Abstract
Purpose
Low socioeconomic status (SES) is associated with advanced stage, lower-quality care, and higher mortality among breast cancer patients. The purpose of this study is to examine the association between neighborhood SES (nSES), surgical management, and disease-specific mortality in de novo metastatic breast cancer (MBC) patients in the Surveillance, Epidemiology, and End Results (SEER) Program.
Methods
MBC patients ages 18 to 85+ years diagnosed from 2010 through 2016 were identified in SEER. The cohort was divided into low, middle, and high nSES based on the NCI census tract-level index. Univariable and multivariable analyses were used to examine the relationship between nSES, surgery, and disease specific mortality in MBC patients.
Results
There were 24,532 de novo MBC patients who met study criteria, with 28.7 % undergoing surgery. Over the study period, surgery utilization decreased across all nSES groups. However, lower nSES was associated with a higher odds of undergoing surgery (low OR 1.25 [1.15–1.36] p < 0.001; middle OR 1.09 [1.01–1.18] p = 0.022; ref high). Living in an area with lower SES was associated with a worse disease specific mortality (low HR 1.24 [1.25, 1.44; ], middle 1.20 [1.1–1.29]: ref high). Specifically, there was a 9.26 month mean survival differences between the lowest (41.02 ± 0.47 months) and highest (50.28 ± 0.47 months) nSES groups.
Conclusion
These results suggest area of residence may contribute to differences in surgical management and clinical outcomes among de novo MBC patients. Future studies should examine the contributions of patient characteristics and preferences within the context of surgeon recommendations.
Keywords: Metastatic breast cancer, Neighborhood socioeconomic index, Surgery, Survival
Highlights
-
•
Utilization of surgical management for de novo metastatic breast cancer is decreasing.
-
•
Low nSES is associated with a higher odds of undergoing surgery among de novo MBC patients.
-
•
MBC patients residing in low SES neighborhoods have a worse disease specific mortality than those in high SES areas.
1. Introduction
Neighborhood is a significant social determinant of health as it provides access to food, transportation, employment opportunities, health care and affects environmental exposures [1]. In the United States, the relationship between neighborhood and health is so intertwined that zip code can predict life expectancy, health status, and clinical outcomes [2]. For example, breast cancer incidence is lower among women living in neighborhoods with low socioeconomic status (SES) than those in areas with high SES [3]. Nevertheless, despite a low breast cancer incidence, non-metastatic breast cancer patients living in low SES neighborhoods are more likely to present with advanced stages of disease, more aggressive subtypes, and have a higher disease-specific mortality compared to their counterparts in areas with high SES [4]. Additionally, breast cancer patients living in neighborhoods with low SES are less likely to receive guideline-concordant surgical care than patients in areas with high nSES [5]. Possible explanations for these nSES disparities in incidence, treatment, and mortality are most likely an interplay between mutiple social determinants of health. [[6], [7], [8], [9]]. Cumulatively, these results suggest neighborhood has significant implications for treatment and survival among breast cancer patients.
To date, there are a paucity of studies on the interplay between neighborhood socioeconomic status (nSES), patterns of surgical management, and survival among MBC patients. This deficit in the literature is significant as de novo MBC patients account for approximately 6 % of all breast cancer patients in the United States [10]. Additionally, although systemic therapy is the main treatment modality among MBC patients, approximately 25 % of MBC patients undergo surgery [[11], [12], [13]]. Informed by the inconsistent evidence of the benefits of surgery, current National Comprehensive Cancer Center (NCCN) guidelines discourage routine surgical resection in de novo stage IV breast cancer patients but indicate surgery can be considered for local control or in the setting of a response to systemic treatment [[14], [15], [16]]. Unfortunately, the guidelines do not explicate surgery type (breast conservation surgery versus mastectomy), management of the axilla or reconstruction use in the metastatic setting if local control is pursued. The purpose of this study is to fill current knowledge gaps on the relationship between nSES, surgical management, and disease-specific mortality among de novo MBC patients using the Surveillance Epidemiology and End Result (SEER) Program. Despite the uncertainty of the benefits of surgery in MBC patients, understanding patterns of surgical care within the context of social determinants of health such as nSES provide stakeholders and policymakers useful information on access to and receipt of surgical care in this population. Moreover, results from this study will provide currently unavailable information on trends in surgical care among MBC patients based on area of residence.
2. Materials and methods
2.1. Database
Data from the SEER Program were used to identify females ages 18 to 85+ years diagnosed from January 01, 2010 through 12/31/2016 with distant stage breast cancer. Patients diagnosed at autopsy or with an unknown stage were excluded from the study. SEER is a collection of high-quality population-based cancer registries with high estimated completeness of reporting. These registries capture data covering approximately 28 % of the U.S. population [17]. Data reflecting demographic and clinical factors (including surgery and survival/death) of patients diagnosed with distant stage breast cancer and included in 18 SEER registries were obtained from case listing sessions using SEER∗Stat software (version 8.3.8) [18]. First-course treatment data reflecting radiation therapy and chemotherapy factors were obtained as a result of permission from the SEER Program to access custom data (please see Discussion Section for limitations and biases associated with these data). Additionally, specialized census tract-level SES and rurality data were obtained as a result of permission from the SEER Program. Due to our interests in nSES, only patients with an nSES index were included in the final analysis; Neighborhood SES was operationalized using the NCI census tract-level index. The NCI census tract-level index is a time-dependent composite score comprised of an education index, household income, percent below 150 % of poverty line, median house value, percent unemployed, median rent, and percent working class [19,20]. The scores have been categorized into tertiles, with the lowest tertile representing a low nSES and the highest tertile representing a high nSES. Due to their small numbers individuals who did not identify as White or Black were aggregated in the other category.
