian pathogenic wild-type and mutant zanamivir-resistant strains and again, complete inactivation of these strains was achieved. The inactivation was confirmed by ELISA and SDS-PAGE analysis of the PEI-exposed viral solutions, which indicated the disappearance of viral particles from solution and a 94% reduction in influenza proteins [173]. This disappearance provides clues as to the specific mechanism of PEI in disabling enveloped viruses like influenza. Namely, when viral particles come into contact with the PEI coating, they become permanently adhered to the polycationic structure via electrostatic and hydrophobic interactions. A subsequent qRT-PCR probing for influenza RNA in the PEI-treated solution found substantial quantities of viral RNA, suggesting the leakage of core RNA from trapped virions. SEM imaging confirmed the rupture of virions.
A complete model of disinfection by PEI can thus be explained as contact immobilization of virions to the polycationic surface. The lipid membrane of the virus then loses integrity and ruptures through the reversal of phospholipids caused by hydrophobic and electrostatic interactions ( Fig. 21) [173]. The effectiveness of PEI based coatings against influenza also presents a promising option for inactivating SARS-CoV-2 and other enveloped viruses, given the mechanism of targeting the envelope lipid bilayer.
Fig. 21.
The proposed mechanism of influenza virus inactivation by N,N-dodecyl methyl-polyethyleneimine coatings. (a) An influenza virion diffuses to the polycation-coated surface from solution and (b) adheres to it. Significant degradation to the viral envelope is induced by the hydrophobic polycations causing (c) the viral genomic RNA to leak from the envelope into solution leaving a ruptured virion inactivated on the surface. Bottom right: SEM images comparing intact (a) and ruptured virion (b). Reproduced from Ref. [173], Copyright (2011) National Academy of Sciences.
Advantages and disadvantages of metal-based nanoparticles
Advantages
Metal and metal based nanoparticles exhibit a versatile range of antiviral mechanisms that target conserved viral components such as structural proteins and the lipid envelope with broad spectrum efficacy against many species of viruses.
While viruses may be able to develop specific resistances through a limited number of mutations in response to a traditional antiviral drug, the probability of being able to concurrently develop multiple resistance mutations that directly counter the multiple mechanisms of a metal-based NP is unlikely [174], [27]. Different NPs could attack viruses through diverse pathways such as oxidative stress, protein disruption, as well as lipid envelope and capsid damage [27]. For instance, the common mechanism of reactive oxygen species production circumvents bacterial and viral resistance by simultaneously damaging multiple targets on the pathogen and compromising its integrity [38], [42], [175]. The ability to treat resistant pathogens is a significant advantage of nanoparticles over antiviral and antibiotic drugs which often target one component of a pathogen and thus are susceptible to resistance [139], [25]. In addition to antiviral activity, the antibacterial properties of metal nanoparticles provide utility in thoroughly sterilizing environments and may reduce the risk of secondary bacterial infections caused by COVID-19 [176].
Further, the physicochemical properties of metal nanoparticles can be more finely controlled by modifying their size and surface functionalization compared to organic lipid or polymer-based NPs [177]. These advantages in microbicidal activity are complemented by the practical benefits of long-term stability of metal nanoparticle products and their relative ease of manufacturing [178]. The chemical and colloidal stability of metal nanoparticles makes them viable for applications where sustained activity is needed such as surface coatings, sterilizing sprays, biomedical products, and reusable fabrics [179]. Metal nanoparticles can also be produced in a cost-effective manner with a range of techniques from green synthesis to electric plasma discharge to reverse micelle deposition [180], [26], [181], [182], [183], [184], [185]. Compounds such as titanium and zinc oxides exhibit unique optical properties such as generating antiviral ROS upon exposure to UV-wavelengths; therefore, their incorporation would synergize well with UV disinfection regimes and sunlit environments [114], [43]. Finally, the magnetic properties of metal compounds such as iron oxides enable the targeting of nanoparticles and enhanced recovery from the environment to minimize pollution using magnetic field manipulation [114], [80], [81].
Disadvantages and considerations
Although metal nanoparticles hold significant potential for combating SARS-CoV-2 and other viruses, there remain limitations that must be considered. For instance, it is challenging to produce nanoparticles at industrial scale and difficult to synthesize pure and uniform batches [186]. Consistent reproducibility is important as batch-to-batch variation in nanoparticles can lead to unforeseen changes in physicochemical and pharmacokinetic properties [187]. Increasing the complexity of nanoparticle systems with multiple drug encapsulation or surface ligands will require multiple production steps and further the difficulty of large-scale manufacturing [188]. Additionally, in therapeutic and imaging applications, many nanoparticle products have been unable to fulfill the promise of selective targeting to specific tissues and further development is needed for ligand-mediated targeting [189], [190].
The most significant limitation that must be considered is the safety and cytotoxicity of such materials. Nanoparticles typically have higher toxicity than bulk materials given their small size and ability to penetrate biological membranes which could lead to persistence in multicellular organisms, if exposure is long term and in high concentrations [191]. Nanoparticles with virucidal mechanisms, particularly reactive oxygen species generation, pose a risk of damage to not only viruses, but also human cells if left unregulated in close proximity. If used in vivo, metal-based NPs can pose a risk of accumulation and toxicity to tissues and organs, as some are non-biodegradable by enzymes in the body. Thus, it is important to design nanoparticles from a safety perspective that prioritizes biodegradability or optimized clearance from the body [192].
Non-metallic nanoparticles, such as those composed completely of polymers or integrating lipids and cell membranes, usually exhibit superior biocompatibility [193], [194]. An alternative to metal nanoparticles for viral inhibition are nanosponges made up of nanoparticles with a poly-lactic-co glycolic acid (PLGA) core coated in either human lung epithelial type II cell or macrophage cell membranes [194]. Incorporating human cell membranes that are natural targets of SARS-CoV-2, such biomimetic decoys can competitively bind the virus. Zhang et al. [194] showed that these nanosponges can attract and sequester SARS-CoV-2 to prevent host cell entry, by displaying the same receptors that the virus uses for infection. Efficacy was dose-dependent and a concentration of 5 mg/mL caused SARS-CoV-2 infectivity inhibition of 88–93% with good safety when administered to lungs in mice. Importantly, the effectiveness of cell membrane-coated nanosponges may be resistant to viral mutations as all known SARS-CoV-2 variants target the same host cells to initiate infection [194]. With the use of respiratory cell membranes, it is also possible that nanosponges could broadly inhibit other respiratory viruses which infect the same cells. Although promising in vivo, it remains to be seen if nanosponges can be utilized in applications outside the body.
It is, however, possible to decrease the cytotoxicity and secondary effects of ROS-associated oxidative damage to healthy cells. The size of nanoparticles plays a role in their safety, with larger nanoparticles (>100 nm) generally exhibiting lower cytotoxicity than smaller nanoparticles [195], [196]. However, there is often a trade-off in efficacy as smaller particles tend to have greater anti-viral potency [65], [74], [27], [25], [94], [110], [95], [97], [96], [102], [119]. However, for some particles such as Ag, smaller sizes exhibited lower cytotoxicity possibly due to their ability to more easily bind virions [27]. Therefore, careful studies on the effect of nanoparticle size on both anti-viral activity and cytotoxicity need to be undertaken. Biocompatible coatings and surface modifications with polymeric compounds such as polyethylene glycol or polyvinyl pyrrolidone also reduce cytotoxicity by preventing nuclear condensation and bioaccumulation [90], [195], [74], [75], [161], [163]. These polymer ligands often have the secondary effect of increasing the anti-viral potency of the functionalized nanoparticles [26], [70], [161], [163], [90], making them an effective strategy in designing safe anti-viral metal based nanoparticles.
Although the safety of nanoparticles is an important consideration, when they are properly embedded in surfaces and polymers to confer antiviral properties, nanotoxicity is less of a concern given the reduced likelihood for NP entry into the human body compared to NP applications such as drug delivery. Nevertheless, the potential toxicity of metal nanoparticles must be considered and further investigated with applications such as facemasks and other PPE where inhalation and close contact may provide routes of NP entry into the body. These safety considerations also give rise to significant regulatory hurdles for any potential nanoparticle product [188], [197].
Prospective nanoparticle applications to SARS-Cov-2 inhibition
Although there have been few direct studies exploring SARS-CoV-2 inactivation by nanomaterials due to its recency, the many antiviral mechanisms observed for other viruses can be analyzed for applicability to the novel coronavirus based on shared characteristics.
First, the generation of excess ROS is a broad biocidal mechanism exhibited by many metal oxide particles, such as TiO2 and ZnO nanoparticles through UV photocatalysis [24], [41], [66], [67], [43] or directly from iron oxide and Cu based materials [131], [138], [84], [85]. Throughout the studies reviewed, ROS such as hydroxyl radicals have been demonstrated to destructively bind with biomolecules in the process of resolving their unpaired electrons. This mechanism causes oxidative damage to the lipid membranes, protein capsids, and genetic material of viruses that impairs their ability to enter hosts and replicate [38], [42], [39], [41], [84]. SARS-CoV-2 is an enveloped positive-sense single-stranded RNA virus with all the mentioned components. Therefore it would be susceptible to ROS-mediated photodegradation by TiO2 and ZnO [47], [76]. Nanoparticle surface coatings based on TiO2 and ZnO have potential for the sterilization of sunlit outdoor contact surfaces or for indoor applications under fluorescent light as described by Park et al. [24]. Additionally, studies such as that by Fujimori et al. demonstrate that copper-based nanoparticles such as CuI NPs can partake in Fenton-like reactions that generate ROS [127]. These free radicals were shown to effectively degrade the hemagglutinin and neuraminidase glycoproteins that influenza uses to bind its host cells. Abo-zeid et al. recently showed that iron oxides are predicted to directly interact with S1-RBD on the SARS-Cov-2 spike protein, leading to irreversible conformational changes that would prevent viral binding [88]. As respiratory viruses, influenza and SARS-CoV-2 both utilize spike glycoproteins to infect similar host cells [158]. Therefore, the observed degradation of influenza glycoproteins by metal generated ROS could also result in the inactivation of SARS-CoV-2's main virulence mechanism.
