Abstract
Background
Effective treatments are still needed to reduce the severity of symptoms, time of hospitalization, and mortality of COVID-19. SARS-CoV-2 specific memory T-lymphocytes obtained from convalescent donors recovered can be used as passive cell immunotherapy.
Methods
Between September and November 2020 a phase 1, dose-escalation, single centre clinical trial was conducted to evaluate the safety and feasibility of the infusion of CD45RA− memory T cells containing SARS-CoV-2 specific T cells as adoptive cell therapy against moderate/severe cases of COVID-19. Nine participants with pneumonia and/or lymphopenia and with at least one human leukocyte antigen (HLA) match with the donor were infused. The first three subjects received the lowest dose (1 × 105 cells/kg), the next three received the intermediate dose (5 × 105 cells/kg) and the last three received the highest dose (1 × 106 cells/kg) of CD45RA− memory T cells. Clinicaltrials.gov registration: NCT04578210.
Findings
All participants’ clinical status measured by National Early Warning Score (NEWS) and 7-category point ordinal scales showed improvement six days after infusion. No serious adverse events were reported. Inflammatory parameters were stabilised post-infusion and the participants showed lymphocyte recovery two weeks after the procedure. Donor microchimerism was observed at least for three weeks after infusion in all patients.
Interpretation
This study provides preliminary evidence supporting the idea that treatment of COVID-19 patients with moderate/severe symptoms using convalescent CD45RA− memory T cells is feasible and safe.
Funding
Clinical Trial supported by Spanish Clinical Research Network PT17/0017/0013. Co-funded by European Regional Development Fund/European Social Fund. CRIS CANCER Foundation Grant to AP-M and Agencia Valenciana de Innovación Grant AVI-GVA COVID-19-68 to BS.
Research in context.
Evidence before this study
We searched PubMed, preprinted servers and other channels through Google search from November 2020 to February 2021 for publications and reports for “COVID-19″ “cell therapy”, “clinical trials”, “COVID-19 and T cell responses”, “SARS-CoV-2 specific T cells”, “SARS-CoV-2 specific memory T cells”. We found one interventional (NCT04457726) study using SARS-CoV-2 specific T cells with no results yet. We didn't find any clinical trial using SARS-CoV-2 specific memory T cells. We have previously published that the procedure for obtaining these cells is feasible, easy to implement for small-scale manufacture, quick and cost-effective, involves minimal manipulation, and has no GMP requirements. We found several reports highlight the importance of robust and durable T cell memory for SARS-CoV-2 for the management of the current pandemic.
Added value of this study
We report the outcomes of the first report of human passive adoptive cell therapy for COVID-19 using allogeneic, off-the-shelf CD45RA− memory T cells obtained from a convalescent COVID-19 donor. A phase 1, dose-escalation, single centre clinical trial was conducted in Madrid, Spain, including 9 patients. We describe the safety and feasibility of the infusion. No serious adverse effects were reported. Inflammatory parameters were stabilised post-infusion. All participants’ clinical status showed improvement six days after infusion, lymphocyte recovery two weeks after the procedure and donor microchimerism was observed at least for three weeks after infusion.
Implications of all the available evidence
So far effective treatments are still needed to reduce the severity of symptoms, time of hospitalisation, and mortality of COVID-19. Our hypothesis is that SARS-CoV-2 specific memory T-lymphocytes obtained from convalescent donors recovered from COVID-19 can be used as a passive cell immunotherapy to treat pneumonia and lymphopenia in moderate/severe patients. We have shown safety of the procedure, we are now developing the phase 2 clinical trial to show treatment efficacy.
Alt-text: Unlabelled box
1. Introduction
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) emerged in December 2019 causing a global pandemic. Older patients and patients with pre-existing comorbidities are at higher risk for complications, including pneumonia and T cell lymphopenia, an indicator of disease severity and higher death risk [1]. Until an effective and available vaccine is distributed, physical distancing and isolation are critical measures to control the infection. So far, effective treatments are still needed to reduce the severity of symptoms, time of hospitalisation, and mortality. Antiviral treatments and administration of convalescent plasma have not shown the expected impact in mortality [2], [3], [4]. Currently, only treatment with steroids has been reported to decrease mortality in patients with severe and critical Coronavirus disease 2019 (COVID-19) [5]. COVID-19 progression is generally biphasic, initially viral and afterwards inflammatory [6], [7], [8]. Since the COVID-19-induced lymphopenia constitutes a therapeutic window that may facilitate donor engraftment and viral protection until recovery, we hypothesised that SARS-CoV-2 specific memory T-lymphocytes obtained from convalescent donors recovered from COVID-19 can be used as passive cell immunotherapy to treat pneumonia and lymphopenia in moderate/severe patients [9,10]. In addition, the broad donor memory T cell repertoire may protect these vulnerable patients from other common viral co-infections as it has been previously reported in hematopoietic stem cell transplant [11], [12], [13], [14], [15].
We previously reported the existence of a SARS-CoV-2 specific T cell population within the CD45RA−memory T cells from the blood of convalescent donors [10]. A wide number of doses can be easily manufactured using CliniMACS Miltenyi® device without the need of a Good Manufacturing Practice (GMP) facility [16]. These cells can be stored and be immediately available as an “off-the-shelf” COVID-19 convalescent donor-derived biobank [10].
The RELEASE trial (NCT04578210) is a phase I/II clinical trial that uses adoptive cell therapy based on NK cells or memory T cells derived from patients that suffered from COVID-19; being the goal of phase I to evaluate the safety, feasibility, and recommended phase II dose (RP2D) of a single infusion.
Here, the outcomes of the memory T cell arm of the RELEASE Phase I clinical trial are reported. The NK cell arm of the study was closed due to a lack of financing, therefore, there are no significant outcomes.
2. Methods
2.1. Study design
RELEASE Phase I clinical trial was conducted at La Paz University Hospital, Madrid, Spain between September and November 2020. The trial was approved by the institutional review board and was conducted following Good Clinical Practice guidelines and the Declaration of Helsinki. All patients provided written informed consent. The investigators designed the trial, collected the data, and performed the analysis. The authors vouch for the accuracy and completeness of the data and the fidelity of the trial to the protocol, with the full text of this article under preparation. The protocol of the trial (Appendix 1) was approved by the Ethics Committee of Hospital Universitario La Paz (identifier: Clinical Ethical Approval No. HULP:5579) and overseen by a data and safety monitoring board. All the authors participated in writing the submitted manuscript. Clinicaltrials.gov registration: NCT04578210.