2.2. Statistical analysis
The study cohort was divided into tertiles (low nSES, middle nSES, high nSES) established by the SEER Program. Sociodemographic variables (age, race, ethnicity, marital status, insurance status, region of care delivery), clinical variables (tumor size, grade, cancer subtype, histology, metastasis site, number of metastatic sites), treatment variables (primary surgery, reconstructive surgery, lymph node surgery, radiation therapy, chemotherapy) and the outcome variable (disease-specific survival) were tabulated as frequencies for the categorical variables and means, with their standard deviation (S.D.), for continuous variables. Pearson's Chi-Square tests and analysis of variance were used, as appropriate, for intergroup bivariate analysis. Lymph node evaluation was dichotomized as no lymph node surgery versus lymph node surgery.
A multivariable analysis was performed using age, race, insurance status, marital status, neighborhood socioeconomic status, region of care delivery, chemotherapy status, and number of metastatic sites as independent variables to determine the odds of undergoing surgery. Independent variables were selected based on their association or recommendations to be considered for surgical decision making [6,14,[21], [22], [23], [24], [25]]. Odds ratios were reported along with the Wald Chi-Square tests. Disease-specific mortality is defined by SEER as the date of diagnosis to date of death due to distant stage breast cancer [26]. A multivariable Cox Proportional Hazards model was created using age, race, neighborhood socioeconomic status, marital status, insurance status, grade, cancer subtype, histology, number of metastases, radiation therapy status, and surgery status, as independent variables and disease-specific mortality as the dependent variable. Cancer subtypes are defined by SEER as luminal A (hormone receptor [HR] positive, human epidermal growth factor [HER 2] negative), Luminal B (HR+/HER 2-), HER2 enriched (HR-/HER 2+) and triple negative (HR-/HER 2-) [27]. Hazard ratios were reported along with the Wald Chi-Square tests. A p-value of 0.05 was considered statistically significant.
A backward stepwise regression approach was used for the multivariate logistic regression model and the Cox proportional hazard model to check for parsimony. First, the full model with all the variables was considered, and then gradually, other variables were eliminated from the model based on a cut-off p-value of 0.1. This study's statistical analysis was performed in Stata software Version 16.0 (Stata Corporation, College Station, TX). The Ohio State University Office of Responsible Research Practices deemed this study IRB exempt.
3. RESULTS
3.1. Description of study population
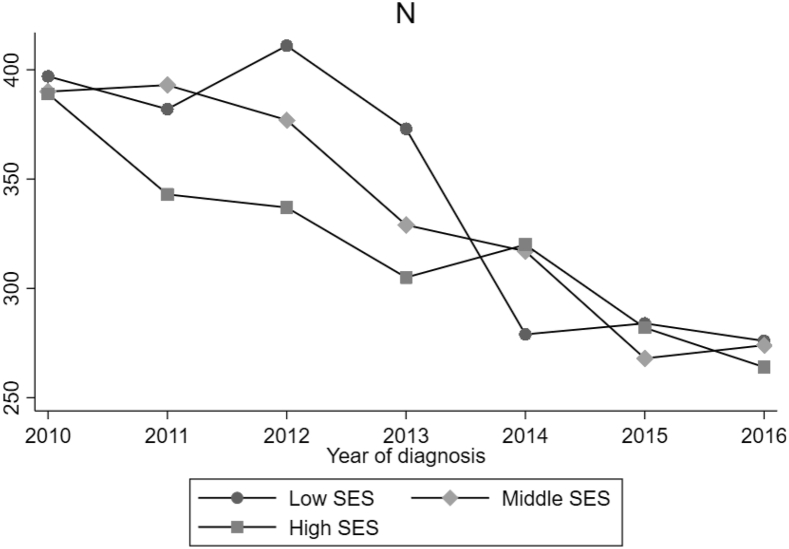
24,532 patients met the study criteria with a mean age of 62 years (SD ± 13.97). 28.7 % of patients in the study cohort underwent surgery. Compared to patients in neighborhoods with middle and high socioeconomic status, a higher percentage of MBC patients living in areas of low nSES were younger, identified as Black race, were of Hispanic ethnicity, separated or divorced, had care delivered in the Eastern region and insured through Medicaid coverage (Table 1). Additionally, there were also statistically significant differences between the nSES groups on clinical factors such as tumor size (p < 0.001), grade (p < 0.001), tumor subtype (p < 0.001), and the number of distant metastasis sites (p < 0.001) (Table 1). Over the study period, surgical management for breast cancer decreased among all nSES groups (Fig. 1).
Table 1.
Description of study sociodemographic and clinical variablesa.