On the topic of glycoproteins, ROS catalyzed by nanoparticles can also cause the cleavage of oligosaccharides from glycoproteins as demonstrated by Yasuda et al. and Eguchi et al. [49], [157] for Cu and by Kumar et al. [85] for Fe. Notably, for Cu compounds, the hydroxyl radicals generated by copper ions in Fenton-like reactions could break N-linked glycosidic linkages at N-Acetylglucosamine (GlcNAc) residues [49], [157]. GlcNAc residues attach oligosaccharides of the glycan shield to the spike protein, which suggests that nanoparticle generated ROS can influence deglycosylation of SARS-CoV-2 via cleavage of these oligosaccharides [1], [34], [158]. The SARS-CoV-2 spike protein depends on its glycosylation for immune evasion and proper binding to ACE-2. For instance, in their detailed model of the spike protein, Watanabe et al. identified glycosylation sites N165, N234, and N3 [94], which shield the receptor-binding domain ( Fig. 22) [1]. These sites contain oligosaccharides with GlcNAc residues that could be cleaved by hydroxyl radicals. A disruption of the glycan shield in the receptor-binding domain could expose the spike protein to antibodies and compromise host cell binding. Thus, an antiviral surface that employs this mechanism could preemptively reduce or disable the infectivity of SARS-CoV-2 and make it more easily destroyed by a host’s immune response. Consequently, copper-based nanomaterials are a promising candidate for SARS-CoV-2 inactivation by targeting its oligosaccharides. However, the inactivation mechanisms require further research to determine the conditions under which they can occur and whether hydroxyl radicals produced without copper can achieve the same results. If hydroxyl radicals are the only requirement, then other photodynamic materials that generate ROS, such as ZnO and TiO2, or even cation producing photoactive molecules like ZnTMPyP4+ photosensitizer [198], would also be able to target and damage the glycan oligosaccharide shield on the spike protein of SARS-CoV-2 [24], [41], [66], [67]. In total, the ROS-mediated mechanisms discussed play a vital role in the antiviral efficacies of specific nanoparticles and may inactivate SARS-CoV-2 through oxidative damage to its glycan shield, proteins, and lipid membrane.
In addition to ROS-driven antiviral mechanisms, nanoparticles interfere with viral viability through direct physical interactions and the formation of chemical bonds. As shown by Lara et al., one of the mechanisms by which silver nanoparticles disable HIV and influenza A is through the formation of complexes with disulfide bonds on its gp120 envelope glycoprotein [25], [94], [100]. Like SARS-CoV-2, HIV uses an envelope-based glycoprotein to bind to target receptors on host cells and disabling it inhibits the entry stage of the viral life cycle [99]. Similarly, iron oxide nanoparticles have been shown to bind with the sulfur-bearing residues (-SH groups) of influenza proteins to prevent viral-host cell entry [85]. The SARS-CoV-2 spike protein also has disulfide bond regions between cysteines in its receptor-binding domain that are essential to binding the ACE-2 receptor [120]. AgNPs, IO-NPs or both together might work to inactivate the SARS-CoV-2 spike protein by binding in these vulnerable disulfide regions. Specifically, Siriwardana et al. proved that silver nanoparticles bind to cysteine residues as a thiolate before eventually replacing the S—S bonds between cysteines with S—Ag bonds [101]. Minoshima et al. substantiated this mechanism and demonstrated that Cu2+ ions could undergo the same pathway to disrupt disulfide links in influenza glycoproteins [106]. Nangmenyi et al. [91] showed significant broad spectrum activity against both bacteria and viruses by producing a hybrid material composed of Ag-modified iron oxide nanoparticles. Since disulfide bridges are crucial to the tertiary and quaternary structure of proteins, the formation of cysteine-silver complexes would change the fold of the glycoprotein, which could inhibit its ability to complete host cell attachment and fusion. The disulfide bonding mechanism may not be solely limited to metal cations as the hybrid surface coating developed by Hodek et al., which also released zinc ions, was effective against HIV-1, influenza and dengue virus, all of which feature disulfide bridges between cysteine in their glycoproteins [168].
Further, the literature on gold, iron oxide and zinc oxide nanoparticles showcases a tendency to form complexes with biomolecules and bond with viral surface structures to inhibit their function. Studies by Mishra et al. Papp et al., Meléndez-Villanueva et al., Kumar et al. and Abo-zeid et al., for example, illustrate how metal nanostructures can bind to viral surface receptors through electrostatic interactions to block their ability to identify and attach to corresponding receptors on their target cells [26], [70], [161], [88], [85]. Zinc, iron and gold can also be easily functionalized with polymers such as polyethylene glycol on their exterior to provide lower cytotoxicity, stability, and improved virucidal characteristics [74], [161], [163], [90]. By inhibiting herpes simplex virus, influenza, and measles virus, which all share the same fundamental envelope structure with SARS-CoV-2, it can be inferred that the metal nanostructures would remain effective against the novel coronavirus [26], [70], [161], as shown explicitly for iron oxides by Abo-zeid et al. [88].
Finally, nanoparticles of zinc oxide, iron oxide, silver, and copper have also been demonstrated using SEM and TEM to directly interact with and compromise bacterial membranes [59], [63], [64], [110], [122], [138], [84]. This occurs through the diffusion of nanoparticles and metal ions through the membrane, which causes cell death by lysis [59], [63]. Given that both cell membranes and viral envelopes are based on the same lipid bilayer structure, it can be inferred that nanoparticles smaller than individual virions could act with a similar bilayer penetration mechanism to cause viral inactivation through damage to the envelope. On the topic of the envelope as an antiviral target, PEI-based polycationic coatings exhibit potent destruction of influenza through envelope rupture and RNA leakage within minutes of contact [172], [173]. Further, iron oxide nanoparticles can catalyze lipid peroxidation of viral envelopes to compromise their integrity while degrading surface proteins [84]. With a lipid envelope encasing a central core of its RNA, SARS-CoV-2 would also be vulnerable to these mechanisms. Combining nanoparticle approaches with polycationic coatings could result in highly effective self-sterilizing surfaces through immobilization and lysis.
In summary, metal and metal based nanoparticles may work to disable SARS-CoV-2 early in its pathogenesis by targeting its spike proteins through competitive inhibition or disulfide cleavage to prevent the virus from entering and infecting cells. Further, direct physical damage to viral membranes of enveloped viruses such as SARS-CoV-2 is exhibited by many nanoparticles due to their high specific surface area and small size. Titanium dioxide, zinc oxide, iron oxide, and cuprous compounds have been proven to inactivate viruses in solid-state surface coatings or in polymers with a high degree of effectiveness. Notably, these compounds share the blanket antiviral pathway of ROS generation to degrade essential viral structures and deglycosylate spike proteins. Therefore, ROS generating nanomaterials are optimal prophylactic agents for self-sterilizing surface coatings and polymers. These are summarized in Table 1 and Fig. 23.
Table 1.
Summary of possible anti-viral metal and metal oxide nanoparticles with the primary viral inhibition mechanisms and the viruses and some bacteria as discussed against which they have already shown efficacy.
| Nanoparticle | Antiviral mechanism | Tested viruses and bacteria |
|---|---|---|
| Titanium Dioxide (TiO2) | Reactive oxygen species generation through photocatalytic reactions driven by UV–vis light. Membrane interactions that damage viral envelopes. Physical deformation and conformational changes to interfere with viral entry. | Influenza A (H1N1), Human Norovirus, feline calicivirus, herpes simplex virus-1 (HSV-1) Bacteria: E. coli, P. putida, S. aureus. |
| Zinc Oxide (ZnO) | Disruption of the lipid membrane via electrostatic interactions. Mimicry of heparan sulfate to capture virions before attachment. UV photocatalysis of reactive oxygen species. Dissociated zinc ions inhibit RNA-dependent RNA polymerase to impair viral replication | Influenza A (H1N1), Herpes simplex virus type-1 Bacteria: E. coli, P. aeruginos, K. pneumonia, P. vulgaris, C. jejuni |
| Iron Oxide (Fe3O4) | Lipid peroxidation of the viral envelope to degrade it and neighboring proteins. Binding with the sulfur-bearing residues (-SH groups) of influenza proteins to prevent viral-host cell entry. | Influenza A (H1-H12 subtypes) Bacteria: E. coli, P. vulgaris, and S. aureus |
| Silver (Ag) | Variable based on virus: Disulfide bond cleavage, binding amino acids, and protein denaturation Reactive oxygen species generation that damages viral biomolecules. Direct interactions with virions to compromise structure Chemisorption of virions to suppress viral infectivity Degradation of viral nucleic acids to inhibit replication | African swine fever virus, Hepatitis B virus (HBV), influenza A, HIV-1, poliovirus, adenovirus type-3, porcine epidemic diarrhea virus, murine norovirus, feline calicivirus, adenovirus serotype 2, bacteriophage ϕX17, bacteriophage Qβ Bacteria: S. enterica, E.coli, MRSA, P. aeruginosa, V. cholerae, S. pyogenes, L. monocytogenes, and Coliform |
| Copper and Copper Oxides (CuO and Cu2O) | Release of bioactive copper ions and reactive oxygen species generation to cause oxidative damage. Direct membrane and protein interactions. | Influenza A (H1N1), feline calicivirus, human norovirus-like particles, hepatitis C virus, herpes simplex virus type-1, bacteriophage Qβ |
| Gold (Au) | Inhibitory binding of viral glycoproteins to prevent viral attachment and entry. Mimicry of heparan sulfate proteoglycans to prevent virus-cell binding. Electrostatic interaction and functional inactivation. Physical deformation and conformational changes to interfere with viral entry. | Measles virus, influenza A, vesicular stomatitis virus, respiratory syncytial virus (RSV), herpes simplex virus (HSV), human papillomavirus (HPV) |
Fig. 23.
Summary of possible viral inhibition mechanisms for nanoparticles on self-sterilizing surfaces.
Conclusion
To conclude, research on metal, metal oxide and metal-based nanoparticles indicates several potential options for antiviral surface coatings, each with distinct mechanisms. From ROS-driven degradation to blocking spike proteins to capsid destruction, nanoparticles have demonstrated their effectiveness in disabling a broad range of viruses through a variety of targets. For the application of sterilizing contaminated surfaces, nanoparticles exhibit potent antiviral and antibacterial activity. If applied to contact surfaces and polymers, metal nanoparticles can confer their sterilizing properties to limit viral transmission and improve the safety of human environments. Disinfection is the primary method of inhibiting virulent spread against viruses with no viable vaccine, or ones that rapidly mutate. Likewise, in areas where vaccination rollout face barriers, or to augment vaccination efforts, nanoparticle-based self-sterilizing surfaces could be useful against SARS-CoV-2, and future emerging viruses. This can be amplified by using multiple types of metal and metal oxide nanoparticles together to take advantage of diverse inactivation mechanisms, while preventing the development of resistance. Without appropriate preparation, another pandemic in the future is not a matter of if but when, so adaptable nanotechnologies will play a crucial role in the diagnosis, treatment and prevention of future viral outbreaks.