2.2. Participants
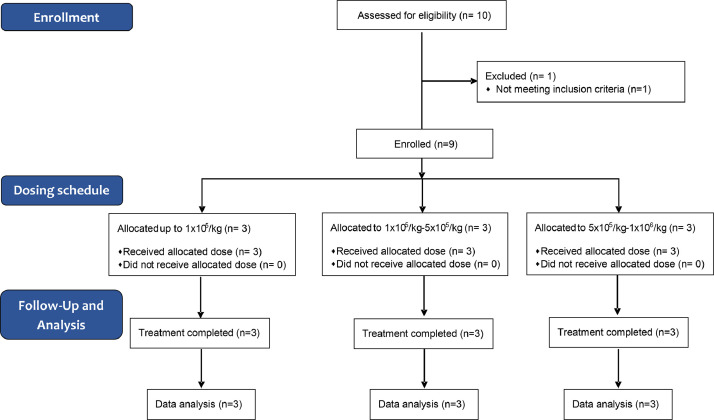
18 years and older hospitalised adults were screened for enrolment if they had a positive reverse-transcriptase–polymerase-chain-reaction (RT-PCR) assay for SARS-CoV-2 from a nasopharyngeal swab, radiologically confirmed pneumonia and/or lymphopenia, O2Sat ≤ 94% on room air at screening, with no oxygen requirement or with an oxygen need of ≤ 2.5 lpm by nasal cannula, and less than ten days since onset of symptoms. Clinical and analytical monitoring was performed. Ten participants were enrolled in the trial. One was excluded due to the lack of an HLA antigen matching donor.
2.3. Intervention
After informed consent, eligible participants were studied for HLA genotype matching between patient and donor. When there was at least one HLA match (one or two fields of resolution for loci A, B or DRB1, DQB1) between donor and patient, a single infusion of convalescent CD45RA− memory T cells containing SARSCoV-2 specific T cells were administered.
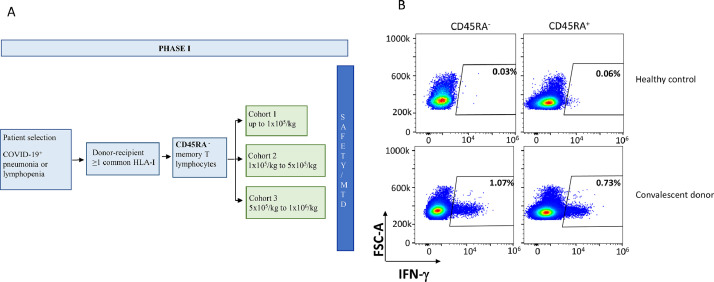
Participants were sequentially enrolled to receive a single infusion in a dose-escalating manner. The first cohort of patients received a single dose of 1 × 105 cells/kg (low dose), the second cohort received a single dose of 5 × 105 cells/kg (intermediate dose) and the third cohort of patients received a single dose of 1 × 106 cells/kg (high dose), as is shown in Fig. 2A.
Fig. 2.
A) RELEASE Study protocol (NCT04578210). Only the first arm of the memory lymphocytes described here. B) Expression of CD45RA+and CD45RA– T on a healthy control and a convalescent donor.
The participants were infused with CD45RA− T cells at their respective doses through a standard blood filter in a gravity-driven system previously medicated with intravenous diphenhydramine 5 mg and acetaminophen 1 gm.
2.4. Donor selection and procedure for memory T cells selection and preservation
A single donor was selected based on the presence of SARS-CoV-2 specific memory T cells [10] (Fig. 2B) and an HLA genotype that could cover around 93.6% of the Spanish population [9]. The percentage of CD45RA−CD3+ cells was 99.8% post depletion, and the total cell number was 1.44 × 109. As expected, most of the cells were CD45RA−CD4+ (99.8%) (Supplemental Table 1).
Blood donor selection was performed by the Regional Blood Transfusion Centre according to the following criteria: ≤ 65 years old, positive SARS-CoV-2 tested by PCR during the disease and complete resolution of symptoms for at least 14 days before donation. The donor had to have at least one SARS-CoV-2 negative test by PCR from a nasopharyngeal swab, or, if available, a negative SARS-CoV-2 viremia tested by quantitative PCR in blood, before the blood donation. The donor blood was HLA typed. Clinical history and physical examination, including venous access, as well as blood count, biochemistry, and serological studies were performed within seven days before the apheresis. Donor´s characteristics and apheresis were obtained as previously described [10]. Briefly, non-mobilized apheresis was obtained from the convalescent donor using Spectra-Optia. CD45RA+ cells were depleted by immunomagnetic separation using CliniMACS CD45RA Reagent and CliniMACS Plus system, both from Miltenyi Biotec, following manufacturer's instructions. CD45RA− cells were aliquoted for cryopreservation in doses adjusted to 100 kg of weight and the three planned doses. Aliquots were cryopreserved in a double bag using donor autologous plasma with a final concentration of 5% dimethyl sulfoxide (DMSO). CD45RA− aliquots were removed from liquid nitrogen storage and thawed in a dry defroster. Infusion of cryopreserved CD45RA− lymphocytes was performed 15 min after thawing. Cell count, viability, and microbiological studies were performed after each aliquot was thawed. CD45RA− fraction viability and purity were analysed by flow cytometry (FCM) and is shown in Supplemental Table 1.
2.5. Study procedures
The study visits and procedures performed are shown in Supplemental Table 2.
Subjects were screened within two days before inclusion and dosing to determine their eligibility to participate in the study. Study subjects who fulfilled all the inclusion criteria and none of the exclusion criteria were immediately offered to participate. Inclusion and dosing occurred on the same day if possible.
Participants must have completed the following assessments before administration of the study drug: Physical examination, documentation of respiratory status, blood samples collections (only before day one, for haematological, chemical, immunological function markers, and donor chimerism analysis), and a record of any adverse events. These assessments, and study of donor chimerism and SARS-CoV-2 PCR were repeated on the study visits. Additional assessments were performed when possible, considering the increased workload. Patients will be followed until day 90, discharge or death.
2.6. Outcomes
The primary outcome was to determine the safety of a single infusion of memory T cells from a healthy donor recovered from COVID-19. The aim was to determine the RP2D and the dose-limiting toxicity (DLT) for a subsequent phase II clinical trial. DLT is defined as any grade 3 or higher adverse event (AE) directly or probably related to the cell infusion and any lower grade AE that increases to a grade 3 or higher as a direct result of the cell infusion.
Adverse Events were assessed and graded according to the Common Terminology Criteria for Adverse Events (CTCAE) version 5.0. If CTCAE grading does not exist for an AE, the severity of mild, moderate, severe, life-threatening, and fatal were used and correspond to the Grade 1–5 respectively.