| Variable | Total sample N = 24,532 | Low nSES N = 8060 (32.9) | Middle nSES N = 8293 (33.8) | High nSES N = 8179 (33.3) | P-value |
|---|---|---|---|---|---|
| Age (years, continuous) Mean (SD) | 62.21 (13.97) | 61.20 (13.90) | 62.59 (13.90) | 62.80 (14.07) | <0.001 |
| Age (years) | |||||
| ≤40 | 1730 (7.1) | 619 (7.7) | 562 (6.8) | 549 (6.7) | <0.001 |
| 41–64 | 12,071 (49.2) | 4174 (51.8) | 4012 (48.4) | 3885 (47.5) | |
| ≥65 | 10,731 (43.7) | 3267 (40.5) | 3719 (44.8) | 3745 (45.8) | |
| Race | |||||
| White | 18,481 (75.3) | 5115 (63.4) | 6556 (79.1) | 6810 (83.3) | <0.001 |
| Black | 4109 (16.8) | 2554 (31.7) | 1065 (12.8) | 490 (6.0) | |
| Other | 1841 (7.5) | 360 (4.5) | 646 (7.8) | 835 (10.2) | |
| Unknown | 101 (0.4) | 31 (0.4) | 26 (0.3) | 44 (0.5) | |
| Hispanic | |||||
| No | 21,823 (89.0) | 6875 (85.3) | 7351 (88.6) | 7597 (92.9) | <0.001 |
| Yes | 2709 (11.0) | 1185 (14.7) | 942 (11.4) | 582 (7.1) | |
| Marital Status | |||||
| Married/Partnered | 10,392 (42.4) | 2788 (34.6) | 3609 (43.5) | 3995 (48.8) | <0.001 |
| Separated/divorced | 3227 (13.2) | 1224 (15.2) | 1088 (13.1) | 915 (11.2) | |
| Single | 5158 (21.0) | 2130 (26.4) | 1627 (19.6) | 1401 (17.1) | |
| Unmarried/Domestic Partner | 70 (0.3) | 18 (0.2) | 21 (0.3) | 31 (0.4) | |
| Widowed | 4256 (17.3) | 1410 (17.5) | 1454 (17.5) | 1392 (17.0) | |
| Unknown | 1429 (5.8) | 490 (6.1) | 494 (6.0) | 445 (5.5) | |
| Year of Diagnosis | |||||
| 2010 | 3329 (13.6) | 1051 (13.0) | 1134 (13.7) | 1144 (14.0) | 0.14 |
| 2011 | 3374 (13.8) | 1172 (14.6) | 1087 (13.1) | 1115 (13.6) | |
| 2012 | 3422 (13.9) | 1148 (14.2) | 1153 (13.9) | 1121 (13.7) | |
| 2013 | 3614 (14.7) | 1235 (15.3) | 1217 (14.7) | 1162 (14.2) | |
| 2014 | 3608 (14.7) | 1151 (14.3) | 1234 (14.9) | 1223 (15.0) | |
| 2015 | 3560 (14.5) | 1153 (14.3) | 1219 (14.7) | 1188 (14.5) | |
| 2016 | 3625 (14.8) | 1150 (14.3) | 1249 (15.0) | 1226 (15.0) | |
| Region | |||||
| East | 10,188 (41.5) | 4040 (50.1) | 3114 (37.5) | 3034 (37.1) | <0.001 |
| Northern Plains | 2369 (9.7) | 914 (11.3) | 1026 (12.4) | 429 (5.2) | |
| Pacific Cost | 10,992 (44.8) | 2796 (34.7) | 3723 (44.9) | 4473 (54.7) | |
| Southwest | 983 (4.0) | 310 (3.8) | 430 (5.2) | 243 (3.0) | |
| Insurance | |||||
| Uninsured | 960 (3.9) | 438 (5.4) | 300 (3.6) | 222 (2.7) | <0.001 |
| Medicaid | 4686 (19.1) | 2378 (29.5) | 1443 (17.4) | 865 (10.6) | |
| Insured | 18,217 (74.3) | 5024 (62.4) | 6311 (76.1) | 6882 (84.1) | |
| Unknown | 669 (2.7) | 220 (2.7) | 239 (2.9) | 210 (2.6) | |
| Tumor size | |||||
| ≤2 cm | 592 (3.1) | 152 (2.4) | 217 (3.4) | 223 (3.5) | <0.001 |
| >2 cm–5cm | 11,173 (58.6) | 3598 (57.8) | 3741 (58.1) | 3834 (60.0) | |
| ≥5 cm | 7289 (38.3) | 2479 (39.8) | 2481 (38.5) | 2329 (36.5) | |
| Grade | |||||
| Well-differentiated | 1505 (6.1) | 447 (5.5) | 535 (6.5) | 523 (6.4) | <0.001 |
| Moderately differentiated | 7527 (30.7) | 2342 (29.1) | 2599 (31.3) | 2586 (31.6) | |
| Poorly differentiated | 8876 (36.2) | 3105 (38.5) | 2957 (35.7) | 2814 (34.4) | |
| Undifferentiated/anaplastic | 155 (0.6) | 51 (0.6) | 61 (0.7) | 43 (0.5) | |
| Unknown | 6469 (26.4) | 2115 (26.3) | 2141 (25.8) | 2213 (27.1) | |
| Breast Cancer Subtype | |||||
| Luminal A | 12,377 (50.4) | 3800 (47.1) | 4243 (51.2) | 4334 (53.0) | <0.001 |
| Luminal B | 3392 (13.8) | 1105 (13.7) | 1115 (13.4) | 1172 (14.3) | |
| HER 2 enriched | 1828 (7.5) | 626 (7.8) | 605 (7.3) | 597 (7.3) | |
| Triple-Negative | 2939 (12.0) | 1125 (14.0) | 1007 (12.1) | 807 (9.9) | |
| Unknown | 3996 (16.3) | 1404 (17.4) | 1323 (16.0) | 1269 (15.5) | |
| Histology | |||||
| Ductal | 15,698 (64.0) | 5320 (66.0) | 5355 (64.6) | 5023 (61.4) | <0.001 |
| Lobular | 2598 (10.6) | 697 (8.7) | 879 (10.6) | 1022 (12.5) | |
| Mixed (ductal and lobular) | 989 (4.0) | 270 (3.3) | 346 (4.2) | 373 (4.6) | |
| Other | 5247 (21.4) | 1773 (22.0) | 1713 (20.6) | 1761 (21.5) | |