Further research must be conducted to determine the efficacy of NPs on SARS-CoV-2 directly as well as the safety evaluations before the possibility of widespread use as a disinfectant. Additionally, the manufacturing challenges remain to be addressed before inorganic NPs can be used at scale. However, given similarities between SARS-CoV-2 and other enveloped viruses, NPs warrant serious consideration for potential applications in sanitation products and self-sterilizing materials. Because of their unique antimicrobial and potential antiviral nature, metal and metal oxide nanoparticles represent a promising tool in the global effort to combat COVID-19, and future epidemics, and safeguard human health.
CRediT authorship contribution statement
Neil Lin: Data curation, Visualization, Writing – original draft. Daksh Verma: Data curation. Nikhil Saini: Writing – review & editing. Ramis Arbi: Writing – review & editing. Muhammad Munir: Writing – review & editing. Marko Jovic: Writing – review & editing. Ayse Turak: Conceptualization, Supervision, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors declare no conflicts of interest. Original figures were created with BioRender.com. We gratefully acknowledge the financial support of the Ontario Ministry of Research and Innovation (Early Researcher Award ER15-11-123 for AT), the Natural Sciences and Engineering Research Council of Canada (Discovery RGPIN-2019-05994, COVID-19 ALLRP 550726-20 for AT), and Sigma Xi Grants-in-Aid-of-Research (NS).
References
- 1.Watanabe Y., Berndsen Z.T., Raghwani J., Seabright G.E., Allen J.D., Pybus O.G., McLellan J.S., Wilson I.A., Bowden T.A., Ward A.B., Crispin M. Vulnerabilities in coronavirus glycan shields despite extensive glycosylation. Nat. Commun. 2020;11:2688. doi: 10.1038/s41467-020-16567-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sportelli M.C., Izzi M., Kukushkina E.A., Hossain S.I., Picca R.A., Ditaranto N., Cioffi N. Can nanotechnology and materials science help the fight against SARS-CoV-2? Nanomaterials. 2020;10:802. doi: 10.3390/nano10040802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tang Z., Kong N., Zhang X., Liu Y., Hu P., Mou S., Liljeström P., Shi J., Tan W., Kim J.S., Cao Y., Langer R., Leong K.W., Farokhzad O.C., Tao W. A materials-science perspective on tackling COVID-19. Nat. Rev. Mater. 2020;5:847–860. doi: 10.1038/s41578-020-00247-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bull R.A., Adikari T.N., Ferguson J.M., Hammond J.M., Stevanovski I., Beukers A.G., Naing Z., Yeang M., Verich A., Gamaarachchi H., Kim K.W., Luciani F., Stelzer-Braid S., Eden J.-S., Rawlinson W.D., van Hal S.J., Deveson I.W. Analytical validity of nanopore sequencing for rapid SARS-CoV-2 genome analysis. Nat. Commun. 2020;11:6272. doi: 10.1038/s41467-020-20075-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luciani F., Bull R.A., Lloyd A.R. Next generation deep sequencing and vaccine design: today and tomorrow. Trends Biotechnol. 2012;30:443–452. doi: 10.1016/j.tibtech.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reichmuth A.M., Oberli M.A., Jaklenec A., Langer R., Blankschtein D. mRNA vaccine delivery using lipid nanoparticles. Ther. Deliv. 2016;7:319–334. doi: 10.4155/tde-2016-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Z., Tang Z., Farokhzad N., Chen T., Tao W. Sensitive, rapid, low-cost, and multiplexed COVID-19 monitoring by the wireless telemedicine platform. Matter. 2020;3:1818–1820. doi: 10.1016/j.matt.2020.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Draz M.S., Shafiee H. Applications of gold nanoparticles in virus detection. Theranostics. 2018;8:1985–2017. doi: 10.7150/thno.23856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang C., Wen T., Shi F.-J., Zeng X.-Y., Jiao Y.-J. Rapid detection of IgM antibodies against the SARS-CoV-2 virus via colloidal gold nanoparticle-based lateral-flow assay. ACS Omega. 2020;5:12550–12556. doi: 10.1021/acsomega.0c01554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Doremalen N., Bushmaker T., Morris D.H., Holbrook M.G., Gamble A., Williamson B.N., Tamin A., Harcourt J.L., Thornburg N.J., Gerber S.I., Lloyd-Smith J.O., de Wit E., Munster V.J. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N. Engl. J. Med. 2020;382:1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chin A.W.H., Chu J.T.S., Perera M.R.A., Hui K.P.Y., Yen H.-L., Chan M.C.W., Peiris M., Poon L.L.M. Stability of SARS-CoV-2 in different environmental conditions. Lancet Microbe. 2020;1 doi: 10.1016/S2666-5247(20)30003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu L., Gao J., Yao L., Zeng L., Liu Q., Zhou Q., Zhang H., Lu D., Fu J., Liu Q.S., Li M., Zhao X., Hou X., Shi J., Liu L., Guo Y., Wang Y., Ying G.-G., Cai Y., Yao M., Cai Z., Wu Y., Qu G., Jiang G. Evidence of foodborne transmission of the coronavirus (COVID-19) through the animal products food supply chain. Environ. Sci. Technol. 2021;55:2713–2716. doi: 10.1021/acs.est.0c06822. [DOI] [PubMed] [Google Scholar]
- 13.Liu P., Yang M., Zhao X., Guo Y., Wang L., Zhang J., Lei W., Han W., Jiang F., Liu W.J., Gao G.F., Wu G. Cold-chain transportation in the frozen food industry may have caused a recurrence of COVID-19 cases in destination: successful isolation of SARS-CoV-2 virus from the imported frozen cod package surface. Biosaf. Health. 2020;2:199–201. doi: 10.1016/j.bsheal.2020.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Han J., Zhang X., He S., Jia P. Can the coronavirus disease be transmitted from food? A review of evidence, risks, policies and knowledge gaps. Environ. Chem. Lett. 2020:1–12. doi: 10.1007/s10311-020-01101-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lewis D. COVID-19 rarely spreads through surfaces. So why are we still deep cleaning? Nature. 2021;590:26–28. doi: 10.1038/d41586-021-00251-4. [DOI] [PubMed] [Google Scholar]
- 16.M.U. Mondelli, M. Colaneri, E.M. Seminari, F. Baldanti, R. Bruno, Low risk of SARS-CoV-2 transmission by fomites in real-life conditions, Lancet Infect. Dis. 〈10.1016/S1473-3099(20)30678-2〉. [DOI] [PMC free article] [PubMed]
- 17.Tang Z., Zhang X., Shu Y., Guo M., Zhang H., Tao W. Insights from nanotechnology in COVID-19 treatment. Nano Today. 2021;36 doi: 10.1016/j.nantod.2020.101019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Talebian S., Wallace G.G., Schroeder A., Stellacci F., Conde J. Nanotechnology-based disinfectants and sensors for SARS-CoV-2. Nat. Nanotechnol. 2020;15:618–621. doi: 10.1038/s41565-020-0751-0. [DOI] [PubMed] [Google Scholar]
- 19.Dey P., Bergmann T., Cuellar-Camacho J.L., Ehrmann S., Chowdhury M.S., Zhang M., Dahmani I., Haag R., Azab W. Multivalent flexible nanogels exhibit broad-spectrum antiviral activity by blocking virus entry. ACS Nano. 2018;12:6429–6442. doi: 10.1021/acsnano.8b01616. [DOI] [PubMed] [Google Scholar]
- 20.Cagno V., Andreozzi P., D’Alicarnasso M., Jacob Silva P., Mueller M., Galloux M., Le Goffic R., Jones S.T., Vallino M., Hodek J., Weber J., Sen S., Janeček E.-R., Bekdemir A., Sanavio B., Martinelli C., Donalisio M., Rameix Welti M.-A., Eleouet J.-F., Han Y., Kaiser L., Vukovic L., Tapparel C., Král P., Krol S., Lembo D., Stellacci F. Broad-spectrum non-toxic antiviral nanoparticles with a virucidal inhibition mechanism. Nat. Mater. 2018;17:195–203. doi: 10.1038/nmat5053. [DOI] [PubMed] [Google Scholar]
- 21.Zoghi S., Khamirani H.J., Dastgheib S.A., Dianatpour M., Ghaffarieh A. An analysis of inhibition of the severe acute respiratory syndrome coronavirus 2 RNA-dependent RNA polymerase by zinc ion: an in silico approach. Future Virol. 2021;16:331–339. doi: 10.2217/fvl-2020-0369. [DOI] [Google Scholar]
- 22.Kaushik N., Subramani C., Anang S., Muthumohan R., Nayak B., Ranjith-Kumar C.T., Surjit M. Zinc salts block hepatitis E virus replication by inhibiting the activity of viral RNA-dependent RNA polymerase. J. Virol. 2017;91 doi: 10.1128/JVI.00754-17. e00754–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bogdan J., Zarzyńska J., Pławińska-Czarnak J. Comparison of infectious agents susceptibility to photocatalytic effects of nanosized titanium and zinc oxides: a practical approach. Nanoscale Res. Lett. 2015;10:309. doi: 10.1186/s11671-015-1023-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park G.W., Cho M., Cates E.L., Lee D., Oh B.-T., Vinjé J., Kim J.-H. Fluorinated TiO2 as an ambient light-activated virucidal surface coating material for the control of human norovirus. J. Photochem. Photobiol. B Biol. 2014;140:315–320. doi: 10.1016/j.jphotobiol.2014.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lara H.H., Ayala-Núñez N.V., Ixtepan Turrent L.d.C., Rodríguez Padilla C. Bactericidal effect of silver nanoparticles against multidrug-resistant bacteria. World J. Microbiol. Biotechnol. 2010;26:615–621. doi: 10.1007/s11274-009-0211-3. [DOI] [Google Scholar]