Secondary outcomes were to evaluate time to lymphopenia recovery and to immune dysregulation, the time needed for a negative SARS-CoV-2 result by PCR, clinical improvement through the NEWS and a 7-category point ordinal scale [17], [18], [19] and length of stay. The NEWS consists of six parameters (respiratory rate, oxygen saturations, temperature, systolic blood pressure, heart rate, level of consciousness) to improve the early detection of and response to clinical deterioration [17]. Each parameter is assigned a score of 0–3 points. NEWS is stratified into three categories: low risk (0–4), medium risk (5–6), and high risk (≥ 7). Besides, we monitored the patient´s clinical index with the use of a 7-category point ordinal scale. Scores on the scale were defined as follows: a score of 1 indicated not hospitalised with no limitations on activities; 2, not hospitalised but with limitations on activities; 3, hospitalised and not receiving supplemental oxygen; 4, hospitalised and receiving supplemental oxygen; 5, hospitalised and receiving oxygen supplementation administered by a high-flow nasal cannula or non-invasive ventilation; 6, hospitalised and receiving mechanical ventilation; and 7, death [19].
Exploratory outcomes included the monitoring of donor chimerism and immune lymphocyte reconstitution after adoptive therapy during the three months following the infusion and the immunological profiling of patients responding to therapy.
2.7. Immune lymphocyte reconstitution and phenotyping
Immunophenotyping of peripheral blood CD3+ T cell subsets (CD4+ and CD8+, CD4+CD45RA+, CD4+CD45RA−, CD8+CD45RA+, CD8+CD45RA−, NKT), NK cells (CD3−CD16+CD56+) cells and B lymphocytes (CD19+) were by a multiparametric flow cytometry analysis at each time point: pre-infusion and weekly during two weeks after infusion.
Peripheral blood was stained for surface markers with the following fluorochrome-conjugated anti-human antibodies (BD Bioscience): anti-CD45 APC, anti-CD3 FITC, anti-CD4 PerCP, anti-CD8 Bv510, anti-CD19 PeCy7, anti-CD45RA APC—H7, anti-CD16 PE, and anti-CD56 PE. Cells were then acquired by flow cytometry on FACSCanto™ II (BD Biosciences). The analysis was performed with BD FACSDiva™ Software v8.0.2 (BD Bioscience).
2.8. Chimerism
For each donor-recipient pair, DNA was isolated using the QIAamp Blood Kit (Qiagen, Hilden, Germany). Chimerism analysis was monitored weekly during three/four consecutive weeks. It was performed based on the detection of insertion–deletion polymorphism (INDELs) by qPCR technology (sensitivity 0.01–0.05%). For that purpose, commercial reagents for screening of informative alleles (Mentype DIPscreen, Biotype, Dresden, Germany) and quantitative chimerism analysis (Mentype DIPquant qPCR, Biotype) were employed. The percentage of donor alleles was calculated based on the ΔΔCt qPCR method by using Chimerism Monitor 2.1 software (Biotype), being β-globin the reference gene.
2.9. HLA typing
The HLA genotype of the convalescent donor and the patients was done at the Regional Blood Transfusion Centre. The HLA genotype of the convalescent donor was performed on two independent samples by Sequence-Specific Oligonucleotide (SSO) and Next-Generation Sequencing (NGS): A*02:01, A*24:02 / B*44:02, B*51:01 / C*16:02, C*16:04 / DRB1×07:01, DRB1×11:03 / DQB1×02:02, DQB1×03:015. The selection was done based on at least one HLA match with the donor with the order: A, B high resolution vs one field, DRB1, DQB1 high resolution vs one field. The HLA shared alleles of the patients (same typing method as the donor) with the convalescent donor are shown in Supplemental Table 3.
2.10. Data management and study monitoring
Categorical data are presented as numbers and percentages when applicable. Descriptive tables and figures are used to enable data visualization.
An electronic case report form (eCRF; appendix 2) was designed using MACRO Electronic Data Capture by Elsevier. This eCRF includes all the variables described. This system anonymizes patients, and the data to analyse it with R software (V.3.5.2 or newer). Data management and statistical plans were approved by the principal investigator and the sponsor. Data collection forms will be included in the final report. Data management was performed by the Spanish Clinical Research Network (SCReN). Trial coordination, management, monitoring, and statistical analysis of the study were performed by the Clinical Trial Unit (Department of Clinical Pharmacology and IdiPAZ) of La Paz University Hospital, belonging to the SCReN. A Data and Safety Monitoring Board reviewed all the data and approved the progression to Phase II using the highest dose of 1 × 106cells/kg.
2.11. Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation or writing of the report. The corresponding author had full access to all data in the study and had final responsibility for the decision to submit for publication.
3. Results
3.1. Patients’ characteristics
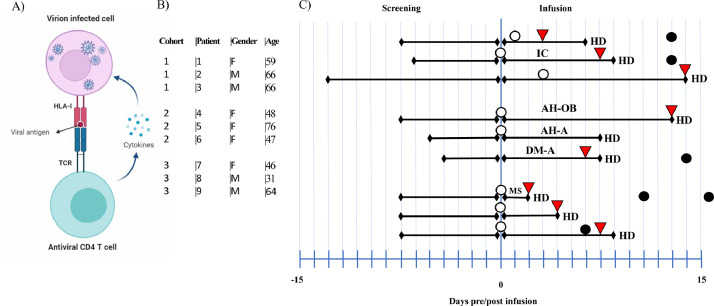
Between September and November 2020, ten participants were enrolled in the trial, all but one received a single dose infusion of memory T cells (Fig. 1). No patients were lost to follow up. Included patients were like so many others admitted to hospital at that time, adult men (4) and women (6), median age 59 (IQR 46.5–66), median weight 80 kg (IQR 63.5–86), with moderate SARS-CoV-2 pneumonia with variable lymphopenia and mild hypertransaminasemia, with hypertension (3 patients), diabetes mellitus (1 patient), obesity (1 patient) or no comorbidities (Table 1). One patient was diagnosed with human immunodeficiency virus (HIV) infection during admission (Patient #8). Patient #10 did not receive the infusion because there was no HLA antigen sharing with the donor. This patient cannot be considered as a control group, but he was screened for the trial, met the inclusion criteria, signed the informed consent, and was tested for HLA match with the study donor. The median oxygen saturation at admission was 91% (IQR 89.5–93). Nine out of ten patients received the infusion of memory T cells in the first 24 h of hospital admission. Accordingly with the trial design the patients received a low, intermediate, or high dose according to CD45RA− memory T cells per kilogram administered (Fig. 2A). Main clinical characteristics and outcomes, comorbidities, clinical status, and concomitant treatment for COVID-19 are shown in Fig. 3, Fig. 4, and Tables 1, 2 and 3, Supplemental Tables 4, 5, and 6. Of the 9 patients, 7 were treated with remdesivir (patients #1, #2, #3, #4, #5, #6 and #9) according to the Spanish national guidelines, all patients were treated with dexamethasone 6 mg per day until clinical improvement, and low molecular weight heparin as thromboprophylaxis (all but patient #3) or treatment (patient #3) according to body weight and need. No other experimental or repurposed drugs were given for COVID-19. Oxygen support was given as needed for peripheral oxygen saturation above 94%, through a nasal cannula or rebreathing mask. No patient needed any other kind of respiratory support including a high-flow nasal cannula. Symptomatic treatment consisted of acetaminophen, metamizole, and dextromethorphan. In our cohort, NEWS had a median score of 4 at screening and a decrease to 2 on day 5 and 0 on day 14. (Supplemental Table 5). NEWS´s score improvement has shown a robust predicting clinical scoring system to identify deterioration in infected patients outside the intensive care unit, as it was our cohort of patients [18]. Besides, we monitored the patient´s clinical index with the use of a 7-level ordinal scale. In our cohort patients with 7-level ordinal scale higher than 3 started to improve this score at day 5 after infusion decreasing to 1 two weeks post-infusion (Supplemental Table 6). Also, we observed a decrease in the pro-inflammatory parameter C-reactive protein from 76.95 mg/L (IQR 53.47–137.72) at recruitment to 37.8 mg/L (IQR 15–103) on day 2 and 10.25 mg/L (IQR 4.6–47.2) on day 6 and 2.3 mg/L (IQR 0.75–98.5) on day 14 post-infusion.