| Metastasis | |||||
| Bone only | 8222 (33.5) | 2560 (31.8) | 2793 (33.7) | 2869 (35.1) | <0.001 |
| Liver only | 1501 (6.1) | 485 (6.0) | 468 (5.6) | 548 (6.7) | |
| Lung only | 2311 (9.4) | 806 (10.0) | 798 (9.6) | 707 (8.6) | |
| Brain only | 308 (1.3) | 126 (1.6) | 104 (1.3) | 78 (1.0) | |
| ≥2 metastasis | 7282 (29.7) | 2450 (30.4) | 2465 (29.7) | 2367 (28.9) | |
| Other | 4553 (18.6) | 1520 (18.8) | 1546 (18.7) | 1487 (18.2) | |
| Unknown | 355 (1.4) | 113 (1.4) | 119 (1.4) | 123 (1.5) | |
| Surgery | |||||
| No | 17,332 (71.3) | 5567 (69.9) | 5888 (71.5) | 5877 (72.4) | 0.001 |
| Yes | 6990 (28.7) | 2402 (30.1) | 2348 (28.5) | 2240 (27.6) | |
| Radiation therapy | |||||
| No/unknown | 17.145 (69.9) | 5719 (71.0) | 5710 (68.9) | 5716 (69.9) | 0.014 |
| yes | 7387 (30.1) | 2341 (29.0) | 2583 (31.1) | 2463 (30.1) | |
| Chemotherapy | |||||
| No/unknown | 11,707 (47.7) | 3794 (47.1) | 3993 (48.1) | 3920 (47.9) | 0.35 |
| Yes | 12,825 (52.3) | 4266 (52.9) | 4300 (51.9) | 4259 (52.1) | |
| Mean survival (S.E.) months | 45.41 (0.27) | 41.02 (0.47) | 44.77 (0.47) | 50.28 (0.47) | <0.001 |
Datapoints reported as n (%) unless specified otherwise. Data may not add up to 100 % due to rounding.
Fig. 1.
Surgical Management Over Study Period Stratified by nSES.
3.2. Systemic and locoregional management
On univariate analysis there appeared to be a trend of increasing surgery use as nSES decreased (low nSES 30.1 %; middle nSES 28.5 %; high nSES 27.6 %; p 0.001) (Table 1). Among patients who had surgery, mastectomy use increased as nSES decreased (low nSES 72.7 %; middle nSES 71.6 %; high nSES 69.2 %; p = 0.028) (Table 2). A slightly lower percentage of MBC patients living in low nSES (29 %) areas received radiation therapy compared to those in middle (31.1 %) and high (30.1 %) nSES (p = 0.014) communities (Table 1). On subset analysis, 41.8 % of patients residing in neighborhoods with low nSES who underwent breast conservation surgery received radiation therapy compared to 49.2 % of patients living in middle nSES areas and 45.6 % of patients in high nSES areas (p = 0.030). Reconstruction use increased with nSES (low nSES (8 %), middle nSES (15.2 %) and high nSES (22.2 %); p < 0.001) (Table 2). Notably, there was no significant difference between the groups on the use of chemotherapy (p = 0.35) (Table 1).
Table 2.
Description of surgical management in study populationa.
| Total N = 6926 |
Low nSES N = 2381 (34.4) |
Middle nSES N = 2324 (33.5) |
High nSES N = 2221 (32.1) |
P-value | |
|---|---|---|---|---|---|
| Surgery Type | 0.028 | ||||
| BCSb | 1993 (28.8) | 650 (27.3) | 659 (28.4) | 684 (30.8) | |
| Mastectomy | 4933 (71.2) | 1731 (72.7) | 1665 (71.6) | 1537 (69.2) | |
| BCTc | 0.029 | ||||
| No/Unknown | 1085 (54.4) | 378 (58.2) | 335 (50.8) | 372 (54.4) | |
| Yes | 908 (45.6) | 272 (41.8) | 324 (49.2) | 312 (45.6) | |
| Mastectomy-Radiation | 0.150 | ||||
| None/Unknown | 2772 (56.2) | 1004 (58.0) | 913 (54.8) | 855 (55.6) | |
| Yes | 2161 (43.8) | 727 (42.0) | 752 (45.2) | 682 (44.4) | |
| Reconstruction | <0.001 | ||||
| No | 4200 (85.1) | 1592 (92.0) | 1412 (84.8) | 1196 (77.8) | |
| Yes | 733 (14.9) | 139 (8.0) | 253 (15.2) | 341 (22.2) | |
| BCS-lymph node Surgeryd | 0.004 | ||||
| No | 771 (39.7) | 285 (44.9) | 232 (36.5) | 254 (37.7) | |
| Yes | 1173 (60.3) | 350 (55.1) | 404 (63.5) | 419 (62.3) | |
| Mastectomy-lymph node surgerye | 0.890 | ||||
| No | 867 (18.1) | 302 (18.1) | 298 (18.4) | 267 (17.7) | |
| Yes | 3924 (81.9) | 1364 (81.9) | 1322 (81.6) | 1238 (82.3) |
Datapoints reported as n (%) unless specified otherwise. Data may not add up to 100 % due to rounding.
Breast Conservation Surgery (BCS).
Breast Conservation therapy (BCS + radiation).
This data point only includes patients who underwent breast conservation surgery.
This data point only includes patients who underwent mastectomy.