- 26.Meléndez-Villanueva M.A., Morán-Santibañez K., Martínez-Sanmiguel J.J., Rangel-López R., Garza-Navarro M.A., Rodríguez-Padilla C., Zarate-Triviño D.G., Trejo-Ávila L.M. Virucidal activity of gold nanoparticles synthesized by green chemistry using garlic extract. Viruses. 2019;11:1111. doi: 10.3390/v11121111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Galdiero S., Falanga A., Vitiello M., Cantisani M., Marra V., Galdiero M. Silver nanoparticles as potential antiviral agents. Molecules. 2011;16:8894–8918. doi: 10.3390/molecules16108894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prasher P., Singh M., Mudila H. Oligodynamic effect of silver nanoparticles: a review. BioNanoScience. 2018;8:951–962. doi: 10.1007/s12668-018-0552-1. [DOI] [Google Scholar]
- 29.Nangmenyi G., Economy J. In: Nanotechnology Applications for Clean Water, Micro and Nano Technologies. Savage N., Diallo M., Duncan J., Street A., Sustich R., editors. William Andrew Publishing; Boston: 2009. Chapter 1 – nanometallic particles for oligodynamic microbial disinfection; pp. 3–15. [DOI] [Google Scholar]
- 30.Rai M., Deshmukh S.D., Ingle A.P., Gupta I.R., Galdiero M., Galdiero S. Metal nanoparticles: the protective nanoshield against virus infection. Crit. Rev. Microbiol. 2016;42:46–56. doi: 10.3109/1040841X.2013.879849. [DOI] [PubMed] [Google Scholar]
- 31.Lu L., W.-Y. Sun R., Chen R., Hui C.-K., Ho C.-M., Luk J.M., Lau G.K.K., Che C.-M. Silver nanoparticles inhibit hepatitis B virus replication. Antivir. Ther. 2008;13:253–262. [PubMed] [Google Scholar]
- 32.Rai M., Kon K., Ingle A., Duran N., Galdiero S., Galdiero M. Broad-spectrum bioactivities of silver nanoparticles: the emerging trends and future prospects. Appl. Microbiol. Biotechnol. 2014;98:1951–1961. doi: 10.1007/s00253-013-5473-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bawage S.S., Tiwari P.M., Singh A., Dixit S., Pillai S.R., Dennis V.A., Singh S.R. Gold nanorods inhibit respiratory syncytial virus by stimulating the innate immune response. Nanomed. Nanotechnol. Biol. Med. 2016;12:2299–2310. doi: 10.1016/j.nano.2016.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Watanabe Y., Allen J.D., Wrapp D., McLellan J.S., Crispin M. Site-specific glycan analysis of the SARS-CoV-2 spike. Science. 2020;369:330–333. doi: 10.1126/science.abb9983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shakeel M., Jabeen F., Shabbir S., Asghar M.S., Khan M.S., Chaudhry A.S. Toxicity of nano-titanium dioxide (TiO2-NP) through various routes of exposure: a review. Biol. Trace Elem. Res. 2016;172:1–36. doi: 10.1007/s12011-015-0550-x. [DOI] [PubMed] [Google Scholar]
- 36.Nosaka Y., Nosaka A.Y. Generation and detection of reactive oxygen species in photocatalysis. Chem. Rev. 2017;117:11302–11336. doi: 10.1021/acs.chemrev.7b00161. [DOI] [PubMed] [Google Scholar]
- 37.Nakano R., Ishiguro H., Yao Y., Kajioka J., Fujishima A., Sunada K., Minoshima M., Hashimoto K., Kubota Y. Photocatalytic inactivation of influenza virus by titanium dioxide thin film. Photochem. Photobiol. Sci. 2012;11:1293–1298. doi: 10.1039/C2PP05414K. [DOI] [PubMed] [Google Scholar]
- 38.Abdal Dayem A., Hossain M.K., Lee S.B., Kim K., Saha S.K., Yang G.-M., Choi H.Y., Cho S.-G. The role of reactive oxygen species (ROS) in the biological activities of metallic nanoparticles. Int. J. Mol. Sci. 2017;18:120. doi: 10.3390/ijms18010120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.de Dicastillo C.L., Correa M.G., Martínez F.B., Streitt C., Galotto M.J. Antimicrobial Resistance – A One Health Perspective. IntechOpen; 2020. Antimicrobial effect of titanium dioxide nanoparticles. [DOI] [Google Scholar]
- 40.Rattanakul S., Oguma K. Analysis of hydroxyl radicals and inactivation mechanisms of bacteriophage MS2 in response to a simultaneous application of UV and chlorine. Environ. Sci. Technol. 2017;51:455–462. doi: 10.1021/acs.est.6b03394. [DOI] [PubMed] [Google Scholar]
- 41.Hajkova P., Spatenka P., Horsky J., Horska I., Kolouch A. Photocatalytic effect of TiO2 films on viruses and bacteria. Plasma Process. Polym. 2007;4:S397–S401. doi: 10.1002/ppap.200731007. [DOI] [Google Scholar]
- 42.Hong Y., Zeng J., Wang X., Drlica K., Zhao X. Post-stress bacterial cell death mediated by reactive oxygen species. PNAS. 2019;116:10064–10071. doi: 10.1073/pnas.1901730116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jalvo B., Faraldos M., Bahamonde A., Rosal R. Antimicrobial and antibiofilm efficacy of self-cleaning surfaces functionalized by TiO2 photocatalytic nanoparticles against Staphylococcus aureus and Pseudomonas putida. J. Hazard. Mater. 2017;340:160–170. doi: 10.1016/j.jhazmat.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 44.Srivastava D.N., Perkas N., Gedanken A., Felner I. Sonochemical synthesis of mesoporous iron oxide and accounts of its magnetic and catalytic properties. J. Phys. Chem. B. 2002;106:1878–1883. doi: 10.1021/jp015532w. [DOI] [Google Scholar]
- 45.Akhtar S., Shahzad K., Mushtaq S., Ali I., Rafe M.H., Fazal-ul-Karim S.M. Antibacterial and antiviral potential of colloidal titanium dioxide (TiO2) nanoparticles suitable for biological applications. Mater. Res. Express. 2019;6 doi: 10.1088/2053-1591/ab3b27. [DOI] [Google Scholar]
- 46.Mazurkova N.A., Spitsyna Y.E., Shikina N.V., Ismagilov Z.R., Zagrebel’nyi S.N., Ryabchikova E.I. Interaction of titanium dioxide nanoparticles with influenza virus. Nanotechnol. Russ. 2010;5:417–420. doi: 10.1134/S1995078010050174. [DOI] [Google Scholar]
- 47.Bianchi M., Benvenuto D., Giovanetti M., Angeletti S., Ciccozzi M., Pascarella S. Sars-CoV-2 envelope and membrane proteins: structural differences linked to virus characteristics? BioMed Res. Int. 2020;2020:4389089. doi: 10.1155/2020/4389089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fischetti M., Hays V.F., Glaunsinger B., Christiansen J. A visual guide to the SARS-CoV-2 coronavirus. Sci. Am. 2020;323:32–37. doi: 10.1038/scientificamerican0720-32. [DOI] [PubMed] [Google Scholar]
- 49.Yasuda J., Eguchi H., Fujiwara N., Ookawara T., Kojima S., Yamaguchi Y., Nishimura M., Fujimoto J., Suzuki K. Reactive oxygen species modify oligosaccharides of glycoproteins in vivo: a study of a spontaneous acute hepatitis model rat (LEC rat) Biochem. Biophys. Res. Commun. 2006;342:127–134. doi: 10.1016/j.bbrc.2006.01.118. [DOI] [PubMed] [Google Scholar]
- 50.L. Casalino, Z. Gaieb, J.A. Goldsmith, C.K. Hjorth, A.C. Dommer, A.M. Harbison, C.A. Fogarty, E.P. Barros, B.C. Taylor, J.S. McLellan, E. Fadda, R.E. Amaro, Beyond shielding: the roles of glycans in SARS-CoV-2 spike protein, bioRxiv, 2020. 〈10.1101/2020.06.11.146522〉. [DOI] [PMC free article] [PubMed]
- 51.S. Khaiboullina, T. Uppal, N. Dhabarde, V.R. Subramanian, S.C. Verma, In vitro inactivation of human coronavirus by titania nanoparticle coatings and UVC radiation: throwing light on SARS-CoV-2, bioRxiv, 2020. 〈10.1101/2020.08.25.265223〉. [DOI] [PMC free article] [PubMed]
- 52.Teunis P.F.M., Moe C.L., Liu P., Miller S.E., Lindesmith L., Baric R.S., Le Pendu J., Calderon R.L. Norwalk virus: how infectious is it? J. Med. Virol. 2008;80:1468–1476. doi: 10.1002/jmv.21237. [DOI] [PubMed] [Google Scholar]
- 53.Liu P., Yuen Y., Hsiao H.-M., Jaykus L.-A., Moe C. Effectiveness of liquid soap and hand sanitizer against norwalk virus on contaminated hands. Appl. Environ. Microbiol. 2010;76:394–399. doi: 10.1128/AEM.01729-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Park G.W., Barclay L., Macinga D., Charbonneau D., Pettigrew C.A., Vinjé J. Comparative efficacy of seven hand sanitizers against murine norovirus, feline calicivirus, and GII.4 norovirus. J. Food Prot. 2010;73:2232–2238. doi: 10.4315/0362-028X-73.12.2232. [DOI] [PubMed] [Google Scholar]
- 55.Liu P., Chien Y.-W., Papafragkou E., Hsiao H.-M., Jaykus L.-A., Moe C. Persistence of human noroviruses on food preparation surfaces and human hands. Food Environ. Virol. 2009;1:141. doi: 10.1007/s12560-009-9019-4. [DOI] [Google Scholar]
- 56.Bartsch S.M., Lopman B.A., Ozawa S., Hall A.J., Lee B.Y. Global economic burden of norovirus gastroenteritis. PLOS One. 2016;11 doi: 10.1371/journal.pone.0151219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Halioua B., Dermatologist Formerly Director of Medical Practice Bruno Halioua, Ziskind B. Harvard University Press; 2005. Medicine in the Days of the Pharaohs. [Google Scholar]