Fig. 1.
CONSORT flow diagram.
Table 1.
Baseline characteristics of the participants in the Arm 1 Phase I RELEASE Clinical Trial. M: male, F: female; N/A Not Applicable; N/D Not Determined; *Patient not infused, no HLA Match; **Time to discharge from hospital admission.
| Dose cohort |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable | 1 × 105 cells/kg |
5 × 105 cells/kg |
1 × 106 cells/kg |
|||||||
| Patient number | #1 | #2 | #3 | #4 | #5 | #6 | #7 | #8 | #9 | #10* |
| Age | 59 | 66 | 66 | 48 | 76 | 47 | 46 | 31 | 64 | 70 |
| Gender (M,F) | F | M | M | F | F | F | F | M | F | M |
| Weight (kg) | 80 | 80 | 92 | 130 | 75 | 62 | 65 | 80 | 56 | |
| Comorbidities | ||||||||||
| Arterial hypertension | No | Yes | No | Yes | Yes | No | No | No | No | No |
| Chronic heart disease | No | No | No | No | No | No | No | No | No | No |
| Diabetes mellitus | No | No | No | No | No | Yes | No | No | No | No |
| Obesity | No | No | No | Yes | No | No | No | No | No | No |
| Asthma | No | No | No | No | No | No | No | No | No | No |
| HIV infection | No | No | No | No | No | No | No | Yes | No | No |
| Treatment regimens, (days) | ||||||||||
| Remdesivir | 4 | 5 | 1 | 5 | 5 | 5 | 0 | 0 | 5 | |
| Dexamethasone | 6 | 5 | 8 | 12 | 7 | 6 | 4 | 3 | 8 | |
| Heparin | Prophylaxis | Prophylaxis | Therapeutic | Prophylaxis | Therapeutic | Prophylaxis | Prophylaxis | Prophylaxis | Prophylaxis | |
| Tocilizumab | No | No | No | Yes | No | No | No | No | No | |
| Sat O2% basal at hospitalization | 89 | 91 | 84 | 91 | 94 | 93 | 93 | 90 | 93 | 92 |
| Time from disease onset to hospital admission (days) | 7 | 6 | 13 | 7 | 5 | 4 | 7 | 7 | 7 | 7 |
| Time from hospitalization to infusion (days) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | N/A |
| Time of hospitalization after infusion (days) | 6 | 8 | 14 | 13 | 7 | 7 | 2 | 4 | 8 | 20** |
| Time to SARS-CoV-2 negative PCR after infusion | 13 | 13 | 11 | 11 | N/D | 14 | 16 | 13 | 6 | N/D |
Fig. 3.
A) Interaction of virus infected cell with CD4+ T cell by HLA-I complex. B) Patients’ characteristics and doses cohort. C) Time-course, clinical outcomes and patients’ co-morbidities. HD: Hospital Discharge,:No fever,: No extra oxygen needs,: Negative PCR, IC: Ischemic cardiopathy, AH: Arterial hypertension, OB: Obesity, DM: Diabetes mellitus, MS: multiple sclerosis.
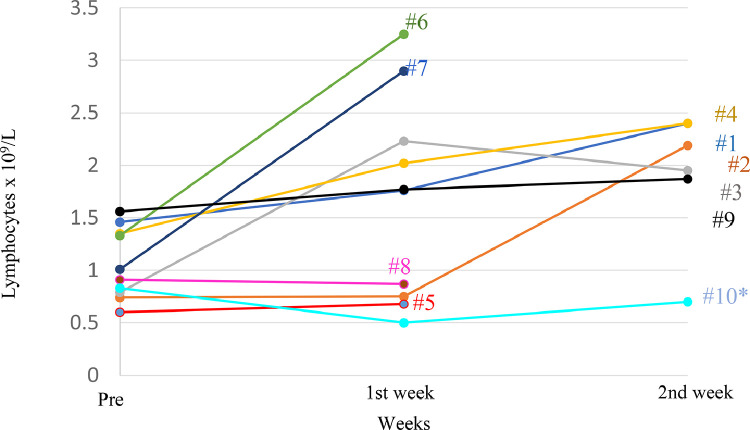
Fig. 4.
Recovery from lymphopenia after CD45RA− lymphocyte infusion. Lymphocyte recovery (x109/L) one and two weeks after CD45RA− memory T cell infusion. #: Patient number.
Table 2.
Immune recovery of Lymphocytes, CD3+, CD4+, CD8+, CD56+, CD19+ cells and NKT−subpopulations after infusion of CD45RA- memory T cells. Data is shown as median, [IQR] N/D: N/D Not Determined not data; Pre: Pre infusion.