On multivariable analysis, the probability of undergoing surgery was higher for groups residing in neighborhoods with low (OR 1.25, 95 % CI 1.15–1.36; p < 0.001) and middle (OR 1.10, 95 % CI 1.01–1.18; p = 0.022) SES compared to patients from neighborhoods with high nSES. Conversely, increasing age, Black race, uninsured or Medicaid insurance status, ≥two metastatic sites, and single marital status (separated/divorced, single, unmarried/Domestic partner, widowed) reduced the probability of undergoing surgery (Table 3).
Table 3.
Multivariable logistic regression model to predict the probability of surgery.
| Variable | Odds Ratio | 95 % Confidence Interval | P-Value |
|---|---|---|---|
| Age (continuous) | 0.98 | (0.97 0.98) | <0.001 |
| Race | |||
| White | Ref | ||
| Black | 0.95 | (0.87 1.04) | 0.302 |
| Other | 1.04 | (0.92 1.17) | 0.550 |
| Insurance | |||
| Insured | Ref | ||
| Uninsured | 0.58 | (0.49 0.69) | <0.001 |
| Medicaid | 0.80 | (0.74 0.87) | <0.001 |
| Marital Status | |||
| Married/Partnered | Ref | ||
| Separated/divorced | 0.88 | (0.80 0.96) | 0.007 |
| Single | 0.74 | (0.68 0.80) | <0.001 |
| Unmarried/Domestic Partner | 0.52 | (0.29 0.92) | 0.026 |
| Widowed | 0.97 | (0.88 1.06) | 0.499 |
| nSES | |||
| Low | 1.25 | (1.15 1.36) | <0.001 |
| Middle | 1.09 | (1.01 1.18) | 0.022 |
| High | Ref | ||
| Region | |||
| East | Ref | ||
| Northern Plains | 1.13 | (1.02 1.26) | 0.025 |
| Pacific Coast | 1.08 | (1.01 1.16) | 0.026 |
| Southwest | 1.25 | (1.07 1.46) | 0.005 |
| Chemotherapy | |||
| Yes | Ref | ||
| No/Unknown | 0.43 | (0.40 0.46) | <0.001 |
| Number of Metastasis | |||
| 1 | Ref | ||
| ≥2 | 0.36 | (0.34 0.39) | <0.001 |
3.3. Survival
A Cox proportional hazard model analysis showed living in neighborhoods with low (HR 1.34, 95 % CI 1.25–1.44; p < 0.001) and middle (HR 1.20, 95 % CI 1.13–1.29; p < 0.001) nSES was associated with an increased disease specific mortality compared to patients residing in areas of high nSES. Furthermore, Black race (HR 1.16 [1.08–1.25] p < 0.001; ref White), single marital status (separated/divorced HR 1.15 [1.06–1.24] p = 0.001; single HR 1.15 [1.07–1.24] p < 0.001, widowed HR 1.29 [1.19–1.40] p < 0.001; ref married/partnered); uninsured status or Medicaid insured status (Uninsured HR 1.43 [1.26–1.63]; p < 0.001], Medicaid insurance HR 1.21 [1.13–1.30; p < 0.001]; Ref Insured) were associated with increased disease specific mortality. Also, increasing tumor grade (moderately differentiated HR 1.40, [1.25–1.58] p < 0.001; poorly differentiated HR 2.03 [1.80–2.29] p < 0.001; ref well differentiated) were associated with a worse disease specific mortality. Receipt of surgery was associated with a reduction in the relative risk of disease specific mortality (HR 0.52, CI 0.49–0.56; p < 0.001; REF no surgery) (Table 4).
Table 4.
Multivariate cox proportional hazard model to predict disease-specific survival.
| Variable | Hazards Ratio | 95 % Confidence Interval | P-Value |
|---|---|---|---|
| Age (continuous) | 1.01 | (1.01 1.02) | <0.001 |
| Race | |||
| White | Ref | ||
| Black | 1.16 | (1.08 1.25) | <0.001 |
| Other | 0.99 | (0.89 1.10) | 0.961 |
| nSES | |||
| Low | 1.34 | (1.25 1.44) | <0.001 |
| Middle | 1.20 | (1.13 1.29) | <0.001 |
| High | Ref | ||
| Marital Status | |||
| Married/Partnered | Ref | ||
| Separated/divorced | 1.15 | (1.06 1.24) | 0.001 |
| Single | 1.15 | (1.07 1.24) | <0.001 |
| Unmarried/Domestic Partner | 0.94 | (0.57 1.57) | 0.828 |
| Widowed | 1.29 | (1.19 1.40) | <0.001 |
| Year of Diagnosis | |||
| 2010 | Ref | ||
| 2011 | 1.00 | (0.92 1.09) | 0.919 |
| 2012 | 0.99 | (0.90 1.08) | 0.758 |
| 2013 | 0.97 | (0.89 1.06) | 0.533 |
| 2014 | 0.87 | (0.79 0.97) | 0.009 |
| 2015 | 0.89 | (0.79 0.99) | 0.049 |
| 2016 | 0.84 | (0.72 0.99) | 0.039 |
| Insurance | |||
| Insured | Ref | ||
| Uninsured | 1.43 | (1.26 1.63) | <0.001 |
| Medicaid | 1.21 | (1.13 1.30) | <0.001 |
| Grade | |||
| Well-differentiated | Ref | ||
| Moderately differentiated | 1.40 | (1.25 1.58) | <0.001 |
| Poorly differentiated | 2.03 | (1.80 2.29) | <0.001 |
| Undifferentiated | 1.87 | (1.39 2.51) | <0.001 |
| Breast Cancer Subtype | |||
| Luminal A | Ref | ||
| Luminal B | 0.78 | (0.72 0.85) | <0.001 |
| HER 2 enriched | 0.95 | (0.85 1.05) | 0.313 |
| Triple-Negative | 3.19 | (2.31 2.68) | <0.001 |
| Histology | |||
| Ductal | Ref | ||
| Lobular | 1.26 | (1.15 1.38) | <0.001 |
| Mixed (ductal and lobular) | 1.07 | (0.94 1.22) | 0.301 |
| Other | 1.18 | (1.07 1.30) | 0.001 |
| Number of Metastasis | |||
| 1 | Ref | ||
| ≥2 | 1.68 | (1.59 1.79) | <0.001 |
| Surgery status | |||
| No | Ref | ||
| Yes | 0.52 | (0.49 0.56) | <0.001 |
| Radiation Therapy | |||
| No/Unknown | Ref | ||
| Yes | 1.09 | (1.03 1.16) | 0.001 |
3.4. Supplementary analysis
On supplementary analysis, when stratified by tumor size, there was no significant difference between the three neighborhood groups on the use of breast conservation surgery versus mastectomy (supplementary table 1). Additionally, there was no difference in surgical management among rural patients by nSES. However, there was an increasing trend of surgery use among patients living in urban areas as nSES decreased (p = 0.013). When stratified by age, among patients age <45, there was an increasing trend in surgery use with increasing nSES (p = 0.041). Conversely, among patients ≥65 years, there was a decreasing trend in surgery use with increasing nSES (p < 0.001). Evaluation of surgery use by SEER region suggests increasing surgery use with decreasing nSES in the East SEER region (p < 0.001). While in the Northern Plains and Southwest, increasing nSES was associated with increasing surgery use (p < 0.015) (Appendix A).