- 58.Stoimenov P.K., Klinger R.L., Marchin G.L., Klabunde K.J. Metal oxide nanoparticles as bactericidal agents. Langmuir. 2002;18:6679–6686. doi: 10.1021/la0202374. [DOI] [Google Scholar]
- 59.Siddiqi K.S., ur Rahman A., Husen A. Properties of zinc oxide nanoparticles and their activity against microbes. Nanoscale Res. Lett. 2018;13:141. doi: 10.1186/s11671-018-2532-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gupta M., Mahajan V.K., Mehta K.S., Chauhan P.S. Zinc therapy in dermatology: a review. Dermatol. Res. Pract. 2014;2014:1–11. doi: 10.1155/2014/709152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mohammed Y.H., Holmes A., Haridass I.N., Sanchez W.Y., Studier H., Grice J.E., Benson H.A.E., Roberts M.S. Support for the safe use of zinc oxide nanoparticle sunscreens: lack of skin penetration or cellular toxicity after repeated application in volunteers. J. Investig. Dermatol. 2019;139:308–315. doi: 10.1016/j.jid.2018.08.024. [DOI] [PubMed] [Google Scholar]
- 62.Chang Y.-N., Zhang M., Xia L., Zhang J., Xing G. The toxic effects and mechanisms of CuO and ZnO nanoparticles. Materials. 2012;5:2850–2871. doi: 10.3390/ma5122850. [DOI] [Google Scholar]
- 63.Xie Y., He Y., Irwin P.L., Jin T., Shi X. Antibacterial activity and mechanism of action of zinc oxide nanoparticles against Campylobacter jejuni. Appl. Environ. Microbiol. 2011;77:2325–2331. doi: 10.1128/AEM.02149-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Castro-Mayorga J.L., Fabra M.J., Pourrahimi A.M., Olsson R.T., Lagaron J.M. The impact of zinc oxide particle morphology as an antimicrobial and when incorporated in poly(3-hydroxybutyrate-co-3-hydroxyvalerate) films for food packaging and food contact surfaces applications. Food Bioprod. Process. 2017;101:32–44. doi: 10.1016/j.fbp.2016.10.007. [DOI] [Google Scholar]
- 65.Raghupathi K.R., Koodali R.T., Manna A.C. Size-dependent bacterial growth inhibition and mechanism of antibacterial activity of zinc oxide nanoparticles. Langmuir. 2011;27:4020–4028. doi: 10.1021/la104825u. [DOI] [PubMed] [Google Scholar]
- 66.Ong C.B., Ng L.Y., Mohammad A.W. A review of ZnO nanoparticles as solar photocatalysts: synthesis, mechanisms and applications. Renew. Sustain. Energy Rev. 2018;81:536–551. doi: 10.1016/j.rser.2017.08.020. [DOI] [Google Scholar]
- 67.Premanathan M., Karthikeyan K., Jeyasubramanian K., Manivannan G. Selective toxicity of ZnO nanoparticles toward gram-positive bacteria and cancer cells by apoptosis through lipid peroxidation. Nanomed. Nanotechnol. Biol. Med. 2011;7:184–192. doi: 10.1016/j.nano.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 68.Haraguchi Y., Sakurai H., Hussain S., Anner B.M., Hoshino H. Inhibition of HIV-1 infection by zinc group metal compounds. Antivir. Res. 1999;43:123–133. doi: 10.1016/S0166-3542(99)00040-6. [DOI] [PubMed] [Google Scholar]
- 69.Schwartz V.B., Thétiot F., Ritz S., Pütz S., Choritz L., Lappas A., Förch R., Landfester K., Jonas U. Antibacterial surface coatings from zinc oxide nanoparticles embedded in poly(N-isopropylacrylamide) hydrogel surface layers. Adv. Funct. Mater. 2012;22:2376–2386. doi: 10.1002/adfm.201102980. [DOI] [Google Scholar]
- 70.Mishra Y.K., Adelung R., Röhl C., Shukla D., Spors F., Tiwari V. Virostatic potential of micro–nano filopodia-like ZnO structures against herpes simplex virus-1. Antivir. Res. 2011;92:305–312. doi: 10.1016/j.antiviral.2011.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.O’Donnell C.D., Shukla D. The importance of heparan sulfate in herpesvirus infection. Virol. Sin. 2008;23:383–393. doi: 10.1007/s12250-008-2992-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wu J.M., Chen Y.-R. Ultraviolet-light-assisted formation of ZnO nanowires in ambient air: comparison of photoresponsive and photocatalytic activities in zinc hydroxide. J. Phys. Chem. C. 2011;115:2235–2243. doi: 10.1021/jp110320h. [DOI] [Google Scholar]
- 73.El-Megharbel S.M., Alsawat M., Al-Salmi F.A., Hamza R.Z. Utilizing of (zinc oxide nano-spray) for disinfection against “SARS-CoV-2” and testing its biological effectiveness on some biochemical parameters during (COVID-19 pandemic)—”ZnO nanoparticles have antiviral activity against (SARS-CoV-2) Coatings. 2021;11:388. doi: 10.3390/coatings11040388. [DOI] [Google Scholar]
- 74.Ghaffari H., Tavakoli A., Moradi A., Tabarraei A., Bokharaei-Salim F., Zahmatkeshan M., Farahmand M., Javanmard D., Kiani S.J., Esghaei M., Pirhajati-Mahabadi V., Monavari S.H., Ataei-Pirkooh A. Inhibition of H1N1 influenza virus infection by zinc oxide nanoparticles: another emerging application of nanomedicine. J. Biomed. Sci. 2019;26:70. doi: 10.1186/s12929-019-0563-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tavakoli A., Ataei-Pirkooh A., MM Sadeghi G., Bokharaei-Salim F., Sahrapour P., Kiani S.J., Moghoofei M., Farahmand M., Javanmard D., Monavari S.H. Polyethylene glycol-coated zinc oxide nanoparticle: an efficient nanoweapon to fight against herpes simplex virus type 1. Nanomedicine. 2018;13:2675–2690. doi: 10.2217/nnm-2018-0089. [DOI] [PubMed] [Google Scholar]
- 76.Schoeman D., Fielding B.C. Coronavirus envelope protein: current knowledge. Virol. J. 2019;16:69. doi: 10.1186/s12985-019-1182-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Peng X., Chen H., Huang J., Mao H., Shin D.M. Biomedical Engineering – From Theory to Applications. IntechOpen; 2011. Targeted magnetic iron oxide nanoparticles for tumor imaging and therapy; pp. 203–224. [DOI] [Google Scholar]
- 78.Malik A., Tahir Butt T., Zahid S., Zahid F., Waquar S., Rasool M., Qazi M.H., Qazi A.M. Use of magnetic nanoparticles as targeted therapy: theranostic approach to treat and diagnose cancer. J. Nanotechnol. 2017;2017 doi: 10.1155/2017/1098765. [DOI] [Google Scholar]
- 79.Vallabani N.V.S., Singh S. Recent advances and future prospects of iron oxide nanoparticles in biomedicine and diagnostics. 3 Biotech. 2018;8:279. doi: 10.1007/s13205-018-1286-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Reddy L.H., Arias J.L., Nicolas J., Couvreur P. Magnetic nanoparticles: design and characterization, toxicity and biocompatibility, pharmaceutical and biomedical applications. Chem. Rev. 2012;112:5818–5878. doi: 10.1021/cr300068p. [DOI] [PubMed] [Google Scholar]
- 81.Chang D., Lim M., M. Goos J.A.C., Qiao R., Ng Y.Y., Mansfeld F.M., Jackson M., Davis T.P., Kavallaris M. Biologically targeted magnetic hyperthermia: potential and limitations. Front. Pharmacol. 2018;9:831. doi: 10.3389/fphar.2018.00831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bobo D., Robinson K.J., Islam J., Thurecht K.J., Corrie S.R. Nanoparticle-based medicines: a review of FDA-approved materials and clinical trials to date. Pharm. Res. 2016;33:2373–2387. doi: 10.1007/s11095-016-1958-5. [DOI] [PubMed] [Google Scholar]
- 83.Abo-zeid Y., Williams G.R. The potential anti-infective applications of metal oxide nanoparticles: a systematic review. WIREs Nanomed. Nanobiotechnol. 2020;12 doi: 10.1002/wnan.1592. [DOI] [PubMed] [Google Scholar]
- 84.Qin T., Ma R., Yin Y., Miao X., Chen S., Fan K., Xi J., Liu Q., Gu Y., Yin Y., Hu J., Liu X., Peng D., Gao L. Catalytic inactivation of influenza virus by iron oxide nanozyme. Theranostics. 2019;9:6920–6935. doi: 10.7150/thno.35826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kumar R., Nayak M., Sahoo G.C., Pandey K., Sarkar M.C., Ansari Y., Das V., Topno R., Madhukar M., Das P. Iron oxide nanoparticles based antiviral activity of H1N1 influenza A virus. J. Infect. Chemother. 2019;25:325–329. doi: 10.1016/j.jiac.2018.12.006. [DOI] [PubMed] [Google Scholar]
- 86.Gutierrez L., Li X., Wang J., Nangmenyi G., Economy J., Kuhlenschmidt T.B., Kuhlenschmidt M.S., Nguyen T.H. Adsorption of rotavirus and bacteriophage MS2 using glass fiber coated with hematite nanoparticles. Water Res. 2009;43:5198–5208. doi: 10.1016/j.watres.2009.08.031. [DOI] [PubMed] [Google Scholar]
- 87.Murugan K., Wei J., Alsalhi M.S., Nicoletti M., Paulpandi M., Samidoss C.M., Dinesh D., Chandramohan B., Paneerselvam C., Subramaniam J., Vadivalagan C., Wei H., Amuthavalli P., Jaganathan A., Devanesan S., Higuchi A., Kumar S., Aziz A.T., Nataraj D., Vaseeharan B., Canale A., Benelli G. Magnetic nanoparticles are highly toxic to chloroquine-resistant Plasmodium falciparum, dengue virus (DEN-2), and their mosquito vectors. Parasitol. Res. 2017;116:495–502. doi: 10.1007/s00436-016-5310-0. [DOI] [PubMed] [Google Scholar]