| Dose | 1 × 105 cells/kg Low dose |
5 × 105 cells/kg Medium dose |
1 × 106 cells/kg High dose |
|---|---|---|---|
| Variable | |||
| Lymphocytex109/L (median [IQR]) | |||
| Pre | 0.79 [0.72] | 1.33 [0.75] | 1.01 [0.65] |
| Week 1 | 1.76 [1.48] | 2.02 [2.57] | 1.77 [2.03] |
| Week 2 | 2.19 [0.45] | 2.40 | 1.87 |
| CD3+ cells x109/L (median [IQR]) | |||
| Pre | N/D | 0.85 [0.57] | 0.77 [0.26] |
| Week 1 | 1.11 [0.26] | 1.34 [2.06] | 1.32 [1.44] |
| Week 2 | 1.68 [0.27] | 1.54 | 1.51 |
| CD4+ cells x109/L (median [IQR]) | |||
| Pre | N/D | 0.61 [0.42] | 0.61 [0.51] |
| Week 1 | 0.68 [0.20] | 1.04 [1.5] | 1.16 [1.25] |
| Week 2 | 0.77 [0.28] | 1.21 | 1.21 |
| CD8+ cells x109/L (median [IQR]) | |||
| Pre | N/D | 0.13 [0.08] | 0.15 [0.24] |
| Week 1 | 0.32 [0.12] | 0.26 [0.44] | 0.21 [0.28] |
| Week 2 | 0.81 [0.69] | 0.26 | 0.32 |
| NK cells x109/L (median [IQR]) | |||
| Pre | N/D | 0.27 [0.13] | 0.24 [0.33] |
| Week 1 | 0.14 [0.06] | 0.13 [0.21] | 0.21 [0.57] |
| Week 2 | 0.27 [0.15] | 0.15 | 0.18 |
| B cells x109/L (median [IQR]) | |||
| Pre | N/D | 0.07 [0.20] | 0.07 [0.16] |
| Week 1 | 0.49 [0.30] | 0.31 [0.59] | 0.31 [0.25] |
| Week 2 | 0.27 [0.06] | 0.71 | 0.17 |
| NKT (%) (median [IQR]) | |||
| Pre | N/D | 2.90 [8.70] | 1.20 [6.60] |
| Week 1 | 2.50 [5.40] | 1.70 [1.20] | 0.70 [4.50] |
| Week 2 | 6.80 [16.60] | 4.30 | 1.8 |
Table 3.
Inflammatory parameters after infusion of CD45RA- memory T cells. NR: normal range. N/D: Not determined; *data are from time to hospitalization.
| Dose Cohort |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 × 105 cells/kg |
5 × 105 cells/kg |
1 × 106 cells/kg |
||||||||
| Laboratory findings | #1 | #2 | #3 | #4 | #5 | #6 | #7 | #8 | #9 | #10* |
| C-reactive protein, mg/L (NR 0–5) | ||||||||||
| Day of infusion | 68.9 | 67.7 | 222.7 | 109.4 | 63 | 90.9 | <0.05 | 85 | 24.9 | 274.3 |
| Day 2 post infusion | 40.4 | 18.2 | 127.8 | 95.2 | 35.2 | 66.2 | 1.2 | 4.6 | 22.8 | 227.4 |
| Day 6 post infusion | 10.6 | 55.2 | 44.6 | 13 | 9.9 | 9.8 | <0.5 | 5.1 | 3.2 | 208.6 |
| Day 13 post infusion | 1.5 | 2.3 | 2.4 | <0.5 | N/D | N/D | N/D | N/D | <0.5 | 173.1 |
| Fibrinogen, mg/dl (NR 150–450) | ||||||||||
| Day of infusion | 490 | 598 | >1200 | 778 | 541 | 600 | 598 | 789 | 807 | 1103 |
| Day 2 post infusion | 507 | 394 | 1117 | 722 | 408 | 607 | 703 | 488 | 589 | 1004 |
| Day 6 post infusion | 388 | 519 | 860 | 372 | 440 | 498 | 430 | 415 | 429 | >1200 |
| Day 13 post infusion | 430 | 334 | 421 | 217 | N/D | N/D | N/D | N/D | 410 | N/D |
| Ferritin, ng/ml (NR 10–291) | ||||||||||
| Day of infusion | 1563 | 705 | 1712 | 468 | 304 | 111 | 206 | 497 | 582 | N/D |
| Day 2 post infusion | 1527 | 687 | 1392 | 690 | 283 | 109 | 254 | 327 | 343 | 695 |
| Day 6 post infusion | 1351 | 528 | 980 | 509 | 204 | 60 | 253 | 408 | 279 | 970 |
| Day 13 post infusion | 1139 | 419 | 625 | 333 | N/D | N/D | N/D | N/D | 250 | 865 |
| D-Dimer, ng/ml (NR 0–500) | ||||||||||
| Day of infusion | 1590 | 320 | 820 | 1470 | 540 | 670 | 300 | 870 | 440 | 510 |
| Day 2 post infusion | 1590 | 230 | 1710 | 1940 | 320 | 910 | 650 | 1050 | 440 | 440 |
| Day 6 post infusion | 1200 | 200 | 820 | 5090 | 660 | 670 | 310 | 570 | 200 | 1700 |
| Day 13 post infusion | 600 | N/D | 420 | 2340 | N/D | N/D | N/D | N/D | 330 | 830 |
| IL-6, pg/ml (NR < 3,4) | ||||||||||
| Day of infusion | 2.6 | 25.6 | N/D | 58.3 | 66.2 | N/D | N/D | 25.6 | 0.7 | N/D |
| Day 2 post infusion | 2.6 | 10.9 | 131 | 66.6 | 7.1 | 33 | 29.3 | 6.5 | 1.7 | N/D |
| Day 6 post infusion | 2.7 | <2 | 21.5 | 202 | 10.6 | N/D | 17.3 | N/D | <2.7 | 12.1 |
| Day 13 post infusion | N/D | N/D | 2.8 | 59.8 | N/D | N/D | N/D | N/D | <2.7 | 12.2 |
The median time needed for a negative SARS-CoV-2 result by PCR was 13 days (IQR 11–13) in the low dose cohort, 12.5 days (IQR 11–14) in the medium dose cohort and 13 days (IQR 6–16) in the high dose cohort. After monitoring, three patients did not meet the inclusion criteria. Those incompatibilities (time from PCR to inclusion in the trial and time from the onset of symptoms to the inclusion in the study) did not interfere with the safety evaluation of the study and therefore, the patients were maintained during the analysis of the study results.
3.2. Safety
The primary outcome was to determine the DLT to define the RP2D for the phase 2 trial. There were no immediate reaction complications or septic events in none of the infusions. The only mild side effect reported occurred in patient #7 (high dose cohort), considered directly related to the experimental treatment and grade 1. The patient described a taste for 10 h after infusion [20]. No adverse events including transfusion-related acute lung injury (TRALI), cytokine release syndrome (CRS), graft-versus-host disease (GvHD), fever, or sudden respiratory failure were observed. Therefore, DLT was not identified with doses up to 1 × 106 cells/Kg and the maximum tolerated doses (MTD) were not reached.