4. Discussion
Our examination of de novo MBC patients in SEER indicates the use of surgical management is decreasing in this population. Additionally, study results suggest there is an association between nSES, surgical management, and survival. Although there were statistically significant differences in the utilization of mastectomy, surgical lymph node evaluation and post mastectomy reconstruction among the nSES groups the clinical implications of these findings are unclear. Specifically, the lack of clearly explicated guidelines on locoregional management of the axilla, surgery type utilization (mastectomy vs lumpectomy) and reconstruction make it difficult to contextualize these findings. Nevertheless, study results suggest disparities in disease specific mortality by nSES among de novo MBC patients’ parallel findings in non-metastatic patients.
Currently, surgical management among MBC patients is mostly palliative [24]. The literature on the impact of surgical management on survival in MBC patients has been inconsistent. Retrospective studies suggest surgery improves survival for MBC patients with limited disease (i.e., oligometastatic metastasis) [28,29]. Conversely, recent prospective trials evaluating surgical management among MBC patients showed no survival benefit [16,30]. We anticipate the likely drivers of the decreasing utilization of surgery in the study cohort and differences in patterns of surgical care by nSES are a combination of inconclusive study results on surgery's benefit in conjunction with disparate interpretations of evolving national guidelines on surgical management.
Of note, our supplementary analysis suggests age and geography (rural/urban status or SEER region) may also contribute to differences in surgical management by nSES. Consequently, a plausible explanation for the higher rates of surgical management in patients from low SES areas could be a complex interplay between differences in the extent of locally advanced disease, age and geography. Patients living in areas of low SES historically face barriers in accessing healthcare and may present with locally advanced tumors, such as fungating masses, that require surgical management to improve quality of life with no curative intent.
The reasons why those who identified as Black race, uninsured or Medicaid insured, and were of single marital status were less likely to receive surgical management is most likely due to a combination of disease presentation, patient preference, and surgeon recommendations. Black, uninsured, and Medicaid patients face barriers in receiving surgical care in the nonmetastatic setting, and we anticipate factors driving surgical access in MBC patients are similar [6,31].
Multivariable analysis suggests residing in a low SES area was associated with a 35 % increased relative risk of death compared to those residing in neighborhoods with high SES. The association between low nSES and increased disease-specific mortality is unsurprising. Low individual and neighborhood socioeconomic status have both been implicated in higher mortality among breast cancer patients [4]. Ren et al.'s review of racial differences in outcomes among MBC patients in SEER showed socioeconomic factors contributed 50 % of the excess relative risk of mortality [32]. In the aforementioned study, socioeconomic factors were operationalized as insurance status, marital status, and the NCI-census level tract index. We anticipate that the differences in disease-specific mortality based on nSES are most likely an interplay between multiple social determinants of health (e.g., transportation, personal finances, workplace issues such as sick paid leave, social networks, stress, etc.) that are currently unavailable in SEER and tumor biology. Additionally, as noted earlier, surgical management among those in neighborhoods with low SES may be primarily for palliative reasons instead of being done with curative intent within the context of a response to chemotherapy. Consequently, these patients may not be receiving the full benefit of surgical management afforded to their counterparts in wealthier neighborhoods who may be undergoing surgery after response to chemotherapy.
Overall, study results highlight the continued influence of social determinants of health such as neighborhood, geography, marital status, and insurance even in the metastatic setting. Additionally, results on the differences in the use of treatment modalities such as breast conservation therapy, reconstruction and disease specific mortality suggest lower neighborhood SES, similar to the non-metastatic setting, drives disparities in access to locoregional therapies and subsequent clinical outcomes.
A limitation of this study is the lack of comorbidity data in SEER, which is significant as comorbidities are important in the decision to perform surgery. However, it should be noted that we focused on disease-specific mortality to eliminate the impact of death from other causes. Another limitation is the lack of clarification if surgical management was performed for purely palliative reasons or was conducted after a response to chemotherapy with curative intent. The use of an nSES index may not be representative of individual SES. However, this study's SES index used census tract information that represents a more homogenous population than county or zip code [19]. Of note, SEER combines the no and unknown group for radiation and chemotherapy, making it difficult to discern if radiation or chemotherapy was indeed omitted. Furthermore, SEER points out there may be biases associated with unmeasured reasons for receipt of radiation and chemotherapy and further stipulates those variables may not be complete [33]. As a result, the radiation and chemotherapy data should be viewed with caution.