- 88.Abo-zeid Y., Ismail N.S.M., McLean G.R., Hamdy N.M. A molecular docking study repurposes FDA approved iron oxide nanoparticles to treat and control COVID-19 infection. Eur. J. Pharm. Sci. 2020;153 doi: 10.1016/j.ejps.2020.105465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Thakor A.S., Jokerst J.V., Ghanouni P., Campbell J.L., Mittra E., Gambhir S.S. Clinically approved nanoparticle imaging agents. J. Nucl. Med. 2016;57:1833–1837. doi: 10.2967/jnumed.116.181362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kumar S.R., Paulpandi M., ManivelRaja M., Mangalaraj D., Viswanathan C., Kannan S., Ponpandian N. An in vitro analysis of H1N1 viral inhibition using polymer coated superparamagnetic Fe3O4 nanoparticles. RSC Adv. 2014;4:13409–13418. doi: 10.1039/C3RA47542E. [DOI] [Google Scholar]
- 91.Nangmenyi G., Li X., Mehrabi S., Mintz E., Economy J. Silver-modified iron oxide nanoparticle impregnated fiberglass for disinfection of bacteria and viruses in water. Mater. Lett. 2011;65:1191–1193. doi: 10.1016/j.matlet.2011.01.042. [DOI] [Google Scholar]
- 92.Ryan J.N., Harvey R.W., Metge D., Elimelech M., Navigato T., Pieper A.P. Field and laboratory investigations of inactivation of viruses (PRD1 and MS2) attached to iron oxide-coated quartz sand. Environ. Sci. Technol. 2002;36:2403–2413. doi: 10.1021/es011285y. [DOI] [PubMed] [Google Scholar]
- 93.Morones J.R., Elechiguerra J.L., Camacho A., Holt K., Kouri J.B., Ramírez J.T., Yacaman M.J. The bactericidal effect of silver nanoparticles. Nanotechnology. 2005;16(10):2346–2353. doi: 10.1088/0957-4484/16/10/059. doi:20050827031309. [DOI] [PubMed] [Google Scholar]
- 94.Lara H.H., Garza-Treviño E.N., Ixtepan-Turrent L., Singh D.K. Silver nanoparticles are broad-spectrum bactericidal and virucidal compounds. J. Nanobiotechnol. 2011;9:30. doi: 10.1186/1477-3155-9-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dung T.T.N., Nam V.N., Nhan T.T., Ngoc T.T.B., Minh L.Q., Nga B.T.T., Le V.P., Quang D.V. Silver nanoparticles as potential antiviral agents against African swine fever virus. Mater. Res. Express. 2020;6:1250g9. doi: 10.1088/2053-1591/ab6ad8. [DOI] [Google Scholar]
- 96.Dung T.T.N., Buu N.Q., Quang D.V., Ha H.T., Bang L.A., Chau N.H., Ly N.T., Trung N.V. Synthesis of nanosilver particles by reverse micelle method and study of their bactericidal properties. J. Phys. Conf. Ser. 2009;187 doi: 10.1088/1742-6596/187/1/012054. [DOI] [Google Scholar]
- 97.Xiang D.-x., Chen Q., Pang L., Zheng C.-l. Inhibitory effects of silver nanoparticles on H1N1 influenza A virus in vitro. J. Virol. Methods. 2011;178(1):137–142. doi: 10.1016/j.jviromet.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 98.Hui C., Cheung W.W.W., Zhang H., Au W., Yueng Y., Leung A.Y.H., Leung N., Luk J.M., Lie A.K.W., Kwong Y., Liang R., Lau G.K.K. Kinetics and risk of de novo hepatitis b infection in HBsAg–negative patients undergoing cytotoxic chemotherapy. Gastroenterology. 2006;131:59–68. doi: 10.1053/j.gastro.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 99.Lekutis C., Olshevsky U., Furman C., Thali M., Sodroski J. Contribution of disulfide bonds in the carboxyl terminus of the human immunodeficiency virus type I gp120 glycoprotein to CD4 binding. J. Acquir. Immune Defic. Syndr. 1992;5:78–81. [PubMed] [Google Scholar]
- 100.Kim J., Yeom M., Lee T., Kim H.-O., Na W., Kang A., Lim J.-W., Park G., Park C., Song D., Haam S. Porous gold nanoparticles for attenuating infectivity of influenza A virus. J. Nanobiotechnol. 2020;18:54. doi: 10.1186/s12951-020-00611-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Siriwardana K., Wang A., Gadogbe M., Collier W.E., Fitzkee N.C., Zhang D. Studying the effects of cysteine residues on protein interactions with silver nanoparticles. J. Phys. Chem. C Nanomater. Interfaces. 2015;119:2910–2916. doi: 10.1021/jp512440z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Huy T.Q., Hien Thanh N.T., Thuy N.T., Chung P.V., Hung P.N., Le A.-T., Hong Hanh N.T. Cytotoxicity and antiviral activity of electrochemical–synthesized silver nanoparticles against poliovirus. J. Virol. Methods. 2017;241:52–57. doi: 10.1016/j.jviromet.2016.12.015. [DOI] [PubMed] [Google Scholar]
- 103.Chen N., Zheng Y., Yin J., Li X., Zheng C. Inhibitory effects of silver nanoparticles against adenovirus type 3 in vitro. J. Virol. Methods. 2013;193:470–477. doi: 10.1016/j.jviromet.2013.07.020. [DOI] [PubMed] [Google Scholar]
- 104.Du T., Liang J., Dong N., Lu J., Fu Y., Fang L., Xiao S., Han H. Glutathione-capped Ag2S nanoclusters inhibit coronavirus proliferation through blockage of viral RNA synthesis and budding. ACS Appl. Mater. Interfaces. 2018;10:4369–4378. doi: 10.1021/acsami.7b13811. [DOI] [PubMed] [Google Scholar]
- 105.Fensterl V., Sen G.C. Interferon-induced ifit proteins: their role in viral pathogenesis. J. Virol. 2015;89:2462–2468. doi: 10.1128/JVI.02744-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Minoshima M., Lu Y., Kimura T., Nakano R., Ishiguro H., Kubota Y., Hashimoto K., Sunada K. Comparison of the antiviral effect of solid-state copper and silver compounds. J. Hazard. Mater. 2016;312:1–7. doi: 10.1016/j.jhazmat.2016.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Koide T., Ikenaka T. Studies on soybean trypsin inhibitors. Eur. J. Biochem. 1973;32:417–431. doi: 10.1111/j.1432-1033.1973.tb02624.x. [DOI] [PubMed] [Google Scholar]
- 108.Russell A.D., Hugo W.B. In: Progress in Medicinal Chemistry. Ellis G.P., Luscombe D.K., editors. Volume 31. Elsevier; 1994. Antimicrobial activity and action of silver; pp. 351–370. [DOI] [PubMed] [Google Scholar]
- 109.Gevondyan N.M., Volynskaia A.M., Gevondyan V.S. Four free cysteine residues found in human IgG1 of healthy donors. Biochemistry. 2006;71:279–284. doi: 10.1134/S0006297906030072. (Moscow) [DOI] [PubMed] [Google Scholar]
- 110.Castro-Mayorga J.L., Randazzo W., Fabra M.J., Lagaron J.M., Aznar R., Sánchez G. Antiviral properties of silver nanoparticles against norovirus surrogates and their efficacy in coated polyhydroxyalkanoates systems. LWT Food Sci. Technol. 2017;79:503–510. doi: 10.1016/j.lwt.2017.01.065. [DOI] [Google Scholar]
- 111.Wiegand C., Völpel A., Ewald A., Remesch M., Kuever J., Bauer J., Griesheim S., Hauser C., Thielmann J., Tonndorf-Martini S., Sigusch B.W., Weisser J., Wyrwa R., Elsner P., Hipler U.-C., Roth M., Dewald C., Lüdecke-Beyer C., Bossert J. Critical physiological factors influencing the outcome of antimicrobial testing according to ISO 22196/JIS Z 2801. PLOS One. 2018;13 doi: 10.1371/journal.pone.0194339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Suzuki S., Imai S., Kourai H. Background and evidence leading to the establishment of the JIS standard for antimicrobial products. Biocontrol Sci. 2006;11:135–145. doi: 10.4265/bio.11.135. [DOI] [PubMed] [Google Scholar]
- 113.Martínez-Abad A., Ocio M.J., Lagarón J.M., Sánchez G. Evaluation of silver-infused polylactide films for inactivation of Salmonella and feline calicivirus in vitro and on fresh-cut vegetables. Int. J. Food Microbiol. 2013;162:89–94. doi: 10.1016/j.ijfoodmicro.2012.12.024. [DOI] [PubMed] [Google Scholar]
- 114.Park S., Park H.H., Kim S.Y., Kim S.J., Woo K., Ko G. Antiviral properties of silver nanoparticles on a magnetic hybrid colloid. Appl. Environ. Microbiol. 2014;80:2343–2350. doi: 10.1128/AEM.03427-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Park H.H., Park S., Ko G., Woo K. Magnetic hybrid colloids decorated with Ag nanoparticles bite away bacteria and chemisorb viruses. J. Mater. Chem. B. 2013;1:2701–2709. doi: 10.1039/C3TB20311E. [DOI] [PubMed] [Google Scholar]
- 116.Xu H., Qu F., Xu H., Lai W., Andrew Wang Y., Aguilar Z.P., Wei H. Role of reactive oxygen species in the antibacterial mechanism of silver nanoparticles on Escherichia coli O157:H7. Biometals. 2012;25:45–53. doi: 10.1007/s10534-011-9482-x. [DOI] [PubMed] [Google Scholar]
- 117.Chen Y.-N., Hsueh Y.-H., Hsieh C.-T., Tzou D.-Y., Chang P.-L. Antiviral activity of graphene–silver nanocomposites against non-enveloped and enveloped viruses. Int. J. Environ. Res. Public Health. 2016;13:430. doi: 10.3390/ijerph13040430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rai M., Yadav A., Gade A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol. Adv. 2009;27:76–83. doi: 10.1016/j.biotechadv.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 119.Elechiguerra J.L., Burt J.L., Morones J.R., Camacho-Bragado A., Gao X., Lara H.H., Yacaman M.J. Interaction of silver nanoparticles with HIV-1. J. Nanobiotechnol. 2005;3:6. doi: 10.1186/1477-3155-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Shang J., Ye G., Shi K., Wan Y., Luo C., Aihara H., Geng Q., Auerbach A., Li F. Structural basis of receptor recognition by SARS-CoV-2. Nature. 2020;581:221–224. doi: 10.1038/s41586-020-2179-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kim J.S., Kuk E., Yu K.N., Kim J.-H., Park S.J., Lee H.J., Kim S.H., Park Y.K., Park Y.H., Hwang C.-Y., Kim Y.-K., Lee Y.-S., Jeong D.H., Cho M.-H. Antimicrobial effects of silver nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2007;3:95–101. doi: 10.1016/j.nano.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 122.Grass G., Rensing C., Solioz M. Metallic copper as an antimicrobial surface. Appl. Environ. Microbiol. 2011;77:1541–1547. doi: 10.1128/AEM.02766-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tamayo L., Azócar M., Kogan M., Riveros A., Páez M. Copper-polymer nanocomposites: an excellent and cost-effective biocide for use on antibacterial surfaces. Mater. Sci. Eng. C. 2016;69:1391–1409. doi: 10.1016/j.msec.2016.08.041. [DOI] [PubMed] [Google Scholar]