3.3. Immune reconstitution and chimerism
The exploratory outcomes, immune reconstitution and chimerism were evaluated. Lymphocyte counts, stratified by CD3+ T lymphocytes and T cell subsets (CD4+ and CD8+), NK (CD56+), B (CD19+) lymphocytes, and the CD4/CD8 ratio pre-infusion and one and two weeks after infusion appear in Fig. 4, Table 2, Supplemental Table 4, and Supplemental Figure 1. In general, we observed an increase in lymphocyte counts from 0.96 × 109 /L (IQR 0.77–1.37) at recruitment to 1.76 109 /L (IQR 0.81–2.39) one week post-infusion and 2.19 109 /L (IQR 1.32–2.44) two weeks post-infusion. Of the 9 patients infused with CD45RA− T cells, 5 patients showed lymphocyte recovery compared to the pre-infusion day two weeks post-infusion. Data from patients #5, #6, #7and #8 were not recorded two weeks post infusion. Patients #6 and #7 showed lymphocyte recovery one-week post-infusion, both patients were discharged on days 8 and 2 respectively. The two patients were followed up by phone showing good clinical recovery. Patients #5 and #8 were discharged from the hospital on days 7 and 4 respectively. Patient #5 had a good clinical recovery and patient #8 was at home with no symptoms. (Fig. 4). Patient #10 did not show lymphocyte recovery two weeks after hospital admission. Two weeks after infusion, a recovery in the CD3+, CD4+, and CD8+ cells were observed for patients #1, #2, #3, #4, #6, #7, and #9. The data for patients #5 and #8 was not recorded after the first week for the reasons mentioned above. Patients #5 and #6 showed an increase in the percentage of CD8+RA+ T cells one week after infusion. (Supplemental Table 4). The number of NK cells increased after two weeks in patients #1, #2, #3, #4, and #7. Table 2 shows a recovery one- or two-weeks post-infusion in the different immune subsets, total lymphocytes, CD3+, CD4+, CD8+, CD19+−, and NKT.
Donor microchimerism was followed for four weeks after infusion of CD45RA− memory T cells. As is shown in Table 4, microchimerism was detected in all patients for at least three weeks after infusion.
Table 4.
Donor chimerism after infusion of CD45RA- memory T cells from the convalescent donor. The indicated chimerism values were obtained as the mean value of the analysis of two different INDEL systems. Pre-infusion patient samples were analysed, and results were employed as negative background control. The data are shown by weeks after infusion.
| Dose cohort |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Variables | 1 × 105 cells/kg |
5 × 105 cells/kg |
1 × 106 cells/kg |
||||||
| Donor Chimerism (%) | #1 | #2 | #3 | #4 | #5 | #6 | #7 | #8 | #9 |
| D0-D7 | 0.011 | 1.445 | 0.405 | 0.065 | 0.023 | 0.192 | 0.989 | 0.450 | <0.01 |
| D7-D14 | <0.01 | 0.310 | <0.01 | 0.097 | 0.185 | 0.086 | 3.090 | 0.163 | 0 |
| D14–21 | 0.084 | 0.163 | <0.01 | 0.135 | <0.01 | <0.01 | <0.01 | <0.01 | 0.2 |
| D21–28 | N/D | N/D | N/D | N/D | N/D | N/D | N/D | N/D | 0.011 |
4. Discussion
In this study, we show the outcomes of nine hospitalised patients suffering from COVID-19 pneumonia and/or lymphopenia requiring supplemental oxygen after treatment with a single dose of an “off-the-shelf” partially matched allogeneic memory T lymphocyte infusion containing SARS-CoV-2 specific T lymphocytes from a COVID-19 convalescent donor. This study is a phase I clinical trial focused on dose and schedule determination, and patient safety, with a conventional 3 + 3 design [21]. The product development process is feasible, cost-effective, and easy to develop in centres with experience in HSCT [22]. None of these COVID-19 patients developed any serious side effects after treatment. Adoptive T cell therapy displays almost no toxicity, as shown, and the cell product can be stored in a lymphocyte biobank to provide off-the-shelf access for subsequent viral pandemics [10]. As we had just one donor for the study, the rationale for the donor-recipient histocompatibility scheme was done based on first, most of HLA sharing between them, second, HLA class I share or, third, HLA class II sharing. Four of nine of our pairs share at least one HLA class I and 4/9 HLA class I and HLA class II. Only 1/9 pairs share only HLA class II. We include donor-recipient sharing in HLA class I but also class II because the optimal antigen-presenting cell/effector cell HLA mediated presentation is still unknown and most of the CD45RA− T cells are CD4+ T cells [16].
All participants had sustained recovery after 28 days of follow-up, and no allogeneic toxic effect has been reported using memory (CD45RA−) lymphocytes. This is probably because alloreactivity resides mainly within the naïve (CD45RA+) T-cell compartment [12].
CRS is a common side effect of T-cell transplantation; however, we do not observe any indication of CRS after CD45RA− T cell infusion. Baseline data on C-reactive protein, serum ferritin or d-dimer in our group was similar to the large group (2226 patients) reported by our hospital [23], but in contrast, in our group C-reactive protein decreases from day cero to 14 days post-infusion, none of our patients needed to be transferred to ICU and mortality was null. It would be tempting to speculate that the antiviral effect of memory T cells diminishes the SARS-CoV-2 induced cytokine release storm. However, the scarcity of data and the limited knowledge on the pathogenesis of the COVID-19 CRS response prevent us from any conclusion. Nevertheless, previous reports with allogeneic haematopoietic stem cell transplantation (HSCT) shows that grafts containing CD45RA− T cells rise 2-year overall survival up to 78% (95% CI 59–89%), in allogeneic adult myeloablative HSCT with a grade II–IV aGVHD (66%; 95% CI 41–74%) [24]. Probably the most similar scenario to our adoptive cell therapy for COVID-19 patients is the use of CD45RA− donor lymphocyte infusion (DLI) in haploidentical setting to boost immune recovery controlling virus reactivation and avoiding GvHD [22,25]. Given the appropriate conditions it should be a safe approach even with inflammatory disease and mismatch setting.
We did not observe the general adverse effects associated with the cryopreserved cell product in DMSO such as nausea, vomiting or abdominal cramps [20]. One patient reported a metallic taste for a few hours just after the infusion that we attributed to the cryopreservant and we considered it a mild side effect but not an adverse effect. Many factors that can contribute to the adverse reactions caused by DMSO such as age, weight, gender, even the specific disease of the patient. Also, the procedure, speed of injection and time from thawing of frozen cells to injection can influence adverse effects [20].
Although three patients did not meet the inclusion criteria, we do not consider that cell therapy could be harmful to these patients. We hypothesize that it could be less effective, but it should not interfere with the outcome of safety which is the main aim of this study. These patients presented with a PCR confirmation time longer than in the inclusion criteria of the first version of the protocol or with symptoms longer than 10 days. These inclusion criteria were modified in subsequent versions of the protocol where no more than 3 days of hospitalisation before infusion of cell therapy was eliminated, and the onset of symptoms was replaced from 10 to 12 days.