5. Conclusions
This study is one of the first to evaluate the relationship between nSES, surgical management, and survival in de novo metastatic breast cancer patients. Consistent with findings in non-metastatic populations, low nSES is associated with a worse disease specific mortality. Unfortunately, at this juncture, the clinical implications of differences in the management of the breast, axilla and utilization of reconstructive surgery are unclear. Nevertheless, results highlight probable barriers to locoregional treatment faced by MBC patients residing in neighborhoods with low SES. Future studies should focus on understanding how patient and provider-related factors influence surgical decision-making among MBC patients. Additionally, disparities in mortality based on nSES warrant further investigation into the causative agents.
Declaration of competing interest
Oindrila Bhattacharyya declares that she has no conflict of interest. Yaming Li declares that she has no conflict of interest. James L. Fisher declares that he has no conflict of interest. Allan Tsung declares that he has no conflict of interest. Mariam Eskander declares that she has no conflict of interest. Ahmad Hamad declares that he has no conflict of interest. Samilia Obeng-Gyasi declares that she has no conflict of interest.
Acknowledgements
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.breast.2021.08.003.
Funding
Samilia Obeng-Gyasi is supported by the National Institutes of Health [K12 CA133250].
Ethical approval
The Ohio State University Office of Responsible Research Practices considered this study IRB exempt.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Saini G., Ogden A., McCullough L.E., Torres M., Rida P., Aneja R. Disadvantaged neighborhoods and racial disparity in breast cancer outcomes: the biological link. Cancer Causes Control. Jul 2019;30(7):677–686. doi: 10.1007/s10552-019-01180-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arcaya M.C., Tucker-Seeley R.D., Kim R., Schnake-Mahl A., So M., Subramanian S.V. Research on neighborhood effects on health in the United States: a systematic review of study characteristics. Soc Sci Med. Nov 2016;168:16–29. doi: 10.1016/j.socscimed.2016.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akinyemiju T.F., Genkinger J.M., Farhat M., Wilson A., Gary-Webb T.L., Tehranifar P. Residential environment and breast cancer incidence and mortality: a systematic review and meta-analysis. BMC Canc. Mar 28 2015;15:191. doi: 10.1186/s12885-015-1098-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abdel-Rahman O. Impact of NCI socioeconomic index on the outcomes of nonmetastatic breast cancer patients: analysis of SEER census tract-level socioeconomic database. Clin Breast Canc. Dec 2019;19(6):e717–e722. doi: 10.1016/j.clbc.2019.06.013. [DOI] [PubMed] [Google Scholar]
- 5.Dreyer M.S., Nattinger A.B., McGinley E.L., Pezzin L.E. Socioeconomic status and breast cancer treatment. Breast Cancer Res Treat. Jan. 2018;167(1):1–8. doi: 10.1007/s10549-017-4490-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Obeng-Gyasi S., Timsina L., Miller K.D., Ludwig K.K., Fisher C.S., Haggstrom D.A. The implications of insurance status on presentation, surgical management, and mortality among nonmetastatic breast cancer patients in Indiana. Surgery. Dec 2018;164(6):1366–1371. doi: 10.1016/j.surg.2018.07.012. [DOI] [PubMed] [Google Scholar]
- 7.Altice C.K., Banegas M.P., Tucker-Seeley R.D., Yabroff K.R. Financial hardships experienced by cancer survivors: a systematic review. J Natl Cancer Inst. Feb. 2017;109(2) doi: 10.1093/jnci/djw205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Price M.A., Tennant C.C., Butow P.N. The role of psychosocial factors in the development of breast carcinoma: Part II. Life event stressors, social support, defense style, and emotional control and their interactions. Cancer. Feb 15. 2001;91(4):686–697. [PubMed] [Google Scholar]
- 9.Singh G.K., Williams S.D., Siahpush M., Mulhollen A. Socioeconomic, rural-urban, and racial inequalities in US cancer mortality: Part I-all cancers and lung cancer and Part II-colorectal, prostate, breast, and cervical cancers. J Cancer Epidemiol. 2011;2011:107497. doi: 10.1155/2011/107497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.America Cancer Society . 2019. Breast cancer facts & figures 2019-2020.https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/breast-cancer-facts-and-figures/breast-cancer-facts-and-figures-2019-2020.pdf [Google Scholar]
- 11.Stahl K., Wong W., Dodge D. Benefits of surgical treatment of stage IV breast cancer for patients with known hormone receptor and HER2 status. Ann Surg Oncol. Oct 30 2020 doi: 10.1245/s10434-020-09244-5. [DOI] [PubMed] [Google Scholar]
- 12.Lane W.O., Thomas S.M., Blitzblau R.C. Surgical resection of the primary tumor in women with de novo stage IV breast cancer: contemporary practice patterns and survival analysis. Ann Surg. Mar. 2019;269(3):537–544. doi: 10.1097/sla.0000000000002621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Corona S.P., Sobhani N., Ianza A. Advances in systemic therapy for metastatic breast cancer: future perspectives. Med Oncol (Northwood) Jul 2017;34(7):119. doi: 10.1007/s12032-017-0975-5. [DOI] [PubMed] [Google Scholar]