- 124.Rai M., Ingle A.P., Pandit R., Paralikar P., Shende S., Gupta I., Biswas J.K., da Silva S.S. Copper and copper nanoparticles: role in management of insect-pests and pathogenic microbes. Nanotechnol. Rev. 2018;7:303–315. doi: 10.1515/ntrev-2018-0031. [DOI] [Google Scholar]
- 125.Ermini M.L., Voliani V. Antimicrobial nano-agents: the copper age. ACS Nano. 2021;15:6008–6029. doi: 10.1021/acsnano.0c10756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Turnlund J.R. Human whole-body copper metabolism. Am. J. Clin. Nutr. 1998;67:960S–964S. doi: 10.1093/ajcn/67.5.960S. [DOI] [PubMed] [Google Scholar]
- 127.Fujimori Y., Sato T., Hayata T., Nagao T., Nakayama M., Nakayama T., Sugamata R., Suzuki K. Novel antiviral characteristics of nanosized copper(I) iodide particles showing inactivation activity against 2009 pandemic H1N1 influenza virus. Appl. Environ. Microbiol. 2012;78:951–955. doi: 10.1128/AEM.06284-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Shionoiri N., Sato T., Fujimori Y., Nakayama T., Nemoto M., Matsunaga T., Tanaka T. Investigation of the antiviral properties of copper iodide nanoparticles against feline calicivirus. J. Biosci. Bioeng. 2012;113:580–586. doi: 10.1016/j.jbiosc.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 129.Masarwa M., Cohen H., Meyerstein D., Hickman D.L., Bakac A., Espenson J.H. Reactions of low-valent transition-metal complexes with hydrogen peroxide. Are they “Fenton-like” or not? 1. The case of Cu+aq and Cr2+aq. J. Am. Chem. Soc. 1988;110:4293–4297. doi: 10.1021/ja00221a031. [DOI] [Google Scholar]
- 130.Cross J.B., Currier R.P., Torraco D.J., Vanderberg L.A., Wagner G.L., Gladen P.D. Killing of bacillus spores by aqueous dissolved oxygen, ascorbic acid, and copper ions. Appl. Environ. Microbiol. 2003;69:2245–2252. doi: 10.1128/AEM.69.4.2245-2252.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Burkitt M.J. A critical overview of the chemistry of copper-dependent low density lipoprotein oxidation: roles of lipid hydroperoxides, α-tocopherol, thiols, and ceruloplasmin. Arch. Biochem. Biophys. 2001;394:117–135. doi: 10.1006/abbi.2001.2509. [DOI] [PubMed] [Google Scholar]
- 132.Broglie J.J., Alston B., Yang C., Ma L., Adcock A.F., Chen W., Yang L. Antiviral activity of gold/copper sulfide core/shell nanoparticles against human norovirus virus-like particles. PLOS One. 2015;10 doi: 10.1371/journal.pone.0141050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Broglie J.J., Moore M.D., Jaykus L.-A., Yang L. Design and evaluation of three immuno-based assays for rapid detection of human norovirus virus-like particles. J. Anal. Bioanal. Tech. 2014;5:1–8. doi: 10.4172/2155-9872.1000220. [DOI] [Google Scholar]
- 134.Warnes S.L., Summersgill E.N., Keevil C.W. Inactivation of murine norovirus on a range of copper alloy surfaces is accompanied by loss of capsid integrity. Appl. Environ. Microbiol. 2015;81:1085–1091. doi: 10.1128/AEM.03280-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Tavakoli A., Hashemzadeh M.S. Inhibition of herpes simplex virus type 1 by copper oxide nanoparticles. J. Virol. Methods. 2020;275 doi: 10.1016/j.jviromet.2019.113688. [DOI] [PubMed] [Google Scholar]
- 136.Lakshmanan S.B., Zou X., Hossu M., Ma L., Yang C., Chen W. Local field enhanced Au/CuS nanocomposites as efficient photothermal transducer agents for cancer treatment. J. Biomed. Nanotechnol. 2012;8:883–890. doi: 10.1166/jbn.2012.1486. [DOI] [PubMed] [Google Scholar]
- 137.Maruzuru Y., Shindo K., Liu Z., Oyama M., Kozuka-Hata H., Arii J., Kato A., Kawaguchi Y. Role of herpes simplex virus 1 immediate early protein ICP22 in viral nuclear egress. J. Virol. 2014;88:7445–7454. doi: 10.1128/JVI.01057-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Chatterjee A.K., Chakraborty R., Basu T. Mechanism of antibacterial activity of copper nanoparticles. Nanotechnology. 2014;25 doi: 10.1088/0957-4484/25/13/135101. [DOI] [PubMed] [Google Scholar]
- 139.Hang X., Peng H., Song H., Qi Z., Miao X., Xu W. Antiviral activity of cuprous oxide nanoparticles against hepatitis C virus in vitro. J. Virol. Methods. 2015;222:150–157. doi: 10.1016/j.jviromet.2015.06.010. [DOI] [PubMed] [Google Scholar]
- 140.Chevaliez S., Pawlotsky J.-M. In: Hepatitis C Viruses: Genomes and Molecular Biology. Tan S.-L., editor. Horizon Bioscience; Norfolk (UK): 2006. HCV genome and life cycle; pp. 5–47. [Google Scholar]
- 141.Voisset C., Dubuisson J. Functional hepatitis C virus envelope glycoproteins. Biol. Cell. 2004;96:413–420. doi: 10.1016/j.biolcel.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 142.Banerjee N., Mukhopadhyay S. Viral glycoproteins: biological role and application in diagnosis. VirusDisease. 2016;27:1–11. doi: 10.1007/s13337-015-0293-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Y. Tong, D. Lavillette, Q. Li, J. Zhong, Role of hepatitis C virus envelope glycoprotein E1 in virus entry and assembly, Front. Immunol., p. 9. 〈10.3389/fimmu.2018.01411〉. [DOI] [PMC free article] [PubMed]
- 144.Evans M.J., von Hahn T., Tscherne D.M., Syder A.J., Panis M., Wölk B., Hatziioannou T., McKeating J.A., Bieniasz P.D., Rice C.M. Claudin-1 is a hepatitis C virus co-receptor required for a late step in entry. Nature. 2007;446:801–805. doi: 10.1038/nature05654. [DOI] [PubMed] [Google Scholar]
- 145.Mazurkow J.M., Yüzbasi N.S., Domagala K.W., Pfeiffer S., Kata D., Graule T. Nano-sized copper (oxide) on alumina granules for water filtration: effect of copper oxidation state on virus removal performance. Environ. Sci. Technol. 2020;54:1214–1222. doi: 10.1021/acs.est.9b05211. [DOI] [PubMed] [Google Scholar]
- 146.Sunada K., Minoshima M., Hashimoto K. Highly efficient antiviral and antibacterial activities of solid-state cuprous compounds. J. Hazard. Mater. 2012;235–236:265–270. doi: 10.1016/j.jhazmat.2012.07.052. [DOI] [PubMed] [Google Scholar]
- 147.Castro-Mayorga J.L., Rovira M.J.F., Mas L.C., Moragas G.S., Cabello J.M.L. Antimicrobial nanocomposites and electrospun coatings based on poly(3-hydroxybutyrate-co-3-hydroxyvalerate) and copper oxide nanoparticles for active packaging and coating applications. J. Appl. Polym. Sci. 2018;135:45673. doi: 10.1002/app.45673. [DOI] [Google Scholar]
- 148.Borkow G., Zhou S.S., Page T., Gabbay J. A novel anti-influenza copper oxide containing respiratory face mask. PLOS One. 2010;5 doi: 10.1371/journal.pone.0011295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Gabbay J., Borkow G., Mishal J., Magen E., Zatcoff R., Shemer-Avni Y. Copper oxide impregnated textiles with potent biocidal activities. J. Ind. Text. 2006;35:323–335. doi: 10.1177/1528083706060785. [DOI] [Google Scholar]
- 150.Borkow G., Gabbay J. Putting copper into action: copper-impregnated products with potent biocidal activities. FASEB J. 2004;18:1728–1730. doi: 10.1096/fj.04-2029fje. [DOI] [PubMed] [Google Scholar]
- 151.Hostynek J.J., Maibach H.I. Copper hypersensitivity: dermatologic aspects. Dermatol. Ther. 2004;17:328–333. doi: 10.1111/j.1396-0296.2004.04035.x. [DOI] [PubMed] [Google Scholar]
- 152.Ready T. FDA clears first antiviral surgical mask. Medscape. 2011 [Google Scholar]
- 153.O. US EPA, EPA Registers Copper Surfaces for Residual Use Against Coronavirus, 2021. 〈https://www.epa.gov/newsreleases/epa-registers-copper-surfaces-residual-use-against-coronavirus〉.
- 154.V. Prather, CDA Press Releases: 25 March 2008, U.S. EPA Approves Registration of Antimicrobial Copper Alloys, 2008. 〈https://www.copper.org/about/pressreleases/2008/pr2008_Mar_25.html〉.
- 155.Cupron, Cupron Reusable Face Masks – Copper Infused Reusable Face Masks, 2021. 〈https://cupron.com/cupron-reusable-face-masks/〉.
- 156.Cupron, Medical Textiles – Cupron, 2021. 〈https://cupronmedicaltextiles.com/medical-textiles/〉.