Despite this study was not addressing treatment efficacy, we have observed an improvement in clinical parameters measured by NEWS and 7-point scale two weeks post-infusion in both scales. Remarkable, patients with medium and high NEWS score at screening presented an improvement in the score to low risk and medium risk, respectively, at day 2 after infusion. Also, the increase in lymphocyte counts from the day of recruitment to one- and two-weeks post-infusion along with the microchimerism data where the patients keep a percentage of allogenic T cell population for at least 3 weeks that is getting lost while total lymphocyte counts is increasing, and the decreased in the pro-inflammatory parameter C-reactive protein from the day of recruitment untilday 6 post-infusion could reflect the improvement related to the recovery of the immune-inflammatory parameters. As mentioned, a previous study from our institution, with a longer cohort with similar patient´s characteristics, reported how high C- reactive protein and low lymphocyte count were related to a high probability of death [23]. In our cohort, these parameters improved after infusion. Unfortunately, we cannot compare with the previous historical cohort, so we cannot assume any improvement related to this cell therapy approach. We will be able to make a statement after the results of phase II where high dose CD45RA−memory T cell infusion plus the standard of care (SoC) will be compared with SoC for COVID-19 pneumonia requiring supplemental oxygen. For the time needed for a negative SARS-CoV-2 result by PCR, our data shows values similar to those already published [26,27]. PCR was measured in the oropharyngeal mucosa. More studies are needed to better determine whether viral load decrease after memory T cell intravenous infusion.
Several reasons led us to believe that memory T cells from COVID-19 convalescents may be effective in patients with moderate or severe COVID-19. First, adoptive infusion of memory T lymphocytes has been previously reported to successfully improve pathogen-specific immune response after HSCT [11], [12], [13], [14], [15]. Second, adoptive transfer of memory T lymphocytes (which are predominantly CD4+ T cells) could accelerate immune cell recovery, which could be helpful during virus-induced lymphopenia [1]. In addition, donor chimerism was detected in patients three weeks after infusion.
To our knowledge, this is the first report of human passive adoptive cell therapy using allogeneic, off-the-shelf CD45RA− memory T cells obtained from a convalescent COVID-19 donor. There is increasing evidence that T cells play a major role in the amelioration of COVID-19 [28], [29], [30]. Phase II clinical trial is ongoing to evaluate clinical outcomes and eventually confirm the benefit of the investigated treatment, upon a match between donor and patient.
Declaration of Competing Interest
AP-M filed patent EP20382850 on Memory T cells as adoptive cell therapy for viral diseases.
CF filed patent EP20382850 on Memory T cells as adoptive cell therapy for viral diseases.
BS received salary from University Miguel Hernandez, a grant from Agencia Valenciana de Innovacion, support from Al-Andalus Biopharma and medical writing from Comunidad de Madrid, since the initial planning of the work. He received grant AVI-GVA CO19–068 and consulting fees from Gilead-Weber. BS is on the board at Institut de Bioengineria de Catalunya and Laminar Pharmaceuticals. BS filed patent EP20382850 on Memory T cells as adoptive cell therapy for viral diseases. BS reports an unpaid role with FAID. BS reports the receipt of equipment, materials, drugs, medical writing, gifts or other services from Al-Andalus BioPharmaSL.
CS received fees from MSD, Pfizer and Gilead not related to this topic, and reports payments made by Gilead, MSD, Janssen and Therakos to suppliers for support for attending meetings and/or travel.
IG reports a “Rio Hortega” Grant (Plan Estatal de I + D + I 2013–2016. Plan Estatal de Investigación Científica y Técnica y de Innovación (2017–2020). Co-funded by European Regional Development Fund/European Social Fund "A way to make Europe"/"Investing in your future").
All other authors have nothing to declare.
Acknowledgments
Author contributions
The authors’ contributions were as follow:
Methodology: AP-M, AMB, AJC, PG-G, IG
Investigation: AP-M, CF, BP-M, CM-D, JLV, AB, ES-Z
Participants’ enrolment: MM-R, JRA, RMB, RM,
Cell preparations and administration: MG, RDP, AM,
Data Collection: AP-M, MM-R and CF
Management of clinical data and interpretation: AP-M, MM-R, PG-G, BS
Wrote the original draft figures and tables: AP-M, MM-R, CF, BS
HLA studies: JLV, AB, MAM
Conceptualization and supervision: AP-M, BS
Monitorization: JQ-P, PG-G
Data analysis and project administration: JQ-P, PG-G, AMB
All authors critically edited and reviewed the manuscript. All authors had full access to the data in the study and accept responsibility to submit for publication. The underlying data has been verified by AP-M, JQ-P and PG-G
Data sharing statement
All relevant data are included in this manuscript. The data that support the findings of this study are available from the corresponding authors upon reasonable request.
Acknowledgements
We are grateful to the convalescent donor of the study. We also thank the Medical Writing services provided by Geistek Pharma, particularly the writers, Marina Ochando, Sara Aljama, and Victoria Menéndez.
Funding
This Clinical Trial has been supported by Plataforma Española de Investigación Clínica y Ensayos Clínicos, SCReN (Spanish Clinical Research Network), funded by ISCIII-Subdirección General de Evaluación y Fomento de la Investigación, research project PT17/0017/0013. Plan Estatal de I + D + I 2013–2016. Plan Estatal de Investigación Científica y Técnica y de Innovación (2017–2020). Co-funded by European Regional Development Fund/European Social Fund "A way to make Europe"/"Investing in your future". It has also been funded by CRIS CANCER Foundation Grant to AP-M and Agencia Valenciana de Innovación Grant AVI-GVA COVID-19-68 to BS.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.eclinm.2021.101086.