- 14.National Comprehensive Cancer Network . June 28, 2021. NCCN clinical practice guidelines in oncology (NCCN guidelines) breast cancer (version 5.2021). 2021.https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf [Google Scholar]
- 15.Neuman H.B., Morrogh M., Gonen M., Van Zee K.J., Morrow M., King T.A. Stage IV breast cancer in the era of targeted therapy: does surgery of the primary tumor matter? Cancer. Mar 1. 2010;116(5):1226–1233. doi: 10.1002/cncr.24873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khan S.A., Zhao F., Solin L.J. A randomized phase III trial of systemic therapy plus early local therapy versus systemic therapy alone in women with de novo stage IV breast cancer: a trial of the ECOG-ACRIN Research Group (E2108) J Clin Oncol. 2020;38(18_suppl) doi: 10.1200/JCO.2020.38.18_suppl.LBA2. LBA2-LBA2. [DOI] [Google Scholar]
- 17.Program S.R. 2020. Overview of the SEER Program. NCI, DCCPS.https://seer.cancer.gov/about/overview.html Accessed 06/30/2020. [Google Scholar]
- 18.National Cancer Institute . 2020. SEER∗Stat software latest release.https://seer.cancer.gov/seerstat/ Version 8.3.8 - September 2, 2020. [Google Scholar]
- 19.Yost K., Perkins C., Cohen R., Morris C., Wright W. Socioeconomic status and breast cancer incidence in California for different race/ethnic groups. Cancer Causes Control. Oct 2001;12(8):703–711. doi: 10.1023/a:1011240019516. [DOI] [PubMed] [Google Scholar]
- 20.Liu L., Deapen D., Bernstein L. Socioeconomic status and cancers of the female breast and reproductive organs: a comparison across racial/ethnic populations in Los Angeles County, California (United States). Cancer causes & control : CCC. Aug 1998;9(4):369–380. doi: 10.1023/a:1008811432436. [DOI] [PubMed] [Google Scholar]
- 21.Osborne C., Ostir G.V., Du X., Peek M.K., Goodwin J.S. The influence of marital status on the stage at diagnosis, treatment, and survival of older women with breast cancer. Breast cancer research and treatment. Sep. 2005;93(1):41–47. doi: 10.1007/s10549-005-3702-4. [DOI] [PubMed] [Google Scholar]
- 22.Jackson D.K., Li Y., Eskander M.F. Racial disparities in low-value surgical care and time to surgery in high-volume hospitals. J Surg Oncol. 2021;123(2):676–686. doi: 10.1002/jso.26320. [DOI] [PubMed] [Google Scholar]
- 23.Zhang S., Liu Y., Yun S., Lian M., Komaie G., Colditz G.A. Impacts of neighborhood characteristics on treatment and outcomes in women with ductal carcinoma in situ of the breast. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for cancer research, cosponsored by the American Society of preventive oncology. Nov. 2018;27(11):1298–1306. doi: 10.1158/1055-9965.Epi-17-1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khan S.A., Stewart A.K., Morrow M. Does aggressive local therapy improve survival in metastatic breast cancer? Surgery. Oct 2002;132(4):620–626. doi: 10.1067/msy.2002.127544. discussion 626-7. [DOI] [PubMed] [Google Scholar]
- 25.Gu J., Groot G., Boden C., Busch A., Holtslander L., Lim H. Review of factors influencing women's choice of mastectomy versus breast conserving therapy in early stage breast cancer: a systematic review. Clin Breast Cancer. Aug. 2018;18(4):e539–e554. doi: 10.1016/j.clbc.2017.12.013. [DOI] [PubMed] [Google Scholar]
- 26.National Cancer Institute . 2020. SEER cause-specific death classification.https://seer.cancer.gov/causespecific/ [Google Scholar]
- 27.SEER Cancer Stat Facts . National Cancer Institute; 2021. Female breast cancer subtypes.https://seer.cancer.gov/statfacts/html/breast-subtypes.html [Google Scholar]
- 28.Petrelli F., Barni S. Surgery of primary tumors in stage IV breast cancer: an updated meta-analysis of published studies with meta-regression. Med Oncol (Northwood) Dec 2012;29(5):3282–3290. doi: 10.1007/s12032-012-0310-0. [DOI] [PubMed] [Google Scholar]
- 29.Si Y., Yuan P., Hu N. Primary tumor surgery for patients with de novo stage IV breast cancer can decrease local symptoms and improve quality of life. Ann Surg Oncol. Apr. 2020;27(4):1025–1033. doi: 10.1245/s10434-019-08092-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Badwe R., Hawaldar R., Nair N. Locoregional treatment versus no treatment of the primary tumour in metastatic breast cancer: an open-label randomised controlled trial. Lancet Oncol. Oct 2015;16(13):1380–1388. doi: 10.1016/s1470-2045(15)00135-7. [DOI] [PubMed] [Google Scholar]
- 31.Jackson D.K., Li Y., Eskander M.F. Racial disparities in low-value surgical care and time to surgery in high-volume hospitals. J Surg Oncol. 2021;123(2):676–686. doi: 10.1002/jso.26320. [DOI] [PubMed] [Google Scholar]
- 32.Ren J.X., Gong Y., Ling H., Hu X., Shao Z.M. Racial/ethnic differences in the outcomes of patients with metastatic breast cancer: contributions of demographic, socioeconomic, tumor and metastatic characteristics. Breast Canc Res Treat. Jan 2019;173(1):225–237. doi: 10.1007/s10549-018-4956-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Noone A.M., Lund J.L., Mariotto A. Comparison of SEER treatment data with medicare claims. Med Care. Sep 2016;54(9):e55–e64. doi: 10.1097/mlr.0000000000000073. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.