- 157.Eguchi H., Ikeda Y., Koyota S., Honke K., Suzuki K., Gutteridge J.M., Taniguchi N. Oxidative damage due to copper ion and hydrogen peroxide induces glcnac-specific cleavage of an Asn-linked oligosaccharide1. J. Biochem. 2002;131:477–484. doi: 10.1093/oxfordjournals.jbchem.a003124. [DOI] [PubMed] [Google Scholar]
- 158.Watanabe Y., Bowden T.A., Wilson I.A., Crispin M. Exploitation of glycosylation in enveloped virus pathobiology. Biochim. Biophys. Acta (BBA) Gen. Subj. 2019;1863:1480–1497. doi: 10.1016/j.bbagen.2019.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kimling J., Maier M., Okenve B., Kotaidis V., Ballot H., Plech A. Turkevich method for gold nanoparticle synthesis revisited. J. Phys. Chem. B. 2006;110:15700–15707. doi: 10.1021/jp061667w. [DOI] [PubMed] [Google Scholar]
- 160.De Clercq E., Li G. Approved antiviral drugs over the past 50 years. Clin. Microbiol. Rev. 2016;29:695–747. doi: 10.1128/CMR.00102-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Papp I., Sieben C., Ludwig K., Roskamp M., Böttcher C., Schlecht S., Herrmann A., Haag R. Inhibition of influenza virus infection by multivalent sialic-acid-functionalized gold nanoparticles. Small. 2010;6:2900–2906. doi: 10.1002/smll.201001349. [DOI] [PubMed] [Google Scholar]
- 162.Bowman M.-C., Ballard T.E., Ackerson C.J., Feldheim D.L., Margolis D.M., Melander C. Inhibition of HIV fusion with multivalent gold nanoparticles. J. Am. Chem. Soc. 2008;130:6896–6897. doi: 10.1021/ja710321g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Vonnemann J., Sieben C., Wolff C., Ludwig K., Böttcher C., Herrmann A., Haag R. Virus inhibition induced by polyvalent nanoparticles of different sizes. Nanoscale. 2014;6:2353–2360. doi: 10.1039/C3NR04449A. [DOI] [PubMed] [Google Scholar]
- 164.Finkelshtein D., Werman A., Novick D., Barak S., Rubinstein M. LDL receptor and its family members serve as the cellular receptors for vesicular stomatitis virus. Proc. Natl. Acad. Sci. USA. 2013;110:7306–7311. doi: 10.1073/pnas.1214441110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Carja G., Grosu E.F., Petrarean C., Nichita N. Self-assemblies of plasmonic gold/layered double hydroxides with highly efficient antiviral effect against the hepatitis B virus. Nano Res. 2015;8:3512–3523. doi: 10.1007/s12274-015-0851-6. [DOI] [Google Scholar]
- 166.Yao Q., Masters P.S., Ye R. Negatively charged residues in the endodomain are critical for specific assembly of spike protein into murine coronavirus. Virology. 2013;442:74–81. doi: 10.1016/j.virol.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Lan J., Ge J., Yu J., Shan S., Zhou H., Fan S., Zhang Q., Shi X., Wang Q., Zhang L., Wang X. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature. 2020;581:215–220. doi: 10.1038/s41586-020-2180-5. [DOI] [PubMed] [Google Scholar]
- 168.J. Hodek, V. Zajícová, I. Lovětinská-Šlamborová, I. Stibor, J. Müllerová, J. Weber, Protective hybrid coating containing silver, copper and zinc cations effective against human immunodeficiency virus and other enveloped viruses, BMC Microbiol., p. 16. 〈10.1186/s12866-016-0675-x〉. [DOI] [PMC free article] [PubMed]
- 169.Bondarenko O., Juganson K., Ivask A., Kasemets K., Mortimer M., Kahru A. Toxicity of Ag, CuO and ZnO nanoparticles to selected environmentally relevant test organisms and mammalian cells in vitro: a critical review. Arch. Toxicol. 2013;87:1181–1200. doi: 10.1007/s00204-013-1079-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Šlamborová I., Zajícová V., Karpíšková J., Exnar P., Stibor I. New type of protective hybrid and nanocomposite hybrid coatings containing silver and copper with an excellent antibacterial effect especially against MRSA. Mater. Sci. Eng. C Mater. Biol. Appl. 2013;33:265–273. doi: 10.1016/j.msec.2012.08.039. [DOI] [PubMed] [Google Scholar]
- 171.Ditta I.B., Steele A., Liptrot C., Tobin J., Tyler H., Yates H.M., Sheel D.W., Foster H.A. Photocatalytic antimicrobial activity of thin surface films of TiO2, CuO and TiO2/CuO dual layers on Escherichia coli and bacteriophage T4. Appl. Microbiol. Biotechnol. 2008;79:127–133. doi: 10.1007/s00253-008-1411-8. [DOI] [PubMed] [Google Scholar]
- 172.Haldar J., An D., de Cienfuegos L.Á., Chen J., Klibanov A.M. Polymeric coatings that inactivate both influenza virus and pathogenic bacteria. PNAS. 2006;103:17667–17671. doi: 10.1073/pnas.0608803103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Hsu B.B., Yinn Wong S., Hammond P.T., Chen J., Klibanov A.M. Mechanism of inactivation of influenza viruses by immobilized hydrophobic polycations. Proc. Natl. Acad. Sci. USA. 2011;108:61–66. doi: 10.1073/pnas.1017012108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Wang L., Hu C., Shao L. The antimicrobial activity of nanoparticles: present situation and prospects for the future. Int. J. Nanomed. 2017;12:1227–1249. doi: 10.2147/IJN.S121956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Raghunath A., Perumal E. Metal oxide nanoparticles as antimicrobial agents: a promise for the future. Int. J. Antimicrob. Agents. 2017;49:137–152. doi: 10.1016/j.ijantimicag.2016.11.011. [DOI] [PubMed] [Google Scholar]
- 176.Feldman C., Anderson R. The role of co-infections and secondary infections in patients with COVID-19. Pneumonia. 2021;13:5. doi: 10.1186/s41479-021-00083-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Khalid K., Tan X., Zaid H.F.M., Tao Y., Chew C.L., Chu D.-T., Lam M.K., Ho Y.-C., Lim J.W., Wei L.C. Advanced in developmental organic and inorganic nanomaterial: a review. Bioengineered. 2020;11:328–355. doi: 10.1080/21655979.2020.1736240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Neouze M.-A. Nanoparticle assemblies: main synthesis pathways and brief overview on some important applications. J. Mater. Sci. 2013;48:7321–7349. doi: 10.1007/s10853-013-7542-z. [DOI] [Google Scholar]
- 179.Wu W., Jiang C.Z., Roy V.A.L. Designed synthesis and surface engineering strategies of magnetic iron oxide nanoparticles for biomedical applications. Nanoscale. 2016;8:19421–19474. doi: 10.1039/c6nr07542h. [DOI] [PubMed] [Google Scholar]
- 180.Tripathi D., Modi A., Narayan G., Rai S.P. Green and cost effective synthesis of silver nanoparticles from endangered medicinal plant Withania coagulans and their potential biomedical properties. Mater. Sci. Eng. C. 2019;100:152–164. doi: 10.1016/j.msec.2019.02.113. [DOI] [PubMed] [Google Scholar]
- 181.Frey N.A., Peng S., Cheng K., Sun S. Magnetic nanoparticles: synthesis, functionalization, and applications in bioimaging and magnetic energy storage. Chem. Soc. Rev. 2009;38:2532–2542. doi: 10.1039/B815548H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Nikam A.V., Prasad B.L.V., Kulkarni A.A. Wet chemical synthesis of metal oxide nanoparticles: a review. CrystEngComm. 2018;20:5091–5107. doi: 10.1039/C8CE00487K. [DOI] [Google Scholar]
- 183.Liang K., Shu Hui L., Turak A. Probing the multi-step crystallization dynamics of micelle templated nanoparticles: structural evolution of single crystalline γ-Fe2O3. Nanoscale. 2019;11:9076–9084. doi: 10.1039/C9NR00148D. [DOI] [PubMed] [Google Scholar]
- 184.R. Arbi, A. Ibrahim, L. Goldring-Vandergeest, K. Liang, G. Hanta, L.S. Hui, A. Turak, Role of hydration and micellar shielding in tuning the structure of single crystalline iron oxide nanoparticles for designer applications, Nano Sel. n/a. 〈10.1002/nano.202100085〉.
- 185.Lee S.I., Yun G.J., Kim J.W., Hanta G., Liang K., Kojvic L., Hui L.S., Turak A., Kim W.Y. Improved hole injection for blue phosphorescent organic light-emitting diodes using solution deposited tin oxide nano-particles decorated ITO anodes. Sci. Rep. 2019;9:2411. doi: 10.1038/s41598-019-39451-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Stavis S.M., Fagan J.A., Stopa M., Liddle J.A. Nanoparticle manufacturing–heterogeneity through processes to products. ACS Appl. Nano Mater. 2018;1:4358–4385. doi: 10.1021/acsanm.8b01239. [DOI] [Google Scholar]
- 187.Tinkle S., McNeil S.E., Mühlebach S., Bawa R., Borchard G., Barenholz Y.C., Tamarkin L., Desai N. Nanomedicines: addressing the scientific and regulatory gap. Ann. N. Y. Acad. Sci. 2014;1313:35–56. doi: 10.1111/nyas.12403. [DOI] [PubMed] [Google Scholar]
- 188.S. Hua, M.B.C. de Matos, J.M. Metselaar, G. Storm, Current trends and challenges in the clinical translation of nanoparticulate nanomedicines: pathways for translational development and commercialization, Front. Pharmacol., p. 9. 〈10.3389/fphar.2018.00790〉. [DOI] [PMC free article] [PubMed]
- 189.G. Pasut, Grand challenges in nano-based drug delivery, Front. Med. Technol., p. 1. 〈10.3389/fmedt.2019.00001〉. [DOI] [PMC free article] [PubMed]
- 190.Crist R.M., Dasa S.S.K., Liu C.H., Clogston J.D., Dobrovolskaia M.A., Stern S.T. Challenges in the development of nanoparticle-based imaging agents: characterization and biology. WIREs Nanomed. Nanobiotechnol. 2021;13 doi: 10.1002/wnan.1665. [DOI] [PubMed] [Google Scholar]
- 191.Xia T., Li N., Nel A.E. Potential health impact of nanoparticles. Annu. Rev. Public Health. 2009;30:137–150. doi: 10.1146/annurev.publhealth.031308.100155. [DOI] [PubMed] [Google Scholar]
- 192.Vlasova I.I., Kapralov A.A., Michael Z.P., Burkert S.C., Shurin M.R., Star A., Shvedova A.A., Kagan V.E. Enzymatic oxidative biodegradation of nanoparticles: mechanisms, significance and applications. Toxicol. Appl. Pharmacol. 2016;299:58–69. doi: 10.1016/j.taap.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Essa D., Kondiah P.P.D., Choonara Y.E., Pillay V. The design of poly(lactide-co-glycolide) nanocarriers for medical applications. Front. Bioeng. Biotechnol. 2020;8:48. doi: 10.3389/fbioe.2020.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Zhang Q., Honko A., Zhou J., Gong H., Downs S.N., Vasquez J.H., Fang R.H., Gao W., Griffiths A., Zhang L. Cellular nanosponges inhibit SARS-CoV-2 infectivity. Nano Lett. 2020;20:5570–5574. doi: 10.1021/acs.nanolett.0c02278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Wang X., Ji Z., Chang C.H., Zhang H., Wang M., Liao Y.-P., Lin S., Meng H., Li R., Sun B., Winkle L.V., Pinkerton K.E., Zink J.I., Xia T., Nel A.E. Use of coated silver nanoparticles to understand the relationship of particle dissolution and bioavailability to cell and lung toxicological potential. Small. 2014;10:385–398. doi: 10.1002/smll.201301597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Yen H.-J., Hsu S.-h., Tsai C.-L. Cytotoxicity and immunological response of gold and silver nanoparticles of different sizes. Small. 2009;5(13):1553–1561. doi: 10.1002/smll.200900126. [DOI] [PubMed] [Google Scholar]
- 197.Desai N. Challenges in development of nanoparticle-based therapeutics. AAPS J. 2012;14:282–295. doi: 10.1208/s12248-012-9339-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Peddinti B.S., Scholle F., Ghiladi R.A., Spontak R.J. Photodynamic polymers as comprehensive anti-infective materials: staying ahead of a growing global threat. ACS Appl. Mater. Interfaces. 2018;10:25955–25959. doi: 10.1021/acsami.8b09139. [DOI] [PubMed] [Google Scholar]