Contributor Information
A. Pérez-Martínez, Email: bernat.soria@umh.es.
B. Soria, Email: bernat.soria@umh.es.
Appendix. Supplementary materials
References
- 1.Tan L., Wang Q., Zhang D. Lymphopenia predicts disease severity of COVID-19: a descriptive and predictive study. Signal Transduct Target Ther. 2020;5:33. doi: 10.1038/s41392-020-0148-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beigel J.H., Tomashek K.M., Dodd L.E. Remdesivir for the treatment of Covid-19 — final report. N Engl J Med. 2020;383:1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cao B., Wang Y., Wen D. A trial of lopinavir–ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382:1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simonovich V.A., Burgos Pratx L.D., Scibona P. A randomized trial of convalescent plasma in Covid-19 severe pneumonia. N Engl J Med. 2021 Feb 18;384(7):619–629. doi: 10.1056/NEJMoa2031304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.The WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group Association between administration of systemic corticosteroids and mortality among critically Ill patients with COVID-19: a meta-analysis. JAMA. 2020;324:1330–1341. doi: 10.1001/jama.2020.17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tay M.Z., Poh C.M., Rénia L., MacAry P.A., Ng L.F.P. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol. 2020;20:363–374. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sánchez-Guijo F., García-Arranz M., López-Parra M. Adipose-derived mesenchymal stromal cells for the treatment of patients with severe SARS-CoV-2 pneumonia requiring mechanical ventilation. A proof of concept study. EClinicalMedicine. 2020 Aug;25 doi: 10.1016/j.eclinm.2020.100454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization . World Health Organization and R&D Blueprint strategy for COVID-19; 2021. R&D blueprint and COVID-19.https://www.who.int/teams/blueprint/covid-19 [Google Scholar]
- 9.Leung W., Soh T.G., Linn Y.C. Rapid production of clinical-grade SARS-CoV-2 specific T cells. Adv Cell Gene Ther. 2020;3:e101. doi: 10.1002/acg2.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferreras C., Pascual-Miguel B., Mestre-Durán C. SARS-CoV-2 specific memory T lymphocytes from COVID-19 convalescent donors: identification, biobanking and large-scale production for Adoptive Cell Therapy. Front Cell Dev Biol. 25 February 2021 doi: 10.3389/fcell.2021.620730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bleakley M., Heimfeld S., Jones L.A. Engineering human peripheral blood stem cell grafts that are depleted of naïve T cells and retain functional pathogen-specific memory T cells. Biol Blood Marrow Transplant. 2014;20:705–716. doi: 10.1016/j.bbmt.2014.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Teschner D., Distler E., Wehler D. Depletion of naive T cells using clinical grade magnetic CD45RA beads: a new approach for GVHD prophylaxis. Bone Marrow Transplant. 2014;49:138–144. doi: 10.1038/bmt.2013.114. [DOI] [PubMed] [Google Scholar]
- 13.Bleakley M., Heimfeld S., Loeb K.R. Outcomes of acute leukemia patients transplanted with naive T cell–depleted stem cell grafts. J Clin Invest. 2015;125:2677–2689. doi: 10.1172/JCI81229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Triplett B.M., Shook D.R., Eldridge P. Rapid memory T-cell reconstitution recapitulating CD45RA-depleted haploidentical transplant graft content in patients with hematologic malignancies. Bone Marrow Transplant. 2015;50:968–977. doi: 10.1038/bmt.2014.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Triplett B.M., Muller B., Kang G. Selective T-cell depletion targeting CD45RA reduces viremia and enhances early T-cell recovery compared with CD3-targeted T-cell depletion. Transpl Infect Dis. 2018;20:e12823. doi: 10.1111/tid.12823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fernández L., Fernández A., Mirones I. GMP-compliant manufacturing of NKG2D CAR memory T cells using CliniMACS prodigy. Front Immunol. 2019 Oct 10;10:2361. doi: 10.3389/fimmu.2019.02361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McGinley A., Pearse R.M. A national early warning score for acutely ill patients. BMJ. 2012 Aug 8;345:e5310. doi: 10.1136/bmj.e5310. [DOI] [PubMed] [Google Scholar]
- 18.Churpek M.M., Snyder A., Han X., Sokol S., Pettit N., Howell M.D., Edelson D.P. Quick sepsis-related organ failure assessment, systemic inflammatory response syndrome, and early warning scores for detecting clinical deterioration in infected patients outside the intensive care unit. Am J Respir Crit Care Med. 2017 Apr 1;195(7):906–911. doi: 10.1164/rccm.201604-0854OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cavalcanti A.B., Zampieri F.G., Rosa R.G. Coalition Covid-19 Brazil I investigators. Hydroxychloroquine with or without azithromycin in mild-to-moderate Covid-19. N Engl J Med. 2020 Nov 19;383(21):2041–2052. doi: 10.1056/NEJMoa2019014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shu Z., Heimfeld S., Gao D. Hematopoietic SCT with cryopreserved grafts: adverse reactions after transplantation and cryoprotectant removal before infusion. Bone Marrow Transplant. 2014;49:469–476. doi: 10.1038/bmt.2013.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ivy S.P., Siu L.L., Garrett-Mayer E., Rubinstein L. Approaches to phase 1 clinical trial design focused on safety, efficiency, and selected patient populations: a report from the clinical trial design task force of the national cancer institute investigational drug steering committee. Clin Cancer Res. 2010 Mar 15;16(6):1726–1736. doi: 10.1158/1078-0432.CCR-09-1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maschan M., Blagov S., Shelikhova L. Low-dose donor memory T-cell infusion after TCR alpha/beta depleted unrelated and haploidentical transplantation: results of a pilot trial. Bone Marrow Transplant. 2018;53:264–273. doi: 10.1038/s41409-017-0035-y. [DOI] [PubMed] [Google Scholar]
- 23.Borobia A.M., Carcas A.J., Arnalich F. On behalf of the Covid Hulp working group. A cohort of patients with COVID-19 in a major teaching hospital in Europe. J Clin Med. 2020 Jun 4;9(6):1733. doi: 10.3390/jcm9061733. PMID: 32512688; PMCID: PMC7356883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bleakley M., Heimfeld S., Loeb K.R. Outcomes of acute leukemia patients transplanted with naive T cell-depleted stem cell grafts. J Clin Invest. 2015 Jul 1;125(7):2677–2689. doi: 10.1172/JCI81229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blagov S., Zvyagin I.V., Shelikhova L. T-cell tracking, safety, and effect of low-dose donor memory T-cell infusions after αβ T cell-depleted hematopoietic stem cell transplantation. Bone Marrow Transplant. 2021;56:900–908. doi: 10.1038/s41409-020-01128-2. [DOI] [PubMed] [Google Scholar]
- 26.Wang J., Yang W., Chen P. The proportion and effect of corticosteroid therapy in patients with COVID-19 infection: a systematic review and meta-analysis. PLoS One. 2021 Apr 21;16(4) doi: 10.1371/journal.pone.0249481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zha L., Li S., Pan L. Corticosteroid treatment of patients with coronavirus disease 2019 (COVID-19) Med J Aust. 2020 May;212(9):416–420. doi: 10.5694/mja2.50577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sekine T., Perez-Potti A., Rivera-Ballesteros O. Robust T cell immunity in convalescent individuals with asymptomatic or mild COVID-19. Cell. 2020 Oct 1;183(1):158–168. doi: 10.1016/j.cell.2020.08.017. e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peng Y., Mentzer A.J., Liu G. Broad and strong memory CD4+ and CD8+ T cells induced by SARS-CoV-2 in UK convalescent individuals following COVID-19. Nat Immunol. 2020;21:1336–1345. doi: 10.1038/s41590-020-0782-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cañete P.F., Vinuesa C.G. COVID-19 makes B cells forget, but T cells remember. Cell. 2020;183:13–15. doi: 10.1016/j.cell.2020.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.